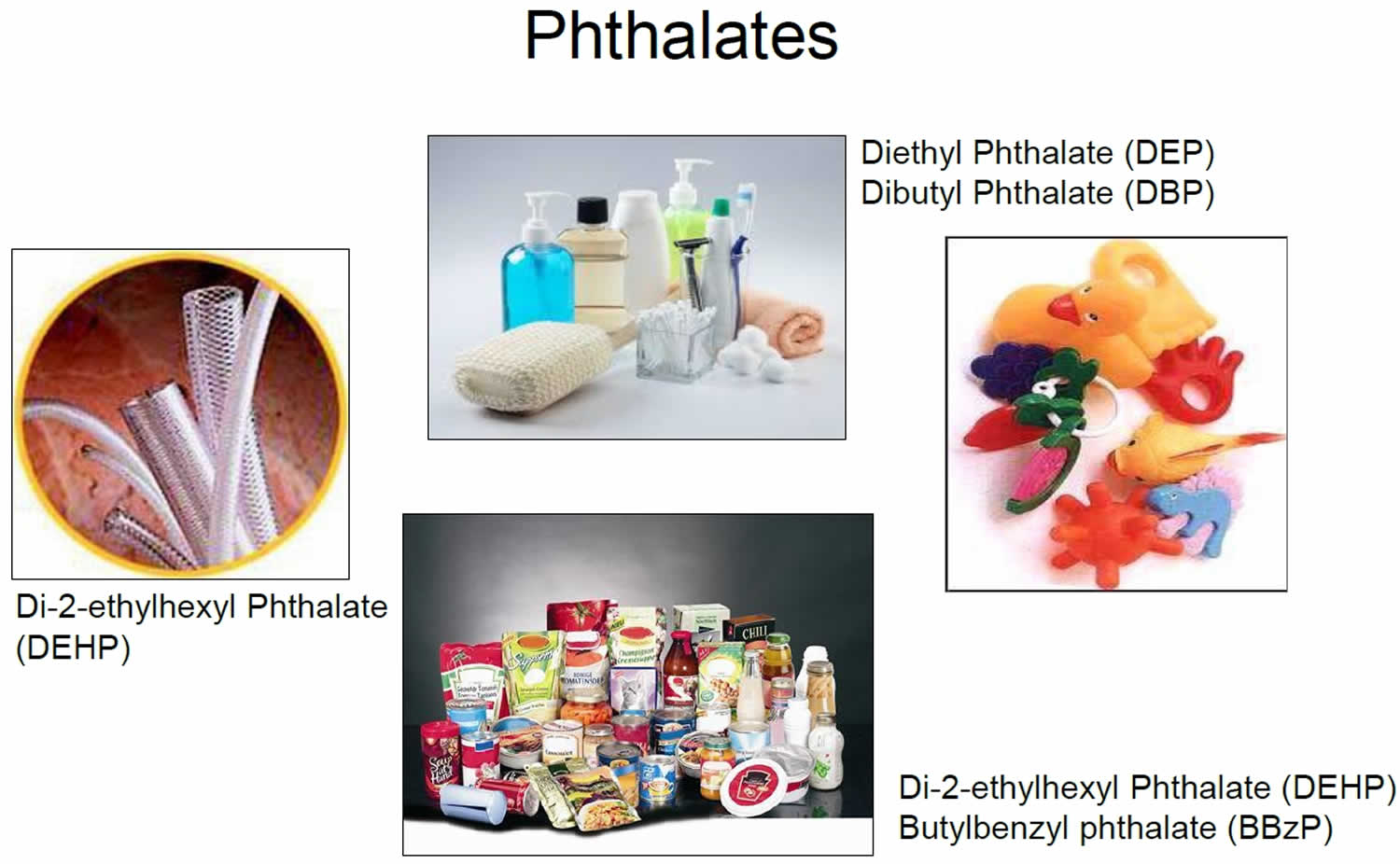
What are phthalates
Phthalates are diesters of phthalic acids or phthalate esters, is a family of man-made industrial chemicals extensively used since the early 20th century as softeners of plastics, solvents in perfumes, and additives to hairsprays and lubricants and as insect repellents 1. Phthalates are a class of manufactured chemicals commonly used to increase the flexibility of plastics in a wide array of consumer products. High-molecular-weight phthalates, such as butylbenzyl phthalate (BBzP), di-2-ethylhexyl phthalate (DEHP) and mixtures of di-n-octyl phthalates (DnOP), are most well-known for their use as plasticizers in polyvinyl chloride (PVC) materials such as food packaging, children’s toys, vinyl flooring, and medical devices 2. Whereas low-molecular-weight phthalates, such as dibutyl phthalate (DBP), are used primarily as solvents in items such as shampoo and nail polish 3. More than 470 million pounds of phthalates are produced or imported in the United States each year 4. Human exposure to phthalates is nearly unavoidable 5. Phthalates are used in hundreds of products, such as toys, vinyl flooring and wall covering, detergents, lubricating oils, food packaging, pharmaceuticals, blood bags and tubing, and personal care products, such as nail polish, hair sprays, aftershave lotions, soaps, shampoos, perfumes and other fragrance preparations. By far the most common use of phthalates is in the production of polyvinyl chloride (PVC) products 6. Polyvinyl chloride (PVC) is the second most commonly used plastic in the world, and is present in pipes and tubing, construction materials, packaging, electrical wiring, and thousands of consumer goods 7. Biomonitoring samples collected from women of reproductive age as part of the National Human and Nutrition Examination Survey (NHANES) identified metabolites from >13 different phthalates and detected at least 1 phthalate metabolite in all available samples 8. The aggregated dietary exposure for di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), di-2-ethylhexyl phthalate (DEHP) and di-isononylphthalate (DINP) was estimated to be 0.9–7.2 and 1.6–11.7 μg/kg body weight per day for mean and high consumers, respectively, thus contributing up to 23% of the group‐tolerable daily intake (TDI) in the worst‐case scenario 9. For di-isodecylphthalate (DIDP), not included in the group‐tolerable daily intake (TDI), dietary exposure was estimated to be always below 0.1 μg/kg body weight per day and therefore far below the tolerable daily intake (TDI) of 150 μg/kg body weight per day.
Di-2-ethylhexyl phthalate (DEHP) is used primarily as a plasticizer for polyvinyl chloride (PVC) and can therefore be found in a variety of products such as floor and wall coverings, vinyl gloves, toys, child care articles, food packaging materials, and medical devices 10. DEHP is one of the most environmentally abundant endocrine‐disrupting chemicals that cause reproductive abnormalities in humans, and DEHP can leach out of plastic beverage or food containers and readily enter the blood following oral ingestion. After absorption, the parent diester phthalates are rapidly hydrolyzed to the corresponding monoesters, some of which are then further metabolized, with the metabolites excreted in urine and feces. In humans, phthalates are eliminated mostly within hours, with excretion complete by a day or two; half-lives in the body are in hours 11. For phthalates with short alkyl chains, monoesters represent the major human metabolite, but in the case of phthalates with long alkyl chains, including DEHP, diisononyl phthalate (DINP) and diisodecyl phthalate (DIDP), the monoesters are further metabolized via omega- and omega-1-oxidation of the aliphatic side chain 12.
Developmental exposures to DEHP has been associated with increased body fat and impaired glucose tolerance in rodents 13. DEHP has been shown to produce a wide range of adverse effects in experimental animals, notably liver toxicity and testicular atrophy 14. Although the toxic and carcinogenic effects of DEHP have been well established in experimental animals, the ability of this compound to produce adverse effects in humans is controversial. The specific mechanisms underlying the toxic effects of DEHP are an area that is largely unexplored. As a result, the ability of DEHP and other phthalate esters to produce adverse effects in humans has been a topic of active discussion and debate in the scientific and regulatory communities.
Despite indications that human exposures to DEHP are decreasing, exposure to other phthalates such as DINP, are increasing 8, there is a lack of in vivo data regarding metabolic impacts of developmental exposures to phthalates other than DEHP. Animal studies examining metabolic health outcomes resulting from exposures to phthalates other than DEHP are needed to understand whether other phthalates are also capable of interfering with metabolic processes.
Table 1. Phthalate parent compounds and their metabolites
| Phthalate name | Abbreviation | Urinary metabolite | Abbreviation |
|---|---|---|---|
| Dimethyl phthalate | DMP | Mono-n-methyl phthalate | MnMP |
| Diethyl phthalate | DEP | Mono-ethyl phthalate | MEP |
| Di-isobutyl phthalate | DiBP | Mono-isobutyl phthalate | MiBP |
| Di-n-butyl phthalate | DnBP | Mono-n-butyl phthalate | MnBP |
| Di-n-octyl phthalate | DnOP | Mono-(3-carboxypropyl) phthalate | MCPP |
| Di-isononyl phthalate | DiNP | Mono-carboxyoctyl phthalate | MCOP |
| Di-isodecyl phthalate | DiDP | Mono-carboxynonyl phthalate | MCNP |
| Benzylbutyl phthalate | BzBP | Mono-benzyl phthalate | MBzP |
| Di-2-ethylhexyl phthalate | DEHP | Mono-2-ethylhexyl phthalate | MEHP |
| Mono-(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | ||
| Mono-(2-ethyl-5-oxohexyl) phthalate | MEOHP | ||
| Mono-(2-ethyl-5-carboxypentyl) phthalate | MECPP |
Phthalates are or have been used in wall coverings, tablecloths, floor tiles, furniture upholstery, carpet backings, shower curtains, garden hoses, rainwear, pesticides, some toys, shoes, automobile upholstery, food packaging, medical tubing, and blood storage bags 16. Phthalates are not strongly bound in these products and can therefore leach out 17. Some phthalates are also present in cosmetics, nail polish, hair products, skin care products, and some medications 18. In recent years, di-nonyl phthalate (DiNP) and di-decyl phthalate (DiDP) have increasingly replaced DEHP in these applications 19. Alternatively, low-molecular weight phthalates, dimethyl phthalate (DMP), diethyl phthalate (DEP) and dibutyl phthalate (DBP), are primarily added to cosmetics and personal care products as solvents, fixatives and adhesives 20. Due to non-covalent bonds between the phthalate chemicals and their parent materials, there can be significant leaching and volatilization leading to environmental contamination and thus ubiquitous exposures in the general population. In fact, a recent report showed that metabolite biomarkers of eight major phthalates have been detected in 89% to 98% of the United States (US) population 19.
Phthalates are used in many consumer products, including:
- Cosmetics and personal care products
- Plastic and vinyl toys
- Shower curtains
- Miniblinds and wallpaper
- Vinyl flooring
- Raincoats
- Food packaging and wraps
- Detergents
- Adhesives
- Plastic pipes
- Medical equipment and devices
- Polyvinyl chloride (PVC) plastics
Phthalates commonly enter(s) the body through:
- Ingestion (swallowing): Eating food or water packaged in plastic, or drinking water contaminated with phthalates; for children, chewing on soft vinyl toys or products made with phthalates
- Inhalation (breathing): Breathing dust in rooms with plastic miniblinds, wallpaper, or flooring that contain phthalates
- Skin contact: Touching or using products made with phthalates
For most phthalates, the major route of exposure is food ingestion 7. However, personal care product use and inhalation are major routes of exposure for certain phthalates 21. Some phthalates have been found at higher levels in fatty foods such as dairy products, fish, seafood, and oils 22. Phthalates in a mother’s body can enter her breast milk. Ingestion of that breast milk and infant formula containing phthalates may also contribute to infant phthalate exposure 23. The phthalates that may be present in dust can be ingested by infants and children through hand-to-mouth activities 24. Finally, infants and small children can be exposed to phthalates by sucking on toys and objects made with phthalate-containing plastics 17.
