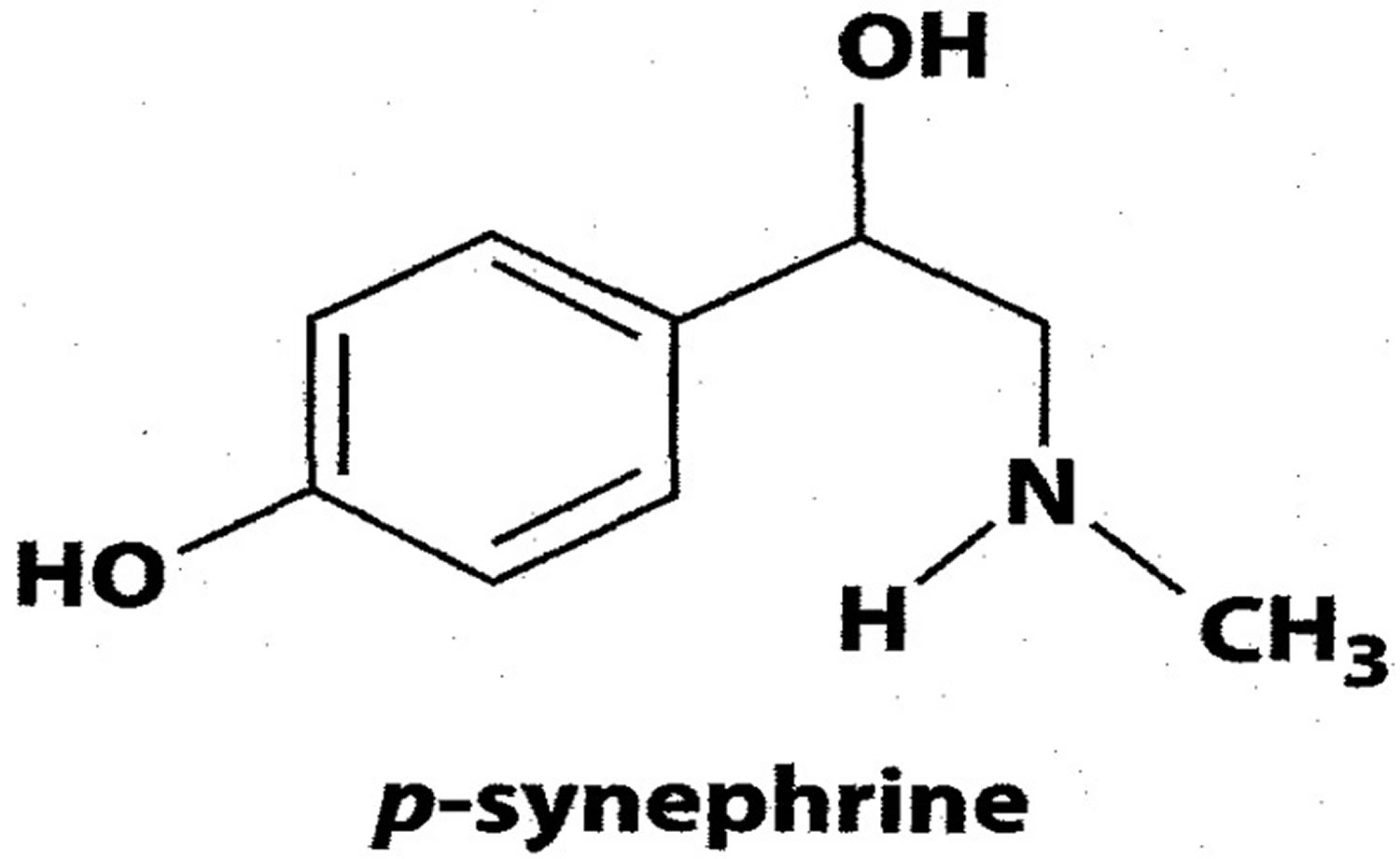
What is synephrine
Synephrine also known as p-synephrine or oxedrine, is naturally occurring alkaloid (phenethylamine alkaloid) in bitter orange extracts (also called Seville orange, sour orange or Citrus aurantium L.) and other citrus species, that is widely used for weight loss or weight management, sports performance, appetite control, energy and mental focus and cognition 1. Bitter orange extracts contain synephrine (p-synephrine), which comprises about 90% or more of the total protoalkaloids, are water and ethanol extracts of the dried immature fruits of bitter orange that are harvested in May and June 2. In China, the immature fruits of bitter orange are also known as Fructus aurantii immaturus 3. Bitter orange synephrine have been used as dietary supplements for approximately 20 years for weight management, energy production, and sports performance, as well as appetite control and energy. Bitter orange has been used in traditional Chinese medicine for indigestion, nausea, and constipation. Today, various bitter orange products are promoted for heartburn, nasal congestion, weight loss, appetite stimulation or suppression, and athletic performance. Bitter orange is also applied to the skin for pain, bruises, fungal infections, and bedsores. Bitter orange is used in cooking and for adding flavor to beer and spirits. At the present time, apart from applying bitter orange oil to the skin which may help with ringworm, jock itch, and athlete’s foot infections, there’s not enough scientific evidence to show whether bitter orange is helpful for other health purposes, such as weight loss, anxiety, and premenstrual syndrome.
