What is orexin
Orexins also known as hypocretins are two excitatory neuropeptides, orexin peptides A and B also called hypocretin 1 and 2 respectively, which are derived from a single polypeptide precursor (prepro-orexin) produced by a cluster of neurons in the lateral and posterior hypothalamus and perifornical area 1. The neuropeptides orexins have been associated with numerous physiological functions, including sleep-wake cycle, appetite, cognition, energy homeostasis, endocrine, visceral functions and pathological states, such as narcolepsy and drug abuse, all of which are affected in stress-related mental illness 2. Furthermore, orexins are known to play a role many of the phenotypes associated with stress-related mental illness such as changes in cognition, sleep-wake states, and appetite 3. Orexins are altered in anxious and depressed patients 4. Interestingly, orexins are altered in stress-related psychiatric disorders such as Major Depressive Disorder and Anxiety Disorders. Thus, orexins may be a potential target for treatment of these disorders.
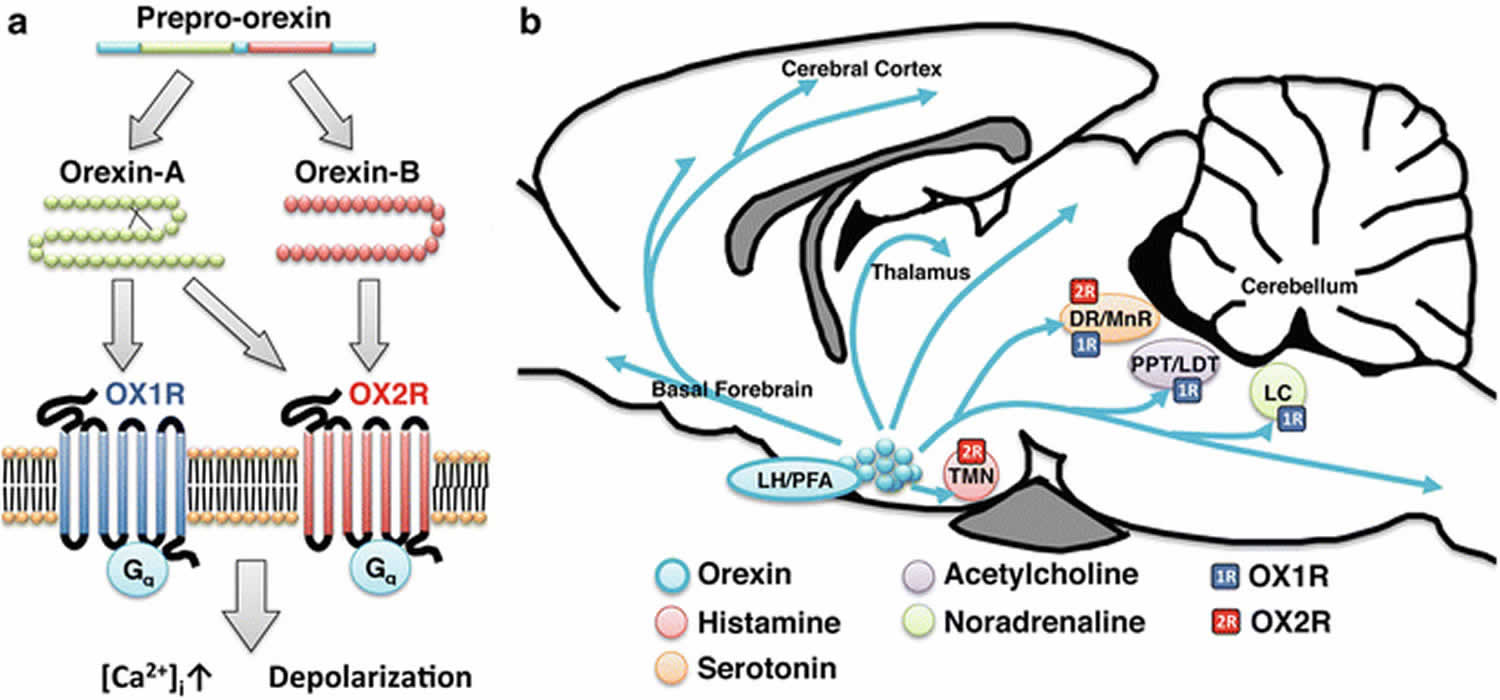
Prepro-orexin is cleaved into two highly structurally related and highly conserved peptides, Orexin A (composed of 33 amino acids) and orexin B (composed of 28 amino acids) 5. There are approximately 10,000–20,000 orexinergic neurons in the human brain 6.
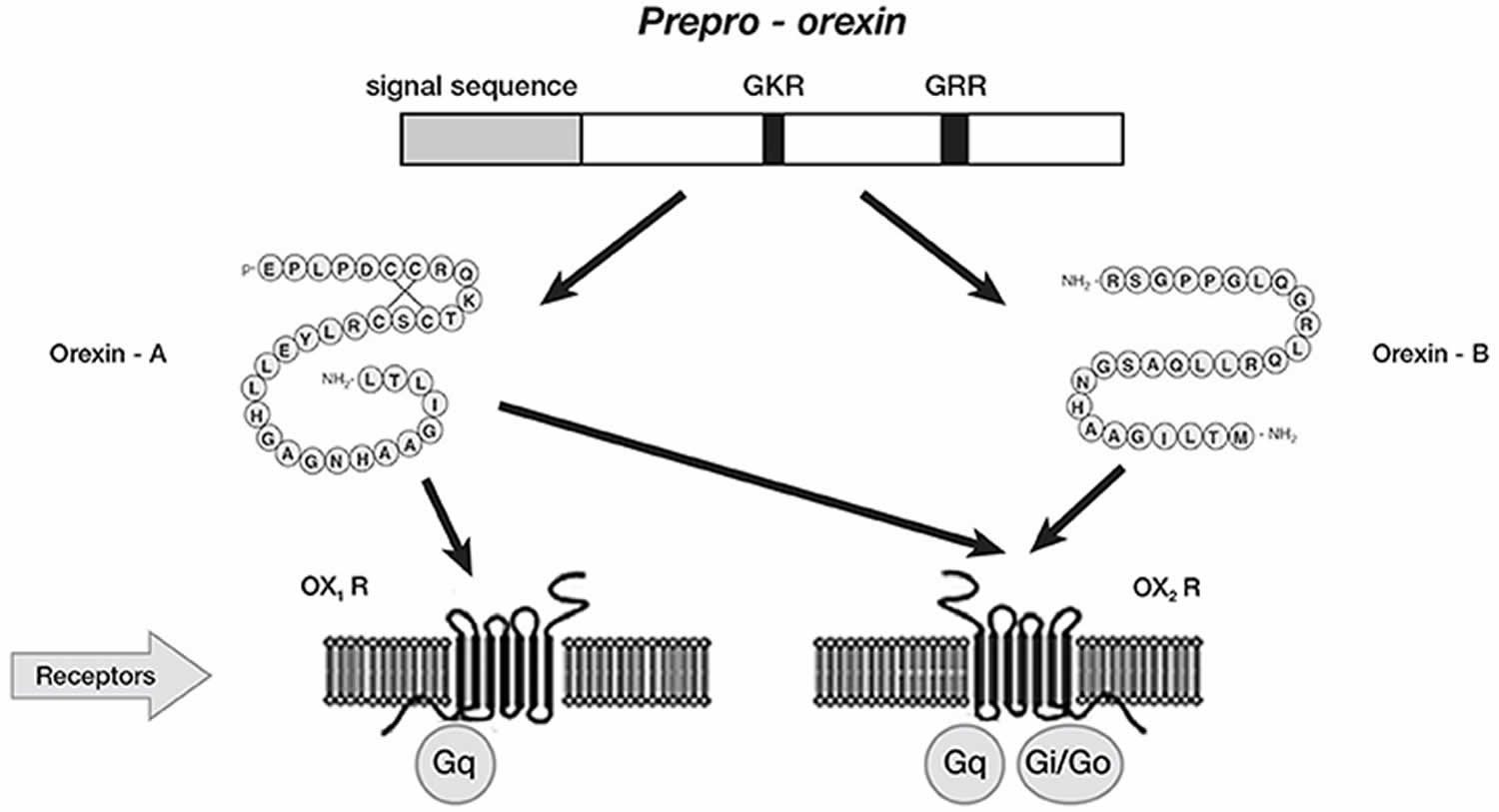
Orexin A is a neuropeptide composed of 33 amino acids with an amino(N)-terminal pyroglutamyl residue, two intra-chain disulphide bonds and carboxy (C)-terminal amidation, while Orexin B is a linear neuropeptide sized 28 amino acids, C-terminally amidated. The N-terminal portion presents more variability, whilst the C-terminal portion is similar between the two subtypes. The orexins activity is modulated by their specific receptors (OX1R, OX2R). OX1R presents higher affinity for orexin A than B and transmits signals throughout the G-protein class activating a cascade that leads to an increase in intracellular calcium concentration. By contrast, OX2R binds the two subtypes of orexin with similar affinities, probably associated to a G inhibitory protein class 7. These differences seem to suggest different physiological roles for OX1R and OX2R 8. Different physiological roles of OX1R and OX2R seem to be supported by the observation that mRNAs receptors show complementary distribution patterns (Figures 1 and 2):
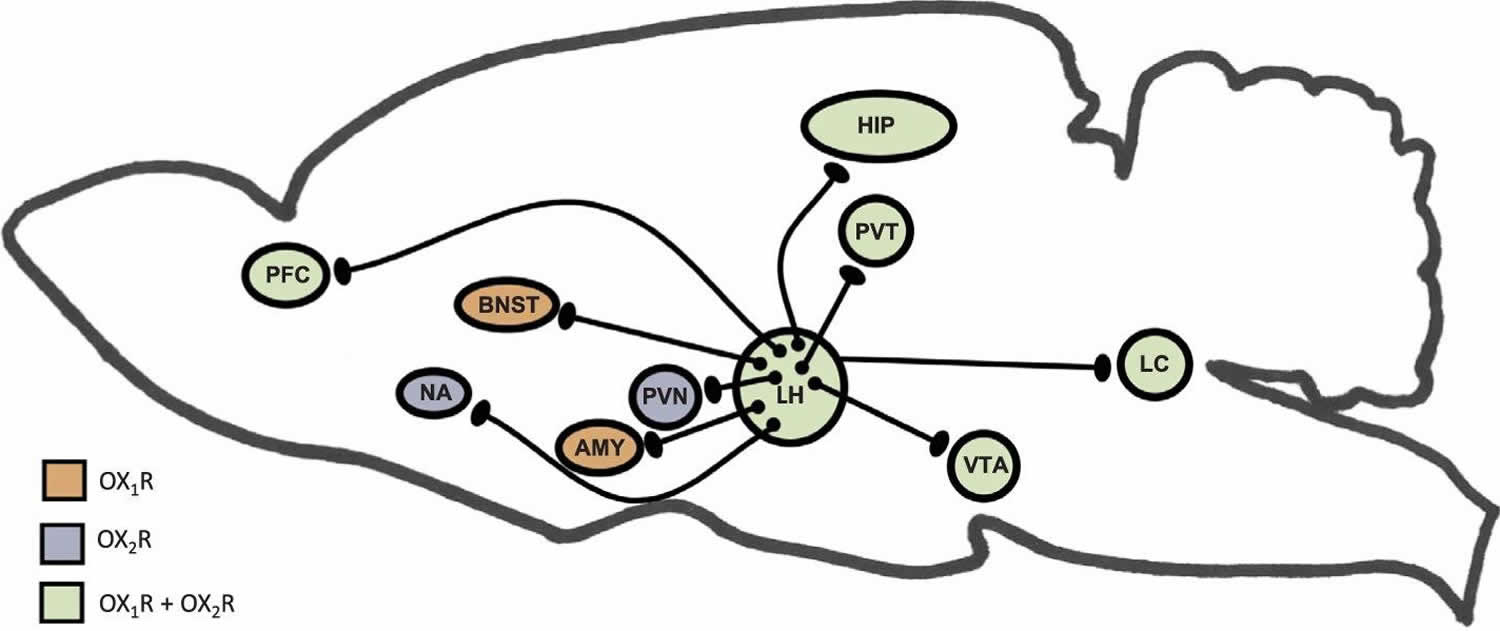
- OX1R is distributed in Prefrontal cortex (PFC) and infralimbic cortex, Hippocampus (HIP), Amygdala (AMY), Bed Nucleus of the Stria Terminalis (BNST), paraventricular nucleus of the thalamus (PVT), anterior hypothalamus, dorsal raphe (DR), Ventral Tegmental Area (VTA), locus coeruleus (LC), and laterodorsal tegmental nucleus (LDT)/pedunculopontine nucleus (PPT) 9;
- OX2R is distributed in amygdala, tuberomammillary nucleus (TMN), arcuate nucleus, dorsomedial hypothalamic nucleus (DMH), paraventricular nucleus (PVN), lateral hypothalamic area (LHA), Bed Nucleus of the Stria Terminalis (BNST), paraventricular thalamus (PVT), dorsal raphe, Ventral Tegmental Area (VTA), laterodorsal tegmental nucleus (LDT)/pedunculopontine nucleus (PPT), CA3 in the hippocampus, and medial septal nucleus 9.
