Neovascularization
Neovascularization means “new blood vessels”.
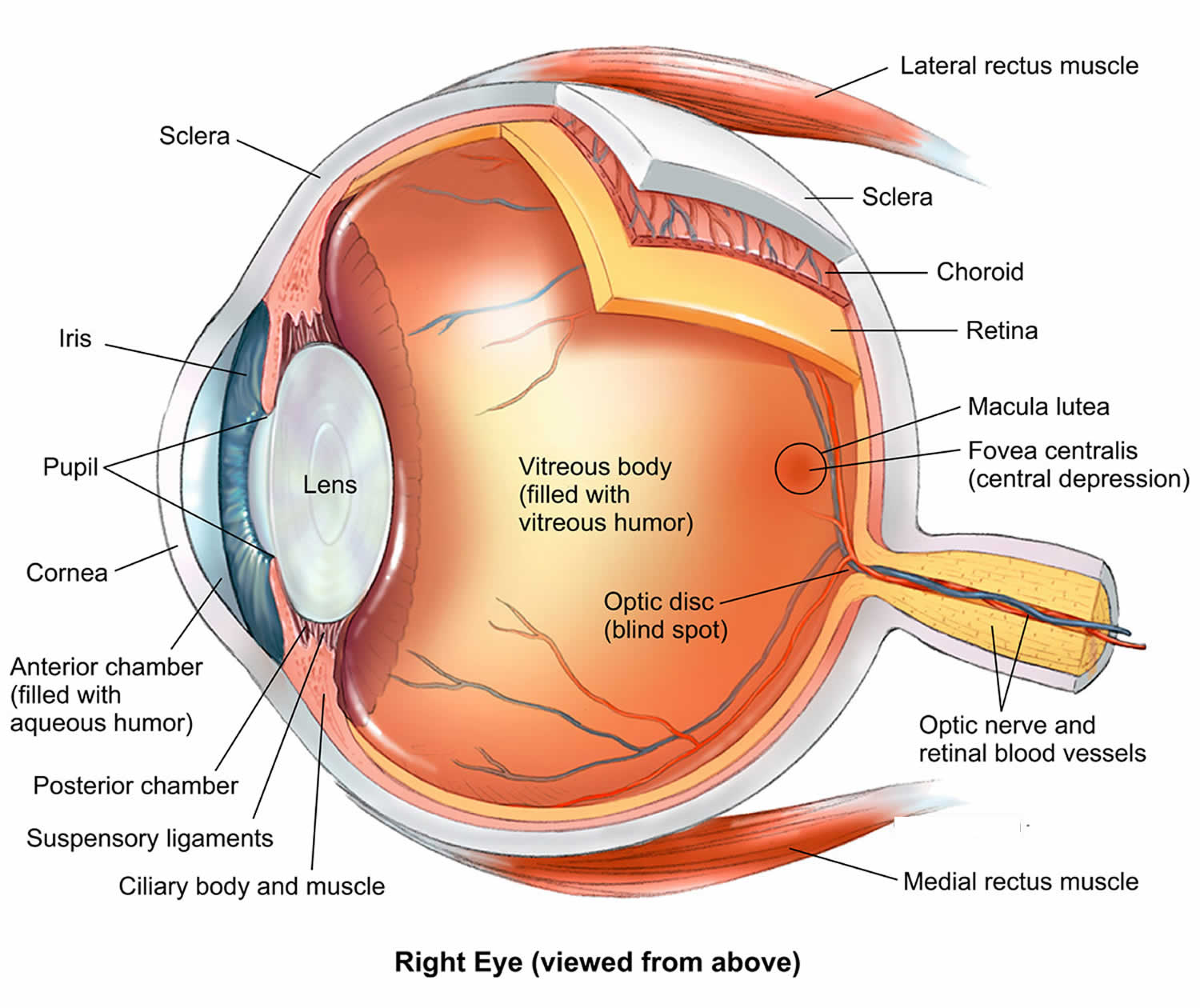
Anatomy of the eye
Your eye is connected to the brain and dependent upon the brain to interpret what you see. How you see depends upon the transfer of light. Light passes through the front of the eye (cornea) to the lens. The cornea and the lens help to focus the light rays onto the back of the eye (retina). The cells in the retina absorb and convert the light to electrochemical impulses which are transferred along the optic nerve and then to the brain.
The eye works much the same as a camera. The shutter of a camera can close or open depending upon the amount of light needed to expose the film in the back of the camera. The eye, like the camera shutter, operates in the same way. The iris and the pupil control how much light to let into the back of the eye. When it is very dark, your pupils are very large, letting in more light. The lens of a camera is able to focus on objects far away and up close with the help of mirrors and other mechanical devices. The lens of the eye helps you to focus but sometimes needs some additional help in order to focus clearly. Glasses, contact lenses, and artificial lenses all help you to see more clearly.
- Cornea: The clear front window of the eye which transmits and focuses (i.e., sharpness or clarity) light into the eye. Corrective laser surgery reshapes the cornea, changing the focus.
- Pupil: The dark center opening in the middle of the iris. The pupil changes size to adjust for the amount of light available (smaller for bright light and larger for low light). This opening and closing of light into the eye is much like the aperture in most 35 mm cameras which lets in more or less light depending upon the conditions.
- Iris: The colored part of the eye which helps regulate the amount of light entering the eye. When there is bright light, the iris closes the pupil to let in less light. And when there is low light, the iris opens up the pupil to let in more light.
- Sclera: The white outer coat of the eye, surrounding the iris.
- Ciliary Body: Structure containing muscle and is located behind the iris, which focuses the lens.
- Lens: Focuses light rays onto the retina. The lens is transparent, and can be replaced if necessary. Our lens deteriorates as we age, resulting in the need for reading glasses. Intraocular lenses are used to replace lenses clouded by cataracts.
- Choroid: Layer containing blood vessels that lines the back of the eye and is located between the retina (the inner light-sensitive layer) and the sclera (the outer white eye wall).
- Vitreous Humor: The, clear, gelatinous substance filling the central cavity of the eye.
- Retina: The nerve layer lining the back of the eye. The retina senses light and creates electrical impulses that are sent through the optic nerve to the brain.
- Macula: The area in the retina that contains special light-sensitive cells. In the macula these light-sensitive cells allow us to see fine details clearly in the center of our visual field. The deterioration of the macula is a common condition as we get older (age related macular degeneration or ARMD).
- Fovea: The center of the macula which provides the sharp vision.
- Optic Nerve (optic disc): A bundle of more than a million nerve fibers carrying visual messages from the retina to the brain. (In order to see, we must have light and our eyes must be connected to the brain.) Your brain actually controls what you see, since it combines images. The retina sees images upside down but the brain turns images right side up. This reversal of the images that we see is much like a mirror in a camera. Glaucoma is one of the most common eye conditions related to optic nerve damage.
