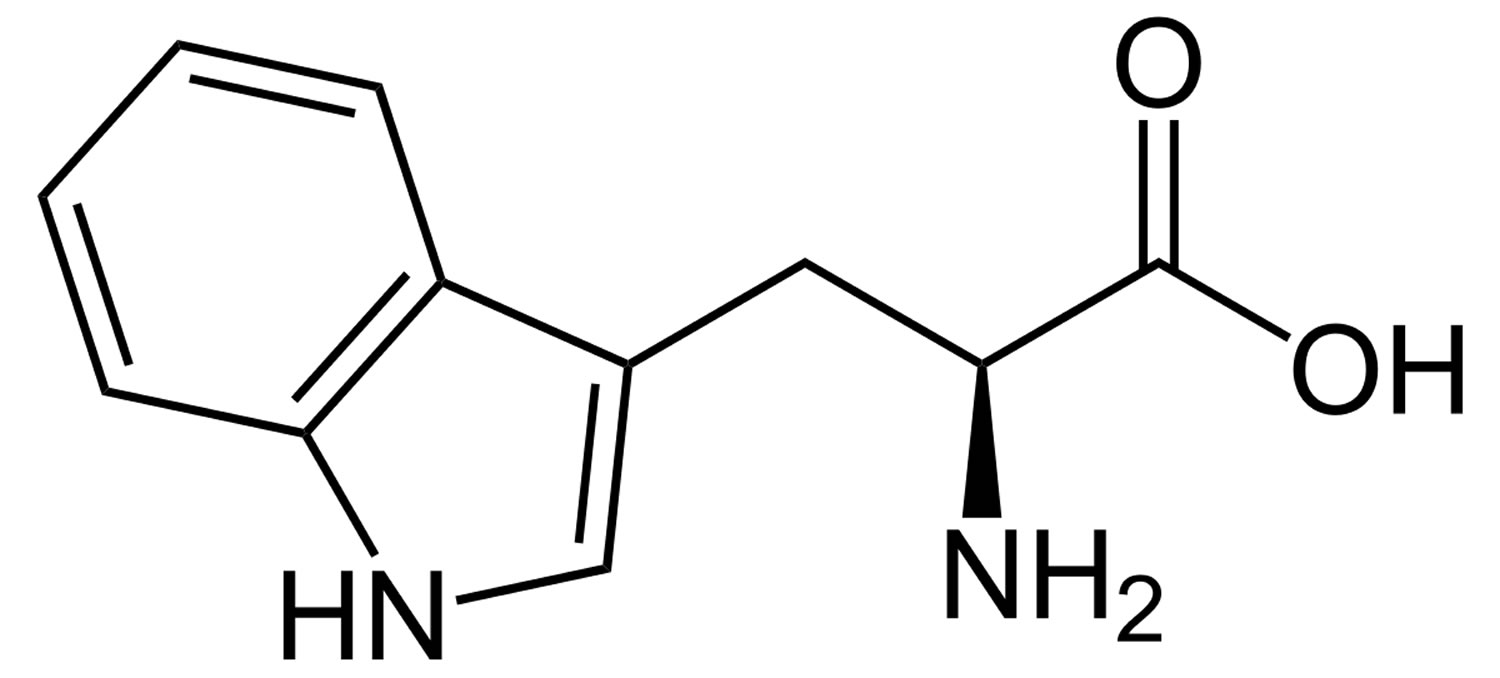
What is tryptophan
Tryptophan or L-tryptophan is an essential amino acid and a precursor of serotonin (5-hydroxytryptamine) and consequently melatonin; substances that are considered important in the modulation of several essential behaviors and psychological functions including sleep, mood, cognition, and circadian rhythms 1. Tryptophan is an essential amino acid, which means your body cannot produce it, so you must get tryptophan from your diet (see Tables 1 and 2 below). Tryptophan is an essential amino acid found in many protein-based foods and dietary proteins 2 including meats, dairy, fruits, and seeds. High-glycemic index (high GI) and high-glycemic load (high GL) meals also increase the availability of tryptophan 3. Levels of plasma tryptophan are determined by a balance between dietary intake 4 and its removal from the plasma as a part of its essential role in protein biosynthesis 5. Aside from its role in protein formation, tryptophan is a precursor for a number of metabolites, most notably kynurenine and the neurotransmitters, serotonin and melatonin.
Melatonin is a hormone that is produced by the pineal gland in human, which regulates sleep and wakefulness. Serotonin is a brain neurotransmitter, platelet clotting factor, and neurohormone found in organs throughout your body. Metabolism of tryptophan into serotonin requires nutrients such as vitamin B6, niacin, and glutathione. Niacin (also known as vitamin B3) is an important metabolite of tryptophan. It is synthesized via kynurenine and quinolinic acids, which are products of tryptophan degradation 6.
For all amino acids, including L-tryptophan, only the L isomer is used in protein synthesis 7 and can pass across the blood-brain barrier 8. In humans, tryptophan has relatively low tissue storage 9 and the overall tryptophan concentration in the body is the lowest among all amino acids 10, although only small amounts are necessary for general healthy nutrition 11. While typical intake for many individuals is approximately 900 to 1000 mg daily, the recommended daily allowance for adults is estimated to be between 250 mg/day 11 and 425 mg/day 12, which translates to a dietary intake of 3.5 to 6.0 mg/kg of body weight per day. Some common sources of tryptophan are oats, bananas, dried prunes, milk, tuna fish, cheese, bread, chicken, turkey, peanuts, and chocolate (see Table 1).
Table 1. L-tryptophan found in common foods
| L-tryptophan*(mg) | Sum of Competing Amino Acids (CAAs)** (mg) | Ratio | |
| Turkey, Skinless, Boneless, Light Meat (per pound, raw) | 410 | 9525 | 0.043 |
| Chicken, Skinless, Boneless, Light Meat (per pound, raw) | 238 | 5122 | 0.046 |
| Turkey, Skinless, Boneless, Dark Meat (per pound, raw) | 303 | 7036 | 0.043 |
| Chicken, Skinless, Boneless, Dark Meat (per pound, raw) | 256 | 5492 | 0.047 |
| Whole Milk (per quart) | 732 | 8989 | 0.081 |
| 2% Milk (per quart) | 551 | 12516 | 0.044 |
| Wheat Bread (per slice) | 19 | 317 | 0.06 |
| White Bread (per slice) | 22 | 439 | 0.05 |
| Semisweet Chocolate (per ounce) | 18 | 294 | 0.061 |
| Sweet Chocolate (per ounce) | 16 | 270 | 0.059 |
| Canned Tuna (per ounce) | 472 | 10591 | 0.045 |
| Cheddar Cheese (per ounce) | 91 | 2298 | 0.04 |
| Peanuts (per ounce) | 65 | 1574 | 0.041 |
| Oats for Oatmeal (per cup) | 147 | 2617 | 0.056 |
| Dried Prune (one) | 2 | 27 | 0.074 |
| Banana (one medium) | 11 | 237 | 0.046 |
| Apple (one medium) | 2 | 70 | 0.029 |
Footnotes:
The L-tryptophan/competing amino acid (CAA) ratio represents the relative availability of plasma L-tryptophan for crossing the blood-brain barrier and is thought to be the best indicator of brain serotonin synthesis.
*e.g. The recommended daily allowance for a 79 kg (175 lb) adult is 278 to 476 mg.
**CAAs = Isoleucine, Leucine, Phenylalanine, Tyrosine, and Valine, the five large neutral amino acids typically included in the tryptophan/competing amino acid (CAA) ratio.
In humans acute tryptophan depletion inhibits serotonin synthesis 14 and also lowers cerebrospinal fluid concentrations of tryptophan 15 and 5-hydroxyindoleacetic acid (5-HIAA), the major serotonin metabolite 16.
Tryptophan is the sole precursor of peripherally and centrally produced serotonin (5-hydroxytryptamine) 13. However, the second most prevalent metabolic pathway of tryptophan after protein synthesis is the synthesis of kynurenine, which accounts for approximately 90% of tryptophan metabolism 17. Kynurenine is the precursor of kynurenic acid, an antagonist at glutamate ionotropic receptors. There is strong evidence implicating the kynurenines in behavioral and cognitive symptoms of neurological disease 18, however the relationship between the central effects of tryptophan depletion/supplementation and the kynurenine pathway is as yet not clear 19.