Other minor routes of phthalate exposure include inhalation, drinking contaminated water, and absorption through the skin 25. Phthalates can be released in small amounts to the air people breathe inside homes or schools from the many consumer products that contain them 26. People living near phthalate-producing factories or hazardous waste sites may be exposed to phthalates released into the air or ground water where they live 22. Individuals may be exposed to phthalates during the use of many personal care products containing phthalates, such as hair products, cosmetics, and lotions 27. Phthalates in these products may be absorbed through contact with the skin or may be inhaled if some of the product is present in the air. In addition, certain medical devices, such as intravenous tubing or flexible bags containing blood, medications, or nutritional products, contain phthalates. These can be a source of phthalate exposure to children and women of child-bearing age when the tubing or bags are used to administer medications, nutritional products, or blood to the individual. This can be a very significant route of exposure, especially for premature infants in intensive care units 28.
Phthalate exposures, assessed from urinary concentrations of phthalate metabolites (i.e., breakdown products), appear to be higher for children compared with adolescents and adults. Studies of phthalate metabolites in children’s urine are limited, but the few that have been published have found children’s urinary phthalate metabolite levels to be higher than levels in adults and to decrease with age (i.e., younger children had more phthalate metabolites in their urine than older children did) 29. The exception is monoethyl phthalate (MEP), a metabolite of diethyl phthalate, which has been found to be present in higher levels in adult urine compared with children’s urine 29. Levels of monoethyl phthalate (MEP) are most likely associated with use of consumer products that contain diethyl phthalate, such as detergents, soaps, cosmetics, shampoos, and perfumes 29.
What are endocrine disruptors (endocrine disrupting chemicals)?
The Endocrine Society defined endocrine disrupting chemical as “an exogenous chemical, or mixture of chemicals, that interfere with any aspect of hormone action” 30. In other words, the chemical substances that can affect the endocrine system resulting in adverse effects are called Endocrine Disruptor Chemicals 31. These chemicals often bind to the endogenous receptors (e.g., estrogen receptor, steroid receptor) and interfere with the normal function of brain, reproductive organs, development, immune system, and other organs 32. However, endocrine disrupting chemicals are not the same as hormones but they can mimic hormones, and produce ill effects in the body 33.
Endocrine disruptors are found in many everyday products, including some plastic bottles and containers, liners of metal food cans, food, beverages, detergents, flame retardants, food, toys, cosmetics, pesticides and air. The common endocrine disrupting chemicals are bisphenol A (BPA), perchlorate, dioxins, phthalates, phytoestrogens, polychlorinated biphenyls (PCB), polybrominated diphenyl ethers (PBDE), triclosan, perfluoroalkyl and polyfluoroalkyl substances (PFAS), pesticides like dichlorodiphenyldichloroethylene (DDT) and its metabolite dichlorodiphenyldichloroethylene (DDE), organophosphorus compounds, alkylphenols(surfactants), parabens, methoxychlor, diethylstilbestrol (DES), fungicide vinclozolin, and natural hormones 34. Among these, BPA (bisphenol A) is the most commonly encountered endocrine disrupting chemical, which has both estrogenic and antiandrogenic properties. endocrine disrupting chemicals are mostly lipophilic in nature and resistant to metabolism 35. People who get exposed to any of these endocrine disrupting chemicals may have hormonal imbalance. Even a small amount of endocrine disrupting chemical consumed can result in hormonal imbalance especially in children 31. Sometimes they are stored in body fats, and transferred to the developing fetus via the placenta 35.
Studies on animal models and humans reveal that the mechanisms through which the endocrine disrupting chemicals act involve many complex pathways. The endocrine disrupting chemical can act like endogenous hormones and thereby increase or decrease the cellular response. Also, they can block the effects of hormones and stimulate or inhibit the production of hormones. They can thus interfere with synthesis, transport, action, and degradation of hormones 36. endocrine disrupting chemicals can act via nuclear receptors, nonsteroidal receptors, transcription coactivators, and certain enzymatic pathways 34.
Endocrine disrupting chemicals can affect several systems in our body resulting in many ill health effects. There is evidence showing various diseases are linked to endocrine disrupting chemicals as shown in table below.
| Endocrine disrupting chemicals | Main Sources | Possible Mechanism | Clinical condition |
|---|---|---|---|
| Alkylphenols | Detergents Shampoos Pesticide | Mimics estrogen | Breast cancer |
| Phthalates | Plastic products Personal care products (perfume, moisturizer) | Not yet known | Testicular and ovarian toxicants |
| Polychlorinated biphenyls (chlorinated/ halogenated/ TBBPA) | Paints Plastics Lubricants Electrical applications | Estrogenic and anti-androgenic activity Indirectly regulate circulating gonadal hormones. Inducers of CYP1A and CYPIIB Decreased NMDA receptor binding in striatum, frontal cortex and hippocampus, cerebellum Reduced glutamate and dopamine Acts at AhR signaling pathways resulting in cytotoxic effects | Neurobehavioral defects like cognitive deficits in children Neurotoxicity Thyroid toxicity Susceptibility to infections Cancers (especially Breast Cancer) Infertility |
Abbreviations: TBBPA = Tetrabromobisphenol A; CYP = cytochrome P450 enzymes; NMDA = N-methyl-D-aspartate; AhR = aryl hydrocarbon receptor
The difference between hormone and endocrine disruptor chemicals
| Hormones | Endocrine disrupting chemicals |
|---|---|
| 1. Hormones are chemical substances produced by the body and transported via bloodstream to the cells and organs which carry receptors for the hormone and on which it has a specific regulatory effect. | 1. Endocrine disrupting chemicals are exogenous substance that alters function(s) of the endocrine system and consequently causes adverse effects in an intact organism, or its progeny, or populations. |
| 2. They act via specific receptors and produces class effects | 2. They act via hormone and other receptors and produces abnormal functions and interactions. |
| 3. No bio accumulation | 3. Results in bioaccumulation |
| 4. Non-linear dose response with saturable kinetics | 4. Non-linear dose response with saturable kinetics |
| E.g.; steroid hormones, thyroid hormones | Eg; Perchlorate, Dioxins, Phthalates |
Phthalates health effects
Some phthalates are suspected endocrine disruptors 37. Endocrine disruptors act by interfering with the biosynthesis, secretion, action, or metabolism of naturally occurring hormones 37. Given the importance of hormones in human physiology, there is concern in the scientific community over the potential for endocrine disruptors to adversely affect children’s health, particularly in reproduction, development, and behavior. Male laboratory animals exposed to high doses of some phthalates have been known to display elements of “phthalate syndrome,” which includes infertility, decreased sperm count, cryptorchidism (undescended testes), hypospadias (malformation of the penis in which the urethra does not open at the tip of the organ), and other reproductive tract malformations 7. A number of animal studies have reported associations between exposure to certain phthalates and changes in male hormone production, altered sexual differentiation, and changes to reproductive organs, including hypospadias 38. These findings in animal studies, although typically occurring at exposure levels much higher than what the general population may be exposed to, suggest a potential concern for health effects in children as well. The National Research Council has concluded that prenatal exposure to certain phthalates produces reproductive tract abnormalities in male rats, and also concluded that the same effects could plausibly occur in humans 7.
There are only a limited number of human studies looking at the relationship between phthalate exposure and hormonal and reproductive health changes. In one study, prenatal exposure to some phthalates (DEP, diisobutyl phthalate (DiBP), di-n-butyl phthalate (DnBP) and DEHP) at typical U.S. population levels was associated with changes in physical measures of the distance between the anus and the genitals (anogenital distance) in male infants, a marker of androgenization 39. A shorter anogenital distance has been associated with decreased fertility in animal experiments 40 and a recent human study reported that a shorter anogenital distance in men was associated with decreased semen quality and low sperm count 41. Another study reported an association between increased concentrations of phthalate metabolites in breast milk and altered reproductive hormone levels in newborn boys. The same study did not find an association between breast milk phthalate metabolite concentrations and cryptorchidism 42.
Exposure to some phthalates has been associated with neurodevelopmental problems in children in some studies. Two studies of a group of New York City children ages 4 to 9 years reported associations between prenatal exposure to certain phthalates and behavioral deficits, including effects on attention, conduct, and social behaviors 43. Studies conducted in South Korea of children ages 8 to 11 years reported that children with higher levels of certain phthalate metabolites in their urine were more inattentive and hyperactive, displayed more symptoms of attention-deficit/hyperactivity disorder (ADHD), and had lower IQ compared with those who had lower levels 44. The exposure levels in these studies are comparable to typical exposures in the U.S. population.
A handful of studies have reported associations between prenatal exposure to some phthalates and preterm birth, shorter gestational length, and low birth weight 45; however, one study reported phthalate exposure to be associated with longer gestational length and increased risk of delivery by Cesarean section 46.
In adult populations, various epidemiological studies support an association between phthalate exposure and markers of testicular function in men, particularly decreased semen quality 47. There is also evidence linking endometriosis in women with high phthalate metabolite levels 48. Increases in waist circumference and body mass index (BMI) have been linked to DEHP, BzBP, DBP and DEP exposure in men and DEP exposure in adolescent and adult females 49. One of the replacements for the DEHP, DiNP, has recently been designated as a carcinogen in the State of California 50.
Finally, some researchers have hypothesized that phthalate exposure in homes may contribute to asthma and allergies in children. Two research groups have conducted studies, primarily in Europe, and reported associations between surrogates for potential phthalate exposure in the home and risk of asthma and allergies in children 51. Examples of the exposure indicators and outcomes considered in these studies include an association between some phthalates in surface dust and increased risk of runny nose, eczema, and asthma 52 and increased risk of bronchial obstruction associated with the presence of PVC in the home 53.
In 2006, the National Toxicology Program (NTP) concluded that there is “concern” for effects on reproductive tract development in male infants less than one year old exposed to DEHP. In addition, the National Toxicology Program also concluded that there is “some concern” (the midpoint on a five-level scale ranging from “negligible” to “serious” concern) for effects on reproductive tract development in male children older than one year old exposed to DEHP, and also that there is “some concern” for effects of prenatal DEHP exposure on reproductive tract development in males 54. Concern was greater for males exposed to high levels of DEHP in the womb or early in life. These conclusions were based primarily on findings from animal studies, as human data are limited and were determined to be insufficient for evaluating the reproductive effects of DEHP 55. Some studies have also reported associations of DEHP exposure with increased risk of asthma and bronchial obstruction, increased risk of ADHD symptoms, and altered pregnancy durations 52. Human health studies have reported associations between exposures to DBP and altered reproductive hormone levels in newborn boys, and shifts in thyroid hormone levels in pregnant women 56. Signs of feminization in young boys (as measured by reduced anogenital distance), altered hormone levels in newborn boys, and increased risk of rhinitis and eczema are health effects that have been associated with BBzP exposure in some studies 52. The exposure levels in these studies are comparable to typical exposures in the U.S. population. It is important to note that while the following indicators present data on individual phthalate metabolites, evidence suggests that exposures to multiple phthalates may contribute to common adverse outcomes. The National Research Council has concluded that multiple phthalates may act cumulatively to adversely impact male reproductive development 7.
