Pyeloplasty
Pyeloplasty is a surgery where the ureteropelvic junction obstruction is removed and the ureter is reattached to the renal pelvis to create a wide opening. Pyeloplasty lets the urine drain quickly and easily. Pyeloplasty also relieves symptoms and the risk of urinary tract infection. The surgeon’s cut is usually 2 to 3 inches long, just below the ribs. Pyeloplasty procedure usually takes a few hours with a great success rate (95% success). The patient may have to stay in the hospital for a day or 2 after surgery. Drainage tubes can be used to promote healing.
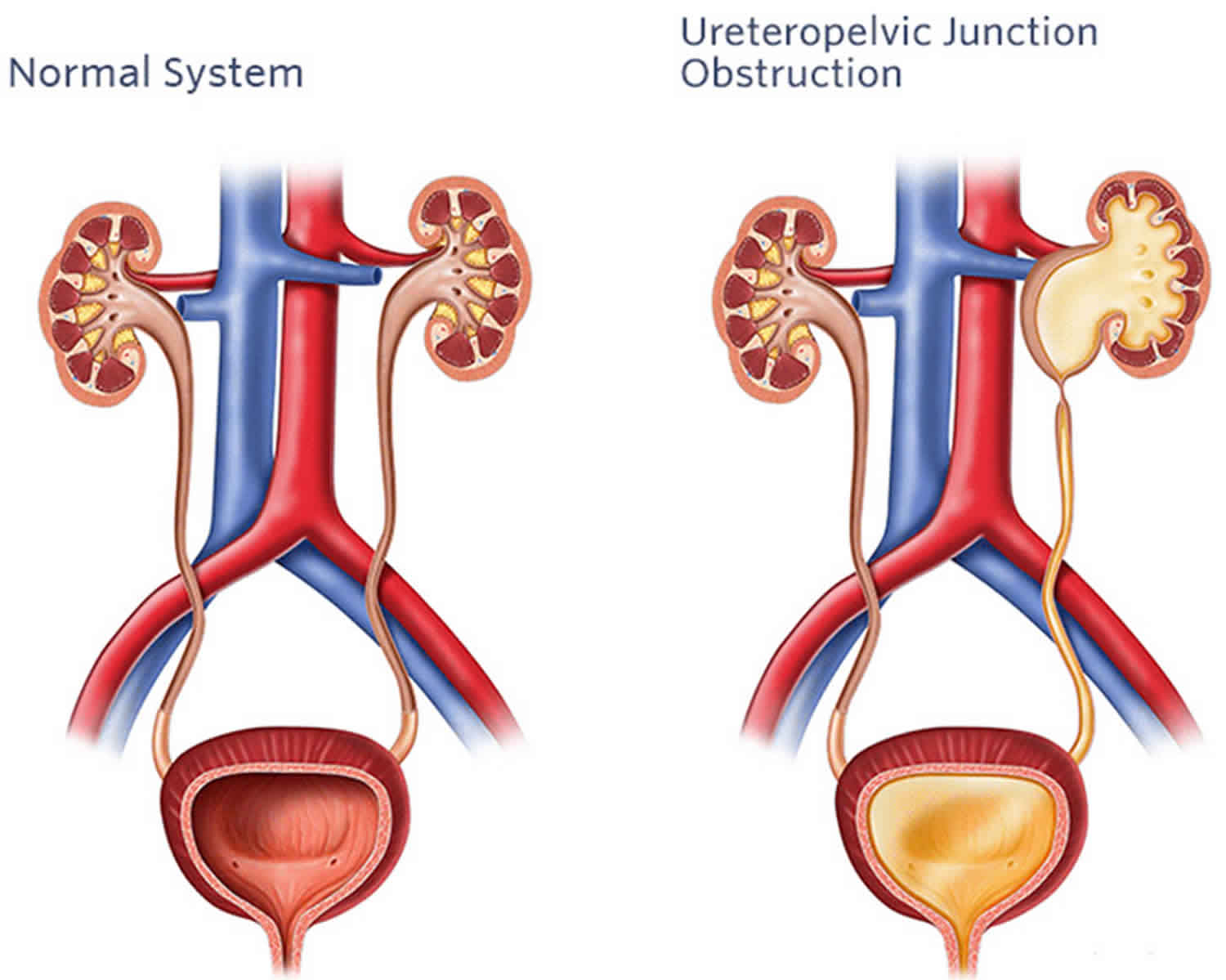
Urologic texts define ureteropelvic junction obstruction as an impediment to urinary flow from the renal pelvis into the ureter, which may result in symptoms or renal damage. Ureteropelvic junction obstruction is the most common cause of prenatal hydronephrosis (a congenital abnormality of the urinary tract), accounting for 80% of the cases. Ureteropelvic junction obstruction often happens when a baby is still growing in the womb. This is called a congenital condition (present from birth). The frequency of births with unilateral ureteropelvic junction obstruction is estimated to be 1 case in 5000-8000 live births, and it is bilateral in 6% of these cases. Studies have indicated that ureteropelvic junction obstruction is more common on the left side than on the right by a left-to-right ratio of 5:2 and that it is has a male-to-female ratio of 5:2. Another study found bilateral ureteropelvic junction obstruction in 32 of 89 cases. This same study found that the diagnosis was made prenatally in 74 of 89 cases and that most patients diagnosed postnatally presented with an abdominal mass.
