Solitary thyroid nodule
Thyroid nodule is a discrete lesion or abnormal growth of thyroid cells that forms a lump within the thyroid gland that can be delineated on imaging studies from the adjacent thyroid parenchyma 1. Thyroid nodule may be solitary, multiple, cystic, or solid 2. Approximately 23% of solitary thyroid nodules represent a dominant nodule within a multinodular goiter 3. Thyroid nodules can represent a range of benign or malignant conditions. They are more common in females (4:1 Female: Male) and have an increasing prevalence with increasing age and reduced iodine intake 4. True solitary thyroid nodules occur in 0.22-1.35% of the pediatric population and in close to 4% of the adult population 5. The incidence is estimated at 0.1% with a lifetime prevalence of 5 to 10% 6, however, high resolution ultrasound has revealed thyroid nodules in 19-68% of randomly selected individuals 7. Autopsy data have shown a 50% prevalence of thyroid nodules larger than one centimeter in patients without previously diagnosed thyroid disease 8. Thyroid nodules are found with increasing frequency, likely due to the widespread use of modern imaging modalities, particularly ultrasound (US), but also computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) 2.
There are different types of thyroid nodules:
- Colloid nodules: These are one or more overgrowths of normal thyroid tissue. These growths are benign (not cancer). They may grow large, but they do not spread beyond the thyroid gland.
- Thyroid cysts: These are growths that are filled with fluid or partly solid and partly filled with fluid.
- Inflammatory nodules: These nodules develop as a result of chronic (long-term) inflammation (swelling) of the thyroid gland. These growths may or may not cause pain.
- Multinodular goiter: Sometimes an enlarged thyroid (goiter) is made up of many nodules (which are usually benign).
- Hyperfunctioning thyroid nodules: These nodules autonomously produce thyroid hormone without regard for normal feedback control mechanisms, which may lead to the development of hyperthyroidism. Hyperthyroidism can affect the heart and cause such problems as sudden cardiac arrest, high blood pressure, arrhythmias (abnormal heart rhythm), osteoporosis and other health problems.