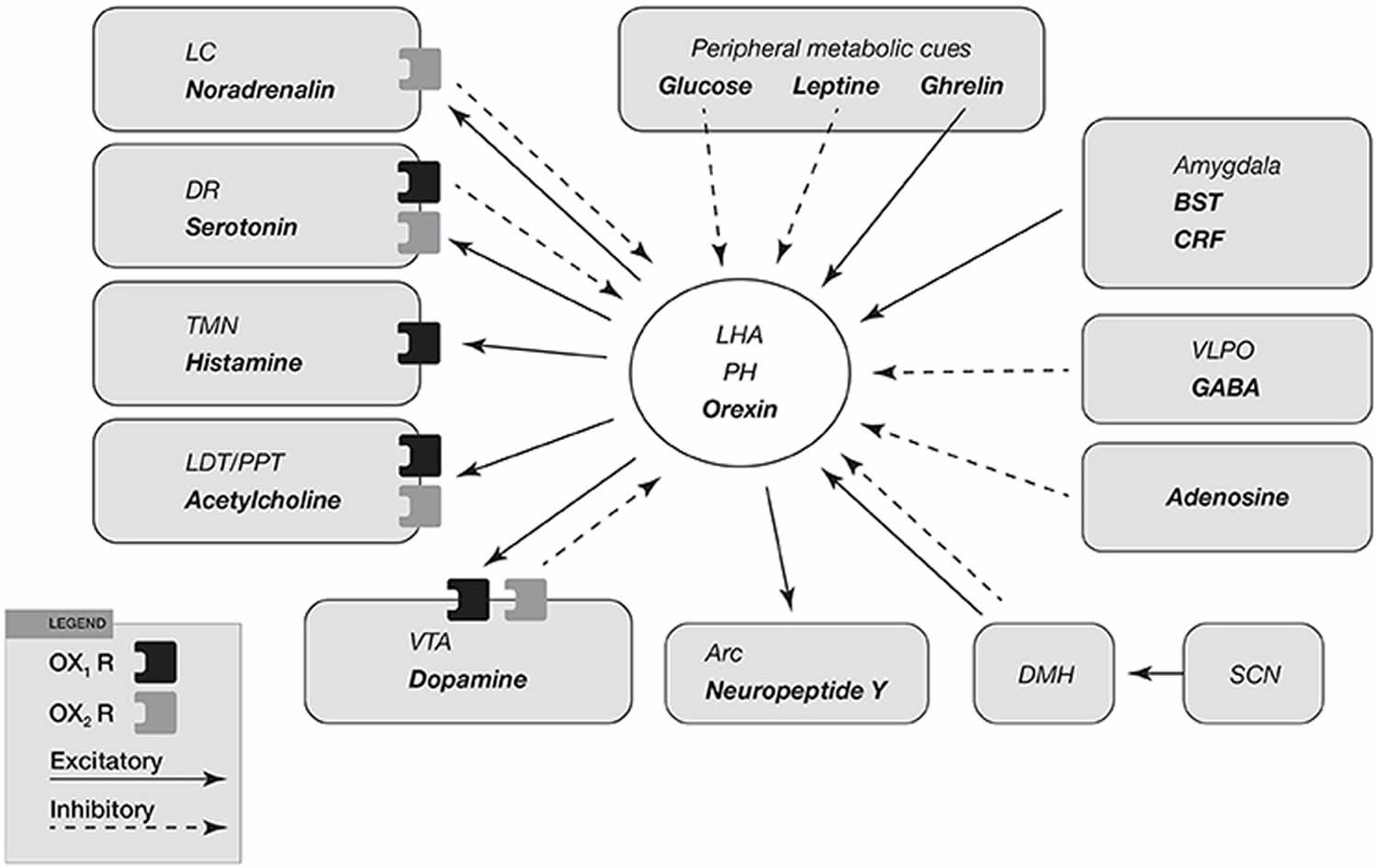
Figure 1. Orexin receptor
Figure 2. Orexin neurons
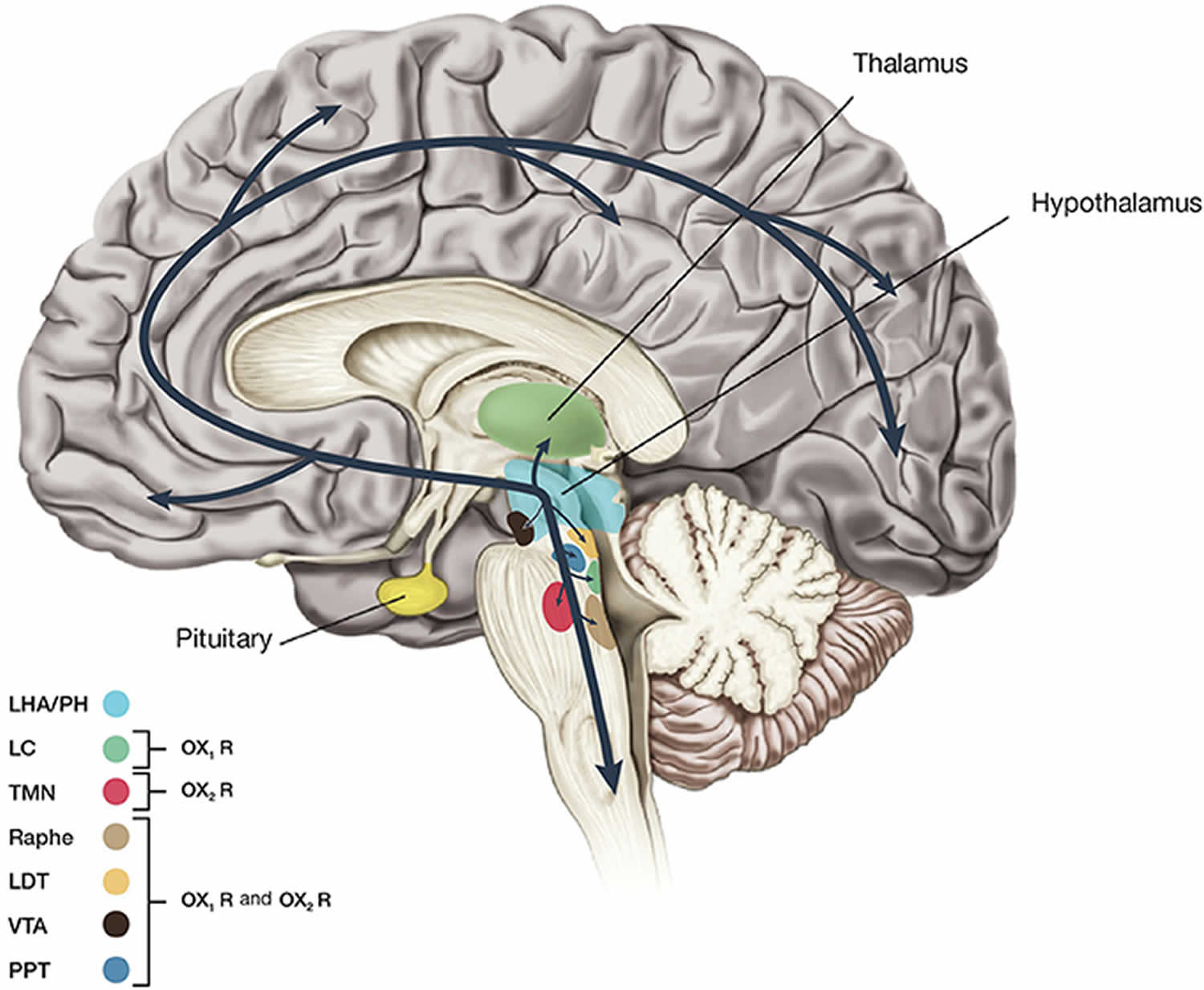
Footnotes: Input and output of orexin neurons at interface of sleep, stress, reward, and energy homeostasis. Orexin neurons in the lateral hypothalamic area (LHA) and posterior hypothalamus (PH) are placed to provide a link among limbic system, energy homeostasis, brainstem and other systems. Arrows show excitatory projections and broken arrows inhibitory projections. Gray semicircles indicate OX1R and black semicircles indicate OX2R. Neurotransmitters/modulators are underlined. locus ceruleus, dorsal raphe, and tuberomammillary nucleus are wake-active regions, ventrolateral preoptic area is sleep-active region, and laterodorsal tegmental nucleus/pedunculopontine tegmental nucleus is REM-active region. Orexin neurons promote wakefulness through monoaminergic nuclei that are wake-active. Stimulation of dopaminergic centers by orexins modulates reward systems (VTA). Peripheral metabolic signals influence orexin neuronal activity to coordinate arousal and energy homeostasis. Stimulation of neuropeptide Y neurons by orexin increases food intake. The suprachiasmatic nucleus, the central body clock, sends input to orexin neurons via the dorsomedial hypothalamus. Input from the limbic system (amygdala and bed nucleus of the stria terminalis) might be important to regulate the activity of orexin neurons upon emotional stimuli to evoke emotional arousal or fear-related responses.
Abbreviations: BST = bed nucleus of the stria terminalis; VLPO = ventrolateral preoptic area; LC = locus ceruleus; DR = dorsal raphe; TMN = tuberomammillary nucleus; LDT = laterodorsal tegmental nucleus; PPT = pedunculopontine tegmental nucleus; VTA = ventral tegmental area; SCN = suprachiasmatic nucleus; DMH = dorsomedial hypothalamus; Arc = arcuate nucleus.
Figure 3. Orexin projections to stress-relevant brain regions
Footnotes: This illustration depicts orexin neurons in the lateral hypothalamus (LH) and several stress-relevant projection sites. The expression of orexin receptors within these brain regions is also shown. Orexins are known directly innervate the prefrontal cortex (PFC) and Hippocampus (HIP), and orexin activation during stress underlie cognitive impairments. Orexin receptors in the Nucleus Accumbens (NA) and Ventral Tegmental Area (VTA) mediate stress-induced reward seeking. Orexins regulate activity in the hypothalamic pituitary adrenal (HPA) axis through projections to the paraventricular nucleus of the hypothalamus (PVN), where neurons expressing Corticotropin Releasing Hormone (CRH) are located. Further, orexin neurons densely project to the paraventricular nucleus of the thalamus (PVT), which plays a role in regulating neuroendocrine and behavioral adaptations to repeated or chronic stress. Orexins mediate behaviors relevant to affect and mood in humans (depression, anxiety, fear) through actions in the Bed Nucleus of the Stria Terminalis (BNST) and the Amygdala (AMY). Orexins also have inputs to brain regions important for arousal, such as the locus coeruleus (LC), which in turn can regulate activity in the limbic, thalamic and hypothalamic structure that are directly regulated by orexins.
[Source 3 ]Where is orexin produced?
Orexin-A (hypocretin-1) and orexin-B (hypocretin-2) are produced from the polypeptide precursor (prepro-orexin) precursor that is exclusively localized in cells of the lateral and posterior hypothalamic region 10 and perifornical area 1. There are approximately 10,000–20,000 orexinergic neurons in the human brain 6.
Orexin neurons can be round, elliptical, or fusiform, and either bipolar or unipolar 11. In addition, orexin neurons are large, with a diameter of 21 μm on average 11. Orexin neurons are intrinsically in a depolarized state due to constitutively active non-selective cation currents 12. Orexin activity, including peptide levels and neural function, varies across the circadian cycle, supporting the involvement of orexins in regulating arousal 13. Almost all orexin neurons (94%) also synthesize the peptide dynorphin, an opioid receptor agonist 14, while about 50% of orexin neurons are thought to also contain the excitatory neurotransmitter glutamate 15. Some studies have suggested that there is functional dichotomy for orexin neurons; specifically, that orexin neurons in the perifornical and dorsomedial hypothalamic areas are involved in stress and arousal, while those in the lateral hypothalamus are more involved in addiction 16. However, this anatomical specificity has not been investigated in the majority of the current literature.