It is estimated that 95% of mammalian serotonin is found within the gastrointestinal tract 20 and only 3% of dietary tryptophan is used for serotonin synthesis throughout the body 21. Nevertheless, serotonin synthesis is one of the most important tryptophan pathways and a topic of intense research. It is estimated that only 1% of dietary tryptophan is used for serotonin synthesis in the brain 22, but despite the relatively low concentration of brain serotonin compared to that in the rest of the body, it has a broad impact as a neurotransmitter and neuromodulator and has been implicated in numerous psychiatric conditions and psychological processes.
After protein synthesis, the second most prevalent metabolic pathway of tryptophan is for the synthesis of kynurenine, which accounts for approximately 90% of tryptophan catabolism 23. Kynurenine is a key component in the synthesis of a number of metabolites, but most importantly, it is the precursor of kynurenic and quinolinic acids. Each of these metabolites has the potential to affect other neurotransmitters; specifically kynurenic acid is a glutamate receptor antagonist, while quinolinic acid is a glutamate receptor agonist 24. Among other pathways, kynurenine is known to be involved in acting as an ultra violet (UV) filter which protects the retina of the eye from UV damage 25. The effectiveness of this protection deteriorates with age, contributing to the normal changes in coloration and fluorescence of the lens that interfere with visual function and may, in some individuals, play a role in cataract formation.
In addition to tryptophan’s three major activities of protein, kynurenine, and serotonin synthesis, tryptamine is another biologically active compound that is derived from tryptophan. The immediate decarboxylation of tryptophan results in the synthesis of trace amounts of tryptamine (i.e. ng/g), which is an important neuromodulator of serotonin.45 Numerous animal studies have indicated that tryptamine acts as a control for the balance between excitatory and inhibitory functions of serotonin, and in other instances, tryptamine acts as a neurotransmitter with specific receptors that are independent of serotonin function 26.
Melatonin is a hormone produced in the tryptophan/serotonin pathway, which regulates diurnal rhythms and influences the reproductive and immune systems,47 as well as digestive processes and gastrointestinal motility.48 Melatonin synthesis is regulated by the blue light spectrum (i.e. 446 to 477 nm) in both artificial and sun light 27. During periods of darkness, it is actively secreted from the pineal gland to induce neural and endocrine effects that regulate circadian rhythms of behavior, physiology, and sleep patterns 28.
Tryptophan also plays a role as a substrate for synthesis of the coenzymes nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP) 29. NAD and NADP are coenzymes essential for electron transfer reactions (i.e. redox reactions) in all living cells. These enzymes can be synthesized de novo from ingested tryptophan, or from ingestion of niacin (i.e. vitamin B3).
There are a number of conditions or diseases that are characterized tryptophan deficiencies. For instance, fructose malabsorption causes improper absorption of tryptophan in the intestine, which reduces levels of tryptophan in the blood and leads to depression. High corn or other tryptophan-deficient diets can cause pellagra, which is a niacin-tryptophan deficiency disease with symptoms of dermatitis, diarrhea, and dementia 6. Hartnup’s disease is a disorder in which tryptophan and other amino acids are not absorbed properly. Symptoms of Hartnup’s disease include skin rashes, difficulty coordinating movements (cerebellar ataxia), and psychiatric symptoms such as depression or psychosis 6. Tryptophan supplements may be useful for treating Hartnup’s disease. Assessment of tryptophan deficiency is done through studying excretion of tryptophan metabolites in the urine or blood. Blood may be the most sensitive test because the amino acid tryptophan is transported in a unique way. Increased urination of tryptophan breakdown products (such as kynurenine) correlates with increased tryptophan degradation, which occurs with oral contraception, depression, mental retardation, hypertension, and anxiety states 6.
The requirement for tryptophan and protein decreases with age 6. The minimum daily requirement for adults is 3 mg/kg body weight per day or about 200 mg a day. There is 400 mg of tryptophan in a cup of wheat germ 6. A cup of low fat cottage cheese contains 300 mg of tryptophan and chicken and turkey contain up to 600 mg of tryptophan per pound (see Table 1 below).
Tryptophan plays a role in “feast-induced” drowsiness. Ingestion of a meal rich in carbohydrates triggers the release of insulin. Insulin, in turn, stimulates the uptake of large neutral branched-chain amino acids (BCAAs) into muscle, increasing the ratio of tryptophan to branched-chain amino acid in the bloodstream. The increased tryptophan ratio reduces competition at the large neutral amino acid transporter (which transports both branched-chain amino acids and tryptophan), resulting in greater uptake of tryptophan across the blood-brain barrier into the cerebrospinal fluid (CSF). Once in the cerebrospinal fluid (CSF), tryptophan is converted into serotonin and the resulting serotonin is further metabolized into melatonin by the pineal gland, which promotes sleep 6.
Under certain situations, tryptophan can be a neurotoxin and a metabotoxin 6. A neurotoxin is a compound that causes damage to the brain and nerve tissues. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of tryptophan can be found in glutaric aciduria type I (glutaric acidemia type I or GA1). GA1 is an inherited disorder in which the body is unable to completely break down the amino acids lysine, hydroxylysine, and tryptophan 6. Babies with glutaric acidemia type I are often born with unusually large heads (macrocephaly). Affected individuals may also have difficulty moving and may experience spasms, jerking, rigidity or decreased muscle tone, and muscle weakness. High levels of tryptophan have also been implicated in eosinophilia-myalgia syndrome, an incurable and sometimes fatal flu-like neurological condition linked to the ingestion of large amounts of L-tryptophan. The risk of developing eosinophilia-myalgia syndrome increases with larger doses of tryptophan and increasing age. Some research suggests that certain genetic polymorphisms may be related to the development of eosinophilia-myalgia syndrome. The presence of eosinophilia is a core feature of eosinophilia-myalgia syndrome, along with unusually severe myalgia (muscle pain). It is thought that both tryptophan and certain unidentified tryptophan contaminants may contribute to eosinophilia-myalgia syndrome 30. It has also been suggested that excessive tryptophan or elevation of its metabolites could play a role in amplifying some of the pathological features of eosinophilia-myalgia syndrome 31. This pathological damage is further augmented by metabolites of the kynurenine pathway (a tryptophan degradation pathway).
Tryptophan and depression
Tryptophan depletion studies in never-depressed individuals are variable, with no or little overall effect on lowering of mood 32. Interestingly, reports of moderate mood lowering are seen more often in studies with healthy women than in studies with healthy men 33. However in never-depressed healthy volunteers who are at high risk for depression through a familial risk factor, acute tryptophan depletion produces clear abnormalities in mood control 34. Finally, in depressed patients in remission, temporarily lowering tryptophan levels can result in an acute depressive relapse 35 with transient exacerbation of symptoms associated with patients taking serotonergic anti-depressants 36. These studies reveal that subjects with a pre-existing vulnerability in the serotonergic system may be most susceptible to a tryptophan challenge. Moreover, low serotonin can indeed contribute to a lowered mood state, however this cannot occur in isolation—it must be in concert with some other unknown system (perhaps neurotransmitter or genetic) that interacts with the reduced serotonin to decrease mood 37.
A comprehensive meta-analysis of over fifty tryptophan depletion studies in man from 1966 through to 2008 was published by Mendelsohn and colleagues in 2009 38. The effects of acute tryptophan depletion on psychomotor processing, declarative memory, working memory, executive functions, and attention were evaluated with the most robust finding that lowering tryptophan impaired the consolidation of episodic memory for verbal information 39. Semantic memory appeared to be unaffected by acute tryptophan depletion as were verbal, spatial, and affective working memory, executive function, and attention 38.