- Thyroid cancer: Less than 5 percent of thyroid nodules are cancerous.
Although the vast majority (more than 90%) of thyroid nodules are benign (noncancerous) 8, a small proportion (in approximately 4.0% to 6.5% of cases) of thyroid nodules do contain thyroid cancer 6. A retrospective study by Keh et al 9 of 61 patients found 75.4% of solitary thyroid nodules to have a neoplastic pathology and 34.4% to be malignant.
In order to diagnose and treat thyroid cancer at the earliest stage, most thyroid nodules need some type of evaluation 1.
Treatment depends on the type of thyroid nodule. Treatment options include:
- No treatment/”watchful waiting.” If the nodules are not cancerous, you and your doctor may decide that you don’t need to be treated at this time. You will see your doctor on a regular basis so he or she can watch for any changes in the nodules.
- Radioactive iodine. Your doctor may use radioactive iodine to treat hyperfunctioning thyroid nodules and goiters with several nodules. The radioactive iodine is absorbed into the thyroid gland, causing the nodules to shrink. Pregnant women and women trying to become pregnant should not have this treatment.
- Surgery. Surgery to take out the nodules is the best treatment for nodules that are cancerous, cause “obstructive symptoms” (for example, are so large that they make breathing or swallowing difficult), and are “suspicious” (they cannot be diagnosed without being taken out and examined).
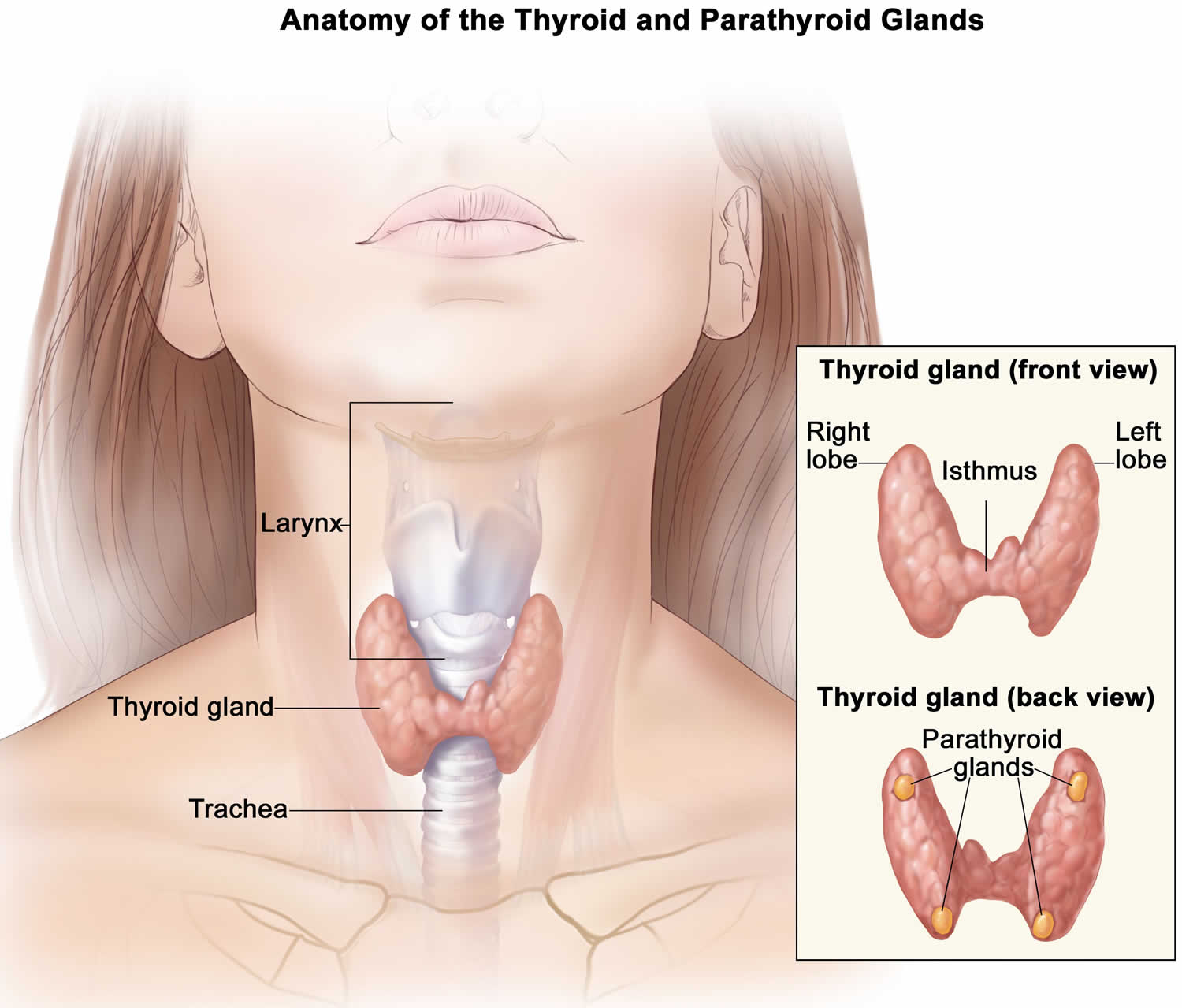
Figure 1. Thyroid gland
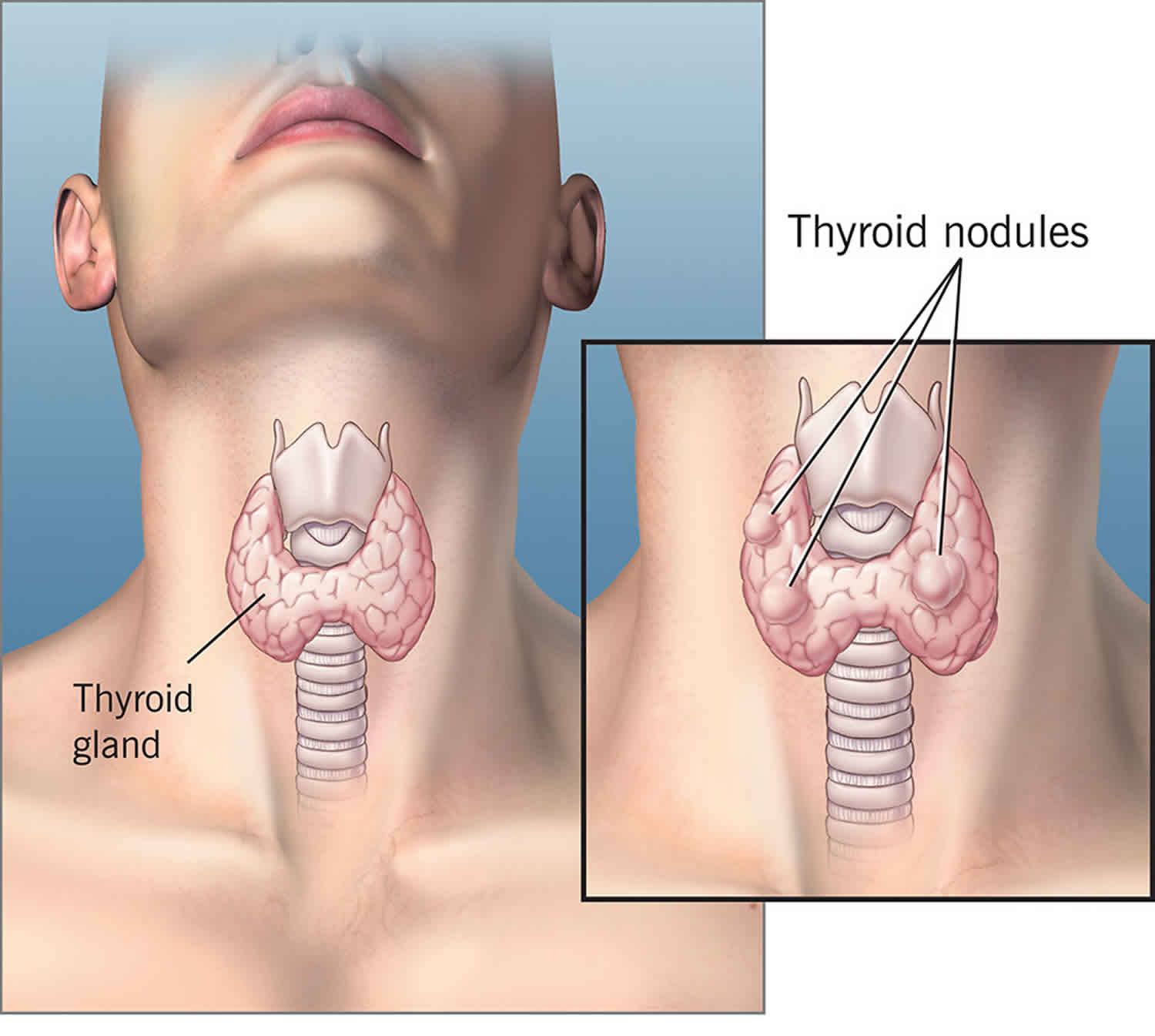
Figure 2. Solitary thyroid nodule
How can my doctor tell if I have a thyroid nodule that is cancerous?
Your doctor can do several different tests. One test is called fine-needle aspiration biopsy (FNAB). Your doctor will take a tissue sample from your thyroid gland and examine it under a microscope to see if it is cancerous. The tissue sample is taken with a very small needle.
Another test your doctor may do is an ultrasound. This test uses sound waves to make a picture of the thyroid’s shape and the size of the nodules. It can help your doctor determine whether the nodule is a solid tumor or a cyst filled with fluid.
