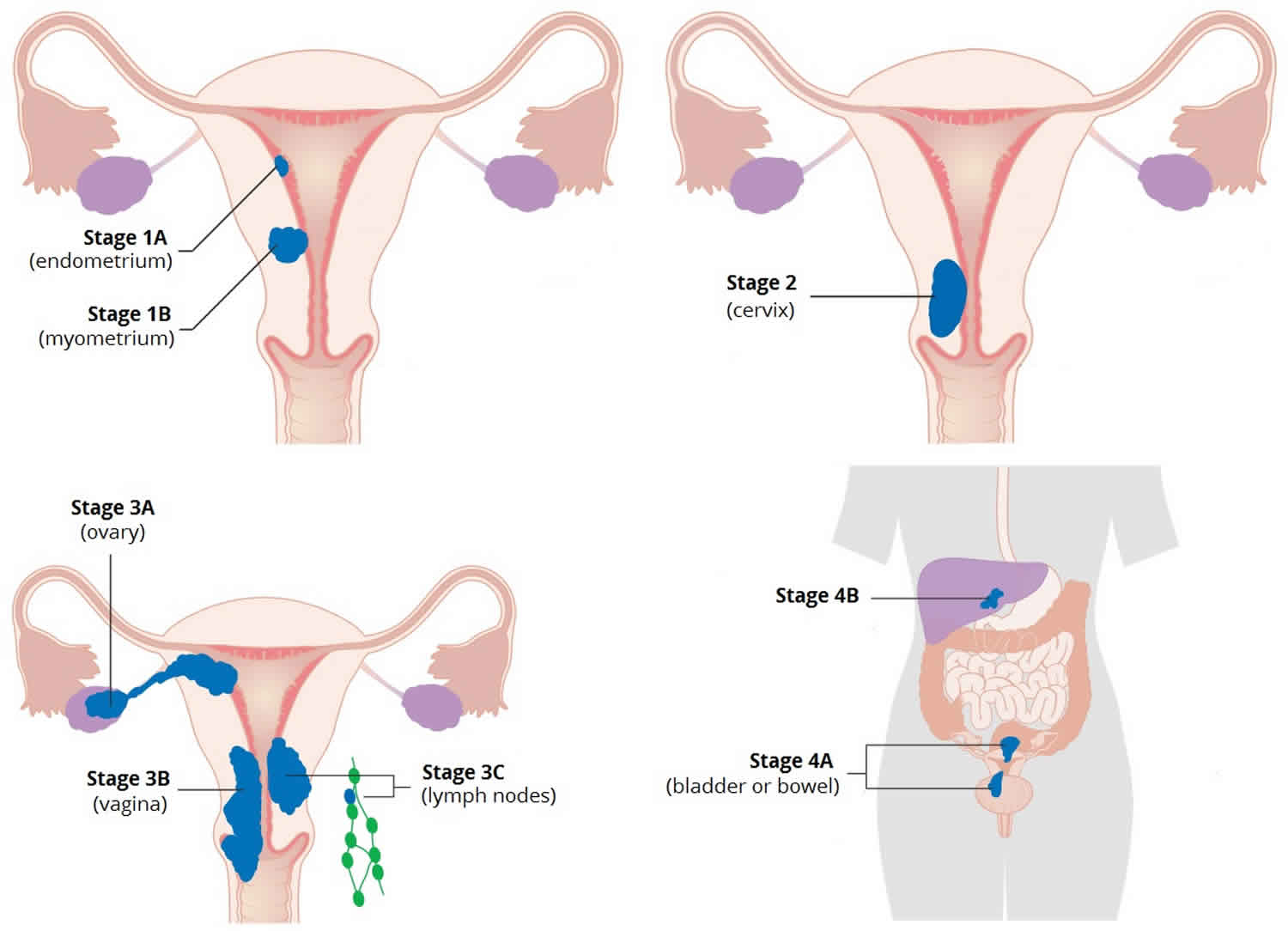
Uterine cancer
Uterine cancer is cancer that starts in the cells of the uterus or the womb. The uterus (womb) is part of the female reproductive system. The uterus (womb) is a hollow, pear-shaped, muscular organ in the pelvis, where a fetus grows. Uterine cancers can be of two types: endometrial cancer (common) and uterine sarcoma (rare).
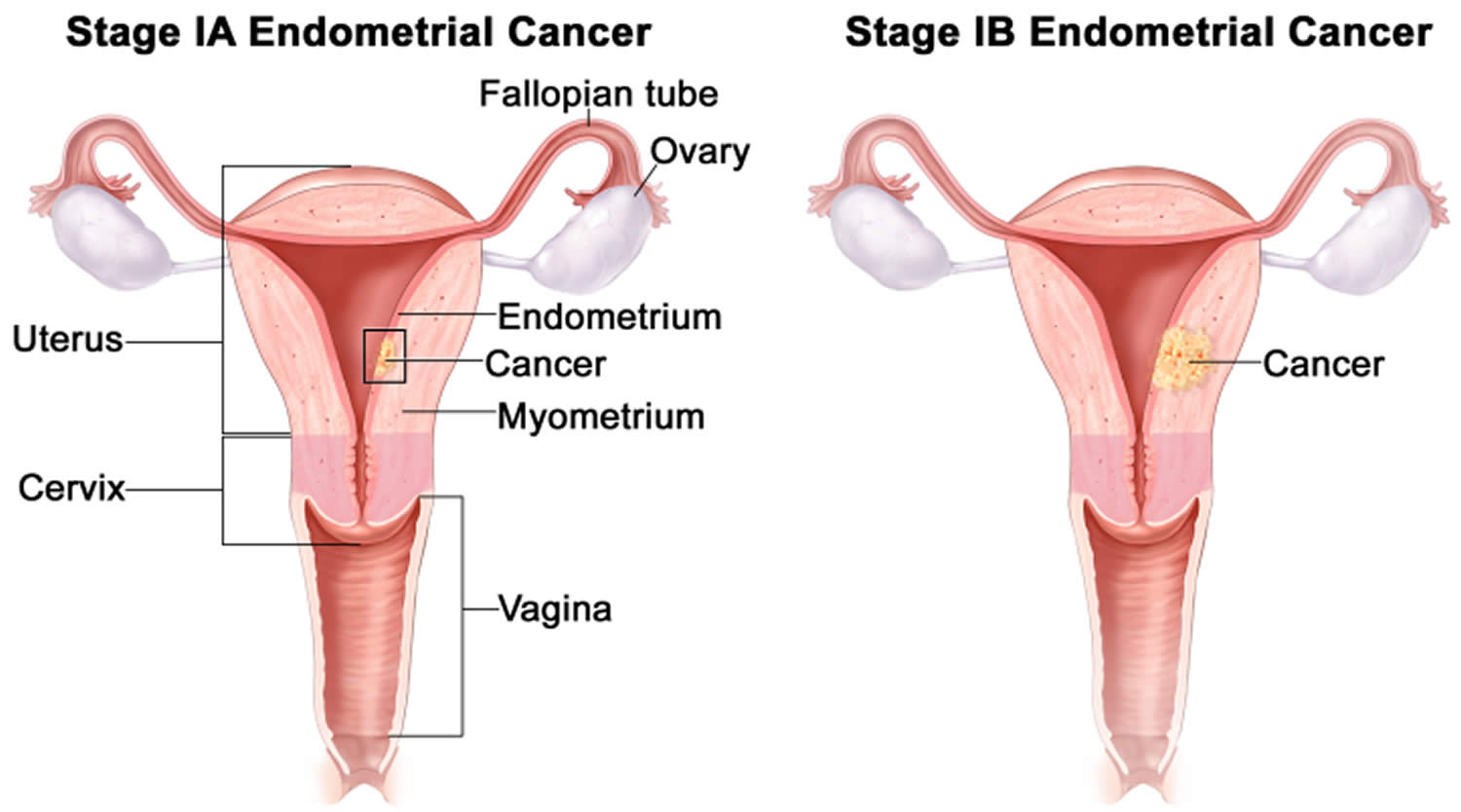
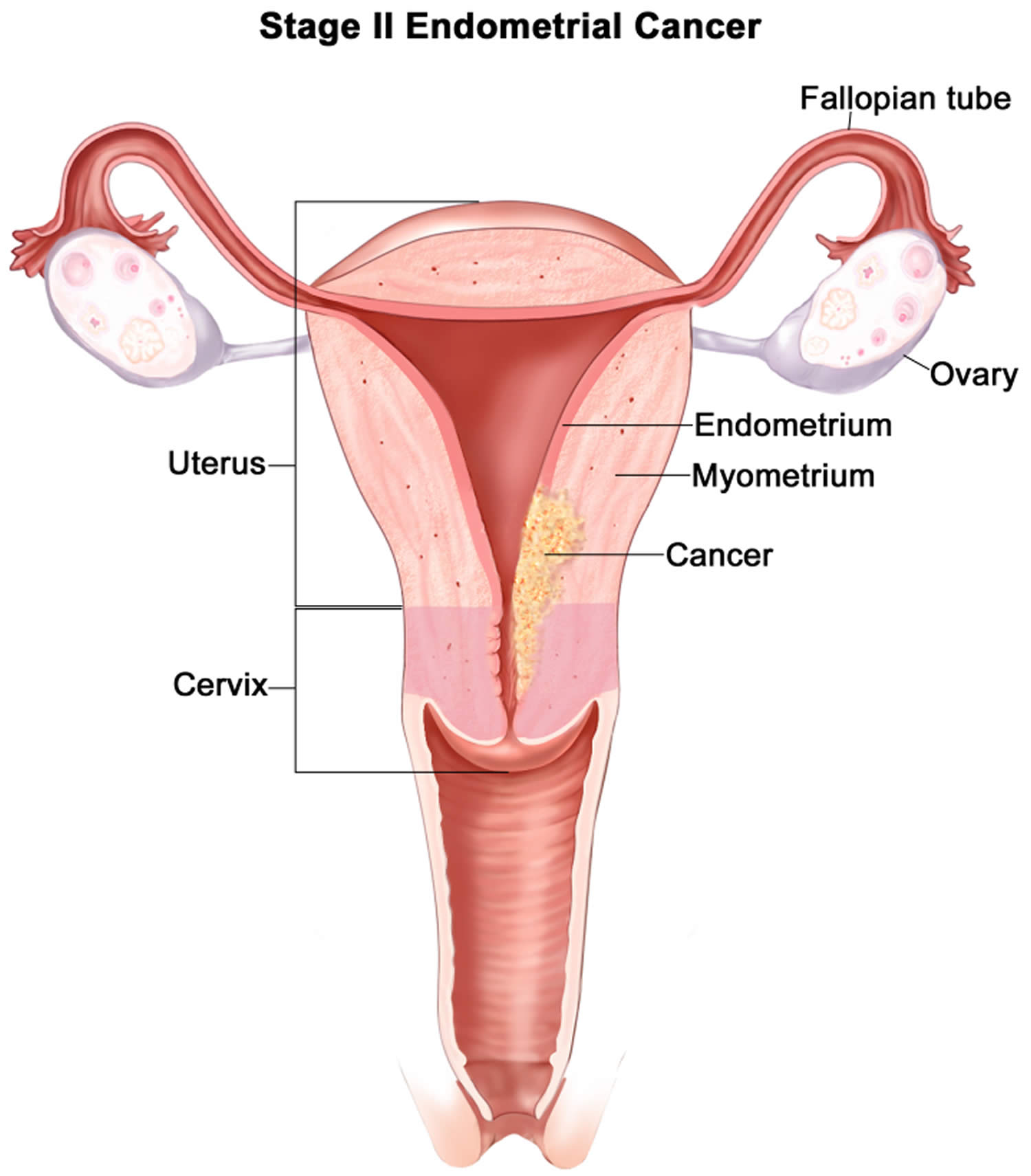
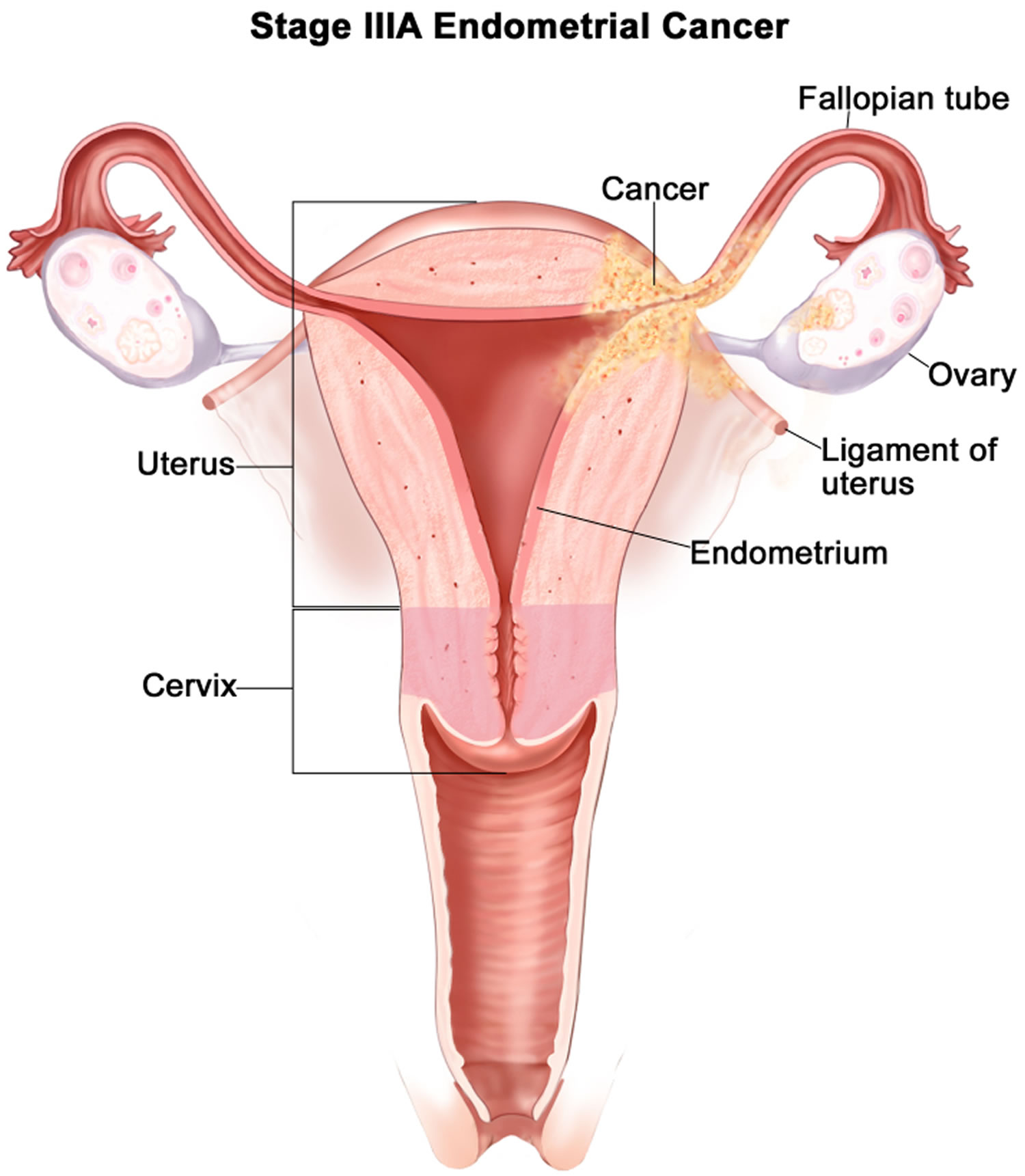
