What is Transferrin saturation
Transferrin is a blood-plasma glycoprotein with a molecular weight of 79570 daltons and transferrin is the iron transport protein in serum. Transferrin plays a central role in iron metabolism and is responsible for ferric (Fe3+) iron delivery 1. Transferrin is the main protein in the blood that binds to iron and transports it throughout the body. A transferrin test directly measures the transferrin level in your blood. Alternatively, transferrin may be measured indirectly (or converted by calculation) so that its level is expressed as the amount of iron it is capable of binding. This is called the total iron binding capacity (TIBC). Transferrin saturation is the percentage of transferrin and other iron binding proteins. Transferrin saturation is calculated by dividing the iron concentration by the total iron-binding capacity (TIBC) produces an estimate of how many of transferrin iron-binding sites are occupied; this is called the transferrin saturation. Under normal conditions, transferrin is typically one-third saturated with iron. This means that about two-thirds of its capacity is held in reserve. Less commonly, the iron concentration may be divided by the transferrin concentration, not the total iron-binding capacity (TIBC). This similar estimate is usually called the transferrin index. Transferrin saturation is helpful to find the cause of abnormal iron and total iron binding capacity (TIBC) level.
Transferrin saturation is calculated as follows:
- Transferrin saturation calculation (%) = [Serum iron level (µg/dL)/total iron-binding capacity (TIBC) (µg/dL)] x 100
Transferrin functions as the most critical ferric pool in the body. Transferrin transports iron through the blood to various tissues such as the liver, spleen and bone marrow. Transferrin is an essential biochemical marker of body iron status. Transferrin is a free peptide (apotransferin) that undergoes a conformation change after binding with iron. Iron circulates in the plasma until it attaches to a transferrin receptor on a target cell. A carbonate has to be present to help attract iron to transferrin by creating opposing repulsive charges. Transferrin can bind to two atoms of ferric iron (Fe3+) with high affinity. The carbonate needed also serves as a ligand to stabilize iron in the binding site of transferrin. Clathrin/receptor-mediated endocytosis mediates the uptake of iron by transferrin receptors 2. An acidic environment of Ph5.6 reduces iron-transferrin affinity, which encourages the release of iron from its binding site and endocytosed into a cell 3.
Transferrin can be used to assess the iron level in the body along with other markers in the body. Transferrin level testing is used to determine the cause of anemia, examine iron metabolism and determine the iron-carrying capacity of the blood 1. Transferrin saturation levels cannot be solely read in isolation but in conjunction with other laboratory tests such as serum ferritin and TIBC. Ferritin is the first marker to become low, therefore more sensitive than transferrin in diagnosing Iron deficiency anemia 4. Total or Transferrin iron binding capacity (TIBC) is a test which measures the blood capacity to bind iron with transferrin.
Serum iron, total iron-binding capacity (TIBC) and percent transferrin saturation are useful only in screening for chronic iron overload diseases, particularly hereditary hemochromatosis. Although serum iron, total iron-binding capacity and percent transferrin saturation are widely used for the diagnosis of iron deficiency, serum ferritin is a much more sensitive and reliable means of demonstrating iron deficiency.
In hereditary hemochromatosis, serum iron is usually above 150 mcg/dL and percent transferrin saturation exceeds 60%.
In advanced iron overload states, the percent transferrin saturation often exceeds 90%.
Measurement of serum iron, iron-binding capacity, and percent transferrin saturation should not be used as the primary test for iron deficiency. It may be helpful when used in conjunction with ferritin and soluble transferrin receptor testing, especially in patients with inflammation.
Transferrin divides into subgroups; these are serum transferrin, lactotransferrin, and melanotransferrin 5. Hepatocytes produce serum transferrin found in the serum, CSF, and semen. Mucosal epithelial cells produce lactotransferrin seen in bodily secretions such as milk. Lactotransferrin has antioxidants, antimicrobial and anti-inflammatory properties. All plasma iron is bound to transferrin 6. The transferrin-bound iron complex turnover rate is about ten times a day, which is essential to meet the daily demands of erythropoiesis 7. Therefore, transferrin acts as a balance between reticuloendothelial iron release and bone marrow uptake. Once, iron is bound to transferrin it is transported by transferrin to the bone marrow for production of hemoglobin and portions of erythrocytes. The human body loses iron through perspiration, epithelial cell desquamation, and menstruation. Iron loss is obligatory, and there are no specific means to regulate it. Therefore, iron homeostasis is hugely dependent on the tight regulation of absorption, which occurs mostly in the proximal intestine 8. The iron-bound transferrin is vital to distribute iron to the different cells of the body.
