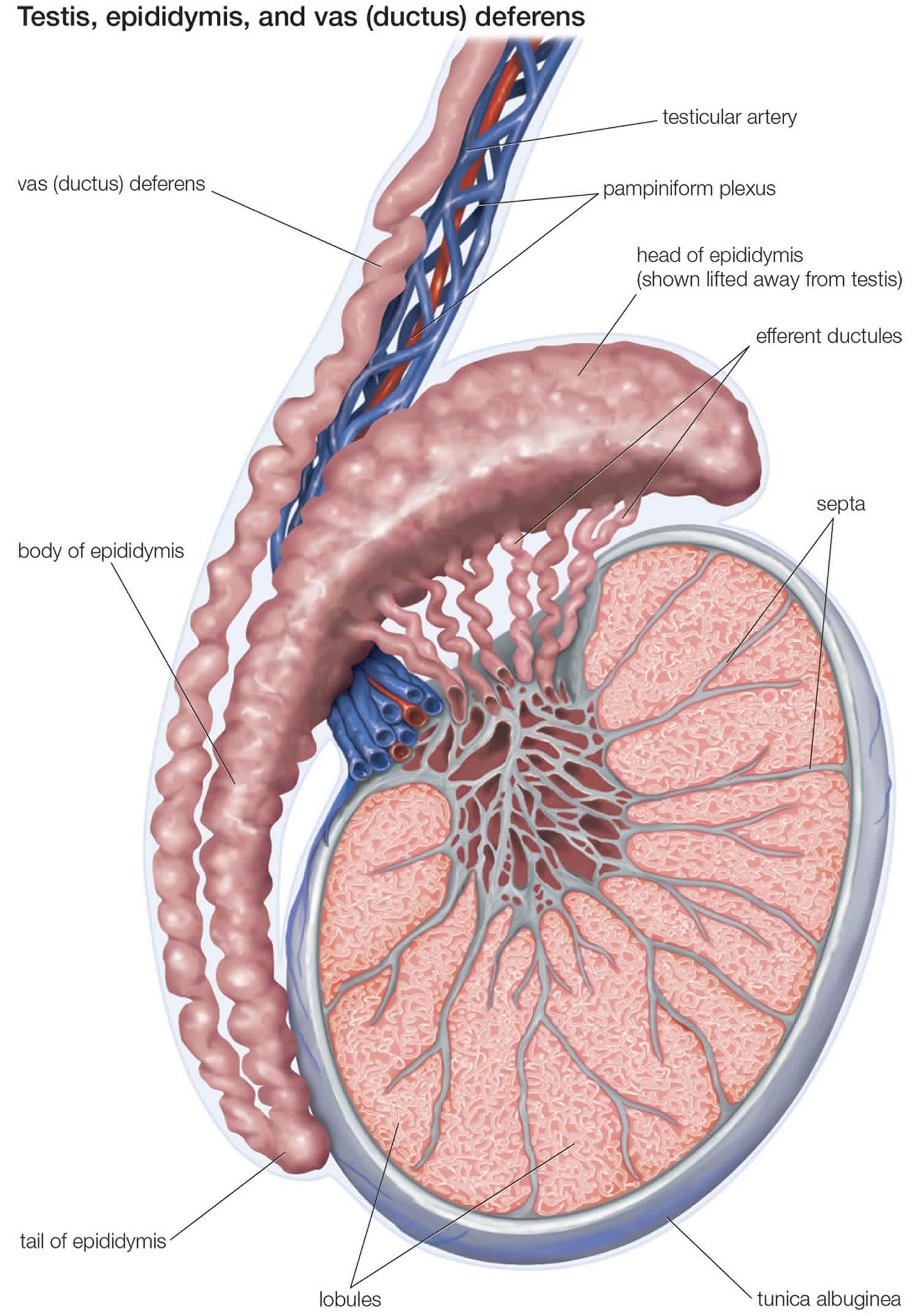
Testicular microlithiasis
Testicular microlithiasis is a relatively common condition that represents the deposition of multiple tiny calcifications throughout both testes. The most common criterion for diagnosis of testicular microlithiasis is that of five microcalcifications in one testicle, although definitions have varied in the past. In the majority of cases testicular microlithiasis is bilateral. Testicular microlithiasis is seen in up to 0.6% of patients undergoing scrotal ultrasound. Some reports suggest that it may present in up to 5.6% of the general population between 17 and 35 years of age 1. However, the prevalence of testicular microlithiasis varied in the past data, depending on the study group. In symptomatic adults, it oscillated between 0.6% and 9.0% 2 and from 2.4% to 5.6% in adults without symptoms 2. In a group with genetic disorders, the prevalence of testicular microlithiasis has been reported much more frequently compared to the general population. The frequency of testicular microlithiasis is as high as 17.5% in men with Klinefelter syndrome 3 and 36% in men with Down syndrome 4.