Orexin neurons receive a variety of signals related to environmental, physiological and emotional stimuli, and project broadly to the entire central nervous system (CNS). Orexin neurons are “multi-tasking” neurons regulating a set of vital body functions, including sleep-wake cycle, feeding behavior, energy homeostasis, reward systems, cognition and mood 1. Furthermore, a dysfunction of orexinergic system may underlie different pathological conditions. A selective loss orexin neurons was found in narcolepsia, supporting the crucial role of orexins in maintaining wakefulness. In animal models, orexin deficiency lead to obesity even if the consume of calories is lower than wildtype counterpart. Reduced physical activity appears the main cause of weight gain in these models resulting in energy imbalance. Orexin signaling promotes obesity resistance via enhanced spontaneous physical activity and energy expenditure regulation and the deficiency/dysfunction in orexins system lead to obesity in animal models despite of lower calories intake than wildtype associated with reduced physical activity. Interestingly, orexinergic neurons show connections to regions involved in cognition and mood regulation, including hippocampus. Orexins enhance hippocampal neurogenesis and improve spatial learning and memory abilities, and mood. Conversely, orexin deficiency results in learning and memory deficits, and depression.
Figure 4. Orexin production
Footnote: Schematic representation of orexin system. Orexin A and orexin B are derived from a common precursor peptide, prepro-orexin. The actions of orexins are mediated via two G protein-coupled receptors named orexin-1 (OX1R or hypocretin 1 receptor) and orexin-2 (OX2R or hypocretin 2 receptor) receptors. OX1R is selective for orexin A, whereas orexin-2 (OX2R or hypocretin 2 receptor) is a non-selective receptor for both orexin A and orexin B. OX1R is coupled exclusively to the Gqsubclass of heterotrimeric G proteins, whereas OX2R couples to Gi/oand/or Gq.
[Source 1 ]Factors that influence the secretion of orexins
The orexin neurons are glucose-sensitive neurons 17. Their functions are suppressed by elevated blood glucose levels, whereas reduced blood glucose levels excite these neurons 17. In cultured rat hypothalamic neurons undergoing a reduction in glucose concentration from 8.3 to 2.8 mmol/L, 41% showed significant increases in Ca2+ influx. These results indicate that orexin neurons are glucose-sensitive and that they can translate energy status into neural signals.70 Fasting causes low blood glucose, upregulates the expression of orexin mRNA in the hypothalamus, and increases the phosphorylation of cAMP-response element binding protein in orexin-responsive neurons. This suggests that these neurons have functional responses to feeding status. Intraperitoneal injections of insulin and 2-deoxy-d-glucose (2-DG) cause hypoglycemia in animals and lower glucose levels in cells; these two starving stimuli can both induce the expression of orexin-A. The function of orexin-A is closely associated with the type of stimulation. For example, acute stimulation, such as intraperitoneal injection of 2-deoxy-d-glucose (2-DG), is suitable for studying neuronal activation, whereas chronic stimulation, such as fasting, is suitable for studying changes in orexin-A expression after neuronal activation 18. However, Iqbal et al. 19 demonstrated that long-term feeding restrictions in sheep did not affect the expression of prepro-orexin. This finding suggests that orexin neurons may have increased sensitivities to reduced blood glucose, but are insensitive to chronic hypoglycemia.
A high-fat diet stimulates the expression of orexins in the perifornical hypothalamus, and this is highly correlated with increases in the concentrations of triglycerides in the blood 20. Intravenous injection of triglycerides in normal rats with fixed levels of leptin, blood glucose, and insulin stimulates the expression of orexin mRNA. This result demonstrates that orexin neurons are sensitive to triglycerides 20. Orexins may explain high-fat diet-induced eating, and the consequent development of obesity. Orexins are stimulated by increased circulating lipids during a positive energy balance, and are also stimulated by decreased blood glucose levels during a negative energy balance.
Leptin is a polypeptide hormone secreted by adipose tissues that reduces energy intake and increases energy metabolism. It alleviates symptoms of obesity by enhancing the secretion of endogenous insulin and lowering blood glucose levels 21. Orexin neurons express the leptin receptor, and exogenous leptin can downregulate the level of orexins in rat lateral hypothalamus, which suggests that leptin inhibits the expression of orexins. However, increased levels of leptin are often accompanied by an upregulation of orexins in high-fat DIO rats 20. Thus, it is possible that leptin is not the key factor regulating the expression of orexins. Ghrelin is an acylated polypeptide secreted by both the hypothalamus and the stomach, which regulates appetite. It has been shown that ghrelin enhances the expression of orexins via neuropeptide Y (NPY) and agouti-related protein (AgRP) 22. Additionally, exercise also stimulates the activities of orexin neurons in mice 23.
Modulation of orexin neurons
Electrophysiological studies on transgenic mice have identified several neurotransmitters and neuromodulators influencing the activation or inhibition in orexin neurons activity. Specifically, GABA 24, noradrenaline and serotonin seem to inhibit the activity of orexin neurons 25, as dopamine acts through activation of α2-adrenoceptors 26. Moreover, agonists of ionotropic glutamate receptors tend to excite orexin neurons, while glutamate antagonists inhibit their activity 27 indicating that glutamatergic neurons can tonically activate orexin neurons. Cholecystokinin, neurotensin, oxytocin, and vasopressin enhance orexin neurons activity 28 by modulation of adenosine and CO2 concentrations 29.
Orexin function
The first physiological function attributed to the orexin system was the modulation of the feeding behavior. Orexins (hypocretins) were originally identified as appetite-stimulating peptides produced in the lateral hypothalamus 5. Sakurai et al. 5 firstly described hypocretins 1 (orexin-A) and 2 (orexin-B) as regulators of feeding and appetite behavior, produced in a specific hypothalamic region 30. This was suggested by two major studies: (i) the hypothalamic expression of orexin precursor was increased during fasting 5, 31 and (ii) the pharmacological central administration of orexin in rats induced food intake (food consumption being dependent of the orexin injected dose) 32. This feeding-promoting effect is not as robust as the one induced by neuropeptide Y (NPY) and agouti-related protein (AgRP), but it is considered as similar as the one initiated by melanin-concentrating hormone (MCH) and galanin 33. Although the precise effect of Orexin-B on feeding remains unclear, its orexigenic potential was described to be less potent than orexin-A 34, which could be explained by differences in their secondary structures: Orexin-A being maintained by two disulphide bonds conferring resistance to peptidase actions 35.
Subsequent research has indicated that Orexin-A (hypocretin-1) and orexin-B (hypocretin-2) are excitatory hypothalamic neuropeptides playing a relevant role in different physiologic functions such as sleep-wake cycle and thermoregulation, control of energy metabolism, cardiovascular responses, feeding behavior, and spontaneous physical activity 1, analgesia, alcoholism, learning, and memory 36.
The neurons responsible for producing orexin rapidly perceive the body’s nutritional status by responding to metabolic signals such as peripheral blood glucose, as well as leptin and ghrelin levels 37. In previous studies, both mice deficient in orexin-producing neurons and orexin-knockout mice demonstrated symptoms of paroxysmal narcolepsy and delayed obesity, despite reductions in food intake 38. Kakizaki et al. 39 also implied that orexin neurons are involved in body weight gain via the interactive effects of exercise and diet, with each orexin receptor playing a unique role. These studies suggest that orexin functions as a regulator of obesity. It is well known that obesity is a result of an imbalance between caloric intake and energy expenditure, manifesting as excess adipose tissue development, decreased lipolysis, and high fat storage 39. However, the function of orexins in metabolism pathways are far from being completely understood.
More recent studies focused the attention on the role of the orexins in mood and emotional regulation, energetic homeostasis, reward mechanisms, drug addiction, arousal system, and sleep and wakefulness 40.
Sleep-Wake regulation
Orexin system seems to be crucial for maintenance of wakefulness state, as demonstrated by narcolepsy caused by orexin deficiency in human and animals 41. Preclinical data supports the hypothesis that orexin hyperactivity plays a role in insomnia 42. (1) Central administration of exogenous orexin promotes arousal and wakefulness 43. (2) Optogenetic activation of orexin neurons increases the likelihood of sleep-wake transitions 44. (3), Endogenous orexin levels increase during wakefulness and decrease during sleep 45. (4) Orexin receptors antagonists are an effective treatment for insomnia, further supporting the idea that orexin hyperactivity contributes to insomnia 46. In contrast, loss of orexins (as in narcolepsy) results in hypersomnia, including disrupted REM sleep, excessive daytime sleepiness, and cataplexy 47. Indeed, decreased levels or orexins in the cerebrospinal fluid levels can be used as diagnostic criteria for narcolepsy 48.
Narcolepsy is a neurological disease affecting ~1 in 2,000 individuals in the United States 49, characterized by chronic daytime sleepiness, sleep attacks, and possibility of cataplexy, hypnagogic hallucinations and sleep paralysis. These symptoms are not necessary to be present all together and narcolepsy may be identified and diagnosed by standard polysomnography at all ages, including childhood. Narcolepsy is the results of orexin-containing neurons loss, which tend to increase their activity during wakefulness activating aminergic nuclei such as locus coeruleus, raphe nuclei, and tuberomamillary nucleus with maintaining wake state and preventing of inappropriate transitions into sleep, particularly REM sleep phases 50. Narcolepsy is usually classified into two subtypes: (a) Narcolepsy with cataplexy (type 1); and (b) Narcolepsy without cataplexy (type 2). On the other hand, the orexin in sleep-wake regulation and pathophysiology of narcolepsy may be not limited to the activation/deactivation of cataplexic phenomena and/or sleep attacks. In fact, affected children and adolescents present specific cognitive impairments 51, which pinpoint the close relationship between sleep and cognition 52. Moreover, probably due to the same role in sleep modulation, orexin seems to be also involved in the pathogenesis of migraine 53 as suggested by the link between NREM sleep instability and risk of cognitive impairments and behavioral problems 54.
Cognition
Given that orexins promote arousal, and changes in arousal can modulate cognitive function 55, these neuropeptides likely play an important role in cognition. In support of this, narcoleptic patients show specific cognitive impairments 56. Consistently, much of the preclinical literature suggests that orexins enhance cognitive function. Accordingly, when orexin antagonists are used, different types of cognitive function, such as attention, spatial memory, and social memory, are impaired. For example, studies in rodents demonstrate that orexin administration into the prefrontal cortex increases attentional performance in a self-paced task of visuospatial sustained and divided attention, while systemic administration of orexin receptor 1 antagonist SB334867 decreases performance in a sustained attention task 57. Moreover, transnasal delivery of orexin A in monkeys ameliorates sleep deprivation-induced cognitive deficits in a delayed match-to-sample memory task assessing short term memory 58. Previous studies have demonstrated via both lesion experiments and neural activity measurements that the medial prefrontal cortex (mPFC) is required for attention-related tasks and short-term memory tasks 59. Moreover, the literature indicates that mRNA for both orexin 1 and 2 receptors are expressed in the medial prefrontal cortex 60. Thus, orexin actions in the medial prefrontal cortex (mPFC) likely promote attention.
Inactivation of orexin 1 receptors in the hippocampus impairs acquisition, consolidation, and retrieval in the Morris Water Maze, a spatial memory task 61. However, systemic administration of the dual orexin antagonist almorexant failed to impact spatial memory in the Morris Water Maze 62. As the latter study used systemic administration, it is possible that the antagonist was not used at a high enough dose to prevent orexin action in the hippocampus of these rats. Additionally, the sleep-promoting properties of almorexant may have indirectly elicited a beneficial effect on cognitive function, but this has not been extensively tested. In sum, there is some evidence that orexins promote spatial memory through actions in the hippocampus.
Orexin-A also appears to enhance social memory, as Orexin/ataxin-3-transgenic mice, in which orexin neurons degenerate by 3 months of age, display deficits in long-term social memory, which was measured using an automated “two-enclosure homecage” social test (where mice had to choose between approaching a previously investigated mouse or a novel mouse after a 60 min delay) 63. Nasal administration of exogenous orexin-A restored social memory and enhanced synaptic plasticity in the hippocampus in these ataxin mice. This experiment, studying how orexins contribute to social memory, along with the previously discussed experiments, focusing on the role of orexins in attention and spatial memory, support the idea that orexin activation enhances several different types of cognitive function.
However, when orexin activity is increased during stress, it is possible that cognition might be negatively impacted. For example, researchers recently found that stimulating orexins (by administering CNO, which binds to Gs-coupled DREADDs expressed in orexin neurons) during social defeat stress leads to subsequent impairments in the novel object recognition test (unpublished). This test assays spatial and recognition memory and depends on hippocampal and entorhinal cortex structures, which express both OX1R and OX2R 64. Interestingly, stimulating orexins did not impair novel object recognition in non-stressed rats, thus, the specific interaction between increased orexins and stress caused cognitive impairment. It may be that the combination of CNO-induced increases in orexin activity and stress-induced increases in orexin activity leads to excessive orexin-induced arousal, and as a result, cognition is impaired. The relationship between orexin activity and cognitive function may be an inverted U curve, consistent with the understanding of GPCR regulation 65. Specifically, when orexin levels are too low, as in narcolepsy, cognition is impaired; however, when orexin levels are too high, as in conditions of stress, cognition may also be impaired, possibly due to receptor desensitization or downregulation 65. An appropriate amount of orexins is required to maintain optimal cognitive ability. One possible mechanism by which stress might interact with orexins to impair cognitive function is through its actions in the prefrontal cortex. Particularly, several laboratories have shown that repeated restraint causes atrophy in the apical dendrites in layer II/III pyramidal cells in the prefrontal cortex (PFC), which are directly innervated by orexin 66. This pathway only explains the role of orexins in cognitive tasks that are prefrontal cortex (PFC)-dependent (and likely not the hippocampally-mediated novel object recognition task). Future research should investigate other brain regions involved in orexin-mediated changes in cognition after stress. In sum, orexins play an important role in stress-induced cognitive impairment, which is an important phenotype in stress-related psychiatric disorders.
