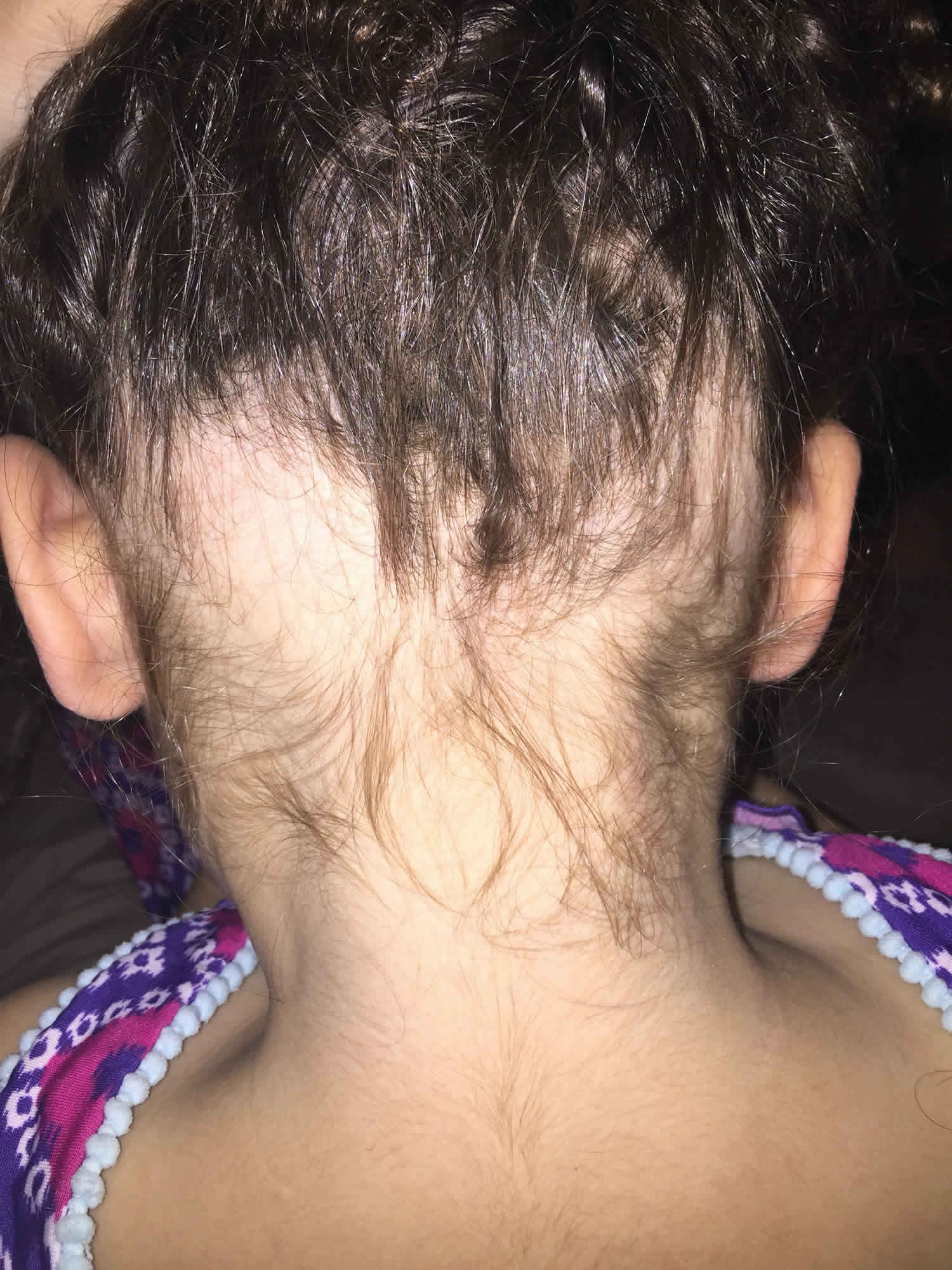
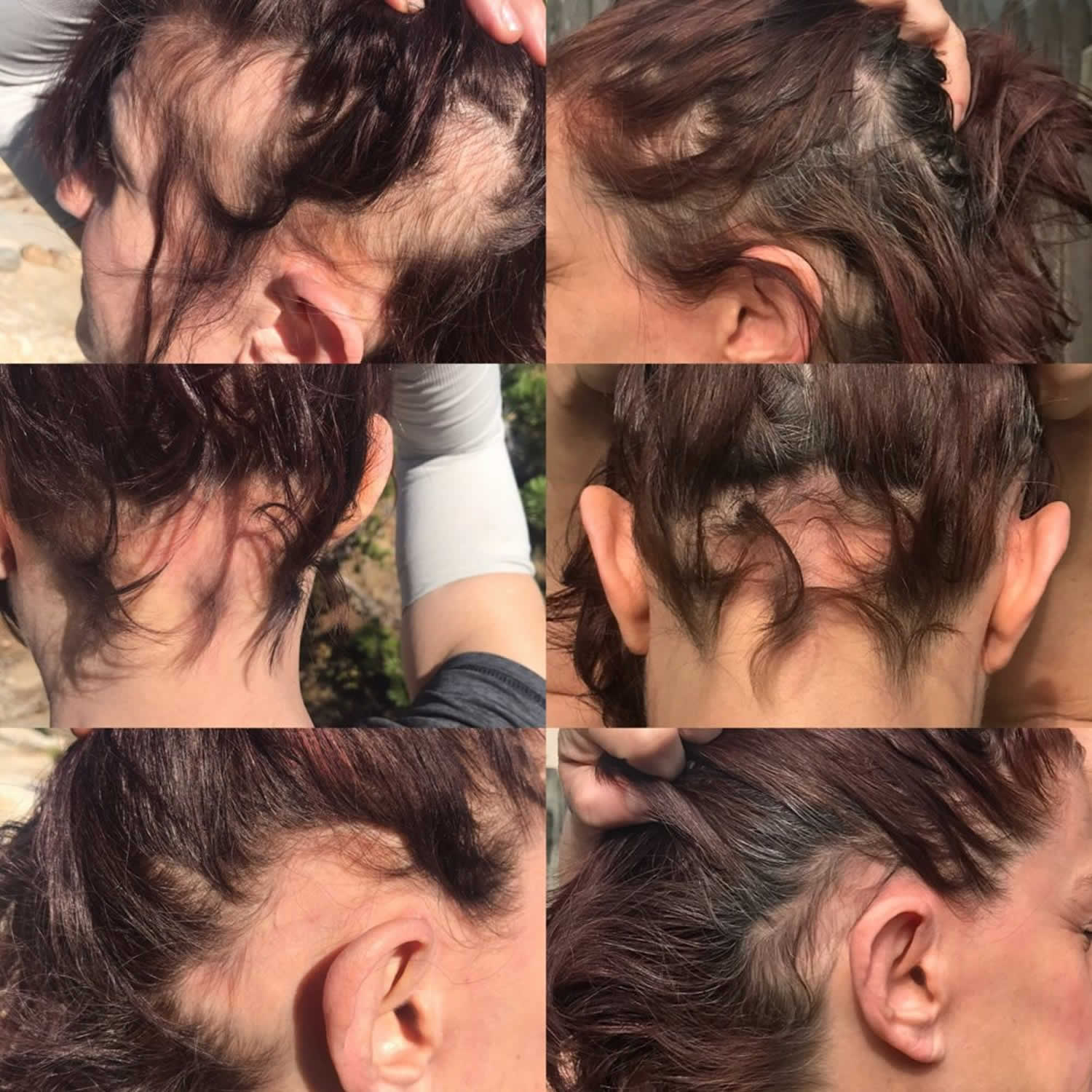
Ophiasis
Ophiasis is a subtype of alopecia areata that is characterized as a symmetric, band-like hair loss pattern of the occipital, temporal and parietal regions of the scalp 1. The bald area can encircle the scalp. The term alopecia means hair loss. Alopecia areata also called autoimmune alopecia, is a chronic inflammatory disease that affects the hair follicle causing patchy non-scarring hair loss on the scalp. Alopecia areata is a T cell–mediated autoimmune disease resulting in the destruction of hair follicles 2. In alopecia areata, one or more round bald patches appear suddenly, most often on the scalp.
