What are psychedelics?
Psychedelics also known as “classic hallucinogens” or more specifically “serotonergic hallucinogens”, are a class of psychoactive substances that produce changes in all your senses, mood, thinking, sense of time and emotions 1. Psychedelics are substances that exert their effects primarily by an agonist (or partial agonist) action on brain serotonin 5-hydroxytryptamine (5-HT2A) receptors (because drugs of other pharmacological classes can also induce hallucinations) 1. Moreover, the full spectrum of a given hallucinogen’s action may well entail actions at other serotonin receptors as well as receptors of dopamine, noradrenaline, histamine, trace amines, and neurotransmitter uptake sites 2. Psychedelics affect all your senses, altering your thinking (introspection, self-consciousness, mystical experiences, altered time passage), sense of time and your mood or emotions (blissful state, euphoria, and joy) 3. Psychedelics can also cause a person to hallucinate (mainly visual effects)—seeing or hearing things that do not exist or are distorted. The intensity and nature of these effects are highly influenced by the set (your personality and internal expectations of the subject), setting (environment in which the substance is used), and dosage 4. Subjects participating in clinical trials often describe mystical experiences and improved mood while under the effects of these substances 5, 6. Although there is accumulating evidence that psychedelics are generally safe in humans 7, it is important to note that these substances can produce side effects and/or unpleasant experiences popularly known as “bad trips”, which involve transitory anxious and psychotic symptoms, confusion, dissociation and depersonalization 4. Nevertheless, careful screening of volunteers and a supportive environment seem to reduce the occurrence and severity of these experience in controlled settings 8, 7.
There are many different kinds of psychedelics. Some occur naturally, in trees, vines, seeds, fungi and leaves (such as psilocybin, present in mushrooms of the genus Psilocybe and many others; dimethyltryptamine [DMT], present in the botanical preparations ayahuasca and jurema; and mescaline, present in peyote and San Pedro cacti) 9, 10. Others are made in laboratories (such as the diethylamide of lysergic acid diethylamide [LSD]) 10.
The name psychedelics was coined by Humphrey Osmond in 1957, connoting that they have a mind-manifesting capability, revealing useful or beneficial properties of the mind 11. Botanical psychedelics have been used for centuries as part of the traditional medicine of many cultures in the Americas 12. Mescaline has its traditional use based in northern Mexico (peyote) and Peru (San Pedro), and psilocybin (Psilocybe spp. mushrooms) in Central Mexico 13. DMT (Diemethyltryptamine) is the main hallucinogenic component present in jurema (traditionally used in Northeast Brazil) and ayahuasca (traditionally used in Northwestern Amazon in countries, such as Brazil, Peru, Ecuador, and Colombia) 13. Psychedelic drugs were popularized in the 1960s by researchers such as Timothy Leary, Ram Dass, Terrence McKenna and other enthusiasts 14. Between the 1960’s and the 1980’s a number of scientific studies were being conducted on psychedelic substances including biochemical, pharmacological and psychological studies. Therapists were experimenting with using psychedelics as adjuncts to various psychotherapy methods. Due to radical activism and drug propaganda, psychedelics were scheduled as Schedule 1 substances