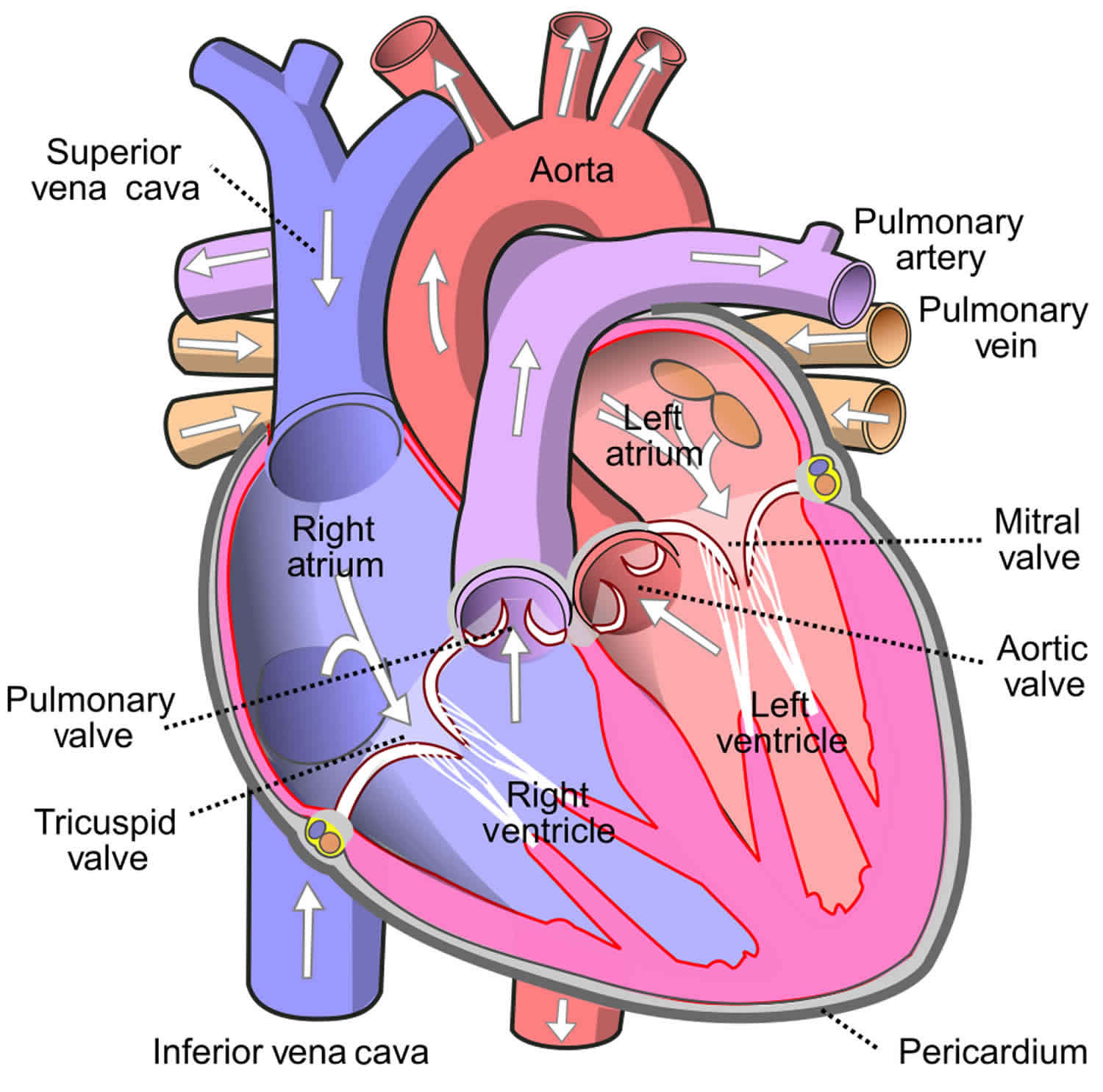
Pulmonary insufficiency
Pulmonary insufficiency also known as pulmonary regurgitation, pulmonic regurgitation or pulmonic valvular regurgitation, is a condition in which the pulmonary valve — located between your heart’s lower right heart chamber (right ventricle) and the artery that delivers blood to the lungs (pulmonary artery) — doesn’t work properly by allowing backflow of blood from the pulmonary artery to the right ventricle of the heart during diastole. Pulmonary insufficiency can interrupt blood flow from your heart to your lungs.