Ophiasis gets its name from “ophis”, the Greek word for snake—referring to its snake-like distribution, particularly above the ears on the lateral aspects of the scalp 3. Ophiasis has a poorer prognosis than other forms of alopecia areata and is often refractory to conventional treatments, such as intralesional or topical corticosteroids, minoxidil, or topical immunotherapy 1.
Alopecia areata can affect males and females at any age. Alopecia areata starts in childhood in about 50% and before the age of 40 years in 80%, but the mean age of onset appears to be between 25 and 36 years 4. Early onset alopecia areata (between 5 to 10 years-old) predominantly presents as a more severe subtype 5. Data shows no demonstrable sex predilection. Lifetime risk is 1–2% and is independent of ethnicity. The overall incidence is approximately 20.2 per 100,000 person-years 6. The prevalence of adult patients with a family history is estimated to be between 0 and 8.6%.
Alopecia areata carries associations with an increased overall risk of other autoimmune diseases (16%), including lupus erythematosus, vitiligo, and autoimmune thyroid disease. Additionally, an association with atopic dermatitis exists in 39% of cases 7.
- A family history of alopecia areata and/or of other autoimmune disease are present in 10–25% of patients.
- At least 8 susceptibility genes have been detected.
- Patients with alopecia areata have higher than expected rates of thyroid disease, vitiligo and atopic eczema.
- There is an increased prevalence in patients with chromosomal disorders such as Down syndrome.
- It’s possibly drug-induced when arising in patients on biologic medicines.
Figure 1. Ophiasis pattern alopecia areata
Ophiasis causes
The true cause of alopecia areata remains unknown. Alopecia areata is classified as an autoimmune disorder that is most likely to occur in genetically predisposed individuals 8. It is histologically characterized by T cells around the hair follicles. These CD8+ NK group 2D-positive (NKG2D+) T cells release pro-inflammatory cytokines and chemokines that reject the hair. The exact mechanism is not yet understood.
About 20% of patients have a family history or genetic basis. A 55% concordance rate between identical twins has been observed 9. Recent genome-wide association studies metanalysis have localized the HLA signal of alopecia areata mostly to the HLA-DRB1. One locus harboring the genes that encode the natural killer cell receptor D (NKG2D) was implicated in alopecia areata and not in other autoimmune diseases, which suggests a key role in pathogenesis. Therefore, CD8+ NKG2D T cells have been a subject of study and found to be the major effectors in alopecia areata 10.
Alopecia areata is a disorder of hair follicle-cycling, where inflammatory cells attack the hair follicle matrix epithelium that is undergoing early cortical differentiation (anagen hair follicles), which are then prematurely induced into the catagen phase. However, since no destruction of hair-follicle stem cells occurs, the hair follicle retains its capacity to regenerate and continue cycling. Thereby, follicles re-enter the anagen phase normally but do not develop beyond the anagen III/IV phase.
Presumably, alopecia areata develops in a previously healthy hair follicle because its immune privilege collapses. Therefore, it could occur in a genetically predisposed person only when proinflammatory signals (i.e., IFN gamma, substance P) known to upregulate MHC class Ia in human hair-follicle epithelium expose previously unrevealed follicle-associated autoantigens to preexisting autoreactive CD8+ T cells.
Since only anagen hair follicles undergo attack, those autoantigens may generate and then be presented only during anagen.
The precise event that precipitates alopecia areata is unknown. Some triggers have been reported, most commonly emotional or physical stress, vaccines, viral infections, and drugs 7.
The onset or recurrence of hair loss is sometimes triggered by:
- Viral infection
- Trauma
- Hormonal change
- Emotional/physical stressors. The exact role of stressful events remains unclear, but they most likely trigger a condition already present in susceptible individuals, rather than acting as the true primary cause.