In the last few years, some investigations have aimed to determine the efficacy of synephrine to enhance fat utilization during exercise. This knowledge may be essential for those individuals seeking body fat reduction as the participation in regular exercise is one of the best practices to produce effective body composition changes. Understanding the additive action of synephrine intake and exercise may help to integrate synephrine as weight-loss supplement in exercise programs for weight and obesity management 4.
Humans are widely exposed to varied concentrations of synephrine (p-synephrine) on a daily basis from various juices, and food and beverage (orange flavored liqueurs) products from bitter orange, as well as Marrs sweet oranges (Citrus sinensis), grapefruits (Citrus paradisi), mandarins (Citrus reticulata), clementines (Citrus clementina) and other orange‐related species that contain synephrine (p-synephrine) 5. Mandarin oranges juice may contain more than 20 mg and as much as 40 mg synephrine (p-synephrine) per eight fluid oz glass 6
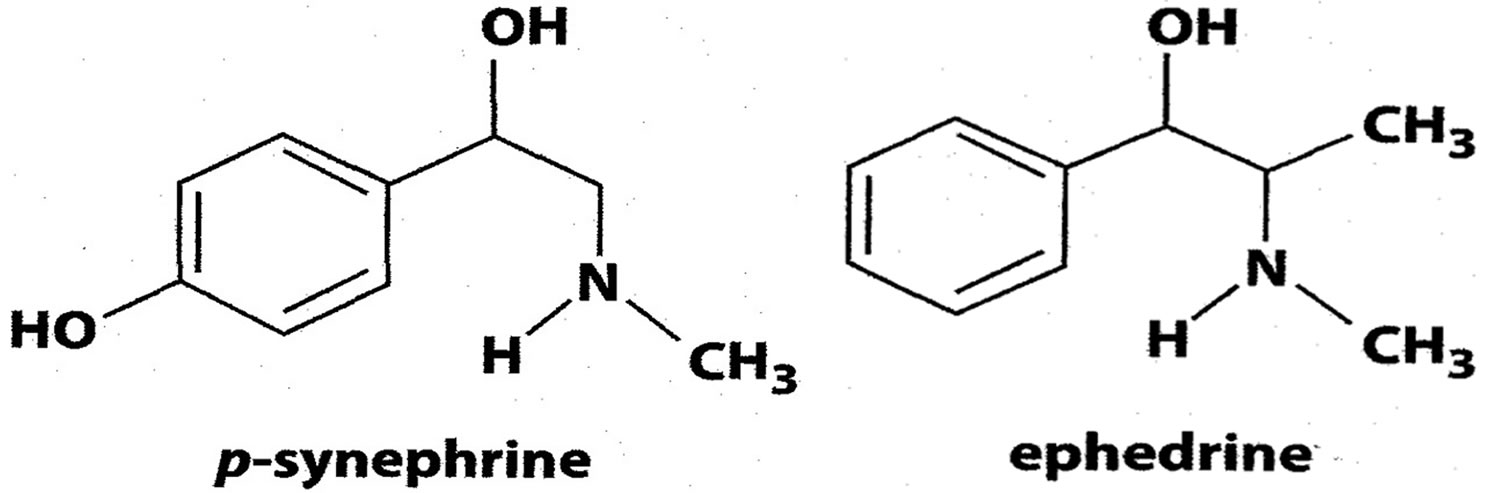
Questions have been raised regarding the safety of synephrine because it has some structural similarity to ephedrine (the main component in the herb ephedra) and synephrine is widely referred to as a stimulant and is assumed to exhibit cardiovascular activity because of its structural similarity to ephedrine (see Figure 1) 7. However, the chemical differences between synephrine and ephedrine greatly alter the stereochemistry, pharmacokinetic, adrenergic receptor binding, and physiological/pharmacological properties 8. Therefore, the effects observed with ephedrine cannot be extrapolated to synephrine (p‐synephrine) and bitter orange extracts. Synephrine has different pharmacologic properties (how the component acts) when compared to ephedrine. The U.S. Food and Drug Administration (FDA) banned the use of Ephedra in dietary supplements because it raises blood pressure and is linked to heart attack and stroke. Synephrine (p-synephrine) became popular as an active ingredient for thermogenics and weight-loss supplements due to the ban of Ephedra species by the U.S. Food and Drug Administration in 2004 9. Several dietary supplements companies substituted Ephedra with bitter orange or p-synephrine because they purportedly have the capacity to increase the metabolic rate at rest and enhance lipolysis 10. It is believed that, in the long term, the chronic ingestion of synephrine (p-synephrine) may reduce fat mass through increased thermogenesis and fat oxidation, although there is no clinical or research evidence to support this notion by using supplements containing only synephrine (p-synephrine) 11. Despite the lack of evidence, synephrine (p-synephrine) is widely present in slimming, weight loss, thermogenics, and supplements for meal replacement and its presence in the market of weight-loss products is comparable to other stimulants with well-contrasted efficacy such as caffeine 12. The National Collegiate Athletic Association (NCAA) has placed “synephrine (bitter orange)” on its current list of banned drugs, listing it as a stimulant 13.
