What is Omega 6 Fatty Acids
Omega-6 fatty acids are essential fatty acids —also known as n-6 polyunsaturated fatty acids (PUFAs)—are important for maintaining proper cellular function in the human body and, in particular, maintaining bone health, regulating metabolism, in stimulating skin and hair growth. play a role in immunity and inflammation. They are necessary for human health, but the body cannot make them and must obtain it in your diet.
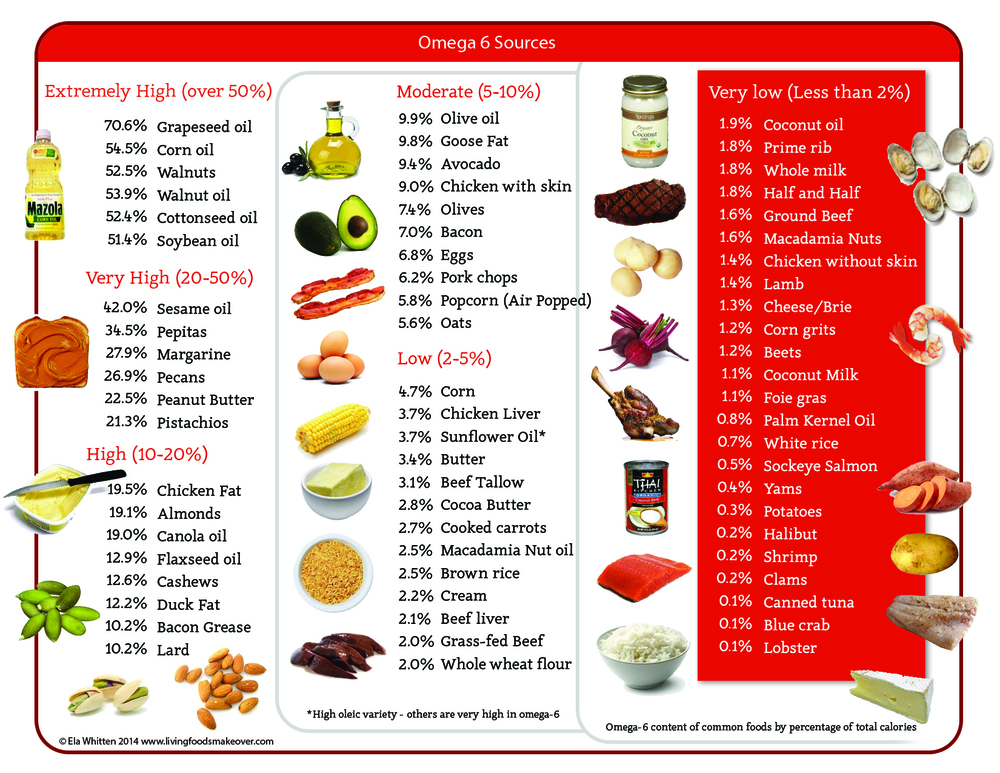
Omega 6 can be obtained from a variety of dietary sources, such as vegetable oil and nuts. There are several different types of omega-6 fatty acids, and not all promote inflammation. Most omega-6 fatty acids in the diet come from vegetable oils, such as linoleic acid (LA), not to be confused with alpha-linolenic acid (ALA), which is an omega-3 fatty acid. The distinction between omega-6 and omega-3 fatty acids is based on the location of the first double bond, counting from the methyl end of the fatty acid molecule (see Figure 3). Omega-6 fatty acids are represented by Linoleic acid (LA) (18:2ω-6) and Arachidonic acid (AA) (20:4ω-6) and omega-3 fatty acids by Alpha-linolenic acid (ALA) (18:3ω-3), Eicosapentaenoic acid (EPA) (20:5ω-3) and Docosahexaenoic acid (DHA) (22:6ω-3). Linoleic acid (LA) is converted to gamma-linolenic acid (GLA) in the body. It can then break down further to arachidonic acid (AA). GLA is found in several plant-based oils, including evening primrose oil (EPO), borage oil, and black currant seed oil.
Mammalian cells cannot convert omega-6 to omega-3 fatty acids because they lack the converting enzyme, omega-3 desaturase. Omega-6 and omega-3 fatty acids are not interconvertible, are metabolically and functionally distinct, and often have important opposing physiological effects, therefore their balance in the diet is important 1.
Linoleic acid (LA) is plentiful in nature and is found in the seeds of most plants except for coconut, cocoa, and palm 1. Alpha-linolenic acid (ALA), on the other hand, is found in the chloroplasts of green leafy vegetables, and in the seeds of flax, rape, chia, perilla and walnuts. Both essential fatty acids are metabolized to longer-chain fatty acids of 20 and 22 carbon atoms. Linoleic acid (LA) is metabolized to arachidonic acid (AA) (20:4ω6) while ALA is metabolized to eicosapentaenoic acid (EPA) (20:5ω3) and docosahexaenoic acid (DHA) (22:6ω3). This is achieved by increasing the chain length and the degree of unsaturation by adding extra double bonds to the carboxyl end of the fatty acid molecule (Figure 4) 2. Arachidonic acid (AA) is found predominantly in the phospholipids of grain-fed animals, dairy and eggs. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are found in the oils of fish, particularly fatty fish.