Figure 1. Anatomy of the eye
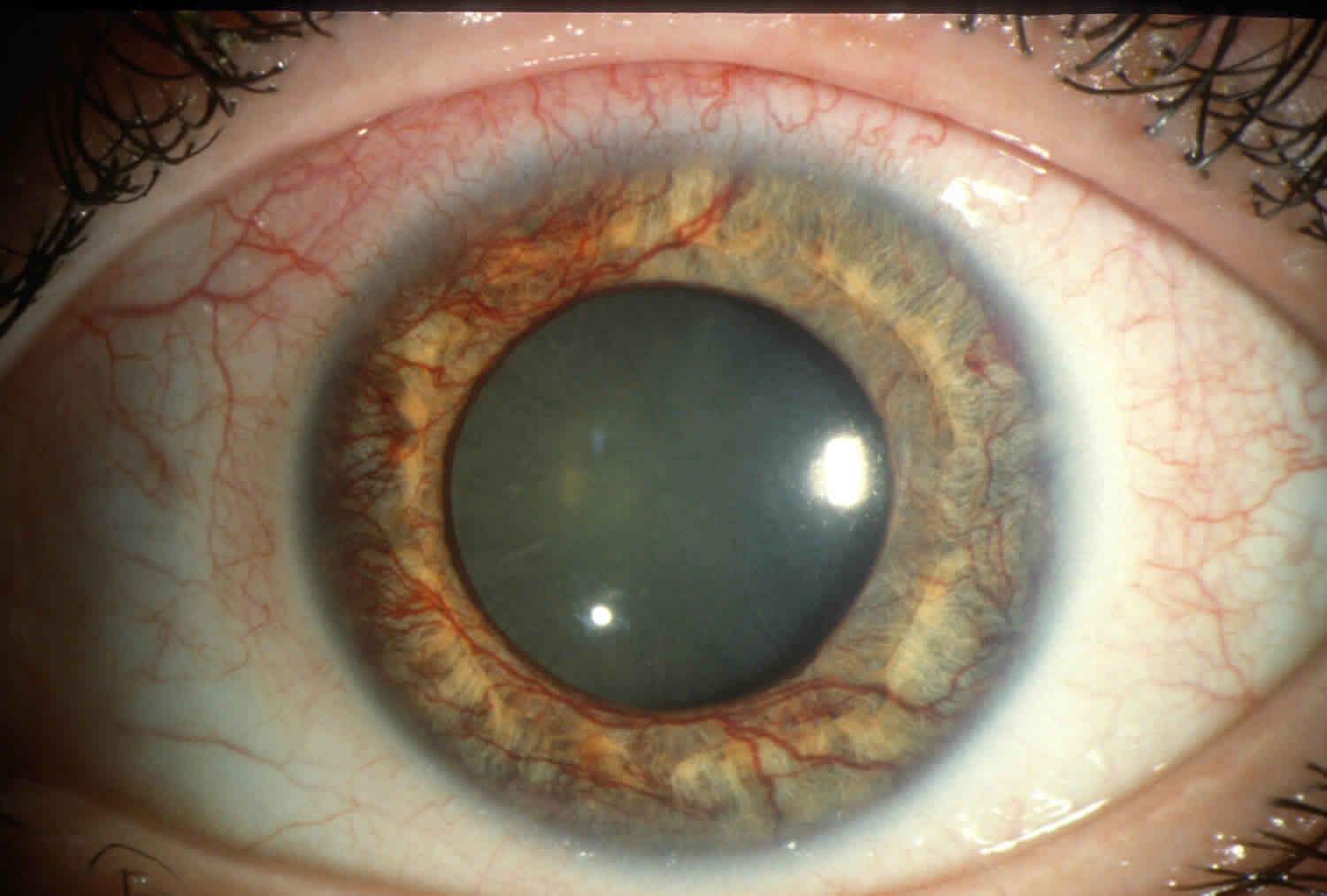
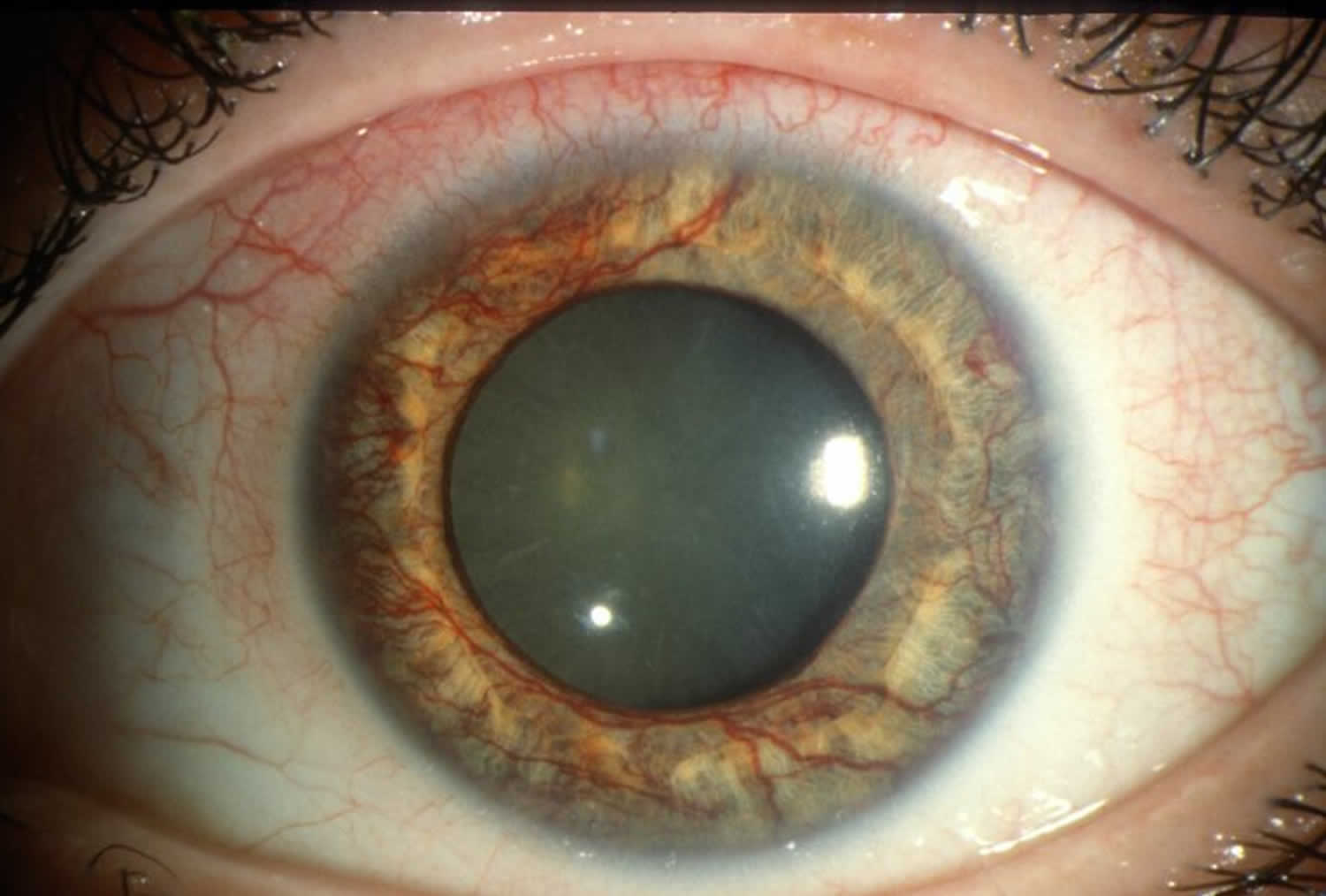
Corneal neovascularization
Corneal neovascularization is characterized by the invasion of new blood vessels into the cornea caused by an imbalance between angiogenic and antiangiogenic factors that preserve corneal transparency as a result of various ocular insults and hypoxic injuries 1. Risk factors that have been implicated in the pathogenesis of corneal neovascularization include contact lens wear, ocular surface disease, trauma, previous surgery and herpes 2. Left untreated, corneal neovascularization can lead to tissue scarring, edema, lipid deposition, and persistent inflammation that may significantly affect visual prognosis and quality of life.
The cornea is unique because it is completely avascular and alymphatic, which is essential for its clarity and optimal vision in healthy individuals; however, under specific pathological circumstances, new capillaries can grow within the cornea. There are three categories of neovascularization based on severity:
- superficial neovascularization,
- vascular pannus,
- deep stromal vascularisation.
