Uremic pruritus
Uremic pruritus also known as “chronic kidney disease-associated pruritus” (CKD-associated pruritus) or uraemic pruritus, remains a frequent and compromising symptom in patients with chronic kidney disease (CKD) and end-stage kidney disease (ESKD) 1. Uremia refers to excessive urea in the blood and occurs when both kidneys stop working (renal failure). Pruritus refers to an unpleasant itch sensation on the skin that provokes the desire to scratch 2. Pruritus results from a systemic condition in which the cross talk between keratinocytes, immune cells, and neurons is perturbed 3. Uremic pruritus or uremic itch, is a common problem for patients with chronic renal failure (CKD) or end stage renal disease (ESKD). Uremic pruritus affects about one-third of patients on dialysis and is more common with hemodialysis than continuous ambulatory peritoneal dialysis (CAPD). On the basis of a large-scale investigation published a few years ago, more than 40% of hemodialysis patients suffer from chronic pruritus 4. Uremic pruritus does not arise when uremia is due to acute renal failure.
Interestingly, severe pruritus is very rare in pediatric patients on dialysis. This could be shown by a systematic review of all German pediatric dialysis centers involving 199 children, where only 9.1% of the children on dialysis complained of pruritus 5. Moreover, the intensity was not very severe in the affected patients 6.
Sex is increasingly recognized as a key variable in the study of diseases, including chronic renal failure (CKD) 7 and several skin disorders 8. Pisoni et al 9 observed that males showed higher odds of having moderate to extreme pruritus. Male sex was also identified as a predictor of pruritus in a study of 6137 patients on hemodialysis across 7 countries 10 and as an independent risk factor for severe uremic pruritus in prospective studies of 1773 11 and 341 patients on hemodialysis 12. Other studies defend that female sex predisposes to uremic pruritus 13, 14, but most were limited by small sample size. By contrast, 7 studies concluded that sex does not influence its development 15. Six of these studies were limited by few patients on dialysis, unadjusted analyses, and/or imbalanced male-to-female ratio 16.
Data on the prevalence of uremic pruritus in peritoneal dialysis patient are rather scarce. The few reports available, however, permit the conclusion that patients undergoing peritoneal dialysis are affected as much by pruritus as hemodialysis patients are 17.
Uremic pruritus is characterized by daily bouts of itching that tend to worsen at night and may prevent sleep. The itch may be generalized or localized to one area, most often the back, abdomen, head and /or arms. In hemodialysis patients, the pruritus is lowest the day after dialysis and peaks 2 days afterwards. The skin may appear normal or dry (xerosis), with few to numerous scratch marks and/or picked sores.
In patients with chronic kidney disease (CKD) and, particularly, patients with end-stage kidney disease (ESKD), uremic pruritis is one of the most common and bothersome manifestations of uremia that signals the ensuing need for renal replacement therapy 18. Of more than 6000 hemodialysis patients across 17 countries from 2012 to 2015 found that 18% of patients reported being very much or extremely bothered by itching 19. Among those patients very much or extremely bothered by itching, 58% reported being depressed about the itching, 45% reported the itching made it hard to work, and 35% reported the itching reduced their desire to be with other people 19. Yet, despite the significant impact on work and social life, 18% of patients very much or extremely bothered by itching reported taking no medication to relieve their symptoms 19.
During the early days of dialysis treatment, uremic pruritus was a very common problem, it appears that its incidence has declined over the past 20 years. In the early 1970s, Young et al. 20 reported that about 85% of patients were affected by uremic pruritus. This number decreased to 50% to 60% in the late 1980s 21. An investigation in Germany showed that only 22% of all dialysis patients complained about moderate to severe pruritus at the time they were studied 22.
The pathophysiology of uremic pruritus involves a complex interplay of uremic toxins, systemic inflammation, mast cell activation, and imbalance of opioid receptors. High-quality evidence in the management of uremic pruritus remains lacking. Most recommendations are based on expert opinion or studies involving small numbers of patients. Most therapeutic trials have shown only limited success. Several times in the past a new treatment option has been reported to be effective, but very soon thereafter conflicting results appear 22. The main obstacle in the effort to create effective treatment modalities is the incomplete knowledge of the underlying pathophysiological mechanisms behind uremic pruritus 5. Furthermore, given the great clinical heterogeneity of patients with kidney failure, systematically performed studies are hard to undertake and therefore sparse.
Classic treatment strategies for uremic pruritus include optimization of dialysis parameters, amelioration of chronic kidney disease -related mineral and bone disease, topical emollients and analgesics, antihistamines, the anticonvulsant medications gabapentin and pregabalin, and ultraviolet light B (UV-B) phototherapy. Strong data to support many of these classical treatments for uremic pruritus are limited. Newly evolving treatment approaches for uremic pruritus include opioid receptor modulators, neurokinin-1 inhibitors, and cannabinoids. Further studies regarding their efficacy, pharmacodynamics, and safety in the chronic kidney disease and end-stage kidney disease population are needed before these agents are accepted into widespread use. Additional nonpharmacological strategies aimed at treating uremic pruritus include psychotherapy, acupuncture, omega-3 fatty acids, and exercise. Finally, sex differences may exist regarding uremic pruritus, but studies directly addressing sex-specific mechanisms of uremic pruritus remain absent.
