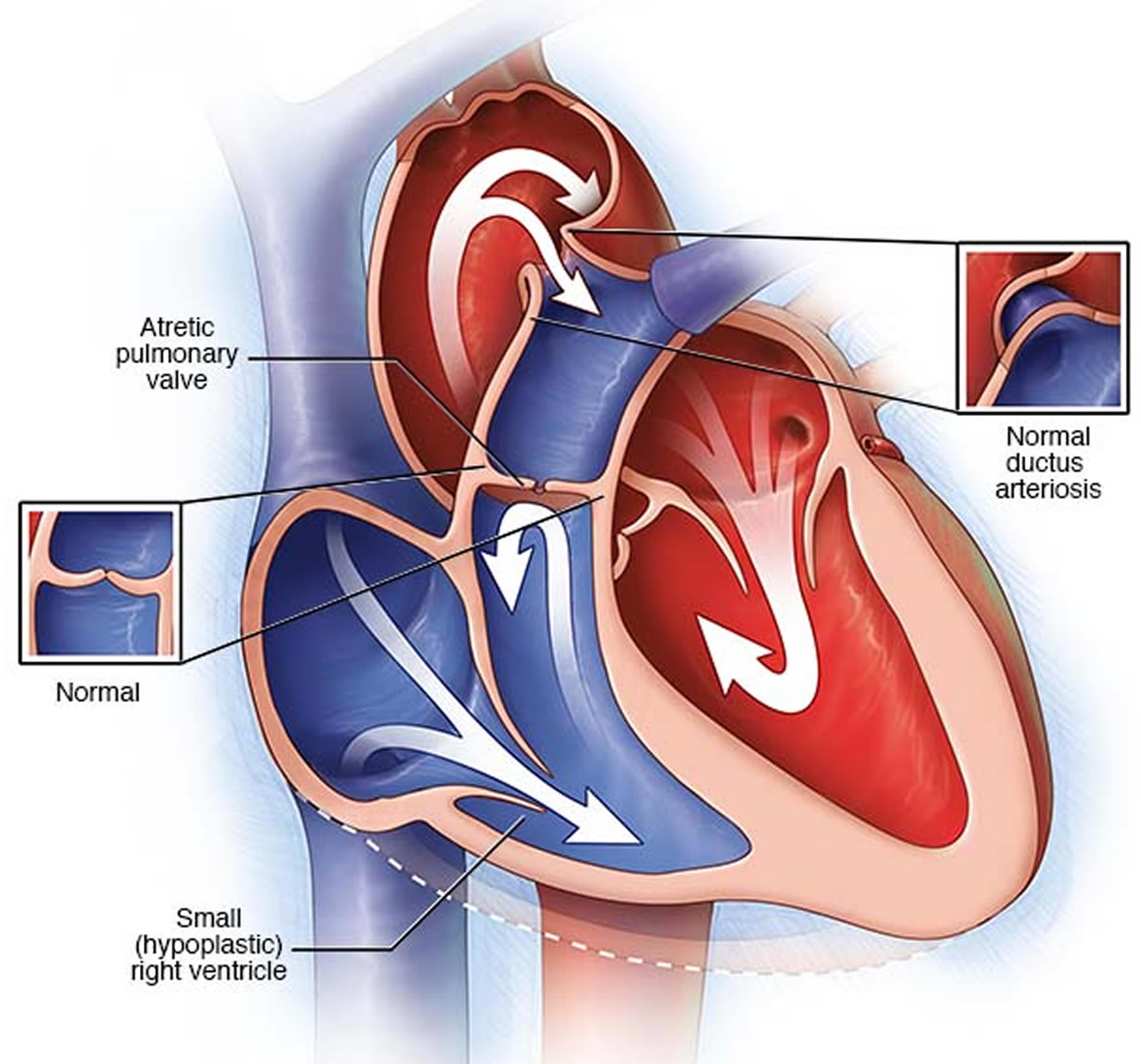
What is pulmonary atresia
Pulmonary atresia is a heart defect present at birth (congenital) that’s normally diagnosed within the first few hours or days of life. The word “atresia” means “no opening.” The pulmonary valve is an opening on the right side of the heart that regulates blood flow from the right ventricle (right side pumping chamber) to the lungs. With pulmonary atresia, the valve that lets blood flow from the lower-right chamber (the right ventricle) to the lungs has not formed or is closed by a solid sheet of tissue where the valve opening should be and the valve stays closed (see Figure 5). So blood can’t travel by its normal route to pick up oxygen from the lungs. Instead, some blood travels to the lungs through other natural passages within the heart and its arteries via a temporary connection (ductus arteriosus) between the baby’s aorta and the pulmonary artery. These passages are necessary when your baby is developing in the womb and they normally close soon after birth.