Autoimmunity
Much evidence supports the hypothesis that alopecia areata is an autoimmune condition. The process appears to be T-cell mediated, but antibodies directed to hair follicle structures also have been found with increased frequency in alopecia areata patients compared with control subjects. Using immunofluorescence, antibodies to anagen-phase hair follicles were found in as many as 90% of patients with alopecia areata compared with less than 37% of control subjects. The autoantibody response is heterogeneous and targets multiple structures of the anagen-phase hair follicle. The outer root sheath is the structure targeted most frequently, followed by the inner root sheath, the matrix, and the hair shaft. Whether these antibodies play a direct role in the pathogenesis or whether they are an epiphenomenon is not known.
Histologically, lesional biopsy findings of alopecia areata show a perifollicular lymphocytic infiltrate around anagen-phase hair follicles. The infiltrate consists mostly of T-helper cells and, to a lesser extent, T-suppressor cells. CD4+ and CD8+ lymphocytes likely play a prominent role because the depletion of these T-cell subtypes results in complete or partial regrowth of hair in the Dundee experimental bald rat model of alopecia areata. The animals subsequently lose hair again once the T-cell population is replete. The fact that not all animals experience complete regrowth suggests that other mechanisms likely are involved. Total numbers of circulating T lymphocytes have been reported at both decreased and normal levels.
Studies in humans also reinforce the hypothesis of autoimmunity. Studies have shown that hair regrows when affected scalp is transplanted onto severe combined immunodeficiency (SCID) mice that are devoid of immune cells. Autologous T lymphocytes isolated from an affected scalp were cultured with hair follicle homogenates and autologous antigen-presenting cells. Following initial regrowth, injection of the T lymphocytes into the grafts resulted in loss of regrown hairs. Injections of autologous T lymphocytes that were not cultured with follicle homogenates did not trigger hair loss.
A similar experiment on nude (congenitally athymic) mice failed to trigger hair loss in regrown patches of alopecia areata after serum from affected patients was injected intravenously into the mice. However, the same study showed that mice injected with alopecia areata serum showed an increased deposition of immunoglobulin and complement in hair follicles of both grafted and nongrafted skin compared with mice injected with control serum, which showed no deposition.
In addition, research has shown that alopecia areata can be induced using transfer of grafts from alopecia areata–affected mice onto normal mice. Transfer of grafts from normal mice to alopecia areata–affected mice similarly resulted in hair loss in the grafts.
Clinical evidence favoring autoimmunity suggests that alopecia areata is associated with other autoimmune conditions, the most significant of which are thyroid diseases and vitiligo. For instance, in a retrospective cross-sectional review of 2115 patients with alopecia areata who presented to academic medical centers in Boston over an 11-year period, comorbid autoimmune diagnoses included thyroid disease (14.6%), diabetes mellitus (11.1%), inflammatory bowel disease (6.3%), systemic lupus erythematosus (4.3%), rheumatoid arthritis(3.9%), and psoriasis and psoriatic arthritis (2.0%). Other comorbid conditions found included atopy (allergic rhinitis, asthma, and/or eczema; 38.2%), contact dermatitis and other eczema (35.9%), mental health problems (depression or anxiety; 25.5%), hyperlipidemia (24.5%), hypertension (21.9%), and gastroesophageal reflux disease (GERD) (17.3%) 11.
In conclusion, the beneficial effect of T-cell subtype depletion on hair growth, the detection of autoantibodies, the ability to transfer alopecia areata from affected animals to nonaffected animals, and the induction of remission by grafting affected areas onto immunosuppressed animals are evidence in favor of an autoimmune phenomenon. Certain factors within the hair follicles, and possibly in the surrounding milieu, trigger an autoimmune reaction. Some evidence suggests a melanocytic target within the hair follicle. Adding or subtracting immunologic factors profoundly modifies the outcome of hair growth.