by the Controlled Substances Act in 1970 1 and were made illegal to consume, possess or distribute. After a several decades hiatus, psychedelics such as LSD (Lysergic acid diethylamide), psilocybin, ketamine, MDMA (3,4-methylenedioxymethamphetamine or ecstasy), DMT (Diemethyltryptamine), ibogaine and mescaline are increasingly being studied for the treatment of a range of mental illnesses 15. Within the past 2 years, the US Food and Drug Administration (FDA) has recommended 2 compounds [3,4-methylenedioxymethamphetamine (MDMA or ecstasy) and psilocybin], which are still on the US Drug Enforcement Administration’s most restrictive schedule, a breakthrough therapy designation, and approved esketamine for treatment-resistant depression 16.
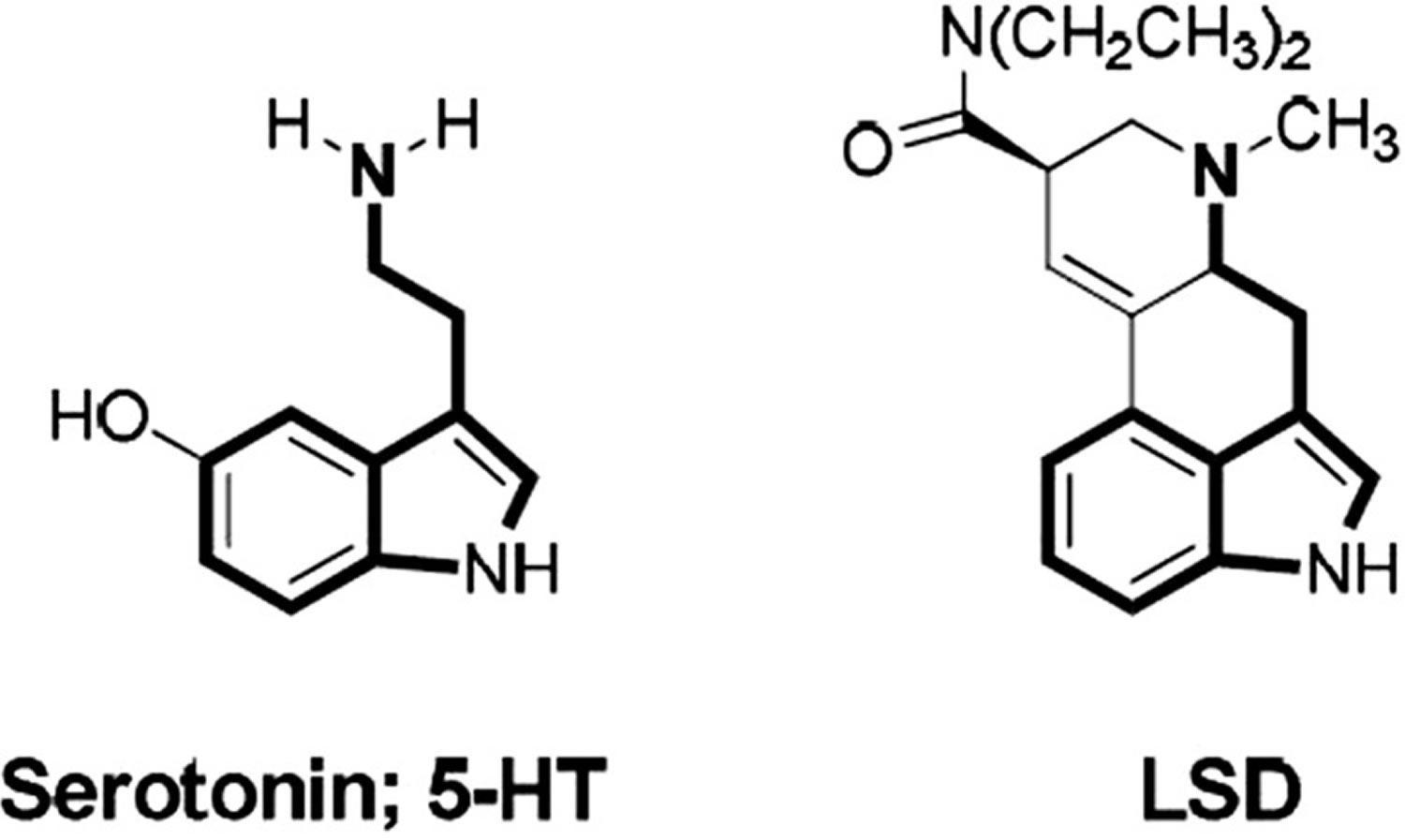
Figure 1. Chemical structures of serotonin and lysergic acid diethylamide (LSD)
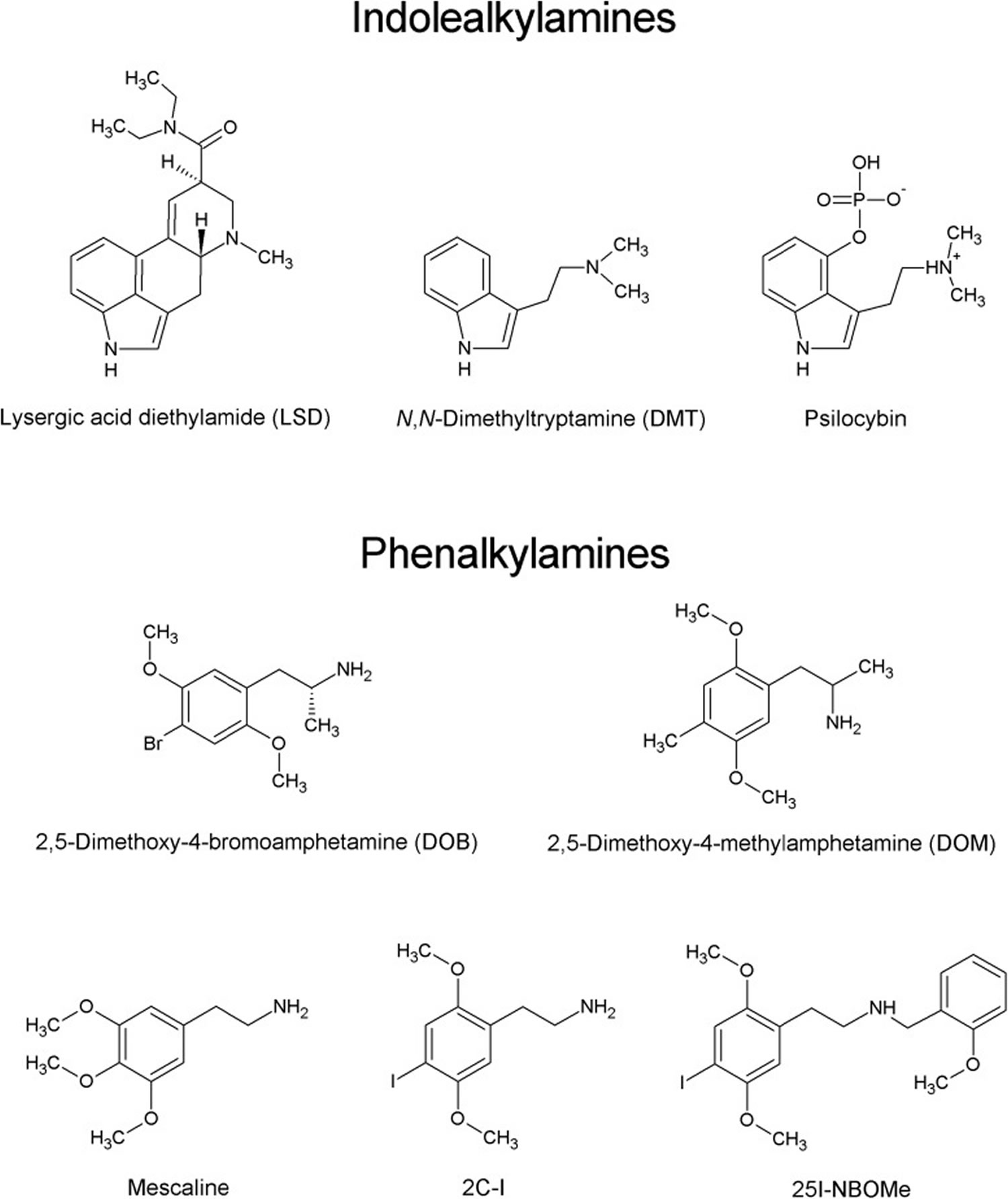
[Source 1 ]Figure 2. Psychedelics chemical structures
[Source 17 ]Types of psychedelics
Psychedelics (serotoninergic hallucinogens or classical hallucinogens) are divided into two main groups based on their chemical structure:
- Indoleamines also known as indolealkylamines that can be further divided into ergolines like the semisynthetic lysergic acid diethylamide (LSD), and the simple tryptamines, including N,N-dimethyltryptamine (DMT) and the phosphorylated counterpart 4-phosphoryloxy-N,N-DMT (psilocybin) 18.
- Phenylalkylamines such as the well-known mescaline obtained from the peyote cactus (Lophophora williamsii) (see Figure 2) 17, 19.
These psychedelics share a similar mode of action, acting as agonists (or partial agonists) of the serotonin (5-HT) receptor 5-HT2A, through which they exert their effects in the central nervous system (CNS) 20, 21, 22.
Phenylalkylamines are highly selective for serotonin 5-HT2 receptors, while indoleamines are relatively non-selective for 5-HT receptors, displaying moderate to high affinity for 5-HT1 and 5-HT2 receptor subtypes 17. The phenylalkylamines can be further divided into two subgroups, one group being the phenylisopropylamines (analogs of amphetamine), e.g., 2,5-dimethoxy-4-bromoamphetamine (DOB) and 2,5-dimethoxy-4-methylamphetamine (DOM), and the other being the phenethylamines, including mescaline, 2C-X compounds and their derivatives (Figure 2) 23. The name “2C” refers to an acronym created by the ‘godfather’ of psychedelic drugs Alexander Shulgin to describe their chemical structure, where two carbon atoms separate the amine group from the phenyl ring 24. The prototype of the 2C series, 2C-B, was synthesized by Shulgin in 1974. Since 2010, a new group of 2C compounds containing an N-(2-methoxy)benzyl (N-benzoylmethoxy) substituent, known as N-(2-methoxybenzyl)phenethylamines (aka 25X-NBOMes or simply NBOMes), has emerged in the illicit drug market 17. Structure-activity studies indicate that this substituent significantly increases the affinity of the drug toward the 5-HT2A (serotonin or 5-hydroxytryptamine) receptor and its pharmacological activity 25. It is important to note that stimulation of the 5-HT2A (serotonin or 5-hydroxytryptamine) receptors is required for the psychedelic effects of compounds such as LSD, mescaline, and psilocybin 2. The first NBOMes were originally synthesized by Ralf Heim at the Free University of Berlin in a search for pharmacological tools to study the 5-HT2A receptor 26. Since then, [11C]25I-NBOMe and [11C]25C-NBOMe have been used to map the distribution of 5-HT2A receptors in the brain by positron emission tomography (PET) imaging 27.