Phthalates in food
Given the increasing scientific evidence base linking phthalate exposure with harmful health outcomes, it is important to understand major sources of exposure. A recent and well-designed study by Koch et al. 57 that monitored urinary phthalate excretion in individuals fasting for 48 hours, found that diet was the most significant pathway for exposures to DEHP, DiNP and DiDP while DMP, DEP, DiBP, DnBP and BBzP were primarily linked to non-food exposures. According to a review by Cao 58, phthalates can migrate into food from plasticized PVC materials such as tubing typically used in the milking process, lid gaskets, food-packaging films, gloves used in the preparation of foods, and conveyor belts. These compounds are also found in printing inks and adhesives on food wrappers as well as coatings on cookware that have been contaminated by packaging 59. Foods high in fat are contaminated by higher weight phthalates that are more lipophilic such as DEHP 58. In the United States, phthalates have been approved by the Food and Drug Administration (FDA) as plasticizers in food packaging materials and food contact substances used during processing and storage while the European Commission and Chinese authorities have limited phthalates in food contact materials made of plastic since 2008–2009 60. Thus, there can be substantial variability in phthalate concentrations within food groups based on the region of food production, processing practices, presence and type of packaging and lipid content 61. With an ever increasing global market, phthalate contamination is a food safety issue that crosses international borders. Dietary phthalate exposure assessment has become a topic of great interest given the significance of the dietary pathway and health impacts associated with the specific phthalate species found in food.
The review of the literature revealed that poultry, some dairy products (cream) and fats are routinely contaminated with high concentrations of DEHP than other foods 15. Milk, yogurt, eggs, fruits, vegetables, pasta, noodles, rice, beverages and water were found to contain low concentrations of phthalates as a whole 15.
Foods with High DEHP Concentrations (>300 μg/kg)
- Poultry
- Cream
- Cooking Oils/Fats
Foods with Low DEHP Concentrations (<50 μg/kg)
- Yogurt, Eggs
- Pasta, Rice, Noodles
- Fruits/vegetables
- Beverages
Given the chemistry of high molecular weight phthalates like DEHP, higher concentrations in lipid rich foods were expected. There was significant variability in concentration observed between dairy products based on typical fat content. Among the dairy products tested, cream and cheese were more heavily contaminated across studies in comparison to yogurt 15. Poultry consistently had higher phthalate content than other meats, however it is unclear what factors impacted these results since details as to the fat content of products was not always reported. Noteworthy, phthalates in non-fatty foods including bread and cereal products were observed in variable concentrations. This is of importance since two recent studies conducted in Belgium 62 and Germany 63 reported bread as a significant source of DEHP and highest contributor to total exposures in the general adolescent and adult population at 31.4% and 14.06%, respectively. Sources of contamination may be present in the processing of grains, though this is unclear. As a whole, food monitoring data also suggests that the consumption of fruit and vegetables is associated with limited phthalate exposures. However, processed fruit and vegetable products found in jars appear to contribute to greater exposures given the high concentrations reported.
Figure 1. Phthalates exposures through diet per capita
[Source 64 ]As expected, the epidemiology literature reported that dairy products, meats and discretionary fat intake, in fact, were associated with increases in DEHP urinary metabolite levels in adolescent and adult populations. Furthermore, consumption of these products were found to be associated with MnBP levels in one epidemiology study and elimination of some of these products from the diet (dairy and meat) led to a decrease in MnBP and DEHP metabolites in the Temple Stay intervention 65. It is important to note that although results from Ji and others suggest that discontinuing meat and dairy from the diet may be largely responsible for decreases in metabolite levels, there may have been other factors in the environment that impacted results since details of the diet as well as daily practices in the Temple Stay program were not available 66. It is possible that decreases in low molecular weight phthalates, given their primary source, could be attributed to reduced use of personal care products rather than changes in the diet 66.
Results between food monitoring and epidemiological data were not completely consistent. Two epidemiology studies reported an association between fish consumption and MiBP; however the food monitoring data did not support this result as all DiBP levels in seafood were found to be low across studies. Additionally, increased levels of MMP (a metabolite of DMP) were associated with consumption of fruit while the food monitoring data did not show DMP at significant levels for this food group 67. Finally, Colacino et al. 68 as well as Trasande et al. 69 reported positive associations between vegetables and MEP. However, the food monitoring data does not support this finding and two other epidemiology studies 70, 71 suggest that diets with high consumption of fruits and vegetables may be associated with decreases in DEP exposure.
How much of the five phthalates is safe in food contact materials?
According to the 2019 experts opinion at the European Food Safety Authority 72 – a group Tolerable Daily Intake (TDI) – for four of the five phthalates (di-butylphthalate [DBP], butyl-benzyl-phthalate [BBP], di-2-ethylhexyl phthalate [DEHP] and di-isononylphthalate [DINP]) of 50 micrograms per kilogram of body weight (50 µg/kg body weight) per day based on their effects on the reproductive system. The Tolerable Daily Intake (TDI) is an estimate of the amount of a substance that people can ingest daily during their whole life without any appreciable risk to health 72. The key effect on which this group-Tolerable Daily Intake (TDI) is based is a reduction in testosterone in fetuses. The fifth phthalate in the assessment, di-isodecyl phthalate (DiDP), does not affect testosterone levels in fetuses, therefore the European Food Safety Authority experts set a separate Tolerable Daily Intake (TDI) of 150 µg/kg body weight per day based on its effects on the liver 72.
Nota that the European Food Safety Authority experts have set all these Tolerable Daily Intakes (TDIs) on a temporary basis due to uncertainties about health effects other than the reproductive ones and about the contribution of plastic food contact materials to overall consumer exposure of phthalates. The European Food Safety Authority experts have also added the need to address these uncertainties by considering the whole body of scientific evidence.
Are there any safety concerns of phthalates in food?
According to the 2019 European Food Safety Authority experts opinion at the current exposure to these five phthalates (di-butylphthalate [DBP], butyl-benzyl-phthalate [BBP], di-2-ethylhexyl phthalate [DEHP] and di-isononylphthalate [DINP]) from food is not a concern for public health 72. Dietary exposure to the group of DBP, BBP, DEHP and DINP for average consumers is 7 µg/kg body weight (7 micrograms per kilogram of body weight) or seven times below the safe level, while for high consumers it is 12 µg/kg bw, which is four times lower 72. For di-isodecylphthalate (DIDP), the dietary exposure for high consumers is 1,500 times below the safe level 72.
This 2019 assessment of the five phthalates (DBP, BBP, DEHP, DINP and DIDP) is in line with the 2005 European Food Safety Authority assessment in terms of their most sensitive effects and the individual tolerable daily intakes. The main differences concern an improved estimate of dietary exposure to phthalates and the introduction of the group-Tolerable Daily Intake (TDI) for four of the phthalates to account for combined exposure to several phthalates at the same time. This is a common occurrence and confirmed by data from studies with humans, e.g. traces found in urine.
Phthalates in cosmetics
Historically, the primary phthalates used in cosmetic products have been dibutylphthalate (DBP), used as a plasticizer in products such as nail polishes (to reduce cracking by making them less brittle); dimethylphthalate (DMP), used in hair sprays (to help avoid stiffness by allowing them to form a flexible film on the hair); and diethylphthalate (DEP), used as a solvent and fixative in fragrances. According to FDA’s latest survey of cosmetics, conducted in 2010, DBP and DMP are now used rarely. DEP is the only phthalate still commonly used in cosmetics 73.
In 2002, the Cosmetic Ingredient Review Expert Panel 74 reaffirmed its original conclusion (reached in 1985), finding that DBP, DMP, and DEP were safe as used in cosmetic products. Looking at maximum known concentrations of these ingredients in cosmetics, the Cosmetic Ingredient Review Expert Panel 74 evaluated phthalate exposure and toxicity data, and conducted a safety assessment for dibutylphthalate in cosmetic products. The Cosmetic Ingredient Review Expert Panel 74 found that exposures to phthalates from cosmetics were low compared to levels that would cause adverse effects in animals. The Cosmetic Ingredient Review Expert Panel is an industry-sponsored organization that reviews cosmetic ingredient safety and publishes its results in open, peer-reviewed literature. The FDA participates in Cosmetic Ingredient Review on a non-voting basis and may or may not accept Cosmetic Ingredient Review Expert Panel findings 73.
The FDA reviewed the safety and toxicity data for phthalates, including the Centers for Disease Control and Prevention (CDC) data from 2001 75, as well as the Cosmetic Ingredient Review Expert Panel 74 conclusions based on reviews in 1985 and 2002. While the Centers for Disease Control and Prevention (CDC) report noted elevated levels of phthalates excreted by women of child-bearing age, neither this report nor the other data reviewed by FDA established an association between the use of phthalates in cosmetic products and a health risk. Based on this information, FDA determined that there wasn’t a sound, scientific basis to support taking regulatory action against cosmetics containing phthalates 73.
The FDA continues to monitor levels of phthalates in cosmetic products. The FDA have developed an analytical method for determining the levels of phthalates in cosmetic products and conducted surveys of products to determine these levels in cosmetics on the market.
What we know about infant exposure to phthalates
Infants, like all consumers, are exposed daily to phthalates from a number of sources, including air, drugs, food, plastics, water, and cosmetics.
The American Academy of Pediatrics has published an article stating that infants exposed to infant care products, specifically baby shampoos, baby lotions, and baby powder, showed increased levels of phthalate metabolites in their urine 76.
Like the CDC report, this study did not establish an association between these findings and any health effects. In addition, levels of phthalates, if any, in the infant care products were not determined.
The FDA included 24 children’s products intended for infants and children in the survey we completed in 2006, and nearly 50 products for infants and children in the survey we completed in 2010. What the FDA have learned was that the use of phthalates in cosmetics intended for people of all ages, including infants and children, has decreased considerably since the surveys began in 2004.
How to know if there are phthalates in the cosmetics you use
Under the authority of the Fair Packaging and Labeling Act, the FDA requires an ingredient declaration on cosmetic products sold at the retail level to consumers. Consumers can tell whether some products contain phthalates by reading the ingredient declaration on the labels of such products.
However, the regulations do not require the listing of the individual fragrance ingredients; therefore, the consumer will not be able to determine from the ingredient declaration if phthalates are present in a fragrance. Also, because the Fair Packaging and Labeling Act does not apply to products used exclusively by professionals–for example, in salons–the requirement for an ingredient declaration does not apply to these products. Based on available safety information, DEP does not pose known risks for human health as it is currently used in cosmetics and fragrances. Consumers who nevertheless do not want to purchase cosmetics containing DEP may wish to choose products that do not include “Fragrance” in the ingredient listing.
Results of FDA’s 2010 Survey of Cosmetics for Phthalate Content
The products listed below represent a sample of cosmetics on the market at the time the survey was conducted, and products may have been reformulated since then. This survey was intended to monitor trends in the use of phthalates in cosmetics, not as a comprehensive analysis of all cosmetics on the market. The law does not require cosmetic firms to file their formulations with FDA. Also, note that some so-called “personal care products,” such as diaper creams and nipple creams, are regulated as drugs, or in some cases both cosmetics and drugs.
