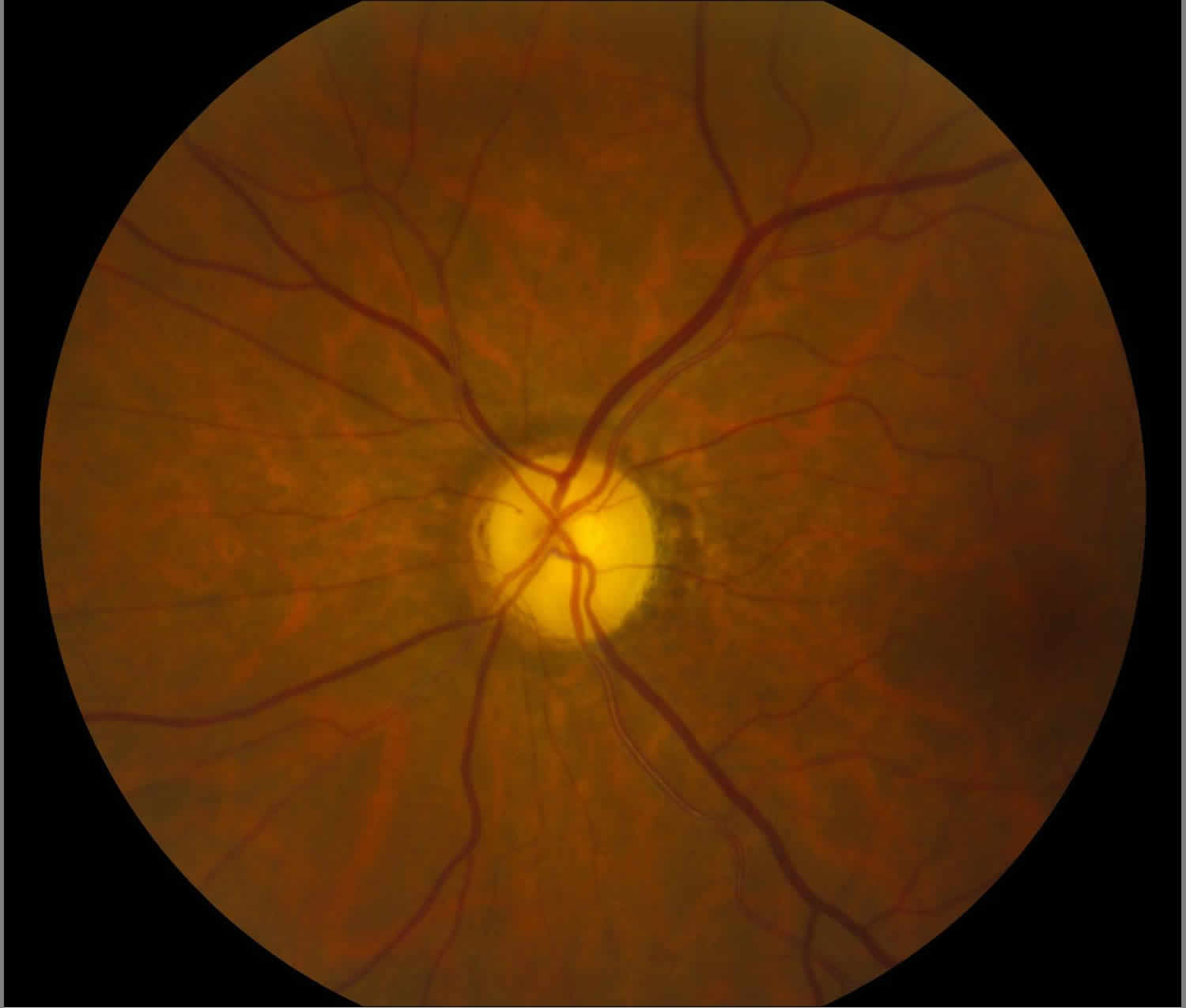
What is optic atrophy
Optic atrophy also called optic nerve atrophy, refers to the death of the retinal ganglion cell axons that comprise the optic nerve with the resulting picture of a pale optic nerve on funduscopy. Optic atrophy is an end stage that arises from myriad causes of optic nerve damage anywhere along the path from the retina to the lateral geniculate. Since the optic nerve transmits retinal information to the brain, optic atrophy is associated with vision loss. Clinically, optic atrophy manifests as changes in the color and the structure of the optic disc (cupping) associated with variable degrees of visual dysfunction. The term “atrophy” is a misnomer, since, in its strict histologic definition, atrophy implies involution of a structure due to prolonged disuse. Optic atrophy is somewhat of a misnomer as atrophy implies disuse and optic nerve damage is better termed optic neuropathy.
