Spinal dysraphism
Spinal dysraphism also called spina bifida is a condition in which the neural tube, a layer of cells that ultimately develops into the brain and spinal cord, fails to close completely during the first few weeks of embryonic development 1, 2. “Spinal” means the bones of the spinal column or the vertebrae and “dysraphism” means incomplete fusion. As a result, when the spine forms, the bones of the spinal column or the vertebrae do not close completely around the developing nerves of the spinal cord. Part of the spinal cord may stick out through an opening in the spine, leading to permanent nerve damage. Because spinal dysraphism is caused by abnormalities of the neural tube, it is classified as a neural tube defect 3. Spinal dysraphism is one of the most common types of neural tube defect, affecting an estimated 1 in 2,500 to 2.7 to 3.8 per 10,000 newborns worldwide 4. Furthermore, 90-95% of those with spinal dysraphism have no previous family history 5. Spinal dysraphism is slightly more common in females than males. For unknown reasons, the prevalence of spinal dysraphism varies among different geographic regions and ethnic groups. In the United States, spinal dysraphism or spina bifida occurs more frequently in Hispanics and non-Hispanic whites than in African Americans.
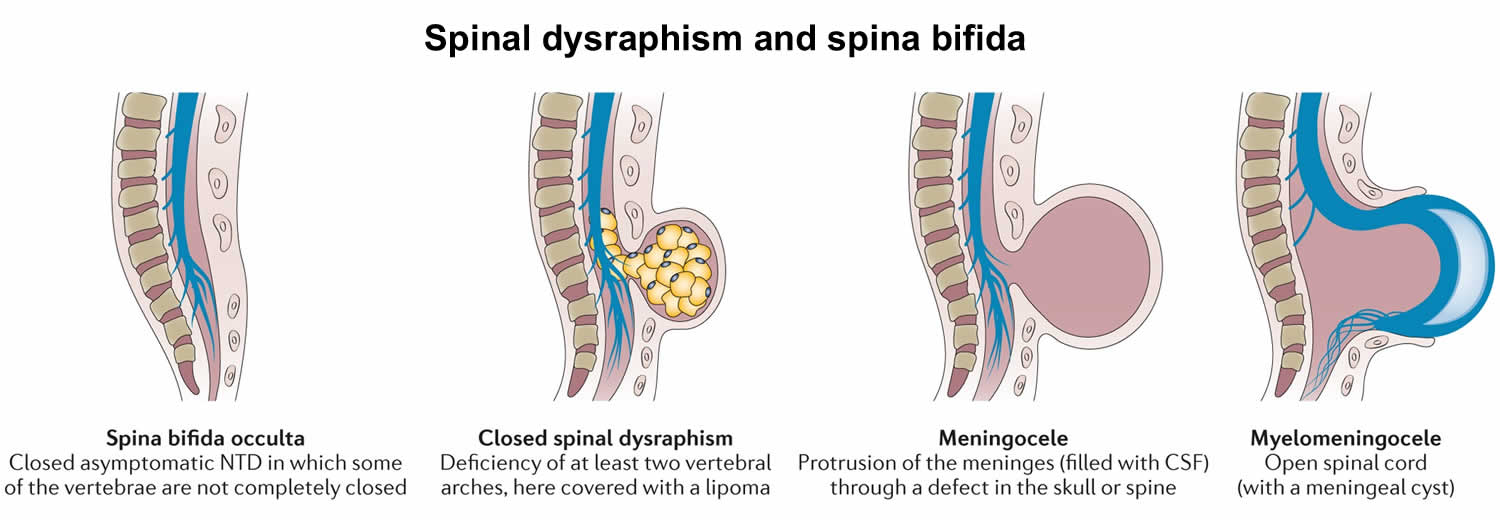
Spinal dysraphism is a congenital (present at birth) abnormality that results in an abnormal structure in the spine, including the bony structure, the spinal cord, and the nerve roots. Spinal dysraphism can be broadly divided into two different clinicoradiological entities 6, 7:
- Open spinal dysraphism previously called spina bifida aperta or cystica: occurs when the cord and its covering communicate with the outside; no skin or tissues cover the sac:
- Myelomeningocele (98% of open spinal dysraphism). Myelomeningocele is a spinal dysraphism in which the spinal cord and its contents herniate through a congenital bony defect in the posterior elements.
- Myelocele
- Hemimyelomeningocele
- Hemimyelocele
- Closed spinal dysraphism previously called spina bifida occulta: occurs when the spinal cord is covered by other normal mesenchymal elements
- With subcutaneous mass
- lipoma with dural defect
- lipomyelomeningocele
- lipomyelocele/lipomyeloschisis
- terminal myelocystocele
- meningocele
- limited dorsal myeloschisis
- Without subcutaneous mass
- posterior spina bifida (isolated defect of the posterior neural arch of vertebra)
- intradural lipoma
- filar lipoma
- tight filum terminale
- persistent terminal ventricle
- disorders of midline notochordal integration
- dorsal dermal sinus
- dorsal enteric fistula
- neurenteric cyst 5,6
- split cord malformations
- diastematomyelia
- diplomyelia
- disorders of notochordal formation
- caudal regression syndrome: a spectrum of structural defects of the caudal region. Malformations vary from isolated partial agenesis of the coccyx to lumbosacral agenesis 8
- Type 1: the conus medullaris is blunt and terminates above the normal level; there is sometimes an associated dilated central canal or a cerebrospinal fluid-filled cyst at the lower end of the conus; these patients have major sacral deformities
- Type 2: the conus medullaris is elongated and tethered by a thickened filum terminale or intraspinal lipoma and ends below the normal level. Neurologic disturbances are more severe in this group
- segmental spinal dysgenesis
- caudal regression syndrome: a spectrum of structural defects of the caudal region. Malformations vary from isolated partial agenesis of the coccyx to lumbosacral agenesis 8
- With subcutaneous mass
Spinal dysraphism encompasses congenital problems that result in an abnormal bony formation of the spine and/or the spinal cord 9. Spinal dysraphism is caused by the maldevelopment of the ectodermal, mesodermal, and neuroectodermal tissues. The two major types of spinal dysraphism are based on the appearance, i.e., aperta (open) if the lesion is visible and occulta (closed) if the lesion is not visible on the surface 10. Common manifestations are meningocele, myelomeningocele, lipomeningocele, lipomyelomenigocele, myeloschisis, and rachischisis 10.
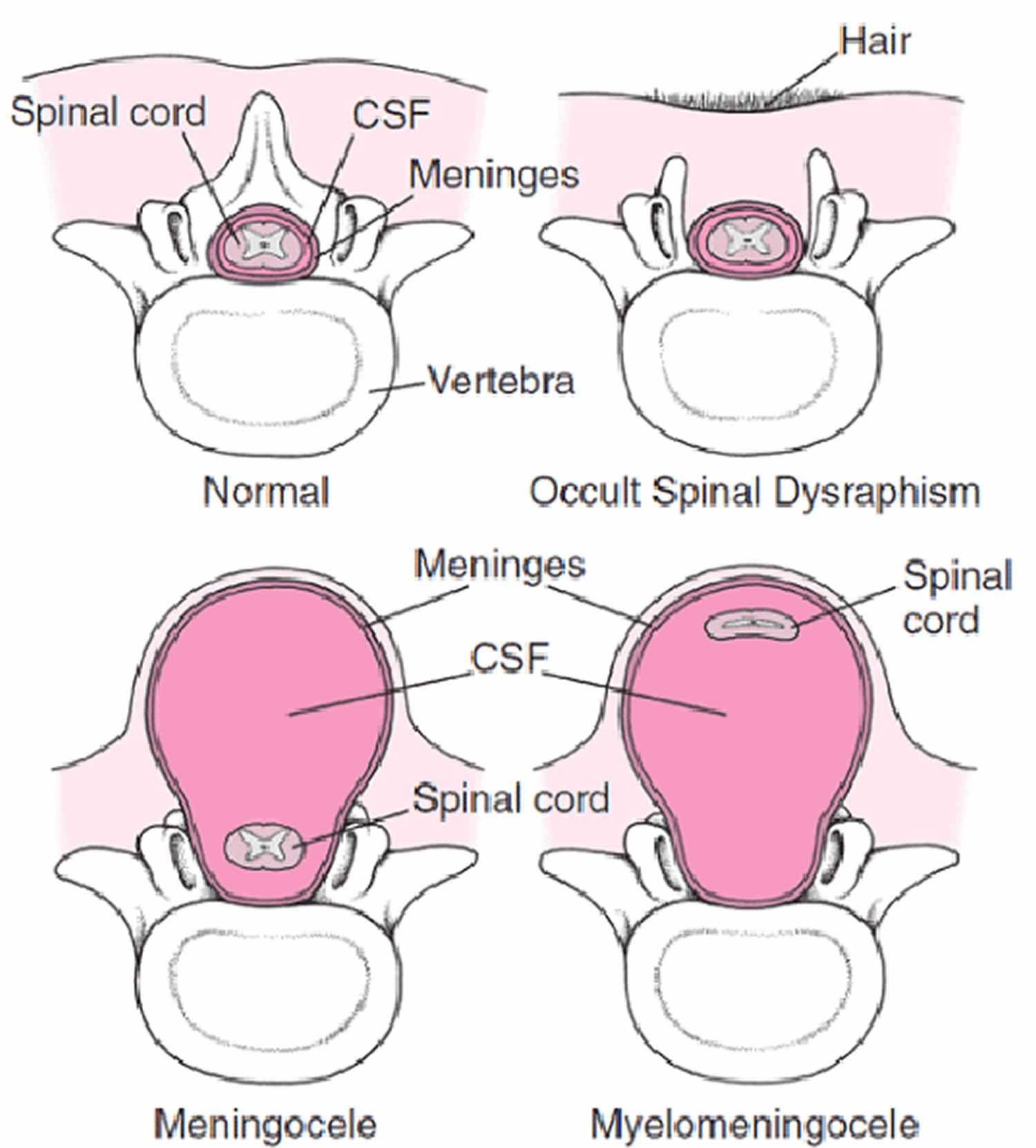
Children born with spinal dysraphism often have a fluid-filled sac on their back that is covered by skin, called a meningocele. If the sac contains part of the spinal cord and its protective covering, it is known as a myelomeningocele. The signs and symptoms of these abnormalities range from mild to severe, depending on where the opening in the spinal column is located and how much of the spinal cord is contained in the sac. Related problems can include a loss of feeling below the level of the opening, weakness or paralysis of the feet or legs, and problems with bladder and bowel control. Some affected individuals have additional complications, including a buildup of excess fluid around the brain (hydrocephalus) and learning problems. With surgery and other forms of treatment, many babies with spinal dysraphism live into adulthood.