Table 2. 2010 Survey of Cosmetics for Phthalate Content
| Product Type | Brand | Lot # | Phthalates (ppm) | ||
|---|---|---|---|---|---|
| DMP | DEP | DBP | |||
Nail Polish | Pure Ice – Spit Fire | 991CP | |||
| Dora the Explorer Mega Nail Polish Kit – Townley | WO90817 | ||||
| Scherer Nail Polish CQ #143 Cabernet | Not visible | ||||
| Rimmel Lycra Wear 10 Days Nail Polish #303 Vintage | 732318 | ||||
| In a New York Color Minute 224B | 8MBCK | ||||
| Sally Hansen Diamond Strength #45 Fuchsia | 8MSHK | ||||
| Hard Candy Just Nails (Glitter) | 9226 | ||||
| Petites Pink Crush 270 – CQ | Not visible | ||||
| Revlon Nail Enamel Red Hot Tamale 908 | Not visible | ||||
| Maybelline Express Finish Grape Times 608 | WF224 | ||||
| Nicole by OPI Razzle Dazzler | 09295AAG | ||||
| Scherer Nail Polish CQ #107 Crystal Clear | Not visible | ||||
| LA Colors Nail Lacquer Red Stilettos CBLQ 389 | Not visible | ||||
| LA Colors Art Deco Polish Silver Glitter CBNA 502 | Not visible | ||||
| LA Colors Nail Hardener-Strengthener | 9796 | ||||
| Hot Topic Nail Polish Black | Not visible | ||||
| Hot Topic Nail Polish Green | Not visible | ||||
| Hot Topic Nail Polish Purple | Not visible | ||||
| Hot Topic Nail Polish (Skull) Green | Not visible | 4,800 | |||
| Hot Topic Nail Polish (Skull) Black | Not visible | 4.4 | |||
| Hot Topic Nail Polish (Skull) Yellow | Not visible | 4 | |||
| Simple Pleasures Nail Polish “Peace” (Glitter) | Not visible | ||||
| Simple Pleasures Nail Polish “Love” (Pink) | Not visible | ||||
| WetnWild Wild Shine 410A | 918201 | ||||
| Sinful Colors Professional Nail Polish Enamel Pinky Glitter 830 | Not visible | ||||
| Sinful Colors Under 18 | Not visible | ||||
| Borghese Nail Lacquer Vernis Botticelli Nude | 6KXJKC | 3.4 | |||
| Sally Hansen High Definition 04 (Green) | 9MHYK | ||||
| Cover Girl Boundless Base Coat Nail Color Red Revolution 553 | 7239HV | ||||
| Cover Girl Boundless Base Coat Nail Color Gold Rush 415 | 9139HV | ||||
| Sally Hansen Hard As Nails Xtreme Wear Hot Magenta 03 | 6MTXK | ||||
| Sally Hansen Salon Lacquer Nail Polish Orange You Cute? 450 | 8JFDK | 6.6 | |||
| Sally Hansen Insta-Dri Rose-a-go-go 06 | AKVDKN | ||||
| Sally Hansen Diamond Strength 33 Champagne Toast | 8M8OK | ||||
| Avon Nailwear Pro Nail Enamel Polish Midnight Plum | VEK19 | ||||
Skin Cream and Lotion | Red Velvet Body Lotion – Gift Pack | H8920109 | |||
| Dove Deep Moisture Nourishing Body Wash with NutrimMoisture | 10079PP23S | ||||
| Eucerin Plus Intensive Repair Hand Crème with Dry Skin Therapy | 90125515 | ||||
| Aveeno Active Naturals Daily Moisturizing Lotion | 1129LK | ||||
| Vaseline Sheer Infusion Vitamin Burst Body Lotion | 08149UM42 | ||||
| Palmer’s Cocoa Butter Formula Concentrated Cream | N9215A | ||||
| Jergens Ultra Healing Extra Dry Skin Moisturizer | Y225106ZZA | ||||
| Ponds Dry Skin Cream | 08289HU87 | ||||
| Dollar General Guarantee Skin Rescue Moisture Lock Lotion | C9A129 | ||||
| Noxzema The Original Deep Cleansing Cream with Eucalyptus Oil | Rubbed out | ||||
| Suave Powder Fresh Body Lotion | 10289JU41 | ||||
| Celine Dion Sensational Shimmering Body Lotion | 92251 | ||||
| Scentsations by Body Source Cherry Blossom Body Lotion | M9290AL18 | ||||
| Walgreens Advanced Care (Fragrance Free) | 0401609 | ||||
| Curel Continuous Comfort Original Formula Moisturizer | X140106ZZA | ||||
| Lubriderm Daily Moisture Lotion, Normal to Dry Skin, Fragrance Free | 0118C | ||||
| Nivea Soft Refreshingly Soft Moisturizing Crème | 73729160 | 100 | |||
| Bath, Body, etc… Organic Soothing Aloe Vera Body Lotion | 29473 | 260 | |||
| J.R. Watkins Natural Apothecary Hand & Body Lotion | 0374579 | ||||
| Jergens Original Scent Cherry-Almond Moisturizer | W106125ZZ | 110 | |||
| Palmer’s Cocoa Butter Formula with Vitamin E Skin Therapy Oil | Rubbed out | ||||
| St. Ives Hydrating Vitamin E Advanced Body Moisturizer | 09327021103 | ||||
| Vaseline for Men Hand Lotion | 05279HU09 | ||||
| Corn Huskers Heavy Duty Oil-Free Hand Treatment Lotion | 2969G | ||||
| Malibu Hemp Moisturizer Body Lotion for Dry Skin | 5434 | ||||
| Keri Original Dry Skin Lotion | 23654901 | ||||
| Avon Haiku Perfumed Skin Softener | MK091 | ||||
| Avon Jet Femme Body Lotion | MLP81 | ||||
| Avon Candid Perfumed Skin Softener | MAN91 | ||||
| Avon Moisture Therapy Intensive Extra Strength Cream | MIV91 | ||||
Fragrance | Dove Go Fresh Body Mist | 090495U49 | |||
| Jovan Island Gardenia Cologne Spray | 9218 | 14,000 | |||
| Love’s Baby Soft Cologne Spray | 0906D48 | ||||
| A Little Sexy Body Spray by Parfums de Coeur | 09324 | ||||
| Axe Instinct Body Spray | 03119KK09 | ||||
| Curve Crush Body Mist | 9JA02 | ||||
| Bodycology Sweet Petals Body Mist | S9J27AK | ||||
| Cotton Candy Body Spray Prince Matchabelli | 09267 | ||||
| Degree Classic Romance Body Mist | 4068 | ||||
| Hannah Montana Cologne Spray | 3169Y | ||||
| Wanna Play Body Spray Parfums de Coeur | 09294 | 3,800 | |||
| Chantilly – Walmart Gift Pack | 090723A | 7,300 | |||
| Tabu – Walmart Gift Pack | 090721A | 6,200 | |||
| Heaven Sent – Walmart Gift Pack | 090622B | 1,300 | |||
| Navy – Walmart Gift Pack | 090722C | 40,000 | |||
| English Leather – Walmart Gift Pack | 090721A | 3,900 | |||
| British Sterling – Walmart Gift Pack | 090526A | 480 | |||
| Canoe – Walmart Gift Pack | 0907248 | 2,000 | |||
| English Leather Black – Walmart Gift Pack | 0907916 | ||||
| Johnson’s Baby Cologne | 1919COB128135 | ||||
| Barbasol After Shave Pacific Rush | 60161 | ||||
| Aqua Velva Classic Ice Blue | R09K174 | 760 | |||
| Tattooed by Inky | Not visible | ||||
| BOD Really Ripped Abs | 8TZ60 | 6,200 | |||
| i Carly | 168961B | ||||
Baby Cream and Lotion | Johnson’s Baby Lotion | 2519T | |||
| Dollar General Sleepy Time Baby Lotion | 8KF1025 | ||||
| Baby Avalon Organics Protective A,D & E Ointment | 6H01 | ||||
| Huggies Naturally Refreshing (Green Tea & Cucumber) Lotion | CU6287282 | ||||
| Baby Magic Gentle Baby Lotion | 9237 | ||||
| Burt’s Bees Baby Bee Buttermilk Lotion | 0850801 | ||||
| Aveeno Baby Soothing Relief Moisture Cream | 0209D | ||||
| Parent’s Choice Baby Lotion | 0034311 | ||||
| Johnson’s Bedtime Lotion | 1759G | ||||
| Johnson’s Head-to-Toe Fragrance Free Baby Lotion | 0049LK | ||||
| Johnson’s Shea and Cocoa Butter Baby Cream | 0069VB | ||||
| Susan Brown’s Baby Sensitive Baby Lotion-to-powder | 10577A | ||||
| California Baby Calming Everyday Lotion | CB9303A2 | ||||
Deodorant | Brut 24-Hour Protection deodorant | 11029TR53 | 22 | ||
| Tom’s of Maine Natural Care Lavender Deodorant Stick | LD1370 | ||||
| Old Spice High Endurance Deodorant | 9307TN | ||||
| Axe Fresh Action Essence Deodorant | 08129UR16 | ||||
| Degree Men Deodorant Silver Ion Intense Sport | 11209UR39 | 2.9 | |||
| Speed Stick Ocean Surf Deodorant | 9270502 | ||||
| Personal Care Clear Stick Deodorant | 08123A | ||||
| Kiss My Face Active Enzyme Lavender Deodorant | KO73008B | ||||
| Dove Powder Invisible Solid | 09229UR86 | ||||
| Secret Powder Fresh | 9068TN | 34 | |||
Hair Products | Biosilk Rock Hard Gelee Firm Hold | C8200 | |||
| Garnier Fructis Style Body Boost Volumizing Gel | 48F6030 | ||||
| TRESemmé No Frizz Shine Spray | 09308TA20SDSIL2506 | 22 | |||
| Suave Professionals Styling Foam Extra Hold | 11129KK61 | 52 | |||
| Rave 4X Mega Unscented Hair Spray | 11199HU63 | 16 | |||
| White Rain Unscented Extra Hold Hair Spray | 287CP3475403SDSIL15001 | 61 | |||
| Short Sexy Hair Quick Change Shaping Balm | 09219 | ||||
| American Crew Forming Cream | F952CIOC | 50 | |||
| Dep Sport Endurance Styling Gel | R2029932A2 | 6.8 | |||
| TRESemme Tres Two Extra Hold Hair Spray | 09313AA1416 | 37 | |||
| John Frieda Collection Frizz-Ease Mousse | Y223DK17DDT2Q | ||||
| Aussi Catch the Wave Mousse & Conditioner | 92755398F | ||||
| Catwalk Extra Strong Mousse | 233748783122142 | 23 | |||
| Johnson’s No More Tangles Detangling Spray | 0489VA | ||||
| Manic Panic Amplified Semi-Permanent Hair Color Cream | 10922 | ||||
| Color Fiend Blood Red Temporary Comb-In Color | RN0709 | ||||
| Color Fiend Pink Pop Water Based Semi-Permanent Hair Color | Not visible | ||||
Shampoo | Johnson’s Baby Shampoo | 2118T | |||
| Suave Kids 2 in 1 Shampoo Smoothers Cowabunga Coconut | 081491J18 | ||||
| Ave Dual 2 in 1 Shampoo + Conditioner | 01139TJ27 | 17 | |||
| Pantene Pro-v Moisture Renewal Hydration Quotidienne Shampoo | 93005401E1 | ||||
| Aveeno Baby Essential Moisture Shampoo | 0099VA | ||||
| Big Sexy Hair Big Volume Shampoo | 09205150 | 210 | |||
| Garnier Fructis Fortifying Shampoo Color Shield | EF036 | ||||
| Dove Intense Damage Therapy Shampoo | 08299JU39 | ||||
| VO5 Normal Balancing Shampoo | 09316020758 | 440 | |||
| Suave Professionals Sleek Shampoo | 07109JU41 | ||||
| Finesse Self Adjusting Moisturizing Shampoo | 9234M | ||||
| Herbal Essences Hello Hydration Moisturizing Shampoo | 93145395LF | ||||
| TRESemmé Smooth and Silky Touchable Softness | 09231C1130 | ||||
| Advance Techniques Color Reviving Shampoo | MK191 | 82 | |||
| Avon High School Musical Raspberry Roarin’ 2-in-1 Shampoo | MKW91 | ||||
Body Wash | Caress Daily Silk Silkening Body Wash | 10159UR022010 | |||
| Equate Tropical Fresh Body Wash with Exfoliating Pomegranate Seed | A50298-9328C1 | ||||
| Caress Tahitian Renewal Silkening Body Wash | 08069TJ37 | ||||
| Olay Body Ultra Moisture with Shea Butter Body Wash | 92595395WA | ||||
| Suave Naturals Cucumber Melon Rejuvenating Body Wash | 11109J038 | ||||
| Suave Men Body Wash Active Sport | 09169CU05 | 10 | |||
| White Rain All Day Moisturizing Body Wash | 093203 | ||||
| Dove Sensitive Skin Beauty Body Wash | 03049PP05 | ||||
| Natural Concepts Sensitive Skin Body Wash | A48292926583 | 340 | |||
| Dial Clean and Soft Moisturizing Body Wash | Q89M0310806091102 | ||||
| St. Ives Renewing Collagen Elastin Moisturizing Body Wash | 09345181020141 | ||||
| Natural Concepts Natural Blends Body Wash Violet & Pea | A40128-8353C1 | ||||
| Avon Naturals Strawberry & Guava Shower Gel | Rubbed out | ||||
| Avon Bubble Bath Bain-mousse Vanilla Cream for Dry Skin | MLK91 | ||||
| High School Musical Strawberry Sudsin’ Body Wash | MLC91 | ||||
Nipple Cream | Lansinoh HPA Lanolin for Breastfeeding Mothers | 39982 | |||
| Gerber Breast Therapy Moisturizing Balm | BK07A02UU | ||||
Children’s Makeup | Kiss Me 2 Gift Pack Blush – Markwins | ||||
| Kiss Me 2 Gift Pack Eye Shadow – Markwins | 1090075 | ||||
| Hard Candy in the Shadows Eye Shadow Collection | 9260 | ||||
| Claire’s Cosmetics Eye Shadow | 11/09 | ||||
| Claire’s Cosmetics Eye Glitter | DR911333 | ||||
Diaper Cream | Butt Butter Organics Herbal Diaper Rash Treatment | 31823 | |||
| Burts Bees Baby Bee Diaper Ointment | 1070801 | ||||
| Baby’s Bliss Diaper Cream | 1386 | 130 | |||
| Lavera Baby & Kinder Neutral | 2897 | ||||
| Weleda Baby Calendula Baby Cream | 902212 | ||||
Wet Wipes | Pull-ups Flushable Moist Wipes | MK929202A | |||
| Equate Flushable Wipes All Purpose | 09295D209651416 | ||||
