What is photobiomodulation therapy
Photobiomodulation, also known as low level laser (or light) therapy, low-intensity laser therapy, low-power laser therapy, cold laser, soft laser, red light therapy, biostimulation and photobiostimulation 1. Photobiomodulation describes the ability to stimulate or inhibit cellular functions by using light at specific wavelengths, intensities and dosing regimens. The classically described photobiomodulation treatment window is between 600 and 1,200 nm 2. Light in this portion of the spectrum readily penetrates skin and tissues via the so-called optical window. Light is absorbed by various structures and molecules, and primarily molecules that are instrumental in energy production and oxygen delivery. The primary site of light absorption in mammalian cells has been identified as the mitochondria and more specifically, cytochrome C oxidase (CCO). Photobiomodulation has been shown to influence a wide variety of cellular functions, including gene expression, growth and proliferation, survival, and differentiation 3. It is hypothesized cytochrome C oxidase appears to be the primary photoacceptor and transducer of photosignals in these regions of the light spectrum 4. The accepted light energy activates the cytochrome c oxidase and triggers a series of biochemical cascades that improve cellular functions 5. These functions are primarily mediated by raising the levels of adenosine triphosphate (ATP), which increases the mitochondrial membrane potential, cyclic adenosine monophosphate, calcium (Ca2+), and reactive oxygen species (ROS) and activates transcription factors 6. The mechanistic basis for the outcomes observed after using photobiomodulation therapy are a result of the upregulation of intracellular metabolism by increasing production of adenosine triphosphate (ATP), augmenting other metabolic pathways, and the induction or reduction of reactive oxygen species (ROS) and other free radical production 2.
The interaction of photons with cells and cellular structures is a necessary condition for photobiomodulation. Scientists have learned that all cells and tissues don’t respond to photobiomodulation and that one size does not fit all when determining the dose or course of treatment 7. Different photobiomodulatory effects have been described depending upon the specific cell lines and species being investigated. One laboratory demonstrated that cell proliferation and metabolism in vitro can be influenced by varying the dose frequency or treatment interval of the photobiomodulation therapy 8. Scientists have also demonstrated this same phenomenon as regards wound healing in a mice pressure ulcer model 7. These investigations underscore the concept that a unique dose frequency combination exists for tissues and cell lines and that this specific treatment paradigm must be determined to optimize outcomes and maximally stimulate cellular metabolism and proliferation.
Figure 1. Photobiomodulation mechanisms of action (hypothesis)

The therapeutic use of light began with the invention of laser technology in the early 1960s 9. Mester et al. 10 noted that laser light at low doses demonstrated increased hair growth at an accelerated rate in mice and promoted excisional wound healing. However the use of the term low-level laser therapy has been replace with less ambiguous word photobiomodulation, because the words “low” and “level” are vague and not accurately definable, whereas the word “laser” is no longer appropriate, as other types of light devices such as LEDs and broadband light sources are currently used for photobiomodulation therapy 11.
Photobiomodulation (low level laser) therapy is a safe form of light/heat treatment under investigation for a variety of health indications. It is being used to treat the genetic forms of hair loss common in men and women, androgenetic alopecia or pattern balding 12, to reduce pain, inflammation, edema, and to regenerate damaged tissues such as wounds, bones and tendons 13, 14.
Figure 2. Photobiomodulation clinical effects

Types of Photobiomodulation
Photobiomodulation can be classified into two modes by its continuity:
- Continuous wave (CW) and
- Pulsed wave light (PW).
