Reiter’s syndrome
Reiter’s syndrome is now known as reactive arthritis, is inflammatory arthritis which manifests after several days to weeks after a gastrointestinal or genitourinary infection 1. Reiter’s syndrome or reactive arthritis occurs in reaction to an infection by certain bacteria. Most often, these bacteria are in the genitals (Chlamydia trachomatis) or the bowel (Campylobacter, Salmonella, Shigella and Yersinia). Chlamydia trachomatis most often transmits by sex. Chlamydia trachomatis often has no symptoms, but can cause a pus-like or watery discharge from the genitals. The bowel bacteria can cause diarrhea. If you develop arthritis within one month of diarrhea or a genital infection – especially with a discharge – see a doctor. You may have Reiter’s syndrome. It is believed that Reiter’s syndrome is due to an aberrant autoimmune response to a gastrointestinal or genitourinary infection caused by salmonella, shigella, campylobacter, or chlamydia 2. The bacteria induce (cause) arthritis by distorting your body’s defense against infections, as well as your genetic environment. How exactly each of these factors plays a role in the disease likely varies from patient to patient. However, this is a focus of research.
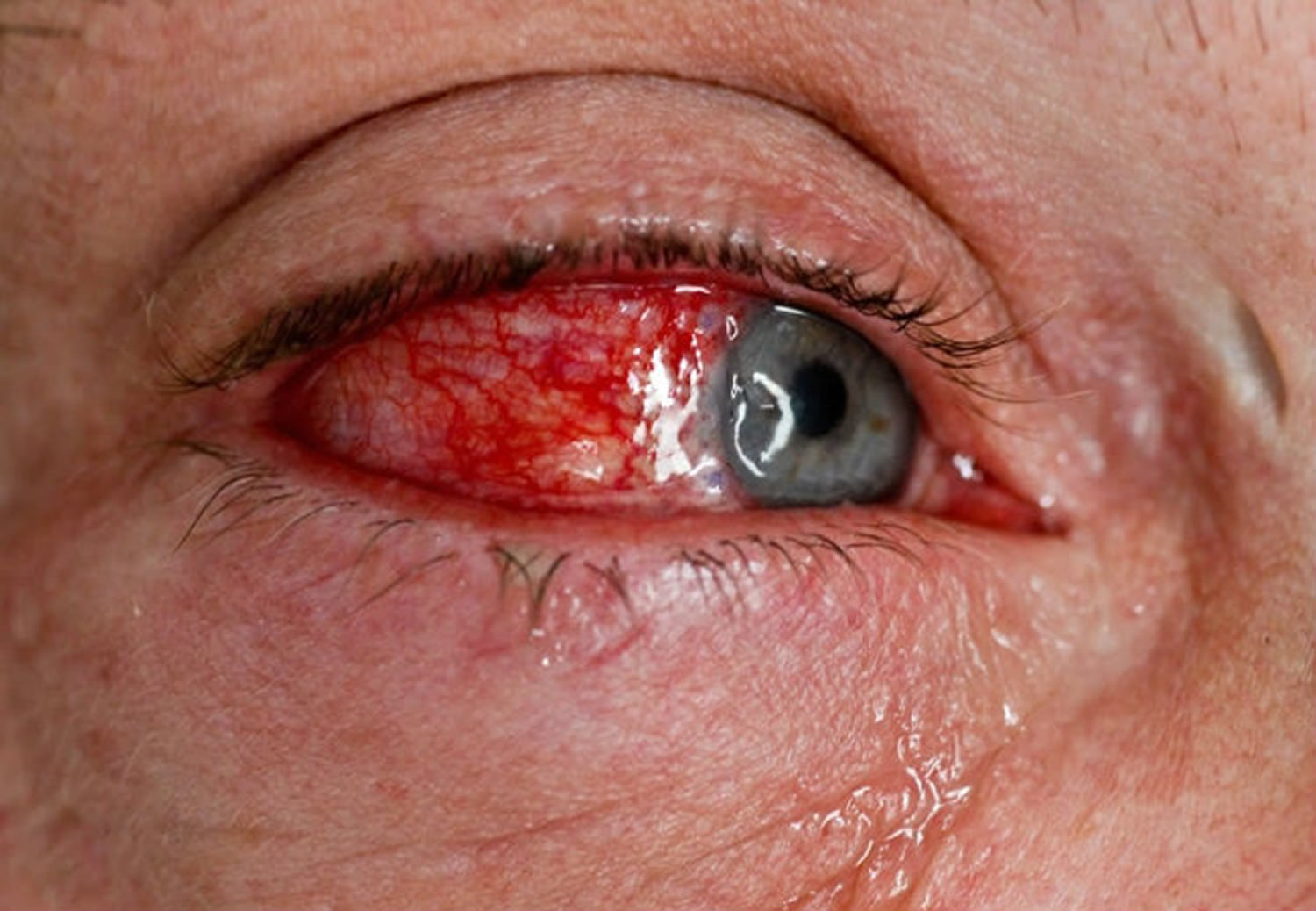
Reiter’s syndrome is also described as a classic triad of arthritis, urethritis and, conjunctivitis. However, a majority of patients do not present with the classic triad. Reiter’s syndrome usually targets your knees and the joints of your ankles and feet. Inflammation also can affect your eyes, skin and urethra.
The name, Reiter syndrome was dismissed because it is believed that Hans Reiter was a member of The National Socialist German Workers’ Party or the “Nazis” and the director of the Kaiser Wilhelm Institute of Experimental Therapy under whose leadership the war prisoners were subject to many inhumane experiments 1. Today, it is believed that Reiter’s syndrome is due to an aberrant autoimmune response to the gastrointestinal infection caused by Salmonella, Shigella, Campylobacter or chlamydia 3.
Reiter’s reactive arthritis falls within the subclass of seronegative spondyloarthropathies that affect the axial skeleton. Other members of that group are ankylosing spondylitis and psoriatic arthritis. Joint involvement is oligoarticular and asymmetrical 1.
Reiter’s reactive arthritis is relatively rare, and the incidence in population-based studies is reported to be 0.6 to 27 per 100,000. Reactive arthritis is more common in adult males in the second and third decades of their life 4. For most people, signs and symptoms come and go, eventually disappearing within 12 months.
About 1-3% of patients with nonspecific urethritis will develop an episode of arthritis. Overall, higher disease activity and worse functional capacity are seen in the lower socioeconomic populations.
Reiter’s syndrome typically begins within 2 to 4 weeks after infection. Reiter’s syndrome is not contagious, but the bacterium that triggers the disease can pass from person to person.
Men age 40 and younger are most commonly affected. Evidence shows that they are nine times more likely than women to get Reiter’s syndrome due to a sexually transmitted infection. However, both sexes are equally likely to get it from a food-related infection.
The diagnosis of Reiter’s syndrome is clinical, based on the history and physical examination findings. A high index of suspicion is required. No laboratory study or imaging finding is diagnostic of Reiter’s syndrome. No specific tests or markers are indicated. Indicators of inflammation are usually abnormal.
Reiter’s syndrome diagnosis is largely based on symptoms of the inducing infections and appearance of typical musculoskeletal (joint and muscle) involvement. If indicated, doctors might order a test for Chlamydia infection or test for the HLA-B27 gene. The test for Chlamydia uses a urine sample or a swab of the genitals.
Reactive arthritis is a multiorgan disorder that is best managed by a team of healthcare professionals that includes a rheumatologist, ophthalmologist, gastroenterologist, physical therapist, nurse, and pharmacist. While evaluating, general physicians should not shy away from exploring the detailed history of sexual contacts and genital symptoms.
There is no cure for reactive arthritis and the treatment is supportive. With proper treatment, most people with Reiter’s syndrome recover fully and can resume normal activities a few months after initial symptoms. However, arthritis symptoms may last up to a year, but they are usually mild and do not interfere with daily life. Some people with Reiter’s syndrome will have long-term, but mild, arthritis. Studies show that between 15 and 50 percent of patients will develop symptoms again, possibly due to re-infection. Back pain and arthritis are the symptoms that most commonly reappear. A few patients will have chronic, severe arthritis that is difficult to control with treatment and may cause joint damage.
All patients should be encouraged to become physically active and a physical therapy consult should be obtained.
A consult with a dermatologist is recommended to assess skin lesions and recommend treatment.
Finally, all patients with reactive arthritis should follow up with an ophthalmologist since they remain at high risk for visual problems 5.