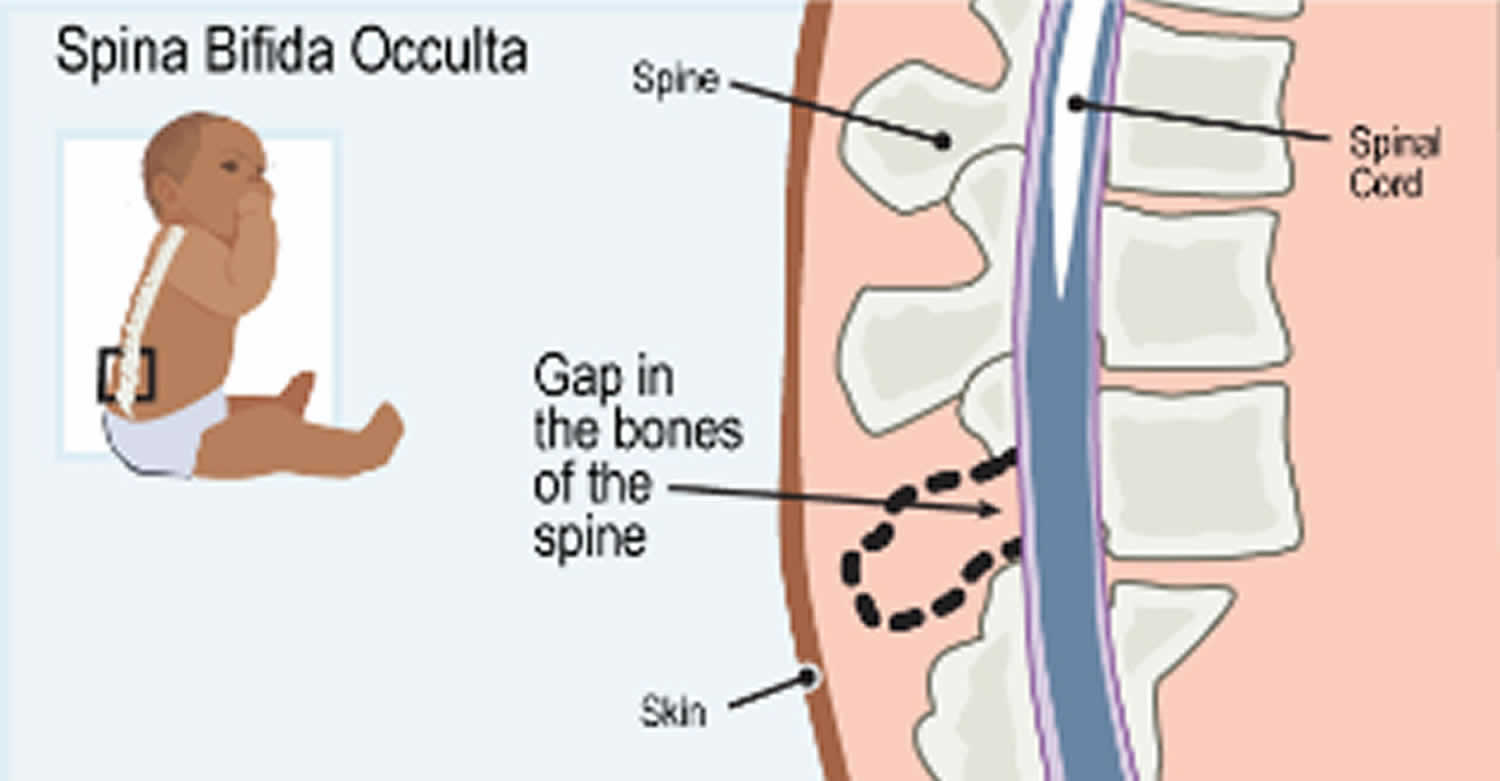
In a milder form of the condition, called spinal dysraphism occulta or occult spinal dysraphism, the bones of the spinal column are abnormally formed, but the nerves of the spinal cord usually develop normally. Unlike in the more severe form of spinal dysraphism, the spinal cord does not stick out through an opening in the spine. Occult spinal dysraphism most often causes no health problems, although rarely it can cause back pain or changes in bladder function.
Spinal dysraphism most commonly affects the lumbar and sacral region of the spine and, least commonly, the cervical spine 9. About 0 to 5% affects the cervical spine, 5% to 10% the thoracic spine, 20% to 30% the thoracolumbar spine, 20 to 30% the lumbar spine, 30 to 50% the lumbosacral spine and 5 to 15% the sacral spine 11. Cervicothoracic spinal dysraphism is relatively rare, with an incidence of 1% to 6.5% 12.
Spinal dysraphism can be diagnosed during pregnancy or after the baby is born. Occult spinal dysraphism might not be diagnosed until late childhood or adulthood, or might never be diagnosed.
If you’re pregnant, you’ll be offered prenatal screening tests to check for spina bifida and other birth defects, but typically the diagnosis is made with ultrasound. The tests aren’t perfect. Some mothers who have positive blood tests have babies without spina bifida. Even if the results are negative, there’s still a small chance that spina bifida is present. Talk to your doctor about prenatal testing, its risks and how you might handle the results.
Not all people born with spinal dysraphism have the same severity and needs, so treatment will be different for each person. Some people have problems that are more serious than others. People with occult spinal dysraphism often doesn’t require any treatment at all, but people with myelomeningocele and meningocele will need more treatments.
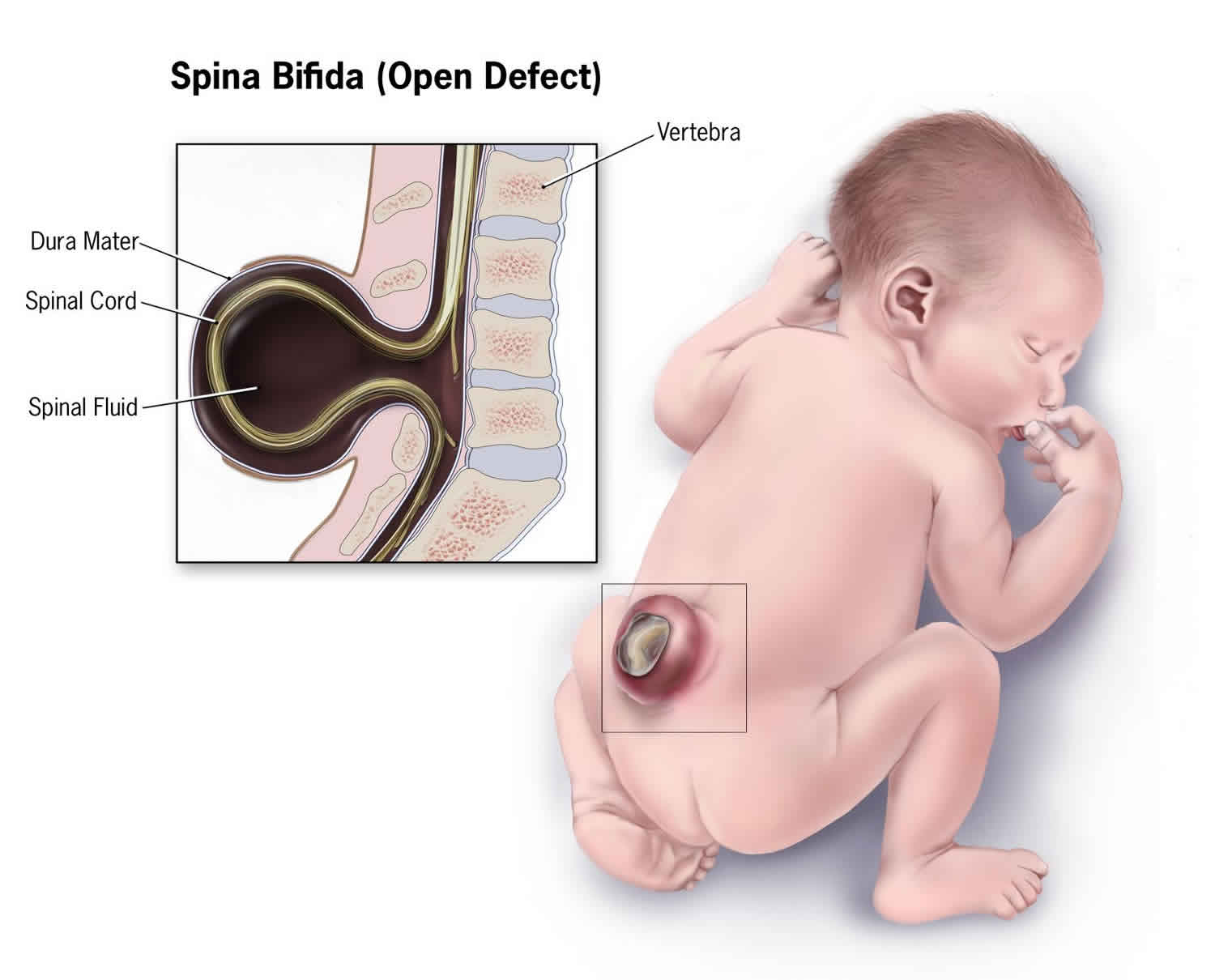
Figure 1. Spinal dysraphism
[Source 13, 14 ]Spinal dysraphism types
The three most common types of spinal dysraphism are:
Myelomeningocele
When people talk about spinal dysraphism, most often they are referring to myelomeningocele or open spina bifida. The spinal canal is open along several vertebrae in the lower or middle back. Myelomeningocele is the most serious type of spinal dysraphism in which the membranes and the spinal nerves protrude through an opening in the baby’s back, forming a sac on the baby’s back. Part of the spinal cord and nerves are in this sac and are damaged. The exposed nervous system may become infected and may also cause paralysis and bladder and bowel dysfunction, so prompt surgery is needed after birth.
Myelomeningocele is the most common presentation of spinal dysraphism and constitutes about 80% of the cases 15, 16. Myelomeningocele occurs in approximately 1 in 1200 to 1400 births 16.
Myelomeningocele type of spinal dysraphism causes moderate to severe disabilities, such as problems affecting how the person goes to the bathroom, loss of feeling in the person’s legs or feet, and not being able to move the legs.
