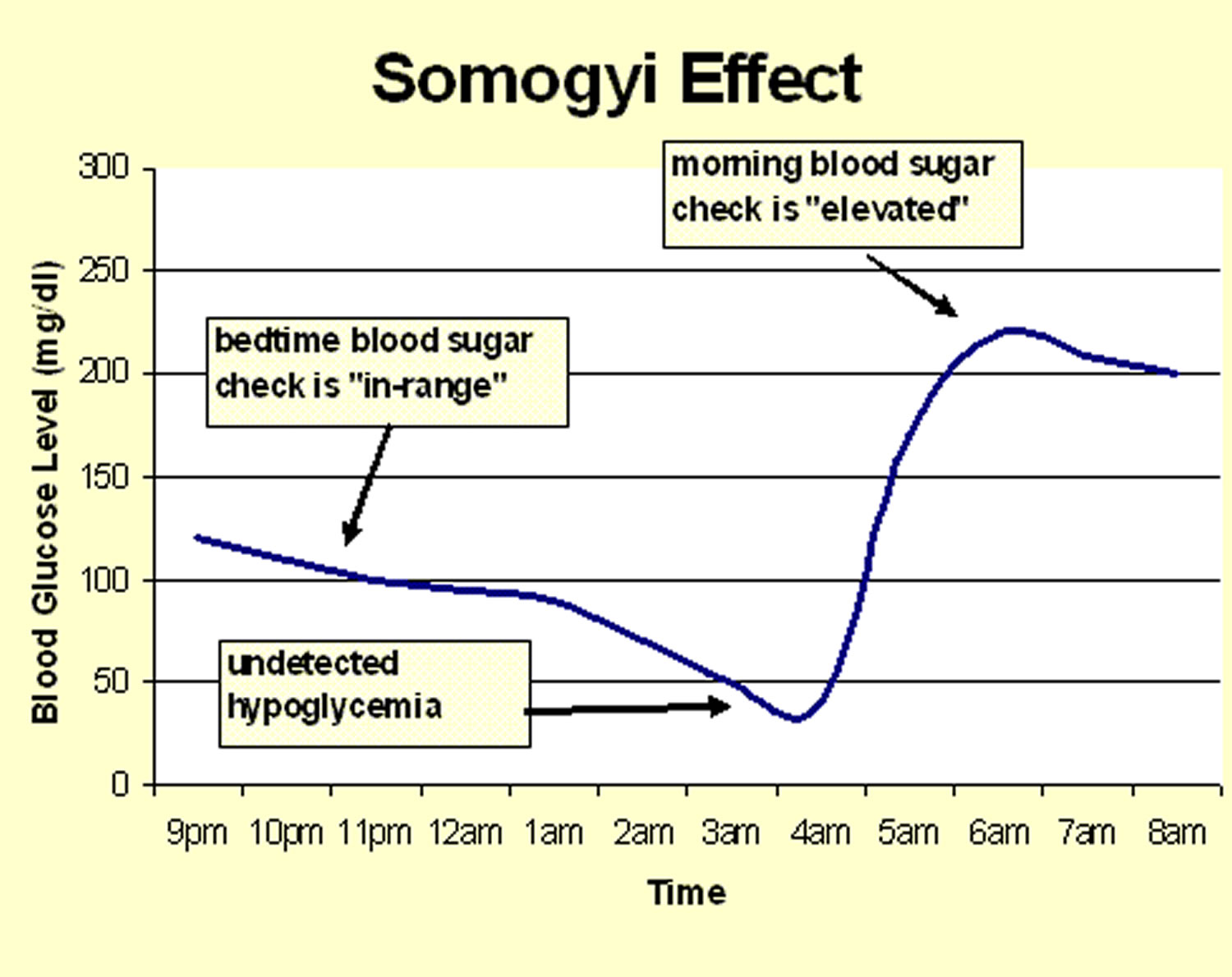
What is Somogyi effect
Somogyi effect also known as post-hypoglycemic hyperglycemia, Somogyi phenomenon or chronic Somogyi rebound, describes a rebound high blood glucose (rebound hyperglycemia) level first thing in the morning in response to low blood glucose at nighttime, often induced by excess insulin or inadequate calorie intake with insulin therapy 1. Somogyi effect may follow an untreated hypoglycemic episode during the night and is caused by the release of stress hormones. Amongst those people with diabetes who manage their blood glucose using insulin injections, this may take the form of high blood sugar in the morning due to an excess amount of insulin during the night. Somogyi effect was the most common cause of fasting hyperglycemia in type 1 diabetes patients with poor glycemic control 2. Exclusion of overnight hypoglycemia by use of continuous glucose monitoring is an effective way of ruling this out when evaluating early morning hyperglycemia. However, the Somogyi effect is still a matter of debate for some authorities.
Morning hyperglycemia in patients with type 1 diabetes may be caused by the Dawn phenomenon, the Somogyi effect or poor glycemic control, diagnoses that lead to different clinical management in each case. Poor glycemic control in the overall picture of diabetes is evident by the persistent elevation of blood glucose levels, without an obvious prominence of early morning hyperglycemia.