| Pure’n Gentle Fragrance Free Wipes | 09272C230400512 | ||||
| Huggies Soft Skin Baby Wipes | MK914804A | ||||
| My Fair Baby Baby Wipes | 38091679169 | ||||
Infant Soap, Shampoo, Body Wash | Johnson’s Head to Toe Baby Wash | Rubbed out | |||
| Body Sense Baby Wash with Shea & Cocoa butter | OU28781 | ||||
| My Fair Baby Baby Wash with Camomile | 608015001/A2 | 60 | |||
| Burts Bees Baby Bees Shampoo and Wash | 2280801 | ||||
| Avon Bubble Bath Bain-mousse for Kids | MKY91 | ||||
| Aubrey Organics – Natural Baby & Kids Bath Soap | 11249 | ||||
Baby Oil | Johnson’s Baby Oil | 2339G | |||
| Equate Delicate Baby Oil | 0031646 | ||||
| Burt’s Bees Baby Bee Apricot Baby Oil | 0460801 | ||||
Face & Body Paint | Claire’s Cosmetics Body Glitter | 07/09 | |||
| Claire’s Cosmetics Vanilla Glitter Body Mist | 0929301 | 390 | |||
| Alex Face Paint Studio – Face Paint | 9012LP | ||||
| Snazaroo Face Painting Kit | T190803 | ||||
Glitter Gel | Hard Candy Glitteratzi Eye Glitter Gel | 9301 | |||
| Claire’s Club Scented Body Glitter | 4926 | 167 | |||
| Alex Face Paint Studio – Pink Glitter Gel | 9B2351 | ||||
Baby Powder | Johnson’s Baby Powder | 2528RA | |||
| Body Sense Baby Powder | 9DG0797 | ||||
| Burt’s Bees Baby Bee Dusting Powder | Not visible | ||||
DEHP
DEHP also known as di-2-ethylhexyl phthalate or bis(2-ethylhexyl)phthalate, which is a phthalate ester, is extensively used as a plasticizer in many products, especially for polyvinyl chloride (PVC) medical devices, furniture materials, cosmetics, and personal care products 14, 77. DEHP is continuously released from plastic products and directly infiltrates food, water and air; thus, humans are exposed to DEHP daily via ingestion, inhalation, and skin absorption 78. Patients undergoing medical procedures such as IV therapy, enteral and parenteral nutrition support, blood transfusion, hemodialysis and peritoneal dialysis, cardiopulmonary bypass and extracorporeal membrane oxygenation (ECMO) can be exposed to DEHP 14. The human body is reported to be exposed to DEHP at concentrations of up to 30 mg/kg/day, and occupational exposure levels of up to 600 mg/kg/day have been reported 79, 80. Estimates of mean daily intake in adults in the US population range from approximately 0.0006–0.002 mg/kg-day 81 to 0.011 mg/kg-day 82. However, intake is not necessarily reflective of biologically active dose, due to potential differences in pharmacokinetics 83.
DEHP is an endocrine‐disrupting chemical that has been shown in animal studies to disrupt the function of reproductive system in both females and males 84. DEHP disrupts normal mice reproductive and ovarian function 85, alters follicular development during weaning and maturity 86, impairs the steroidogenesis of ovarian follicular cells 87 and induces premature ovarian failure 88. In addition, prenatal exposure to DEHP exerts multigenerational and transgenerational effects on female mice reproduction 89. This systematic review 83 showed that prenatal exposure to diethylhexyl phthalate (DEHP) is associated with decreased anogenital distance in male offspring and DEHP is presumed to be a reproductive hazard to humans.
In recent years, DEHP and its active metabolic product, mono-2-ethylhexyl phthalate (MEHP), have been successively detected in many tissues, including the liver, blood, cord blood, breast milk, placenta, amniotic fluid, and early gestation villi 90, 91. According to previous studies, DEHP is associated with liver, kidney and neural injures or diseases in mice 92, 93, 94. Developmental exposures to DEHP has been associated with increased body fat and impaired glucose tolerance in rodents 13. DEHP has been shown to produce a wide range of adverse effects in experimental animals, notably liver toxicity and testicular atrophy 14. Although the toxic and carcinogenic effects of DEHP have been well established in experimental animals, the ability of this compound to produce adverse effects in humans is controversial. As a result, the ability of DEHP and other phthalate esters to produce adverse effects in humans has been a topic of active discussion and debate in the scientific and regulatory communities.
Several animal studies have confirmed that the toxicity of DEHP is mediated by its effects on gonadal steroidogenesis and an accompanying decrease in reproductive function and fertility 79. Prenatal exposure to DEHP induces premature reproductive senescence in male mice 95. The average exposure of DEHP in humans ranges from 3 to 30 mg/kg body weight per day and occupational exposure levels have been calculated to even reach up to 300 to 600 mg/kg/day 89. Based on epidemiological data, decreased conception rates, increased miscarriage rates, decreased estrogen levels, and abnormal ovulation are associated with the occupational exposure of female workers in India to DEHP 96. Thus, the health risks of DEHP exposure in humans have attracted increasing attention.
The presence of DEHP metabolites in urine is associated with adiposity and insulin resistance in children 97. Previous studies have reported that perinatal exposure to DEHP may induce obesity and metabolic disorders in mice 98, 99; however, the mechanisms underlying these associations are yet to be investigated.
The ovary is the primary reproductive organ in the female and it regulates female endocrinology and provides a microenvironment for follicle development 100. DEHP and MEHP (mono-2-ethylhexyl phthalate) have been shown to induce a depletion of the primordial follicles and a decrease in sex hormone production in mice 101. Previous studies have also reported that DEHP is able to induce a dose-dependent decrease in mouse fertility 102 and affect reproductive outcomes in female mice 103. The results from the female mice study 103 indicate that prenatal DEHP exposure increased male-to-female ratio compared to controls. Prenatal DEHP exposure also increased preantral follicle numbers at postnatal day 21 compared to controls 103. Furthermore, 22.2% of the 20 μg/kg/day treated animals took longer than 5 days to get pregnant at 3 months and 28.6% of the 750 mg/kg/day treated animals lost some of their pups at 6 months 103. Therefore, prenatal DEHP exposure alters male-to-female sex ratio, increases preantral follicle numbers, and causes some breeding abnormalities in female mice 103. However, the precise mechanisms by which DEHP affects female reproduction remain unclear. The human gastrointestinal tract contains 1013–1014 microbiota that consist of 1500 microbial species and are characterized by more than 3 million genotypes, and numerous studies have shown that the intestinal microbiota is inextricably linked to human health 104. Approximately 1000 bacterial species and 7000 bacterial strains have been identified using the currently available advanced sequencing technology 105. Recent studies have revealed the bidirectional relationship between the estrogen level and gut microbiota in females with diseases induced by abnormal estrogen levels; estrogens are potentially regulated by the gut microbiota through secretion of beta‐glucuronidase, which deconjugates estrogens into active forms, and estrogens regulate the gut microbiota through immunoregulation 106. In addition, gut microbiota dysbiosis may increase intestinal permeability and alter the levels of host gut metabolites, allowing bacterial endotoxins, such as lipopolysaccharide, to be transported into the circulation and activate the inflammatory response to promote disease development in females 107. Therefore, scientists hypothesized that women of child‐bearing age who are exposed to DEHP would exhibit abnormal follicular development and infertility that may be mediated by alterations in the gut bacterial composition and metabolite profiles 84. In ths study 84, DEHP exposure altered the estrous cycle and estrogen level, accelerated primordial follicle recruitment and induced follicular atresia. In terms of toxicological mechanisms, DEHP induces DNA damage and apoptosis of the ovarian somatic cells, and alters the ovarian oxidative stress status 108. DEHP interferes with PI3K signaling and induces an acceleration of primordial follicle development; it also affects oocyte maturation and the embryogenesis process 109. DEHP downregulates the expression of the germ cell markers Stra8, Dazl, and Nobox, delays the fetal oogenesis processes, inhibits the expression of the Gdf9 and Atm genes, and leads to abnormal follicular growth and cell division 110. Additionally, DEHP induces oxidative stress by promoting reactive oxygen species (ROS) generation and inhibits steroid synthesis by modulating the expression of steroidogenic responsive genes in rat ovarian granulosa cells; it also activates the Bax/Bcl‐2 and the caspase‐3‐mediated mitochondrial apoptotic pathway to induce apoptosis 111. Thus, DEHP induces oxidative stress in ovary and alters ovarian function to induce female rodents reproductive toxicity. However, the specific mechanisms underlying the toxic effects of DEHP are an area that is largely unexplored.
In another mice study, no significant decline in fertility was exhibited by pregnant female mice treated with 0.05 mg/kg DEHP; however, the abortion rate was 100% in the 500 mg/kg DEHP dose group compared with 0% in the control and 0.05 mg/kg DEHP groups 13. These findings indicate that exposure to DEHP at the tolerable daily intake (TDI) level did not affect the reproductive outcomes of mice; however, a high dose of DEHP may damage the reproductive capacity 13. In addition, 0.05 mg/kg DEHP exposure did not significantly affect the food intake or reproductive outcome of female mice, although it did induce metabolic disorders in the offspring. Previous studies have reported a correlation between in utero exposure to endocrine-disrupting chemicals and the development of metabolic disorders in adulthood 99. In the mice study, serum leptin, insulin, serum lipid, and glucose concentrations were significantly elevated in male and female offspring at postnatal week nine, indicating that in utero exposure to DEHP may influence metabolic function in adulthood 13.
Are children at increased risk for the adverse effects of DEHP, relative to adults?
The FDA’s Center for Devices and Radiological Health has examined this issue and has concluded that children undergoing certain medical procedures may represent a population at increased risk for the effects of DEHP 14. This decision is supported by three findings:
- Children undergoing some medical procedures receive a greater dose of DEHP, on a mg/kg basis, than adults do,
- Pharmacokinetic differences between children and adults may result in greater absorption of DEHP, greater conversion of DEHP to mono-2-ethylhexyl phthalate (MEHP) (the toxic
metabolite of DEHP), and reduced excretion of MEHP in children compared to adults, - Children may be more pharmacodynamically sensitive to the adverse effects of DEHP than adults are.