A third test is a thyroid scan. Your doctor will inject harmless radioactive iodine into a vein in your arm. The iodine is absorbed by your thyroid gland and makes it “glow” as your doctor takes a special picture. Your doctor can learn about the nodule depending on how much or how little of the iodine shows in the picture.
Can thyroid nodules be prevented or avoided?
Doctors are not sure what causes most thyroid nodules, so most likely, you cannot prevent them. You can make sure you eat enough food that contains iodine (table salt, dairy products, seafood, meat, etc.), which can prevent one cause of thyroid nodules.
Solitary thyroid nodule causes
Doctors aren’t always sure why some people get thyroid nodules, but they are very common. The nodules are sometimes associated with other medical conditions, including:
- Iodine deficiency
- Hyperthyroidism (overactive thyroid)
- Hashimoto’s disease (hypothyroidism, or underactive thyroid)
- Thyroid cancer
Ionizing radiation is a known risk factor for both benign and malignant nodules of the thyroid 10. This population may develop thyroid nodules at a rate of 2% annually 3. The incidence of malignancy has been documented as high as 20% to 50% in palpable nodules of previously irradiated thyroids glands 11.
Other factors that lead to an increased risk of thyroid nodules and goiter include smoking, obesity, metabolic syndrome, alcohol consumption, increased levels of insulin-like growth factor-1, and uterine fibroids. Factors associated with possible decreased risk may include the use of oral contraceptives and statins 11.
Thyroid nodules may be classified as neoplastic and non-neoplastic. Neoplastic thyroid nodules may be benign or malignant, with benign neoplastic including non-functioning and functioning nodules. Non-neoplastic thyroid nodules include hyperplastic and inflammatory nodules 2.
Colloid nodules represent adenomatous benign neoplasms, are the most common thyroid nodules and do not pose an increased risk of malignancy. While most follicular adenomas are benign, they share characteristics with follicular carcinomas 3.
Thyroid carcinomas may be classified as non-medullary thyroid cancers, arising from epithelial cells and constituting approximately 95% of all thyroid malignancies, or medullary thyroid cancer (MTC) arising from the calcitonin-producing parafollicular cells of the thyroid. Twenty percent of medullary thyroid cancers are familiar and may occur as part of multiple endocrine neoplasia (MEN) syndromes 11.
Risk factors for thyroid nodules
Risk factors for developing thyroid nodules include:
- Family history. Having parents or siblings who have had thyroid nodules or thyroid or other endocrine cancers increases your chance of developing nodules.
- Age: The chance of developing nodules increases as you get older.
- Gender: Women are more likely than men to develop thyroid nodules.
- Radiation exposure: A history of radiation exposure to the head and neck (from medical treatments, but not from diagnostic procedures, such as a CT scan) increases your risk of developing nodules.
Risk factors for developing cancerous thyroid nodules include:
- Family history of thyroid cancer
- A nodule that is hard or is stuck to a nearby structure
- Male gender
- Age younger than 20 and older than 70
- Radiation exposure
Solitary thyroid nodule symptoms
Most thyroid nodules do not cause symptoms. Some people might have trouble swallowing or have a feeling of fullness, pain or pressure in the throat or neck. Some people might notice a lump in their neck when they look in the mirror, but this is uncommon. Some people experience rapid unintended weight loss, feelings of nervousness or irregular heartbeat.
Hyperfunctioning thyroid nodules also known as hyperthyroidism can lead to overproduction of thyroid hormones. Symptoms of hyperthyroidism include:
- Irritability/nervousness
- Muscle weakness/tremors
- Light or missed menstrual periods
- Weight loss
- Difficulty sleeping
- Enlarged thyroid gland
- Vision problems or eye irritation
- Heat sensitivity (trouble dealing with heat)
- Increase or decrease in appetite
- Shortness of breath
- Itchy skin/clammy skin
- Thinning hair
- Skin flushing (sudden reddening of face, neck or upper chest)
- Heart palpitations (rapid or irregular heartbeat)
Thyroid nodules may also be associated with low thyroid hormone levels, or hypothyroidism. Symptoms of hypothyroidism include:
- Fatigue (feeling tired)
- Frequent, heavy menstrual periods
- Forgetfulness
- Weight gain
- Dry, coarse skin and hair, and hair loss
- Hoarse voice
- Trouble dealing with cold temperatures
- Weakness/irritability
- Constipation
- Depression
- Generalized edema (swelling)
Most patients will present an incidental nodule that is found on imaging studies performed for other reasons. Most thyroid nodules are asymptomatic, and most individuals with thyroid nodules are euthyroid, with less than 1% of nodules causing thyroid disease. Some patients may complain of neck pressure or pain, particularly when spontaneous hemorrhage occurs 3.
Palpation of the thyroid is the easiest but least sensitive method for detecting thyroid nodules, with a prevalence of 4% to 7% 12. Physical examination findings that should raise concern for malignancy include nodules larger than 4 cm in size (approximately 19% risk for malignancy), firmness on palpation, fixation to adjacent tissues, cervical lymphadenopathy, and vocal fold paralysis. In some patients, the physical examination may be limited by body habitus 13.
The combined physical examination findings of a solitary nodule along in addition to cervical lymphadenopathy (greater than 1 cm) and vocal fold paralysis have an approximate positive predictive value of 100% for thyroid malignancy 13.
Appropriate social history is important and may aid in identifying patients with multiple endocrine neoplasia, type 2 (MEN II). These are at higher risk for pheochromocytomas and need appropriate workup before surgical intervention 11.
Benign versus malignant thyroid nodules
Benign masses are usually movable, soft, and nontender. Malignancy is associated with a hard nodule, fixation to surrounding tissue, and regional lymphadenopathy (enlarged lymph node) 5.
Reported rapid growth or recurrent laryngeal nerve dysfunction found on examination may indicate malignancy and local infiltration.