The first recreationally used drug from this group was 25I-NBOMe (2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine), identified in seven green blotters seized by the Swedish police in May 2012 28. It is likely that 25I-NBOMe was the first NBOMe to be used recreationally in the United States 29. Following this, several potent NBOMes were synthesized and introduced into the illegal drug market. In these, the iodine atom was exchanged for other halogens: e.g., bromine (25B-NBOMe) or chlorine (25C-NBOMe), a hydrogen atom (25H-NBOMe), a nitro group (25N-NBOMe) or an organic functional group, – methyl (25D-NBOMe), -ethyl (25E-NBOMe), or –isopropyl (25iP-NBOMe) 30. Three compounds from this group, namely 25I-NBOMe, 25B-NBOMe, and 25C-NBOMe, accounted for 0.03% of the total quantity of hallucinogens (other than ketamine) seized globally between 2011 and 2017 31. In the United States, 2,129 reports for 25I-NBOMe, 1,273 reports for 25C-NBOMe, and 924 reports for 25B-NBOMe were collected by the System to Retrieve Information from Drug Evidence and the National Forensic Laboratory Information System between January 2014 and April 2018 32.
- LSD (Lysergic acid diethylamide) is made from a substance found in ergot, which is a fungus that infects rye.
- Psilocybin is a naturally occurring substance found in mushrooms and is found in many parts of the world.
- Mescaline is derived from the Mexican peyote and San Pedro cactus and produces similar effects to LSD.
- DMT (Diemethyltryptamine or N,N-dimethyltryptamine) is structurally similar to psilocin, an alkaloid found in psilocybin mushrooms. DMT (Diemethyltryptamine or N,N-dimethyltryptamine) can be synthesized in the laboratory but is also a naturally occurring component of several plants (from bark of the Banisteriopsis caapi vine [containing beta-carboline alkaloids] and leaves of the Psychotria viridis bush, a traditional Amazonian psychoactive drink commonly known as Ayahuasca) 33. In 1970, DMT was classified as a Schedule I drug under the US Controlled Substances Act 34.
- DOM (2,5-dimethoxy-4-methylamphetamine) is a member of the DOx family of compounds which are known for their high potency, long duration, and mixture of psychedelic and stimulant effects.
- 2C-B (4-Bromo-2,5-dimethoxyphenethylamine) is a psychedelic drug first synthesized in 1974. 2C-B (4-Bromo-2,5-dimethoxyphenethylamine) is considered both a psychedelic and a mild entactogenic. ‘Entactogen’ means ‘touching within’ and is a term used by psychiatrists to classify MDMA and related drugs.
- North American Peyote cactus (Lophophora williamsii) is the most well-known and potent psychedelic cactus, although the smallest and slowest growing 35. Instead of growing upward to form a column, it grows as ‘buttons’ low to the ground. Peyote mescaline (3,4,5-trimethoxyphenethylamine) is a class 1 hallucinogenic alkaloid, and although it is chemically unrelated to lysergic acid diethyl amide (LSD), the hallucinogenic effects of mescaline are similar to those of LSD, albeit longer lasting 36. For centuries, North American indigenous peoples have used mescaline as a medicine and as a part of hallucinogenic religious sacrament 37. The ceremonial use of peyote alkaloids has masked and mythologized the potential use of peyote in modern medicine. For example, some of the illnesses treated with peyote by Mexican Natives are tuberculosis, pneumonia, scarlet fever, intestinal ills, diabetes, rheumatic pains, colds, grippe, fevers, and venereal diseases, which is why peyote is officially listed in the Mexican pharmacopoeia 38.
- 25X-NBOMe or NBOMes (N-methoxybenzyl or N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine) are synthetic psychedelics that have appeared on the illegal drug market 17. Reports indicate that there are a number of different versions of NBOMe available (25I-NBOMe, 25B-NBOMe, and 25C-NBOMe) – all with differing effects 39. NBOMes are also referred to as a New Psychoactive Substances (NPS) because they are designed to mimic or produce similar effects to common illicit drugs such as the so-called ‘classical psychedelics’ like mescaline 40. While they belong to the same drug type, their chemical structures have differences 40. NBOMes are sold as blotter papers, or in powder, liquid, or tablet form, and they are administered sublingually/buccally, intravenously, via nasal insufflations, or by smoking. Since their introduction in the early 2010s, numerous reports have been published on clinical intoxications and fatalities resulting from the consumption of NBOMe compounds. Commonly observed adverse effects include visual and auditory hallucinations, confusion, anxiety, panic and fear, agitation, uncontrollable violent behavior, seizures, excited delirium, and sympathomimetic signs such mydriasis, tachycardia, hypertension, hyperthermia, and diaphoresis. Rhabdomyolysis, disseminated intravascular coagulation, hypoglycemia, metabolic acidosis, and multiorgan failure were also reported 17.
Psychedelics mechanisms of action
The therapeutic effects of psychedelics are related primarily to their agonist action at the serotonergic receptor 5-HT2A (5-hydroxytryptamine) and to a minor extent at 5-HT1A and 5-HT2C receptors 41. Agonists of 5-HT1A receptors are thought to be responsible for passive adaptive response (tolerability to a source of stress), while 5-HT2A agonists are thought to be responsible for the active adaptive response (dealing with the source of the stress actively) 42. Considering that psychedelics are agonists of both receptors, the increase in overall adaptability could be related to their therapeutic effects 42. Furthermore, 5-HT2A agonists also induce the release of glutamate, modulating the activation of the amygdala, hippocampus, and prefrontal cortex 43. The increase of glutamate in these brain areas also stimulates the synthesis of brain-derived neurotrophic factor (BDNF), increasing neuroplasticity. 44. Reduced activity of the default mode network (DMN) and enhanced functional connectivity between otherwise distinct brain networks also indicate enhanced neuroplasticity induced by psychedelics 45.
Regarding ayahuasca, modulation of BDNF (brain-derived neurotrophic factor), cortisol, and inflammatory biomarkers seem to be related to its antidepressive effects in depressive patients 46. During an imagination task with the eyes closed, ayahuasca produced a brain activation pattern in occipital areas similar to the pattern observed when looking at an object with open eyes 47. This could be related to its therapeutic effects by giving a status of reality to inner experiences, such as biographical memories 47.