This conclusion is consistent with that reached by the expert panel that was recently convened by the Center for the Evaluation of Risks to Human Reproduction of the National Toxicology Program. Specifically, the panel noted that: “The available reproductive and developmental toxicity data and the limited but suggestive human exposure data indicate that human exposures in this situation approach toxic doses in rodents, which causes the Panel serious concern that exposure may adversely affect male reproductive tract development.”
Dicyclohexyl phthalate
Dicyclohexyl phthalate (DCHP) is one of a family of synthetic compounds known as phthalates. Dicyclohexyl phthalate (DCHP) is as a plasticizer primarily used in adhesives and plastic and rubber products and resins 112. Other uses of dicyclohexyl phthalate include:
- As a plasticizer in adhesive, paint and coating, plastic product, rubber product, and plastic resin manufacturing;
- As a phlegmatizer (to improve safety and stability) in a variety of peroxide curing agent mixtures used in industrial and commercial applications, such as roofing systems, road markings, coatings, adhesives and other composites;
- In industrial and commercial automobile and aerospace products;
- For laboratory chemicals; and
- In commercial and consumer products, such as adhesives and sealants, and plastic and rubber products.
Several animal studies about the effects of dicyclohexyl phthalate (DCHP) on mammalian development and reproductive system have been published 113, 114. Hoshino et al. 115 investigated the two-generation effects of dicyclohexyl phthalate (DCHP) exposure at doses of 240, 1200, and 6000 ppm (about 14.3, 69.8, and 349 mg/kg) per day in the diet from gestational day 1–21 of females in Sprague Dawley rats. They found that the atrophy of seminiferous tubules of male offspring occurred at 6000 ppm dose 115. When Sprague Dawley rats were exposed in utero to dicyclohexyl phthalate (DCHP) at 250, 500, and 750 mg/kg per day by gavage from gestational day 6 to 20, male fetuses at gestational day 21 had low body weight at 750 mg/kg and reduced anogenital distance at 500 and 750 mg/kg doses, suggesting that the anti-androgenic effect of dicyclohexyl phthalate (DCHP), since anogenital distance is the biomarker of androgen-dependent action 116. Previous study also demonstrated that dicyclohexyl phthalate (DCHP) directly inhibited the activities of two important Leydig cell androgen-biosynthetic enzymes, 3β-hydroxysteroid dehydrogenase 1 (HSD3B1, encoded by Hsd3b1) and 17β-hydroxysteroid dehydrogenase 3 (HSD17B3, encoded by Hsd17b3) 117, suggesting that dicyclohexyl phthalate (DCHP) exerts its action at least in part via suppression of many androgen-biosynthetic enzyme activities. Lower androgen activity is one of the typical manifestations of phthalate-mediated testicular dysgenesis syndrome 118. Other phthalate-mediated manifestations of testicular dysgenesis syndrome include the abnormal aggregations of fetal Leydig cells and formation of multinucleated gonocytes 119. The term “testicular dysgenesis syndrome” was coined referring to a spectrum of reproductive disorders that originate in male fetal life 119. Testicular dysgenesis syndrome includes cryptorchidism (undescended testes) and hypospadias (abnormal formation of the urethral meatus) in newborn boys and testicular cancer and reduced fertility in adult males 119. The occurrence of multinucleated gonocytes, abnormal fetal Leydig cell aggregation, decreased intratesticular testosterone levels, and reduced insulin-like factor 3 (Insl3) mRNA/protein (INSL3) expression in the fetal testis have been well documented in some phthalates including DEHP and dibutyl phthalate (DBP) 120. Therefore, these phthalate-mediated manifestions in rodents are similar to those of human testicular dysgenesis syndrome in human males, and are often referred as “phthalate syndrome” 119.
Di-n-pentyl phthalate (DnPP)
Di-n-pentyl phthalate (DnPP) is no longer in use in the United States, because DnPP (di-n-pentyl phthalate) has been shown to cause developmental and/or reproductive effects in laboratory animals 121.
Di-isononyl phthalate (DINP)
Di-isononyl phthalate (DINP) is used in the following products: adhesives and sealants, coating products, lubricants and greases and polymers. DINP is used in the following areas: building and construction work. The release to the environment of di-isononyl phthalate (DINP) is likely to occur from: indoor use (e.g. machine wash liquids/detergents, automotive care products, paints and coating or adhesives, fragrances and air fresheners), outdoor use, indoor use in close systems with minimal release (e.g. cooling liquids in refrigerators, oil-based electric heaters) and outdoor use in close systems with minimal release (e.g. hydraulic liquids in automotive suspension, lubricants in motor oil and break fluids).
How to reduce exposure to phthalates and other endocrine disrupting chemicals?
While you cannot completely avoid exposure to endocrine disrupting chemicals, you can take some simple steps to reduce exposure to them. This is especially important for women and men who plan to have children. Talk to your doctor when planning a family to discuss what precautions you can take to limit the risks of endocrine disrupting chemicals.
Here are some practical tips for reducing your exposure to endocrine disrupting chemicals:
- Washing fruit and vegetables and buying them from known (local) sources reduces your intake of pesticides, fungicides, herbicides and chemicals that may have been sprayed on the plants
• eating fewer processed, canned, pre-packaged foodsreduces your intake of phthalates, BPA (bisphenol A) and plasticizers that coat the inside of cans or those absorbed from plastic wrappings or cling wrap. - Limiting your intake of fatty meats reduces your consumption of persistent organic pollutants, pesticides, heavy metals and fat-soluble chemicals that can accumulate in animals.
- Avoiding handling sales receipts or storing them in your wallet. The thermal coating contains BPA (bisphenol A) to give them their shiny plastic texture.
- Drinking water/soft drinks out of glass or hard plastic bottles, not soft plastic bottles. BPA, phthalates and other plasticisers are used to make plastics in bottles flexible.
- Never heating food in soft plastic takeaway containers or those covered with cling wrap or foil. Instead, place food in a china or glass bowl and cover it with paper towel or a china plate before heating. When they are heated, phthalates and bisphenols in plastic can easily be absorbed into the food, especially if it is fatty. The heating process also releases dioxins from the plastics that can be absorbed into the food.
- Avoiding air fresheners, smoke, strong chemicals, heavily perfumed products, plastic smells and fumes.
- Airing your home frequently to reduce the amount of inhalable chemical particles.
- Avoiding use of pesticides and herbicides in your garden, at work or in the home. Instead, try using ‘green chemicals’, which use non-toxic agents to reduce pests and weeds.
- Avoiding potent household products like detergents, hand sanitizers, cleaning agents, and carpet cleaners or strong chemicals like glues, paints, and varnishes which have numerous chemicals classes in them. Use ‘green products’ which use alternative non-toxic agents.
- Reading the labels on all personal care products such as cosmetics, shampoos, conditioners, hair colorings and body washes etc and choosing those that are free of parabens. Try to avoid using heavily perfumed or scented products where possible.
- Reading the labels on all food products and avoid those with additives, preservatives and anti-bacterial agents.
- Being aware of marketing ploys – some products that are advertised as ‘BPA free’ for example often have replacement chemicals such (bisphenol S, commonly used in curing fast-drying epoxy resin adhesives) which can be just as harmful as BPA.
- Schecter A, Lorber M, Guo Y, et al. Phthalate Concentrations and Dietary Exposure from Food Purchased in New York State. Environmental Health Perspectives. 2013;121(4):473-479. doi:10.1289/ehp.1206367. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3620091
- Phthalates. https://www.epa.gov/sites/production/files/2017-08/documents/phthalates_updates_live_file_508_0.pdf
- SCHETTLER, T. (2006), Human exposure to phthalates via consumer products. International Journal of Andrology, 29: 134-139. https://doi.org/10.1111/j.1365-2605.2005.00567.x
- U.S. Environmental Protection Agency. 2012. Phthalates Action Plan. Washington, DC: U.S. EPA. http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/phthalates_actionplan_revised_2012-03-14.pdf
- Neier, K., Cheatham, D., Bedrosian, L. D., Gregg, B. E., Song, P., & Dolinoy, D. C. (2019). Longitudinal Metabolic Impacts of Perinatal Exposure to Phthalates and Phthalate Mixtures in Mice. Endocrinology, 160(7), 1613–1630. https://doi.org/10.1210/en.2019-00287
- Thornton, J. 2000. Pandora’s Poison: Chlorine, Health, and a New Environmental Strategy. Cambridge, Massachusetts: MIT Press.
- National Research Council. 2008. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington, DC: The National Academies Press. http://www.nap.edu/catalog/12528/phthalates-and-cumulative-risk-assessment-the-tasks-ahead
- Carlson, Kent & Garland, Sarah. (2015). Estimated Phthalate Exposure and Risk to Pregnant Women and Women of Reproductive Age as Assessed Using Four NHANES Biomonitoring Data Sets (2005/2006, 2007/2008, 2009/2010, 2011/2012).
- EFSA (European Food Safety Authority), Volk, K, Castle, L., 2019. Technical report of the public consultation on the ‘Draft update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis(2-ethylhexyl)phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials’. EFSA supporting publication 2019: 16( 12): EN-1747. 232 pp. doi: 10.2903/sp.efsa.2019.EN-1747 https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/sp.efsa.2019.EN-1747
- Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, Huttner K, Hu H. Environ Health Perspect. 2005 Sep; 113(9):1222-5.
- Human body burdens of chemicals used in plastic manufacture. Koch HM, Calafat AM. Philos Trans R Soc Lond B Biol Sci. 2009 Jul 27; 364(1526):2063-78.
- Agency for Toxic Substances and Disease Registry. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2002. Toxicological Profile for Di-(2-ethylhexyl) Phthalate [DEHP]
- Gu, H., Liu, Y., Wang, W., Ding, L., Teng, W., & Liu, L. (2016). In utero exposure to di-(2-ethylhexyl) phthalate induces metabolic disorder and increases fat accumulation in visceral depots of C57BL/6J mice offspring. Experimental and therapeutic medicine, 12(6), 3806–3812. https://doi.org/10.3892/etm.2016.3820
- Safety Assessment of Di(2-ethylhexyl)phthalate (DEHP) Released from PVC Medical Devices. https://www.fda.gov/media/114001/download
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environmental Health. 2014;13:43. doi:10.1186/1476-069X-13-43. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4050989/
- Agency for Toxic Substances and Disease Registry (ATSDR). 2001. Toxicological Profile for Di-n-butyl Phthalate. Update. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/toxprofiles/tp135.pdf
- Sathyanarayana, S. 2008. Phthalates and children’s health. Current Problems in Pediatric and Adolescent Health Care 38 (2):34-49.
- Kwapniewski, R., S. Kozaczka, R. Hauser, M.J. Silva, A.M. Calafat, and S.M. Duty. 2008. Occupational exposure to dibutyl phthalate among manicurists. Journal of Occupational and Environmental Medicine 50 (6):705-11.
- Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Zota AR, Calafat AM, Woodruff TJ. Environ Health Perspect. 2014 Mar; 122(3):235-41.
- Phthalates and children’s health. Sathyanarayana S. Curr Probl Pediatr Adolesc Health Care. 2008 Feb; 38(2):34-49.
- Colacino, J.A., T.R. Harris, and A. Schecter. 2010. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environmental Health Perspectives 118 (7):998-1003.