If medullary carcinoma is suspected in conjunction with multiple endocrine neoplasia (MEN) 2B, multiple mucosal neuromas, marfanoid body habitus, and skeletal defects may be evident.
Hormonal imbalance
Although most patients with thyroid nodules are asymptomatic 14, some exhibit signs and symptoms of altered levels of thyroid hormone, as follows:
- Hyperthyroidism (overactive thyroid gland) – Nervousness, heat intolerance, diarrhea, muscle weakness, and loss of weight and appetite; in rare cases, exophthalmos may be present in a person with a hyperfunctioning nodule
- Hypothyroidism (underactive thyroid gland) – May result in cold intolerance, constipation, fatigue, and weight gain, which, in children, is primarily caused by the accumulation of myxedematous fluid.
Nerve involvement
Signs and symptoms of local nerve involvement should trigger rapid investigation, because such involvement may be indicative of local invasiveness from malignancy. The most important of these signs are dysphagia and hoarseness.
Solitary thyroid nodule diagnosis
Often, a doctor finds the lump during a routine checkup or during other tests. He or she can feel it. Ultrasound is the first-line imaging modality for assessment of thyroid nodules found on clinical examination or incidentally on another imaging modality. Controversy surrounds the efficacy of imaging studies and fine-needle aspiration biopsy (FNAB) because precision and accuracy vary among centers. In general, when in doubt, perform excisional biopsy, especially in pediatric patients because of the higher rates of malignancy and aggressiveness of disease in this population.
Laboratory studies
- Thyroid function tests – An elevated thyroid-stimulating hormone (TSH) level may indicate agenesis of a thyroid lobe or thyroiditis; a very low TSH level indicates an autonomous or hyperfunctioning nodule
- Antithyroid antibodies – Helpful in diagnosing chronic lymphocytic thyroiditis (ie, Hashimoto thyroiditis)
- Complete blood count (CBC) – If abscess is suspected
- Calcium levels – Should be monitored immediately postoperatively to assess parathyroid function and the need for supplementation
Several laboratory studies are helpful in assessing the nature of a thyroid nodule and in monitoring response to surgical treatment.
Initially, thyroid function tests can determine whether a nodule is functioning or autonomous. However, a large percentage of malignant nodules cause no change in thyroid function tests. An elevated thyroid-stimulating hormone (TSH) level may indicate agenesis of a thyroid lobe or thyroiditis. A very low TSH level indicates an autonomous or hyperfunctioning nodule. Levels of free thyroxine (T4), triiodothyronine (T3), and TSH are used to direct medical therapy.
Some centers also find antithyroid antibodies helpful in diagnosing chronic lymphocytic thyroiditis (ie, Hashimoto thyroiditis); however, a positive antibody test result does not exclude the possibility of malignancy (it actually may indicate a higher risk).
A complete blood count (CBC) count may be obtained if abscess is suspected.
Calcium levels should be monitored immediately postoperatively to assess parathyroid function and the need for supplementation. In addition, certain laboratory findings should be monitored for therapeutic purposes and for the recurrence of disease after surgical excision.
TSH levels should be completely suppressed, but clinical euthyroidism should be maintained if the patient is on replacement therapy following total thyroidectomy for malignancy. This approach prevents TSH stimulation of any remaining tumor cells. As the child grows, periodic thyroid hormone levels help determine thyroid hormone replacement adequacy and any changing needs due to development.
Some laboratory tests can also be used to monitor for recurrence of disease. Thyroglobulin levels may be useful but only after total thyroidectomy is performed. Thyroglobulin levels exceeding 1 ng/mL in patients on thyroxine therapy or 10 ng/mL in patients off thyroxine therapy indicate recurrence. Calcitonin should be monitored after thyroidectomy for medullary thyroid carcinoma. Measurable calcitonin after surgery may indicate return of disease.
Imaging studies
- Ultrasonography – To determine whether the nodule is cystic, solid, or mixed 15
- Radioiodine scintigraphy – To determine whether the nodule is cold, warm, or hot
- Chest radiography – If malignancy is suspected, given the high incidence of early metastases to the lungs
- Computed tomography (CT) scanning and magnetic resonance imaging (MRI) – To analyze the extent of disease by scanning the neck and chest
Ultrasonography
Ultrasound examination of the nodule is helpful for determining the nature of the nodule, whether cystic, solid, or mixed 15. In addition, knowing the exact location of the nodule and the size can be helpful when planning fine-needle aspiration biopsy (FNAB). Ultrasonography can also be used to exclude the presence of other nodules, which indicates a multinodular disease process.
The radiologist should also assess the overall anatomy of the gland, searching for anatomic defects or developmental anomalies that can determine diagnosis or affect surgical excision. Discovery of a developmental error that explains physical findings, such as a thyroglossal duct cyst or agenesis of one lobe, may prevent the child having to undergo invasive biopsy or surgery.
Scintigraphy
Once a nodule has been confirmed as having a solid component, radioiodine scintigraphy is used to determine the activity of the nodule as cold, warm, or hot.
Scintigraphy can also be used to detect ectopic thyroid tissue or identify the thyroid tissue that will be lost with thyroglossal duct cyst removal, requiring lifelong thyroid hormone replacement.
The activity of the nodule determines the next step in therapy. Hot nodules often require antithyroid medications before surgery, whereas cold nodules have a much higher incidence of malignancy. Scintigraphy is also used postoperatively to exclude the presence of metastases, especially after total thyroidectomy.
Chest radiography, CT scanning, and MRI
Other useful imaging tests include chest radiography, CT scanning, and MRI. If malignancy is suspected, chest radiography should be performed, given the high incidence of early metastases to the lungs. However, chest radiography has only 60% sensitivity in this setting and, therefore, should be confirmed with postoperative CT scanning and scintigraphy.
If the tumor is large, and invasion of the airway or mediastinum is suspected, an MRI or CT scanning of the neck and chest may be used to analyze the extent of disease.