The rate of transferrin synthesis in the liver can be altered in accordance with the body’s iron requirements and iron reserves. In cases of iron deficiency, the degree of transferrin saturation appears to be an extremely sensitive indicator of functional iron depletion. The ferritin levels are depressed when there is a deficiency of storage iron. In sideropenia (iron deficiency in the blood serum), an iron deficiency can be excluded if the serum transferrin concentration is low, as in inflammation or less commonly, in cases of ascorbic acid deficiency. In screening for hereditary hemochromatosis, transferrin saturation provides a better indication of the homozygous genotype than does ferritin. The treatment of anemia with erythropoietin in patients with renal failure is only effective when sufficient depot iron is present. The best monitoring procedure is to determine transferrin saturation during therapy. Transferrin saturation in conjunction with ferritin gives a conclusive prediction of the exclusion of iron overloading in patients with chronic liver disease.
Iron physiology
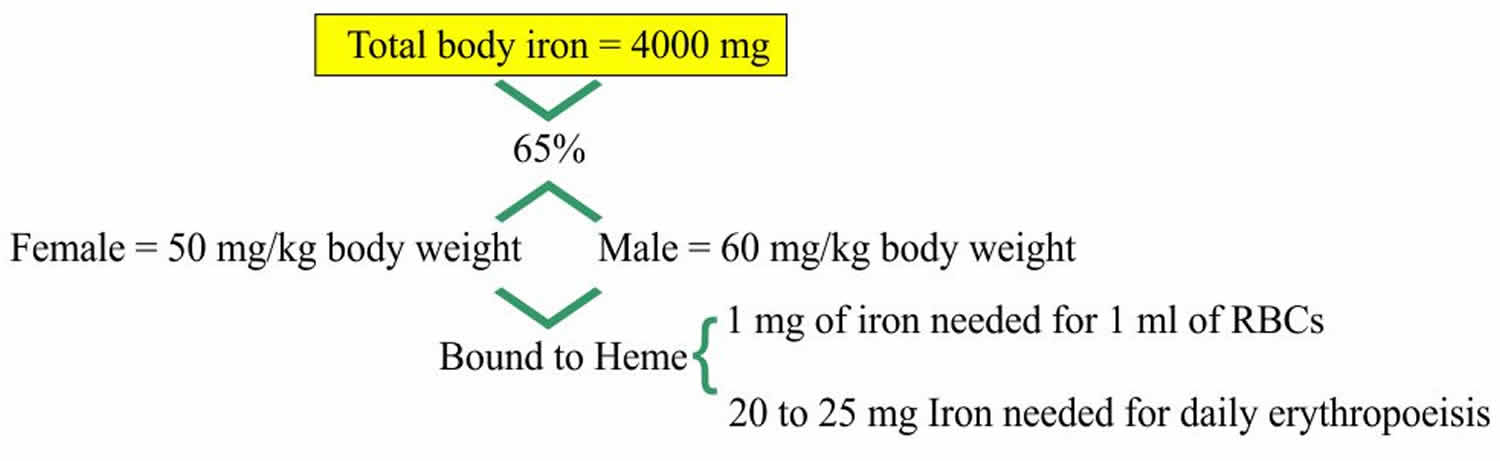
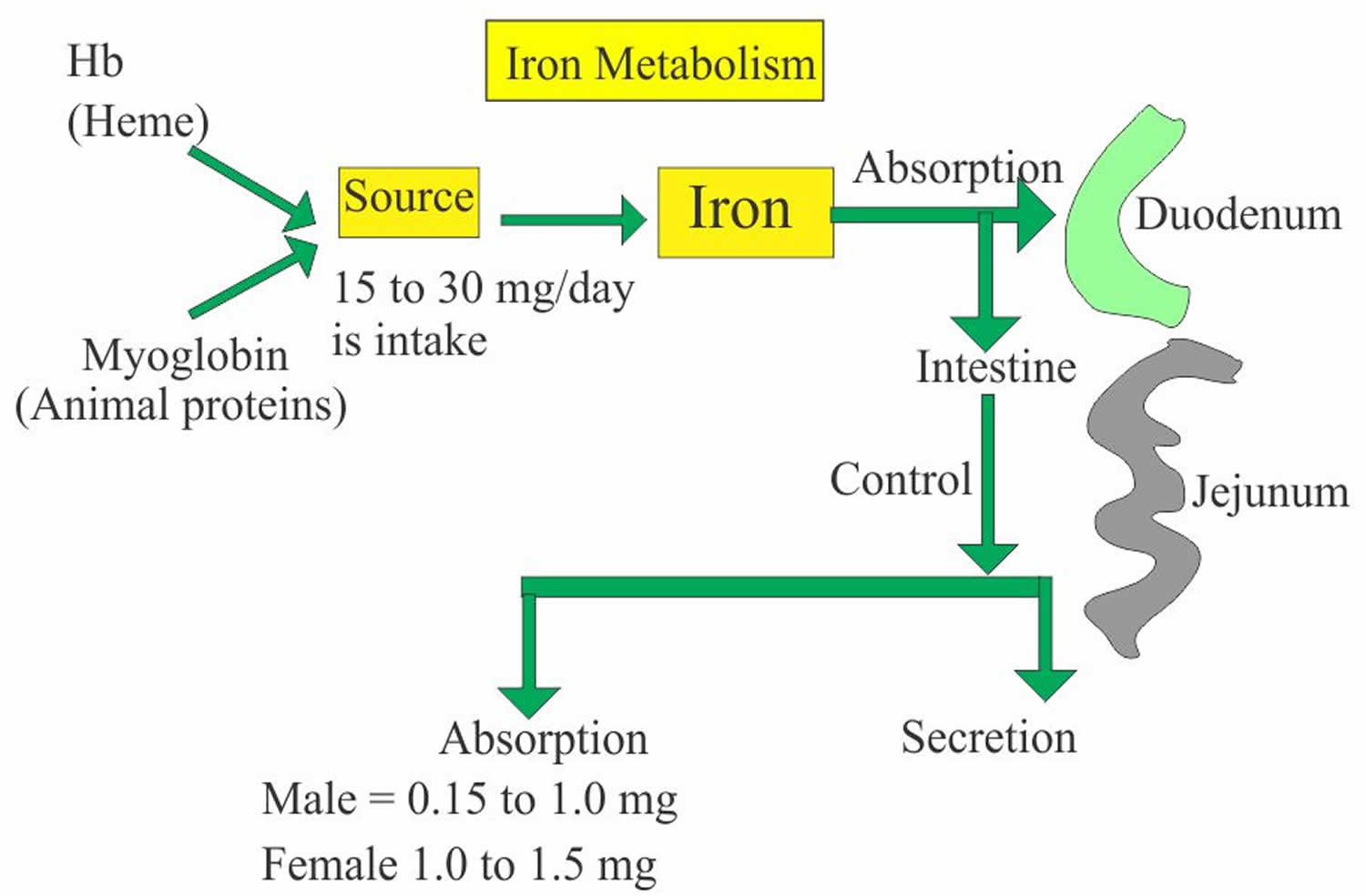
Iron is a most abundant trace element in your body and iron is an essential nutrient that, among other functions, is necessary for the production of healthy red blood cells (RBCs). Iron is a critical part of hemoglobin, the protein in red blood cells that binds