What are polyunsaturated fatty acids
Fats are essential for living organisms. Fatty acid molecules have a variable length carbon chain with a methyl terminus and a carboxylic acid head group 3. They can be categorized based on the degree of saturation of their carbon chains. Saturated fatty acids possess the maximal number of hydrogen atoms, while monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) have one, or two or more, double bonds, respectively.
Figure 1. Monounsaturated Fatty Acids Structure

Figure 2. Polyunsaturated Fatty Acids Structure

Polyunsaturated fatty acids (PUFAs) can be further subdivided on the basis of the location of the first double bond relative to the methyl terminus of the chain. For example, n-3 and n-6 fatty acids are two of the most biologically significant polyunsaturated fatty acid classes, and have their first double bond on either the third or sixth carbon from the chain terminus, respectively. The final carbon in the fatty acid chain is also known as the omega carbon, hence the common reference to these fatty acids as omega-3 or omega-6 PUFAs.
Figure 3. Omega-3 fatty acids and Omega-6 fatty acids structure
Figure 4. Conversion of dietary ALA to EPA and DHA via Desaturation, elongation and retroconversion of polyunsaturated fatty acids.
[Source 4]Long-chain n-3 and n-6 PUFAs are synthesized from the essential fatty acids: alpha-linolenic acid (ALA) and linoleic acid (LA), respectively. Basic structures of these two parent PUFAs are shown in Figure 3. An essential fatty acid cannot be made by the body and must be obtained through dietary sources. Animals and humans have the capacity to metabolize essential fatty acids to long-chain derivatives. Because the n-6 and n-3 pathways compete with one another for enzyme activity, the ratio of n-6 to n-3 PUFAs is very important to human health. An overabundance of fatty acids from one family will limit the metabolic production of the longer chain products of the other. The typical Western diet provides n-6 and n-3 PUFAs in a ratio ranging from 8:1 to 25:1 3, values in severe contrast with the recommendations from national health agencies of approximately 4:1 5. Lowering the n-6:n-3 ratio would reduce competition for the enzymes and facilitate the metabolism of more downstream products of ALA.
The very high intake of n-6 PUFA, mostly as linoleic acid (LA) (Fig. 3) in our diet (12–15 g/day) from common vegetable oils (corn, safflower, soybean) and other sources, yields an overall n-6:n-3 dietary ratio (total omega-6 fatty acids in the diet: total omega-3 fatty acids in the diet) of about 8:1. Health Canada has recommended that this ratio be as low as 4:19 to reduce the competitive influence of high LA intakes on ALA metabolism to its longer chain products (such as EPA and DHA). Although high intakes of linoleic acid (LA) can provide some modest blood cholesterol lowering, experimental studies in animals have raised concerns regarding the enhancing effect of these high intakes on certain cancers 6. This association has not been established in human studies 7.
For general health, there should be a balance between omega-6 and omega-3 fatty acids. The ratio should be in the range of 2:1 to 4:1, omega-6 to omega-3, and some health educators advocate even lower ratios. Omega-6 fatty acids can be found in sunflower, safflower, soy, sesame, and corn oils. The average diet provides plenty of omega-6 fatty acids, so supplements are usually not necessary. People with specific conditions, such as eczema, psoriasis, arthritis, diabetes, or breast tenderness (mastalgia) may want to ask their doctors about taking omega-6 supplements.
Gamma-linolenic acid (GLA) may actually reduce inflammation. Much of the gamma-linolenic acid (GLA) taken as a supplement is converted to a substance called DGLA that fights inflammation. Having enough of certain nutrients in the body (including magnesium, zinc, and vitamins C, vitamin B3, and vitamin B6) helps promote the conversion of GLA to DGLA.
You have to get them through food. Along with omega-3 fatty acids, omega-6 fatty acids play a crucial role in brain function, and normal growth and development. As a type of polyunsaturated fatty acid (PUFA), omega-6s help stimulate skin and hair growth, maintain bone health, regulate metabolism, and maintain the reproductive system.
A healthy diet contains a balance of omega-3 and omega-6 fatty acids. Omega-3 fatty acids help reduce inflammation, and some omega-6 fatty acids tend to promote inflammation. In fact, some studies suggest that elevated intakes of omega-6 fatty acids may play a role in complex regional pain syndrome. The typical American diet tends to contain 14 to 25 times more omega-6 fatty acids than omega-3 fatty acids.
The Mediterranean diet, on the other hand, has a healthier balance between omega-3 and omega-6 fatty acids. Studies show that people who follow a Mediterranean-style diet are less likely to develop heart disease. The Mediterranean diet does not include much meat (which is high in omega-6 fatty acids, though grass fed beef has a more favorable omega-3 to omega-6 fatty acid ratio), and emphasizes foods rich in omega-3 fatty acids, including whole grains, fresh fruits and vegetables, fish, olive oil, garlic, as well as moderate wine consumption.