Currently, the vast majority of ureteropelvic junction obstructions (90%) are detected with prenatal ultrasonography. As many as 80% of children identified with prenatal hydronephrosis have no signs or symptoms of their urologic abnormality after birth. An epidemiological study of 11,986 pregnant women who underwent prenatal ultrasound evaluations demonstrated that the overall frequency of congenital abnormalities is 0.5% and that urinary tract abnormalities represent 50% of these cases 1. Furthermore, others have estimated that the cause of prenatal hydronephrosis is ureteropelvic junction obstruction in 50-67% of the cases. Taken together, this suggests that each mother who undergoes prenatal ultrasound examination has a 0.2-0.4% chance of having a neonate with hydronephrosis and these children have a 50% chance of eventually having the diagnosis of ureteropelvic junction obstruction after birth.
Ureteropelvic junction obstruction is associated with a number of anomalies. A known association exists between ureteropelvic junction obstruction and horseshoe kidneys 2. Ureteropelvic junction obstruction has also been found to occur in 17 of 82 patients with ectopic kidneys. A strong relationship also exists between ureteropelvic junction obstruction and nephrolithiasis (kidney stone) 3. One study found a 20% incidence rate of stones in patients with ureteropelvic junction obstruction. Finally, no strong evidence exists indicating a hereditary pattern. Only one study has suggested that ureteropelvic junction obstruction has a genetic cause 4 and thus, most cases are believed to be spontaneous in nature.
Open pyeloplasty has been the criterion standard for pyeloplasty, achieving excellent long-term success rates of over 90% 5, despite the disadvantages of longer hospital stay, increased postoperative pain, and slower return to normal activities as compared with laparoscopic renal surgery 6.
Newer less invasive pyeloplasty surgical options are available:
- laparoscopic pyeloplasty with or without a surgical robot, or
- internal incision of the ureteropelvic junction using a camera and scope inserted through the bladder (retrograde endopyelotomy)
Open pyeloplasty
Open pyeloplasty remains the criterion standard for the treatment of ureteropelvic junction obstruction. Although many variations exist in the methodology, this procedure typically involves the surgical excision of the narrowed segment of the ureteropelvic junction and performance of a spatulated reanastomosis of the renal pelvis to the ureter. If significant dilation of the renal pelvis occurs, it is often reduced in size by trimming off redundant tissue, and then it is tailored in such a fashion that it funnels down towards the anastomosis. If an accessory or aberrant vessel exists near the ureteropelvic junction, the anastomosis is positioned anterior to the vessel.
An open pyeloplasty can be performed through a variety of incisions but is most likely performed through an extraperitoneal flank incision. Depending on the surgeon’s preference and the function of the kidney postoperatively, a nephrostomy tube is occasionally left in place, or, more often, a ureteral stent that passes from the renal pelvis to the bladder is placed. Some surgeons leave a drain near the anastomosis; this is removed postoperatively when the output becomes minimal. The rationale behind this is to help detect fluid leakage from the anastomosis and to prevent its accumulation in the retroperitoneum. If a drain is left, it is usually removed after the patient’s bladder Foley catheter is removed and after trial of having the nephrostomy tube (if present) clamped to ascertain that neither maneuver will increase drainage from the anastomosis.
The advantages of this operation include excellent exposure of the ureteropelvic junction, familiar anatomy for essentially all urologists, the ability to tailor the renal pelvis as needed, and the performance of a watertight anastomosis. The disadvantages include the large surgical incisions and the associated postoperative pain and convalescence.
Laparoscopic pyeloplasty
In this method the surgeon works through a small cut in the abdominal wall. A surgical robot can help guide the tools. The clear advantages of this method are less pain and nausea, especially in older children and adults. But scarring in the abdomen can result. This treatment has led to very successful results.
The first laparoscopic pyeloplasty was reported in 1993 7. Many series showed that laparoscopic pyeloplasty was comparable to open pyeloplasty 8. Long-term series with a minimum of 2 years’ follow-up reported excellent success rates (96-98%) 9. Laparoscopic pyeloplasty also achieved good success (eg, 83% in 36 patients) after previous failed procedures (eg, antegrade or retrograde endopyelotomy, balloon dilatation, and open pyeloplasty) 10.