Testicular microlithiasis is a finding incidental to the ultrasound examination of the scrotum 5. Although testicular microlithiasis is present in ~50% of men with a germ cell tumor, it is very common in patients without cancer, and a direct relationship between the two has been debated. The testicular microcalcifications are likely a marker of tubular degeneration, but not a risk factor for tubular degeneration 6. A link between testicular microlithiasis and testicular cancer as well as male infertility has been analyzed. Follow-up is only recommended where risk factors of testicular cancer other than testicular microlithiasis are present 7.
The typical ultrasound appearance of testicular microlithiasis is characterized by multiple small, same-sized echogenic non-shadowing foci observed throughout the testicles 2. Testicular microlithiasis can be either unilateral or bilateral. The number of calcifications counted on any single image may vary considerably, ranging from five to more than sixty 8. When evaluating the testes, ultrasound should be performed, as a minimum, with a 15 MHz high-frequency transducer 5. The detection of testicular microlithiasis has low inter-observer variability by ultrasound 9. The microcalcifications are not visible on magnetic resonance imaging (MRI) 10. The microliths do not bring about pain or symptoms and are impalpable 5. The Scrotal Imaging Subcommittee of the European Society of Urogenital Radiology published a consensus report on testicular microlithiasis in 2015, proposing 2 definitions of testicular microlithiasis 10: five or more microliths per field of view, or five or more microliths in the whole testis. In testicular microlithiasis’s ultrasound appearance, particular attention should be paid to clustering. A cluster (a few microliths per field in a cluster) may be more worrying than testicular microlithiasis scattered throughout the testis. It may indicate a dysgenic area in the testis, in which carcinoma in situ (CIS) may develop 10.
Testicular microlithiasis is in itself asymptomatic and benign. A relationship with testicular tumors, in particular germ cell tumors is controversial. An ~8 fold increased risk of germ cell tumor in symptomatic testicles with microlithiasis has been reported with microlithiasis found in approximately 50% of germ cell tumor cases, however, no increased risk has been found in asymptomatic testicles. It is also unclear whether early detection confers any benefit over self-exam. As such, screening is unlikely to be beneficial 11.
Some publications advise routine self-examination rather than sonographic surveillance 12, while others recommend annual ultrasound follow up when it is accompanied by other premalignant factors 13.
The European Society of Urogenital Radiology 14 advises annual ultrasound follow-up until age 55, only if a risk factor is present which include:
