Osmolar gap
Osmolar gap is the difference between the osmolality (mOsm/kg) which is a measure of the number of dissolved particles in a fluid and the osmolarity (mOsm/liter) which is the concentration of a solution expressed as the total number of solute particles per liter 1.
- Osmolar gap = Osmolality – Osmolarity
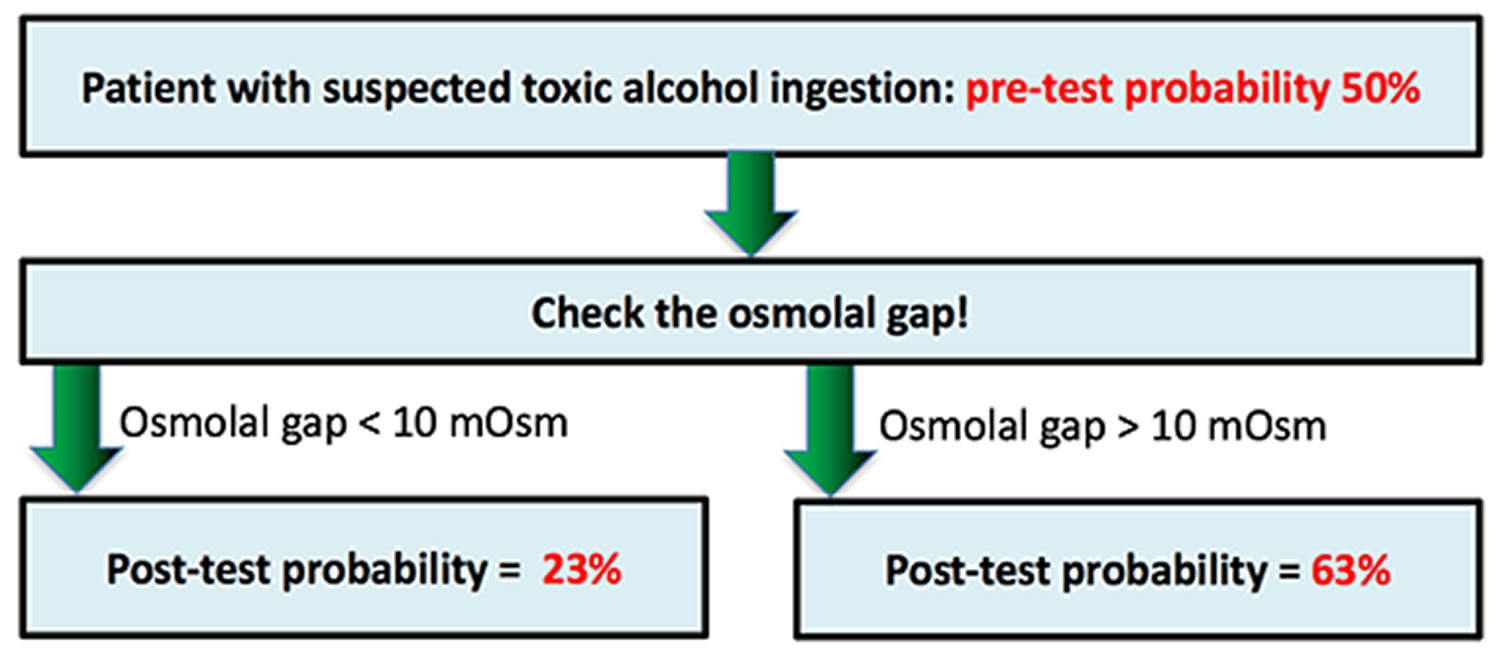
The osmolar gap is the difference between the measured osmolality and the calculated osmolarity. The calculated osmolarity is given as: 2[Na] + [Glucose] + [Urea] (all in mmol/L). Clinically, the osmolar gap may be used to detect the presence of an osmotically active particle that is not normally present in plasma. Measurement of the osmolar gap is used routinely as a screening test for toxic alcohol poisonings (ethylene glycol, diethylene glycol, butanol and methanol) as a rapid means to estimate exposure particularly when the ability to measure serum concentrations of the substances is not available and can be performed in virtually all hospital laboratories 2, 3. While the osmolar gap is not a definitive diagnostic test by any means, particularly when toxic alcohol concentrations are low, it has been shown to be a useful adjunct to estimate toxic alcohol concentrations in select clinical settings 4. To estimate toxic alcohol serum concentrations, the osmolar gap value is multiplied by one tenth of the molecular weight of the compound in question, thereby providing an estimate of the serum toxic alcohol concentration in appropriate units (mg/dl) 2.