Many of the studies covered in the Mendelsohn et al. review 38 focus on healthy volunteers, or those with susceptibility to depression. Latter work published after Mendelsohn’s review has demonstrated some interesting findings concerning emotional processing. In a small study of depressed patients, a bimodal symptom response to acute tryptophan depletion was shown to be preceded by a bimodal emotional processing bias in the same direction; that is, patients whose depressive symptoms improved 24 hour after depletion showed more positive emotional processing bias 5 hour after depletion, while the reverse was true for patients whose mood symptoms worsened 40. Asymptomatic individuals at high familial risk for depression also showed abnormalities in emotional processing while undergoing acute tryptophan depletion 34. Interestingly in normal subjects, acute tryptophan depletion elicited significantly lower intensity and arousal ratings for angry faces in an unconscious perception task 41. In another study involving tryptophan depletion in postmenopausal women, there was an increase in brain activation in the orbital frontal cortex and bilateral amygdala, as measured by functional magnetic resonance imaging, during an emotional processing task as compared to untreated controls 42.
Manipulating central tryptophan levels using acute tryptophan depletion is also used as a tool to investigate the role of serotonin in neurological disorders. In Parkinson’s disease patients, a demonstrable reduction in global cognitive function and verbal recognition during acute tryptophan depletion is observed compared with placebo and control patients suggesting an interaction between serotonergic and cholinergic impairment 43. No deficits in memory were observed in tryptophan-depleted young persons with attention deficit hyperactivity disorder 44, in reward response tests with alcoholic males 45, or in cognitive testing of Alzheimer’s patients that could not be attributed to old age 46. Interestingly it was observed that the detrimental effects of acute tryptophan depletion on working memory were more common in an elderly, compared to young, group of healthy volunteers 32.
Manipulating tryptophan levels using acute tryptophan depletion has also been used to investigate the role of serotonin in other disorders. Kennedy and colleagues have used acute tryptophan depletion to demonstrate that impaired hippocampal-mediated cognitive performance in irritable bowel syndrome 47 is modulated by peripheral tryptophan levels 48. Moreover, in breast cancer survivors, acute tryptophan depletion was used to model serotonin loss which is a common side effect of oestrogen withdrawal in this disease population. This study demonstrated specific impairment in episodic memory and motor speed suggesting a critical role for serotonin in cognitive impairment in these patients 49.
L-tryptophan and depression
In 13 adults with recovered depression, tryptophan depletion by a tryptophan-free drink was associated with shorter rapid eye movement (REM) latency (i.e. time span between bedtime and start of REM sleep) as compared to the control drink 50. The evidence relating tryptophan to mental well-being is presently mixed. A meta-analysis 51 and a 2002 Cochrane review 52 concluded that supplementation with tryptophan or serotonin significantly improved depressive symptoms as compared to the placebo treatment in patients with depression. This was further confirmed in a mega-analysis of five trials concluding that acute tryptophan depletion evokes depressive symptoms in 50% of the remitted depressed patients 53. However, a separate review of 13 trials in healthy and depressed adults, indicated mixed results and no clear conclusion 1.
Studies of tryptophan in combination with electroconvulsive therapy (ECT) have also produced inconsistent findings. One study demonstrated that patients with depression who received combined doses of tryptophan (3 g/day) and nicotinamide for 4 weeks reported significantly lower ratings of depression compared to those who received electroconvulsive therapy (ECT) twice weekly 54. Conversely, in another study, patients with a severe primary depressive illness treated with ECT improved significantly compared to those receiving tryptophan (6 to 8 g) plus pyridoxine daily 55. Similarly, patients receiving ECT twice daily improved significantly compared to patients receiving daily doses of combined tryptophan (6 g) and pyridoxine 56. In yet another study, there were no significant differences in depressed patients treated with tryptophan alone (6 g/day) compared to ECT alone 57.
In contrast to the mixed results of the effects of tryptophan with tricyclic antidepressants or ECT, tryptophan has been shown to be more effective in combination with monoamine oxidase inhibitors (MAOI). For example, depressed patients who were unresponsive to a 60 mg/day dose of the MAOI phenelzine received supplements of 12, 15, or 18 g of tryptophan or placebo. A significantly higher percentage of the patients on the combined therapy (i.e. MAOI plus tryptophan) improved compared to those receiving the MAOI alone or with placebo 58. Patients who received a combined therapy of tryptophan (6 g/day) and a different MAOI, nialamide, also improved significantly compared to those who received nialamide alone.185 In another study, compared to patients who received doses of the MAOI tranylcypromine plus placebo for one week, those who received a 214 mg/kg/day supplement of tryptophan reported a significant decrease in depression ratings during that week, as well as during the 2 weeks after tryptophan supplements were discontinued 59.
Although the results of the therapeutic combination of tryptophan with MAOIs have demonstrated the most successful results for treatment of depression, most clinical studies have produced mixed results as to the efficacy of tryptophan for treatment of depression 60. These mixed results are due, in part, to flawed study designs and trials using insufficient lengths of time to allow determination of efficacy. Methodological differences such as inconsistent diagnostics within and across studies 21 have also produced mixed results. Taken together, there is evidence that tryptophan is effective for ameliorating mild to moderate depressive symptoms, but not severe depressive symptoms 61.
L-tryptophan and sleep
The relationship between tryptophan and sleep quality has, to date, only been investigated in intervention trials. A review including 21 trials in humans with and without sleep disorders concluded that increasing brain tryptophan by dietary treatments appeared to improve subjective measures of sleepiness and reduce sleep latency (i.e. time span between bedtime and sleep onset) 1. This improved quality of sleep is associated with an improvement in hedonic and cognitive measures 62, improved morning alertness and brain measures of attention 63. In addition, a 7-day trial with tryptophan-enriched cereals at breakfast and dinner showed beneficial effects on sleep/wake cycle in 35 elderly with sleep problems 64.
Acute tryptophan depletion studies in humans demonstrate inhibition of rapid eye movement (REM) latency and prolonged REM sleep 65, with further work from animal studies demonstrating the importance of serotonin in this association [88]. Serotonin is also a precursor to melatonin in the pineal gland.
Patients with depression suffer from poor sleep quality 66, with associated antidepressant treatment often exacerbating sleep inefficiency with insomnia and decreased total sleep time being common side-effects 67. The effect of tryptophan depletion on sleep in depression has largely focused on remitted patients, who were still taking antidepressants, tryptophan depletion resulted in reduced sleep and REM latencies but increased density 68, demonstrating that depleting tryptophan did not alter the antidepressant side-effects. Interestingly, in a population of patients with obsessive compulsive disorder (OCD), tryptophan depletion induced a worsening of sleep continuity, but no changes of REM or slow wave sleep 69.
Tryptophan supplement benefits
L-tryptophan supplement is possibly effective for:
- Premenstrual dysphoric disorder. Taking 6 grams of L-tryptophan per day seems to decrease mood swings, tension, and irritability in women with premenstrual dysphoric disorder.
- To help people quit smoking. Taking L-tryptophan seems to help people quit smoking when used with conventional treatment.
L-tryptophan supplement is possibly ineffective for:
- Teeth grinding (bruxism). Taking L-tryptophan by mouth doesn’t help treat teeth grinding.
- Facial pain. Taking L-tryptophan by mouth doesn’t help reduce facial pain.
Insufficient evidence to rate effectiveness for:
- Improving athletic ability. Some research shows that taking L-tryptophan for 3 days before exercising can improve power during exercise. This improvement in power helps increase the distance an athlete can go in the same amount of time. But other early research shows that taking L-tryptophan during exercise doesn’t improve endurance during a cycling exercise. Reasons for the conflicting results are not clear. It is possible that L-tryptophan improves some measures of athletic ability but not others. On the other hand, L-tryptophan might need to be taken for a few days before exercise in order to see any benefit.
- Attention deficit-hyperactivity disorder (ADHD). There is some evidence that L-tryptophan levels are lower in children with ADHD. But taking L-tryptophan supplements does not appear to improve ADHD symptoms.
- Problems with mental function in the elderly. Taking a mixture of L-tryptophan and other ingredients can slightly improve mental function in older people. But the improvement is very small, so it might not be meaningful. Also, it’s not known if any potential benefit is due to L-tryptophan or another ingredient.
- Depression. Early research suggests that L-tryptophan might improve the effectiveness of common medications for depression.
- Healing ulcers caused by the bacteria Helicobacter pylori (H pylori). Research shows that taking L-tryptophan in combination with the ulcer medication omeprazole improves ulcer healing rates compared to taking omeprazole alone.