It has been proposed that the term “corneal vascularization” is appropriate in contrast to “corneal neovascularization”, as the latter refers to a condition in which new blood vessels arise from pre-existing ones 3. To avoid confusion with choroidal neovascularization and due to the absence of pre-existing blood vessels in the cornea, the term “corneal vascularization” should be used for corneal vessel formation 4. However, in reviewing the literature, majority of studies term this pathology “corneal neovascularization” 3.
Corneal neovascularization is a sight-threatening condition and a growing public health concern. One study reported the estimated incidence rate of 1.4 million people per year, 12% of whom suffered subsequent loss of vision 5. Moreover, 20% of corneal specimens taken from corneal transplant procedures have shown evidence of corneal neovascularization 6.
Figure 2. Corneal neovascularization
Corneal neovascularization causes
It is known that a number of diseases and conditions can lead to the development of corneal neovascularization. Corneal neovascularization is caused by a wide variety of ocular insults, including infection, inflammation, ischemia, degeneration, trauma, and loss of the limbal stem cell barrier. Corneal pathologies that can lead to neovascularization include lipid keratopathy, corneal ulcers and scars, herpes eye disease, infectious keratitis, chemical burns, graft rejections and hypoxic insults from contact lens wear 6. The most common causes highlighted have been the wearing of contact lenses, inflammation of the eyelid, trauma, previous surgery and herpes 7.
Vasculogenesis comprises the de novo formation of vessels from vascular endothelial precursor cells (i.e. hemangioblasts and angioblasts) which are derived from mesodermal precursors (via mesodermal induction).9 In contrast, angiogenesis is a process in which endothelial cells of pre-existing vessels proliferate and form new vessels 8. In corneal neovascularization, the endothelial cells of newly formed corneal vessels originate from pre-existing limbal vessels (i.e. angiogenesis). However, pericytes, another crucial cell type in blood vessel formation, originate from bone-marrow derived precursors (i.e. vasculogenesis) 9. Ozerdem and colleagues 9 believe that both angiogenesis and vasculogenesis are involved in corneal neovascularization and that targeting both mechanisms would be most effective in managing this condition. Similar to blood vessels, lymphatic vessels may arise de novo from bone-marrow derived cells (i.e. CD11b-positive macrophages) or they may extend from pre-existing limbal lymphatic vessels 10.
The mechanisms of corneal neovascularization are observed in significant detail in animal models. It has been hypothesized from these models that corneal neovascularization commences as a result of insult or injury. When the cornea is damaged, the epithelial defects are normally healed by the corneal and limbal epithelium. The corneal limbus is located at the corneoscleral junction. The limbal epithelium is rich in stem cells with the capacity to differentiate from normal corneal epithelium. However, defects can occur, leading to these cells undergoing apoptosis and repaired abnormally by the conjunctival epithelium 11. The problem arises as the conjunctival epithelium is rich in goblet cells and highly vascularized. Consequently, the resulting phenotype is optically inferior and leads to the deterioration of vision 12. Furthermore, the process also leads to an irregular optical surface, weakened tensile strength, and incompetent barrier function.
Research suggests that IL-8 may also contribute to the manifestation of corneal neovascularization 13. Strieter et al. 14 demonstrated the relationship to be dose dependent. High doses of 400 ng per cornea did not give rise to neovascularization, whereas doses in the range of 2-40ng per cornea resulted in neovascularization. Furthermore, the study interestingly found regression of vascularity after 14 days, which suggested that IL-8 angiogenesis underwent dynamic modulation as it was observed in normal wound healing, suggesting a dynamic relationship between inflammation and wound healing.
Herpes stromal keratitis can lead to the development of corneal neovascularization. Herpes stromal keratitis is classed as an immune mediated disease and due to the eye being immune privileged, has been considered a target tissue for herpes stromal keratitis. It is believed that vascular endothelial growth factor (VEGF) has a significant role in the development of corneal neovascularization as a result of herpes stromal keratitis. It has been suggested that the presence of herpes stromal keratitis leads to inhibition of VEGF receptor (sVEGFR-1) synthesis at a higher rate compared to vascular endothelial growth factor (VEGF) resulting in a ratio imbalance between sVEGFR-1 and VEGF and therefore, the release of VEGF is accelerated to consequently cause angiogenesis 15. Another source of VEGF is infected cells stimulating the production of VEGF as a result of IL-6 expression 16. A similar relationship has been observed in response to infected cells expressing IL-7, which also stimulates nearby cells to release VEGF 17. The excessive release of VEGF leads to the development of fragile blood vessels in the cornea.
Corneal neovascularization can have a significant negative impact on vision. The physical presence of the vessels blocking and diffracting light being the main mechanism of impact, with further influence from the deposition of lipids and proteins on the corneal stromal as well as damage to the structural integrity of the cornea.
The hypothesized pathophysiology is extrapolated from animal studies therefore leaving some uncertainty as to whether the relationships described can be transferred to a human model.
Corneal neovascularization diagnosis
The cornea can be easily assessed in the clinical setting for examination. Slit lamp biomicroscopy can be used to determine changes to the cornea including topographical ones. Slit lamp aids are also particularly useful in determining the thickness of the cornea, which can also provide evidence of endothelial cell function. Diffuse illumination can be used to assess the cornea in terms of gross alterations, whereas indirect and retro-illumination can be used to detect lesions such as neovascularization. Neovascularization can occur very rapidly, and may be challenging to detect in early stages.
Corneal neovascularization treatment
The treatment of corneal neovascularization is currently problematic. Corneal transplantation is at present the only successful universal treatment for this disease process. However, there are various treatment procedures that have an effect, such as topical treatments, injections and laser/ phototherapy. One therapeutic aim of these treatments is to initiate antiangiogenesis and stop the neoangiogenesis at early stages, whereas the other treatment modality aims to achieve angioregression by inducing reversion of immature vessels.
Corneal transplantation
Meta-analysis on 24,000 corneal grafts revealed that rejection of transplanted corneas is higher in patients with neovascularization. The analysis estimates that “presence of corneal neovascularization before surgery is 30% more likely that the transplant will fail, and more than doubles the risk of graft rejection”, in other words, the greater the neovascularization the higher risk of rejection 18. Therefore, preparing and conditioning the vascularized cornea before transplantation is a hopeful potential therapeutic development.
Laser or Phototherapy
Argon laser therapy for corneal neovascularization is the use of an argon laser beam, which passes through a clear cornea, but, when there are many vessels present, the haemoglobin (within the blood) absorbs the argon energy allowing corneal vessels to coagulate, which causes reversal of the corneal neovascularization 19. Studies have shown its efficacy in regression of corneal neovascularization 20. Photodynamic therapy involves a photosensitizing compound, light and oxygen. The compound is absorbed by the neovascular tissue and is activated through laser treatment, which causes free radicals to be released thus destroying the surrounding neovascular tissue and reversing corneal neovascularization 21. It has been shown that photodynamic therapy is safe and has a high efficacy within humans; however, it is a very costly method of treatment as well as time consuming 21.
Both laser and phototherapy need further study to determine their efficacy when compared to other therapeutic strategies. Currently, safety concerns associated with laser therapy and the cost and time of phototherapy have been the negative issues coupled with this innovative treatment, resulting in the relatively low uptake in clinical practice. However, a recent study by Gerten et al. has shown that the combination therapy of bevacizumab with argon laser-therapy causes a marked decrease in corneal neovascularization, this being because the argon laser-induced coagulation closes the mature pathological blood vessels whilst the bevacizumab prevents new angiogenesis 22. Therefore, the hope is that these therapies will be introduced as an adjunct and usage will increase.
Injections
As described previously, treatment can be administered in many ways, also including the administration of steroids and anti-VEGF agents through subconjunctival injections with similar efficacy to topical treatment. Petsogulu C et al. 23 carried out a randomized control trial looking at the outcomes of subconjunctival bevacizumab in 30 eyes of 30 patients with corneal neovascularization. 15 eyes randomized to receive 2.5mg/ 0.1ml subconjunctival injections and 15 eyes randomized to 0.9% saline. A standard therapy of preservative-free dexamethasone 0.1% drops four times a day was prescribed for all patients at baseline.