Fine-needle aspiration biopsy
Fine-needle aspiration biopsy (FNAB) is used for definitive diagnosis. Fine-needle aspiration biopsy has attracted much attention in the adult population in the evaluation of thyroid nodules. However, because of the uncertain results with FNAB in many centers, no clear-cut protocol regarding its use in pediatric patients has been established. Fine-needle aspiration biopsy can decrease rates of surgery by 25-50% in adults and has demonstrated promise in children; however, consistency in results is lacking.
It is suggested to perform a ultrasound guided fine-needle aspiration biopsy (FNAB) in patients with 2:
- Nonpalpable thyroid nodules larger than 1 cm
- Palpable nodules smaller than 1.5 cm
- Deeply found nodules
- Nodules in proximity to blood vessels
- Nodules after nondiagnostic conventional FNA cytology
- Cystic or mixed nodules, especially if a previous conventional FNA was nondiagnostic
- Coexistence of nonpalpable lymphadenopathy
Cystic or spongiform lesions are considered to pose a low-risk for malignancy and are either monitored or biopsied if larger than 2 centimeters 6.
Fine-needle aspiration biopsy remains unpopular in children because of their smaller neck sizes, the need for heavier amounts of anesthesia and sedation, and the amount of specimen needed. Furthermore, because the higher rates of malignancy in children, definitive pathologic diagnosis is much more in demand.
Results with fine-needle aspiration biopsy are much more consistent at centers with staff that are skilled in aspiration in children and with experienced cytopathologists. However, even in these centers, distinguishing benign follicular adenomas and Hürthle cell hyperplasia from their malignant counterparts is difficult because of the amount of specimen obtained using fine-needle aspiration biopsy. Results are more accurate with papillary and undifferentiated carcinomas.
Despite of the difficulties associated with fine-needle aspiration biopsy, in a series involving 41 children, Al-Shaikh et al 16 reported 100% sensitivity, 86% specificity, and a 59% decrease in surgery rates. However, Lugo-Vicente et al 17 found no decrease in rates of surgery, 80% sensitivity, and 60% specificity; they used results to treat individuals with a frankly malignant gland more aggressively.
Current recommendations suggest the removal of nodules in children younger than 13 years. The risk of cancer in adolescents more closely approximates that of adults; therefore, fine-needle aspiration biopsy is more useful. Fine-needle aspiration biopsy is not necessary or recommended in the case of toxic nodules. Thyrotoxicosis should be controlled with antithyroid medications, and nodules should be removed, regardless of pathology.
Clinical suspicion of cancer because of a history of ionizing radiation, a family history of thyroid cancer, or clinical signs and symptoms of malignancy also should preclude the use of FNAB in favor of excisional biopsy.
Fine-needle aspiration biopsy can be very useful in centers with the appropriate experience level. In the individual with a suspected benign cyst, surgery can be avoided if aspiration causes resolution without recurrence and cytopathology of the cyst wall demonstrates no malignant cells. Cytopathology of cyst fluid is not useful in the assessment of malignancy. Fine-needle aspiration biopsy may also prevent surgery in adolescents with hypofunctioning and isofunctioning nodules in the absence of clinical suspicion of malignancy.
Each child should have close clinical follow-up to confirm the absence of malignancy. Remember that the incidence of malignancy in pediatric thyroid nodules is high, and the risk of surgical complications can be significant.
As documentation and experience with fine-needle aspiration biopsy in children continues, its use will continue to expand. If any doubt surrounds the pathologic diagnosis, surgical excision is recommended.
Histology
Once a biopsy is performed, whether by fine-needle aspiration or excision, close examination of the tissue obtained is necessary to determine diagnosis and definitive treatment. Findings may be benign or malignant.
The most common histology is follicular adenoma. These tumors maintain the follicular architecture of the gland and are usually encapsulated without evidence of infiltration. Follicular adenomas are not associated with the development of malignancy.
In contrast, many papillary adenomas, which exhibit papillary structures within follicular and cystic spaces, have microscopic evidence of invasion and malignancy on further examination, raising doubts about the benign nature of this tumor.
Hashimoto thyroiditis is often associated with nodule development; therefore, it may be observed on biopsy. Histologic findings include lymphocytic infiltration and replacement of gland architecture with lymphocytes, plasma cells, and macrophages.
Hürthle cells, characteristic of Hashimoto thyroiditis, are also observed, having characteristic bright eosinophilic cytoplasm.
More rarely, Graves disease may present with a nodule. This condition reveals a hypercellular picture (cells increased in height and number with the formation of pseudopapillary beds), which may be confused with cancer.
Another benign condition that may be found on biopsy of a midline neck mass, especially in children, is a residual thyroglossal duct cyst. This structure retains the thyroid acinar epithelium and may be surrounded by lymphocytic infiltrate. It may also become infected and progress to abscess formation.
The Bethesda system diagnostic categories for reporting thyroid cytopathology describes 18:
- Non-diagnostic: Represents an inadequate sample with an insufficient number of follicular cells
- Benign, normal thyroid tissue, showing nodules of adenomatous or multinodular goiters, common entities include adenomatous nodules, Hashimoto thyroiditis, and subacute granulomatous thyroiditis
- Follicular lesion of undetermined significance (FLUS) or atypia of undetermined significance (AUS): proposed for lesions that are not convincingly benign. AUS shows lesions with nuclear atypia and lesions with extensive oncocytic changes, although not enough to be classified as a Hürthle cell neoplasm. FLUS show a combined microfollicular and macrofollicular pattern.