LSD and psilocybin reduce the recognition of negative emotions through amygdala modulation, while increasing positive affect and mood 48. The anxiolytic and antidepressant effects reported in the literature seem to be related to this mechanism of action in brain areas related to emotional processing 49. Psychological mechanisms also seem to be involved in the therapeutic effects of hallucinogens. Decentering and mindfulness-related processes have been associated with positive therapeutic outcomes in studies of acute drug administration 50 and in long-term users 51. Positive change in personality, such as increas in the traits of openness to experience and self-transcendence, is also related to the therapeutic effects of these compounds 52
It is still not clear what is the role of psychotherapy in clinical trials with psychedelics. Psychological interventions may or may not be used combined with psychedelics. In the open-label and controlled ayahuasca trials of ayahuasca for treatment-resistant depression, no psychotherapy was used 53, while in the open-label trials of psilocybin for treatment-resistant depression 42, major depressive disorder 54 and alcohol dependence 55 and tobacco addiction 56, and in the LSD 57 and psilocybin 58 trials for anxiety and depression in cancer patients, more elaborate techniques were used. Further research is needed to better understand the possible beneficial role of different types of psychotherapy in trials with psychedelics.
Effects of psychedelics
Psychedelics affect everyone differently, based on:
- your size, weight and health
- whether the person is used to taking it
- whether other drugs are taken around the same time
- the amount taken
- the strength of the drug (varies from batch to batch).
The effects of psychedelics can last several hours and vary considerably, depending on the specific type of psychedelic. The following may be experienced during this time:
- feelings of euphoria
- sense of relaxation and wellbeing
- seeing and hearing things that aren’t there
- confusion and trouble concentrating
- dizziness
- blurred vision
- clumsiness
- fast or irregular heart beat
- breathing quickly
- vomiting
- sweating and chills
- numbness.
Bad trips
Sometimes you can experience a ‘bad trip’, which is frightening and disturbing hallucinations. This can lead to panic and unpredictable behavior, like running across a road or attempting suicide.
If you take a large amount or have a strong batch, you are likely to experience negative effects of psychedelics.
Flashbacks
The most common long-term effect of psychedelic use is the ‘flashback’. Flashbacks are a re-experience of the drug and can occur days, weeks, months and even years later.
Flashbacks can be triggered by the use of other drugs or by stress, fatigue or physical exercise. The flashback experience can range from being pleasant to causing severe feelings of anxiety. They are usually visual and last for a minute or two.
Psychedelics health benefits
Between the late 1950s and early 1970s several studies were conducted to investigate the possible therapeutic effects of psychedelics (especially LSD) in the treatment of anxiety, depression, and substance use disorders 59, 4. Despite the promising results of these studies and the good safety profile of these drugs in controlled contexts 60, important methodological limitations in these studies (lack of placebo or control groups, absence of standardization in the monitoring of volunteers and interventions used) and the popularization of the recreational use of these drugs culminated in the prohibition of human studies until the early 1990s 61.
In recent years, several research groups have been investigating the possible antidepressant, anti-anxiety and anti-addictive effects of psychedelics. Recent open-label studies showed that psilocybin reduced depressive and anxious symptoms in treatment-resistant depression 42 and in major depressive disorder 54. Depression and anxiety symptoms were also reduced in controlled trials in terminal cancer patients 62. Psilocybin produced positive effects in open-label trials for the treatment of alcohol and tobacco dependence 55, 56. Although there is less recent research with LSD, a recent meta-analysis of early studies in the 1960–70s provided evidence that it can be useful to treat of alcohol use disorder 63 and other studies indicate its possible anxiolytic properties in life-threatening diseases 57. Regarding ayahuasca, a controlled trial showed that it reduced panic-like and hopelessness symptoms in healthy volunteers 64. Furthermore, an open-label 65 and a placebo-controlled trial 53 showed that ayahuasca administration reduced depression, anxiety, and suicidality in patients with treatment-resistant depression 66.
In most of these clinical trials single or few doses were administered and the beneficial effects were fast-acting (hours/days) and enduring (weeks/months) 67. Notably, all substances showed a good safety and tolerability profile, producing mostly mild/moderate and transient effects, such as vomiting and nausea, anxiety, confusion, and headache 67.
In the 1980s, another type of serotonergic drugs with mind-altering and therapeutic properties appeared, the “entactogens” 68. These substances enhance empathy and emotional openness, and the most intensely studied drug of this class is MDMA (3,4-methylenedioxymethamphetamine), popularly known as “ecstasy”. MDMA is being researched for the treatment of social anxiety in autistic adults 69, in the treatment of end-of-life anxiety 70 and is being developed as a prescription medicine for the treatment of Post-Traumatic Stress Disorder (PTSD) 71. Both MDMA and psilocybin have been designated by the FDA as “breakthrough therapies” and in the case of MDMA both the FDA and the Israeli Agency of Medicines have authorized its use as a compassionate medicine 72. MDMA has different mechanisms of action from those of classic hallucinogens. Instead of agonism at cortical 5-HT2A receptors, MDMA inhibits monoamine (serotonin>norepinephrine>dopamine) reuptake and enhances oxytocin release 73. The therapeutic effects of MDMA on PTSD seem to be related to memory reconsolidation and fear extinction 74. Although MDMA is not a truly “classical hallucinogen”, it is of interest here because it is in Phase 3 of clinical trials for the treatment of PTSD and is also a scheduled substance. Therefore, the challenges related to its medical use are similar to those of classic hallucinogens.
Psychedelic side effects
There is no safe level of drug use. Use of any drug always carries some risk. It’s important to be careful when taking any type of drug. Psychedelics are generally considered physiologically safe and do not lead to dependence or addiction 1, 7. However, there is no safe way to use psychedelic drugs. It is important to note that psychedelics can produce side effects and/or unpleasant experiences popularly known as “bad trips”, which involve transitory anxious and psychotic symptoms, confusion, dissociation and depersonalization 4. Nevertheless, careful screening of volunteers and a supportive environment seem to reduce the occurrence and severity of these experience in controlled settings 8, 7.
Use of psychedelics is likely to be more dangerous when:
- taken in combination with alcohol or other drugs, particularly stimulants such as crystal methamphetamine (‘ice’) or ecstasy
- driving or operating heavy machinery
- judgement or motor coordination is required
- alone (in case medical assistance is required)
- the person has mental health issues.
If you do decide to use psychedelics, it’s important to consider the following:
- It is difficult to predict the strength and effects of psychedelics (even if they have been taken before), as the strength and potency can vary from batch to batch.
- People with mental health conditions or a family history of these conditions should avoid using psychedelics.
- Taking psychedelics in a familiar environment in the company of people who are known and trusted may alleviate any unpleasant emotional effects. Anxiety can be counteracted by taking deep, regular breaths while sitting down.