According to Tielsch et al 1, the prevalence of blindness attributable to optic atrophy was 0.8%. According to Munoz et al 2, the prevalence of visual impairment and blindness attributable to optic atrophy was 0.04% and 0.12%, respectively.
The optic nerve comprises approximately 1.2 million axons that originate at the ganglion cell layer of the retina. The axons of the optic nerve are heavily myelinated by oligodendrocytes, and the axons, once damaged, do not regenerate. Thus, the optic nerve behaves more like a white matter tract rather than a true peripheral nerve.
The optic nerve is divided into the following 4 parts:
- Intraocular part (1 mm) (optic nerve head)
- Intraorbital part (25 mm)
- Intracanalicular part (5-9 mm)
- Intracranial part (10-16 mm)
The average optic nerve head is 1 mm deep, 1.5 mm wide, 1.8 mm high at the retinal level, and a little wider posteriorly. The optic nerve head sits at a major transition between an area of high pressure to an area of low pressure (intracranial pressure) and is composed of 4 types of cells: ganglion cell axons, astrocytes, capillary-associated cells, and fibroblasts.
Light incident from the ophthalmoscope undergoes total internal reflection through the axonal fibers, and subsequent reflection from the capillaries on the disc surface gives rise to the characteristic yellow-pink color of a healthy optic disc. Degenerated axons lose this optical property, explaining the pallor in optic atrophy.
The blood supply at the optic nerve head is provided by pial capillaries arising from the circle of Zinn-Haller. These capillaries exhibit autoregulation and are not leaky. Alternatively, the loss of these capillaries leads to a pale-appearing disc. The Kestenbaum capillary number index is the number of capillaries observed on the optic disc. The normal count is approximately 10. In optic atrophy, the number of these capillaries reduces to less than 6, while more than 12 suggests a hyperemic disc.
Histopathologic changes noted in optic atrophy include the following:
- Shrinkage or loss of both myelin and axis cylinders
- Gliosis
- Deepening of the physiologic cup with barring of the lamina cribrosa
- Widening of the subarachnoid space with redundant dura
- Widening of the pial septa
- Severed nerve leads to bulbous axonal swellings (Cajal end bulbs); may be observed at the anterior cut end of the fibers.
