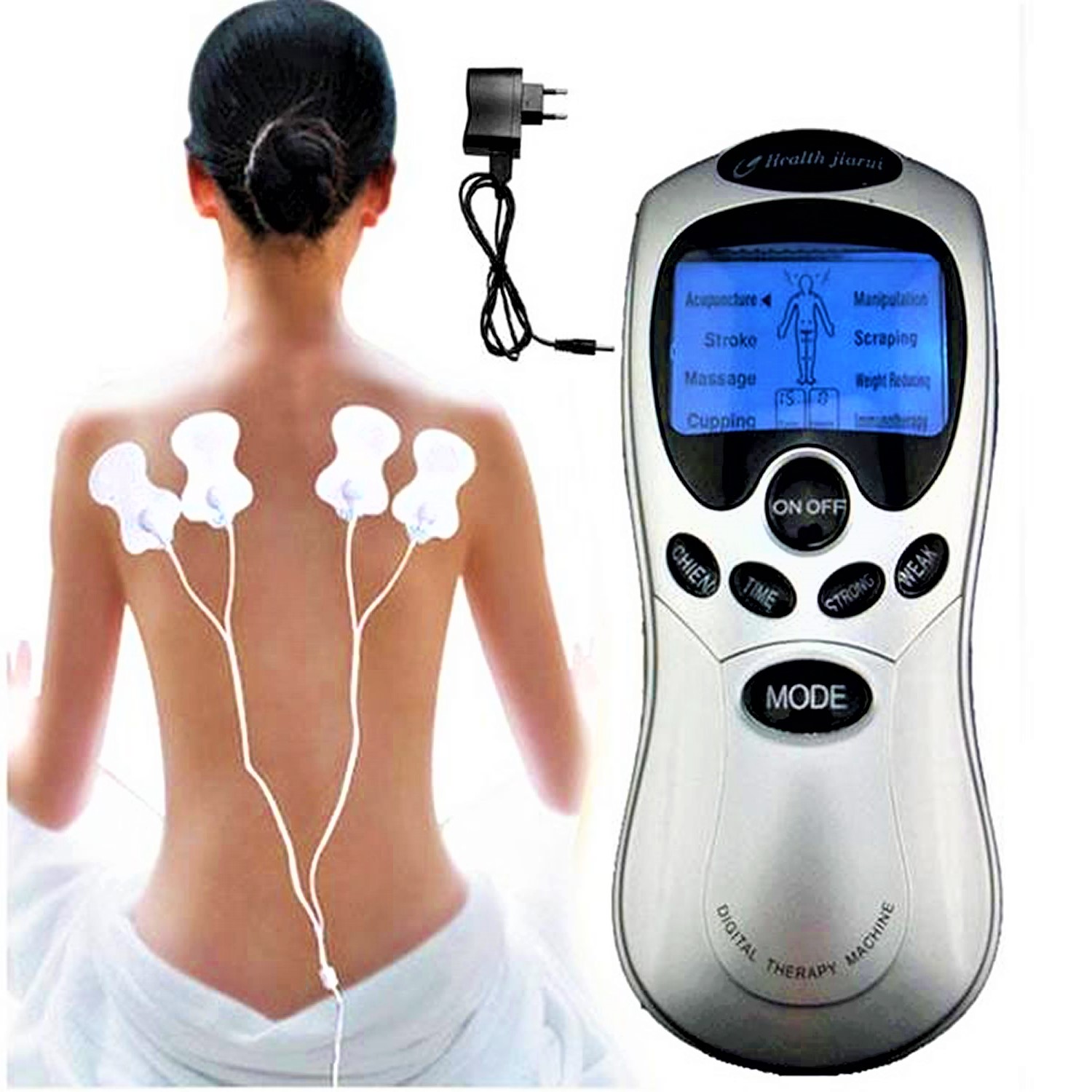
What is a TENS unit
TENS is the abbreviation for transcutaneous electrical nerve stimulation and TENS is also called electrotherapy. Transcutaneous means across the skin. A TENS unit passes electricity across your skin to stimulate your nerves and relieve your pain 1. TENS unit consists of a battery operated stimulator (about the size of your palm), lead wires and 2 or more electrodes (pads) which stick to your skin. By adjusting control knobs on the stimulator you are able to start or stop the electrical impulses and you can vary the type and intensity of each electrical impulse. The electrical current, which produces a mild tingling sensation, travels from the TENS unit through the lead wires to the electrodes which are placed near the painful areas. The exact electrode placement may be anywhere along this path, but often 1 pair of electrodes is located either at the pain site or near the spine where the nerve pathway connects to the spinal cord. A physiotherapist can show you how to correctly place the electrodes and then trial it to see if it works for you.
TENS therapy is a method of pain relief that is used to treat localized or regional pain. During TENS (transcutaneous electrical nerve stimulation) therapy, a TENS machine delivers a small electrical current to nearby nerve pathways through electrodes attached to your skin — which can help control or relieve some types of pain. TENS is often used to treat osteoarthritis, arthritis, migraine headaches, back and neck injuries, pulled muscles, and postoperative pain. TENS machine is also used for people with chronic pain or women in labor. TENS has been used in childbirth since the 1970s 2.
A TENS unit runs on batteries. You put small electrodes on your skin, and the electrodes are connected to the TENS machine. The machine sends pulses of gentle electric current to the electrodes. The current stimulates the nerves near your pain.
Scientists aren’t clear how TENS works. It’s possible that TENS blocks pain signals by stimulating different nerves in your spinal cord. TENS might also cause the release of endorphins – the body’s natural pain relievers. Some people find TENS gives some pain relief. TENS therapy uses no medicines, no needles and no injections.
TENS interventions tend to be described according to technical characteristics as either high frequency, low intensity (conventional TENS) or low frequency, high intensity (acupuncture-like TENS, AL-TENS). The physiological intention when administering conventional TENS is to activate selectively non-noxious low threshold afferent nerve fibers in the skin (Aβ-fibres) which are claimed to inhibit transmission of nociceptive information at the level of the spinal cord (i.e. segmental modulation) 3. In practice, Aβ nerve fiber activity is recognized by the user reporting strong electrical paresthesia (pins and needles) beneath the electrodes. The physiological intention of acupuncture-like TENS (AL-TENS) is to generate a muscle twitch which is believed to increase activity in small diameter afferent nerve fibers in muscles (Aδ) leading to activation of descending pain inhibitory pathways. In practice, acupuncture-like TENS (AL-TENS) is achieved by administering low frequency and high intensity, but non-painful, currents over muscles 4. Interestingly, experimental evidence to establish the roles of different afferent fibers in TENS outcome is inconclusive 5.
Healthcare professionals have reported that TENS seems to help some people, although how well TENS works depends on the individual and the condition being treated.
TENS isn’t a cure for pain and often only provides short-term relief while the TENS machine is being used.
However, the treatment is generally very safe and you may feel it’s worth trying instead of, or in addition to, the usual medical treatments.
If you’re thinking about trying TENS, it’s a good idea to speak to your doctor about a referral to a physiotherapist or pain clinic.
A physiotherapist or pain specialist may be able to loan you a TENS machine for a short period if they think it could help.
You can choose to buy your own TENS machine without getting medical advice, but it’s generally better to have a proper assessment first, so you can find out whether a TENS machine is appropriate for you and be taught how to use it properly.
To get the most benefit from TENS, it’s important that the settings are adjusted correctly for you and your individual condition.
If you find TENS effective, you can buy a TENS machine from a pharmacy. They range in price from about $15 to $200. More expensive machines aren’t necessarily any better than lower-priced ones, so it’s best to do some research before you buy.
Figure 1. TENS unit
TENS unit benefits
TENS first received serious consideration from the medical community in the 1960’s. At that time surgeons started implanting electrodes in back pain sufferers. The doctors soon discovered that electrodes taped to the skin produced similar pain relief. A few years later the first commercial TENS unit was introduced.
TENS has not become widely used in the medical community. In fact many doctors have never tried TENS. However the method is widely used by health care professionals whose primary job is to treat patients with pain.
