Reversible cerebral vasoconstriction syndrome
Reversible cerebral vasoconstriction syndrome (RCVS) is a rare cerebrovascular disorder characterized by severe headaches with or without focal neurological deficits or seizures, and a reversible segmental and multifocal vasoconstriction of cerebral arteries, that resolves spontaneously within 3 months 1. Reversible cerebral vasoconstriction syndrome mimics an aneurysmal subarachnoid hemorrhage 1. The main symptom of RCVS is sudden, severe, and disabling headaches that are sometimes called “thunderclap headaches”. Strokes or a bleeding into the brain may or may not be present.
RCVS occurs predominantly in females from the ages of 20 to 50 and more than half of cases occur in the puerperium (postpartum period of about six weeks after childbirth during which the mother’s reproductive organs return to their original non-pregnant condition) or after exposure to vasoactive substances. Typically, RCVS is self-limited and has a benign course, although in more than 10% of patients it may have more serious complications with permanent neurologic complications and death if not promptly diagnosed and treated 1. There is no specific laboratory investigation and cerebrospinal fluid (CSF) analysis is normal in most cases. Cerebral angiography shows alternating segmental constrictions and dilations. However, computed tomography (CT), magnetic resonance imaging (MRI) and angiography may be normal until the first week of evolution of the disease.
There is no established treatment for RCVS 2 and there is no form of treatment which clearly modifies the natural history of vasoconstriction 3. Reversible cerebral vasoconstriction syndrome treatment is predominantly supportive and directed to the symptoms includes analgesics, antiepileptic drugs for seizures, blood pressure monitoring and admission to the intensive care unit in severe cases 4.
Is reversible cerebral vasoconstriction syndrome dangerous?
Most patients with RCVS recover completely. A minority of patients have neurological problems. Reversible cerebral vasoconstriction syndrome does not usually come back.
Reversible cerebral vasoconstriction syndrome causes
RCVS happens when persistent contraction of the blood vessels (vasoconstriction) causes arteries to narrow. This reduces blood flow and oxygen delivery to the affected area of the body. When vasoconstriction affects the blood vessels of the brain, it is called cerebral vasoconstriction. RCVS can occur spontaneously, without apparent cause, or be secondary to endogenous or exogenous precipitating factors 1, the most common being associated with the changes that happen in the body immediately after giving birth (postpartum), changing birth control pills and exposure to various vasoactive substances such as illicit drugs and selective serotonin-reuptake inhibitors (SSRIs). A history of migraine is nearly always found in patients with RCVS.
The exact pathophysiology of reversible cerebral vasoconstriction syndrome (RCVS) remains unknown and the prevailing hypothesis involves a transient disturbance in the control of cerebral vascular tone 5. A rapid change of vascular tone can lead to vasoconstriction and vasodilatation of segmental small vessels and the sudden stretching of arterial walls can cause the thunderclap headache in the initial phase 6. This appears to be induced by sympathetic stimulation, endothelial dysfunction and oxidative stress 7. The association of RCVS with outbreaks of hypertension, vasoactive substance intake and phaeochromocytoma support the role of sympathetic stimulation. On the other hand, the overlap with posterior reversible encephalopathy syndrome (PRES) supports the role of endothelial dysfunction. Some hormones and biochemical factors have been involved in vascular tone dysregulation, namely endothelin-1, estrogen, serotonin, nitric oxide and prostaglandins. For example, levels of 8-iso-prostaglandin F2alfa, a marker of oxidative stress and a potent vasoconstrictor, seem to be related to the severity of RCVS, thus supporting the role of oxidative stress. Other factors, including the placental growth factor, soluble placental growth factor receptor and soluble endoglin, play a role in angiogenesis and have been implicated in postpartum RCVS 5.
Genetic factors may be the basis of individual susceptibility to develop RCVS and in the severity of its clinical course 5. Recently, the Brain-derived neurotrophic factor gene (BDNF) polymorphism (Val66Met), which is important for neuronal survival, neurogenesis and synaptic plasticity, has been associated with severe vasoconstriction in patients with RCVS 5.