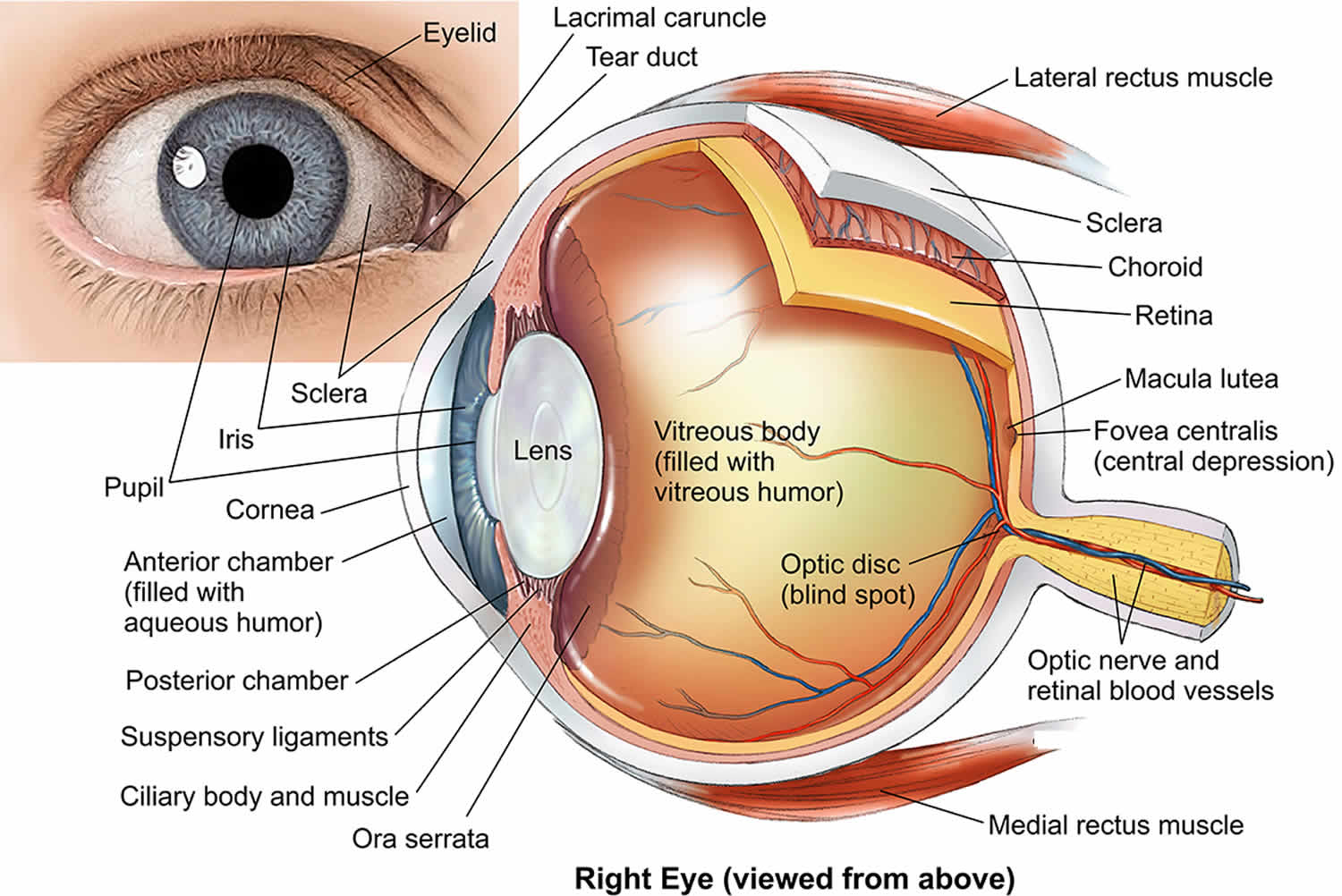
Figure 1. Human eye anatomy
Optic atrophy causes
Anything that can compromise ganglion cell function can cause (over time) optic atrophy (and more broadly optic neuropathy). Risk factors run the gamut from increased intraocular pressure (glaucoma), ischemia, compression (tumors), inflammation, infection, etc.
Optic atrophy is classified as pathologic, ophthalmoscopic, or etiologic.
Pathologic optic atrophy
Anterograde degeneration (Wallerian degeneration)
- Degeneration begins in the retina and proceeds toward the lateral geniculate body (eg, toxic retinopathy, chronic simple glaucoma). Larger axons disintegrate more rapidly than smaller axons.
Retrograde degeneration
- Degeneration starts from the proximal portion of the axon and proceeds toward the optic disc (eg, optic nerve compression via intracranial tumor).
Trans-synaptic degeneration
- In trans-synaptic degeneration, a neuron on one side of a synapse degenerates as a consequence of the loss of a neuron on the other side (eg, in individuals with occipital damage incurred either in utero or during early infancy).