The authors demonstrated a reduction in the mean area of corneal neovascularization by 36% in the 15 eyes that received bevacizumab compared with an increase of 90% in eyes that received saline placebo 23. After exclusion of one outlier with an exaggerated response, the placebo arm treated with topical dexamethasone 0.1% over 3 months showed only a 3% decrease in corneal neovascularization.
Moreover, this method of treatment also allows the incorporation of gene therapy strategies. Gene therapy involves transferring therapeutic genes to the cornea through different vectors. There are safety concerns regarding viral vectors (adenoviruses, retroviruses or lentiviruses) but they are the most efficient in infecting the corneal epithelial cells with infection rates of 80-100%, allowing higher gene transfer rates compared to non-viral vectors 24. The safety concerns include the potential of replication-deficient viral vectors such as adenoviruses and retroviruses to become replication-competent and pathogenic again. Furthermore, retroviral vectors randomly integrate their genome into host cells, which can lead to insertional mutagenesis to occur 25. Gene therapies that influence angiogenic factors like VEGF have been investigated, for example Lai and colleagues transduced corneal epithelial cells with an adenovirus vector containing the VEGFR-1 gene in a rodent model and found that it successfully inhibited corneal neovascularization 24. Gene therapy can also occur through intrasomal or subconjunctival injections or via electroporation and gene gun 26. However, the use of viral vectors has the highest efficiency in transduction of genes 27. Furthermore, when the adenovirus vector containing VEGFR-1 was subconjunctivally injected in a rat model of corneal neovascularization there was inhibition of the corneal neovascularization 28. Likewise, when an adeno-associated viral vector containing the gene for human angiostatin (protein-angiogenesis inhibitor) was subconjunctivally injected in a rat model, the rats showing a significant decrease in corneal neovascularization 29. Although gene therapy has shown promise in effectiveness there are still technical and safety issues which have to be overcome first 30.
Topical treatments
Steroids and anti-VEGF agents are currently the mainstay initial treatment for corneal neovascularization 30. Topical steroids such as cortisone, dexamethasone and prednisolone have all been shown to have an antiangiogenic effect and hence inhibit corneal neovascularization 31. However, there are studies suggesting that steroids do not inhibit the development of corneal vascularisation 32. This was however demonstrated in response to corneal neovascularization post chemical injury, with recent research suggesting positive outcomes in other scenarios 33. Klintworth has shown that steroid use is most effective in suppressing angiogenesis when applied directly after or before corneal injury and if applied any later it has no effect on the development of corneal vascularisation 32. It is thought that steroids work by inhibiting cell chemotaxis and by inhibiting pro-inflammatory cytokines like interleukin-1 and -6 34. They also cause lymphocytes to be killed and inhibit vascular dilation, which all amounts to their antiangiogenic effect 34. The use of steroids (such as cortisone) in conjunction with heparin and cyclodextrins causes a greater antiangiogenic effect, this leading to the development of ‘angiostatic steroids’, which are thought to modulate collagen metabolism that can completely disintegrate the basement membrane of the blood vessels 35. Heparin modulates the expression of anti-angiogenic and pro-angiogenic factors 30. However, steroids have a considerable side effect profile with negative associations such as glaucoma and increased infection susceptibility due to their immune suppressive effect.
VEGF has been shown to be crucial in inflammatory corneal neovascularization through the rat experimental model 36. The eye is a site which has ‘angiogenic privilege’ meaning it has a balance of pro-angiogenic and anti-angiogenic factors. Pro-angiogenic factors include VEGF, FGF and PDGF 30. Selectively targeting these angiogenic growth factors is desirable over steroids due to their side effect profile and more selective action. Anti-VEGF drugs work by inhibiting VEGF which prevents new blood vessel formation through down regulation of endothelial cell proliferation. Bevacizumab is a humanized monoclonal antibody which binds to all VEGF isoforms 37.
Another study has shown that bevacizumab does have an immediate inhibitory effect on corneal neovascularization and inflammation, but the effects are very short-lived 38. Lin and colleagues 39 have similarly shown that early treatment with bevacizumab inhibits corneal neovascularization but late treatment does not display these features. This shows that anti-VEGF therapy is not as effective in individuals who have mature blood vessels as they do not rely on pro-angiogenic factors 39. Anti-VEGF treatment is important during active vessel growth which is characterized by the presence of immature blood vessels relying on pro-angiogenic factors for proliferation 40. This is in line with the findings by Lin that anti-VEGF treatment (bevacizumab) is effective when used in early treatment of patients with corneal neovascularization 39. Anti-VEGF treatment can have undesirable effects, including suppression of wound healing, corneal nerve regeneration and can systemically cause hypertension and cardiovascular disease 30. Krizova showed that the use of bevacizumab is effective and very safe in treating active corneal neovascularization whether applied topically or given as a subconjunctival injection 41. However, they also show that bevacizumab does not have the same effect on mature corneal neovascularization and this treatment does not cure the disorder.
New topical therapeutic advancement
It has been shown that the activation of the IRS-1 proteins is vital in angiogenesis and it is overexpressed in corneal neovascularization sites 42. Aganirsen is an antisense oligonucleotide, which inhibits the expression of IRS-1 mRNA, mRNA of interleukin-1beta and the mRNA of VEGF 42. Cursiefen et al. 43 conducted a phase 3 trial and found that topical administration of Aganirsen eye drops massively inhibits corneal neovascularization in patients with keratitis and that the need for future transplantation is not needed. They have also shown that Aganirsen is very safe and well tolerated in individuals 43.
Another novel advance in the treatment of corneal vascularisation is the use of matrix metalloproteinase inhibitors such as use of tetracyclines. Doxycycline is a tetracycline analogue, which has an antibiotic effect but is also a matrix metalloproteinase inhibitor. Matrix metalloproteinase are enzymes that degrade collagen, basement membranes and the extracellular matrix. Doxycycline also inhibits angiogenesis in a non-metalloproteinase-dependent mechanism, and has an anti-inflammatory effect 44. Jovanovic and Nikolic 45 have shown that the use of topical doxycycline on human corneal neovascularization is effective in reducing the neovascularization and effective in the healing process without any side effects. Combination therapy of anti-VEGF agents, steroids and doxycycline have been investigated and have been shown to have higher efficiency in inhibiting corneal neovascularization compared to solitary use 35. The theory behind combination therapy is that this method will target various mechanisms involved in maintaining corneal neovascularization and hence will be much more efficient in inhibiting the disease and its reoccurrence.
New injectable therapeutic advancements
Pillai et al. first described the technique of fine needle diathermy, which involves using a needle to cauterize individual vessels; this method is effective in occluding mature vessels that are not dependent on angiogenic growth factors 46. This technique has been modified through using an electrolysis needle that is much more flexible and precise 47. Trikha et al. carried out a 5 year retrospective study on individuals who underwent fine needle diathermy and found that this treatment is safe and very effective in regressing corneal neovascularization 48. This suggests that this method of treatment can be used in conjunction with anti-VEGF drugs to allow an angioregressive treatment of corneal neovascularization 49.
Gene therapy targeting VEGF has had successful results, for example Lai et al. 25 have shown that an adenovirus vector carrying VEGFR genes caused regression of the corneal neovascularization. Furthermore, when the adenovirus vector was subconjunctivally injected in a rat model, there was inhibition of the corneal neovascularization 28. Likewise, when an adeno-associated viral vector containing the gene for human angiostatin (protein-angiogenesis inhibitor) was subconjunctivally injected in a rat model, the rats showed a significant decrease in corneal neovascularization 29. Although gene therapy has shown promise in effectiveness, there are still current technical concerns.
