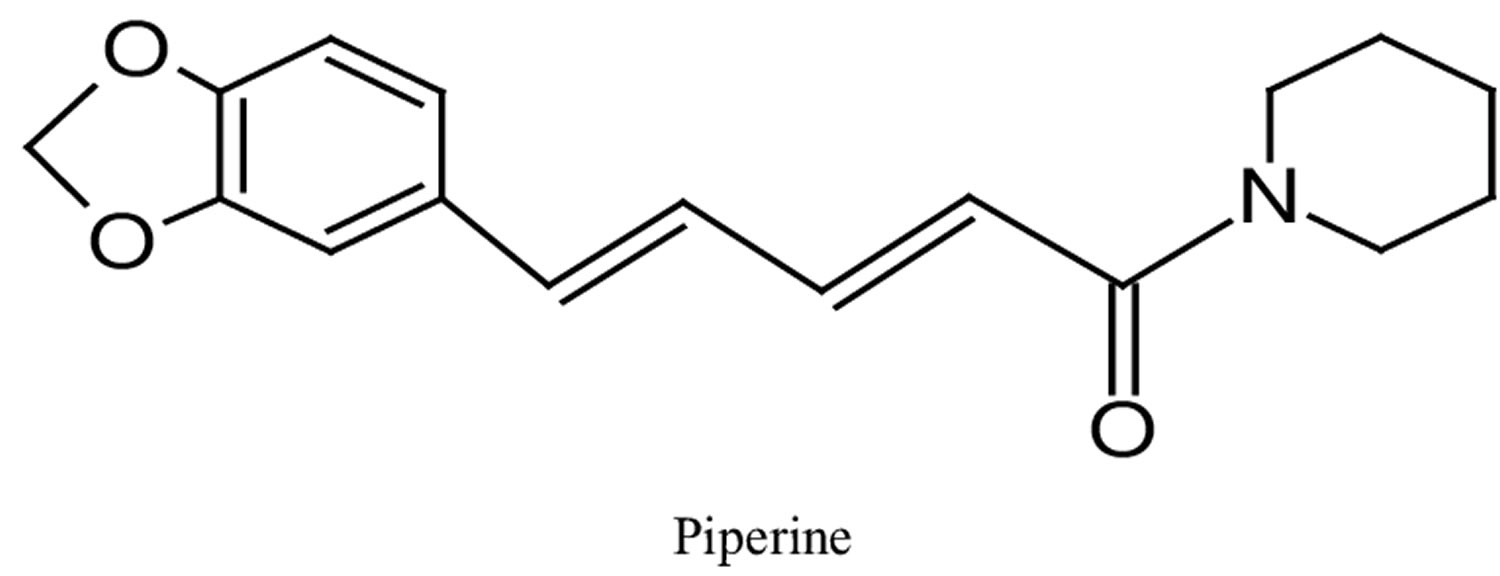
What is piperine
Piperine (1‐piperoyl piperidine) is a naturally occurring alkaloid of black pepper (Piper nigrum), long pepper (Piper longum) and other Piper species 1. Piperine constitutes approximately 5 to 9 percent of commercial black or white pepper. The sharp flavor of freshly ground pepper is attributed to the compound chavicine, a geometric isomer (having the same molecular formula but differing in structure) of piperine. The loss of pungency of ground pepper on storage is associated with slow transformation of chavicine into piperine. Piperine is ‘generally recognized as safe’ (GRAS) by the United States Food and Drug Administration (FDA) 2. Piperine is also known as a bioavailability enhancer or booster for promoting bioavailability of other drugs and has been shown to increase the bioavailability and pharmacological effects of some drugs in the body 3 and decrease the cytotoxicity of aflatoxin B1 and genotoxicity in vitro without leaving residues or its derivatives in the host 4 . Suresh and Srinivasan 5 showed that the amount of piperine in rat tissues (0.3%) reduced significantly after 48 hours, and was no longer detectable in the blood, liver, and intestine after 96 hours.