The temporary connection (ductus arteriosus) between the baby’s aorta and the pulmonary artery allows the oxygen-poor blood to travel directly from the upper-right chamber (the right atrium) to the left side of the heart – instead of following the normal route through the lungs. This oxygen-poor blood is then pumped back into the body. The lack of oxygen-rich blood makes the fingers, toes, and lips appear blue, a condition called cyanosis. Babies with pulmonary atresia typically have a bluish cast to their skin because they aren’t getting enough oxygen.
Some babies born with pulmonary atresia may also have a small (hypoplastic) right ventricle.
Pulmonary atresia is a life-threatening situation. Procedures to correct your baby’s heart condition and medications to help your baby’s heart work more effectively are the first steps to treat pulmonary atresia.
Babies with pulmonary atresia may seem normal before birth. This is because a passageway called the ductus arteriosus connects the pulmonary artery to the aorta in the fetal heart. The ductus arteriosus begins to close naturally after birth.
In a 2012 study using data from birth defects tracking systems across the United States, researchers estimated that about 1 out of every 10,000 babies is born with pulmonary atresia 1.
How the heart works
The heart is divided into four hollow chambers, two on the right and two on the left. In performing its basic job — pumping blood throughout the body — the heart uses its left and right sides for different tasks.
The right side of the heart moves blood to the lungs through vessels called pulmonary arteries. In the lungs, blood picks up oxygen then returns to the heart’s left side through the pulmonary veins. The left side of the heart then pumps the blood through the aorta and out to the rest of the body to supply your baby’s body with oxygen.
Blood moves through your baby’s heart in one direction through valves that open and close as the heart beats. The valve that allows blood out of your baby’s heart and into the lungs to pick up oxygen is called the pulmonary valve.
In pulmonary atresia, the pulmonary valve doesn’t develop properly, preventing it from opening. Blood can’t flow from the right ventricle to the lungs.
Before birth, the improperly formed valve isn’t life-threatening, because the placenta provides oxygen for your baby instead of the lungs. Blood entering the right side of your baby’s heart passes through a hole (foramen ovale) between the top chambers of your baby’s heart, so the oxygen-rich blood can be pumped out to the rest of your baby’s body through the aorta.
After birth, your baby’s lungs must provide oxygen for his or her body. In pulmonary atresia, without a working pulmonary valve, blood must find another route to reach your baby’s lungs.
The foramen ovale usually shuts soon after birth, but may stay open in pulmonary atresia. Newborn babies also have a temporary connection (ductus arteriosus) between the aorta and the pulmonary artery.
This passage allows some of the oxygen-poor blood to travel to the lungs where it can pick up oxygen to supply your baby’s body. The ductus arteriosus normally closes within a few hours or days after birth, but can be kept open with medications.
In some cases, there may be a second hole in the tissue that separates the main pumping chambers of your baby’s heart, called a ventricular septal defect (VSD).
The VSD allows a pathway for blood to pass through the right ventricle into the left ventricle. Children with pulmonary atresia and a VSD often have additional abnormalities of the lungs and the arteries that bring blood to the lungs. If there’s no VSD, the right ventricle receives little blood flow before birth and often doesn’t develop fully. This is a condition called pulmonary atresia with intact ventricular septum.
Figure 1. The anatomy of the heart valves
Figure 2. Top view of the 4 heart valves
Figure 3. Normal heart blood flow
Figure 4. Heart valves function
 Figure 5. Pulmonary atresia
Figure 5. Pulmonary atresia
Pulmonary atresia types
- Pulmonary atresia with intact ventricular septum
- Pulmonary atresia with VSD
Pulmonary atresia with intact ventricular septum
In this form of pulmonary atresia, the wall, or septum, between the ventricles remains complete and intact. Pulmonary atresia with intact ventricular septum is the combination of obstruction of the pulmonary outflow tract from pulmonary valve atresia without a ventricular septal defect. Blood returns to the left heart via a patent foramen ovale, and a patent ductus arteriosus is required for pulmonary blood flow. The right ventricle is variably hypoplastic, and the right atrium is typically dilated.