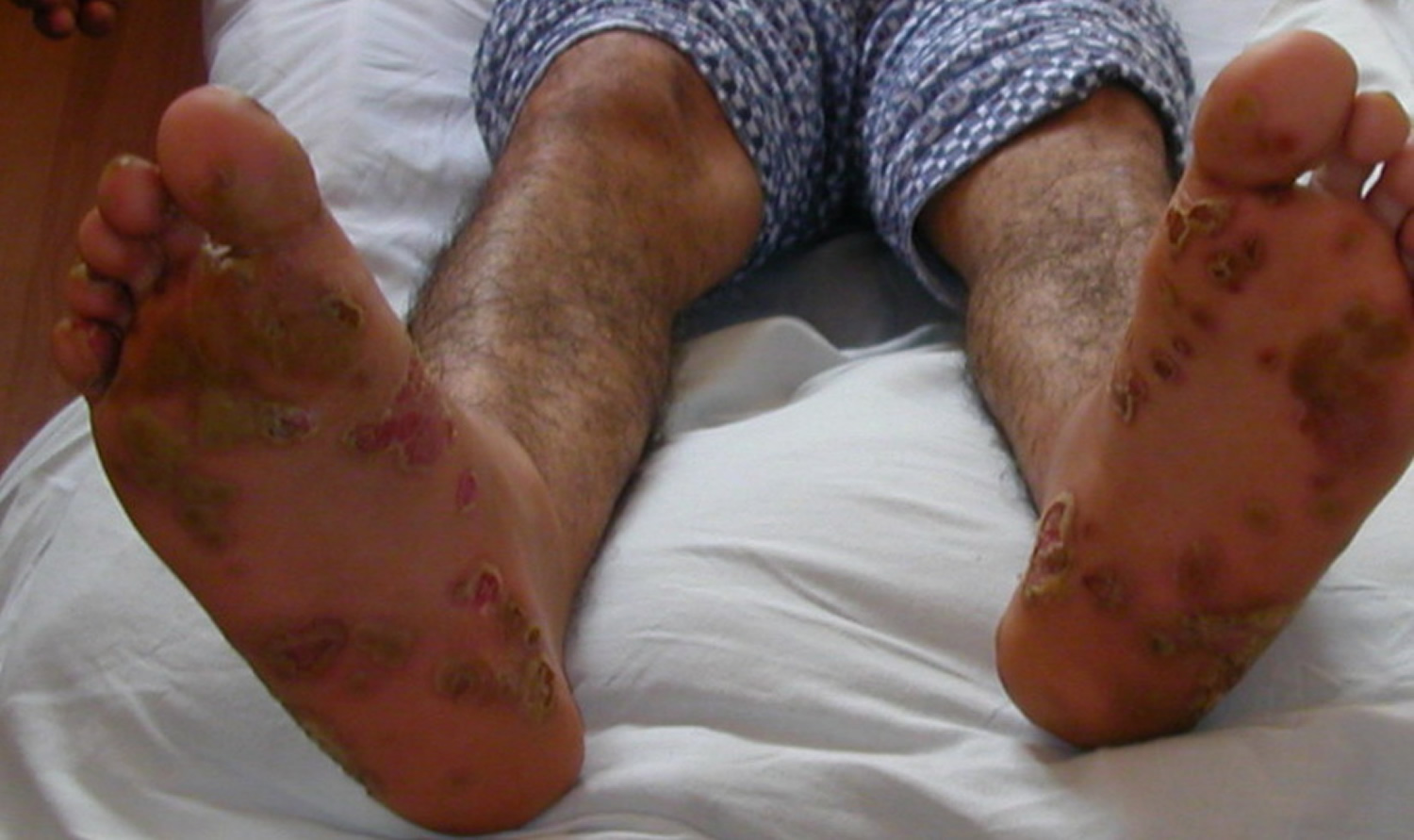
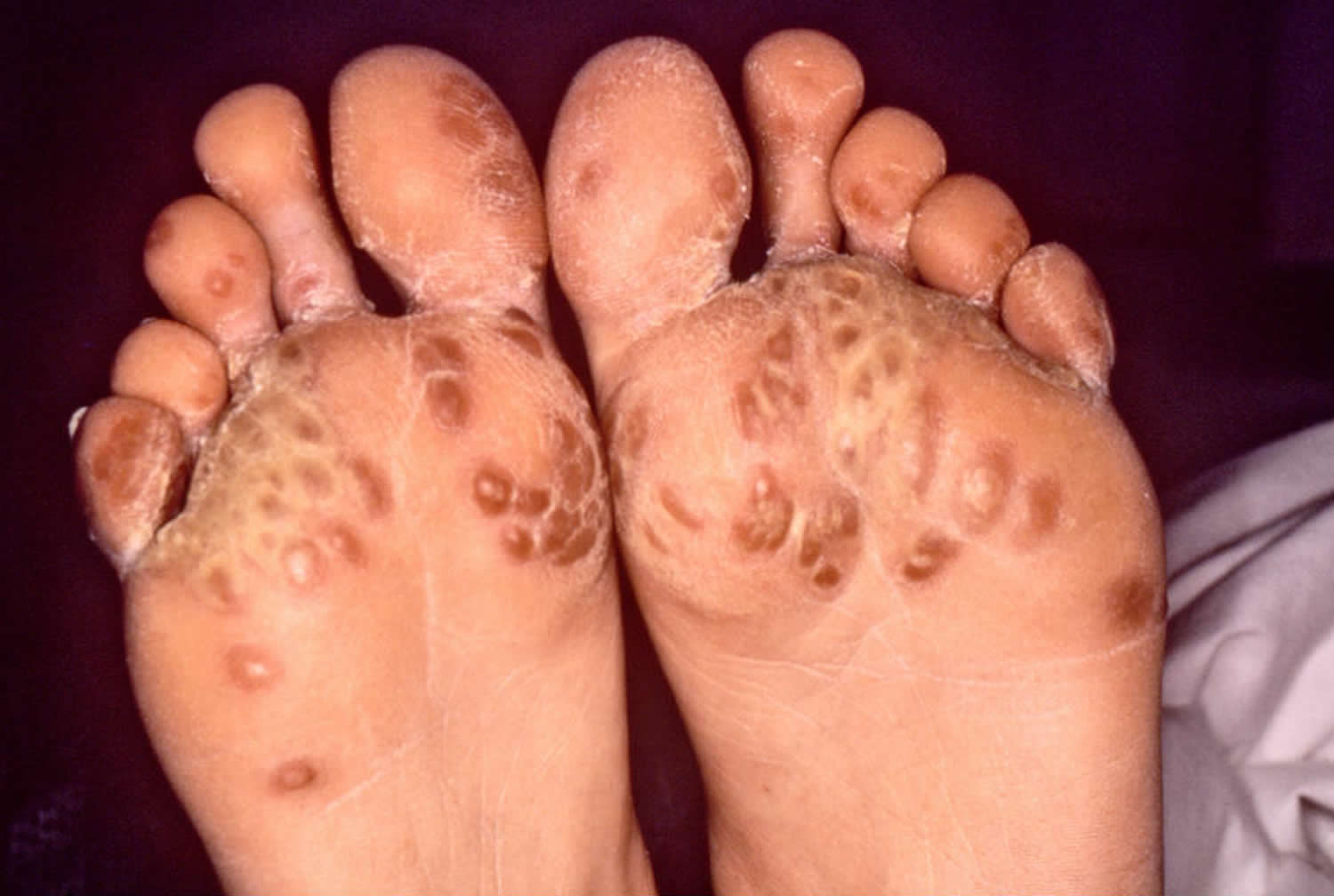
Figure 1. Reiter’s syndrome rash feet (keratoderma blenorrhagica or psoriasiform hyperkeratosis)
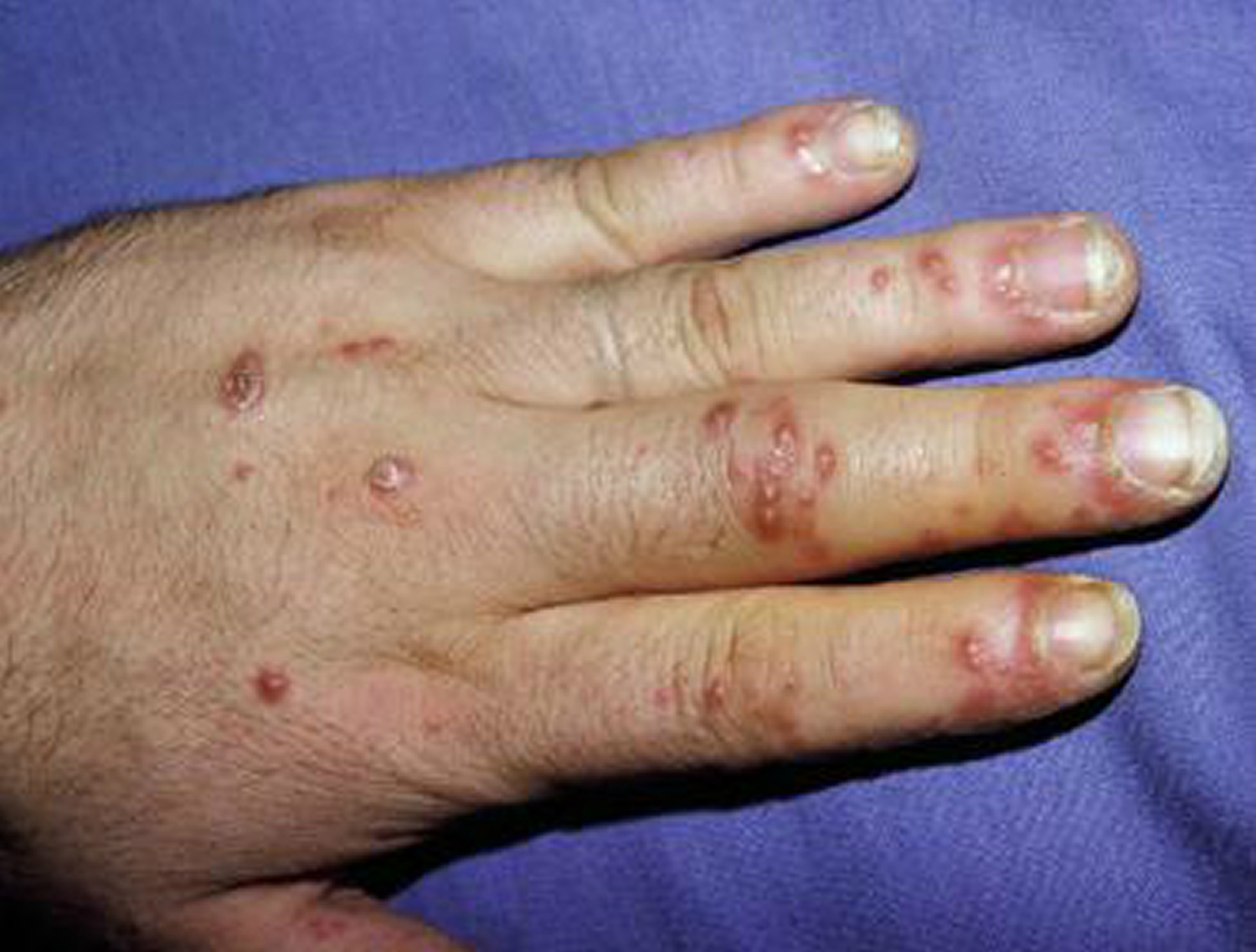
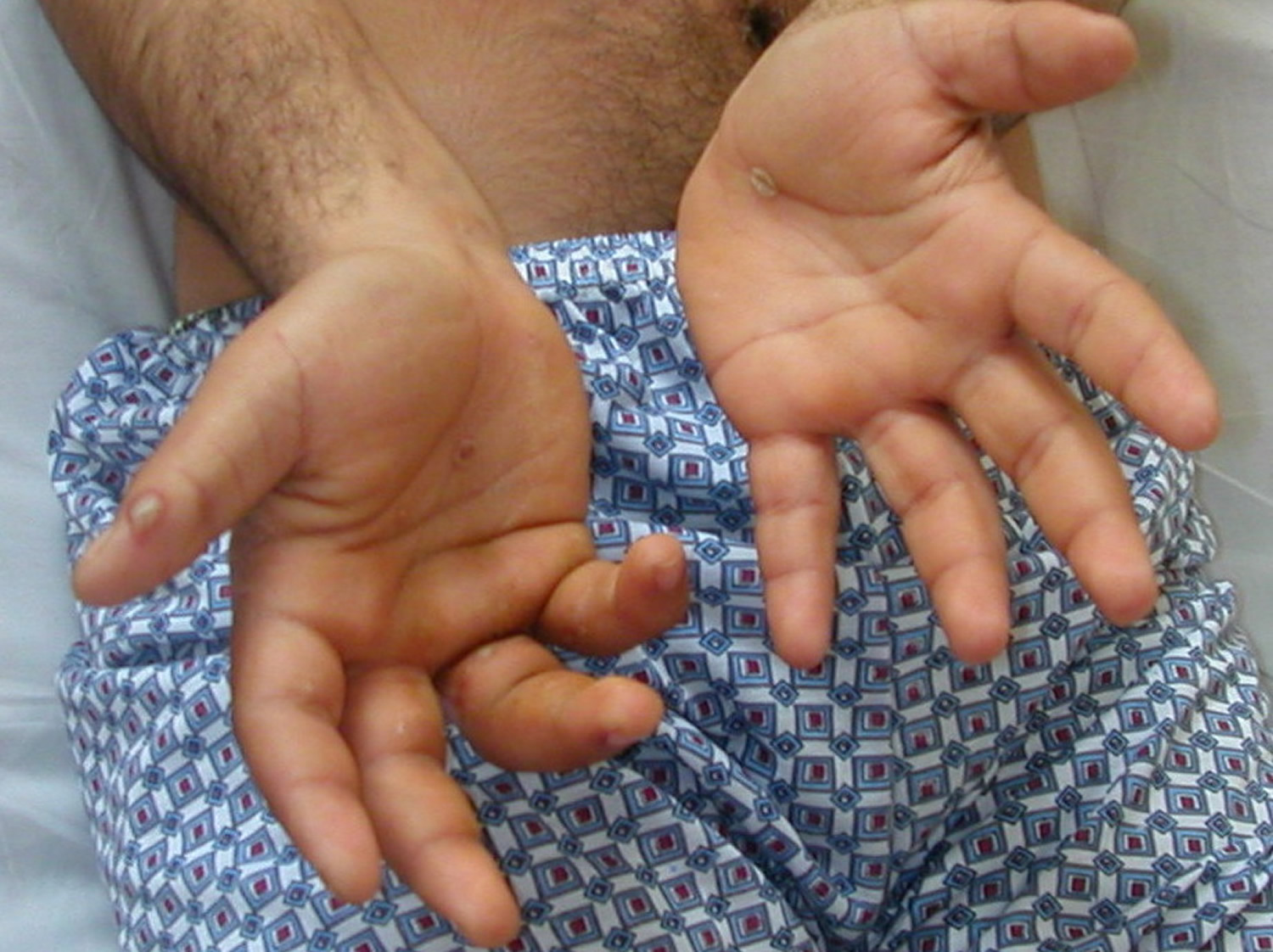
Figure 2. Reiter’s syndrome rash hands (palmoplantar pustulosis)
Who gets Reiter’s syndrome?
The bacteria that cause Reiter’s syndrome are very common. In theory, anyone who becomes infected with these bacteria might develop Reiter’s syndrome. Yet very few people with bacterial diarrhea actually go on to have serious Reiter’s syndrome. What remains unclear is the role of Chlamydia infection that has no symptoms. It is possible that some cases of arthritis of unknown cause are due to Chlamydia.
Reiter’s syndrome tends to occur most often in men between ages 20 and 50. Some patients with Reiter’s syndrome carry a gene called HLA-B27. Patients who test positive for HLA-B27 often have a more sudden and severe onset of symptoms. They also are more likely to have chronic (long-lasting) symptoms. Yet, patients who are HLA-B27 negative (do not have the gene) can still get Reiter’s syndrome after exposure to an organism that causes it.
Patients with weakened immune systems due to acquired immunodeficiency syndrome (AIDS) and human immunodeficiency virus (HIV) can also develop Reiter’s syndrome. Reiter’s syndrome is very common in HIV individuals, and hence patients with the new-onset Reiter’s syndrome must have HIV ruled out. Individuals with HIV who develop Reiter’s syndrome often develop severe psoriasiform dermatitis on the scalp, soles, palms, and flexures.
How common is Reiter’s syndrome?
Reiter’s syndrome is relatively rare, and the incidence in population-based studies is reported to be 0.6 to 27 per 100,000 2. Reiter’s syndrome is more common in adult males in the second and third decades of their life 6.