- Treating sleep disorders. Taking L-tryptophan might decrease the amount of time it takes to fall asleep and improve mood in healthy people with sleep problems.
- Seasonal affective disorder (SAD). Early research suggests L-tryptophan might be helpful in SAD.
- Treating sleep apnea. There is some evidence that taking L-tryptophan might decrease episodes in some people who periodically stop breathing during sleep (sleep apnea).
- Anxiety.
- Other conditions.
More evidence is needed to rate L-tryptophan for these uses.
L-tryptophan dosage
The following doses have been studied in scientific research:
Adults By mouth:
- Premenstrual dysphoric disorder: Doses of 6 grams of L-tryptophan have been taken daily from ovulation to the third day of the period.
- To help people quit smoking: Doses of 50 mg/kg of L-tryptophan have been taken daily.
- For depression: Adults 8 to 12 grams per day, given in 3 to 4 equally divided doses
- Evening oral doses of tryptophan as low as 250 mg have been shown to improve sleep quality, although the typical dosage range for sleep disorders is 1-3 g daily. Safe and effective dosages for other disorders range from 0.5-4 g daily, while potentially higher doses (50 mg/kg/day) have been used short term as a smoking cessation intervention.
The intraperitoneal median lethal dose of tryptophan was determined to be 1600 mg/kg body weight in rats but was drastically decreased to a median lethal dose of 11–25 mg/kg body weight when corticosteroid production was inhibited 70. These observations suggested that the rate of tryptophan catabolism is a factor in the excess dietary intake that results in adverse effects. Thus, the upper limit of tryptophan catabolism may be a possible marker of the intake above which increasing intake increases the risk of adverse effects. In the rat experiment 70, the urinary excretory ratio of anthranilic acid:kynurenic acid was specified as a possible marker of the intake above which increasing intake increases the risk of adverse effects.
In rat 71 and pig 70 studies with tryptophan, symptoms of excess tryptophan intake in pigs 70 include reduced food intake and growth rate, which were described as manifestations of amino acid imbalance. In growing animals, it appears that tryptophan intakes of >10 times the requirement are necessary before there are detrimental effects on growth performance 72. Symptoms of tryptophan excess may include development of fatty liver and fibrotic changes in muscles, lung, and pancreas and the serotonin syndrome (in rats: tremors, hyper-reactivity, hyper-tonicity). Similar to leucine 73, the effects of tryptophan excess are attenuated by higher protein intake. Although the dietary tryptophan requirement appears to vary widely across species when compared on the basis of g/kg body weight or g/kg of diet, the ratio of tryptophan:lysine requirement is fairly similar in many mono-gastric species. The small differences among species are more likely due to differences in techniques and outcome measurements than a true difference in the ratio of tryptophan:lysine in body proteins 72.
Tryptophan supplement side effects
L-tryptophan can cause some side effects such as heartburn, stomach pain, belching and gas, nausea, vomiting, diarrhea, and loss of appetite. L-tryptophan can also cause headache, lightheadedness, drowsiness, dry mouth, visual blurring, muscle weakness, dry mouth, blurred vision, sedation, euphoria, and nystagmus (involuntary eye movements) and sexual problems 74.
It was also noted, that because tryptophan raises brain serotonin production, its ingestion with drugs that stimulate serotonin function (e.g., certain antidepressants, monoamine oxidase inhibitors) can produce a medically important condition termed the “serotonin syndrome,” for which medical attention is required. Serotonin syndrome is a potentially life-threatening drug reaction. It causes the body to have too much serotonin 75.
You can develop serotonin syndrome if you take tryptophan supplement together with antidepressants called selective serotonin reuptake inhibitors (SSRIs), selective serotonin/norepinephrine reuptake inhibitors (SSNRIs) and monoamine oxidase inhibitors.
Common selective serotonin reuptake inhibitors (SSRIs) include citalopram (Celexa), sertraline (Zoloft), fluoxetine (Prozac), paroxetine (Paxil), and escitalopram (Lexapro). Selective serotonin/norepinephrine reuptake inhibitors (SSNRIs) include duloxetine (Cymbalta) and venlafaxine (Effexor). Common monoamine oxidase inhibitors include isocarboxazid (Marplan), phenelzine (Nardil), selegiline (Emsam) and tranylcypromine (Parnate).
If you take these medicines, be sure to read the warning on the packaging. It tells you about the potential risk of serotonin syndrome. However, do not stop taking your medicine. Talk to your doctor about your concerns first.
People with serotonin syndrome will likely stay in the hospital for at least 24 hours for close observation.
Serotonin syndrome treatment may include:
- Benzodiazepine medicines, such as diazepam (Valium) or lorazepam (Ativan) to decrease agitation, seizure-like movements, and muscle stiffness
- Cyproheptadine (Periactin), a drug that blocks serotonin production
- Intravenous (through the vein) fluids
- Withdrawal of medicines that caused the syndrome
In life-threatening cases, medicines that keep the muscles still (paralyze them), and a temporary breathing tube and breathing machine will be needed to prevent further muscle damage.
Serotonin syndrome prognosis
People may get slowly worse and can become severely ill if not quickly treated. Untreated, serotonin syndrome can be deadly. With treatment, symptoms usually go away in less than 24 hours.
Uncontrolled muscle spasms can cause severe muscle breakdown. The products produced when the muscles break down are released into the blood and eventually go through the kidneys. This can cause severe kidney damage if serotonin syndrome isn’t recognized and treated properly.
Serotonin syndrome symptoms occur within minutes to hours, and may include 75:
- Agitation or restlessness
- Diarrhea
- Fast heartbeat and high blood pressure
- Hallucinations
- Increased body temperature
- Loss of coordination
- Nausea and vomiting
- Overactive reflexes
- Rapid changes in blood pressure
Special precautions and warnings
Pregnancy and breast-feeding: L-tryptophan is likely UNSAFE in pregnancy because it may harm the unborn child. Not enough is known about the safety of L-tryptophan during breast-feeding. Avoid using L-tryptophan during pregnancy and breast-feeding.
A white blood cell disorder called eosinophilia: L-tryptophan might make this condition worse. L-tryptophan has been associated with the development of eosinophilia-myalgia syndrome (EMS).
Liver or kidney disease: L-tryptophan might make these conditions worse since it has been associated with the development of eosinophilia-myalgia syndrome (EMS).
Eosinophilia myalgia syndrome
In 1989, reports began appearing that over-the-counter tryptophan use was associated with a serious condition, eosinophilia myalgia syndrome 74. High levels of tryptophan have also been implicated in eosinophilia-myalgia syndrome, an incurable and sometimes fatal flu-like neurological condition linked to the ingestion of large amounts of L-tryptophan. Eosinophilia myalgia syndrome is a neurological condition with symptoms that include fatigue; intense muscle pain; nerve pain; skin changes; baldness; rash; and pain and swelling affecting the joints, connective tissue, lungs, heart, and liver. Symptoms tend to improve over time, but some people may still experience symptoms up to 2 years after they develop eosinophilia myalgia syndrome. Some people report that their symptoms have never gone away completely. The risk of developing eosinophilia-myalgia syndrome increases with larger doses of tryptophan and increasing age. Some research suggests that certain genetic polymorphisms may be related to the development of eosinophilia-myalgia syndrome. The presence of eosinophilia is a core feature of eosinophilia-myalgia syndrome, along with unusually severe myalgia (muscle pain). It is thought that both tryptophan and certain unidentified tryptophan contaminants may contribute to eosinophilia-myalgia syndrome 30. It has also been suggested that excessive tryptophan or elevation of its metabolites could play a role in amplifying some of the pathological features of eosinophilia-myalgia syndrome 31. This pathological damage is further augmented by metabolites of the kynurenine pathway (a tryptophan degradation pathway).