Risk factors for developing reversible cerebral vasoconstriction syndrome
Female gender appears to be a risk factor in the development of RCVS 2, which is also more severe in women than in men 8.
Physiological changes during pregnancy and the postpartum period appear to increase susceptibility to develop reversible cerebral vasoconstriction syndrome 2, as well as hormonal therapy 6, pharmaceutical vasoconstrictors, including those used in the treatment of migraine 9, which is more common in women, and the use of recreational drugs 2; genetic factors appear to be the basis of individual susceptibility or can affect severity and clinical course 6.
Other risk factors associated with RCVS include:
- Use of drugs
- Use of alcohol, especially binge drinking
- Use of certain prescription medications, such as anti-depressants
- Use of nasal decongestants
- Use of nicotine patches
- Certain tumors
- Elevated calcium levels in the blood (hypercalcemia)
- Head trauma
RCVS triggers
Some possible external factors related to RCVS may include the use of prescription, over the counter, or illegal drugs that can cause constriction of the arteries. RCVS also may be linked to internal factors such as tumors, which secrete substances that, in turn, constrict blood vessels.
Prescription medications associated with reversible vasoconstriction syndrome include the following:
- Antidepressants: Selective serotonin reuptake inhibitors (SSRIs) such as Prozac®, Paxil®, and Zoloft®
- Medications to treat migraines: triptans (Imitrex®, Maxalt®), isometheptene (Amidrine®, Midrin®), and ergotamines (Migergot®, Ergomar®, Cafergot®)
- Immunosuppressants: cyclophosphamide (Cytoxan®) and tacrolimus (FK-506®)
- Drugs to prevent bleeding after childbirth
- Anti-Parkinson’s medications: bromocriptine and lisuride (Dopergin®, Proclacam®,and Revanil®)
Common over-the-counter (OTC) drugs and supplements that can cause constriction of cerebral arteries include the following:
- Nasal decongestants (pseudoephedrine, ephedrine, phenylpropanolamine)
- Nicotine patches
- Caffeine-containing energy drinks
- Ginseng
Illegal drugs associated with RCVS are:
- Marijuana
- Cocaine
- Ecstasy
- Amphetamine derivatives
- Lysergic acid diethylamide (LSD)
Others factors related to RCVS can include blood and intravenous immunoglobulin (IVIG) transfusions as well as vasoactive secreting tumors. These tumors include phaeochromocytoma, bronchial carcinoid, and glomus tumors.
Reversible cerebral vasoconstriction syndrome symptoms
The most common symptom in 95–100% of cases of reversible cerebral vasoconstriction syndrome is severe acute headache also known as thunderclap headache, which reaches maximum intensity in seconds, mimicking the onset of an aneurysm ruptured headache 1. On average, the headache lasts 3 hours 10 and repeats four times over a period of 1–4 weeks 11, often triggered by sexual activity, stress, Valsalva manoeuvre and/or emotions. The typical headache is bilateral, although it may be unilateral, starting in the occipital region, followed by diffuse pain 11 and often accompanied by nausea, vomiting, photophobia and phonophobia. In some cases, the headache does not correspond to the definition of thunderclap headache, being less intense or more progressive; the presentation of other symptoms without the headache is exceptional 11. If accompanied by neck pain, it is especially necessary to exclude cervical artery dissection 12. Although patients may have migraine as a comorbidity, they report the thunderclap headache of RCVS as having location, degree and quality different from their usual headache 10. RCVS may also be associated with focal neurological deficits, transient or permanent, and seizures, as reported in three major series, in 8–43% and 1–17% of cases, respectively 11, 13, 14.