About 1-3% of patients with nonspecific urethritis will develop an episode of arthritis. Overall, higher disease activity and worse functional capacity are seen in the lower socioeconomic populations.
How long does Reiter’s syndrome last?
Clinical course of Reiter’s syndrome is variable. Reiter’s syndrome can spontaneously resolve or progress to chronic arthritis which is defined as persistence of symptoms for greater than six months. Reiter’s syndrome is usually temporary, but treatment can help to relieve your symptoms and clear any underlying infection. Most people start returning to normal activities after 3 to 6 months. Symptoms don’t usually last longer than 12 months, but a small number of people experience long-term joint problems.
More than half of Reiter’s syndrome cases that have a chronic course are usually triggered by genitourinary organisms as compared to enteric organisms. This can be secondary to increased rates of reinfection with genitourinary organisms such as Chlamydia trachomatis and Neisseria gonorrhea 7 .
Certain factors on presentation are indicative of poor prognosis as they point towards a more severe spectrum of the disease. These factors include male sex, age of disease onset less than 16 years, HLA-B27 positivity, heel and foot involvement, arthritis of the hip, lumbar spine stiffness, dactylitis, oligoarthritis, ESR greater than 30 mm in the first hour, and poor response to nonsteroidal anti-inflammatory drugs (NSAIDs) 7 .
Will I develop Reiter’s syndrome if I have food poisoning or chlamydia?
Most people have these infections without developing Reiter’s syndrome. Researchers aren’t sure why some people get Reiter’s syndrome while others don’t. Some people carry a gene called HLA B27 (human leukocyte antigen B27) that increases their chance of developing Reiter’s syndrome. Human leukocyte antigen B27 (HLA B27) seems to be associated with more severe and chronic forms of the “classical triad” of Reiter’s syndrome 8. About 30–50% patients of Reiter’s syndrome are HLA B27 positive 9.
Is Reiter’s syndrome contagious?