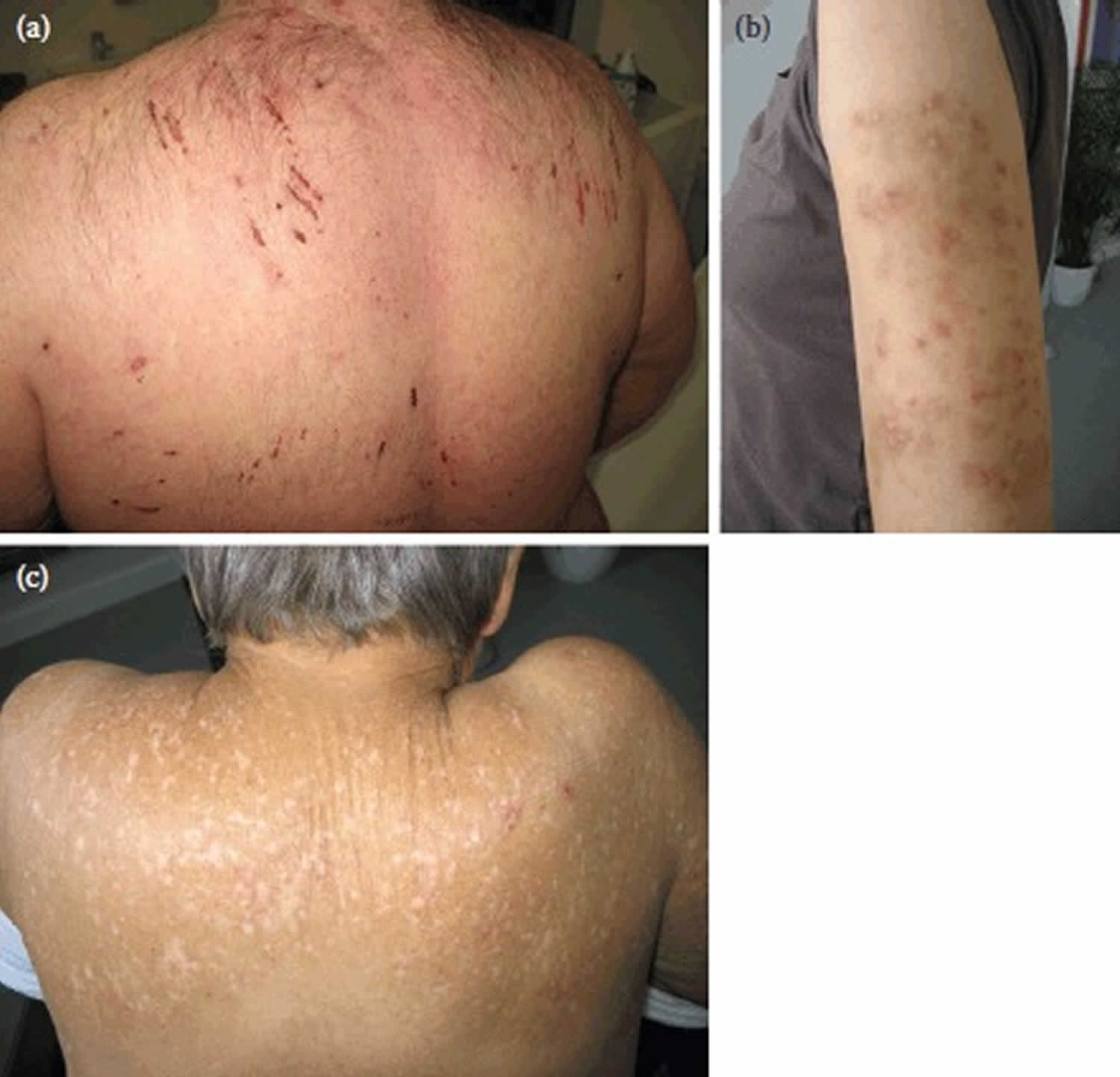
Figure 1. Uremic pruritus
Footnote: Typical skin changes due to uremic pruritus. (a) Scratch marks on the back with excoriations. (b) Typical hyperkeratotic partly excoriated nodules (prurigo nodularis) on the arm. (c) Deep scars on the shoulders and back of a female patient on hemodialysis.
Uremic pruritus causes
So far, there have been no clear ideas regarding the pathogenesis of uremic pruritus 23. Uremic pruritus is thought to be due to a combination of factors including:
- Dry skin (xerosis or xeroderma)
- Reduced sweating
- Abnormal metabolism of calcium and phosphorus / raised parathyroid hormone
- Accumulation of toxins
- Sprouting of new nerves
- Systemic inflammation
- Co-existing medical problems, particularly diabetes and liver disease.
Some patients have acquired perforating collagenosis.
Several clinical factors have been associated with uremic pruritus, including hyperparathyroidism, allergic sensitization, neuropathy, and abnormal levels of magnesium, calcium, iron, bile acids, nitric oxide, vitamin A, and parathyroid hormone 16. The development of itch has largely been attributed to systemic immune responses to hemodialysis, leading to nociceptive responses. Uremic toxins and increased serum levels of C-reactive protein, interleukin (IL)-6, and IL-31 in patients on hemodialysis with pruritus support the inflammatory nature of the disease 24. Proliferation, degranulation, and histamine release by mast cells are considered key events triggering the itch response 25. Interestingly, dialysis membranes may also stress blood cells and induce the release of pruritogenic cytokines, further contributing to uremic pruritus 26.
In pruritus, stimulation of dermal itch receptors or peripheral nerve endings generates impulses that are transmitted centrally via C-fibers 27. Endogenous opioids activate mast cells, promoting histamine release and undesirable effects such as urticaria and tachycardia 28. An imbalance in the levels and activation of opioid receptors in dermal cells, lymphocytes, peripheral nerves, and brain accentuates itch and predisposes patients to scratch 29. Antihistamine medications often fail to attenuate itch, suggesting a role of histamine-independent mechanisms in uremic pruritus 27. In this regard, receptors for morphine, endothelin-1, chloroquine, and IL-13/31 may mediate the itch sensation 28.