Other symptoms may include:
- Strokes or transient ischemic attacks (TIAs or “mini-strokes”)
- Weakness
- Problems with eyesight, changes in vision
- Difficulty understanding others when they are speaking
- Difficulty speaking
- Seizures
- Weakness on one side of the body
The seizures can be at the onset; their recurrence is rare 11. Transient focal deficits are present in little more than 10% of patients, last 1 minute to 4 hours, they are often visual, but can also be motor, sensory or dysphasic 11. The majority have sudden onset and are similar to a transient ischemic attack (TIA), though they can mimic positive symptoms of a migraine aura 15.
Neurological deficits exceeding 12 hours are not likely to improve. Permanent deficits suggest a stroke and include hemiplegia, aphasia, hemianopia or cortical blindness 11. Cerebellar strokes may also occur.
In more than a third of patients, hypertensive peaks (systolic blood pressure >160mm Hg) occur during headache crisis 16. It is not clear whether this is due to the pain, a response to the vasoconstriction of arteries, or integrates the manifestations of the disease.
The major complication of RCVS is ischemic or hemorrhagic stroke.
RCVS patients are at risk of experiencing a stroke or transient ischemic attack (TIA). Signs of a stroke include:
- Sudden numbness or weakness of the face, arm or leg, especially on one side of the body
- Sudden confusion
- Sudden trouble speaking
- Sudden trouble seeing in one or both eyes
- Sudden trouble walking
- Sudden dizziness, loss of balance or coordination
- Sudden, severe headache with no known cause
The effects of an acute ischemic stroke may cause additional symptoms in women including:
- Face, arm or leg pain
- Hiccups or nausea
- Chest pain or palpitations
- Shortness of breath
If you notice one or more of these signs of stroke in another person or in yourself, do not wait to seek help. Call your local emergency services number immediately.
Reversible cerebral vasoconstriction syndrome complications
Hemorrhagic complications (parenchymal and subarachnoid hemorrhage [SAH]), posterior reversible encephalopathy syndrome (PRES) and seizures often occur in the first week of reversible cerebral vasoconstriction syndrome 17. In contrast, ischemic events and resulting focal neurological deficits occur later, with a peak between the first and second week of RCVS onset. Brain ischamia can occur even later in the course of the syndrome, occasionally after the resolution of symptoms such as headache, reflecting the well documented apparent delay in resolving cerebral vasoconstriction 17.