Genetic factors
Many factors favor a genetic predisposition for alopecia areata. The frequency of positive family history for alopecia areata in affected patients has been estimated to be 10-20% compared with 1.7% in control subjects 8. The incidence is higher in patients with more severe disease (16-18%) compared with patients with localized alopecia areata (7-13%). Reports of alopecia areata occurring in twins also are of interest. No correlation has been found between the degree of involvement of alopecia areata and the type of alopecia areata seen in relatives.
Several genes have been studied and a large amount of research has focused on human leukocyte antigen. Two studies demonstrated that human leukocyte antigen DQ3 (DQB1*03) was found in more than 80% of patients with alopecia areata, which suggests that it can be a marker for general susceptibility to alopecia areata. The studies also found that human leukocyte antigen DQ7 (DQB1*0301) and human leukocyte antigen DR4 (DRB1*0401) were present significantly more in patients with alopecia totalis and alopecia universalis 12.
Another gene of interest is the interleukin 1 receptor antagonist gene, which may correlate with disease severity. Finally, the high association of Down syndrome with alopecia areata suggests involvement of a gene located on chromosome 21.
In summary, genetic factors likely play an important role in determining susceptibility and disease severity. Alopecia areata is likely to be the result of polygenic defects rather than a single gene defect. The role of environmental factors in initiating or triggering the condition is yet to be determined.
Cytokines
Interleukin 1 (IL-1) and tumor necrosis factor (TNF) were shown to be potent inhibitors of hair growth in vitro. Subsequent microscopic examination of these cultured hair follicles showed morphologic changes similar to those seen in alopecia areata.
Innervation and vasculature
Another area of interest concerns the modification of perifollicular nerves. The fact that patients with alopecia areata occasionally report itching or pain on affected areas raises the possibility of alterations in the peripheral nervous system. Circulating levels of the neuropeptide calcitonin gene-related peptide (CGRP) were decreased in 3 patients with alopecia areata compared with control subjects. Calcitonin gene-related peptide (CGRP) has multiple effects on the immune system, including chemotaxis and inhibition of Langerhans cell antigen presentation and inhibition of mitogen-stimulated T-lymphocyte proliferation.
Calcitonin gene-related peptide (CGRP) also increases vasodilatation and endothelial proliferation. Similar findings were reported in another study, in which decreased cutaneous levels of substance P and of CGRP but not of vasoactive intestinal polypeptide were found in scalp biopsy specimens. The study also noted a lower basal blood flow and greater vasodilatation following intradermal CGRP injection in patients with alopecia areata compared with control subjects. More studies are needed to shed light on the significance of these findings.
Viral cause
Other hypotheses have been proposed to explain the pathophysiology of alopecia areata, but more evidence is needed to support them. Alopecia areata was believed to possibly have an infectious origin, but no microbial agent has been isolated consistently in patients. Many efforts have been made to isolate cytomegalovirus, but most studies have been negative 13.
Ophiasis signs and symptoms
Ophiasis signs and symptoms:
- A pattern of alopecia areata affecting the occipital and lateral scalp
- The bald area can encircle the scalp.
Ophiasis diagnosis
Ophiasis alopecia areata is diagnosed clinically. Although usually straightforward, additional tests are sometimes needed to confirm the diagnosis.
- Trichoscopy (use of a dermatoscope to examine hair and scalp). The key dermoscopic findings are yellow dots, black dots, broken hairs, exclamation point hairs and short vellus hairs, which are a sign of early regrowth.
- Skin biopsy (histopathology).
Despite the association with other autoimmune diseases, (i.e., thyroid disease) current evidence does not support the need to screen for these diseases without a clinical history that suggests its presence 5.
Histopathology
Histologic examination demonstrates a characteristic “bee-swarm pattern” of dense lymphocytic infiltrates surrounding the bulbar region of anagen hair follicles 7. The lymphocytic infiltrate consists of CD8+ T cells in the follicular epithelium and CD4+ T cells around the hair follicles 14.
Ophiasis treatment
There is not yet any reliable cure for alopecia areata and other forms of autoimmune hair loss. Because spontaneous regrowth is common in alopecia areata, especially in the early stages of the disease, and research has often been of poor quality, the effectiveness of reported treatments is mostly unknown. Leaving alopecia areata untreated is a legitimate option for many patients 15.