oxygen in the lungs and releases oxygen as blood circulates to other parts of the body. The body cannot produce iron and must absorb it from the foods you eat or from supplements.
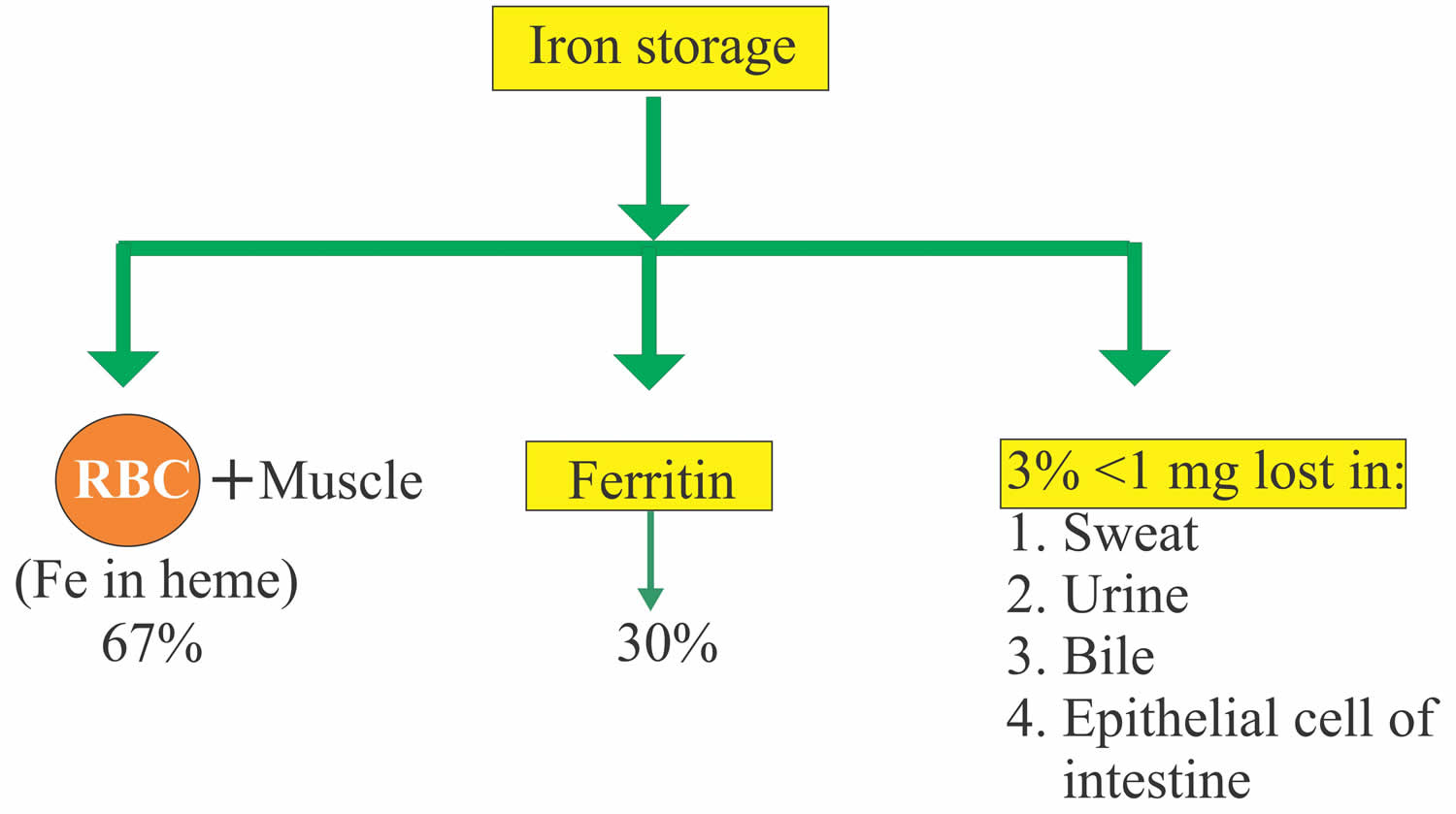
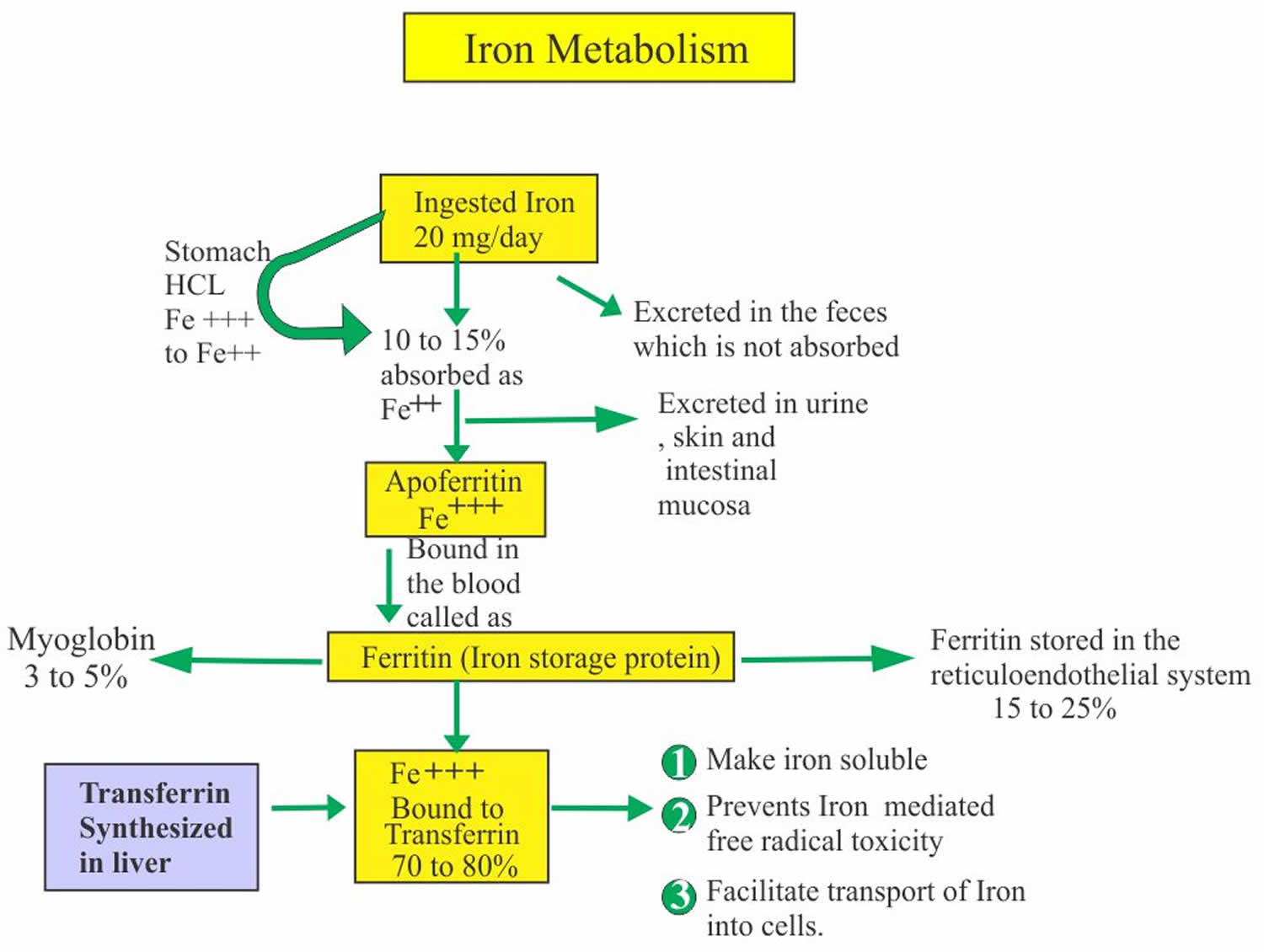
Iron in the food is absorbed by the intestinal epithelium. Iron is ingested as Fe3+ (ferric iron) form and is converted to Fe2+ (ferrous iron) form for the absorption. The conversion of Fe3+ (ferric iron) to Fe2+ (ferrous iron) form takes place in the stomach where gastric hydrochloric acid provides the acidity to reduce the iron. Ferric (Fe3+) iron is reduced to Ferrous (Fe2+) iron by vitamin C and this ferrous (Fe2+) iron form is absorbed very easily. Milk