The precise molecular underpinnings that drive the pathophysiology of uremic pruritus remain unclear. The lack of reliable experimental models has resulted in a paucity of research studies investigating the cross talk between mast cells, keratinocytes, and neurons in a more physiological “uremic-like” setting. Thus, it is not currently possible to investigate uremic-specific mechanisms in the laboratory and to differentiate them from mechanisms more generally involved in other itch-related disorders. Encouragingly, some of the “anti-itch” pharmacological and nonpharmacological therapeutic approaches that are described in the following section have shown promising effects in patients with uremic disease. The optimization of dialysis conditions and CKD-related mineral and bone disease parameters is currently the only treatment that directly targets itch pathogenesis. Most of these promising strategies focus on the consequence rather than the cause of uremic pruritus and aim to target the nociceptive phase of the disease. None have shown the capacity to fully ameliorate inflammation, which is likely the trigger to the perpetuation of itch. A better understanding of the current therapeutic approaches to uremic pruritus at the bedside, and the molecular mechanisms targeted by these strategies, is required to identify the key mechanisms and cell types warranting further investigation at the bench.
Uremic pruritus signs and symptoms
The intensity and spatial distribution of uremic pruritus vary significantly over time and some patients are affected to a varying degree throughout the duration of their renal disease. The intensity of uremic pruritus ranges from sporadic discomfort to complete restlessness during day- and nighttime 5. The skin of hemodialysis patients with chronic itch looks quite similar to that of patients without itch. However, there is evidence of secondary skin changes, most likely due to scratching. Excoriation by scratching with or without impetigo can occur as a secondary phenomenon and rarely even prurigo nodularis is observed (Figure 1a through c). There are interindividual differences in the spatial distribution of uremic pruritus: 25%–50% of patients with uremic pruritus complain about generalized pruritus 30. In the remaining patients, uremic pruritus seems to affect predominantly the back, the face, and the forearm, respectively 31. In about 25% of patients with uremic pruritus, pruritus was most severe during or immediately after dialysis 32. Once patients develop uremic pruritus, it can last for months or years 33.
Scratching may lead to impetigo (skin infection), prurigo (papules) and chronic, lichenified dermatitis / eczema.
Uremic pruritus can be very unpleasant; about half of affected individuals become agitated or depressed. Uremic pruritus in hemodialysis patients is associated with a 17% increase in mortality.
Uremic pruritus diagnosis
The first steps of evaluation of an itchy patient are medical history and examination. A thorough history can identify constitutional symptoms that may point towards an underlying systemic disease. The doctor evaluates whether the person has kidney failure and, very importantly, its cause. Drug triggers such as opioids may be identified, especially if the commencement of the drug relates to the itch.
A careful examination can identify dermatological causes for the itch (eg scabies, lichen simplex, pemphigoid) or evidence of chronic skin changes related to the itch. In dermatological causes of pruritus, primary skin lesions will usually suggest the diagnosis. Patients without primary skin lesions and little evidence of chronic scratching should be investigated for systemic, neuropathic and psychogenic causes.
Physical examination
The physical examination findings may help doctors identify the cause of kidney failure. For example, enlarged or tender kidneys may indicate obstruction of the urinary tract causing hydronephrosis.
The panel of investigations could include:
- Full/complete blood count
- Creatinine and renal function tests
- Liver function tests
- Thyroid function tests
- Erythrocyte sedimentation rate
- Chest radiography
- HIV serology.
Blood tests
Blood tests that measure levels of creatinine and urea nitrogen in the blood are needed to confirm kidney failure diagnosis. A progressive daily rise in creatinine indicates acute kidney injury.
The level of creatinine is also the best indicator of the degree or severity of kidney function decline. The higher the level, the more severe the decline in kidney function is likely to be.
Other blood tests detect metabolic imbalances that occur if the decline in kidney function is severe, such as an increase in blood acidity (acidosis, which means low bicarbonate level), a high potassium level (hyperkalemia), a low sodium level (hyponatremia), and a high phosphorus level (hyperphosphatemia).
Urine tests
Urine tests, such as urinalysis and measurement of certain electrolytes (sodium, potassium, calcium, phosphate), may enable doctors to determine whether the cause of kidney injury is insufficient blood flow to the kidneys, damage to the kidneys, or urinary obstruction.
Imaging
Imaging of the kidneys using ultrasonography or computed tomography (CT) is helpful, sometimes by identifying hydronephrosis or an enlarged bladder. Imaging can also reveal the size of the kidneys.
X-rays of the arteries or veins that lead to and from the kidneys (angiography) may be done if obstruction of blood vessels is the suspected cause. However, angiography is done only when other tests do not provide enough information, because angiography uses an intravenous contrast agent that contains iodine, which carries a risk of additional kidney damage.
Magnetic resonance angiography (MRA) can provide information similar to that provided by angiography. However, MRA has traditionally used gadolinium, a substance that, in people who have severely reduced kidney function, rarely causes a disorder that triggers production of scar tissue in the body (nephrogenic fibrosing dermopathy). Thus, use of MRA is now more restricted and done very carefully. If other tests do not reveal the cause of kidney injury, a biopsy may be necessary to determine the diagnosis and the prognosis.
Uremic pruritus treatment
High-quality evidence in the management of uremic pruritus remains lacking, with many recommendations based on expert opinion or studies involving small numbers of patients (see Table 1 below).
The first step in treatment is optimizing dialysis efficacy. It is also important to attempt to reduce serum parathyroid hormone to normalize calcium and phosphorus.
Dry skin can be managed by using non-soap cleansers and applying emollients such as sorbolene cream or petrolatum several times daily.
Menthol and camphor may be added to an emollient to cool the skin and relieve the itch. Any localized itch may be reduced by frequent applications of topical capsaicin if tolerated.
UVB phototherapy is the mainstay of treatment for severe uremic pruritus. Oral antihistamines and systemic steroids are generally not effective.