Use of high doses of psychedelics can lead to vascular problems because the 5-hydroxytryptamine (5-HT2A) receptor is associated with vascular smooth muscle contraction, platelet aggregation, thrombus formation, and coronary artery spasms 75. Acute vasoconstriction caused by serotonin is usually shared by activation of 5-HT1B and 5-HT2A receptors; however, in intracranial arteries, only the 5-HT1B receptor mediates constriction 76. Both 5-HT1B and 5-HT2A receptors can mediate coronary artery spasm. 5-HT2A receptors also constrict the portal venous system, including esophageal collaterals in cirrhosis. Data from studies by Ootsuka et al. 77 suggest that spinal 5-HT2A receptors contribute to sympathetically induced cutaneous vasoconstriction regulated by the raphe/parapyramidal neurons in the brainstem.
Balíková 78 reports a fatal and nonfatal overdose after ingestion of the psychedelic phenethylamine DOB (2,5-dimethoxy-4-bromoamphetamine) by two male individuals. Gas chromatography–mass spectrometry was used to detect the presence of DOB in both gastric and urine samples of the two men. Although one subject survived, the other suffered convulsions and metabolic acidosis and died 6 days after admission.
Psilocybin, when administered in a controlled setting, has frequently been reported to cause transient, delayed headache, with incidence, duration, and severity increased in a dose-related manner 79. Bickel et al. 80 reported the case of a 25-year-old hepatitis-C infected man, who presented with severe rhabdomyolysis and acute renal failure after Psilocybe mushroom ingestion. He later developed encephalopathy with cortical blindness. Respiratory and cardiovascular support, mechanical ventilation, continuous venovenous hemodialysis, and corticosteroid treatment led to improvement and the patient recovered completely over several months.
Psilocin was identified in the urine of a subject who was investigated for driving under the influence 81. The subject apparently did not exhibit any response to the crash of his automobile, seemingly unaware of the severity of his situation or immediate surroundings.
Although very rare, there have been reports of rhabdomyolysis after ingestion of LSD 82. A newer tryptamine, 5-methoxy-N,N-diisopropyltryptamine (“foxy”) also produced rhabdomyolysis and transient acute renal failure in an otherwise healthy 23-year-old man 83.
Although many ergot alkaloids are known to produce vasospasm, especially after chronic use, LSD has rarely been associated with this adverse effect. Raval et al. 84 reported on a 19-year-old woman who experienced severe lower-extremity ischemia related to a single use of LSD 3 days prior to presentation. After intra-arterial nitroglycerin and verapamil failed, balloon percutaneous transluminal angioplasty therapy led to rapid clinical improvement in lower-extremity perfusion. As of the date of the report, the patient had not required a major amputation.
Sunness 85 described a 15-year-old female patient with a 2-year history of afterimages and photophobia after a history of drug use that included LSD, marijuana, and other illicit drugs. She had discontinued LSD 1 year prior to examination. Although the author connected her visual problems with her prior LSD use, it is not at all clear from the report that her LSD use was the cause of her visual problem.
Bernhard and Ulrich 86 reported a case of cortical blindness in a 15-year-old girl. She had headache and nausea 5 days after taking LSD and suddenly developed complete blindness in both eyes. The blindness persisted for 48 hours. Over the next 3 months, the subject had three more episodes of complete blindness that lasted 12–36 hours, with no visual disturbances between episodes. The authors suggested that the temporary blindness might be a correlate of “flashbacks” caused by LSD.
Toxicity also has been noted for several of the so-called designer drugs. For example, Jovel et al. 87 reported the case of a healthy young male individual who ingested 5-methoxy-N,N-diallytryptamine, one of the emerging new tryptamine-type research chemicals. The patient was admitted with extreme agitation, tachycardia, diaphoresis, and combativeness that required physical restraint and intravenous sedation, but the patient did recover.
Andreasen et al. 88 reported a fatality involving the potent synthetic psychedelic phenethylamine compound 1-(8-bromobenzo[1,2-b; 4,5-b′]difuran-4-yl)-2-aminopropane, known commonly as Bromo-Dragonfly. An 18-year-old woman was found dead after ingesting 1 ml of a “hallucinogenic liquid.” She and her boyfriend had ingested it between 10 and 11 PM on the previous evening and then they both fell asleep. On awakening at 5 AM the next morning, the woman’s boyfriend discovered that she was dead. Autopsy findings 3 days after her death included edema of the lungs, slight edema of the brain, spleen enlargement, irritation of the mucous membrane in the stomach, and ischemic changes in the kidneys. Her femoral blood concentration of the drug was 4.7 μg/kg. The bottle containing the hallucinogenic liquid was recovered and analyzed by ultraperformance liquid chromatography time-of-flight mass spectrometry, high-performance liquid chromatography diode array detection, 1H nuclear magnetic resonance, and 13C nuclear magnetic resonance and found to contain a solution of almost pure Bromo-Dragonfly. Based on the solution concentration and the amount of solution consumed, it was estimated that she had ingested approximately 700 μg. Although that would seem to be a relatively small dose, no other drugs were discovered in her system, including the absence of ethanol.
Data for 2005 to 2006 from the Texas Poison Control Centers were reviewed for mushroom exposures 89. There were a total of 742 exposures, which were all acute and intentional. Of those, 59 individuals were admitted to a hospital, with 17 requiring admission to a critical care unit. Nonetheless, only 10 of the admissions that were identified involved psilocybin. Of all of the admissions, major toxic reactions were uncommon, with no deaths reported.
Using psychedelics with other drugs
The effects of mixing psychedelics with other drugs, including alcohol, prescription medications and over-the-counter medicines, are often unpredictable.
Mixing psychedelics with stimulant drugs increases the stimulant effects and can further increase heart rate and place the body under extreme stress. Stimulants can also increase anxiety which can lead to a negative experience.
Combing psychedelics with depressant drugs such as alcohol may further reduce coordination and increases the chances of vomiting. Alcohol may also decrease the effects of the psychedelic.
Psychedelics dependence and tolerance
Most psychedelics produce tolerance rapidly and psychological dependence can occur in some people. The development of physical dependence is not well supported by evidence and there are no withdrawal symptoms even after chronic use.
References- Nichols D. E. (2016). Psychedelics. Pharmacological reviews, 68(2), 264–355. https://doi.org/10.1124/pr.115.011478
- Rickli A, Moning OD, Hoener MC, Liechti ME. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol. 2016 Aug;26(8):1327-37. doi: 10.1016/j.euroneuro.2016.05.001
- Dos Santos RG, Hallak JEC. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci Biobehav Rev. 2020 Jan;108:423-434. doi:10.1016/j.neubiorev.2019.12.001
- Bogenschutz MP, Ross S. Therapeutic Applications of Classic Hallucinogens. Curr Top Behav Neurosci. 2018;36:361-391. doi: 10.1007/7854_2016_464
- Dos Santos RG, Bouso JC, Alcázar-Córcoles MÁ, Hallak JEC. Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: a systematic review of systematic reviews. Expert Rev Clin Pharmacol. 2018 Sep;11(9):889-902. doi: 10.1080/17512433.2018.1511424
- Timmermann, C., Roseman, L., Williams, L., Erritzoe, D., Martial, C., Cassol, H., Laureys, S., Nutt, D., & Carhart-Harris, R. (2018). DMT Models the Near-Death Experience. Frontiers in psychology, 9, 1424. https://doi.org/10.3389/fpsyg.2018.01424
- Dos Santos RG, Hallak JEC. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci Biobehav Rev. 2020 Jan;108:423-434. doi: 10.1016/j.neubiorev.2019.12.001
- Dos Santos, R. G., Osório, F. L., Crippa, J. A., & Hallak, J. E. (2016). Antidepressive and anxiolytic effects of ayahuasca: a systematic literature review of animal and human studies. Revista brasileira de psiquiatria (Sao Paulo, Brazil : 1999), 38(1), 65–72. https://doi.org/10.1590/1516-4446-2015-1701
- Escobar JÁ, Roazzi A. Panorama contemporâneo do uso terapêutico de substâncias psicodélicas: ayahuasca e psilocibina. Neurobiologia. 2010;73(3):159–172.