The female gender and a history of migraine are independent risk factors for hemorrhagic complications 8. On the contrary, a history of hypertension and peaks of elevation in systolic blood pressure are not associated with the risk of hemorrhage 8. The identification of migraine, mainly with aura, as a risk factor for hemorrhagic complications, is particularly relevant, since previous studies have not highlighted migraine as a risk factor for global RCVS 8. More studies are needed to re-evaluate migraine as a risk factor for RCVS hemorrhagic complications, for RCVS, or both 8.
Reversible cerebral vasoconstriction syndrome diagnosis
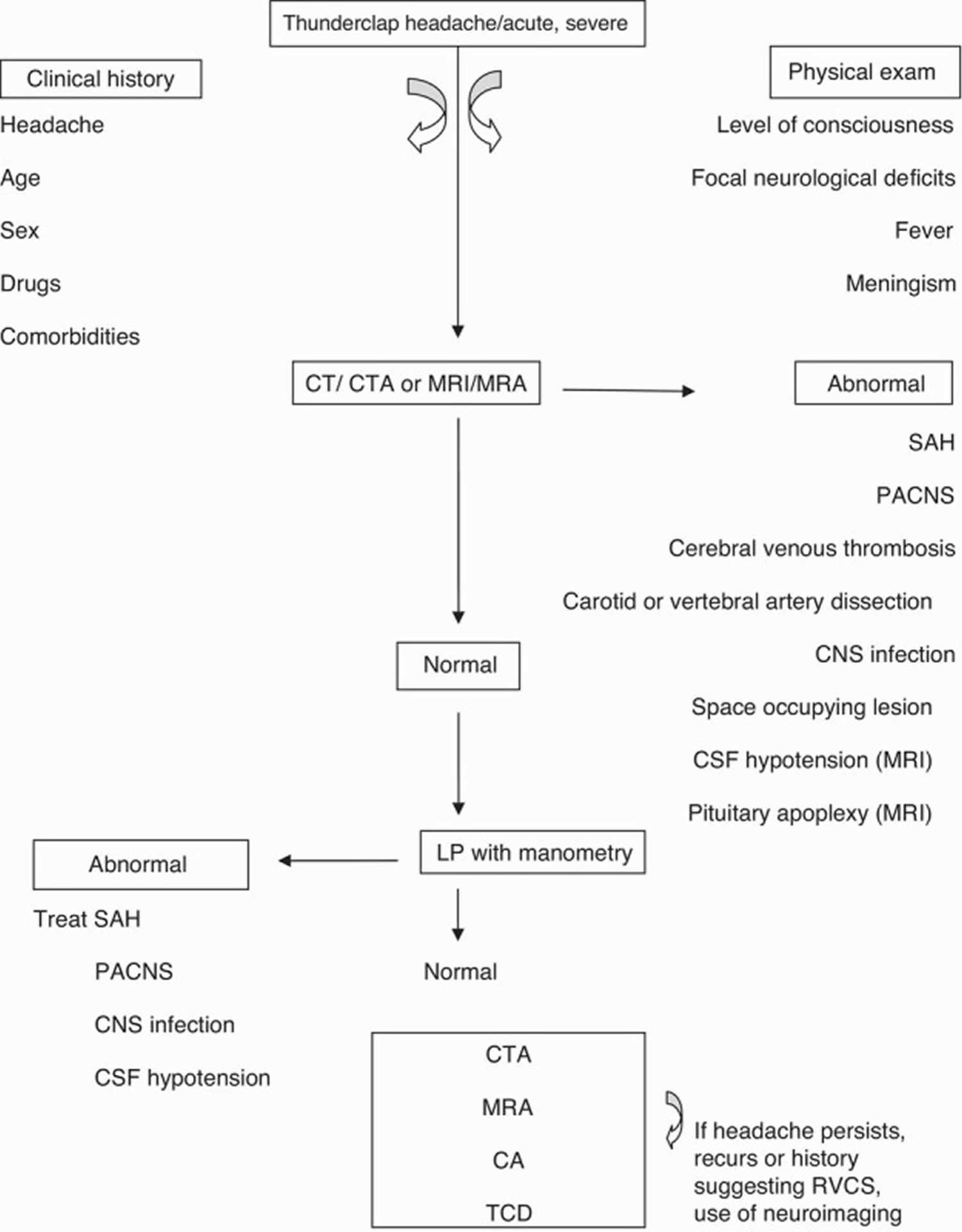
Reversible cerebral vasoconstriction syndrome is diagnosed based on a person’s medical history, symptoms, a complete physical exam, and the results of vascular brain imaging (see Figure 1 below). Such imaging may be in the form of an angiogram, magnetic resonance angiogram (MRA) or computed tomography angiography (CTA) scan that can show spasms in blood vessels that are narrowed. These imaging tests look at the soft tissue and blood vessels within the body and can determine if the condition is associated with a stroke or other underlying issues. It is necessary to keep in mind that the arterial vasoconstriction may not be evident in the first angiographic study; in cases with high clinical suspicion, the repetition of these exams is indicated 18. As suggested by Ducros et al. 15, the segmental vasomotor dysregulation seems to start in the most peripheral arterioles, progressing centripetally to the medium and large calibre cerebral arteries, which are viewed more easily 5. This centripetal progression can explain the time lag between headaches and vasoconstriction, given that headaches appear before any detectable vasoconstriction and this extends on average 14 days after headache resolution. Therefore, headache may be due to the involvement of small distal arteries, whose rapid changes of calibre (constriction or dilation) stimulate perivascular pain-sensitive fibres 15. However, given the association with migraine, however, not all patients with suspected RCVS will require advanced imaging tests.
Cerebrospinal fluid testing can help to rule out other conditions that appear to be similar to RCVS. It is important to repeat the vascular imaging to ensure that the spasm that resulted in the diagnosis can be confirmed.