The advantages of the laparoscopic approach include less postoperative pain, a shorter hospital stay, and a more rapid recovery. As with its open counterpart, laparoscopy allows for excision of the strictured segment, reduction pyeloplasty, transposition of the ureteropelvic junction over crossing vessels, and even extraction of concomitant renal calculi. The primary disadvantage is the advanced skill level required for intracorporeal suturing and knot tying. Although the development of specialized laparoscopic instruments has facilitated these tasks, the technical difficulty of the procedure has limited its widespread adoption.
Excellent results notwithstanding, laparoscopic pyeloplasty did not replace open pyeloplasty the way laparoscopic nephrectomy replaced open nephrectomy—mainly because of the steep learning curve required to master advanced laparoscopic skills such as intracorporal suturing, which can be time-consuming and imprecise in the initial learning stages. The development of robotic laparoscopic pyeloplasty has reduced the obstacles to learning intracorporeal suturing, which is the main reconstructive step in pyeloplasty.
Robot-assisted laparoscopic pyeloplasty
Robotic pyeloplasty is very similar to laparoscopic pyeloplasty, however, instead of the surgeon using their own hands, the surgical instruments are attached to a robotic system that is controlled by the surgeon via a computer interface. The dexterity of the robotic system enhances precision, control and flexibility of the surgeon’s movements. Robotic surgery is similar to traditional laparoscopic surgery in that instruments are introduced into the body through several small incisions and manipulated under video guidance. Unlike conventional laparoscopy, in which the surgeon’s hands are directly linked to the instruments, in robotic surgery, the surgeon’s movements are made in a console that is remote from the patient and translated by the robotic arms.
The advantages provided by the robot include a magnified 3-dimensional view, increased articulation with 6° of freedom to mimic wrist movements, tremor reduction, and motion scaling. These make intracorporeal suturing less formidable and more efficient, giving more surgeons the tools necessary to perform a minimally invasive pyeloplasty. Thus, robotics is rapidly becoming the preferred approach to pyeloplasty for an increasing number of surgeons. In 2009, robotic laparoscopic pyeloplasty surpassed open surgery as the most widely used approach for pyeloplasty in the United States 11. Now robotic laparoscopic pyeloplasty has been found to achieve success rates similar to those of open pyeloplasty while also providing the benefits of minimally invasive surgery, it is fast becoming the operative technique of choice for patients presenting with ureteropelvic junction obstruction.
A retrospective cohort study by Varda et al 12 that included data from 11,899 pyeloplasties from 2003 to 2015 reported that 75% were open, 10% laparoscopic and 15% robotic. Even though the total number of cases decreased 7% annually, robotic pyeloplasty grew 29% annually, accounted for 40% of the cases in 2015 and reduced the likelihood of prolonged length of stay, however, increased likelihood of prolonged operative time.
Internal incision pyeloplasty (retrograde endopyelotomy)
With retrograde endopyelotomy procedure a ureteroscope is passed through the bladder and into the ureter in a retrograde fashion. The scope is advanced up to the region of the ureteropelvic junction. Under direct vision, the ureteropelvic junction is incised through the full thickness using a variety of devices such as electrocautery or laser. The incision is usually carried proximal and distal to the strictured area until periureteral fat is seen. Following incision, a special ureteral drain or stent is left in place for a few weeks and then removed.
Retrograde endopyelotomy procedure has the advantage of being minimally invasive; no skin incision is required and also include less pain and nausea. It can often be performed in an outpatient setting. The ureteropelvic junction heals in a more open manner but the surgery may need to be repeated. Potential disadvantages include slightly lower success rates compared to its open pyeloplasty or minimally invasive laparoscopic pyeloplasty; the inability to pass the ureteroscope to the region of stricture, thus requiring additional procedures; the discomfort associated with the postoperative stent; and the need for a skilled endoscopist to accurately and safely perform the procedure.
Acucise endopyelotomy
An additional retrograde approach to the treatment of ureteropelvic junction obstruction has been developed. This technique uses a balloon catheter with a cutting wire. The Acucise catheter is passed in a retrograde fashion over a safety guidewire under fluoroscopic guidance to the level of the ureteropelvic junction obstruction. The cutting wire of the catheter is positioned so that it traverses the strictured area. The balloon portion of the catheter is inflated with contrast; when the appropriate position is confirmed fluoroscopically, the cutting wire of the catheter is activated, which incises the strictured area. The incision is meant to be of full thickness and usually results in the extravasation of contrast material. Following incision, the Acucise catheter is removed and a ureteral stent is placed. This stent is left in place for approximately 6 weeks to allow reepithelialization of the ureter and then is removed cystoscopically.
This procedure has the advantages that it is relatively easy to perform, can be performed in an outpatient setting, has low patient morbidity, and has overall success rates of approximately 75%. Disadvantages include the reliance on fluoroscopy rather than continuous and direct vision, potential bleeding complications that may require angiographic or other intervention, and the discomfort associated with the indwelling ureteral stent.