Reiter’s syndrome isn’t contagious. However, the bacteria that cause it can be transmitted sexually or in contaminated food. Only a few of the people who are exposed to these bacteria develop Reiter’s syndrome.
Reiter’s syndrome causes
Reiter’s syndrome develops in reaction to an infection in your body, often in your intestines, genitals or urinary tract. You might not be aware of the triggering infection if it causes mild symptoms or none at all.
Typically, Reiter’s syndrome is caused by a sexually transmitted infection (STI), such as Chlamydia trachomatis, or an infection of the bowel, such as food poisoning.
You may also develop Reiter’s syndrome if you, or someone close to you, has recently had glandular fever or slapped cheek syndrome.
The body’s immune system seems to overreact to the infection and starts attacking healthy tissue, causing it to become inflamed. But the exact reason for this is unknown.
Doctors are not sure why some people exposed to these bacteria get the disease and others don’t. However, researchers have identified a gene, called human leukocyte antigen (HLA) B27, that makes a person more likely to get Reiter’s syndrome. Not everyone who inherits this gene will get the disease.
Reiter’s syndrome isn’t contagious. However, the bacteria that cause it can be transmitted sexually or in contaminated food. Only a few of the people who are exposed to these bacteria develop Reiter’s syndrome.
Exposure to certain bacteria has been linked to Reiter’s syndrome. The ones most commonly associated with Reiter’s syndrome are:
- Chlamydia trachomatis, Neisseria gonorrhea, Mycoplasma genitalium, Mycoplasma hominis and Ureaplasma urealyticum. These bacteria are spread through sexual contact. The infection may begin in the vagina, bladder or the urethra.
- Salmonella enteritidis, Shigella flexneri, and Shigella dysenteriae, Yersinia enterocolitica, Clostridium difficile, Escherichia coli, and Campylobacter jejuni. These bacteria typically infect the gastrointestinal tract.
Reiter’s syndrome incidence is about 2% to 4% after a urogenital infection, mainly with Chlamydia trachomatis, and varies from 0% to 15% after gastrointestinal infections with Salmonella, Shigella, Campylobacter, or Yersinia 2. This might be affected by the epidemiological, environmental factors, the pathogenicity of the bacteria, and differences in the study designs. The enteric Reiter’s syndrome occurs commonly following enteric infections. However, chlamydia associated Reiter’s syndrome is endemic, especially in developed countries 10. Data suggest that chlamydial Reiter’s syndrome is underdiagnosed and that asymptomatic chlamydial infections might be a common cause 11. An important difference between Chlamydia-induced (postvenereal) Reiter’s syndrome and postenteric Reiter’s syndrome is the presence of viable but aberrant chlamydial organisms in the synovial fluid 12 (so-called Chlamydia persistence) 13. Polymerase chain reaction (PCR) assay to detect Chlamydia trachomatis DNA in synovial samples may be a good method for establishing the diagnosis of Chlamydia-induced arthritis in patients with Reiter’s syndrome 14.
In rare cases, the bacterium Chlamydia pneumoniae, which causes respiratory infections, may also cause Reiter’s syndrome 9.
There is also evidence that Reiter’s syndrome can also be caused by pathogens like: parvovirus B19, Gonococcus species, mycobacterial, and streptococcal infections, as well as parasitic diseases such as Entamoeba, Giardia, and Strongyloides 15.
Rare cases of Reiter’s syndrome have also been reported after administering the Bacillus Calmette Guerin vaccine (BCG) intravesically for bladder cancer therapy 16.
The triad of arthritis, urethritis, and conjunctivitis represents a small part of the spectrum of the clinical manifestations of Reiter’s syndrome and only a minority of patients present with this “classical triad” of symptoms. Human leukocyte antigen B27 (HLA B27) seems to be associated with more severe and chronic forms of the “classical triad” of reactive arthritis. About 30–50% patients of Reiter’s syndrome are HLAB27 positive 9. Arthritis is usually asymmetric and additive, with involvement of new joints occurring over few days to 1–2 weeks. Arthritis typically persists for 3–5 months, but more chronic courses do occur. Chronic joint symptoms persist in about 15% patients and in up to 60% patients in hospital-based series, but these tend to be less severe than in the acute stage. Recurrences of the acute syndrome are also common. Low back pain, sacroiliitis, and frank ankylosing spondylitis are also common sequelae. In most studies, HLA B27 positive patients have shown a worse outcome than HLA B27 negative patients 9.
Risk factors for Reiter’s reactive arthritis
Certain factors increase your risk of Reiter’s syndrome:
- Age. Reiter’s syndrome occurs most frequently in adults between the ages of 20 and 40.
- Sex. Women and men are equally likely to develop Reiter’s syndrome in response to foodborne infections. However, men are more likely than are women to develop Reiter’s syndrome in response to sexually transmitted bacteria.
- Hereditary factors. A specific genetic marker has been linked to Reiter’s syndrome. But many people who have this marker never develop the condition.
Genetic factors
Reiter’s syndrome has an important genetic component; it tends to cluster in certain families and almost exclusively affects males, and HLA-B27 is identified in 70-80% of patients 17. HLA-B27 may share molecular characteristics with bacterial epitopes, facilitating an autoimmune cross-reaction instrumental in pathogenesis. HLA-B27 contributes to the pathogenesis of the disease and reportedly increases the risk of Reiter’s syndrome 50-fold 18. HLA-B51 and HLA-DRB1 alleles have also been shown to be associated with Reiter’s syndrome 14.
Rihl et al 19 found a high proportion of proangiogenic factors accounting for a genetically determined susceptibility to Reiter’s syndrome. Sun et al 20 reported increased susceptibility to Reiter’s syndrome associated with the HLA-C1C1 genotype, which indicates the absence of the HLA ligands for the inhibitory killer cell immunoglobulin-like receptor (KIR) KIR2DL1; Imbalance between activating and inhibitory KIR signals may allow pathogens to trigger cytokine overproduction.
Reiter’s syndrome pathophysiology
Reiter’s syndrome is an immune-mediated syndrome in which environmental factors interact with genetic factors 21. Pathogen infection plays a key role in Reiter’s syndrome. Studies using polymerase chain reaction (PCR) and immunocytochemical staining have proven that definite bacterial triggers, such as lipopolysaccharides, or bacterial products can be found in the synovial tissue or fluid, and the persistence of these components is an important factor, resulting in Reiter’s syndrome turning into chronic arthritis 22. After local bacterial infection, the bacterial antigens or peptides are transported from the primary site into the synovial membrane by antigen-presenting cells (APC), leading to the activation of T-lymphocytes against bacterial antigens or peptides and the release of a large number of inflammatory cytokines, finally resulting in synovial inflammation. Chlamydia (Chlamydia trachomatis) infection is the most common factor causing Reiter’s syndrome, which can persist in the host and cause typical reactive arthritis 23. Approximately 5% of patients with acute Chlamydia infection will suffer from Reiter’s syndrome 24. When Chlamydia infects certain host cells, such as monocytes, the body releases pro-inflammatory cytokines, which induce persistent Chlamydia infection. This is mainly because Chlamydia in some way inhibits the combination of phagosomes and lysosomes, which makes Chlamydia live in cells; it is closely related to entering the stage of persistent infection 25. Briefly, Reiter’s syndrome development is dependent on live infection and it is correlated with cytokines, tissue damage and inflammation 26.
HLA-B27 plays an important supporting role in Reiter’s syndrome and the most closely related one is the free heavy chain of HLA-B27 27. Studies have shown that HLA-B27 test results are positive in 50–80% of Reiter’s syndrome patients 28. However, HLA-B27 is not the only determinant of Reiter’s syndrome. It has been proven that HLA-B51, B60 and other genes may encode susceptibility to Reiter’s syndrome. HLA-B27 has multiple alleles that may affect the host response and disease susceptibility; among these, HLA-B*2703 increases the risk of the typical clinical triad of Reiter’s syndrome 29. Other data suggest that HLA-B27 may contribute to the persistence of bacteria in the host, especially Chlamydia and Salmonella 30. So how does the susceptible HLA-B27 gene participate in the occurrence and development of Reiter’s syndrome? It was found that, (1) HLA-B27 folds more slowly than other types of HLA in endoplasmic reticulum assembly, which leads to the accumulation of the HLA-B27 homologous dimer and b2-microglobulin, as well as activating the inflammatory process; (2) the HLA-B27 heavy chain can activate natural killer cells, T-cells and B-cells, thus causing an inflammatory reaction; (3) microbial peptides mimic certain autopeptides, which increase the specificity of HLA-B27 and the reactivity of CD8+T lymphocytes, thus leading to autoimmune and inflammatory tissue damage 31.