There are structural similarities between synephrine (p‐synephrine) and the catecholamines epinephrine (adrenaline) and norepinephrine (noradrenaline) that contain hydroxyl groups at both the meta and para positions of the benzene ring. Several studies have concluded that it is the hydroxyl group in the meta position of the ring that primarily promotes adrenergic receptor binding and the subsequent cardiovascular effects, while the single hydroxyl group in the para position, as in the case of synephrine, decreases adrenergic receptor binding 14. Therefore, cardiovascular effects observed with epinephrine and norepinephrine cannot be directly extrapolated to synephrine (p‐synephrine) 1.
Figure 1. Structures of synephrine (p‐synephrine) and ephedrine
Footnote: P-synephrine has some structural similarity to ephedrine, from which it differs by the presence of a hydroxyl group in the para position on the benzene ring 5.
Synephrine benefits
The most common commercial source of synephrine (p-synephrine) is bitter orange (Citrus aurantium L.). Various preparations of bitter oranges have been widely used for hundreds of years in foods and folk medicine 15. Bitter orange extracts are now extensively used in dietary supplements and as a flavoring and acidifying agent for food 16. Animal studies have shown that synephrine stimulates beta‐adrenoreceptors, causing thermogenesis and lipolysis 17.
Carpene’ et al. 18 examined the lipolytic activity of a number of potential beta-3 adrenergic receptor agonists including synephrine (p‐synephrine), p‐octopamine and noradrenaline (norepinephrine) in white fat cells from hamsters, rats, dogs, humans and guinea pigs. p‐Octopamine was the most selective for beta‐3 adrenergic receptors, stimulating lipolysis in rat, hamster and dog adipocytes. p‐Octopamine was the only amine the authors studied that fully stimulated lipolysis in rat, hamster and dog fat cells, but was ineffective in human and guinea pig fat cells. Synephrine (p‐synephrine) was partially active in stimulating lipolysis in all species while tyramine, dopamine, and β‐phenylethylamine exhibited no activity. The authors concluded that p‐octopamine was the most selective agonist for β‐3 adrenergic receptors. These studies demonstrated marked differences in adrenergic receptor binding among the various biogenic amines that were assessed.
In a subsequent study, the lipolytic activity of synephrine (p‐synephrine), p‐octopamine, tyramine and N‐methyltyramine were compared in rat and human adipocytes based on β‐3 adrenergic receptor binding 19. In rat fat cells, at a concentration of 10 μg/ml both synephrine (p‐synephrine) and p‐octopamine exhibited approximately 60% of the lipolytic activity of 1 nM/ml of isoprenaline while tyramine and N‐methyltyramine exhibited no effect or were weakly antagonistic. In human adipocytes, 10 μg/ml of both synephrine (p‐synephrine) and p‐octopamine exhibited approximately 10% of the lipolytic activity of 1 μM/ml of isoprenaline. Various studies indicate that N‐methyltyramine acts as an alpha‐adrenergic receptor antagonist while promoting appetite and inhibiting lipolysis, effects counter to those of ephedrine, synephrine (p‐synephrine) and p‐octopamine 20.
An extension of previous studies affirmed that the adrenergic receptor binding of synephrine (p‐synephrine) and p‐octopamine in rodents was at least 10‐fold greater than in humans while tyramine and N‐methyltyramine exhibited no binding activity 21. In fact, half‐maximal lipolysis stimulation was achieved with a 100‐fold lower dose of p‐octopamine in mouse adipocytes as compared to human adipocytes. These results indicate that mice may be much more responsive to p‐octopamine than synephrine (p‐synephrine) and support previous observations that effects produced in rodents at specific doses cannot be directly extrapolated to humans 19. In this study, high concentrations synephrine (p‐synephrine) and p‐octopamine were shown to activate glucose transport in human fat cells.
Several studies have examined the effects of synephrine (p‐synephrine) on carbohydrate metabolism in perfused rat liver 22. Synephrine increased glycogenolysis, glycolysis, oxygen uptake, glucose output and perfusion pressure. These effects were shown to be at least in part mediated by alpha‐ and beta‐adrenergic signaling, while requiring the simultaneous participation of both cAMP and Ca2+ 22. The authors concluded that most of the actions of synephrine (p‐synephrine) were catabolic.
Neuromedin U2 receptor (NMUR2) is present in the hypothalamic regions of the brain and is involved in the regulation of energy balance, food intake, nociception and stress 23. As was demonstrated in NMUR2 negative and short hairpin RNA knockdown HEK293 cell lines, synephrine (p‐synephrine) binds to this receptor with high efficacy and potency. The ability of synephrine (p‐synephrine) to suppress appetite and enhance eating control has been affirmed in humans 24 and animals 25. How well synephrine (p‐synephrine) can cross the blood brain barrier to achieve functional concentrations and bind to NMUR2 has not been specifically determined, nor have studies been reported regarding the ability of ephedrine, m‐synephrine and p‐octopamine to across the blood brain barrier. However, ephedrine can be detected in rat brain following its administration 26 and the neurological effects of ephedrine are well known, thus demonstrating that it is able to cross the blood brain barrier.