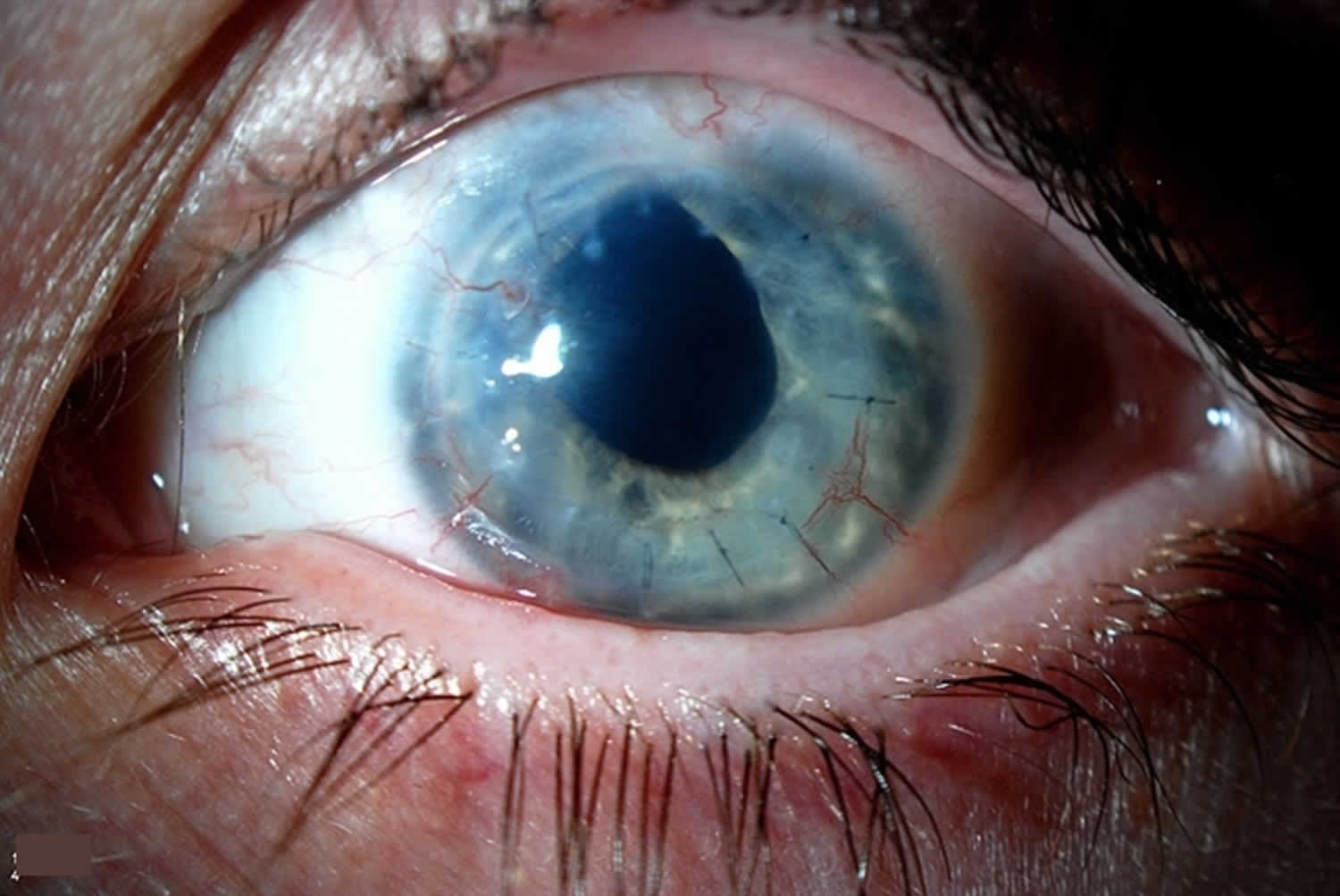
Iris neovascularization
Iris neovascularization also called rubeosis iridis, is defined as blood vessel proliferation along the surface of the iris. There are many causes of iris neovascularization, including most commonly diabetic retinopathy and central retinal venous occlusion 50. Untreated, it can result in neovascular glaucoma, which is often difficult to treat and vision threatening.
Figure 3. Iris neovascularization
Iris neovascularization causes
The pathophysiology of iris neovascularization is complex, involving dysregulation of pro- and anti-angiogenic factors in the setting of posterior segment ischemia. Other causes of iris neovascularization, such as intraocular tumors or anterior chamber ischemia, also result in factor dysregulation 51. Imbalance of vascular endothelial growth factor (VEGF), interleukin-6 (IL6), and pigment epithelium-derived factor (PEDF) results in proliferation of friable capillaries 51. These blood vessels grow along the surface of the iris, first visible at the pupillary margin, and can extend to the iridocorneal angle.
Iris neovascularization symptoms
Iris neovascularization is initially painless and asymptomatic. In early stages, a fine network of capillaries is visible at the pupillary margin on slit lamp examination. Capillaries then extend to the iridocorneal angle, known as neovascularization of the angle, appreciable on gonioscopy. When suspicion for iris neovascularization is high but not seen on slit lamp, iris fluorescein angiography can be performed to further elucidate the presence of iris neovascularization in a patient.
Iris neovascularization complications
Untreated, iris neovascularization can progress to neovascular glaucoma, a form of secondary glaucoma. There are four stages involved in this process: prerubeosis, rubeosis, open angle glaucoma, and lastly closed angle glaucoma 52. The time from iris neovascularization to developing neovascular glaucoma varies. Following CRVO, neovascular glaucoma can develop within 1 to 6 months, while it takes at least 1 year in diabetic patients 53. As iris neovascularization progresses to neovascularization of the angle, the vessels impair aqueous humor outflow, resulting in open angle glaucoma. As fibrotic membranes develop and contract, the iridocorneal angle closes, resulting in closed angle glaucoma. In untreated cases, neovascular glaucoma will cause blindness and pain, often ending in enucleation.
Iris neovascularization treatment
The gold standard treatment of iris neovascularization is pan retinal photocoagulation to reduce neovascularization. Pan retinal photocoagulation can also be used if patients progress to neovascular glaucoma. In recent years, intravitreal bevacizumab, an anti-VEGF agent, has proven to be effective for short term management at reducing neovascularization at the iris and angle and controlling intraocular pressure 53. Once neovascular glaucoma has developed, treatment is often complicated. For example, in later stages of neovascular glaucoma, topical agents that work on aqueous flow tend not to improve intraocular pressure. Surgery, such as trabeculectomy, can be complicated by intraoperative hemorrhage from friable vessels and excessive post-surgical scarring 54.