In addition to genetic factors, immune cells, such as dendritic cells and T-cells, also play important roles in Reiter’s syndrome. In genetically sensitive individuals, abnormal physiological and pathological processes exist in the affected patient, including Th1 and Th17 cell differentiation, enhancement of the IL-17 response, the activation of IL-17-related T-cells and the release of various cytokines in intestinal lymph nodes. All these could promote the host’s immune response and the infiltration of immune cells (Figure 1). One study focused on whether there were cytokines in the joints of 11 Reiter’s syndrome patients after infections with Chlamydia, Pseudomonas aeruginosa or Salmonella, and showed that under the stimulation of specific bacteria, mononuclear cells in the synovial fluid secrete a small amount of IFN-γ and TNF-α, while secreting a large amount of IL-10. After anti-IL-10 antibody was added or exogenous IL-12 was increased, the secretion of IFN-γ and TNF-α increased significantly in these cultures 32. This proves that IL‐10–IL‐12 balance plays a key role in regulating the release of cytokines in the joints of patients with Reiter’s syndrome. Meador et al. showed that TNF-α was involved in Reiter’s syndrome, and an effective anti-TNF treatment also indirectly confirmed this point 33. One study showed that iNKT cells, a unique subgroup of T lymphocytes, could provide protection from Reiter’s syndrome induced by Salmonella through down-regulating the IL-17 produced by γδ T cells 34. The level of IFN-γ was reduced in the peripheral blood of Reiter’s syndrome patients 35. The decreased ratio of Th1 to Th17 will lead to decreased clearance of Chlamydia 36. Local migration of myeloid cells is activated by chlamydia pathogen-associated molecular patterns and transport of Chlamydia antigens spreads the inflammatory response from the genital tract to diseased tissue, and the myeloid antigen-presenting cells deliver Chlamydia antigens to antigen-specific TNF-producing T-cells locally. It is consistent with the detection of Chlamydia antigen-specific CD4+ and CD8+ T-cells in the joints of patients with Chlamydia-induced Reiter’s syndrome 26. In addition, myeloid cells are increased in infected SKG mice, predisposing them to higher levels of inflammation.
Reiter’s syndrome prevention
Genetic factors appear to play a role in whether you’re likely to develop Reiter’s syndrome. Though you can’t change your genetic makeup, you can reduce your exposure to the bacteria that may lead to Reiter’s syndrome.
Make sure your food is stored at proper temperatures and is cooked properly to help you avoid the many foodborne bacteria that can cause reactive arthritis, including salmonella, shigella, yersinia and campylobacter. Some sexually transmitted infections can trigger Reiter’s syndrome. Using condoms might lower your risk.
Reiter’s syndrome symptoms
Clinical presentation of Reiter’s syndrome varies widely ranging from the absence of symptoms to multi-systemic involvement. The signs and symptoms of Reiter’s syndrome generally start one to four weeks after exposure to a triggering infection 37. They might include:
- Pain and stiffness. The joint pain associated with Reiter’s syndrome most commonly occurs in your knees, ankles and feet. You also might have pain in your heels, low back or buttocks.
- Eye inflammation. Many people who have Reiter’s syndrome also develop eye inflammation (conjunctivitis).
- Urinary problems. Increased frequency and discomfort during urination may occur, as can inflammation of the prostate gland or cervix.
- Inflammation of soft tissue where it enters bone (enthesitis). This might include muscles, tendons and ligaments.
- Swollen toes or fingers. In some cases, your toes or fingers might become so swollen that they resemble sausages.
- Skin problems. Reactive arthritis can affect your skin a variety of ways, including a rash on your soles and palms and mouth sores.
- Low back pain. The pain tends to be worse at night or in the morning.
The most common symptoms of Reiter’s syndrome are inflammation in the joints, eyes, bladder and urethra (the tube that helps remove urine from the body). Sometimes, mouth sores and skin rashes may occur.
Summarized in Table 1 are the potential clinical manifestations of Reiter’s syndrome. Other causes of acute arthritis must be ruled out prior to the diagnosis of Reiter’s syndrome (see Table 2 below).
Reiter’s syndrome symptoms can be very mild and come and go over several weeks to months. So they may not be noticeable in the early stages. Urinary symptoms usually appear first but may absent in women. This symptom may occur with, or be followed by conjunctivitis. Arthritis is usually the last symptom to appear. But sometimes the main and only symptom of reactive arthritis is pain, stiffness and swelling in the joints and tendons.
Table 1. Clinical features of Reiter’s syndrome represented by organ system
| Organ Involvement | Typical Clinical Presentations |
|---|---|
| Musculoskeletal | Asymmetric lower limb oligoarthritis Dactylitis Sacroiliitis Enthesitis |
| Skin and Nail | Circinate balanitis Keratoderma Blennorrhagica Psoriatic onychodystrophy Painless ulcers in the mouth |
| Eye | Conjunctivitis Corneal ulceration Episcleritis Keratitis Uveitis |
| Genitourinary | Cervicitis/Salpingitis/Vulvovaginitis in women Urethritis and Prostatitis in men |
| Gastrointestinal | Acute diarrhea resembling inflammatory bowel disease |
| Heart (Rare) | Ascending aortitis Conduction abnormalities |
These symptoms manifest several days to weeks after the initial infection. Diarrhea or other symptoms caused by the offending agents are usually resolved by the time the patient develops arthritis. A detailed history and physical examination to investigate any recent illness such as urethritis, diarrhea, etc, should be performed. Reiter’s syndrome can be self-limiting, recurrent or continuous and about 20% to 25% of the patients may progress to have chronic articular, ocular and cardiac complications.
For sexually acquired Reiter’s syndrome, there is a history of sexual intercourse, usually with a new partner, within 3 months of arthritis symptoms. Genital symptoms precede arthritis by about 2 weeks on an average. It may include dysuria, discharge, testicular pain in men, and intermenstrual or postcoital bleeding, or deep pelvic pain apart from vaginal discharge in women 38.
Reiter’s syndrome is very common in HIV individuals and hence patients with the new-onset disease must have HIV ruled out. Individuals with HIV who develop reactive arthritis often develop severe psoriasiform dermatitis on the scalp, soles, palms, and flexures.
The physical exam may reveal:
- Sausage shaped finger, toe or heel pain
- Asymmetric oligoarthritis- usually of the lower extremities
- Conjunctivitis or iritis
- Acute diarrhea or cervicitis within 4 weeks of the onset of arthritis
- Urethritis or genital ulcers
Two or more of the above features plus involvement of the skeletal system establishes the diagnosis.
Joint and entheses
Patients typically present with acute onset oligo-arthritis, mainly involving the lower extremities, sacroiliac joint, and the lumbar spine. Not more than 6 large joints are affected at a time and knee and ankle are the most commonly affected. Joint pain is classically nocturnal with early morning stiffness. Involvement is asymmetric and affects the weight-bearing joint. The joints are often warm, painful and swollen. Tendinitis is a common feature of the disease. About 30% of patients suffer from associated enthesitis in the form of plantar fascitis or Achilles tendinitis 39.
Extra-articular manifestations
Extra-articular manifestations may involve the skeletal system (enthesitis, dactylitis), eye (conjunctivitis, anterior uveitis episcleritis, and keratitis), genitourinary (urethritis, cervicitis, prostatitis, salpingo-oophoritis, cystitis or circinate balanitis), mucosal and skin involvement (mucosal ulcers, keratoderma blennorrhagica and erythema nodosum), cardiac (carditis, aortic, conduction and valvular abnormalities), and nail changes (onycholysis, subungual keratosis, or nail pits) also are seen
Skin and mucocutaneous changes are common and may include hyperkeratotic skin and erythematous dermatitis. Nail dystrophy is common. Other involvements include pustular psoriasis on the sole ( keratoderma blenorrhagica), geographic tongue, circinate balanitis, or oral ulceration.
Eye involvement is common and may include conjunctivitis (30%) or uveitis. In patients with visual symptoms, recognition of uveitis is of paramount importance as it can rapidly lead to visual loss.
Rare cases can involve the cardiovascular system causing conduction abnormalities in early-stage and aortic regurgitation when advanced. Myelopathy, as well as non-specific gastrointestinal features of diarrhea and colitis, can also persist 40.
Figure 3. Reiter’s syndrome uveitis
Reiter’s syndrome complications
Complications of Reiter’s syndrome include:
- Recurrent arthritis (15-50%)
- Chronic arthritis or sacroiliitis
- Ankylosing spondylitis (30-50% if the patient is also HLA-B27–positive)
- Urethral stricture
- Aortic root necrosis
- Cataracts
- Cystoid macular edema
Reiter’s syndrome diagnosis
Reiter’s syndrome can be difficult to diagnose because there is no specific laboratory test to confirm that a person has it. The patient may be referred to a rheumatologist, depending on the severity of symptoms.
During the physical exam, your doctor is likely to check your joints for signs and symptoms of inflammation, such as swelling, warmth and tenderness, and test range of motion in your spine and affected joints. Your doctor might also check your eyes for inflammation and your skin for rashes.
Blood tests
Your doctor might recommend that a sample of your blood be tested for:
- Evidence of past or current infection
- Signs of inflammation
- Antibodies associated with other types of arthritis
- A genetic marker linked to reactive arthritis
Joint fluid tests
Your doctor might use a needle to withdraw a sample of fluid from within an affected joint. This fluid will be tested for:
- White blood cell count. An increased number of white blood cells might indicate inflammation or an infection.
