What are phytoestrogens
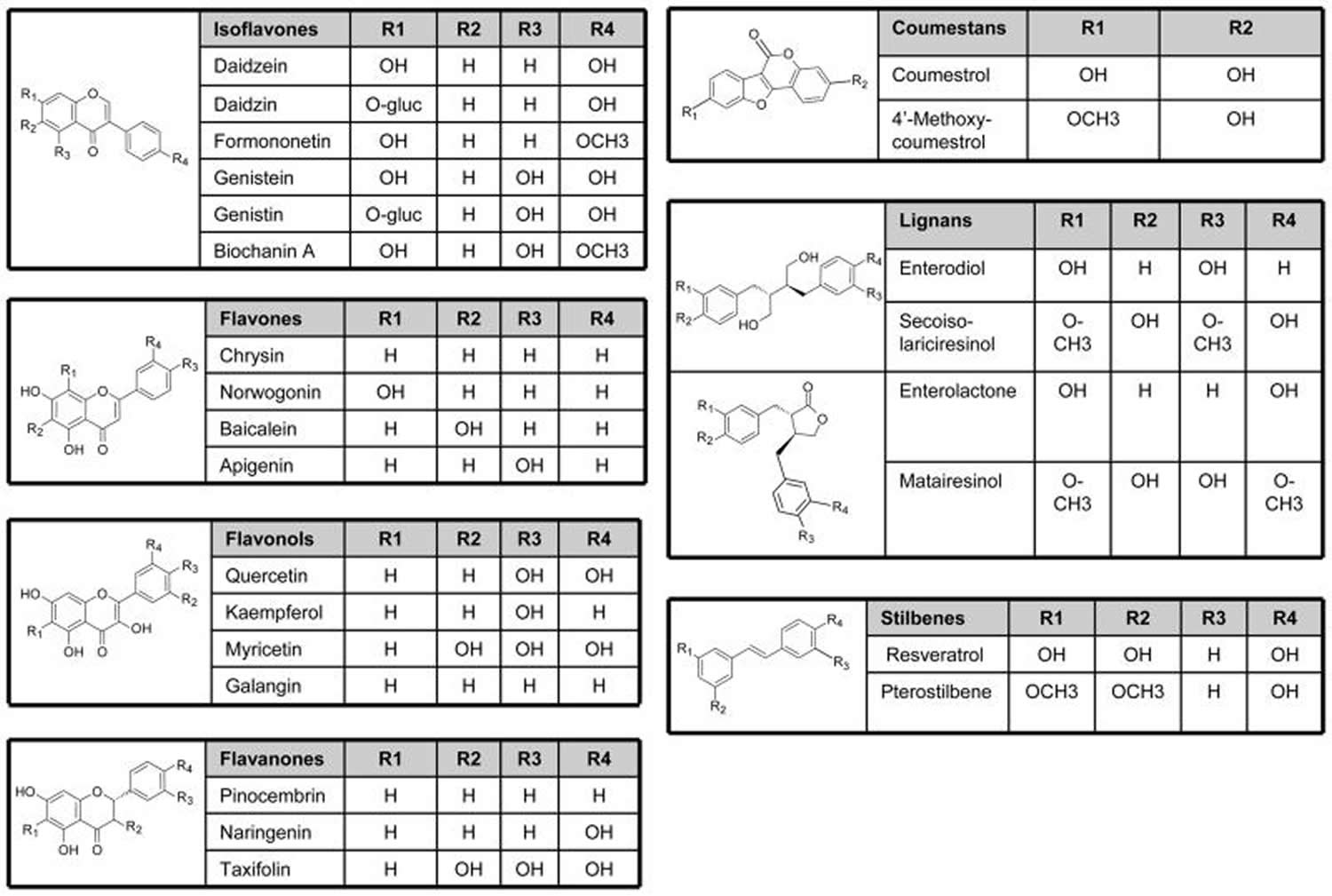
Phytoestrogens are estrogenic compounds of plant origin classified into different groups including isoflavones, lignans, coumestans and stilbenes, the structure of which is similar to that of 17-β-estradiol 1. They are called estrogen-like molecules or non-steroidal estrogens. In spite of the structural similarity with estradiol, phytoestrogens are diphenolic yet non-steroidal compounds. Currently the group of phytoestrogens includes more than 100 molecules, divided according to their chemical structure into:
- Isoflavones (genistein, daidzein, glycitein, formononetin, and biochanin A),
- Lignans (matairesinol, secoiso-lariciresinol-diglucoside, pinoresinol, and sesamin),
- Coumestans (coumestrol, 4-methoxycoumestrol), and
- Stilbens (resveratrol). Some of these substances (e.g. resveratrol) act as natural antioxidants.
Isoflavones such as genistein and daidzein are the most studied and most potent phytoestrogens, and are found mainly in soy based foods 1. The effects of phytoestrogens are partly mediated via estrogen receptors (ERs): ERα, ERβ and possibly GPER. Estrogens influence the growth and functioning of female and male reproductive tissues, maintain both the skeletal and central nervous systems, and provide cardioprotective effects in the cardiovascular system 2. Considering phytoestrogens similarity to estrogens and the numerous effects estrogens have on the human body, it is not surprising to see the rapid increase of their consumption especially in the last decade, no matter what supporting scientific evidence was available on their biological effects. The amount of isoflavones (genistein, daidzein, glycitein, formononetin, and biochanin A) found in soybeans and their processed products needed to give health effects is around 40-70 mg/day or an average of 50 mg/day 3. The average consumption of isoflavones in Asian society is 15-50 mg per day, while in Western countries only about 2 mg per day 4.
Estrogen (E2) deficiency during menopause is associated with perimenopausal symptoms such as hot flushes and night sweats which prompt women to seek menopausal hormone therapy. In addition to the relief of menopausal hot flushes and vaginal dryness, menopausal hormone therapy may improve sleep quality and social well-being, retard bone loss and minimize osteoporotic fractures 5.
The risk of cardiovascular disease (CVD) also increases after menopause, suggesting vascular benefits of endogenous estrogen (E2) 6. Estrogen receptors (ERs) have been identified in the vasculature, and estrogen has been shown to promote beneficial effects on the endothelium, vascular smooth muscle and extracellular matrix 7. The vascular benefits of estrogen (E2) observed in experimental studies have suggested potential benefits of menopausal hormone therapy in cardiovascular disease.
Epidemiological evidence suggests potential protective effects of phytoestrogens. Results from human studies suggest that phytoestrogens may lower the risk of osteoporosis, some cardiometabolic diseases, cognitive dysfunction, breast and prostate cancer, and menopausal symptoms by modulating the endocrine system 8. The incidence of cardiovascular disease, diabetes, obesity, breast cancer and endometrial cancers are less in Asian than Western populations 9. Also, the incidence of hot flushes is 70-80% in menopausal Western women compared to 14-15% in Asian women 10. Migration studies of the Japanese population moving to the United States showed that they developed an increased incidence in “Western Diseases” – mainly cardiovascular- after two generations due to their diets to include more protein and fat and reduce their fibre and soy intake 11. These observations suggest that the factors contributing to cardiovascular disease are not only genetic, but could also involve environmental factors such as the diet. One important difference between Asian and Western diets is the high content of soy-rich in phytoestrogens- in the Asian diet (20-150 mg/d) compared to the Western diet (1-3 mg/d) 12. In a study examining the relation between coronary artery disease (coronary heart disease) and dietary habits of 61 populations in 25 countries, the 24 hour urinary excretion of taurine and isoflavones, which are abundant in fish and soybean diets, was inversely related to mortality rates from coronary artery disease 13. These dietary differences may contribute to the lower incidence of coronary heart disease among the Asian populations.
However, phytoestrogens are a diverse group of compounds with different modes of metabolism, bioavailability and in the body effects. Some authors describe phytoestrogens as endocrine disruptors and believe their beneficial effects have been overestimated 14, 15, 16. This ambiguity could be partially due to the variability of published studies, as the beneficial or harmful effects of phytoestrogens depend on the exposure (type, amount consumed, and bioavailability), ethnicity, hormonal status (age and sex and physiological condition), and health status of the consumer 14, 15, 16. Therefore, after decades of research there is no definitive agreement as to the vascular effects of phytoestrogens and their benefit in cardiovascular disease. In effect, some studies have suggested that phytoestrogens may not have any benefit in cardiovascular disease, and other studies attributed the benefits of the soy-rich diet to food components other than phytoestrogens 17. Also, most of the clinical studies of phytoestrogens have been limited in terms of the number of subjects enrolled, the compounds studied, the duration of dietary intake and the long-term follow-up of the participants.
Sources and Classification of Phytoestrogens
Phytoestrogens are polyphenolic non-steroidal compounds of estrogenic activity 18. Major classes of phytoestrogens include isoflavones, lignans and coumestans. Other phytoestrogens include stilbenes, flavanones, flavonols, and flavones (see Figure 1). The most abundant, most studied and most potent phytoestrogens are isoflavones 18. There are more than 1000 types of isoflavones including genistein, daidzein, genistin, daidzin, formononetin, biochanin-A and equol. The most commonly studied isoflavones are genistein, daidzein and its metabolite equol.
Figure 1. Classification of phytoestrogens
Note: Phytoestrogens include isoflavones, flavanones, flavonols, flavones, lignans, coumestans, and stilbenes with different core structure and side chains.
[Source 19]Isoflavones
Isoflavones are a subgroup of phytoestrogens, natural plant substances with structure similar to 17-β-estradiol and capable of binding to estrogen receptors (ERs). Isoflavones possess higher affinity to estrogen receptors-β (ERβ) than to estrogen receptors-α (ERα) and may have a potency to activate both genomic and non-genomic estrogen signaling pathways. In addition, isoflavones interact with the metabolism of steroid hormones. Therefore, the actions of isoflavones are rather complex and may be related to large number of factors, which are not satisfactorily identified yet. Recently, isoflavones have come into focus of interest due to several reports about their positive effect on human health, in particular prevention of hormone-dependent cancers, cardiovascular diseases, osteoporosis, adverse menopausal manifestations and age-related cognitive decline.
Isoflavones are found in legumes such as soy, chickpeas, clover, lentils and beans (Table 1) 20. Unextracted soy protein contains on average 1.105 mg genistein and 0.365 mg daidzein/g soy proteins isolate. However, total isoflavone content may vary up to 3-fold with growth of the same soy cultivar in different geographical areas and different years. In addition, the contents of isoflavones in different soy products (e.g. tofu and soy protein concentrates) vary substantially. For example, processed soy products, such as soy hot dogs and tofu yogurt, may contain only 1/10th the isoflavone content of whole soy beans (0.2– 0.3 vs. 2–4 mg isoflavone/g) 21. Biochanin-A and formononetin are precursors of genistein and daidzein, respectively, and also have estrogenic properties. Formononetin is abundant in Astragalus mongholicus Bunge and Curcuma comosa Roxb. Glycitein and its conjugates are minor isoflavones in soybean cotyledons, but are major components in dietary supplements and foods made from the soybean hypocotyls.
Table 1. Foods high in phytoestrogens
| Isoflavones | Coumestans | Lignans | ||
|---|---|---|---|---|
| Food | Daidzein | Genistein | Coumestrol | Secoisolariciresinol |
| Soy based foods | ||||
| Black bean sauce | 2304.0 | 2486.6 | tr | tr |
| Miso soup | 430.2 | 1009.8 | nd | tr |
| Soy beans | 56621.4 | 44213.4 | tr | 79.1 |
| Soy bean sprouts | 268.3 | 514.6 | nd | tr |
| Soy milk | 921.3 | 1852.2 | tr | tr |
| Soy nuts | 28351.2 | 36264.0 | tr | tr |
| Soy sauce | tr | 100.6 | tr | tr |
| Soy yogurt | 3364.4 | 6565.1 | tr | tr |
| Tempeh | 6974.8 | 10729.6 | tr | tr |
| Tofu | 9337.5 | 17050.2 | tr | tr |
| Veggie burger | 461.5 | 1111.5 | tr | tr |
| Vegetables and legumes | ||||
| Alfalfa sprouts | 151.7 | 117.6 | 105.3 | tr |
| Broccoli | tr | tr | tr | 414.0 |
| Clover sprouts | 71.3 | 70.9 | 97.7 | nd |
| Mung bean sprouts | 91.4 | 135.2 | 136.6 | 97.0 |
| Beans, green | tr | 32.9 | nd | 30.9 |
| Beans, white | tr | 25.3 | tr | 29.9 |
| Nuts and oil seeds | ||||
| Almonds | tr | tr | tr | 70.3 |
| Chestnuts | tr | tr | tr | 172.7 |
| Flaxseed | 58.2 | 173.2 | 46.8 | 375321.9 |
| Hazelnuts | tr | tr | tr | 60.5 |
| Pistachios | 73.1 | 103.3 | tr | tr |
| Sunflower seeds | nd | nd | nd | 127.8 |
| Walnuts | 35.2 | tr | tr | 78.0 |
| Peanut butter | tr | 38.2 | tr | 28.6 |
| Fruits | ||||
| Dried apricots | tr | tr | tr | 147.6 |
| Dried dates | tr | tr | tr | 106.2 |
| Dried prunes | tr | tr | tr | 103.8 |
| Strawberries | tr | tr | tr | 1210.0 |
| Cranberries | tr | tr | nd | 1500.0 |
| Blackberries | tr | tr | nd | 3710.0 |
| Breads | ||||
| Bread, flax | 85.0 | 212.3 | tr | 7208.3 |
| Bread, rye | tr | tr | nd | 122.0 |
| Bread, multigrain | tr | tr | tr | 4770.4 |
| Bread, whole wheat | 155.8 | 141.8 | tr | tr |
| Beverages | ||||
| Tea, black | na | na | na | 159.0 |
| Tea, green | na | na | na | 246.0 |
| Wine, red | tr | tr | nd | 29.4 |
Footnotes: Phytoestrogen levels are indicated in μg/100 g; tr, trace defined as ≤ 25 μg/100 g; nd = none detected; na = information not available.