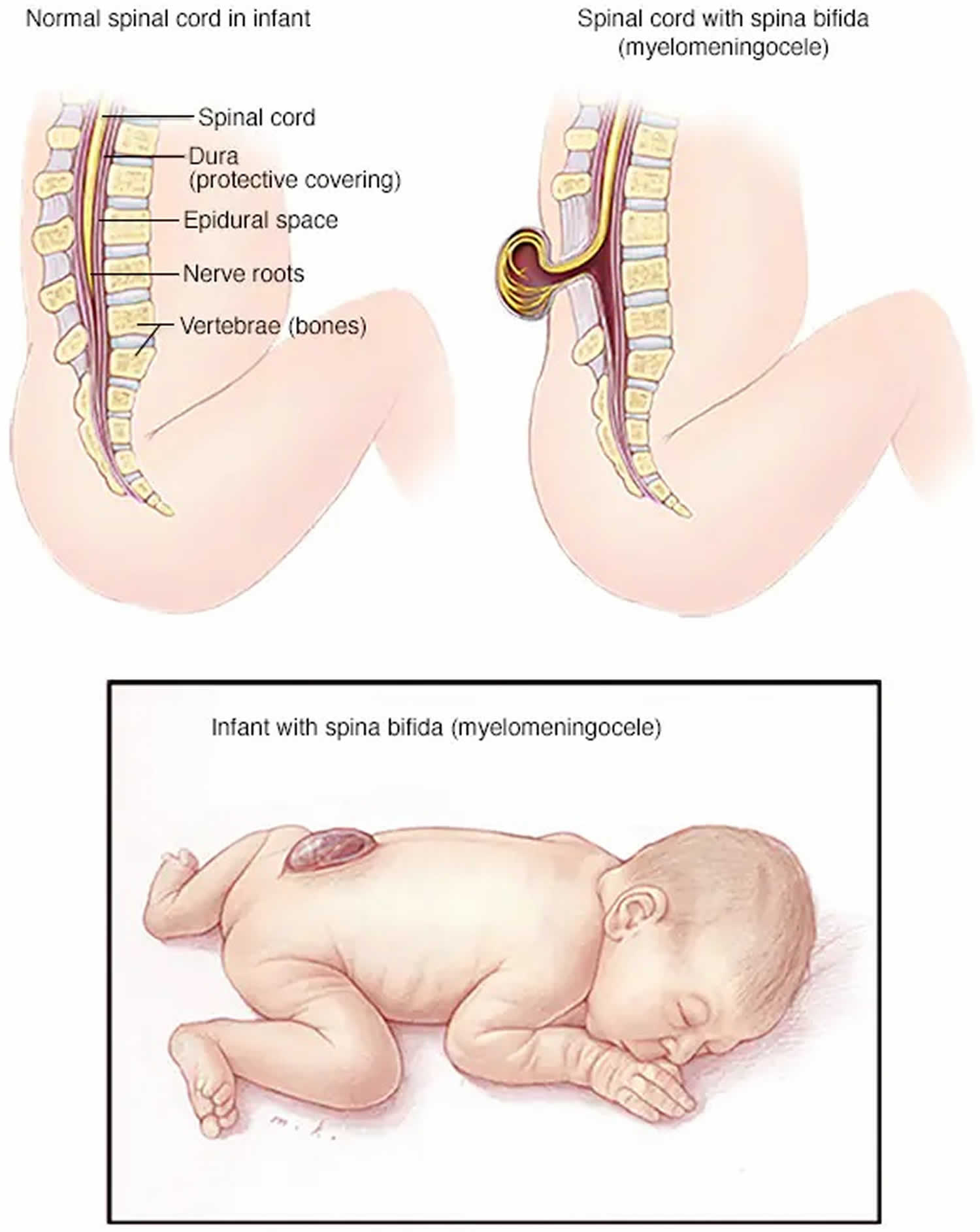
Figure 2. Myelomeningocele
Meningocele
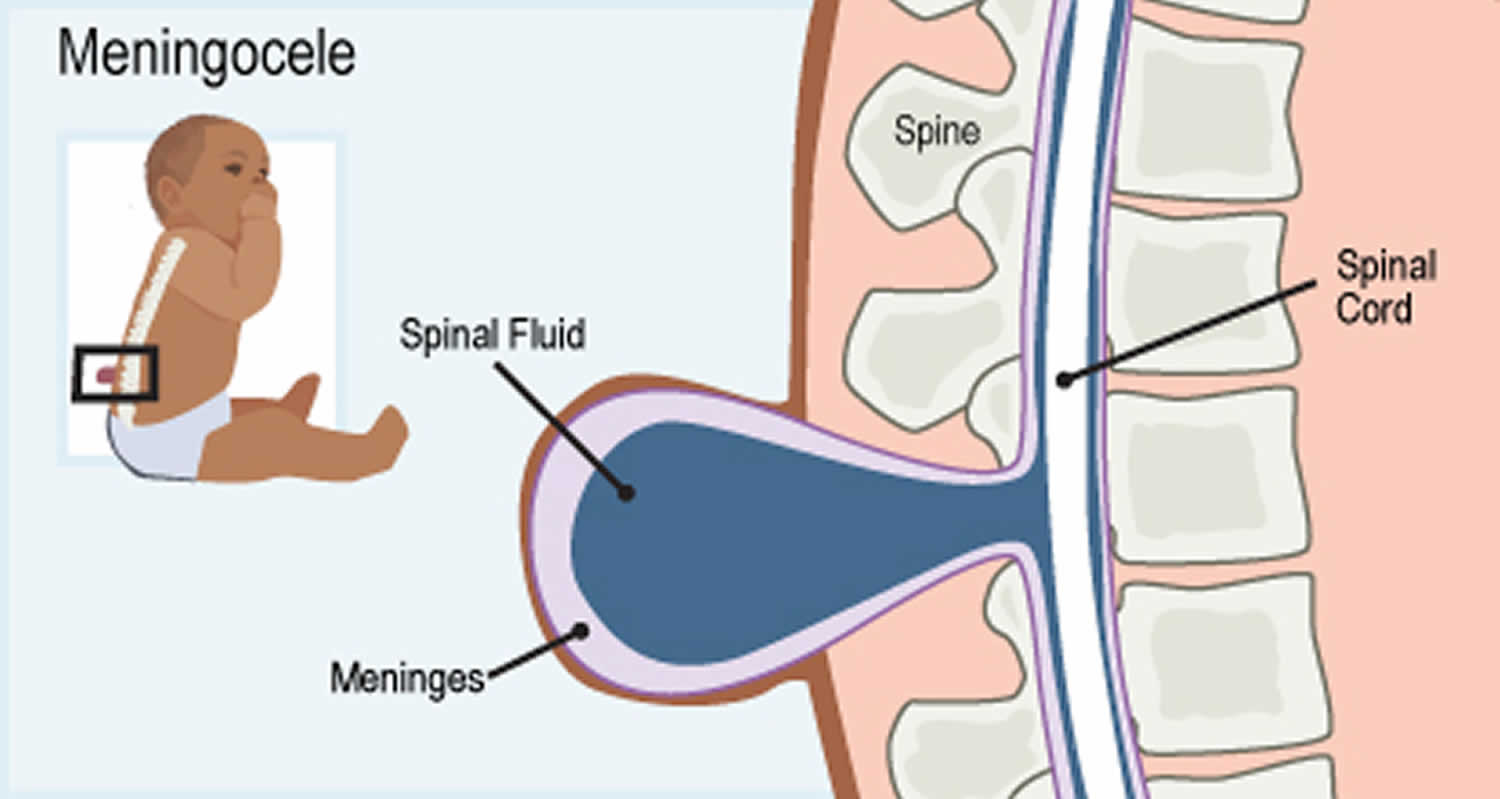
Another type of spinal dysraphism is meningocele. With meningocele a sac of spinal fluid comes through an opening in the baby’s back. But, the spinal cord is not in this fluid sac. There is usually little or no nerve damage. This type of spinal dysraphism can cause minor disabilities. Babies with meningocele may have some minor problems with functioning, including those affecting the bladder and bowels.
Figure 3. Meningocele
Occult spinal dysraphism
Occult spinal dysraphism or spina bifida occulta is the mildest type of spinal dysraphism and most common type. Occulta means hidden. Occult spinal dysraphism or spina bifida occulta is sometimes called “hidden” spinal dysraphism. With occult spinal dysraphism or spina bifida occulta, there is a small separation or a small gap in one or more of the bones of the spine (vertebrae), but no opening or sac on the back. The spinal cord and the nerves usually are normal. Many people who have occult spinal dysraphism or spina bifida occulta don’t even know it, unless the condition is discovered during an imaging test done for unrelated reasons. This type of spinal dysraphism usually does not cause any disabilities.
Figure 4. Occult spinal dysraphism
Spinal dysraphism causes
Scientists do not know all of the causes of spinal dysraphism, however they believe that spina bifida is caused by a combination of genetic and environmental factors including the mother’s nutrition 17, 18, 9. Research has shown that getting enough folic acid (vitamin B9) or folate before and during pregnancy can prevent most cases of spinal dysraphism or spina bifida 19.
If you are pregnant or planning to get pregnant, use the following tips to help prevent your baby from having spinal dysraphism 19:
- Take 400 micrograms (mcg) of folic acid every day. If you have already had a pregnancy affected by spinal dysraphism, you may need to take a higher dose of folic acid (folate) before pregnancy and during early pregnancy. Talk to your doctor to discuss what’s best for you.
- Talk to your doctor or pharmacist about any prescription and over-the-counter drugs, vitamins, and dietary or herbal supplements you are taking.
- If you have a medical condition―such as diabetes or obesity―be sure it is under control before you become pregnant.
- Avoid overheating your body, as might happen if you use a hot tub or sauna.
- Treat any fever you have right away with acetaminophen (paracetamol).
Spinal dysraphism happens in the first few weeks of pregnancy during fetal development, at approximately 28 days (3-4 weeks) of gestation, often before a woman knows she’s pregnant 20, 5. Although folic acid (folate) is not a guarantee that a woman will have a healthy pregnancy, taking folic acid (folate) can help reduce a woman’s risk of having a pregnancy affected by spinal dysraphism 19. Because half of all pregnancies in the United States are unplanned, it is important that all women who can become pregnant take 400 mcg of folic acid daily.
In cases of myelomeningocele, perinatal folic acid intake has shown to reduce the incidence of myelomeningocele significantly 9.
Other potential causes of spinal dysraphism include genetic inheritance and racial backgrounds 9. Usually, infants who are born with spinal dysraphism are born to mothers who have given birth to children with no history of spinal dysraphism 9. However, after one child with spinal dysraphism is born, the risk will increase to 1 in 20 for subsequent childbirth 9. Some infants born with spinal dysraphism can have chromosomal anomalies such as as trisomies, duplications, deletions, and some single-gene mutations 21. Some reports show that pregestational diabetes increases the risk of central nervous system malformations, including spinal dysraphism 16.