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004 Feb;101(2):131-81. doi: 10.1016/j.pharmthera.2003.11.002
- OSMOND H. A review of the clinical effects of psychotomimetic agents. Ann N Y Acad Sci. 1957 Mar 14;66(3):418-34. doi: 10.1111/j.1749-6632.1957.tb40738.x
- Ott J. Pharmacotheon: drogas enteogénicas, sus fuentes vegetales y su historia (Pharmacotheon: entheogenic drugs, their plant sources and history). La Liebre de Marzo, Barcelona; 2004. Spanish.
- Schultes RE, Hofmann A. Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powers. Rochester: Healing Arts Press; 1992.
- Thompson C, Szabo A. Psychedelics as a novel approach to treating autoimmune conditions. Immunol Lett. 2020 Dec;228:45-54. doi: 10.1016/j.imlet.2020.10.001
- Kyzar EJ, Nichols CD, Gainetdinov RR, Nichols DE, Kalueff AV. Psychedelic Drugs in Biomedicine. Trends Pharmacol Sci. 2017 Nov;38(11):992-1005. doi: 10.1016/j.tips.2017.08.003
- Krediet, E., Bostoen, T., Breeksema, J., van Schagen, A., Passie, T., & Vermetten, E. (2020). Reviewing the Potential of Psychedelics for the Treatment of PTSD. The international journal of neuropsychopharmacology, 23(6), 385–400. https://doi.org/10.1093/ijnp/pyaa018
- Zawilska, J. B., Kacela, M., & Adamowicz, P. (2020). NBOMes-Highly Potent and Toxic Alternatives of LSD. Frontiers in neuroscience, 14, 78. https://doi.org/10.3389/fnins.2020.00078
- Araujo A.M., Carvalho F., Bastos Mde L., de Pinho G.P., Carvalho M. The hallucinogenic world of tryptamines: An updated review. Arch. Toxicol. 2015;89:1151–1173. doi: 10.1007/s00204-015-1513-x
- Dinis-Oliveira R.J., Pereira C.L., da Silva D.D. Pharmacokinetic and pharmacodynamic aspects of peyote and mescaline: Clinical and forensic repercussions. Curr. Mol. Pharmacol. 2019;12:184–194. doi: 10.2174/1874467211666181010154139
- Nichols D.E. Hallucinogens. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002
- Nichols D.E. Psychedelics. Pharmacol. Rev. 2016;68:264–355. doi: 10.1124/pr.115.011478
- Dos Santos R.G., Hallak J.E.C. Therapeutic use of serotoninergic hallucinogens: A review of the evidence and of the biological and psychological mechanisms. Neurosci. Biobehav. Rev. 2020;108:423–434. doi: 10.1016/j.neubiorev.2019.12.001
- Halberstadt AL. Pharmacology and Toxicology of N-Benzylphenethylamine (“NBOMe”) Hallucinogens. Curr Top Behav Neurosci. 2017;32:283-311. doi: 10.1007/7854_2016_64
- Shulgin A., Shulgin A. (eds). (1991). Phenethylamines I Have Known and Loved (PiHKAL). Berkeley, CA: Transform Press.
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL. Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci. 2014 Mar 19;5(3):243-9. doi: 10.1021/cn400216u
- Heim R. (2003). Synthese und Pharmakologie Potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Ph.D. thesis, Free Universität Berlin, Berlin.
- Poulie CBM, Jensen AA, Halberstadt AL, Kristensen JL. DARK Classics in Chemical Neuroscience: NBOMes. ACS Chem Neurosci. 2019 Nov 12. doi: 10.1021/acschemneuro.9b00528
- EMCDDA (2014). Report on the Risk Assessment of 2-(4-iodo-2,5-Dimethoxyphenyl)-N-(2-Methoxybenzyl)Ethanamine (25I-NBOMe) in the Framework of the Council Decision on New Psychoactive Substances. https://www.emcdda.europa.eu/system/files/publications/772/TDAK14001ENN_480887.pdf
- Palamar JJ, Le A. Use of new and uncommon synthetic psychoactive drugs among a nationally representative sample in the United States, 2005-2017. Hum Psychopharmacol. 2019 Mar;34(2):e2690. doi: 10.1002/hup.2690
- Wood DM, Sedefov R, Cunningham A, Dargan PI. Prevalence of use and acute toxicity associated with the use of NBOMe drugs. Clin Toxicol (Phila). 2015 Feb;53(2):85-92. doi: 10.3109/15563650.2015.1004179
- United Nations (2019). United Nations Office on Drugs and Crime. World Drug Report 2019. Booklet 5. Cannabis and Hallucinogens. https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_5_CANNABIS_HALLUCINOGENS.pdf
- Drug Enforcement Administration (2018). 25I-NBOMe, 25C-NBOMe, and 25B-NBOMe (Street Names: N-bomb, Smiles, 25I, 25C, 25B). https://www.deadiversion.usdoj.gov/drug_chem_info/nbome.pdf
- Hamill, J., Hallak, J., Dursun, S. M., & Baker, G. (2019). Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness. Current neuropharmacology, 17(2), 108–128. https://doi.org/10.2174/1570159X16666180125095902
- Araújo AM, Carvalho F, Bastos Mde L, Guedes de Pinho P, Carvalho M. The hallucinogenic world of tryptamines: an updated review. Arch Toxicol. 2015 Aug;89(8):1151-73. doi: 10.1007/s00204-015-1513-x
- Dinis-Oliveira, R. J., Pereira, C. L., & da Silva, D. D. (2019). Pharmacokinetic and Pharmacodynamic Aspects of Peyote and Mescaline: Clinical and Forensic Repercussions. Current molecular pharmacology, 12(3), 184–194. https://doi.org/10.2174/1874467211666181010154139
- Bruhn J, Holmstedt B. Early peyote research an interdisciplinary study. Econ Bot. 1973;28(4):353–390. doi: 10.1007/BF02862854
- McLaughlin JL. Peyote: an introduction. Lloydia. 1973 Mar;36(1):1-8.