[Sources 20, 22, 23]Table 2. Isoflavone content of a representative sample of food products including soy based products
| Food product | Genistein (mg/100g) | Daidzein (mg/100g) | Total isoflavones (mg/100 g) |
|---|---|---|---|
| Soy Infant Formula (powder) | 13.5 | 6.32 | 26.3 |
| Edamame (raw green soybeans) | 22.6 | 20.3 | 48.9 |
| Miso | 23.2 | 16.4 | 41.5 |
| Silken tofu | 8.4 | 9.2 | 18.0 |
| Raw tofu, regular | 13 | 9 | 23 |
| Textured soy flour | 89.4 | 67.7 | 172.6 |
| Soy protein isolate | 57 | 31 | 91 |
| Soy-based sliced cheese | 6.5 | 5.1 | 14.5 |
| Soy-based bacon bits | 45.8 | 64.4 | 118.5 |
| Soy-based burgers | 5.0 | 2.4 | 6.4 |
| Red clover | 10 | 11 | 21 |
| Multigrain bread | 0.2 | 0.2 | 0.4 |
| KASHI Go Lean cereal | 7.7 | 8.4 | 17.4 |
| Green tea, Japanese | 0.02 | 0.01 | 0.02 |
| Flaxseeds | 0.04 | 0.02 | 0.07 |
| Raw broccoli | 0.00 | 0.04 | 0.25 |
Lignans
Lignans are common in the plant kingdom and are the building block of lignin found in the plant cell wall. Food containing lignans include flaxseed, lentils, bran (wheat, oat, rye), poppy seeds, sesame, whole grains (rye, oats, barley), beans, fruits (particularly berries) and cruciferous vegetables (broccoli and cabbage) 25 (see Table 1 above). Tea and coffee also have some lignans. Flaxseed (Linum usatissimum) is by far the richest dietary source of plant lignans, and lignan bioavailability can be improved by crushing or milling flaxseed 26. The concentration of secoisolariciresinol diglucoside in flaxseeds is 75–800 times higher than that in other foods, and thus intake of flaxseed causes the highest mammalian lignan production 27. Much lower amounts are contained in sesame seeds, the second most lignan rich food.
Enterolactone and enterodiol are major lignans produced by the action of intestinal bacteria on matairesinol and secoisolariciresinol, respectively 28.
The biological activity of flaxseed and other plant lignans depends on the presence of certain bacteria in the gut. Some humans appear to lack either the right type or a sufficient number of gut bacteria to convert secoisolariciresinol diglucoside and other lignans to mammalian lignans. The use of antibiotics may abolish the ability of intestinal flora to produce active phytoestrogen metabolites for several weeks 29. Smoking and obesity are also associated with a reduction in enterolactone production, while coffee, tea, and of course fibre intake are noted to enhance the production of enterolactone 30.
The main flax lignan SDG is also an antioxidant. It scavenges for certain free radicals like the hydroxyl ion (•OH) 31.
Coumestans
Coumestans show oestrogenic activity, possess a coumarin structure, and are biosynthetically related to isoflavones 32. Coumestans such as coumestrol and 4-methoxycoumestrol are found in mung bean sprouts, brussel sprouts and spinach 33. Coumestrol, the most important coumestan consumed by humans, is found in clover sprouts, alfalfa sprouts, and other legumes. Legumes such as split peas, pinto beans, lima beans, and soybean sprouts also contain small amounts of coumestrol. Furthermore, it has been observed that coumestrol concentrations in legumes increase after insect and fungal attack 34.
Stilbenes
The stilbenes family of phytoestrogens includes reseveratrol and pterostilbene which are commonly found in skin of grapes (Vitis vinifera), other brightly pigmented fruit juices and red wine. Peanuts (Arachis), particularly the papery skin around the nut, and pistachios also contain resveratrol. Resveratrol, the most common and most studied stilbene, functions as a phytoalexin to protect against fungal infections. While resveratrol is most often discussed in terms of its vascular effects (similar to flavonoids), it has been shown to possess some phytoestrogenic activity as well, only reported for the trans isomers of this compound 35, 36.
Flavanones
Flavanones include eriodictyol, naringenin, pinocembrin and are mainly found in citrus fruits. Flavonols include kaempferol, myricetin, quercetin, and quercetagetin, and are found abundantly in green tea and to a less extent in dark tea and chocolate. Flavones include apigenin, baicalain, chyrisin, norwogenin and are found mainly in cereals and herbs.
Phytoestrogens Metabolism
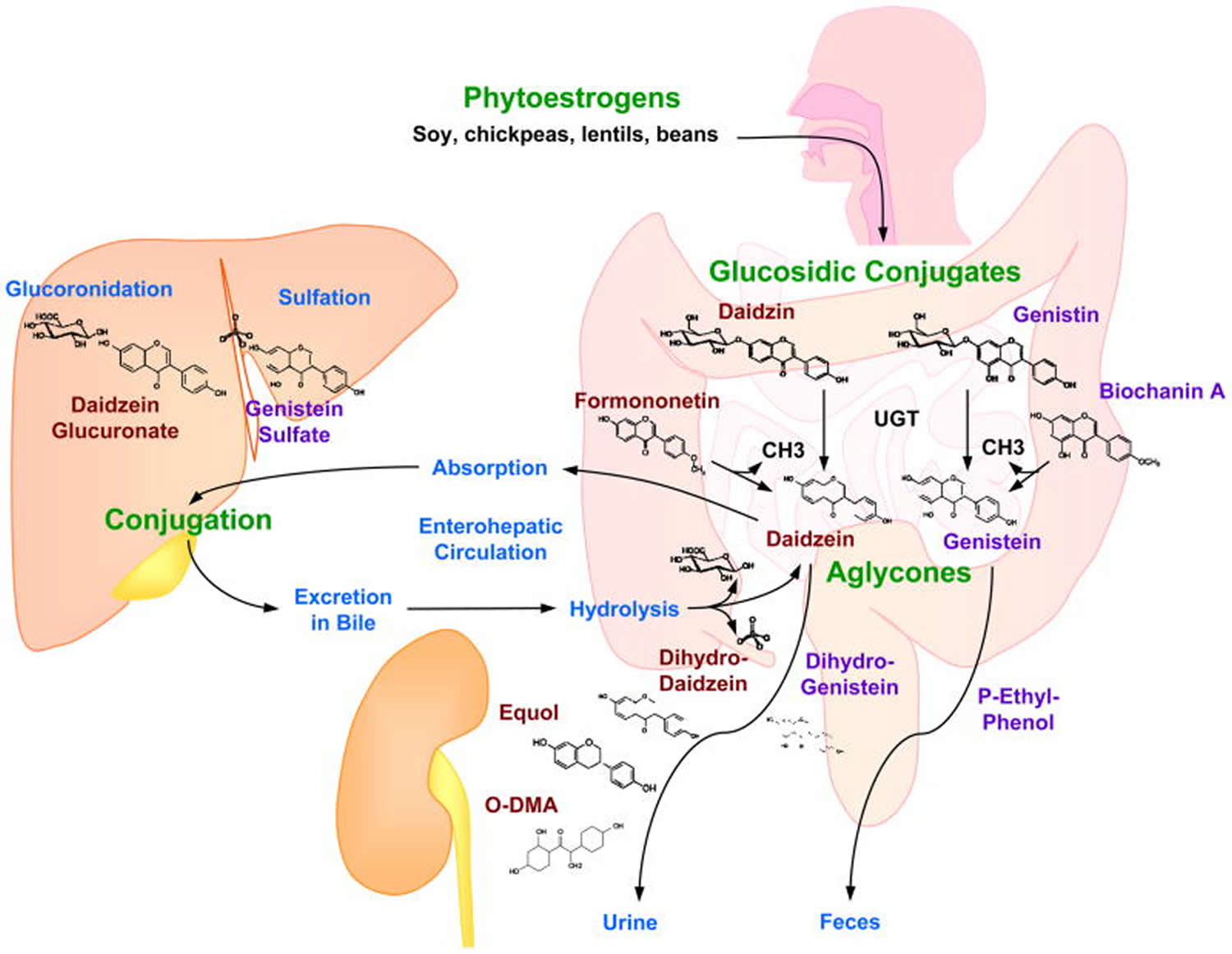
Phytoestrogens are present in plants as inactive glycosidic conjugates. In the intestine, they are hydrolyzed by the action of UDP-glucuronosyltransferase secreted by intestinal bacteria to the active forms aglycones (see Figure 2). The aglycones are then absorbed by the intestinal tract. On entering the circulation, aglycones may undergo extensive metabolism to other compounds through various reactions including demethylation, methylation, hydroxylation, chlorination, iodination, and nitration 37. These metabolites are then transported to the liver where they undergo conjugation to form β-glucuronides and to a less extent sulfate esters. In the liver some glucuronides undergo further fermentation into other metabolites that vary depending on the class of phytoestrogen. The glucuronides are excreted in bile and partially reabsorbed via the enterohepatic circulation. Phytoestrogens are excreted in bile and urine as conjugated glucuronides and in feces in the unconjugated form (see Figure 2) 38.
As with other phytoestrogens, isoflavones in food are bound to glucose. When ingested, they are enzymatically cleaved in the gut into active aglycones. Genistein and daidzein, the most active forms of isoflavones, are produced both by hydrolysis of their biologically inactive glucoconjugates, as well as from the demethylation of their precursors biochanin A and formononetin, respectively. The aglycone forms of isoflavones are easily transported across the intestinal epithelial cells to the blood or are further metabolized in the intestine 38. In humans consuming soy-free diets, the plasma concentration of isoflavones is usually in the nanomolar range, ≤40 nM. Acute ingestion of dietary soy leads to a rapid increase in the plasma concentration of isoflavones up to the micromolar range 39. The isoflavone serum concentration shows variability in different populations. In serum samples of Japanese men, the average concentration of genistein is 276 nmol/L and of daidzein is 107 nmol/L 40.
The majority of the genistein and daidzein consumed is eliminated from the body within 24 hours 38. Genistein is transformed to dihydrogenistein and is further metabolized in the colon to 4-ethyl phenol. Daidzein is metabolized to dihydrodaidzein, which is further metabolized to both equol and O-desmethylangolensin (O-DMA). Genistein, daidzein, equol and O-DMA are the major isoflavones detected in blood and urine of humans and animals 41. Interestingly, only 30-40% of humans –mostly Asians and vegetarians – are able to metabolize daidzein into equol, and the ability to produce equol may be associated with an increased benefit of isoflavones on bone mineral density and a lower risk of breast cancer 42.
Figure 2. Phytoestrogens Metabolism
Note: Absorption, metabolism and excretion of isoflavones. Phytoestrogens, found in diet as glucoconjugates (daidzin, genistin), are hydrolyzed in the intestine, by the action of UDP-glucuronosyltransferase (UGT) secreted by intestinal bacteria, into the active forms aglycones (daidzein and genistein). Genistein and daidzein are also produced from the demethylation of their precursors biochanin A and formononetin, respectively. The aglycones are absorbed from the intestinal tract to the liver where they are mainly conjugated with glucuronic acid and sufates. Some of the conjugated aglycones are excreted in the bile where they are hydrolyzed, and some of the unconjugated aglycones are excreted in the feces, while some are reabsorbed to the liver via enterohepatic circulation. In blood, Isoflavones are metabolized mainly into equol and O-desmethylangolensin (O-DMA) which are excreted in urine.
[Source 19]Factors Affecting the Metabolism of Phytoestrogens
The metabolism and excretion of isoflavones after soy consumption show considerable variation among individuals. The average time taken after ingesting the aglycones to reach peak plasma concentration is 4–7 hr, and is delayed to 8–11 hr for the corresponding glycosidic conjugates. This suggests that the rate-limiting step for absorption is the initial hydrolysis of the glycosidic moiety. The half-lives of genistein and daidzein are 7.1 and 9.3 hr, respectively 43.