Most previous studies have used continuous wave-photobiomodulation to aggressively promote the proliferation and differentiation of stem cells 15, beginning with dental treatment 16. Continuous wave-photobiomodulation typically uses low power density, from 5 mW/cm2 to 5 W/cm2 25, to prevent thermal effects in intracellular molecules. However, pulsed wave-photobiomodulation is more effective in maintaining an a-thermal environment due to the quenching periods, that is, OFF times. PW-PBM also enables the light to penetrate more deeply into a biological system than continuous wave-photobiomodulation because it uses higher peak power while keeping the total energy the same26. In addition, pulsed wave-photobiomodulation can promote light–biological system interactions. Some fundamental frequencies in biological systems, in the range of tens to hundreds Hz, are similar to the pulsing frequencies used in pulsed wave-photobiomodulation 17.
On the other side, the responsiveness of biological systems to photobiomodulation can be identified using delayed luminescence, which is measured in the form of optical photons emitted after the illumination source is switched off. Thus, delayed luminescence is a spectral emission from the optical range to near-infrared (780 to 1,100 nm), and its intensity is various orders of magnitude 18. Delayed luminescence demonstrates cellular reduction/oxidation (redox) states in relation to cytochrome C oxidase, which produces ROS in the mitochondrial respiratory chain28,29. Because the cellular redox state appears to differ in the proliferation and differentiation phases of a cell, delayed luminescence can be used to determine cellular phases 19. The cellular phase is associated with further transient increases in cellular ROS production, which also affects delayed luminescence 20.
How Photobiomodulation Therapy for Hair Loss is supposed to work ?
The hair growth cycle consists of three phases: growth (anagen phase), resting (telogen phase) and shedding (catagen phase). Hair loss in androgenetic alopecia depends on a testosterone derivative in the skin, dihydrotestosterone (DHT). Low level laser therapy is believed to increase blood flow in the scalp and stimulate metabolism in catagen or telogen follicles, resulting in the production of anagen hair. In theory:
- The photons of light act on cytochrome C oxidase leading to the production of adenosine triphosphate (ATP). This is converted to cyclic AMP in the hair follicle cells, releasing energy and stimulating metabolic processes necessary for hair growth.
- Release of nitric oxide from cells leads to increased vascularisation to the scalp distributing nutrients and oxygen to the hair roots.
- Excessive build-up of dihydrotestosterone (DHT) is prevented.
What is the clinical evidence to show Photobiomodulation Therapy for Hair Loss is effective ?
Physicians have varying views on whether or not low level laser therapy is effective. While some physicians reject its use entirely, others believe that low level laser therapy can provide benefit for some men and women suffering from androgenic alopecia (genetic baldness). It has also been suggested that it may assist a hair transplant patient’s postoperative wound healing process and expedite hair growth.
- Results of a double-blind, sham device-controlled, randomised multicentre trial have shown that 110 male patients with Norwood-Hamilton classes IIa-V androgenic alopecia, exhibited a significantly greater increase in mean terminal hair density compared with subjects in the sham device group after 26 weeks therapy with the laser light comb 21.
- Macro photographs of the scalp were captured of all of the subjects at the beginning and end of the trial and sophisticated hair counting software was used to determine the number of normal-sized hairs that grew as a result of using either device.
- Hair growth in subjects who used laser therapy increased by an average of 19 normal-size hairs per square centimeter, while it decreased by an average of 7 normal-size hairs per square centimeter in those using the placebo device.
- Consistent with this evidence for primary effectiveness, significant improvements in overall hair regrowth were demonstrated in terms of patients’ subjective assessment at 26 weeks over baseline.
- Treated subjects also experienced more favorable hair attributes, such as thicker, shinier, and more manageable hair compared to those who used the placebo device.
- No statistical improvement was noted on global investigator assessment.
- Similar study results have been reported in a double-blind device controlled study in women with androgenic alopecia 22.
In a second study of 103 males and 122 females with pattern alopecia that completed the study, HairMax® LaserComb (with 12, 9 and 7 beams) was reported to result in increase in terminal hair density compared to similar trial subjects treated with a sham device 23.
Trials are underway to study the efficacy of LaserCap™ (Transdermal Cap, Gates Mills, Ohio), TopHat 655 Rejuvenation System (Apira Science, Newport Beach, California) and Erchronia ML Scanner (Erchronia Corporation, McKinney, Texas) in pattern alopecia and other forms of hair loss 24.