The Importance of Omega-6/Omega-3 Fatty Acid Ratio and Obesity Risk
In the past three decades, total fat and saturated fat intake as a percentage of total calories has continuously decreased in Western diets, while the intake of omega-6 fatty acid increased and the omega-3 fatty acid decreased, resulting in a large increase in the omega-6/omega-3 ratio from 1:1 during evolution to 20:1 today or even higher 1. This change in the composition of fatty acids parallels a significant increase in the prevalence of overweight and obesity. Experimental studies have suggested that omega-6 and omega-3 fatty acids elicit divergent effects on body fat gain through mechanisms of adipogenesis, browning of adipose tissue, lipid homeostasis, brain-gut-adipose tissue axis, and most importantly systemic inflammation 1. Prospective studies clearly show an increase in the risk of obesity as the level of omega-6 fatty acids and the omega-6/omega-3 ratio increase in red blood cell membrane phospholipids, whereas high omega-3 red blood cell membrane phospholipids decrease the risk of obesity. Recent studies in humans show that in addition to absolute amounts of omega-6 and omega-3 fatty acid intake, the omega-6/omega-3 ratio plays an important role in increasing the development of obesity via both AA eicosanoid metabolites and hyperactivity of the cannabinoid system, which can be reversed with increased intake of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). A balanced omega-6/omega-3 ratio is important for health and in the prevention and management of obesity 1.
Omega-6 and omega-3 polyunsaturated fatty acids (PUFAs) are essential fatty acids that must be derived from the diet, cannot be made by humans, and other mammals because of the lack of endogenous enzymes for omega-3 desaturation 8, 9. Due to agribusiness and modern agriculture western diets contain excessive levels of omega-6 PUFAs but very low levels of omega-3 PUFAs, leading to an unhealthy omega-6/omega-3 ratio of 20:1, instead of 1:1 that was during evolution in humans 8, 10.
Eicosanoid products derived from omega-6 PUFAs (such as prostaglandin (PG) E2 and leukotriene (LT) B4 synthesized from arachidonic acid (AA)) are more potent mediators of thrombosis and inflammation than similar products derived from omega-3 PUFAs (PGE3 and LTB5 synthesized from eicosapentaenoic acid (EPA)) 8, 9, 10.
Thus, an unbalanced omega-6/omega-3 ratio in favor of omega-6 PUFAs is highly prothrombotic and proinflammatory, which contributes to the prevalence of atherosclerosis, obesity, metabolic syndrome and diabetes 11, 12, 13. In fact, regular consumption of diets rich in omega-3 PUFAs have been associated with low incidence of these diseases, particularly in Icelandic populations, Inuit indigenous people, and Native Americans in Alaska 14, 15, 16. However, using fish oil as the primary source of omega-3 PUFAs to treat type 2 diabetes has not always met with success 13, 17, 18. Although nutritional studies suggest that high omega-6/omega-3 ratios have contributed significantly to the “obesity epidemic” 19, 20, clinical trials using omega-3 PUFAs as weight-reducing agents have produced conflicting findings of both positive 21, 22, 23 and negative effects 24, 25, 26 due to many factors.
In mammals, including humans, the cerebral cortex, retina, testis and sperm are particularly rich in DHA. DHA is one of the most abundant components of the brain’s structural lipids. DHA, like EPA, can be derived only from direct ingestion or by synthesis from dietary EPA or ALA: humans and other mammals, except for certain carnivores such as lions, can convert LA to AA and ALA to EPA and DHA, although the process is slow 27, 28. There is competition between omega-6 and omega-3 fatty acids for the desaturation enzymes. Both fatty acid desaturase 1 (FADS1) and fatty acid desaturase 2 (FADS2) prefer ALA to LA 27, 29, 30 and docosahexaenoic acids (22:6(n-3)) in isolated liver cells. Hagve TA, Christophersen BO. Biochim Biophys Acta. 1986 Feb 12; 875(2):165-73. https://www.ncbi.nlm.nih.gov/pubmed/2935195/)). However a high LA intake, such as that characterizing Western diets, interferes with the desaturation and elongation of ALA 30 and docosahexaenoic acids (22:6(n-3)) in isolated liver cells. Hagve TA, Christophersen BO. Biochim Biophys Acta. 1986 Feb 12; 875(2):165-73. https://www.ncbi.nlm.nih.gov/pubmed/2935195/)), 31. Similarly, trans fatty acids interfere with the desaturation and elongation of both LA and ALA 1.
When humans ingest fish or fish oil, the Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) from the diet partially replace the omega-6 fatty acids, especially Arachidonic acid (AA), in the membranes of probably all cells, but especially in the membranes of platelets, erythrocytes, neutrophils, monocytes, and liver cells 32. Arachidonic acid (AA) and Eicosapentaenoic acid (EPA) are the parent compounds for eicosanoid production. Because of the increased amounts of omega-6 in the Western diet, the eicosanoid metabolic products from Arachidonic acid (AA), specifically prostaglandins, thromboxanes, leukotrienes, hydroxy fatty acids, and lipoxins, are formed in larger quantities than those derived from omega-3 fatty acids, specifically Eicosapentaenoic acid (EPA) 10. The eicosanoids from AA are biologically active in very small quantities and, if they are formed in large amounts, they contribute to the formation of thrombus and atheromas; to allergic and inflammatory disorders, particularly in susceptible people; and to proliferation of cells 33. Thus, a diet rich in omega-6 fatty acids shifts the physiological state to one that is proinflammatory, prothrombotic, and proaggregatory, with increases in blood viscosity, vasospasm, vasoconstriction and cell proliferation 1.