Figure 6. Pulmonary atresia with intact ventricular septum
Pulmonary atresia with intact ventricular septum associations
- Tricuspid regurgitation +/- Ebstein anomaly 2
- Aortopulmonary collaterals (rare) 2
- Down syndrome (rare). The association of pulmonary atresia with intact ventricular septum and Down’s syndrome is very rare but occurred in 3 of 240 (1.3%) cases in this study 3
During pregnancy when the heart is developing, very little blood flows into or out of the right ventricle (RV), and therefore the right ventricle (RV) doesn’t fully develop and remains very small. If the right ventricle is under-developed, the heart can have problems pumping blood to the lungs and the body. The artery which usually carries blood out of the right ventricle, the main pulmonary artery, remains very small, since the pulmonary valve doesn’t form.
Usually, some blood travels to the lungs through other passages within the heart and its arteries. Before birth, blood entering the right side of your baby’s heart passes through a hole between the top chambers of your baby’s heart (foramen ovale), so the oxygen-rich blood can be pumped out to the rest of your baby’s body. After birth, the foramen ovale normally closes, but may stay open in pulmonary atresia. Another temporary opening (ductus arteriosus) may allow some blood flow to the lungs after birth, but your baby will need medication, procedures or surgery to correct the defect.
Pulmonary atresia with intact ventricular septum treatment
Your baby’s doctor may suggest several different approaches to caring for your baby, including:
- Medication. Your baby’s doctor may give your baby a medication called prostaglandin by IV to prevent the ductus arteriosus from closing before the doctor performs a procedure.
- Procedures. Your baby most likely will require one or more procedures to restore heart function and blood flow. Some procedures are done in the first days to weeks of your baby’s life, and others are done later.
Procedures in the first days to weeks of life
- Balloon valvotomy. If the leaflets in your baby’s pulmonary valve are fused, your doctor might create a small hole at the center of the valve using a small amount of energy (radiofrequency ablation) or a wire and then open the leaflets using a balloon.
- Ductal stent placement. Placement of a rigid tube (stent) in the channel that connects the aorta and pulmonary artery (ductus arteriosus) permits blood to travel to the lungs.
- Balloon atrial septostomy. In rare circumstances, your doctor also may use a balloon to open up the foramen ovale, which typically may close soon after birth or becomes restrictive. This increases the amount of blood that can be pumped to the lungs.
- Systemic-to-pulmonary artery shunt. This is a surgical procedure that may be needed in the first few days of life to increase blood flow to the lungs by creation of a connection between one of the arteries and the pulmonary artery (shunt) using a small tube of synthetic material. One example of such procedure is the Blalock-Taussig shunt. This represents the first-stage palliation till your baby is big enough to undergo the second stage.
Later surgical procedures
- Bidirectional Glenn procedure. In this procedure, your baby’s surgeon attaches the blood vessel that handles blood from the upper body (superior vena cava) to the pulmonary artery. This procedure is usually done between 4 and 6 months of age. This can be performed without the need the use of the heart-lung machine (off-pump Glenn).
- Fontan procedure. During this procedure, which is typically done when your child is 2 or 3 years old, the surgeon will connect the blood vessel that handles blood from the lower body (inferior vena cava) to the pulmonary artery. This serves to restore the oxygen level back to normal and represents the final stage. This also can be performed without the use of the heart-lung machine (off-pump Fontan).
- One and one-half ventricle repair. In this procedure, the surgeon connects the superior vena cava to the pulmonary artery (Bidirectional Glenn). This can be the final procedure if the right ventricle in your baby’s heart is big enough and will not require the Fontan procedure. This approach reduces the likelihood of the long-term issues that can occur with the Fontan procedure.
- Hybrid procedures. Hybrid procedures involve a surgeon and a cardiologist who specializes in catheter procedures. In a hybrid procedure, surgical and catheter procedures are performed at the same operation, sometimes without the use of the heart-lung machine.