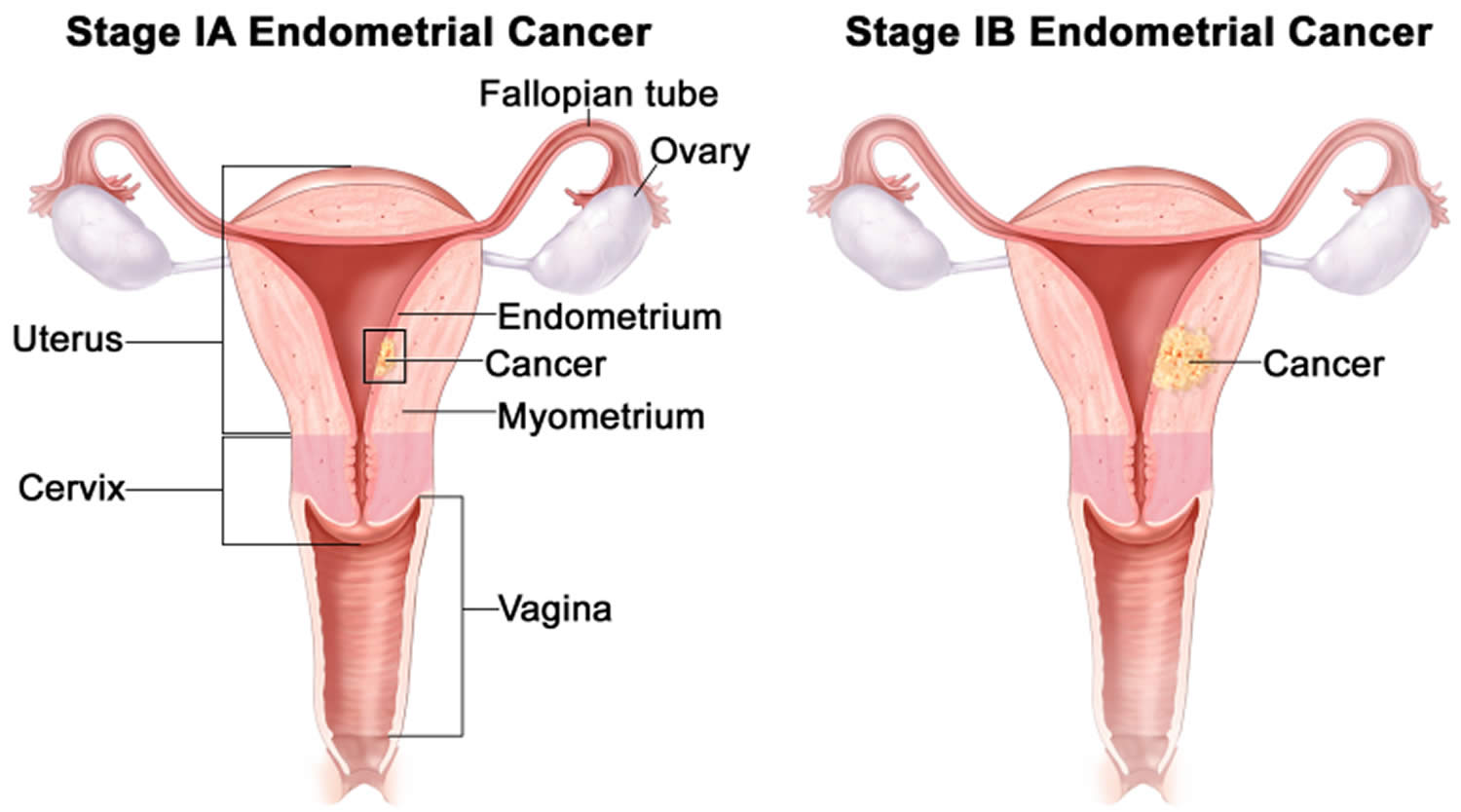
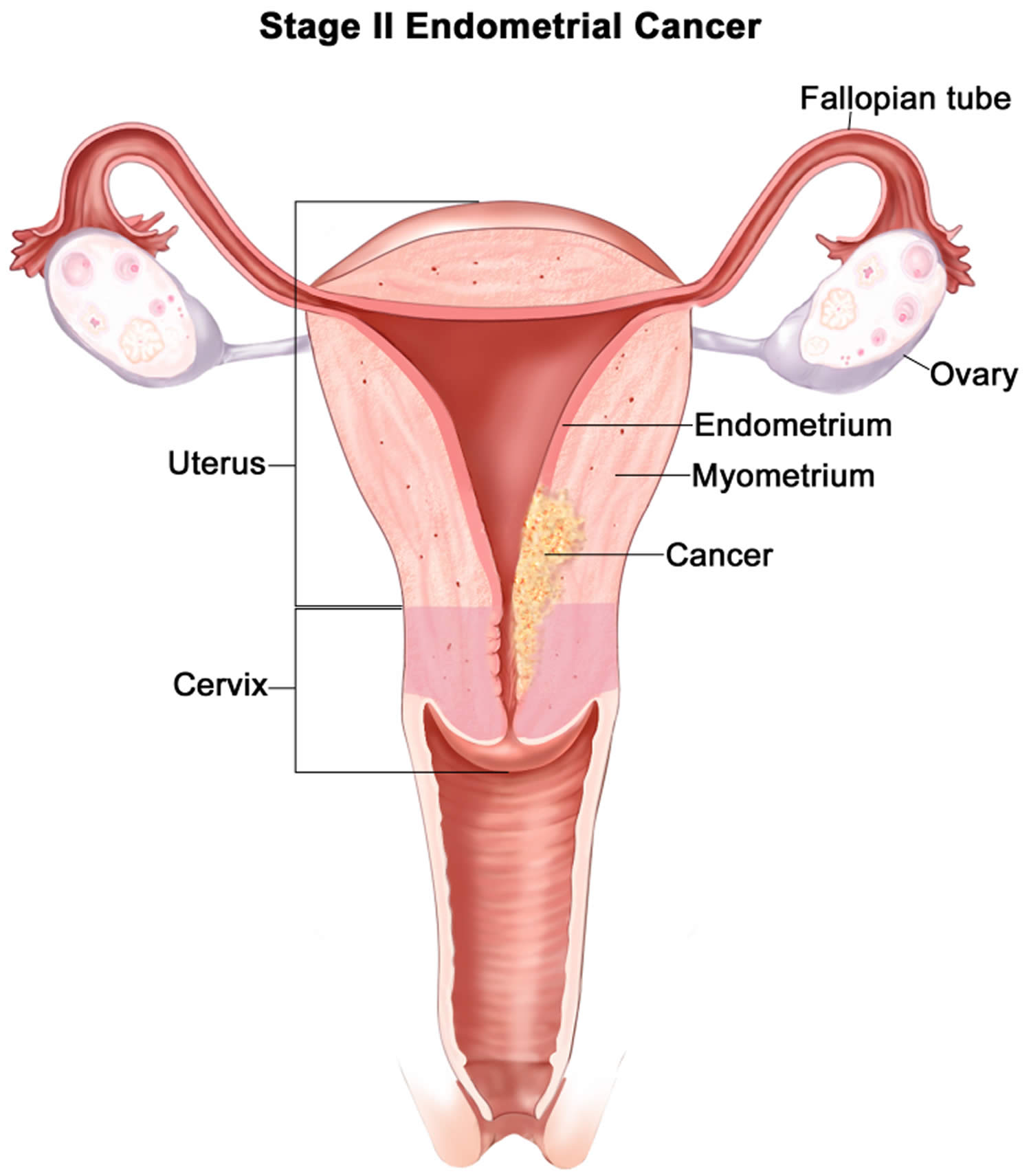
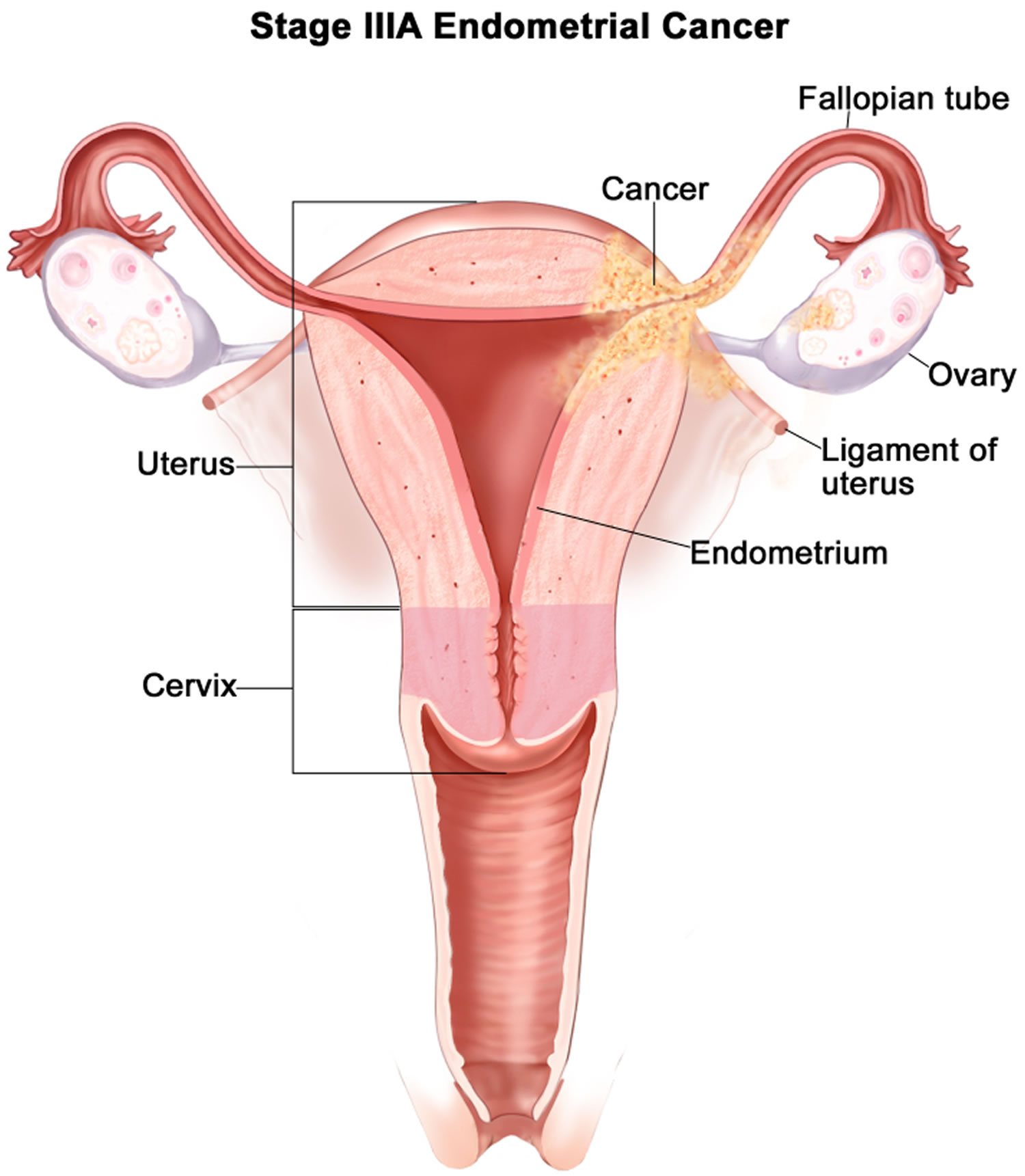
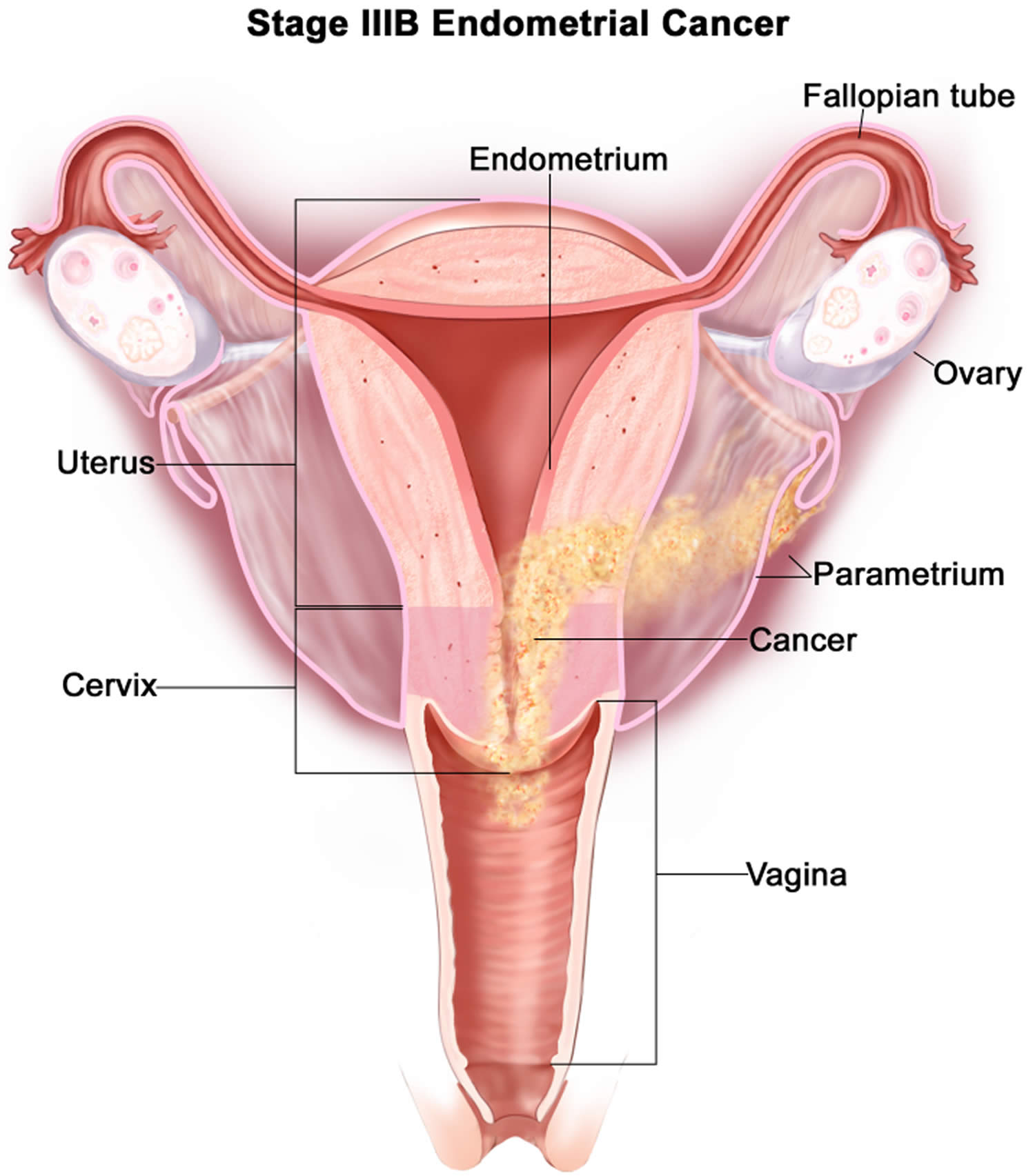
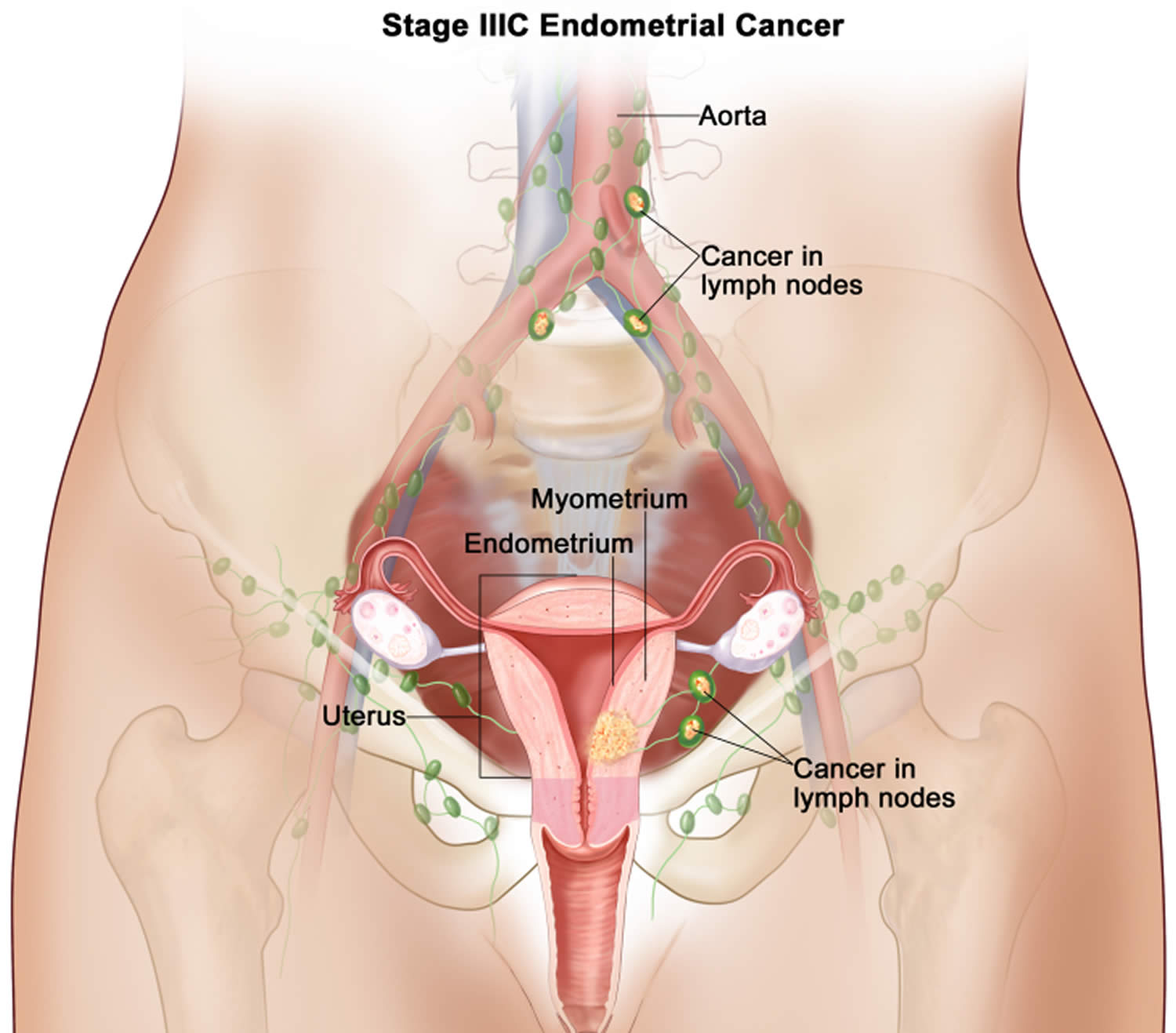
- Endometrial cancer (endometrial carcinoma) is cancer that begins in the layer of cells that form the lining (the endometrium) of the uterus (see Figures 1 and 3). Endometrial cancer makes up the majority of uterine cancers. Endometrial cancer is the fourth most common cancer in women in the United States after breast, lung, and colorectal cancers 1. Cancer of the endometrium is the most common gynecologic malignancy in the United States and accounts for 7% of all cancers in women. Endometrial cancer is often detected at an early stage, because it frequently produces abnormal vaginal bleeding such as vaginal bleeding after menopause or bleeding between periods, which prompts women to see their health care providers. If endometrial cancer is discovered early, removing the uterus surgically often cures endometrial cancer. The majority of endometrial cancer cases are diagnosed at an early stage and are amenable to treatment with surgery alone 2.
- Uterine sarcoma is a rarer type of uterine cancer that develops in the muscles of the uterus (the myometrium) or other tissues that support the uterus such as fat, bone, and fibrous tissue (the material that forms tendons and ligaments). Sarcoma accounts for about 2% to 4% of uterine cancers. Subtypes of endometrial sarcoma include uterine leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated sarcoma.