Table 1. Opposing Effects of Omega-6 and Omega-3 Fatty Acids in Obesity.
| Conditions | Omega-6 | Omega-3 |
|---|---|---|
| Adipogenesis (Pre-adipocyte-Adipocyte) | High AA via the PI2 receptor activates the cAMP protein kinase A, signaling pathway leads to proliferation and differentiation of WAT, prevention of its browning through inhibition of PPARy target genes including UCPI, decrease mitochondrial biogenesis [74], increasing triglycerides [73], insulin resistance, leptin resistance, decreased adiponectin levels, decreased fatty acid oxidation and hepatic steatosis [4]. | High EPA and DHA partially inhibit cAMP signaling pathways triggered by AA at levels upstream of PKA [71] block COX-2 metabolites PGI2 and PGEF2a that stimulate white adipogenesis and inhibit the browning process respectively, prevent increased triglycerides and adipose tissue proliferation through UCP-I and PPARy activation, increased mitochondrial biogenesis, increased fatty acid oxidation, and apoptosis [71,75]. |
| Inflammation | AA metabolites prostaglandin 2 thromboxane 2 and leukotriene 4 are prothrombotic and proinflammatory leading to increased production of IL-1, IL-6, NFKB and TNF and inflammation [1,64]. | High dietary intake of EPA and DHA blocks the metabolites of AA and prevents inflammation, which is the hallmark of obesity [1,64,67]. |
| Insulin Resistance Leptin Resistance Adiponectin | AA leads to insulin resistance, leptin resistance, lower adiponectin and hepatic steatosis. AA blunts PI3-Akt pathway leading to leptin resistance in the brain and deregulation of food intake [19,76,77]. | EPA and DHA regulate glucose utilization, insulin sensitivity (Akt phosphorylation) in part mediated by PPARy and AMPK activation [19]. EPA and DHA regulate the secretion of adipokines involved in energy homeostasis and intermediate metabolism and in glucose and lipid metabolism. DHA restores insulin sensitivity in skeletal muscle by preventing lipotoxicity and inflammation [78,79]. |
| Cannabinoids | AA increases the concentration of (2-AG) and (AEA) leading to excessive endocannabinoid signaling, and dysregulation of the cannabinoid system, weight gain, larger adipocytes and more macrophages in adipose tissue [80], inflammation and a metabolic profile associated with obesity [81,82]. | EPA and DHA decrease 2-AG and AA in the brain while increasing DHA, decreasing the dysregulation of the cannabinoid system, improving insulin sensitivity and decreasing central body fat. |
High intake of omega-6 fatty acids during the perinatal period is associated with increased adiposity in the offspring. In human studies the level of AA in adipose tissue is associated with the BMI and overweight status of children. High omega-6/omega-3 fatty acids in umbilical cord red blood cell membrane phospholipids was associated with high subscapular skin-fold thickness at 3 years of age 34.
Animal and human studies have shown that EPA and DHA supplementation may be protective against obesity, and may reduce weight gain in already obese animals and humans 35. Specifically, studies demonstrated a reduction in visceral (epididymal and/or retroperitoneal) fat in rats fed high lipid diets that incorporate omega-3 PUFAs 36, 37 and the effect was dose-dependent 38. The reduction in visceral fat was associated with a decrease in adipocyte size 38 and number 37. High fat diets rich in omega-6 fatty acids have been shown to increase the risk of leptin resistance, diabetes, and obesity in humans and rodents 39, 40. AA impairs hypothalamic leptin signaling and energy homeostasis in mice 41. The inhibitory role of AA has been suggested in both basal and insulin-stimulated leptin expression and production 40.
Endocannabinoids are lipids, derived from the omega-6 AA. Their concentrations are regulated by dietary intake of omega-6 and omega-3 fatty acids 8 and by the activity of biosynthetic and catabolic enzymes involved in the endocannabinoid pathway 42, which is an important player in regulation of appetite and metabolism 43, 44. The endocannabinoid system is involved in regulation of energy balance and sustained hyperactivity of the endocannabinoid system contributes to obesity 43, 45.
A diet high in the omega-6/omega-3 ratio causes an increase in the endocannabinoid signaling and related mediators, which lead to an increased inflammatory state, energy homeostasis, and mood 1. In animal experiments a high omega-6 acid intake leads to decreased insulin sensitivity in muscle and promotes fat accumulation in adipose tissue. Nutritional approaches with dietary omega-3 fatty acids reverse the dysregulation of this system, improve insulin sensitivity and control body fat 1.
Adipose tissue is the main peripheral target organ handling fatty acids, and AA is required for adipocyte differentiation (adipogenesis). The increased LA and AA content of foods has been accompanied by a significant increase in the AA/EPA + DHA ratio within adipose tissue, leading to increased production in AA metabolites, PGI2 which stimulates white adipogenesis and PGF2α which inhibits the browning process, whereas increased consumption of EPA and DHA leads to adipose tissue homeostasis through adipose tissue loss and increased mitochondrial biogenesis.