Despite limited evidence for the efficacy of therapeutic agents, intralesional and topical corticosteroids are considered first-line treatment for most patients with patchy alopecia areata 9.
Patients with extensive disease, often defined as greater than 50% scalp hair loss, may be treated with topical immunotherapy. This approach avoids the large number of injections that would be otherwise required when using intralesional corticosteroids. Moreover, one retrospective study 16 reported superior efficacy of topical immunotherapy over intralesional corticosteroids for patients with patches of hair loss exceeding 50 cm2 in size. A potent contact allergen such as diphenylcyclopropenone (diphencyprone) or squaric acid dibutyl ester (SADBE) is applied weekly to the scalp to precipitate hair regrowth. Recently, a meta analysis looked at clinical outcomes of contact immunotherapy for alopecia areata 17. The rate of hair regrowth was 74.6% in the patchy alopecia subgroup, and 54.4% in the alopecia totalis/universalis subgroups. Recurrence rates were 38.2% in patients receiving maintenance treatment vs. 49% among those not receiving maintenance treatment.
Second-line therapies include minoxidil, anthralin, and PUVA (photochemotherapy). PUVA is a combination treatment which consists of Psoralens (P) and then exposing the skin to UVA (long wave ultraviolet radiation). Cases in the literature have described successful treatment of ophiasis alopecia areata with unconventional techniques such as khellin-excimer, platelet-rich plasma, psoralen and ultraviolet A (PUVA) and fexofenadine 18.
Systemic therapies are generally only for patients with severe alopecia areata. Systemic glucocorticoids may induce hair growth. However, they are not widely used, mainly because of their side effects. Additionally, relapse occurs within a year in one-third of responsive patients, and the number of relapses increases with time 7. Other systemic therapies include methotrexate, cyclosporine, azathioprine, and etanercept, all of which have shown variable clinical responses.
Systemic therapy is reserved for patients with:
- More than 20% of scalp hair loss
- Rapid hair loss
- Chronic hair loss
- Severe distress.
Current investigational treatments include platelet-rich plasma, recombinant IL-2, hydroxychloroquine, JAK inhibitors (tofacitinib), simvastatin with ezetimibe, and excimer laser 19.
Topical treatments
Several topical treatments used for alopecia areata are reported to result in temporary improvement in some people. Their role and efficacy are unknown. The hair may fall out when they are stopped. These include:
- Potent or ultrapotent topical steroids
- Minoxidil solution or foam
- Dithranol (anthralin) ointment.
Potent topical glucocorticoids find frequent utilization in the treatment of alopecia areata; however, evidence of effectiveness is limited. Topical steroids can be a reasonable therapeutic option in patients unlikely to tolerate intralesional injections. Utilization of occlusive dressings confers a higher response, leading to improvement in greater than 25% of patients. Glucocorticoid-induced folliculitis is a relatively common adverse effect of this approach 7.
Intralesional corticosteroid injections
Injections of triamcinolone acetonide 2.5–10 mg/ml into patchy scalp, beard or eyebrow alopecia areata may speed up regrowth of hair. Triamcinolone acetonide 5-10 mg per milliliter given every 2 – 6 weeks, stimulates localized re-growth in 60 – 67% of cases 7. Its effect is temporary. If bald patches reappear, they can be reinjected.
A study comparing different concentrations of intralesional triamcinolone acetonide (2.5 mg/ml, 5 mg/ml, 10 mg/ml) for the treatment of alopecia areata on the scalp, demonstrated similar rates of hair regrowth regardless of the concentration. However, the risk of cutaneous atrophy was higher at a higher concentration (10mg/ml) 20. The use of intralesional betamethasone has been proposed, however, further studies are needed to evaluate its efficacy 21. Side effects include localized skin atrophy, pain, and depigmentation. Local skin atrophy might resolve within a few months. Relapses are frequent after cessation of treatment.
Systemic corticosteroids
Oral and pulse intravenous steroids in high dose can lead to temporary regrowth of hair. Most physicians agree that long-term systemic steroid treatment is not justified because of potential and actual adverse effects.