and antacid bind iron and reduce its absorption. Iron 1 mg/day is lost in the urine, sweat, bile and epithelial cells. In the blood, this absorbed Ferric (Fe3+) iron attaches with the transport protein (Transferrin). So transferrin may indirectly represent the total iron-binding capacity (TIBC).
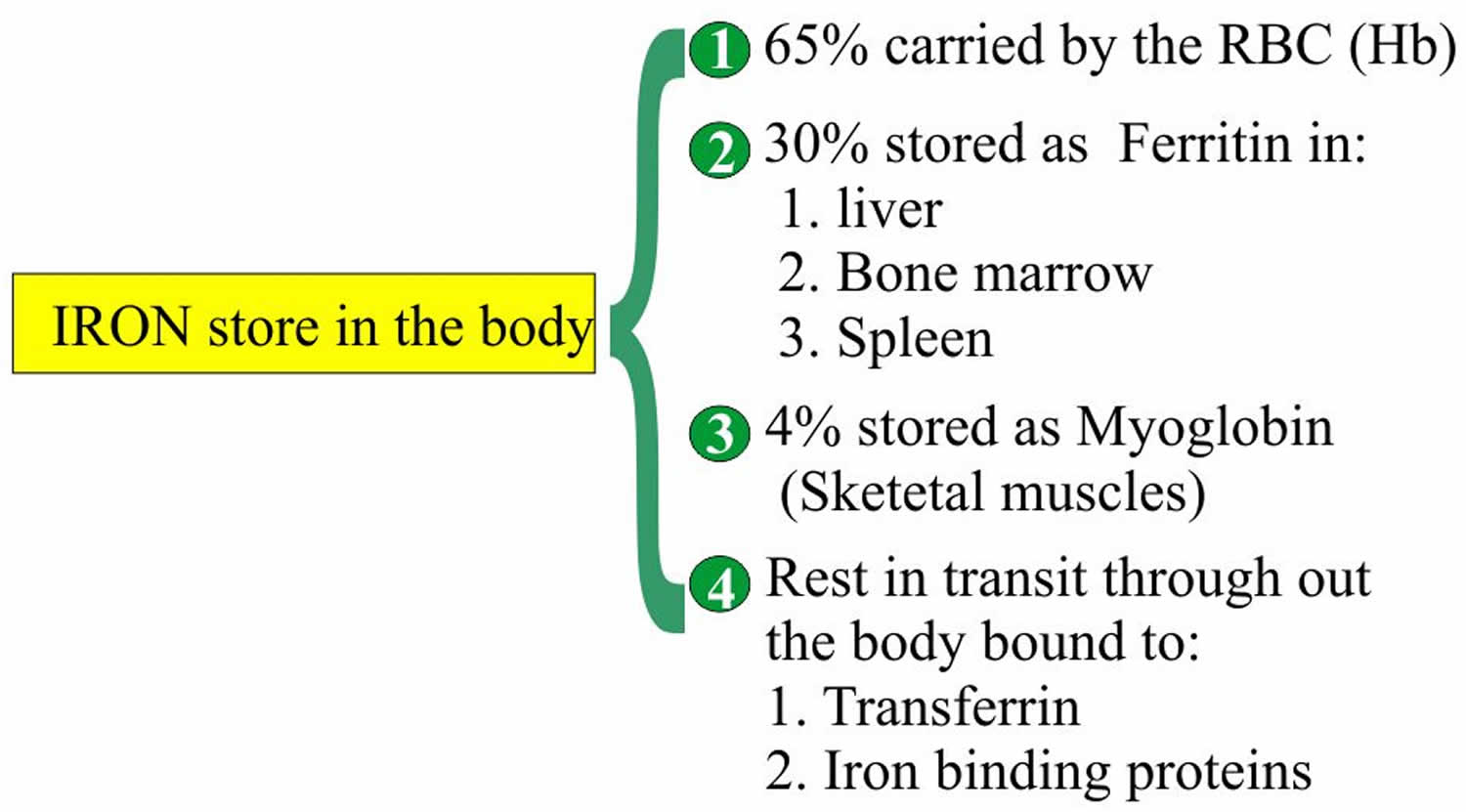
Normally, iron is transported throughout the body by transferrin, which is produced by the liver. In healthy people, most iron is incorporated into the hemoglobin within red blood cells. The remainder is stored in the tissues as ferritin or hemosiderin, with additional small amounts used for other purposes (e.g., to produce other proteins such as myoglobin and some enzymes).