No specific individual factor has been identified to be related to open and closed spinal dysraphism 16. Nutritional deficiency is the most common risk factor reported for any kind of spinal dysraphism, causing up to 50% of all cases. In addition to folic acid deficiency, zinc deficiency is also found to be associated with spinal dysraphism 9. Excessive intake of some elements can also lead to spinal dysraphism. Vitamin A deficiency or excess can be associated with the formation of spinal dysraphism 9. Moreover, nitrates are present in canned meat and groundwater and are known to cause spinal dysraphism 9. Cytochalasin is a fungal metabolite, and its intake can also cause spinal dysraphism in children 16. Some medications are also found to be associated with spinal dysraphism. For example, sodium valproate, a well-known antiepileptic drug, is related to 1% to 2% chances of myelomeningocele formation when used in pregnancy 22, 23.
Risk factors for spinal dysraphism
Spinal dysraphism is more common among white people and Hispanics, and females are affected more often than males 24. Although doctors and researchers don’t know for sure why spinal dysraphism occurs, they have identified some risk factors 24:
- Folate deficiency. Folate or folic acid, the natural form of vitamin B9, is important to the development of a healthy baby. The synthetic form, found in supplements and fortified foods, is called folic acid. A folate deficiency increases the risk of spinal dysraphism and other neural tube defects.
- Family history of neural tube defects. Couples who’ve had one child with a neural tube defect have a slightly higher chance of having another baby with the same defect. That risk increases if two previous children have been affected by the condition. In addition, women who were born with a neural tube defect have a greater chance of giving birth to a child with spinal dysraphism than someone who doesn’t have a neural tube defect. However, most babies with spinal dysraphism are born to parents with no known family history of the condition.
- Some medications. Anti-seizure medications, such as valproic acid seem to cause neural tube defects when taken during pregnancy. This might happen because they interfere with the body’s ability to use folate and folic acid.
- Diabetes. Women with diabetes who don’t have well-controlled blood sugar have a higher risk of having a baby with spinal dysraphism.
- Obesity. Pre-pregnancy obesity is associated with an increased risk of neural tube birth defects, including spinal dysraphism.
- Increased body temperature. Some evidence suggests that increased body temperature (hyperthermia) in the early weeks of pregnancy may increase the risk of spinal dysraphism. Increases in core body temperature, due to fever or use of a sauna or hot tub, have been associated with a slightly increased risk of spinal dysraphism.
- Genetic conditions. Very rarely, spinal dysraphism can occur alongside a genetic condition such as Patau’s syndrome, Edwards’ syndrome or Down’s syndrome. If your baby is found to have spina bifida and it’s thought they may also have one of these syndromes, you’ll be offered a diagnostic test, such as amniocentesis or chorionic villus sampling that can tell for certain if your baby has one of these genetic conditions.
If you have known risk factors for spinal dysraphism, talk with your doctor to determine if you need a larger dose or prescription dose of folic acid, even before a pregnancy begins.
If you take medications, tell your doctor. If you plan ahead, some medications can be adjusted to diminish the potential risk of spinal dysraphism.
Spinal dysraphism prevention
Folic acid, taken in supplement form starting at least one month before conception and continuing through the first trimester of pregnancy, greatly reduces the risk of spinal dysraphism and other neural tube defects.
Folic acid (folate)
Having enough folic acid in your system by the early weeks of pregnancy is critical to prevent spinal dysraphism. Because many women don’t discover that they’re pregnant until this time, experts recommend that all women of childbearing age should take a daily supplement of 400 micrograms (mcg) of folic acid.
Folate is naturally present in many foods, and folic acid is added to some foods. You can get recommended amounts by eating a variety of foods, including the following.
- Folate is naturally present in:
- Beef liver
- Vegetables (especially asparagus, brussels sprouts, and dark green leafy vegetables such as spinach and mustard greens)
- Fruits and fruit juices (especially oranges and orange juice)
- Nuts, beans, and peas (such as peanuts, black-eyed peas, and kidney beans)
- Folic acid is added to the following foods:
- Enriched bread, flour, cornmeal, pasta, and rice
- Fortified breakfast cereals
- Fortified corn masa flour (used to make corn tortillas and tamales, for example)
To find out whether a food has added folic acid, look for “folic acid” on its Nutrition Facts label. Folic acid may be listed on food packages as folate, which is the natural form of folic acid found in foods.
Table 1. Folate and Folic Acid content of selected foods
| Food | Micrograms (mcg) DFE per serving | Percent DV* |
|---|---|---|
| Beef liver, braised, 3 ounces | 215 | 54 |
| Spinach, boiled, ½ cup | 131 | 33 |
| Black-eyed peas (cowpeas), boiled, ½ cup | 105 | 26 |
| Breakfast cereals, fortified with 25% of the DV† | 100 | 25 |
| Rice, white, medium-grain, cooked, ½ cup† | 90 | 22 |
| Asparagus, boiled, 4 spears | 89 | 22 |
| Brussels sprouts, frozen, boiled, ½ cup | 78 | 20 |
| Spaghetti, cooked, enriched, ½ cup† | 74 | 19 |
| Lettuce, romaine, shredded, 1 cup | 64 | 16 |
| Avocado, raw, sliced, ½ cup | 59 | 15 |
| Spinach, raw, 1 cup | 58 | 15 |
| Broccoli, chopped, frozen, cooked, ½ cup | 52 | 13 |
| Mustard greens, chopped, frozen, boiled, ½ cup | 52 | 13 |
| Bread, white, 1 slice† | 50 | 13 |
| Green peas, frozen, boiled, ½ cup | 47 | 12 |
| Kidney beans, canned, ½ cup | 46 | 12 |
| Wheat germ, 2 tablespoons | 40 | 10 |
| Tomato juice, canned, ¾ cup | 36 | 9 |
| Crab, Dungeness, 3 ounces | 36 | 9 |
| Orange juice, ¾ cup | 35 | 9 |
| Turnip greens, frozen, boiled, ½ cup | 32 | 8 |
| Peanuts, dry roasted, 1 ounce | 27 | 7 |
| Orange, fresh, 1 small | 29 | 7 |
| Papaya, raw, cubed, ½ cup | 27 | 7 |
| Banana, 1 medium | 24 | 6 |
| Yeast, baker’s, ¼ teaspoon | 23 | 6 |
| Egg, whole, hard-boiled, 1 large | 22 | 6 |
| Cantaloupe, raw, cubed, ½ cup | 17 | 4 |
| Vegetarian baked beans, canned, ½ cup | 15 | 4 |
| Fish, halibut, cooked, 3 ounces | 12 | 3 |
| Milk, 1% fat, 1 cup | 12 | 3 |
| Ground beef, 85% lean, cooked, 3 ounces | 7 | 2 |
| Chicken breast, roasted, 3 ounces | 3 | 1 |
Footnotes:
* DV = Daily Value. The FDA developed DVs (Daily Values) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The Daily Value for folate is 400 mcg DFE for adults and children aged 4 years and older 25, where mcg DFE = mcg naturally occurring folate + (1.7 x mcg folic acid). The labels must list folate content in mcg DFE per serving and if folic acid is added to the product, they must also list the amount of folic acid in mcg in parentheses. The FDA does not require food labels to list folate content unless folic acid has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
† Fortified with folic acid as part of the folate fortification program.
[Source 26 ]Planning pregnancy
Adult women who are planning pregnancy or who could become pregnant should be advised to get 400 to 800 mcg of folic acid a day.
Your body doesn’t absorb folate as easily as it absorbs synthetic folic acid, and most people don’t get the recommended amount of folate through diet alone, so vitamin supplements are necessary to prevent spinal dysraphism. And it’s possible that folic acid will also help reduce the risk of other birth defects, including cleft lip, cleft palate and some congenital heart defects.
Folic acid is available in multivitamins and prenatal vitamins, supplements containing other B-complex vitamins, and supplements containing only folic acid. Common doses range from 680 to 1,360 mcg DFE (400 to 800 mcg folic acid) in supplements for adults and 340 to 680 mcg DFE (200 to 400 mcg folic acid) in children’s multivitamins 27.
About 85% of supplemental folic acid, when taken with food is bioavailable (that is around 85% of supplemental folic acid taken by mouth can be absorbed and used by the body) 28, 29. When consumed without food, nearly 100% of supplemental folic acid is bioavailable.
It’s also a good idea to eat a healthy diet, including foods rich in folate or enriched with folic acid. This vitamin is present naturally in many foods, including:
- Beans and peas
- Citrus fruits and juices
- Egg yolks
- Milk
- Avocados
- Dark green vegetables, such as broccoli and spinach
When higher folic acid doses are needed
If you have spinal dysraphism or if you’ve previously given birth to a child with spinal dysraphism, you’ll need extra folic acid before you become pregnant. If you’re taking anti-seizure medications or you have diabetes, you may also benefit from a higher dose of this B vitamin. Check with your doctor before taking additional folic acid supplements.
Spinal dysraphism signs and symptoms
Signs and symptoms of spinal dysraphism vary by type and severity, and also between individuals.
Occult spinal dysraphism signs and symptoms
Most people with occult spinal dysraphism or spina bifida occulta do not even know they have it because the spinal nerves aren’t involved. But you can sometimes see signs on the newborn’s skin above the spinal problem, including a patch of hair, a small dimple or a birthmark or a red mark at the base of the spine. Sometimes, these skin marks can be signs of an underlying spinal cord issue that can be discovered with MRI or spinal ultrasound in a newborn.
Some people with occult spinal dysraphism or spina bifida occulta also have a tethered cord. A tethered cord is a spinal cord that can’t move freely inside the spinal canal. Sometimes a tethered spinal cord needs to be released with surgery. Otherwise, it can stretch (especially during a growth spurt) and lead to pain, trouble walking, and loss of bladder (pee) control.
A tethered spinal cord may go undiagnosed until adulthood. Delayed presentation of symptoms can be insidious, meaning that symptoms come on slowly over time, but can be complex and severe. Back pain, brought on or worsened by activity and relieved with rest, can be a sign of spinal cord tethering.
Sometimes back pain is also associated with leg pain, even in areas that have decreased or no sensation. Changes in leg strength, deterioration in gait (walking), progressive or repeated muscle contractures, orthopedic deformities of the legs, scoliosis, and changes in bowel or bladder function may be signs of spinal cord tethering.
