Testosterone replacement therapy
Testosterone replacement therapy (TRT) is an important treatment option for men with low testosterone and symptomatic hypogonadism. Male hypogonadism also known as testosterone deficiency, is defined as having one or more symptoms attributable to low circulating levels of testosterone (serum total testosterone <300 ng/dL) 1, 2. Although controversy remains regarding indications for testosterone replacement therapy (TRT) in aging men due to lack of large-scale, long-term studies assessing the benefits and risks of testosterone-replacement therapy in men, reports indicate that TRT has been shown to increase serum testosterone to physiologic levels, which may produce a wide range of benefits for men with hypogonadism that include improve libido, improve erectile dysfunction, improve overall sexual function, increase bone mineral density, decrease body fat mass, and increase lean body muscle mass, increase energy, improve mood, erythropoiesis, cognition, quality of life and cardiovascular disease 3. The TIMES2 (Testosterone replacement In hypogonadal men with either MEtabolic Syndrome or type 2 diabetes) study 4 showed that testosterone replacement therapy (TRT) improved glycemic control, lipid levels, sexual function and libido in men with type 2 diabetes and/or metabolic syndrome, with no corresponding increase in adverse events.
The most controversial area for TRT is the issue of risk, especially possible stimulation of prostate cancer by testosterone, even though no evidence to support this risk exists. A 2021 study 5 concluded that compared to TRT-untreated patients, TRT-treated patients may not have increased risks for disease progression in prostate cancer. However, the quality of currently available evidence is extremely poor. TRT may be harmful in men with advanced prostate cancer, in those with untreated prostate cancer undergoing active surveillance, and in those with successfully treated prostate cancer but having high-risk disease 5. The United States Food and Drug Administration (FDA) stated, in all testosterone package inserts, that TRT is contraindicated in men with known or suspected prostate cancer, but it did not substantiate this contraindication 6. The clinical guidelines by Endocrine Society recommend against treating hypogonadism in men with prostate cancer, citing lack of sufficient data to make a general recommendation in men previously treated for prostate cancer (‘recommendation with low quality evidence’) 7. Meanwhile, the European Association of Urology stated that there is no conclusive evidence that TRT increases the risk of prostate cancer (‘level of evidence=4’), and that men with prostate cancer can receive TRT with careful monitoring for prostate safety (‘level of evidence=3’) 8. Similarly, the recent treatment guideline by the American Urologic Association stated that patients should be informed that there is inadequate evidence for TRT (‘expert opinion’), but TRT can be considered in men who have undergone radical prostatectomy with favorable pathology (e.g., negative margins, negative seminal vesicles, negative lymph nodes), without PSA recurrence 1.
Currently, there are a variety of widely available testosterone formulations, including topical gels and patches, intramuscular injections, subcutaneous pellets, and oral/buccal formulations that provide clinicians and male patients the opportunity to personalize replacement therapy to reestablish testosterone levels and improve testosterone deficiency symptoms 9:
- Exogenous Testosterone Replacement Therapy
- Mechanism of action: Restore testosterone levels by supplementing various preparations of natural testosterone
- Types: Oral, buccal, intramuscular, transdermal, subdermal, and nasal preparations
- Gel. There are several gels and solutions available, with different ways of applying them. Depending on the brand, you rub the testosterone into your skin on your upper arm or shoulder (AndroGel, Testim, Vogelxo) or apply it to the front and inner thigh (Fortesta). Your body absorbs testosterone through your skin. Don’t shower or bathe for several hours after a gel application, to be sure it gets absorbed. Side effects include skin irritation and the possibility of transferring the medication to another person. Avoid skin-to-skin contact until the gel is completely dry, or cover the area after an application.
- Injection. Testosterone cypionate (Depo-Testosterone) and testosterone enanthate are given in a muscle or under the skin. Your symptoms might waver between doses depending on the frequency of injections. You or a family member can learn to give testosterone injections at home. If you’re uncomfortable giving yourself injections, member of your care team can give the injections. Testosterone undecanoate (Aveed) is given by deep intramuscular injection, typically every 10 weeks. It must be given at your provider’s office and can have serious side effects.
- Patch. A patch containing testosterone (Androderm) is applied each night to your thighs or torso. A possible side effect is severe skin reaction.
- Gum and cheek (buccal cavity). A small putty-like substance, gum-and-cheek testosterone replacement delivers testosterone through the natural depression above your top teeth where your gum meets your upper lip (buccal cavity). This product, taken three times a day, sticks to your gumline and allows testosterone to be absorbed into your bloodstream. It can cause gum irritation.
- Nasal. This testosterone gel (Natesto) can be pumped into the nostrils. This option reduces the risk that medication will be transferred to another person through skin contact. Nasal-delivered testosterone must be applied twice in each nostril, three times daily, which might be more inconvenient than other delivery methods.
- Implantable pellets. Testosterone-containing pellets (Testopel) are surgically implanted under the skin every three to six months. This requires an incision.
- Advantages:
- Multiple routes of administration depending on patient preference and needs
- Improves low testosterone levels and maintains virilization in hypogonadal men
- Particularly useful for treating hypergonadotropic hypogonadism in which testosterone levels are low but LH levels are high
- Limitations:
- Because it mimics functions of endogenous testosterone, it also exerts negative feedback on the hypothalamus and pituitary gland, reducing LH and decreasing endogenous intratesticular testosterone production
- Decreases intratesticular testosterone, impairs spermatogenesis, and results in male infertility
- Therapies that Increase Endogenous Testosterone
- Mechanism of action: Multiple mechanisms of action by affecting various points along the hypothalamic-pituitary-gonadal axis to increase
- Types: Selective estrogen receptor modulators (SERMs), Gonadotropins, Aromatase inhibitors, Selective androgen receptor modulators (SARMs), Leydig stem cell transplantation
- Advantages:
- Able to increase serum and intratesticular testosterone levels
- Greater likelihood of preserving hypothalamic-pituitary-gonadal axis axis function and, subsequently, spermatogenesis and fertility
- Limitations:
- Potential unintended decline in quality of semen and complications related to changes in estrogen levels
- None have been approved by the US FDA specifically for men with hypogonadism or infertility.
Table 1. Testosterone replacement therapy formulations
| Names | Strengths | Limitations | Comparative Efficacy | References |
|---|---|---|---|---|
| Testosterone undecanoate |
|
|
| Nieschlag 2015 11, Swerdloff et al. 2020 12 |
| Striant (testosterone buccal) |
|
| Tsametis & Isidori 2018 15, Dinsmore & Wyllie 2012 16 | |
Long acting testosterone:
Extra-long acting testosterone:
|
|
|
| Nieschlag 2015 11, Tsametis & Isidori 2018 15, Middleton et al. 2015 18 |
Testosterone Gel:
Testosterone Solution:
Testosterone Patch:
|
|
|
| Tsametis & Isidori 2018 15 |
Testosterone implant:
|
|
| Tsametis & Isidori 2018 15 | |
Testosterone nasal:
|
|
|
| Tsametis & Isidori 2018 15, Gronski et al. 2019 23, Rogol et al. 2018 24 |
Oral testosterone
Currently, the most commonly available oral preparation is testosterone undecanoate 9. This preparation is able to avoid first passing through the liver due to its aliphatic side chain which allows it to be absorbed by the lymph system and directly enter the circulatory system 25. This formulation requires more frequent and larger doses in order to effectively replete testosterone therapy 26. Resent study reported that although oral testosterone undecanoate is a safe and effective means to treat hypogonadal men, it was related to a small but significant increased systolic blood pressure 12. While it has shown efficacy, pharmacokinetic analysis has proven that it is difficult to predict absorption due to high intra- and inter-individual variability in serum concentrations. Oral Testosterone undecanoate also includes a black box warning regarding blood pressure increases and risk of cardiac events, which makes its use a major concern for older men. An additional group of oral agents exist that have been available for decades; these are 17-alpha alkylated androgens (methyltestosterone and fluoxymesterone). However, many reports have implicated these oral preparations in cholestatic jaundice, a hepatic cystic disease called peliosis hepatis, and hepatoma 10. Therefore, oral testosterone undecanoate are no longer suggested for use due to the discovery of extreme liver toxicity and minimal effectiveness in resolving hypogonadal symptoms.
Buccal testosterone
Currently, the most commonly available buccal preparation is a buccal tablet (Striant®) which exists as a potential therapeutic option for patients who can tolerate it 9. The tablets are mucoadhesive and are applied to a depression in the gum above the upper incisors, slowly releasing testosterone into the circulatory system. Twice daily applications of 30 mg tablets result in stable serum levels of testosterone. The benefits of this method are that the effects can quickly be reversed and the release of medication mimics circadian rhythms 27. However, it has also been shown that 3-15% of patients may experience irritation, inflammation, or gingivitis while also having difficulty from complaints due to the unique method of delivery 15. These potential side effects should be taken into consideration when opting for this method of TRT.
Testosterone intramuscular injections
Intramuscular injection of testosterone has proven to be effective in initiating and maintaining virilization in all hypogonadal men 27. Current options include long-acting (testosterone enanthate and testosterone cypionate) and extra-long-acting (testosterone undecanoate) variants. The long-acting options are esters of testosterone which make the molecule more lipophilic than the natural variant. This results in their storage and gradual release, thereby prolonging the presence of testosterone in the blood 28. Administration of long-acting intramuscular testosterone usually occurs as 50–100 mg every week, 100–200 mg every 2 weeks, or 250 mg every 3 weeks and has shown consistent results 27. The advantages of long-acting options are that they are extremely effective, easily accessible, and cost effective, and they free patients from daily administration. Conversely, the disadvantages are the need for patients to be comfortable with deep intramuscular administration of the testosterone solution and fluctuations in energy, mood, and libido in many patients.
An extra-long-acting option exists with testosterone undecanoate in oil, which is also one of the common oral options. When injected alongside castor oil, a half-life of 34 days is observed 25. Each dose is 750 mg in 3mL of oil. The recommendations for administration in the United States are to administer a dose into the buttocks, followed by a second dose 4 weeks later, and then subsequent doses every 10 weeks. Access to this option is regulated by a restricted program called the AVEED Risk Evaluation and Mitigation Strategy (REMS) Program and is not recommended consistently. Although the injectable preparation is generally considered safe, patients must be monitored for pulmonary oil micro-embolism (POME) 29. Symptoms of POME (pulmonary oil micro-embolism), such as sudden onset of non-productive cough associated with faintness following injections, have been observed in 1.5% of patients 30. Additionally, anaphylactic responses have been observed in some patients, requiring 30-min post-dose monitoring to rule out this side effect 27. The advantages of less frequent administration come at the cost of various systemic side effects that require regular monitoring.
Transdermal testosterone
Transdermal testosterone preparations are known to provide very stable and effective improvements in serum testosterone levels due to their pharmacokinetic profile which mimics physiological diurnal variations. Transdermal testosterone preparations include preparations that incorporate solutions, gels, and patches. Three testosterone gels (AndroGel®, Testim®, and Fortesta®), one solution (Axiron®), and one patch (Androderm®) are available in the United States 27. Transdermal testosterone substitution has the following advantages: a) since they have a short duration of action, the preparations can be quickly discontinued if any adverse effect occurs and the testosterone levels will then quickly decline, and b) they are easy to use and easily accessible to many. Conversely, the disadvantages include the need for daily administration, relatively high cost, skin irritation, and the risk of contact transfer to another person 16. The high risk of unintentional transfer led the FDA to require testosterone gel products to include a Blackbox warning. They have also been shown to be less effective in obese individuals and require higher doses 27. However, due to the ease of use, accessibility, and reversibility, these are often a first-line choice for patients who require testosterone substitution.
Subdermal testosterone
Subdermal pellet implants (Testopel®) are a viable option for testosterone replacement and result in stable testosterone levels. The pellets are implanted into the subdermal fat of the buttocks, lower abdominal wall, or thigh with a tunneling technique using a local anesthetic 31. It is recommended that three to six 75 mg pellets should be implanted every 4-6 months (150 mg-450 mg) to achieve stable testosterone levels in the normal range 27. The benefits of this method include guaranteed compliance and lack of transference to persons who may come in contact with it 27. Adverse effects include pellet extrusion, infection, and fibrosis 32. Due to the need for a surgical procedure for implantation and the potential side effects, this form of TRT is usually not recommended as first-line treatment 18.
Nasal testosterone
A nasal testosterone gel, known as Natesto®, is now available in the United States for treatment of male hypogonadism. Studies have shown successful treatment with this new method and have exhibited consistent improvements in testosterone deficiency 33. This gel is administered in the nostrils via a metered dose pump which delivers 5.5mg per actuation. The recommended dosage is to administer one actuation in each nostril three times daily, resulting in a daily dosage of 33 mg/day. This method has shown a reduction in gel transfer to a partner or child due to the inconspicuous application site, and it provides ease of use as a non-invasive option. Most importantly, preliminary studies have demonstrated that Natesto® increases serum testosterone while also maintaining FSH, LH, and semen parameters 34. Natesto nasal testosterone gel can maintain spermatogenesis in >95% of men, in contrast to injections or gels 22. Also, significant differences have been observed between duration of action, frequency of administration and clinical pharmacokinetic profile of Natesto compared to long-acting testosterone injections, gels and pellets 24. On the other hand, more than 3% of men have reported experiencing rhinorrhea, epistaxis, nasopharyngitis, sinusitis, and nasal scab as a result of this medication 27. Unique among the formulations of exogenous TRT, nasal formulations of testosterone are showing promise in alleviating symptoms of low testosterone without adversely affecting the hypothalamic-pituitary-gonadal (HPG) axis.
Table 2. Therapies that increase endogenous testosterone
| Class | Mechanism of Action | Strengths | Limitations | References |
|---|---|---|---|---|
| Selective Estrogen Receptor Modulators (SERMs) |
|
|
| El Meliegy et al. 2018 35, Chehab et al. 2015 36 |
| Gonadotropins |
|
|
| Lee & Ramasamy 2018 37, El Meliegy et al. 2018 35, Dohle et al. 2016 38 |
| Aromatase Inhibitors |
|
|
| Crosnoe et al. 2013 39, El Meliegy et al. 2018 35, Ribeiro et al. 2016 40 |
| Selective Androgen Receptor Modulators (SARMs) |
|
|
| Narayanan et al. 2018 41, Solomon et al. 2018 42 |
| Leydig Stem Cell Transplantation |
|
|
| Zang et al. 2017 43, Beattie et al. 2015 44, Arora et al. 2019 45 |
Selective estrogen receptor modulators
Selective estrogen receptor modulators (SERMs) are a group of pharmaceuticals that act as competitive inhibitors of estrogen receptors in the hypothalamus and pituitary with the increasing release of GnRH and gonadotrophins, subsequently increasing production of intra-testicular testosterone and improving spermatogenesis 46. Clomiphene citrate is a SERM that has been used in the treatment of men with azoospermia, oligozoospermia, and unexplained infertility, in addition to treating male hypogonadism. It has been demonstrated that Clomiphene citrate leads to a moderate increase in FSH, LH, total testosterone, and the concentration of sperm in these men 47. Also, in men with late-onset hypogonadism, long-term Clomiphene citrate therapy can be prescribed as a testosterone replacement 46. Although thromboembolic events and carcinogenesis have been reported as adverse effects of SERMs 48, current ongoing research shows that this group of pharmaceuticals has great potential for future applications 49.