TENS has been used to control acute and chronic pain in a wide variety of cases. TENS can give pain relief in labor. TENS unit is also used for chronic pain in people who have conditions such as cancer or arthritis. Physiotherapists sometime use it to treat muscle pain.
What TENS is used for
TENS may be able to help reduce pain and muscle spasms caused by a wide range of conditions including:
- arthritis
- period pain
- knee pain
- neck pain
- back pain
- sports injuries
It’s also sometimes used as a method of pain relief during labor.
The Spinal cord
The spinal cord consists of thirty-one segments, each of which gives rise to a pair of spinal nerves. Although the spinal cord is not visibly segmented, the part supplied by each pair of nerves is called a segment. The cord exhibits longitudinal grooves on its anterior and posterior sides—the anterior median fissure and posterior median sulcus, respectively. These nerves (part of the peripheral nervous system) branch to various body parts and connect them with the central nervous system.
The spinal cord is divided into cervical, thoracic, lumbar, and sacral regions. It may seem odd that it has a sacral region when the cord itself ends well above the sacrum. These regions, however, are named for the level of the vertebral column from which the spinal nerves emerge, not for the vertebrae that contain the cord itself.
In two areas, the spinal cord is a little thicker than elsewhere. In the inferior cervical region, a cervical enlargement gives rise to nerves of the upper limbs. In the lumbosacral region, there is a similar lumbar enlargement that issues nerves to the pelvic region and lower limbs. Inferior to the lumbar enlargement, the cord tapers to a point called the medullary cone. Arising from the lumbar enlargement and medullary cone is a bundle of nerve roots that occupy the vertebral canal from L2 (lumbar vertbra L2) to S5 (sacral vertbra S5). This bundle, named the cauda equina for its resemblance to a horse’s tail, innervates the pelvic organs and lower limbs.
Figure 2. Spinal cord segments
Spinal Nerves
A nerve is a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue. If you compare a nerve fiber to a wire carrying an electrical current in one direction, a nerve would be comparable to an electrical cable composed of thousands of wires carrying currents in opposite directions. A nerve contains anywhere from a few nerve fibers to (in the optic nerve) a million. Nerves usually have a pearly white color and resemble frayed string as they divide into smaller and smaller branches. As we move away from the spinal nerves proper, the smaller branches are called peripheral nerves, and their disorders are collectively called peripheral neuropathy.
There are 31 pairs of spinal nerves: 8 cervical (C1–C8), 12 thoracic (T1–T12), 5 lumbar (L1–L5), 5 sacral (S1–S5), and 1 coccygeal (Co1). The first cervical nerve emerges between the skull and atlas, and the others emerge through intervertebral foramina, including the anterior and posterior foramina of the sacrum and the sacral hiatus. Thus, spinal nerves C1 through C7 emerge superior to the correspondingly numbered vertebrae (nerve C5 above vertebra C5, for example); nerve C8 emerges inferior to vertebra C7; and below this, all the remaining nerves emerge inferior to the correspondingly numbered vertebrae (nerve L3 inferior to vertebra L3, for example).
Proximal Branches
Each spinal nerve arises from two points of attachment to the spinal cord. In each segment of the cord, six to eight nerve rootlets emerge from the anterior surface and converge to form the anterior (ventral) root of the spinal nerve. Another six to eight rootlets emerge from the posterior surface and converge to form the posterior (dorsal) root. A short distance away from the spinal cord, the posterior root swells into a posterior (dorsal) root ganglion, which contains the somas (neuron bodies) of sensory neurons. There is no corresponding ganglion on the anterior root.
Slightly distal to the ganglion, the anterior and posterior roots merge, leave the dural sheath, and form the spinal nerve proper. The nerve then exits the vertebral canal through the intervertebral foramen. The spinal nerve is a mixed nerve, carrying sensory signals to the spinal cord by way of the posterior root and ganglion, and motor signals out to more distant parts of the body by way of the anterior root.
The anterior and posterior roots are shortest in the cervical region and become longer inferiorly. The roots that arise from segments L2 to Co1 of the cord form the cauda equina. Some viruses can invade the CNS by way of the spinal nerve roots (e.g varicella-zoster virus of shingles and herpes simplex virus of core sores or genital herpes).
Figure 3. Spinal nerve
Distal Branches
Distal to the vertebrae, the branches of a spinal nerve are more complex. Immediately after emerging from the intervertebral foramen, the nerve divides into an anterior ramus, posterior ramus, and a small meningeal branch. Thus, each spinal nerve branches on both ends—into anterior and posterior roots approaching the spinal cord, and anterior and posterior rami leading away from the vertebral column.
The meningeal branch reenters the vertebral canal and innervates the meninges, vertebrae, and spinal ligaments with sensory and motor fibers. The posterior ramus innervates the muscles and joints in that region of the spine and the skin of the back. The larger anterior ramus innervates the anterior and lateral skin and muscles of the trunk, and gives rise to nerves of the limbs.
The anterior ramus differs from one region of the trunk to another. In the thoracic region, it forms an intercostal nerve, which travels along the inferior margin of a rib and innervates the skin and intercostal muscles (thus contributing to breathing). Sensory fibers of the intercostal nerve branches to the skin are the most common routes of viral migration in the painful disease known as shingles. Motor fibers of the intercostal nerves innervate the internal oblique, external oblique, and transverse abdominal muscles. All other anterior rami form the nerve plexuses.
The anterior ramus also gives off a pair of communicating rami, which connect with a string of sympathetic chain ganglia alongside the vertebral column. These are seen only in spinal nerves T1 through L2. They are components of the sympathetic nervous system.
Figure 4. Rami of the spinal nerve
Figure 5. Spinal nerve fiber anatomy
If a nerve resembles a thread, a ganglion resembles a knot in the thread. A ganglion is a cluster of neurosomas outside the central nervous system. It is enveloped in an epineurium continuous with that of the nerve. Among the neurosomas are bundles of nerve fibers leading into and out of the ganglion. Figure 9 shows a type of ganglion associated with the spinal nerves.
Figure 6. Spinal nerve ganglion
 Footnote: The posterior root ganglion contains the somas of unipolar sensory neurons conducting signals from peripheral sense organs toward the spinal cord. Below this is the anterior root of the spinal nerve, which conducts motor signals away from the spinal cord, toward peripheral effectors. Note that the anterior root is not part of the ganglion.
Footnote: The posterior root ganglion contains the somas of unipolar sensory neurons conducting signals from peripheral sense organs toward the spinal cord. Below this is the anterior root of the spinal nerve, which conducts motor signals away from the spinal cord, toward peripheral effectors. Note that the anterior root is not part of the ganglion.Cutaneous Innervation and Dermatomes
Each spinal nerve except C1 receives sensory input from a specific area of skin called a dermatome. A dermatome map is a diagram of the cutaneous regions innervated by each spinal nerve. Such a map is oversimplified, however, because the dermatomes overlap at their edges by as much as 50%. Therefore, severance of one sensory nerve root does not entirely deaden sensation from a dermatome. It is necessary to sever or anesthetize three sequential spinal nerves to produce a total loss of sensation from one dermatome. Spinal nerve damage is assessed by testing the dermatomes with pinpricks and noting areas in which the patient has no sensation.
Figure 7. Dermatome (spinal nerves sensory innvervation)

Footnote: Each zone of the skin is innervated by sensory branches of the spinal nerves indicated by the labels. Nerve C1 does not innervate the skin.
Spinal Cord Tracts
Ascending tracts carry sensory information up the cord, and descending tracts conduct motor impulses down. All nerve fibers in a given tract have a similar origin, destination, and function. Many of these fibers have their origin or destination in a region called the brainstem. Described more fully in the human brain article.
Several of these tracts undergo decussation as they pass up or down the brainstem and spinal cord—meaning that they cross over from the left side of the body to the right, or vice versa. As a result, the left side of the brain receives sensory information from the right side of the body and sends motor commands to that side, while the right side of the brain senses and controls the left side of the body. Therefore, a stroke that damages motor centers of the right side of the brain can cause paralysis of the left limbs and vice versa.