In an in vitro study, the effect of synephrine (p‐synephrine) on glucose consumption and its mechanism of action were determined in L6 skeletal muscle cells in culture 27. Synephrine dose‐dependently increased basal glucose consumption by over 50% relative to controls, and had no effect on cell viability. The increased glucose consumption by synephrine (p‐synephrine) involved Glut4‐dependent glucose uptake that in turn was dependent upon synephrine (p‐synephrine) stimulation of AMP‐activated protein kinase phosphorylation.
The effects of synephrine (p‐synephrine) on lipid accumulation and glucose production have been assessed in H411E rat liver cells 28. Synephrine dose‐dependently decreased glucose production, and α‐ and β‐adrenergic receptor antagonists did not alter this effect. These results indicated that the effects of synephrine on gluconeogenesis did not require involvement of adrenergic receptors.
Several studies have demonstrated the anti‐inflammatory activity of synephrine (p‐synephrine). Synephrine suppressed lipopolysaccharide‐induced acute lung injury in mice by reducing the number of inflammatory cells in the lungs, decreasing the levels of reactive species, enhancing superoxide dismutase activity, decreasing tumor necrosis alpha and interleukin‐6 (IL‐6), and increasing IL‐10 29. In normal human fibroblasts and NIH/3 T3 mouse fibroblasts in culture, synephrine inhibited IL‐4‐induced eotaxin‐1 expression through the inhibition of signal transducer and activator of transcription (STAT6) phosphorylation which acts as a signal transducer immediately downstream from IL‐4 (Roh et al., 2014). Eotaxin‐1 is a potent chemoattractant and mediator for eosinophils which are associated with inflammation. STAT6 is critical in activating cytokine gene expression and cytokine signaling in immune and target tissue cells. p‐Synephrine also inhibited eosinophil recruitment induced by eotaxin‐1 overexpression. m‐Synephrine had little effect on eotaxin‐1 induction and therefore little anti‐inflammatory activity. These results indicated that p‐synephrine exerts anti‐inflammatory effects at least in part by inhibiting eotaxin‐1 expression 30. Arbo et al. 25 reported that in mouse livers synephrine exhibited antioxidant and tissue protective activities by enhancing reduced glutathione content, decreasing glutathione peroxidase activity and increasing catalase activity.
Synephrine and weight loss
The use of bitter orange extract and its constituent synephrine for the treatment of obesity in 360 subjects, was reviewed 31. More than 50% of the subjects involved in these clinical studies were overweight and approximately two-thirds of them consumed caffeine (132–528 mg/day) and synephrine (p-synephrine) (10–53 mg/day) 31. Approximately 44% of the subjects used a bitter orange or synephrine (p-synephrine) product, while the remaining consumed a combination product containing multiple ingredients with synephrine (p-synephrine). The results showed that bitter orange extract alone or in combination with other ingredients did not cause significant adverse effects including an increase in heart rate or blood pressure or change in electrocardiographic (ECG) data, serum chemistry, blood cell counts, or urinalysis. Synephrine, alone or in combination products, was demonstrated to enhance metabolic rate and energy expenditure and to promote weight loss when given for six to 12 weeks 32.
The study by Verpeut et al. 33 investigated the effect of the combination of bitter orange (standardized to 6% synephrine) and Rhodiola rosea L. (golden root) (standardized to 3% rosavins and 1% salidroside) on diet-induced obesity in Sprague-Dawley rats. Acute administration of bitter orange (1–10 mg/kg) or Rhodiola rosea (2–20 mg/kg) alone did not decrease food intake in normal weight animals; however, the combination of bitter orange (5.6 mg/kg) and Rhodiola rosea (20 mg/kg) provided a 10.5% feeding suppression. On the other hand, 10 days of treatment with bitter orange (5.6 mg/kg) or Rhodiola rosea (20 mg/kg) alone, or in combination, to the animals fed on a high-fat diet (60% fat) during the 13-week period led to a 30% decline in visceral fat weight, compared with other treatments 33. Coadministration of bitter orange and Rhodiola rosea also resulted in an elevation in hypothalamic norepinephrine and frontal cortex dopamine, indicating the beneficial role of bitter orange and Rhodiola rosea in the treatment of obesity 33.
Bitter orange extracts’ efficacy in inducing weight loss has been shown in some clinical studies 34 and literature revisions including studies conducted on subjects who received products containing synephrine (p-synephrine) alone or in combination with other supplements concluded that the consumption of this dietary supplement is overall safe and may induce modest weight loss 35. The most recent review, involving approximately 30 human studies and over 600 subjects, have confirmed that evidences supporting the anti-obesogenic role of bitter orange extracts are limited and uncertain, as they are often studied in combination with other molecules. Synephrine (p-synephrine) does not appear to produce cardiovascular effects at doses up to 100 mg 36. Human clinical trials evaluating the effects of bitter orange extracts on weight outcomes have not been published since 2016, although some recent preclinical study showed that Citrus peel extracts attenuated obesity and modulated gut microbiota in a high-fat diet-induced obesity mice 37 and regulated in vitro adipogenesis and thermogenesis via AMPK activation 38.