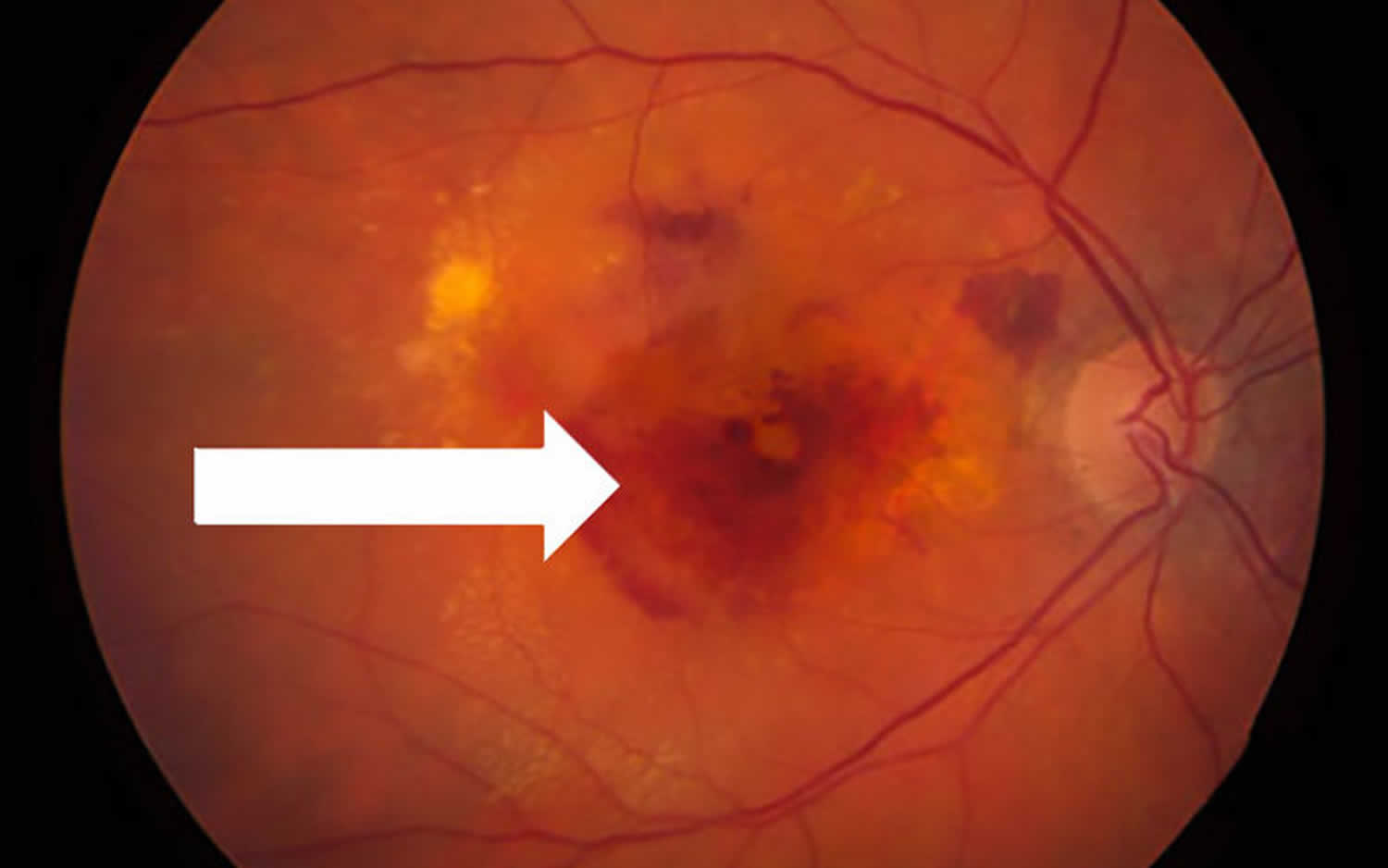
Choroidal neovascularization
Choroidal neovascularization also called wet age-related macular degeneration, is a less common but more serious form of age related macular degeneration (AMD or ARMD). These new, abnormal blood vessels originate in the choroid, a vessel-containing layer under the retina. When the retinas of people with age related macular degeneration produce too much vascular endothelial growth factor (VEGF), new blood vessels sprout from the choroid, then grow into the retina. The new abnormal blood vessels, unlike normal ones, are unusually delicate and leaky and they allow fluid from the blood, and sometimes even red blood cells, to enter the retina. This fluid builds up beneath the retina causing it to bulge or lift up (forms a “blister”) from the back of the eye, which is normally flat, can immediately distort your vision. Over the course of days to months, this fluid can damage the retina, killing the light-sensing cells, called photoreceptors. The eye is damaged as a result, causing central vision to appear blurred, wavy, or distorted.
Choroidal neovascularization or wet age-related macular degeneration can progress rapidly, leading to severe vision problems in the affected eye and causing permanent vision loss. Early diagnosis of coroidal neovascularization is critical if caught. Early treatment options exist which may delay or reduce damage to the eye and decrease the severity of vision loss.
Figure 4. Choroidal neovascularization
Choroidal neovascularization causes
Conditions that may cause choroidal neovascularization:
- Age-related macular degeneration is the most common disease causing choroidal neovascularization, but other diseases that “stress” the retina, causing it to produce excess VEGF, or disrupting the barrier between the retina and choroid, can also cause choroidal neovascularization.
- In patients with pathologic myopia (extreme nearsightedness), the eye is longer than normal, and this lengthening stretches and stresses the retina.
- Ocular histoplasmosis is a fungal infection that can cause choroidal neovascularization.
- Eye trauma and angioid streaks (small breaks in one of the retina’s layers) can break the barrier between the retina and choroid, resulting in choroidal neovascularization.
- Severe ocular inflammation, a condition called uveitis, can also cause choroidal neovascularization.
Choroidal neovascularization symptoms
The symptoms of choroidal neovascularization include a distortion or waviness of central vision or a gray/black/void spot in the central vision. This should prompt a call to an ophthalmologist right away to get a priority emergency visit. The ophthalmologist can halt the growth and leakage of the blood vessels by injecting a anti-VEGF drug, a drug blocking a protein called vascular endothelial growth factor (VEGF) into the eye, but only if they can deliver the drug as soon as possible, within hours or days or so from the time you notice the change in vision. Time lost is vision lost!
Choroidal neovascularization diagnosis
The ophthalmologist can detect choroidal neovascularization using a combination of techniques. First, during the dilated eye exam, she/he may see a blister of fluid or bleeding in the retina. Then, using specialized imaging called optical coherence tomography (OCT), a cross-section picture of the retina is obtained. This image can detect even small amounts of fluid that have leaked into the retina from choroidal neovascularization.
Additional imaging techniques called fluorescein or Indocyanine Green (ICG) angiography involve injection of a dye into a vein somewhere else in your body (where the dye gradually diffuses into the vessels in the back of the eye), followed by retinal imaging that shows the dye leaking from the blood vessels into the retina. These images sometimes provide additional information about the choroidal neovascularization.
Choroidal neovascularization treatment
Most patients with choroidal neovascularization benefit from injection into the eye of anti-VEGF drugs (Lucentis®, Eylea®, or Avastin®), since VEGF promotes the choroidal neovascularization growth and leakage in most cases.
New research is bringing longer lasting anti-VEGF drugs, like brolucizumab, and anti-VEGF gene therapy, which should enable the anti-VEGF effect to last longer, so patients will not need to have injections as frequently.