In addition to the inter-individual variations in phytoestrogen metabolism, sex may also play a role, with women metabolizing phytoestrogens more efficiently than men 44. Other factors that could influence isoflavone bioavailability include the chemical composition, the administered dose, intestinal transit time, intestinal microflora and the individual ability to produce equol 40. The source of the isoflavones and hence the food matrix in which the compound is delivered plays a minor role in their bioavailability. The effect of age on the bioavailability of isoflavones was also investigated, but no difference was found in the pharmacokinetics of either genistein or daidzein between Pre- and Post-Menopausal women 45. Also, the frequency of ingestion does not appear to cause significant difference in the bioavailability of isoflavones 46.
Phytoestrogens Mechanism of Action
The phytoestrogens are believed to work by binding to estrogen receptors (ERs) on cell membranes, much like the body’s own steroid estrogens do. Coumestrol and genistein have been shown to be more potent than any other known phytoestrogen when their in vitro (test tube study) estrogenic activity was compared with that of the primary female hormone, 17β-estradiol (E2) 47. However, not all the biological effects of phytoestrogens involve estrogen receptors. Phytoestrogens can also activate serotonergic and insulin-like growth factor receptors 1 (IGF-1) , induce free radical binding and modify tyrosine kinases, cycle adenosine monophosphate (cAMP), phosphatidylinositol-3 kinase (PI3K)/Akt, mitogen-activated protein (MAP) kinases, transcription of nuclear factor-kappa β (NF-Kβ), as well as promote DNA methylation and affect histone and RNA expression 8. In addition, phytoestrogens can act as intracellular regulators of the cell cycle and apoptosis. Thus, due to their antioxidant, antiproliferative, antimutagenic, and antiangiogenic roles, phytoestrogens can improve health 3. In addition, some authors observed that estrogen and androgen seem to be involved in breast and prostate cancer regulating proliferative and migratory signaling, such as Src/PI3K. Hormonal therapy response may vary depend on interactions between estrogen or androgen receptors and proteins, according to hormone levels 48.
Most phytoestrogens are found to offer benefits for menopausal symptoms and bone density without carrying the risks of heart disease, coronary artery damage, or peripheral vascular issues 49.
In addition to their function as phytoestrogens, isoflavones act as potent antioxidants and help in neutralizing the harmful effects of free radicals in tissues. Furthermore, genistein possesses a number of biochemical features that may have influence on cancer cells. In particular, it inhibits protein tyrosine kinase, thereby disrupting signal transduction and inducing cell differentiation, and topoisomerase II, leading to DNA fragmentation and apoptosis. These anti-cancer properties of genistein were also considered in 1995 by the National Cancer Institute (NCI) that recommended genestein for clinical development as a cancer chemopreventive agent. Recently, Klein and King 50 claimed that the concentrations at which such effects occurred were often much higher than the physiologically relevant doses achievable by dietary or pharmacologic intake of soy foods or supplements. The inhibitory activities of intracellular enzymes displayed by genistein are not expressed by other isoflavones. This seems to be due to the absence of the hydroxyl group in position 5, which is present in the structure of genistein and appears to be essential for inhibitory activity 51.
The mammalian lignans are not as potent as the endogenous ligand in their estrogenic activities, they can act as either weak oestrogens or they can oppose the actions of oestrogen, depending on the presence of stronger oestrogens like estradiol. During women’s reproductive years, when blood levels of endogenous oestrogens are at their highest, the lignans can bind to the estrogen receptor and block the actions of endogenous oestrogens. In this case, they act as antagonists. After menopause, the levels of endogenous oestrogens in the blood naturally decrease because the ovaries release less natural oestrogens. In this case, the lignans act like weak oestrogens 52.
Many phytoestrogens such as lignans, coumestrol, and isoflavonoids are known to be aromatase inhibitors 53. Aromatase is a member of the cytochrome P450 enzyme family that converts androgens (androstenedione and testosterone) into oestrogens (estrone and estradiol, respectively); high levels of this enzyme are associated with breast, adrenal, and prostate cancers.
Phytoestrogens benefits
Numerous epidemiological and clinical studies have now evaluated the relationship between phytoestrogen consumption and human disease outcomes, but the results have not yielded a clear picture as to whether or not these compounds have therapeutic potential 54, 55. Dose, dietary composition, phytoestrogens administered, and duration of use vary considerably across epidemiological studies making them difficult to intercompare. Some feeding trials are limited by markedly small sample sizes, and several widely popularized studies were funded, at least in part, by the manufacturers of soy and soy-based supplements leading to some mistrust. Nonetheless, there is considerable public interest in the legitimacy of the claims being made regarding benefits to heart, bone, breast and menopausal symptoms. Unfortunately, the data supporting these claims are not strong in most cases, thus the degree to which phytoestrogen consumption confers meaningful health benefits remains unresolved (Figure 3).
Figure 3. Summary of the pros and cons of genistein
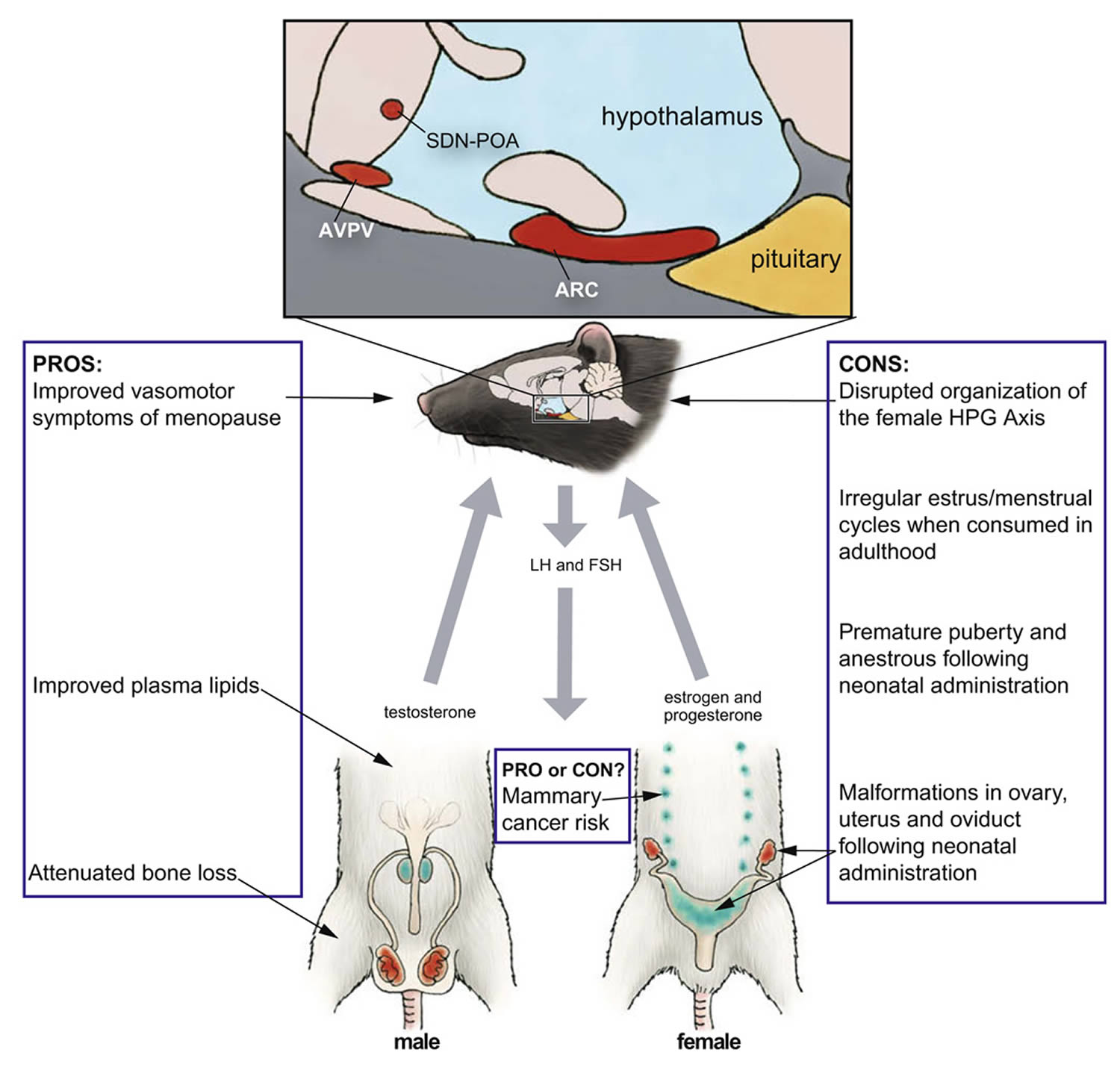
Note: Summary of the pros and cons of genistein. The illustration depicts the rat hypothalamic–pituitary–gonadal (HPG) axis, indicating key components (in red) known to be vulnerable to disruption by genistein administration during critical windows of development. Developmental exposure to genistein can also result in abnormal development of the accessory sex glands (blue). Artwork by Barbara Aulicino. Edited and reprinted by permission of American Scientist, magazine of Sigma Xi, The Scientific Research Society.
[Source 24]In the European Union, any health claim must be pre-approved before it can be used: approval can be obtained via its submission to the European Food Safety Authority (EFSA) in the generic procedure that is intended to produce a list of claims [the so-called list of claims under article 13.1 of the Regulation 56], such as those regarding the role of a nutrient or other substance in growth, development and body functions or psychological and behavioural functions.
The EFSA (European Food Safety Authority) Panel concluded that there was INSUFFICIENT evidence to substantiate these claims:
- In 2012, a scientific opinion on health claims related to soy isoflavones and maintenance of bone mineral density and reduction of vasomotor symptoms associated with menopause 57;
- In 2011, a scientific opinion on health claims related to soy isoflavones and protection of DNA, proteins and lipids from oxidative damage, maintenance of normal blood LDL-cholesterol concentrations, reduction of vasomotor symptoms associated with menopause, maintenance of normal skin tonicity, contribution to normal hair growth, “cardiovascular health”, treatment of prostate cancer and “upper respiratory tract” 58.
Several products from a botanical source (including phytoestrogen) have been already submitted to the EFSA pursuant to article 14.
Examples are:
- In 2008, the EFSA Panel assessed the scientific substantiation of a health claim related to Femarelle® (mixture of DT56a soy derivative and ground flaxseed at a ratio of 3:1, for oral administration; each capsule contains 344 mg soy and 108 mg flaxseed – altogether 430mg powder) and “induces bone formation and increases bone mineral density reducing the risk for osteoporosis and other bone disorders”. The Panel concluded that “a cause and effect relationship has not been established between the consumption of Femarelle® and increased BMD, increased bone formation, or decreased risk of osteoporosis or other bone disorders in post-menopausal women” 59;
- In 2010, the EFSA Panel was asked an opinion on a health claim related to soy protein and reduction of blood cholesterol concentrations. The Panel concluded that “a cause and effect relationship has not been established between the consumption of soy protein and the reduction of LDL-cholesterol concentrations” 60;
- Finally, in 2012, the scientific opinion on a health claim of isolated soy protein and reduction of blood LDL-cholesterol concentrations was, again, negative and stated that “a cause and effect relationship has not been established between the consumption of isolated soy protein (as defined by the applicant) and a reduction in blood LDL-cholesterol concentrations” 61.
Menopausal symptoms
The first widely attributed health benefit of phytoestrogen consumption was relief from vasomotor perimenopausal symptoms, including hot flushes and night sweats. For some women, the severity of these symptoms can markedly diminish their quality of life and interfere with daily activities. Although pharmaceutical hormone replacement therapy is effective in most cases, this option has fallen out of favor because of fears that its use increases the risk of developing breast cancer risk 62, 63. Incidence of vasomotor symptoms is higher in Western countries (70–80% of women) than in Asian countries (10–20%) 64, an observation which has led to the now popularly held belief that soy phytoestrogens may bring relief. Unfortunately, demonstrable evidence for such an association is weak at best, with most clinical trials showing no or minimal relief. One feature that stands out in nearly all studies is a large placebo effect 65. In 2004 the North American Menopause Society issued a position statement which read, in part, “Among nonprescription remedies, clinical trial results are insufficient to either support or refute efficacy for soy foods and isoflavone supplements (from either soy or red clover), black cohosh, or vitamin E 66.” Despite this uncertainty, dietary supplements continue to be popular, particularly among women seeking a “natural” alternative to hormone replacement therapy 67. Recently, a 2016 meta-analysis of clinical trials showed that specific phytoestrogen supplementation led to relief of menopausal symptoms 68. Furthermore, phytoestrogens are becoming progressively common constituents of human diets due to the latest dietary guidelines on substituting animal protein with soy-based foods 69. Therefore, there is an increased interest in the potential health effects of phytoestrogens beyond menopausal symptoms.