High intake of omega-6 fatty acids during the perinatal period is associated with increased adiposity in the offspring. This study also showed that a higher n−6:n−3 PUFA ratio in the maternal diet, in maternal blood, or in cord blood was associated with higher child adiposity at age 3 years 46. In this cohort study of children followed since pregnancy, higher n−3 PUFA concentrations in the maternal diet and in umbilical cord plasma phospholipids were associated with lower adiposity in children at age 3 y as measured by the sum of skinfold thicknesses, odds of obesity and the concentration of leptin, which is a biomarker directly correlated with adiposity. Fish intake is a primary source of n−3 PUFAs, and a greater maternal prenatal fish intake was also associated with lower child adiposity 46.
Omega 6 intake to prevent Cardiovascular Disease
Some evidence suggests that a proportionally higher intake of omega 6 fatty acids along with a low intake of saturated fat is associated with significant reductions in coronary heart disease. In contrast, there is concern that high levels of omega 6 fatty acids may worsen cardiovascular risk. There appears to be inconclusive evidence from observational studies and meta-analyses on the benefits of omega 6 intake on cardiovascular disease outcomes.
In this context, careful examination of the dietary patterns of large numbers of individuals followed for many years as they relate to coronary heart disease can help bring perspective into this controversy surrounding omega-6 PUFAs and cardiovascular disease. The contribution of Farvid et al. 47 who performed the largest systematic review and meta-analysis to date examining the relations between omega-6 fatty acid (essentially LA) intake and coronary heart disease morbidity and mortality. Using data from both published and unpublished studies (via direct investigator contact), Farvid et al. 47 included 12 cohort studies involving ≈290,000 individuals, among whom there were ≈11,000 coronary heart disease events and ≈4,500 coronary heart disease deaths. Intakes of omega 6 linoleic acid (LA) were estimated by a variety of dietary questionnaires, and follow-up ranged from 5 to 30 years. Comparing the highest- with the lowest-intake groups, risk for coronary heart disease events was lower by 14% and for coronary heart disease death by 17%, both statistically significant. The effects on total mortality rates would have been of interest as well, but such data were not available. The authors note that several studies have sought effects of dietary LA on cancer outcomes but have found none 47.
However, in the most recent Cochrane Review, the evidence is current to 23 September 2014 48 concluded there is no differences in effects of increased or decreased omega 6 intake were seen on blood lipids and blood pressure, but this is based on very few studies. There is insufficient evidence to date from randomized controlled trials to recommend increasing or reducing omega 6 for the prevention of cardiovascular disease. There is a need for larger well conducted randomized controlled trials assessing cardiovascular events as well as cardiovascular risk factors 48.
Omega-6 fatty acids may be useful for the following health conditions:
- Diabetic neuropathy
Some studies show that taking gamma linolenic acid (GLA) for 6 months or more may reduce symptoms of nerve pain in people with diabetic neuropathy. People who have good blood sugar control may find GLA more effective than those with poor blood sugar control. - Rheumatoid arthritis
Studies are mixed as to whether evening primrose oil (EPO) helps reduce symptoms of RA. Preliminary evidence suggests EPO may reduce pain, swelling, and morning stiffness, but other studies have found no effect. When using GLA for symptoms of arthritis, it may take 1 to 3 months for benefits to appear. It is unlikely that EPO would help stop progression of the disease. So joint damage would still occur. - Allergies
Omega-6 fatty acids from food or supplements, such as GLA from EPO or other sources, have a longstanding history of folk use for allergies. Women who are prone to allergies appear to have lower levels of GLA in breast milk and blood. However, there is no good scientific evidence that taking GLA helps reduce allergy symptoms. Well-conducted research studies are needed.Before you try GLA for allergies, work with your doctor to determine if it is safe for you. Then follow your allergy symptoms closely for any signs of improvement. - Attention deficit/hyperactivity disorder (ADHD)
Clinical studies suggest that children with ADHD have lower levels of EFAs, both omega-6s and omega-3s. EFAs are important to normal brain and behavioral function. Some studies indicate that taking fish oil (containing omega-3 fatty acids) may help reduce ADHD symptoms, though the studies have not been well designed. Most studies that used EPO have found it was no better than placebo at reducing symptoms. - Breast cancer
One study found that women with breast cancer who took GLA had a better response to tamoxifen (a drug used to treat estrogen-sensitive breast cancer) than those who took only tamoxifen. Other studies suggest that GLA inhibits tumor activity among breast cancer cell lines. There is some research suggesting that a diet rich in omega-6 fatty acids may promote breast cancer development. DO NOT add fatty acid supplements, or any supplements, to your breast cancer treatment regimen without your doctor’s approval. - Breast pain (mastalgia)
Some evidence suggests that EPO may reduce breast pain and tenderness in people with cyclic mastalgia. It may also help reduce symptoms to a lesser extent in people with noncyclic mastalgia. However, it does not seem to be effective for severe breast pain. - Premenstrual syndrome (PMS)
Although most studies have found no effect, some women report relief of PMS symptoms when using GLA. The symptoms that seem to improve the most are breast tenderness and feelings of depression, as well as irritability and swelling and bloating from fluid retention. - Eczema
Evidence is mixed as to whether EPO can help reduce symptoms of eczema. Preliminary studies showed some benefit, but they were not well designed. Later studies that examined people who took EPO for 16 to 24 weeks found no improvement in symptoms. If you want to try EPO, talk to your doctor about whether it is safe for you. - High blood pressure (hypertension)
Preliminary evidence suggests that GLA may help reduce high blood pressure, either alone or in combination with omega-3 fatty acids found in fish oil, namely eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). In one study, men with borderline high blood pressure who took 6g of blackcurrant oil had a reduction in diastolic blood pressure compared to those who took placebo.Another study examined people with intermittent claudication, which is pain in the legs while walking that is caused by blockages in the blood vessels. Those who took GLA combined with EPA had a reduction in systolic blood pressure compared to those who took placebo.More research is needed to see whether GLA is truly effective for hypertension.