Gonadotropins
Gonadotropin therapy is used to manage infertile patients with low testosterone concentrations, with the aim of recovering normal sperm production. In such patients, stimulating spermatogenesis requires human chorionic gonadotropin (hCG) therapy alone or in combination with human menopausal gonadotrophins (hMGs), urinary FSH, or recombinant FSH 46. It has been recommended that TRT with concomitant use of hCG can be prescribed in hypogonadal men who seek fertility treatment 50. Treatment with hCG can preserve normal sperm production in men undergoing TRT by maintaining intratesticular testosterone concentrations 51. Potential side effects of gonadotropin therapy include gynecomastia, headache, fatigue, and mood changes 52. A retrospective study found that treatment of hypogonadal men with TRT and co-administration of low dose hCG maintains semen parameters in these men 53. Coviello et al. 54 evaluated intratesticular testosterone concentrations from men with normal reproductive physiology who received testosterone enanthate in combination with hCG (125, 250, or 500 IU). A linear increase in intratesticular testosterone concentrations with increased hCG confirms that moderately low dose hCG preserves testicular function in endogenous gonadotropin-suppressed healthy men 54. A systematic review showed that hypogonadal men desiring fertility preservation while benefiting from TRT can be prescribed SERMs or testosterone plus a low dose of hCG. Prescription of hCG alone or in combination with FSH preparations can be recommended to hypogonadotropic hypogonadal patients who need to be treated for infertility 46. In younger men with low serum testosterone levels, treatment with hCG or with Clomiphene citrate will increase serum and intra-testicular testosterone levels without resulting in declining gonadotropin levels via feedback inhibition 39. Overall, human chorionic gonadotropin (hCG) is an efficient and safe alternative or adjunct to TRT in men desiring both fertility and treatment for symptoms of hypogonadism. However, a drawback of hCG is that this is an injectable agent.
Aromatase inhibitors
Aromatase inhibitors are a class of drugs that act by blocking aromatase P450 enzymes, consequently normalizing the testosterone/E2 ratio and improving spermatogenesis 35. It has been shown that an aromatase inhibitor (Testolactone) is an effective drug for alleviating infertility in hypogonadotropic hypogonadal obese men 36. However, Testolactone is no longer commercially available. Some men with severe spermatogenic failure who have too much aromatase activity, decreased serum levels of testosterone, increased E2 levels, or impaired testosterone/E2 ratio might benefit from the use of aromatase inhibitors in treating infertility 46. Although no important side effects have been observed with consumption of an aromatase inhibitor for elevating serum testosterone concentrations (46), one study has reported a slight insignificant increase in the level of serum prostate-specific antigen (PSA) in elderly men with low or borderline hypogonadism 55. However, the use of aromatase inhibitors is not suggested due to the absence of long-term data and a limited amount of published literature 56.
Selective androgen receptor modulators
The pathological effects arising from testosterone has resulted in the search for tissue-selective agonists of the androgen receptor which could potentially trigger the androgen receptor in a tissue-selective manner 41. Selective androgen receptor modulators (SARMs) are a new class of androgen receptor ligands that bind to androgen receptors, depending on the chemical structure of each SARM. Consequently, SARMs lead to anabolic activity while avoiding most of the side effects caused by anabolic steroids 42. The tissue specificity of SARMs has led to the notion that they may be a promising treatment for a wide variety of diseases 42. SARMs have been studied in the treatment of male hypogonadism and their use is being investigated in clinical trials (6, 60). Miner et al. (2007), in a rat model of sexual behavior, showed that SARMs can lead to increased sexual function. In this model treatment of male rats with 100 mg/kg of a synthetic SARM, LGD226, increased number of mounts, intromissions, and ejaculation 38. Jones et al. 57 demonstrated that administration of S-23 with estradiol benzoate in male rats led to inhibition in spermatogenesis and the suppression of FSH and LH levels. However, these effects are completely reversible. Chen et al. 58 reported that C-6, a novel SARM, can mimic in vivo endocrine effects of testosterone. Administration of C-6 significantly inhibited spermatogenesis and decreased testosterone concentration, epididymal and testicular size (63). Another study showed that SARM-2f, a novel SARM compound, can restore the sexual behavior in castrated male rats 59. Although SARMs represent an attractive therapeutic approach, further studies are required for the safe use of these medications 42.
Leydig stem cell transplantation
Adult Leydig cells found in the interstitial space of the testis, are the primary source of testosterone production in males 60. Adult Leydig cells undergo a four-stage process of differentiation, beginning with undifferentiated mesenchymal cells (also known as Leydig stem cells [LSCs]) and progressing to progenitor Leydig cells, immature Leydig cells, and finally to adult Leydig cells 61. Leydig stem cells do not have steroidogenic capabilities; however, they are unique in that they are capable of replicating indefinitely and differentiating to functioning adult Leydig cells 61. This suggests the possibility of having an endless pool of progenitor cells capable of replacing dysfunctional adult Leydig cells and restoring testosterone production in men with low testosterone 62. Arora et al. 45 showed that Leydig stem cells in combination with Sertoli and myoid cells underwent self-renewal as well as differentiation into mature Leydig cells. Adult Leydig cells act in the hypothalamic-pituitary-gonadal axis: an increase in LH stimulates the first step of testosterone synthesis in adult Leydig cells; conversely, an increase in testosterone production by adult Leydig cells decreases LH production by the pituitary 63. Dysfunction at any point along the hypothalamic-pituitary-gonadal axis axis can lead to testosterone deficiency 43.
Beattie et al. 44 demonstrated increased testosterone production in rat models by directly stimulating aged adult Leydig cells with translocator protein, a ligand involved in transporting cholesterol from the cytosol to mitochondria. This process was independent of LH regulation and treated aged adult Leydig cells had equivalent testosterone production to young adult Leydig cells. Other studies have shown great promise in increasing testosterone production at more upstream regulation points. Jiang et al. 64 showed that transplantation of nestin-positive Leydig stem cells into the testis of mice with impaired adult Leydig cells caused differentiation into functional adult Leydig cells and an increase in testosterone production. Zhang et al. 13 identified that transplanting human p75+ Leydig stem cells into EDS-treated rats (inducing ALC dysfunction) successfully replaced the impaired adult Leydig cells and partially restored testosterone production. Arora et al. 45 demonstrated a novel form of Leydig stem cell transplantation by autografting Leydig stem cells in combination with Sertoli cells and peritubular myoid cells into the subcutaneous, rather than the testicular, tissue of hypogonadal mouse models. This also resulted in differentiation of Leydig stem cells into adult Leydig cells and increased testosterone production 45.
What is Testosterone?
Testosterone is the major male sex hormone and is produced by the male testes in men and to a lesser extent by the adrenal glands in both men and women. In men, testosterone is thought to regulate sex drive (libido), bone mass, fat distribution, promotes gains in muscle mass and strength when combined with resistance training, the production of red blood cells and controlling fertility and the development of sperm. Testosterone also plays an important part in the development of ‘male sex characteristics’ such as a deeper voice and certain patterns of muscle development and hair growth. Testosterone helps bring on the physical changes that turn a boy into a man. This time of life is called puberty. Adolescent boys with too little testosterone may not experience normal masculinization. For example, the genitals may not enlarge, facial and body hair may be scant and the voice may not deepen normally. In women or anyone with ovaries, testosterone impacts overall growth as well as development of muscle and reproductive tissue.
A small amount of circulating testosterone is converted to estradiol, a form of estrogen. As men age, they often make less testosterone, and so they produce less estradiol as well. Thus, changes often attributed to testosterone deficiency might be partly or entirely due to the accompanying decline in estradiol.
Testosterone is now widely prescribed to men whose bodies naturally produce low levels. But the levels at which testosterone deficiency become medically relevant still aren’t well understood. Normal testosterone production varies widely in men, so it’s difficult to know what levels have medical significance. Testosterone hormone’s mechanisms of action are also unclear.
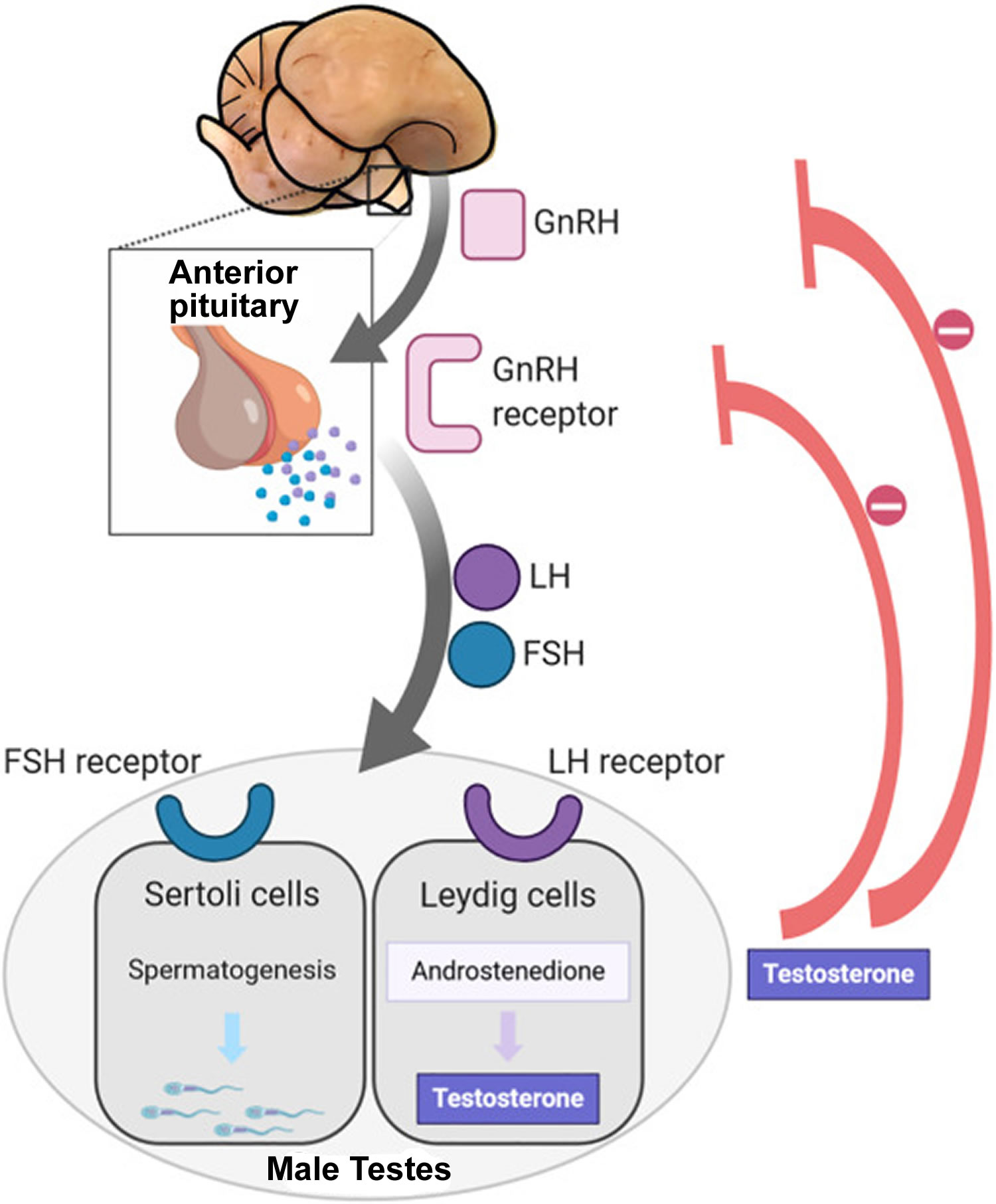
The amount of testosterone synthesized is regulated by the hypothalamic–pituitary–testicular axis (Figure 1) 65. The brain and pituitary gland, a small gland at the base of the brain, control production of testosterone by the testes. In males, testosterone is synthesized primarily in the testes (testicles) by the Leydig cells. The number of Leydig cells in turn is regulated by luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In addition, the amount of testosterone produced by existing Leydig cells is under the control of LH, which regulates the expression of 17β-hydroxysteroid dehydrogenase 66. When testosterone levels are low, gonadotropin-releasing hormone (GnRH) is released by the hypothalamus, which in turn stimulates the pituitary gland to release FSH (follicle-stimulating hormone) and LH (luteinizing hormone). These latter two hormones stimulate the testis to synthesize testosterone. Finally, increasing levels of testosterone through a negative feedback loop act on the hypothalamus and pituitary to inhibit the release of GnRH and FSH/LH, respectively (Figure 1).
Prior to puberty, there is no sex difference in circulating testosterone concentrations or athletic performance, but from puberty onward a clear sex difference in athletic performance emerges as circulating testosterone concentrations rise in men because testes (testicles) produce 30 times more testosterone than before puberty with circulating testosterone exceeding 15-fold that of women at any age 67. There is a wide sex difference in circulating testosterone concentrations and a reproducible dose-response relationship between circulating testosterone and muscle mass and strength as well as circulating hemoglobin in both men and women. These dichotomies largely account for the sex differences in muscle mass and strength and circulating hemoglobin levels that result in at least an 8% to 12% performance-enhancing advantage in men.
Testosterone is the major sex hormone in males and testosterone makes a man look and feel like a man. In a man, testosterone hormone helps 68:
- The development of the penis and testes (testicles)
- The deepening of the voice during puberty
- The appearance of facial and pubic hair starting at puberty; later in life, it may play a role in balding (androgenic alopecia)
- Promote gains in muscle mass and strength when combined with resistance training 69.
- Bone growth and strength
- Growth spurts in puberty
- Sex drive (libido) and erections
- Sperm production (spermatogenesis)
- Fat distribution
- Make red blood cells
- Maintain normal mood and boost energy
There may be other important functions of testosterone hormone that have not yet been discovered.
Testosterone in the blood can be either bound or free:
- Bound testosterone is attached to proteins such as sex-hormone-binding-globulin (SHBG) and albumin. Most testosterone (approximately 60–70%) is transported in the blood to target tissues bound to sex hormone-binding globulin (SHBG), which in men is also called testosterone-binding globulin. About 30–40% of testosterone is loosely bound to albumin. This majority supply of protein-bound testosterone acts as a surplus of testosterone hormone for the body 70.
- Free testosterone, the active form, is all the remaining testosterone that is not bound to other substances. The small amounts of free testosterone (approximately 0.5–2%) in the blood act at the level of the tissues, primarily the seminal vesicles, bone, muscle, and prostate gland 70.
Historically, only free testosterone was thought to be the biologically active component. However, testosterone is weakly bound to serum albumin and dissociates freely in the capillary bed, thereby becoming readily available for tissue uptake. All non-SHBG-bound testosterone is therefore considered bioavailable.
Decreased testosterone levels indicate partial or complete hypogonadism. Hypogonadism is a clinical syndrome that is characterized by low serum testosterone levels and the presence of symptoms including low libido, fatigue and sexual dysfunction, among others 71, 72, 73. The incidence of hypogonadism increases with age and affects approximately 4-5 million men in the United States 74.
Some men with low testosterone do not have any symptoms. Early symptoms (changes you feel) and signs (abnormalities that your doctor finds) of low testosterone in men include 75:
- A drop in sex drive. It’s normal to feel less interested in sex as you get older. But, it is not usually normal to have no interest in sex.
- Problems having an erection
- Low sperm count
- Enlarged or tender breasts
Later, low testosterone can lead to decreased muscle size and strength, bone loss, less energy, sleep problems such as insomnia, lower fertility, increase in body fat, depression and trouble concentrating. Some things can temporarily lower testosterone, for instance, too much exercise, poor nutrition, or serious illness. Living a healthy lifestyle with regular exercise and a good diet helps maintain normal testosterone levels.
Factors that may decrease production of testosterone can occur with:
- Aging: Testosterone levels gradually reduce as men age, by 1%–2% per year after the age of 30 -40 years 76, 77. This effect is sometimes referred to as andropause or late-onset hypogonadism (LOH) 78. However, on average serum levels of testosterone still remain within the normal range of young men 79. Furthermore, decreases in testosterone concentrations with age are gradual and vary between individuals, with higher rates of decline in men with adiposity and comorbid diseases 80. The age-dependent reference range for testosterone has not been defined and the criteria on cutoff levels for hypogonadism remain somewhat controversial 77.