- Agency for Toxic Substances and Disease Registry (ATSDR). 2002. Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. http://www.atsdr.cdc.gov/toxprofiles/tp9.pdf
- Mortensen, G.K., K.M. Main, A.M. Andersson, H. Leffers, and N.E. Skakkebaek. 2005. Determination of phthalate monoesters in human milk, consumer milk, and infant formula by tandem mass spectrometry (LC-MS-MS). Anal Bioanal Chem 382 (4):1084-92.
- U.S. Environmental Protection Agency. 2008. Child-specific Exposure Factors Handbook (Final Report). Washington, DC: U.S. Environmental Protection Agency. EPA/600/R-06/096F.
- Calafat, A.M., and R.H. McKee. 2006. Integrating biomonitoring exposure data into the risk assessment process: phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environmental Health Perspectives 114 (11):1783-9.
- Otake, T., J. Yoshinaga, and Y. Yanagisawa. 2004. Exposure to phthalate esters from indoor environment. Journal of Exposure Analysis and Environmental Epidemiology 14 (7):524-8.
- Romero-Franco, M., R.U. Hernandez-Ramirez, A.M. Calafat, M.E. Cebrian, L.L. Needham, S. Teitelbaum, M.S. Wolff, and L. Lopez-Carrillo. 2011. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environment International 37 (5):867-71.
- Weuve, J., B.N. Sanchez, A.M. Calafat, T. Schettler, R.A. Green, H. Hu, and R. Hauser. 2006. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environmental Health Perspectives 114 (9):1424-31.
- Silva, M.J., D.B. Barr, J.A. Reidy, N.A. Malek, C.C. Hodge, S.P. Caudill, J.W. Brock, L.L. Needham, and A.M. Calafat. 2004. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environmental Health Perspectives 112 (3):331-8.
- Anne B, Raphael R. Endocrine Disruptor Chemicals. [Updated 2021 Mar 16]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK569327
- Endocrine Disruptors. https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm
- Endocrine Disruptor Knowledge Base. https://www.fda.gov/science-research/bioinformatics-tools/endocrine-disruptor-knowledge-base
- Effects of human exposure to hormone-disrupting chemicals examined in landmark UN report. https://www.who.int/news/item/19-02-2013-effects-of-human-exposure-to-hormone-disrupting-chemicals-examined-in-landmark-un-report
- Endocrine Disrupting Chemicals.Encyclopedia of Analytical Science (Third Edition), Academic Press, 2019,Pages 31-38, ISBN 9780081019849. https://doi.org/10.1016/B978-0-12-409547-2.14512-3
- Elobeid, M. A., & Allison, D. B. (2008). Putative environmental-endocrine disruptors and obesity: a review. Current opinion in endocrinology, diabetes, and obesity, 15(5), 403–408. https://doi.org/10.1097/MED.0b013e32830ce95c
- Schneider, M., Pons, J. L., Labesse, G., & Bourguet, W. (2019). In Silico Predictions of Endocrine Disruptors Properties. Endocrinology, 160(11), 2709–2716. https://doi.org/10.1210/en.2019-00382
- Diamanti-Kandarakis, E., J.P. Bourguignon, L.C. Giudice, R. Hauser, G.S. Prins, A.M. Soto, R.T. Zoeller, and A.C. Gore. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine Reviews 30 (4):293-342.
- Christiansen, S., J. Boberg, M. Axelstad, M. Dalgaard, A.M. Vinggaard, S.B. Metzdorff, and U. Hass. 2010. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reproductive Toxicology 30 (2):313-21.
- Swan, S.H., K.M. Main, F. Liu, S.L. Stewart, R.L. Kruse, A.M. Calafat, C.S. Mao, J.B. Redmon, C.L. Ternand, S. Sullivan, et al. 2005. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives 113 (8):1056-61.
- Macleod, D.J., R.M. Sharpe, M. Welsh, M. Fisken, H.M. Scott, G.R. Hutchison, A.J. Drake, and S. van den Driesche. 2010. Androgen action in the masculinization programming window and development of male reproductive organs. International Journal of Andrology 33 (2):279-87.
- Mendiola, J., R.W. Stahlhut, N. Jorgensen, F. Liu, and S.H. Swan. 2011. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environmental Health Perspectives 119 (7):958-63.
- Main, K.M., G.K. Mortensen, M.M. Kaleva, K.A. Boisen, I.N. Damgaard, M. Chellakooty, I.M. Schmidt, A.M. Suomi, H.E. Virtanen, D.V. Petersen, et al. 2006. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives 114 (2):270-6.
- Miodovnik, A., S.M. Engel, C. Zhu, X. Ye, L.V. Soorya, M.J. Silva, A.M. Calafat, and M.S. Wolff. 2011. Endocrine disruptors and childhood social impairment. Neurotoxicology 32 (2):261-7.
- Cho, S.C., S.Y. Bhang, Y.C. Hong, M.S. Shin, B.N. Kim, J.W. Kim, H.J. Yoo, I.H. Cho, and H.W. Kim. 2010. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environmental Health Perspectives 118 (7):1027-32.
- Whyatt, R.M., J.J. Adibi, A.M. Calafat, D.E. Camann, V. Rauh, H.K. Bhat, F.P. Perera, H. Andrews, A.C. Just, L. Hoepner, et al. 2009. Prenatal Di(2-ethylhexyl) phthalate exposure and length of gestation among an inner-city cohort. Pediatrics 124 (6):e1213-20.
- Adibi, J.J., R. Hauser, P.L. Williams, R.M. Whyatt, A.M. Calafat, H. Nelson, R. Herrick, and S.H. Swan. 2009. Maternal urinary metabolites of Di-(2-Ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. American Journal of Epidemiology 169 (8):1015-24.
- Phthalate excretion pattern and testicular function: a study of 881 healthy Danish men. Joensen UN, Frederiksen H, Blomberg Jensen M, Lauritsen MP, Olesen IA, Lassen TH, Andersson AM, Jørgensen N. Environ Health Perspect. 2012 Oct; 120(10):1397-403.
- Phthalates and risk of endometriosis. Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, Holt VL. Environ Res. 2013 Oct; 126():91-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3905445/
- Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Environ Health. 2008 Jun 3; 7():27.
- Chemical Listed Effective December 20, 2013 as Known to the State of California to Cause Cancer: Diisononyl Phthalate (DINP). https://oehha.ca.gov/proposition-65/crnr/chemical-listed-effective-december-20-2013-known-state-california-cause-cancer
- Jaakkola, J.J., and T.L. Knight. 2008. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environmental Health Perspectives 116 (7):845-53.
- Bornehag, C.G., J. Sundell, C.J. Weschler, T. Sigsgaard, B. Lundgren, M. Hasselgren, and L. Hagerhed-Engman. 2004. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environmental Health Perspectives 112 (14):1393-7.
- Jaakkola, J.J., L. Oie, P. Nafstad, G. Botten, S.O. Samuelsen, and P. Magnus. 1999. Interior surface materials in the home and the development of bronchial obstruction in young children in Oslo, Norway. American Journal of Public Health 89 (2):188-92.
- All About Phthalates. https://www.niehs.nih.gov/research/supported/translational/peph/podcasts/phthalates/index.cfm
- National Toxicology Program. 2006. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Di(2-Ethylhexyl) Phthalate (DEHP), edited by U.S. Department of Health and Human Services. Research Triangle Park, NC: NIH.
- Huang, P.C., P.L. Kuo, Y.L. Guo, P.C. Liao, and C.C. Lee. 2007. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Human Reproduction 22 (10):2715-22.
- Identifying sources of phthalate exposure with human biomonitoring: results of a 48h fasting study with urine collection and personal activity patterns. Koch HM, Lorber M, Christensen KL, Pälmke C, Koslitz S, Brüning T. Int J Hyg Environ Health. 2013 Nov; 216(6):672-81. https://www.ncbi.nlm.nih.gov/pubmed/23333758/
- Cao X. Phthalate esters in foods: sources, occurrence, and analytical methods. Compr Rev Food Sci Food Saf. 2010;9:21–43. doi: 10.1111/j.1541-4337.2009.00093.x
- Indirect food additives: polymers. Indirect food additives: polymers”. Title 21 Code of Federal Regulations; 2014. Part 177.
- Phthalates and food-contact materials: enforcing the 2008 European Union plastics legislation. Petersen JH, Jensen LK. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010 Nov; 27(11):1608-16.
- Phthalate concentrations and dietary exposure from food purchased in New York State. Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D, Colacino JA, Birnbaum LS. Environ Health Perspect. 2013 Apr; 121(4):473-94.
- Phthalates dietary exposure and food sources for Belgian preschool children and adults. Sioen I, Fierens T, Van Holderbeke M, Geerts L, Bellemans M, De Maeyer M, Servaes K, Vanermen G, Boon PE, De Henauw S. Environ Int. 2012 Nov 1; 48():102-8.
- Estimation of dietary intake of bis(2-ethylhexyl)phthalate (DEHP) by consumption of food in the German population. Heinemeyer G, Sommerfeld C, Springer A, Heiland A, Lindtner O, Greiner M, Heuer T, Krems C, Conrad A. Int J Hyg Environ Health. 2013 Jul; 216(4):472-80.
- Phthalates Exposures through Diet: Lessons Learned. NIEHS/EPA: Children’s Health Environmental Health Research Centers Webinars. June 10, 2015. https://www.epa.gov/sites/production/files/2016-11/documents/cehc_june2015_sathyanarayana.pdf
- Phthalate exposure through food and consumers’ risk perception of chemicals in food. Dickson-Spillmann M, Siegrist M, Keller C, Wormuth M. Risk Anal. 2009 Aug; 29(8):1170-81.
- Ji K, Kho YL, Park Y, Choi K. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: A pilot study with “Temple Stay” participants. Environ Res. 2010;110:375–382. doi: 10.1016/j.envres.2010.02.008.
- What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. Risk Anal. 2006 Jun; 26(3):803-24.
- Colacino JA, Harris TR, Schecter A. Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect. 2010;118:998–1003. doi: 10.1289/ehp.0901712
- Trasande L, Sathyanarayana S, Messito MJ, Gross RS, Attina TM, Mendelsohln AL. Phthalates and the diets of US children and adolescents. Environ Res. 2013;126:84–90
- Dickson-Spillmann M, Siegrist M, Keller C, Wormuth M. Phthalate exposure through food and consumers’ risk perception of chemicals in food. Risk Anal. 2009;29(8):1170–1181. doi: 10.1111/j.1539-6924.2009.01233.x
- Ji K, Kho YL, Park Y, Choi K. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: A pilot study with “Temple Stay” participants. Environ Res. 2010;110:375–382. doi: 10.1016/j.envres.2010.02.008
- FAQ: phthalates in plastic food contact materials. https://www.efsa.europa.eu/en/news/faq-phthalates-plastic-food-contact-materials
- Phthalates. https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128250.htm
- Annual Review of Cosmetic Ingredient Safety Assessments 2002/2003. International Journal of Toxicology (Supplement 1), 1-102, 2005.