- Infections. Bacteria in your joint fluid might indicate septic arthritis, which can result in severe joint damage.
- Crystals. Uric acid crystals in your joint fluid might indicate gout. This very painful type of arthritis often affects the big toe.
Imaging tests
X-rays of your low back, pelvis and joints can indicate whether you have any of the characteristic signs of reactive arthritis. X-rays can also rule out other types of arthritis.
A combination of genitourinary symptoms, metatarsophalangeal joint involvement, elevated C reactive protein, and positive HLA- B27 renders a 69% sensitivity and 93.5% specificity to the diagnosis of Reiter’s syndrome 41.
Although Reiter’s syndrome is a clinical diagnosis, laboratory tests to detect the offending pathogens to confirm concomitant or preceding infections are usually performed to support the diagnosis. Nucleic acid amplification tests from an early morning urine sample or urogenital swab are utilized to detect Chlamydia trachomatis and Neisseria gonorrhea. Nucleic acid amplification test for Mycoplasma genitalium is also available nowadays and is relevant in men with urethritis. Positive evidence of Chlamydia by polymerase chain reaction (PCR) in the joint is probably strongly diagnostic, but the current methods used for the detection of chlamydia in the urine are not validated for diagnostic purposes for synovial samples. Serological testing for Chlamydia trachomatis is of limited importance due to serological cross-reactivity between Chlamydia trachomatis and Chlamydia pneumoniae, inability to distinguish past and present infection by the persistence of antibodies, lower or absent antibody response in lower urinary tract infections. Serological testing is available for Salmonella, Yersinia, and Campylobacter but is not useful in clinical practice. There are also gastrointestinal infections, for example, Shigella, in which no reliable serological methods exist. A stool culture may be helpful to detect enteric pathogens 42.
Certain complications, like uveitis, are important to identify. The slit-lamp exam is helpful to diagnose cells in the anterior chamber in acute iritis. Therefore, the presence of ocular symptoms in a suspected patient should generate a prompt referral to an ophthalmologist. The usual presentation of uveitis will involve acute pain, photophobia, visual impairment, scleral injection, and hypopyon.
Acute phase reactants such as the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) may be elevated. Joint aspiration must be performed when possible to rule out other arthritis. Aspiration of the joint is often done to rule out septic arthritis and crystalline arthritis. The findings in synovial fluid are nonspecific and are characteristic of inflammatory arthritis, with elevated leukocyte counts (typically 2000 to 4000 white blood cell per ml), with neutrophil predominance.
HLA B 27 can be measured as it correlates with the severity of the disease but is not diagnostic. It is also important in the localization of arthritis. Sacroiliitis occurs more commonly in HLA B 27 positive patients 43.
In a patient from an endemic population, the tuberculin skin test should be performed.
Plain radiographs may reveal nonspecific inflammatory joint findings in the acute phase. Ultrasonography or magnetic resonance imaging (MRI) can be used to diagnose peripheral synovitis, enthesitis, or sacroiliitis. Scintigraphy can reveal the early stages of enthesitis.
American College of Rheumatology diagnostic criteria
American College of Rheumatology came up with diagnostic criteria for reactive arthritis in 1999. The criteria were divided into 44:
- Major criteria
- Arthritis, with two of the following three findings:
- Asymmetric
- Monoarthritis or oligoarthritis
- Predominantly affecting lower limbs
- Preceding symptomatic infection 3 days to 6 weeks before the onset of arthritis with one or two of the following findings:
- Enteritis (defined as diarrhea for at least 1 day) or
- Urethritis (dysuria or discharge for at least 1 day)
- Arthritis, with two of the following three findings:
- Minor criteria (at least 1 of the following):
- Evidence of triggering infection:
- Nucleic acid amplification test (NAAT) test from morning urine or urethral/cervical swab for Chlamydia trachomatis
- Positive stool culture for enteric pathogens associated with reactive arthritis
- Evidence of persistent synovial infection:
- Positive immunohistology or polymerase chain reaction (PCR) assay for Chlamydia
- Evidence of triggering infection:
A “definite” diagnosis of reactive arthritis is based on the fulfillment of both major criteria and a relevant minor criterion, while a “probable” diagnosis is characterized by both major criteria but no relevant minor criterion or one major criterion and one or more of the minor criteria. The identification of the trigger infection is also required 44.
Reiter’s syndrome differential diagnosis
Table 2. Reiter’s syndrome differential diagnosis
| Diagnosis | Characteristic Features |
|---|---|
| Septic Arthritis | Mono or oligoarticular involvement with joint effusion showing elevated WBC counts (e.g. > 50,000 cells/microL) and positive synovial fluid cultures for causative organism. |
| Gout | Monosodium urate crystals present on synovial fluid analysis with elevated WBC counts |
| Pseudogout | Calcium pyrophosphate crystals present on synovial fluid analysis with elevated WBC counts |
| Rheumatoid Arthritis Flare | Symmetric polyarticular involvement with positive serology (RF/anti-CCP antibodies), elevated acute phase reactants (ESR/CRP) |
| Psoriatic Arthritis | Oligo- or polyarthritis with onycholysis and psoriatic skin lesion |
| Lyme Arthritis | Mono- or oligoarthritis with positive Lyme serology |
| STI related arthritis | Based on the serology of the virus |
| Lupus Arthritis | Migratory, polyarticular and symmetric involvement with systemic features of SLE |
| Inflammatory bowel disease-associated arthritis | Peripheral arthritis with spondylitis and history of inflammatory bowel disease |
| Sarcoid arthropathy | Oligo or polyarthritis associated with hilar adenopathy and erythema nodosum |
Abbreviations: WBC- white blood cell; RF- rheumatoid Factor; CCP- cyclic citrullinated peptide; ESR- erythrocyte sedimentation rate; CRP- C-reactive protein; STI- sexually transmitted infection; SLE- systemic lupus erythematosus
[Source 7 ]Reiter’s syndrome treatment
The goal of treatment in Reiter’s syndrome is to manage your symptoms, prevent chronic complications and treat an infection that could still be present.
Healthcare Team
Since Reiter’s syndrome may affect different parts of the body, more than one doctor may be involved in the patient’s care. A rheumatologist (a doctor with specialized training in arthritis treatment) will likely be the primary doctor. Other specialists may include:
- Dermatologist to treat skin symptoms
- Gynecologist to treat genital symptoms in women
- Ophthalmologist to treat eye disease
- Orthopaedist to perform surgery if joints are severely damaged
- Physical therapist or physiatrist to oversee the patient’s exercise routine
- Urologist to treat genital symptoms in men and women
The type of treatment for Reiter’s syndrome depends on the stage of reactive arthritis.
- The early stage of reactive arthritis is considered acute (early). Acute inflammation can be treated with nonsteroidal anti-inflammatory drugs (often referred to as NSAIDs). These drugs suppress swelling and pain. They include naproxen (Aleve), diclofenac (Voltaren), indomethacin (Indocin) or celecoxib (Celebrex). The exact effective dose varies from patient to patient. The risk of side effects of these drugs, such as gastrointestinal (often called GI) bleeding, also varies. Your doctor will consider your risk of GI bleeding in suggesting an NSAID.
- The late stage of reactive arthritis is considered chronic. Chronic reactive arthritis may require treatment with a disease-modifying antirheumatic drug (sometimes called a DMARD) such as sulfasalazine or methotrexate. Sulfasalazine may be more useful when the reactive arthritis is triggered by a gastrointestinal (GI) infection. In some cases, very inflamed joints may benefit from corticosteroid injections (cortisone shots). In more severe cases, stronger immune lowering medications called “biologics” may be used such as Etanercept (Enbrel) or Adalimumab (Humira).
Medications
If an infectious agent has been identified as a trigger for Reiter’s syndrome, antibiotic therapy is strongly recommended often for a long term of 3 to 6 months. It can significantly shorten the time to remission 45. Treatment of the underlying concomitant infection, if present should be initiated without delay. Patients who do not have active infection do not benefit from antibiotic therapy 46. Vasey et al 47 reported the results of a double-blinded prospective triple placebo trial in which chlamydia positive patients by PCR were treated for 6 months with a combination of doxycycline and rifampin or azithromycin and rifampin. The treatment arm did achieve statistically significant symptom remission as well as PCR negativity although the study was underpowered for identification of the preferred combination of antibiotics.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These are often the first type of medicine used and include aspirin, ibuprofen and naproxen. All NSAIDs work similarly by blocking substances called prostaglandins that contribute to inflammation and pain. They are available as tablets, capsules and powders.
- Corticosteroids. These medicines help to quickly reduce inflammation. For people with severe joint inflammation, they may be injected into the affected joint. Doctors usually prescribe these injections when NSAIDs have not helped ease symptoms. Corticosteroids also are available in topical forms, which are cream or lotion applied directly to the skin.