- Follicular neoplasm or suspicion for follicular neoplasm includes microfollicular or cellular adenomas. Since FNA only samples a portion of the nodule, surgical excision is required to determine if a microfollicular lesion is benign or malignant; microfollicles are identified, the colloid is absent or scant, and cells are more crowded than in macrofollicular nodules.[19]
- Suspicious for malignancy: Includes lesions with features of malignancy which are not definite for thyroid cancer
- Malignancy: Cytology will differ on the different types of possible thyroid malignancies. For papillary cancers, microscopy shows large cells with ground-glass cytoplasm, prominent nucleoli, and intranuclear cytoplasmic inclusions. Medullary cancer shows disperse cells showing eccentrically displaced nuclei and slightly granular cytoplasm usually configured as a teardrop.
- Anaplastic cancer shows marked pleomorphism, bizarre giant cells, and spindle cells.
Major thyroid gland malignancies
The 4 major malignancies that may be found in the thyroid gland are:
- Papillary carcinoma,
- Follicular carcinoma,
- Anaplastic carcinoma,
- Medullary thyroid cancer.
Remember that the incidence of malignancy in childhood thyroid nodules is high. Papillary and follicular carcinomas occur more frequently and carry a better prognosis than anaplastic carcinoma and medullary thyroid cancer.
The most common thyroid gland malignancies, papillary carcinomas, are infiltrative, often with multiple centers of development. They rarely invade blood vessels, preferring early spread to regional lymph nodes. They tend to grow slowly and may display follicular elements. However, these tumors have certain unique identifiers. Psammoma bodies are pathognomonic for papillary cancer and may be surrounded by calcified rings. In addition, the nuclei, often called “Orphan Annie eyes,” are hypochromatic, sometimes with nuclear grooves and eosinophilic nuclear inclusions, representing the invasion of cytoplasm. Ischemic necrosis and cystic changes may be present.
In contrast, follicular carcinomas demonstrate no nuclear features and lack psammoma bodies. These tumors prefer capsular and vessel invasion; therefore, they metastasize earlier to bone, lung, and liver, sometimes with no lymph node involvement. These tumors can be difficult to distinguish from their adenomatous counterparts because microscopic capsular invasion may be the only evidence of malignancy and may be missed by FNAB.
Less common are the anaplastic and medullary cancers, each comprising approximately 5% of all thyroid cancers and carrying a much worse prognosis. Anaplastic thyroid carcinomas present in 3 patterns. Spindle cell tumors have a sarcomatoid appearance. Small cell cancers closely resemble other small cell cancers and may be difficult to distinguish from bronchogenic cancer metastases and lymphoma. Giant cell carcinoma is the most anaplastic variety, characterized by huge bizarre cells, often with multiple nuclei and visible mitoses.
Medullary thyroid cancer of the parafollicular cells rarely presents as a single nodule, often affecting both lobes of the thyroid, especially in association with multiple endocrine neoplasia (MEN) 2A and MEN 2B. However, when found on biopsy, medullary thyroid cancer is characterized by large deposits of amyloid substance, surrounded by sheets of pleomorphic epithelial cells.
Finally, the thyroid may be affected by metastases from occult malignancies, most commonly lymphoma and leukemia but including many other cancers. In these incidents, histology obviously depends on the primary tumor. However, metastatic disease in the thyroid gland in children is not common.
Solitary thyroid nodule treatment
- Benign thyroid nodule – A presumed benign nodule, especially in an adolescent, may simply be observed. Close observation and follow-up care is essential. The patient should be closely monitored for change of size and the development of symptoms.
- Autoimmune thyroiditis – Treatment of autoimmune thyroiditis involves hormone replacement to maintain a euthyroid state.
- Infection – Treat infection appropriately. Abscesses should be drained and antibiotics should be administered. In patients with immunocompromise, be aware of the remote possibility of local spread to mediastinal structures.
- Warm thyroid nodule – A warm thyroid nodule without physical signs of malignancy is usually benign and may be observed with close follow-up for growth or change in the nodule
- Hot thyroid nodule – A hot toxic thyroid nodule may require medical therapy before surgical removal; the patient should receive suppressive doses of antithyroid medications. Once physiologic stability is obtained, the surgeon can proceed with removal of the gland or lobe.
The American Thyroid Association 19 issued management guidelines for children with thyroid nodules and differentiated thyroid cancer. The guidelines included some of the following recommendations:
- For children at high risk for thyroid neoplasia, an annual physical examination is recommended and if the examination finds palpable nodules, thyroid asymmetry, and/or abnormal cervical lymphadenopathy, additional imaging is warranted.
- Instead of size alone, ultrasound characteristics and clinical context should be used to identify nodules that merit fine-needle aspiration which should be implemented using ultrasound guidance.
- Lobectomy may be done in patients with compressive symptoms and cosmetic concerns or if the patient or parent prefers. Consider lobectomy in all benign appearing solid thyroid nodules >4 cm, the lesions that have grown significantly, or if malignancy is a concern.
- The indications for 131I (iodine 131) treatment are unresectable iodine-avid persistent locoregional or nodal disease and also for identified or assumed iodine-avid distant metastases.
Lobectomy
In the presence of a small, asymptomatic nodule, the surgeon may elect to perform a simple lobectomy with close follow-up observation. In such cases, full thyroid suppression also is recommended as lifetime postoperative therapy for the patient.
Nodule removal
All toxic nodules in children should be removed. In addition, if the presence of malignancy is still in question after diagnostic tests and procedures have been completed, perform surgical excision.
Total thyroidectomy
If any metastases are present, total thyroidectomy is the recommended treatment 20. Thyroidectomy (near-total or total) may also be performed if Graves disease is diagnosed; thyroid hormone replacement therapy may not be needed if some tissue remains.
Routine preoperative and postoperative care includes the maintenance of nutrition and hydration as well as the observation for signs of complications.
After thyroidectomy, thyroid hormone replacement is necessary. This therapy is continued for your lifetime. Thyroid hormone levels should be monitored periodically so that adequate therapy is maintained during growth and changing needs.
Calcium supplementation may be necessary in the individual with parathyroid compromise, whether temporarily or permanently. Closely monitor laboratory findings to determine the initial and ongoing requirements.