Other tests look exclusively at the blood vessels within the body. An angiogram may also be used to view the arteries. A transcranial Doppler ultrasound is used to measure the blood that is flowing through the arteries at the base of the brain.
Blood and urine tests may also be used to examine how well the liver and kidneys are functioning. Additionally, it is important to screen serum and urine for vasoactive drugs.
The complete blood count and biochemistry are generally normal. Exclusion of vasculitis and vasoactive tumours (carcinoid and pheochromocytoma) is useful.
The diagnosis is based on angiographic exams and requires the initial demonstration of diffuse segmental vasoconstriction of cerebral arteries, as well as its reversibility (complete or marked normalization of arteries) in 12 weeks after onset of the disease 19.
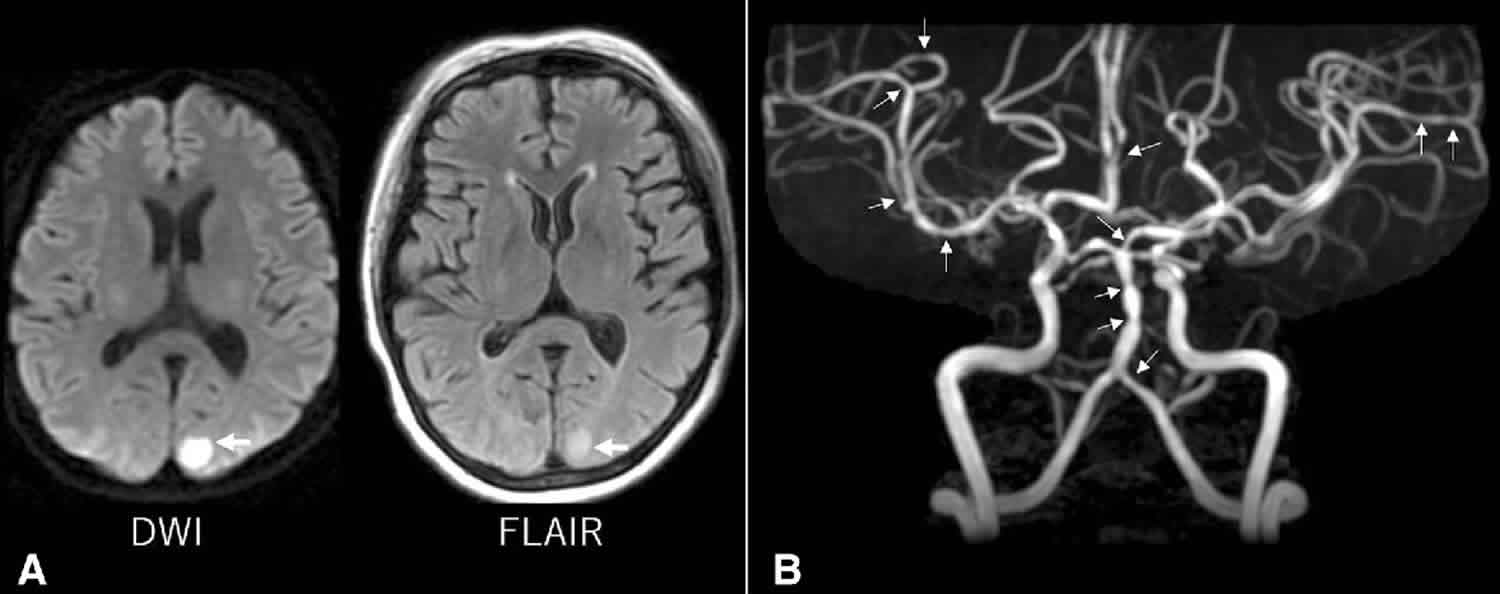
Initially, 55–80% of patients have a normal CT or MRI. However, in subsequent images, 12–81% of patients have changes consistent with subarachnoid hemorrhage (SAH), convexity intracerebral hemorrhage, cerebral infarction and posterior reversible encephalopathy syndrome (PRES). Hemorrhagic complications are the most frequent.