- Iron is 65% bound to Heme.
- Measurement of the iron concentration refers specifically to the Fe3+ (ferric iron) bound to the transferrin and not to the iron circulating as free hemoglobin in the blood.
- Iron is constituents of:
- Heme.
- Hemoglobin.
- Methemoglobin.
- Myoglobin.
- Several enzymes.
Ferritin represents stored iron.
- 10 to 20% to 30% of the total iron is stored as Ferritin.
- Ferritin Adult male = 12 to 300 ng/mL (12 to 300 µg/L).
- Ferritin Adult female = 10 to 150 ng/mL (10 to 150 µg/L).
- Ferritin Children
- Newborn = 25 to 200 ng/mL.
- One month old = 200 to 600 ng/mL.
- 2 to 5 months old = 50 to 200 ng/mL.
- 6 months = 7 to 142 ng/mL.
- Iron is stored as ferritin in the body in liver, spleen and bone marrow.
- Or stored as Ferric (Fe3+) iron bound to an apoferritin protein molecule.
- When iron is needed then it is released from the Ferritin and is then bound to β1 globulin molecule, transferrin.
- Serum Ferritin is the best diagnostic test for the iron deficiency anemia.
- Because ferritin is the measure of the iron stores in the body.
- In an iron deficiency anemia, the ferritin level is <15 µg/L as compared to the normal level of 20 to 250 µg/L.
- In children <6 µg/L compared to the normal value of 7 to140 µg/L.
- In baby <12 µg/L compared to the normal values of 50 to 200 µg/L.
- Ferritin is the acute phase protein, so its value may increase in infections, systemic lupus erythematosus (SLE), liver diseases, malignancies, and chronic renal failure.
Total iron binding capacity (TIBC) is the capacity of transferrin to bind to iron:
- Total iron binding capacity (TIBC) is an indirect measurement of Transferrin concentration.
- TIBC measure the total amount of iron that apotransferrin has the capacity to bind.
- TIBC refer to the amount of iron that could be bound by saturation of transferrin and other minor iron-binding proteins present in the serum or plasma.
- TIBC is the sum of all protein bound to iron.
- TIBC increases in 70% of the patients with iron deficiency anemia.
- When serum iron falls then the total iron binding capacity (TIBC) increases.
- TIBC is increased in the presence of iron deficiency but may be normal or low in chronic diseases.
- TIBC may be calculated from the direct measurement of serum transferrin by the following formula:
- TIBC µg/dL = serum transferrin mg/dL x 1.25.
- Total iron binding capacity (TIBC) in Adult = 250 to 425 µg/dL (this value varies from one reference to other)
- Total iron binding capacity (TIBC) decreases in the older people around = 250 µg/dL (this value varies from one reference to other)
- TIBC µg/dL = serum transferrin mg/dL x 1.25.
- TIBC may be calculated from the direct measurement of serum transferrin by the following formula:
- A small proportion of the iron is bound to other proteins, so the above equation underestimates the total iron binding capacity (TIBC).
- The unsaturated iron-binding capacity, the amount of apotransferrin is still available to bind the iron, can be measured.
- Increased total iron binding capacity (TIBC) is seen in:
- Pregnancy.
- Iron deficiency.
- Acute hepatitis.
- Acute and chronic blood loss.
- The decreased total iron binding capacity (TIBC) is seen in:
- Hemochromatosis.
- Hypoproteinemia in malabsorption.
- Burns.
- Cirrhosis.
- Renal diseases like nephrosis etc.
- Thalassemia.