Screening and diagnostic osmolar gap tests are generally used to classify asymptomatic patients with respect to the likelihood of the presence of a disease 5. Screening tests are ideally suited to detect diseases with a latent period between onset of disease (or time of exposure) and the development of overt symptoms, especially when the early diagnosis and initiation of therapy improves prognosis 6. Toxic alcohol exposure meets these criteria given that serious toxicity is preventable with early diagnosis and initiation of antidotal therapy. The rapid and accurate diagnosis of toxic alcohol poisoning is therefore crucial to prevent serious adverse outcomes.
Note: The units of osmolality (mOsm/kg) and osmolarity (mOsm/litre) are different so strictly they cannot be subtracted from one another. That said though, the value of the difference is clinically useful so this problem is usually ignored.
In healthy persons, the osmolar gap is small as the osmolarity (calculated using the formula below) is a fairly good estimate of the osmolality. But in some conditions, there are significant amounts of abnormal substances present which contribute to the total osmolality and then the osmolarity will underestimate the osmolality. Consequently the osmolar gap will necessarily be increased. A given concentration of abnormal other solutes (in mg/dl) will contribute more particles (mOsm/kg) if they have a low molecular weight. It follows then that if the osmolar gap is significantly elevated, this provides indirect evidence that there must be a significant concentration of one or more low molecular substances present. It does not identify these abnormal solutes but alerts you to their presence.
Important reservations need to be made about the clinical utility of the osmolar gap, in particular:
- Osmolar gap calculation depends on measurement of three substances and an osmolality measurement, so the error is the sum of the errors of all these measurements
- Many formulae are available to calculate osmolarity and the calculated value varies significantly depending on which one is used
- The osmolar gap has a wide normal range in the population
- The osmolar gap may be normal with ethylene glycol ingestion because of its higher molecular weight (in comparison to methanol). The sensitivity of the test in detecting toxic ingestion of ethylene glycol is not high
- As ethylene glycol and methanol are metabolized, the osmolar gap decreases and the anion gap increases, so a ‘normal’ osmolar gap value is more likely if the patient presents late.
An elevated osmolar gap indicates an unknown solute but does not identify it. It is important to follow-up and determine what substance or substances is responsible. As an example, consider the following situation:
- Consider a patient who has ingested ethanol as well as ethylene glycol or methanol. The ethanol will increase the osmolar gap and you can miss the presence of the more toxic substances if you make the assumption that the osmolar gap is due to the ethanol alone. This mistake could have serious adverse consequences for the patient.
- Solution 1: For this reason, it is advisable to request an ethanol level whenever you request a measured osmolality. You can then correct the osmolar gap for any ethanol present and determine a ‘corrected’ osmolar gap. This approach is generally readily available in hospitals and has the advantage of indirectly detecting the presence of ANY other such low molecular weight toxin and not just ethanol. You won’t know what this other solute is yet but your suspicions are raised and you can proceed to more specifc analyses.
- [Note: To convert ethanol levels in mg/dl to mmol/l divide by 4.6. For example, an ethanol level of 0.05% is 50mg/dl. Divide by 4.6 gives 10.9mmols/l]
- Solution 2: Another way to sort this out is if there is clinical suspicion AND your laboratory has the facilities, is to request specific assays for methanol or ethylene glycol. However, depending on the technique your laboratory uses, you may or may not detect other rare ingestions. You can miss the specific toxins that you are trying to measure if time has passed and they have already been extensively metabolised to their toxic products. In this latter case, you would be misled as to the toxic potential lurking in your patient. The problem with this solution is that many laboratories do not measure these levels so your specimen may need to be sent to a distant large laboratory.