Antegrade endopyeloplasty
A modification of the antegrade endopyelotomy is termed an endopyeloplasty. The beginning of this procedure is similar to that of an antegrade endopyelotomy. After establishing percutaneous access to the kidney, the ureteropelvic junction is incised full thickness, as with a traditional endopyelotomy. The incision is enlarged with laparoscopic Endoshears. The vertical incision is then closed in a Heineke-Mikulicz (horizontal) fashion with a laparoscopic suture device placed through the working channel of the nephroscope. The experience with this procedure is limited to only a few large centers; thus, it has not gained widespread acceptance.
Pyeloplasty procedure
All patients should have an anatomic study illustrating the extent of the obstruction and a functional study to assess the function of the affected kidney and contralateral kidney. Some urologists recommend that all patients have a Lasix renal scan prior to surgery to confirm the presence of obstruction and to ascertain function of the affected and contralateral kidney.
Every attempt should be made to sterilize the urine of any of these patients preoperatively. Baseline kidney function, hemoglobin values, and bleeding parameters should be checked. Informed consent that lists alternative treatments and potential intraoperative and postoperative complications obtained. Although transfusion after pyeloplasty or endopyelotomy is rare, all patients need to sign a blood consent preoperatively. Conversion to an open procedure or the need for additional procedures is a possibility with all of the treatments for ureteropelvic junction and thus should be addressed in detail with the patient preoperatively.
Immediately prior to the procedure, the patient should receive a dose of a broad-spectrum antibiotic such as cefazolin. As an alternative, ampicillin and gentamicin can be given concurrently.
Open pyeloplasty
If the patient’s ureteral anatomy has not been defined preoperatively with intravenous urogram (IVU) images, most physicians advocate performance of a retrograde pyelogram prior to the pyeloplasty. Findings illustrate the length of the stricture and help confirm that no evidence of obstruction exists distal to the ureteropelvic junction. After performance of the retrograde pyelogram, a Foley catheter is placed and the patient is placed in the flank position. The incision used is based on surgeon preference and the patient’s particular anatomy, but typically, an extraperitoneal flank or subcostal approach is used.
Following incision and division of the muscles, the peritoneum is swept medially, the kidney is identified, and Gerota fascia is opened. Perinephric fat is cleaned off until the renal pelvis and proximal ureter are completely identified. Control of the renal hilum is generally not necessary. Accessory renal arteries are often identified, supplying the lower pole of the kidney that crosses the ureter at the point of the ureteropelvic junction obstruction. Make every effort to spare these vessels because ligation can devascularize a segment of kidney.
Once the region at the ureteropelvic junction is dissected out, holding stitches are placed on the renal pelvis and the ureter distal to the ureteropelvic junction obstruction. The region of the ureteropelvic junction is then excised out sharply. Following this, free flow of urine from the renal pelvis should occur. The ureter is often injected with sterile saline to confirm free flow down the remainder (distal portion) of the ureter. Next, the ureter is spatulated laterally at a distance of approximately 1 cm. If the renal pelvis is largely hydronephrotic, it is frequently reduced with the hope of funneling the urine down towards the newly created anastomosis.
The next step is the anastomosis. If an accessory renal vessel is identified, perform the anastomosis anterior to the vessel. Using small absorbable sutures such as 5-0 Monocryl or polydioxanone on a small tapered needle, the anastomosis is performed between the spatulated ureter and renal pelvis.
The first stitch is usually placed in the lateral aspect of the ureter and the lateral most aspect of the renal pelvis. The remainder of the anastomosis is then performed using either interrupted or running sutures. If a ureteral stent is to be placed, it is placed prior to completion of the anastomosis. After performance of the anastomosis, usually a redundant pelvis must be closed. This is usually performed in a running fashion. If a Penrose or a closed-suction drain is placed near the anastomosis, it is brought up through a separate incision. The abdomen is closed in a routine fashion.
Laparoscopic pyeloplasty
Prior to proceeding with pyeloplasty, many surgeons perform retrograde pyelography to more precisely define the stricture length and location and to rule out obstruction distal to the ureteropelvic junction. A double-J ureteral stent may be placed at this time. The patient is then repositioned on the operating table, and pneumoperitoneum is established. The dissection is begun by mobilizing the colon on the affected side medially by incising along the avascular line of Toldt. The kidney, with overlying perinephric fat, is usually easily identified. Gerota fascia is opened, and dissection is carried down to the level of the kidney.
Once the ureteropelvic junction is identified, the renal pelvis and proximal ureter are dissected. As with the open pyeloplasty, dissection of the ureter is minimized in an attempt to preserve its blood supply. The strictured region is then excised sharply. At this point, the ureteral stent should be clearly visible. The ureter is spatulated on its lateral aspect, and, if necessary, the redundant renal pelvis is excised. The goal is to place the ureteropelvic junction in a dependent position to maximize drainage without placing the anastomosis under tension. If a crossing vessel is present and appears to be obstructive in nature, the renal pelvis and ureter may be transposed anterior to the vessel. It is important to preserve accessory arteries because they often supply blood to a significant portion of the kidney, as well as to the renal pelvis and proximal ureter.