Other treatments that have been reported to help some individuals include:
- Gabapentin and pregabalin in small doses (eg, 100-300 mg gabapentin, three times weekly)
- Nalfurafine (opioid agonist)
- Activated charcoal
- Thalidomide
- Cholestyramine
- Ondansetron
- Dupilumab.
In 2019, in a Phase III clinical trial, an agonist of kappa opioid receptors, difelikefalin, was reported to relieve the itch in hemodialysis patients significantly better than placebo.
Kidney transplantation usually results in resolution of uremic pruritus.
Table 1. Treatment options for uremic pruritus
| Treatment | Mechanism of action | Limitations/drawbacks |
| Dialysis optimization | ||
| ↑ Dialysis dose (↑ Kt/V) | ↑ Clearance of uremic toxins | |
| High flux dialyzer | ↑ Clearance of uremic toxins | |
| Optimization of CKD-mineral and bone disorder parameters | ||
| Parathyroidectomy | Reduction in parathyroid hormone and calcium-phosphate product; mechanism remains unclear | |
| Topical therapies | ||
| Emollients | Reduce xerosis | |
| Analgesics (eg, Capsaicin, Pramoxine) | Analgesia | Insufficient evidence of efficacy of Capsaicin in CKD/ESKD patients |
| Immunosuppressant (eg, Tacrolimus) | Suppression of immune-mediated exacerbation of dry skin, inflammation, and pruritus | Evidence indicates Tacrolimus is ineffective in CKD/ESKD patients FDA warning (risk of dermatological malignancies) |
| Cannabinoids (THC, CBD) | Analgesia, ↓ histamine-independent inflammation; exert effects on ionotropic TRPV1-4, TRPA1, and TRPM8 channels | Insufficient evidence of efficacy in CKD/ESKD patients Inconsistent CBD/THC content pharmacokinetics not well understood |
| Systemic pharmacological interventions | ||
| Antihistamines | Block effects of histamine, reducing its contribution to itch | Evidence indicates ineffective in CKD/ESKD patients |
| Anticonvulsants (Gabapentin, Pregabalin) | Negatively modulate voltage-gated calcium channels and calcitonin gene–related peptide release; possible modulation of μ-opioid receptors | Neurological side effects such as dizziness and somnolence reported |
| Opioid receptor modulators | ||
| μ-antagonist (Naltrexone, Naloxone) | Inhibits μ-opioid receptor, a mediator of itch | Effective in a subset of patients Sedation, gastrointestinal complications, among other side effects Dependency risk |
| Selective κ-agonist (Nalfurafine) | Selective central activation of the κ-receptor, which contributes to anti-itch sensation (research underway to determine mechanism of this biased agonism) | Only approved in Japan, US randomized controlled trial terminated due to insufficient enrollment |
| Peripheral κ-agonist (Difelikefalin) | Activation of peripheral κ-receptors (does not penetrate the blood-brain barrier) | Increased diarrhea, dizziness, vomiting No independent trials, not FDA approved |
| Dual κ-agonist/μ-antagonist (Nalbuphine, Butorphanol) | Dual targeting reduces adverse dysphoria that κ-agonism can contribute to or the sedation associated with μ-antagonism | Absent or limited number of controlled, randomized, placebo-controlled trials |
| Neurokinin-1 inhibitors (Aprepitant, Serlopitant) | Blocks substance P-mediated itch sensation in histamine-independent pruritus | Interactions of Aprepitant with other medications restrict use in some patients Limited number of studies in uremic pruritus patients |
Abbreviations: CKD = chronic kidney disease; MBD = mineral and bone disorder; ESKD = end-stage kidney disease; FDA = Federal Drug Agency; THC = tetrahydrocannabinol; CBD = cannabidiol; TRP = transient receptor potential.
Uremic pruritus natural treatment
Research on nonpharmacological therapies for uremic itch in CKD has focused on phototherapy, acupuncture, omega-3 fatty acid intake, aromatherapy, and exercise, although evidence is generally limited to small studies with methodological issues that limit conclusions to be drawn. Phototherapy is believed to be beneficial due to cutaneous immunosuppression at the cellular level, which is beneficial for skin diseases (eg, psoriasis) that have T-cell hyperactivity 34. A systematic review identified 4 randomized controlled trials examining the effects of phototherapy in patients with CKD stage ≥3; the 2 trials examining broadband UV-B therapy found it to be effective compared with ultraviolet light A (UV-A), whereas the other 2 trials examining narrow-band UV-B therapy or far-infrared ray thermal therapy did not report similar benefit 35. Evidence suggesting UV-B therapy is a safe treatment with no increased risk of skin cancer 34 indicates it can be safely used in populations with CKD. However, larger randomized controlled trials that address limitations in existing evidence, such as inadequate blinding of participants and personnel and selective reporting of results, are needed to confirm its efficacy.
Acupuncture and acupressure are alternative treatments used to reduce pruritus, possibly via parasympathetic activation and positive functional connectivity of the putamen-posterior midcingulate cortex 36. A systematic review found that, although several randomized controlled trials have reported positive uremic pruritus outcomes associated with acupuncture or acupressure, there was a high risk of bias in the trials 37 and a need for further trials with adequate blinding, appropriate control groups, and systematic allocation.
Omega-3 fatty acid supplementation has been proposed to target uremic pruritus by reducing essential fatty acid deficiency and inflammation 38. Four studies have reported beneficial effects of omega-3 fatty acid supplementation, including 3 small randomized controlled trials, suggesting larger trials are warranted to better understand its efficacy 38. In addition, a small number of exploratory studies have investigated the effects of aromatherapy on uremic pruritus severity and reported beneficial effects84-86; however, randomized controlled trials are needed to address a lack of randomization and blinding in existing evidence.