- Solution 1: For this reason, it is advisable to request an ethanol level whenever you request a measured osmolality. You can then correct the osmolar gap for any ethanol present and determine a ‘corrected’ osmolar gap. This approach is generally readily available in hospitals and has the advantage of indirectly detecting the presence of ANY other such low molecular weight toxin and not just ethanol. You won’t know what this other solute is yet but your suspicions are raised and you can proceed to more specifc analyses.
Osmolality
Osmolality is a measure of the number of dissolved particles in a fluid. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent (mOsm/kg). The units of osmolality are mOsm/kg of solute. An osmole is the amount of a substance that yields, in ideal solution, that number of particles (Avogadro’s number, this is the number of molecules in one mole of a substance i.e., 6.022 x 1023) that would depress the freezing point of the solvent by 1.86K. Osmolality is independent of the size, shape or weight of the particles. A test for osmolality measures the amount of dissolved substances such as sodium, potassium, chloride, glucose, and urea in a sample of blood and sometimes in urine. Alternatively, it can be estimated from the major solutes expected to be in the blood or urine.
Water balance in the body is a dynamic process that is regulated by controlling the amount of water eliminated in the urine by the kidneys and by increasing or decreasing water drinking by regulating “thirst.” In a healthy person, the body perceives and reacts to changes in the amount of water and particles in the blood.
- When blood osmolality increases with a decrease in the amount of water in the blood or an increase in the number of particles such as sodium, chloride, and glucose, a gland called the hypothalamus releases antidiuretic hormone (ADH). The kidneys respond to antidiuretic hormone by conserving water and producing urine that is more concentrated. The retained water dilutes the blood and lowers blood osmolality back to normal. This also increases blood volume and blood pressure. If this is not sufficient to restore the water balance, then thirst is also stimulated so that the affected person will drink more water.
- When blood osmolality decreases, the release of ADH (antidiuretic hormone) is suppressed, the kidneys release more dilute urine, the amount of water in the body decreases, thirst is diminished, and blood osmolality increases back toward normal.
A blood (serum) osmolality test is primarily a measure of sodium dissolved in the serum (the liquid portion of blood). Sodium is the major electrolyte in the blood and urine. It works with potassium, chloride, and CO2 (in the form of bicarbonate) to maintain electrical neutrality in the body and acid-base balance. Sodium comes into the body in the diet and is normally conserved or eliminated in the urine by the kidneys to maintain its concentration in the blood within a healthy range.
In addition to electrolytes, glucose and urea contribute to osmolality. Normally their contributions are small, but when someone has high blood glucose (hyperglycemia, as found in untreated diabetes) or high blood urea (seen in diseases such as kidney failure), their influence can be significant.
Serum osmolality is often used in cases of suspected poisoning or overdose. Toxins such as methanol, isopropyl alcohol, ethylene glycol, propylene glycol, and acetone, and drugs such as salicylates (aspirin) can also affect osmolality when ingested in sufficiently large amounts.
A urine osmolality test primarily measures the waste products urea and creatinine. Urea and creatinine are produced and removed by the body at a relatively constant rate.
A serum osmolal gap (osmotic gap) may also be calculated. It is the difference between measured and calculated (estimated) osmolality results. In order to calculate the osmolal gap, tests for blood sodium, blood urea nitrogen (BUN), and glucose must be performed to calculate the expected osmolality. Some versions of the expected osmolality calculation also include the measurement of ethanol. An increase in the osmolal gap (greater than 10) indicates the presence of substances such as toxins, aspirin (salicylates), or mannitol.