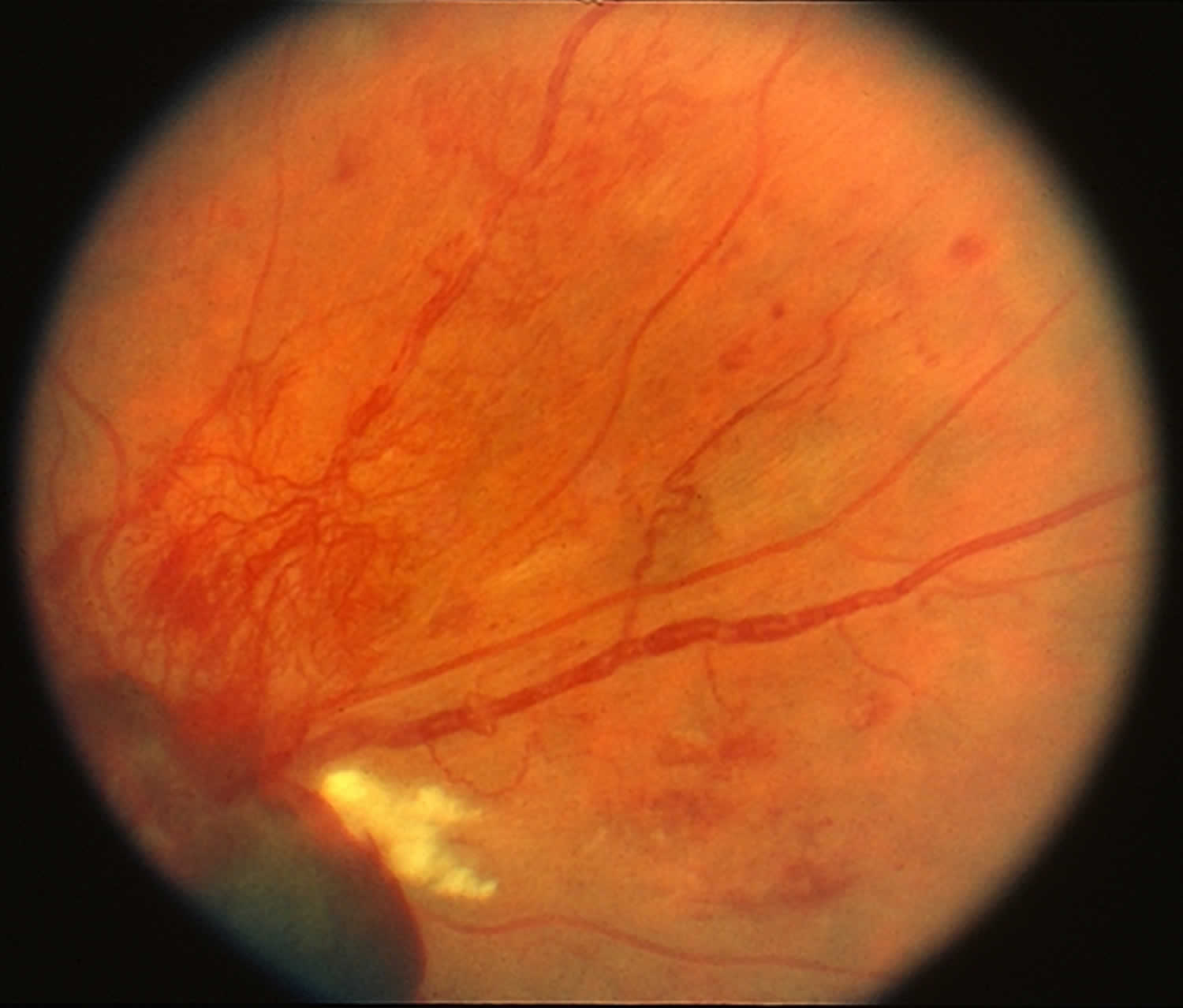
Retinal neovascularization
Retinal neovascularization are growth of tangle new blood vessels on retinal surface as a compensatory mechanism to overcome an earlier phase of microvessel degeneration and reinstate metabolic equilibrium in response to vascular endothelial growth factor (VEGF) liberated by hypoxic retina 55. Retinal neovascularization are divided into those that appear on optic disc surface (new vessels on the optic disc) and on retina (new vessels elsewhere), mostly within view of direct ophthalmoscope. Lacking integrity and bifurcating pattern of normal vessels, these new vessels bleed spontaneously or with minimal trauma. Retinal and vitreous hemorrhages attract fibroglial elements that form fibrovascular stalks. These stalks induce vitreous contraction that tugs on retina until it bleeds and detaches. New blood vessels form net of small curls in places where no blood vessels belong.
Retinal neovascularization is a pathological feature of many retinal diseases, such as retinopathy of prematurity and diabetic retinopathy, and can lead to severe vision loss and even blindness 56. It is known that ischemia or hypoxia contributes to the initiation and development of retinal neovascularization 57. One of the key oxygen homeostasis regulators, hypoxia-inducible factor 1-alpha (HIF-1α), is a transcription factor that regulates a variety of proangiogenic factors, such as VEGF, stromal-derived factor-1 (SDF), stem cell factor (SCF), platelet-derived growth factor B (PDGFB), and placental growth factor (PlGF), and erythropoietin (EPO) 58. It has been reported that increased expression of HIF-1α is sufficient to induce neovascularization 59. Therefore, inhibition of HIF-1α may block several proangiogenic signaling pathways, thus possessing advantages over monotherapy that targets a single proangiogenic factor. Several studies have demonstrated that inhibition of HIF-1α reduces retinal neovascularization in the murine model of oxygen-induced retinopathy 60. Therefore, identifying agents that inhibit HIF-1α represents a therapeutic strategy for the treatment of retinal neovascularization diseases.
Figure 5. Retinal neovascularization
Retinal neovascularization causes
Commonest cause of retinal neovascularization is diabetes, also sickle cell disease, retinopathy of prematurity, retinal vein occlusion and severe carotid stenosis.
Retinal neovascularization treatment
Retinal neovascularization is treated with intravitreal injections of anti-vascular endothelial growth vactor (VEGF) and retinal photocoagulation.
If diabetes is cause, strict blood sugar control is imperative.
If poor carotid artery flow is cause, endarterectomy or stenting of that vessel may be helpful.
References- Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Beebe DC. Semin Cell Dev Biol. 2008 Apr; 19(2):125-33.
- Sharif Z, Sharif W. Corneal neovascularization: updates on pathophysiology, investigations & management. Rom J Ophthalmol. 2019;63(1):15–22. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6531773
- Roshandel D, Eslani M, Baradaran-Rafii A, et al. Current and emerging therapies for corneal neovascularization. Ocul Surf. 2018;16(4):398–414. doi:10.1016/j.jtos.2018.06.004 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6461401
- Evaluation of corneal neovascularisation. Faraj LA, Said DG, Dua HS. Br J Ophthalmol. 2011 Oct; 95(10):1343-4.
- Ocular neovascularization: an epidemiologic review. Lee P, Wang CC, Adamis AP. Surv Ophthalmol. 1998 Nov-Dec; 43(3):245-69.
- Clinical correlates of common corneal neovascular diseases: a literature review. Abdelfattah NS, Amgad M, Zayed AA, Salem H, Elkhanany AE, Hussein H, Abd El-Baky N. Int J Ophthalmol. 2015; 8(1):182-93.
- Abdelfattah NS, Amgad M, Zayed AA, Salem H, Elkhanany AE, Hussein H, Abd El-Baky N. Clinical correlates of common corneal neovascular diseases: A literature review. Int J Ophthalmol. 2015;8:182–193.
- Risau W Mechanisms of angiogenesis. Nature. 1997;386(6626):671–4.
- Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci. 2005;46(10):3502–6.
- Park PJ, Chang M, Garg N, et al. Corneal lymphangiogenesis in herpetic stromal keratitis. Surv Ophthalmol. 2015;60(1):60–71.
- Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Survey of Ophthalmology. 2000;44:415–425.
- Clements JL, Dana R. Inflammatory corneal neovascularization: Etiopathogenesis. Semin Ophthalmol. 2011;26:235–245.
- Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM. Roles of IL-8 in ocular inflammations: A review. Ocul Immunol Inflamm. 2011;19:401–412.
- Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992;141:1279–1284.
- Suryawanshi A, Mulik S, Sharma S, Reddy PB, Sehrawat S, Rouse BT. Ocular neovascularization caused by herpes simplex virus type 1 infection results from breakdown of binding between vascular endothelial growth factor a and its soluble receptor. J Immunol. 2011;186:3653–3665.
- Zahir-Jouzdani F, Atyabi F, Mojtabavi N. Interleukin-6 participation in pathology of ocular diseases. Pathophysiology. 2017;24(3):123–131.
- Kim SY, Yeo A, No H. Downregulation of IL-7 and IL-7R Reduces Membrane-Type Matrix Metalloproteinase 14 in Granular Corneal Dystrophy Type 2 Keratocyte. Invest Ophthalmol Vis Sci. 2018;59(13):5693–5703.
- Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: An evidence-based meta-analysis. Ophthalmology. 2010;117:1300–1305.