The pulmonary valve normally acts like a one-way door from your heart’s right ventricle to the lungs. Blood flows from the right ventricle through the pulmonary valve to the pulmonary artery and then into the lungs, where it picks up oxygen to deliver to your body.
When the heart squeezes, the right ventricle (the lower right chamber) pumps blood out into the pulmonary artery. The pulmonary artery then takes the blood to the lungs. The pulmonic or pulmonary valve is located between the right ventricle and the main pulmonary artery. Its job is to prevent blood from leaking back into the heart between beats.
Pulmonary regurgitation occurs when the pulmonary valve doesn’t completely close and allows some blood to leak back into the heart. This condition is also known as pulmonic or pulmonary insufficiency. Pulmonary regurgitation can be categorized as mild, moderate or severe.
Pulmonary valve anatomy and function
The pulmonary valve is the semilunar valve that separates the right ventricle from the pulmonary trunk 1. Anatomically, the annulus (ring-like connective tissue) of pulmonary valve delimits the right ventricle chamber at the junction of the pulmonary arterial trunk 2. The annulus and the cardiac fibrous skeleton, a structure which connects the pulmonary valve to other heart valves, plays an essential role in anchoring all the heart valves in the myocardium. The pulmonary valve consists of three cusps; anterior, left, and right cusp. Each of these cusps is separated from one another by a commissure. Below the free margin, these cusps overlap one another for about several millimeters to ensure adequate closure of the lumen. The overlap area is called lunula. At the central portion of the lunula, there is a local fibrous thickening that forms a nodule (nodule of Arantius) that maximize closure of the lumen. At the superior border of the pulmonary valve, there is a pocket formed by the valve cusp and adjacent arterial wall called the sinus of Valsalva 3.
The pulmonary valve is separated from the tricuspid valve by a muscular fold, known as the ventriculoinfundibular fold. At its septal margin, the fold forms the supraventricular crest and this fold inserts between the anterior and posterior limbs of the septomarginal trabeculation. This same fold also forms the subpulmonary infundibulum of the right ventricular outlet 2.
Histologically, the pulmonary valve consists of stratified extracellular matrix compartments of four layers which are the arterialis, fibrosa, spongiosa, and the ventricularis layer. The arterialis faces the artery, and the ventricularis layer faces the ventricle 4. Each of these layers has a different composition and function. The arterialis layer is the thinnest layer whose function remains. The fibrosa layer is the backbone of the semilunar valves and consists of circumferentially arranged dense collagen networks that merge with the annulus and the cardiac fibrous skeleton. The spongiosa layer is the layer that allows shear stress between layers during flexure and provides compressive strength because it consists of proteoglycans and glycosaminoglycans. The ventricularis layer contained the most elastic fibers and assists with the elastic recoil of the cusps 5.
The pulmonary valve opens at the systolic phase of the cardiac cycle enabling the deoxygenated blood to be pumped from the right ventricle to the pulmonary circulation. It closes at the diastolic phase of the cardiac cycle, allowing sufficient filling of the right ventricle. The pulmonary valve has a diameter of about 20 mm 1.
Pulmonary valve regurgitation classification
Pulmonary valve regurgitation is classified into the following types based on the morphology of the valve and severity of the disease:
Pulmonary valve morphology
Pulmonary valve regurgitation is classified into primary and secondary types based on the involvement of the pulmonary valve
- Primary pulmonary regurgitation: The pulmonary valve morphology is affected. Isolated pulmonary insufficiency is very rare and is most commonly associated with other congenital heart diseases 6
- Secondary or functional pulmonary regurgitation: The pulmonary valve function is normal. Conditions such as pulmonary hypertension and pulmonary artery aneursym cause dilation of the valve annulus resulting in regurgitation 7
Pulmonary regurgitation severity
Pulmonary valve regurgitation is classified into three categories based on the severity into the following 8:
- Mild pulmonary regurgitation: normal valve morphology and usually asymptomatic
- Moderate pulmonary regurgitation: normal to abnormal valve morphology with mild symptoms
- Severe pulmonary regurgitation: abnormal valve morphology with significant symptomatology
Pulmonary insufficiency causes
The most common causes for a leaky pulmonary valve is pulmonary hypertension or a congenital heart defect most specifically, a defect called tetralogy of Fallot.
Less common causes are:
- Infective endocarditis
- Complications after surgery to repair tetralogy of Fallot
- Carcinoid syndrome
- Rheumatic fever and complications after catheterization are rare causes in the United States
Primary and pulmonary hypertension
Primary pulmonary hypertension occurs in approximately 1 per 500,000 cases. This diagnosis can be made only after all other causes have been excluded. Primary causes include iatrogenic, infective endocarditis, systemic (carcinoid disease), immune-mediated (rheumatic heart disease), and congenital heart disease 9.