Feeding Behaviors and Energy Homeostasis
Orexin A seems to regulate feeding behaviors and energy expenditure as evidenced by the intracerebroventricular (icv) injection of orexins effects during the light period, which induces feeding behavior in rodents and zebrafishes, probably for a direct action on lateral hypothalamic area containing neurons modulated by glucose concentration. In fact, the high concentrations of glucose and leptin tend to hyperpolarize orexinergic neurons, while low concentrations of glucose and ghrelin depolarize them. Therefore, the orexinergic system discriminate physiological variation in glucose levels due to meals modulating in this way energy balance according to food intake 67. Moreover, transgenic mice with gradual and then loss of hypothalamic orexin-containing neurons show feeding abnormalities and dysregulation in energy homeostasis determining obesity despite the reduction of food intake/calories. Interestingly, it has been reported an increased prevalence of obesity in narcoleptic subjects in all ages 68.
Shiuchi et al. 69 observed that the regulation mediated by orexinergic system on muscle glucose metabolism is due to activation of β2-adrenergic signaling and consequently peripheral energy expenditure. A persistent wake-state mediated by orexins could also be important for food intake motivation. When facing reduced food availability, animals adapt with a longer awake period, revolutionizing their normal pattern of activity 70. During starvation the activation of orexin neurons mediated by low leptin and glucose levels, might modulate their activity according to energy expenditure and stores to maintain wakefulness, whilst orexin neuron-ablated mice fail to respond to fasting with increased wakefulness and activity 71. These data confirm that orexin neurons mediate energy balance and arousal, maintaining a consolidated state of wakefulness in hungry animals in order to promote alertness.
Orexin and Obesity
Obesity is a complex multifactorial condition lowering health quality and many effects such as metabolic syndrome, type 2 diabetes mellitus, coronary heart disease, sleep apnea syndrome, and reduced in life expectancy 72. In the last decades, the incidence of obesity has increased both in children and adults worldwide 73. Environmental and genetic factors cause large variations among human susceptibility to obesity. Physical activity and the so called “non-exercise induced thermogenesis” (NEAT) are factors determining this variability and susceptibility. The term NEAT includes all types of energy expenditure not associated with formal exercise, such as standing and fidgeting 74. A complementary concept to that of NEAT is the spontaneous physical activity (SPA) describing any type of physical activity not qualified as voluntary exercise. Together NEAT and SPA are hereditable but not interchangeable, because NEAT refers to energy expenditure while SPA describes the types of physical activity resulting in NEAT. Therefore, SPA induces an important variability in sensitivity to obese subject that spend less time standing than leans 75. Orexin signaling would promote obesity resistance via enhanced SPA and energy expenditure regulation and the deficiency/dysfunction in orexins system lead to obesity in animal models despite of lower calories intake than wildtype associated with reduced physical activity. On the other hand, the body weight regulation seems to be complex according to the lack of orexin neurons. In 2012, Perez-Leighton et al. 76 highlighted the protective role of intratecal administration orexin A against obesity in mice models.
Orexin A has also been discovered to promote spontaneous physical activity (SPA) and non-exercise induced thermogenesis (NEAT) as effect of administration into specific cerebral areas (i.e., rostral LH, hypothalamic paraventricular nucleus, nucleus accumbens, locus coeruleus, dorsal raphe nucleus, tuberomamillary nucleus, substantia nigra) 77. In this light, orexinergic neurotransmission may be an interesting and new pharmacologic target for obesity therapy 78. Low levels of orexin in CNS and peripheral tissues were found in animal models of obesity diet-induced 77, and adipose tissue in obese humans subjects showed lower concentrations of orexin and reduced in its receptors activity 77. A study conducted by Levin et al. 79 on Sprague Dawley rats showed that models fed with a high-fat diet gained no more weight than chow-fed controls. Obesity prone (OP) and obesity resistant (OR) models present different profiles in weight gaining despite of no differences in energy intake 80. The obesity resistant group showed lower body weight and fat mass on a low-fat diet and gain less weight when fed with high-fat diet than obesity prone group. Furthermore, obesity resistant rats lean group suggest that the negative caloric benefit of orexin-A induced spontaneous physical activity appears to outweigh the positive calories due to orexin-A induced hyperphagia 81. Orexin-A action on spontaneous physical activity had a longer duration when compared with that above food intake 82; obesity resistant rats have higher endogenous spontaneous physical activity thus reflecting their higher sensitivity to spontaneous physical activity – promoting stimuli such as lower caloric intake. By contrast obesity prone rats displayed lower spontaneous physical activity endogenous levels after a high-fat diet administration if compared to their obesity resistant group counterpart 83. Conversely, obesity tends to increase also the prevalence of migraine in all ages of life 84.
Reward system
Orexin system seems to play a unified role in coordinating motivational activation under numerous behavioral conditions 85 as showed, for example, by its involving in alcohol use and drug-addiction 86. Olds and Milner 87 showed that rats would self-stimulate current when an electrode was placed in the lateral hypothalamic region, in close proximity to orexin neurons. This effect was thought to be mediated by the medial forebrain bundle, which contains fibres of passage to the dopaminergic mesencephalic neurons from several neighbouring nuclei. Indeed, anatomical data have shown that orexin cells project to dopaminergic neurons in the ventral tegmental area (VTA) 88, although few synapses have been observed and many of these projections are fibres of passage.
Recent studies focused their attention on reward system modulation by orexin system. The functional role of orexin in the reward process was demonstrated by Boutrel et al. 89, who showed that intracerebroventricularly infusion of orexin-A elevated intracranial self-stimulation thresholds in rats, which suggests that it decreases brain reward function by an action different from dopamine excitation. Indeed, there is much evidence connecting orexin with the effects of opioids and corticotrophin-releasing factor (CRF). Furthermore, orexin-A has been shown to facilitate glutamatergic synapses in dopaminergic neurons in the ventral tegmental area (VTA), providing a cellular basis for its behavioural effects 90. Many other authors have now shown a direct role for orexin in the re-instatement of opioid 91, cocaine 92, alcohol 93 and nicotine-seeking behavior 94. The orexin system may be differentially involved in stress- compared to cue-induced re-instatement of drug seeking behaviour. Interactions with non-dopaminergic systems such as corticotrophin-releasing factor (CRF) or noradrenergic signalling may account for these neuromodulatory effects on drug-seeking behaviour following its eradication.
Treating narcoleptic patients with amphetamine-like drugs 95 did not lead to addiction to these drugs 96. Wild-type mice are more susceptible to developing morphine dependence in comparison with orexin knockout mice 97. Furthermore, reward brain circuits in humans affected by narcolepsy were identified as abnormal 98. However, the mismatch between predicted reward and reward subsequently received was significantly higher in Parkinson’s disease compared to narcoleptic, independent of reward magnitude and valence as showed by cataplexy that may be triggered by both positive and negative emotions 99.
Regulatory mechanisms at the base of reward system are shown in Figure 2. It seems to be clear that orexin neurons modulate reward system and play a predominant role in mechanisms of drug addiction. Many reports suggest a critical role of orexin signaling in neural plastic effects at glutamatergic synapses in the ventral tegmental area (VTA) (Figure 2).
Also, it has been hypothesized that orexins have a role in sexual behaviour 100. Although the mechanisms of increased sexual drive may include the circuits involved in natural reward, there is no data directly linking orexin-induced hypersexuality and dopaminergic transmission. Detailed reviews on the role of orexin in brain reward and addiction have been recently published elsewhere 101.
Orexins and the stress response
Orexins are also important regulators of the stress response. The role orexins play in acute stress is relatively straightforward, with numerous studies providing evidence of orexins increasing Hypothalamic Pituitary Adrenal (HPA) and sympathetic activity, as well as stress-related behaviors 3. A link between orexin and stress-related pathologies, such as panic disorder, has recently been established 102. Panic attacks involve activation of the hypothalamic pituitary adrenal (HPA) axis and the autonomic system. Intrahypothalamic administration of an RNAi to orexin or an OX1 receptor antagonist has been shown to block these panic responses in rats injected with sodium lactate, and elevated levels of orexin-A have been detected in humans with panic disorder 103. Moreover, acute stress produces reliable increases in orexin actions, thus, this relationship between orexins and acute stress is reciprocal. While orexins modulate the response to repeated stress, less data is available on this topic. Currently, it appears that the involvement of the orexin system in repeated stress depends on the type, intensity, and duration of the stressor, and whether one adapts to this repeated stress. More research should be done on the particular brain regions that orexins modulate during repeated stress. Additionally, it would be ideal to measure orexin function in the same individuals before, during, and after stress in order to determine exactly how this system changes in response to repeated stress.
Hypothalamic-pituitary-adrenal axis
In general, orexins have been shown to promote the hypothalamic-pituitary-adrenal axis (HPA axis) response to acute stress at all levels of the HPA axis 104. For example, central infusion of orexins activates Corticotropin Releasing Hormone (CRH) neurons. Additionally, optogenetic stimulation of orexin neurons increases cFos expression in the paraventricular nuclei of the hypothalamus (PVN) 105. Moreover, Corticotropin Releasing Hormone (CRH) neurons in the paraventricular nuclei of the hypothalamus (PVN) abundantly express the OX2R 106. Central administration of orexins also increases downstream hypothalamic-pituitary-adrenal (HPA) hormones ACTH and corticosterone, and this can be reversed with a corticotropin releasing hormone (CRH) antagonist 107. Orexins promote release of ACTH through its actions on both OX1R and OX2R in the pituitary 108. Interestingly, narcolepsy patients with low levels of orexins have blunted ACTH levels, which suggests that orexins are important in promoting this branch of the hypothalamic-pituitary-adrenal (HPA) response 109. Orexins stimulate glucocorticoid secretion via OX1R in the adrenal gland, though studies in both rat and human adrenal glands have indicated that both orexin receptors are present 110. While both orexin 1 and 2 receptors have been implicated in acute hypothalamic-pituitary-adrenal (HPA) activity, the role of each receptor appears dependent on the paradigm of stress used. For example, while the orexin 1 receptor modulates ACTH release by restraint stress 111, it does not modulate ACTH release induced by cage exchange stress (where the rodent is placed in a dirty cage previously occupied by another mouse) 112. The role each receptor plays during each type of acute stress is likely dependent upon the circuitry activated, as the relative expression of each orexin receptor differs between brain regions.
While orexins are known to promote hypothalamic-pituitary-adrenal (HPA) activity, it has also been shown that hypothalamic-pituitary-adrenal (HPA) activation induces orexin activity. Specifically, electron microscopy has demonstrated that corticotropin releasing hormone (CRH) boutons contact orexin neurons 113, and that orexin neurons express both CRH2R and CRH-BP mRNA and protein 114. Moreover, electrophysiological data demonstrate that corticotropin releasing hormone (CRH) administration increases orexin neuron activity 113. These studies also found that orexin neurons were less sensitive to foot shock or restraint stress in CRH1R knockout mice. These data, along with previous receptor localization data, suggest that both CRH1R and CRH2R play a role in activating orexins during stress.
Sympathetic nervous system
Orexins are known to acutely promote sympathetic nervous system activity. For example, central orexin administration increases blood pressure, heart rate, and renal sympathetic nerve activity 115. However, orexins have been shown to be involved in promoting the sympathetic response to only certain types of stressors. For example, contextual fear conditioning-induced increases in heart rate and blood pressure are reduced with the dual orexin receptor antagonist almorexant, but restraint- and cold exposure-induced pressor and tachycardic responses are not 116. Moreover, sympathetic outflow during defense responses are clearly regulated by orexins, as orexin knockout mice display attenuated blood pressure and heart rate in response to emotional stress in the resident-intruder test compared with controls 117. Recent experiments using specific receptor antagonists (rather than dual receptor antagonists) have indicated that both the OX1R and OX2R promote cardiovascular and locomotive responses to stress 118. Orexin neurons exert much of their effects on the sympathetic response through actions on the medulla and nucleus of the solitary tract (NTS) in the brainstem 119.