Finally, a prospective pre-post study investigated the effects of a 12-week aerobic exercise program on symptom burden and reported improvements in symptoms, including pruritus, after the program 39. Randomized controlled trials are therefore needed to better understand the impact of exercise on uremic pruritus, given its various other known benefits in patients with CKD. For example, it would be prudent to investigate the impact of intradialytic exercise on uremic pruritus in hemodialysis patients, given that intradialytic exercise is a more feasible approach to exercise for patients that is associated with a reduction in other symptoms, such as restless legs syndrome and fatigue 40. Studies are also needed to understand the mechanisms of the potential effects of exercise.
There are also several unexplored interventions that may have the potential to improve symptom severity and quality of life in people with uremic pruritus. For example, the amplifying effect of stress on the perception of symptoms, including itch, has previously been noted 41. Psychotherapeutic techniques such as cognitive-behavioral therapy, mindfulness meditation, and relaxation training are purported to reduce symptom burden by interrupting maladaptive automatic thought and behavioral reactions that amplify the experience of an unpleasant symptom stimulus.90 These approaches have been studied for their effects on symptoms, including pain, fatigue, and pruritus in non-CKD populations, with several studies suggesting positive effects 42. The possibility that stress reduction techniques might be able to reduce the saliency and distress of pruritus therefore warrants further investigation in individuals with CKD. The implementation of routine, systematic symptom assessments in CKD has also been proposed as a way to address underreporting of symptoms in general, identify patients in need of intervention, and trigger more timely and consistent intervention 43. Studies are currently underway that will shed light on the impact of routine symptom assessment protocols on patient outcomes, including pruritus and other common symptoms of CKD 1.
Dialysis optimization
As it is likely that uremic toxins considerably contribute to the development of uremic pruritus, ensuring that patients are adequately dialyzed often leads to a modest improvement in symptoms. For patients who have progressed to ESKD requiring dialysis, increasing the dose of hemo- or peritoneal dialysis may reduce itch 44. For instance, a prospective study among 111 patients on maintenance hemodialysis showed that achieving a Kt/V ≥ 1.5 was associated with a reduction in pruritus intensity compared with a Kt/V < 1.5.22 The use of high-flux versus low-flux dialyzers can further alleviate symptoms 45. Finally, the use of bioincompatible hemodialysis membranes may contribute to uremic pruritus in some patients. In these instances, transition to a biocompatible membrane (eg, polymethylmetacrylate) may reduce its severity 46.
Several small studies have suggested that an elevated calcium-phosphate product and secondary or tertiary hyperparathyroidism contribute to uremic itch 47. The largest study included a relatively small number of patients on hemodialysis who underwent parathyroidectomy and experienced significant reductions in both the calcium-phosphate product and parathyroid hormone, with significant reduction in pruritus intensity within 1 week of surgery 47. There remains a lack of evidence that other standard CKD-related mineral and bone disease treatments such as phosphate binders, activated vitamin D analogues, or Cinacalcet are effective in reducing uremic pruritus.
Kidney transplantation
Kidney transplantation, which substantially increases clearance of uremic toxins and improves CKD-related mineral and bone disease parameters beyond that of dialysis in most cases, relieves pruritus symptoms in the vast majority of cases. For instance, a prospective cohort study of 49 patients with uremic pruritus (and associated histological skin changes) who underwent successful kidney transplantation showed consistent resolution of the uremic pruritus skin changes following restoration of kidney function 48. Thus, kidney transplantation should be considered in eligible patients suffering from uremic pruritus.
Topical therapies
Emollients and analgesics
Dry skin is exceedingly common in CKD and ESKD 49. Emollients, particularly those with a high water content, are effective in reducing pruritus symptoms and improving quality of life 50. In addition to emollients, topical analgesics, such as the neuropeptide-releasing agents Capsaicin and Pramoxine, are often prescribed to alleviate pruritus 51. These function by blocking both the initiation and conduction of nerve impulses, leading to numbness. However, a systemic review of interventional trials, including 3 of which were for the treatment of hemodialysis-related pruritus, provided insufficient data for the efficacy of Capsaicin as a treatment 52. Similarly, although early evidence suggested that topical Tacrolimus, an immunosuppressant, may be effective in reducing pruritus, its use is now discouraged due to the evidence of lack of efficacy 53 and a black box warning from the US Food and Drug Administration related to a potential increased risk of dermatological malignancies.
Cannabinoids
With the recent legalization of recreational marijuana in Canada and several US states, patients increasingly have access to cannabidiol (CBD)- and tetrahydrocannabinol (THC)-containing compounds, making this an important area for new research. A single study of 21 ESKD patients with uremic pruritus suggests a potential benefit of topical creams containing cannabinoids 54. This study reported that after 3 weeks of therapy, 38% of patients experienced complete resolution of their pruritus symptoms while 81% experienced an improvement in their symptoms 54. The mechanism of action of cannabinoids in treating itch appears to be their ability to target inflammation and pain. The THC and CBD bind ionotropic transient receptor potential (TRP) ion channels (TRPV1-4, TRPA1, and TRPM8), which have been shown to play a role in the complex cutaneous intercellular communication network between epidermal keratinocytes, immune cells, and sensory nerves, leading to itch sensation 55. Although there is promise in the possibility that antagonizing or desensitizing such TRP channels (using well-selected topically applied phytocannabinoids), it is not yet possible to draw conclusions from the few studies performed due to limitations in the reporting percentages of THC and cannabidiol tested, small samples sizes, and short duration of studies 56. Furthermore, as the long-term effect of cannabis use, especially in CKD, remains unclear, caution should be made in recommendation of use of its medicinal properties until further independent and controlled studies are undertaken.
Systematic pharmacological therapies
Antihistamines
Despite their common use, there is a lack of data on the efficacy of oral antihistamines in treating uremic pruritus. In fact, the limited data available suggest that these medications may be less effective in the CKD/ESKD population 57. In these studies, oral antihistamines provided no benefit above emollients alone, and their antipruritic effects were diminished with advanced renal disease. This is likely due to the increasingly accepted hypothesis that uremic itch is a histamine-independent phenomenon.