- Phthalates Factsheet. https://www.cdc.gov/biomonitoring/phthalates_factsheet.html
- Baby Care Products: Possible Sources of Infant Phthalate Exposure,” disclaimer icon S. Sathyanarayana, Pediatrics, 2008, vol. 121, pp. 260-268
- Rowdhwal, S., & Chen, J. (2018). Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. BioMed research international, 2018, 1750368. https://doi.org/10.1155/2018/1750368
- Wójtowicz, A. K., Sitarz-Głownia, A. M., Szczęsna, M., & Szychowski, K. A. (2019). The Action of Di-(2-Ethylhexyl) Phthalate (DEHP) in Mouse Cerebral Cells Involves an Impairment in Aryl Hydrocarbon Receptor (AhR) Signaling. Neurotoxicity research, 35(1), 183–195. https://doi.org/10.1007/s12640-018-9946-7
- Gray, L. E., Jr, Barlow, N. J., Howdeshell, K. L., Ostby, J. S., Furr, J. R., & Gray, C. L. (2009). Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicological sciences : an official journal of the Society of Toxicology, 110(2), 411–425. https://doi.org/10.1093/toxsci/kfp109
- Loff S, Kabs F, Witt K, Sartoris J, Mandl B, Niessen KH, Waag KL. Polyvinylchloride infusion lines expose infants to large amounts of toxic plasticizers. J Pediatr Surg. 2000 Dec;35(12):1775-81. doi: 10.1053/jpsu.2000.19249
- Lorber M, Angerer J, Koch HM. A simple pharmacokinetic model to characterize exposure of Americans to di-2-ethylhexyl phthalate. J Expo Sci Environ Epidemiol. 2010 Jan;20(1):38-53. doi: 10.1038/jes.2008.74
- Lorber, M., & Calafat, A. M. (2012). Dose reconstruction of di(2-ethylhexyl) phthalate using a simple pharmacokinetic model. Environmental health perspectives, 120(12), 1705–1710. https://doi.org/10.1289/ehp.1205182
- Dorman, D. C., Chiu, W., Hales, B. F., Hauser, R., Johnson, K. J., Mantus, E., Martel, S., Robinson, K. A., Rooney, A. A., Rudel, R., Sathyanarayana, S., Schantz, S. L., & Waters, K. M. (2018). Systematic reviews and meta-analyses of human and animal evidence of prenatal diethylhexyl phthalate exposure and changes in male anogenital distance. Journal of toxicology and environmental health. Part B, Critical reviews, 21(4), 207–226. https://doi.org/10.1080/10937404.2018.1505354
- Fu, X., Han, H., Li, Y., Xu, B., Dai, W., Zhang, Y., Zhou, F., Ma, H., & Pei, X. (2021). Di-(2-ethylhexyl) phthalate exposure induces female reproductive toxicity and alters the intestinal microbiota community structure and fecal metabolite profile in mice. Environmental toxicology, 36(6), 1226–1242. https://doi.org/10.1002/tox.23121
- Hannon, P. R., Peretz, J., & Flaws, J. A. (2014). Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biology of reproduction, 90(6), 136. https://doi.org/10.1095/biolreprod.114.119032
- Liu J, Wang W, Zhu J, Li Y, Luo L, Huang Y, Zhang W. Di(2-ethylhexyl) phthalate (DEHP) influences follicular development in mice between the weaning period and maturity by interfering with ovarian development factors and microRNAs. Environ Toxicol. 2018 May;33(5):535-544. doi: 10.1002/tox.22540
- Lai FN, Liu JC, Li L, Ma JY, Liu XL, Liu YP, Zhang XF, Chen H, De Felici M, Dyce PW, Shen W. Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice. Arch Toxicol. 2017 Mar;91(3):1279-1292. doi: 10.1007/s00204-016-1790-z
- Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, Huynh E, Shi QH, De Felici M, Shen W. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol Mutagen. 2013 Jun;54(5):354-61. doi: 10.1002/em.21776
- Brehm, E., Rattan, S., Gao, L., & Flaws, J. A. (2018). Prenatal Exposure to Di(2-Ethylhexyl) Phthalate Causes Long-Term Transgenerational Effects on Female Reproduction in Mice. Endocrinology, 159(2), 795–809. https://doi.org/10.1210/en.2017-03004
- Lu Q, Shen H, Wang X, et al. Exposure to phthalic acid esters in early human pregnancy. Fertility Sterility. 2010;94(4‐supp‐S):S230‐S231.
- Matsumoto M, Hirata-Koizumi M, Ema M. Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction. Regul Toxicol Pharmacol. 2008 Feb;50(1):37-49. doi: 10.1016/j.yrtph.2007.09.004
- Dombret, C., Capela, D., Poissenot, K., Parmentier, C., Bergsten, E., Pionneau, C., Chardonnet, S., Hardin-Pouzet, H., Grange-Messent, V., Keller, M., Franceschini, I., & Mhaouty-Kodja, S. (2017). Neural Mechanisms Underlying the Disruption of Male Courtship Behavior by Adult Exposure to Di(2-ethylhexyl) Phthalate in Mice. Environmental health perspectives, 125(9), 097001. https://doi.org/10.1289/EHP1443
- Sircar D, Albazi SJ, Atallah Y, Pizzi W. Validation and application of an HPLC method for determination of di (2-ethylhexyl) phthalate and mono (2-ethylhexyl) phthalate in liver samples. J Chromatogr Sci. 2008 Aug;46(7):627-31. doi: 10.1093/chromsci/46.7.627
- Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006 Feb;290(2):F262-72. doi: 10.1152/ajprenal.00099.2005
- Barakat, R., Lin, P. P., Rattan, S., Brehm, E., Canisso, I. F., Abosalum, M. E., Flaws, J. A., Hess, R., & Ko, C. (2017). Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice. Toxicological sciences : an official journal of the Society of Toxicology, 156(1), 96–108. https://doi.org/10.1093/toxsci/kfw248
- Reddy BS, Rozati R, Reddy BV, Raman NV. Association of phthalate esters with endometriosis in Indian women. BJOG. 2006 May;113(5):515-20. doi: 10.1111/j.1471-0528.2006.00925.x
- Smerieri, A., Testa, C., Lazzeroni, P., Nuti, F., Grossi, E., Cesari, S., Montanini, L., Latini, G., Bernasconi, S., Papini, A. M., & Street, M. E. (2015). Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PloS one, 10(2), e0117831. https://doi.org/10.1371/journal.pone.0117831
- Chanjuan Hao, Xuejia Cheng, Jian Guo, Hongfei Xia, Xu Ma. Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Frontiers in Bioscience-Elite. 2013. 5(2); 725-733. https://www.fbscience.com/Elite/articles/pdf/Elite653.pdf
- Schmidt, J. S., Schaedlich, K., Fiandanese, N., Pocar, P., & Fischer, B. (2012). Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environmental health perspectives, 120(8), 1123–1129. https://doi.org/10.1289/ehp.1104016
- Edson, M. A., Nagaraja, A. K., & Matzuk, M. M. (2009). The mammalian ovary from genesis to revelation. Endocrine reviews, 30(6), 624–712. https://doi.org/10.1210/er.2009-0012
- Hannon, P. R., Brannick, K. E., Wang, W., & Flaws, J. A. (2015). Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biology of reproduction, 92(5), 120. https://doi.org/10.1095/biolreprod.115.129148
- Lamb JC 4th, Chapin RE, Teague J, Lawton AD, Reel JR. Reproductive effects of four phthalic acid esters in the mouse. Toxicol Appl Pharmacol. 1987 Apr;88(2):255-69. doi: 10.1016/0041-008x(87)90011-1
- Niermann, S., Rattan, S., Brehm, E., & Flaws, J. A. (2015). Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reproductive toxicology (Elmsford, N.Y.), 53, 23–32. https://doi.org/10.1016/j.reprotox.2015.02.013
- Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., & Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature, 489(7415), 220–230. https://doi.org/10.1038/nature11550
- Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. 2017 Oct;74(20):3769-3787. doi: 10.1007/s00018-017-2550-9
- Rizzetto L, Fava F, Tuohy KM, Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J Autoimmun. 2018 Aug;92:12-34. doi: 10.1016/j.jaut.2018.05.008
- Xue J, Li X, Liu P, Li K, Sha L, Yang X, Zhu L, Wang Z, Dong Y, Zhang L, Lei H, Zhang X, Dong X, Wang H. Inulin and metformin ameliorate polycystic ovary syndrome via anti-inflammation and modulating gut microbiota in mice. Endocr J. 2019 Oct 28;66(10):859-870. doi: 10.1507/endocrj.EJ18-0567
- Li, L., Liu, J. C., Lai, F. N., Liu, H. Q., Zhang, X. F., Dyce, P. W., Shen, W., & Chen, H. (2016). Di (2-ethylhexyl) Phthalate Exposure Impairs Growth of Antral Follicle in Mice. PloS one, 11(2), e0148350. https://doi.org/10.1371/journal.pone.0148350
- Dalman A, Eimani H, Sepehri H, Ashtiani SK, Valojerdi MR, Eftekhari-Yazdi P, Shahverdi A. Effect of mono-(2-ethylhexyl) phthalate (MEHP) on resumption of meiosis, in vitro maturation and embryo development of immature mouse oocytes. Biofactors. 2008;33(2):149-55. doi: 10.1002/biof.5520330207
- Liu J, Yue S, Yang Z, Feng W, Meng X, Wang A, Peng C, Wang C, Yan D. Oral hydroxysafflor yellow A reduces obesity in mice by modulating the gut microbiota and serum metabolism. Pharmacol Res. 2018 Aug;134:40-50. doi: 10.1016/j.phrs.2018.05.012
- Tripathi, A., Pandey, V., Sahu, A. N., Singh, A., & Dubey, P. K. (2019). Di-(2-ethylhexyl) phthalate (DEHP) inhibits steroidogenesis and induces mitochondria-ROS mediated apoptosis in rat ovarian granulosa cells. Toxicology research, 8(3), 381–394. https://doi.org/10.1039/c8tx00263k
- Risk Evaluation for Dicyclohexyl Phthalate. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-evaluation-dicyclohexyl-phthalate
- Aydoğan Ahbab M, Barlas N. Influence of in utero di-n-hexyl phthalate and dicyclohexyl phthalate on fetal testicular development in rats. Toxicol Lett. 2015 Mar 4;233(2):125-37. doi: 10.1016/j.toxlet.2015.01.015
- Aydoğan Ahbab M, Barlas N. Developmental effects of prenatal di-n-hexyl phthalate and dicyclohexyl phthalate exposure on reproductive tract of male rats: Postnatal outcomes. Food Chem Toxicol. 2013 Jan;51:123-36. doi: 10.1016/j.fct.2012.09.010
- Hoshino N, Iwai M, Okazaki Y. A two-generation reproductive toxicity study of dicyclohexyl phthalate in rats. J Toxicol Sci. 2005 Dec;30 Spec No.:79-96. doi: 10.2131/jts.30.s79
- Saillenfait A.M., Gallissot F., Sabate J.P. Differential developmental toxicities of di-n-hexyl phthalate and dicyclohexyl phthalate administered orally to rats. J. Appl. Toxicol. 2009;29:510–521. doi: 10.1002/jat.1436
- Yuan K., Zhao B., Li X.W., Hu G.X., Su Y., Chu Y., Akingbemi B.T., Lian Q.Q., Ge R.S. Effects of phthalates on 3 beta-hydroxysteroid dehydrogenase and 17 beta-hydroxysteroid dehydrogenase 3 activities in human and rat testes. Chem. Biol. Interact. 2012;195:180–188. doi: 10.1016/j.cbi.2011.12.008
- Fisher J.S., Macpherson S., Marchetti N., Sharpe R.M. Human ‘testicular dysgenesis syndrome’: A possible model using in-utero exposure of the rat to dibutyl phthalate. Hum. Reprod. 2003;18:1383–1394. doi: 10.1093/humrep/deg273
- Hu, G. X., Lian, Q. Q., Ge, R. S., Hardy, D. O., & Li, X. K. (2009). Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends in endocrinology and metabolism: TEM, 20(3), 139–145. https://doi.org/10.1016/j.tem.2008.12.001
- Lin, H., Ge, R. S., Chen, G. R., Hu, G. X., Dong, L., Lian, Q. Q., Hardy, D. O., Sottas, C. M., Li, X. K., & Hardy, M. P. (2008). Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proceedings of the National Academy of Sciences of the United States of America, 105(20), 7218–7222. https://doi.org/10.1073/pnas.0709260105
- Fact Sheet: Di-n-pentyl phthalate (DnPP). https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-di-n-pentyl-phthalate-dnpp