Prior to completion of the anastomosis, the proximal end of the double-J stent is passed into the renal pelvis. Most surgeons perform the anastomosis in a running fashion. Lapra-Ty clips may be used to minimize knot tying, and specialized instruments such as the Endostitch device may facilitate suturing. Important principles include the creation of a tension-free watertight anastomosis with preservation of the periureteral blood supply. Once the anastomosis is completed, a closed-suction drain is placed through one of the trocar sites. The pneumoperitoneum is reduced, and the abdomen is exited in the standard fashion. All trocar sites larger than 10 mm are closed in an effort to reduce herniation of abdominal contents through the fascial defect.
Robot-assisted laparoscopic pyeloplasty
The steps of the robotic pyeloplasty are similar to those of a traditional laparoscopic pyeloplasty. Retrograde pyelography and stent placement may be performed initially with the patient in the lithotomy position. However, the specialized instruments used during robotic surgery facilitate antegrade stent placement, leading some surgeons to forego the initial cystoscopic procedure. The patient is then placed in the lateral decubitus position with the ipsilateral kidney superior. Pneumoperitoneum is established with either a Veress needle or the Hasson technique. A 12-mm camera port and two 8-mm robotic ports are placed in a triangular fashion, with the camera in the center. Assistant ports are placed as needed.
After the colon has been reflected medially, the ureteropelvic junction is mobilized and then divided, taking care to preserve the periureteral blood supply and any accessory lower-pole vessels. Careful manipulation of the proximal ureter may be accomplished by using the redundant ureteropelvic junction tissue as a handle or with the aid of a stay suture. The redundant pelvis is excised along with the strictured segment, and the ureter is spatulated along its lateral aspect. A running anastomosis is performed over a stent, most commonly with 4-0 polyglactin suture, along the posterior and anterior walls. A drain may be placed near the anastomosis and brought out through one of the port sites, although many surgeons do not routinely leave a drain.
Antegrade endopyelotomy
Ureteral anatomy must be defined. If needed, a retrograde pyelogram is performed. A stricture longer than 2 cm is generally a contraindication to endopyelotomy. The role of a crossing vessel in the region of the ureteropelvic junction is controversial. Some authors believe a crossing vessel is a contraindication to endopyelotomy because success rates are reportedly lower and bleeding complications higher. However, others have challenged these contentions and believe that if the incision is performed in a true lateral position, endopyelotomy is safe and has an acceptable success rate even in the presence of a crossing vessel.
A Foley catheter is placed at the start of the case. The patient is positioned in a prone position with appropriate padding. Access to the kidney is obtained through either a mid- or upper-pole posterior calyx. This can be performed at the start of the procedure or prior to the endopyelotomy if the interventional radiologist establishes access at the surgeon’s hospital. A guidewire is then passed through the calyx, negotiated beyond the ureteropelvic junction, and continued down into the bladder. The inability to pass a guidewire through the strictured area is a contraindication to endopyelotomy. Then, a second safety wire is usually placed. The nephrostomy tract is dilated using either a dilating balloon or serial dilators.
A working sheath is passed from the skin into the collecting system. A scope is then passed through the working sheath and into the renal pelvis. The renal pelvis is inspected. If any calculi are found, they are treated and removed. The region of the ureteropelvic junction is then inspected. Contrast material can be injected in an antegrade fashion to further delineate the anatomy. Next, the endopyelotomy is performed.
Some surgeons typically use a cold knife; however, a hot knife (electrocautery or laser) can be used to make the incision. Making the incision in a truly lateral position is critical. This position can be confirmed using a combination of direct visualization and fluoroscopic guidance. The incision is generally begun 0.5-1 cm distal to the obstruction, carried through the strictured area, and continued 0.5-1 cm proximal to the strictured area. The incision should be of full thickness until periureteral fat is visible. Following the incision of the strictured segment, a ureteral stent is placed. The size of the stent depends on surgeon preference. An endopyelotomy stent can be used. The stent is passed down over one of the safety guidewires, and the position is confirmed with fluoroscopy so that one curl is in the renal pelvis and the distal curl is in the urinary bladder.
After confirming satisfactory stent position, the scope is removed and a nephrostomy tube is placed through the working sheath into the renal pelvis. The working sheath is then removed. The nephrostomy tube is generally hooked to gravity drainage, and the patient is recovered from anesthesia.