- personal or family history of germ cell tumor
- testicular maldescent
- orchidopexy
- testicular atrophy
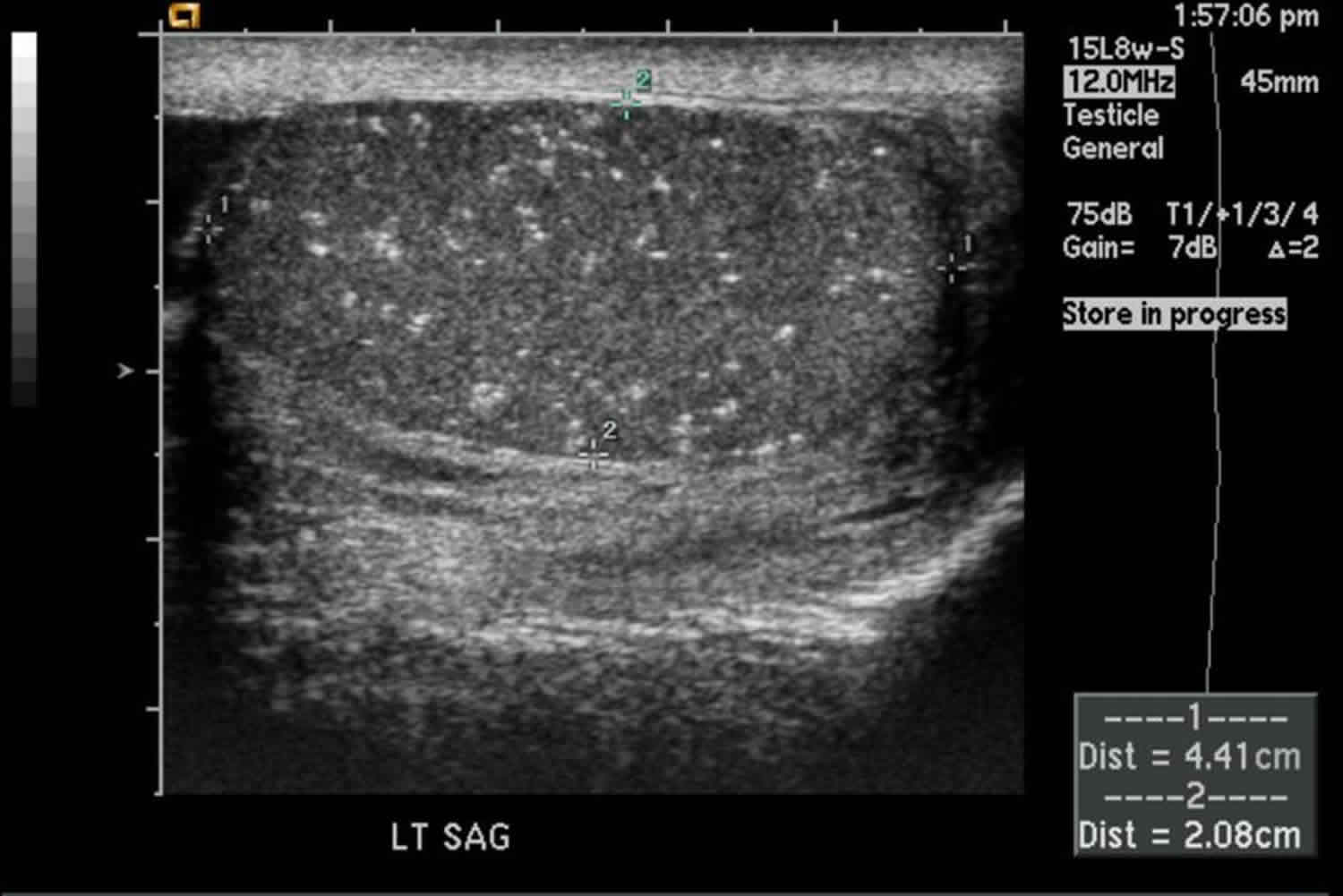
Figure 1. Testicular microlithiasis ultrasound
Testicular microlithiasis association with testicular cancer
In recent years, numerous studies have reported a relationship between testicular microlithiasis and the risk of testicular cancer but provided ambiguous results. Currently, the most reliable data is reported by Wang et al 15. The meta-analyses were based on data from 12 cohort studies and 2 case-control studies (involving 35,578 participants). The authors found that compared with non-testicular microlithiasis individuals or the general population, testicular microlithiasis men might have more than a 12-fold higher incidence of testicular cancer (relative tisk = 12.70) 15. On the other hand, data published as part of a follow-up program showed controversial results. DeCastro et al. 16 published a 5-year follow-up study of 63 asymptomatic men with testicular microlithiasis, of whom only one participant (1.6%) developed testicular cancer after 64 months of observation.
Patel et al. 17 investigated a follow-up program in a single centre for a period of 14 years with 442 men with testicular microlithiasis among more than 20,000 participants. In the follow-up period only 2 men (0.5%) developed testicular cancer. Afterwards, Pederson et al. 18 concluded – based on the two-year follow-up program – that none of the investigated men had developed testicular cancer within the minimum time frame of 50 months.
In 2015, Sharmeen et al. 19 investigated the relationship between testicular microlithiasis and the histologic subtypes of germ cell tumor to determine whether microliths correlate with tumor stage at diagnosis. The authors suggest that testicular microlithiasis may be associated positively with seminomas and negatively with embryonal cell carcinomas 19. What is more, they reported a link between a higher testicular microlithiasis count and a lower initial stage at diagnosis, which suggests that testicular microlithiasis may be associated with less aggressive tumors 19. No association was found between testicular microlithiasis and age, tumor size and the presence of lymphovascular / rete testis invasion 19. The foregoing studies had not found elevated tumor markers in those with incidental testicular microlithiasis, hence monitoring serum tumor markers in follow-up is not appropriate 15.
Risk factors for germ cell tumours
Known risk factors for germ cell tumors include history of a previous germ cell tumour in the contralateral testis, history of maldescended testis or orchidopexy, a history of a germ cell tumor in a first degree relative, atrophic testis and Klinefelter’s syndrome.