- Schultes RE. The appeal of peyote (Lophophora williamsii) as a medicine. Am Anthropol. 1938;40(4):698–715. doi: 10.1525/aa.1938.40.4.02a00100
- Poklis, J. L., Dempsey, S. K., Liu, K., Ritter, J. K., Wolf, C., Zhang, S., & Poklis, A. (2015). Identification of Metabolite Biomarkers of the Designer Hallucinogen 25I-NBOMe in Mouse Hepatic Microsomal Preparations and Human Urine Samples Associated with Clinical Intoxication. Journal of analytical toxicology, 39(8), 607–616. https://doi.org/10.1093/jat/bkv079
- Poulie C, Jensen A, Halberstadt A, Kristensen J. DARK Classics in chemical neuroscience: NBOMes. ACS Chemical Neuroscience. 2019.
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stämpfli P, Liechti ME, Seifritz E, Vollenweider FX. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol. 2017 Feb 6;27(3):451-457. doi: 10.1016/j.cub.2016.12.030
- Carhart-Harris, R. L., & Nutt, D. J. (2017). Serotonin and brain function: a tale of two receptors. Journal of psychopharmacology (Oxford, England), 31(9), 1091–1120. https://doi.org/10.1177/0269881117725915
- Marek GJ. Interactions of Hallucinogens with the Glutamatergic System: Permissive Network Effects Mediated Through Cortical Layer V Pyramidal Neurons. Curr Top Behav Neurosci. 2018;36:107-135. doi: 10.1007/7854_2017_480
- Ly, C., Greb, A. C., Cameron, L. P., Wong, J. M., Barragan, E. V., Wilson, P. C., Burbach, K. F., Soltanzadeh Zarandi, S., Sood, A., Paddy, M. R., Duim, W. C., Dennis, M. Y., McAllister, A. K., Ori-McKenney, K. M., Gray, J. A., & Olson, D. E. (2018). Psychedelics Promote Structural and Functional Neural Plasticity. Cell reports, 23(11), 3170–3182. https://doi.org/10.1016/j.celrep.2018.05.022
- Pasquini L, Palhano-Fontes F, Araujo DB. Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J Psychopharmacol. 2020 Jun;34(6):623-635. doi: 10.1177/0269881120909409
- Galvão-Coelho NL, de Menezes Galvão AC, de Almeida RN, Palhano-Fontes F, Campos Braga I, Lobão Soares B, Maia-de-Oliveira JP, Perkins D, Sarris J, de Araujo DB. Changes in inflammatory biomarkers are related to the antidepressant effects of Ayahuasca. J Psychopharmacol. 2020 Oct;34(10):1125-1133. doi: 10.1177/0269881120936486
- de Araujo, D. B., Ribeiro, S., Cecchi, G. A., Carvalho, F. M., Sanchez, T. A., Pinto, J. P., de Martinis, B. S., Crippa, J. A., Hallak, J. E., & Santos, A. C. (2012). Seeing with the eyes shut: neural basis of enhanced imagery following Ayahuasca ingestion. Human brain mapping, 33(11), 2550–2560. https://doi.org/10.1002/hbm.21381
- Rocha, J. M., Osório, F. L., Crippa, J., Bouso, J. C., Rossi, G. N., Hallak, J., & Dos Santos, R. G. (2019). Serotonergic hallucinogens and recognition of facial emotion expressions: a systematic review of the literature. Therapeutic advances in psychopharmacology, 9, 2045125319845774. https://doi.org/10.1177/2045125319845774
- Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012 Dec 1;72(11):898-906. doi: 10.1016/j.biopsych.2012.04.005
- González, D., Cantillo, J., Pérez, I., Farré, M., Feilding, A., Obiols, J. E., & Bouso, J. C. (2020). Therapeutic potential of ayahuasca in grief: a prospective, observational study. Psychopharmacology, 237(4), 1171–1182. https://doi.org/10.1007/s00213-019-05446-2
- Franquesa A, Sainz-Cort A, Gandy S, Soler J, Alcázar-Córcoles MÁ, Bouso JC. Psychological variables implied in the therapeutic effect of ayahuasca: A contextual approach. Psychiatry Res. 2018 Jun;264:334-339. doi: 10.1016/j.psychres.2018.04.012
- Bouso JC, Dos Santos RG, Alcázar-Córcoles MÁ, Hallak JEC. Serotonergic psychedelics and personality: A systematic review of contemporary research. Neurosci Biobehav Rev. 2018 Apr;87:118-132. doi: 10.1016/j.neubiorev.2018.02.004
- Palhano-Fontes, F., Barreto, D., Onias, H., Andrade, K. C., Novaes, M. M., Pessoa, J. A., Mota-Rolim, S. A., Osório, F. L., Sanches, R., Dos Santos, R. G., Tófoli, L. F., de Oliveira Silveira, G., Yonamine, M., Riba, J., Santos, F. R., Silva-Junior, A. A., Alchieri, J. C., Galvão-Coelho, N. L., Lobão-Soares, B., Hallak, J., … Araújo, D. B. (2019). Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychological medicine, 49(4), 655–663. https://doi.org/10.1017/S0033291718001356
- Davis, A. K., Barrett, F. S., May, D. G., Cosimano, M. P., Sepeda, N. D., Johnson, M. W., Finan, P. H., & Griffiths, R. R. (2021). Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA psychiatry, 78(5), 481–489. https://doi.org/10.1001/jamapsychiatry.2020.3285
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015 Mar;29(3):289-99. doi: 10.1177/0269881114565144
- Johnson, M. W., Garcia-Romeu, A., Cosimano, M. P., & Griffiths, R. R. (2014). Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of psychopharmacology (Oxford, England), 28(11), 983–992. https://doi.org/10.1177/0269881114548296
- Gasser, P., Holstein, D., Michel, Y., Doblin, R., Yazar-Klosinski, B., Passie, T., & Brenneisen, R. (2014). Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. The Journal of nervous and mental disease, 202(7), 513–520. https://doi.org/10.1097/NMD.0000000000000113
- Griffiths, R. R., Johnson, M. W., Carducci, M. A., Umbricht, A., Richards, W. A., Richards, B. D., Cosimano, M. P., & Klinedinst, M. A. (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of psychopharmacology (Oxford, England), 30(12), 1181–1197. https://doi.org/10.1177/0269881116675513
- Barker S. A. (2018). N, N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function. Frontiers in neuroscience, 12, 536. https://doi.org/10.3389/fnins.2018.00536
- Cohen S. The Beyond Within, the LSD Story. New York: Atheneum; 1967.