Over a period of several months, the Centers for Disease Control and Prevention (CDC) identified 1531 eosinophilia-myalgia syndrome cases and 37 deaths in the U.S. population; given the seriousness of the condition, the FDA banned over-the-counter tryptophan 76. Once the ban was in place, eosinophilia-myalgia syndrome occurrence dropped to zero. Eosinophilia-myalgia syndrome symptoms were a high peripheral eosinophil count and disabling myalgias (note that the side effect profile associated with tryptophan use prior to 1989 does not overlap with these symptoms.) It subsequently became evident that almost all cases were traceable to the tryptophan produced by a single company (about 95% of all eosinophilia-myalgia syndrome cases were traced to L-tryptophan produced by a single manufacturer in Japan). Cases of eosinophilia-myalgia syndrome were not associated with the use of tryptophan from other manufacturers 77. A contaminant in the tryptophan, rather than tryptophan itself, was therefore considered the likely cause. Multiple contaminants were found in the suspected lot of tryptophan 78, but none faithfully reproduced eosinophilia-myalgia syndrome in animals. Currently, under the Dietary Supplement Health and Education Act (DSHEA) of 1994, L-tryptophan is available and marketed as a dietary supplement.
Interactions with medications
Major interactions
Do NOT take this combination.
Sedative medications (CNS depressants)
L-tryptophan might cause sleepiness and drowsiness. Medications that cause sleepiness are called sedatives. Taking L-tryptophan along with sedative medications might cause too much sleepiness.
Some sedative medications include clonazepam (Klonopin), lorazepam (Ativan), phenobarbital (Donnatal), zolpidem (Ambien), and others.
Moderate interactions
Be cautious with this combination.
Dextromethorphan (Robitussin DM, and others)
- L-tryptophan can affect a brain chemical called serotonin. Dextromethorphan (Robitussin DM, others) can also affect serotonin. Taking L-tryptophan along with dextromethorphan (Robitussin DM, others) might cause there to be too much serotonin in the brain and serious side effects including heart problems, shivering and anxiety could occur. Do not take L-tryptophan if you are taking dextromethorphan (Robitussin DM, others).
Medications for depression (Antidepressant drugs)
- L-tryptophan increases a brain chemical called serotonin. Some medications for depression also increase the brain chemical serotonin. Taking L-tryptophan along with these medications for depression might increase serotonin too much and cause serious side effects including heart problems, shivering, and anxiety. Do not take L-tryptophan if you are taking medications for depression.
Some of these medications for depression include fluoxetine (Prozac), paroxetine (Paxil), sertraline (Zoloft), amitriptyline (Elavil), clomipramine (Anafranil), imipramine (Tofranil), and others.
Medications for depression (MAOIs)
- L-tryptophan increases a chemical in the brain. This chemical is called serotonin. Some medications used for depression also increase serotonin. Taking L-tryptophan with these medications used for depression might cause there to be too much serotonin. This could cause serious side effects including heart problems, shivering, and anxiety.
Some of these medications used for depression include phenelzine (Nardil), tranylcypromine (Parnate), and others.
Meperidine (Demerol)
L-tryptophan increases a chemical in the brain called serotonin. Meperidine (Demerol) can also increase serotonin in the brain. Taking L-tryptophan along with meperidine (Demerol) might cause too much serotonin in the brain and serious side effects including heart problems, shivering, and anxiety.
Pentazocine (Talwin)
L-tryptophan increases a brain chemical called serotonin. Pentazocine (Talwin) also increases serotonin. Taking L-tryptophan along with pentazocine (Talwin) might cause serious side effects including heart problems, shivering, and anxiety. Do not take L-tryptophan if you are taking pentazocine (Talwin).
Phenothiazines
Taking L-tryptophan with phenothiazines can cause serious side effects including movement disorders.
Some phenothiazines include chlorpromazine (Thorazine), fluphenazine (Prolixin), trifluoperazine (Stelazine), thioridazine (Mellaril), and others.
Sedative medications (Benzodiazepines)
Sedative medications can affect the nervous system. L-tryptophan can also affect the nervous system. Taking L-tryptophan along with sedative medications can cause serious side effects. Do not take L-tryptophan if you are taking sedative medications.
Some of these sedative medications include clonazepam (Klonopin), diazepam (Valium), lorazepam (Ativan), and others.
Tramadol (Ultram)
Tramadol (Ultram) can affect a chemical in the brain called serotonin. L-tryptophan can also affect serotonin. Taking L-tryptophan along with tramadol (Ultram) might cause too much serotonin in the brain and side effects including confusion, shivering, and stiff muscles could result.
Interactions with herbs and supplements
Herbs and supplements that act like sedatives
- L-tryptophan can cause drowsiness and relaxation. Using it along with other herbs and supplements that also have sedative effects might cause too much drowsiness. Some of these herbs and supplements include 5-HTP, calamus, California poppy, catnip, hops, Jamaican dogwood, kava, St. John’s wort, skullcap, valerian, yerba mansa, and others.
Herbs and supplements that increase serotonin levels
- L-tryptophan seems to raise the level of serotonin, a hormone that transmits signals between nerve cells and affects mood. There is a concern that using it with other herbs and supplements that increase serotonin, might increase the effects and side effects of those herbs and supplements. Some of those include 5-HTP, Hawaiian baby woodrose, and S-adenosylmethionine (SAMe).
St. John’s wort
- Combining L-tryptophan with St. John’s wort might increase the risk of serotonin syndrome, a possibly fatal condition that occurs when there is too much serotonin in the body. There is a report of serotonin syndrome in a patient who took L-tryptophan and high doses of St. John’s wort.
Tryptophan rich foods
Table 2. Foods high in tryptophan (ordered from highest to low)
| Description | Tryptophan (g) Value Per 100 grams |
| Egg, white, dried, stabilized, glucose reduced | 1.43 |
| Egg, white, dried, powder, stabilized, glucose reduced | 1.27 |
| Egg, white, dried, flakes, stabilized, glucose reduced | 1.18 |
| Soy protein isolate | 1.12 |
| Soy protein isolate, potassium type | 1.12 |
| Seeds, sesame flour, low-fat | 1.1 |
| Egg, white, dried | 1 |
| Seaweed, spirulina, dried | 0.93 |
| Seeds, sesame flour, partially defatted | 0.88 |
| Soy protein concentrate, produced by alcohol extraction | 0.83 |
| Soy protein concentrate, produced by acid wash | 0.83 |
| Whale, beluga, meat, dried (Alaska Native) | 0.8 |
| Egg, whole, dried | 0.78 |
| Egg, whole, dried, stabilized, glucose reduced | 0.77 |
| Winged beans, mature seeds, raw | 0.76 |
| Seeds, cottonseed flour, low fat (glandless) | 0.75 |
| Tofu, dried-frozen (koyadofu) | 0.75 |
| Tofu, dried-frozen (koyadofu), prepared with calcium sulfate | 0.75 |
| Seeds, cottonseed meal, partially defatted (glandless) | 0.74 |
| Seeds, sunflower seed flour, partially defatted | 0.73 |
| Beverages, Protein powder soy based | 0.72 |
| Fish, cod, Atlantic, dried and salted | 0.7 |
| Soy flour, defatted | 0.68 |
| Seeds, sesame flour, high-fat | 0.67 |
| Soy meal, defatted, raw | 0.65 |
| Pork, fresh, variety meats and by-products, pancreas, cooked, braised | 0.62 |
| Seeds, cottonseed flour, partially defatted (glandless) | 0.62 |
| Mollusks, whelk, unspecified, cooked, moist heat | 0.62 |
| Soybeans, mature seeds, raw | 0.59 |
| Seeds, pumpkin and squash seed kernels, dried | 0.58 |
| Soybeans, mature seeds, dry roasted | 0.57 |
| Meat extender | 0.57 |
| Seeds, pumpkin and squash seed kernels, roasted, without salt | 0.57 |
| Seeds, pumpkin and squash seed kernels, roasted, with salt added | 0.57 |
| Cheese, parmesan, shredded | 0.56 |
| Cheese, mozzarella, low moisture, part-skim | 0.55 |
| Cheese, cheddar (Includes foods for USDA’s Food Distribution Program) | 0.55 |
| Game meat, elk, cooked, roasted | 0.55 |