- Disease-modifying antirheumatic drugs (DMARDs). A small number of patients with reactive arthritis have severe symptoms that cannot be controlled with the above-mentioned treatments. In this case, the doctor may prescribe medicines called disease-modifying antirheumatic drugs (DMARDs). Such drugs suppress the immune system. There are two main types: traditional DMARDs and a newer class called biologics. The most commonly used DMARDs are methotrexate and sulfasalazine. It can take a few months before you notice a DMARD is working, so it’s important to keep taking it even if you don’t see immediate results. Common side effects of methotrexate and sulfasalazine include feeling sick, diarrhea, loss of appetite and headaches, although these usually improve once your body gets used to the medication. DMARDs may also cause changes in your blood or liver, so it’s important to have regular blood tests while taking these medicines.
Non-steroidal anti-inflammatory drugs (NSAIDs) are the initial treatment of choice in the acute phase. Intra-articular or local glucocorticoids as in case of enthesitis or bursitis can be used if the patient has mono/oligoarthritis. Mechanical devices like orthotics and insoles can be useful. Systemic use of glucocorticoids is limited to severe polyarthritis, cardiac and ocular manifestations. Disease-modifying antirheumatic drugs (DMARDs), mainly Sulphasalazine have been shown to be effective in both acute and chronic ReA. Other agents such as methotrexate and azathioprine have shown to be useful in chronic arthritis. They are indicated in patients who have failed Nonsteroidal anti-inflammatory drug (NSAID) therapy. Biologicals such as tumor necrosis factor (TNF) blocking agents (e.g., and infliximab and etanercept have been suggested in the treatment of reactive arthritis. However, further studies are needed to determine their definitive indications 48.
Physical therapy
All patients should be urged to become physically active. A physical therapist can provide you with targeted exercises for your joints and muscles. Strengthening exercises develop the muscles around your affected joints, which increase the joint’s support and to prevent muscle wasting. Range-of-motion exercises can increase your joints’ flexibility and reduce stiffness.
Exercise
If your arthritis is painful, you may not feel like exercising. However, being active can help reduce and prevent pain. Regular exercise can also:
- improve your range of movement and joint mobility
- increase muscle strength
- reduce stiffness
- boost your energy
As long as you do the right type and level of exercise for your condition, your arthritis won’t get any worse. Combined with a healthy, balanced diet, regular exercise will help you lose weight and place less strain on your joints.
An occupational therapist can help if you have severe arthritis that’s affecting your ability to move around your home and carry out everyday tasks, such as cooking and cleaning.
Home remedy
Self care for Reiter’s syndrome includes making sure food is stored at proper temperatures and cooked properly. These helps prevent foodborne bacteria that can cause reactive arthritis. Some sexually transmitted infections can trigger Reiter’s syndrome. Using condoms may lower one’s risk.
Staying physically active is the key to keeping joints flexible. Too little movement can lead to joint stiffness. Strong muscles help to protect joints. But it’s important to talk to a doctor before beginning an exercise program. Managing weight, eating a nutritious diet and getting a good balance of rest and activity each day are important, too.
As your symptoms improve, you should begin to do exercises to stretch and strengthen the affected muscles, and improve the range of movement in your affected joints.
You might also find ice packs and heat pads useful in reducing joint pain and swelling. Wrap them in a clean towel before putting them against your skin.
Splints, heel pads and shoe inserts (insoles) may also help.
Reiter’s syndrome diet
Eating a healthy, balanced diet is an important part of maintaining good health, and can help you feel your best.
This means eating a wide variety of foods in the right proportions, and consuming the right amount of food and drink to achieve and maintain a healthy body weight.
Reiter’s syndrome prognosis
Reiter’s syndrome has a variable natural history but typically follows a self-limited course, with resolution of symptoms by 3-5 months, even in patients who are acutely incapacitated 49. Symptoms lasting beyond 6 months indicate a chronic element of the disease. Sacroiliitis is the most common chronic joint involvement. The presence of hip involvement, unresponsiveness to nonsteroidal anti-inflammatory drugs (NSAIDs) and ESR greater than 30 portend a worse outcome 2.
A fatal outcome is seldom reported, but death can occur, and it is usually related to the adverse effects of treatment 50. Postdysenteric cases are associated with a better prognosis than postvenereal cases. Patients who are HLA-B27 positive have a higher risk of recurrence of Reiter’s syndrome and may predict a more prolonged course and severe outcome, as may infections triggered by Yersinia, Salmonella, Shigella, or Chlamydia 51.
Reiter’s syndrome has a high tendency to recur (15-50% of cases), particularly in individuals who are HLA-B27–positive. A new infection or other stress factor could cause reactivation of the disease.
Approximately 15-30% of patients with Reiter’s syndrome can develop a long-term, sometimes destructive arthritis or enthesitis or spondylitis. A 1994 study analyzed 7 factors as predictors of long-term outcome in spondyloarthropathies 52. The number of patients with Reiter’s syndrome in this study was low, and a valid subgroup analysis was impossible. The presence of hip-joint involvement, an ESR higher than 30, and unresponsiveness to nonsteroidal anti-inflammatory drugs (NSAIDs) probably portend a severe outcome or chronicity in Reiter’s syndrome.
References- Cheeti A, Chakraborty RK, Ramphul K. Reactive Arthritis (Reiter Syndrome) [Updated 2019 Dec 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499831
- Cheeti A, Chakraborty RK, Ramphul K. Reactive Arthritis. [Updated 2021 Oct 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499831
- Arévalo M, Gratacós Masmitjà J, Moreno M, Calvet J, Orellana C, Ruiz D, Castro C, Carreto P, Larrosa M, Collantes E, Font P., REGISPONSER group. Influence of HLA-B27 on the Ankylosing Spondylitis phenotype: results from the REGISPONSER database. Arthritis Res. Ther. 2018 Oct 03;20(1):221.
- Muilu P, Rantalaiho V, Kautiainen H, Virta LJ, Eriksson JG, Puolakka K. Increasing incidence and shifting profile of idiopathic inflammatory rheumatic diseases in adults during this millennium. Clin. Rheumatol. 2019 Feb;38(2):555-562.
- Maravic M, Bozonnat MC, Sevezan A, Gasqueres D, Pastor J, Péré M, Neil V, Roch-Bras F, Daures JP, Sany J. Preliminary evaluation of medical outcomes (including quality of life) and costs in incident RA cases receiving hospital-based multidisciplinary management. Joint Bone Spine. 2000;67(5):425-33.
- Muilu P, Rantalaiho V, Kautiainen H, Virta LJ, Eriksson JG, Puolakka K. Increasing incidence and shifting profile of idiopathic inflammatory rheumatic diseases in adults during this millennium. Clin Rheumatol. 2019 Feb;38(2):555-562. doi: 10.1007/s10067-018-4310-0
- Alexander, S. A., Kim, E., & Mandhadi, R. (2021). Approaching Reactive Arthritis Associated With Poor Prognostic Factors: A Case Report and Literature Review. Cureus, 13(2), e13555. https://doi.org/10.7759/cureus.13555
- Dey, S., Sipani, A. K., & Das, R. (2021). Case Report of a Rare Cause of Reactive Arthritis: Leptospirosis. Journal of orthopaedic case reports, 11(3), 79–84. https://doi.org/10.13107/jocr.2021.v11.i03.2098
- Harrison TR, Kasper DL, Fauci AS. Harrison’s Principles of Internal Medicine. 19th ed. New York: McGraw-Hill; 2015.
- Spyridakis E, Gerber JS, Schriver E, Grundmeier RW, Porsch EA, St Geme JW, Downes KJ. Clinical Features and Outcomes of Children with Culture-Negative Septic Arthritis. J Pediatric Infect Dis Soc. 2019 Jul 1;8(3):228-234. doi: 10.1093/jpids/piy034
- Kousa M, Saikku P, Richmond S, Lassus A. Frequent association of chlamydial infection with Reiter’s syndrome. Sex Transm Dis. 1978 Apr-Jun. 5(2):57-61.
- Berlau J, Junker U, Groh A, Straube E. In situ hybridisation and direct fluorescence antibodies for the detection of Chlamydia trachomatis in synovial tissue from patients with reactive arthritis. J Clin Pathol. 1998 Nov. 51(11):803-6.
- Carter JD, Hudson AP. The evolving story of Chlamydia-induced reactive arthritis. Curr Opin Rheumatol. 2010 Jul. 22(4):424-30.
- Siala M, Mahfoudh N, Fourati H, Gdoura R, Younes M, Kammoun A, et al. MHC class I and class II genes in Tunisian patients with reactive and undifferentiated arthritis. Clin Exp Rheumatol. 2009 Mar-Apr. 27(2):208-13.
- Pappas G, Akritidis N, Christou L, Mastora M, Tsianos E. Unusual causes of reactive arthritis: Leptospira and Coxiella burnetii. Clin Rheumatol. 2003 Oct;22(4-5):343-6. doi: 10.1007/s10067-003-0730-5
- Kim PS, Klausmeier TL, Orr DP. Reactive arthritis: a review. J Adolesc Health. 2009 Apr;44(4):309-15. doi: 10.1016/j.jadohealth.2008.12.007
- Savolainen E, Kettunen A, Närvänen A, Kautiainen H, Kärkkäinen U, Luosujärvi R, et al. Prevalence of antibodies against Chlamydia trachomatis and incidence of C. trachomatis-induced reactive arthritis in an early arthritis series in Finland in 2000. Scand J Rheumatol. 2009. 38(5):353-6.