When the origin and destination of a tract are on opposite sides of the body, anatomists say they are contralateral to each other. When a tract does not decussate, its origin and destination are on the same side of the body and anatomists say they are ipsilateral. Bear in mind that each tract is repeated on the right and left sides of the spinal cord.
Figure 8. Spinal cord tracts
Figure 9. Processing of sensory input and motor output by the spinal cord
Note: Sensory input is conveyed from sensory receptors to the posterior gray horns of the spinal cord, and motor output is conveyed from the anterior and lateral gray horns of the spinal cord to effectors (muscles and glands).
Ascending Tracts
Ascending tracts carry sensory signals up the spinal cord. Sensory signals typically travel across three neurons from their origin in the receptors to their destination in the brain: a first-order neuron that detects a stimulus and transmits a signal to the spinal cord or brainstem; a second-order neuron that continues as far as a “gateway” called the thalamus at the upper end of the brainstem; and a third-order neuron that carries the signal the rest of the way to the cerebral cortex. The axons of these neurons are called the first- through third-order nerve fibers.
Figure 10. Spinal cord ascending tracts to the brain
The major ascending tracts are as follows. The names of most of them consist of the prefix spino- followed by a root denoting the destination of its fibers in the brain, although this naming system does not apply to the first two.
Gracile fasciculus
The gracile fasciculus carries signals from the midthoracic and lower parts of the body. Below vertebra T6, it composes the entire posterior column. At T6, it is joined by the cuneate fasciculus, discussed next. It consists of first-order nerve fibers that travel up the ipsilateral side of the spinal cord and terminate at the gracile nucleus in the medulla oblongata of the brainstem. These fibers carry signals for vibration, visceral pain, deep and discriminative touch (touch whose location one can precisely identify), and especially proprioception from the lower limbs and lower trunk. Proprioception is the nonvisual sense of the position and movements of the body.
Cuneate fasciculus
The cuneate fasciculus joins the gracile fasciculus at the T6 level. It occupies the lateral portion of the posterior column and forces the gracile fasciculus medially. It carries the same type of sensory signals, originating from T6 and up (from the upper limbs and chest). Its fibers end in the cuneate nucleus on the ipsilateral side of the medulla oblongata. In the medulla, second-order fibers of the gracile and cuneate systems decussate and form the medial lemniscus, a tract of nerve fibers that leads the rest of the way up the brainstem to the thalamus. Third-order fibers go from the thalamus to the cerebral cortex. Because of decussation, the signals carried by the gracile and cuneate fasciculi ultimately go to the contralateral cerebral hemisphere.
Spinothalamic tract
The spinothalamic tract and some smaller tracts form the anterolateral system, which passes up the anterior and lateral columns of the spinal cord. The spinothalamic tract carries signals for pain, temperature, pressure, tickle, itch, and light or crude touch. Light touch is the sensation produced by stroking hairless skin with a feather or cotton wisp, without indenting the skin; crude touch is touch whose location one can only vaguely identify.
In this pathway, first-order neurons end in the posterior horn of the spinal cord near the point of entry. Here they synapse with second-order neurons, which decussate and form the contralateral ascending spinothalamic tract. These fibers lead all the way to the thalamus. Third-order neurons continue from there to the cerebral cortex. Because of decussation, sensory signals in this tract arrive in the cerebral hemisphere contralateral to their point of origin.
Spinoreticular tract
The spinoreticular tract also travels up the anterolateral system. It carries pain signals resulting from tissue injury. The first-order sensory neurons enter the posterior horn and immediately synapse with second-order neurons. These decussate to the opposite anterolateral system, ascend the cord, and end in a loosely organized core of gray matter called the reticular formation in the medulla and pons. Third-order neurons continue from the pons to the thalamus, and fourth-order neurons complete the path from there to the cerebral cortex.
Posterior and anterior spinocerebellar tracts
The posterior and anterior spinocerebellar tracts travel through the lateral column and carry proprioceptive signals from the limbs and trunk to the cerebellum at the rear of the brain. Their first-order neurons originate in muscles and tendons and end in the posterior horn of the spinal cord. Second-order neurons send their fibers up the spinocerebellar tracts and end in the cerebellum.
Fibers of the posterior tract travel up the ipsilateral side of the spinal cord. Those of the anterior tract cross over and travel up the contralateral side but then cross back in the brainstem to enter the ipsilateral side of the cerebellum. Both tracts provide the cerebellum with feedback needed to coordinate muscle action.
What is pain?
Pain is the body’s warning system when you are sick or injured. Pain leads people to take action, a good thing, and has been important in humans’ ability to evolve and survive. This type of pain is acute pain (nociceptive pain) and is a reaction to a noxious stimulus. Acute pain is generally simple to treat and tends to fade away as you begin to feel better.
Chronic pain is pain that persists after the body should have healed, usually about three months. This pain may not be warning you of damage occurring in the body so there is no longer a direct link between pain and harm being caused by the (preceding) injury or disease. The terms chronic pain and persistent pain are often used interchangeably. Pain is said to be chronic if it persists beyond the normal healing time of about three months. ‘Chronic’ simply means ongoing and doesn’t tell us much about the severity or quality of the pain. Chronic pain is a complex phenomenon and may not be easy to treat, especially with analgesia alone.
Sometimes, it is not possible for doctors to pin point the cause of the pain and it can be frustrating not to have a diagnosis. Chronic pain is complex because it involves the nerves and nervous systems, including the central nervous system made up of the brain and spinal cord.
Chronic pain occurs because of changes to the nerves or nervous system which keeps the nerves firing and signaling pain. However, there are likely to be other precipitating factors with chronic pain including genetics, gender and previous episodes of acute pain. Chronic pain can be intense and unrelenting, and lead to various degrees of disability if it is not managed well.
Chronic pain is a condition in its own right because of the changes in the nervous system unrelated to the original diagnosis or injury, if there was one. Medical scientists are able to map pain centers in the brain using brain imaging, bringing hope to the many Americans who have not had their pain properly believed, assessed or treated in the past.
How does a TENS unit work?
The exact mechanism for TENS is not known, Some scientists believe that the electrical impulses override the pain messages traveling along the nerve pathway to the brain. Others theorize that the current triggers the brain to release its own pain killing chemicals. Recent studies have shown that both these theories are probably involved, plus several others.
TENS induced analgesia is thought to be multifactorial and encompasses likely peripheral, spinal and supraspinal mechanisms 6. In one animal study, the increased mechanical sensitivity caused by peripheral injection of serotonin (a substance naturally produced following injury/inflammation) was decreased by application of TENS 7. Importantly, it was demonstrated that this analgesia was partly mediated by peripheral mechanisms as preinjection of a peripheral opioid receptor blocker decreased the analgesia produced, implying the TENS effect was mediated via activation of these peripheral receptors 7. A spinal effect for electrical stimulation was initially demonstrated by Wall 1967, and was suggested to work via the ‘pain-gate’ mechanism proposed in 1965 8. The pain gate theory proposes that large diameter (Aβ) afferent fibers (carrying sensations such as vibration, touch, etc.) inhibit nociceptive activity in the dorsal horn of the spinal cord, with a resultant decrease in pain perception 8. TENS application and its stimulation of peripheral neural structures is a source of considerable large diameter afferent activity and this is therefore a plausible means of TENS induced analgesia. TENS is also thought to have additional spinal segmental effects; decreased inflammation-induced dorsal horn neuron sensitization 9, altered levels of neurotransmitters such as gamma-aminobutyric acid (GABA) and glycine, which are thought to be involved in inhibition of nociceptive traffic 10, and modulation of the activity of the cells that provide support/surround neurons (glial cells) in the spinal cord 11, have all been suggested as means by which TENS may produce analgesia at a spinal segmental level.