In patients with risk factors for germ cell tumour who are found to have testicular microlithiasis on ultrasound, the European Society of Urogenital Radiology Scrotal Imaging Working Group guidelines state that ultrasound should be performed annually until the patient reaches the age of 55 years, after which the risk of developing a germ cell tumor decreases.
In patients with no risk factors, regular self-examination is advised and if the patient develops a palpable mass, ultrasound should then be performed. In patients with no risk factors for germ cell tumour who are found to have microlithiasis, routine ultrasound surveillance is not recommended, as the risk for developing a tumor remains very small.
If a focal lesion is seen in either testis, then referral to a specialist centre for further investigation is advised. This may include tumor markers, further imaging with repeat ultrasound or magnetic resonance imaging (MRI), or orchidectomy depending on the clinical and ultrasound findings.
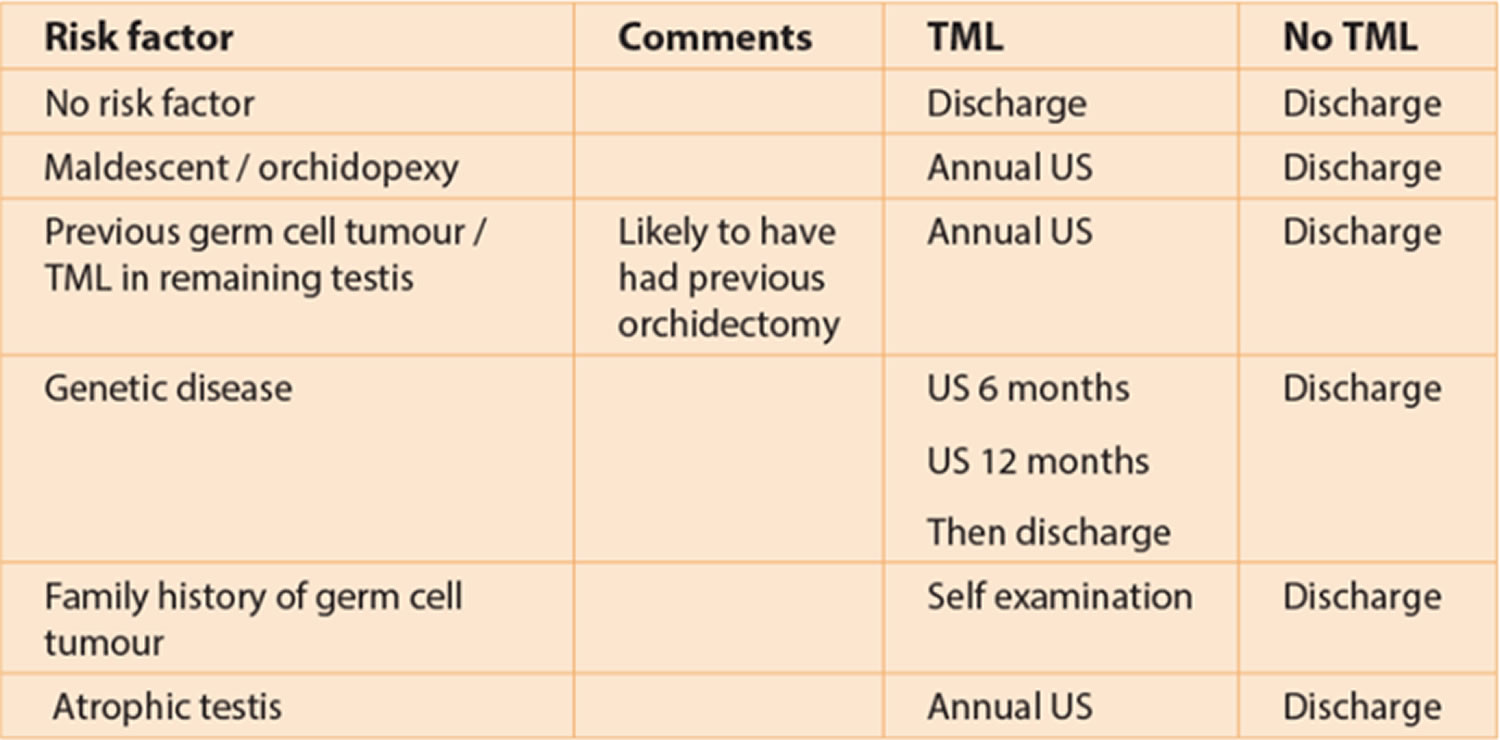
The following table has been designed by the European Society of Urogenital Radiology Scrotal Working Group to aid follow-up advice given to patients.