However, published trials of low level laser light have been criticized as not being independent and anecdotal individual reports of using these devices appears disappointing.
Benefits of laser therapy for hair loss
- Low level laser thereapy can be used in both men and women
- No adverse effects have been reported
- It is clean and painless
- Low level laser hair therapy is relatively inexpensive
- It requires minimal time commitment
- Some low level laser therapy devices are portable
- Hair growth may occur on the top of the head/crown and along the hairline of forehead
Improvement is reported in at least some users after 12 to 26 weeks of use, with reduced hair fall and noticeable hair growth.
How is low level laser hair therapy administered ?
Laser hair therapy may be delivered in a salon by professionals trained in its administration, or at home.
Two to three times weekly treatments are typically recommended, and consist of a 8 to 15-minute exposure of the scalp to light-emitting diodes under a bonnet or head cap or using a handheld comb or brush.
Scalp treatment and massages that promote blood circulation may be used additionally as part of the program.
Proprietors of low level laser therapy services speak about the importance of regularity, which includes frequent appointments (twice a week, more or less) over a long duration (typically one year).
Warnings and caution
Laser therapy should not be used concomitantly with medications or products that are photosensitising.
Transcranial photobiomodulation
Transcranial photobiomodulation is a non‐invasive low‐level laser therapy, where a laser with near‐infrared light (620–1,100 nm) is used to stimulate the brain is a novel form of non‐invasive photobiomodulation that has shown therapeutic potential in a variety of neurological and psychological conditions 25.
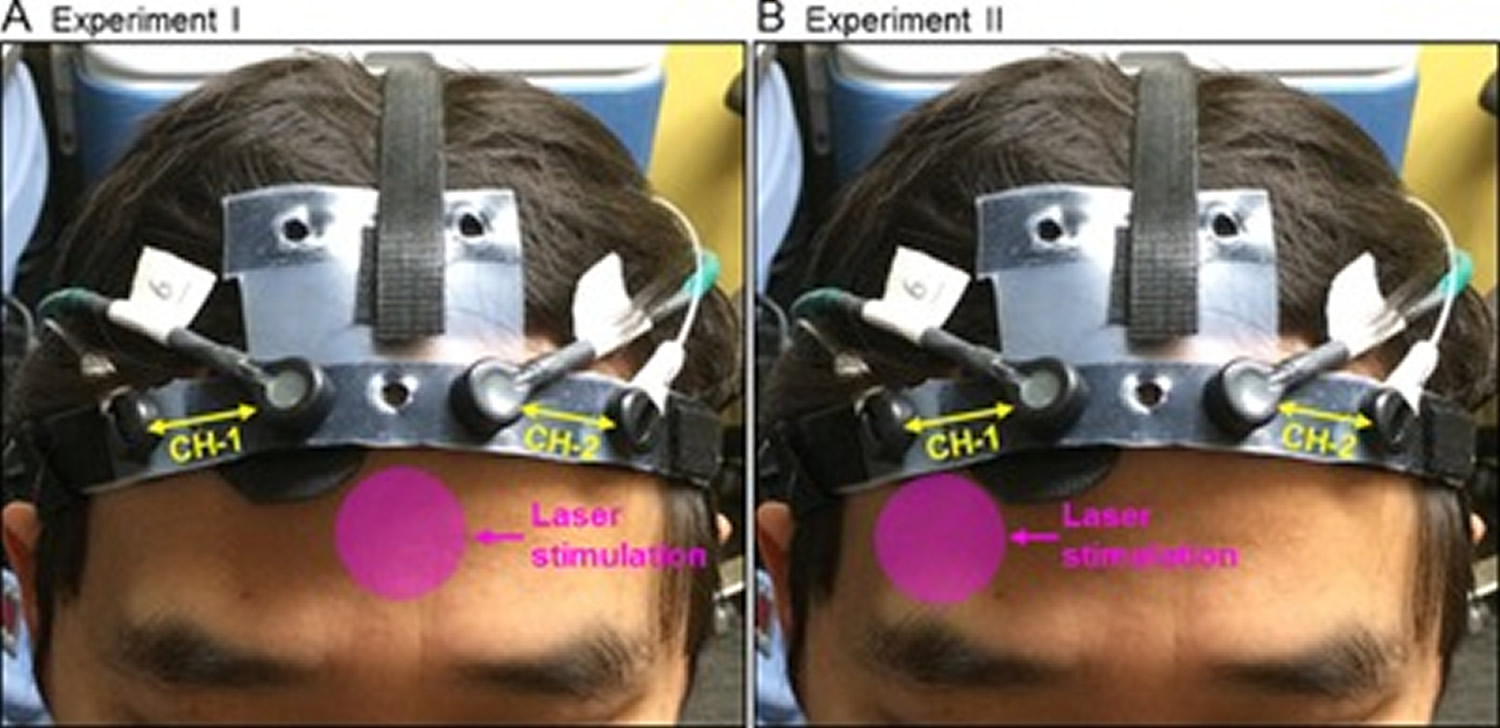
Figure 3. Transcranial photobiomodulation

Note: Positions of the transcranial laser stimulation and configuration of the functional near‐infrared spectroscopy (fNIRS) probe in (A) Experiment I, and (B) Experiment II. In each graph, the pink circle indicates the approximate position where the treatment laser (in 4.16‐cm diameter) was shined on. The fNIRS probe consisted of two measurement channels (CH‐1 and CH‐2), one channel over each cerebral hemisphere.
The laser stimulation session was divided into 10 one‐minute cycles, 55‐second stimulation laser on and 5‐second stimulation laser off per cycle. The fNIRS data acquisition included 1‐minute baseline, 10‐minute laser stimulation, and 6‐minute recovery.