Secondary pulmonary hypertension (multiple causes) is the most common cause of pulmonic regurgitation in adults. Secondary or functional pulmonary valve regurgitation occurs in patients with nromal pulmonic valve who have severe pulmonary arterial hypertension and/or pulmonary artery dilatation 10.
Tetralogy of Fallot
Tetralogy of Fallot, especially with congenital absence of the pulmonary valve, or postoperative following surgical repair of this condition (eg, pulmonary valvotomy), commonly cause significant pulmonary valve regurgitation 11.
Infective endocarditis
In rare cases, infective endocarditis results in significant pulmonary valve regurgitation. It may occur in an intravenous/injection drug user or in an individual with an atrial septal defect and a large left-to-right intracardiac shunt.
Rheumatic and carcinoid heart diseases
In rheumatic heart disease leading to significant pulmonary valve regurgitation, the pulmonary valve is affected following mitral, aortic, and tricuspid valve involvement.
Medications
Medications that act via serotoninergic pathways may result in significant pulmonary valve regurgitation (eg, methysergide, pergolide, fenfluramine).
Disorders that dilate the pulmonic valve ring to create valvular incompetence
Disorders that dilate the pulmonic valve ring to create valvular incompetence are the most common cause of pulmonary valve regurgitation and include primary or secondary pulmonary hypertension, dilatation of the pulmonary trunk in Marfan syndrome or Takayasu arteritis, and idiopathic causes.
Acquired disorders that alter pulmonic valve morphology
Acquired conditions that alter pulmonic valve morphology include the following:
- Rheumatic heart disease: In most cases, the other valves (ie, mitral, aortic, tricuspid) are also substantially affected.
- Trauma from a Swan-Ganz catheter: This cause is unusual, but it can result if the catheter tip is withdrawn across the pulmonic valve with the balloon inflated.
- Complications related to therapeutic balloon catheter dilation of a stenotic pulmonic valve (eg, pulmonary balloon valvuloplasty): Such complications are not uncommon; however, in most cases, the degree of regurgitation is clinically insignificant, rendering pulmonic valve balloon catheter dilation a safe and effective treatment for moderate to severe pulmonic stenosis in adult and pediatric patients.
- Complications of surgical repair of pulmonic stenosis 12 or congenital heart disease, such as tetralogy of Fallot 13
- Syphilis infection
- Carcinoid heart disease: The heart is affected in up to 60% of patients in whom carcinoid heart disease has metastasized to the liver, most commonly manifesting as valvular disease. In a series of 74 patients, the pulmonic valve was involved in 88% 14. Of those affected, 49% exhibited significant pulmonic stenosis, and 81% had significant pulmonary valve regurgitation.
Congenital disorders that produce an incompetent pulmonic valve
These include complete absence of the pulmonic valve and valvular abnormalities (eg, fenestrations or redundant leaflets).
Pulmonary insufficiency symptoms
Patients with pulmonary regurgitation are typically asymptomatic prior to the onset of right ventricular dysfunction. Signs that can be detected in a medical exam include a certain type of murmur heard when the heart is between heart beats. The murmur is in early diastole and it may increase in intensity with inspiration. With more signicant pulmonary valve regurgitation, an ejection murmur in systole may be heard and a third heart sound may be present.
Eventually, whether due to the pulmonary valve problem or the pulmonary hypertension that may have caused the pulmonary valve problem, the lower right chamber of the heart can become enlarged. Rarely, pulmonary insufficiency can progress to heart failure which can create more noticeable symptoms such as chest pain or discomfort, fatigue, lightheadedness or fainting.
With the progression of right ventricular dysfunction, the onset of atrial and ventricular arrhythmias, palpitations, and syncope may occur.
Significant pulmonary valve regurgitation can be associated with the following symptoms:
- Distended neck veins
- Palpable pulmonary artery pulsation
- Pedal edema
- Hepatosplenomegaly
- Ascites
- Syncope
- Lightheadedness
In children abnormal signs and symptoms may appear, including:
- Feeling tired
- Fainting with exercise or other activity
- Abnormal heart rhythms
- A heart murmur (an extra heart sound when a doctor listens with a stethoscope)
Pulmonary insufficiency diagnosis
Echocardiography generally confirms the diagnosis of pulmonic valve insufficiency, which also provides an evaluation of the mechanism, cause, and severity of the valve disease. In addition, echocardiography provides information about the hemodynamic effects and the assessment of associated disorders, such as pulmonary artery hypertension. Pulmonary valve regurgitation severity is determined by jet width, density, and deceleration rate. Patients with moderate or greater pulmonary valve regurgitation should also undergo evaluation with cardiovascular magnetic resonance imaging (CMRI) to provide a quantitative assessment of pulmonary valve insufficiency and of right ventricle size and function.