The American Cancer Society estimates for uterine cancer in the United States for 2022 are 3, 4:
- New cases: About 65,950 new cases of cancer of the body of the uterus (uterine body or corpus) will be diagnosed.
- Deaths: About 12,550 women will die from cancers of the uterine body.
- 5-Year Relative Survival: 81.3%. Relative survival is an estimate of the percentage of patients who would be expected to survive the effects of their cancer. It excludes the risk of dying from other causes. Because survival statistics are based on large groups of people, they cannot be used to predict exactly what will happen to an individual patient. No two patients are entirely alike, and treatment and responses to treatment can vary greatly.
- Endometrial cancer deaths as a percentage of All Cancer Deaths: 2.1%.
- Rate of New Cases and Deaths per 100,000: The rate of new cases of uterine cancer was 27.8 per 100,000 women per year. The death rate was 5.0 per 100,000 women per year. These rates are age-adjusted and based on 2015–2019 cases and deaths.
- Lifetime Risk of Developing Cancer: Approximately 3.1 percent of women will be diagnosed with uterine cancer at some point during their lifetime, based on 2017–2019 data.
- In 2019, there were an estimated 822,388 women living with uterine cancer in the United States. There are more than 600,000 survivors of endometrial cancer in the US today.
These estimates include both endometrial cancers and uterine sarcomas. Up to 10% of uterine body cancers are sarcomas, so the actual numbers for endometrial cancer cases and deaths are slightly lower than these estimates.
Endometrial cancer affects mainly post-menopausal women. The average age of women diagnosed with endometrial cancer is 60. It’s uncommon in women under the age of 45.
Endometrial cancer is more common in Black women than white women, and Black women are more likely to die from it.
Uterus and endometrium
The uterus is a hollow, muscular organ shaped somewhat like an inverted pear. The uterus receives the embryo that develops from an oocyte fertilized in the uterine tube, and sustains its development.
In its nonpregnant, adult state, the uterus is about 7 centimeters long, 5 centimeters wide (at its broadest point), and 2.5 centimeters in diameter. The size of the uterus changes greatly during pregnancy and it is somewhat larger in women who have been pregnant. The uterus is located medially in the anterior part of the pelvic cavity, superior to the vagina, and usually bends forward over the urinary bladder (see Figure 3).
The uterus has 2 main parts:
- The upper part of the uterus is called the body or the corpus. Corpus is the Latin word for body.
- The cervix is the lower end of the uterus that joins it to the vagina.
When people talk about cancer of the uterus, they usually mean cancers that start in the body of the uterus, not the cervix. Cervical cancer is a separate kind of cancer.
The upper two-thirds or body (corpus), of the uterus has a domeshaped top called the fundus (see Figure 1). The uterine tubes (also called Fallopian tubes) connect at the upper lateral edges of the uterus. The lower third of the uterus is called the cervix. This tubular part extends downward into the upper part of the vagina. The cervix surrounds the opening called the cervical orifice, through which the uterus opens to the vagina.
The uterine wall is thick and has 3 layers (Figure 1):
- The endometrium, the inner mucosal layer, is covered with columnar epithelium and contains abundant tubular glands (see Figure 3). During a woman’s menstrual cycle, hormones cause the endometrium to change. Estrogen causes the endometrium to thicken so that it could nourish an embryo if pregnancy occurs. If there is no pregnancy, estrogen is produced in lower amounts and more of the hormone called progesterone is made. This causes the endometrial lining to shed from the uterus and become the menstrual flow (period). This cycle repeats until menopause.
- The myometrium, a thick, middle, muscular layer, consists largely of bundles of smooth muscle cells. This thick layer of muscle is needed to push the baby out during birth. During the monthly female menstrual cycles and during pregnancy, the endometrium and myometrium change extensively.
- The perimetrium also called the serosa consists of an outer serosal layer, which covers the body of the uterus and part of the cervix.
During a woman’s menstrual cycle, hormones cause the endometrium to change. During the early part of the cycle, before the ovaries release an egg (ovulation), the ovaries produce hormones called estrogens. Estrogen causes the endometrium to thicken so that it could nourish an embryo if pregnancy occurs. If there is no pregnancy, estrogen is produced in lower amounts and more of the hormone called progesterone is made after ovulation. This prepares the innermost layer of the lining to shed. By the end of the cycle, the endometrial lining is shed from the uterus and becomes the menstrual flow (period). This cycle repeats until the woman’s goes through menopause (change of life).
The uterus is supported by the muscular floor of the pelvis and folds of peritoneum that form supportive ligaments around the organ, as they do for the ovary and uterine tube. The broad ligament has two parts: the mesosalpinx mentioned earlier and the mesometrium on each side of the uterus. The cervix and superior part of the vagina are supported by cardinal (lateral cervical) ligaments extending to the pelvic wall. A pair of uterosacral ligaments attaches the posterior side of the uterus to the sacrum, and a pair of round ligaments arises from the anterior surface of the uterus, passes through the inguinal canals, and terminates in the labia majora.
As the peritoneum folds around the various pelvic organs, it creates several dead-end recesses and pouches (extensions of the peritoneal cavity). Two major ones are the vesicouterine pouch, which forms the space between the uterus and urinary bladder, and rectouterine pouch between the uterus and rectum (see Figure 2).
The uterine blood supply to the uterus is particularly important to the menstrual cycle and pregnancy. A uterine artery arises from each internal iliac artery and travels through the broad ligament to the uterus (Figure 4). It gives off several branches that penetrate into the myometrium and lead to arcuate arteries. Each arcuate artery travels in a circle around the uterus and anastomoses with the arcuate artery on the other side. Along its course, it gives rise to smaller arteries that penetrate the rest of the way through the myometrium, into the endometrium, and give off spiral arteries. The spiral arteries wind tortuously between the endometrial glands toward the surface of the mucosa. They rhythmically constrict and dilate, making the mucosa alternately blanch and flush with blood.
Figure 1. Uterus
Figure 2. Uterus location
Figure 3. Endometrium of the uterus and its blood supply
Figure 4. Blood supply to the uterus

Endometrium function
The endometrium changes throughout the menstrual cycle in response to hormones. During the first part of the cycle, the hormone estrogen is made by the ovaries. Estrogen causes the lining to grow and thicken to prepare the uterus for pregnancy. In the middle of the cycle, an egg is released from one of the ovaries (ovulation).
Following ovulation, levels of another hormone called progesterone begin to increase. Progesterone prepares the endometrium to receive and nourish a fertilized egg. If pregnancy does not occur, estrogen and progesterone levels decrease. The decrease in progesterone triggers menstruation, or shedding of the lining. Once the lining is completely shed, a new menstrual cycle begins.
Figure 5. Ovarian activity during the Menstrual cycle
Footnote: Major events in the female menstrual cycle. (a) Plasma hormonal concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) affect follicle maturation in the ovaries. (b) Plasma hormonal concentrations of estrogen and progesterone influence changes in the uterine lining.
Endometrial hyperplasia
Endometrial hyperplasia is a common condition in which the endometrium (lining of the uterus) is abnormally thick. The uterus is part of the female reproductive system. It is a hollow, pear-shaped, muscular organ in the pelvis, where a fetus grows. Endometrial hyperplasia is defined as a proliferation of endometrial glands of irregular size and shape that results in a greater than normal gland-to-stroma ratio 5. Endometrial hyperplasia is caused by too much exposure to exogenous or endogenous estrogen along with a relative deficiency of progesterone 6. Both estrogen and progesterone hormones play roles in the menstrual cycle. Estrogen makes the cells grow, while progesterone signals the shedding of the cells. A hormonal imbalance estrogen and progesterone hormones in can produce too many cells or abnormal cells. Endometrial hyperplasia is not cancer, but in some cases, if it’s not treated, has the propensity to develop into endometrial cancer or cancer of the uterus 7. Endometrial hyperplasia is believed to be a precursor of cancer of the uterus or endometrial carcinoma, which is the most common gynecologic cancer and the fourth most common cancer in women in the United States 8.
Endometrial hyperplasia is most frequently diagnosed in postmenopausal women, but women of any age can be at risk if they are exposed to a source of unopposed estrogen 9. Endometrial hyperplasia is increasingly seen in young women with chronic anovulation due to polycystic ovary syndrome (PCOS) or obesity 10.
Endometrial hyperplasia usually occurs after menopause, when ovulation stops and progesterone is no longer made 11. Endometrial hyperplasia can also develop during perimenopause, when ovulation may not occur regularly. There may be high levels of estrogen and not enough progesterone in other situations, including when a woman 11:
- uses medications that act like estrogen, such as tamoxifen for cancer treatment
- uses estrogen for hormone therapy and does not use progesterone or progestin if she still has a uterus
- has irregular menstrual periods, especially associated with polycystic ovary syndrome (PCOS) or infertility
- has obesity – body mass index (BMI) of 27 or more
The main symptom of endometrial hyperplasia is abnormal menstrual bleeding. However, abnormal uterine bleeding can be a symptom for many things. Your doctor can perform an exam and tests to diagnose the cause of the abnormal uterine bleeding. A transvaginal ultrasound measures your endometrium. It uses sound waves to see if the layer is average or too thick. A thick layer can indicate endometrial hyperplasia. Endometrial hyperplasia is typically diagnosed by endometrial biopsy or curettage when a woman is noted as suffering from abnormal uterine bleeding 12. Your doctor will take a biopsy of your endometrium cells to determine if cancer is present.