Prevention of osteoporosis
Another consequence of aging is the progressive loss of bone-mineral density, a process that accelerates during perimenopause and increases fracture risk. Estrogens help maintain normal bone density, and it has been hypothesized that phytoestrogens are structurally similar to estrogens, can bind to estrogen receptors (ERs) in bone and exert estrogenic actions that could reduce the risk of osteoporosis 70. Most studies examining the impact of phytoestrogens on bone health measure osteocalcin, a metabolic regulatory hormone secreted by osteoblasts, as it is a sensitive biomarker for bone formation 71. Parathyroid hormone (PTH), secreted by the parathyroid glands, plays an important role in calcium and phosphate metabolism. As well as stimulating bone turnover, there is increasing evidence that PTH may also promote bone formation 72.
Results from animal studies, although inconsistent and negative in some cases, are nonetheless encouraging. Numerous phytoestrogens including coumestrol, genistein, daidzein and others have been reported to have bone sparing effects in the rat 73, but efficacy appears to depend on dose, route and duration of administration, and, to some degree, the animal model employed. In ovariectomized post-menopausal monkeys, soy phytoestrogens were ineffective, even after 3 years of intake 74. Evidence for measurable effects in humans is equally mixed. At least one study has found that post-menopausal women consuming high quantities of soy foods have better femoral and/or lumbar spine density compared to women who consume less soy 75. A 2009 meta-analysis of randomized clinical trials conducted in humans, however, found only a weak association between increased consumption of soy isoflavones and improved bone-mineral density, leading the authors to conclude that soy isoflavones were unlikely to meaningfully reduce the risk of osteoporosis 76.
Bone sparing benefits may depend on the ability to bioconvert daidzein to equol. Equol production may also at least partially explain why the beneficial effects of isoflavones observed in laboratory rodents have not been easily recapitulated in humans, because while the most laboratory species (rats, mice and monkeys) consistently produce high levels of equol, only approximately a third to half of the human population appears capable of doing so 77. Equol is a chiral molecule with the natural enantiomer, S-equol having a 13-fold higher relative binding affinity for ERβ than ERα 78. In contrast, the R-enantiomer has a stronger affinity for ERα. S-equol also binds ERβ better than its parent compound, daidzein 78. It is not yet clear which enantiomer has more potent bone sparing effects. In ovariectomized rats, daily consumption of 200 mg/kg racemic equol for 8 weeks moderately elevated femoral calcium concentrations but also increased uterine weight 79. Lower doses also appear to be effective in bone without inducing uterine proliferation, yielding hope that it might be beneficial for human bone without unwanted estrogenic side effects 80. To date, few clinical trials have considered equol production as a potentially important variable, but at least one found that consumption of isoflavones (18 g soy protein powder or 105 mg isoflavone aglycone equivalents) for a year failed to improve bone-mineral density in post-menopausal women, even among equol producers 81. This finding, however, conflicts with prior studies 73 including one which found a 2.4% increase in lumbar spine bone-mineral density among equol producers following ingestion of an isoflavone-rich soy milk over 2 years 82. Dose, duration of therapy and subject age may have contributed to the incongruous results emphasizing the need to understand more about the mechanism by which equol and other phytoestrogens act to enhance bone density. Interest in the potential for equol to improve bone and other aspects of human health remains a hot topic of investigation and a potentially fruitful area of research 83. For many women, adding soy to an already healthful diet may be an appealing choice to help stave off bone loss in mid-life.
Children
Early-life exposure to soy protein formula did not produce any change in osteocalcin and parathyroid hormone (PTH) in a clinical study of 48 children 84. Even though the available data suggest that phytoestrogen intake does not affect bone-related hormones in early stages of life, more studies are needed to clarify this relationship.
Premenopausal women
The reported effects of dietary phytoestrogen on bone health in premenopausal women are inconsistent. Kwak H.S. et al. 85 found an increase in serum osteocalcin after the administration of 120 mg/day of soy-isoflavones for three menstrual cycles. They also observed that high genistein-excretors in the soy group had higher concentrations of osteocalcin, suggesting that individual variation may affect the metabolism and functions of isoflavones 86. However, previous studies report unaltered osteocalcin levels 87, indicating a need for more research on the phytoestrogen effects on bone metabolism in premenopausal women.
Postmenopausal women
After menopause, estrogen concentrations decrease dramatically, triggering a greater risk of osteoporosis 88. Phytoestrogens might improve bone health due to their estrogenic effects, and it has been hypothesized that they could reduce the risk of osteoporosis. Chiechi L.M. et al. 89 and Scheiber M.D. et al. 90 report an increase in osteocalcin concentrations in postmenopausal women who consumed a soy-rich diet for 6 and 3 months, respectively. Although uncorroborated by the majority of studies, these results indicate a stimulation of osteoblast activity and suggest that soy may have beneficial effects on bone health. It has been suggested that longer treatments may be necessary to produce any change in bone metabolism, but to date neither shorter nor longer studies have reported any alterations in osteocalcin related to phytoestrogen intake 8.
In contrast, beneficial effects on bone metabolism through mechanisms of action not involving osteocalcin have been described in healthy postmenopausal women 91. Lambert M.N.T. et al. 91 demonstrated that red clover-derived isoflavones combined with probiotics attenuated estrogen-deficient bone mineral density loss and improved bone turnover even in postmenopausal women with osteopenia. Moreover, a recent meta-analysis and systematic review of randomized control trial with perimenopausal and postmenopausal women concluded that isoflavones can be effective in preserving bone mineral density and attenuating accelerated bone resorption 92. A possible explanation for these contrasting results could be that estrogens are predominantly antiresorptive agents, so the beneficial effects of phytoestrogens may arise from decreased bone resorption by osteoclasts rather than increased bone formation by osteoblasts.
Lastly, administration of isoflavones or genistein alone for 1 to 24 months did not alter parathyroid hormone (PTH) in postmenopausal women 93. Only a cross-sectional study carried out with Chinese women found that postmenopausal women with a high intake of isoflavone had lower serum parathyroid hormone (PTH) levels 94.
Cardiovascular health and the prevention of heart disease
The purported health benefit that has arguably received the greatest attention and, consequently, stimulated the rapid proliferation and adoption of soy foods in Western countries, is a reduced risk of cardiovascular disease. The 1999 approval by FDA of the health claim that daily consumption of soy is effective in reducing the risk of coronary artery disease 95 has undoubtedly cemented the idea in the minds of many that soy is beneficial for human health. It is impossible to walk among US grocery store aisles without spotting this claim which reads, “Diets low in saturated fat and cholesterol that include 25 grams of soy protein a day may reduce the risk of heart disease. One serving of (this food) provides (this amount) of soy protein.” Similar claims and labeling policies quickly followed in other countries including the United Kingdom, Japan, Korea and South Africa. However, because of mounting evidence that such a claim might be spurious, in December of 2007, the FDA announced its intent to reevaluate the data justifying this claim 96. In June 30, 2016 the FDA completed its evaluation of the totality of the current scientific evidence regarding the relationship between soy protein and coronary heart disease and is developing a proposed rule with respect to a health claim authorized in 1999. “The agency has reviewed more than 700 publications identified through literature searches, comments and other information, including information solicited by the FDA in 2007. The FDA plans to publish a proposed rule in the Federal Register and solicit public comments and scientific information concerning the proposal. During the rulemaking process, food manufacturers may continue to use the authorized health claim” 97. Similarly discouraging, the American Heart Association issued a statement in August of 2005 warning that “Earlier research indicating that soy protein as compared with other proteins has clinically important favorable effects on low density lipoprotein (LDL) cholesterol and other cardiovascular disease risk factors has not been confirmed by many studies reported during the past 10 years” 98. A recent review of the animal data also concluded that although marginal benefits have been observed, the impact of soy consumption on LDL “bad cholesterol” levels and other cardiovascular risk factors are smaller than previously hoped 99.
There are numerous risk factors for cardiovascular disease including high blood pressure, obesity, C-reactive protein levels, systemic arterial compliance and the ratio of “good” (high density lipoprotein, HDL) to “bad” (LDL) cholesterol. Of these, only marginally reduced LDL levels are a consistent feature of human and animal studies of soy intervention. The most significant reductions in LDL cholesterol levels are generally small (3% or less) and only seen in individuals with high cholesterol who replace a substantial portion of their animal protein intake with soy, consuming between 40 and 318 mg isoflavones per day 98. For example, daily administration of soy supplements providing 0.7–1.5 mg/kg isoflavones over 5 or 12 weeks did not alter serum lipids in men or women with average cholesterol levels 100, but lowered LDL levels have been reported in hypercholesterolemic women following soy isoflavone therapy 101.
Intriguingly, removal of the isoflavones from the soy protein does not eliminate the modest impact on LDL levels, suggesting that the soy protein itself, rather than the isoflavones, is producing the modest benefit 102. An important consequence of this finding is the implication that use of phytoestrogen supplements, rather than substituting soy protein for animal protein as part of a balanced diet, is unlikely to confer meaningful cardiovascular benefits. Whether or not the individual isoflavones or another aspect of soy protein itself is responsible for producing the modest improvement in cardiovascular health must be disentangled. Regardless, people at risk for heart disease may want to consider replacing at least a portion of their meat intake with soy.
Breast cancer: pro or con ?
Determining if phytoestrogens increase or reduce the risk of developing breast cancer has proven to be one of the most challenging human health impacts to address. It is well established that estrogens promote breast tumorigenesis, and that parameters which increase lifetime estrogen exposure (such as early menarche, short duration breastfeeding, and low parity) are associated with elevated breast cancer risk. Because they bind ERs with relatively high affinity, some researchers and clinicians are concerned that high phytoestrogen intake may increase the risk of carcinogenesis and put breast cancer survivors at risk for reoccurrence. Others have proposed that the opposite is true, citing traditionally low cancer rates in Asia as evidence 103. Depending on the assay used, levels of endogenous estrogen present, life stage, and tumor type, genistein can act as both a proliferative and an antiproliferative agent 104. For example, in vitro, genistein can inhibit proliferation of ER-positive and ER-negative breast cancer cells at high doses (>10 M), but, paradoxically, promote tumor growth at lower, more physiological doses 105. Tamoxifen and other selective estrogen receptor modulators (SERMs) used for breast cancer therapy can also have mixed effects depending on dose and tissue type 106. The SERM-like activity of soy phytoestrogens makes dietary guidelines particularly difficult to issue with confidence.
A relatively large number of studies have taken an epidemiological approach to address these concerns, but the results have differed by region and patient population. A Dutch study comparing plasma isoflavone levels in women with and without breast cancer found that high plasma levels of genistein were associated with a 32% reduction in breast cancer risk 107. Most studies, however, have failed to corroborate such a profoundly beneficial effect of genistein. A meta-analysis, supported in part by the Susan G Koman Breast Cancer Foundation, concluded that, for Asian women, the risk of developing breast cancer drops as soy intake rises. As little as 10 mg of soy per day was sufficient to decrease breast cancer risk by 12% 108. This association was not found for Caucasian women, but average daily isoflavone intake in this group was considerably lower (under 1 mg per day). Thus, it is unclear, if higher intake levels would have been beneficial for Caucasian as well as Asian women. Paradoxically, a different meta-analysis of 18 studies published between 1978 and 2004 found a protective effect of soy in pre-menopausal Caucasian women, but not women of Asian descent 109.
Dietary intervention studies have generally produced negative results. One of the largest found that consumption of 50–100 mg isoflavones per day for 1–2 years did not reduce mammographic density, a biomarker of increased risk 110. Administration of a dietary supplement containing red clover derived isoflavones also failed to alter mammographic breast density after 1 year 111. The impact of soy on breast cancer survivors is also unclear and appears to differ by ethnicity. The most recent study on breast cancer survivors examined 5042 Chinese women aged 20–75, and found that soy intake was significantly associated with a decreased risk of death and/or recurrence 112. These results are consistent with a prior study, also done in Chinese women, which found chemopreventitive effects of soy consumption, particularly among pre-menopausal women 113. As described previously, equol production has emerged as an important variable for achieving bone sparing effects and it may also prove to be an important predictor of cancer protection. An association between equol production and reduced breast cancer risk has been observed in at least one study of Caucasian women 114. Additional studies are needed to further explore the relationship between equol production and breast cancer risk.
Phytoestrogens may have the biggest impact on lifetime risk when exposure occurs prior to puberty and possibly before birth. Although not an initial goal of the study, a Hawaiian research group found an association between high soy intake during early life and increased breast density, a risk factor for breast cancer 110. The study consisted of 220 pre-menopausal women and was designed to determine if consumption of approximately 50 mg of isoflavones over 2 years in adulthood could reduce breast density. This intervention failed but life history data obtained during the process led the authors to conclude that Caucasian women who ate more soy over their lifetime had denser breast tissue than those who did not. This observation is not consistent with an earlier study, which found that, in Chinese women, high intake over a lifetime is directly correlated with reduced risk of cancer 115.