- Exercise: Resistance training increases testosterone levels 81, however, in older men, that increase can be avoided by protein ingestion 82. Chronic stress from too much exercise (overtraining syndrome) and endurance training in men may lead to lower testosterone levels 83.
- Nutrients:
- Vitamin A deficiency may lead to sub-optimal plasma testosterone levels 84.
- Vitamin D supplementation might increase testosterone levels 85. However, results from preliminary randomized controlled trials are conflicting. Vitamin D treatment had no effect on testosterone levels in middle-aged healthy men with normal baseline testosterone 86. In male patients with advanced heart failure and low vitamin D, a daily vitamin D3 supplement of 4000 IU for 3 years did not prevent the decline in testosterone indices 87.
- Zinc deficiency lowers testosterone levels 88, but over-supplementation has no effect on serum testosterone 89.
- Diets: There is limited evidence that low-fat diets may reduce total and free testosterone levels in men 90 and moderate evidence that very high protein diets (≥35% protein) decrease total testosterone levels in men (∼5.23 nmol/L) 91.
- Weight loss: Reduction in weight may result in an increase in testosterone levels, as well as sex-hormone binding globulin (SHBG) 92, 93, 94. However, it’s unclear whether the improvement in semen quality is a result of the reduction in body weight per se or improved lifestyles remains unknown 92. Fat cells synthesize the enzyme aromatase, which converts testosterone, the male sex hormone, into estradiol, the female sex hormone. A 2021 meta-analysis indicated that overweight and/or obesity were associated with lower sperm quality (i.e., semen volume, sperm count and concentration, sperm vitality, total motility and normal morphology), and underweight was likewise associated with low sperm normal morphology 95. In conclusion, these results suggest that maintaining a healthy body weight is important for increasing sperm quality parameters and potentially male fertility.
- Too much body fat (obesity). Ask your doctor whether you need a test called free testosterone.
- Sleep: REM (rapid-eye movement) sleep increases nocturnal testosterone levels 96. Peak testosterone levels coincide with rapid-eye movement (REM) sleep onset. The decreasing sleep efficiency and numbers of REM sleep episodes with altered REM sleep latency observed in older men are associated with lower concentrations of circulating testosterone 96.
- Behavior: Dominance challenges can, in some cases, stimulate increased testosterone release in men 97.
- Antiandrogens: Natural or man-made antiandrogens including spearmint tea reduce testosterone levels 98. Licorice can decrease the production of testosterone and this effect is greater in females 99.
- Medicine side effects, such as from chemotherapy.
- Diseases of the testicles (trauma, cancer, infection, immune, iron overload and undescended testicle [cryptorchidism])
- Problems with glands in the brain (hypothalamus and pituitary) that control hormone production. The cause is either primary or secondary/tertiary (pituitary/hypothalamic) testicular failure.
- Primary testicular failure is associated with increased luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels, and decreased total, bioavailable, and free testosterone levels. Causes include:
- Genetic causes (eg, Klinefelter syndrome, XXY males)
- Developmental causes (eg, testicular maldescent)
- Testicular trauma or ischemia (eg, testicular torsion, surgical mishap during hernia operations)
- Infections (eg, mumps)
- Autoimmune diseases (eg, autoimmune polyglandular endocrine failure)
- Metabolic disorders (eg, hemochromatosis, liver failure)
- Orchidectomy
- Secondary/tertiary hypogonadism, also known as hypogonadotrophic hypogonadism, shows low testosterone and low, or inappropriately “normal,” LH/FSH levels; causes include:
- Inherited or developmental disorders of hypothalamus and pituitary (eg, Kallmann syndrome, congenital hypopituitarism)
- Pituitary or hypothalamic tumors
- Hyperprolactinemia of any cause
- Malnutrition or excessive exercise
- Cranial irradiation
- Head trauma
- Medical or recreational drugs (eg, estrogens, gonadotropin releasing hormone [GnRH] analogs, cannabis)
- Primary testicular failure is associated with increased luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels, and decreased total, bioavailable, and free testosterone levels. Causes include:
- Low thyroid function.
- Genetic disorders such as Klinefelter syndrome and Kallmann syndrome. Klinefelter syndrome is the most common congenital abnormality that results in primary hypogonadism. In Klinefelter syndrome, there is dysgenesis of seminiferous tubules and loss of Sertoli cells which leads to a decrease in inhibin levels and a resultant increase in follicle-stimulating hormone (FSH). FSH upregulates aromatase leading to increased conversion of androgens to estrogens. In Klinefelter, there is also Leydig cell dysfunction which leads to decreased testosterone levels and an increase in luteinizing hormone (LH) due to loss of negative feedback. In Kallmann syndrome, failed migration of gonadotropin-releasing hormone (GnRH)-producing neurons leads to lack of GnRH (gonadotropin-releasing hormone). No GnRH results in a decrease in luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, and sperm count. Specific to Kallmann syndrome, in comparison to other causes of hypogonadotropic hypogonadism, is defects in the sensation of smell (hyposmia or anosmia) 100.
- Other disorders, chronic diseases, treatments, or infection.
Increased total testosterone levels may be due to:
- Resistance to the action of male hormones (androgen resistance)
- Tumor of the ovaries
- Adrenal tumors
- Cancer of the testes
- Congenital adrenal hyperplasia (CAH). In classic CAH (95% of cases), due to 21 hydroxylase deficiency, newborns usually present with ambiguous genitalia and later develop salt wasting, vomiting, hypotension, and acidosis. A marked increase in 17-hydroxyprogesterone is diverted towards adrenal androgen synthesis and leads to hyperandrogenism. Hyperandrogenism impairs hypothalamic sensitivity to progesterone leading to a rapid rise in GnRH synthesis and thus increased LH and FSH. Elevations in LH and FSH lead to increased gonadal steroid production (17-hydroxyprogesterone, dehydroepiandrosterone [DHEA], testosterone, LH, and FSH). Diagnosis is with adrenocorticotropic hormone stimulation test showing exaggerated 17 hydroxyprogesterone response 101.
- Taking medicines or drugs that increase testosterone level (including some supplements)
During childhood, excessive production of testosterone induces premature puberty (puberty too early before age 9) in boys and masculinization in girls 75. Some rare conditions, such as certain types of tumors, cause boys to make testosterone earlier than normal. Young boys also can have too much testosterone if they touch testosterone gel that an adult man is using for treatment.
In females, the ovaries produce most of the testosterone. The adrenal glands can also produce too much of other androgens that are converted to testosterone. In adult women, excess testosterone production results in varying degrees of virilization, including hirsutism, acne, oligo-amenorrhea, or infertility. Levels are most often checked to evaluate signs of higher testosterone levels, such as:
- Acne, oily skin
- Change in voice
- Decreased breast size
- Excess hair growth (dark, coarse hairs in the area of the moustache, beard, sideburns, chest, buttocks, inner thighs)
- Increased size of the clitoris
- Irregular or absent menstrual periods
- Male-pattern baldness or hair thinning.
Mild-to-moderate testosterone elevations are usually asymptomatic in males but can cause distressing symptoms in females. The exact causes for mild-to-moderate elevations in testosterone often remain obscure. Common causes of pronounced elevations of testosterone include genetic conditions (eg, congenital adrenal hyperplasia); adrenal, testicular, and ovarian tumors; polycystic ovarian syndrome (PCOS) and abuse of testosterone or gonadotrophins by athletes. Testosterone-producing ovarian or adrenal neoplasms often produce total testosterone values greater than 200 ng/dL. High estrogen values also may be observed, and LH and FSH are low or “normal.” In polycystic ovarian syndrome (PCOS), hirsutism, acne, menstrual disturbances, insulin resistance and, frequently, obesity, form part of this syndrome. Total testosterone levels may be normal or mildly elevated and, uncommonly, greater than 200 ng/dL.
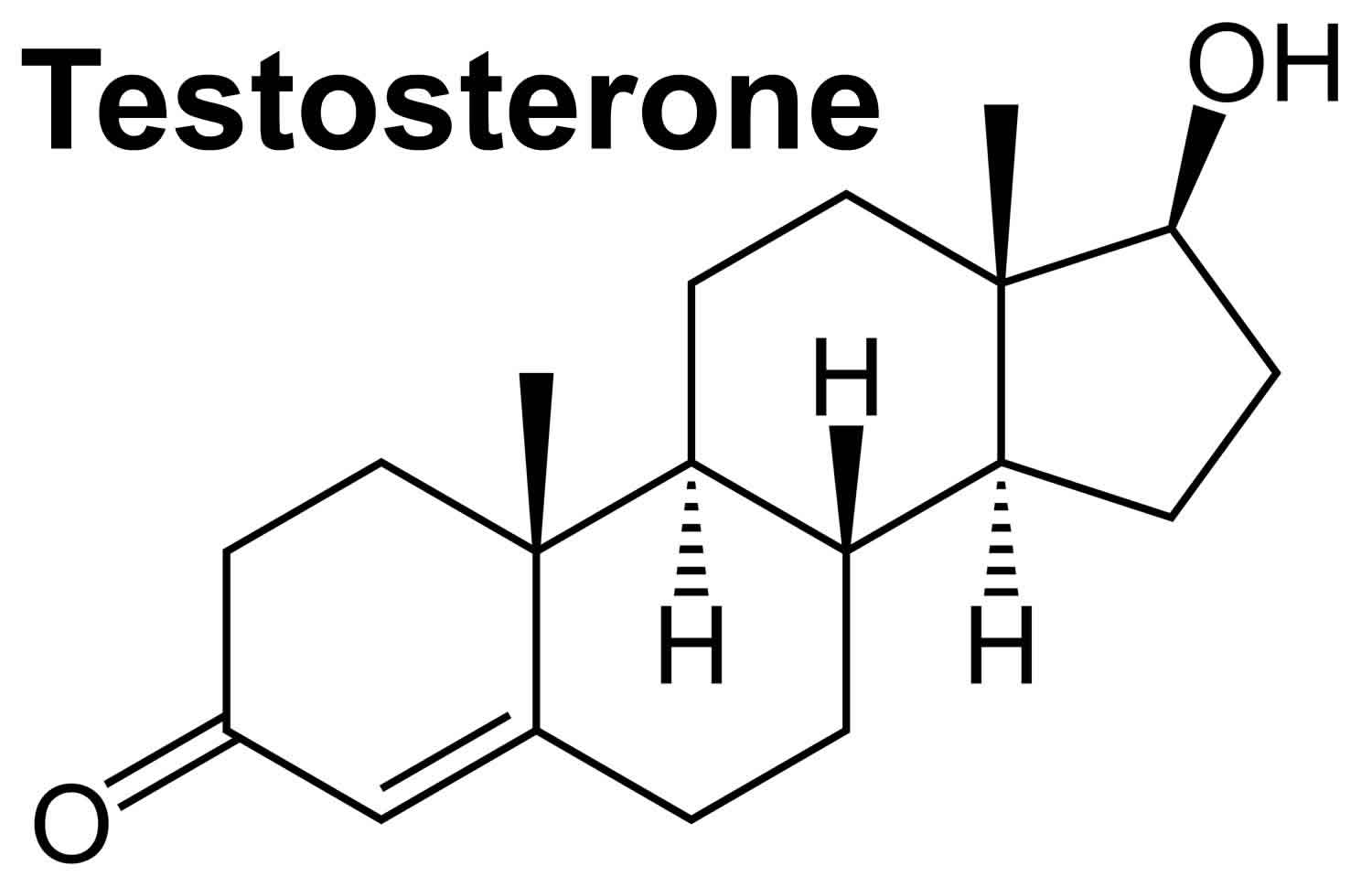
Figure 1. Testosterone chemical structure
Figure 2. Hypothalamic–pituitary–gonadal axis
Footnotes: In puberty, the hypothalamic-pituitary-gonadal axis plays a major role in regulating testosterone levels and gonadal function. Gonadotropin-releasing hormone (GnRH) is secreted from the hypothalamus by GnRH-expressing neurons. The GnRH travels down the hypothalamohypophyseal portal system to the anterior pituitary, which secretes luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH are two gonadotropic hormones that travel through the blood and act on receptors in the gonads. The largest amounts of testosterone (>95%) are produced by the testes in men, while the adrenal glands account for most of the remainder. In the testes, testosterone is produced by the Leydig cells 102. The male testes also contain Sertoli cells, which require testosterone for spermatogenesis (sperm cell development). Luteinizing hormone (LH), in particular, acts on the Leydig cells to increase testosterone production. Testosterone limits its own secretion via negative feedback. High levels of testosterone in the blood feedback to the hypothalamus to suppress the secretion of GnRH and also feedback to the anterior pituitary, making it less responsive to GnRH stimuli 103. Throughout the reproductive life of males, the hypothalamus releases GnRH in pulses every 1 to 3 hours. Despite this pulsatile release, however, average plasma levels of FSH and LH remain fairly constant from the start of puberty, where levels spike, to the third decade of life, where levels peak and slowly begin to decline. Prior to puberty, testosterone levels are low, reflecting the low secretion of GnRH and gonadotropins. Changes in neuronal input to the hypothalamus and brain activity during puberty cause a dramatic rise in GnRH secretion.