Acute stress
Administration of orexins affects behaviors impacted by stress and induces both Hypothalamic Pituitary Adrenal (HPA) and sympathetic activity. For example, central administration of orexins induces grooming, face washing, and burrowing 120. These behavioral effects are blocked by an OX1R antagonist 121. Moreover, stimulation of orexins (by administration of clozapine-N-oxide (CNO), which binds to Gs-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) expressed in orexin neurons) increases struggling behavior during restraint stress, and this is prevented by an OX2R antagonist 122. Interestingly, orexin-deficient mice have a diminished behavioral response to stress, as measured by decreased activity in a resident-intruder paradigm 117, further highlighting the importance of this neuropeptide in producing stress-related behaviors.
The interaction between orexins and stress is reciprocal. Consequently, in addition to orexin increasing stress-related behaviors, acute stress activates orexins neurons. For example, acute psychological stressors such as forced swim stress 123 and restraint stress 122 produce increases in both the percentage of orexin neurons dual labeled with cFos (a measure of neural activation) and orexin A levels in the cerebrospinal fluid. Restraint stress was also found to increase orexin neuron activity as measured by increased calcium signals via fiber optometry 124. Acute physical stressors such as intraperitoneal injections and exercise have also been shown to increase orexin neural activation 125. Remarkably, acute stress can have long lasting effects on orexin gene expression, as increased prepro-or-exin can be observed 2 weeks following an acute 2-minute foot shock stress 126.
Repeated stress
While the role of orexins in acute stress appears to be straightforward (orexins promote the acute behavioral and neuroendocrine response to stress, and acute stress activates orexin neurons), the role of the orexin system in regulating responses to repeated stress is not as clearly defined. Whether orexin function is increased or decreased with repeated stress may depend on the type, intensity, and duration of the stressor, and whether habituation and other adaptations take place. With repeated exposure to moderately intense stressors, individuals typically habituate to that stress as indicated by decreasing responsivity in behavioral, HPA and autonomic measures 127. Failure to habituate to a stressor is a hallmark of stress-related illnesses such as post-traumatic stress disorder (PTSD) and panic disorder 104. Interestingly, patients with panic anxiety symptoms have higher levels of orexin in their cerebrospinal fluid (CSF) 4.
In studies using repeated restraint stress, both hypoactivity and hyperactivity of the orexin system have been reported. For example, both orexin neuronal activation (as measured by dual orexin and cFos immunohistochemistry) and orexin A levels in the cerebrospinal fluid decreased after hypothalamic-pituitary-adrenal (HPA) habituation to repeated restraint in male rats 122. Thus, in this case, habituation to a repeated stressor is associated with decreased orexin function. Another study found that orexins were upregulated in the basolateral amygdala of male mice after 14 days of repeated restraint stress 128. As this paradigm did not examine HPA habituation to repeated restraint, and used 2 h of restraint per day (compared with 30-min per day in the previously mentioned restraint study), it is possible that these rodents did not habituate, which would explain their increased orexin activity. In addition, the Kim et al. 128 study reported upregulation of orexin mRNA transcripts specifically in the amygdala, whereas the Grafe et al. 122 study examined orexin neuronal activation in the lateral hypothalamus and overall orexin levels in the cerebrospinal fluid. Thus, these different types of assessments may yield quite different results. Ultimately, it will be important to examine orexin function in a variety of locations during repeated stress (e.g. both in orexinergic neurons in the lateral hypothalamus and in specific projection regions) in order to truly understand the role these neuropeptides play.
Both decreased and increased orexin function have been observed in rodents exposed to repeated social defeat. Specifically, decreased prepro-orexin mRNA and orexin A and B peptides were observed in the hypothalamus after social defeat paradigms in both mice and rats 129. Though HPA habituation was not assessed in these paradigms, it has been suggested that when a stressor is chronic, predictable, and inescapable, this leads to cessation of coping behaviors, orexin system hypoactivity, and may lead to depressive-like behavior 2. However, previous literature has noted that there are naturally occurring differences in response to social defeat, where some rodents display a passive coping strategy while others display an active coping strategy 130. The passive coping strategy, assessed by a short latency to defeat, is typically associated with delayed habituation to repeated defeat and depressive-like behavior, whereas the active coping strategy is not 131. In a recent study, scientists found active coping behaviors in social defeat are associated with lower orexin function in male rats. Moreover, scientists found that inhibiting orexins (by administering clozapine-N-oxide, which binds to Gi-coupled DREADDs expressed in orexin neurons) promotes resilience to repeated social defeat in previously passive coping rodents 132. Consistent with our data, another social defeat study found that lower orexin function may be indicative of resilience, rather than vulnerability 133. Thus, it appears that orexins may contribute to individual differences in response to social defeat stress, whereupon lower orexins exhibited by actively coping animals is associated with resilience to social defeat. However, in situations where animals are exposed to inescapable stress (and exhibit reduced coping), lower orexins are associated with vulnerability to stress.
A study using 8 weeks of unpredictable chronic mild stress found that orexin neurons were activated (using dual immunohistochemical staining for orexin and cFos) 134. Typically, unpredictable chronic mild stress prevents habituation through variation in the type of stressor, which might explain the increase in orexin activation after repeated stress in this case. Indeed, in a study of male and female repeated restraint, female rats did not habituate as fully as males, and thus, had higher levels of orexin neuronal activation (as measured by dual orexin and cFos immunohistochemical labeling) 135. In this simplistic view of the orexin system in repeated stress, if habituation does not take place, orexin function is increased, whereas if habituation does take place, orexin function is decreased.
Visceral functions
The orexin system has also been found to effect visceral functions, in addition to its roles in energy homeostasis and endocrine function, mentioned previously.
Circulatory system
Orexin-deficient mice exhibit lower arterial blood pressure, heart rate and sympathetic tone 136. Furthermore, intracerebroventricularly, intracisternally or intrathecally applied orexin increases the mean arterial pressure (MAP), heart rate, renal sympathetic nerve activity and plasma catecholamine or vasopressin levels 137, effects that are blocked or attenuated by the OX1 receptor antagonist SB-334867 138. However, i.v. injections of orexin-A have no effect on sympathetic activity 139, suggesting that the cardiac effects of orexin are mediated centrally. Consistently, microinjections of orexin-A into the rostral ventrolateral medulla 140 or rostral ventromedial medulla 141 elicit cardiovascular excitatory responses through the activation of both OX1 and OX2 receptors 140.
However, orexin-A signalling in the nucleus ambiguus (NA) 141 and subfornical organ 142 has been shown to produce bradycardia responses, which are, respectively, mediated by an elevation of vagal excitation and a reduction of sympathetic tone. In contrast, it has also been found that orexin-A enhances the inhibitory input and attenuates excitatory synapses to vagal neurons in the nucleus ambiguus 143. Moreover, the cardiac effects of orexin in the nucleus tractus solitarius are both dose- and site-dependent, as orexin increases MAP and HR at higher doses (>20 pmol) but reduces these variables at a lower dose (5 pmol). Furthermore, microinjections of orexin into the caudal lateral and medial subnuclei of the nucleus tractus solitarius decrease both the MAP and heart rate 144, whereas pressor and tachycardiac effects were obtained when orexin was injected into the commissural nucleus of nucleus tractus solitarius 145.
Respiratory effects
Several studies with preproorexin-knockout and orexinergic neuron-ablated mice have demonstrated the essential role orexin plays in the hypercapnic chemoreflex response, as well as in phrenic and ventilatory long-term facilitation 146. Also, pharmacological experiments have shown that intracerebroventricularly, intracisternal or intrathecal administration of orexin has the ability to elevate respiratory frequency, tidal volume and minute ventilation 138. Furthermore, orexin signalling on OX1 receptors in the retrotrapezoid nucleus contributes to the control of the hypercapnic chemoreflex 147. In addition, when applied to the pre-Botzinger region and phrenic nuclei, orexin augments the phrenic nerve discharge and, subsequently, the electromyographic activity of the diaphragm 148. Moreover, injection of orexin-B into pontine Kölliker-Fuse nucleus results in an increase in respiratory frequency and facilitation of upper airway patency 149. In parallel, it may be worth noting that orexinergic neurons are strongly inhibited by anaesthetics and orexin-KO mice show delayed emergence from isofluorane anaesthesia 150. However, ambient levels of H+ and CO2 can significantly enhance the activation of orexinergic neurons 151. Unlike in mice, chemoresponsiveness in humans is independent of OX1 receptors, as the different ventilatory responses to hypoxia observed in humans with narcolepsy-cataplexy have been associated with the HLA-DQB1*0602 allele that segregates with narcolepsy, but not an orexin deficiency 152. Therefore, in humans ventilatory responses to hypoxia may be mediated by other factors or immune components independent of orexin.
It has been suggested that orexinergic neurons are involved in sleep apnoea syndrome, as patients show increased plasma levels of orexin-A 153. Hypercapnia and associated reflexes may increase the activity of orexinergic neurons during sleep, facilitating microarousals and a cascade of sympathetic activity that results in elevated blood pressure during the night. Thus, OX receptor antagonists could be used to prevent these peaks of blood pressure in mild sleep apnoea.
Regulation of digestive activity
Early work showed that orexin immunoreactivity is present in intestinal tissue 154. Intracisternal or intraventromedial hypothalamus, but not i.p., injections of orexin-A stimulate gastric acid secretion by activating the vagal system through OX1 receptors 155. Moreover, activation of OX1 receptors in the dorsal motor nucleus of the vagus results in facilitation of vagal pancreatic efferent nerve activities 156, stimulating pancreatic exocrine secretion 157. Administration of orexin-A, i.a., increases duodenal secretion in normal fed but not in fasted animals, by an effect that is independent of cholinergic pathways 158. In addition, orexin-A can modify gastrointestinal motility, including gastric emptying, gastric interdigestive motility 159 and enteric peristalsis 160, as well as colonic motility 161. It is noteworthy that orexin exerts region-specific effects on gastric contractility and relaxation both at the central and peripheral levels, by mechanisms involving ACh and NO respectively 162. Furthermore, orexin-A shows gastroprotective effects against stress-induced 163, ischemia-reperfusion-induced 164 or ethanol-induced 165 gastric damage.
Urinary activity
The presence of orexin-A and its receptors has been shown in human kidneys and urine 166, as well as in the bovine urethroprostatic complex 167. These findings are supported by results from physiological studies, which demonstrated that orexin-A is involved in the pelvic-urethral reflex 168 and the micturition reflex 169.
Sensory system
So far, the involvement of orexin in sensory modulation has focused on its role in nociception, in addition to an emerging role in olfaction.
Pain
The analgesic properties of orexin peptides have been well-established with i.v., intrathecal or intracerebroventricularly injection approaches in mouse and rat models of thermal (hot-plate, tail-flick, paw-withdrawal), mechanical (tail pressure, partial sciatic nerve ligation), chemical (formalin, carrageenan, capsaicin and abdominal stretch) nociception and (or) hyperalgesia 170. In all of those models, the orexin-induced analgesic effects were suppressed by the OX1 receptor antagonist SB-334867 but not by naloxone, an opioid receptor inverse agonist, suggesting that regulation of nociception by orexin is independent of the opiate system. In addition to the direct projection of orexin neurons to the spinal cord 171, orexin signalling in the posterior hypothalamic area 172, the pontine reticular nucleus, oral part 173 and periaqueductal gray matter have been shown to be important for its antinociceptive effects. Moreover, orexin plays a significant role in the regulation of stress-induced analgesia (SIA), coordinating with the nociceptin/orphanin FQ systems 174. Consistent with these data, preproorexin knockout mice exhibit hyperalgesia and less stress-induced analgesia 175. The involvement of orexin in pain is also supported by clinical observations, which have shown there is an association between changes in the orexin receptors and headaches 176 and a recent multicentre case-control study revealed that chronic pain is more common in patients with narcolepsy with cataplexy than in the controls 177.
Olfaction
The participation of orexin in olfactory function is suggested by the presence of orexin neurons and receptors at all levels of the olfactory system, and their ability to modulate the excitability of olfactory sensory and relay neurons 178. Indeed, intracerebroventricularly injection of orexin-A increases the olfactory sensitivity to isoamyl acetate 179 and food odour 180, although it is unclear whether these increases in sensory perception are related to modulation of brain reward function. Importantly, in studies in humans, it has been established that olfactory dysfunction is a feature of narcolepsy with or without cataplexy 181 and that this state could be reversed by intranasal orexin-A 182.
Locomotion
The relationship between the orexin system and locomotion was highlighted initially in behavioural tests; intracerebroventricularly administration of orexin was shown to enhance locomotor activity 183, which involves dopamine D1 and D2 receptors 184, 5-HT 185 and central α1-adrenoceptors 186, whereas the selective OX1 receptor antagonist SB-334867 reversed this effect of orexin 187. Orexin-A injected into multiple structures stimulates locomotor activity, but the effect of orexin-A in the LH is independent of its feeding effect 188. Moreover, both orexin-A and -B, injected into the nucleus accumbens shell (AccSh), potentiated the dopamine-dependent pivoting in rats 189. However, the OX1 receptor antagonist SB-334867 decreased the orexin-A-induced spontaneous physical activity when injected into the paraventricular nucleus (PVN) 190, but did not have any effects when injected into the nucleus accumbens shell 191. Consistent with its motor stimulating effect, an injection of orexin into the medioventral medullary alpha parts, LC 192, pedunculopontine nuclei (PPN), substantia nigra pars reticulata 193 or trigeminal motor nucleus 194 increased muscle tone, but inhibited muscle tone when injected in the gigantocellular nucleus sites, dorsal paragigantocellular nucleus and nucleus pontis oralis 192.