- Kim PS, Klausmeier TL, Orr DP. Reactive arthritis: a review. J Adolesc Health. 2009 Apr. 44(4):309-15.
- Rihl M, Barthel C, Klos A, Schmidt RE, Tak PP, Zeidler H, et al. Identification of candidate genes for susceptibility to reactive arthritis. Rheumatol Int. 2009 Oct. 29(12):1519-22.
- Sun HS, Liu DX, Bai YY, Hu NW. Disease-association of different killer cell immunoglobulin-like receptors (KIR) and HLA-C gene combinations in reactive arthritis. Mod Rheumatol. 2018 Jul 23. 1-7.
- Inman, R. D., & Chiu, B. (2012). Nafamostat mesylate, a serine protease inhibitor, demonstrates novel antimicrobial properties and effectiveness in Chlamydia-induced arthritis. Arthritis research & therapy, 14(3), R150. https://doi.org/10.1186/ar3886
- Nikkari S, Rantakokko K, Ekman P, Möttönen T, Leirisalo-Repo M, Virtala M, Lehtonen L, Jalava J, Kotilainen P, Granfors K, Toivanen P. Salmonella-triggered reactive arthritis: use of polymerase chain reaction, immunocytochemical staining, and gas chromatography-mass spectrometry in the detection of bacterial components from synovial fluid. Arthritis Rheum. 1999 Jan;42(1):84-9. doi: 10.1002/1529-0131(199901)42:1<84::AID-ANR11>3.0.CO;2-C
- Carter J.D. and Hudson A.P. (2009) Reactive arthritis: clinical aspects and medical management. Rheum. Disease Clin. 35, 21–44 10.1016/j.rdc.2009.03.010
- Carter J.D., Rehman A., Guthrie J.P., Gerard H.C., Stanich J. and Hudson A.P. (2013) Attack rate of Chlamydia-induced reactive arthritis and effect of the CCR5-Delta-32 mutation: a prospective analysis. J. Rheumatol. 40, 1578–1582 10.3899/jrheum.130136
- Carter J.D. and Hudson A.P. (2017) Recent advances and future directions in understanding and treating Chlamydia-induced reactive arthritis. Exp. Rev. Clin. Immunol. 13, 197–206 10.1080/1744666X.2017.1233816
- Baillet AC, Rehaume LM, Benham H, O’Meara CP, Armitage CW, Ruscher R, Brizard G, Harvie MC, Velasco J, Hansbro PM, Forrester JV, Degli-Esposti MA, Beagley KW, Thomas R. High Chlamydia Burden Promotes Tumor Necrosis Factor-Dependent Reactive Arthritis in SKG Mice. Arthritis Rheumatol. 2015 Jun;67(6):1535-47. doi: 10.1002/art.39041
- Allen RL, O’Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999 May 1;162(9):5045-8.
- Sieper J. Pathogenesis of reactive arthritis. Curr Rheumatol Rep. 2001 Oct;3(5):412-8. doi: 10.1007/s11926-996-0012-8
- Díaz-Peña R, Blanco-Gelaz MA, Njobvu P, López-Vazquez A, Suárez-Alvarez B, López-Larrea C. Influence of HLA-B*5703 and HLA-B*1403 on susceptibility to spondyloarthropathies in the Zambian population. J Rheumatol. 2008 Nov;35(11):2236-40. doi: 10.3899/jrheum.080395
- Ge, S., He, Q., & Granfors, K. (2012). HLA-B27 modulates intracellular growth of Salmonella pathogenicity island 2 mutants and production of cytokines in infected monocytic U937 cells. PloS one, 7(3), e34093. https://doi.org/10.1371/journal.pone.0034093
- Colbert, R. A., Tran, T. M., & Layh-Schmitt, G. (2014). HLA-B27 misfolding and ankylosing spondylitis. Molecular immunology, 57(1), 44–51. https://doi.org/10.1016/j.molimm.2013.07.013
- Yin Z, Braun J, Neure L, Wu P, Liu L, Eggens U, Sieper J. Crucial role of interleukin-10/interleukin-12 balance in the regulation of the type 2 T helper cytokine response in reactive arthritis. Arthritis Rheum. 1997 Oct;40(10):1788-97. doi: 10.1002/art.1780401010
- Meador R, Hsia E, Kitumnuaypong T, Schumacher HR. TNF involvement and anti-TNF therapy of reactive and unclassified arthritis. Clin Exp Rheumatol. 2002 Nov-Dec;20(6 Suppl 28):S130-4.
- Noto Llana, M., Sarnacki, S. H., Morales, A. L., Aya Castañeda, M., Giacomodonato, M. N., Blanco, G., & Cerquetti, M. C. (2017). Activation of iNKT Cells Prevents Salmonella-Enterocolitis and Salmonella-Induced Reactive Arthritis by Downregulating IL-17-Producing γδT Cells. Frontiers in cellular and infection microbiology, 7, 398. https://doi.org/10.3389/fcimb.2017.00398
- Simon, A. K., Seipelt, E., & Sieper, J. (1994). Divergent T-cell cytokine patterns in inflammatory arthritis. Proceedings of the National Academy of Sciences of the United States of America, 91(18), 8562–8566. https://doi.org/10.1073/pnas.91.18.8562
- Singh R, Aggarwal A, Misra R. Th1/Th17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathy. J Rheumatol. 2007 Nov;34(11):2285-90.
- Hamdulay, S. S., Glynne, S. J., & Keat, A. (2006). When is arthritis reactive?. Postgraduate medical journal, 82(969), 446–453. https://doi.org/10.1136/pgmj.2005.044057
- Carlin E, Flew S. Sexually acquired reactive arthritis. Clin Med (Lond). 2016 Apr;16(2):193-6.
- Stavropoulos PG, Soura E, Kanelleas A, Katsambas A, Antoniou C. Reactive arthritis. J Eur Acad Dermatol Venereol. 2015 Mar;29(3):415-24.
- Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J. Am. Acad. Dermatol. 2008 Jul;59(1):113-21.
- Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014 Apr-May;13(4-5):546-9. doi: 10.1016/j.autrev.2014.01.005
- Shohat N, Goswami K, Fillingham Y, Tan TL, Calkins T, Della Valle CJ, George J, Higuera C, Parvizi J. Diagnosing Periprosthetic Joint Infection in Inflammatory Arthritis: Assumption Is the Enemy of True Understanding. J Arthroplasty. 2018 Nov;33(11):3561-3566. doi: 10.1016/j.arth.2018.07.016
- Ikeda M, Yu DT. The pathogenesis of HLA-B27 arthritis: role of HLA-B27 in bacterial defense. Am J Med Sci. 1998 Oct;316(4):257-63. doi: 10.1097/00000441-199810000-00006
- Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014 Apr-May;13(4-5):546-9. https://doi.org/10.1016/j.autrev.2014.01.005
- Bojović J, Strelić N, Pavlica L. Reiter’s syndrome–disease of young men– analysis of 312 patients. Med. Pregl. 2014 Jul-Aug;67(7-8):222-30.
- Carter JD, Espinoza LR, Inman RD, Sneed KB, Ricca LR, Vasey FB, Valeriano J, Stanich JA, Oszust C, Gerard HC, Hudson AP. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010 May;62(5):1298-307.
- Carter J.D., Valeriano J., Vasey F.B. (2004) A prospective, randomized 9-month comparison of doxycycline vs. doxycycline and rifampin in undifferentiated spondyloarthritis– with special reference to Chlamydia-induced arthritis. J Rheumatol 31: 1973–1980
- Glintborg B, Lindström U, Aaltonen K, Kristianslund EK, Gudbjornsson B, Chatzidionysiou K, Askling J, Nordström D, Hetland ML, Di Giuseppe D, Dreyer L, Kristensen LE, Jørgensen TS, Eklund K, Grondal G, Ernestam S, Joensuu J, Törmänen M, Skydsgaard H, Hagfors J, Kvien TK, Lie E, Fagerli K, Geirsson AJ, Jonsson H, Provan SA, Krogh NS, Jacobsson L. Biological treatment in ankylosing spondylitis in the Nordic countries during 2010-2016: a collaboration between five biological registries. Scand. J. Rheumatol. 2018 Nov;47(6):465-474.
- Rohekar S, Pope J. Epidemiologic approaches to infection and immunity: the case of Reiter’s syndrome. Curr Opin Rheumatol. 2009 Jul. 21(4):386-90.
- Reactive Arthritis Prognosis. https://emedicine.medscape.com/article/331347-overview#a6
- Wechalekar MD, Rischmueller M, Whittle S, Burnet S, Hill CL. Prolonged remission of chronic reactive arthritis treated with three infusions of infliximab. J Clin Rheumatol. 2010 Mar. 16(2):79-80.
- Amor B, Santos RS, Nahal R, Listrat V, Dougados M. Predictive factors for the longterm outcome of spondyloarthropathies. J Rheumatol. 1994 Oct. 21(10):1883-7.