Treatment options for endometrial hyperplasia depend on what type you have. The most common treatment is progestin. This can be taken in several forms, including pill, shot, vaginal cream, or intrauterine device.
Atypical types of endometrial hyperplasia, especially complex, increase your risk of getting cancer. If you have these types, you might consider a hysterectomy. This is a surgery to remove your uterus. Doctors recommend this if you no longer want to become pregnant.
There are also a number of more conservative treatments for younger women who do not wish to have a hysterectomy. Your doctor will help you decide which treatment option is best for you.
Figure 6. Endometrial hyperplasia ultrasound
Footnote: Endometrial thickness is 12 mm. Multiple cystic spaces are noted within. Solid areas are echogenic. Margins of endometrial lining was well-defined. No significant flow signals seen. Myometrium does not show focal lesion. Either of ovaries could not be localized.
[Source 13 ]Types of endometrial hyperplasia
The types of endometrial hyperplasia vary by the amount of abnormal cells and the presence of cell changes. According to the World Health Organization endometrial hyperplasia classification system in 1994 (WHO94), endometrial hyperplasia can be divided into 4 categories 14:
- Simple endometrial hyperplasia – Increased number of endometrial glands but regular glandular architecture.
- Complex endometrial hyperplasia – Crowded irregular endometrial glands.
- Simple atypical endometrial hyperplasia – Simple hyperplasia with presence of cytologic atypia (prominent nucleoli and nuclear pleomorphism).
- Complex atypical endometrial hyperplasia – Complex hyperplasia with cytologic atypia
The World Health Organization endometrial hyperplasia classification system 1994 (WHO94) was based on the original retrospective study of 170 patients by Kurman et al 14 who found that lesions with varying degrees of complexity and presence of atypia, when left untreated for a mean of 13 years, progressed to adenocarcinoma at different rates. Simple endometrial hyperplasia was associated with a 1% rate of progression to cancer, complex endometrial hyperplasia 3% rate of progression, simple atypical endometrial hyperplasia 8% rate of progression, whereas complex atypical endometrial hyperplasia had a 29% rate of progression to cancer 14.
Not only does the concern exist for atypical endometrial hyperplasia progressing to invasive cancer if untreated, but numerous studies found coexisting carcinoma at rates ranging from 17-56% 12, 15. A prospective Gynecologic Oncology Group study found that 306 patients with preoperative biopsies that diagnosed atypical endometrial hyperplasia had concurrent invasive adenocarcinoma in 42.6% of hysterectomy specimen 16. Part of the difficulty in diagnosing concurrent carcinoma is due to lack of reproducibility in diagnosing hyperplasia, especially atypical hyperplasia versus adenocarcinoma among even expert gynecologic pathologists. Studies report only 40-69% interobserver agreement for endometrial hyperplasia or endometrial cancer 17.
Due to the poor reproducibility of diagnosis, and confusion regarding optimal clinical management, gynecologic pathologists proposed a simpler classification of endometrial hyperplasia versus endometrial intraepithelial neoplasia (EIN) using a computerized morphometric analysis. Endometrial hyperplasia is benign hyperplasia and correlates closely to simple hyperplasia, whereas endometrial intraepithelial neoplasia (EIN) is a pre-malignant condition 18. Endometrial intraepithelial neoplasia (EIN) is defined as when the volume of glandular crowding is greater than the stromal volume, the presence of cytologic alterations, a lesion larger than 1 mm, and exclusion of mimics or carcinoma 18. Classification as complex atypical hyperplasia (WHO’94) or as endometrial intraepithelial neoplasia (EIN) had similar sensitivities and negative predictive values for coexisting endometrial cancer 19. Others found the endometrial intraepithelial neoplasia (EIN) classification to be better at predicting progression to cancer. Thus in 2014 the WHO formally adopted the simplified classification of endometrial hyperplasia into 2 categories.
World Health Organization endometrial hyperplasia classification system in 2014 (Table 1) 20:
- Hyperplasia without atypia or benign endometrial hyperplasia. Hyperplasias without atypia (benign endometrial hyperplasia) exhibit no relevant genetic changes. They are benign changes and will regress again after the endocrine milieu (physiological gestagen levels) has normalized. In a few cases (1–3 %), progression to invasive disease may occur if the endocrine disorder (long-term estrogen dominance or relative or absolute gestagen deficiency) persists over the long term.
- Endometrial Intraepithelial Neoplasia (EIN) or atypical endometrial hyperplasia. Atypical endometrial hyperplasias exhibit many of the mutations typical for invasive endometrioid endometrial cancer 21. In up to 60 % of cases, patients have coexisting invasive cancer or are at extremely high risk of developing invasive cancer (see Table 1).
This reduction to 2 categories was not only due to the need to do away with the confusing multitude of terms currently in use. Rather, it reflects a new understanding of molecular genetic changes.
The American College of Obstetricians and Gynecologists and the Society of Gynecologic Oncology Committee opinion 2015 also recommend the use of endometrial intraepithelial neoplasia (EIN) schema for a more clear terminology to distinguish the two clinicopathologic categories 8, 22. Not only does this classification reflect clinical outcome, but also underlying genetic and molecular changes 23.
The implications for treatment are obvious: hyperplasias without atypia should generally be treated conservatively (normalization of the cycle through weight loss, metformin; oral contraceptives; cyclical gestagens; gestagen IUD). Preventive hysterectomy should only be considered in exceptional cases (e.g., extreme obesity without any prospect of weight loss) 24. The surgery should be done as a total hysterectomy, i.e., it must include removal of the cervix 24.
Treatment of atypical hyperplasia or endometrioid intraepithelial neoplasia should generally consist of total (not supracervical) hysterectomy 24. Conservative treatment with high-dose gestagens and close histological monitoring should only be considered in exceptional cases (when the patient wants to have children, satisfactory compliance) 25, 24.
Table 1. New World Health Organization (WHO) classification of endometrial hyperplasia
| New term | Synonyms | Genetic changes | Coexistent invasive endometrial carcinoma | Progression to invasive carcinoma |
|---|---|---|---|---|
| Hyperplasia without atypia | Benign endometrial hyperplasia; simple non-atypical endometrial hyperplasia; complex non-atypical endometrial hyperplasia; simple endometrial hyperplasia without atypia; complex endometrial hyperplasia without atypia | Low level of somatic mutations in scattered glands with morphology on HE staining showing no changes | < 1 % | RR: 1.01–1.03 |
| Atypical hyperplasia or endometrioid intraepithelial neoplasia | Complex atypical endometrial hyperplasia; simple atypical endometrial hyperplasia; endometrial intraepithelial neoplasia (EIN) | Many of the genetic changes typical for endometrioid endometrial cancer are present, including: micro satellite instability; PAX2 inactivation; mutation of PTEN, KRAS and CTNNB1 (β-catenin) | 25–33 % 20 59 % 26 | RR: 14–45 |
Footnotes: RR = Relative Risk. Relative risk is a ratio of the probability of an event occurring in the exposed group versus the probability of the event occurring in the non-exposed group. Relative Risk = (Probability of event in exposed group) / (Probability of event in not exposed group) 27. For example, atypical hyperplasia or endometrioid intraepithelial neoplasia (EIN) has a Relative Risk of 14 to 45 times more likely to progress to invasive endometrial cancer in this study compared to those without atypical hyperplasia or endometrioid intraepithelial neoplasia (EIN) in the study.
[Source 23 ]Endometrial hyperplasia signs and symptoms
The most common of endometrial hyperplasia is abnormal menstrual bleeding. If you have any of the following, you should see your doctor or an obstetrician–gynecologist (ob-gyn):
- Menstrual bleeding that is heavier or longer lasting than usual.
- Menstrual cycles (amount of time between periods) that are shorter than 21 days (counting from the first day of the menstrual period to the first day of the next menstrual period).
- Menstrual bleeding between menstrual periods.
- Not having a period (pre-menopause).
- Any bleeding after menopause.
Endometrial hyperplasia causes
Endometrial hyperplasia is the result of chronic unopposed estrogenic stimulation of the endometrial tissue with a relative deficiency of the counterbalancing effects of progesterone 28. The causes of estrogen excess could be endogenous or exogenous. For example, if ovulation does not occur, progesterone is not made, and the lining is not shed. The endometrium may continue to grow in response to estrogen. The cells that make up the lining may crowd together and may become abnormal. This condition, called hyperplasia, can lead to cancer.
You are more likely to have endometrial hyperplasia if you have gone through menopause. This is because your body’s hormones and menstrual cycles change. Other risk factors for endometrial hyperplasia are:
- Long-term use of medicines that contain high levels of estrogen or chemicals that act like estrogen.