Table 1. European Society of Urogenital Radiology guidelines for testicular microlithiasis managment
Abbreviations: TML = testicular microlithiasis; US = ultrasound
Testicular microlithiasis association with male infertility
Testicular microlithiasis association with male infertility is still debated. Previous studies showed incidence of testicular microlithiasis ranging from to 20% in a subfertile population 20. Evidence showed that testicular microlithiasis was a testicular dysgenesis syndrome, which was postulated to underpin abnormalities related to male reproductive disorders 21. Reduction in sperm count and sperm motility in a man with microliths is attributable to testicular microlithiasis-related obstruction of seminiferous tubules present in 30 to 60% of patients with testicular microlithiasis 22. The obstruction of seminiferous tubules may cause secondary inflammation, increased intraseminiferous pressure and change the blood supply of testicles. Inflammation and calcification in the seminiferous tubules area bring about deterioration in sperm quality and cause subinfertility 22. Thomas et al. 20 reported a relationship between the degree of calcification and poor sperm function. The study showed a statistical difference between the number of investigations in those patients with minimal degrees of calcification and those with marked testicular microlithiasis [(analysed parameters: sperm migration test, namely sperm migration and sperm motility. Xu et al. 22 investigated the association between testicular microlithiasis and semen parameters in Chinese adult men with infertility intention. testicular microlithiasis is associated with worse semen parameters in adult men with infertility. The authors showed significant changes between testicular microlithiasis group versus non-testicular microlithiasis group in semen volume, sperm concentration and total motility 22. Testicular microlithiasis was reported to be more prevalent in patients with spermatogenic defects such as severe oligospermia and reduced testicular volume 22.
Subfertility is reported to be a risk factor for a testicular tumor. Bilateral testicular microlithiasis is indicative of CIS (carcinoma in situ) in subfertile men. De Gouveia Brazao et al. 23 reported that 20% of patients with bilateral testicular microlithiasis were diagnosed with CIS (carcinoma in situ). Therefore, the prevalence of CIS in subfertile men with bilateral testicular microlithiasis is significantly higher than in patients without testicular microlithiasis (0.5%) and with unilateral testicular microlithiasis (0%) 23. Thus, men with carcinoma in situ are at particular risk for invasive testicular germ cell tumor. An assessment of testicular microlithiasis is a valuable tool for the early diagnosis of this disease. Approximately 50% of carcinoma in situ progresses to germ cell tumor within 5 years 24. Nearly 20% of patients with a previous testicular germ cell tumor have testicular microlithiasis in their contralateral testes. Those patients have an increased risk ratio of 8.9 for concurrent carcinoma in situ compared with patients who do not have testicular microlithiasis 25.
Testicular microlithiasis causes
Testicular microlithiasis is a condition of unknown cause where calcium deposits form in the lumina of seminiferous tubules or arise from the tubular basement membrane components. The microliths are asymptomatic, do not cause pain, and are so small that they are impalpable. Shanmugasundaram et al. 26 reported a number of theories proposed in an attempt to explain the origin of testicular microlithiasis. Among them were hypotheses variously attributing testicular microlithiasis to a range of causes, including liquefaction of protoplasmic dendrites of a spermatocyte, ectopic oocytes in dysgenetic testes, displaced spermatogonia, undifferentiated or desquamated calcified cells, deposition of glycoprotein around the nidus of cell material sloughed into the tubular lumen and abnormal Sertoli cells 26.
Microliths can be seen in the testis as well as in extra-testicular structures such as the lungs and the central nervous system, with genetic factors also thought to play a role in their development. Mutation in the SLC34A2 gene (4p15) has been found to occur in patients with pulmonary alveolar microliths. Patients with this mutation are found to have testicular microlithiasis as well 27.
Based on the Renshew et al. 28 study, two types of testicular microliths have been described: hematoxylin bodies and lamellated calcifications. Under the optical and electron microscopes, microliths are found to consist of two zones, namely a central calcified zone and multi-layered envelope-stratified collagen fibers, both of which are covered with a thin fibrous capsule of spermatogenic epithelium. Microliths may occupy 30 to 40% of the seminiferous tubules and range in size from 50 to 400 μm. They do not typically affect Leydig cells and the majority of the uninvolved seminiferous tubules often have abnormal spermatogonia and reduced luminal diameters.