Summary
The health benefits of omega-6 PUFA intake are still contentious; concern has been raised over the hypothesized pro-inflammatory and pro-thrombotic effects of omega-6 PUFA 49.
A high omega-6 fatty acid intake and a high omega-6/omega-3 ratio are associated with weight gain in both animal and human studies, whereas a high omega-3 fatty acid intake decreases the risk for weight gain. Lowering the LA/ALA ratio in animals prevents overweight and obesity.
High omega-6 fatty acids increase leptin resistance and insulin resistance, whereas omega-3 fatty acids lead to homeostasis and weight loss.
High intake of omega-6 fatty acids during the perinatal period is associated with increased adiposity in the offspring. In this cohort study of children followed since pregnancy, higher n−3 PUFA concentrations in the maternal diet and in umbilical cord plasma phospholipids were associated with lower adiposity in children at age 3 y as measured by the sum of skinfold thicknesses, odds of obesity and the concentration of leptin, which is a biomarker directly correlated with adiposity. Fish intake is a primary source of n−3 PUFAs, and a greater maternal prenatal fish intake was also associated with lower child adiposity 46.
A diet high in the omega-6/omega-3 ratio causes an increase in the endocannabinoid signaling and related mediators, which lead to an increased inflammatory state, energy homeostasis, and mood 1.
Because a high omega-6/omega-3 ratio is associated with overweight/obesity, whereas a balanced ratio decreases obesity and weight gain, it is essential that every effort is made to decrease the omega-6 fatty acids in the diet, while increasing the omega-3 fatty acid intake. This can be accomplished by (1) changing dietary vegetable oils high in omega-6 fatty acids (corn oil, sunflower, safflower, cottonseed, and soybean oils) to oils high in omega-3s (flax, perilla, chia, rapeseed), and high in monounsaturated oils such as olive oil, macadamia nut oil, hazelnut oil, or the new high monounsaturated sunflower oil; and (2) increasing fish intake to 2–3 times per week, while decreasing meat intake.
References- Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8(3):128. doi:10.3390/nu8030128. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4808858/
- Omega-3 fatty acids in health and disease and in growth and development. Simopoulos AP. Am J Clin Nutr. 1991 Sep; 54(3):438-63. https://www.ncbi.nlm.nih.gov/pubmed/1908631/
- Salem N., Jr Introduction to polyunsaturated fatty acids. Backgrounder. 1999;3:1–8.
- Holub BJ. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. Hoffer LJ, Jones PJ, eds. CMAJ: Canadian Medical Association Journal. 2002;166(5):608-615. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC99405/
- Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. Holub BJ. CMAJ. 2002 Mar 5; 166(5):608-15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC99405/
- Breast cancer risk in rats fed a diet high in n-6 polyunsaturated fatty acids during pregnancy. Hilakivi-Clarke L, Onojafe I, Raygada M, Cho E, Clarke R, Lippman ME. J Natl Cancer Inst. 1996 Dec 18; 88(24):1821-7. https://www.ncbi.nlm.nih.gov/pubmed/8961971/
- Linoleic acid intake and cancer risk: a review and meta-analysis. Zock PL, Katan MB. Am J Clin Nutr. 1998 Jul; 68(1):142-53. https://www.ncbi.nlm.nih.gov/pubmed/9665108/
- Simopoulos A.P. Evolutionary aspects of diet and essential fatty acids. In: Hamazaki T., Okuyama H., editors. Fatty Acids and Lipids—New Findings. Volume 88. Karger; Basel, Switzerland: 2001. pp. 18–27.