[Source 26]In recent years, transcranial photobiomodulation has gained attention for its therapeutic potential in a variety of neurological and psychological conditions. Transcranial photobiomodulation has been shown to be safe for treating ischemic stroke patients in a few controlled clinical trials, but did not significantly improve patient outcomes 27. Two studies by Naeser et al. 28, 29 reported that daily use of near‐infrared light to the forehead may improve cognitive functions in patients with chronic traumatic brain injuries. Schiffer et al. 30 also found that a single near‐infrared light treatment to the forehead using LEDs may have psychological benefits in ten patients with major depression and anxiety. Stimulating with the same 0.25 W/cm2 irradiance as Schiffer et al. 30, but using a laser with a longer wavelength (1,064 nm), Barrett and Gonzalez‐Lima 31 conducted the first controlled study in 40 healthy human participants and demonstrated that transcranial laser stimulation improves cognitive and emotional functions. A subsequent controlled study by Blanco et al. 32 also demonstrated that transcranial laser stimulation with 0.25 W/cm2 irradiance and 1,064‐nm laser improves executive functions in healthy human participants.
The mechanism of action of near‐infrared light rests on photon absorption by cytochrome oxidase 33, which is the terminal enzyme in the mitochondrial respiratory chain that plays a key role in cerebral oxygen utilization for energy metabolism 34. The more the activity of cytochrome oxidase increases, the more oxygen consumption and metabolic energy is produced via mitochondrial oxidative phosphorylation 35. This photonics‐bioenergetics mechanism results in metabolic and hemodynamic alterations in the brain that facilitate both neuroprotection and cognitive enhancement 36. In 2012, Rojas et al. 37 were the first to report that near‐infrared light increased oxygen consumption in the rat prefrontal cortex in vivo. However, most of the human studies have evaluated the effects of low‐level laser therapy by observing the changes in behavioral and psychological measures and postulating the underlying neurophysiological mechanism that causes them. To date, only the study by Schiffer et al. 30 has looked at the effects of near‐infrared LEDs on human cerebral hemodynamics by measuring the total hemoglobin changes with a cerebral oximeter.