Antegrade endopyeloplasty
The technique for pyeloplasty is very similar to that of a standard endopyelotomy. Antegrade access is established into the kidney in an upper or midpole calix. The site of obstruction is identified and incised the full thickness, as with a standard endopyelotomy. Laparoscopic scissors are passed through the nephroscope, and the distal edge of the endopyelotomy incision is mobilized. The vertical defect is then closed in a horizontal fashion (Heineke-Mikulicz) using a laparoscopic suture assist device. A ureteral stent and nephrostomy tube are placed in the standard fashion.
Retrograde endopyelotomy
In this procedure, the patient is placed in the lithotomy position. Retrograde pyelography is performed to help delineate the ureteral anatomy. A guidewire is passed in a retrograde fashion up the ureter and curled in the renal pelvis. A second safety wire is then placed. Generally, the procedure is performed using a flexible ureteroscope, although in certain individuals, a rigid ureteroscope can be passed to the region of the ureteropelvic junction. The scope is passed to the level of the ureteropelvic junction. Again, the incision is made in a true lateral position. This is critical to reduce the risk of bleeding complications. The strictured segment is incised 0.5-1 cm both proximal and distal to the strictured area. The device used for incision is based on surgeon preference but can include a hook electrode hooked to electrocautery or, more recently, the holmium:YAG laser. The incision should be of full thickness until periureteral fat is visible.
Following the incision, the ureteroscope is removed and a double-J stent is placed in a routine fashion. The position of the proximal curl in the renal pelvis is confirmed, as is the distal curl in the bladder. A Foley catheter is generally placed at least overnight, and the patient is recovered from anesthesia.
Acucise endopyelotomy
This procedure is performed without the passage of an ureteroscope and is essentially performed under fluoroscopic guidance only. With the patient in the lithotomy position, a retrograde pyelogram is performed to help delineate the ureteropelvic junction anatomy. Next, a safety wire is passed up the ureter, beyond the ureteropelvic junction, and into the renal pelvis. The Acucise endopyelotomy catheter is then passed over the guidewire and to the region of the ureteropelvic junction. The cutting-wire portion of the catheter is readily visible on fluoroscopy images. Inflate the balloon portion of the catheter with contrast under fluoroscopic guidance. Identify a clear “waist” (ie, narrowing) in the region of the ureteropelvic junction. Once this is confirmed, the balloon is then deflated again.
Using fluoroscopic guidance, a true lateral position of the cutting wire is confirmed. The balloon is then reinflated with contrast, and the cutting wire is activated with 75 W of pure cutting current for 5 seconds. With activation of the cutting wire, the waist should immediately disappear. After cutting, the balloon is left inflated for an additional 5 minutes. If extravasation of contrast is identified outside of the collecting system, the balloon is deflated and the Acucise catheter is removed.
If no obvious extravasation is identified, more contrast material is injected in a retrograde fashion to further fill the renal pelvis. If extravasation is still not identified, the catheter can be repositioned and a second incision performed in a similar fashion to the first. Perform no more than 2 cuts. Following removal of the Acucise catheter, a ureteral stent is placed in a routine fashion over the safety wire, under fluoroscopic guidance. The safety guidewires are removed, a Foley catheter is placed, and the patient is recovered from anesthesia.
Pyeloplasty risks and complications
Each of the different surgical treatments for the correction of ureteropelvic junction obstruction has potential complications. Recurrent obstruction can develop after any of the techniques described. Specific success rate for the various procedures are described in pyeloplasty prognosis.
As with all surgical procedures, a potential for bleeding exists at the time of surgery and in the immediate postoperative period. Adherence to proper surgical technique and proper patient selection should decrease the potential for significant bleeding complications. The endourological techniques of antegrade and retrograde endopyelotomy and the Acucise endopyelotomy are different because bleeding may not be as readily apparent as in the open or laparoscopic pyeloplasty. Adequate monitoring of vital signs, hematocrit measurements when indicated, and the degree of hematuria after the procedure can alert the surgeon to the possibility of significant bleeding.
Urinary tract infection can occur after any treatment for ureteropelvic junction obstruction. Make every effort to sterilize the urine of patients prior to the procedure. In addition, use preoperative and perioperative antibiotics in all patients. For antibiotic coverage, use broad-spectrum antibiotics with particular emphasis on coverage against gram-negative rod pathogens. A study by Ferroni et al 13 found that the administration of extended prophylactic antibiotics following minimally invasive pyeloplasty showed no significant impact on the rate of urinary tract infection.
See your doctor now or seek immediate medical care if:
- You have new or worse symptoms of a kidney infection. These may include:
- Pain or burning when you urinate.
- A frequent need to urinate without being able to pass much urine.
- Pain in the flank, which is just below the rib cage and above the waist on either side of the back.
- Blood in the urine.
- A fever.
- You are vomiting or nauseated.
- Your tube leaks.
- Urine does not collect in the drainage bag.
- You have pain or bleeding around the tube.
Watch closely for changes in your health, and be sure to contact your doctor if:
- You do not get better as expected.