Pulmonary atresia with VSD (ventricular septal defect)
In this form of pulmonary atresia, a ventricular septal defect (VSD) allows blood to flow into and out of the right ventricle (RV). Therefore, blood flowing into the right ventricle can help the ventricle develop during pregnancy, so it is typically not as small as in pulmonary atresia with an intact ventricular septum. Pulmonary atresia with a VSD is similar to another condition called Tetralogy of Fallot. However, in tetralogy of Fallot, the pulmonary valve (PV) does form, although it is small and blood has trouble flowing through it – this is called pulmonary valve stenosis. Thus, pulmonary atresia with a VSD is like a very severe form of tetralogy of Fallot.
Figure 7. Pulmonary atresia with VSD
Pulmonary atresia with VSD treatment
Medication and several procedures may be needed to treat your baby’s pulmonary atresia with ventricular septal defect.
Procedures. Your baby will need one or more procedures to improve heart function and blood flow. There are several treatment pathways depending on the anatomy of the pulmonary arteries and the presence or absence of the major aortopulmonary collateral arteries.
- Stent placement. Placement of a rigid tube (stent) between the aorta and pulmonary artery (ductus arteriosus) allows blood to flow to your infant’s lungs.
- Systemic-to-pulmonary artery shunt. In this procedure, your infant’s surgeon uses a part of an artery from another part of the body to create a passage for blood flow (shunt).
Complete repair
- Neonatal complete repair. If your baby’s heart has well-developed pulmonary arteries and no major aortopulmonary collateral arteries, your baby’s surgeon can perform the complete repair in one stage during the neonatal period. This will include closure of the ventricular septal defect and placement of a valve conduit between the right ventricle and the pulmonary artery.
- One-stage complete repair. In this procedure, your baby’s surgeon connects all the major aortopulmonary collateral arteries together to create the new pulmonary arteries and then completes the repair with closure of the VSD and placement of the conduit. This is usually performed between 4 and 6 months of age. This procedure may also be called unifocalization.
Staged repair
- Staged unifocalization. If the major aortopulmonary collateral arteries are small or have multiple areas of narrowing, they may be connected in stages. This allows the arteries to grow to adequate size prior to the complete repair. The source of blood flow to the lungs will be provided through a small shunt from the aorta to the newly constructed pulmonary arteries. After a few months, your baby’s heart will be evaluated with a cardiac catheterization and cardiac CT scan to determine the suitability and timing of the complete repair.
- Catheter interventions for the pulmonary artery branches. This procedure is done to assess the anatomy of your baby’s heart and reconstruct pulmonary artery branches and dilate or stent the areas that have narrowing in them.
Pulmonary atresia outlook (prognosis)
Most cases can be helped with surgery. How well a baby does depends on:
- Size and connections of the pulmonary artery (the artery that takes blood to the lungs)
- How well the heart is beating
- How well the other heart valves are formed or how much they are leaking
Outcome varies because of the different forms of this defect. A baby may need only a single procedure or could need 3 or more surgeries and have only a single working ventricle.
Pulmonary atresia possible complications
Complications may include:
- Delayed growth and development
- Seizures
- Stroke
- Infectious endocarditis
- Heart failure
- Death
Even after surgical repairs, you’ll need to carefully monitor your child’s health for any changes that could signal a problem.
People with structural heart problems, such as pulmonary atresia, are at a higher risk of infectious endocarditis than the general population. Infectious endocarditis is an inflammation of the valves and inner lining of the heart caused by a bacterial infection.
Pulmonary atresia causes
The causes of heart defects, such as pulmonary atresia, among most babies are unknown. Some babies have heart defects because of changes in their genes or chromosomes. Heart defects also are thought to be caused by a combination of genes and other factors, such as the things the mother comes in contact with in the environment, or what the mother eats or drinks, or certain medicines she uses.
Pulmonary atresia is linked with another type of congenital heart defect called a patent ductus arteriosus (PDA).
Pulmonary atresia may occur with or without a ventricular septal defect (VSD).
- If the person does not have a VSD, the condition is called pulmonary atresia with intact ventricular septum.
- If the person has both problems, the condition is called pulmonary atresia with VSD. This is an extreme form of tetralogy of Fallot.
Although both conditions are called pulmonary atresia, they are actually different defects. This article discusses pulmonary atresia without a VSD.
Persons with pulmonary atresia with intact ventricular septum may also have a poorly developed tricuspid valve. They may also have an underdeveloped right ventricle and abnormal blood vessels feeding the heart.