Pulmonary insufficiency diagnosis may require some or all of these tests:
- Pulse oximetry – a painless way to monitor the amount of oxygen in the blood
- Chest X-ray
- Echocardiogram (also called “echo” or cardiac ultrasound) – sound waves used to see the heart
- Electrocardiogram (ECG) – measures the electrical activity of the heart
- Cardiac MRI (CMRI) – a three-dimensional picture of the heart arteries and veins
- Cardiac catheterization – a thin tube is inserted into the heart through a vein and/or artery in either the leg or through the umbilicus (“belly button”)
Pulmonary insufficiency treatment
Treatment for pulmonary regurgitation is usually focused on the underlying cause that created the valve problem (i.e. pulmonary hypertension). The need to replace the pulmonary valve is very rare.
Mild pulmonary regurgitation is very common and may not require any treatment. If the pulmonary valve is normal, there may not even be a need for regular checkups. However, if there is moderate or severe pulmonary regurgitation, doctors will monitor the patient with regular checkups.
Currently, medications are not used to help with pulmonary regurgitation itself, but medications may be used to help the heart to pump more efficiently.
In severe cases of pulmonary regurgitation, surgery may be needed to repair or to replace the pulmonary valve. Surgeons will consider your child’s age, gender and particular needs, as well as the valve anatomy, before attempting to repair the valve (or at least improve its function) with a type of surgery called a valvuloplasty.
Another treatment option includes the insertion of an artificial pulmonary valve, which can be done either by a surgeon or a cardiologist.
The following are indications for surgical pulmonic valve replacement 15:
- Severe, symptomatic pulmonary valve regurgitation
- Asympatomatic severe pulmonary valve regurgitation with severe right ventricular dilatation and/or dysfunction, or symptomatic atrial and/or ventricular arrythmias; dysfunction on cardiovascular magnetic resonance imaging with a right ventricular end-diastolic volume above 150 mL/m², an end-systolic volume over 80 mL/m², and an ejection fraction below 47%
- Severe pulmonary valve regurgitation and progressive tricuspid valve regurgitation
Follow-up care for pulmonary regurgitation
Through 18
Children with moderate or severe pulmonary regurgitation require regular checkups with a pediatric cardiologist. Our pediatric cardiologists follow patients until they are young adults, coordinating care with the primary care physicians.
Into adulthood
Pulmonary regurgitation can cause heart problems in adults. It is very important that adults with pulmonary insufficiency are monitored by a cardiologist regularly.
Pulmonary insufficiency prognosis
The prognosis for patients with severe pulmonary or pulmonic regurgitation (pulmonary valve regurgitation) depends on the presence or absence of right ventricular dysfunction, pulmonary artery dilatation, and symptoms.
Most patients with with pulmonary valve regurgitation following repair of tetralogy of Fallot carry an excellent prognosis. However there is a late mortality that is related to right ventricular dysfunction 16. Residual pulmonary valve regurgitation is an important determinant of outcome, as it may contribute to right ventricular hypertrophy and dysfunction, a propensity for arrhythmias, and an increased risk for sudden cardiac death 17.
In general, survival is not significantly affected by mild to moderate pulmonary valve regurgitation. If the pulmonary valve regurgitation is severe, the right ventricle is initially able to compensate for the volume overload state, and the state may remain well compensated for years. However, persistently elevated right ventricular volumes may eventually cause right ventricular dilatation and, finally, failure.
As previously stated, the various disorders causing pulmonary hypertension are the most common causes of clinically significant pulmonary valve regurgitation. The principal prognostic indicators of mortality in pulmonary valve regurgitation associated with pulmonary hypertension are (1) the severity and duration of the pulmonary hypertension at the time of diagnosis and (2) the right ventricular response to the state of volume overload.
In all etiologies of pulmonary hypertension, early diagnosis that allows for intervention to slow or reverse the cause of pulmonary hypertension is essential, although, in many cases, diagnosis is difficult and requires a high degree of clinical suspicion.