Health status, lifestyle characteristics and ethnicity of men with testicular microlithiasis
In general, there were limited differences in health and lifestyle among men with testicular microlithiasis and those who did not suffer from the disease. New data suggests some differences. Men with testicular microlithiasis reported significantly less physical exercise than men without microliths (38.6% vs. 48.2%) 29. The authors also suggest some discrepancies in food intake. Men with testicular microlithiasis consumed more crisps and popcorn than men without testicular microlithiasis (35.6% vs. 22.0%) 29. Crisps and popcorn contain acrylamide, which is known for its potential health hazards, but according to the American Cancer Society, it is not clear whether acrylamide consumption increases the risk of developing cancer 30. Mothers smoking during pregnancy have been associated with testicular cancer in the male offspring 31. Pederson et al. found a negligibly more widespread tendency for men affected with testicular microlithiasis to have been exposed to maternal smoking than men without it 29. Another potential analysed factor of testicular microlithiasis is men’s height (known risk factor of testicular cancer). Pederson et al. reported no differences in height between men with and without testicular microlithiasis 29. There exists an interesting piece of research concerning testicular microlithiasis and its relation to ethnicity and socioeconomic status. Based on the Pederson et al. study, black men had increased prevalence of testicular microlithiasis (odds ratio = 2.17) compared with white men. Whereas men from the most deprived socioeconomic groups had higher prevalence of testicular microlithiasis (odds ratio = 1.17) than men in the most affluent groups.
Testicular microlithiasis associations
- testicular germ cell tumor
- Klinefelter syndrome
- cryptorchidism
- testicular infarct
- testicular granulomatous disease
- infertility
- Down syndrome
- alveolar microlithiasis
Testicular microlithiasis symptoms
Testicular microlithiasis per se is asymptomatic and is usually found incidentally when the scrotal content is examined with ultrasound, or found in association with symptomatic conditions.
Testicular microlithiasis diagnosis
Testicular microlithiasis is most commonly diagnosed by ultrasound, as the microliths are clearly identified as hyperechoic foci which do not demonstrate posterior acoustic shadowing, less than 3mm in size. Different definitions exist about the diagnosis of microlithiasis, but the most universally accepted definition is that five of more microliths are seen per field of view on ultrasound (Figure 1). When testicular microlithiasis is widespread and seen throughout the testis, it is described as a ‘snow-storm’ appearance 32.
Although MRI is increasingly used in imaging of the testes, microliths are not clearly visible on this imaging modality as calcification, even when large in size.
With recent advances, ultrasound machines are able to produce higher resolution images, making testicular microlithiasis more clearly visible. Earlier literature reported that the incidence of microlithiasis on scrotal ultrasounds is approximately 0.6% and is usually an incidental finding 33.
Previously, there was evidence that suggested that testicular microlithiasis increased a patient’s risk of developing a germ cell tumour and annual ultrasound surveillance had been recommended. A study performed by Cast et al. 34 on 4819 patients suggested that microlithiasis is strongly associated with germ cell tumors, but other studies have failed to confirm this association, which therefore suggests that the condition is benign 35.
No formal guidelines existed regarding the need and frequency of ultrasound follow-up in patients found to have microlithiasis and different departments adopted local policies.
Testicular microlithiasis ultrasound
Ultrasound is the modality of choice for examining the testicular microlithiasis. Microlithiasis appears as small non-shadowing hyperechoic foci ranging in diameter from 1-3 mm. These foci occur within the testicular parenchyma and although usually distributed uniformly, may be distributed peripherally or segmentally 36.
One arbitrary grading system used on ultrasound is 37:
- Grade 1: 5-10 microcalcifications
- Grade 2: 11 to 20 microcalcifications
- Grade 3: 21 to 30 microcalcifications
- Grade 4: >30 microcalcifications
It is unclear if a grading system adds prognostic value.
Testicular microlithiasis treatment
Based on European data on the incidence of testicular cancer by age in the male population, follow-up for patients with testicular microlithiasis is recommended up to the age of 55 years 25. Management depends on the existing risk factors which are described by the European Society of Urogenital Radiology scrotal imaging subcommittee and European Association of Urology. The presence of testicular microlithiasis alone (without any coexistence risk factors) is not an indication for a regular scrotal ultrasound, it does not require biopsy or further ultrasound screening 38. When testicular microlithiasis is detected in conjunction with any risk factors (regardless of whether it is unilateral or bilateral) and provided that there is no focal mass within either testis, annual follow-up with ultrasound and monthly self-examination should be advised 10. Recommendation for testicular biopsy in testicular microlithiasis is still a hotly debated topic. Patients with small or atrophic testes with microliths are at increased risk of carcinoma in situ (CIS) 39. At orchidectomy in patients with germ cell tumor, if there is testicular microlithiasis in the contralateral testis, or if the contralateral testis is atrophic, biopsy of contralateral testis may be recommended in order to look for CIS (carcinoma in situ) 10.