Risk factors for pulmonary atresia
In most cases, the exact cause of a congenital heart defect, such as pulmonary atresia, is unknown. However, several factors may increase the risk of a baby being born with a congenital heart defect, including:
- A mother who had German measles (rubella) or another viral illness during early pregnancy
- A parent who has a congenital heart defect
- Drinking alcohol during pregnancy
- Smoking before or during pregnancy
- A mother who has poorly controlled diabetes
- A mother who has lupus, an autoimmune disorder
- Use of some types of medications during pregnancy, such as the acne drug isotretinoin (Claravis, Amnesteem, others), some anti-seizure medications and some bipolar disorder medications
- The presence of Down syndrome, a genetic condition that results from an extra 21st chromosome
Pulmonary atresia prevention
There is no known way to prevent this condition.
All pregnant women should get routine prenatal care. Many congenital defects can be found on routine ultrasound exams.
There are some things you can do that might reduce your child’s overall risk of congenital heart defects, such as:
- Get a German measles (Rubella) vaccine. If you develop German measles during pregnancy, it may affect your baby’s heart development. Being vaccinated before you try to conceive likely eliminates this risk.
- Control chronic medical conditions. If you have diabetes, keeping your blood sugar in check can reduce the risk of heart defects. If you have other chronic conditions, such as epilepsy, that require the use of medications, discuss the risks and benefits of these drugs with your doctor.
- Don’t smoke or drink. Smoking cigarettes and drinking alcohol during pregnancy may increase the risk of heart defects in your baby.
If the defect is found before birth, medical specialists (such as a pediatric cardiologist, cardiothoracic surgeon, and neonatologist) can be present at the birth, and ready to help as needed. This preparation can mean the difference between life and death for some babies.
Pulmonary atresia symptoms
Symptoms usually occur in the first few hours of life, although it may take up to a few days.
Pulmonary atresia symptoms may include:
- Bluish colored skin (cyanosis)
- Fast breathing or shortness of breath
- Fatigue
- Poor eating habits (babies may get tired while nursing or sweat during feedings)
- Shortness of breath
- Pale, clammy skin that may feel cool to the touch
Pulmonary atresia diagnosis
Pulmonary atresia may be diagnosed during pregnancy or soon after a baby is born.
During Pregnancy
During pregnancy, there are screening tests (also called prenatal tests) to check for birth defects and other conditions. Pulmonary atresia might be seen during an ultrasound (which creates pictures of the body). Some findings from the ultrasound may make the healthcare provider suspect a baby may have pulmonary atresia. If so, the healthcare provider can request a fetal echocardiogram to confirm the diagnosis. A fetal echocardiogram is an ultrasound specifically of the baby’s heart and major blood vessels that is performed during the pregnancy. This test can show problems with the structure of the heart and how well the heart is working.
After the Baby is Born
Babies born with pulmonary atresia will show symptoms at birth or very soon afterwards. They may have a bluish looking skin color, called cyanosis, because their blood doesn’t carry enough oxygen. Infants with pulmonary atresia can have additional symptoms such as:
- Problems breathing
- Ashen or bluish skin color
- Poor feeding
- Extreme sleepiness
During a physical examination, a doctor can see the symptoms, such as bluish skin or problems breathing. Using a stethoscope, a doctor will check for a heart murmur (an abnormal “whooshing” sound caused by blood not flowing properly). However, it is not uncommon for a heart murmur to be absent right at birth.
If a doctor suspects that there might be a problem, the doctor can request one or more tests to confirm the diagnosis of pulmonary atresia. The most common test is an echocardiogram. This test is an ultrasound of the baby’s heart that can show problems with the structure of the heart, like holes in the walls between the chambers, and any irregular blood flow. Cardiac catheterization (inserting a thin tube into a blood vessel and guiding it to the heart) also can confirm the diagnosis by looking at the inside of the heart and measuring the blood pressure and oxygen levels. An electrocardiogram (ECG), which measures the electrical activity of the heart, and other medical tests may also be used to make the diagnosis.
Pulmonary atresia is a critical congenital heart defect (critical congenital heart defect) that may be detected with newborn screening using pulse oximetry (also known as pulse ox). Pulse oximetry is a simple bedside test to estimate the amount of oxygen in a baby’s blood. Low levels of oxygen in the blood can be a sign of a critical congenital heart defect. Newborn screening using pulse oximetry can identify some infants with a critical congenital heart defect, like pulmonary atresia, before they show any symptoms.