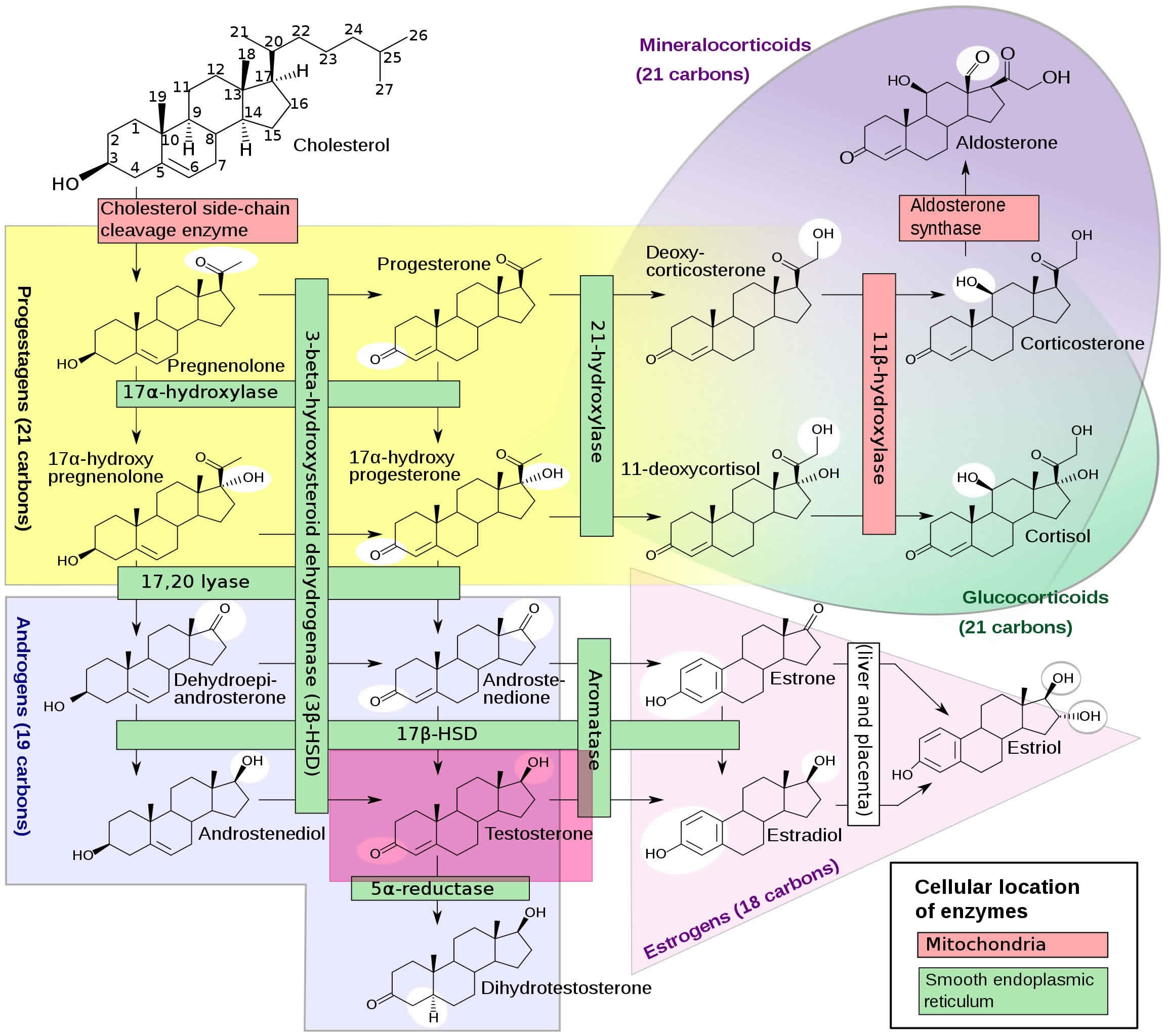
Figure 3. Testosterone production
Footnotes: Figure 2 illustrates all of the enzymes involved in the biosynthesis of the adrenal steroid hormones, corticosterone, cortisol, and aldosterone; and the gonadal steroid hormones, progesterone, estradiol, and testosterone. Leydig cells in the testes function to turn cholesterol into testosterone 104. Luteinizing hormone (LH) regulates the initial step in this process. Two important intermediates in this process are dehydroepiandrosterone (DHEA) and androstenedione. Androstenedione is converted to testosterone by the enzyme 17-beta-hydroxysteroid dehydrogenase. At the cellular level, testosterone gets converted to dihydrotestosterone (DHT) by the enzyme 5-alpha-reductase. Dihydrotestosterone (DHT) is an androgen, which means it is a hormone that triggers the development of male characteristics 105. About 10% of the testosterone in the bodies of both men and women is converted into dihydrotestosterone (DHT) in adults, with a much higher amount in puberty. This may be why it is so closely related to the triggering of puberty. The dihydrotestosterone hormone (DHT) is much more powerful than testosterone. Dihydrotestosterone initiates the start of puberty in boys. It causes the genitals to develop and can cause the growth of pubic and body hair. It also causes the prostate to grow during puberty and may work together with testosterone to begin the expression of sexual desires and behavior. Women also have dihydrotestosterone (DHT), but its role in their bodies is not as well known. Some research has shown that it can lead to pubic hair growth after puberty in girls. It may also play a role in determining when puberty will start for a girl. Both men and women also produce weak acting androgens in the zona reticularis of the adrenal cortex. These weak-acting androgens are known as dehydroepiandrosterone (DHEA and androstenedione. They bind to testosterone receptors with weaker affinity but can also be converted to testosterone in the peripheral tissues if produced at high amounts 105.
5-alpha reductase is an enzyme that converts testosterone to dihydrotestosterone (DHT). Male patients with 5-alpha reductase deficiency present with normal female or male genitalia or ambiguous genitalia at birth due to lack of dihydrotestosterone (DHT). These patients have a male internal urogenital tract (anti-Mullerian hormone is still present). At puberty, adolescents with 5-alpha reductase deficiency, who may have been raised as girls due to lack of secondary male characteristics, begin to develop male secondary sex characteristics and have primary amenorrhea. These patients will have normal testosterone and LH, low dihydrotestosterone (DHT), and an increased testosterone-to-DHT ratio. In contrast to 5-alpha reductase deficiency, androgen insensitivity is a condition in which patients lack functional androgen receptors resulting in under-virilization. These patients, like those with 5-alpha reductase deficiency, have a 46 XY karyotype. In contrast, however, these patients have normal female external genitalia and usually undescended testes. In adolescence, they experience primary amenorrhea and breast development but have no pubic or axillary hair and lack the deepening voice changes that occur with puberty. They will have a blind vaginal pouch and abnormal internal reproductive organs (fallopian tubes, uterus, and the upper portion of the vagina) due to the production of the Mullerian inhibiting factor. These patients will have high levels of testosterone and LH 106.
Impaired testosterone metabolism can occur in certain cases of congenital adrenal hyperplasia (CAH). In classic CAH (95% of cases), due to 21 hydroxylase deficiency, newborns usually present with ambiguous genitalia and later develop salt wasting, vomiting, hypotension, and acidosis. A marked increase in 17-hydroxyprogesterone is diverted towards adrenal androgen synthesis and leads to hyperandrogenism. Hyperandrogenism impairs hypothalamic sensitivity to progesterone leading to a rapid rise in GnRH synthesis and thus increased LH and FSH. Elevations in LH and FSH lead to increased gonadal steroid production (17-hydroxyprogesterone, dehydroepiandrosterone [DHEA], testosterone, LH, and FSH). Diagnosis is with adrenocorticotropic hormone stimulation test showing exaggerated 17 hydroxyprogesterone response 101.
Testosterone function
Testosterone is responsible for the development of primary sexual development, which includes testicular descent, spermatogenesis, enlargement of the penis and testes, and increasing libido 104. Testosterone’s effects are first seen in the fetus. During the first 6 weeks of development, the reproductive tissues of males and females are identical. At around week 7 in utero, the sex-related gene on the Y chromosome (SRY gene) initiates the development of the testicles. Sertoli cells from the testis cords (fetal testicles) eventually develop into seminiferous tubules. Sertoli cells produce a Mullerian-inhibiting substance (MIS), which leads to the regression of the Fallopian tubes, uterus, and upper segment of the vagina (Mullerian structures normally present in females). Fetal Leydig cells and endothelial cells migrate into the gonad and produce testosterone, which supports the differentiation of the Wolffian duct (mesonephric duct) structures that go on to become the male urogenital tract 104. Testosterone also gets converted to dihydrotestosterone (DHT) in the periphery and induces the formation of the prostate and male external genitalia. Testosterone is also responsible for testicular descent through the inguinal canal, which occurs in the last 2 months of fetal development. When an embryo lacks a Y chromosome and thus the SRY gene, ovaries develop. Fetal ovaries do not produce adequate amounts of testosterone, thus the Wolffian ducts do not develop. There is also an absence of Mullerian-inhibiting substance (MIS) in these individuals, leading to the development of the Mullerian ducts and female reproductive structures 107.
The testes usually begin the descent into the scrotum around 7 months of gestation, where the testes begin secreting reasonable quantities of testosterone. If a male child is born with undescended but normal testes that do not descend by 4 to 6 months of age, administration of testosterone can help the testes descend through the inguinal canals 108.
Testosterone is also involved in regulating secondary male characteristics, which are those responsible for masculinity. These secondary sex characteristics include male hair patterns, vocal changes, and voice deepening, anabolic effects, which include growth spurts in puberty (testosterone increases tissue growth at the epiphyseal plate early on and eventual closure of plate later in puberty) and skeletal muscle growth (testosterone stimulates protein synthesis). Testosterone also stimulates erythropoiesis, which results in a higher hematocrit in males versus females. Testosterone levels tend to drop with increasing age; because of this, men tend to experience a decrease in testicular size, a drop in libido, lower bone density, muscle mass decline, increased fat production, and decreased red blood cells production (erythropoiesis), which leads to possible anemia.
Normal testosterone levels
A total testosterone test measures both bound and free testosterone in a sample of blood. Total testosterone is the most commonly used test for diagnostic and monitoring purposes and levels are commonly reported in nanograms per deciliter of blood (ng/dL). Less often, a testosterone test may be performed for free testosterone, which is reported in picograms per deciliter of blood (pg/dL). Another less common test is for bioavailable testosterone, which is testosterone that can be used more readily by the body. Bioavailable testosterone includes all testosterone that is not bound to SHBG, including free testosterone and albumin-bound testosterone. Bioavailable testosterone is also commonly reported in nanograms per deciliter of blood (ng/dL).
To measure your testosterone level, your doctor can order a blood test. The blood test should be done in the morning between 7:00 AM and 10:00 AM.
Normal testosterone levels
- Normal total testosterone levels in males: 300 to 1,000 nanograms per deciliter (ng/dL) or 10 to 35 nanomoles per liter (nmol/L). The harmonized normal serum total testosterone range in a healthy nonobese population of European and American men, 19 to 39 years, is 264 to 916 ng/dL 109. Furthermore, in randomized testosterone trials, the men with testosterone levels below the harmonized threshold would be more likely to respond to testosterone therapy than those with testosterone levels above the harmonized threshold.
- 0-5 months: 75-400 ng/dL
- 6 months-9 years: <7-20 ng/dL
- 10-11 years: <7-130 ng/dL
- 12-13 years: <7-800 ng/dL
- 14 years: <7-1,200 ng/dL
- 15-16 years: 100-1,200 ng/dL
- 17-18 years: 300-1,200 ng/dL
- Over 19 years: 240-950 ng/dL
- Normal total testosterone levels in females: 15 to 70 ng/dL or 0.5 to 2.4 nmol/L
- 0-5 months: 20-80 ng/dL
- 6 months to 9 years: <7-20 ng/dL
- 10-11 years: <7-44 ng/dL
- 12-16 years: <7-75 ng/dL
- 17-18 years: 20-75 ng/dL
- Over 19 years: 8-60 ng/dL
- Normal free testosterone levels in males:
- 20-25 years: 5.25-20.7 ng/dL
- 25-30 years: 5.05-19.8 ng/dL
- 30-35 years: 4.85-19.0 ng/dL
- 35-40 years: 4.65-18.1 ng/dL
- 40-45 years: 4.46-17.1 ng/dL
- 45-50 years: 4.26-16.4 ng/dL
- 50-55 years: 4.06-15.6 ng/dL
- 55-60 years: 3.87-14.7 ng/dL
- 60-65 years: 3.67-13.9 ng/dL
- 65-70 years: 3.47-13.0 ng/dL
- 70-75 years: 3.28-12.2 ng/dL
- 75-80 years: 3.08-11.3 ng/dL
- 80-85 years: 2.88-10.5 ng/dL
- 85-90 years: 2.69-9.61 ng/dL
- 90-95 years: 2.49-8.76 ng/dL
- 95-100+ years: 2.29-7.91 ng/dL
- Normal free testosterone levels in females:
- 20-25 years: 0.06-1.08 ng/dL
- 25-30 years: 0.06-1.06 ng/dL
- 30-35 years: 0.06-1.03 ng/dL
- 35-40 years: 0.06-1.00 ng/dL
- 40-45 years: 0.06-0.98 ng/dL
- 45-50 years: 0.06-0.95 ng/dL
- 50-55 years: 0.06-0.92 ng/dL
- 55-60 years: 0.06-0.90 ng/dL
- 60-65 years: 0.06-0.87 ng/dL
- 65-70 years: 0.06-0.84 ng/dL
- 70-75 years: 0.06-0.82 ng/dL
- 75-80 years: 0.06-0.79 ng/dL
- 80-85 years: 0.06-0.76 ng/dL
- 85-90 years: 0.06-0.73 ng/dL
- 90-95 years: 0.06-0.71 ng/dL
- 95-100+ years: 0.06-0.68 ng/dL
Talk to your doctor about the meaning of your specific test results. Testosterone levels change from hour to hour. Testosterone levels tend to be highest in the morning and lowest at night. Testosterone levels are highest by age 20 to 30 and slowly go down after age 30 to 35. In addition, normal testosterone level ranges may vary slightly among different laboratories. Some labs use different measurements or test different specimens.
If the result is not normal, you should repeat the test to make sure of the result. In healthy men, testosterone levels can change a lot from day to day, so a second test could be normal.
What is the role of testosterone in men’s health?
Testosterone is a hormone produced primarily in the testicles. Testosterone is the most important sex hormone that men have. It is responsible for the typical male characteristics, such as facial, pubic, and body hair as well as muscle. Testosterone hormone also helps maintain sex drive, sperm production, and bone health. The brain and pituitary gland (a small gland at the base of the brain) control the production of testosterone by the testes.
Testosterone helps maintain men’s:
- Bone density
- Fat distribution
- Muscle strength and mass
- Facial and body hair
- Red blood cell production
- Sex drive
- Sperm production
Testosterone deficiency signs and symptoms may include 110:
- Physical
- Anemia
- Reduced energy
Reduced endurance - Diminished work performance
- Diminished physical performance
- Fatigue
- Reduced lean muscle mass
- Obesity
- Loss of body hair
- Reduced beard growth
- Development of breast tissue (gynecomastia)
- Loss of bone mass (osteoporosis)
- Hot flashes
- Cognitive
- Depressive symptoms
- Cognitive dysfunction
- Reduced motivation
- Poor concentration
- Poor memory
- Irritability
- Sexual
- Reduced sex drive
- Reduced erectile function
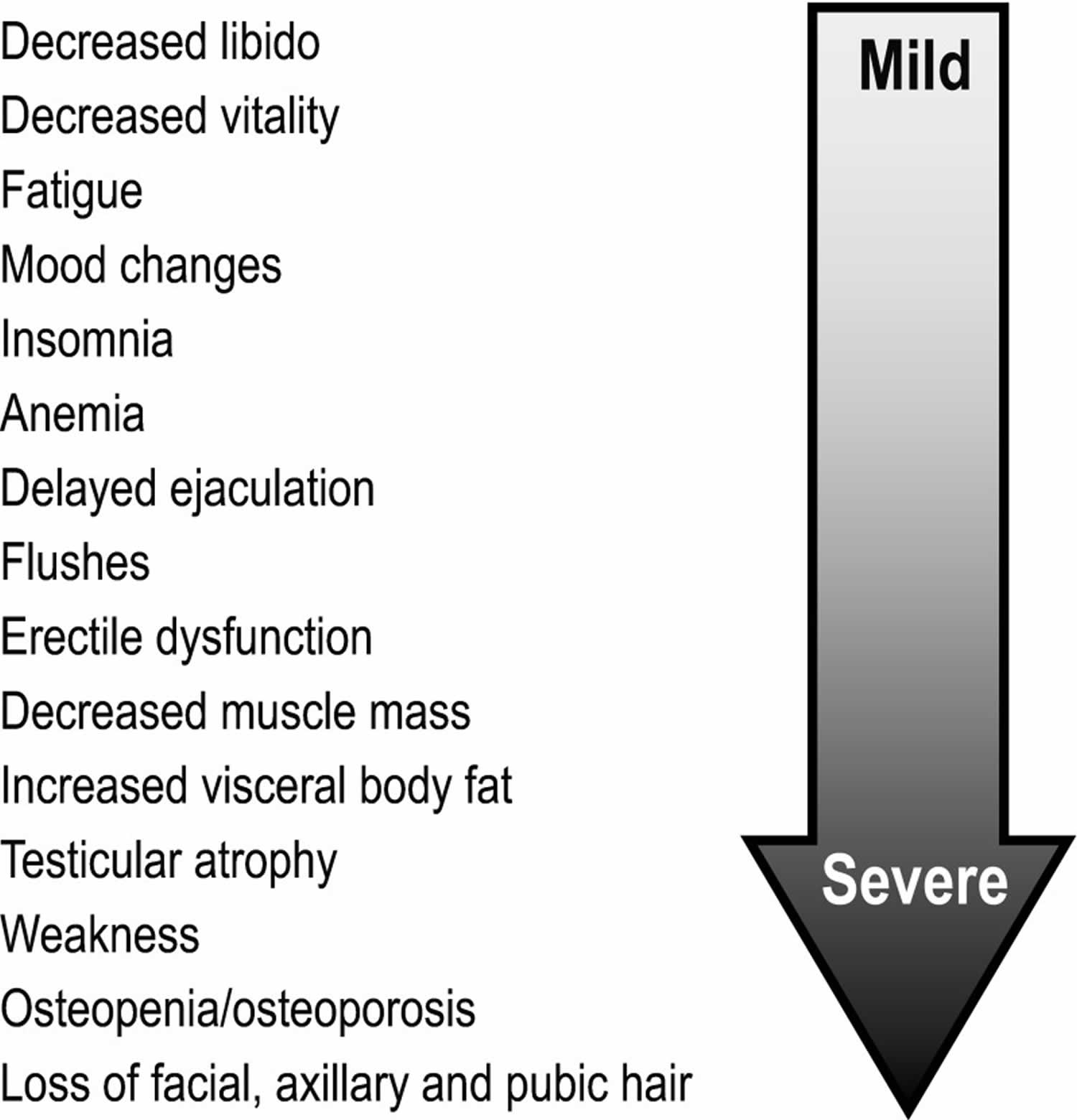
Symptoms of hypogonadism may be categorized as sexual or non-sexual. Sexual symptoms include erectile dysfunction, diminished frequency of morning erections, and decrease in sexual thoughts (low libido), as well as difficulty in achieving orgasm and reduced intensity of orgasm 111. Non-sexual symptoms include fatigue, impotence, impaired concentration, depression, and decreased sense of vitality and/or well-being. Signs of hypogonadism also include anemia, osteopenia and osteoporosis, abdominal obesity, and metabolic syndrome 112.