Results from perinatal exposure in animals have also been mixed. For example, one early study of this hypothesis found that rat pups born to mothers that consumed genistein (25 or 250 mg/kg diet) during gestation and lactation developed fewer breast tumors 116. A more recent study, however, found that neonatal, subcutaneous administration of 5 or 50 mg/kg genistein stunted mammary gland development and the animals, particularly those given the higher dose, exhibited abnormal ductal morphology including reduced lobular alveolar development, and focal areas of “beaded” ducts lined with hyperplastic ductal epithelium 117. Subcutaneous administration of a lower dose (0.5 mg/kg genistein), produced the opposite effect. In these animals mammary gland development was advanced and no significant ductal malformations were observed in adulthood suggesting that accelerated differentiation might reduce cancer risk. This biphasic effect of genistein on breast tissue development and differentiation indicates that dose may be an important factor when considering risk. The hypothesis that exposure to soy phytoestrogens early in life can alter the timing and character of breast development is supported by a 2008 cross-sectional study of 694 girls in Israel, which found increased prevalence of breast buds in 2-year old girls fed soy formula as infants 118. It is unclear how this may impact their lifetime risk of developing breast cancer but argues for a more thorough investigation of the possible relationship between early life phytoestrogen exposure, premature thelarche, and breast cancer risk.
Overall, although research in this area has been intense over the past two decades, results from both in vivo and in virto studies have been frustratingly incongruous 119. Recent, comprehensive reviews of the human studies suggest a modest inverse association between risk and high soy intake 108 but this trend is generally not supported by data from the animal literature 120. To date, no clear consensus has been reached on whether or not phytoestrogens are helpful or harmful, or when they might be contraindicated for some groups. Unfortunately, despite the need for guidance, in many published reviews of the topic too many authors shy away from making definitive recommendations and instead suggest that women “discuss the issue with their health care provider.” This directive is unhelpful because it abdicates responsibility to clinicians, who are no more capable of giving informed opinions on the subject than research scientists. Although a myriad of factors such as patient age, hormone receptor status of breast tumors, ethnicity, alcohol consumption, and other dietary habits likely all interact and complicate the potential impact of soy consumption on breast tumor proliferation, movement towards a clear consensus-based set of guidelines is badly needed 121. Given the evidence that adding soy foods to an already healthy diet may have modest but measurable benefits on bone and cardiovascular health, women without serious risk factors for breast cancer or a family history of breast cancer could likely incorporate soy into their diet without significant concern.
Endocrine disrupting properties of phytoestrogens in the adult brain and reproductive tract
In a 2008 clinical case report, physicians at SUNY Downstate Medical Center treated three women (aged 35–56) for a similar suite of symptoms including abnormal uterine bleeding, endometrial pathology and dysmenorrhea. In all three cases, symptoms ameliorated after soy intake was reduced or eliminated, demonstrating that consumption of particularly high isoflavone levels can compromise female reproductive health 122. The youngest of the three had been on a soy-rich diet since age 14 and was experiencing secondary infertility, a condition that resolved and resulted in a pregnancy once she reduced her soy consumption. Isoflavone intake was not quantified, but estimated to exceed 40 g per day in the oldest of the three patients. It remains to be determined if these cases are atypical or sentinels of a legitimate public health concern. Because soy consumption is increasing so rapidly, and so many products now contain soy, along with its isoflavones and other phytoestrogens, this possibility clearly warrants greater attention.
Disruption of endogenous hormone levels and the ovulatory cycle
Animal and human studies evaluating phytoestrogen effects on the adult hypothalamic–pituitary–gonadal (HPG) axis following adult exposures have been fairly consistent and reveal the potential for suppression. Multiple studies have documented the estrogenic activity of phytoestrogens in ovariectomized rodents 123 and, in humans, it is generally accepted that consumption of isoflavones-rich soy foods suppresses circulating estrogen and progesterone levels and can attenuate the preovulatory surge of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) 124. Notably, however, a handful of studies have found no impact of isoflavones on female hormone levels at all. One of the most recent of these followed 34 women consuming 100 mg of isoflavones per day for a year and found no changes in luteal phase levels of estradiol, estrone, progesterone, SHBG, FSH or LH at months 1, 3, 6 or 12 125. Another also failed to find altered circulating gonadal hormone levels in 14 women given soy cookies containing 52 mg of isoflavones or isoflavone-free cookies for 5 days. Interestingly, at least one study found suppressed luteal estradiol levels following increased soy intake, but only in women of Asian descent 124, indicating ethnicity could be an underappreciated factor when considering the potential human health effects of soy isoflavones. With such small samples sizes in all of these studies, however, they may have been too underpowered to detect effects. A 2009 meta-analysis concluded that, in pre-menopausal women, isoflavone intake increases cycle length and suppresses LH and FSH levels 126. This conclusion is consistent with the clinical case report from SUNY Downstate Medical Center 122 and indicates that use of soy foods should be approached with caution in women attempting to become pregnant or experiencing menstrual cycle irregularities.
Behavior
Our research has revealed that isoflavone intake can suppress female sex behavior in rats. Administration of a commercially prepared phytoestrogen supplement to adult female rats, at a dose that results in serum levels between those seen in Western and Asian (human) adults, attenuated lordosis to the same degree as the SERM tamoxifen 127. The supplement treated group displayed significantly fewer proceptive behaviors than the tamoxifen treated group, demonstrating the potential for soy isoflavones to suppress sexual motivation. Intriguingly, administration of genistein alone did not recapitulate these effects on sexual behavior [205] suggesting that a compound, or mixture of compounds, other than genistein was responsible for the suppressed sexual behavior. Studies using ERαKO mice have shown that ERα is required for the normal expression of both male and female sexual behavior 128 indicating that activity through ERα may be mediating this behavioral change.
Other behaviors may be affected as well including social, aggressive, and anxiety-related behaviors. Increased aggression and circulating testosterone levels have been reported in male Syrian hamsters maintained for 5 weeks on the soy-rich Purina 5001 diet compared to control animals fed a soy-free diet 129. Animals on the phytoestrogen- rich diet also had lower vasopressin receptor (V1a) expression in the lateral septum but higher V1a expression in lateral hypothalamus indicating that the altered behavior might result from changes within vasopressin signaling pathways. Similarly, male rats maintained on a diet containing 150 µg/g genistein and daidzein displayed increased anxiety and elevated stress-induced plasma vasopressin and corticosterone levels 130. Elevated hypothalamic vasopressin has also been reported in rats fed a diet containing 1250 ppm genistein across the lifespan 131. Anxiolytic effects of phytoestrogen-rich diets have also been reported in gonadally intact male and female rats exposed over their entire lifetimes but not when administered briefly in adulthood 132. Phytoestrogen intake can also affect behavior in non-human primates. Male cynomolgus monkeys fed soy protein isolate containing 1.88 mg isoflavones/g protein over 18 months demonstrated higher frequencies of intense aggressive (67% higher) and submissive (203% higher) behaviors as well as a decreased proportion of time (68% reduction) spent in physical contact with other monkeys 133.
Evidence for endocrine disruption during development
A possibility of increasing concern is that phytoestrogens may interfere with the organizational role of estrogen in the developing brain and reproductive system. Regardless of animal model used, manipulation of estrogen during specific critical windows of development throughout gestation and early infancy leads to a myriad of adverse health outcomes including malformations in the ovary, uterus, mammary gland and prostate, early puberty, reduced fertility, disrupted brain organization, and reproductive tract cancers 134. These effects mirror some very disturbing public health trends in Western nations. For example, in the United States and Europe median age at menarche, first breast development, and sexual precocity has steadily advanced, especially among minority populations 135. Similar trends have been documented among children adopted from developing countries by Western parents 136. There are also indications that female fecundity is declining, even among young women, although the rate and degree to which this is occurring has been difficult to quantify. Among men, sperm counts in the United States and Europe appear to have declined by roughly half over the past 50 years 137. In Demark, it is now estimated that more than 10% of men have sperm counts in the infertile range and up to 30% are in the subfertile range 138. Rates of testicular cancer also appear to be increasing. Another study showed that infant boys born to vegetarian mothers had increased incidence of hypospadias (malformation of the male external genitalia) 139 suggesting that dietary components (perhaps phytoestrogens) cross the placenta and cause adverse effects on the developing fetus. The causes of these reproductive health trends are likely complex and multi-faceted, but rapidity of the increase in reproductive and behavioral disorders suggests an environmental, endocrine disrupting component 140. Whether or not isoflavone phytoestrogens could be one such component is now the subject of rigorous debate and has caught the attention of public health officials in the US and abroad.
Soy-based infant formulas: prevalence and phytoestrogen content
Isoflavones can pass from mother to fetus through the placenta, and have been found in human umbilical cord blood and amniotic fluid at levels comparable to concentrations seen in maternal plasma, indicating that fetal exposure is possible 141. These levels are considerably lower, however, than blood levels in infants exclusively fed soy formula. Initially developed as an alternative to bovine milk formulas for babies with a milk allergy, use of soy infant formula in the US has steadily risen in popularity. An estimated 25% of US infants, approximately one million each year, are now raised on soy formula, largely because of perceived health benefits or to maintain a vegetarian lifestyle, rather than concerns about cow milk allergies, colic, or other health concerns 142. The widespread prevalence of popular media articles touting the beneficial effects of soy have undoubtedly contributed to its selection by mothers trying to make the most healthful choice for their babies.
Total isoflavone content in soy infant formula varies widely due to environmental and genetic differences between batches and sources, but is consistently higher than in most other food sources 143. Infants on soy formula consume approximately 6–9 mg isoflavones per kg body weight per day, an amount, when adjusted for body weight, that is up to seven times higher than for adults meeting the FDA soy consumption guideline, or Asians consuming a traditional soy-based diet (0.3–1.2 mg/kg per day) 143. It was initially thought that, because the gastrointestinal tract of infants is undeveloped compared to adults, they would not be able to completely absorb the isoflavones. At least one study, however, reports that infants as young as 4 weeks can digest, absorb and excrete isoflavones as effectively as adults 144. Moreover, plasma isoflavone levels are an order of magnitude higher in infants than adults, even when levels of intake are similar 145. Infants fed soy formula have circulating phytoestrogen concentrations of approximately 1000 ng/ml, 13,000–22,000 times higher than their own endogenous estrogen levels, 50–100 times higher than estradiol levels in pregnant women and 3000 times higher than estradiol levels at ovulation 145. These blood levels are high enough to produce many of the physiological effects observed in research animals and human adults. In addition, they are at least a level of magnitude higher than those reported for other endocrine disruptors including BPA and the phthalates 134. A recent prospective study in human infants observed that female infants on soy-based infant formulas exhibit estrogenized vaginal epithelium at times when their breast fed or cow based formula counterparts did not, suggesting estrogenic activity of the soy infant formula 146. Determining if use of soy infant formulas can have long term reproductive health effects is a public health imperative.
Evidence for long term health consequences of soy infant formula in humans
The question of whether or not soy formula is safe has been extensively debated across the globe for more than a decade, and a litany of review articles and position papers have been published on the subject 143. The National Toxicology Program recently completed its most recent safety assessment of soy infant formula available at 147 and concluded there is “minimal concern for adverse developmental effects.” To put this classification in context, the NTP uses a 5 level scale and this is level 2 (with 5 being the most serious level of concern). It is the same levels of concern initially expressed for BPA until mounting evidence for wide ranging health effects pressed the FDA to elevate that advisory to “some concern” in January, 2010. The isoflavone phytoestrogens genistein and daidzien, as well as many others, have far greater relative binding affinities for both ERα and ERβ than BPA, raising concern that these compounds pose an underappreciated threat to infant development.
In 2008 the American Academy of Pediatricians (AAP) concluded that in terms of nutritional quality “isolated soy protein-based formula has no advantage over cow milk protein-based formula” and that soy formula has “no proven value in the prevention or management of infantile colic or fussiness” but stopped short of recommending against its use. Internationally, use of soy formula is viewed more cautiously than in the US. For example, the United Kingdom, Australia and New Zealand all caution against indiscriminate use 148. Other nations offer it only by prescription and, consequently, use of soy formula is customarily lower in these countries. These types of advisories are confusing for parents and have done little to quash the idea that soy formula is a healthful alternative to breastfeeding.
An apparent lack of notable long term effects is one reason why so many consumers, clinicians and public health agencies consider regular use of soy formula to be safe, even beneficial. This near absence of documented effects in soy-fed infants is not entirely reassuring, however, because although soy infant formula has been widely available for more than four decades, surprisingly little work has been done regarding its potential long term effects on reproduction, fertility or behavior. Historically, epidemiological studies have mainly focused on nutritional status, growth parameters, and impacts on the thyroid system because soy has long been recognized to induce hypothyroidism and goiter when not counteracted with elevated iodine intake 149. Very few have explored the possibility that soy formula use can impact reproductive development or function and those which have, are hampered by insufficient sample sizes and the absence of appropriately sensitive measures. The task is not easy because, as with many endocrine disrupting compounds, isoflavone effects, if present, may not manifest for years, even decades, and are likely mild enough to escape clinical detection.