Risk factors that need follow-up of patients with testicular microlithiasis:
- Previous germ cell tumor
- History of testicular maldescent
- History of orchidopexy
- Testicular atrophy <12 ml volume (the normal mean testicular volume is estimated at 18 ml) 40
- History of germ cell tumor in 1st degree relative (standardised incidence ratios for familial risk are 3.8-fold when a father and 7.6-fold when a brother have testicular cancer) 41.
- Costabile RA. How worrisome is testicular microlithiasis?. Curr Opin Urol. 2007;17 (6): 419-23. doi:10.1097/MOU.0b013e3282f0ffea
- Winter TC, Kim B, Lowrance WT, Middleton WD. Testicular Microlithiasis: What should you recommend? Am J Roentgenol. 2016;206:1164–1169.
- Pasquali D, Accardo G, Esposito D, et al. Testicular parenchymal abnormalities in Klinefelter syndrome: a question of cancer? Examination of 40 consecutive patients. Asian J Androl. 2015;17:154.
- Cebeci AN urcan, Aslanger A, Ozdemir M. Should patients with Down syndrome be screened for testicular microlithiasis? Eur J Pediatr Surg. 2015;25:177–180.
- Balawender K, Orkisz S, Wisz P. Testicular microlithiasis: what urologists should know. A review of the current literature. Cent European J Urol. 2018;71(3):310–314. doi:10.5173/ceju.2018.1728 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6202617
- Winter TC, Kim B, Lowrance WT, Middleton WD. Testicular Microlithiasis: What Should You Recommend?. AJR. American journal of roentgenology. 206 (6): 1164-9. doi:10.2214/AJR.15.15226
- Balawender K, Orkisz S, Wisz P. Testicular microlithiasis: what urologists should know. A review of the current literature. Cent European J Urol. 2018;71(3):310–314. doi:10.5173/ceju.2018.1728 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6202617/
- Backus ML, Mack LA, Middleton WD, King BF, Winter TC, True LD. Testicular microlithiasis: imaging appearances and pathologic correlation. Radiology. 1994;192:781–785.
- Pedersen MR, Graumann O, Hørlyck A, et al. Inter-and intraobserver agreement in detection of testicular microlithiasis with ultrasonography. Acta Radiol. 2016;57:767–772.
- Richenberg J, Belfield J, Ramchandani P, et al. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol. 2014;25:323–330.
- Tan IB, Ang KK, Ching BC et-al. Testicular microlithiasis predicts concurrent testicular germ cell tumors and intratubular germ cell neoplasia of unclassified type in adults: a meta-analysis and systematic review. 2010;doi:10.1002/cncr.25231
- DeCastro BJ, Peterson AC, Costabile RA. A 5-year followup study of asymptomatic men with testicular microlithiasis. J. Urol. 2008;179 (4): 1420-3. doi:10.1016/j.juro.2007.11.080
- Dagash H, Mackinnon EA. Testicular microlithiasis: what does it mean clinically?. BJU Int. 2007;99 (1): 157-60. doi:10.1111/j.1464-410X.2006.06546.x
- Richenberg J, Belfield J, Ramchandani P et-al. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol. 2015;25 (2): 323-30. doi:10.1007/s00330-014-3437-x
- Wang T, Liu LH, Luo JT, Liu TS, Wei AY. A meta-analysis of the relationship between testicular microlithiasis and incidence of testicular cancer. Urol J. 2015;12:2057–2064.
- DeCastro BJ, Peterson AC, Costabile RA. A 5-Year Followup Study of Asymptomatic Men With Testicular Microlithiasis. J Urol. 2008;179:1420–1423.
- Patel K V, Navaratne S, Bartlett E, et al. Testicular Microlithiasis: Is Sonographic Surveillance Necessary? Single Centre 14 Year Experience in 442 Patients with Testicular Microlithiasis. Ultraschall der Medizin. 2016;37:68–73.
- Pedersen MR, Osther PJS, Soerensen FB, Rafaelsen SR. Testicular Microlithiasis: Patient Compliance in a Two-Year Follow-Up Program. Ultrasound Int Open. 2016;2:E113–E116.
- Sharmeen F, Rosenthal MH, Wood MJ, Tirumani SH, Sweeney C, Howard SA. Relationship between the pathologic subtype/initial stage and microliths in testicular germ cell tumors. J Ultrasound Med. 2015;34:1977–1982.