Secondary ureteropelvic obstruction
Secondary ureteropelvic junction obstruction occurs when a previous attempt at surgical treatment has failed. Scar tissue formation due to surgical dissection or to fluid extravasation during endoscopic treatment may present an additional challenge during treatment of secondary ureteropelvic junction obstruction. Traditionally, the treatment of choice following a previous open repair has been antegrade endopyelotomy, while open pyeloplasty is usually attempted after an initial endoscopic approach has been unsuccessful. Jabbour and colleagues 14 report an 88% success rate with endopyelotomy following failed open pyeloplasty. Conversely, the success rate of endoscopic treatment of secondary obstruction following a previous attempt at endoscopic management may be as low as 38%.
More recently, centers with considerable experience in laparoscopy have achieved good success rates with secondary laparoscopic pyeloplasty. Sundaram and colleagues 15 reported an overall success rate of 83% among 36 patients, most of whom had undergone previous endourologic procedures, including 28 who underwent previous cutting balloon retrograde endopyelotomy. Laparoscopic pyeloplasty is certainly a reasonable option following previous attempts at endourologic management. However, more authors are proceeding directly to laparoscopic or robotic pyeloplasty for secondary obstruction after an initial pyeloplasty. Basiri and colleagues 16 treated 18 patients with laparoscopic pyeloplasty following failed open surgery, with a success rate exceeding 75%.
Pyeloplasty recovery
After the pyeloplasty surgery patient are taken to recovery where they are monitored until the anaesthetic wears off, then they are taken to a hospital room to rest. Patients are typically required to stay for two to three nights.
After pyeloplasty surgery a catheter is usually put in place but may be removed the following day. Pain at the incision site is common. Patients that underwent laparoscopic surgery may also experience some mild shoulder pain as a result of the gas used to inflate the abdomen during surgery.
Patients can usually return to work two to four weeks after the surgery. Heavy lifting and other strenuous activity is discouraged during this time. Driving is also discouraged until the patient is feeling 100 percent.
The ureteral stent can cause some discomfort, especially during urination where pain can occur in the back on the side near where the kidneys are located. Blood in the urine is also common.
The stent is usually taken out after 6 weeks. The procedure is fairly straightforward. It is performed under local aesthetic; the stent is removed using a cystoscope, which is inserted into the bladder via the urethra. This is a day procedure and doesn’t require an overnight stay in hospital.
Patients usually recover quickly. After pyeloplasty surgery the ureter may be swollen for a while. Some have pain for a few days after pyeloplasty surgery. Kidney drainage can also remain poor, but often gets better as the area heals. Your doctor will use ultrasound a few weeks after your pyeloplasty surgery to check for hydronephrosis (swollen kidney). The appearance of the kidney will improve with time, though it will not look normal.
If the other kidney is normal, children can participate in sports or other activities when the blocked kidney responds well to treatment. Once the ureteropelvic junction obstruction is fixed, it almost never comes back.
Note that patients who have had ureteropelvic junction obrstruction may have a slightly greater risk of future kidney stones or infection. This is because the kidneys may still contain some pooled urine, even though overall drainage is improved. Sometimes a child who had some obstruction as an infant, which improved naturally, will later have signs of obstruction that call for surgery.
Pyeloplasty prognosis
Success rates for the different treatments for ureteropelvic junction obstruction vary depending on a number of factors, including, but not limited, to the degree of obstruction; whether it is a primary or secondary obstruction; the function of the affected kidney; anatomic considerations, such as the presence of crossing vessels; and the expertise of the treating surgeon.
Open pyeloplasty
Often considered the criterion standard for the treatment of ureteropelvic junction obstruction, open pyeloplasty has success rates consistently greater than 90% based on subjective patient symptoms and objective radiographic evidence.
Laparoscopic pyeloplasty
Although technically more difficult than open pyeloplasty, this technique shares similar success rates with its open counterpart. Success rates range from 88%-100%, with most series citing rates well over 90%. In the largest series to date of 170 patients undergoing dismembered pyeloplasty, Moon and colleagues 17 report a symptomatic success rate of 96%.
Robotic pyeloplasty
The enthusiasm for robotic pyeloplasty is supported by a mounting body of evidence documenting its efficacy. Several single-center series report symptomatic and radiographic success rates of 94%-100%, and, recently, a multi-institutional review reported that 96% of patients undergoing robotic dismembered pyeloplasty at 3 centers demonstrated resolution of obstruction on imaging studies 18. In addition, most series report significantly shorter operative times for robotic compared with laparoscopic pyeloplasty.
Antegrade endopyelotomy
The success rates of antegrade endopyelotomy are 75-95%. Different factors contributing to the success or failure include whether the obstruction is primary or secondary, whether high insertion of the ureter into the renal pelvis occurred, the degree of hydronephrosis and the function of the affected kidney, and the presence of a crossing vessel near the ureteropelvic junction.