The earliest evidence for reproductive health effects came from two studies, conducted in the mid 1980’s, which associated neonatal phytoestrogen exposure with thelarche before age 2 in a population of Puerto Rican girls. A number of confounding factors, however, including the consumption of chicken that had been fattened with potent estrogens, make the data problematic and difficult to interpret 150. Within the last decade, a retrospective cohort study of 952 women found that young women fed soy-based infant formula (248 women) as part of a controlled, University of Iowa feeding study, reported longer menstrual bleeding and menstrual discomfort than those who were fed a non-soy-based formula (563 women) 151. At the time the study was conducted, the women were too young to comprehensively examine pregnancy or fertility outcomes, but, now that nearly a decade has past, this area is ripe for reevaluation. Most recently, soy formula consumption has been linked to a greater risk of developing uterine fibroids 152. The study population included over 19,000 women enrolled in the Sister Study, making it one of the largest and most highly powered epidemiological evaluations of the long term reproductive health impacts of soy formula.
When considering the potential safety of soy formula, one argument that frequently comes up is that Asian populations have been consuming soy for a long time, with no obvious consequences. This argument fails to recognize, however, that intake levels between Asians consuming a traditional soy-rich diet and Caucasians eating a typical “Western” diet differ dramatically over the lifespan. This temporal divergence may explain why there appear to be differences in both the pros and cons of phytoestrogen exposure between the two populations. In Asian populations, soy consumption is high across the entire lifespan, except for a brief 6–8 month neonatal breastfeeding window. In Westerners feeding their babies soy infant formula the pattern is just the opposite, and the highest consumption levels occur in the first year of life then drop to near zero. In Asia, soy is consumed mostly in the form of tofu, tempeh, and other unprocessed foods, not as dietary supplements or products enriched with soy protein isolate. Asian populations also eat considerably higher levels of seafood and low levels of animal fat than Western populations. These variables make the two populations quite distinct in terms of lifestyle, dietary habits, and lifetime phytoestrogen exposure. Thus, phytoestrogen effects may differ between the two groups, a possibility that should be taken into account when interpreting epidemiological data.
Phytoestrogens side effects
As Selective Estrogen Receptor Modulators (SERMs), the effects of phytoestrogens on the endometrium warrant vigilant scrutiny. As a matter of fact, the risk of endometrial hyperplasia and carcinoma appeared increased from clinical trials 153. The most updated meta-analysis performed on 174 randomized clinical trials was designed to compare side effects of phytoestrogens with placebo or no treatment.
It was shown that phytoestrogen supplements had only a moderately elevated rates of gastrointestinal side effects such as abdominal pain as well as myalgia and sleepiness 154. Regional difference was found, with Asian studies showing higher side effect rates than western studies. Side effects were also more common in women over age 55. No association emerged between duration of study and incidence of side effects suggesting no cumulative dose effect with time. Although these key findings are reassuring, the long-term safety profile is still largely unknown. In addition to routine reporting of suspected adverse reactions, active pharmacovigilance activities should be encouraged to clarify the pattern of use and perception of both benefit and risks by women.
The impact of phytoestrogens can vary according to the life stage 8. There is particular concern about how phytoestrogens may affect pregnant women, as this has been poorly studied 8. Although phytoestrogens transfer from maternal blood to the fetus, no effects have been observed in early life. Nor have endocrine changes been found in infants fed with soy formula, except in a retrospective study carried out in the first year of life of infants with congenital hypothyroidism, which reported an increase of thyroid stimulating hormone (TSH) but no conclusive effects on thyroid function. Congenital hypothyroidism is an endocrine disorder that is caused by under-activity of the thyroid gland, which results in low levels of thyroid hormones. These hormones play an important role in body metabolism and their deficiency can cause intellectual and physical disability. Consumption of phytoestrogens in conditions of insufficient iodine and hypothyroidism may negatively affect thyroid function and favor endocrine imbalance, although such effects have not been observed in euthyroid (normal) individuals living in areas with enough supply of iodine. In later stages of childhood, an increase of androgens and decrease of estrogens associated with dietary phytoestrogens have been observed in girls and boys, respectively.
Phytoestrogens summary
In general, the available evidence for an association between dietary phytoestrogens and endocrine biomarkers is inconclusive 8. The disparity in results may be due to differences in the type and concentration of the compounds administered and the variety of matrices, which could influence phytoestrogen bioavailability and consequently the effect on hormonal function. Also, while most studies analyze circulating hormones, others report the urinary excretion of metabolites. There is a clear need for further carefully designed studies to elucidate the effects of phytoestrogen consumption on the endocrine system.
Phytoestrogens are intriguing because, although they behave similarly to numerous synthetic compounds in laboratory models of endocrine disruption, society embraces these compounds at the same time it rejects, often with vigor, use of synthetic endocrine disruptors in household products. Results from human studies suggest that phytoestrogens may lower the risk of osteoporosis, some cardiometabolic diseases, cognitive dysfunction, breast and prostate cancer, and menopausal symptoms by modulating the endocrine system 8. However, some authors describe phytoestrogens as endocrine disruptors and believe their beneficial effects have been overestimated 15, 14, 16. This ambiguity could be partially due to the variability of published studies, as the beneficial or harmful effects of phytoestrogens depend on the exposure (type, amount consumed, and bioavailability), ethnicity, hormonal status (age and sex and physiological condition), and health status of the consumer 15, 14, 16.
Preclinical studies have suggested that phytoestrogens influence sexual function and the incidence of cancer associated with the reproductive system such as ovarian and breast cancer 155, but the results of cross-sectional studies and clinical trials are conflicting 156. In addition to factors such as dose, type, and bioavailability, the effects of phytoestrogens on sexual function could also depend on the life stage of the consumer.
Recent studies have pointed to a protective role of estrogens in prostate cancer development and progression, alone or in synergy with androgens 157. Several studies have focused on the beneficial effect of soy isoflavones, specifically genistein and daidzein, as these components can act as weak estrogens. A 6-month randomized controlled study evaluating the effects of isoflavone on men at high risk of developing advanced prostate cancer found an increase in concentrations of the estrogen hormones estrone (E1), E2, 2-hydroxi-estradiol (2-OH-E2), and 16α-OH-E1. An increase in the 2:16α-OH-E1 ratio was also reported, which is related to a reduced risk of estrogen-mediated cancer. No differences were observed for 2-methoxyestradiol (2-ME2), 1-methoxyestrone (2-ME1), E3 and 2-OH-E1 158. Conversely, Bylund A. et al. 159 reported that levels of estradiol (E2), follicle stimulating hormone (FSH) and luteinizing hormone (LH) in prostate cancer patients remained unaltered after a 3-week rye bran bread intervention.
In premenopausal women, usually studied separately from postmenopausal women, uncertain results have been obtained regarding sex hormones, breast cancer protection, and bone remodeling 8. Nor has evidence been provided for phytoestrogens affecting insulin-like growth factor (IGF) levels. Whereas no significant changes in thyroid function were observed, a decrease of free-triiodothyronine (T3) was found in healthy young females. Among stress response-related hormones, no significant changes in cortisol are described in healthy women or in those at cardiometabolic risk, but a lower production of cortisol is reported in equol-excretors. In postmenopause, the results reported for sex hormones are also ambiguous. However, possible goitrogenic activity derived from phytoestrogen consumption opens up a path for future research. Apart from that, an ameliorative effect has been observed in the cardiometabolic profile of hyperinsulinemic patients, individuals with metabolic syndrome and diabetes. Regarding bone remodeling, the effects of phytoestrogens on osteocalcin concentrations are unclear, and their beneficial impact may arise instead from reducing bone resorption by osteoclasts. The results obtained for parathyroid hormone (PTH) and insulin-like growth factor (IGF) are unconvincing, precluding the drawing of any conclusions.
While the potentially beneficial effects of phytoestrogen consumption have been eagerly pursued, and frequently overstated, the potentially adverse effects of these compounds are likely underappreciated. The opposite situation exists for synthetic endocrine disruptors, most of which have lower binding affinities for classical estrogen receptors than any of the phytoestrogens but can sometime produce similar biological effects. Animal data reveal that the isoflavones have a wide range of molecular, cellular and behavioral effects at doses and plasma concentrations attainable in humans. In vivo isoflavone responses have been reported for a wider range of tissues and processes than the endpoints generally used to evaluate most synthetic endocrine disrupting compounds 160, yet only minimal concern has been raised about their increasing use. Infants fed soy formula have the highest exposure to any nonpharmacological source of estrogen-like compounds, yet scientists know virtually nothing about how the use of these phytoestrogen-rich formulas might impact their future reproductive health. Although relative few adverse effects have been detected, that may simply be because a surprising paucity of large-scale, comprehensive studies have been undertaken to address this issue, especially in boys. That may change in the near future because the health effects of endocrine disrupting compounds in general are receiving more attention from public health agencies, and the public at large.
As with many other compounds, like alcohol or caffeine, there are many pros and cons associated with moderate soy intake. Consumers should be aware that soy contains endocrine disrupting compounds and make dietary choices accordingly. For a typical consumer, alarm over soy products is likely unnecessary but so is the belief that a soy-rich diet will alleviate all ills. Women who are pregnant, nursing, or attempting to become pregnant should use soy foods with caution and be aware that soy formula may not be the best option for their babies. Older individuals, especially those with high cholesterol, may experience modest benefits including improved bone and cardiovascular health, and perhaps a decreased risk of carcinogenesis. Moderation is likely key and the incorporation of real foods, as opposed to supplements or processed foods to which soy protein is added, is probably essential for maximizing health benefits. Finally, the relative importance of the soy protein itself, compared to the isoflavones, on health outcomes such as lipid levels, reduced risk of carcinogenesis, and fracture risk must be resolved. If something other than the isoflavone phytoestrogens is producing the mild but measurable health benefits of soy foods, this would considerably help shape the development of dietary guidelines for both adults and children.
References- Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular Effects of Phytoestrogens and Alternative Menopausal Hormone Therapy in Cardiovascular Disease. Mini Reviews in Medicinal Chemistry. 2012;12(2):149-174. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3288319
- Production and actions of estrogens. Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. N Engl J Med. 2002 Jan 31; 346(5):340-52. https://www.ncbi.nlm.nih.gov/pubmed/11821512/
- Desmawati, D., & Sulastri, D. (2019). Phytoestrogens and Their Health Effect. Open access Macedonian journal of medical sciences, 7(3), 495–499. https://doi.org/10.3889/oamjms.2019.044
- Bedell S, Nachtigall M, Naftolin F. The pros and cons of plant estrogens for menopause. J Steroid Biochem Mol Biol. 2014 Jan;139:225-36. doi: 10.1016/j.jsbmb.2012.12.004
- Effects of estrogen plus progestin on health-related quality of life. Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, Aragaki AK, Shumaker SA, Brzyski RG, LaCroix AZ, Granek IA, Valanis BG, Women’s Health Initiative Investigators. N Engl J Med. 2003 May 8; 348(19):1839-54.
- Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels. Smiley DA, Khalil RA. Curr Med Chem. 2009; 16(15):1863-87.
- Genomic and nongenomic effects of estrogen in the vasculature. Mendelsohn ME. Am J Cardiol. 2002 Jul 3; 90(1A):3F-6F.
- Domínguez-López, I., Yago-Aragón, M., Salas-Huetos, A., Tresserra-Rimbau, A., & Hurtado-Barroso, S. (2020). Effects of Dietary Phytoestrogens on Hormones throughout a Human Lifespan: A Review. Nutrients, 12(8), 2456. https://doi.org/10.3390/nu12082456
- Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, Shiau AC, Goldstein J, Davis P, Perez-Stable EJ. Am J Epidemiol. 2001 Sep 1; 154(5):434-41. https://www.ncbi.nlm.nih.gov/pubmed/11532785/
- Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29(2):95–120.
- How to reduce the risk factors of osteoporosis in Asia. Kao PC, P’eng FK. Zhonghua Yi Xue Za Zhi (Taipei). 1995 Mar; 55(3):209-13. https://www.ncbi.nlm.nih.gov/pubmed/7780876/
- Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med. 2001;161(9):1161–1172.
- Yamori Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp Clin Cardiol. 2006;11(2):94–98.