Recently, Zhang et al. 195 demonstrated that orexin-A signalling in the rat lateral vestibular nucleus (LVN) is involved in the vestibular-mediated motor, postural control and negative geotaxis, and intriguingly, whereas microinjection of SB-334867 into the lateral vestibular nucleus usually has no effect on this condition, it makes a difference when the rat is facing a major motor challenge. These results suggest that the motor effects of orexin are independent of its function in arousal and emotion, and provide a possible mechanism for the loss of muscle tone in cataplexy attacks. Interestingly, administration of orexin-A into the paraventricular nucleus of the midline thalamus reduces distance travelled, yet enhances the grooming and freezing behaviours in animals, whereas the orexin receptor antagonist SB-334867 has no effects on these variables 196. Consistent with the elevated levels of activity of the orexin system during active wakefulness being associated with high muscle tone and stirring movements 197, the orexin system has been shown to be involved in the maintenance of food anticipatory activity 198.
References- Orexin System: The Key for a Healthy Life. Front. Physiol., 31 May 2017 https://doi.org/10.3389/fphys.2017.00357
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, 2014. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci 17, 1298–1303. 10.1038/nn.3810
- Grafe LA, Bhatnagar S. Orexins and stress. Front Neuroendocrinol. 2018;51:132-145. doi:10.1016/j.yfrne.2018.06.003 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6345253
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A, 2010. A key role for orexin in panic anxiety. Nat. Med 16, 111–115. 10.1038/nm.2075
- Sakurai T.; Amemiya A.; Ishii M.; Matsuzaki I.; Chemelli R. M.; Tanaka H.; Williams S. C.; Richardson J. A.; Kozlowski G. P.; Wilson S.; Arch J. R. S.; Buckingham R. E.; Haynes A. C.; Carr S. A.; Annan R. S.; McNulty D. E.; Liu W.-S.; Terrett J. A.; Elshourbagy N. A.; Bergsma D. J.; Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. 10.1016/s0092-8674(00)80949-6
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E, 2000. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355, 39–40. 10.1016/S0140-6736(99)05582-8
- Xu, T. R., Yang, Y., Ward, R., Gao, L., and Liu, Y. (2013). Orexin receptors: multi-functional therapeutic targets for sleeping disorders, eating disorders, drug addiction, cancers and other physiological disorders. Cell. Signal. 25, 2413–2423. doi: 10.1016/j.cellsig.2013.07.025
- Trivedi P.; Yu H.; MacNeil D. J.; Van der Ploeg L. H. T.; Guan X.-M. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998, 438, 71–75. 10.1016/s0014-5793(98)01266-6
- Lu, X. Y., Bagnol, D., Burke, S., Akil, H., and Watson, S. J. (2000). Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm. Behav. 37, 335–344. doi: 10.1006/hbeh.2000.1584
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, 1998. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A 95, 322–327.
- Cheng S-B, Kuchiiwa S, Gao H-Z, Kuchiiwa T, Nakagawa S, 2003. Morphological study of orexin neurons in the hypothalamus of the Long-Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci. Res 46, 53–62.
- Horvath TL, Gao X-B, 2005. Input organization and plasticity of hypocretin neurons: possible clues to obesity’s association with insomnia. Cell Metab. 1, 279–286. 10.1016/j.cmet.2005.03.003
- Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, Rossi M, Ghatei M, Bloom S, 2000. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci. Lett 279, 109–112.
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE, 2001. Orexin (hypocretin) neurons contain dynorphin. J. Neurosci 21, RC168.
- Henny P, Brischoux F, Mainville L, Stroh T, Jones BE, 2010. Immunohistochemical evidence for synaptic release of glutamate from orexin terminals in the locus coeruleus. Neuroscience 169, 1150–1157. 10.1016/j.neuroscience.2010.06.003
- Harris GC, Aston-Jones G, 2006. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 29, 571–577. 10.1016/j.tins.2006.08.002
- Liu L, Wang Q, Liu A, et al. Physiological Implications of Orexins/Hypocretins on Energy Metabolism and Adipose Tissue Development. ACS Omega. 2019;5(1):547-555. Published 2019 Dec 27. doi:10.1021/acsomega.9b03106 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6964296
- Okumura T.; Takakusaki K. Role of orexin in central regulation of gastrointestinal functions. J. Gastroenterol. 2008, 43, 652–660. 10.1007/s00535-008-2218-1
- Iqbal J.; Henry B. A.; Pompolo S.; Rao A.; Clarke I. J. Long-term alteration in bodyweight and food restriction does not affect the gene expression of either preproorexin or prodynorphin in the sheep. Neuroscience 2003, 118, 217–226. 10.1016/s0306-4522(02)00815-1
- Wortley K. E.; Chang G.-Q.; Davydova Z.; Leibowitz S. F. Orexin gene expression is increased during states of hypertriglyceridemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1454–R1465. 10.1152/ajpregu.00286.2002
- Benoit S. C.; Clegg D. J.; Seeley R. J.; Woods S. C. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 2004, 59, 267–285. 10.1210/rp.59.1.267
- Toshinai K.; Date Y.; Murakami N.; Shimada M.; Mondal M. S.; Shimbara T.; Guan J.-L.; Wang Q.-P.; Funahashi H.; Sakurai T.; Shioda S.; Matsukura S.; Kangawa K.; Nakazato M. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003, 144, 1506–1512. 10.1210/en.2002-220788
- Shiuchi T.; Miyatake Y.; Otsuka A.; Chikahisa S.; Sakaue H.; Séi H. Role of orexin in exercise-induced leptin sensitivity in the mediobasal hypothalamus of mice. Biochem. Biophys. Res. Commun. 2019, 514, 166–172. 10.1016/j.bbrc.2019.04.145
- Xie, X., Crowder, T. L., Yamanaka, A., Morairty, S. R., LeWinter, R. D., and Sakurai, T. (2006). GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J. Physiol. 574, 399–414. doi: 10.1113/jphysiol.2006.108266
- Yamanaka, A., Muraki, Y., Tsujino, N., Goto, K., and Sakurai, T. (2003b). Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem. Biophys. Res. Commun. 303, 120–129. doi: 10.1016/S0006-291X(03)00299-7
- Yamanaka, A., Muraki, Y., Ichiki, K., Tsujino, N., Kilduff, T. S., Goto, K., et al. (2006). Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J. Neurophysiol. 96, 284–298. doi: 10.1152/jn.01361.2005
- Li, Y., Gao, X. B., Sakurai, T., and van den Pol, A. N. (2002). Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36, 1169–1181. doi: 10.1016/S0896-6273(02)01132-7
- Tsunematsu, T., Fu, L. Y., Yamanaka, A., Ichiki, K., Tanoue, A., Sakurai, T., et al. (2008). Vasopressin increases locomotion through a V1a receptor in orexin/hypocretin neurons: implications for water homeostasis. J. Neurosci. 28, 228–238. doi: 10.1523/JNEUROSCI.3490-07.2008
- Liu, Z. W., and Gao, X. B. (2007). Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J. Neurophysiol. 97, 837–848. doi: 10.1152/jn.00873.2006
- Haynes, A. C., Jackson, B., Chapman, H., Tadayyon, M., Johns, A., Porter, R. A., et al. (2000). A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul. Pept. 96, 45–51. doi: 10.1016/S0167-0115(00)00199-3
- López M, Seoane L, García MC, Lago F, Casanueva FF, Senarís R, et al. . Leptin regulation of prepro-orexin and orexin receptor mRNA levels in the hypothalamus. Biochem Biophys Res Commun. (2000) 269:41–5. 10.1006/bbrc.2000.2245
- López M, Seoane LM, García MC, Diéguez C, Señarís R. Neuropeptide Y, but not agouti-related peptide or melanin-concentrating hormone, is a target Peptide for orexin-a feeding actions in the rat hypothalamus. Neuroendocrinology. (2002) 75:34–44. 10.1159/000048219
- Ida T, Nakahara K, Kuroiwa T, Fukui K, Nakazato M, Murakami T, et al. . Both corticotropin releasing factor and neuropeptide Y are involved in the effect of orexin (hypocretin) on the food intake in rats. Neurosci Lett. (2000) 293:119–22. 10.1016/S0304-3940(00)01498-1
- Sartin JL, Dyer C, Matteri R, Buxton D, Buonomo F, Shores M, et al. . Effect of intracerebroventricular orexin-B on food intake in sheep. J Anim Sci. (2001) 79:1573–7. 10.2527/2001.7961573x
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. (2001) 24:429–58. 10.1146/annurev.neuro.24.1.429
- Tanaka S.; Honda Y.; Takaku S.; Koike T.; Oe S.; Hirahara Y.; Yoshida T.; Takizawa N.; Takamori Y.; Kurokawa K. Involvement of PLAGL1/ZAC1 in hypocretin/orexin transcription. Int. J. Mol. Med. 2019, 43, 2164–2176. 10.3892/ijmm.2019.4143
- Chang X.; Suo L.; Xu N.; Zhao Y. Orexin-A Stimulates Insulin Secretion Through the Activation of the OX1 Receptor and Mammalian Target of Rapamycin in Rat Insulinoma Cells. Pancreas 2019, 48, 568–573. 10.1097/mpa.0000000000001280
- Hara J.; Yanagisawa M.; Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci. Lett. 2005, 380, 239–242. 10.1016/j.neulet.2005.01.046
- Kakizaki M.; Tsuneoka Y.; Takase K.; Kim S. J.; Choi J.; Ikkyu A.; Abe M.; Sakimura K.; Yanagisawa M.; Funato H. Differential Roles of Each Orexin Receptor Signaling in Obesity. iScience 2019, 20, 1–13. 10.1016/j.isci.2019.09.003
- Narita, M., Nagumo, Y., Hashimoto, S., Narita, M., Khotib, J., Miyatake, M., et al. (2006). Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J. Neurosci. 26, 398–405. doi: 10.1523/JNEUROSCI.2761-05.2006
- Chemelli, R. M., Willie, J. T., Sinton, C. M., Elmquist, J. K., Scammell, T., Lee, C., et al. (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451. doi: 10.1016/S0092-8674(00)81973-X
- Prober DA, Rihel J, Onah AA, Sung R-J, Schier AF, 2006. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J. Neurosci 26, 13400–13410. 10.1523/JNEUROSCI.4332-06.2006
- Nagahara T, Saitoh T, Kutsumura N, Irukayama-Tomobe Y, Ogawa Y, Kuroda D, Gouda H, Kumagai H, Fujii H, Yanagisawa M, Nagase H, 2015. Design and synthesis of non-peptide, selective orexin receptor 2 agonists. J. Med. Chem 58, 7931–7937. 10.1021/acs.jmedchem.5b00988
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L, 2007. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424. 10.1038/nature06310
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N, 1999. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U. S. A 96, 10911–10916
- Nakamura M, Nagamine T, 2017. Neuroendocrine, autonomic, and metabolic responses to an orexin antagonist, suvorexant, in psychiatric patients with insomnia. Innov. Clin. Neurosci 14, 30–37.
- Nakamura M, Kanbayashi T, Sugiura T, Inoue Y, 2011. Relationship between clinical characteristics of narcolepsy and CSF orexin-A levels. J. Sleep Res 20, 45–49. 10.1111/j.l365-2869.2010.00870.x
- Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S, 2002. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol 59, 1553–1562.