Gabapentin or pregabalin
The neuropathic/anticonvulsant agents Gabapentin and Pregabalin are the mostly widely studied systemic medications for treating uremic pruritus. They were initially designed to mimic the neurotransmitter gamma-aminobutyric acid (GABA); however, they do not bind to GABA receptors. Rather, their mechanism of action likely involves negative modulation of the alpha 2 delta subunit of voltage-gated calcium channels and/or inhibition of the release of calcitonin gene–related peptide (a mediator of itch) from primary afferent neurons 58. It has also been hypothesized that modulation of μ-opioid receptors (MORs) may be involved in its anti-itch properties 59. Across numerous studies, Gabapentin and Pregabalin, administered in reduced doses (due to renal insufficiency), have repeatedly been shown to be effective in reducing pruritus in patients undergoing dialysis 53. When compared with one another, there is no significant difference in efficacy between Gabapentin and Pregabalin60; however, if one of these medications is ineffective, patients may receive benefit from being switched to the other 60. Adherence to these reduced doses in patients with renal insufficiency are essential, as side effects from somnolence and unsteadiness on the feet to mononucleosis have been reported in these populations 61.
Opioid receptor modulators
Opioid-based interventions are increasingly recognized as effective in reducing pruritus symptoms, and their refinement remains a frontier in uremic pruritus treatment. Opioid receptors collectively contribute to a wide range of physiological and pathophysiological activities, including mediation of neurological sensations such as pain modulation 62, which occurs collectively through MOR, δ-opioid receptor (DOR), and κ-opioid receptor (KOR) (reviewed thoroughly elsewhere) 63. An imbalance of μ and κ receptors has been hypothesized to contribute to uremic pruritus 64 as well as other forms of chronic itch 65. When stimulated, the μ-receptor promotes pruritus, whereas the κ-receptor inhibits it. More recently, selective KOR agonists (Nalfurafine [TRK-820]; approved in Japan) 66 and Difelikefalin (CR845; stage III clinical trial complete) 67 have become an attractive target for inhibition of itch over MOR antagonists (such as Naltrexone, Naloxone), which appear to be effective in only a subset of patients with frequent adverse effects (primarily gastrointestinal) 68. A major benefit to the use of KOR agonists is that they are physiologically safe and do not promote euphoria 69, limiting the likelihood of abuse. Recently, a large double-blind, placebo-controlled, randomized controlled, phase 3 trial of intravenous Difelikefalin was completed. Results demonstrated a significant decrease in pruritus based on a numerical rating scale compared with placebo (52% vs 31% improvement) as well as an improvement in quality of life in the Difelikefalin group, garnering optimism within the field about its potential impact on treating uremic pruritus 67. However, independent verification of efficacy and long-term safety data for Difelikefalin is still needed before mainstream use.
The molecular mechanism underlying KOR activity and itch relief is not well understood, but basic research is emerging around the signaling events that contribute to anti-itch action downstream of KOR activation. It has been suggested that the activation of KOR induces an anti-inflammatory response through the downregulation of cytokine, chemokine, and chemokine receptor expression, which may contribute to their antipruritic effects 70. A recent study also found evidence that KOR activation attenuates histamine-independent acute and chronic itch in mice in a mechanism that involves another G protein-coupled receptor (GPCR) that functions in itch sensation, the gastrin-releasing peptide receptor 71, via a calcium-independent phospholipase C-protein kinase C-δ pathway 71. The contribution of gastrin-releasing peptide receptor signaling to the development of uremic pruritus remains fully unexplored and represents a new avenue for future investigations into its treatment.
NK-1 inhibitors
The substance P (SP) or neurokinin-1 (NK-1) pathway is important in histamine-independent pruritus 72. Inhibition of NK-1 receptors, the primary receptor of substance P, has been shown to decrease the perception of itch. Aprepitant, an NK-1 inhibitor, has demonstrated efficacy in the treatment of other pruritus-related disorders, and it was at one point identified as a promising new option for the treatment of uremic pruritus. However, interactions with other medications restrict its use in some patients. Serlopitant, another NK-1 inhibitor, was successful in a recent phase II clinical trial of reducing pruritus in patients with chronic itch with minimal adverse effects 73. However, enrollment of patients with uremic or cholestatic pruritus was minimized to reduce possible confounding effects from these comorbidities. Thus, it remains unknown whether Serlopitant might be successful for alleviating uremic pruritus. An earlier, smaller study using Serlopitant in patients, including several with CKD 74, showed a strong inhibition of pruritus with few adverse effects. However, the study was a nonrandomized trial and had very few participants, making it difficult to draw conclusions. Increased investigation of the efficacy of NK-1 inhibitors in uremic pruritus will hopefully shed light on its therapeutic potential in the near future.
References- Martin CE, Clotet-Freixas S, Farragher JF, Hundemer GL. Have We Just Scratched the Surface? A Narrative Review of Uremic Pruritus in 2020. Can J Kidney Health Dis. 2020;7:2054358120954024. Published 2020 Oct 15. doi:10.1177/2054358120954024 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7573751
- Shanley KJ. Pathophysiology of pruritus. Vet Clin North Am Small Anim Pract. 1988;18:971-981.
- Lavery MJ, Kinney MO, Mochizuki H, Craig J, Yosipovitch G. Pruritus: an overview. What drives people to scratch an itch? Ulster Med J. 2016;85:164-173.
- Pisoni R. L, Wikström B, Elder S. J, Akizawa T, Asano Y, Keen M. L, Saran R, Mendelssohn D. C, Young E. W, Port F. K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2006;21:3495–3505.