- Reed JW, Fromer C, Klintworth GK. Induced corneal vascularization remission with argon laser therapy. Arch Ophthalmol. 1975;93:1017–1019.
- Cherry PM, Faulkner JD, Shaver RP, Wise JB, Witter SL. Argon laser treatment of corneal neovascularization. Ann Ophthalmol. 1973;5:911–920.
- Gomer CJ, Ferrario A, Hayashi N, Rucker N, Szirth BC, Murphree AL. Molecular, cellular, and tissue responses following photodynamic therapy. Lasers Surg Med. 1988;8:450–463.
- Gerten G. Bevacizumab (avastin) and argon laser to treat neovascularization in corneal transplant surgery. Cornea. 2008;27:1195–1199.
- Petsoglou C, Balaggan K, Dart JK, Bunce C, Xing W, Ali RR, Tuft SJ. Subconjunctival bevacizumab induces regression of corneal neovascularisation: a pilot randomised placebo-controlled, double-masked trial. Br J Ophthalmol. 2013;97(1):28–32.
- Williams KA, Jessup CF, Coster DJ. Gene therapy approaches to prolonging corneal allograft survival. Expert Opin Biol Ther. 2004;4:1059–1071.
- Lai CM, Spilsbury K, Brankov M, Zaknich T, Rakoczy PE. Inhibition of corneal neovascularization by recombinant adenovirus mediated antisense vegf rna. Exp Eye Res. 2002;75:625–634.
- Mohan RR, Tovey JC, Sharma A, Tandon A. Gene therapy in the cornea: 2005–present. Prog Retin Eye Res. 2012;31:43–64.
- He Z, Pipparelli A, Manissolle C, Acquart S, Garraud O, Gain P, Thuret G. Ex vivo gene electrotransfer to the endothelium of organ cultured human corneas. Ophthalmic Res. 2010;43:43–55.
- Mwaikambo BR, Sennlaub F, Ong H, Chemtob S, Hardy P. Activation of cd36 inhibits and induces regression of inflammatory corneal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4356–4364.
- Cheng HC, Yeh SI, Tsao YP, Kuo PC. Subconjunctival injection of recombinant aav-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol Vis. 2007;13:2344–2352.
- Maddula S, Davis DK, Burrow MK, Ambati BK. Horizons in therapy for corneal angiogenesis. Ophthalmology. 2011;118:591–599.
- Hos D, Saban DR, Bock F, Regenfuss B, Onderka J, Masli S, Cursiefen C. Suppression of inflammatory corneal lymphangiogenesis by application of topical corticosteroids. Arch Ophthalmol. 2011;129:445–452.
- Klintworth GK. Corneal angiogenesis a comprehensive critical review. New York: Springer; 1991.
- Hoffart L, Matonti F, Conrath J, Daniel L, Ridings B, Masson GS, Chavane F. Inhibition of corneal neovascularization after alkali burn: Comparison of different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin Experiment Ophthalmol. 2010;38:346–352.
- Schleimer RP, Freeland HS, Peters SP, Brown KE, Derse CP. An assessment of the effects of glucocorticoids on degranulation, chemotaxis, binding to vascular endothelium and formation of leukotriene b4 by purified human neutrophils. J Pharmacol Exp Ther. 1989;250:598–605.
- Aydin E, Kivilcim M, Peyman GA, Esfahani MR, Kazi AA, Sanders DR. Inhibition of experimental angiogenesis of cornea by various doses of doxycycline and combination of triamcinolone acetonide with low-molecular-weight heparin and doxycycline. Cornea. 2008;27:446–453.
- Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22.
- Ferrara N, Hillan KJ, Novotny W. Bevacizumab (avastin), a humanized anti-vegf monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335.
- Awadein A. Subconjunctival bevacizumab for vascularized rejected corneal grafts. J Cataract Refract Surg. 2007;33:1991–1993.
- Lin CT, Hu FR, Kuo KT, Chen YM, Chu HS, Lin YH, Chen WL. The different effects of early and late bevacizumab (avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Invest Ophthalmol Vis Sci. 2010;51:6277–6285.
- Jo N, Mailhos C, Ju M, Cheung E, Bradley J, Nishijima K, Robinson GS, Adamis AP, Shima DT. Inhibition of platelet-derived growth factor b signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168:2036–2053.
- Krizova D, Vokrojova M, Liehneova K, Studeny P. Treatment of corneal neovascularization using anti-vegf bevacizumab. J Ophthalmol. 2014;2014:178132.
- Al-Mahmood S, Colin S, Farhat N, Thorin E, Steverlynck C, Chemtob S. Potent in vivo antiangiogenic effects of gs-101 (5′-tatccggagggctcgccatgctgct-3′), an antisense oligonucleotide preventing the expression of insulin receptor substrate-1. J Pharmacol Exp Ther. 2009;329:496–504.
- Cursiefen C, Viaud E, Bock F, Geudelin B, Ferry A, Kadlecová P, Lévy M, Al Mahmood S, Colin S, Thorin E, Majo F, Frueh B, Wilhelm F, Meyer-Ter-Vehn T, Geerling G, Böhringer D, Reinhard T, Meller D, Pleyer U, Bachmann B, Seitz B. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: The i-can study. Ophthalmology. 2014;121:1683–1692.
- Gilbertson-Beadling S, Powers EA, Stamp-Cole M, Scott PS, Wallace TL, Copeland J, Petzold G, Mitchell M, Ledbetter S, Poorman R. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother Pharmacol. 1995;36:418–424.
- Jovanovic V, Nikolic L. The effect of topical doxycycline on corneal neovascularization. Curr Eye Res. 2014;39:142–148.
- Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41:2148–2153.
- Wertheim MS, Cook SD, Knox-Cartwright NE, Van DL, Tole DM. Electrolysis-needle cauterization of corneal vessels in patients with lipid keratopathy. Cornea. 2007;26:230–231.
- Trikha S, Parikh S, Osmond C, Anderson DF, Hossain PN. Long-term outcomes of fine needle diathermy for established corneal neovascularisation. Br J Ophthalmol. 2014;98:454–458.
- Faraj LA, Elalfy MS, Said DG, Dua HS. Fine needle diathermy occlusion of corneal vessels. Br J Ophthalmol. 2014;98:1287–1290.
- Neovascularization of the Iris (Rubeosis Iridis). http://morancore.utah.edu/section-10-glaucoma/neovascularization-of-the-iris-rubeosis-iridis/
- Wang JW, et al. Short-term effect of intravitreal ranibizumab on intraocular concentrations of vascular endothelial growth factor-A and pigment epithelium-derived factor in neovascular glaucoma. Clinical and Experimental Ophthalmology 2015;43:415-421. Doi: 10.1111/ceo.12477
- Shazly TA, Latina MA. Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol2009;24(2):113-121. DOI: 10.1080/08820530902800801
- Rodrigues GB, et al. Neovascular glaucoma: a review. International Journal of Retina and Vitreous 2016;2:26. https://doi.org/10.1186/s40942-016-0051-x
- Neovascular Glaucoma. https://eyewiki.aao.org/Neovascular_Glaucoma
- Retinal neovascularization. http://kellogg.umich.edu/theeyeshaveit/opticfundus/retinal_neovascularization.html
- Proliferative retinopathies: angiogenesis that blinds. Sapieha P, Hamel D, Shao Z, Rivera JC, Zaniolo K, Joyal JS, Chemtob S. Int J Biochem Cell Biol. 2010 Jan; 42(1):5-12.
- Hypoxia-induced angiogenesis: good and evil. Krock BL, Skuli N, Simon MC. Genes Cancer. 2011 Dec; 2(12):1117-33.
- Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–33.
- Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–81.
- Yoshida T, Zhang H, Iwase T, Shen J, Semenza GL, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24:1759–67.