The Somogyi phenomenon was named after a Hungarian-born professor called Dr. Michael Somogyi. He prepared the first insulin treatment given to a child with diabetes in the USA, and also showed that too much insulin would make diabetes management unstable and more difficult.
What causes Somogyi effect?
Somogyi theorized that prolonged levels of untreated hypoglycemia could lead to stress due to low blood sugar and a high blood sugar levels rebound.
This is a defensive response by the body as it released endocrine hormone glucagon, backed up by the stress hormones cortisol and epinephrine.
This means an instant increase in blood glucose and stress hormones cause insulin resistance for several hours, and this in turn leads to elevated blood sugar.
Somogyi effect prevention
Somogyi phenomenom is avoidable in several ways. Firstly, intense blood glucose testing allows the individual experiencing Somogyi effect to detect and then prevent the circumstances leading to it.
Testing blood sugar regularly using a traditional blood glucose meter helps to catch low blood sugar levels before any rebound occurs.
Night testing of blood glucose levels is also important, and adjusting insulin in response may also be appropriate.
Somogyi rebound can be a challenge to avoid. The need to keep blood sugar levels stable whilst still adjusting insulin to take account of a complex lifestyle involving stress and exercise can be really difficult.
Somogyi effect symptoms
The main 3 symptoms of high blood sugar levels (hyperglycemia) are increased urination, increased thirst and increased hunger.
High blood sugar levels can also contribute to the following symptoms:
- Regular/above-average urination
- Weakness or feeling tired
- Loss of weight
- Increased thirst
- Vision blurring
Hyperglycemia can be serious if:
- Blood glucose levels stay high for extended periods of time – this can lead to the development of long term complications
- Blood glucose levels rise dangerously high – this can lead to short term complications
In the short term
Short term complications of very high blood sugar levels include ketoacidsosis and hyperosmolar hyperglycemic nonketotic syndrome.
Ketoacidosis is a dangerous complication that mainly affects people with type 1 diabetes but can also affect some people with type 2 diabetes that are dependent on insulin. The risk of ketoacidosis becomes significant if blood glucose levels rise above 15 mmol/l (270 mg/dl).
There is a higher risk of ketoacidosis if a dose of insulin is missed or during periods of illness.
A dangerous complication known as hyperosmolar hyperglycemic nonketotic syndrome can affect people with diabetes if blood glucose levels remain very high, above 33 mmol/l (600 mg/dl) for an extended period of time.
In the longer term
Regularly having high blood glucose levels for long periods of time increases the risk of organ damage occurring which can lead to health problems that are commonly referred to as the long term complications of diabetes.
Try to keep as close to the HbA1c target of 48 mmol/mol (6.5%) as this will reduce the chances of developing diabetes complications.
When aiming to achieve or get close to this target, ensure you do not put yourself at a high risk of regular or severe hypos.
Is Somogyi phenomenon the same as Dawn phenomenon?
No, although they are often confused by healthcare professionals 3.
The Dawn effect or Dawn phenomenon, is a morning rise in blood sugar which occurs as a response to waning levels of insulin and a surge in growth hormones 4. The Dawn phenomenon differs from the Somogyi effect in that it is not preceded by an episode of hypoglycemia 5.
Diabetic patients will manifest the Dawn phenomenon clinically with persistent and worsening early morning hyperglycemia, which is difficult to control. Often found early in the disease process, this is associated with worsening HbA1c levels. The Dawn phenomenon is not associated with nocturnal hypoglycemic episodes, and no specific physical findings are present.
The Dawn phenomenon, and more recently the extended Dawn phenomenon (persistence of hyperglycemia into the later morning hours), have been studied extensively with numerous articles published on the subject. Both entities are responsible for morning glucose elevations which are difficult to control. The Dawn phenomenon has been documented in both type 1 and type 2 diabetes and has been demonstrated in all age groups, even type 2 diabetics over 70 years of age. For both type 1 and type 2 diabetes mellitus, prevalence is estimated to exceed 50 percent 6. This obviously affects a large patient population over a wide age range, and the Dawn phenomenon will be an important consideration for any clinician who manages diabetic patients.
Studies in nondiabetic populations have shown that blood glucose, and plasma insulin levels remain steady through the night, with only a small increase in insulin secretion before dawn, which serves to depress hepatic glucose production. Hyperglycemia is prevented by this physiologic surge of insulin. Hence, the Dawn phenomenon does not occur in nondiabetic subjects because they can secrete normal amounts of insulin to prevent it. In type 1 diabetics, nocturnal spikes of growth hormone are the most likely mechanism to explain the Dawn phenomenon, as growth hormone exerts insulin-antagonistic effects 7. Furthermore, exogenous insulin activity frequently begins to wane during the early morning hours (depending on the type of insulin and route of administration), so there is not enough opposition to hepatic activity to prevent hyperglycemia. Type 2 diabetics are more likely affected by early morning dysregulation of hepatic glucose production because of the inability to produce compensatory insulin secretion 3.