Several tests may be used to diagnose pulmonary atresia, including:
- Echocardiogram. In an echocardiogram, sound wave movement shows the features of your child’s heart. Your doctor might also use an echocardiogram of your abdomen before you deliver your baby (fetal echocardiogram) to diagnose pulmonary atresia.
- Electrocardiogram (ECG). In this test, sensor patches with wires attached (electrodes) measure the electrical impulses given off by your baby’s heart. An ECG reveals abnormal heart rhythms (arrhythmias or dysrhythmias) and may show other heart problems.
- Cardiac catheterization. In catheterization, your baby’s doctor inserts a thin, flexible tube (catheter) into a blood vessel in your baby’s groin and guides it to your baby’s heart. This test shows your baby’s heart structure and the blood pressure and oxygen levels in your baby’s heart, pulmonary artery and aorta. The doctor may inject a dye into the catheter to make your baby’s arteries visible under X-ray.
- Pulse oximetry. This test indicates how much oxygen your baby is getting through his or her blood.
- X-ray. An X-ray shows your baby’s doctor the size of your baby’s pulmonary atresia, as well as bones and other tissues.
Pulmonary atresia treatment
Newborns with pulmonary atresia are usually given an intravenous medicine called prostaglandin E1 to keep the ductus arteriosus open after birth. Keeping this blood vessel open will help with blood flow to the lungs until the pulmonary valve can be repaired. This is not a permanent solution, but it can buy your doctors enough time to determine what type of surgery or procedure might be best for your child.
Procedures via catheterization
In some cases, repairs can be made via a long, thin tube (catheter) inserted into a large vein in your baby’s groin and threaded up to the heart. These procedures include:
- Radiofrequency ablation and balloon valvotomy. If your baby’s pulmonary valve features fused cusps, your doctor might apply a small amount of energy (radiofrequency ablation) or use a wire to create a small hole at the center of the valve and then use a balloon to open the cusps.
- Balloon atrial septostomy. A balloon can also be used to enlarge the natural hole (foramen ovale) in the wall between the upper two chambers of the heart. This hole usually closes shortly after birth. Enlarging it increases the amount of blood available to travel to the lungs.
- Stent placement. Your baby’s doctor may place a rigid tube (stent) in the natural connection between the aorta and pulmonary artery (ductus arteriosus). This opening also usually closes a few days after birth. Keeping it open allows blood to travel to the lungs.
Heart surgery
The type of surgical repair needed will depend on the size of your child’s right ventricle and pulmonary artery. Babies with pulmonary atresia often require a series of heart operations over time. Some examples include:
- Shunting. Creating a bypass (shunt) from the main blood vessel leading out of the heart (aorta) to the pulmonary arteries allows for adequate blood flow to the lungs. However, babies usually outgrow this shunt within a few months.
- Glenn procedure. In this surgery, one of the large veins that normally returns blood to the heart is connected directly to the pulmonary artery instead. Another large vein continues to provide blood to the right side of the heart, which pumps it through the surgically repaired pulmonary valve. This can help the right ventricle grow larger.
- Fontan procedure. If the right ventricle remains too small to be useful, surgeons may use a Fontan procedure to create a pathway that allows most, if not all, of the blood coming to the heart to flow directly into the pulmonary artery.
- Heart transplant. In some cases, the heart is too damaged to repair and a heart transplant may be necessary.
In some cases, blood flow can be improved by using cardiac catheterization (inserting a thin tube into a blood vessel and guiding it to the heart). During this procedure, doctors can expand the valve using a balloon or they may need to place a stent (a small tube) to keep the ductus arteriosus open.
In most cases of pulmonary atresia, a baby may need surgery soon after birth. During surgery, doctors widen or replace the pulmonary valve and enlarge the passage to the pulmonary artery. If a baby has a ventricular septal defect, the doctor also will place a patch over the ventricular septal defect to close the hole between the two lower chambers of the heart. These actions will improve blood flow to the lungs and the rest of the body. If a baby with pulmonary atresia has an underdeveloped right ventricle, he or she might need staged surgical procedures, similar to surgical repairs for hypoplastic left heart syndrome.