- Bennetau-Pelissero C. Risks and benefits of phytoestrogens: where are we now? Curr Opin Clin Nutr Metab Care. 2016 Nov;19(6):477-483. doi: 10.1097/MCO.0000000000000326
- Patisaul, H. B., & Jefferson, W. (2010). The pros and cons of phytoestrogens. Frontiers in neuroendocrinology, 31(4), 400–419. https://doi.org/10.1016/j.yfrne.2010.03.003
- Rietjens, I., Louisse, J., & Beekmann, K. (2017). The potential health effects of dietary phytoestrogens. British journal of pharmacology, 174(11), 1263–1280. https://doi.org/10.1111/bph.13622
- Phytoestrogen supplement use by women. Kurzer MS. J Nutr. 2003 Jun; 133(6):1983S-1986S.
- The physiological actions of isoflavone phytoestrogens. Pilšáková L, Riečanský I, Jagla F. Physiol Res. 2010; 59(5):651-64. http://www.biomed.cas.cz/physiolres/pdf/59/59_651.pdf
- Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular Effects of Phytoestrogens and Alternative Menopausal Hormone Therapy in Cardiovascular Disease. Mini Reviews in Medicinal Chemistry. 2012;12(2):149-174. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3288319/
- Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N. Nutr Cancer. 2006; 54(2):184-201. https://www.ncbi.nlm.nih.gov/pubmed/16898863/
- Wang H, Murphy PA. Isoflavone Content in Commercial Soybean Foods. J. Agric. Food Chem. 1994;42(8):1666–1673
- Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65(8):995–1016. https://www.ncbi.nlm.nih.gov/pubmed/15110680
- Mazur W. Phytoestrogen content in foods. Baillieres Clin Endocrinol Metab. 1998;12(4):729–742. https://www.ncbi.nlm.nih.gov/pubmed/10384822
- Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Frontiers in neuroendocrinology. 2010;31(4):400-419. doi:10.1016/j.yfrne.2010.03.003. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3074428/
- Meagher LP, Bentz EK. Assessment of data on the lignan content of foods. J Food Compos Anal. 2000;13(6):935–947.
- The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. Kuijsten A, Arts IC, van’t Veer P, Hollman PC. J Nutr. 2005 Dec; 135(12):2812-6.
- ChungJa J, VasanthaRupasinghe H. Tables of Isoflavone, Coumestan, and Lignan Data. In Phytoestrogens and Health ed. Edited by Anderson J.J.B. Sarwar Gilani G. AOCS Publishing. 2002.
- Intestinal metabolism of rye lignans in pigs. Glitsø LV, Mazur WM, Adlercreutz H, Wähälä K, Mäkelä T, Sandström B, Bach Knudsen KE. Br J Nutr. 2000 Oct; 84(4):429-37.
- Enterolignans. Raffaelli B, Hoikkala A, Leppälä E, Wähälä K. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Sep 25; 777(1-2):29-43.
- Stansbury JE. Phytoestrogen Review.
- Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Prasad K. Mol Cell Biochem. 1997 Mar; 168(1-2):117-23.
- Martin M, Dewick PM. Biosynthesis of pterocarpan isoflavan and coumestan metabolites of Medicago sativa. The role of an isoflav-3-ene. Phytochemistry. 1980;19:2341–2346.
- Naturally occurring oestrogens in foods–a review. Price KR, Fenwick GR. Food Addit Contam. 1985 Apr-Jun; 2(2):73-106.
- Fristsche S, Steinhart H. Occurrence of Hormonally Active Compounds in Food. A Review. Eur. Food Res. Techn. 1999;209:153–179.
- Benefits of resveratrol in women’s health. Bagchi D, Das DK, Tosaki A, Bagchi M, Kothari SC. Drugs Exp Clin Res. 2001; 27(5-6):233-48.
- Hormonal and genotoxic activity of resveratrol. Schmitt E, Lehmann L, Metzler M, Stopper H. Toxicol Lett. 2002 Dec 15; 136(2):133-42.
- D’Alessandro TL, Boersma-Maland BJ, Peterson TG, Sfakianos J, Prasain JK, Patel RP, Darley-Usmar VM, Botting NP, Barnes S. Metabolism of phytoestrogen conjugates. Methods Enzymol. 2005;400:316–342.
- Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131(4 Suppl):1362S–1375S.
- Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. King RA, Bursill DB. Am J Clin Nutr. 1998 May; 67(5):867-72.
- Plasma concentrations of phyto-oestrogens in Japanese men. Adlercreutz H, Markkanen H, Watanabe S. Lancet. 1993 Nov 13; 342(8881):1209-10.
- Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Setchell KD. Am J Clin Nutr. 1998 Dec; 68(6 Suppl):1333S-1346S.
- Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Duncan AM, Merz-Demlow BE, Xu X, Phipps WR, Kurzer MS. Cancer Epidemiol Biomarkers Prev. 2000 Jun; 9(6):581-6. http://cebp.aacrjournals.org/content/9/6/581.long
- Dietary phytoestrogens. Kurzer MS, Xu X. Annu Rev Nutr. 1997; 17():353-81.
- A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Glazier MG, Bowman MA. Arch Intern Med. 2001 May 14; 161(9):1161-72.
- Urinary isoflavone kinetics: the effect of age, gender, food matrix and chemical composition. Faughnan MS, Hawdon A, Ah-Singh E, Brown J, Millward DJ, Cassidy A. Br J Nutr. 2004 Apr; 91(4):567-74.
- Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. Setchell KD, Cole SJ. J Agric Food Chem. 2003 Jul 2; 51(14):4146-55.
- Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. Milligan SR, Kalita JC, Heyerick A, Rong H, De Cooman L, De Keukeleire D. J Clin Endocrinol Metab. 1999 Jun; 84(6):2249-52.
- Di Donato, M., Giovannelli, P., Cernera, G., Di Santi, A., Marino, I., Bilancio, A., Galasso, G., Auricchio, F., Migliaccio, A., & Castoria, G. (2015). Non-genomic androgen action regulates proliferative/migratory signaling in stromal cells. Frontiers in endocrinology, 5, 225. https://doi.org/10.3389/fendo.2014.00225
- Selective estrogen receptor modulators and phytoestrogens. Oseni T, Patel R, Pyle J, Jordan VC. Planta Med. 2008 Oct; 74(13):1656-65.
- Genistein genotoxicity: critical considerations of in vitro exposure dose. Klein CB, King AA. Toxicol Appl Pharmacol. 2007 Oct 1; 224(1):1-11.
- Biological properties of genistein. A review of in vitro and in vivo data. Polkowski K, Mazurek AP. Acta Pol Pharm. 2000 Mar-Apr; 57(2):135-55.
- Hutchins AM, Slavin JL. In Flaxseed in Human Nutrition . 2nd ed. Champaign Illinois.: Edited by Thompson L.U.C.S.C. AOCS Press. ; 2003. Effects of flaxseed on sex hormone metabolism. pp. 126–149.
- The binding of lignans, flavonoids and coumestrol to CYP450 aromatase: a molecular modelling study. Karkola S, Wähälä K. Mol Cell Endocrinol. 2009 Mar 25; 301(1-2):235-44.
- Phytoestrogens: end of a tale? Sirtori CR, Arnoldi A, Johnson SK. Ann Med. 2005; 37(6):423-38. https://www.ncbi.nlm.nih.gov/pubmed/16203615/
- Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Cassidy A, Albertazzi P, Lise Nielsen I, Hall W, Williamson G, Tetens I, Atkins S, Cross H, Manios Y, Wolk A, Steiner C, Branca F. Proc Nutr Soc. 2006 Feb; 65(1):76-92. https://www.ncbi.nlm.nih.gov/pubmed/16441947/
- The european parliament and the council of the european union. regulation (ec) no 1924/2006 of the european parliament and of the council.
- EFSA Panel on Dietetic Products N..A.N. . Scientific Opinion on the substantiation of health claims related to soy isoflavones and maintenance of bone mineral density (ID 1655) and reduction of vasomotor symptoms associated with menopause [ID 1654. 1704. 2140. 3093. 3154. 3590) (further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006.
- EFSA Panel on Dietetic Products N..A.N. . Scientific Opinion on the substantiation of health claims related to soy isoflavones and protection o DNA proteins and lipids from oxidative damage (ID 1286. 4245). maintenance of normal blood LDL-cholesterol concentrations (ID 1135. 1704a, 3093a). reduction of vasomotor symptoms associated with menopause (ID 1654. 1704b. 2140. 3093b. 3154. 3590). maintenance of normal skin tonicity (ID 1704a) contribution to normal hair growth (ID 1704a 4254) “cardiovascular health” (ID 3587) treatment of prostate cancer (ID 3588) and “upper respiratory tract” (ID 3589) pursuant to Article 13(1) of Regulation. (EC) No 1924/20061.
- Panel on Dietetic Products N..A. . Scientific substantiation of a health claim related to “Femarelle®” and “induces bone formation and increases bone mineral density reducing the risk for osteoporosis and other bone disorders” pursuant to Article 14 of the Regulation (EC) No 1924/2006.
- EFSA Panel on Dietetic Products N..A. Scientific Opinion on the substantiation of a health claim related to soy protein and reduction of blood cholesterol concentrations pursuant to Article 14 of the Regulation (EC) No 1924/2006.
- EFSA Panel on Dietetic Products N..A. . Scientific Opinion on the substantiation of a health claim related to isolated soy protein and reduction of blood LDL cholesterol concentrations ursuant to Article 14 of Regulation (EC) No 1924/2006.
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427.
- Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491.
- Kurzer MS. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology. 2008;16:227–229.
- Kurzer MS. Phytoestrogen supplement use by women. J. Nutr. 2003;133:1983S–1986S.
- Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11:11–33. https://www.ncbi.nlm.nih.gov/pubmed/14716179
- Speroff L. Alternative therapies for postmenopausal women. Int. J. Fertil. Womens Med. 2005;50:101–114.
- Franco OH, Chowdhury R, Troup J, Voortman T, Kunutsor S, Kavousi M, Oliver-Williams C, Muka T. Use of Plant-Based Therapies and Menopausal Symptoms: A Systematic Review and Meta-analysis. JAMA. 2016 Jun 21;315(23):2554-63. doi: 10.1001/jama.2016.8012
- Wocławek-Potocka, I., Mannelli, C., Boruszewska, D., Kowalczyk-Zieba, I., Waśniewski, T., & Skarżyński, D. J. (2013). Diverse effects of phytoestrogens on the reproductive performance: cow as a model. International journal of endocrinology, 2013, 650984. https://doi.org/10.1155/2013/650984
- Chiang SS, Pan TM. Beneficial effects of phytoestrogens and their metabolites produced by intestinal microflora on bone health. Appl Microbiol Biotechnol. 2013 Feb;97(4):1489-500. doi: 10.1007/s00253-012-4675-y
- Seibel MJ. Biochemical markers of bone remodeling. Endocrinol Metab Clin North Am. 2003 Mar;32(1):83-113, vi-vii. doi: 10.1016/s0889-8529(02)00077-4
- Goltzman D. Physiology of Parathyroid Hormone. Endocrinol Metab Clin North Am. 2018 Dec;47(4):743-758. doi: 10.1016/j.ecl.2018.07.003
- Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro amd in vivo, human observational, and dietary intervention studies. Am. J. Clin. Nutr. 2003;78:593S–609S.
- Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J. Clin. Endocrinol. Metabol. 2003;88:4362–4370.
- Yamori Y, Moriguchi EH, Teramoto T, Miura A, Fukui Y, Honda KI, Fukui M, Nara Y, Taira K, Moriguchi Y. Soybean isoflavones reduce postmenopausal bone resorption in female Japanese immigrants in Brazil: a ten-week study. J. Am. Coll. Nutr. 2002;21:560–563.
- Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone. 2009;44:948–953.
- Lampe J, Karr S, Hutchins A, Slavin J. Urinary equol excretion with a soy challenge: influence of habitual diet. PSEBM. 1998;217:335–339.
- Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004;12:1559–1567.
- Legette LL, Martin BR, Shahnazari M, Lee WH, Helferich WG, Qian J, Waters DJ, Arabshahi A, Barnes S, Welch J, Bostwick DG, Weaver CM. Supplemental dietary racemic equol has modest benefits to bone but has mild uterotropic activity in ovariectomized rats. J. Nutr. 2009;139:1908–1913.
- Mathey J, Mardon J, Fokialakis N, Puel C, Kati-Coulibaly S, Mitakou S, Bennetau-Pelissero C, Lamothe V, Davicco MJ, Lebecque P, Horcajada MN, Coxam V. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: the case for equol. Osteopor. Int. 2007;18:671–679.
- Kenny AM, Mangano KM, Abourizk RH, Bruno RS, Anamani DE, Kleppinger A, Walsh SJ, Prestwood KM, Kerstetter JE. Soy proteins and isoflavones affect bone mineral density in older women: a randomized controlled trial. Am. J. Clin. Nutr. 2009;90:234–242.
- Lydeking-Olsen E, Jensen JBE, Setchell KDR, Daamhus M, Jensen TH. Isoflavone-rich soymilk prevents bone-loss in the lumbar spine of postmenopasual women. A 2 year study. J. Nutr. 2002;132:581S.