- Mettang T. Pruritus in Renal Disease. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK200918
- Schwab M, Mikus G, Mettang T, Pauli-Magnus C, Kuhlmann U. Arbeitsgemeinschaft für Pädiatrische Nephrologie. Urämischer Pruritus im Kindes- und Jugendalter. Monatszeitschrift Kinderheilkunde. 1999;147:232.
- Bairey Merz CN, Dember LM, Ingelfinger JR, et al. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol. 2019;15(12):776-783.
- Hägg D, Sundström A, Eriksson M, Schmitt-Egenolf M. Severity of psoriasis differs between men and women: a study of the clinical outcome measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol. 2017;18(4):583-590.
- Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495-3505.
- Wikström B. Itchy skin—a clinical problem for haemodialysis patients. Nephrol Dial Transplant. 2007;22(suppl 5):v3-v7.
- Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626-1632.
- Mistik S, Utas S, Ferahbas A, et al. An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol. 2006;20(6):672-678.
- Ramakrishnan K, Bond TC, Claxton A, et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis. 2013;7:1-12.
- Ersoy NA, Akyar İ. Multidimensional pruritus assessment in hemodialysis patients. BMC Nephrol. 2019;20:42.
- Hu T, Wang B, Liao X, Wang S. Clinical features and risk factors of pruritus in patients with chronic renal failure. Exp Ther Med. 2019;18(2):964-971.
- Akhyani M, Ganji M-R, Samadi N, Khamesan B, Daneshpazhooh M. Pruritus in hemodialysis patients. BMC Dermatol. 2005;5:7.
- Tessari G, Dalle Vedove C, Loschiavo C, Tessitore N, Ruguiu C, Lupo A, Girolomoni G. The impact of pruritus on the quality of life of patients undergoing dialysis: A single centre cohort study. J. Nephrol. 2009;22 (2):241–248.
- Zemaitis MR, Foris LA, Chandra S, Bashir K. Uremia. Treasure Island, FL: StatPearls Publishing; 2020.
- Rayner HC, Larkina M, Wang M, et al. International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol. 2017;12:2000-2007.
- Young A. W, Sweeney E. W, David D. S, Cheigh J, Hochgelerent E. L, Sakai S, Stenzel K. H, Rubin A. L. Dermatologic evaluation of pruritus in patients on hemodialysis. N. Y. State J. Med. 1973;73:2670–2674.
- Mettang T, Fritz P, Weber J, Machleidt C, Hübel E, Kuhlmann U. Uremic pruritus in patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD). The role of plasma histamine and skin mast cells. Clin. Neprol. 1990;34:136–141.
- Pauli-Magnus C, Mikus G, Alscher D. M, Kirschner T, Nagel W, Gugeler N, Risler T, Berger E. D, Kuhlmann U, Mettang T. Naltrexone does not relieve uremic pruritus: Results of a randomized, placebo-controlled crossover-study. J. Am. Soc. Nephrol. 2000b;11:514–515.
- Mettang T, Pauli-Magnus C, Alscher D. M. Uraemic pruritus—New perspectives and insights from recent trials. Nephrol. Dial. Transplant. 2002;17:1558–1563.
- Malekmakan L, Malekmakan A, Sayadi M, Pakfetrat M, Sepaskhah M, Roozbeh J. Association of high-sensitive C-reactive protein and dialysis adequacy with uremic pruritus. Saudi J Kidney Dis Transpl. 2015;26(5):890-895.
- Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2015;6:620.
- Fallahzadeh MK, Roozbeh J, Geramizadeh B, Namazi MR. Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrol Dial Transplant. 2011;26(10):3338-3344.
- Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin. Nephrol. 2015;35:383-391.
- Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta. 2014;1842(7):869-892.
- Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87:685-691.
- Ponticelli C, Bencini P. L. Uremic pruritus: A review. Nephron. 1992;60:1–5
- Gilchrest G. A, Stern R. S, Steinman T. I, Brown R. S, Arndt K. A, Anderson W. W. Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch. Dermatol. 1982;118:154–156.
- Dawn A. G, Yosipovitch G. Butorphanol for treatment of intractable pruritus. Am. Acad. Dermatol. 2006;54:527–531.
- Mathur V. S, Lindberg J, Germain M, Block G, Tumlin J, Smith M, Grewal M, McGuire D. ITCH National Registry Investigators. A longitudinal study of uremic pruritus in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010;5:1410–1419.