Meningocele signs and symptoms
Meningocele is when a sac made up of the meninges (the membranes that cover the brain and spinal cord) that contains spinal fluid pushes through the gap in the spine. The spinal cord is in its normal place in the spinal canal. The skin over the meningocele often is open. A meningocele can be seen on the baby’s head, neck, or back.
Most babies with a meningocele do not have any symptoms. Although it doesn’t happen very often, sometimes the nerves around the spine are damaged. This can lead to problems with movement and controlling when urine and poop comes out and other medical issues.
Myelomeningocele signs and symptoms
In myelomeningocele the sac that contains the meninges, spinal fluid, and part of the spinal cord pushes through the gap in the spine and the skin. You can see it on the baby’s back. In this severe type of spinal dysraphism:
- The spinal canal remains open along several vertebrae in the lower or middle back
- Both the membranes and the spinal cord or nerves protrude at birth, forming a sac
- Tissues and nerves usually are exposed, though sometimes skin covers the sac
The signs and symptoms of a myelomeningocele depend on where it is. A myelomeningocele can lead to 30:
- weakness, loss of feeling, or trouble moving body parts below the level of the myelomeningocele
- problems with bladder (pee) and bowel (poop) control or incontinence
- too much spinal fluid in the brain (hydrocephalus)
- problem with how the brain is formed (Chiari malformation)
- learning problems
- seizures
Sometimes babies with a myelomeningocele are born with other medical problems like clubfoot, curvature of the spine (scoliosis), hip problems (dislocation of the hip), heart problems, or kidney problems.
Spinal dysraphism complications
Spinal dysraphism may cause minimal symptoms or minor physical problems. But severe spinal dysraphism can lead to more significant physical conditions. Severity is affected by:
- The size and location of the neural tube defect
- Whether skin covers the affected area
- Which spinal nerves come out of the affected area of the spinal cord
This list of possible complications may seem overwhelming, but not all children with spinal dysraphism get all of these complications. Many of these complications can be treated.
- Walking and mobility problems. The nerves that control the leg muscles don’t work properly below the area of the spinal dysraphism defect. This can cause muscle weakness of the legs and sometimes paralysis or paraplegia. Whether a child can walk typically depends on where the defect is, its size, and the care received before and after birth. Bracing using external orthosis can help to maximize their mobility and ensure a near-normal developmental progression. In children over 1-year-old, utilizing a standing frame can reduce the risk of osteoporosis and the formation of contractures in lower extremities. A wheelchair can provide mobility for older children and adults.
- Orthopedic complications. Children with myelomeningocele can have a variety of problems in the legs and spine because of weak muscles in the legs and back. The types of problems depend on the location of the defect. Possible problems include orthopedic issues such as:
- Curved spine (scoliosis)
- Abnormal growth
- Dislocation of the hip
- Bone and joint deformities
- Muscle contractures
- Bowel and bladder problems. Nerves that supply the bladder and bowels usually don’t work properly when children have myelomeningocele. This is because the nerves that supply the bowel and bladder come from the lowest level of the spinal cord.
- Most patients with myelomeningocele have some degree of bladder incontinence. Preventive goals are directed toward preventing infection with the implementation of bladder drainage utilizing intermittent catheterization or indwelling catheters. Bladder stimulation has shown to improve bladder emptying and reduce infection.
- Myelomeningocele is associated with anal sphincter dysfunction that results in bowel incontinence. Assisted bowel emptying reduces barriers associated with social activities, including attending school and personal relationships.
- Accumulation of fluid in the brain or hydrocephalus. Babies born with myelomeningocele commonly experience accumulation of fluid in the brain, a condition known as hydrocephalus. Extra fluid can cause the head to swell and put pressure on the brain. Hydrocephalus can cause intellectual and developmental disabilities. These are problems with how the brain works that can cause a person to have trouble or delays in physical development, learning, communicating, taking care of himself or getting along with others. In some cases, a surgeon needs to drain the extra fluid from a baby’s brain.
- Shunt malfunction. Shunts placed in the brain to treat hydrocephalus can stop working or become infected. Warning signs may vary. Some of the warning signs of a shunt that isn’t working include:
- Headaches
- Vomiting
- Sleepiness
- Irritability
- Swelling or redness along the shunt
- Confusion
- Changes in the eyes (fixed downward gaze)
- Trouble feeding
- Seizures
- Chiari malformation type 2. Chiari malformation type 2 is a common problem with the brain in children who have the myelomeningocele type of spinal dysraphism. The brainstem is the lowest part of the brain above the spinal cord. In Chiari malformation type 2, the brainstem is elongated and positioned lower than usual. This can cause problems with breathing and swallowing. Rarely, compression on this area of the brain occurs and surgery is needed to relieve the pressure.
- Infection in the tissues surrounding the brain (meningitis). Some babies with myelomeningocele may develop meningitis, an infection in the tissues surrounding the brain. This potentially life-threatening infection may cause brain injury.
- Shunt infections. Shunts are also prone to infections. When shunts are placed, infections can occur superficially at the skin or intraabdominal, as many of these patients have multiple abdominal procedures.
- Tethered spinal cord. Tethered spinal cord results when the spinal nerves bind to the scar where the defect was closed surgically. The spinal cord is less able to grow as the child grows. This progressive tethering can cause loss of muscle function to the legs, bowel or bladder. Surgery can limit the degree of disability.
- Sleep-disordered breathing. Both children and adults with spinal dysraphism, particularly myelomeningocele, may have sleep apnea or other sleep disorders. Assessment for a sleep disorder in those with myelomeningocele helps detect sleep-disordered breathing, such as sleep apnea, which warrants treatment to improve health and quality of life.
- Skin problems. Children with spinal dysraphism may get wounds on their feet, legs, buttocks or back. They can’t feel when they get a blister or sore. Sores or blisters can turn into deep wounds or foot infections that are hard to treat. Children with myelomeningocele have a higher risk of wound problems in casts.
- Latex allergy. Children with spinal dysraphism have a higher risk of latex allergy, an allergic reaction to natural rubber or latex products. Latex allergy may cause rash, sneezing, itching, watery eyes and a runny nose. It can also cause anaphylaxis, a potentially life-threatening condition in which swelling of the face and airways can make breathing difficult. So it’s best to use latex-free gloves and equipment at delivery time and when caring for a child with spinal dysraphism.
- Other complications. More problems may arise as children with spinal dysraphism get older, such as urinary tract infections, gastrointestinal disorders and depression. Children with myelomeningocele may develop learning disorders, such as problems paying attention, and difficulty learning reading and math.
Spinal dysraphism diagnosis
Spinal dysraphism can be diagnosed during pregnancy or after the baby is born. Occult spinal dysraphism might not be diagnosed until late childhood or adulthood, or might never be diagnosed.
If you’re pregnant, you’ll be offered prenatal screening tests to check for spina bifida and other birth defects. The tests aren’t perfect. Some mothers who have positive blood tests have babies without spina bifida. Even if the results are negative, there’s still a small chance that spina bifida is present. Talk to your doctor about prenatal testing, its risks and how you might handle the results.
During pregnancy
During pregnancy there are screening tests (prenatal tests) to check for spinal dysraphism and other birth defects, but typically the diagnosis is made with ultrasound. Talk with your doctor about any questions or concerns you have about this prenatal testing.
- Alpha-fetoprotein (AFP) – AFP (alpha-fetoprotein) is a protein the unborn baby produces. Maternal serum alpha-fetoprotein (MSAFP) test is a simple blood test that measures how much AFP has passed into the mother’s bloodstream from the baby. It’s normal for a small amount of AFP to cross the placenta and enter the mother’s bloodstream. But unusually high levels of AFP suggest that the baby has a neural tube defect, such as spinal dysraphism or spina bifida, though high levels of AFP don’t always occur in spina bifida. An AFP test might be part of a test called the “triple screen” that looks for neural tube defects and other issues.
- Test to confirm high AFP levels. Varying levels of AFP can be caused by other factors — including a miscalculation in fetal age or multiple babies — so your doctor may order a follow-up blood test for confirmation. If the results are still high, you’ll need further evaluation, including an ultrasound exam.
- Other blood tests. Your doctor may perform the maternal serum alpha-fetoprotein (MSAFP) test with two or three other blood tests. These tests are commonly done with the MSAFP test, but their objective is to screen for other conditions, such as trisomy 21 (Down syndrome), not neural tube defects.
- Fetal ultrasound – An ultrasound is a type of picture of the baby. Fetal ultrasound is the most accurate method to diagnose spina bifida in your baby before delivery. In some cases, your doctor can see if the baby has spinal dysraphism or find other reasons that there might be a high level of AFP. Frequently, spinal dysraphism can be seen with ultrasound test. Ultrasound can be performed during the first trimester (11 to 14 weeks) and second trimester (18 to 22 weeks). Spina bifida can be accurately diagnosed during the second trimester ultrasound scan. Therefore, this examination is crucial to identify and rule out congenital anomalies such as spina bifida. An advanced ultrasound also can detect signs of spina bifida, such as an open spine or particular features in your baby’s brain that indicate spina bifida. In expert hands, ultrasound is also effective in assessing severity.
- Amniocentesis – If the prenatal ultrasound confirms the diagnosis of spina bifida, your doctor may request amniocentesis. For amniocentesis test, your doctor takes a small sample of the amniotic fluid surrounding the baby from the amniotic sac in the womb. Higher than average levels of AFP in the amniotic fluid might mean that your baby has spinal dysraphism. Amniocentesis may be important to rule out genetic diseases, despite the fact that spina bifida is rarely associated with genetic diseases. Discuss the risks of amniocentesis, including a slight risk of loss of the pregnancy, with your doctor.
After your baby is born
In some cases, spinal dysraphism might not be diagnosed until after your baby is born. Sometimes there is a hairy patch of skin or a dimple on the baby’s back that is first seen after the baby is born. A doctor can use an image scan, such as an, X-ray, MRI, or CT, to get a clearer view of the baby’s spine and the bones in the back.
Sometimes spinal dysraphism is not diagnosed until after the baby is born because the mother did not receive prenatal care or an ultrasound did not show clear pictures of the affected part of the spine.
Spinal dysraphism treatment
No two people with spinal dysraphism are exactly alike 31. Health issues and treatments for people with spinal dysraphism will be different for each person 31. Some people have issues that are more severe than other people 31. Those born with “open” spinal dysraphism usually have more health issues and need more types of treatments 32. The Spina Bifida Association has written guidelines for spina bifida click here https://www.spinabifidaassociation.org/wp-content/uploads/Guidelines-for-the-Care-of-People-with-Spina-Bifida-2018.pdf
Surgery before birth
Sometimes when a baby has open spinal dysraphism, or myelomeningocele, doctors will perform surgery to close the spine before the baby is born. This surgery is a major procedure for the mother and the baby, and may not be available where you live. Contact a doctor who works regularly with spinal dysraphism babies about the pros and cons of this option.