- Mignot, E. (1998). Genetic and familial aspects of narcolepsy. Neurology 50, S16–S22. doi: 10.1212/WNL.50.2_Suppl_1.S16
- España, R. A., and Scammell, T. E. (2011). Sleep neurobiology from a clinical perspective. Sleep 34, 845–858. doi: 10.5665/SLEEP.1112
- Posar, A., Pizza, F., Parmeggiani, A., and Plazzi, G. (2014). Neuropsychological findings in childhood narcolepsy. J. Child Neurol. 29, 1370–1376. doi: 10.1177/0883073813508315
- Esposito, M., and Carotenuto, M. (2014). Intellectual disabilities and power spectra analysis during sleep: a new perspective on borderline intellectual functioning. J. Intellect. Disabil. Res. 58, 421–429. doi: 10.1111/jir.12036
- Rainero, I., De Martino, P., and Pinessi, L. (2008). Hypocretins and primary headaches: neurobiology and clinical implications. Expert Rev. Neurother. 8, 409–416. doi: 10.1586/14737175.8.3.409
- Carotenuto, M., Esposito, M., Cortese, S., Laino, D., and Verrotti, A. (2016). Children with developmental dyslexia showed greater sleep disturbances than controls including problems initiating and maintaining sleep. Acta Paediatr. 105, 1079–1082. doi: 10.1111/apa.13472
- Maran T, Sachse P, Martini M, Weber B, Pinggera J, Zuggal S, Furtner M, 2017. Lost in time and space: states of high arousal disrupt implicit acquisition of spatial and sequential context information. Front. Behav. Neurosci 11, 206 10.3389/fnbeh.2017.00206
- Blackwell JE, Alammar HA, Weighall AR, Kellar I, Nash HM, 2017. A systematic review of cognitive function and psychosocial well-being in school-age children with narcolepsy. Sleep Med. Rev 34, 82–93. 10.1016/j.smrv.2016.07.003
- Boschen KE, Fadel JR, Burk JA, 2009. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology 206, 205–213. 10.1007/s00213-009-1596-2
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE, 2007. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J. Neurosci 27, 14239–14247. 10.1523/JNEUROSCI.3878-07.2007
- Miner LA, Ostrander M, Sarter M, 1997. Effects of ibotenic acid-induced loss of neurons in the medial prefrontal cortex of rats on behavioral vigilance: evidence for executive dysfunction. J. Psychopharmacol 11, 169–178. 10.1177/026988119701100210
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol 435, 6–25.
- Akbari E, Naghdi N, Motamedi F, 2006. Functional inactivation of orexin 1 receptors in CA1 region impairs acquisition, consolidation and retrieval in Morris water maze task. Behav. Brain Res 173, 47–52. http://dx.doi.Org/10.1016/j.bbr.2006.05.028
- Dietrich H, Jenck F, 2010. Intact learning and memory in rats following treatment with the dual orexin receptor antagonist almorexant. Psychopharmacology 212, 145–154. 10.1007/s00213-010-1933-5
- Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, Xie J, Sakurai T, Xie XS, 2013. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J. Neurosci 33, 5275–5284. 10.1523/JNEUROSCI.3200-12.2013
- Stackman RW, Cohen SJ, Lora JC, Rios LM, 2016. Temporary inactivation reveals that the CA1 region of the mouse dorsal hippocampus plays an equivalent role in the retrieval of long-term object memory and spatial memory. Neurobiol. Learn. Mem 133, 118–128. 10.1016/j.nlm.2016.06.016
- Black JB, Premont RT, Daaka Y, 2016. Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin. Cell Dev. Biol 50, 95–104. 10.1016/j.semcdb.2015.12.015
- Lambe EK, Olausson P, Horst NK, Taylor JR, Aghajanian GK, 2005. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: correlation with improved attention in rat. J. Neurosci 25, 5225–5229.
- Messina, G., Dalia, C., Tafuri, D., Monda, V., Palmieri, F., Dato, A., et al. (2014). Orexin-A controls sympathetic activity and eating behavior. Front. Psychol. 5:997. doi: 10.3389/fpsyg.2014.00997
- Yokobori, E., Kojima, K., Azuma, M., Kang, K. S., Maejima, S., Uchiyama, M., et al. (2011). Stimulatory effect of intracerebroventricular administration of orexin A on food intake in the zebrafish, Danio rerio. Peptides 32, 1357–1362. doi: 10.1016/j.peptides.2011.05.010
- Shiuchi, T., Haque, M. S., Okamoto, S., Inoue, T., Kageyama, H., Lee, S., et al. (2009). Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 10, 466–480. doi: 10.1016/j.cmet.2009.09.013
- Viggiano, A., Vicidomini, C., Monda, M., Carleo, D., Carleo, R., Messina, G., et al. (2009). Fast and low-cost analysis of heart rate variability reveals vegetative alterations in noncomplicated diabetic patients. J. Diabetes Complicat. 23, 119–123. doi: 10.1016/j.jdiacomp.2007.11.009
- Villano, I., Messina, A., Valenzano, A., Moscatelli, F., Esposito, T., Monda, V., et al. (2017). Basal forebrain cholinergic system and orexin neurons: effects on attention. Front. Behav. Neurosci. 11:10. doi: 10.3389/fnbeh.2017.00010
- Must, A., Spadano, J., Coakley, E. H., Field, A. E., Colditz, G., and Dietz, W. H. (1999). The disease burden associated with overweight and obesity aviva. JAMA 282, 1523–1529. doi: 10.1001/jama.282.16.1523
- Flegal, K. M., Carroll, M. D., Ogden, C. L., and Curtin, L. R. (2010). Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303, 235–241. doi: 10.1001/jama.2009.2014
- Levine, J. A. (2002). Non-exercise activity thermogenesis (NEAT). Best Pract. Res. Clin. Endocrinol. Metab. 16, 679–702. doi: 10.1053/beem.2002.0227
- Levine, J. A., Lanningham-Foster, L. M., McCrady, S. K., Krizan, A. C., Olson, L. R., Kane, P. H., et al. (2005). Interindividual variation in posture allocation: possible role in human obesity. Science 307, 584–586. doi: 10.1126/science.1106561
- Perez-Leighton, C. E., Boland, K., Teske, J. A., Billington, C., and Kotz, C. M. (2012). Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. Am. J. Physiol. Endocrinol. Metab. 303, E865–E874. doi: 10.1152/ajpendo.00119.2012
- Hara, J., Yanagisawa, M., and Sakurai, T. (2005). Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci. Lett. 380, 239–242. doi: 10.1016/j.neulet.2005.01.046
- Zink, A. N., Perez-Leighton, C. E., and Kotz, C. M. (2014). The orexin neuropeptide system: physical activity and hypothalamic function throughout the aging process. Front. Syst. Neurosci. 8:211. doi: 10.3389/fnsys.2014.00211
- Levin, B. E., Dunn-Meynell, A. A., Balkan, B., and Keesey, R. E. (1997). Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 273, R725–R730.
- Messina, A., De Fusco, C., Monda, V., Esposito, M., Moscatelli, F., Valenzano, A., et al. (2016). Role of the orexin system on the hypothalamus-pituitary-thyroid axis. Front. Neural Circuits 10:66. doi: 10.3389/fncir.2016.00066
- Messina G.; Monda V.; Moscatelli F.; Valenzano A. A.; Monda G.; Esposito T.; De Blasio S.; Messina A.; Tafuri D.; Barillari M. R. Role of orexin system in obesity. Biol. Med. 2015, 7, 248.10.4172/0974-8369.1000248
- Bellini, B., Arruda, M., Cescut, A., Saulle, C., Persico, A., and Carotenuto, M. (2013). Headache and comorbidity in children and adolescents. J. Headache Pain. 14:79. doi: 10.1186/1129-2377-14-79
- Esposito, M., Pascotto, A., Gallai, B., Parisi, L., Roccella, M., Marotta, R., et al. (2012a). Can headache impair intellectual abilities in children? an observational study. Neuropsychiatr. Dis. Treat. 8, 509–513. doi: 10.2147/NDT.S36863
- Verrotti, A., Carotenuto, M., Altieri, L., Parisi, P., Tozzi, E., Belcastro, V., et al. (2015). Migraine and obesity: metabolic parameters and response to a weight loss programme. Pediatr. Obes. 10, 220–225. doi: 10.1111/ijpo.245
- James, M. H., Mahler, S. V., Moorman, D. E., and Aston-Jones, G. (2016). Decade of orexin/hypocretin and addiction: where are we now? Curr. Top. Behav. Neurosci. doi: 10.1007/7854_2016_57. [Epub ahead of print].
- Walker, L. C., and Lawrence, A. J. (2016). The role of orexins/hypocretins in alcohol use and abuse. Curr. Top Behav. Neurosci. doi: 10.1007/7854_2016_55. [Epub ahead of print].
- Olds J. Hypothalamic substrates of reward. Physiol Rev. 1962;42:554–604.
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684.
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173.
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601.
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559.
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56 Suppl 1(Suppl 1):112-121. doi:10.1016/j.neuropharm.2008.06.060
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759.
- Plaza-Zabala A, Martin-Garcia E, de Le cea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310.
- Di Bernardo, G., Messina, G., Capasso, S., Del Gaudio, S., Cipollaro, M., Peluso, G., et al. (2014). Sera of overweight people promote in vitro adipocyte differentiation of bone marrow stromal cells. Stem Cell Res. Ther. 5:4. doi: 10.1186/scrt393
- Monda, M., Messina, G., Scognamiglio, I., Lombardi, A., Martin, G. A., Sperlongano, P., et al. (2014). Short-term diet and moderate exercise in young overweight men modulate cardiocyte and hepatocarcinoma survival by oxidative stress. Oxid. Med. Cell. Longev. 2014:131024. doi: 10.1155/2014/131024
- Chieffi, S., Iavarone, A., Iaccarino, L., La Marra, M., Messina, G., De Luca, V., et al. (2014b). Age-related differences in distractor interference on line bisection. Exp. Brain Res. 232, 3659–3664. doi: 10.1007/s00221-014-4056-0
- Viggiano, A., Chieffi, S., Tafuri, D., Messina, G., Monda, M., and De Luca, B. (2014). Laterality of a second player position affects lateral deviation of basketball shooting. J. Sports Sci. 32, 46–52. doi: 10.1080/02640414.2013.805236
- Mensen, A., Poryazova, R., Huegli, G., Baumann, C. R., Schwartz, S., and Khatami, R. (2015). The roles of dopamine and hypocretin in reward: a electroencephalographic study. PLoS ONE 10:e0142432. doi: 10.1371/journal.pone.0142432
- Bai YJ, Li YH, Zheng XG, Han J, Yang XY, Sui N. Orexin A attenuates unconditioned sexual motivation in male rats. Pharmacol Biochem Behav. 2009;91:581–589.
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121.
- Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, et al. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav. 2012;107:726–732.
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115.
- Jöhnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A, 2012. Orexin, stress, and anxiety/panic states. Prog. Brain Res 198, 133–161. 10.1016/B978-0-444-59489-1.00009-4
- Bonnavion P, Jackson AC, Carter ME, de Lecea L, 2015. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat. Commun 6, 6266 10.1038/ncomms7266
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM, 1998. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438, 71–75.
- Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG, 2006. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol. Rev 58, 46–57. 10.1124/pr.58.1.4
- Date Y, Mondal MS, Matsukura S, Ueta Y, Yamashita H, Kaiya H, Kangawa K, Nakazato M, 2000. Distribution of orexin/hypocretin in the rat median eminence and pituitary. Brain Res. Mol. Brain Res 76, 1–6.
- Kok SW, Roelfsema F, Overeem S, Lammers GJ, Strijers RL, Frolich M, Meinders AE, Pijl H, 2002. Dynamics of the pituitary-adrenal ensemble in hypocretin-deficient narcoleptic humans: blunted basal adrenocorticotropin release and evidence for normal time-keeping by the master pacemaker. J. Clin. Endocrinol. Metab 87, 5085–5091. 10.1210/jc.2002-020638
- Ziolkowska A, Spinazzi R, Albertin G, Nowak M, Malendowicz LK, Tortorella C, Nussdorfer GG, 2005. Orexins stimulate glucocorticoid secretion from cultured rat and human adrenocortical cells, exclusively acting via the 0X1 receptor. J. Steroid Biochem. Mol. Biol 96, 423–429. 10.1016/j.jsbmb.2005.05.003
- Samson WK, Bagley SL, Ferguson AV, White MM, 2007. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am. J. Physiol. Regul. Integr. Comp. Physiol 292, R382–R387. 10.1152/ajpregu.00496.2006
- Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, Lebold TP, Nepomuceno D, Lord B, Wennerholm M, Shelton J, Carruthers N, Lovenberg T, Dugovic C, 2015. A Selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J. Pharmacol. Exp. Ther 352, 590–601. 10.1124/jpet.114.220392
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L, 2004. Interaction between the corticotropinreleasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci 24, 11439–11448. 10.1523/JNEUROSCI.3459-04.2004
- Slater PG, Noches V, Gysling K, 2016. Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur. J. Neurosci 43, 220–229. http://dx.doi.org/10.l11l/ejn.13113
- Smith PM, Connolly BC, Ferguson AV, 2002. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 950, 261–267.
- Furlong TM, Vianna DML, Liu L, Carrive P, 2009. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur. J. Neurosci 30, 1603–1614. 10.1111/j.1460-9568.2009.06952.x
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T, 2003. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 285, R581–R593. 10.1152/ajpregu.00671.2002
- Beig MI, Dampney BW, Carrive P, 2015. Both Ox1r and Ox2r orexin receptors contribute to the cardiovascular and locomotor components of the novelty stress response in the rat. Neuropharmacology 89, 146–156. http://dx.doi.Org/10.1016/j.neuropharm.2014.09.012
- Zheng H, Patterson LM, Berthoud H-R, 2005. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J. Comp. Neurol 485, 127–142. 10.1002/cne.20515
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N, 2000. Possible involvement of orexin in the stress reaction in rats. Biochem. Biophys. Res. Commun 270, 318–323. 10.1006/bbrc.2000.2412
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter RA, Upton N, 2001. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (0X1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology 153, 203–209.