Further, it appears that TENS may have an effect on endogenous analgesia. Descending activity relayed via the midbrain periaqueductal grey and the rostral ventral medulla in the brainstem may have inhibitory effects at the segmental level 12. This periaqueductal grey-rostral ventral medulla relayed segmental inhibition is mediated in part via opioidergic pathways 13. TENS induced analgesia has been shown to be reversible with preinjection of opioid receptor blockers in both the periaqueductal grey and rostral ventral medulla in rats with experimentally induced peripheral inflammation implying that this may be an operational pathway by which TENS contributes to analgesia 14. This descending mechanism may also exist in humans with pain. An enhanced conditioned pain modulation (descending modulation) response has been observed in people with fibromyalgia during active TENS application compared to no TENS or placebo TENS 15. The descending modulation of pain is apparently not related to frequency of TENS stimulation employed 14, rather it is the intensity of stimulation that appears to be critical in TENS analgesia 16.
Low frequency and high frequency TENS effects have been shown to be mediated via µ- and δ-opioid receptor classes, respectively and as such low frequency TENS effects may be limited in people using opioids for pain relief as they primarily act via µ-opioid receptor pathways 17.
These descending inhibitory mechanisms have also been implicated in placebo analgesia (the phenomena of improvements in pain that follow the delivery of an inert treatment) 18; therefore, it is possible that the suggested mechanisms of TENS induced analgesia described above may not necessarily represent specific effects of electrical stimulation but could possibly result purely from the therapeutic ritual of providing a TENS unit.
What does a TENS unit do
TENS devices create pulsed currents with asymmetrical biphasic rectangular or symmetrical biphasic rectangular waveforms. TENS devices are designed so that users can adjust the electrical characteristics of the currents including: pulse frequency (usually less than 200 Hz), pulse amplitude (usually less than 70 mA), pulse duration (usually 50 μseconds to 250 μseconds), and pulse pattern (sometimes termed ‘mode’ and including continuous, burst, and modulated). Modulated pulse patterns may help to reduce tolerance to TENS caused by repeated use and include modulated frequency, modulated amplitude, and modulated duration 19.
The International Association for the Study of Pain defined two TENS techniques which are commonly used in the literature 20: conventional TENS administered using high-frequency, low-intensity currents to produce a strong non-painful TENS sensation; and acupuncture-like TENS (AL-TENS) using low-frequency, high-intensity currents to produce strong non-painful pulsate sensations, phasic muscle contractions (twitching), or both 21. Low-frequency TENS is consistently defined as the delivery of pulsed current of 10 Hz or less or low-frequency trains (bursts) of high-frequency pulsed current (i.e. burst mode TENS). High-frequency TENS is often described as pulsed current between about 50 Hz and 100 Hz, although this neglects frequencies between 11 Hz and 49 Hz and frequencies above 100 Hz. The term medium-frequency TENS is rarely used in the literature so high-frequency TENS should be used to describe frequencies greater than 10 Hz to the maximum setting on the TENS device, which is usually 150 Hz to 200 Hz 22. High-frequency TENS is not always applied at a low intensity and low-frequency TENS is not always applied at a high intensity. Low-frequency TENS applied 10% below motor threshold generates analgesia in humans and reduces primary and secondary joint inflammation in animal models of nociception 19. The critical factor for response to TENS is the perceptual experience of the intensity of currents during stimulation regardless of frequency. Evidence suggests that optimal hypoalgesia is achieved using pulse amplitudes (mA) that generate a strong, non-painful TENS sensation and therefore pulse amplitude should be titrated during treatment to maintain this intensity level 23.
Response to TENS is also influenced by site of stimulation according to the placement of electrodes. Best practice guidelines suggest that electrodes should be placed on healthy sensate skin so that the TENS sensation covers (permeates) the painful area. This is achieved by placing electrodes directly over or ‘bracketing’ the painful site. This may not always be possible because, for example, skin sensation is altered, there is a skin lesion, or a body part is absent. In these circumstances, electrodes are placed over the main nerves proximal to the site of pain, close to vertebrae of spinal segments, over contralateral dermatomes, over acupuncture points (acu-TENS), or over myofascial trigger points. Research findings on the effect of the site of stimulation on treatment outcome are ambiguous 22. Consideration also needs to be given to the duration and regularity of treatment and the timing of outcome measurements. In particular, evidence suggests that the effects of TENS are maximal during stimulation or immediately after stimulation 19 and that some studies have failed to measure outcome during stimulation 24.
How to use a TENS unit
The information below is a general guide on how to use a TENS unit. You should always follow the manufacturer’s specific instructions.
Technological advances have produced a variety of TENS devices with a wide range of stimulation parameters for clinicians and patients to choose from (e.g. pulse frequency, pulse amplitude, pulse duration and electrode placement site).
TENS units are small and lightweight, so you can use them while you’re working or on the move. You can put it in your pocket, clip it to your belt or hold it in your hand.
You can use TENS throughout the day for as long as you like, although it shouldn’t be used while you’re driving, operating machinery, or in the bath or shower.
TENS units typically use adhesive electrodes applied to the skin surface to apply pulsed electrical stimulation that can be modified in terms of frequency (stimulation rate), intensity and duration 25.
Several types of TENS applications, differing in frequency, amplitude, pulse width and waveform, are used in clinical practice. The two most common application modes include:
- High frequency or conventional TENS (frequencyranging up to 50 Hz or 100 Hz and above, pulse width less than 150 μsec, low intensity sufficient to produce a comfortable tingling sensation) 7 and
- Low frequency or so called acupuncture-like TENS (frequency of 10 Hz or less, pulse width greater than 150 μsec, high intensity sufficient to elicit muscle twitching) 16.
Low frequency TENS is often used at higher intensities eliciting motor contraction, while high frequency TENS has traditionally been used at lower intensities 26. Modulated TENS applies stimulation across a range of frequencies and may help ameliorate development of tolerance to TENS 19.
Acupuncture-like TENS is associated with a slower onset and longer duration of analgesia compared to conventional TENS 27. However, whether there is a significant difference in clinical effectiveness between high frequency and low frequency modes is unclear and not well defined 27. Indeed, patient preference for, and response to, different stimulation settings may be highly individualized 28.
Three other standard modes of TENS include:
- Brief-Intense TENS (frequency greater than 80Hz, pulse width greater than 150 μsec, brief duration of stimulation, very high intensity sufficient to activate nociceptive fibres in addition to motor fibres and primary sensory afferents),
- Burst TENS (bursts of high frequency pulses delivered at low frequency (less than 10 Hz) and at a high enough intensity sufficient to activate both motor fibres and primary sensory afferents) and
- Modulation TENS (one or more parameters are randomly modulated during therapy).
Intensity appears to be a critical factor in optimizing TENS efficacy and increasingly it is thought that regardless of frequency of application, the intensity needs to produce a strong, non-painful sensation that ideally is titrated during treatment to maintain the intensity level 19. Placement of electrodes may influence response, although this issue is somewhat ambiguous with local, related spinal segment and contralateral electrode placement demonstrating an effect in both animal and human studies 15. Theory predicts that the TENS analgesia induced should peak during or immediately after use 19.
Positioning the TENS pads
Make sure the TENS unit is switched off before you attach the pads to your skin. Position the pads either side of the painful area, at least 2.5cm (1 inch) apart.
NEVER place the TENS pads over:
- the front or sides of your neck
- your temples
- your mouth or eyes
- your chest and upper back at the same time
- irritated, infected or broken skin
- varicose veins
- numb areas
Turning TENS unit on and adjusting the strength
- Turn on the TENS machine when the pads are attached in the correct places. You’ll feel a slight tingling sensation pass through your skin.
- The TENS unit has a dial that allows you to control the strength of the electrical impulses.
- Start on a low setting and gradually increase it until the sensation feels strong but comfortable. If the tingling sensation starts to feel painful or uncomfortable, reduce it slightly.
Switch the TENS machine off after you’ve finished using it and remove the electrodes from your skin.
Is TENS effective?
TENS helps ease pain for some people, but not for others. There isn’t enough good-quality scientific evidence to say for sure whether TENS is a reliable method of pain relief. More research is needed and clinical trials for TENS are ongoing.
The success of TENS depends in part on how easily the nerve pathway carrying the painful signals can be identified and how accessible they are for placing the electrodes. Patient attitude and clinical history also play an important part. That is why patients must undergo a thorough examination by a knowledgeable clinician before TENS treatment can begin.