Figure 4. Testosterone deficiency signs and symptoms
[Source 113 ]Low testosterone / testosterone deficiency
Testosterone deficiency also known as male hypogonadism, is defined as having one or more symptoms (e.g. decreased libido, decreased erections, decreased energy, decreased physical stamina, decreased lean muscle mass) attributable to low circulating levels of testosterone (morning serum total testosterone less than 300 ng/dL on two separate occasions) 1, 2, 114. An estimated 25% of men have low levels of testosterone 115. Besides being fundamental for the development and maintenance of male characteristics and male sexual organs, testosterone also has effects on most major organs, such as the brain, muscle, kidney, bone, liver and skin 113. Recent studies have also suggested that testosterone deficiency is independently and robustly associated with various obesity-related chronic diseases in men, including type 2 diabetes, hypertension, cardiovascular disease, hyperlipidemia, asthma, chronic obstructive pulmonary disease (COPD) 116, 117. However, there is uncertainty as to what constitutes optimal physiological levels of total testosterone among men across different age categories, and to the effects that varying total testosterone levels have on disease risk 118. This debate is partially fueled by inconsistent findings in the literature and differences across expert clinical recommendations, as well as an incomplete understanding of the underlying mechanisms and temporal sequence of events leading to and stemming from testosterone deficiency. For example, the authors of a well-known systematic review 119 assert that although testosterone deficiency is robustly associated with obesity, insulin resistance, low high-density lipoprotein (HDL) cholesterol and elevated triglycerides, low-density lipoprotein (LDL) cholesterol, and plasminogen activator type 1, there is insufficient evidence to demonstrate a causal role of endogenous testosterone deficiency on coronary artery disease-the leading cause of preventable death among men in the U.S 120.
Incidence of hypogonadism (symptomatic low testosterone) increases with advancing male age such that levels of circulating testosterone decline annually by approximately 1% from the age of 40 years according to the European Male Ageing Study 80. When hypogonadism occurs in an older man, the condition is often called andropause or androgen deficiency of the aging male or late onset hypogonadism (LOH) 121. The most easily recognized clinical signs of late onset hypogonadism (andropause) in older men are a decrease in muscle mass and strength, a decrease in bone mass and osteoporosis, decreased libido, erectile dysfunction, anemia, increase in central body fat, and impaired memory, mood, concentration, and sleep 122, as well as increased mortality 123. Sexual symptoms and fatigue are the earliest and most common presentations 124. Other symptoms include depression, sleep alterations, poor concentration, and metabolic disorders are seen at borderline testosterone levels 125, 126. However, symptoms such as a decrease in libido and sexual desire, forgetfulness, loss of memory, anemia, difficulty in concentration, insomnia, and a decreased sense of well-being are more difficult to measure and differentiate from hormone-independent aging 127. Androgen deficiency of the aging male may result in significant detriment to quality of life and adversely affect the function of multiple organ systems 128.
The pathophysiology of male hypogonadism can be characterized into two types 129:
- Primary hypogonadism also known as primary testicular failure or hypergonadotropic hypogonadism. Primary hypogonadism is a testosterone deficiency due to a testicular abnormality impairing testosterone production; for example, Klinefelter syndrome, testicular tumors, infection, trauma, impaired Leydig cell function, or those caused by certain medications 130.
- Secondary hypogonadism also known as hypogonadotropic hypogonadism. Secondary hypogonadism is a testosterone deficiency due to a problem in the hypothalamus or the pituitary gland, parts of the brain that signal the testicles to produce testosterone (i.e., luteinizing hormone (LH) and follicle-stimulating hormone (FSH)); for example, hypothalamic or pituitary lesions, GnRH deficiency, or hyperprolactinemia 130. Another important cause of hypogonadism is late-onset hypogonadism, an age-related (i.e., >40 years) decline in testosterone accompanied by clinical symptoms that is often associated with obesity, diabetes, and other chronic health conditions 131.
Risk factors for hypogonadism include:
- HIV/AIDS
- Previous chemotherapy or radiation therapy
- Aging
- Obesity
- Malnutrition
Late-onset hypogonadism (andropause) has the combined features of both primary and secondary hypogonadism 132. The diagnosis of andropause (late-onset hypogonadism) requires the presence of characteristic signs and symptoms, in combination with decreased serum total testosterone. Based on the recent guidelines by the International Society for the Study of Aging Male (ISSAM), the European Association of Urology (EAU), the European Society of Endocrinology (ESE), the European Academy of Andrology (EAA), and the American Association of Urology (AUA), a total testosterone of 250–350 ng/dL is the proper threshold value to define low testosterone. The optimal indication for TRT in late-onset hypogonadism (andropause) is the presence of signs and symptoms of hypogonadism, and low testosterone without contraindications for TRT 110.
Low testosterone causes
Testosterone deficiency also known as male hypogonadism, is defined as having one or more symptoms (e.g. decreased libido, decreased erections, decreased energy, decreased physical stamina, decreased lean muscle mass) attributable to low circulating levels of testosterone (morning serum total testosterone less than 300 ng/dL on two separate occasions) 1, 2, 114.
There are two basic types of hypogonadism:
- Primary. This type of hypogonadism — also known as primary testicular failure — originates from a problem in the testicles.
- Secondary. This type of hypogonadism indicates a problem in the hypothalamus or the pituitary gland — parts of the brain that signal the testicles to produce testosterone. The hypothalamus produces gonadotropin-releasing hormone, which signals the pituitary gland to make follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Luteinizing hormone then signals the testes to produce testosterone.
Either type of hypogonadism can be caused by an inherited (congenital) trait or something that happens later in life (acquired), such as an injury or an infection. At times, primary and secondary hypogonadism occur together.
Primary hypogonadism
Common causes of primary hypogonadism include:
- Klinefelter syndrome also known as 47,XXY or XXY male. Klinefelter syndrome results from a congenital abnormality of the sex chromosomes, X and Y. A male normally has one X and one Y chromosome. In Klinefelter syndrome, two or more X chromosomes are present in addition to one Y chromosome. The Y chromosome contains the genetic material that determines the sex of a child and related development. The extra X chromosome typically affects physical, neurodevelopmental, behavioral, and neurocognitive functioning. Common physical features may include tall stature, reduced muscle tone, abnormal development of the testicles (hypogonadism), which in turn results in underproduction of testosterone with delayed pubertal development and lack of secondary male sex characteristics such as decreased facial and body hair. Increased breast growth (gynecomastia) may occur later in puberty without appropriate biological care.
- Undescended testicles (cryptorchidism). Before birth, the testicles develop inside the abdomen and normally move down into their permanent place in the scrotum. Sometimes one or both of the testicles aren’t descended at birth. This condition often corrects itself within the first few years of life without treatment. If not corrected in early childhood, it can lead to malfunction of the testicles and reduced production of testosterone.
- Mumps orchitis. A mumps infection involving the testicles that occurs during adolescence or adulthood can damage the testicles, affecting the function of the testicles and testosterone production.
- Hemochromatosis. Too much iron in the blood can cause testicular failure or pituitary gland dysfunction, affecting testosterone production.
- Injury to the testicles. Because they’re outside the abdomen, the testicles are prone to injury. Damage to both testicles can cause hypogonadism. Damage to one testicle might not impair total testosterone production.
- Cancer treatment. Chemotherapy or radiation therapy for the treatment of cancer can interfere with testosterone and sperm production. The effects of both treatments often are temporary, but permanent infertility may occur. Although many men regain their fertility within a few months after treatment, preserving sperm before starting cancer therapy is an option for men.
Secondary hypogonadism
In secondary hypogonadism, the testicles are normal but don’t function properly due to a problem with the pituitary or hypothalamus. A number of conditions can cause secondary hypogonadism, including:
- Kallmann’s syndrome. This is an abnormal development of the area of the brain that controls the secretion of pituitary hormones (hypothalamus). This abnormality can also affect the ability to smell (anosmia) and cause red-green color blindness.
- Pituitary disorders. An abnormality in the pituitary gland can impair the release of hormones from the pituitary gland to the testicles, affecting normal testosterone production. A pituitary tumor or other type of brain tumor located near the pituitary gland may cause testosterone or other hormone deficiencies. Also, treatment for a brain tumor, such as surgery or radiation therapy, can affect the pituitary gland and cause hypogonadism.
- Inflammatory disease. Certain inflammatory diseases, such as sarcoidosis, histiocytosis and tuberculosis, involve the hypothalamus and pituitary gland and can affect testosterone production.
- HIV/AIDS. HIV/AIDS can cause low levels of testosterone by affecting the hypothalamus, the pituitary and the testes.
- Medications. The use of certain drugs, such as opiate pain medications and some hormones, can affect testosterone production.
- Obesity. Being significantly overweight at any age might be linked to hypogonadism.
- Aging. As men age, there’s a slow, progressive decrease in testosterone production. The rate varies greatly.
Low testosterone is common in older men. An accurate blood test needs to be done in the morning between 7am-10am.
What happens to testosterone levels with age?
Testosterone levels generally peak during adolescence and early adulthood. As you get older, your testosterone level gradually declines — typically about 1 percent a year after age 30 or 40. It is important to determine in older men if a low testosterone level is simply due to the decline of normal aging or if it is due to a disease (hypogonadism).
Hypogonadism is a disease in which the body is unable to produce normal amounts of testosterone due to a problem with the testicles or with the pituitary gland that controls the testicles. Testosterone replacement therapy can improve the signs and symptoms of low testosterone in these men. Doctors may prescribe testosterone as injections, pellets, patches or gels.
In the short term, low testosterone (also called hypogonadism) can cause:
- A drop in sex drive
- Poor erections
- Low sperm count
- Enlarged breasts
Over time, low testosterone may cause a man to lose body hair, muscle bulk, and strength and to gain body fat. Chronic (long-term) low testosterone may also cause weak bones (osteoporosis), mood changes, less energy, and smaller testes. Signs and symptoms (what you see and feel) vary from person to person.
Does a naturally declining testosterone level cause the signs and symptoms of aging?
Not necessarily. Men can experience many signs and symptoms as they age. Some may occur as a result of lower testosterone levels and can include:
- Changes in sexual function. This may include reduced sexual desire, fewer spontaneous erections — such as during sleep — and infertility.
- Changes in sleep patterns. Sometimes low testosterone causes insomnia or other sleep disturbances.
- Physical changes. Various physical changes are possible, including increased body fat, reduced muscle bulk and strength, and decreased bone density.
- Swollen or tender breasts (gynecomastia) and body hair loss are possible. You may have less energy than you used to.
- Emotional changes. Low testosterone may contribute to a decrease in motivation or self-confidence. You may feel sad or depressed, or have trouble concentrating or remembering things.
Some of these signs and symptoms can be caused by various underlying factors, including medication side effects, obstructive sleep apnea, thyroid problems, diabetes and depression. It’s also possible that these conditions may be the cause of low testosterone levels, and treatment of these problems may cause testosterone levels to rise. A blood test is the only way to diagnose a low testosterone level.
Low testosterone diagnosis
Your doctor will conduct a physical examination and note whether your sexual development, the amount and distribution of body hair (including beard growth and pubic hair), muscle mass and size and consistency of your testes, is consistent with your age. The prostate should be examined in older patients for size, consistency, symmetry, and presence of nodules or induration; it should be noted that the prostate may be enlarged in older men, despite a low testosterone level 133. Weight, height, body mass index (BMI), and waist circumference should also be measured, since signs and symptoms potentially indicative of testosterone deficiency in men include height loss, reduced muscle bulk and strength, and increased body fat, particularly increased BMI and abdominal fat accumulation 7.
Your doctor will test your blood level of testosterone if you have signs or symptoms of hypogonadism. Because testosterone levels vary and are generally highest in the morning, blood testing is usually done early in the day, before 10 a.m. (8 AM to 10 AM), possibly on more than one day. If both measurements are low, then certain studies should be ordered to rule out secondary hypogonadism. Further testing includes FSH (follicle-stimulating hormone), LH (luteinizing hormone), prolactin, TSH (thyroid-stimulating hormone), complete blood count (CBC), and comprehensive metabolic panel. In cases of low normal testosterone with clinical symptoms, further testing to assess free or bioavailable testosterone should be done. These tests include sex hormone binding globulin (SHBG) and albumin to calculate the bioavailable testosterone which can be affected by obesity, type 2 diabetes, hypothyroidism, and liver disease. Furthermore, semen analysis, pituitary MRI, testicular ultrasound and biopsy, and genetic studies can be ordered, if there is clinical suspicion of a secondary cause.
Testosterone in blood is mainly bound to serum proteins with only 2% of the hormone circulating as free testosterone. Sex hormone binding globulin (SHBG) accounts for 60-70% of testosterone binding, and 30-40% of the total testosterone is bound by albumin or other proteins. Bioavailable testosterone, referring to free and albumin-bound testosterone together, is thought to reflect an individual’s biologically active, circulating testosterone 134. Biochemical parameters commonly used to assess androgen deficiency include total testosterone (TT), free testosterone, calculated free testosterone, bioavailable testosterone (bT), and free androgen index 135. Theoretically, bioavailable testosterone (bT) is the most accurate; however, a standard method to measure bioavailable testosterone (bT) has not been established 134. Practically, morning total testosterone is the most widely accepted parameter, but results have been found to be misleading when SHBG is elevated. The diagnosis of low testosterone should be made only after two total testosterone measurements have been taken on separate occasions between 8 and 11 AM due to the circadian rhythm of testosterone production by the testicles 134. Free testosterone measurement is recommended when total testosterone measurement is not diagnostic. In individuals with clinically suspected testosterone deficiency, SHBG levels should be assessed if total testosterone is low to normal or borderline, especially in obese or older men 136. Calculation of free testosterone based on serum levels of testosterone and SHBG may be used to avoid inaccuracies in assessing the individual degree of androgenicity in a cost-effective manner 137. Since normal total testosterone ranges vary significantly between laboratories depending on the methods used and/or the assay kits employed, it is better to measure testosterone in the same laboratory 138.
Recently, other hormones are also being measured as per recommendations. In patients with low testosterone, serum luteinizing hormone (LH) should be measured to establish the cause of testosterone deficiency and may be an important factor in determining if adjunctive tests should be ordered 129. Serum prolactin levels should be measured in patients with low testosterone levels combined with low or low/normal LH levels to screen for hyperprolactinemia. Persistently elevated prolactin levels can indicate the presence of pituitary tumors, such as prolactinomas 139. Men with total testosterone levels <150 ng/dL in combination with a low or low/normal LH should undergo a pituitary MRI regardless of prolactin levels, as this may indicate non-secreting adenomas 140.