- Grafe LA, Eacret D, Luz S, Gotter AL, Renger JJ, Winrow CJ, Bhatnagar S, 2017b. Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience 348, 313–323. 10.1016/j.neuroscience.2017.02.038
- Chang H, Saito T, Ohiwa N, Tateoka M, Deocaris CC, Fujikawa T, Soya H, 2007. Inhibitory effects of an orexin-2 receptor antagonist on orexin A- and stress-induced ACTH responses in conscious rats. Neurosci. Res 57, 462–466. 10.1016/j.neures.2006.11.009
- González JA, Iordanidou P, Strom M, Adamantidis A, Burdakov D, 2016. Awake dynamics and brain-wide direct inputs of hypothalamic MCH and orexin networks. Nat. Commun 7, 11395 10.1038/ncomms11395
- Messina G, Di Bernardo G, Viggiano A, De Luca V, Monda V, Messina A, Chieffi S, Galderisi U, Monda M, 2016. Exercise increases the level of plasma orexin A in humans. J. Basic Clin. Physiol. Pharmacol 27, 611–616. 10.1515/jbcpp-2015-0133
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, Vrontakis ME, Kirouac GJ, 2013. Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct. Funct 10.1007/s00429-013-0626-3
- Grissom N, Bhatnagar S, 2009. Habituation to repeated stress: get used to it. Neurobiol. Learn. Mem 92, 215–224. 10.1016/j.nlm.2008.07.001
- Kim T-K, Kim J-E, Park J-Y, Lee J-E, Choi J, Kim H, Lee E-H, Kim S-W, Lee J-K, Kang H-S, Han P-L, 2015. Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol. Dis 79, 59–69. 10.1016/j.nbd.2015.04.004
- Nocjar C, Zhang J, Feng P, Panksepp J, 2012. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience 218, 138–153. 10.1016/j.neuroscience.2012.05.033
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S, 2010. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 151, 1795–1805.
- Finnell JE, Lombard CM, Melson MN, Singh NP, Nagarkatti M, Nagarkatti P, Fadel JR, Wood CS, Wood SK, 2017. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain. Behav. Immun 59, 147–157. 10.1016/j.bbi.2016.08.019
- Grafe LA, Eacret D, Dobkin J, Bhatnagar S, 2018. Reduced orexin system function contributes to resilience to repeated social stress. eneuro 5, ENEURO.0273–17.2018. 10.1523/ENEURO.0273-17.2018
- Chung H-S, Kim J-G, Kim J-W, Kim H-W, Yoon B-J, 2014. Orexin administration to mice that underwent chronic stress produces bimodal effects on emotion-related behaviors. Regul. Pept 194–195, 16–22. 10.1016/j.regpep.2014.11.003
- Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S, 2011. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 61, 336–346. 10.1016/j.neuropharm.2011.04.022
- Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S, 2017a. Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol. Psychiatry 81, 683–692. 10.1016/j.biopsych.2016.10.013
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593.
- Samson WK, Taylor MM, Ferguson AV. Non-sleep effects of hypocretin/orexin. Sleep Med Rev. 2005;9:243–252.
- Shahid IZ, Rahman AA, Pilowsky PM. Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol. 2011;162:961–973.
- Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension. 2001;37:1382–1387.
- Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334:522–529.
- Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1611–R1620.
- Smith PM, Samson WK, Ferguson AV. Cardiovascular actions of orexin-A in the rat subfornical organ. J Neuroendocrinol. 2007;19:7–13.
- Dergacheva O, Wang X, Huang ZG, Bouairi E, Stephens C, Gorini C, et al. Hypocretin 1 (orexin A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther. 2005;314:1322–1327.
- Oliveira CV, Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Cardiovascular effects of hypocretin-1 in nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2003;284:H1369–H1377.
- Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–267.
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, et al. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507.
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587(Pt 9):2059–2067.
- Liu ZB, Song NN, Geng WY, Jin WZ, Li L, Cao YX, et al. Orexin-A and respiration in a rat model of smoke-induced chronic obstructive pulmonary disease. Clin Exp Pharmacol Physiol. 2010;37:963–968.
- Dutschmann M, Kron M, Morschel M, Gestreau C. Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol. 2007;159:232–235.
- Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–1314.
- Williams RH, Burdakov D. Hypothalamic orexins/hypocretins as regulators of breathing. Expert Rev Mol Med. 2008;10:e28.
- Han F. Respiratory regulation in narcolepsy. Sleep Breath. 2012;16:241–245.
- Busquets X, Barbe F, Barcelo A, de la Pena M, Sigritz N, Mayoralas LR, et al. Decreased plasma levels of orexin-A in sleep apnea. Respiration. 2004;71:575–579.
- Kirchgessner AL, Liu M. Orexin synthesis and response in the gut. Neuron. 1999;24:941–951.
- Kermani M, Eliassi A. Gastric acid secretion induced by paraventricular nucleus microinjection of orexin A is mediated through activation of neuropeptide Yergic system. Neuroscience. 2012;226:81–88.
- Wu X, Gao J, Yan J, Owyang C, Li Y. Hypothalamus-brain stem circuitry responsible for vagal efferent signaling to the pancreas evoked by hypoglycemia in rat. J Neurophysiol. 2004;91:1734–1747.
- Miyasaka K, Masuda M, Kanai S, Sato N, Kurosawa M, Funakoshi A. Central Orexin-A stimulates pancreatic exocrine secretion via the vagus. Pancreas. 2002;25:400–404.
- Bengtsson MW, Makela K, Sjoblom M, Uotila S, Akerman KE, Herzig KH, et al. Food-induced expression of orexin receptors in rat duodenal mucosa regulates the bicarbonate secretory response to orexin-A. Am J Physiol Gastrointest Liver Physiol. 2007;293:G501–G509.
- Bulbul M, Babygirija R, Ludwig K, Takahashi T. Central orexin-A increases gastric motility in rats. Peptides. 2010;31:2118–2122.
- Satoh Y, Okishio Y, Azuma YT, Nakajima H, Hata F, Takeuchi T. Orexin A affects ascending contraction depending on downstream cholinergic neurons and descending relaxation through independent pathways in mouse jejunum. Neuropharmacology. 2006;51:466–473.
- Nozu T, Kumei S, Takakusaki K, Ataka K, Fujimiya M, Okumura T. Central orexin-A increases colonic motility in conscious rats. Neurosci Lett. 2011;498:143–146.
- Baccari MC. Orexins and gastrointestinal functions. Curr Protein Pept Sci. 2010;11:148–155.
- Brzozowski T, Konturek PC, Sliwowski Z, Drozdowicz D, Burnat G, Pajdo R, et al. Gastroprotective action of orexin-A against stress-induced gastric damage is mediated by endogenous prostaglandins, sensory afferent neuropeptides and nitric oxide. Regul Pept. 2008;148:6–20.
- Bulbul M, Tan R, Gemici B, Ongut G, Izgut-Uysal VN. Effect of orexin-a on ischemia-reperfusion-induced gastric damage in rats. J Gastroenterol. 2008;43:202–207.
- Yamada H, Tanno S, Takakusaki K, Okumura T. Intracisternal injection of orexin-A prevents ethanol-induced gastric mucosal damage in rats. J Gastroenterol. 2007;42:336–341.
- Takahashi K, Arihara Z, Suzuki T, Sone M, Kikuchi K, Sasano H, et al. Expression of orexin-A and orexin receptors in the kidney and the presence of orexin-A-like immunoreactivity in human urine. Peptides. 2006;27:871–877.
- Russo F, Pavone LM, Tafuri S, Avallone L, Staiano N, Vittoria A. Expression of orexin A and its receptor 1 in the bovine urethroprostatic complex. Anat Rec. 2008;291:169–174.
- Peng HY, Chang HM, Chang SY, Tung KC, Lee SD, Chou D, et al. Orexin-A modulates glutamatergic NMDA-dependent spinal reflex potentiation via inhibition of NR2B subunit. Am J Physiol Endocrinol Metab. 2008;295:E117–E129.
- Kobayashi M, Nomura M, Fujihara H, Suzuki H, Otsubo H, Nishii H, et al. Involvement of orexin-A on micturition reflex in normal and cyclophosphamide-induced cystitis bladder in rat. Peptides. 2009;30:2348–2356.
- Yamamoto T, Saito O, Shono K, Hirasawa S. Activation of spinal orexin-1 receptor produces anti-allodynic effect in the rat carrageenan test. Eur J Pharmacol. 2003b;481:175–180.
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 1999;19:3171–3182.
- Bartsch T, Levy MJ, Knight YE, Goadsby PJ. Differential modulation of nociceptive dural input to [hypocretin] orexin A and B receptor activation in the posterior hypothalamic area. Pain. 2004;109:367–378.
- Watson SL, Watson CJ, Baghdoyan HA, Lydic R. Thermal nociception is decreased by hypocretin-1 and an adenosine A1 receptor agonist microinjected into the pontine reticular formation of Sprague Dawley rat. J Pain. 2010;11:535–544.
- Gerashchenko D, Horvath TL, Xie XS. Direct inhibition of hypocretin/orexin neurons in the lateral hypothalamus by nociceptin/orphanin FQ blocks stress-induced analgesia in rats. Neuropharmacology. 2011;60:543–549.
- Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 2005;16:5–8.
- Rainero I, Gallone S, Valfre W, Ferrero M, Angilella G, Rivoiro C, et al. A polymorphism of the hypocretin receptor 2 gene is associated with cluster headache. Neurology. 2004;63:1286–1288.
- Dauvilliers Y, Bayard S, Shneerson JM, Plazzi G, Myers AJ, Garcia-Borreguero D. High pain frequency in narcolepsy with cataplexy. Sleep Med. 2011;12:572–577.
- Gorojankina T, Grebert D, Salesse R, Tanfin Z, Caillol M. Study of orexins signal transduction pathways in rat olfactory mucosa and in olfactory sensory neurons-derived cell line Odora: multiple orexin signalling pathways. Regul Pept. 2007;141:73–85.
- Julliard AK, Chaput MA, Apelbaum A, Aime P, Mahfouz M, Duchamp-Viret P. Changes in rat olfactory detection performance induced by orexin and leptin mimicking fasting and satiation. Behav Brain Res. 2007;183:123–129.
- Prud’homme MJ, Lacroix MC, Badonnel K, Gougis S, Baly C, Salesse R, et al. Nutritional status modulates behavioural and olfactory bulb Fos responses to isoamyl acetate or food odour in rats: roles of orexins and leptin. Neuroscience. 2009;162:1287–1298.
- Buskova J, Klaschka J, Sonka K, Nevsimalova S. Olfactory dysfunction in narcolepsy with and without cataplexy. Sleep Med. 2010;11:558–561.
- Baier PC, Weinhold SL, Huth V, Gottwald B, Ferstl R, Hinze-Selch D. Olfactory dysfunction in patients with narcolepsy with cataplexy is restored by intranasal orexin A (hypocretin-1) Brain. 2008;131(Pt 10):2734–2741.
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916.
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, et al. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–187.
- Matsuzaki I, Sakurai T, Kunii K, Nakamura T, Yanagisawa M, Goto K. Involvement of the serotonergic system in orexin-induced behavioral alterations in rats. Regul Pept. 2002;104:119–123.
- Stone EA, Lin Y, Ahsan MR, Quartermain D. Evidence of roles of central alpha1-adrenoceptors and epinephrine in orexin A-induced hyperactivity in mice. Neurosci Lett. 2005;381:325–328.
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, et al. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin- 1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–209.
- Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol Behav. 2006;88:294–301.
- Kotani A, Ikeda H, Koshikawa N, Cools AR. Role of orexin receptors in the nucleus accumbens in dopamine-dependent turning behaviour of rats. Neuropharmacology. 2008;54:613–619.
- Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–E559.
- Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050:156–162.
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Muscle tone facilitation and inhibition after orexin-A (hypocretin-1) microinjections into the medial medulla. J Neurophysiol. 2002;87:2480–2489.
- Takakusaki K, Takahashi K, Saitoh K, Harada H, Okumura T, Kayama Y, et al. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol. 2005;568(Pt 3):1003–1020.
- Peever JH, Lai YY, Siegel JM. Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol. 2003;89:2591–2600.
- Zhang J, Li B, Yu L, He YC, Li HZ, Zhu JN, et al. A role for orexin in central vestibular motor control. Neuron. 2011;69:793–804.
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol Biochem Behav. 2010b;95:121–128.
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;26:25–28.
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. Eur J Neurosci. 2004;20:3054–3062.