Functional near‐infrared spectroscopy (fNIRS) 38 is an emerging neuroimaging technology that measures the changes in cerebral hemodynamics and oxygenation related to neuronal activities. Because both fNIRS and transcranial laser stimulation use light in the near‐infrared range, they share similar optical pathways through the tissues. Thus, fNIRS is a suitable tool for in vivo mechanistic study of transcranial laser stimulation. Furthermore, both transcranial laser stimulation and fNIRS are safe, compact and easy to implement. A combination of these two non‐invasive, near‐infrared technologies can potentially provide an effective treatment‐with‐imaging approach for neurological and psychological applications.
However, due to the limitation in continuous‐wave fNIRS, the long‐term duration of effects of transcranial laser stimulation remains unknown. Some previous studies have suggested the benefits could last for several weeks. For example, Barrett and Gonzalez‐Lima 31 found a significant benefit as compared to the placebo group in positive and negative affective states in healthy volunteers two weeks after a single 8‐minute laser stimulation as described here. Schiffer et al. 30 reported psychological benefits at 2 and 4 weeks after a single treatment in patients with anxiety and depression. Light power density (0.25 W/cm2) and energy density (60 J/cm2) used in these two studies were the same, but Schiffer et al. 30 used 810‐nm LEDs instead of 1,064‐nm laser. Naeser et al. 29 used similar LEDs in patients with mild traumatic brain injury for 18 treatments (three treatments per week for 6 weeks), and measured cognitive performance after one week, and 1 and 2 months after the 18th treatment. They found a significant linear trend for the effect of LED treatment over time for various cognitive tests. While these pioneering studies are promising, there are no placebo‐controlled human studies investigating long‐term neuronal or cognitive effects after single or repeated transcranial photobiomodulation treatments.
References- Anders JJ, Lanzafame RJ, Arany PR. Low-Level Light/Laser Therapy Versus Photobiomodulation Therapy. Photomedicine and Laser Surgery. 2015;33(4):183-184. doi:10.1089/pho.2015.9848. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4390214/
- Photobiomodulation, tissue effects and bystanders. Lanzafame RJ. Photomed Laser Surg. 2011 Aug; 29(8):519-20.
- Zhang, Y. et al. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol. 120, 849–57 (2003).
- Karu, T. I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62, 607–10 (2010).
- Karu, T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 49, 1–17 (1999).
- Wu, S. et al. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal. 20, 733–46 (2014).
- Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lanzafame RJ, Stadler I, Kurtz AF, Connelly R, Peter TA Sr, Brondon P, Olson D. Lasers Surg Med. 2007 Jul; 39(6):534-42.
- A study of the effects of phototherapy dose interval on photobiomodulation of cell cultures. Brondon P, Stadler I, Lanzafame RJ. Lasers Surg Med. 2005 Jun; 36(5):409-13.
- Schawlow AL. C. H. Townes infrared and optical masers physical review. Phys Rev X 1958;112:1940–1949
- [The effect of laser beams on the growth of hair in mice]. Mester E, Szende B, Gärtner P. Radiobiol Radiother (Berl). 1968; 9(5):621-6. https://www.ncbi.nlm.nih.gov/pubmed/5732466/
- Photobiomodulation: poised from the fringes. Arany PR. Photomed Laser Surg. 2012 Sep; 30(9):507-9. http://online.liebertpub.com/doi/abs/10.1089/pho.2012.9884
- https://www.dermnetnz.org/topics/low-dose-laser-therapy-for-hair-loss/
- Hamblin, M. R., Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol. Accepted Author Manuscript. doi:10.1111/php.12864
- Pulse frequency dependency of photobiomodulation on the bioenergetic functions of human dental pulp stem cells. Scientific Reports 7, Article number: 15927 (2017). doi:10.1038/s41598-017-15754-2. https://www.nature.com/articles/s41598-017-15754-2
- Emelyanov, A. N. & Kiryanova, V. V. Photomodulation of proliferation and differentiation of stem cells by the visible and infrared light. Photomed Laser Surg. 33, 164–74 (2015).