Nerve function in babies with spinal dysraphism can worsen after birth if spinal dysraphism isn’t treated. Prenatal surgery for spinal dysraphism (fetal surgery) takes place before the 26th week of pregnancy. Surgeons expose the pregnant mother’s uterus surgically, open the uterus and repair the baby’s spinal cord. In select patients, this procedure can be performed less invasively with a special surgical tool (fetoscope) inserted into the uterus.
Research suggests that children with spinal dysraphism who had fetal surgery may have reduced disability and be less likely to need crutches or other walking devices. Fetal surgery may also reduce the risk of hydrocephalus. Ask your doctor whether this procedure may be appropriate for you. Discuss the potential benefits and risks, such as possible premature delivery and other complications, for you and your baby.
It’s important to have a comprehensive evaluation to determine whether fetal surgery is feasible. This specialized surgery should only be done at a health care facility that has experienced fetal surgery experts, a multispecialty team approach and neonatal intensive care. Typically the team includes a fetal surgeon, pediatric neurosurgeon, maternal-fetal medicine specialist, fetal cardiologist and neonatologist.
Cesarean birth
Many babies with myelomeningocele tend to be in a feet-first (breech) position. If your baby is in this position or if your doctor has detected a large cyst or sac, cesarean birth may be a safer way to deliver your baby.
Surgery after birth
Myelomeningocele requires surgery to close the opening in the baby’s back within 72 hours of birth. Performing the surgery early can help minimize the risk of infection associated with the exposed nerves. It may also help protect the spinal cord from more trauma.
During the procedure, a neurosurgeon places the spinal cord and exposed tissue inside the baby’s body and covers them with muscle and skin. At the same time, the neurosurgeon may place a shunt in the baby’s brain to control hydrocephalus.
Hydrocephalus
Many babies born with spinal dysraphism get hydrocephalus (water on the brain). This means that there is extra cerebrospinal fluid (CSF) in and around the brain. The extra cerebrospinal fluid (CSF) can cause the spaces in the brain, called ventricles, to become too large and the head can swell. Hydrocephalus needs to be followed closely and treated properly to prevent brain injury.
If a baby with spinal dysraphism has hydrocephalus, a neurosurgeon can put in a shunt. A shunt is a small hollow tube that will help drain excess cerebrospinal fluid (CSF) from the baby’s brain to another place where the body can remove it naturally and protect it from too much pressure. Additional surgery might be needed to change the shunt as the child grows up or if it becomes clogged or infected.
Shunts have valves that regulate both the direction and amount of fluid that is drained. Shunts have three parts:
- A ventricular catheter to reach the area where there is too much fluid
- A valve to control flow (there are many types)
- Tubing to carry the fluid from one place in the body to another.
The most common type of shunt is the ventriculo-peritoneal (VP) shunt. This shunt drains from the ventricle in the brain to the abdomen.
Most babies with myelomeningocele will need a surgically placed tube that allows fluid in the brain to drain into the abdomen (ventriculo-peritoneal (VP) shunt). This tube might be placed just after birth, during the surgery to close the sac on the lower back or later as fluid accumulates. A less invasive procedure, called endoscopic third ventriculostomy, may be an option. But candidates must be carefully chosen and meet certain criteria. During the procedure, the surgeon uses a small video camera to see inside the brain and makes a hole in the bottom of or between the ventricles so cerebrospinal fluid can flow out of the brain.
Other types of shunt that are less common are:
- Ventriculoatrial (VA) shunts—VA shunts move the to a vein, usually in the neck or under the collarbone
- Ventriculo-pleural shunts—These shunts move fluid to the chest around the lungs
- Ventriculo-gall bladder shunts—These shunts move to the gall bladder
There are several types of shunt valves. All of them work by controlling the amount of cerebrospinal fluid (CSF) that is drained. Most are made to work automatically when fluid pressure in the head gets too high. Some valves also may have special devices to keep too much fluid from draining. Experts have not yet learned which type of shunt is best for whom.
Neurosurgeons usually pick ones that they think are best. Shunts can be put into one of these places in the head:
- The edge of the soft spot
- Above and behind the ear
- The back of the head
Experts don’t know if one place is better than another. So where to put the shunt also is up to what the surgeon thinks is best. Almost all shunts are put in during the first days or weeks after birth. Sometimes the shunt will be inserted at the time of the initial back closure. A child who doesn’t need a shunt by the time they are 5 months old probably will never need one.
Signs of hydrocephalus or of shunt malfunction in infants
The signs of shunt problems in people with spinal dysraphism are different for each person. This can make it hard for families and health care providers to know what’s going on. The most common sign of a shunt problem is a headache. Vomiting and nausea can happen, too, but not always.
Signs of hydrocephalus or of shunt malfunction in infants may include:
- Rapid head growth
- Full or tense soft spot (fontanelle)
- Unusual irritability
- Repeated vomiting
- Crossed eyes
- An inability to look up
- Periods in which the baby stops breathing (called apnea) swallowing
- A hoarse or weak cry in keeping the infant awake
- Any worsening brain function
Less common signs of a shunt problem include:
- Seizures (either the onset of new seizures or an increase in the frequency of existing seizures)
- A change in intellect, school performance or personality
- Back pain at the spine closure site
- Worsening arm or leg function (increasing weakness or loss of sensation, worsening coordination or balance and/ or worsening orthopedic deformities)
- Increasing scoliosis
- Worsening speech or swallowing
- Changes in bowel or bladder function
Shunt malfunction can look like any of the signs of a Chiari malformation or spinal cord tethering. When the brain or spinal cord function gets worse, and there is no other clear cause, health care providers should check to see if there are shunt problems.
A head ultrasound, computed tomography (CT) scan or a magnetic resonance imaging (MRI) scan will show cerebrospinal fluid (CSF) build-up, but a shunt still may not be working right even if it doesn’t show up on a CT or MRI scan.
To see if there is a problem with a shunt, doctors will study images of the brain (usually a CT scan or, for children under one year, a head ultrasound).
Most people with spinal dysraphism and shunted hydrocephalus will need the shunt for life. The most common problem with shunts is that they can get blocked up, break or come apart. About 40 percent of shunts will fail and need changing or revision within one year, 60 percent within years and 80–85 percent within 10 years. About 20 percent of people with spinal dysraphism will need more than one shunt revision.
New, long-term treatments using small endoscopes may eliminate the need for a shunt. All patients with hydrocephalus should be seen by a neurosurgeon at least every one to two years.
When ventricles start to get too big, it is a strong sign that the shunt is not working right. It is important to know that some people (between 5 and 15 percent) with spinal dysraphism may have very few signs or even no visible change in the size of the ventricles when the shunt is not working correctly. On the other hand, some people with shunted hydrocephalus can develop the slit (or stiff) ventricle syndrome. For these people, too much fluid drainage leads to very small (or slit) ventricles. In these cases, experts think that the walls of the ventricles temporarily block the shunt catheter. This leads to a series of temporary shunt malfunctions without any visible increase in the size of the ventricles.
Families and health care providers must pay close attention to a person’s symptoms, especially if they are similar to those that were present with previous shunt problems.
Infections
Infection is a major problem that can happen with shunt operations. Between 5 and 10 percent of people will have this problem. Shunt infections are higher in babies than in older children and adults. Seventy percent of shunt infections happen within the first two months after a shunt operation. Eighty percent of these infections develop within the first six months. Skin bacteria (Staphylococcus epidermis) are the most common causes of shunt infection. Half of people with shunt infections show signs of a shunt malfunction. Additional signs of an infection include:
- Fever
- Neck stiffness
- Pain
- Tenderness
- Redness
- Drainage from the shunt incisions or tract
- Abdominal pain
The diagnosis can be checked by putting a small needle into the valve or a chamber of the shunt and taking out fluid for study. Infections are commonly treated with antibiotics and with removal and replacement of the shunt system. There are two ways of doing this. The first is to take out the shunt system and then put in a temporary external drainage tube at the same time that antibiotics are given. When the treatment is done, the tube is taken out, and a new shunt is put back in. This almost always stops the infection, but it takes two operations.
The second (assuming that the shunt is working) is to keep the infected shunt in until the end of the antibiotic treatment. Then the infected shunt is removed and replaced with a new one. The second way only takes one operation, but it does not get rid of the infection as often as the first.
Tethered spinal cord
Many people with open spinal dysraphism have tethered spinal cords. A tethered spinal cord is attached to the spinal canal via surrounding structures. Normally, the bottom of the spinal cord floats around freely in the spinal canal, freely bending and stretching and moving up and down as the body grows. However, a tethered spinal cord does not move; it is pulled tightly at the end. This reduces blood flow to the spinal nerves and damages the spinal cord from the stretching and the decreased blood supply. Because the spinal cord stretches as a child grows, a tethered spinal cord can permanently damage the spinal nerves. Your child might have back pain, scoliosis (crooked spine), leg and foot weakness, changes in bladder or bowel control, and other problems. A tethered spinal cord can be treated with surgery.
A tethered spinal cord can happen before or after birth in children and adults with spinal dysraphism or spina bífida, and most often occurs in the lower (lumbar) level of the spine 33. A tethered cord may go undiagnosed until adulthood when sometimes complex and severe symptoms come on slowly over time. While all forms of spinal dysraphism can be accompanied by spinal cord tethering, it rarely occurs with occult spinal dysraphism 33.
What is tethered spinal cord syndrome?
Tethered spinal cord syndrome is the presence of several clinically recognizable signs (observed by a physician), or symptoms (reported by the patient) that occur together as a result of spinal cord tethering. These signs and symptoms can include 33:
- sensory disturbance,
- significant muscle weakness (as determined by neurological assessment),
- pain, and
- urinary or bowel incontinence.
How does tethered spinal cord occur in myelomeningocele?
During the early stages of a pregnancy, the spinal cord of the fetus extends from the brain all the way down to the tailbone (coccygeal) region of the spine. As the pregnancy progresses, the bony spine grows faster than the spinal cord, so the end of the spinal cord appears to rise relative to the adjacent bony spine. By the time a child is born, the spinal cord is normally located opposite the disc between the first (L1) and second (L2) lumbar vertebrae, in the upper part of the lower back. In a baby with spinal dysraphism, the spinal cord is still attached to the surrounding skin, and is prevented from ascending normally.