Immunotherapy
The sensitizers diphenylcyclopropenone (diphencyprone) and dinitrochlorobenzene provoke contact allergic dermatitis in treated areas. These sensitizers can be reapplied once weekly to bald areas on the scalp. The resultant dermatitis is irritating and may be unsightly. It is often accompanied by a swollen lymph gland.
Other treatments
A combination of the lipid-lowering medications simvastatin and ezetimibe (which have immunomodulating effects) has been reported to be effective.
A single case is reported of substantial hair growth in a patient with longstanding alopecia totalis following the use of dupilumab for her concomitant severe atopic eczema.
There is no convincing data to support the use of methotrexate, sulfasalazine, azathioprine, ciclosporin or phototherapy.
JAK/STAT inhibitors
Several patients with severe alopecia areata have had improvement when treated with oral tofacitinib or oral ruxolitinib, which are Janus kinase (JAK) inhibitors. It is thought they may act by blocking interleukin (IL)-15 signalling and gamma interferon (IFNγ). Watch out for the results of clinical trials of these biologic medicines.
Camouflaging hair loss
Scalp
A hairpiece is often the best solution to disguise the presence of hair loss. These cover the whole scalp or only a portion of the scalp, using human or synthetic fibers tied or woven to a fabric base.
- A full wig is a cap that fits over the whole head.
- A partial wig must be clipped or glued to existing hair.
- A hair integration system is a custom-made hair net that provides artificial hair where required, normal hair being pulled through the net.
- Hair additions are fibers glued to existing hair and removed after 8 weeks
Styling products include gels, mousses and sprays to keep hair in place and add volume. They are reapplied after washing or styling the hair.
Eyelashes
Artificial eyelashes come as singlets, demilashes and complete sets. They can be trimmed if necessary. The lashes can irritate the eye and eyelids. They are stuck on with methacrylate glue, which can also irritate and sometimes causes contact allergic dermatitis.
Eyeliner tattooing is permanent and should be undertaken by a professional cosmetic tattooist. The color eventually fades and may move slightly from the original site. It is extremely difficult to remove the pigment, should the result turn out to be unsatisfactory.
Eyebrows
Artificial eyebrows are manufactured from synthetic or natural human hair on a net that is glued in place.
An eyebrow pencil can be obtained in a variety of colors made from inorganic pigments.
Tattooing can also be undertaken to disguise the loss of eyebrows but tends to look rather unnatural because of the shine of hairless skin.
Counseling
Alopecia areata is a benign condition and most patients are asymptomatic; however, it can cause emotional and psychosocial distress in affected individuals. Self-consciousness concerning personal appearance can become important. Openly addressing these issues with patients is important in helping them cope with the condition. Some people with alopecia areata seek and benefit from professional counseling to come to terms with the disorder and regain self-confidence.
Ophiasis prognosis
The potential for hair regrowth remains since the inflammatory process does not destroy hair follicles, especially stem cells. In many patients with a single bald patch, spontaneous regrowth occurs within a year. However, induction of regrowth by current treatments seems to be difficult to achieve.
Up to 34-50% of patients may recover spontaneously within one year, although most patients will experience multiple episodes of alopecia, and 14-25% of patients will progress to alopecia totalis or alopecia universalis, from which full recovery is infrequent (<10% of patients) 7.
Even in the most severe cases of alopecia totalis and alopecia universalis, recovery may occur at some future date. Research has shown:
- 40% of patients with a single patch of hair loss have full hair regrowth within 6 months.
- 27% of patients with multiple patches of hair loss have full regrowth within 12 months.
- 33% of patients with alopecia areata have chronic hair loss.
The extent of hair loss and patient age when first diagnosed appears to be a prognostic factor, being a less favorable prognosis with childhood-onset alopecia areata and ophiasis, and a later stage of onset correlates with less extensive alopecia 5. A family history of alopecia areata, nail dystrophy, a history of atopy, or concomitant autoimmune disease are also potential indicators of poor prognosis 22.