Table 3. Cutoff values of testosterone for low testosterone (testosterone deficiency) diagnosis
| Cutoff Values | Year of Release and Update | |
|---|---|---|
| Expert opinion | Total testosterone or free testosterone below the lower limits of normal | Before Official Guideline |
| International Society for the Study of the Aging Male (ISSAM) | Total testosterone < 231 ng/dL (8 nmol/L) Total testosterone: 231–346 ng/dL (8–12 nmol/L) or free testosterone < 52 pg/mL | 2005 |
| Total testosterone < 230 ng/dL (8 nmol/L) total testosterone: 230–350 ng/dL (8–12 nmol/L) or free testosterone < 52 pg/mL, SHBG | 2008 | |
| Total testosterone < 350 ng/dL (12 nmol/L) or free testosterone < 65 pg/mL | 2015 | |
| Endocrine Society | Total testosterone < 300 ng/dL or free testosterone < 5 ng/dL | 2006 |
| Total testosterone < 280–300 ng/dL or free testosterone < 5–9 ng/dL | 2010 | |
| Total testosterone < 300 ng/dL or free testosterone < 5 ng/dL | 2018 | |
| International Society for Sexual Medicine (ISSM) | Total testosterone < 350 ng/dL (12 nmol/L) | International Consultation for Sexual Medicine (ICSM) 2015 |
| American Urological Association (AUA) | Total testosterone < 300 ng/dL | 2018 |
Low testosterone treatment
Testosterone replacement therapy can improve sexual interest, erections, mood and energy, body hair growth, bone density, and muscle mass. There are several ways to replace testosterone:
- Gel or patches that you put on your skin
- Injections (shots)
- Tablets that stick to the gums
- Pellets inserted under the skin or pills (in some countries outside the United States)
The best method will depend on your preference and tolerance, and the cost.
There are potential risks with long-term use of testosterone. You should discuss with your physician how to monitor for prostate cancer and other risks to your prostate. Men with known or suspected prostate cancer, or with breast cancer, should not receive testosterone treatment.
Other possible risks of testosterone treatment include:
- A high red blood cell count
- Acne
- Breast enlargement
- An increase in prostate size
- Sleep apnea—the occasional stopping of breathing during sleep (rarely)
- Fluid buildup (edema) in ankles, feet and legs (rarely)
Can testosterone replacement therapy promote youth and vitality?
Testosterone replacement therapy can help reverse the effects of hypogonadism, but it’s unclear whether testosterone replacement therapy would have any benefit for older men who are otherwise healthy.
Although some men believe that taking testosterone medications may help them feel younger and more vigorous as they age, few rigorous studies have examined testosterone therapy in men who have healthy testosterone levels. And some small studies have revealed mixed results. For example, in one study healthy men who took testosterone medications increased muscle mass but didn’t gain strength.
Should I talk to my doctor about testosterone replacement therapy?
If you wonder whether testosterone therapy might be right for you, talk with your doctor about the risks and benefits. Your doctor will likely measure your testosterone levels at least twice before discussing whether testosterone replacement therapy is an option for you.
A medical condition that leads to an unusual decline in testosterone may be a reason to take supplemental testosterone. However, treating normal aging with testosterone therapy is not currently advisable.
Your doctor will also likely suggest natural ways to boost testosterone, such as losing weight and increasing muscle mass through resistance exercise.
Benefits of testosterone replacement therapy for men
- Low testosterone comes with age — testosterone levels naturally decrease by 1% each year after age 30, though don’t severely deplete, even in advanced age
- Testosterone production may be disrupted by disorders of the testicles, pituitary gland, or brain
- Testosterone levels change from hour to hour — highest in the morning; lowest at night
- Testosterone levels can temporarily lower due to too much exercise, poor nutrition, severe illness, and with certain medications
- Normal testosterone levels should be between 300–1,000 ng/dL (nanograms per deciliter), depending on age and lab used
- Testosterone must be measured more than once for accurate assessment
Testosterone therapy is only recommended for hypogonadism patients. Boosting testosterone is NOT approved by the US Food and Drug Administration (FDA) to help improve your strength, athletic performance, physical appearance, or to treat or prevent problems associated with aging. Using testosterone for these purposes may be harmful to your health.
Male hypogonadism is a combination of low testosterone levels and the presence of any of these symptoms:
- Drop in sex drive (libido)
- Erectile dysfunction (ED — inability to get or keep an erection) and loss of spontaneous erections
- Lowered sperm count and infertility (inability to have children)
- Breast enlargement or tenderness
- Reduced energy
- Reduced muscle mass
- Shrinkage of testes
- Increased irritability, inability to concentrate, and depressed mood
- Hot flashes (when testosterone levels are very low)
You should NOT receive testosterone therapy if you have:
- Prostate or breast cancer (or suspected)
- Enlarged prostate causing difficulty with urination
- Elevated prostate specific antigen (PSA) levels
- High number of red blood cells
- Untreated sleep apnea (obstructed breathing during sleep)
- Planning to have children
- Heart attack or stroke within the last 6 months
- Blood clots
Testosterone replacement therapy contraindications
Contraindications to testosterone replacement therapy include 141:
- History of breast cancer
- Prostate cancer
- Uncontrolled heart failure
- Myocardial infarction or cerebrovascular accident within the past 6 months
- Untreated obstructive sleep apnea
- Hematocrit over 48%
- Men planning fertility
- A palpable undiagnosed prostate nodule
- An elevated PSA above 4 ng/mL
- An elevated PSA level above 3vng/mL in high-risk patients including African Americans and men with a first-degree relative with prostate cancer.
Testosterone replacement therapy dosage
Testosterone undecanoate injection (Aveed) may cause serious breathing problems and allergic reactions, during or immediately after the injection. The injection should be given by a doctor or nurse in a healthcare setting where these problems or reactions can be treated. You will need to remain in the healthcare setting for at least 30 minutes after you receive your injection. Tell your doctor or nurse immediately if you experience any of the following symptoms during or after your injection: tightening of your throat, difficulty breathing, difficulty swallowing, shortness of breath, cough or urge to cough, chest pain, dizziness, fainting, sweating, rash, hives, or itching.
A program has been set up to limit the use of testosterone undecanoate injection (Aveed) and to inform people about the increased risk of breathing problems and allergic reactions while receiving this medication. The program also makes sure that everyone who received this medication understands the risks and benefits from this medication and receives the medication in a setting where they can be monitored for serious reactions.
Your doctor or pharmacist will give you the manufacturer’s patient information sheet (Medication Guide) when you begin treatment with testosterone undecanoate injection. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration (FDA) website (http://www.fda.gov/Drugs/DrugSafety/ucm085729.htm) or the manufacturer’s website to obtain the Medication Guide.
Adult Male Dose for Hypogonadism
Uses:
- Primary hypogonadism (congenital or acquired): Testicular failure due to conditions such as cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter Syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (FSH, LH) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): Gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range.
IM INJECTION:
- Testosterone Undecanoate: 750 mg (3 mL) IM injection followed by 750 mg (3 mL) injected after 4 weeks, then 750 mg (3 mL) every 10 weeks thereafter
- Testosterone Enanthate and Cypionate: 50 to 400 mg IM injection every 2 to 4 weeks
IMPLANT:
- 2 to 6 pellets (75 mg each) implanted subcutaneously every 3 to 6 months.
- The number of pellets to be implanted depends upon the minimal daily requirements of testosterone propionate administered parenterally. Thus, implant two 75 mg pellets for each 25 mg testosterone propionate required weekly.
Comments:
- The chronological and skeletal ages must be taken into consideration, both in determining the initial dose and in adjusting the dose.
- This drug should be used only if the benefits outweigh the serious risks of pulmonary oil microembolism and anaphylaxis.
- Injections more frequently than every two weeks are not recommended.
- Adequate effect of the implants (pellets) continues for three to four months, sometimes as long as six months.
BUCCAL:
- Mucoadhesive Oral Patch: Apply a 30 mg patch to the gum region twice a day; morning and evening (about 12 hours apart).
TOPICAL:
- Transdermal Film: 2 to 6 mg applied to the back, abdomen, upper arm, or upper thigh once a day, preferably at night.
- Gel (in tubes, packets or spray): 5 g applied once a day, preferably in the morning. Consult the manufacturer product information for specific dosage and additional instructions of use.
- Transdermal Solution: Initial dose is 60 mg of testosterone (1 pump actuation of 30 mg of testosterone to each axilla), applied once a day, at the same time each morning. Consult the manufacturer product information for specific dosage and additional instructions of use.
Comments: Prior to initiating therapy with this drug, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days, and that these serum testosterone concentrations are below the normal range.
Pediatric Male Dose for Delayed Puberty
Use: To stimulate puberty in selected males with clearly delayed puberty
IM INJECTION:
- Testosterone Enanthate: 50 to 200 mg every 2 to 4 weeks for 4 to 6 months
IMPLANT:
- 2 pellets (each pellet contain 75 mg of testosterone) implanted subcutaneously every 3 to 6 months
- Duration of therapy: 4 to 6 months
Comments:
- The chronological and skeletal ages should be taken into consideration when determining the initial dose and when adjusting the dose.
- An X-ray of the hand and wrist to determine bone age should be obtained every six months to assess the effect of treatment on the epiphyseal centers.
- Report frequent or persistent erections.
- Androgen therapy should be used very cautiously in children and only by specialists who are aware of the adverse effects on bone maturation.
Renal Dose Adjustments
- Testosterone Cypionate IM injection: Contraindicated in renal disease
Liver Dose Adjustments
- Testosterone Cypionate IM injection: Contraindicated in liver disease
Dose Adjustments
- The doses of this drug should be adjusted according to the patient’s response and the appearance of adverse reactions.
Testosterone replacement therapy monitoring
Lab tests needed before starting androgen replacement include hemoglobin, hematocrit (red blood cell count), liver function tests, lipid panel, digital rectal exam, PSA level, 2-morning testosterone levels, and consider a DEXA scan.
Monitoring should be done as follows 141:
- One month after treatment: morning testosterone level
- Three to 6 months after treatment during 1 year: morning testosterone level, liver function tests, lipid profile, PSA, digital rectal exam, estradiol, hemoglobin, and hematocrit, blood pressure
- Annually after 1 year: morning testosterone level, liver function tests, lipid profile, digital rectal exam, PSA, estradiol, Hgb, and hematocrit (red blood cell count), blood pressure
Referral to urology is recommended if there is an increase in PSA level greater than 1.4 ng/mL within any 12-month period. If hematocrit rises above 54%, then stop therapy as soon as possible 141. It is important to look out for signs of sleep apnea on annual follow-up visits. DEXA scans need to be repeated 1 to 2 years after initiating therapy in hypogonadal men with osteoporosis 142. Hyperestrogenism can be a side effect of replacement therapy because testosterone can be aromatized to estrogen 143. Aromatase inhibitors may need to be prescribed. Therefore, estradiol levels in men need to be assessed to rule out hyperestrogenism. Physicians need to regularly monitor patients receiving testosterone therapy and should discontinue therapy in those who fail to follow up.
Testosterone replacement therapy risks
Testosterone replacement therapy has various risks. For example, testosterone therapy may:
- Elevate red blood cell count (erythrocytosis), thereby increasing the risk of blood clots or venous thromboembolism 144
- Cause acne or other skin reactions
- Contribute to sleep apnea — a potentially serious sleep disorder in which breathing repeatedly stops and starts
- Stimulate noncancerous growth of the prostate (benign prostatic hyperplasia) and growth of existing prostate cancer
- Enlarge breasts
- Limit sperm production or cause testicle shrinkage
- Increase the risk of a blood clot forming in a deep vein (deep vein thrombosis), which could break loose, travel through your bloodstream and lodge in your lungs, blocking blood flow (pulmonary embolism)
In addition, testosterone therapy may impact your risk of heart disease. Research has had conflicting results, so the exact risk isn’t clear yet. Most notable are the TOM (Testosterone in Older Men) trial and the TEAAM (Testosterone’s Effects on Atherosclerosis Progression in Aging Men) trials. Low testosterone levels have been associated with increased risk of coronary artery disease 145. Published in JAMA 2017, a study 146 found that testosterone replacement was associated with a lower risk of cardiovascular outcomes. In 2015, the FDA concluded a possible increased cardiovascular risk associated with testosterone use requiring labeling change to inform the public. The FDA requires that you are made aware that the possibility of cardiovascular events may exist during treatment.
The American Association of Clinical Endocrinologists 147 issued a guideline in response to the 2015 FDA labeling requirement on cardiovascular risk and stated that there is no compelling evidence that testosterone therapy increases cardiovascular risk. On the other hand, testosterone deficiency has been associated with falls, sarcopenia, frailty, osteopenia, and osteoporosis 148.
Serum PSA levels can increase in response to testosterone treatment, so it is important to rule out prostate cancer before starting therapy as it can worsen the disease process. Patients on replacement therapy need to be reevaluated for prostate cancer at 3 months and 1 year after beginning treatment 149.
There is no firm scientific evidence that long-term testosterone replacement is associated with either prostate cancer or cardiovascular events. Prostate cells are stimulated by testosterone, so be extra vigilant about cancer screenings. African American men over age 45 — especially those with family history of cancer — are already at risk for prostate cancer.
References- Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, Lightner DJ, Miner MM, Murad MH, Nelson CJ, Platz EA, Ramanathan LV, Lewis RW. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol. 2018 Aug;200(2):423-432. doi: 10.1016/j.juro.2018.03.115
- Carrasquillo R, Chu K, Ramasamy R. Novel Therapy for Male Hypogonadism. Curr Urol Rep. 2018 Jun 9;19(8):63. doi: 10.1007/s11934-018-0816-x
- Corona, G., Vignozzi, L., Sforza, A., & Maggi, M. (2013). Risks and benefits of late onset hypogonadism treatment: an expert opinion. The world journal of men’s health, 31(2), 103–125. https://doi.org/10.5534/wjmh.2013.31.2.103
- Jones, T. H., Arver, S., Behre, H. M., Buvat, J., Meuleman, E., Moncada, I., Morales, A. M., Volterrani, M., Yellowlees, A., Howell, J. D., Channer, K. S., & TIMES2 Investigators (2011). Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes care, 34(4), 828–837. https://doi.org/10.2337/dc10-1233
- Kim, M., Byun, S. S., & Hong, S. K. (2021). Testosterone Replacement Therapy in Men with Untreated or Treated Prostate Cancer: Do We Have Enough Evidences?. The world journal of men’s health, 39(4), 705–723. https://doi.org/10.5534/wjmh.190158
- Morgentaler A. Testosterone therapy for men at risk for or with history of prostate cancer. Curr Treat Options Oncol. 2006 Sep;7(5):363-9. doi: 10.1007/s11864-006-0004-y
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018 May 1;103(5):1715-1744. doi: 10.1210/jc.2018-00229
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC; International Society of Andrology; International Society for the Study of Aging Male; European Association of Urology; European Academy of Andrology; American Society of Andrology. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009 Jan;55(1):121-30. doi: 10.1016/j.eururo.2008.08.033
- Khodamoradi, K., Khosravizadeh, Z., Parmar, M., Kuchakulla, M., Ramasamy, R., & Arora, H. (2021). Exogenous testosterone replacement therapy versus raising endogenous testosterone levels: current and future prospects. F&S reviews, 2(1), 32–42. https://doi.org/10.1016/j.xfnr.2020.11.001
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet. 1977 Aug 6;2(8032):262-3.