- Arany, P. R. et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci Transl Med. 6, 238–69 (2014).
- Hashmi, J. T. et al. Effect of pulsing in low-level light therapy. Lasers in surgery and medicine 42, 450–466 (2010).
- Scordino, A. et al. Delayed luminescence to monitor programmed cell death induced by berberine on thyroid cancer cells. J Biomed Opt. 19, 117005 (2014).
- Tafur, J. & Mills, P. J. Low-intensity light therapy: exploring the role of redox mechanisms. Photomed Laser Surg. 26, 323–8 (2008).
- Tafur, J. et al. Biophoton detection and low-intensity light therapy: a potential clinical partnership. Photomed Laser Surg. 28, 23–30 (2010).
- Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29(5):283-92
- Treatment of Androgenetic Alopecia in Females, 9 beam. https://clinicaltrials.gov/ct2/show/NCT00981461
- Efficacy and Safety of a Low-level Laser Device in the Treatment of Male and Female Pattern Hair Loss: A Multicenter, Randomized, Sham Device-controlled, Double-blind Study. Jimenez, J.J., Wikramanayake, T.C., Bergfeld, W. et al. Am J Clin Dermatol (2014) 15: 115. https://doi.org/10.1007/s40257-013-0060-6
- Kalia S, Lui H. Utilizing electromagnetic radiation for hair growth: a critical review of phototrichogenesis. Dermatol Clin. 2013 Jan;31(1):193-200.
- Eells JT, Wong‐Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT. Mitochondrial signal transduction in accelerated wound and retinal healing by near‐infrared light therapy. Mitochondrion 2004; 4:559–567.
- Tian F, Hase SN, Gonzalez‐Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers in Surgery and Medicine. 2016;48(4):343-349. doi:10.1002/lsm.22471. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5066697
- Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J, for the NEST‐2 Investigators. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke 2009; 40:1359–1364. http://stroke.ahajournals.org/content/40/4/1359.long
- Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, Knight JA. Improved cognitive function after transcranial, light‐emitting diode treatments in chronic, traumatic brain injury: Two case reports. Photomed Laser Surg 2011; 29:351–358. https://www.ncbi.nlm.nih.gov/pubmed/21182447
- Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, Knight JA, Meehan WP 3rd, Baker EH. Significant improvements in cognitive performance post‐transcranial, red/near‐infrared light‐emitting diode treatments in chronic, mild traumatic brain injury: Open‐protocol study. J Neurotrauma 2014; 31:1008–1017. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4043367/
- Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: A pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 2009; 5:46. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2796659/
- Barrett DW, Gonzalez‐Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013; 230:13–23. https://www.ncbi.nlm.nih.gov/pubmed/23200785
- Blanco NJ, Maddox WT, Gonzalez‐Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 2015. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4662930/
- Pastore D, Greco M, Passarella S. Specific helium‐neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol 2000; 76:863–870.
- Wong‐Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem 2005; 280:4761–4771.
- Rojas JC, Gonzalez‐Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol 2013; 86:447–457.
- Gonzalez‐Lima F, Auchter A. Protection against neurodegeneration with low‐dose methylene blue and near‐infrared light. Front Cell Neurosci 2015; 9:179. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4428125/
- Rojas JC, Bruchey AK, Gonzalez‐Lima F. Low‐level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis 2012; 32:741–752. https://www.ncbi.nlm.nih.gov/pubmed/22850314
- Ferrari M, Quaresima V. A brief review on the history of human functional near‐infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012; 63:921–935.