- The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. Kang JX. World Rev Nutr Diet. 2003; 92:23-36. https://www.ncbi.nlm.nih.gov/pubmed/14579681/
- The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Simopoulos AP. Exp Biol Med (Maywood). 2008 Jun; 233(6):674-88. https://www.ncbi.nlm.nih.gov/pubmed/18408140/
- Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome, brain metabolic abnormalities, and non-alcoholic fatty liver disease. Simopoulos AP. Nutrients. 2013 Jul 26; 5(8):2901-23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3775234/
- Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Am J Clin Nutr. 2011 Apr; 93(4):780-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3057547/
- Update on cardiometabolic health effects of ω-3 fatty acids. Kromhout D, de Goede J. Curr Opin Lipidol. 2014 Feb; 25(1):85-90. https://www.ncbi.nlm.nih.gov/pubmed/24345990/
- Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. Kromann N, Green A. Acta Med Scand. 1980; 208(5):401-6. https://www.ncbi.nlm.nih.gov/pubmed/7457208/
- Lower prevalence of impaired glucose tolerance and diabetes associated with daily seal oil or salmon consumption among Alaska Natives. Adler AI, Boyko EJ, Schraer CD, Murphy NJ. Diabetes Care. 1994 Dec; 17(12):1498-501. https://www.ncbi.nlm.nih.gov/pubmed/7882827/
- Low fasting insulin levels in Eskimos compared to American Indians: are Eskimos less insulin resistant? Schraer CD, Risica PM, Ebbesson SO, Go OT, Howard BV, Mayer AM. Int J Circumpolar Health. 1999 Oct; 58(4):272-80. https://www.ncbi.nlm.nih.gov/pubmed/10615832/
- n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. Nettleton JA, Katz R. J Am Diet Assoc. 2005 Mar; 105(3):428-40. https://www.ncbi.nlm.nih.gov/pubmed/15746832/
- Fish intake, contaminants, and human health: evaluating the risks and the benefits. Mozaffarian D, Rimm EB. JAMA. 2006 Oct 18; 296(15):1885-99. http://jamanetwork.com/journals/jama/fullarticle/203640
- A randomized controlled trial of long-chain polyunsaturated fatty acid supplementation of formula in term infants after weaning at 6 wk of age. Birch EE, Hoffman DR, Castañeda YS, Fawcett SL, Birch DG, Uauy RD. Am J Clin Nutr. 2002 Mar; 75(3):570-80. https://www.ncbi.nlm.nih.gov/pubmed/11864865/
- Blood lipid concentrations of docosahexaenoic and arachidonic acids at birth determine their relative postnatal changes in term infants fed breast milk or formula. Guesnet P, Pugo-Gunsam P, Maurage C, Pinault M, Giraudeau B, Alessandri JM, Durand G, Antoine JM, Couet C. Am J Clin Nutr. 1999 Aug; 70(2):292-8. https://www.ncbi.nlm.nih.gov/pubmed/10426708/
- Couet C., Delarue J., Ritz P., Antoine J.M., Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int. J. Obes. Relat. Metab. Disord. 1997;21:637–643. doi: 10.1038/sj.ijo.0800451. https://www.ncbi.nlm.nih.gov/pubmed/15481762
- Fontani G., Corradeschi F., Felici A., Alfatti F., Bugarini R., Fiaschi A.I., Cerretani D., Montorfano G., Rizzo A.M., Berra B. Blood profiles, body fat and mood state in healthy subjects on different diets supplemented with omega-3 polyunsaturated fatty acids. Eur. J. Clin. Investig. 2005;35:499–507. doi: 10.1111/j.1365-2362.2005.01540.x.https://www.ncbi.nlm.nih.gov/pubmed/16101670
- Hill A.M., Buckley J.D., Murphy K.J., Howe P.R. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am. J. Clin. Nutr. 2007;85:1267–1274. https://www.ncbi.nlm.nih.gov/pubmed/17490962
- Belury M.A., Mahon A., Banni S. The conjugated linoleic acid (CLA) isomer, t10c12-CLA, is inversely associated with changes in body weight and serum leptin in subjects with type 2 diabetes mellitus. J. Nutr. 2003;133:257S–260S. https://www.ncbi.nlm.nih.gov/pubmed/12514304
- Chan D.C., Watts G.F., Nguyen M.N., Barrett P.H. Factorial study of the effect of n-3 fatty acid supplementation and atorvastatin on the kinetics of HDL apolipoproteins A-I and A-II in men with abdominal obesity. Am. J. Clin. Nutr. 2006;84:37–43. https://www.ncbi.nlm.nih.gov/pubmed/16825679
- Simopoulos A.P. The Impact of the Bellagio Report on Healthy Agriculture, Healthy Nutrition, Healthy People: Scientific and Policy Aspects and the International Network of Centers for Genetics, Nutrition and Fitness for Health. J. Nutrigenet. Nutrigenom. 2015;7:189–209. doi: 10.1159/000375495. https://www.ncbi.nlm.nih.gov/pubmed/25766457
- Oxidative desaturation of alpha-linoleic, linoleic, and stearic acids by human liver microsomes. de Gŏmez Dumm IN, Brenner RR. Lipids. 1975 Jun; 10(6):315-7. https://www.ncbi.nlm.nih.gov/pubmed/1134219/
- Emken E.A., Adlof R.O., Rakoff H., Rohwedder W.K. Metabolism of deuterium-labeled linolenic, linoleic, oleic, stearic and palmitic acid in human subjects. In: Baillie T.A., Jones J.R., editors. Synthesis and Application of Isotopically Labeled Compounds 1988. Elsevier Science Publishers; Amsterdam, The Netherlands: 1989. pp. 713–716.