Bitter orange might slightly increase the number of calories you burn. It might also reduce your appetite a little, but whether it can help you lose weight is unknown. At the moment, bitter orange should not be recommended for the treatment of obesity as the quality of evidence supporting its application for this purpose is low 39.
Synephrine dosage
The acute intake of synephrine (p-synephrine) in a dose of 2–3 mg/kg of body mass, has been effective to enhance the rate of fat oxidation when combined with exercise programs (during incremental and continuous exercise) 11. Although the magnitude of the effect synephrine (p-synephrine) on fat oxidation during exercise is small (increase of fat oxidation ~0.1 g/min), the use of synephrine (p-synephrine) containing supplements may be an option to maximize fat oxidation. However, more research is necessary to determine if the effect of synephrine (p-synephrine) on fat oxidation during exercise is maintained with chronic ingestion, in order to ascertain the utility of this substance in conjunction with exercise programs to produce an effective body fat/weight loss reduction. Lastly, more research is also necessary to ascertain whether the lower carbohydrate use with synephrine produces significant glycogen sparing that may be effective to enhance performance in endurance sports competition.
Synephrine side effects
Bitter orange is likely safe when used orally in amounts commonly found in foods. Studies in humans indicate that the synephrine has a wide margin of safety with an oral Lethal Dose 50 (LD50) greater than 2500 mg/kg in rats 1. LD50 is the amount of a material, given all at once, which causes the death of 50% (one half) of a group of test animals. The LD50 is one way to measure the short-term poisoning potential (acute toxicity) of a material. Approximately 30 human studies involving over 600 subjects have demonstrated that synephrine does not produce cardiovascular effects at doses up to 100 mg. Approximately 45% of the subjects in these studies were overweight or obese and over 40% of the subjects consumed caffeine in conjunction with bitter orange extract (synephrine) 1. Synephrine binds up to 10 times more readily to adrenergic receptors in rodents than humans 40, which can explain small cardiovascular effects in some animal studies at very high doses 41.
It has been recommended that synephrine and bitter orange extract not be used under a variety of conditions 42, 43, 44. The majority of these contraindicated conditions are based on the assumption that synephrine exhibits cardiovascular effects, and these warnings have been extrapolated from warnings that were associated with the use of ephedra products 1. The contraindicated conditions that have been proposed include individuals with high blood pressure, thyroid, kidney, liver, or heart disease; people with psychiatric or epileptic disorders; those using antihypertensive drugs, sympathomimetics, monoamine oxidase inhibitors, thyroid medications, and antidepressants; and children and women who are pregnant or breast feeding. No evidence exists to support these proposed contraindications 1.
Very large numbers of people consume synephrine on a daily basis in the forms of citrus juices and foods as well as dietary supplements with no known or apparent adverse effects 1. There are no indications that synephrine adversely affects the heart, liver, kidneys, or thyroid at doses up to 100 mg per day 45 and as a consequence, there is no evidence supporting the proposed contraindications. Furthermore, studies have shown that no teratogenicity 46 or mutagenicity 47 occurs in response to synephrine and bitter orange extracts. However, some cautionary statements regarding use of bitter orange extract and synephrine may be warranted and are subject to further discussions.
There is one case report of a woman having a faster-than-normal heart rate at rest after taking a dietary supplement that contained only bitter orange. There are other case reports of healthy people experiencing fainting, angina, heart attack, and stroke after taking bitter orange as part of multi-component products. However, because these products contained multiple ingredients, it is difficult to know the role that bitter orange played. Evidence regarding the effects of bitter orange (alone or combined with other substances, such as caffeine and green tea) on the heart and cardiovascular system is inconclusive. Some studies showed that bitter orange raised blood pressure and heart rate, but other studies showed that bitter orange didn’t have this effect at commonly used doses. Some sources list bitter orange as a stimulant whereas other sources say that it’s not a stimulant at commonly used doses. Little is known about whether it’s safe to use bitter orange during pregnancy or while breastfeeding.
Effects on the cardiovascular system
The cardiovascular toxicity of bitter orange extracts with different concentrations of synephrine (4 and 6%) was reported by Calapai et al. in 1999 in rats 48. Authors showed an antiobesity effect by the administration of bitter orange but also possible cardiovascular toxicity. The cardiovascular effects have not been confirmed by studies using much higher doses of synephrine 48.
Another study was carried out to evaluate the cardiovascular effects of different doses of bitter orange and pure synephrine in rats. Bitter orange extract and pure synephrine enhanced the heart rate and blood pressure. Higher activities were obtained with bitter orange extract than synephrine, suggesting that other compounds in the extract can alter physiological parameters 49. The effect of synephrine on heart rate and blood pressure in female Sprague-Dawley rats was assessed. During 28 days, two types of extracts, one of which contained 6% and other 95% synephrine, were administered daily by gavage at 10 or 50 mg/kg, doses for a 60 kg human equal to 600 and 3000 mg/kg. The outcome of the study showed that both synephrine and bitter orange extract resulted in clinically insignificant increases in heart rate and blood pressure at doses many times greater than used in humans. Synephrine and bitter orange extract exhibited little or no effect on the cardiovascular effects of caffeine 49.