Piperine has been investigated extensively as a supportive nutrient for other compounds such as curcumin 6. In a randomized controlled trial that enrolled nonalcoholic fatty liver disease (NAFLD) patients, the supplementation of curcumin with piperine for 8 weeks improved nonalcoholic fatty liver disease (NAFLD), as shown by the ultrasound results of the patients 6. Choi et al. 7 also found that piperine alleviated high-fat diet-induced hepatic steatosis in mice in a dose-dependent manner. Although obesity is a risk factor for nonalcoholic fatty liver disease (NAFLD), the overlap between these two entities is incompletely understood 8. Okumura et al. 9 compared the actions of black pepper and piperine on adiposity suppression in the mice fed with a high-carbohydrate, high-fat diet for four weeks and found that black pepper suppressed the effect of body fat accumulation mainly through the action of piperine. Although several animal studies showed that piperine may have anti-obesity, lipid-lowering, and glucose-lowering properties 7, the mechanism of piperine on lipid metabolism and adipose tissue expansion in fat tissue remains still unclear. An animal study by Du et al 10 showed that piperine protects against obesity and improves lipid metabolism in mice fed with a high fat diet through the regulation of adipose tissue expansion related genes. Regarding the regulation of lipogenic and lipolytic target genes, piperine is more effective in visceral fat than in subcutaneous fat. However, further animal studies on this topic should investigate the potential effect modification by animal model, treatment dosage, intervention time, and administration routes.
Figure 1. Piperine chemical structure
Piperine benefits
Piperine, a naturally occurring alkaloid, has been demonstrated in test tube and animal studies as antifungal, anti-epileptic activities 11, anti-obesity 12, anti-gastric ulcer 13, anti-acute pancreatitis 14, and anti-arthritis 15, insecticidal 16, anti-oxidant 17, anti-inflammatory, antimicrobial 18, antiparasitic 19, antidepressant 20, antipyretic, analgesic, anti-inflammatory 21, anti-apoptotic 22, anti-mutagenic, anti-tumor and anti-proliferative agent. It has also been reported that piperine enhances the bioavailability of other phytochemicals 23 and drugs, for example, rifampcin 24 and resveratrol 25.
Recently, Zhang et al. 26 demonstrated the chemopreventive abilities of piperine against two osteosarcoma cell lines (HOS and U-2OS) metastasis and Greenshields et al. 27 demonstrated its protective properties against cancer cell migration in vitro through the inhibition of matrix metalloproteinase-2 and -9 expression.
Reen et al. 4 showed that piperine was able to significantly decrease the toxicity of aflatoxin B1 in rat hepatocytes in vitro and Selvendiran et al 28 demonstrated the protective effects of piperine against cytotoxic and genotoxic carcinogenesis induced by certain chemical carcinogens and aflatoxin B1.
Previous studies have shown that piperine possesses a number of pharmacological activities. Pharmaceutically, piperine has been reported to protect against hepatotoxicity 29, attenuate depressive disorders 30 and mitigate obesity and diabetes 31.
This naturally available non-toxic molecule has been used for the treatment of leukemia 32, as an insecticide against malaria Anopheles mosquitos 33 as well leshmianasis 34. Piperine also inhibits Akt phosphorylation 35 and suppresses angiogenesis as well as it exerts its anti-cancer effect by inhibiting CREB, NF-kB, c-Fos activities 36.
It is also noteworthy that the administration of piperine and its derivatives resulted in the activation of AMPKα signaling in mice 37. Piperine also decreased the phosphorylation of extracellular signal-regulated kinase (ERK) in vitro 38. These findings raised the possibility that piperine could protect against cardiac hypertrophy.
Piperine uses
A double-blind, randomized placebo-controlled trial involving 55 patients with nonalcoholic fatty liver disease (NAFLD) showed that 500 mg curcumin supplementation (plus 5 mg piperine to increase intestinal absorption) for a period of 8 weeks can improve serum levels of inflammatory cytokines in subjects with nonalcoholic fatty liver disease (NAFLD) and this might be at least partly responsible for the anti-steatotic effects of curcuminoids 6. Curcumin supplementation improved the severity of nonalcoholic fatty liver disease (NAFLD) according to the ultrasound results. Moreover, serum concentrations of TNF-α, MCP-1 and EGF were improved by curcuminoids in nonalcoholic fatty liver disease (NAFLD) patients. The results also showed that supplementation with curcumin could decrease weight compared to the placebo group in patients with nonalcoholic fatty liver disease (NAFLD) 6.
Piperine side effects
Piperine is ‘generally recognized as safe’ (GRAS) by the United States Food and Drug Administration (FDA) 39. Recent studies have shown that the consumption of piperine is relatively safe, with low toxicity to cells of mammals and avian species 40.