Calculating osmolality
In order to calculate the osmolar gap, tests for blood sodium, blood urea nitrogen (BUN), and glucose must be performed to calculate the expected osmolality. Some versions of the expected osmolality calculation also include the measurement of ethanol. An example calculation is:
Serum Osmolality Calculation (ethanol not always included)
- 2 x (Na+) + (Glucose/18) + (BUN/2.8) + (Ethanol/3.8)
Note: Glucose, BUN, and ethanol may be reported in mg/dL (milligrams per deciliter) or mmol/L (millimole per liter). The numbers shown in the equation above are used to convert from mg/dL to mmol/L. For mmol/L, the equation would be:
- 2 x (Na+) + (Glucose) + (BUN) + (Ethanol)
How is osmolality test used?
The blood osmolality test is primarily used to help determine whether a person has ingested a toxin such as methanol or ethylene glycol (antifreeze). Sometimes it may be used to investigate low blood sodium and your body’s water balance. Osmolality may be measured directly or estimated using a calculation.
In addition to osmolality, the osmolal gap (osmotic gap) may be calculated and used to detect and/or measure toxins in the blood, such as methanol, ethylene glycol, isopropyl alcohol, and propylene glycol.
Urine osmolality may be used along with serum osmolality to help evaluate the body’s water balance and to investigate increased and decreased urination. Urine sodium and creatinine are often ordered along with urine osmolality. Sometimes a urine osmolal gap is calculated and used to help evaluate the kidney’s ability to eliminate acid and reabsorb bicarbonate, to detect the presence of osmotically active molecules, and to compare with the serum osmolal gap.
Osmolarity
Osmolarity of a solution is the number of osmoles of solute per liter of solution (mOsm/liter). An osmole is the amount of a substance that yields, in ideal solution, that number of particles (Avogadro’s number, this is the number of molecules in one mole of a substance i.e., 6.022 x 1023) that would depress the freezing point of the solvent by 1.86K. Serum osmolality can be measured by use of an osmometer or it can be calculated as the sum of the concentrations of the solutes present in the solution. The value measured in the laboratory is usually referred to as the osmolality. The value calculated from the solute concentrations is reported by the laboratory as the osmolarity. The Osmolar gap is the difference between these two values. The two values usually don’t match exactly for various reasons: there are a number of formulas that can be used and they all give slightly different results; the formulas typically use the concentrations of only 3 solutes (Na, glucose, urea) in the calculation so contributions from abnormal small MW uncharged substances will be missed so the calculated value will be low; use of osmometers that use the vapour pressure method are unreliable in the presence of volatile chemicals.
Osmolarity is calculated from a formula which represents the solutes which under ordinary circumstances contribute nearly all of the osmolality of the sample. There are many such formulae which have been used. One widely used formula for plasma which is used at my hospital is:
- Calculated osmolarity = (1.86 x [Na+]) + [glucose] + [urea] + 9
Other equations used to calculate the serum osmolarity:
- Calculated osmolarity = 2 x Na (mEq/L) + Blood urea nitrogen (mmol/L) + Glucose (mmol/L) + Ethanol (mmol/L)
- Calculated osmolarity = 2 x Na (mEq/L) + Blood urea nitrogen (mmol/L) + Glucose (mmol/L) + 1.25 x Ethanol (mmol/L)
- To convert from SI units, use the following corrections: Blood urea nitrogen [BUN]/2.8 mg/dl, glucose/18.1 mg/dl, ethanol/0.217 mg/dl
Note regarding units: For the above equation, all concentrations are in mmol/l, and not mg/100mls. The result will then be in mOsm/l of solution. This equation is often expressed differently in North America where glucose and blood urea nitrogen (BUN) are reported in mg/dl. This version is essentially identical as it just includes conversion factors to convert mg/dl to mmol/l:
- Calculated osmolarity = (1.86 x [Na+]) + glucose/18 + BUN/2.8 + 9
What level of osmolar gap is “abnormal”?
A serum osmolar gap greater than 10 mOsm/l is considered abnormal and indicates the presence of an osmotically active substance in the blood. When someone has an increased osmolar gap, a toxic ingestion, such as methanol, is suspected, and the size of the osmolar gap is proportional to the amount of toxin. Other common causes of an elevated osmolar gap are alcoholic ketoacidosis, kidney failure, diabetic ketoacidosis, and shock. During monitoring of treatment, the osmolar gap, and findings such as a low sodium level, return to normal.