Recurrent laryngeal nerve injuries may cause dysphagia, which can endanger nutrition. In such an individual, involve speech pathology early to optimize recovery.
If malignancy is diagnosed, radioablation therapy may be used for any residual disease. Long-term follow-up care remains vital in such individuals to screen for disease progression or late recurrence.
Surgical care
The presence of a thyroid nodule in children presents somewhat of a dilemma, given the less-than-ideal reliability of diagnostic tests. However, the increased incidence of malignancy in pediatric nodules has led to a somewhat more aggressive approach than that used in adults.
Indications for surgical excision
Indications for surgery include physical examination findings consistent with malignancy, persistence of a nodule, progressive increase in size, or the presence of significant risk factors, including family history or history of irradiation exposure. All toxic nodules in children should be removed. If the presence of malignancy is still in question after diagnostic tests and procedures have been completed, perform surgical excision. Some authorities recommend the removal of all nonsuppressible thyroid nodules found in children younger than 13 years.
In the presence of a small asymptomatic nodule, the surgeon may elect to perform a simple lobectomy with close follow-up observation. Complications with this surgery are generally low. In such an individual, full thyroid suppression also is recommended as lifetime postoperative therapy. Many adenomas contain mutations, causing them to be hyperresponsive to thyroid-stimulating hormone (TSH). The presence of such an adenoma may signify the presence of other cells bearing the same mutations.
Total thyroidectomy
With the presence of any metastases on diagnosis, including lymph node involvement, total thyroidectomy is the recommended treatment. [3] This procedure has decreased the rates of local and metastatic recurrence.
Postoperative radioablation is also more effective in this case because tissue to absorb the radioiodine is reduced.
Because the rate of pulmonary metastasis is high, postoperative radioiodine scintigraphy is performed 6 weeks after surgery to exclude the presence of pulmonary tumors. Scintigraphy is only reliable after total or subtotal thyroidectomy because any remaining tissue may hide the presence of metastases.
Total thyroidectomy should be performed in individuals with medullary thyroid cancer, preferably before evidence of disease is obvious. This malignancy is aggressive and metastasizes early.
Prophylactic therapy, with removal of the gland in children with a family history and genetic markers, provides the best outcomes with this malignancy.
If Graves disease is diagnosed, thyroidectomy (near-total to total) may be performed. Thyroid hormone replacement therapy may not be needed in this case if some tissue remains.
Surgical technique
During the procedure, both lobes of the thyroid should be closely examined, because contralateral tumor involvement is common.
Lymph node exploration should include nodes in the jugulodigastric chain and along the recurrent laryngeal nerve in the tracheoesophageal groove. Dissection may also be needed in the superior mediastinum. Care should be taken to avoid injury to the recurrent laryngeal nerve or to the parathyroids. Of the parathyroid glands, 1-2 may be preserved and transplanted into the sternocleidomastoid muscle or nondominant forearm if needed.
Thyroidectomy for familial medullary thyroid carcinoma demands a total thyroidectomy with complete lymph node dissection of the entire central compartment, including the paratracheal nodes, delphian nodes, and superior mediastinal nodes.
Adequate dissection should extend to both carotid sheaths and to the innominate artery. A surgical cure is possible for this disease if the tumor has not spread to jugular or lateral cervical nodes or to distant organs.
Complications
The most common complications of thyroidectomy are injuries to the recurrent laryngeal nerve and parathyroid compromise, causing hypocalcemia. Both of these complications have been divided into temporary (< 6 mo) and permanent categories. Permanent hypocalcemia has occurred in 6-27% of operative cases, whereas, in some studies, temporary hypocalcemia has affected an additional 29%.
In one study, recurrent laryngeal nerve damage temporarily caused difficulties in 12% of cases and permanently caused difficulties in 2%. Overall, permanent damage rates have been estimated at 0-24%. This wide range is most likely the result of differing treatment and surgical techniques.
Some centers remove tumor-invaded recurrent laryngeal nerves, whereas others have achieved good results with careful dissection and subsequent treatment with 131I (iodine 131). Millman et al 21 assert that, with experience and proper technique, the rates of both these complications should approach 1%.
Much more rarely, other major complications can affect recovery. Damage can occur in cranial nerves VII, X, and XI and the superior laryngeal nerve. An occasional postoperative pneumothorax has been noted. Postoperative hemorrhage can be devastating because of possible airway compromise and may cause emergent reoperation. In addition, required tracheostomy and extensive wound necrosis or infection can occur, severely delaying recovery.
Minor complications include hypertrophic scarring, delayed healing, seromas, temporary dysphagia, facial edema, and serous otitis media.
In general, complications are proportional to the amount of gland removed. Simple lobectomy is associated with a low risk of complication, whereas total thyroidectomy may cause more problems. In addition, children with malignant nodules tend to sustain more complications than children with benign disease.
Follow up care
After surgery for a diagnosed thyroid malignancy, outpatient follow-up care is vital to optimize patient survival.
Radioiodine scintiscan may be used 6 weeks postsurgery to monitor for metastases. Uptake in the lungs, lateral neck, or around the recurrent laryngeal nerve indicates metastasis or residual disease. If these are discovered, therapeutic dosing of 131I (iodine 131) is indicated to ablate remaining tumor cells. Thyroxine in full replacement doses to suppress thyroid-stimulating hormone (TSH) stimulation of malignant cells is necessary, even if some thyroid tissue remains.
Pediatric patients require periodic monitoring of thyroid hormone levels as the child grows to ensure adequate dosing. Annual radioiodine scan is recommended to monitor for long-term recurrence of disease.
Thyroglobulin levels may also be used to monitor for recurrence of disease, but only if a total thyroidectomy has been performed. Levels vary based on replacement therapy. Levels more than 1 ng/mL in patients on replacement therapy and 10 ng/mL in patients off thyroxine indicate recurrence of disease.