- Thomas K, Wood SJ, Thompson AJM, Pilling D, Lewis-Jones DI. The incidence and significance of testicular microlithiasis in a subfertile population. Br J Radiol. 2000;73:494–497.
- Tan M-H, Eng C. Testicular microlithiasis: recent advances in understanding and management. Nat Rev Urol. 2011;8:153–163.
- Xu C, Liu M, Zhang FF, et al. The association between testicular microlithiasis and semen parameters in Chinese adult men with fertility intention: Experience of 226 cases. Urology. 2014;84:815–820.
- De Gouveia Brazao CA, Pierik FH, Oosterhuis JW, Dohle GR, Looijenga LHJ, Weber RFA. Bilateral testicular microlithiasis predicts the presence of the precursor of testicular germ cell tumors in subfertile men. J Urol. 2004;171:158–160.
- von der Maase H, Rørth M, Walbom-Jørgensen S, et al. Carcinoma in situ of contralateral testis in patients with testicular germ cell cancer: study of 27 cases in 500 patients. Br Med J (Clin Res Ed). 1986;293:1398–1401.
- Tan IB, Ang KK, Ching BC, Mohan C, Toh CK, Tan MH. Testicular microlithiasis predicts concurrent testicular germ cell tumors and intratubular germ cell neoplasia of unclassified type in adults: A meta-analysis and systematic review. Cancer. 2010;116:4520–4532.
- Kekre N, Shanmugasundaram R, Singh Jc. Testicular microlithiasis: Is there an agreed protocol? Indian J Urol. 2007;23:234.
- Corut A, Senyigit A, Ugur SA, et al. Mutations in SLC34A2 Cause Pulmonary Alveolar Microlithiasis and Are Possibly Associated with Testicular Microlithiasis. Am J Hum Genet. 2006;79:650–656.
- Renshaw AA. Testicular calcifications: Incidence, histology and proposed pathological criteria for testicular microlithiasis. J Urol. 1998;160:1625–1628.
- Pedersen MR, Møller H, Rafaelsen SR, Jørgensen MMB, Osther PJ, Vedsted P. Characteristics of symptomatic men with testicular microlithiasis – A Danish cross-sectional questionnaire study. Andrology. 2017;5:556–561.
- American Cancer Society . Cancer Facts & Figures 2016. Cancer Facts Fig. 2016. 2016. pp. 1–9.
- Pettersson A, Kaijser M, Richiardi L, Askling J, Ekbom A, Akre O. Women smoking and testicular cancer: One epidemic causing another? Int J Cancer. 2004;109:941–944.
- Roberts IS, Loughran CF. Case report: the ultrasound appearances of testicular microlithiasis ‘snow-storm’ testis: a case complicated by testicular seminoma. Clin Radiol 1993;47(L1):65-7.
- Bennett HF, Middleton WD, Bullock AD, Teefey SA. Testicular Microlithiasis: US Follow-up. Radiology 2001;218:359-63.
- Cast JE, Nelson WM, Early AS, et al. Testicular Microlithiasis. Prevalence and Tumour Tisk in a Population Referred for Scrotal Sonography. AJR 2000;175(6):1703-6.
- Richenberg J, Brejt N. Testicular microlithiasis: is there a need for surveillance in the absence of other risk factors? Eur Radiol 2012;22(11):2540-6.
- Cast JE, Nelson WM, Early AS et-al. Testicular microlithiasis: prevalence and tumor risk in a population referred for scrotal sonography. AJR Am J Roentgenol. 2000;175: 1703-1706. 10.2214/ajr.175.6.1751703 https://www.ajronline.org/doi/full/10.2214/ajr.175.6.1751703
- Richenberg J, Brejt N. Testicular microlithiasis: is there a need for surveillance in the absence of other risk factors?. European radiology. 22 (11): 2540-6. doi:10.1007/s00330-012-2520-4
- Montgomery JS, Bloom DA. The diagnosis and management of scrotal masses. Med Clin North Am. 2011;95:235–244.
- Hoei-Hansen CE, Olesen IA, Jorgensen N, et al. Current approaches for detection of carcinoma in situ testis. Int J Androl. 2007;30:398–404.
- Goede J, Hack WWM, Sijstermans K, et al. Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr. 2011;76:56–64.
- Hemminki K, Chen B, Schettler T, Assinder SJ. Familial risks in testicular cancer as aetiological clues. In: Int J Androl. 2006;29:205–210.