Safety of synephrine was investigated by Ratamess et al. 50 on humans, animals, and in vitro. Authors reported over 30 human studies indicating that the cardiovascular effects of synephrine and bitter orange extracts are clinically insignificant 50. Synephrine showed a greater ability to bind adrenergic receptors in rodents than in humans, and the data in literature on its effects on animals cannot be indicative for men at commonly used doses 50.
This review 50 concluded that bitter orange extract and synephrine are safe for use in dietary supplements and foods at commonly used doses. Also, other authors reported similar conclusions 36, 51. While synephrine alone seems to have low toxicity, when it is formulated in combination with other ingredients such as caffeine (Paullinia cupana, Cola nitida, Cola acuminata, and Camellia sinensis), salicin (Salix sp.), and ephedrine (ma huang, Ephedra sinica, and Ephedra sp.) in weight loss products, the mixture could induce some cardiovascular effects. However, the study did not demonstrate that synephrine contributed to cardiovascular effects.
Schmitt et al. 52 reported clear signs of toxicity of this mixture in mice of both sexes as reduction in locomotor activity, ptosis, seizures, salivation, agitation, piloerection, and deaths after acute oral administration of 300, 350, and 400 mg/kg total of synephrine, ephedrine, salicin, plus caffeine in a 10 : 4 : 6 : 80 w/w ratio.
References- Stohs S. J. (2017). Safety, Efficacy, and Mechanistic Studies Regarding Citrus aurantium (Bitter Orange) Extract and p-Synephrine. Phytotherapy research : PTR, 31(10), 1463–1474. https://doi.org/10.1002/ptr.5879
- Carpene’ MA, Testar X, Carpene’ C. 2014. High doses of synephrine and octopamine activate lipolysis in human adipocytes, indicating that amines from citrus might influence adiposity In Citrus, Hayat K. (ed). Nova Science Publishers Inc. Chapter 8; 141–168.
- Chen JK, Chen TT. 2004. Zhi Shi (Fructus aurantii immaturus) In Chinese Medical Herbology and Pharmacology. Art of Medicine Press: City of industry, CA; 485.
- Keating SE, Johnson NA, Mielke GI, Coombes JS. A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity. Obes Rev. 2017 Aug;18(8):943-964. doi: 10.1111/obr.12536
- Stohs, S. J., Shara, M., & Ray, S. D. (2020). p-Synephrine, ephedrine, p-octopamine and m-synephrine: Comparative mechanistic, physiological and pharmacological properties. Phytotherapy research : PTR, 34(8), 1838–1846. https://doi.org/10.1002/ptr.6649
- Uckoo RM, Jayaprakasha GK, Nelson SD, Patil BS. Rapid simultaneous determination of amines and organic acids in citrus using high-performance liquid chromatography. Talanta. 2011 Jan 15;83(3):948-54. doi: 10.1016/j.talanta.2010.10.063
- Bakhiya N, Ziegenhagen R, Hirsch‐Ernst KI, et al. 2017. Phytochemical compounds in sports medicine: synephrine and hydroxycitric acid (HCA) as examples for evaluation of possible health risks. Mol Mutr Food Res. https://doi.org/10.1002/mnfr.201601020
- Stohs SJ, Preuss HG, Shara M. 2011b. A review of the receptor‐binding properties of p‐synephrine as related to its pharmacological effects. Oxid Med Cell Longev 2011: 1–9.
- Ephedra. https://ods.od.nih.gov/HealthInformation/Ephedra.aspx
- Gutiérrez-Hellín J, Del Coso J. Acute p-synephrine ingestion increases fat oxidation rate during exercise. Br J Clin Pharmacol. 2016 Aug;82(2):362-8. doi: 10.1111/bcp.12952
- Ruiz-Moreno, C., Del Coso, J., Giráldez-Costas, V., González-García, J., & Gutiérrez-Hellín, J. (2021). Effects of p-Synephrine during Exercise: A Brief Narrative Review. Nutrients, 13(1), 233. https://doi.org/10.3390/nu13010233
- Müller LS, Moreira APL, Muratt DT, Viana C, de Carvalho LM. An Ultra-High Performance Liquid Chromatography-Electrospray Tandem Mass Spectrometric Method for Screening and Simultaneous Determination of Anorexic, Anxiolytic, Antidepressant, Diuretic, Laxative and Stimulant Drugs in Dietary Supplements Marketed for Weight Loss. J Chromatogr Sci. 2019 Jul 1;57(6):528-540. doi: 10.1093/chromsci/bmz025
- 2020-21 NCAA Banned Substances. https://www.ncaa.org/sport-science-institute/topics/2020-21-ncaa-banned-substances
- Ma G, Bavadekar SA, Schaneberg BT, Khan IA, Feller DR. Effects of synephrine and beta-phenethylamine on human alpha-adrenoceptor subtypes. Planta Med. 2010 Jul;76(10):981-6. doi: 10.1055/s-0029-1240884
- Stohs S.J., Shara M. Review of the safety and efficacy of bitter orange (Citrus aurantium) and its primary protoalkaloid, p-synephrine, in weight management. In: Bagchi D., Preuss H.G., editors. Obesity: Epidemiology, Pathophysiology, and Prevention. 2nd ed. CRC Press; Boca Raton, FL, USA: 2007. pp. 535–554.