In patients with medullary thyroid cancer, calcitonin levels may be used to monitor for recurrence. Late mortality caused by unmonitored recurrence of disease is tragic. Therefore, primary care physicians should be diligent in maintaining long-term follow-up care.
Solitary thyroid nodule prognosis
Because mortality rates for thyroid cancer approach zero, the prognosis of a solitary thyroid nodule is generally quite good, even with diagnosed malignancy. Most pediatric patients with a solitary thyroid nodule can expect a normal life span. Even in patients with malignancy, the progression-free survival rate is 60-70% at 10-20 years.
In a study of 329 pediatric patients with thyroid cancer, Newman et al 22 found that the progression-free survival rate was 67% at 10 years and 60% at 20 years. They reported only 2 disease-related deaths. Factors contributing to less favorable prognosis vary among studies; however, patients younger than 10 years are generally considered to have an increased risk for poor outcomes. Other risk factors for poor prognosis are residual cervical disease after thyroidectomy, extensive pulmonary metastases, and tracheal and laryngeal invasion. Unfortunately, younger patients with thyroid cancer are likely to have more extensive disease on diagnosis than older patients, confusing the independence of these risk factors. Genetic markers indicating poor prognosis include nondiploid DNA, overexpression of p21 ras, and mutations of the n-ras gene. Close follow-up care after treatment is essential because late deaths from extension of residual disease can occur.
Determinants of poor prognosis include younger age (< 10 years), extensive pulmonary metastases, and tracheal and laryngeal invasion. Medullary thyroid cancer and anaplastic cancer also result in poor outcomes.
Patients exhibiting papillary carcinoma or well-differentiated follicular carcinoma with proper treatment can have excellent recovery. Undifferentiated follicular cancer and anaplastic carcinoma cause poor outcomes. Medullary thyroid carcinoma in association with multiple endocrine neoplasia (MEN) has an increased mortality rate, as high as 50% at 10 years with multiple endocrine neoplasia, type 2B (MEN 2B). For this reason, prophylactic thyroidectomy is recommended for patients who have a family history of multiple endocrine neoplasia (MEN) and the proper genetic markers.
Operative morbidity mainly involves parathyroid complications, nerve injuries, and wound complications. Some centers report more complications with extensive operations as opposed to lobectomy; however, this finding is not universal. Most reports indicate that younger patients are more at risk of operative and recovery complications.
The consequence of hypothyroidism in a child can be devastating, whether the child is rendered hypothyroid by surgery, ablation, or by pathology. Growth delays and mental retardation can be severe if hormone deficiency is prolonged. Adequate replacement is fundamental to prevent hypothyroidism. Lifetime treatment from childhood involves adjustment in dosing based on changing size and development needs. Compliance with therapy and follow-up may become an issue.
References- Thyroid Nodules. https://www.thyroid.org/thyroid-nodules
- Pemayun TG. Current Diagnosis and Management of Thyroid Nodules. Acta Med Indones. 2016 Jul;48(3):247-257.
- Welker MJ, Orlov D. Thyroid nodules. Am Fam Physician. 2003 Feb 01;67(3):559-66.
- Xie C, Cox P, Taylor N, LaPorte S. Ultrasonography of thyroid nodules: a pictorial review. Insights Imaging. 2016;7(1):77-86. doi:10.1007/s13244-015-0446-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4729706
- Solitary Thyroid Nodule. https://emedicine.medscape.com/article/924550-overview
- Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349. doi:10.1016/j.mcna.2012.02.002 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3575959
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan. 26 (1):1-133.
- Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, Puxeddu E, Torlontano M, Tumino S, Attard M, Lamartina L, Nicolucci A, Filetti S. The natural history of benign thyroid nodules. JAMA. 2015 Mar 03;313(9):926-35.
- Keh SM, El-Shunnar SK, Palmer T, Ahsan SF. Incidence of malignancy in solitary thyroid nodules. J Laryngol Otol. 2015 Jul. 129 (7):677-81.
- Zamora EA, Khare S, Cassaro S. Thyroid Nodule. [Updated 2020 Jun 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535422
- Yeung MJ, Serpell JW. Management of the solitary thyroid nodule. Oncologist. 2008 Feb;13(2):105-12.
- Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008 Dec;22(6):901-11.
- Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43(2):229-vii. doi:10.1016/j.otc.2010.01.002 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879398
- Hanley P, Lord K, Bauer AJ. Thyroid Disorders in Children and Adolescents: A Review. JAMA Pediatr. 2016 Oct 1. 170 (10):1008-1019.
- Kihara M, Hirokawa M, Masuoka H, Yabuta T, Shindo H, Higashiyama T, et al. Evaluation of cytologically benign solitary thyroid nodules by ultrasonography: A retrospective analysis of 1877 cases. Auris Nasus Larynx. 2012 Oct 23.
- Al-Shaikh A, Ngan B, Daneman A, Daneman D. Fine-needle aspiration biopsy in the management of thyroid nodules in children and adolescents. J Pediatr. 2001 Jan. 138(1):140-2.
- Lugo-Vicente H, Ortiz VN, Irizarry H, et al. Pediatric thyroid nodules: management in the era of fine needle aspiration. J Pediatr Surg. 1998 Aug. 33(8):1302-5.
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017 Nov;27(11):1341-1346.
- Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015 Jul. 25 (7):716-59.
- Raval MV, Browne M, Chin AC, et al. Total thyroidectomy for benign disease in the pediatric patient–feasible and safe. J Pediatr Surg. 2009 Aug. 44(8):1529-33.
- Millman B, Pellitteri PK. Nodular thyroid disease in children and adolescents. Otolaryngol Head Neck Surg. 1997 Jun. 116(6 Pt 1):604-9.
- Newman KD, Black T, Heller G, Azizkhan RG, Holcomb GW 3rd, Sklar C, et al. Differentiated thyroid cancer: determinants of disease progression in patients Ann Surg</i>. 1998 Apr. 227(4):533-41.