- Irregular menstrual cycles, which can be caused by infertility or polycystic ovary syndrome (PCOS).
- Obesity.
- Use of tobacco.
- First menstrual cycle at an early age.
- Going through menopause at an older age.
- Never having been pregnant.
- Family history of uterine, ovarian, or colon cancer.
Endometrial hyperplasia pathology outlines
Endometrial hyperplasia is defined as a proliferation of endometrial glands of irregular size and shape that results in a greater than normal gland-to-stroma ratio 5. This results in varying degrees of architectural complexity and cytologic atypia.
Endometrial hyperplasia results from continuous estrogen stimulation that is unopposed by progesterone 29. This can be due to endogenous estrogen or exogenous estrogenic sources. Endogenous estrogen may be caused by chronic anovulation associated with polycystic ovary syndrome (PCOS). Obesity also contributes to unopposed estrogen exposure due to chronic high levels of estradiol that result from aromatization of androgens in adipose tissue and conversion of androstenedione to estrone. Endometrial hyperplasia and cancer can also result from estradiol-secreting ovarian tumors such as granulosa cell tumors.
Exogenous unopposed estrogen without progesterone has been associated with increased endometrial hyperplasia and adenocarcinoma 30. The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial found that unopposed estrogen exposure with 0.625 mg of conjugated equine estrogen replacement therapy increased the risk of complex hyperplasia by 22.7% and atypical hyperplasia by 11.8% over 3 years of use compared with a less than 1% increase in placebo controls 31. The risk of endometrial cancer was not increased when 2.5 mg of medroxyprogesterone acetate was used in combination with 0.625 mg of conjugated equine estrogens in 8506 women in the Women’s Health Initiative (WHI) study 32. Tamoxifen, with its estrogenic effect on the endometrium, increases the risk of endometrial hyperplasia and endometrial cancer. The risk of progression to cancer is associated with an increased duration of use 33. While unopposed estrogen in oral contraceptive pills or estrogen replacement therapy increases the risk of endometrial hyperplasia and endometrial cancer, combination oral contraceptive pills and combination hormone replacement therapy does not increase and may decrease the risk of endometrial hyperplasia and endometrial cancer 29.
However, the exact mechanism of estrogen’s role in the transformation of normal endometrium to hyperplasia and cancer is unknown 29. Genetic alterations are known to be associated with hyperplasia and type 1 endometrial cancers. Benign endometrial hyperplasia is associated with low levels of somatic mutations, whereas EIN is associated with genetic alternations similar to endometrioid endometrial cancer such as microsatellite instability, and mutations in PTEN and KRAS 34. PTEN tumor suppressor gene mutations have also been found in 55% of endometrial hyperplasia cases and 83% of endometrial hyperplasia cases once it has progressed to endometrial cancer 35.
Endometrial hyperplasia risk factors
Endometrial hyperplasia is more likely to occur in women with risk factors, including 7:
- Age (age older than 53 years)
- Early age when menstruation started (early menarche [a girl’s first period])
- Late menopause (older age at menopause)
- Nulliparity (a woman who hasn’t given birth to a child or never having been pregnant)
- Obesity: body mass index (BMI) of 27 or more
- Genetic
- Cigarette smoking
- History of certain conditions, such as diabetes mellitus, gallbladder disease, or thyroid disease.
- Anovulatory cycles: polycystic ovary syndrome (PCOS), perimenopause
- Ovarian tumors: granulosa cell tumors
- Hormone replacement therapy: estrogen-only therapy can lead to endometrial hyperplasia at even a minimal dose and is contraindicated in women with a uterus. Over the counter/ herbal preparations may have a high amount of estrogen 36
- Immunosuppression (renal graft recipients) and infection may also be involved in the development of endometrial hyperplasia 37
- Hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome – women with this condition are at a greatly increased risk of endometrial hyperplasia 38.
- Family history of ovarian, colon, or uterine cancer
The independent risk factors for predicting when endometrial hyperplasia coexists with cancer include age older than 53 years, postmenopausal status, diabetes, abnormal bleeding, body mass index (BMI) of 27 or more, and atypical hyperplasia 12.
Endometrial hyperplasia prevention
You cannot prevent endometrial hyperplasia, but you can help lower your risk of endometrial hyperplasia by:
- Losing weight, if you are overweight or obese.
- Quit smoking if you smoke cigarette
- Taking a medicine with progestin (synthetic progesterone), if you already are taking estrogen, due to menopause or another condition.
- If your periods are irregular, birth control pills may be recommended. They contain estrogen along with progestin. Other forms of progestin also may be taken.
- Taking birth control or another medicine to regulate your hormones and menstrual cycle.
Endometrial hyperplasia differential diagnosis
The potential differential diagnosis for endometrial hyperplasia are conditions which can result in focal or generalized thickening of the endometrium 6:
- Endometrial cancer: Histopathological examination of the endometrial tissue can show markers of invasions in endometrial cancer.
- Endometrial polyp: Hydrosonography can be helpful in diagnosing endometrial polyp by enhancing visualization. Diagnostic hysteroscopy can confirm the presence of a polyp.
- Endometritis: Irregular endometrium and focal thickness are the hallmarks of endometritis.
Endometrial hyperplasia diagnosis
There are many causes of abnormal uterine bleeding. If you have abnormal bleeding and you are 35 or older, or if you are younger than 35 and your abnormal bleeding has not been helped by medication, your doctor may recommend diagnostic tests for endometrial hyperplasia and cancer.
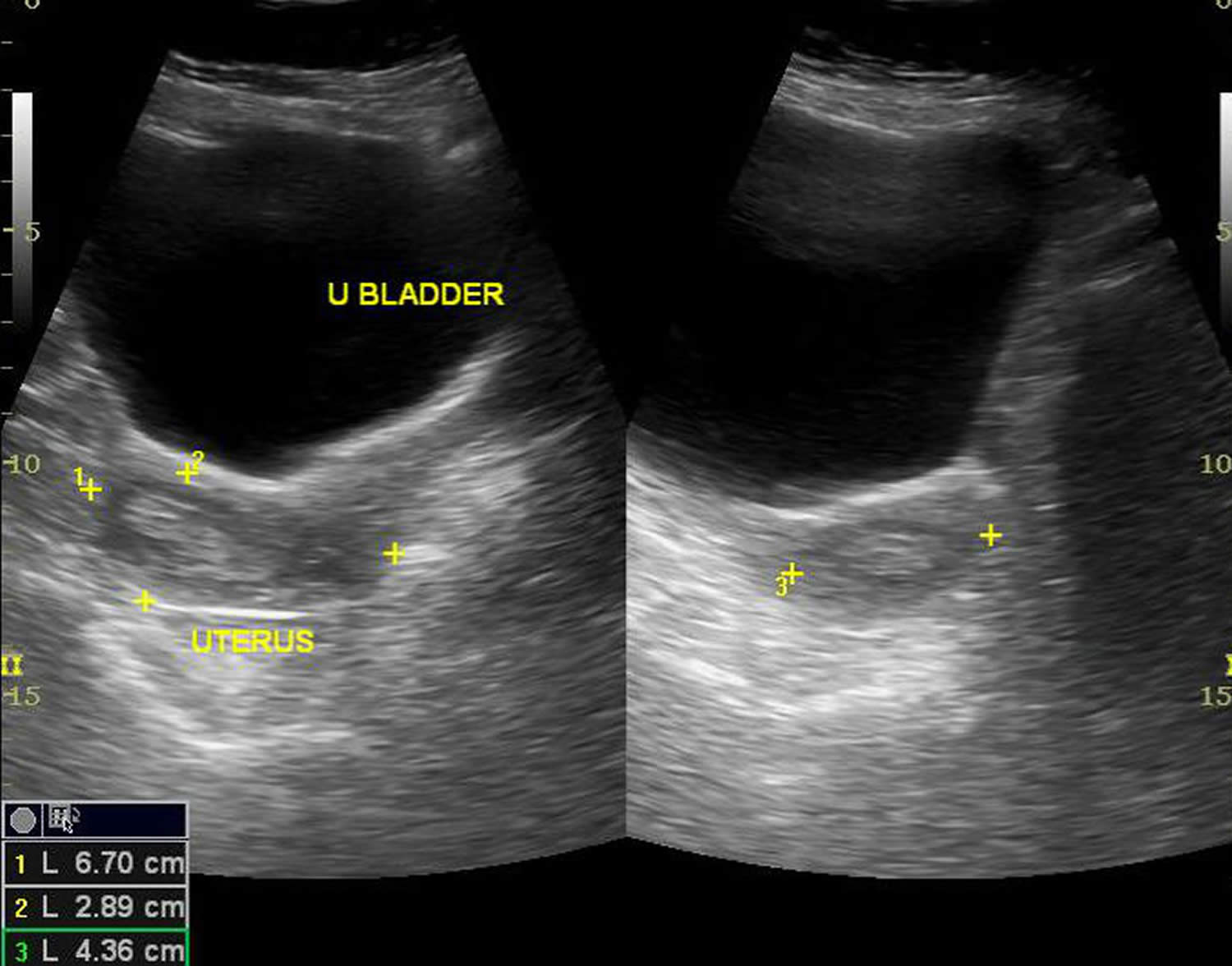
A transvaginal ultrasound exam may be done to measure the thickness of the endometrium. For this test, a small device is placed in your vagina. Sound waves from the device are converted into images of the pelvic organs. If the endometrium is thick, it may mean that endometrial hyperplasia is present.