Ophthalmoscopic optic atrophy
Primary optic atrophy
In conditions with primary optic atrophy (eg, pituitary tumor, optic nerve tumor, traumatic optic neuropathy, multiple sclerosis), optic nerve fibers degenerate in an orderly manner and are replaced by columns of glial cells without alteration in the architecture of the optic nerve head. The disc is chalky white and sharply demarcated, and the retinal vessels are normal. Lamina cribrosa is well defined.
Secondary optic atrophy
In conditions with secondary optic atrophy (eg, papilledema, papillitis), the atrophy is secondary to papilledema (shown in the image below). Optic nerve fibers exhibit marked degeneration, with excessive proliferation of glial tissue. The architecture is lost, resulting in indistinct margins. The disc is grey or dirty grey, the margins are poorly defined, and the lamina cribrosa is obscured due to proliferating fibroglial tissue. Hyaline bodies (corpora amylacea) or drusen may be observed. Peripapillary sheathing of arteries as well as tortuous veins may be observed. On visual fields, progressive contraction of visual fields may be seen.
Consecutive optic atrophy
In consecutive optic atrophy (eg, retinitis pigmentosa, myopia, central retinal artery occlusion), the disc is waxy pale with a normal disc margin, marked attenuation of arteries, and a normal physiologic cup.
Glaucomatous optic atrophy
Also known as cavernous optic atrophy, marked cupping of the disc is observed in glaucomatous optic atrophy. Characteristics include vertical enlargement of cup, visibility of the laminar pores (laminar dot sign), backward bowing of the lamina cribrosa, bayoneting and nasal shifting of the retinal vessels, and peripapillary halo and atrophy. Splinter hemorrhage at the disc margin may be observed.
Temporal pallor
Temporal pallor may be observed in traumatic or nutritional optic neuropathy, and it is most commonly seen in patients with multiple sclerosis, particularly in those with a history of optic neuritis. The disc is pale with a clear, demarcated margin and normal vessels, and the physiologic pallor temporally is more distinctly pale.
Etiologic optic atrophy
Hereditary atrophy
This is divided into congenital or infantile optic atrophy (recessive or dominant form), Behr hereditary optic atrophy (autosomal recessive), and Leber optic atrophy 3. Several hereditary optic neuropathies, including optic atrophy type 1 and Leber optic atrophy, have been attributed to mitochondrial dysfunction in retinal ganglion cells.
Autosomal-dominant optic atrophy type 1 is caused by mutations in the OPA1 gene on chromosome 3q29. The OPA1 protein produced plays a key role in a process called oxidative phosphorylation and in self-destruction of cells (apoptosis). OPA1 is an integral pro-fusion protein within the internal mitochondrial membrane. Mutations in the OPA1 gene lead to vision problems experienced by people with breakdown of structures that transmit visual information from the eyes to the brain. Affected individuals first experience a progressive loss of nerve cells within the retina, called retinal ganglion cells. The loss of these cells is followed by the degeneration (atrophy) of the optic nerve.
X-linked optic atrophy type 2 is caused by mutation in the OPA2 gene with cytogenetic location Xp11.4-p11.21. The patient presents with early-onset childhood vision loss with slow progression of loss.
Hereditary optic atrophy type 3 is caused by mutation in the OPA3 gene with cytogenetic location 19q13.32. The mutation in this gene is associated with childhood-onset vision loss with cataract. It can also be associated with type III methylglutaconic aciduria.
Leber hereditary optic neuropathy results from mitochondrial point mutations in mtDNA 11778G>A, 14484T>C, or 3460G>A mutations.
Consecutive atrophy
Consecutive atrophy is an ascending type of atrophy (eg, chorioretinitis, pigmentary retinal dystrophy, cerebromacular degeneration) that usually follows diseases of the choroid or the retina.