The spinal cord at birth is low-lying, or tethered. Although the myelomeningocele is surgically separated from the skin and closed at birth, the spinal cord, which has grown in this position, stays in roughly the same location after the closure, and quickly scars to the site. As the child and the bony spine continues to grow, the spinal cord can become stretched; damaging the spinal cord both by directly stretching it, and by interfering with the blood supply to the spinal cord. The result can be progressive neurological, urological, or orthopedic deterioration.
How does tethered spinal cord occur in milder forms of spinal dysraphism?
Spinal cord tethering, usually in adults with milder forms of spinal dysraphism may be related to the degree of strain placed on the spinal cord over time, and may appear or be significantly worsened during physical activity, injury, or pregnancy. It may also be caused by narrowing of the spinal column (spinal stenosis) or bony spurs.
A tethered spinal cord may go undiagnosed until adulthood. Delayed presentation of symptoms can be insidious, meaning that symptoms come on slowly over time, but can be complex and severe. Back pain, brought on or worsened by activity and relieved with rest, can be a sign of spinal cord tethering.
Sometimes back pain is also associated with leg pain, even in areas that have decreased or no sensation. Changes in leg strength, deterioration in gait (walking), progressive or repeated muscle contractures, orthopedic deformities of the legs, scoliosis, and changes in bowel or bladder function may be signs of spinal cord tethering.
How is a tethered spinal cord diagnosed?
If a child with myelomeningocele and shunted hydrocephalus presents with clinical worsening, the first issue is to determine whether or not the shunt is working, as shunt malfunction can appear the same as a tethered spinal cord. So, always check the shunt first 33. Accordingly, the first test is usually a computed tomography (CT) or magnetic resonance imaging (MRI) scan of the brain. Once the shunt is found to be working, or for those who do not have a shunt, an MRI of the spine is performed. It is important to know that virtually every child with spinal dysraphism has evidence of tethering on the MRI. Untethering is therefore performed only if there are clinical signs or symptoms of deterioration.
The MRI is done to show the neurosurgeon the anatomy of the spinal cord tethering, and to exclude other abnormalities such as a syringomyelia (syrinx), split cord malformation, or a dermoid cyst (small tag of skin in the area around or within the spinal cord). Additional studies may include spine X-rays or CT scans of the spine to look for other bony abnormalities, or to follow the progress of scoliosis. Other functional studies
may be done, including Manual Muscle Testing (MMT) and urodynamics. Both are compared with previous studies to document changes, and to provide a pre-surgical baseline.
When is surgery performed?
After reviewing the diagnostic studies, the neurosurgeon may decide to untether the spinal cord. The decision to untether the spinal cord requires some clinical judgment. The neurosurgeon considers the patient’s symptoms, signs, and the results of the tests. Virtually every child with spinal dysraphism has evidence of tethering on the MRI. Untethering the spinal cord is generally only done if there is clinical evidence of
deterioration, progressive or severe pain, loss of muscle function, deterioration in gait, or changes in bladder or bowel function. The longer deterioration continues, the less likely it is that function will return.
What happens after surgery?
Recovery in the hospital is generally about 2-5 days, and the patient often returns to normal activity within a few weeks 33. Some surgeons require the patient to remain at in bed for a couple of days to minimize the risk of spinal cerebrospinal fluid (CSF) leakage from the wound. Pain is often not severe, because there is usually some degree of numbness in that area anyway. Recovery of lost muscle and bladder function is variable, and depends on both the degree and length of the neurologic losses before the surgery.
Untethering is designed primarily to prevent further deterioration, not to improve spinal cord damage that has already occurred. Modern microsurgical equipment and techniques have made untethering a relatively routine surgical procedure, in the hands of an experienced neurosurgeon.
What are the complications of surgery?
Untethering the spinal cord is generally a safe procedure. However, the scar can make dissection difficult; and the abnormal anatomy can be confusing for any surgeon.
Complications are few, but may include:
- infection,
- bleeding,
- damage to the spinal cord and myelomeningocele, resulting in worsening of muscle, bladder, or bowel function.
The combined complication rate of surgery is usually only 1-2% 33. Although some have suggested that shunt malfunction may occur secondary to untethering surgery, it is more likely that an occult or unrecognized shunt malfunction was the original cause of the deterioration in the first place.
Is repeat untethering surgery necessary?
Although most children require only one untethering procedure, some (perhaps 10-20%) require repeated untethering operations as the child continues to grow. The most problematic period is the pre-adolescent period (7-12 years) 33. Since all children grow, it is not clear why some develop symptoms and signs of spinal cord tethering, while others do not; perhaps some children’s spinal cords tolerate a greater degree of stretching than others. In adulthood, clinical deterioration from spinal cord tethering becomes much less frequent although it can still occur. In adulthood, re-tethering may occur from scar tissue, spinal deterioration, or injury.
Can tethered spinal cord be prevented?
Many techniques have been tried to prevent or minimize spinal cord tethering, but only surgical untethering has been successful in long term studies. Research continues into this important area. With close observation, it should be possible to diagnose this condition early and untether the spinal cord before progressive and permanent damage occurs.
Treatment of complications
In babies with myelomeningocele, irreparable nerve damage has likely already occurred and ongoing care from a multispecialty team of surgeons, physicians and therapists is usually needed. Babies with myelomeningocele may need more surgery for a variety of complications. Treatment of complications — such as weak legs, bladder and bowel problems, or hydrocephalus — typically begins soon after birth.
Depending on the severity of spinal dysraphism and the complications, treatment options may include:
- Walking and mobility aids. Some babies may start exercises to prepare their legs for walking with braces or crutches when they’re older. Some children may need walkers or a wheelchair. Mobility aids, along with regular physical therapy, can help a child become independent. Even children who need a wheelchair can learn to function very well and become self-sufficient.
- Bowel and bladder management. Routine bowel and bladder evaluations and management plans help reduce the risk of organ damage and illness. Evaluations include X-rays, kidney scans, ultrasounds, blood tests and bladder function studies. These evaluations will be more frequent in the first few years of life but less often as children grow. A specialist in pediatric urology with experience in evaluating and performing surgery on children with spinal dysraphism may offer the most effective management options.
- Bowel management may include oral medications, suppositories, enemas, surgery or a combination of these approaches.
- Bladder management may include medications, using catheters to empty the bladder, surgery or a combination of treatments.
- Treatment and management of other complications. Special equipment such as bath chairs, commode chairs and standing frames may help with daily functioning. Whatever the issue — orthopedic complications, tethered spinal cord, gastrointestinal issues, skin problems or others — most spinal dysraphism complications can be treated or at least managed to improve quality of life.
Mobility and physical activity
People affected by spinal dysraphism get around in different ways. These include walking without any aids or assistance; walking with braces, crutches or walkers; and using wheelchairs.
People with spinal dysraphism higher on the spine (near the head) might have paralyzed legs and use wheelchairs. Those with spinal dysraphism lower on the spine (near the hips) might have more use of their legs and use crutches, braces, or walkers, or they might be able to walk without these devices.
Regular physical activity is important for all people, but especially for those with conditions that affect movement, such as spinal dysraphism. Experts recommends 60 minutes of physical activity a day. There are many ways for people with spinal dysraphism to be active. For example, they can:
- Engage in active play with friends.
- Roll or walk in the neighborhood.
- Participate in community programs, such as the Early Intervention Program for Infants and Toddlers with Disabilities and Special Education Services for Preschoolers with Disabilities, which are free programs in many communities.
- Enjoy parks and recreation areas with playgrounds that are accessible to people with disabilities.
- Do exercises recommended by a physical therapist.
- Attend summer camps and recreational facilities that are accessible for those with disabilities.
- Participate in sports activities (for example, swimming) and teams for people with or those without disabilities.
Using the bathroom
People with spinal dysraphism often cannot control when they go to the bathroom (incontinence). They also can develop urinary tract infections. It is important to develop a plan for going to the bathroom that works and is as simple as possible. This can lead to increased health, participation, and independence, and avoid embarrassment for people with spinal dysraphism. Healthcare providers can help develop a plan for each person. A tube (catheter) inserted in the bladder can help drain urine. In some cases, extra fiber can be added to the diet to keep bowel movements regular. Surgery also might be recommended.
Skin care
Children and adults living with spinal dysraphism may have limited feeling in some areas of their body, leaving them unable to feel cuts, bruises, sores, and dry skin. Since a person with spinal dysraphism may not know they have been hurt, they may be unable to tell a parent or caregiver that they need help.
Pressure sores occur when there is prolonged pressure on soft tissue, skin, and muscle. Healthcare professionals report skin wounds as one of the primary diagnoses associated with the hospitalization1 of people with spinal dysraphism. Pressure sores can lead to infection, amputation, or even death.
By checking skin regularly for redness including under braces, people with spinal dysraphism, along with their parents and caregivers, can identify skin problems before they become pressure sores.
Additional ways to protect the skin:
- Avoid hot bath water, heaters, hot dishes, hot car seats, and metal seatbelt clasps, since they may cause burns.
- Make sure you are wearing properly fitting shoes at all times, even when swimming.
- Use sunscreen, and don’t stay out in the sun too long.
- Do not sit or lie in one position for too long.
Latex (natural rubber) allergy
Many people with spinal dysraphism are allergic to products that contain latex, or natural rubber 34. This means they should not use items made of natural rubber. For babies, this would include rubber nipples and pacifiers. A person with this type of allergy can wear a bracelet to alert other people of the allergy.
Health checks
Every person needs a primary care provider, such as a pediatrician, nurse practitioner, general family doctor, or internist. The primary care provider will want to make sure that he or she is healthy; developing normally; and receiving immunization against diseases and infections, including the flu.
In addition to seeing a primary health care provider, a person with spinal dysraphism will be checked and treated as needed by doctors who specialize in different parts of the body. These doctors might suggest treatments or surgeries to help the person.
These specialists might include:
- An orthopedist, who will work with muscles and bones.
- A urologist, who will check the kidneys and bladder.
- A neurosurgeon, who will check the brain and spine.
Other concerns
Some people with spinal dysraphism have difficulty with:
- Learning
- Relating to others
- Vision
- Staying at a healthy weight
- Depression.
Ongoing care
Children with spinal dysraphism need close follow-up care and observation. Their primary care doctors evaluate growth, the need for vaccinations and general medical issues, and they coordinate medical care.