| Leavening agents, yeast, baker’s, active dry | 0.54 |
| Parsley, freeze-dried | 0.52 |
| Cheese, mozzarella, whole milk | 0.52 |
| Soybeans, mature seeds, roasted, salted | 0.51 |
| Soybeans, mature seeds, roasted, no salt added | 0.51 |
| Milk, dry, nonfat, regular, without added vitamin A and vitamin D | 0.51 |
| Milk, dry, nonfat, regular, with added vitamin A and vitamin D | 0.51 |
| Peanut flour, defatted | 0.51 |
| Soy flour, full-fat, roasted | 0.51 |
| Soy flour, full-fat, raw | 0.5 |
| Milk, dry, nonfat, calcium reduced | 0.5 |
| Milk, dry, nonfat, instant, with added vitamin A and vitamin D | 0.49 |
| Milk, dry, nonfat, instant, without added vitamin A and vitamin D | 0.49 |
| Seeds, cottonseed kernels, roasted (glandless) | 0.49 |
| Milk, buttermilk, dried | 0.48 |
| Cheese, parmesan, hard | 0.48 |
| Spices, parsley, dried | 0.47 |
| Pork, cured, bacon, cooked, microwaved | 0.46 |
| Game meat, caribou, cooked, roasted | 0.46 |
| Seeds, chia seeds, dried | 0.44 |
| Game meat, rabbit, wild, cooked, stewed | 0.44 |
| Cheese, romano | 0.43 |
| Cheese, gruyere | 0.42 |
| Lamb, shoulder, arm, separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.41 |
| T.G.I. FRIDAY’S, classic sirloin steak (10 oz) | 0.41 |
| Game meat, elk, raw | 0.41 |
| CRACKER BARREL, grilled sirloin steak | 0.41 |
| Pork, ground, 96% lean / 4% fat, cooked, pan-broiled | 0.41 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.41 |
| Pork, fresh, variety meats and by-products, pancreas, raw | 0.41 |
| Pork, cured, bacon, pre-sliced, cooked, pan-fried | 0.41 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.41 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 0.41 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.41 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, cooked, braised | 0.4 |
| Goose, domesticated, meat only, cooked, roasted | 0.4 |
| Seeds, safflower seed meal, partially defatted | 0.4 |
| Game meat, goat, cooked, roasted | 0.4 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 0.4 |
| Cheese, swiss | 0.4 |
| Game meat, rabbit, domesticated, composite of cuts, cooked, stewed | 0.4 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.4 |
| Duck, young duckling, domesticated, White Pekin, leg, meat only, bone in, cooked without skin, braised | 0.4 |
| Beef, plate steak, boneless, inside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.4 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, choice, cooked, braised | 0.4 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, select, cooked, braised | 0.4 |
| Lamb, Australian, imported, fresh, shoulder, arm, separable lean only, trimmed to 1/8″ fat, cooked, braised | 0.4 |
| Cereals ready-to-eat, wheat germ, toasted, plain | 0.4 |
| Lamb, New Zealand, imported, frozen, shoulder, whole (arm and blade), separable lean only, cooked, braised | 0.4 |
| Seeds, sesame butter, paste | 0.4 |
| Pork, ground, 96% lean / 4% fat, cooked, crumbles | 0.39 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, grilled | 0.39 |
| Lamb, cubed for stew or kabob (leg and shoulder), separable lean only, trimmed to 1/4″ fat, cooked, braised | 0.39 |
| Seeds, sesame butter, tahini, from unroasted kernels (non-chemically removed seed coat) | 0.39 |
| Restaurant, family style, sirloin steak | 0.39 |
| Spices, fenugreek seed | 0.39 |
| Egg, yolk, dried | 0.39 |
| Chicken, broilers or fryers, breast, meat only, cooked, fried | 0.39 |
| Seeds, watermelon seed kernels, dried | 0.39 |
| Seeds, sesame butter, tahini, from raw and stone ground kernels | 0.39 |
| Beef, top loin filet, boneless, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.39 |
| Seeds, sesame seeds, whole, dried | 0.39 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, select, cooked, braised | 0.39 |
| Beef, loin, top loin steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.39 |
| Chicken, stewing, light meat, meat only, cooked, stewed | 0.39 |
| Pork, fresh, loin, tenderloin, separable lean only, cooked, broiled | 0.39 |
| Beef, loin, top loin steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.39 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, cooked, grilled | 0.39 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, all grades, cooked, braised | 0.39 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 0.39 |
| Beef, round, eye of round steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.39 |
| Game meat, rabbit, domesticated, composite of cuts, cooked, roasted | 0.38 |
| Cheese, parmesan, grated | 0.38 |
| Chicken, broilers or fryers, light meat, meat only, cooked, fried | 0.38 |
| Lamb, shoulder, whole (arm and blade), separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.38 |
| Beef, loin, top sirloin petite roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 0.38 |
| Beef, round, top round, separable lean and fat, trimmed to 1/8″ fat, choice, cooked, braised | 0.38 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, braised | 0.38 |
| Beef, rib, back ribs, bone-in, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.38 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 0.38 |
| Duck, young duckling, domesticated, White Pekin, breast, meat only, boneless, cooked without skin, broiled | 0.38 |
| Game meat, boar, wild, cooked, roasted | 0.38 |
| DENNY’S, top sirloin steak | 0.38 |
| Lamb, shoulder, blade, separable lean only, trimmed to 1/4″ fat, choice, cooked, braised | 0.38 |
| Beef, chuck, mock tender steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, braised | 0.38 |
| Beef, rib eye steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 0.38 |
| Beef, rib eye steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.38 |
| Beef, shank crosscuts, separable lean only, trimmed to 1/4″ fat, choice, cooked, simmered | 0.38 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 0.38 |
| Pork, fresh, loin, tenderloin, separable lean and fat, cooked, broiled | 0.38 |
| Snacks, soy chips or crisps, salted | 0.38 |
| Fish, roe, mixed species, cooked, dry heat | 0.38 |
| Beef, rib eye roast, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, roasted | 0.38 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 0.38 |
| Pork, fresh, leg (ham), whole, separable lean only, cooked, roasted | 0.37 |
| Pork, fresh, loin, center rib (chops), boneless, separable lean only, cooked, broiled | 0.37 |
| Pork, fresh, composite of trimmed retail cuts (loin and shoulder blade), separable lean only, cooked | 0.37 |
| Beef, plate steak, boneless, outside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 0.37 |
| Chicken, broilers or fryers, giblets, cooked, fried | 0.37 |
| Beef, chuck for stew, separable lean and fat, choice, cooked, braised | 0.37 |
| Turkey, retail parts, wing, meat only, cooked, roasted | 0.37 |
| Chicken, broiler or fryers, breast, skinless, boneless, meat only, with added solution, cooked, grilled | 0.37 |
| Ham and cheese spread | 0.37 |
| Seeds, sesame butter, tahini, from roasted and toasted kernels (most common type) | 0.37 |
| Veal, leg (top round), separable lean only, cooked, braised | 0.37 |
| Beef, chuck for stew, separable lean and fat, all grades, cooked, braised | 0.37 |
| Milk, dry, whole, with added vitamin D | 0.37 |
| Milk, dry, whole, without added vitamin D | 0.37 |
| Seeds, sesame seeds, whole, roasted and toasted | 0.37 |
| Seeds, sesame seed kernels, toasted, without salt added (decorticated) | 0.37 |
| Seeds, sesame meal, partially defatted | 0.37 |
| Seeds, sesame seed kernels, toasted, with salt added (decorticated) | 0.37 |
- Effects of tryptophan loading on human cognition, mood, and sleep. Silber BY, Schmitt JA. Neurosci Biobehav Rev. 2010 Mar; 34(3):387-407. https://www.ncbi.nlm.nih.gov/pubmed/19715722/
- Friedman M., Levin C.E. Nutritional and medicinal aspects of d-amino acids. Amino Acids. 2012;42:1553–1582. doi: 10.1007/s00726-011-0915-1
- Herrera C.P., Smith K., Atkinson F., Ruell P., Chow C.M., O’Connor H., Brand-Miller J. High-glycaemic index and -glycaemic load meals increase the availability of tryptophan in healthy volunteers. Br. J. Nutr. 2011;105:1601–1606. doi: 10.1017/S0007114510005192
- Young V.R., Hussein M.A., Murray E., Scrimshaw N.S. Plasma tryptophan response curve and its relation to tryptophan requirements in young adult men. J. Nutr. 1971;101:45–59.