Particular attention must be payed to the intracerebral hemorrhage, which seems to be more common in women and people with migraine 8. It occurs early in the course of RCVS, revealing itself in most cases by a thunderclap headache with a persistent focal deficit. Attention is also important to posterior reversible encephalopathy syndrome (PRES), due to clinical and imaging overlapping with RCVS and the possibility of simultaneous occurrence 20. More than 85% patients with PRES demonstrate cerebral vasoconstriction similar to RCVS in conventional angiography. Reversible cerebral oedema alike PRES may also occur in 9–38% of patients with RCVS 17.
Regarding imaging, catheter cerebral angiography is the gold standard diagnostic exam. It allows a real-time assessment of the calibre and flow of vessels, provides a good view of the small and peripheral vessels, with good spatial and temporal resolution, and allows for acting therapeutically with intra-arterial vasodilators. It has the disadvantage of being an invasive method, with potential vascular damage and stroke,59 and it is impractical for follow-up. In one of the series by Ducros et al. 15, over 9% of patients suffered transient neurologic deficits after cerebral angiography 15. This type of angiography should be reserved for cases whose diagnoses raise additional questions 6.
CT and MRI angiographies (CTA and MRA) allow vascular diagnosis, with the advantage of not being intrusive.
CTA overcomes the limitations of conventional angiography, allowing a good assessment of vasoconstriction in patients with RCVS 21. It has been singled out as a specific and very sensitive tool in the assessment of intracranial vasculature 22, however it is not feasible for sequential follow-up due to exposure to radiation and contrast.
Chen et al. 16 demonstrated that the assessment with MRA is valid. Transcranial Doppler (TCD) imaging has long been used and validated in the study of intracranial vessel spasm 23, allowing sequential monitoring. It is a non-invasive method, innocuous, with high reproducibility, which makes it possible to evaluate the vessel vasoconstriction and monitor haemodynamic changes in patients with RCVS. According to a study, cerebral vasoconstriction indicators, including high mean flow velocity of the middle cerebral artery (>120cm/s) and a high hemispheric index (>3), were associated with an increased risk of developing PRES or ischemic stroke 17. However, the acceleration of the velocity in arteries at the base of the skull do not often reach these values in RCVS as in aneurysmal subarachnoid hemorrhage (SAH) and the focal character and the temporal evolution of the flow velocities should be valued in this case. The Transcranial Doppler has the disadvantage of not allowing the evaluation of small vessels 24.
In imagining, the main differential diagnosis, after aneurysmal subarachnoid hemorrhage (SAH) is ruled out, is CNS vasculitis (primary angiitis of the central nervous system or PACNS) 25. This distinction is important, though difficult, because the treatment is quite different 5. The resolution of the cerebral vasoconstriction in the mid-term angiographic control allows for the differentiation between these two entities. In addition, advanced techniques of MRI that evaluate the arterial wall can help the differential diagnosis at an early stage of the disease, because there are abnormal captures of the contrast product on the wall of the involved arteries in primary angiitis of the central nervous system (PACNS) 18. Additionally, the headache of the vasculitis has an insidious onset, progressing gradually, and thus distinct from that typical of RCVS 26 and it is more common in older men, unlike RCVS that is typical in young/middle aged women 27. There are also differences in the CSF analysis because, unlike RCVS, in vasculitis it is not normal, with proteins and leucocyte counts increase with values generally >100mg/dL and 5–10 cells/mm³, respectively 5. Finally, patients with vasculitis have a fulminant course, with poor prognosis, particularly if treatment with immunosuppressant steroids and cytotoxic agents is delayed; however, lack of these drugs benefits RCVS 5.
Another differential diagnosis to be noted is cervical artery dissection, as it evolves with headache/neck pain and can even be associated with RCVS 10.