Children with spinal dysraphism also often need treatment and ongoing care from:
- Physical medicine and rehabilitation
- Neurology
- Neurosurgery
- Urology
- Orthopedics
- Physical therapy
- Occupational therapy
- Special education teachers
- Social workers
- Dietitians
Parents and other caregivers are a key part of the team. They can learn how to help manage a child’s condition and how to encourage and support the child emotionally and socially.
Spinal dysraphism prognosis
The prognosis of spinal dysraphism varies from case to case. It depends on many factors, such as the extent of neurological defect, presence of congenital malformations, time to treatment, and level of care. Usually, lower and less severe lesions have a better outcome as compared to higher lesions with hydrocephalus. Patients with lower and smaller lesions can be ambulatory. The majority of patients with myelomeningocele have normal intelligence, although 60% have some learning disabilities 16. Those with higher lesions tend to develop significant hydrocephalus and do not perform well academically. Most children with myelomeningocele require lifelong treatment focused on the damaged spinal cord and nerves. Children are usually followed closely with biannual clinic visits during childhood and annually during adulthood 16.
The life expectancy of patients suffering from spinal dysraphism depends on the size of the lesion. Patients with large complex defects have a shorter life expectancy. According to statistics, 40% to 50% of children with severe defects die as infants. Patients with higher and smaller lesions and no hydrocephalus have a longer life expectancy. Renal failure is the most common cause of death among these patients 16. The life expectancy of these patients has improved greatly with time owing to better healthcare services. However, most of these patients remain dependent on their parents and caretakers even in adulthood 16. Nowadays, the majority of patients with myelomeningocele have a near-normal life expectancy if they do not develop systemic complications.
Living with spinal dysraphism
Spinal dysraphism can range from mild to severe. Some people may have little to no disability. Other people may be limited in the way they move or function. Some people may even be paralyzed or unable to walk or move parts of their body. Even so, with the right care, most people affected by spinal dysraphism lead full, productive lives.
For toddlers and preschoolers with spinal dysraphism, there are many ways that parents and other caregivers can help them become more active and independent, such as:
- Teaching the child about his or her body and about spinal dysraphism.
- Encouraging the child to make choices, such as between two items of clothing.
- Asking the child to help with daily tasks, such as putting away toys.
Children with spinal dysraphism might need extra help at times. But it is very important that children be given the opportunity to complete a task before help is given. It is also important that parents give only the help that is needed rather than helping with the entire task. Parents must learn the difficult balance between giving the right amount of help to increase their child’s independence and confidence, while at the same time being careful not to give the child tasks that cannot reasonably be completed―which might decrease their child’s confidence.
Children with spinal dysraphism higher on the spine (near the head) might have paralyzed legs and use wheelchairs. Those with spinal dysraphism lower on the spine (near the hips) might have more use of their legs and use crutches, braces, or walkers, or they might be able to walk without these devices. A physical therapist can work with adolescents and teens to teach them how to exercise their legs to increase strength, flexibility, and movement. Regular physical activity is important for all children, but especially for those with conditions that affect movement, such as spinal dysraphism.
Young adults affected by spinal dysraphism can face challenges, such as:
- Learning to take care of their own health needs.
- Working.
- Going to school.
- Volunteering.
- Finding and using transportation.
- Living outside their parents’ home.
- Developing healthy relationships.
Many children with spinal dysraphism do well in school. But some can experience difficulties, especially children with shunts that are used to treat hydrocephalus (often called water on the brain). These children often have problems with learning. They might have difficulty paying attention or work slowly, be restless, or lose things. They also might have trouble making decisions. There are activities that children can do at home and at school to help with these problems. Healthcare professionals can provide information about these activities.
- Individualized Education Plan (IEP). An Individualized Education Plan is important because it will help children develop skills at school. Children who participate in special education classes will have an individualized education plan (IEP). An IEP is a legal document that lets the school know what kinds of assistance will be needed by a child during the school day. An individualized education plan (IEP) is created by parents and school personnel, such as a psychologist, teachers, a school nurse, and a physical education teacher, as well as any other professionals that parents think might be helpful.
- 504 Plan. If a child does not qualify for an individualized education plan (IEP), parents can request a 504 Plan be developed for their child at school. Usually, a 504 Plan is used by a general education student who is not eligible for special education services. By law, children may be eligible to have a 504 Plan which lists accommodations related to a child’s disability. The 504 Plan accommodations may be needed to give the child an opportunity to perform at the same level as their peers. For example, a 504 Plan may include the child’s assistive technology needs, such as using a tablet or laptop computer to take notes, and making sure they have a wheelchair accessible environment at school.
- Lucy C. Holmes, Veetai Li; Occult Spinal Dysraphism. Pediatr Rev December 2019; 40 (12): 650–652. https://doi.org/10.1542/pir.2018-0155
- Harwood-Nash DC, McHugh K. Diastematomyelia in 172 children: the impact of modern neuroradiology. Pediatr Neurosurg. 1990-1991;16(4-5):247-51. doi: 10.1159/000120535
- Spina bifida. https://medlineplus.gov/genetics/condition/spina-bifida
- Mukherjee S, Pasulka J. Care for Adults with Spina Bifida: Current State and Future Directions. Top Spinal Cord Inj Rehabil. 2017 Spring;23(2):155-167. doi: 10.1310/sci2302-155
- Phillips LA, Burton JM, Evans SH. Spina Bifida Management. Curr Probl Pediatr Adolesc Health Care. 2017 Jul;47(7):173-177. doi: 10.1016/j.cppeds.2017.06.007
- Rufener SL, Ibrahim M, Raybaud CA, Parmar HA. Congenital spine and spinal cord malformations–pictorial review. AJR Am J Roentgenol. 2010 Mar;194(3 Suppl):S26-37. https://www.ajronline.org/doi/10.2214/AJR.07.7141
- Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: a review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology. 2000 Jul;42(7):471-91. doi: 10.1007/s002340000325
- Weerakkody Y, Yap J, Dughly M, et al. Caudal regression syndrome. Reference article, Radiopaedia.org https://doi.org/10.53347/rID-9580
- Iftikhar W, De Jesus O. Spinal Dysraphism And Myelomeningocele. [Updated 2023 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557722
- Venkataramana NK. Spinal dysraphism. J Pediatr Neurosci. 2011 Oct;6(Suppl 1):S31-40. doi: 10.4103/1817-1745.85707
- Bauer SB, Labib KB, Dieppa RA, Retik AB. Urodynamic evaluation of boy with myelodysplasia and incontinence. Urology. 1977 Oct;10(4):354-62. doi: 10.1016/0090-4295(77)90168-6
- Mehrotra A, Singh S, Gupta S, Pandey S, Sardhara J, Das KK, Bhaisora KS, Srivastava AK, Jaiswal AK, Behari S. Cervicothoracic Spinal Dysraphism: Unravelling the Pandora’s Box. J Pediatr Neurosci. 2019 Oct-Dec;14(4):203-210. doi: 10.4103/jpn.JPN_28_19
- Copp, A., Adzick, N., Chitty, L. et al. Spina bifida. Nat Rev Dis Primers 1, 15007 (2015). https://doi.org/10.1038/nrdp.2015.7
- Spina Bifida. https://www.msdmanuals.com/professional/pediatrics/congenital-neurologic-anomalies/spina-bifida
- Sahni M, Alsaleem M, Ohri A. Meningomyelocele. [Updated 2023 Feb 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536959
- Netto JM, Bastos AN, Figueiredo AA, Pérez LM. Spinal dysraphism: a neurosurgical review for the urologist. Rev Urol. 2009 Spring;11(2):71-81. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2725308
- Avagliano L, Massa V, George TM, Qureshy S, Bulfamante GP, Finnell RH. Overview on neural tube defects: From development to physical characteristics. Birth Defects Res. 2019 Nov 15;111(19):1455-1467. doi: 10.1002/bdr2.1380
- Avagliano, L., Doi, P., Tosi, D., Scagliotti, V., Gualtieri, A., Gaston-Massuet, C., Pistocchi, A., Gallina, A., Marconi, A.M., Bulfamante, G. and Massa, V. (2016), Cell death and cell proliferation in human spina bifida. Birth Defects Research Part A: Clinical and Molecular Teratology, 106: 104-113. https://doi.org/10.1002/bdra.23466
- What is Spina Bifida? https://www.cdc.gov/ncbddd/spinabifida/facts.html
- Copp AJ, Adzick NS, Chitty LS, Fletcher JM, Holmbeck GN, Shaw GM. Spina bifida. Nat Rev Dis Primers. 2015 Apr 30;1:15007. doi: 10.1038/nrdp.2015.7
- Preiksaitiene E, Benušienė E, Ciuladaite Z, Šliužas V, Mikštienė V, Kučinskas V. Recurrent fetal syndromic spina bifida associated with 3q26.1-qter duplication and 5p13.33-pter deletion due to familial balanced rearrangement. Taiwan J Obstet Gynecol. 2016 Jun;55(3):410-4. doi: 10.1016/j.tjog.2016.04.018
- Nau H. Valproic acid-induced neural tube defects. Ciba Found Symp. 1994;181:144-52; discussion 152-60. doi: 10.1002/9780470514559.ch9
- Koren G, Nava-Ocampo AA, Moretti ME, Sussman R, Nulman I. Major malformations with valproic acid. Can Fam Physician. 2006 Apr;52(4):441-2, 444, 447. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1481679
- Spina bifida. https://www.mayoclinic.org/diseases-conditions/spina-bifida/symptoms-causes/syc-20377860
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- Folate. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional
- Yeung LF, Cogswell ME, Carriquiry AL, Bailey LB, Pfeiffer CM, Berry RJ. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children: National Health and Nutrition Examination Survey (NHANES), 2003-2006. Am J Clin Nutr. 2011 Jan;93(1):172-85. doi: 10.3945/ajcn.2010.30127
- Carmel R. Folic acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005:470-81.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.
- Myelomeningocele. https://kidshealth.org/en/parents/myelomeningocele.html
- Health Issues & Treatments for Spina Bifida. https://www.cdc.gov/ncbddd/spinabifida/treatment.html
- Spina Bifida Association. Guidelines for the Care of People with Spina Bifida. 2018. http://www.spinabifidaassociation.org/guidelines
- Spinal Cord Tethering. https://www.spinabifidaassociation.org/wp-content/uploads/Spinal-Cord-Tethering1.pdf
- de Jong TP, Boemers TM, Schouten A, van Gool JD, de Maat-Bleeker F, Bruijnzeel-Koomen CA. Peroperatieve anafylactische reacties op basis van latexallergie [Peroperative anaphylactic reactions due to latex allergy]. Ned Tijdschr Geneeskd. 1993 Sep 18;137(38):1934-6. Dutch.