- Effect of dietary fats on arachidonic acid and eicosapentaenoic acid biosynthesis and conversion to C22 fatty acids in isolated rat liver cells. Hagve TA, Christophersen BO. Biochim Biophys Acta. 1984 Nov 14; 796(2):205-17. https://www.ncbi.nlm.nih.gov/pubmed/6093889/
- Evidence for peroxisomal retroconversion of adrenic acid (22:4(n-6
- Indu M., Ghafoorunissa P. N-3 fatty acids in Indian diets—Comparison of the effects of precursor (alpha-linolenic acid) vs. product (long chain n-3 polyunsaturated fatty acids) Nutr. Res. 1992;12:569–582.
- Essential fatty acids in health and chronic disease. Simopoulos AP. Am J Clin Nutr. 1999 Sep; 70(3 Suppl):560S-569S. https://www.ncbi.nlm.nih.gov/pubmed/10479232/
- Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. Simopoulos AP. World Rev Nutr Diet. 2011; 102():10-21. https://www.ncbi.nlm.nih.gov/pubmed/21865815/
- Donahue S.M., Rifas-Shiman S.L., Gold D.R., Jouni Z.E., Gillman M.W., Oken E. Prenatal fatty acid status and child adiposity at age 3 years: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011;93:780–788. doi: 10.3945/ajcn.110.005801. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3057547/
- Buckley J.D., Howe P.R. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes. Rev. 2009;10:648–659. doi: 10.1111/j.1467-789X.2009.00584.x. https://www.ncbi.nlm.nih.gov/pubmed/19460115
- Parrish C.C., Pathy D.A., Angel A. Dietary fish oils limit adipose tissue hypertrophy in rats. Metabolism. 1990;39:217–219. doi: 10.1016/0026-0495(90)90038-E. https://www.ncbi.nlm.nih.gov/pubmed/2308514
- Ruzickova J., Rossmeisl M., Prazak T., Flachs P., Sponarova J., Veck M., Tvrzicka E., Bryhn M., Kopecky J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;39:1177–1185. doi: 10.1007/s11745-004-1345-9. https://www.ncbi.nlm.nih.gov/pubmed/15736913
- Belzung F., Raclot T., Groscolas R. Fish oil n-3 fatty acids selectively limit the hypertrophy of abdominal fat depots in growing rats fed high-fat diets. Am. J. Physiol. 1993;264:R1111–R1118. https://www.ncbi.nlm.nih.gov/pubmed/8322963
- Phillips C.M., Goumidi L., Bertrais S., Field M.R., Ordovas J.M., Cupples L.A., Defoort C., Lovegrove J.A., Drevon C.A., Blaak E.E., et al. Leptin receptor polymorphisms interact with polyunsaturated fatty acids to augment risk of insulin resistance and metabolic syndrome in adults. J. Nutr. 2010;140:238–244. https://www.ncbi.nlm.nih.gov/pubmed/20032477
- Nuernberg K., Breier B.H., Jayasinghe S.N., Bergmann H., Thompson N., Nuernberg G., Dannenberger D., Schneider F., Renne U., Langhammer M., et al. Metabolic responses to high-fat diets rich in n-3 or n-6 long-chain polyunsaturated fatty acids in mice selected for either high body weight or leanness explain different health outcomes. Nutr. Metab. 2011;8:56. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3169453/
- Cheng L., Yu Y., Zhang Q., Szabo A., Wang H., Huang X.F. Arachidonic acid impairs hypothalamic leptin signaling and hepatic energy homeostasis in mice. Mol. Cell. Endocrinol. 2015;5, 412:12–18. doi: 10.1016/j.mce.2015.04.025. https://www.ncbi.nlm.nih.gov/pubmed/25986657
- Kang J.X. The importance of omega-6/omega-3 fatty acid ratio in cell function. The gene transfer of omega-3 fatty acid desaturase. In: Simopoulos A.P., Cleland L.G., editors. Omega-6/Omega-3 Essential Fatty Acid Ratio: The Scientific Evidence. Volume 92. Karger; Basel, Switzerland: 2003. pp. 23–36. https://www.ncbi.nlm.nih.gov/pubmed/14579681
- Banni S., Di Marzo V. Effect of dietary fat on endocannabinoids and related mediators: consequences on energy homeostasis, inflammation and mood. Mol. Nutr. Food Res. 2010;54:82–92. doi: 10.1002/mnfr.200900516. https://www.ncbi.nlm.nih.gov/pubmed/20013888
- Ahima R.S., Antwi D.A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. N. Am. 2008;37:811–823. doi: 10.1016/j.ecl.2008.08.005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2710609/
- Matias I., Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol. Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. https://www.ncbi.nlm.nih.gov/pubmed/17141520
- Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. The American Journal of Clinical Nutrition. 2011;93(4):780-788. doi:10.3945/ajcn.110.005801. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3057547/
- Circulation. 2014;130:1568-1578. Originally published August 26, 2014. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. http://circ.ahajournals.org/content/130/18/1568
- Cochrane review 16 November 2015 – Omega 6 intake to prevent cardiovascular disease – http://www.cochrane.org/CD011094/VASC_omega-6-intake-prevent-cardiovascular-disease
- Harris WS, Shearer GC. Omega-6 fatty acids and cardiovascular disease: friend, not foe? Circulation. 2014;130(18):1562-1564. http://circ.ahajournals.org/content/130/18/1562.long