- Hyperthyroidism.
- Chronic diseases.
- Non- iron deficiency anemia
Figure 1. Iron storage in the body
Figure 2. Ferritin iron storage
Figure 3. Iron absorption and excretion
Figure 4. Iron metabolism
Transferrin function
Transferrin represents the major protein which binds to iron. Majority of the iron is bound to transferrin.
- Transferrin is a beta-globulin (β1-globulin).
- Transferrin capacity to bind iron in normal plasma is 240 to 360 µg/dL.
- Transferrin also acts as an acute phase protein.
- This is a transport protein synthesized in the liver.
- This regulates iron absorption.
- Transferrin also called siderophilin.
- Total iron + total iron-binding capacity (TIBC) + Transferrin when done together help in the differential diagnosis of anemia.
- The cellular uptake of iron is mediated by the cell surface transferrin receptor.
- The number of transferrin receptors depends upon the needs of the cell for the iron.
- In the case of apoferritin deficiency, an excess of the iron is deposited as small granules as Iron-oxide, called hemosiderin.
Transferrin is a protein that may decrease during any inflammatory process and is referred to as a negative acute phase reactant. Chronic inflammation, infections, and malignancies may cause changes in transferrin levels.
Functions of transferrin include:
- Free Fe3+ (ferric iron) is insoluble at a neutral pH, when iron binds to transferrin it becomes soluble.
- Deliver and transfer iron to all the various biological tissues between sites of absorption, utilization, and storage 9.
- Prevent the formation of reactive oxygen species.
- Chelate free toxic iron and act as a protective scavenger.
- Deliver white blood cell macrophages to all tissues 10.
- Transferrin is a part of the innate immune system, the binding of transferrin to iron impedes bacterial survival.
- Transferrin acts as a marker for inflammation; the level of transferrin decreases during inflammation.
The increased Transferrin is seen in:
- Iron deficiency anemia.
- Pregnancy.
- Estrogen therapy.
The decreased Transferrin is seen in:
- Chronic infections.
- Microcytic anemia due to chronic diseases.
- Protein deficiency in malabsorption and burns.
- Liver disease, acute.
- The renal disease like nephrosis.
- Hemochromatosis.
- Genetic deficiency of transferrin.
Transferrin test
The transferrin test, total iron-binding capacity (TIBC), unsaturated iron binding capacity (UIBC) and transferrin saturation, along with other iron tests, help to evaluate the amount of iron circulating in your blood, the total capacity of your blood to transport iron, and the amount of stored iron in your body. These tests are often ordered at the same time and the results interpreted together to help diagnose and/or monitor iron deficiency, iron deficiency anemia or too much iron in the body (iron overload). Testing may also help differentiate various causes of anemia.
- Serum iron test—measures the total amount of iron in the liquid portion of the blood, nearly all of which is bound to transferrin.
- Transferrin test—directly measures the level of transferrin in the blood. The level depends upon liver function and a person’s nutritional status. Transferrin is a protein that may decrease during any inflammatory process and is referred to as a negative acute phase reactant.
- TIBC (total iron-binding capacity)—measures the total amount of iron that can be bound by proteins in the blood. Since transferrin is the primary iron-binding protein, the total iron-binding capacity (TIBC) test is a good indirect measurement of transferrin availability—the amount of transferrin that is available to bind to iron. Note: Though total iron-binding capacity (TIBC) is a reflection of the amount of transferrin available, total iron-binding capacity (TIBC) and transferrin are not synonymous.
- UIBC (unsaturated iron-binding capacity)—Unsaturated iron binding capacity (UIBC) test determines the reserve capacity of transferrin, i.e., the portion of transferrin that has not yet been saturated with iron.
- Transferrin saturation— dividing the iron concentration by the total iron-binding capacity (TIBC) produces an estimate of how many of transferrin iron-binding sites are occupied; this is called the transferrin saturation. Under normal conditions, transferrin is typically one-third saturated with iron. This means that about two-thirds of its capacity is held in reserve. Less commonly, the iron concentration may be divided by the transferrin concentration, not the total iron-binding capacity (TIBC). This similar estimate is usually called the transferrin index.
- Ferritin—measures the level of ferritin, a protein made by almost all cells in response to increased iron. The ferritin level reflects the total body iron. It will be low when there is iron deficiency and high when there is an excess of iron in the body.
When the level of iron is insufficient to meet the body’s needs, the level of iron in the blood drops and iron stores are depleted. This may occur because:
- There is an increased need for iron, for example during pregnancy or childhood, or due to a condition that causes chronic blood loss (e.g., peptic ulcer, colon cancer)
- Not enough iron is consumed (either foods or supplements)
- The body is unable to absorb iron from the foods eaten in conditions such as celiac disease
Insufficient levels of circulating and stored iron may eventually lead to iron deficiency anemia (decreased hemoglobin and hematocrit, smaller and paler red cells). In the early stage of iron deficiency, no physical effects are usually seen and the amount of iron stored may be significantly depleted before any signs or symptoms of iron deficiency develop. If a person is otherwise healthy and anemia develops over a long period of time, symptoms may not appear before the hemoglobin in the blood drops below the lower limit of normal.