- Karabıyıklı Ş., Değirmenci H., Karapınar M. Inhibitory effect of sour orange (Citrus aurantium) juice on Salmonella Typhimurium and Listeria monocytogenes. 2014;55(2):421–425. doi: 10.1016/j.lwt.2013.10.037
- Stohs SJ, Preuss HG, Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res. 2011 Oct;25(10):1421-8. doi: 10.1002/ptr.3490
- Carpéné C, Galitzky J, Fontana E, Atgié C, Lafontan M, Berlan M. Selective activation of beta3-adrenoceptors by octopamine: comparative studies in mammalian fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1999 Apr;359(4):310-21. doi: 10.1007/pl00005357
- Mercader, J. , Wanecq, E. , Chen, J. , & Carpene, C. (2011). Isonorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium . Journal of Physiology and Biochemistry, 67, 443–452. 10.1007/s13105-011-0078-2
- Stohs, S. J. , & Hartman, M. J. (2015). A review of the receptor binding and pharmacological effects of N‐methyltyramine. Phytotherapy Research, 29, 14–16.
- Carpene’, M. A. , Testar, X. , & Carpene’, C. (2014). High doses of synephrine and octopamine activate lipolysis in human adipocytes, indicating that amines from Citrus might influence adiposity In Hayat K. (Ed.), Citrus (pp. 141–168). Hauppauge, NY USA: Nova Science Publishers Inc. Chapter 8.
- de Oliveira, A. L. , Comar, J. F. , de Sa‐Nakanishi, A. B. , Peralta, R. M. , & Bracht, A. (2014). The action of p‐synephrine on hepatic carbohydrate metabolism and respiration occurs via both Ca(2+)‐mobilization and cAMP production. Molecular and Cellular Biochemistry, 388, 135–147.
- Zheng, X. , Guo, L. , Wang, D. , & Deng, X. (2014). p‐Synephrine: A novel agonist of neuromedin U2 receptor. Biological and Pharmaceutical Bulletin, 37, 764–770.
- Kaats, G. R. , Leckie, R. B. , Mrvichin, N. , & Stohs, S. J. (2017). Increased eating control and energy levels associated with consumption of a bitter orange (p‐synephrine) extract chew ‐ a randomized placebo‐controlled study. Nutrition and Dietary Supplements, 9, 29–35.
- Arbo, M. D. , Schmitt, G. C. , Limberger, M. F. , Charão, M. F. , Moro, A. M. , Ribeiro, G. L. , … Limberger, R. P. (2009). Subchronic toxicity of Citrus aurantium L (Rutaceae) extract and p‐synephrine in mice. Regulatory Toxicology and Pharmacology, 54, 114–117.
- Song, Y. , Su, D. , Lu, T. , Mao, C. , Ji, D. , Liu, Y. , … Fan, R. (2014). Differential pharmacokinetics of the brain distribution of morphine and ephedrine constitutional isomers in rats after administration with keke capsule using rapid‐resolution LC‐MS/MS. Journal of Separation Science, 37, 352–359.
- Hong, N. Y. , Cui, Z. G. , Kang, H. K. , Lee, D. H. , Lee, Y. K. , & Park, D. B. (2012). p‐Synephrine stimulates glucose consumption via AMPK in L6 skeletal muscle cells. Biochemical and Biophysical Research Communications, 418, 720–724.
- Cui, Z. , Lee, Y. , Lee, Y. , & Park, D. (2014). p‐Synephrine suppresses glucose production but not lipid accumulation in H4IIE liver cells. Med. Food, 18, 1–7.
- Wu, Q. , Li, R. , Soromou, L. W. , Chen, N. , Yuan, X. , Sun, G. , … Feng, H. (2014). p‐Synephrine suppresses lipopolysaccharide‐induced acute lung injury by inhibition of the NF‐κB signaling pathway. Inflammation Research, 63, 429–439.
- Roh, K. B. , Kim, I. H. , Kim, Y. S. , Lee, M. , Lee, J. A. , Jung, E. , & Park, D. (2014). Synephrine inhibits eotaxin‐1 expression via the STAT6 signaling pathway. Molecules, 19, 11883–11895.