In general, increased serum osmolality may be due to either decreased water in the blood or increased solutes. Examples of conditions in which blood (serum) osmolality may be increased include:
- Toxic ingestion of ethanol, methanol, ethylene glycol, isopropyl alcohol or aspirin (salicylates), for example
- Dehydration
- Diabetes
- Increase blood glucose
- Increased blood sodium
- Increased nitrogen waste products in the blood (uremia)
- Stroke or head trauma that leads to decreased antidiuretic hormone (ADH)
- Kidney damage and disease
- Mannitol therapy–used in the treatment of brain swelling (cerebral edema)
- Shock
In general, a decreased serum osmolality may be due to increased fluids. Examples of conditions causing decreased blood osmolality include:
- Excess hydration (drinking excessive amounts of water, water retention or decreased ability of the kidneys to produce urine)
- Decreased blood sodium
- Increased antidiuretic hormone (ADH) secretion
One study 7 suggested the use of this formula:
- Calculated osmolarity = ( 2 x [Na+] ) + glucose/18 + BUN/2.8 + ethanol/4.6
They found a mean osmolar gap of 2.2 with standard deviation (SD) 5.5 mOsm/l. The 95% range (mean +/- 2SD) was -14 to +10. This study 7 is probably the basis for the >10 value as being abnormal. The range for normal values is very dependent on the particular formula that is used.
An elevated osmolar gap provides indirect evidence for the presence of an abnormal solute which is present in significant amounts. To have much effect on the osmolar gap, the substance needs to have a low molecular weight and be uncharged so it can be present in a concentration (measured in mmol/l) sufficient to elevate the osmolar gap.
Ethanol, methanol, ethylene glycol (used in anti-freeze solutions), isopropanol, and propylene glycol (used as a vehicle with some drugs e.g. lorazepam) are solutes that cause an elevated osmolar gap. If you suspect that your patient may have ingested one of these substances than, as a screening tool, you should determine the osmolar gap 8. This testing is readily available in hospitals. Apart from ethanol levels, determination of the levels of other toxic glycols and alcohols is much less commonly available in pathology laboratories.
When is osmolality test ordered?
Osmolality test may be ordered when it is suspected that someone has ingested a toxin such as methanol or ethylene glycol.
Osmolality testing may be ordered when a person has an unexplained low blood sodium or signs and symptoms that a healthcare practitioner suspects may be due to low blood sodium such as:
- Excessive thirst
- Confusion
- Nausea
- Headache
- Lethargy
- In severe cases, seizures or coma
- Chen JS, Al Khalili Y. Physiology, Osmoregulation and Excretion. [Updated 2019 Apr 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541108
- Holland MG, Nelsen J, Rosano TG. Osmol gap method for the detection of diethylene glycol in human serum. World J Emerg Med. 2010;1(2):104–107. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4129750
- Liamis G, Filippatos TD, Liontos A, Elisaf MS. Serum osmolal gap in clinical practice: usefulness and limitations. Postgrad Med. 2017 May;129(4):456-459.
- Lynd LD, Richardson KJ, Purssell RA, et al. An evaluation of the osmole gap as a screening test for toxic alcohol poisoning. BMC Emerg Med. 2008;8:5. Published 2008 Apr 28. doi:10.1186/1471-227X-8-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2390580
- Bailey B, Amre DK. A toxicologist’s guide to studying diagnostic tests. Clin Toxicol (Phila) 2005;43:171–179.
- Morrison AS. Screening. In: Rothman KJ, Greenland S, editor. Modern epidemiology. 2nd. Lippincott-Raven; 1998. pp. 7–28.
- Osmol gaps revisited: normal values and limitations. J Toxicol Clin Toxicol. 1993;31(1):81-93. DOI:10.3109/15563659309000375
- Krasowski MD. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clinical Pathology. 2012; 12:1