Clinical studies over the years show TENS to be effective in reducing pain among 20% to 40% of patients treated for chronic pain conditions. This success rate is considered to be excellent, since most chronic pain sufferers have already tried a number of drugs and interventions including surgery with little success.
TENS for acute pain in adults
Acute pain is pain of recent onset and limited duration. Acute pain is associated with surgery, physical trauma (e.g. broken bones, burns and cuts) and medical procedures (e.g. venepuncture and sigmoidoscopy). The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” 29. Acute pain is defined as pain “of recent onset and probable limited duration which usually has an identifiable temporal and causal relationship to the injury or disease”. In clinical practice acute pain is categorised as pain of less than three months duration 30. Current approaches to acute pain management include pharmacological agents (drugs) and a number of non-pharmacological agents, one of which is TENS 31.
This 2015 Cochrane Review update (the first update was in 2011) provides tentative evidence that TENS reduces pain intensity over and above that seen with placebo (no current) TENS when administered as a stand-alone treatment for acute pain in adults 26. TENS was better than placebo TENS (delivering no electrical current) at reducing the intensity of acute pain but the reduction in pain was not consistent across all trials. This finding was based on an analysis of only six of the 19 trials. There was an insufficient number patients to make a firm conclusion. Furthermore, the high risk of bias associated with inadequate sample sizes in treatment arms and unsuccessful blinding of treatment interventions makes definitive conclusions impossible. There was incomplete reporting of treatment in many reports making replication of trials impossible.
A small number of patients experienced itching and redness beneath the TENS pads or disliked the sensation produced by TENS.
Overall the study authors concluded that TENS may reduce the intensity of acute pain in some patients but the quality of evidence was weak 26. TENS is inexpensive, safe and can be self-administered. The study authors recommended that TENS should be considered as a treatment option given on its own or in combination with other treatments.
TENS for chronic low-back pain
Low-back pain represents a leading cause for work absenteeism and visits to health care professionals. Sixty to 90% of the adult population is at risk of developing low-back pain. While the majority of episodes appear to resolve within six weeks, recurrences are common. In addition, it is estimated that 10% to 20% of affected adults develop symptoms of chronic low-back pain (persistent pain lasting longer than three months). Chronic low-back pain has a significant impact on everyday life.
A 2008 Cochrane Systematic Review 32, the review authors found conflicting evidence regarding the benefits of TENS for chronic low-back pain, which does not support the use of TENS in the routine management of chronic low-back pain. Further research is encouraged.
TENS for neck pain
Neck pain is common, disabling and costly. In a 2013 Cochrane Systematic Review 33 the study authors cannot make any definite statements on the efficacy and clinical usefulness of electrotherapy modalities for neck pain. Since the evidence is of low or very low quality, the study authors are uncertain about the estimate of the effect. Further research is very likely to change both the estimate of effect and our confidence in the results. Current evidence for TENS shows that this modality might be more effective than placebo. When compared to other interventions the quality of evidence was very low thus preventing further recommendations.
For patients with acute neck pain, TENS possibly relieved pain better than electrical muscle stimulation, not as well as exercise and infrared light, and as well as manual therapy and ultrasound 33. There was no additional benefit when added to infrared light, hot packs and exercise, physiotherapy, or a combination of a neck collar, exercise and pain medication. For patients with acute whiplash, iontophoresis was no more effective than no treatment, interferential current, or a combination of traction, exercise and massage for relieving neck pain with headache.
For patients with chronic neck pain, TENS possibly relieved pain better than placebo and electrical muscle stimulation, not as well as exercise and infrared light, and possibly as well as manual therapy and ultrasound 33. Magnetic necklaces were no more effective than placebo for relieving pain; and there was no additional benefit when electrical muscle stimulation was added to either mobilisation or manipulation.
For patients with myofascial neck pain, TENS, FREMS (FREquency Modulated Neural Stimulation, a variation of TENS) and repetitive magnetic stimulation seemed to relieve pain better than placebo.
TENS for osteoarthritis of the knee
Osteoarthritis (OA) is a disease of the joints, such as your knee. When the joint loses cartilage, the bone grows to try and repair the damage. Instead of making things better, however, the bone grows abnormally and makes things worse. For example, the bone can become misshapen and make the joint painful and unstable. This can affect your physical function or ability to use your knee.
A 2009 Cochrane Systematic Review 34 the review authors could not confirm that TENS is effective for pain relief in adults with osteoarthritis of the knee. The systematic review is inconclusive, hampered by the inclusion of only small trials of questionable quality 34. Appropriately designed trials of adequate power are warranted.
TENS for the treatment of rheumatoid arthritis in the hand
Rheumatoid arthritis (RA) is a chronic, inflammatory, system disease. It commonly affects the small peripheral joints (such as fingers and wrist). The main goals of intervention for rheumatoid arthritis are preventing joint deformity, preserving joint function, and reducing inflammation and pain.
A 2009 Cochrane Systematic Review 35 found conflicting effects of TENS on pain outcomes in patients with rheumatoid arthritis. Acupuncture like-TENS is beneficial for reducing pain intensity and improving muscle power scores over placebo while, conversely, conventional-TENS resulted in no clinical benefit on pain intensity compared with placebo. However conventional-TENS resulted in a clinical benefit on patient assessment of change in disease over acupuncture-like TENS. More well designed studies with a standardized protocol and adequate number of subjects are needed to fully conclude the effect of conventional-TENS and acupuncture-like TENS in the treatment of rheumatoid arthritis of the hand.
TENS for primary dysmenorrhea (period pain)
Dysmenorrhea refers to the occurrence of painful menstrual cramps of uterine origin. It is a common gynecological complaint that can affect as many as 50% of women; 10% of these women suffer severely enough to render them incapacitated for one to three days each menstrual cycle 36. This has a significant impact on personal health and it also has a global economic impact. In the USA alone, it is estimated that annual losses are 600 million work hours and two billion dollars 37.
Dysmenorrhea is commonly defined within two subcategories. When the pelvic pain is associated with an identifiable pathological condition, such as endometriosis, it is considered to be secondary dysmenorrhoea. In contrast, menstrual pain without organic pathology is called primary dysmenorrhoea 38.
The initial onset of primary dysmenorrhea is usually at or shortly (six to 12 months) after menarche (the commencement of menstrual periods), when ovulatory cycles are established. The pain duration is commonly 48 to 72 hours and is associated with the menstrual flow. In contrast, secondary dysmenorrhoea is more likely to occur years after the onset of menarche and occurs premenstrually as well as during menstruation. This distinction is not necessarily robust however as severe primary dysmenorrhoea in young women may indicate endometriosis 39.
A 2002 Cochrane Systematic Review 40 found high-frequency TENS was effective for the treatment of primary dysmenorrhea (menstrual pain without organic pathology) by a number of small trials. The minor adverse effects reported in one trial require further investigation. There is insufficient evidence to determine the effectiveness of low-frequency TENS in reducing primary dysmenorrhea.
TENS for neuropathic pain in adults
Neuropathic pain is defined as “pain caused by a lesion or disease of the somatosensory system” and represents a significant source of chronic pain and loss of function at both an individual and societal level 41. Approximately 20% of adults in the USA and 27% in the EU report chronic pain 42. Within this, it is estimated that 20% of people with chronic pain will have neuropathic pain characteristics, translating to an approximate prevalence of 6% to 7% in the general population 43. This is confirmed by one systematic review that estimated a population prevalence for neuropathic pain of 6.9% to 10% 44. Neuropathic pain is often rated as particularly intense and distressing and can have a significant negative impact on activities of daily living and quality of life 45. Neuropathic pain can be particularly unpleasant and achieving adequate symptom control can be difficult. Non-pharmacological methods of treatment are often employed by people with neuropathic pain and may include TENS (transcutaneous electrical nerve stimulation).