Piperine dosage
There are no official recommendations for consumption of piperine and the maximum tolerable intake has not been identified.
References- Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007;47:735–748.
- H. Jwa, Y. Choi, U.H. Park, S.J. Um, S.K. Yoon, T. Park. Piperine, an LXRalpha antagonist, protects against hepatic steatosis and improves insulin signaling in mice fed a high-fat diet. Biochemical Pharmacology, 84 (11) (2012), pp. 1501-1510, 10.1016/j.bcp.2012.09.009
- Di X., Wang X., Di X., Liu Y. (2015). Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J. Pharm. Biomed. Anal. 115 144–149. 10.1016/j.jpba.2015.06.027
- Reen K.R., Wiebel J.F., Singh J. Piperine inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in V79 Chinese hamster cells genetically engineered to express rat cytochrome P4502B1. J. Ethnopharmacol. 1997;58:165–173. doi: 10.1016/S0378-8741(97)00104-9
- Suresh D., Srinivasan K. Tissue distribution and elimination of capsaicin, piperine and curcumin following oral intake in rats. J. Med. Res. 2010;131:682–691.
- M. Saberi-Karimian, M. Keshvari, M. Ghayour-Mobarhan, L. Salehizadeh, S. Rahmani, B. Behnam, A. Sahebkar. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complementary Therapies in Medicine, 49 (2020), Article 102322, 10.1016/j.ctim.2020.102322
- S. Choi, Y. Choi, Y. Choi, S. Kim, J. Jang, T. Park. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chemistry, 141 (4) (2013), pp. 3627-3635, 10.1016/j.foodchem.2013.06.028
- J.A. Woo Baidal, J.E. Lavine. The intersection of nonalcoholic fatty liver disease and obesity. Science Translational Medicine, 8 (323) (2016), p. 323rv1, 10.1126/scitranslmed.aad8390
- Y. Okumura, M. Narukawa, T. Watanabe. Adiposity suppression effect in mice due to black pepper and its main pungent component, piperine. Bioscience, Biotechnology, and Biochemistry, 74 (8) (2010), pp. 1545-1549, 10.1271/bbb.100117
- Effects of piperine on lipid metabolism in high-fat diet induced obese mice. Journal of Functional Foods Volume 71, August 2020, 104011 https://doi.org/10.1016/j.jff.2020.104011
- Mao Q. Q., Huang Z., Zhong X. M., Xian Y. F., Ip S. P. (2014). Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 261 140–145. 10.1016/j.bbr.2013.12.020
- BrahmaNaidu P., Nemani H., Meriga B., Mehar S. K., Potana S., Ramgopalrao S. (2014). Mitigating efficacy of piperine in the physiological derangements of high fat diet induced obesity in Sprague Dawley rats. Chem. Biol. Interact. 221 42–51. 10.1016/j.cbi.2014.07.008
- Bai Y. F., Xu H. (2000). Protective action of piperine against experimental gastric ulcer. Acta Pharmacol. Sin. 21 357–359.
- Bae G. S., Kim M. S., Jeong J., Lee H. Y., Park K. C., Koo B. S., et al. (2011). Piperine ameliorates the severity of cerulein-induced acute pancreatitis by inhibiting the activation of mitogen activated protein kinases. Biochem. Biophys. Res. Commun. 410 382–388. 10.1016/j.bbrc.2011.05.136
- Murunikkara V., Pragasam S. J., Kodandaraman G., Sabina E. P., Rasool M. (2012). Anti-inflammatory effect of piperine in adjuvant-induced arthritic rats–a biochemical approach. Inflammation 35 1348–1356. 10.1007/s10753-012-9448-3
- Estrela J.L., Guedes R.N., Maltha C.R., Fazolin M. Toxicity of piperine amide analogs to larvae of Ascia monuste orseis Godart (Lepidoptera: Pieridae) and Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) Neotrop. Entomol. 2003;32:343–346. doi: 10.1590/S1519-566X2003000200022
- Mittal R. & Gupta R. L. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 22, 271–274 (2000).
- Zarai Z., Boujelbene E., Ben Salem N., Gargouri Y. & Sayari A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT-Food Sci. Technol. 50, 634–641 (2013).
- Ghoshal S., Krishna P.B.N., Lakshmi V. Antiamoebic activity of Piper longum fruits against Entamoeba histolytica in vitro and in vivo. J. Ethnopharmacol. 1996;50:167–170. doi: 10.1016/0378-8741(96)01382-7
- Li S. et al.. Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J. Asian Nat. Prod. Res. 9, 421–430 (2007).