- Richard D.M., Dawes M.A., Mathias C.W., Acheson A., Hill-Kapturczak N., Dougherty D.M. l-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. IJTR. 2009;2:45–60.
- L-Tryptophan. http://www.hmdb.ca/metabolites/HMDB0000929
- Stryer L. Biochemistry. 4th ed. New York: WH Freeman and Company; 1995.
- Pardridge WM, Oldendorf WH. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta. 1975;401:128–36.
- Reilly JG, McTavish SFB, Young AH. Rapid depletion of plasma tryptophan: A review of studies and experimental methodology. J Psychopharmacol. 1997;11:381–92.
- Young LS, Stoll S. Proteins and amino acids. In: Matarese LE, Gottschlich MM, editors. Contemporary Nutrition Support Practice. 2nd ed. Vol. 1. New York: Saunders; 2003. pp. 94–104.
- Rambali B, Van Andel E, Schenk G, et al. The contribution of cocoa additive to cigarette smoking addiction. RIVM report 650270002/2002. The National Institute for Public Health and the Environment; Netherlands: 2002.
- Young VR. Adult amino acid requirements: The case for a major revision in current recommendations. J Nutr. 1994;124:1517S–23S.
- Richard D.M., Dawes M.A., Mathias C.W., Acheson A., Hill-Kapturczak N., Dougherty D.M. l-tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryptophan Res. IJTR. 2009;2:45–60 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908021/
- Nishizawa S., Benkelfat C., Young S.N., Leyton M., Mzengeza S., de Montigny C., Blier P., Diksic M. Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl. Acad. Sci. USA. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308
- Williams W.A., Shoaf S.E., Hommer D., Rawlings R., Linnoila M. Effects of acute tryptophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J. Neurochem. 1999;72:1641–1647. doi: 10.1046/j.1471-4159.1999.721641.x
- Moreno F.A., Parkinson D., Palmer C., Castro W.L., Misiaszek J., el Khoury A., Mathe A.A., Wright R., Delgado P.L. CSF neurochemicals during tryptophan depletion in individuals with remitted depression and healthy controls. Eur. Neuropsychopharmacol. 2010;20:18–24. doi: 10.1016/j.euroneuro.2009.10.003
- Stone T.W., Darlington L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870
- Stone T.W., Darlington L.G. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013;169:1211–1227. doi: 10.1111/bph.12230 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3831703/
- Crockett M.J., Clark L., Roiser J.P., Robinson O.J., Cools R., Chase H.W., den Ouden H., Apergis-Schoute A., Campbell-Meikeljohn D., Seymour B., et al. Converging evidence for central 5-HT effects in acute tryptophan depletion. Mol. Psychiatr. 2012;17:121–123. doi: 10.1038/mp.2011.106
- Sanger GJ. 5-Hydroxytryptamine and the gastrointestinal tract: Where next. Trends in Pharmacological Sciences. 2008;29:465–71
- van Praag HM, Lemus C. Monoamine precursors in the treatment of psychiatric disorders. In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. New York: Raven Press; 1986. pp. 89–139.
- Sandyk R. L-tryptophan in neuropsychiatric disorders: A review. Int J Neurosci. 1992;67(14):127–44.
- Dougherty DM, Marsh-Richard DM, Mathias CW, et al. Comparison of 50- and 100-g L-tryptophan depletion and loading formulations for altering 5-HT synthesis: Pharmacokinetics, side effects, and mood states. Psychopharmacol (Berl) 2008;198:431–45.
- Moroni F. Tryptophan metabolism and brain function: Focus on kynurenine and other indole metabolites (Review) Eur J Pharmacol. 1999;375:87–100.
- Vazquez S, Parker NR, Sheil M, et al. Protein-bound kynurenine decreases with the progression of age-related nuclear cataract. Invest Ophthalmol Vis Sci. 2004;45:879–83
- Jones RSG. Tryptamine: A neuromodulator or neurotransmitter in mammalian brain. Prog Neurobiol. 1982;19:117–39
- Brainard GC, Sliney D, Hanifin JP, et al. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms. 2008;23:379–86
- Kayumov L, Casper RF, Hawa RJ, et al. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J Clin Endocrinol Metab. 2005;90:2755–61.
- Sainio EL, Pulkki K, Young SN. L-tryptophan: Biochemical, nutritional and pharmacological agents. Amino Acids. 1996;10:21–47.
- Tryptophan toxicity: a pharmacoepidemiologic review of eosinophilia-myalgia syndrome. DICP. 1991 Nov;25(11):1259-62. https://www.ncbi.nlm.nih.gov/pubmed/1763543
- Tryptophan toxicity–time and dose response in rats. Adv Exp Med Biol. 1999;467:507-16. https://www.ncbi.nlm.nih.gov/pubmed/10721094
- Mace J.L., Porter R.J., Dalrymple-Alford J.C., Wesnes K.A., Anderson T.J. The effects of acute tryptophan depletion on neuropsychological function, mood and movement in the healthy elderly. J. Psychopharmacol. 2011;25:1337–1343. doi: 10.1177/0269881110389094
- Ellenbogen M.A., Young S.N., Dean P., Palmour R.M., Benkelfat C. Mood response to acute tryptophan depletion in healthy volunteers: Sex differences and temporal stability. Neuropsychopharmacology. 1996;15:465–474. doi: 10.1016/S0893-133X(96)00056-5
- Feder A., Skipper J., Blair J.R., Buchholz K., Mathew S.J., Schwarz M., Doucette J.T., Alonso A., Collins K.A., Neumeister A., et al. Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol. Psychiatry. 2011;69:804–807. doi: 10.1016/j.biopsych.2010.12.033 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3941748/
- Booij L., van der Does A.J.W., Haffmans P.M.J., Riedel W.J., Fekkes D., Blom M.J.B. The effects of high-dose and low-dose tryptophan depletion on mood and cognitive functions of remitted depressed patients. J. Psychopharmacol. 2005;19:267–275. doi: 10.1177/0269881105051538
- Booij L., van der Does A.J., Haffmans P.M., Riedel W.J. Acute tryptophan depletion in depressed patients treated with a selective serotonin-noradrenalin reuptake inhibitor: Augmentation of antidepressant response? J. Affect. Disord. 2005;86:305–311. doi: 10.1016/j.jad.2005.01.012
- Jenkins TA, Nguyen JCD, Polglaze KE, Bertrand PP. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients. 2016;8(1):56. doi:10.3390/nu8010056. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4728667/
- Mendelsohn D., Riedel W.J., Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: A systematic review. Neurosci. Biobehav. R. 2009;33:926–952. doi: 10.1016/j.neubiorev.2009.03.006 https://www.ncbi.nlm.nih.gov/pubmed/19428501
- Schmitt J.A., Jorissen B.L., Sobczak S., van Boxtel M.P., Hogervorst E., Deutz N.E., Riedel W.J. Tryptophan depletion impairs memory consolidation but improves focussed attention in healthy young volunteers. J. Psychopharmacol. 2000;14:21–29. doi: 10.1177/026988110001400102
- Booij L., van der Does A.J.W. Emotional processing as a predictor of symptom change: An acute tryptophan depletion study in depressed patients. Eur. Neuropsychopharm. 2011;21:379–383. doi: 10.1016/j.euroneuro.2010.09.007
- Beacher F.D.C.C., Gray M.A., Minati L., Whale R., Harrison N.A., Critchley H.D. Acute tryptophan depletion attenuates conscious appraisal of social emotional signals in healthy female volunteers. Psychopharmacology. 2011;213:603–613. doi: 10.1007/s00213-010-1897-5
- Epperson C.N., Amin Z., Ruparel K., Gur R., Loughead J. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrino. 2012;37:372–382. doi: 10.1016/j.psyneuen.2011.07.007
- Mace J.L., Porter R.J., Dalrymple-Alford J.C., Wesnes K.A., Anderson T.J. Effects of acute tryptophan depletion on neuropsychological and motor function in parkinson’s disease. J. Psychopharmacol. 2010;24:1465–1472. doi: 10.1177/0269881109105721
- Zepf F.D., Landgraf M., Biskup C.S., Dahmen B., Poustka F., Wockel L., Stadler C. No effect of acute tryptophan depletion on verbal declarative memory in young persons with adhd. Acta Psychiatr. Scand. 2013;128:133–141. doi: 10.1111/acps.12089
- Crean J., Richards J.B., de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav. Brain Res. 2002;136:349–357. doi: 10.1016/S0166-4328(02)00132-8
- Porter R.J., Lunn B.S., O’Brien J.T. Effects of acute tryptophan depletion on cognitive function in Alzheimer’s disease and in the healthy elderly. Psychol. Med. 2003;33:41–49. doi: 10.1017/S0033291702006906
- Kennedy P.J., Clarke G., O’Neill A., Groeger J.A., Quigley E.M., Shanahan F., Cryan J.F., Dinan T.G. Cognitive performance in irritable bowel syndrome: Evidence of a stress-related impairment in visuospatial memory. Psychol. Med. 2014;44:1553–1566. doi: 10.1017/S0033291713002171
- Kennedy P.J., Allen A.P., O’Neill A., Quigley E.M., Cryan J.F., Dinan T.G., Clarke G. Acute tryptophan depletion reduces kynurenine levels: Implications for treatment of impaired visuospatial memory performance in irritable bowel syndrome. Psychopharmacology. 2015;232:1357–1371. doi: 10.1007/s00213-014-3767-z
- Von Ah D., Skaar T., Unverzagt F., Yu M.G., Wu J.W., Schneider B., Storniolo A.M., Moser L., Ryker K., Milata J., et al. Evaluating the role of serotonin on neuropsychological function after breast cancer using acute tryptophan depletion. Biol. Res. Nurs. 2012;14:5–15. doi: 10.1177/1099800410393273
- Affective state and EEG sleep profile in response to rapid tryptophan depletion in recently recovered nonmedicated depressed individuals. Haynes PL, McQuaid JR, Kelsoe J, Rapaport M, Gillin JC. J Affect Disord. 2004 Dec; 83(2-3):253-62.