Neuropathic pain may be classified as peripheral or central in origin depending on the site of lesion or disease. Peripheral neuropathic pain results from injury or disease of the peripheral nerves and includes conditions such as post-traumatic nerve injury, diabetic peripheral neuropathy (or painful diabetic neuropathy) and postherpetic neuralgia. Central neuropathic pain results from injury or disease affecting the central nervous system (spinal cord, brainstem or brain) and includes central poststroke pain, postspinal cord injury pain and pain related to multiple sclerosis. Regardless of the causal condition or classification there are common features associated with neuropathic pain. Typically, neuropathic pain is associated with positive features such as spontaneous pain, hyperalgesia (excessive pain to a painful stimulus) and allodynia (pain evoked by a normally non-painful stimulus), as well as negative features such as sensory loss, weakness and hypoaesthesia (reduced sense of touch or sensation) 46. For patients, this translates to pain being caused by innocuous stimuli such as light touch or gentle movement, increased pain in response to noxious stimuli, and reduced sensory and motor function 46. Additionally, pain may be perceived in the absence of provoking stimuli 47.
The mechanisms underpinning this persistent pain state are complex. It is most likely that a mix of peripheral and central mechanisms are responsible for ongoing pain perception. Following a lesion or disease in a peripheral somatosensory structure (e.g. peripheral nerve), inflammatory mediators are released that causes sensitisation of nociceptors (nerve receptors that respond to tissue damaging stimuli or threat of damage) resulting in lowered stimulation thresholds and enhanced activity in these receptors 48. Damage to neural structures (at both peripheral nerve and central nervous system levels) can result in longer term changes to their structure and function 49, resulting in abnormal or excessive activity in areas of damaged neural tissue that is thought to lead to ongoing and often severe and intractable pain 48. These changes may also be accompanied by a decreased capacity of the body’s natural pain modulation mechanisms (known as endogenous analgesia), further compounding the pain perceived 50. These multiple, integrated pain mechanisms result in neuropathic pain being particularly difficult to treat and ongoing pain with limited response to treatment is common. First line management of neuropathic pain is primarily pharmacological 51; however, it is also common for management to include non-pharmacological treatments such as psychological or physical interventions including transcutaneous electrical nerve stimulation (TENS). Standard TENS units are portable, widely available, easily self-administered and are a popular adjunct therapy for people with chronic neuropathic pain 25.
In this well conducted 2017 Cochrane review 6, comparing between TENS and sham TENS (placebo). The quality of the evidence was very low meaning the study authors were unable to confidently state whether TENS is effective for pain control in people with neuropathic pain. The very low quality of evidence means the study authors have very limited confidence in the effect estimate reported; the true effect is likely to be substantially different. The study authors concluded for adults with neuropathic pain, it is impossible to confidently state whether TENS is effective in relieving pain when compared to sham TENS.
TENS for pain management in labor
Pain in labor is a complex phenomenon, and it is known that women’s experiences of pain and labour vary enormously 52. Physiological, cognitive and psychological factors all seem to be involved in determining individual experience. The precise mechanisms whereby TENS relieves pain are not known. A number of theories have been proposed.
First is the ‘gate control theory’ of pain 8. According to this theory, the transmission of pain is inhibited by the stimulation of large, afferent nerve fibres which carry impulses towards the central nervous system. When afferent nerves are stimulated, the pathway for other (painful) stimuli is closed by the operation of a ‘gate’ in the spinal cord that controls transmissions to the brain. When applied to the lower back, the TENS unit emits electrical impulses which excite afferent nerves, and thus inhibits the transmission of painful stimuli arising from the uterus, vagina and perineum during labour 2. (According to this theory, the application of heat, cold or massage would be likely to have a similar effect.)
Second, it is suggested that painful stimuli result in chemical changes in the brain, most notably, the release of endorphins which mediate the experience of pain. TENS is thought to complement this chemical process 53. Again, the precise mechanisms are not understood. However, by reducing anxiety, increasing a sense of control, and by providing distraction, TENS is thought to increase women’s sense of well-being and thereby reduce pain in labour 54. It has also been proposed that by decreasing maternal anxiety, TENS may reduce the length of labour by suppressing the release of catecholamines which can inhibit the action of the uterus and thereby delay progress 52.
More recent theories suggest that the varied factors influencing the experience of pain are likely to be interactive 55.
Various models of TENS equipment are available. The TENS unit consists of a hand-held device connected to electrodes which are attached to the skin. During labour the electrodes are usually positioned on the lower back on both sides of the spine at vertebral positions T10 and S2 54, corresponding to the nerve pathways through which painful impulses from the contracting uterus are thought to enter the spinal cord 52. The TENS unit emits low-voltage impulses, the frequency and intensity of which can be controlled by the woman in labour. When using TENS, women experience a tingling or buzzing feeling at the site of the electrodes. At low voltages these sensations are not painful. TENS has also been used to stimulate acupuncture points, and can also be applied to the cranium by trained therapists.
The availability of TENS has increased over the past two decades. The extent of its use by women in different countries and settings, and at different stages in labour, has not been well documented. A UK study suggested that in 1994 approximately 16% of low-risk primiparous women used TENS in labour; invariably TENS was used alongside other methods of pain relief 56. This figure is higher than has been reported in other studies 57. A more recent study of maternity units in the UK suggests that the use of TENS was supported by midwives in all units surveyed, although only approximately a fifth had TENS available. The use of TENS by women admitted to these units was reported to be between 1% and 25% although this information was not always routinely recorded; the extent of its use by women at home in early labour remains uncertain 58.
The use of TENS to relieve pain in labour remains controversial. While there is evidence that the technology is well received by women, it is not clear that this is because it is effective in reducing pain. There is evidence that women’s satisfaction with the experience of childbirth is affected by their sense of control during labour, and in particular, their sense of control during painful contractions 59. The fact that women themselves operate the TENS unit may partly explain its popularity. In addition, the units may be used in a variety of settings, and it has been suggested that using the device at home in early labour may delay admission to hospital.
The intervention does not seem to have serious adverse effects on women or their babies, although there has been only limited research in this area 54. Serious side effects are rare, but the electrodes may cause some local skin irritation. The use of TENS has cost implications, not only in terms of the purchase or hire of the TENS units but also in terms of staff time setting up the equipment and demonstrating its use to women.
In this 2009 Cochrane Systematic Review 60, the study authors found only limited evidence that TENS reduces pain in labour and it does not seem to have any impact (either positive or negative) on other outcomes for mothers or babies. The use of TENS at home in early labour has not been evaluated. TENS is widely available in hospital settings and women should have the choice of using it in labor.
TENS for fibromyalgia in adults
Fibromyalgia is a long-term medical condition that is characterised by chronic widespread pain in the muscles and joints, with sensitivity to pressure stimuli. The symptoms may vary from person to person, but the main symptom is widespread pain throughout the body. This may be worse in certain areas, such as the back or neck. Pain may be described as aching, burning, stabbing, or sharp and may be accompanied by hyperalgesia (heightened sensitivity to pain) and allodynia (pain on very mild stimulus). Pain is often continuous but it may fluctuate in severity depending on various factors including stress, physical activity, and the weather. Exposure to certain environmental stimuli (e.g. smoke, certain foods, and bright lights) may cause flare-ups. Other presenting symptoms may include stiffness, especially in the morning; muscle spasm; depression; fatigue; poor sleep quality, including non-restorative sleep; cognitive difficulties in thinking, learning, attention, and concentration; headaches, including severe migraines; and irritable bowel syndrome 61. Originally, the American College of Rheumatology classification criteria for fibromyalgia were widespread pain (axial pain, left- and right-sided pain, upper and lower segment pain) that lasts for longer than three months, with pain on palpation at 11 or more of 18 specified tender points 62. More recently, a definition of fibromyalgia has been proposed based on symptom severity and the presence of widespread pain, which does not require palpation of tender points for diagnosis 63. Thus, fibromyalgia is diagnosed if the person has: a widespread pain index (WPI) of 7 or greater and a symptom severity scale score of 5 or greater, or a widespread pain index (WPI) of between 3 and 6 and a symptom severity scale score of 9 or greater; symptoms have persisted at a similar level for three months or greater; and the pain cannot be explained by another disorder.