- Suntar, I., Khan, H., Patel, S., Celano, R., & Rastrelli, L. (2018). An Overview on Citrus aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxidative medicine and cellular longevity, 2018, 7864269. https://doi.org/10.1155/2018/7864269
- Stohs S. J., Preuss H. G., Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. 2012;9(7):527–538. doi: 10.7150/ijms.4446
- Verpeut, J. L., Walters, A. L., & Bello, N. T. (2013). Citrus aurantium and Rhodiola rosea in combination reduce visceral white adipose tissue and increase hypothalamic norepinephrine in a rat model of diet-induced obesity. Nutrition research (New York, N.Y.), 33(6), 503–512. https://doi.org/10.1016/j.nutres.2013.04.001
- Cho YG, Jung JH, Kang JH, Kwon JS, Yu SP, Baik TG. Effect of a herbal extract powder (YY-312) from Imperata cylindrica Beauvois, Citrus unshiu Markovich, and Evodia officinalis Dode on body fat mass in overweight adults: a 12-week, randomized, double-blind, placebo-controlled, parallel-group clinical trial. BMC Complement Altern Med. 2017 Jul 28;17(1):375. doi: 10.1186/s12906-017-1871-4
- Stohs SJ, Preuss HG, Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int J Med Sci. 2012;9(7):527-38. doi: 10.7150/ijms.4446
- Stohs SJ. Safety, Efficacy, and Mechanistic Studies Regarding Citrus aurantium (Bitter Orange) Extract and p-Synephrine. Phytother Res. 2017 Oct;31(10):1463-1474. doi: 10.1002/ptr.5879
- Tung YC , Chang WT , Li S , Wu JC , Badmeav V , Ho CT , Pan MH . Citrus peel extracts attenuated obesity and modulated gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2018 Jun 20;9(6):3363-3373. doi: 10.1039/c7fo02066j
- Park J, Kim HL, Jung Y, Ahn KS, Kwak HJ, Um JY. Bitter Orange (Citrus aurantium Linné) Improves Obesity by Regulating Adipogenesis and Thermogenesis through AMPK Activation. Nutrients. 2019 Aug 22;11(9):1988. doi: 10.3390/nu11091988
- Watanabe, M., Risi, R., Masi, D., Caputi, A., Balena, A., Rossini, G., Tuccinardi, D., Mariani, S., Basciani, S., Manfrini, S., Gnessi, L., & Lubrano, C. (2020). Current Evidence to Propose Different Food Supplements for Weight Loss: A Comprehensive Review. Nutrients, 12(9), 2873. https://doi.org/10.3390/nu12092873
- Mercader J, Wanecq E, Chen J, Carpene’ C. 2011. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium . J Physiol Biochem 67: 442–452.
- Hansen DK, George NI, White GE, Abdel-Rahman A, Pellicore LS, Fabricant D. Cardiovascular toxicity of Citrus aurantium in exercised rats. Cardiovasc Toxicol. 2013 Sep;13(3):208-19. doi: 10.1007/s12012-013-9199-x
- Marles R. 2011. Synephrine, octopamine and caffeine health risk assessment (HRA) report. Health Canada Natural Health Products Directorate, File No. 172091, May. pp. 1–49.
- Lynch B. 2013. Review of the safety of p‐synephrine and caffeine. Intertek‐Cantox Report, April. pp. 1–20.
- Natural Medicines Comprehensive Database 2016. Bitter orange. https://naturalmedicines.therapeuticresearch.com
- Kaats GR, Stohs SJ. 2017. Increased eating control and energy levels associated with consumption of a bitter orange (p‐synephrine) extract chew—a randomized placebo controlled study. Nutr Diet Suppl 9: 29–35.
- Hansen DK, Juliar BE, White GE, Pellicore LS. Developmental toxicity of Citrus aurantium in rats. Birth Defects Res B Dev Reprod Toxicol. 2011 Jun;92(3):216-23. doi: 10.1002/bdrb.20308
- Deshmukh NS, Stohs SJ, Magar CC, Kale A, Sowmya B. Bitter orange (Citrus aurantium L.) extract subchronic 90-day safety study in rats. Toxicol Rep. 2017 Nov 12;4:598-613. doi: 10.1016/j.toxrep.2017.11.002
- Calapai G., Firenzuoli F., Saitta A., et al. Antiobesity and cardiovascular toxic effects of Citrus aurantium extracts in the rat: a preliminary report. 1999;70(6):586–592. doi: 10.1016/S0367-326X(99)00093-3
- Hansen DK, George NI, White GE, Pellicore LS, Abdel-Rahman A, Fabricant D; Food and Drug Administration. Physiological effects following administration of Citrus aurantium for 28 days in rats. Toxicol Appl Pharmacol. 2012 Jun 15;261(3):236-47. doi: 10.1016/j.taap.2012.04.006
- Ratamess NA, Bush JA, Stohs SJ, Ellis NL, Vought IT, O’Grady EA, Kuper JD, Hasan SB, Kang J, Faigenbaum AD. Acute cardiovascular effects of bitter orange extract (p-synephrine) consumed alone and in combination with caffeine in human subjects: A placebo-controlled, double-blind study. Phytother Res. 2018 Jan;32(1):94-102. doi: 10.1002/ptr.5953
- Penzak SR, Jann MW, Cold JA, Hon YY, Desai HD, Gurley BJ. Seville (sour) orange juice: synephrine content and cardiovascular effects in normotensive adults. J Clin Pharmacol. 2001 Oct;41(10):1059-63. doi: 10.1177/00912700122012652
- Schmitt GC, Arbo MD, Lorensi AL, Maciel ES, Krahn CL, Mariotti KC, Dallegrave E, Leal MB, Limberger RP. Toxicological effects of a mixture used in weight loss products: p-synephrine associated with ephedrine, salicin, and caffeine. Int J Toxicol. 2012 Mar;31(2):184-91. doi: 10.1177/1091581811435708