- Are tryptophan and 5-hydroxytryptophan effective treatments for depression? A meta-analysis. Shaw K, Turner J, Del Mar C. Aust N Z J Psychiatry. 2002 Aug; 36(4):488-91. https://www.ncbi.nlm.nih.gov/pubmed/12169147/
- Shaw KA, Turner J, Del Mar C. Tryptophan and 5‐Hydroxytryptophan for depression. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No.: CD003198. DOI: 10.1002/14651858.CD003198. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003198/full
- Predictors of mood response to acute tryptophan depletion. A reanalysis. Booij L, Van der Does W, Benkelfat C, Bremner JD, Cowen PJ, Fava M, Gillin C, Leyton M, Moore P, Smith KA, Van der Kloot WA. Neuropsychopharmacology. 2002 Nov; 27(5):852-61. https://www.nature.com/articles/1395951
- MacSweeney DA. Treatment of unipolar depression (letter) Lancet. 1975;2(7933):510–11.
- Carroll BJ, Mowbray RM, Davies B. Sequential comparison of tryptophan with ECT in severe depression. Lancet. 1970;1(7654):967–9.
- Herrington RN, Bruce A, Johnstone EC. Comparative trial of tryptophan and ECT in severe depressive illness. Lancet. 1974;2(7883):731–4.
- D’Elia G, Lehmann J, Raotma H. Evaluation of the combination of tryptophan and ECT in the treatment of depression: I. Clinical analysis. Acta Psychiatr Scand. 1977;56(4):303–18.
- Glassman AH, Platman SR. Potentiation of a monoamine oxidase inhibitor by tryptophan. J Psychiatr Res. 1969;7(2):83–8.
- Coppen A, Shaw DM, Farrell JP. Potentiation of the antidepressive effect of a monoamine-oxidase inhibitor by tryptophan. Lancet. 1963;1(7272):79–81.
- Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. International Journal of Tryptophan Research : IJTR. 2009;2:45-60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2908021/
- Young SN. Behavioral effects of dietary neurotransmitter precursors: Basic and clinical aspects. Neurosci Biobehav Rev. 1996;20:313–23
- Mohajeri M.H., Wittwer J., Vargas K., Hogan E., Holmes A., Rogers P.J., Goralczyk R., Gibson E.L. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br. J. Nutr. 2015;113:350–365. doi: 10.1017/S0007114514003754
- Markus C.R., Jonkman L.M., Lammers J.H., Deutz N.E., Messer M.H., Rigtering N. Evening intake of alpha-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am. J. Clin. Nutr. 2005;81:1026–1033
- Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Bravo R, Matito S, Cubero J, Paredes SD, Franco L, Rivero M, Rodríguez AB, Barriga C. Age (Dordr). 2013 Aug; 35(4):1277-85. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705114/
- Carhart-Harris R.L., Nutt D.J., Munafo M.R., Christmas D.M., Wilson S.J. Equivalent effects of acute tryptophan depletion on rem sleep in ecstasy users and controls. Psychopharmacology. 2009;206:187–196. doi: 10.1007/s00213-009-1595-3
- Nakamaru-Ogiso E., Miyamoto H., Hamada K., Tsukada K., Takai K. Novel biochemical manipulation of brain serotonin reveals a role of serotonin in the circadian rhythm of sleep-wake cycles. Eur. J. Neurosci. 2012;35:1762–1770. doi: 10.1111/j.1460-9568.2012.08077.x
- Beasley C.M., Sayler M.E., Weiss A.M., Potvin J.H. Fluoxetine-activating and sedating effects at multiple fixed doses. J. Clin. Psychopharm. 1992;12:328–333. doi: 10.1097/00002826-199202001-00629
- Landolt H.P., Kelsoe J.R., Rapaport M.H., Gillin J.C. Rapid tryptophan depletion reverses phenelzine-induced suppression of rem sleep. J. Sleep Res. 2003;12:13–18. doi: 10.1046/j.1365-2869.2003.00336.x
- Voderholzer U., Riemann D., Huwig-Poppe C., Kuelz A.K., Kordon A., Bruestle K., Berger M., Hohagen F. Sleep in obsessive compulsive disorder-polysomnographic studies under baseline conditions and after experimentally induced serotonin deficiency. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257:173–182. doi: 10.1007/s00406-006-0708-9
- Moehn S Pencharz PB Ball RO Lessons learned regarding symptoms of tryptophan deficiency and excess from animal requirement studies. J Nutr. 2012;142:2231S–35S.
- Shibata K Fukuwatari T The metabolites in the tryptophan degradation pathway might be useful to determine the tolerable upper intake level of tryptophan intake in rats. J Nutr. 2012;142:2227S–30S.
- Takeshi Kimura, Dennis M. Bier, Christine L. Taylor; Summary of Workshop Discussions on Establishing Upper Limits for Amino Acids with Specific Attention to Available Data for the Essential Amino Acids Leucine and Tryptophan, The Journal of Nutrition, Volume 142, Issue 12, 1 December 2012, Pages 2245S–2248S, https://doi.org/10.3945/jn.112.160846
- Kato H Imamura W Kanamoto R Adverse effects of excessive leucine intake depend on dietary protein intake: a transcriptomic analysis to identify useful biomarkers.. J of Nutritional Science and Vitaminology. 2013
- Summary of workshop discussions on establishing upper limits for amino acids with specific attention to available data for the essential amino acids leucine and tryptophan. J Nutr. 2012 Dec;142(12):2245S-2248S. doi: 10.3945/jn.112.160846. Epub 2012 Oct 17. https://academic.oup.com/jn/article/142/12/2245S/4630869
- Serotonin syndrome. https://medlineplus.gov/ency/article/007272.htm
- CDC. Analysis of L-tryptophan for the etiology of eosinophilia-myalgia syndrome. MMWR 1990;39:589-91.
- Fernstrom JD Effects and side effects associated with the non-nutritional use of tryptophan by humans. J Nutr. 2012;142:2236S–44S.
- Simat T van Wickern B Eulitz K Steinhart EH Contaminants in biotechnologically manufactured L-tryptophan. J Chromatogr B Biomed Appl. 1996;685:41–51.
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/search/list