However, as the iron deficiency progresses, symptoms eventually begin to appear. The most common symptoms of anemia include fatigue, weakness, dizziness, headaches and pale skin.
Conversely, too much iron can be toxic to the body. Iron storage and ferritin levels increase when more iron is absorbed than the body needs. Absorbing too much iron over time can lead to the progressive buildup of iron compounds in organs and may eventually cause their dysfunction and failure. An example of this is hemochromatosis, a rare genetic disease in which the body absorbs and builds up too much iron, even on a normal diet. Additionally, iron overload can occur when a person undergoes repeated blood transfusions.
Normal transferrin saturation
- Normal value for transferrin saturation is 20 to 50%. This may vary with age and sex.
- Male transferrin saturation = 20 to 50 %.
- Female transferrin saturation = 15 to 50 %.
- The reference range for transferrin is 204-360 mg/dL.
Transferrin saturation low
Transferrin saturation is decreased below 15% in a patient with iron deficiency anemia. Iron deficiency is recognized as the most prevalent nutritional deficit in the world. The amount of transferrin in the blood indicates the amount of iron in the body. High transferrin signifies low iron, which means there is less iron bound to transferrin, allowing for a high circulation of non-bound iron transferrin in the body, revealing a possible iron deficiency anemia 1. The liver increases production of transferrin as a form of homeostasis to enable transferrin to bind to iron and transport it to the cells. Upregulation of transferrin receptors occurs in iron deficiency anemia 11. Concerning the percentage of transferrin-iron complex, low iron-bound transferrin indicates low iron levels in the body, which affects hemoglobin and erythropoiesis. The significance of transferrin is that it can detect iron deficiency and can be used to monitor erythropoiesis.
In anemia of chronic disease, there is a decreased transferrin level.
Causes of low transferrin:
- Liver damage leading to reduced production of transferrin
- Kidney insult or injury leads to loss of transferrin in urine.
- Infection
- Malignancy
- Atransferrinemia: A genetic mutation, resulting in the absence of transferrin, which leads to hemosiderosis in the heart and liver, which can lead to heart and liver failure. This condition is treated by plasma infusion.
Low transferrin in plasma indicates iron overload, which means the binding site of transferrin is highly saturated with iron. Iron overload suggests hemochromatosis, which will lead to deposition of iron on tissues.
Other associations with transferrin and its receptors include:
- Diminishing tumor cells when the receptor is used to attract antibodies
Transferrin saturation high
Transferrin saturation is increased in patients:
- Hemolytic anemia.
- Sideroblastic anemia.
- Megaloblastic anemia.
- Patient with iron overload or iron poisoning.
- Hemochromatosis.
High transferrin saturation increased the risk of cardiovascular mortality if patients have high transferrin saturation (>55%) and LDL levels 12.
References- Ogun AS, Adeyinka A. Biochemistry, Transferrin. [Updated 2019 Jan 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532928
- Luck AN, Mason AB. Transferrin-mediated cellular iron delivery. Curr Top Membr. 2012;69:3-35
- Steere AN, Byrne SL, Chasteen ND, Mason AB. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochim. Biophys. Acta. 2012 Mar;1820(3):326-33
- Waldvogel-Abramowski S, Waeber G, Gassner C, Buser A, Frey BM, Favrat B, Tissot JD. Physiology of iron metabolism. Transfus Med Hemother. 2014 Jun;41(3):213-21
- Wally J, Buchanan SK. A structural comparison of human serum transferrin and human lactoferrin. Biometals. 2007 Jun;20(3-4):249-62
- Giansanti F, Panella G, Leboffe L, Antonini G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals (Basel). 2016 Sep 27;9, 4
- Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986 Mar;68(3):375-81
- Anderson ER, Shah YM. Iron homeostasis in the liver. Compr Physiol. 2013 Jan;3(1):315-30
- Tandara L, Salamunic I. Iron metabolism: current facts and future directions. Biochem Med (Zagreb). 2012;22(3):311-28
- Gordon S, Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017 Jun 29;15(1):53.
- Bermejo F, García-López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J. Gastroenterol. 2009 Oct 07;15(37):4638-43
- Wells BJ, Mainous AG, King DE, Gill JM, Carek PJ, Geesey ME. The combined effect of transferrin saturation and low density lipoprotein on mortality. Fam Med. 2004 May;36(5):324-9