- Want E, Sasaki J, Nakamura M, Koo J. Cutaneous carcinogenic risk of phototherapy: an updated comprehensive review. J Psoriasis and Psoriatic Arth. 2015;1:44-51. doi:10.1177/247553031500100107
- Simonsen E, Komenda P, Lerner B, Askin N, Bohm C, Shaw J, Tangri N, Rigatto C. Treatment of Uremic Pruritus: A Systematic Review. Am J Kidney Dis. 2017 Nov;70(5):638-655. doi: 10.1053/j.ajkd.2017.05.018
- Min S, Kim KW, Jung WM, Lee MJ, Kim YK, Chae Y, Lee H, Park HJ. Acupuncture for Histamine-Induced Itch: Association With Increased Parasympathetic Tone and Connectivity of Putamen-Midcingulate Cortex. Front Neurosci. 2019 Mar 12;13:215. doi: 10.3389/fnins.2019.00215
- Kim KH, Lee MS, Kim TH, Kang JW, Choi TY, Lee JD. Acupuncture and related interventions for symptoms of chronic kidney disease. Cochrane Database Syst Rev. 2016 Jun 28;(6):CD009440. doi: 10.1002/14651858.CD009440.pub2
- Panahi Y, Dashti-Khavidaki S, Farnood F, Noshad H, Lotfi M, Gharekhani A. Therapeutic Effects of Omega-3 Fatty Acids on Chronic Kidney Disease-Associated Pruritus: a Literature Review. Adv Pharm Bull. 2016 Dec;6(4):509-514. doi: 10.15171/apb.2016.064
- Wilkinson TJ, Watson EL, Gould DW, Xenophontos S, Clarke AL, Vogt BP, Viana JL, Smith AC. Twelve weeks of supervised exercise improves self-reported symptom burden and fatigue in chronic kidney disease: a secondary analysis of the ‘ExTra CKD’ trial. Clin Kidney J. 2019 Feb;12(1):113-121. doi: 10.1093/ckj/sfy071
- Chang Y, Cheng SY, Lin M, Gau FY, Chao YF. The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: a randomized clinical trial. Int J Nurs Stud. 2010 Nov;47(11):1383-8. doi: 10.1016/j.ijnurstu.2010.05.002
- Schut C, Mollanazar NK, Kupfer J, Gieler U, Yosipovitch G. Psychological Interventions in the Treatment of Chronic Itch. Acta Derm Venereol. 2016 Feb;96(2):157-61. doi: 10.2340/00015555-2177
- Warth M, Zöller J, Köhler F, Aguilar-Raab C, Kessler J, Ditzen B. Psychosocial Interventions for Pain Management in Advanced Cancer Patients: a Systematic Review and Meta-analysis. Curr Oncol Rep. 2020 Jan 21;22(1):3. doi: 10.1007/s11912-020-0870-7
- van der Veer SN, Aresi G, Gair R. Incorporating patient-reported symptom assessments into routine care for people with chronic kidney disease. Clin Kidney J. 2017 Dec;10(6):783-787. doi: 10.1093/ckj/sfx106
- Ko M-J, Wu H-Y, Chen H-Y, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One. 2013;8(8):e71404.
- Chen ZJ, Cao G, Tang WX, et al. A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin Exp Dermatol. 2009;34(6):679-683.
- Lin H-H, Liu Y-L, Liu J-H, et al. Uremic pruritus, cytokines, and polymethylmethacrylate artificial kidney. Artif Organs. 2008;32(6):468-472.
- Chou FF, Ho JC, Huang SC, Sheen-Chen SM. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg. 2000;190(1):65-70.
- Altmeyer P, Kachel HG, Schäfer G, Fassbinder W. [Normalization of uremic skin changes following kidney transplantation]. Hautarzt. 1986;37(4):217-221.
- Gilchrest BA, Stern RS, Steinman TI, Brown RS, Arndt KA, Anderson WW. Clinical features of pruritus among patients undergoing maintenance hemodialysis. Arch Dermatol. 1982;118(3):154-156.
- Balaskas E, Szepietowski JC, Bessis D, et al. Randomized, double-blind study with glycerol and paraffin in uremic xerosis. Clin J Am Soc Nephrol. 2011;6(4):748-752.
- Young TA, Patel TS, Camacho F, et al. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J Dermatolog Treat. 2009;20(2):76-81.
- Gooding SMD, Canter PH, Coelho HF, Boddy K, Ernst E. Systematic review of topical capsaicin in the treatment of pruritus. Int J Dermatol. 2010;49(8):858-865.
- Amirkhanlou S, Rashedi A, Taherian J, Hafezi AA, Parsaei S. Comparison of gabapentin and ketotifen in treatment of uremic pruritus in hemodialysis patients. Pak J Med Sci. 2016;32(1):22-26.
- Szepietowski JC, Szepietowski T, Reich A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: a preliminary study. Acta Dermatovenerol Croat. 2005;13(2):97-103.
- Xie Z, Hu H. TRP channels as drug targets to relieve itch. Pharmaceuticals (Basel). 2018;11:100.
- Ho C, Martinusen D, Lo C. A review of cannabis in chronic kidney disease symptom management. Can J Kidney Health Dis. 2019;6:doi:10.1177/2054358119828391
- Weisshaar E, Dunker N, Röhl F-W, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp Dermatol. 2004;13(5):298-304.
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133-141.
- Yoon MH, Choi JI, Jeong SW. Spinal gabapentin and antinociception: mechanisms of action. J Korean Med Sci. 2003;18(2):255-261.
- Rayner H, Baharani J, Smith S, Suresh V, Dasgupta I. Uraemic pruritus: relief of itching by gabapentin and pregabalin. Nephron Clin Pract. 2012;122(3-4):75-79.
- Yoo L, Matalon D, Hoffman RS, Goldfarb DS. Treatment of pregabalin toxicity by hemodialysis in a patient with kidney failure. Am J Kidney Dis. 2009;54(6):1127-1130.
- Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012;13(2):230-246.
- Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018;43(13):2514-2520.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Inan S, Cowan A. Reduced kappa-opioid activity in a rat model of cholestasis. Eur. J. Pharmacol. 2005;518:182-186.
- Kozono H, Yoshitani H, Nakano R. Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch® capsules 2.5 μg) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renovasc Dis. 2018;11:9-24.
- Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F, KALM-1 Trial Investigators. A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382:222-232.
- Legroux-Crespel E, Clèdes J, Misery L. A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Dermatology. 2004;208(4):326-330.
- Cowan A, Kehner GB, Inan S. Targeting itch with ligands selective for κ opioid receptors. Handb Exp Pharmacol. 2015;226:291-314.
- Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252(1-2):146-154.
- Munanairi A, Liu X-Y, Barry DM, et al. Non-canonical opioid signaling inhibits itch transmission in the spinal cord of mice. Cell Rep. 2018;23:866-877.
- Pojawa-Gołąb M, Jaworecka K, Reich A. NK-1 receptor antagonists and pruritus: review of current literature. Dermatol Ther (Heidelb). 2019;9(3):391-405.
- Yosipovitch G, Ständer S, Kerby MB, et al. Serlopitant for the treatment of chronic pruritus: results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J Am Acad Dermatol. 2018;78(5):882-891.
- Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5:e10968.