In primary pulmonary hypertension, the pathologic process is often insidious, and symptoms manifest at an advanced disease state, resulting in an average survival period of 2.5 years from the time of diagnosis.
In congenital regurgitation of the pulmonic valve, the prognosis depends upon the initial severity, progression of the regurgitation, and the ability of the right ventricle to adapt to volume overload. Usually, the degree of regurgitation in this condition is no more than moderate, so no clinical sequelae occur. Congenital absence of the pulmonic valve, a much rarer condition, confers an increased risk of morbidity and mortality because of more severe regurgitation, and it usually warrants pulmonic valve replacement for improved prognosis.
Morbidity, mortality, and complications
As noted, the morbidity and mortality rates associated with pulmonic regurgitation vary considerably, depending on the underlying cause.
Severe pulmonary valve regurgitation may result in right ventricular enlargement, systolic dysfunction, and death 18. Complications of severe pulmonary valve regurgitation may lead to right-sided heart failure (itself a complication of right ventricular volume overload), thromboembolic events, hepatic congestion, systolic dysfunction, arrhythmias, and death.
Other complications are related to the underlying disease processes resulting in pulmonary valve regurgitation.
After a pulmonic valve replacement, infective endocarditis and structural valve failure are signifcant long-term complications 19.
References- Sundjaja JH, Bordoni B. Anatomy, Thorax, Heart Pulmonic Valve. [Updated 2019 Sep 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547706
- Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart. 2006 Apr;92 Suppl 1:i2-13.
- Stephens EH, Kearney DL, Grande-Allen KJ. Insight into pathologic abnormalities in congenital semilunar valve disease based on advances in understanding normal valve microstructure and extracellular matrix. Cardiovasc. Pathol. 2012 Jan-Feb;21(1):46-58.
- Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ. Res. 2009 Aug 28;105(5):408-21.
- Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008 Oct 28;118(18):1864-80.
- Chaturvedi RR, Redington AN (2007). “Pulmonary regurgitation in congenital heart disease”. Heart. 93 (7): 880–9. doi:10.1136/hrt.2005.075234
- Di Lullo L, Floccari F, Rivera R, Barbera V, Granata A, Otranto G; et al. (2013). “Pulmonary Hypertension and Right Heart Failure in Chronic Kidney Disease: New Challenge for 21st-Century Cardionephrologists”. Cardiorenal Med. 3 (2): 96–103. doi:10.1159/000350952
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K; et al. (2010). “Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography”. J Am Soc Echocardiogr. 23 (7): 685–713, quiz 786-8. doi:10.1016/j.echo.2010.05.010
- [Guideline] Nishimura RA, Otto CM, Bonow RO, et al, for the American College of Cardiology., American College of Cardiology/American Heart Association., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014 Jul. 148 (1):e1-e132.
- Renella P, Aboulhosn J, Lohan DG, et al. Two-dimensional and Doppler echocardiography reliably predict severe pulmonary regurgitation as quantified by cardiac magnetic resonance. J Am Soc Echocardiogr. 2010 Aug. 23 (8):880-6.
- Murphy JG, Gersh BJ, Mair DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993 Aug 26. 329 (9):593-9.
- Liu S, Xu X, Liu G, Ding X, Zhao X, Qin Y. Comparison of immediate and long-term results between the single balloon and inoue balloon techniques for percutaneous pulmonary valvuloplasty. Heart Lung Circ. 2015 Jan. 24 (1):40-5.
- Eyskens B, Brown SC, Claus P, et al. The influence of pulmonary regurgitation on regional right ventricular function in children after surgical repair of tetralogy of Fallot. Eur J Echocardiogr. 2010 May. 11 (4):341-5.
- Pellikka PA, Tajik AJ, Khandheria BK, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993 Apr. 87 (4):1188-96.
- Pulmonary Regurgitation (Pulmonic Regurgitation). https://emedicine.medscape.com/article/157639-overview
- Nollert G, Fischlein T, Bouterwek S, Bohmer C, Klinner W, Reichart B. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997 Nov 1. 30 (5):1374-83.
- Hickey EJ, Veldtman G, Bradley TJ, et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009 Jan. 35 (1):156-64; discussion 164.
- Khairy P, Aboulhosn J, Gurvitz MZ, et al, for the Alliance for Adult Research in Congenital Cardiology (AARCC). Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010 Aug 31. 122 (9):868-75.
- Lee C, Kim YM, Lee CH, et al. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: implications for optimal timing of pulmonary valve replacement. J Am Coll Cardiol. 2012 Sep 11. 60 (11):1005-14.