Circulatory atrophy (vascular)
Circulatory is an ischemic optic neuropathy observed when the perfusion pressure of the ciliary body falls below the intraocular pressure. Circulatory atrophy is observed in central retinal artery occlusion, carotid artery occlusion, and cranial arteritis.
Metabolic atrophy
It is observed in disorders such as thyroid ophthalmopathy, juvenile diabetes mellitus, nutritional amblyopia, toxic amblyopia, tobacco, methyl alcohol, and drugs (eg, ethambutol, sulphonamides).
Demyelinating atrophy
It is observed in diseases such as multiple sclerosis and Devic disease.
Pressure or traction atrophy
It is observed in diseases such as glaucoma and papilledema.
Postinflammatory atrophy
It is observed in diseases such as optic neuritis, perineuritis secondary to inflammation of the meninges, and sinus and orbital cellulites.
Traumatic optic neuropathy
The exact pathophysiology of traumatic optic neuropathy is poorly understood, although optic nerve avulsion and transection, optic nerve sheath hematoma, and optic nerve impingement from a penetrating foreign body or bony fragment all reflect traumatic forms of optic nerve dysfunction that can lead to optic atrophy.
Regardless of etiology, optic atrophy is associated with variable degrees of visual dysfunction, which may be detected by one or all of the optic nerve function tests.
Radiation optic neuropathy
Radiation optic neuropathy more frequently occurs with radiation doses of at least 5,000 centigray. It may be result from radiation damage to the optic nerve vasculature or the optic nerve parenchyma itself.
Optic atrophy prevention
Optic atrophy is the end stage of a process causing damage to the optic nerve. Medical practice is currently unable to return function (regrow axons) to an atrophic optic nerve, and at best is able to stabilize whatever function remains. Primary prevention (removal of the process causing the damage) is the goal to prevent loss of axons and optic atrophy (neuropathy).
Optic atrophy symptoms
The main symptom of optic atrophy is vision loss. Any other symptoms are attributable to the underlying process that caused the disc damage (such as pain with angle closure glaucoma).
Optic atrophy is a sign and typically is noted as optic nerve pallor. This is the end stage of a process resulting in optic nerve damage. Because the optic nerve fiber layer is thinned or absent the disc margins appear sharp and the disc is pale, probably reflecting absence of small vessels in the disc head.
Optic atrophy diagnosis
History is critical in the diagnosis of optic atrophy since the physician needs to know how the eye arrived at this juncture. A careful history with attention to past medical history including all medications, time course of vision loss, associated symptoms etc is critical for arriving at a correct diagnosis. A complete eye exam including visual field, assessing color and contrast vision, intraocular pressures, looking for afferent pupil defect, and funduscopy should be done.
Optic atrophy is usually not difficult to diagnose (characteristic pale optic disc) but the cause for the optic atrophy may be difficult to ascertain. Sometimes the cause of vision loss may be difficult to differentiate between subtle optic neuropathy and disease of the retina (or both). Electrophysiology can be helpful (ERG, mERG) and OCT to assess the thickness of the nerve fiber layer may be helpful in such cases.
Characteristic visual field patterns include papillomacular defect (cecocentral scotoma), arcuate defect (include altitudinal) or temporal wedge defect (nasal fibers) for prechiasmal, bitemporal (superior) field defects for chiasmal lesions, and hemianopsia for post-chiasmal lesions.
The following work up should be considered for patients presenting with unexplained optic atrophy:
- Check for afferent pupil
- Visual fields 30-2,color vision
- MRI of brain and orbit with contrast
- CT with contrast (check bony disease, sinuses)
- Blood pressure and check of cardiovascular health (carotids, etc.), Glucose
Screen for these if history or examination are suggestive:heavy metals, B12, folate, FTA ABS, VDRL, ANA, homocysteine, ACE, Antiphospholipid antibodies, TORCH panel 4.