The type of surgery recommended depends on the size of the right ventricle and the pulmonary artery. If they are normal in size and the right ventricle is able to pump blood, open heart surgery can be done to make blood flow through the heart in a normal pattern.
If the right ventricle is small and unable to act as a pump, doctors may perform an operation called the Fontan procedure. In this procedure, the right atrium is connected directly to the pulmonary artery.
In some cases, if the right ventricle and pulmonary artery are too small, it may be impossible to repair the defect.
- Most babies with pulmonary atresia will need regular follow-up visits with a cardiologist (a heart doctor) to monitor their progress and check for other health conditions that might develop as they get older. As adults, they may need more surgery or medical care for other possible problems.
Home remedies
If your baby is born with pulmonary atresia, it may seem that almost all your time is spent at the hospital or at a doctor’s office. But there will be time spent at home, as well. Here are some tips for caring for your child at home:
- Strive for good nutrition. Your baby may have a difficult time taking in enough calories, both because he or she tires more easily during feeding and because of an increased demand for calories. It’s often helpful to give your baby frequent, small feedings.
- Preventive antibiotics. Your child’s cardiologist will likely recommend that your child take preventive antibiotics before certain dental and other procedures to prevent bacteria from entering the bloodstream and infecting the inner lining of the heart (infective endocarditis). Practicing good oral hygiene — brushing and flossing teeth, getting regular dental checkups — is another good way of preventing infection.
- Help your child stay active. Encourage as much normal play and activity as your child is able to tolerate, with ample opportunity for rest and nap time.
- Staying active helps your child’s heart stay fit. As your child grows, talk with the cardiologist about which activities are best for your child. If some are off-limits, such as competitive sports, encourage your child in other pursuits rather than focusing on what he or she can’t do.
- Keep up with routine well-child care. Standard immunizations are encouraged for children with congenital heart defects, as well as vaccines against the flu, pneumonia and respiratory syncytial virus infections.
- Keep regular follow-up appointments with your child’s doctor. Your child may need regular annual appointments with his or her doctor trained in congenital heart conditions, even throughout his or her adult life.
Coping and support
It’s natural for many parents to feel worried about their child’s health, even after treatment of a congenital heart defect. Although many children who have congenital heart defects can do the same things children without heart defects can, here are a few things to keep in mind if your child has a congenital heart defect:
- Developmental difficulties. Because some children who have congenital heart defects may have had a long recovery time from surgeries or procedures, they may developmentally lag behind other children their age. Some children’s difficulties may last into their school years, and they may have difficulties learning to read or write, as well. Talk to your child’s doctor about ways to help your child through his or her developmental difficulties.
- Emotional difficulties. Many children who have developmental difficulties may feel insecure about their abilities and may have emotional difficulties as they reach school age. Talk to your child’s doctor about ways you can help your child cope with these problems, which may include support groups for parents, or a visit to a therapist or psychologist for your child.
- Support groups. Having a child with a serious medical problem isn’t easy and, depending on the severity of the defect, may be very difficult and frightening. You may find that talking with other parents who’ve been through the same situation brings you comfort and encouragement. Ask your child’s doctor if there are any local support groups.
- Mai CT, Riehle-Colarusso T, O’Halloran A, Cragan JD, Olney RS, Lin A, Feldkamp M, Botto LD, Rickard R, Anderka M, Ethen M, Stanton C, Ehrhardt J, Canfield M; National Birth Defects Prevention Network. Selected Birth Defects Data from Population-based Birth Defects Surveillance Programs in the United States, 2005-2009: Featuring Critical Congenital Heart Defects Targeted for Pulse Oximetry Screening. Birth Defects Research Part A: Clinical and Molecular Teratology. 2012;94: 970-983.
- Alwi M. Management algorithm in pulmonary atresia with intact ventricular septum. Catheter Cardiovasc Interv. 2006;67 (5): 679-86. doi:10.1002/ccd.20672 https://www.ncbi.nlm.nih.gov/pubmed/16572430
- Daubeney PE, Sharland GK, Cook AC et-al. Pulmonary atresia with intact ventricular septum: impact of fetal echocardiography on incidence at birth and postnatal outcome. UK and Eire Collaborative Study of Pulmonary Atresia with Intact Ventricular Septum. Circulation. 1998;98 (6): 562-6. doi:10.1161/01.CIR.98.6.562 http://circ.ahajournals.org/content/98/6/562.long