- Korbonits M, Kipnes M, Grossman AB. Striant SR: a novel, effective and convenient testosterone therapy for male hypogonadism. Int J Clin Pract. 2004 Nov;58(11):1073-80. doi: 10.1111/j.1368-5031.2004.00383.x
- Swerdloff, R. S., Wang, C., White, W. B., Kaminetsky, J., Gittelman, M. C., Longstreth, J. A., Dudley, R. E., & Danoff, T. M. (2020). A New Oral Testosterone Undecanoate Formulation Restores Testosterone to Normal Concentrations in Hypogonadal Men. The Journal of clinical endocrinology and metabolism, 105(8), 2515–2531. https://doi.org/10.1210/clinem/dgaa238
- Zhang, M., Wang, J., Deng, C., Jiang, M. H., Feng, X., Xia, K., Li, W., Lai, X., Xiao, H., Ge, R. S., Gao, Y., & Xiang, A. P. (2017). Transplanted human p75-positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell death & disease, 8(10), e3123. https://doi.org/10.1038/cddis.2017.531
- Dobs AS, Matsumoto AM, Wang C, Kipnes MS. Short-term pharmacokinetic comparison of a novel testosterone buccal system and a testosterone gel in testosterone deficient men. Curr Med Res Opin. 2004 May;20(5):729-38. doi: 10.1185/030079904125003494
- Dinsmore WW, Wyllie MG. The long-term efficacy and safety of a testosterone mucoadhesive buccal tablet in testosterone-deficient men. BJU Int. 2012 Jul;110(2):162-9. doi: 10.1111/j.1464-410X.2011.10837.x
- de Ronde W. Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Hum Reprod. 2009 Feb;24(2):425-8. doi: 10.1093/humrep/den372
- Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004 May;89(5):2085-98. doi: 10.1210/jc.2003-032006
- Fennell C, Sartorius G, Ly LP, Turner L, Liu PY, Conway AJ, Handelsman DJ. Randomized cross-over clinical trial of injectable vs. implantable depot testosterone for maintenance of testosterone replacement therapy in androgen deficient men. Clin Endocrinol (Oxf). 2010 Jul;73(1):102-9. doi: 10.1111/j.1365-2265.2009.03744.x
- Mazer N, Bell D, Wu J, Fischer J, Cosgrove M, Eilers B; BS, RN,. Comparison of the steady-state pharmacokinetics, metabolism, and variability of a transdermal testosterone patch versus a transdermal testosterone gel in hypogonadal men. J Sex Med. 2005 Mar;2(2):213-26. doi: 10.1111/j.1743-6109.2005.20231.x
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R; North American AA2500 T Gel Study Group. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003 Jun;88(6):2673-81. doi: 10.1210/jc.2002-021058
- Grober ED, Khera M, Soni SD, Espinoza MG, Lipshultz LI. Efficacy of changing testosterone gel preparations (Androgel or Testim) among suboptimally responsive hypogonadal men. Int J Impot Res. 2008 Mar-Apr;20(2):213-7. doi: 10.1038/sj.ijir.3901618
- Kim ED, McCullough A, Kaminetsky J. Oral enclomiphene citrate raises testosterone and preserves sperm counts in obese hypogonadal men, unlike topical testosterone: restoration instead of replacement. BJU Int. 2016 Apr;117(4):677-85. doi: 10.1111/bju.13337
- Rogol AD, Tkachenko N, Bryson N. Natesto™ , a novel testosterone nasal gel, normalizes androgen levels in hypogonadal men. Andrology. 2016 Jan;4(1):46-54. doi: 10.1111/andr.12137. Epub 2015 Dec 22. Erratum in: Andrology. 2017 Jul;5(4):844.
- Rogol, A. D., Tkachenko, N., Badorrek, P., Hohlfeld, J. M., & Bryson, N. (2018). Phase 1 pharmacokinetics and phase 3 efficacy of testosterone nasal gel in subjects with seasonal allergies. Canadian Urological Association journal = Journal de l’Association des urologues du Canada, 12(7), E349–E356. https://doi.org/10.5489/cuaj.4898
- Nieschlag E. Current topics in testosterone replacement of hypogonadal men. Best Pract Res Clin Endocrinol Metab. 2015 Jan;29(1):77-90. doi: 10.1016/j.beem.2014.09.008
- Nieschlag E, Mauss J, Coert A, Kićović P. Plasma androgen levels in men after oral administration of testosterone or testosterone undecanoate. Acta Endocrinol (Copenh). 1975 Jun;79(2):366-74. doi: 10.1530/acta.0.0790366
- Tsametis CP, Isidori AM. Testosterone replacement therapy: For whom, when and how? Metabolism. 2018 Sep;86:69-78. doi: 10.1016/j.metabol.2018.03.007
- Behre HM, Abshagen K, Oettel M, Hübler D, Nieschlag E. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies. Eur J Endocrinol. 1999 May;140(5):414-9. doi: 10.1530/eje.0.1400414
- Middleton T, Turner L, Fennell C, Savkovic S, Jayadev V, Conway AJ, Handelsman DJ. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol. 2015 May;172(5):511-7. doi: 10.1530/EJE-14-0891
- Mackey MA, Conway AJ, Handelsman DJ. Tolerability of intramuscular injections of testosterone ester in oil vehicle. Hum Reprod. 1995 Apr;10(4):862-5. doi: 10.1093/oxfordjournals.humrep.a136051
- Seftel A. Testosterone replacement therapy for male hypogonadism: part III. Pharmacologic and clinical profiles, monitoring, safety issues, and potential future agents. Int J Impot Res. 2007 Jan-Feb;19(1):2-24. doi: 10.1038/sj.ijir.3901366
- Kelleher S, Turner L, Howe C, Conway AJ, Handelsman DJ. Extrusion of testosterone pellets: a randomized controlled clinical study. Clin Endocrinol (Oxf). 1999 Oct;51(4):469-71. doi: 10.1046/j.1365-2265.1999.00827.x
- Gronski, M. A., Grober, E. D., Gottesman, I. S., Ormsby, R. W., & Bryson, N. (2019). Efficacy of Nasal Testosterone Gel (Natesto®) Stratified by Baseline Endogenous Testosterone Levels. Journal of the Endocrine Society, 3(9), 1652–1662. https://doi.org/10.1210/js.2019-00183
- Ramasamy R, Masterson TA, Best JC, Bitran J, Ibrahim E, Molina M, Kaiser UB, Miao F, Reis IM. Effect of Natesto on Reproductive Hormones, Semen Parameters and Hypogonadal Symptoms: A Single Center, Open Label, Single Arm Trial. J Urol. 2020 Sep;204(3):557-563. doi: 10.1097/JU.0000000000001078
- Ribeiro MA, Gameiro LF, Scarano WR, Briton-Jones C, Kapoor A, Rosa MB, El Dib R. Aromatase inhibitors in the treatment of oligozoospermic or azoospermic men: a systematic review of randomized controlled trials. JBRA Assist Reprod. 2016 May 1;20(2):82-8. doi: 10.5935/1518-0557.20160019
- Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism. 2003 Sep;52(9):1126-8. doi: 10.1016/s0026-0495(03)00186-0
- Majzoub, A., & Sabanegh, E., Jr (2016). Testosterone replacement in the infertile man. Translational andrology and urology, 5(6), 859–865. https://doi.org/10.21037/tau.2016.08.03
- Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, López FJ, Marschke KB, Rosen J, Schrader W, Turner R, van Oeveren A, Viveros H, Zhi L, Negro-Vilar A. An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology. 2007 Jan;148(1):363-73. doi: 10.1210/en.2006-0793
- Pasqualotto FF, Lucon AM, Sobreiro BP, Pasqualotto EB, Arap S. Effects of medical therapy, alcohol, smoking, and endocrine disruptors on male infertility. Rev Hosp Clin Fac Med Sao Paulo. 2004 Dec;59(6):375-82. doi: 10.1590/s0041-87812004000600011
- Bhasin, S., & Jasuja, R. (2009). Selective androgen receptor modulators as function promoting therapies. Current opinion in clinical nutrition and metabolic care, 12(3), 232–240. https://doi.org/10.1097/MCO.0b013e32832a3d79
- Narayanan, R., Coss, C. C., & Dalton, J. T. (2018). Development of selective androgen receptor modulators (SARMs). Molecular and cellular endocrinology, 465, 134–142. https://doi.org/10.1016/j.mce.2017.06.013
- Solomon, Z. J., Mirabal, J. R., Mazur, D. J., Kohn, T. P., Lipshultz, L. I., & Pastuszak, A. W. (2019). Selective Androgen Receptor Modulators: Current Knowledge and Clinical Applications. Sexual medicine reviews, 7(1), 84–94. https://doi.org/10.1016/j.sxmr.2018.09.006
- Winters SJ, Moore JP Jr, Clark BJ. Leydig cell insufficiency in hypospermatogenesis: a paracrine effect of activin-inhibin signaling? Andrology. 2018 Mar;6(2):262-271. doi: 10.1111/andr.12459
- Beattie, M. C., Adekola, L., Papadopoulos, V., Chen, H., & Zirkin, B. R. (2015). Leydig cell aging and hypogonadism. Experimental gerontology, 68, 87–91. https://doi.org/10.1016/j.exger.2015.02.014
- Arora, H., Zuttion, M., Nahar, B., Lamb, D., Hare, J. M., & Ramasamy, R. (2019). Subcutaneous Leydig Stem Cell Autograft: A Promising Strategy to Increase Serum Testosterone. Stem cells translational medicine, 8(1), 58–65. https://doi.org/10.1002/sctm.18-0069
- El Meliegy, A., Motawi, A., & El Salam, M. (2017). Systematic review of hormone replacement therapy in the infertile man. Arab journal of urology, 16(1), 140–147. https://doi.org/10.1016/j.aju.2017.11.011
- Chehab M, Madala A, Trussell JC. On-label and off-label drugs used in the treatment of male infertility. Fertil Steril. 2015 Mar;103(3):595-604. doi: 10.1016/j.fertnstert.2014.12.122
- Martinkovich, S., Shah, D., Planey, S. L., & Arnott, J. A. (2014). Selective estrogen receptor modulators: tissue specificity and clinical utility. Clinical interventions in aging, 9, 1437–1452. https://doi.org/10.2147/CIA.S66690
- Pickar JH, MacNeil T, Ohleth K. SERMs: progress and future perspectives. Maturitas. 2010 Oct;67(2):129-38. doi: 10.1016/j.maturitas.2010.05.009
- Dohle G, Arver S, Bettocchi C, Jones T, Kliesch S, Punab M. EAU guidelines on male hypogonadism European Association of Urology, Arnhem, The Netherlands, accessed Mar 2016;21:2018.
- Lee, J. A., & Ramasamy, R. (2018). Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men. Translational andrology and urology, 7(Suppl 3), S348–S352. https://doi.org/10.21037/tau.2018.04.11
- Crosnoe-Shipley, L. E., Elkelany, O. O., Rahnema, C. D., & Kim, E. D. (2015). Treatment of hypogonadotropic male hypogonadism: Case-based scenarios. World journal of nephrology, 4(2), 245–253. https://doi.org/10.5527/wjn.v4.i2.245
- Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013 Feb;189(2):647-50. doi: 10.1016/j.juro.2012.09.043
- Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, Sutton PR, Wright WW, Brown TR, Yan X, Zirkin BR, Jarow JP. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005 May;90(5):2595-602. doi: 10.1210/jc.2004-0802
- Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C. Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels. J Clin Endocrinol Metab. 2004 Mar;89(3):1174-80. doi: 10.1210/jc.2003-031467
- Crosnoe, L. E., Grober, E., Ohl, D., & Kim, E. D. (2013). Exogenous testosterone: a preventable cause of male infertility. Translational andrology and urology, 2(2), 106–113. https://doi.org/10.3978/j.issn.2223-4683.2013.06.01
- Jones, A., Chen, J., Hwang, D. J., Miller, D. D., & Dalton, J. T. (2009). Preclinical characterization of a (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide: a selective androgen receptor modulator for hormonal male contraception. Endocrinology, 150(1), 385–395. https://doi.org/10.1210/en.2008-0674
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT. A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther. 2005 Feb;312(2):546-53. doi: 10.1124/jpet.104.075424
- Morimoto, M., Amano, Y., Oka, M., Harada, A., Fujita, H., Hikichi, Y., Tozawa, R., Yamaoka, M., & Hara, T. (2017). Amelioration of sexual behavior and motor activity deficits in a castrated rodent model with a selective androgen receptor modulator SARM-2f. PloS one, 12(12), e0189480. https://doi.org/10.1371/journal.pone.0189480
- Zhou R, Wu J, Liu B, Jiang Y, Chen W, Li J, He Q, He Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol Life Sci. 2019 Jul;76(14):2681-2695. doi: 10.1007/s00018-019-03101-9
- Chen, H., Ge, R. S., & Zirkin, B. R. (2009). Leydig cells: From stem cells to aging. Molecular and cellular endocrinology, 306(1-2), 9–16. https://doi.org/10.1016/j.mce.2009.01.023
- Chen, H., Stanley, E., Jin, S., & Zirkin, B. R. (2010). Stem Leydig cells: from fetal to aged animals. Birth defects research. Part C, Embryo today : reviews, 90(4), 272–283. https://doi.org/10.1002/bdrc.20192
- Neaves WB. Leydig cells. Contraception. 1975 May;11(5):571-606. doi: 10.1016/0010-7824(75)90111-0
- Jiang, M. H., Cai, B., Tuo, Y., Wang, J., Zang, Z. J., Tu, X., Gao, Y., Su, Z., Li, W., Li, G., Zhang, M., Jiao, J., Wan, Z., Deng, C., Lahn, B. T., & Xiang, A. P. (2014). Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell research, 24(12), 1466–1485. https://doi.org/10.1038/cr.2014.149
- Swerdloff RS, Wang C, Bhasin S. Developments in the control of testicular function. Baillieres Clin Endocrinol Metab. 1992 Apr;6(2):451-83. https://doi.org/10.1016/S0950-351X(05)80158-2
- Payne AH, O’Shaughnessy P (1996). “Structure, function, and regulation of steroidogenic enzymes in the Leydig cell”. In Payne AH, Hardy MP, Russell LD (eds.). Leydig Cell. Vienna [Il]: Cache River Press. pp. 260–85. ISBN 978-0-9627422-7-9
- Handelsman, D. J., Hirschberg, A. L., & Bermon, S. (2018). Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocrine reviews, 39(5), 803–829. https://doi.org/10.1210/er.2018-00020
- Cunningham, G. R., Stephens-Shields, A. J., Rosen, R. C., Wang, C., Bhasin, S., Matsumoto, A. M., Parsons, J. K., Gill, T. M., Molitch, M. E., Farrar, J. T., Cella, D., Barrett-Connor, E., Cauley, J. A., Cifelli, D., Crandall, J. P., Ensrud, K. E., Gallagher, L., Zeldow, B., Lewis, C. E., Pahor, M., … Snyder, P. J. (2016). Testosterone Treatment and Sexual Function in Older Men With Low Testosterone Levels. The Journal of clinical endocrinology and metabolism, 101(8), 3096–3104. https://doi.org/10.1210/jc.2016-1645
- Brown GA, Vukovich MD, Sharp RL, Reifenrath TA, Parsons KA, King DS. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. J Appl Physiol (1985). 1999 Dec;87(6):2274-83. doi: 10.1152/jappl.1999.87.6.2274
- Winter, A. G., Zhao, F., & Lee, R. K. (2014). Androgen deficiency and metabolic syndrome in men. Translational andrology and urology, 3(1), 50–58. https://doi.org/10.3978/j.issn.2223-4683.2014.01.04
- Wu FCW, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N Engl J Med 2010;363:123–35. doi:10.1056/NEJMoa0911101
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl 2009;32:1–10. doi:10.1111/j.1365-2605.2008.00924.x
- Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007 Nov;92(11):4241-7. doi: 10.1210/jc.2007-1245
- Seftel AD. Male hypogonadism. Part I: Epidemiology of hypogonadism. Int J Impot Res 2006;18:115–20. doi:10.1038/sj.ijir.3901397
- Testosterone. https://medlineplus.gov/ency/article/003707.htm
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001 Feb;86(2):724-31. doi: 10.1210/jcem.86.2.7219
- Huhtaniemi I. (2014). Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian journal of andrology, 16(2), 192–202. https://doi.org/10.4103/1008-682X.122336
- Huhtaniemi IT. Andropause–lessons from the European Male Ageing Study. Ann Endocrinol (Paris). 2014 May;75(2):128-31. doi: 10.1016/j.ando.2014.03.005
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D; European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008 Jul;93(7):2737-45. doi: 10.1210/jc.2007-1972
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010 Jul 8;363(2):123-35. doi: 10.1056/NEJMoa0911101
- Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010 Dec 1;40(12):1037-53. doi: 10.2165/11536910-000000000-00000
- Hulmi JJ, Ahtiainen JP, Selänne H, Volek JS, Häkkinen K, Kovanen V, Mero AA. Androgen receptors and testosterone in men–effects of protein ingestion, resistance exercise and fiber type. J Steroid Biochem Mol Biol. 2008 May;110(1-2):130-7. doi: 10.1016/j.jsbmb.2008.03.030
- Hackney AC, Moore AW, Brownlee KK. Testosterone and endurance exercise: development of the “exercise-hypogonadal male condition”. Acta Physiol Hung. 2005;92(2):121-37. doi: 10.1556/APhysiol.92.2005.2.3
- Livera G, Rouiller-Fabre V, Pairault C, Levacher C, Habert R. Regulation and perturbation of testicular functions by vitamin A. Reproduction. 2002 Aug;124(2):173-80.
- Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011 Mar;43(3):223-5. doi: 10.1055/s-0030-1269854
- Elisabeth Lerchbaum, Stefan Pilz, Christian Trummer, Verena Schwetz, Oliver Pachernegg, Annemieke C Heijboer, Barbara Obermayer-Pietsch, Vitamin D and Testosterone in Healthy Men: A Randomized Controlled Trial, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 11, 1 November 2017, Pages 4292–4302, https://doi.org/10.1210/jc.2017-01428
- Zittermann A, Ernst JB, Prokop S, Fuchs U, Dreier J, Kuhn J, Knabbe C, Berthold HK, Gouni-Berthold I, Gummert JF, Börgermann J, Pilz S. Vitamin D supplementation does not prevent the testosterone decline in males with advanced heart failure: the EVITA trial. Eur J Nutr. 2019 Mar;58(2):673-680. doi: 10.1007/s00394-018-1666-5
- Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ. Zinc status and serum testosterone levels of healthy adults. Nutrition. 1996 May;12(5):344-8. doi: 10.1016/s0899-9007(96)80058-x
- Koehler K, Parr MK, Geyer H, Mester J, Schänzer W. Serum testosterone and urinary excretion of steroid hormone metabolites after administration of a high-dose zinc supplement. Eur J Clin Nutr. 2009 Jan;63(1):65-70. doi: 10.1038/sj.ejcn.1602899
- Whittaker J, Wu K. Low-fat diets and testosterone in men: Systematic review and meta-analysis of intervention studies. J Steroid Biochem Mol Biol. 2021 Jun;210:105878. doi: 10.1016/j.jsbmb.2021.105878
- Whittaker J, Harris M. Low-carbohydrate diets and men’s cortisol and testosterone: Systematic review and meta-analysis. Nutr Health. 2022 Mar 7:2601060221083079. doi: 10.1177/02601060221083079
- Håkonsen, L. B., Thulstrup, A. M., Aggerholm, A. S., Olsen, J., Bonde, J. P., Andersen, C. Y., Bungum, M., Ernst, E. H., Hansen, M. L., Ernst, E. H., & Ramlau-Hansen, C. H. (2011). Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reproductive health, 8, 24. https://doi.org/10.1186/1742-4755-8-24
- A.A. MacDonald, G.P. Herbison, M. Showell, C.M. Farquhar, The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis, Human Reproduction Update, Volume 16, Issue 3, May-June 2010, Pages 293–311, https://doi.org/10.1093/humupd/dmp047
- Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006 May;85(5):1319-40. doi: 10.1016/j.fertnstert.2005.10.054
- Salas-Huetos A, Maghsoumi-Norouzabad L, James ER, Carrell DT, Aston KI, Jenkins TG, Becerra-Tomás N, Javid AZ, Abed R, Torres PJ, Luque EM, Ramírez ND, Martini AC, Salas-Salvadó J. Male adiposity, sperm parameters and reproductive hormones: An updated systematic review and collaborative meta-analysis. Obes Rev. 2021 Jan;22(1):e13082. doi: 10.1111/obr.13082
- Andersen ML, Tufik S. The effects of testosterone on sleep and sleep-disordered breathing in men: its bidirectional interaction with erectile function. Sleep Med Rev. 2008 Oct;12(5):365-79. doi: 10.1016/j.smrv.2007.12.003
- Schultheiss OC, Campbell KL, McClelland DC. Implicit power motivation moderates men’s testosterone responses to imagined and real dominance success. Horm Behav. 1999 Dec;36(3):234-41. doi: 10.1006/hbeh.1999.1542
- Grant P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother Res. 2010 Feb;24(2):186-8. doi: 10.1002/ptr.2900
- Armanini D, Fiore C, Mattarello MJ, Bielenberg J, Palermo M. History of the endocrine effects of licorice. Exp Clin Endocrinol Diabetes. 2002 Sep;110(6):257-61. doi: 10.1055/s-2002-34587
- Hauser LJ, Jensen EL, Mirsky DM, Chan KH. Pediatric anosmia: A case series. Int J Pediatr Otorhinolaryngol. 2018 Jul;110:135-139. doi: 10.1016/j.ijporl.2018.05.011
- Burdea L, Mendez MD. 21 Hydroxylase Deficiency. [Updated 2021 Aug 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493164
- Saez, J. M., Forest, M. G., Morera, A. M., & Bertrand, J. (1972). Metabolic clearance rate and blood production rate of testosterone and dihydrotestosterone in normal subjects, during pregnancy, and in hyperthyroidism. The Journal of clinical investigation, 51(5), 1226–1234. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC292254/pdf/jcinvest00201-0192.pdf
- Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001 Dec;22(6):764-86. doi: 10.1210/edrv.22.6.0446
- Nassar GN, Leslie SW. Physiology, Testosterone. [Updated 2022 Jan 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526128
- Clark BJ, Prough RA, Klinge CM. Mechanisms of Action of Dehydroepiandrosterone. Vitam Horm. 2018;108:29-73. doi: 10.1016/bs.vh.2018.02.003
- Zhang JR, Zhang PY, Sun LG. [Mild androgen insensitivity syndrome: a case report]. Zhonghua Nei Ke Za Zhi. 2018 Aug 1;57(8):600-602. Chinese. doi: 10.3760/cma.j.issn.0578-1426.2018.08.014
- Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013 Jun;42(2):255-70. doi: 10.1016/j.ecl.2013.02.012
- Kalfa N, Gaspari L, Ollivier M, Philibert P, Bergougnoux A, Paris F, Sultan C. Molecular genetics of hypospadias and cryptorchidism recent developments. Clin Genet. 2019 Jan;95(1):122-131. doi: 10.1111/cge.13432
- Travison, T. G., Vesper, H. W., Orwoll, E., Wu, F., Kaufman, J. M., Wang, Y., Lapauw, B., Fiers, T., Matsumoto, A. M., & Bhasin, S. (2017). Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. The Journal of clinical endocrinology and metabolism, 102(4), 1161–1173. https://doi.org/10.1210/jc.2016-2935
- Shin, Y. S., & Park, J. K. (2019). The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism. Journal of clinical medicine, 8(2), 209. https://doi.org/10.3390/jcm8020209
- Lunenfeld B, Arver S, Moncada I, Rees DA, Schulte HM. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male. 2012 Dec;15(4):187-97. doi: 10.3109/13685538.2012.729110
- Male Hypogonadism. https://d56bochluxqnz.cloudfront.net/media/EAU-Guidelines-on-Male-Hypogonadism-2019v2.pdf
- Grober E. D. (2014). Testosterone deficiency and replacement: Myths and realities. Canadian Urological Association journal = Journal de l’Association des urologues du Canada, 8(7-8 Suppl 5), S145–S147. https://doi.org/10.5489/cuaj.2309
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010 Jun;95(6):2536-59. doi: 10.1210/jc.2009-2354. Erratum in: J Clin Endocrinol Metab. 2021 Jun 16;106(7):e2848.
- Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, Perry HM 3rd. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000 Sep;49(9):1239-42. doi: 10.1053/meta.2000.8625
- Peterson, M. D., Belakovskiy, A., McGrath, R., & Yarrow, J. F. (2018). Testosterone Deficiency, Weakness, and Multimorbidity in Men. Scientific reports, 8(1), 5897. https://doi.org/10.1038/s41598-018-24347-6
- Mulligan, T., Frick, M. F., Zuraw, Q. C., Stemhagen, A., & McWhirter, C. (2006). Prevalence of hypogonadism in males aged at least 45 years: the HIM study. International journal of clinical practice, 60(7), 762–769. https://doi.org/10.1111/j.1742-1241.2006.00992.x
- Aversa A, Morgentaler A. The practical management of testosterone deficiency in men. Nat Rev Urol. 2015 Nov;12(11):641-50. doi: 10.1038/nrurol.2015.238
- Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003 Apr;24(2):183-217. doi: 10.1210/er.2001-0025
- Heron M. Deaths: Leading Causes for 2014. Natl Vital Stat Rep. 2016 Jun;65(5):1-96.
- Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morley JE, Schulman C, Wang C, Weidner W, Wu FC. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2005 Jun;8(2):56-8. doi: 10.1080/13685530500130969
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med. 2011 Jul;124(7):578-87. doi: 10.1016/j.amjmed.2010.12.027
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006 Aug 14-28;166(15):1660-5. doi: 10.1001/archinte.166.15.1660
- Choi, S. W., Jeon, S. H., Kwon, E. B., Zhu, G. Q., Lee, K. W., Choi, J. B., Jeong, H. C., Kim, K. S., Bae, S. R., Bae, W. J., Kim, S. J., Cho, H. J., Ha, U. S., Hong, S. H., Hwang, S. Y., & Kim, S. W. (2019). Effect of Korean Herbal Formula (Modified Ojayeonjonghwan) on Androgen Receptor Expression in an Aging Rat Model of Late Onset Hypogonadism. The world journal of men’s health, 37(1), 105–112. https://doi.org/10.5534/wjmh.180051
- Hackett, G. Type 2 Diabetes and Testosterone Therapy. World J. Mens Health 2019, 37, 31–44.
- Nam, Y.S.; Lee, G.; Yun, J.M.; Cho, B. Testosterone Replacement, Muscle Strength, and Physical Function. World J. Mens Health 2018, 36, 110–122.
- Bassil, N., Alkaade, S., & Morley, J. E. (2009). The benefits and risks of testosterone replacement therapy: a review. Therapeutics and clinical risk management, 5(3), 427–448. https://doi.org/10.2147/tcrm.s3025
- Morales A, Schulman CC, Tostain J, C W Wu F. Testosterone Deficiency Syndrome (TDS) needs to be named appropriately–the importance of accurate terminology. Eur Urol. 2006 Sep;50(3):407-9. doi: 10.1016/j.eururo.2006.07.001
- Kumar, P., Kumar, N., Thakur, D. S., & Patidar, A. (2010). Male hypogonadism: Symptoms and treatment. Journal of advanced pharmaceutical technology & research, 1(3), 297–301. https://doi.org/10.4103/0110-5558.72420
- Seftel A. Male hypogonadism. Part II: etiology, pathophysiology, and diagnosis. Int J Impot Res. 2006 May-Jun;18(3):223-8. doi: 10.1038/sj.ijir.3901365
- Naifar M, Rekik N, Messedi M, Chaabouni K, Lahiani A, Turki M, Abid M, Ayedi F, Jamoussi K. Male hypogonadism and metabolic syndrome. Andrologia. 2015 Jun;47(5):579-86. doi: 10.1111/and.12305
- Shin YS, Park JK. The Optimal Indication for Testosterone Replacement Therapy in Late Onset Hypogonadism. Journal of Clinical Medicine. 2019; 8(2):209. https://doi.org/10.3390/jcm8020209
- Brunton SA, Sadovsky R. Late-onset male hypogonadism and testosterone replacement therapy in primary care. J Fam Pract. 2010 Jul;59(7 Suppl):S1-8.
- Park, H. J., Ahn, S. T., & Moon, D. G. (2019). Evolution of Guidelines for Testosterone Replacement Therapy. Journal of clinical medicine, 8(3), 410. https://doi.org/10.3390/jcm8030410
- Morales A. Andropause (or symptomatic late-onset hypogonadism): facts, fiction and controversies. Aging Male. 2004 Dec;7(4):297-303. doi: 10.1080/13685530400016664
- Yialamas MA, Hayes FJ. Androgens and the ageing male and female. Best Pract Res Clin Endocrinol Metab. 2003 Jun;17(2):223-36. doi: 10.1016/s1521-690x(03)00018-6
- Morales A. Testosterone Deficiency Syndrome: an overview with emphasis on the diagnostic conundrum. Clin Biochem. 2014 Jul;47(10-11):960-6. doi: 10.1016/j.clinbiochem.2013.11.024
- Buvat, J.; Lemaire, A. Endocrine screening in 1022 men with erectile dysfunction: clinical significance and cost-effective strategy. J. Urol. 1997, 158, 1764–1767.
- Mulhall, J.P.; Trost, L.W.; Brannigan, R.E.; Kurtz, E.G.; Redmon, J.B.; Chiles, K.A.; Lightner, D.J.; Miner, M.M.; Murad, M.H.; Nelson, C.J.; et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J. Urol. 2018, 200, 423–432.
- Morales, A.; Bebb, R.A.; Manjoo, P.; Assimakopoulos, P.; Axler, J.; Collier, C.; Elliott, S.; Goldenberg, L.; Gottesman, I.; Grober, E.D.; et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. Can. Med. Assoc. J. 2015, 187, 1369–1377.
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018 May 01;103(5):1715-1744
- Golds G, Houdek D, Arnason T. Male Hypogonadism and Osteoporosis: The Effects, Clinical Consequences, and Treatment of Testosterone Deficiency in Bone Health. Int J Endocrinol. 2017;2017:4602129
- Tan RS, Cook KR, Reilly WG. High estrogen in men after injectable testosterone therapy: the low T experience. Am J Mens Health. 2015 May;9(3):229-34
- Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, Salcido-Montenegro A, Benkhadra R, Prokop LJ, Bhasin S, Brito JP. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: A systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Endocrinol. Metab. 2018 Mar 17
- Rosano GM, Sheiban I, Massaro R, Pagnotta P, Marazzi G, Vitale C, Mercuro G, Volterrani M, Aversa A, Fini M. Low testosterone levels are associated with coronary artery disease in male patients with angina. Int. J. Impot. Res. 2007 Mar-Apr;19(2):176-82
- Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, Quesenberry CP, VanDenEeden SK. Association of Testosterone Replacement With Cardiovascular Outcomes Among Men With Androgen Deficiency. JAMA Intern Med. 2017 Apr 01;177(4):491-499.
- Goodman N, Guay A, Dandona P, Dhindsa S, Faiman C, Cunningham GR., AACE Reproductive Endocrinology Scientific Committee. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY POSITION STATEMENT ON THE ASSOCIATION OF TESTOSTERONE AND CARDIOVASCULAR RISK. Endocr Pract. 2015 Sep;21(9):1066-73.
- Hsu B, Cumming RG, Handelsman DJ. Testosterone, frailty and physical function in older men. Expert Rev Endocrinol Metab. 2018 May;13(3):159-165.
- Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018 May 01;103(5):1715-1744.