Diagnostic procedures
- Visual Field Testing (Humphrey 30-2, Tangent Screen) – to help localize the location of the lesion.
- Optical Coherence Tomography (OCT)- to assess the thickness of peripapillary retinal nerve fiber layer and/or ganglion cell layer.
- Electroretinography (ERG) and multifocal electroretinogram (MERG) to rule out retinal disease.
- Neuro-imaging (MRI, CT)
Optical Coherence Tomography (OCT) has become a valuable tool to verify the status of the nerve fiber layer/ganglion axons. Quantification of the nerve fiber layer height and comparison with normative data can document axon loss and differentiate between optic nerve and retinal disease as a cause for vision loss.
Since the optic nerve is the conduit for information from the retina to the brain, a damaged optic nerve will result in vision loss. Subtle damage might not affect acuity but may lead to a loss of contrast or color vision. Severe damage may lead from legal blindness to no light perception. Damage to a part of the optic nerve results in loss of vision in the corresponding visual field. Occasionally if the process causing damage is removed before apoptosis occurs (as for instance removal of a pituitary tumor compressing the chiasm or reducing inflammation in sarcoid) some improvement in visual function may be noted. A complete diagnosis is based on optic nerve appearance, tests of visual function (visual field, contrast, color, acuity), identifying the causative factor of the damage, and ruling out other causes for vision loss (such as retinal causes).
Certain disc appearances can help to determine the cause for the optic nerve damage. Sector disc pallor in an older individual could have been caused by non-arteritic anterior ischemic optic neuropathy due to loss of blood flow to the optic nerve. Non-arteritic anterior ischemic optic neuropathy typically causes sudden vision loss in one eye, without any pain. Severe optic atrophy with gliosis again in an elderly person could have been due to giant cell arteritis. Damage from papilledema may leave retinal folds and sometimes glistening bodies in the optic nerve head. Cupping is suggestive of glaucoma.
When examining a patient with a pale disc, determine primarily if the pallor is physiologic. Nonpathologic disc pallor is observed in the following:
- Axial myopia: The optic disc has a segmental whitish appearance due to an oblique angle of insertion of the optic nerve and nasal displacement of the optic nerve contents.
- Myelinated nerve fibers: Feathery margins are due to the superficial location, usually adjacent to the disc.
- Optic nerve pit: Small colobomas are most often located in the inferotemporal portion of the disc.
- Tilted disc can cause confusion.
- Optic nerve hypoplasia is characterized by a small disc and peripapillary double ring sign, and the inner ring is actually the optic disc margin.
- Scleral crescent areas are devoid of retinal pigment epithelium.
- Optic disc drusen
- Fundus viewing through an intraocular lens implant
- Brighter-than-normal luminosity: The luminosity of an indirect ophthalmoscope is approximately 2000 lux and that of a direct ophthalmoscope is up to 900 lux. A disc appears pale if the luminosity of the instrument is brighter than normal.
Optic atrophy in young individuals
Hereditary and congenital optic atrophy generally presents in the first or second decade of life. They can be broadly classified into the following 3 major groups:
- Optic atrophy with generalized white matter disease (eg, adrenoleukodystrophy)
- Optic atrophy with seemingly unrelated systemic features (generally associated with OPA1 gene mutation)
- Isolated optic atrophy (may be autosomal dominant or recessive mitochondrial inheritance; eg, Leber hereditary optic neuropathy)
Optic atrophy treatment
No proven treatment reverses optic atrophy. However, treatment that is initiated before the development of optic atrophy can be helpful in saving useful vision.
The role of intravenous steroids is proven in a case of optic neuritis or arteritic anterior ischemic optic neuropathy. Early diagnosis and prompt treatment can help patients with compressive and toxic neuropathies.