Poor prognostic factors include:
- Young age at onset
- Extensive disease
- Bald patches persisting for more than 1 year
- An ophiasis pattern of hair loss
- Alopecia areata of the nails
- The onset of alopecia areata before puberty
- Family members with alopecia areata
- Personal or family history of other autoimmune diseases
- Down syndrome.
New monoclonal antibody biologic agents targeting cytokine pathways offer promise for future treatment of alopecia areata.
References- Asad U, Wallis D, Tarbox M. Ophiasis alopecia areata treated with microneedling. Proc (Bayl Univ Med Cent). 2020;33(3):413-414. Published 2020 Apr 22. doi:10.1080/08998280.2020.1753456 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7340463
- Choi JW, Loh SH, Lew BL, Sim WY. Histopathologic features of ophiasis-type alopecia areata. J Am Acad Dermatol. 2016;76(6):AB156.c
- Troxell MA. Ophiasis: report of two cases. AMA Arch Derm Syphilol. 1954;70(6):812–814. doi:10.1001/archderm.1954.01540240118015
- Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J. Invest. Dermatol. 2014 Apr;134(4):1141-1142.
- Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017 Mar 16;3:17011.
- Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin. Proc. 1995 Jul;70(7):628-33.
- Gilhar A, Etzioni A, Paus R. Alopecia areata. N. Engl. J. Med. 2012 Apr 19;366(16):1515-25.
- van der Steen P, Traupe H, Happle R, Boezeman J, Sträter R, Hamm H. The genetic risk for alopecia areata in first degree relatives of severely affected patients. An estimate. Acta Derm Venereol. 1992 Sep. 72(5):373-5.
- Lepe K, Zito PM. Alopecia Areata. [Updated 2020 Aug 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537000
- Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017 Mar 16;3:17011
- Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, Atopic, and Mental Health Comorbid Conditions Associated With Alopecia Areata in the United States. JAMA Dermatol. 2013 May 22. 1-5.
- Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999 Dec. 4(3):216-9.
- Jackow C, Puffer N, Hordinsky M, Nelson J, Tarrand J, Duvic M. Alopecia areata and cytomegalovirus infection in twins: genes versus environment?. J Am Acad Dermatol. 1998 Mar. 38(3):418-25.
- Rajabi F, Drake LA, Senna MM, Rezaei N. Alopecia areata: a review of disease pathogenesis. Br. J. Dermatol. 2018 Nov;179(5):1033-1048.
- Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br. J. Dermatol. 2012 May;166(5):916-26.
- Ro BI. Alopecia areata in Korea (1982-1994). J. Dermatol. 1995 Nov;22(11):858-64.
- Lee S, Kim BJ, Lee YB, Lee WS. Hair Regrowth Outcomes of Contact Immunotherapy for Patients With Alopecia Areata: A Systematic Review and Meta-analysis. JAMA Dermatol. 2018 Oct 01;154(10):1145-1151.
- Fenniche S, Hammami H, Zaouak A. Association of khellin and 308-nm excimer lamp in the treatment of severe alopecia areata in a child. J Cosmet Laser Ther. 2018;20(3):156–158. doi:10.1080/14764172.2017.1383617
- Putterman E, Castelo-Soccio L. Topical 2% tofacitinib for children with alopecia areata, alopecia totalis, and alopecia universalis. J. Am. Acad. Dermatol. 2018 Jun;78(6):1207-1209.e1
- Chu TW, AlJasser M, Alharbi A, Abahussein O, McElwee K, Shapiro J. Benefit of different concentrations of intralesional triamcinolone acetonide in alopecia areata: An intrasubject pilot study. J. Am. Acad. Dermatol. 2015 Aug;73(2):338-40.
- Melo DF, Dutra TBS, Baggieri VMAC, Tortelly VD. Intralesional betamethasone as a therapeutic option for alopecia areata. An Bras Dermatol. 2018 Mar;93(2):311-312.
- Madani S, Shapiro J. Alopecia areata update. J. Am. Acad. Dermatol. 2000 Apr;42(4):549-66; quiz 567-70.