Retrograde endopyelotomy
Although this approach is typically considered to have lower success rates compared to an antegrade approach and open or laparoscopic pyeloplasty, it can usually be performed with minimal morbidity and, perhaps, at a lower cost compared to alternative treatments. Success rates are reported to be 70-85%. As with an antegrade approach, anatomic and functional factors affect the overall success rates.
Acucise endopyelotomy
The overall success rate of 75% for Acucise retrograde endopyelotomy is similar to that of the antegrade and retrograde approach. Similar anatomic and functional factors can also affect the success rate of this procedure.
References- Helin I, Persson PH. Prenatal diagnosis of urinary tract abnormalities by ultrasound. Pediatrics. 1986 Nov. 78(5):879-83.
- Blanc T, Koulouris E, Botto N, Paye-Jaouen A, El-Ghoneimi A. Laparoscopic pyeloplasty on horseshoe kidney in children. J Urol. 2013 Oct 16.
- Naitoh Y, Kawauchi A, Kamoi K, Soh J, Hongo F, Okihara K, et al. Nephrolithotomy Performed Concurrently With Laparoendoscopic Single-site Pyeloplasty. Urology. 2013 Oct 19.
- Atwell JD. Familial pelviureteric junction hydronephrosis and its association with a duplex pelvicaliceal system and vesicoureteric reflux. A family study. Br J Urol. 1985 Aug. 57(4):365-9.
- Scardino PT, Scardino PL. Obstruction at the ureteropelvic junction. H B, editor. The ureter. 2nd ed. New York: Springer Verlag; 1981. 697.
- Burgess NA, Koo BC, Calvert RC, Hindmarsh A, Donaldson PJ, Rhodes M. Randomized trial of laparoscopic v open nephrectomy. J Endourol. 2007 Jun. 21(6):610-3.
- Schuessler WW, Grune MT, Tecuanhuey LV, Preminger GM. Laparoscopic dismembered pyeloplasty. J Urol. 1993 Dec. 150(6):1795-9.
- Klingler HC, Remzi M, Janetschek G, Kratzik C, Marberger MJ. Comparison of open versus laparoscopic pyeloplasty techniques in treatment of uretero-pelvic junction obstruction. Eur Urol. 2003 Sep. 44(3):340-5.
- Turk IA, Davis JW, Winkelmann B, Deger S, Richter F, Fabrizio MD. Laparoscopic dismembered pyeloplasty–the method of choice in the presence of an enlarged renal pelvis and crossing vessels. Eur Urol. 2002 Sep. 42(3):268-75.
- Sundaram CP, Grubb RL 3rd, Rehman J, Yan Y, Chen C, Landman J. Laparoscopic pyeloplasty for secondary ureteropelvic junction obstruction. J Urol. 2003 Jun. 169(6):2037-40.
- Monn MF, Bahler CD, Schneider EB, Sundaram CP. Emerging trends in robotic pyeloplasty for the management of ureteropelvic junction obstruction in adults. J Urol. 2013 Apr. 189(4):1352-7.
- Varda BK, Wang Y, Chung BI, Lee RS, Kurtz MP, Nelson CP, et al. Has the robot caught up? National trends in utilization, perioperative outcomes, and cost for open, laparoscopic, and robotic pediatric pyeloplasty in the United States from 2003 to 2015. J Pediatr Urol. 2018 Feb 22.
- Ferroni MC, Lyon TD, Rycyna KJ, Dwyer ME, Schneck FX, Ost MC, et al. The Role of Prophylactic Antibiotics after Minimally Invasive Pyeloplasty with Ureteral Stent Placement in Children. Urology. 2015 Dec 9.
- Jabbour ME, Goldfischer ER, Klima WJ, et al. Endopyelotomy after failed pyeloplasty: the long-term results. J Urol. 1998 Sep. 160(3 Pt 1):690-2; discussion 692-3.
- Sundaram CP, Grubb RL 3rd, Rehman J, Yan Y, Chen C, Landman J, et al. Laparoscopic pyeloplasty for secondary ureteropelvic junction obstruction. J Urol. 2003 Jun. 169(6):2037-40.
- Basiri A, Behjati S, Zand S, Moghaddam SM. Laparoscopic pyeloplasty in secondary ureteropelvic junction obstruction after failed open surgery. J Endourol. 2007 Sep. 21(9):1045-51; discussion 1051.
- Moon DA, El-Shazly MA, Chang CM, Gianduzzo TR, Eden CG. Laparoscopic pyeloplasty: evolution of a new gold standard. Urology. 2006 May. 67(5):932-6.
- Mufarrij PW, Woods M, Shah OD, Palese MA, Berger AD, Thomas R, et al. Robotic dismembered pyeloplasty: a 6-year, multi-institutional experience. J Urol. 2008 Oct. 180(4):1391-6.