- Dos Santos RG, Osório FL, Crippa JA, Hallak JE. Antidepressive and anxiolytic effects of ayahuasca: a systematic literature review of animal and human studies. Braz J Psychiatry. 2016 Mar;38(1):65-72. doi: 10.1590/1516-4446-2015-1701
- Ross, S., Bossis, A., Guss, J., Agin-Liebes, G., Malone, T., Cohen, B., Mennenga, S. E., Belser, A., Kalliontzi, K., Babb, J., Su, Z., Corby, P., & Schmidt, B. L. (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. Journal of psychopharmacology (Oxford, England), 30(12), 1165–1180. https://doi.org/10.1177/0269881116675512
- Krebs, T. S., & Johansen, P. Ø. (2013). Psychedelics and mental health: a population study. PloS one, 8(8), e63972. https://doi.org/10.1371/journal.pone.0063972
- Dos Santos RG, Landeira-Fernandez J, Strassman RJ, et al. Effects of ayahuasca on psychometric measures of anxiety, panic-like and hopelessness in Santo Daime members. J Ethnopharmacol. 2007;112(3):507–513. doi:10.1016/j.jep.2007.04.012
- Sanches RF, De Lima Osório F, Dos Santos RG, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a SPECT study. J Clin Psychopharmacol. 2016;36(1):77–81. doi:10.1097/JCP.0000000000000436
- Zeifman RJ, Singhal N, Dos Santos RG, Sanches RF, de Lima Osório F, Hallak JEC, Weissman CR. Rapid and sustained decreases in suicidality following a single dose of ayahuasca among individuals with recurrent major depressive disorder: results from an open-label trial. Psychopharmacology (Berl). 2021 Feb;238(2):453-459. doi: 10.1007/s00213-020-05692-9
- Dos Santos, R. G., Bouso, J. C., Rocha, J. M., Rossi, G. N., & Hallak, J. E. (2021). The Use of Classic Hallucinogens/Psychedelics in a Therapeutic Context: Healthcare Policy Opportunities and Challenges. Risk management and healthcare policy, 14, 901–910. https://doi.org/10.2147/RMHP.S300656
- Nichols DE. Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs. 1986;18(4):305–313. doi:10.1080/02791072.1986.10472362
- Danforth, A. L., Grob, C. S., Struble, C., Feduccia, A. A., Walker, N., Jerome, L., Yazar-Klosinski, B., & Emerson, A. (2018). Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology, 235(11), 3137–3148. https://doi.org/10.1007/s00213-018-5010-9
- MDMA-assisted Therapy in People With Anxiety Related to Advanced Stage Cancer. https://clinicaltrials.gov/ct2/show/NCT00252174
- Mithoefer MC, Mithoefer AT, Feduccia AA, Jerome L, Wagner M, Wymer J, Holland J, Hamilton S, Yazar-Klosinski B, Emerson A, Doblin R. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018 Jun;5(6):486-497. doi: 10.1016/S2215-0366(18)30135-4
- Reiff CM, Richman EE, Nemeroff CB, Carpenter LL, Widge AS, Rodriguez CI, Kalin NH, McDonald WM; the Work Group on Biomarkers and Novel Treatments, a Division of the American Psychiatric Association Council of Research. Psychedelics and Psychedelic-Assisted Psychotherapy. Am J Psychiatry. 2020 May 1;177(5):391-410. doi: 10.1176/appi.ajp.2019.19010035
- Nardou R, Lewis EM, Rothhaas R, et al. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature. 2019;569(7754):116–120. doi:10.1038/s41586-019-1075-9
- Feduccia AA, Mithoefer MC. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms?. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:221–228. doi:10.1016/j.pnpbp.2018.03.003
- Nagatomo T, Rashid M, Abul Muntasir H, Komiyama T. Functions of 5-HT2A receptor and its antagonists in the cardiovascular system. Pharmacol Ther. 2004 Oct;104(1):59-81. doi: 10.1016/j.pharmthera.2004.08.005
- Kaumann AJ, Levy FO. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006 Sep;111(3):674-706. doi: 10.1016/j.pharmthera.2005.12.004
- Ootsuka Y, Nalivaiko E, Blessing WW. Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, “Ecstasy”) and its reversal by clozapine. Brain Res. 2004 Jul 16;1014(1-2):34-44. doi: 10.1016/j.brainres.2004.03.058
- Balíková M. Nonfatal and fatal DOB (2,5-dimethoxy-4-bromoamphetamine) overdose. Forensic Sci Int. 2005 Oct 4;153(1):85-91. doi: 10.1016/j.forsciint.2005.04.022
- Johnson MW, Sewell RA, Griffiths RR. Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug Alcohol Depend. 2012 Jun 1;123(1-3):132-40. doi: 10.1016/j.drugalcdep.2011.10.029
- Bickel M, Ditting T, Watz H, Roesler A, Weidauer S, Jacobi V, Gueller S, Betz C, Fichtlscherer S, Stein J. Severe rhabdomyolysis, acute renal failure and posterior encephalopathy after ‘magic mushroom’ abuse. Eur J Emerg Med. 2005 Dec;12(6):306-8. doi: 10.1097/00063110-200512000-00011
- Tiscione NB, Miller MI. Psilocin identified in a DUID investigation. J Anal Toxicol. 2006 Jun;30(5):342-5. doi: 10.1093/jat/30.5.342
- Berrens Z, Lammers J, White C. Rhabdomyolysis After LSD Ingestion. Psychosomatics. 2010 Jul-Aug;51(4):356-356.e3. doi: 10.1176/appi.psy.51.4.356
- Alatrash G, Majhail NS, Pile JC. Rhabdomyolysis after ingestion of “foxy,” a hallucinogenic tryptamine derivative. Mayo Clin Proc. 2006 Apr;81(4):550-1. doi: 10.4065/81.4.550
- Raval MV, Gaba RC, Brown K, Sato KT, Eskandari MK. Percutaneous transluminal angioplasty in the treatment of extensive LSD-induced lower extremity vasospasm refractory to pharmacologic therapy. J Vasc Interv Radiol. 2008 Aug;19(8):1227-30. doi: 10.1016/j.jvir.2008.05.008
- Sunness JS. Persistent afterimages (palinopsia) and photophobia in a patient with a history of LSD use. Retina. 2004 Oct;24(5):805. doi: 10.1097/00006982-200410000-00022
- Bernhard MK, Ulrich K. Rezidivierende kortikale Blindheit nach LSD-Einnahme [Recurrent cortical blindness after LSD-intake]. Fortschr Neurol Psychiatr. 2009 Feb;77(2):102-4. German. doi: 10.1055/s-0028-1109114
- Jovel A, Felthous A, Bhattacharyya A. Delirium due to intoxication from the novel synthetic tryptamine 5-MeO-DALT. J Forensic Sci. 2014 May;59(3):844-6. doi: 10.1111/1556-4029.12367
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int. 2009 Jan 10;183(1-3):91-6. doi: 10.1016/j.forsciint.2008.11.001
- Barbee G, Berry-Cabán C, Barry J, Borys D, Ward J, Salyer S. Analysis of mushroom exposures in Texas requiring hospitalization, 2005-2006. J Med Toxicol. 2009 Jun;5(2):59-62. doi: 10.1007/BF03161087