- Lampe JW. Is equol the key to the efficacy of soy foods? Am J. Clin. Nutr. 2009;89:1664S–1667S.
- Giampietro PG, Bruno G, Furcolo G, Casati A, Brunetti E, Spadoni GL, Galli E. Soy protein formulas in children: no hormonal effects in long-term feeding. J Pediatr Endocrinol Metab. 2004 Feb;17(2):191-6. doi: 10.1515/jpem.2004.17.2.191
- Kwak, H. S., Park, S. Y., Kim, M. G., Yim, C. H., Yoon, H. K., & Han, K. O. (2009). Marked individual variation in isoflavone metabolism after a soy challenge can modulate the skeletal effect of isoflavones in premenopausal women. Journal of Korean medical science, 24(5), 867–873. https://doi.org/10.3346/jkms.2009.24.5.867
- Zittermann A, Geppert J, Baier S, Zehn N, Gouni-Berthold I, Berthold HK, Reinsberg J, Stehle P. Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur J Nutr. 2004 Apr;43(2):100-8. doi: 10.1007/s00394-004-0447-5
- Drake MT, Clarke BL, Lewiecki EM. The Pathophysiology and Treatment of Osteoporosis. Clin Ther. 2015 Aug;37(8):1837-50. doi: 10.1016/j.clinthera.2015.06.006
- Chiechi LM, Secreto G, D’Amore M, Fanelli M, Venturelli E, Cantatore F, Valerio T, Laselva G, Loizzi P. Efficacy of a soy rich diet in preventing postmenopausal osteoporosis: the Menfis randomized trial. Maturitas. 2002 Aug 30;42(4):295-300. doi: 10.1016/s0378-5122(02)00158-5
- Scheiber MD, Liu JH, Subbiah MT, Rebar RW, Setchell KD. Dietary inclusion of whole soy foods results in significant reductions in clinical risk factors for osteoporosis and cardiovascular disease in normal postmenopausal women. Menopause. 2001 Sep-Oct;8(5):384-92. doi: 10.1097/00042192-200109000-00015
- Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, Jeppesen PB. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr. 2017 Sep;106(3):909-920. doi: 10.3945/ajcn.117.153353
- Lambert MNT, Hu LM, Jeppesen PB. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri- and postmenopausal women. Am J Clin Nutr. 2017 Sep;106(3):801-811. doi: 10.3945/ajcn.116.151464
- Pérez-Alonso M, Briongos LS, Ruiz-Mambrilla M, Velasco EA, Linares L, Cuellar L, Olmos JM, De Luis D, Dueñas-Laita A, Pérez-Castrillón JL. The Effect of Genistein Supplementation on Vitamin D Levels and Bone Turnover Markers during the Summer in Healthy Postmenopausal Women: Role of Genotypes of Isoflavone Metabolism. J Nutrigenet Nutrigenomics. 2017;10(5-6):139-145. doi: 10.1159/000484480
- Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001 Nov;86(11):5217-21. doi: 10.1210/jcem.86.11.8040
- Food and Drug Administration. Food labeling: health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed. Regist. 1999;64:57700–57733. https://www.ncbi.nlm.nih.gov/pubmed/11010706
- Food and Drug Administration. Health claims and qualified health claims; dietary lipids and cancer, soy protein and coronary heart diease, antioxidant vitamins and certain cancers, and selenium and certain cancers. Reevaluation. 2007
- FDA Statement Regarding Soy Protein and Coronary Heart Disease Health Claim Review. https://www.fda.gov/food/ingredientspackaginglabeling/labelingnutrition/ucm509239.htm
- Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044.
- Cooke GM. A review of the animal models used to investigate the health benefits of soy isoflavones. J. AOAC Int. 2006;89:1215–1227.
- Nestel PJ, Pomeroy S, Kay S, Komesaroff P, Behrsing J, Cameron JD, West L. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J. Clin. Endocrinol. Metab. 1999;84:895–898.
- Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW., Jr Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr. 1998;68:1375S–1379S.
- Demonty I, Lamarche B, Jones PJ. Role of isoflavones in the hypocholesterolemic effect of soy. Nutr. Rev. 2003;61:189–203.
- Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J. Nutr. Biochem. 2005;16:641–649.
- Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr. Rev. 2009;67:398–415.
- Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275.
- Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656–1665.
- Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PH. Plasma phytoestrogens and subsequent breast cancer risk. J. Clin. Oncol. 2007;25:648–655.
- Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer. 2008;98:9–14.
- Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006;98:459–471.
- Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J. Nutr. 2004;134:3089–3094.
- Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM, Healey CS, Runswick S, Day NE, Bingham SA. Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165] Breast Cancer Res. 2004;6:R170–R179.
- Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443.
- Zhang C, Ho SC, Lin F, Cheng S, Fu J, Chen Y. Soy product and isoflavone intake and breast cancer risk defined by hormone receptor status. Cancer Sci. 2009.
- Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le Marchand L, Kakazu KK, Nomura AM, Henderson BE, Kolonel LN. Urinary phytoestrogen excretion, postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev. Res. (Phila Pa) 2009;2:887–894.
- Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE. Dietary effects on breast-cancer risk in Singapore. Lancet. 1991;337:1197–1200.
- Fritz WA, Coward L, Wang J, Lamartiniere CA. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19:2151–2158.
- Padilla-Banks E, Jefferson WN, Newbold RR. Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels. Endocrinology. 2006;147:4871–4882.
- Zung A, Glaser T, Kerem Z, Zadik Z. Breast development in the first 2 years of life: an association with soy-based infant formulas. J. Pediatric Gastroenterol. Nutr. 2008;46:191–195.
- Enderlin CA, Coleman EA, Stewart CB, Hakkak R. Dietary soy intake and breast cancer risk. Oncol. Nurs. Forum. 2009;36:531–539.
- Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br. J. Cancer. 2008;98:1485–1493.
- Tomar RS, Shiao R. Early life and adult exposure to isoflavones and breast cancer risk. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008;26:113–173.
- Chandrareddy A, Muneyyirci-Delale O, McFarlane SI, Murad OM. Adverse effects of phytoestrogens on reproductive health: a report of three cases. Complement Ther. Clin. Pract. 2008;14:132–135.
- Schmidt S, Degen GH, Seibel J, Hertrampf T, Vollmer G, Diel P. Hormonal activity of combinations of genistein, Bisphenol A and 17beta-estradiol in the female Wistar rat. Arch. Toxicol. 2006;80:839–845.
- Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, Pike MC. Effects of soy foods on ovarian function in premenopausal women. Br. J. Cancer. 2000;82:1879–1886.
- Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol. Biomarkers Prevent. 2002;11:195–201.
- Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Human Reprod. Update. 2009;15:423–440.
- Patisaul HB, Luskin JR, Wilson ME. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Horm. Behav. 2004;45:270–277.
- Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology. 1996;64:467–470.
- Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, Forsling ML, File SE. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 2003;167:46–53.
- Moore TO, Karom M, O’Farrell L. The neurobehavioral effects of phytoestrogens in male Syrian hamsters. Brain Res. 2004;1016:102–110.
- Dietary exposure to genistein increases vasopressin but does not alter beta-endorphin in the rat hypothalamus. Scallet AC, Wofford M, Meredith JC, Allaben WT, Ferguson SA. Toxicol Sci. 2003 Apr; 72(2):296-300.
- Neurobehavioral effects of dietary soy phytoestrogens.Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurotoxicol Teratol. 2002 Jan-Feb; 24(1):5-16.
- Increased aggressive behavior and decreased affiliative behavior in adult male monkeys after long-term consumption of diets rich in soy protein and isoflavones. Simon NG, Kaplan JR, Hu S, Register TC, Adams MR. Horm Behav. 2004 Apr; 45(4):278-84.
- Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ Jr. Fertil Steril. 2008 Oct; 90(4):911-40.
- The neural and hormonal bases of the reproductive cycle of the rat. Gorski RA, Mennin SP, Kubo K. Adv Exp Med Biol. 1975; 54():115-53.
- Recent decline in age at breast development: the Copenhagen Puberty Study. Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Pediatrics. 2009 May; 123(5):e932-9.
- The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Swan SH, Elkin EP, Fenster L. Environ Health Perspect. 2000 Oct; 108(10):961-6.
- Testicular dysgenesis syndrome and Leydig cell function. Joensen UN, Jørgensen N, Rajpert-De Meyts E, Skakkebaek NE. Basic Clin Pharmacol Toxicol. 2008 Feb; 102(2):155-61.
- A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. North K, Golding J. BJU Int. 2000 Jan; 85(1):107-13.
- How do environmental estrogen disruptors induce precocious puberty? Massart F, Parrino R, Seppia P, Federico G, Saggese G. Minerva Pediatr. 2006 Jun; 58(3):247-54.
- Maternal and neonatal phytoestrogens in Japanese women during birth. Adlercreutz H, Yamada T, Wähälä K, Watanabe S. Am J Obstet Gynecol. 1999 Mar; 180(3 Pt 1):737-43. https://www.ncbi.nlm.nih.gov/pubmed/10076156
- The health consequences of early soy consumption. Badger TM, Ronis MJ, Hakkak R, Rowlands JC, Korourian S. J Nutr. 2002 Mar; 132(3):559S-565S.
- Exposure of infants to phyto-oestrogens from soy-based infant formula. Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Lancet. 1997 Jul 5; 350(9070):23-7.
- Daily intake and urinary excretion of genistein and daidzein by infants fed soy- or dairy-based infant formulas. Irvine CH, Shand N, Fitzpatrick MG, Alexander SL. Am J Clin Nutr. 1998 Dec; 68(6 Suppl):1462S-1465S.
- Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Am J Clin Nutr. 1998 Dec; 68(6 Suppl):1453S-1461S.
- Pilot studies of estrogen-related physical findings in infants. Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ. Environ Health Perspect. 2008 Mar; 116(3):416-20.
- https://ntp.niehs.nih.gov/ntp/ohat/genistein-soy/soyformula/soymonograph2010_508.pdf
- Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Chen A, Rogan WJ. Annu Rev Nutr. 2004; 24():33-54.
- Persistent hypothyroidism in an infant receiving a soy formula: case report and review of the literature. Chorazy PA, Himelhoch S, Hopwood NJ, Greger NG, Postellon DC. Pediatrics. 1995 Jul; 96(1 Pt 1):148-50.
- Premature thelarche in Puerto Rico. A search for environmental factors. Freni-Titulaer LW, Cordero JF, Haddock L, Lebrón G, Martínez R, Mills JL. Am J Dis Child. 1986 Dec; 140(12):1263-7.
- Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA. JAMA. 2001 Aug 15; 286(7):807-14.
- Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the Sister Study. D’Aloisio AA, Baird DD, DeRoo LA, Sandler DP. Environ Health Perspect. 2010 Mar; 118(3):375-81.
- Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Crit Rev. Toxicol. 2011;41(6):463–506. https://www.ncbi.nlm.nih.gov/pubmed/21438720
- Tempfer CB, Froese G, Heinze G, Bentz EK, Hefler LA, Huber JC. Side effects of phytoestrogens a meta-analysis of randomized trials. Am. J. Med. 2009;122(10):939–946. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0027655/
- Zhao, E., & Mu, Q. (2011). Phytoestrogen biological actions on Mammalian reproductive system and cancer growth. Scientia pharmaceutica, 79(1), 1–20. https://doi.org/10.3797/scipharm.1007-15
- Brown BD, Thomas W, Hutchins A, Martini MC, Slavin JL. Types of dietary fat and soy minimally affect hormones and biomarkers associated with breast cancer risk in premenopausal women. Nutr Cancer. 2002;43(1):22-30. doi: 10.1207/S15327914NC431_2
- Ho, S. M., Lee, M. T., Lam, H. M., & Leung, Y. K. (2011). Estrogens and prostate cancer: etiology, mediators, prevention, and management. Endocrinology and metabolism clinics of North America, 40(3), 591–ix. https://doi.org/10.1016/j.ecl.2011.05.002
- Hamilton-Reeves JM, Rebello SA, Thomas W, Slaton JW, Kurzer MS. Soy protein isolate increases urinary estrogens and the ratio of 2:16alpha-hydroxyestrone in men at high risk of prostate cancer. J Nutr. 2007 Oct;137(10):2258-63. doi: 10.1093/jn/137.10.2258
- Bylund A, Lundin E, Zhang JX, Nordin A, Kaaks R, Stenman UH, Aman P, Adlercreutz H, Nilsson TK, Hallmans G, Bergh A, Stattin P. Randomised controlled short-term intervention pilot study on rye bran bread in prostate cancer. Eur J Cancer Prev. 2003 Oct;12(5):407-15. doi: 10.1097/00008469-200310000-00010
- Cross-species and interassay comparisons of phytoestrogen action. Whitten PL, Patisaul HB. Environ Health Perspect. 2001 Mar; 109 Suppl 1():5-20.