While some rheumatologists have thought of fibromyalgia as a specific pain disorder, other investigators have characterized it as a bodily distress syndrome or a physical symptom disorder, or somatoform disorder 61. It is a heterogeneous condition in which there is abnormal processing of the sensation of pain. The cause, or causes, are not well understood, but it has features in common with neuropathic pain, including changes in the central nervous system (CNS). Moreover, people with neuropathic pain and some people with fibromyalgia experience similar sensory phenomena 64. Many people with fibromyalgia are significantly disabled, and experience moderate or severe pain for many years. Chronic painful conditions comprised five of the 11 top-ranking conditions for years lived with disability in 2010 65, and are responsible for considerable loss of quality of life and employment, and increased health costs 66.
Fibromyalgia is common. Numerous studies have investigated prevalence in different settings and countries. The review by Queiroz 2013 67 gave a global mean prevalence of 2.7% (range 0.4% to 9.3%), and a mean in the Americas of 3.1%, in Europe of 2.5%, and in Asia of 1.7%. Fibromyalgia is more common in women, with a female to male ratio of 3:1 (4.2%:1.4%). The change in diagnostic criteria does not appear to have significantly affected estimates of prevalence 68. Estimates of prevalence in specific populations vary greatly, but have been reported to be as high as 9% in female textile workers in Turkey and 10% in metalworkers in Brazil (59% in those with repetitive strain injury) 67. Risk factors for fibromyalgia include: sex (it is more common in women than in men); family history (it is more likely if a relative has the condition); age (it is more common as age increases); and rheumatic disease (rheumatoid arthritis or lupus) 68. The financial burden of fibromyalgia on society is significant. One cross-sectional study on 299 people with fibromyalgia in France and Germany estimated that, on average, people visited their physician 11.6 (France) and 19.6 (Germany) times per year and missed 32.4 (France) and 25.2 (Germany) days of work per year 69. Total annual costs to society based on three-month data from 2008 were EUR 7900 in France and EUR 7256 in Germany per person. Direct costs from physician clinic visits, medications, and out-of-pocket expenses were EUR 910 (France) and EUR 1765 (Germany), and indirect costs from missed days of work and lost productivity were EUR 6990 (France) and EUR 5491 (Germany).
There are no definitive treatments for fibromyalgia. Fibromyalgia pain is difficult to treat effectively, with only a minority of people experiencing a clinically relevant benefit from any one intervention. A multidisciplinary approach is now advocated, with pharmacological interventions being combined with physical or cognitive interventions, or both. Conventional analgesics are usually not effective. Treatment is often by so-called unconventional analgesics, such as antidepressants such as duloxetine and amitriptyline 70, or antiepileptic drugs such as gabapentin or pregabalin 71. The proportion of people who achieve worthwhile pain relief (typically at least a 50% reduction in pain intensity) is small, generally only 10% to 25% more than with placebo 72, with numbers needed to treat for an additional beneficial outcome (NNTB) usually between four and 10 71. People who do experience good levels of pain relief, however, also benefit from substantial reductions in other symptoms, such as fatigue, function, sleep, depression, anxiety, and ability to work, with significant improvement in quality of life 66. Fibromyalgia is not particularly different from other chronic pain in that only a small proportion of study participants have a good response to treatment 72.
A 2017 Cochrane Systematic Review 73 found insufficient high-quality evidence to support or refute the use of TENS for fibromyalgia. The Cochrane Review found a small number of inadequately powered studies with incomplete reporting of methodologies and treatment interventions 73. There were no serious side events reported in any of the studies.
TENS for phantom pain and stump pain following amputation in adults
Pain may present in a body part that has been amputated (phantom pain) or at the site of amputation (stump pain), or both. Phantom pain and stump pain are complex conditions and affect up to 80% of amputees.The underlying causes are not fully understood. Drug therapy is the most common treatment yet the condition remains poorly managed. The need for non-drug interventions has been recognised and TENS may have an important role to play.
There were no randomized controlled trials to judge the effectiveness of TENS for the management of phantom pain and stump pain 74. The published literature on TENS for phantom pain and stump pain lacks the methodological rigour and robust reporting needed to confidently assess its effectiveness. Further randomized controlled trial evidence is required before an assessment can be made. Since publication of the original version of the Cochrane Systematic review back in 2010, the Cochrane review authors have found no new studies and their conclusions remain unchanged in 2015 74.
TENS for cancer pain in adults
Cancer-related pain is complex and multi-dimensional but the mainstay of cancer pain management has predominantly used a biomedical approach. A 2012 Cochrane Systematic Review 75 on the use of TENS for cancer found inconclusive evidence due to a lack of suitable randomized controlled trials. Large multi-centre randomized controlled trials are required to assess the value of TENS in the management of cancer-related pain in adults.
TENS unit side effects
TENS is thought to be safe and for most people with no side effects.
Some people may be allergic to the pads and their skin may become red and irritated, but special pads for people with allergies are available.
TENS unit should NOT be used:
- on an open wound
- if your skin is irritated
- near sensitive areas such as your eyes
- while driving or operating machinery
- in or around water
TENS isn’t safe for everyone to use. DON’T use TENS unit without first seeking medical advice if:
- you have a pacemaker or another type of electrical or metal implant in your body e.g. a cochlear implant
- you’re pregnant, or there’s a chance you might be pregnant – TENS may not be recommended early in pregnancy
- you have epilepsy or a heart problem
TENS shouldn’t be painful, but some people find it uncomfortable. Some people find skin irritation where the electrodes are attached.
References- American Physical Therapy Association. Guide to physical therapist practice. Second Edition. Physical Therapy 2001;81:9-746.
- Augustinsson LE, Bohlin P, Bundsen P, Carlsson CA, Forssman L, Sjoberg P, et al. Pain relief during delivery by transcutaneous electrical nerve stimulation. Pain 1977;4(1):59-65.
- DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Current Rheumatology Reports 2008;10(6):492–9.
- Francis RP, Johnson MI. The characteristics of acupuncture-like transcutaneous electrical nerve stimulation (acupuncture-like TENS): a literature review. Acupuncture and Electrotherapeutics Research 2011;36(3-4):231-58.
- Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-induced antihyperalgesia. Journal of Pain 2005;6(10):673-80.
- Gibson W, Wand BM, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No.: CD011976. DOI: 10.1002/14651858.CD011976.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD011976.pub2/full
- Santos CM, Francischi JN, Lima-Paiva P, Sluka KA, Resende MA. Effect of transcutaneous electrical stimulation on nociception and edema induced by peripheral serotonin. International Journal of Neuroscience 2013;123(7):507-15.
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150(3699):971-9.
- Sabino GS, Santos CM, Francischi JN, de Resende MA. Release of endogenous opioids following transcutaneous electric nerve stimulation in an experimental model of acute inflammatory pain. Journal of Pain 2008;9(2):157-63.
- Somers DL, Clemente FR. Contralateral high or a combination of high- and low-frequency transcutaneous electrical nerve stimulation reduces mechanical allodynia and alters dorsal horn neurotransmitter content in neuropathic rats. Journal of Pain 2009;10(2):221-9.
- Matsuo H, Uchida K, Nakajima H, Guerrero AR, Watanabe S, Takeura N, et al. Early transcutaneous electrical nerve stimulation reduces hyperalgesia and decreases activation of spinal glial cells in mice with neuropathic pain. Pain 2014;155(9):1888-901.
- Gebhart GF. Descending modulation of pain. Neuroscience and Biobehavioral Reviews 2004;27(8):729-37.
- Calvino B, Grilo RM. Central pain control. Joint, Bone, Spine 2006;73(1):10-6.
- DeSantana JM, Da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience 2009;163(4):1233-41.
- Dailey DL, Rakel BA, Vance CG, Liebano RE, Amrit AS, Bush HM, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain 2013;154(11):2554-62.
- Moran F, Leonard T, Hawthorne S, Hughes CM, McCrum-Gardner E, Johnson MI, et al. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. Journal of Pain 2011;12(8):929-35.
- Leonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. Journal of Pain 2011;12(2):213-21.
- Eippert F, Bingel U, Schoell E, Yacubian J, Klinger R, Lorenz J, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009;63:533-43.
- Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Physical Therapy 2013;93(10):1397-402.