- Virinder S.P., Subash C.J., Kirpal S.B., Rajani J. Phytochemistry of genus Piper. Phytochemistry. 1997;46:597–673.
- Shrivastava P. et al.. Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson’s rat model. J. Nutr. Biochem. 24, 680–687 (2013).
- Wadhwa S., Singhal S. & Rawal S. Bioavailability Enhancement by Piperine: A Review, Asian J. Biomed. Pharm. Sci. 4, 1–8 (2014).
- Zutshi R. K., Singh R., Zutshi U., Johri R. K. & Atal C. K. Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. J. Assoc. Physicians India 33, 223–224 (1985).
- Johnson J. J. et al.. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 55, 1169–1176 (2011).
- Zhang J., Zhu X., Li H., Li B., Sun L., Xie T., Zhu T., Zhou H., Ye Z. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharm. 2015;24:50–58. doi: 10.1016/j.intimp.2014.11.012
- Greenshields A.L., Doucette C.D., Sutton K.M., Madera L., Annan H., Yaffe P.B., Allison F.K., Zhongmin D., Hoskin D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015;357:129–140. doi: 10.1016/j.canlet.2014.11.017
- Selvendiran K., Koga H., Ueno T., Yoshida T., Maeyama M., Torimura T., Hirohisa Y., Masamichi K., Sata M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: An implication for the antitumor potential of flavonoids. Cancer Res. 2006;66:4826–4834. doi: 10.1158/0008-5472.CAN-05-4062
- Piyachaturawat P., Kingkaeohoi S., Toskulkao C. Potentiation of carbon tetrachloride hepatotoxicity by piperine. Drug Chem. Toxicol. 1995;18:333–344.
- Bhutani M.K., Bishnoi M., Kulkarni S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009;92:39–43.
- Nogara L, Naber N, Pate E, Canton M, Reggiani C, Cooke R. Piperine’s mitigation of obesity and diabetes can be explained by its up-regulation of the metabolic rate of resting muscle. Proc Natl Acad Sci U S A. 2016;113(46):13009-13014. doi:10.1073/pnas.1607536113 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5135373
- Tawani A, Amanullah A, Mishra A, Kumar A. Evidences for Piperine inhibiting cancer by targeting human G-quadruplex DNA sequences. Sci Rep. 2016;6:39239. Published 2016 Dec 20. doi:10.1038/srep39239 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5171706
- Samuel M., Oliver S. V., Coetzee M. & Brooke B. D. The larvicidal effects of black pepper (Piper nigrum L.) and piperine against insecticide resistant and susceptible strains of Anopheles malaria vector mosquitoes. Parasit. Vectors 9, 238 (2016).
- Ferreira C. et al.. Leishmanicidal effects of piperine, its derivatives, and analogues on Leishmania amazonensis. Phytochemistry 72, 2155–2164 (2011).
- Doucette C. D., Hilchie A. L., Liwski R. & Hoskin D. W. Piperine, a dietary phytochemical, inhibits angiogenesis. J. Nutr. Biochem. 24, 231–239 (2013).
- Pradeep C. R. & Kuttan G. Piperine is a potent inhibitor of nuclear factor-kappaB (NF-kappaB), c-Fos, CREB, ATF-2 and proinflammatory cytokine gene expression in B16F-10 melanoma cells. Int. Immunopharmacol. 4, 1795–1803 (2004).
- Choi S., Choi Y., Choi Y., Kim S., Jang J., Park T. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem. 2013;141:3627–3635.
- Hwang Y.P., Yun H.J., Kim H.G., Han E.H., Choi J.H., Chung Y.C., Jeong H.G. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by piperine via the inhibition of PKCα/ERK1/2-dependent matrix metalloproteinase-9 expression. Toxicol. Lett. 2011;203:9–19.
- GRAS Notice (GRN) No. 474 http://wayback.archive-it.org/7993/20171031042951/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM362936.pdf
- Cardoso V.S., Lima C.A.R., Lima M.E.F., Dorneles L.E.G., Direito G.M., Danelli M.G.M. Piperine as a phytogenic additive in broiler diets. Pesqui. Agropecu. Bras. 2012;47:489–496. doi: 10.1590/S0100-204X2012000400003