Figure 1. Diagnostic algorithm for RCVS
Abbreviations: CTA = computed tomography angiography; MRI = magnetic resonance imaging; MRA = magnetic resonance angiography; PACNS = primary angiitis of the central nervous system (CNS); CA = catheter angiography; TCD = transcranial Doppler.
[Source 24 ]Reversible cerebral vasoconstriction syndrome treatment
There is no known cure for reversible cerebral vasoconstriction syndrome, but reversible cerebral vasoconstriction syndrome can be reversed. If a drug has been associated with RCVS, the patient should talk to his or her doctor about decreasing the dose or stopping the use of the drug. In some cases, the condition clears up without treatment. However, due to the risk of stroke, it is important that patients seek and get immediate medical care. Fluids will be administered intravenously and migraine treatments such as aspirin or Depakote may be given. The use of calcium channel blockers such as Cardizem and Nimodipine may be used to relax the blood vessels and allow more blood to flow through can reduce headaches. Calcium channel blocker medication has been shown to help ease the “thunder clap” headaches, but does not decrease the risk of stroke. Nimodipine is the most used and may have a more elective action on cerebral circulation. Despite reducing the number of episodes and the intensity of the headache, prospective and retrospective studies suggest that it does not affect the time course of cerebral vasoconstriction. It may be given orally or intravenously at a dose used for prevention of vessel spasm in aneurysmal subarachnoid hemorrhage (SAH). The duration of treatment should range from 4 to 12 weeks 11. New bleeding, TIA and stroke are reported in some patients, even if treated for several days 11. Other drugs include nicardipine 28, verapamil 14 and magnesium sulphate (in the treatment of postpartum angiopathy) 29. In some cases, the patient is given intravenous magnesium.
Glucocorticoids should be avoided because they are an independent predictor of poor prognosis 1.
Treatment includes analgesics, antiepileptic drugs for seizures, blood pressure monitoring and admission to the intensive care unit in severe cases 11. In the most severe cases, milrinone, nimodipine and epoprostenol administered intra-arterially, and balloon angioplasty with variable success have been used 11. These are delicate and high risk interventions, and should be restricted to patients who show clear signs of clinical progression 30.
Rest is also very important in the treatment of reversible cerebral vasoconstriction syndrome.
Reversible cerebral vasoconstriction syndrome prognosis
Outcomes for reversible cerebral vasoconstriction syndrome patients range from full recovery in most patients to permanent brain damage in other patients. More than 90% of patients have a good prognosis where the course of this syndrome is self-limited and benign 2. However, 10% of patients have severe complications 14 that can lead to death. Complications include cerebral infarction (4–40%), intracerebral hemorrhage (6–20%), subarachnoid hemorrhage (SAH) (22–34%) and posterior reversible encephalopathy syndrome (PRES) (less than 10%) 2. These values of incidence reported may be the result of bias in recruitment of participants (perhaps with the most symptomatic patients seeking medical help) selection criteria, and the context of each patient 17. For example, the cases of ischemic stroke and intracerebral hemorrhage in patients who developed postpartum RCVS described by Fugate et al. 31 appear to be greater than those described in Ducros et al 11. In the American series, 81% of patients had brain image compatible with lesion. Despite this, the rate of permanent neurological deficits is very low and deaths are rare 14. The recurrence rate was described as being about 8% 16. Cerebral infarct and intracerebral hemorrhage are predictors of poor prognosis 14.
References- Santos L, Azevedo E. Reversible cerebral vasoconstriction syndrome – A narrative revision of the literature. Porto Biomed J. 2016;1(2):65-71. doi:10.1016/j.pbj.2016.04.002 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6806951
- Velez A, McKinney SJ. Reversible cerebral vasoconstriction syndrome: a review of recent research. Curr Neurol Neurosci Rep. 2013;13:319.
- Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol. 2004;61:411-416.
- Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11:906-917. https://doi.org/10.1016/S1474-4422(12)70135-7
- Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible cerebral vasoconstriction syndrome, Part 2: diagnostic work-up, imaging evaluation, and differential diagnosis. AJNR Am J Neuroradiol. 2015;36:1580-1588.
- Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother. 2011;11:1265-1276.
- Paliwal PR, Teoh HL, Sharma VK. Association between reversible cerebral vasoconstriction syndrome and trombotic thrombocytopenic purpura. J Neurol Sci. 2014;338:223-225.
- Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. 2010;41:2505-2511.
- Macgregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843-859.
- Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: an under-recognized clinical emergency. Ther Adv Neurol Disord. 2010;3:161-171.
- Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11:906-917. https://www.thelancet.com/article/S1474-4422(12)70135-7/fulltext
- Chen SP, Fuh JL, Wang SJ, Tsai SJ, Hong CJ, Yang AC. Brain-derived neurotrophic factor gene Val66Met polymorphism modulates reversible cerebral vasoconstriction syndromes. PLoS ONE. 2011;6:e18024.
- Chen SP, Fuh JL, Wang SJ, Tsai SJ, Hong CJ, Yang AC. Brain-derived neurotrophic factor gene Val66Met polymorphism modulates reversible cerebral vasoconstriction syndromes. PLoS ONE. 2011;6:e18024
- Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68:1005-1012.
- Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091-3101.
- Chen SP, Fuh JL, Wang SJ, Chang FC, Lirng JF, Fang YC, et al. Magnetic resonance angiography study in reversible vasoconstriction syndromes. Ann Neurol. 2010;67:648-656.
- Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible cerebral vasoconstriction syndrome, Part 1: epidemiology, pathogenesis, and clinical course. AJNR Am J Neuroradiol. 2015;36:1392-1399.
- Mandell DM, Matouk CC, Farb RI, Krings T, Agid R, terBrugge K, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke. 2012;43:860-862.
- Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34-44.
- Sheikh HU, Mathew PG. Reversible vasoconstriction syndrome: updates and new perspectives. Curr Pain Headache Rep. 2014;18:414.
- Chen SP, Fuh JL, Lirng JF, Chang FC, Wang SJ. Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder? Neurology. 2006;67:2164-2169.
- Aaslid R. Transcranial Doppler assessment of cerebral vasospasm. Eur J Ultrasound. 2002;16:3-10.
- Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, et al. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004;62:1468-1481.
- Tan HL, Flower O. Reversible cerebral vasoconstriction syndrome: an important cause of acute severe headache. Emerg Med Int. 2012. 2012. AN 303152
- Rodrigues T, Loureiro R, Samões R, Alves V, Ramos C, Correia C. Síndrome de vasoconstrição cerebral reversível: uma causa importante de acidentes vasculares cerebrais no puerpério. Acta Med Port. 2014;27:515-518.
- Nouh A, Ruland S, Schneck MJ, Pasquale D, Biller J. Reversible cerebral vasoconstriction syndrome with multivessel cervical artery dissections and a double aortic arch. J Stroke Cerebrovasc Dis. 2014;23:e141-e143.
- Hammad TA, Hajj-Ali RA. Primary angiitis of the central nervous system and reversible cerebral vasoconstriction syndrome. Curr Atheroscler Rep. 2013;15:346.
- Liu HY, Fuh JL, Lirng JF, Chen SP, Wang SJ. Three pediatric patients with reversible cerebral vasoconstriction syndromes. Cephalalgia. 2010;30:354-359.
- McKinney JS, Messe SR, Pukenas BA, Satti SR, Weigele JB, Hurst RW, et al. Intracranial vertebrobasilar artery dissection associated with postpartum angiopathy. Stroke Res Treat. 2010;2010:320627.
- Singhal AB, Kimberley WT, Schaefer PW, Hedley-Whyte ET. Case records of Massachusetts General Hospital. Case 8-2009. A 36-year-old woman with headache, hypertension, and seizure 2 weeks post partum. N Engl J Med. 2009;360:1126-1137.
- Fugate JE, Ameriso SF, Ortiz G, Schottlaender LV, Wijdicks EFM, Flemming KD, et al. Variable presentations of postpartum angiopathy. Stroke. 2012;43:670-676.