- Charlton J. Stimulation-produced analgesia. In: Charlton J editor(s). Task Force on Professional Education. Washington (DC): IASP Press, 2005:93-6.
- Claydon LS, Chesterton LS. Does transcutaneous electrical nerve stimulation (TENS) produce ‘dose’ responses? A review of systematic reviews on chronic pain. Physical Therapy Reviews 2008;13(6):450-63.
- Johnson MI. Transcutaneous Electrical Nerve Stimulation (TENS). Research to Support Clinical Practice. 1st Edition. Oxford(UK): Oxford University Press, 2014.
- Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. European Journal of Pain 2003;7:181-8.
- Bennett MI, Hughes N, Johnson MI. Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: low fidelity may explain negative findings. Pain 2011;156(2):1226-32.
- Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Review of Neurotherapeutics 2011;11(5):735-53.
- Johnson MI, Paley CA, Howe TE, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No.: CD006142. DOI: 10.1002/14651858.CD006142.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD006142.pub3/full
- Belanger AY. Evidence based guide to therapeutic physical agents. Lippincott Williams & Wilkins, 2002.
- Johnson MI, Ashton CH, Thompson JW. An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain 1991;44(3):221-9.
- Merskey H, Bogduk N (editors). Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd Edition. Seattle: IASP Press, 1994.
- Strong J. Chronic pain problems. In: Strong J, Unruh A, Wright A, Baxter GD editor(s). Pain: a Textbook for Therapists. Edinburgh: Churchill Livingstone, 2002:397-410.
- Schug SA, Watson D, Pogatzki-Zahn EM. Acute pain. In: van Griensven H, Strong J, Unrih AM editor(s). Pain. A Textbook for Health Professionals. 2. Edinburgh: Churchill Livingstone Elsevier, 2014:395-405.
- Khadilkar A, Odebiyi DO, BrosseauKhadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No.: CD003008. DOI: 10.1002/14651858.CD003008.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD003008.pub3/full
- Kroeling P, Gross A, Graham N, Burnie SJ, Szeto G, Goldsmith CH, Haines T, Forget M. Electrotherapy for neck pain. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No.: CD004251. DOI: 10.1002/14651858.CD004251.pub5. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD004251.pub5/full
- Rutjes AWS, Nüesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, Brosseau L, Reichenbach S, Jüni P. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No.: CD002823. DOI: 10.1002/14651858.CD002823.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD002823.pub2/full
- Brosseau L, Yonge KA, Welch V, Marchand S, Judd M, Wells GA, Tugwell P. Transcutaneous electrical nerve stimulation (TENS) for the treatment of rheumatoid arthritis in the hand. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No.: CD004377. DOI: 10.1002/14651858.CD004377. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD004377/full
- Dawood MY. Dysmenorrhea. Clinical Obstetrics and Gynecology 1990;33(1):168-78.
- Dawood MY. Ibuprofen and dysmenorrhea. The American Journal of Medicine 1984;77(1A):87-94.
- Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. The Journal of Reproductive Medicine 1987;32:37-41.
- Punnonen RH, Nikkanen VP. Endometriosis in young women. Infertility 1980;3:1-10.
- Proctor M, Farquhar C, Stones W, He L, Zhu X, Brown J. Transcutaneous electrical nerve stimulation for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No.: CD002123. DOI: 10.1002/14651858.CD002123. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD002123/full
- Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, et al. A new definition of neuropathic pain. Pain 2011;152(10):2204-5.
- Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. Journal of Pain 2014;15(10):979-84.
- Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain 2008;136(3):380-7.
- van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155(4):654-62.
- Leadley RM, Armstrong N, Reid KJ, Allen A, Misso KV, Kleijnen J. Healthy aging in relation to chronic pain and quality of life in Europe. Pain Practice 2014;14(6):547-58.
- Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Central Nervous System Agents in Medicinal Chemistry 2012;12(4):304-14.
- Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurology 2012;11(11):999-1005.
- Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ 2014;348:f7656.
- Levinson SR, Luo S, Henry MA. The role of sodium channels in chronic pain. Muscle & Nerve 2012;46(2):155-65.
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurology 2010;9(8):807-19.
- Dworkin RH, O’Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 2013;154(11):2249-61.
- Lowe NK. The nature of labor pain. American Journal of Obstetrics and Gynecology 2002;186(5 Suppl Nature):S16-S24.
- Lechner W, Jarosch E, Solder E, Waitz-Penz A, Mitterschiffthaler G. Beta-endorphins during childbirth under transcutaneous electric nerve stimulation [Verhalten von Beta-Endorphin wahrend der Geburt unter transkutaner elektrischer Nervenstimulation]. Zentralblatt fur Gynakologie 1991;113(8):439-42.
- Simkin P, Bolding A. Update on nonpharmacologic approaches to relieve labor pain and prevent suffering. Journal of Midwifery & Women’s Health 2004;49(6):489-504.
- Holdcroft A, Power I. Recent developments: management of pain. BMJ 2003;326(7390):635-9.
- Williams FL, Florey CV, Ogston SA, Patel NB, Howie PW, Tindall VR. UK study of intrapartum care for low risk primigravidas: a survey of interventions. Journal of Epidemiology & Community Health 1998;52(8):494-500.
- Carroll D, Tramer M, McQuay H, Bye B, Moore A. Transcutaneous electrical nerve stimulation in labour pain: a systematic review. British Journal of Obstetrics and Gynaecology 1997;104:169-75.
- McMunn V, Bedwell C, Neilson J, Jones A, Dowswell T, Lavender T. A national survey of the use of TENs in labour. British Journal of Midwifery 2009;17(8):492-5.
- Green JM, Baston HA. Feeling in control during labor: concepts, correlates, and consequences. Birth 2003;30(4):235-47.
- Dowswell T, Bedwell C, Lavender T, Neilson JP. Transcutaneous electrical nerve stimulation (TENS) for pain management in labour. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No.: CD007214. DOI: 10.1002/14651858.CD007214.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007214.pub2/full
- Wolfe F, Walitt BT, Hauser W. What is fibromyalgia, how is it diagnosed and what does it really mean?. Arthritis Care and Research 2014;66(7):969-71.
- Wolfe F, Symthe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33:160-72.
- Wolfe F, Clauw DJ, Fitzcharles MA, Coldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care and Research 2010;62:600-10.
- Koroschetz J, Rehm SE, Gockel U, Brosz M, Freynhagen R, Tolle TR, et al. Fibromyalgia and neuropathic pain – differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurology 2011;11:55.
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96.
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79-94.
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Current Pain and Headache Reports 2013;17(8):356.
- Wolfe F, Brahler E, Hinz A, Hauser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis and Research Care 2013;645(5):777-85.
- Winklemann A, Perrot S, Schaefer C, Ryan K, Chandran A, Sadosky A, et al. Impact of fibromyalgia severity on health economic costs: results from a European cross-sectional study. Applied Health Economics and Health Policy 2011;9:125-36.
- Lunn MPT, Hughes RAC, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No.: CD007115. DOI: 10.1002/14651858.CD007115.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007115.pub3/full
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice ASC, Lunn MPT, Hamunen K, Haanpaa M, Kalso EA. Antiepileptic drugs for neuropathic pain and fibromyalgia – an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No.: CD010567. DOI: 10.1002/14651858.CD010567.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD010567.pub2/full
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure: pursue analgesic success. BMJ 2013;346:f2690.
- Johnson MI, Claydon LS, Herbison GP, Jones G, Paley CA. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No.: CD012172. DOI: 10.1002/14651858.CD012172.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD012172.pub2/full
- Johnson MI, Mulvey MR, Bagnall AM. Transcutaneous electrical nerve stimulation (TENS) for phantom pain and stump pain following amputation in adults. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No.: CD007264. DOI: 10.1002/14651858.CD007264.pub3 http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007264.pub3/full
- Hurlow A, Bennett MI, Robb KA, Johnson MI, Simpson KH, Oxberry SG. Transcutaneous electric nerve stimulation (TENS) for cancer pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No.: CD006276. DOI: 10.1002/14651858.CD006276.pub3. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD006276.pub3/full