Idebenone, a quinone analog, has been used and is the only clinically proven drug in the treatment of Leber hereditary optic neuropathy. The drug molecule bypasses the defective mitochondrial complex I, leading to improved energy supply and a functional recovery of retinal ganglion cells during the acute stage of the disease, thereby preventing further vision loss and promoting vision recovery 5. So far, the results were noted to be modest and the treatment is quite expensive. Klopstock et al conducted a 24-week multicenter double-blind, randomized, placebo-controlled trial in 85 patients with Leber hereditary optic neuropathy. They did not find a statistically significant visual recovery in the intention-to-treat population. They did find, however, evidence that patients with discordant visual acuities are the most likely to benefit from idebenone treatment, which is safe and well tolerated 6.
Research in stem cell treatment can hold a key in the future treatment of neuronal disorders. Currently, there are no approved treatments for mitochondrial disease, including optic neuropathies caused by primary or secondary mitochondrial dysfunction 7.
de Lima et al were able to restore some depth perception in mice with severe optic nerve damage. In addition, they found that the mice regained the ability to detect overall movement of the visual field and were able to perceive light. They found that using adequate stimulus, the fibers (1) are able to find their way to the correct visual centers in the brain, (2) are wrapped in the conducting insulation known as myelin, and (3) can make connections (synapses) with other neurons, allowing visual circuits to re-form. At present, the best defense is an early diagnosis because if the cause can be found and corrected, further damage can be prevented. de Lima et al discovered a molecule called oncomodulin. They achieved neuroregeneration in mice by simultaneously targeting the protein oncomodulin, elevating levels of the small signaling molecule cyclic adenosine monophosphate (cAMP) and deleting the gene that encodes the enzyme PTEN 8.
The optic nerve fiber is made of axons from the retinal ganglion cells, which usually do not regenerate after injury, resulting in lifelong visual loss. In recent studies using hamster models, anterograde tracing and electrophysiologic responses reveal that a small number of axons can regenerate all the way back to the superior colliculus 9. In other studies, remapping of the retina was noted in the superior colliculus following axon regeneration 10. These findings have given hope to clinically meaningful regeneration of axons, which may become a reality in the near future.
At present, the best defense is early diagnosis, because, if the cause can be found and corrected, further damage can be prevented.
Low-vision aids for patients with some useful vision should be considered for occupational rehabilitation.
Optic atrophy prognosis
Early and intensive treatment in nutritional optic neuropathy can provide patients with near-normal vision. Studies in glaucoma (based on Optical Coherence Tomography (OCT) nerve fiber layer measurements and other methods) have shown that the optic nerve has some reserve (axons) before vision loss is appreciated. After that reserve is depleted small changes in nerve fiber loss lead to significant decrease in vision. Early detection is key since you cannot replace dead axons.
References- Tielsch JM, Javitt JC, Coleman A, et al. The prevalence of blindness and visual impairment among nursing home residents in Baltimore. N Engl J Med. 1995 May 4. 332(18):1205-9.
- Munoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000 Jun. 118(6):819-25.
- Nakaso K, Adachi Y, Fusayasu E, Doi K, Imamura K, Yasui K, et al. Leber’s Hereditary Optic Neuropathy with Olivocerebellar Degeneration due to G11778A and T3394C Mutations in the Mitochondrial DNA. J Clin Neurol. 2012 Sep. 8(3):230-4.
- Lee AG, Chau FY, Golnik KC, Kardon RH, Wall M. The diagnostic yield of the evaluation for isolated unexplained optic atrophy. Ophthalmology 2005; 112(5):757-759
- Gueven N. Idebenone for Leber’s hereditary optic neuropathy. Drugs Today (Barc). 2016. 52(3):173-81.
- Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011 Sep. 134:2677-86.
- Lopez Sanchez MI, Crowston JG, Mackey DA, Trounce IA. Emerging Mitochondrial Therapeutic Targets in Optic Neuropathies. Pharmacol Ther. 2016. Sep 165:132-52
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012 Jun 5. 109(23):9149-54.
- Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M. Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science. 1989 Oct 13. 246(4927):255-7.
- Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001 Dec. 172(2):257-72.