Dawn phenomenon diagnosis
Diagnosis of the Dawn phenomenon is most effectively achieved by use of continuous glucose monitoring, which in recent years has become more widely available to clinicians 8. In addition to documenting elevated early morning glucose levels, continuous glucose monitoring ensures no associated nocturnal episodes of hypoglycemia have occurred, which could indicate a Somogyi effect rather than true Dawn phenomenon. The Dawn phenomenon is quantified by subtracting the overnight glucose nadir from the glucose value observed just before breakfast. An alternative to continuous glucose monitoring has been described by Monnier, et al., utilizing intermittent glucose monitoring to quantify the magnitude of the Dawn phenomenon. A strong correlation between pre-meal glucose values and the change in glucose with the Dawn phenomenon has been identified. This has enabled the development of a formula to calculate the magnitude of early morning hyperglycemia without continuous glucose monitoring 9. By measuring blood glucose pre-breakfast, pre-lunch, and pre-dinner, then taking the difference between the pre-breakfast glucose and the average of the pre-lunch and pre-dinner glucose values to determine “X”, the presence of the Dawn phenomenon in an individual, which has been defined as an upward variation in glucose of 20 mg/dl, can be detected with 71% sensitivity and 68% specificity. The magnitude of the Dawn phenomenon can then be calculated by using the equation 0.49X +15 9.
Dawn phenomenon treatment
When the presence of the Dawn phenomenon is detected, especially when associated with the extended Dawn phenomenon, an individual patient should be considered for earlier and more aggressive control of glucose. The prevention of long-term sequelae by minimizing exposure to hyperglycemia is key early in the disease process. Optimal insulin therapy is important in type 1 diabetes, but also in type 2 diabetes. Oral hypoglycemic agents have failed to show adequate control of the Dawn phenomenon, while insulin therapy has been shown much more effective.
Choosing an insulin regimen must, of course, be individualized for each patient, but research has indicated that the presence of the Dawn phenomenon must be considered in selecting the type of insulin and the mechanism of delivery. In studies which have demonstrated superior glycemic control with continuous insulin infusion as opposed to long-acting glargine formulations, the Dawn phenomenon is likely the reason. The ability for a continuous infusion to provide a bolus in the early morning hours to counteract the Dawn phenomenon is a possible explanation, as long-acting glargine preparations have no ability to provide this. For type 1 diabetes, tight control with insulin must take into account the Dawn phenomenon to avoid nocturnal hypoglycemia before the onset of early morning glucose elevations. If insulin adjustments are made based on early morning fasting glucose levels, a larger dose of insulin might be administered than would be appropriate if the Dawn phenomenon magnitude was considered 10.
Management of morning hyperglycemia should be a part of the overall diabetes control strategy. Lifestyle modification is an important component to be considered. Better control of morning glucose levels has been demonstrated by increasing the amount of exercise in the evening and increasing the protein to carbohydrate ratio of the evening meal. Consuming breakfast is also very important. While it seems counterintuitive, an early morning meal serves to decrease the secretion of insulin-antagonistic hormones 11.
References- The dawn phenomenon and the Somogyi effect – two phenomena of morning hyperglycaemia. Endokrynol Pol. 2011;62(3):276-84. https://journals.viamedica.pl/endokrynologia_polska/article/view/25278/20107
- Minicucci WJ, Maia FF, Neto AM, Zantut-Wittmann DE. Somogyi effect as the most common cause of fasting hyperglycemia in T1D patients. Diabetol Metab Syndr. 2015;7(Suppl 1):A62. Published 2015 Nov 11. doi:10.1186/1758-5996-7-S1-A62 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4653544
- Rybicka M, Krysiak R, Okopień B. The dawn phenomenon and the Somogyi effect – two phenomena of morning hyperglycaemia. Endokrynol Pol. 2011;62(3):276-84.
- Schmidt MI, Hadji-Georgopoulos A, Rendell M, Margolis S, Kowarski A. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care. 1981 Nov-Dec;4(6):579-85.
- O’Neal TB, Luther EE. Dawn Phenomenon. [Updated 2019 May 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430893
- Carroll MF, Schade DS. The dawn phenomenon revisited: implications for diabetes therapy. Endocr Pract. 2005 Jan-Feb;11(1):55-64.
- Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N. Engl. J. Med. 1985 Jun 06;312(23):1473-9.
- Monnier L, Colette C, Dejager S, Owens D. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: is this of concern? Diabetes Care. 2013 Dec;36(12):4057-62.
- Monnier L, Colette C, Dejager S, Owens D. The dawn phenomenon in type 2 diabetes: how to assess it in clinical practice? Diabetes Metab. 2015 Apr;41(2):132-7.
- Hirsch IB, Bode BW, Garg S, Lane WS, Sussman A, Hu P, Santiago OM, Kolaczynski JW., Insulin Aspart CSII/MDI Comparison Study Group. Continuous subcutaneous insulin infusion (CSII) of insulin aspart versus multiple daily injection of insulin aspart/insulin glargine in type 1 diabetic patients previously treated with CSII. Diabetes Care. 2005 Mar;28(3):533-8.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015 Mar;58(3):429-42.