The only way to tell for certain that cancer is present is to take a small sample of tissue from the endometrium and study it under a microscope. This can be done with an endometrial biopsy, dilation and curettage (D&C), or hysteroscopy.
Endometrial hyperplasia ultrasound
Imaging the endometrium on days 5-10 of a woman’s cycle reduces the variability in endometrial thickness 39.
- Premenopausal endometrial thickness
- Normal endometrial thickness depends on the stage of the menstrual cycle, but a thickness of >15 mm is considered the upper limit of normal in the secretory phase
- Endometrial hyperplasia can be reliably excluded in patients only when the endometrium measures less than 8 mm 40
- Postmenopausal endometrial thickness of >5 mm is considered abnormal 39.
The appearance can be non-specific and cannot reliably allow differentiation between endometrial hyperplasia and endometrial carcinoma 41. Usually, there is a homogeneous smooth increase in endometrial thickness, but endometrial hyperplasia may also cause asymmetric/focal thickening with surface irregularity, an appearance that is suspicious for carcinoma. Cystic changes can also be seen in endometrial hyperplasia.
Ultrasound features that are suggestive of endometrial carcinoma as opposed to endometrial hyperplasia include 42:
- heterogeneous and irregular endometrial thickening
- polypoid mass lesion
- intrauterine fluid collection
- frank myometrial invasion
Endometrial hyperplasia biopsy
Diagnosis of endometrial hyperplasia is usually made by sampling the endometrial cavity with an endometrial biopsy in the office. Tissue sampling should be performed in women with risk factors who present with symptoms of abnormal vaginal bleeding or discharge 43. This includes women older than 35 years with abnormal bleeding, women younger than 35 years with bleeding and risk factors, women with persistent bleeding, and women with unopposed estrogen replacement, tamoxifen therapy, and hereditary nonpolyposis colorectal cancer (HNPCC) syndrome or Lynch syndrome.
In addition, a biopsy should be performed in women with atypical glandular cells Pap smear or endometrial cells in Pap smears of women older than 40 years when out of synch with menstrual cycle 44. While no evidence of improved survival has been documented, some also advocate routine screening by endometrial biopsy in asymptomatic women with hereditary nonpolyposis colorectal cancer (HNPCC) syndrome or those on tamoxifen therapy. However, most of this high-risk population present with abnormal vaginal bleeding; thus, other experts recommend work-up only when symptoms are present.
If a patient does not tolerate an office biopsy or has cervical stenosis, endovaginal ultrasonography is an effective method to assess thickness of the endometrial echo complex and to evaluate uterine bleeding. The American College of Obstetricians and Gynecologists recommends transvaginal ultrasonography as a reasonable alternative to endometrial sampling for the evaluation of an initial episode of bleeding in a postmenopausal woman 45.
Endovaginal ultrasonography has a sensitivity of greater than 96% for ruling out endometrial carcinoma if the endometrial echo complex is less than 5 mm. Persistent bleeding despite a thin stripe still warrants tissue biopsy because of the risk of missing a type 2 cancer that is not associated with hyperplasia and thickening of the endometrial echo complex 46. If hyperplasia is diagnosed by office biopsy, one should consider D&C and hysteroscopy to more definitely rule out atypia or cancer prior to conservative medical management. This is because blind dilation and curettage (D&C) and Pipelle endometrial biopsies both sample only 50-60% of the endometrial cavity, and can miss focal lesions 47.
Endometrial hyperplasia treatment
The accurate diagnosis of endometrial hyperplasia type is vital for appropriate treatment based on risk of cancer without over or under treatment 48. Once a tissue diagnosis of endometrial hyperplasia is made, treatment depends on the type of hyperplasia, the patient’s symptoms such as severity of bleeding, surgical risks, and wish for future childbearing.
In many cases, endometrial hyperplasia can be treated with progestin (man made progesterone). Progesterone induce secretory changes in the endometrium and counterbalance the stimulatory effects of estrogen. Progestins can effectively treat endometrial hyperplasia to control bleeding and prevent progression to cancer. They can serve as prevention of recurrence in those with continued risk factors 48. Progestin is given orally, in a shot, in an intrauterine device (IUD), or as a vaginal cream. How much and how long you take it depends on your age and the type of endometrial hyperplasia you have. Treatment with progestin may cause vaginal bleeding like a period.
Multiple regimens of progestin therapy have been found effective in reversing endometrial hyperplasia, including the following 24.:
- Medroxyprogesterone acetate – MPA (Provera), 10-20 mg once daily or cyclic 12-14 days per month
- Depot Medroxyprogesterone (Depo-provera) 150 mg IM every 3 months
- Micronized vaginal progesterone (Prometrium), 100-200 mg once daily or cyclic 12-14 days per month
- Levonorgestrel-containing IUD (Mirena), 20 mcg/day 49
- Megestrol acetate (Megace), 40-200 mg per day, usually reserved for women with atypical hyperplasia
Benign endometrial hyperplasia responds well to progestins. More than 98% of women with endometrial hyperplasia treated with cyclic progestins experienced regression of the disease in 3-6 months 50. However, if you have endometrial intraepithelial neoplasia (EIN) or atypical endometrial hyperplasia changes in the lining of your uterus, the risk of endometrial cancer is increased by 42% 16. Hysterectomy may be a treatment option if you do not want another pregnancy. Talk with your doctor about the right treatment for you.
Interest is increasing in the use of levonorgestrel-releasing intrauterine system (LNG-IUS) as a treatment option for endometrial hyperplasia to deliver local effect with less systemic side-effects 51. A 15-year study using the levonorgestrel-releasing intrauterine system (LNG-IUS) for 34 patients with endometrial hyperplasia found regression to atrophic endometrium in 94% 52. However, this study had only 4 (12%) patients with atypical hyperplasia 52. A meta-analysis of 24 observational studies, including 1,001 cases, indicate that progestins appeared to induce a lower disease regression rate than levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of complex and atypical endometrial hyperplasia. However, a controlled clinical trial is necessary to confirm the observational findings. The authors feel data using the levonorgestrel-releasing intrauterine device (LNG-IUD) as the only treatment modality for atypical hyperplasia or endometrial cancer are still limited 53.
The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial showed a 94% normalization of complex or atypical hyperplasia in 45 women treated with progestins 31. One cohort study found that 115 patients with complex atypical hyperplasia had approximately 30% persistence or progression of disease whether treated with progestins or not. However, of 70 patients with atypical hyperplasia, 67% vs 27% had persistence or progression when not treated with progestins 54.
If the patient has not completed childbearing or is not a surgical candidate, then concurrent cancer must first be ruled out by D&C with hysteroscopy prior to medical management. Experts recommend megestrol acetate for endometrial intraepithelial neoplasia (EIN), with or without levonorgestrel IUD (intrauterine device) for patients wishing to preserve fertility or for those too ill for surgical management. Any progestin should be adequate for treatment of benign hyperplasia or for maintenance after resolution of endometrial intraepithelial neoplasia (EIN). Patient should be sampled to assess for response every 3 to 6 months for regression to normal endometrium. If there is inadequate response in 6 months, consider increasing dose or changing progestins. There is no proven protocol for selection or dosing. Continued surveillance after regression of the lesion is recommended every 6 to 12 months if risk factors persist. Repeat biopsy is also indicated for recurrent abnormal bleeding or discharge. Prevention of recurrence include use of daily or cyclic progesterone, indwelling levonorgestrel IUD, along with weight loss for obese patients.
Due to the large number of young anovulatory women diagnosed with atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) or early endometrial cancer, numerous studies have examined the outcome of fertility-sparing hormonal therapy. A meta-analysis of 24 observational studies that included a total of 151 women with atypical endometrial hyperplasia found that those who had fertility-sparing treatments had 86% regression, 26% relapse, and live birth rate of 26% 55.
Another review of complex atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) patients who underwent progestin therapy found a complete response rate of 66% 56. Median time to response was 6 months. Recurrence rate after initial response was 23%. During study follow-up time of 39 months, 41% of patients with atypical hyperplasia became pregnant 56.
The Gynecologic Oncology Group pathologic study with biopsy diagnosis of atypical hyperplasia found 42.6% concurrent endometrial carcinoma on hysterectomy specimen; 31% of these had myometrial invasion, including 10.6% with outer half myometrial invasion 16. Others also found atypical endometrial hyperplasia on office biopsy or D&C was associated with a 48-56% cancer rate on permanent pathology. Thus, if a hysterectomy is planned for treatment of atypical hyperplasia based on office endometrial biopsy, the authors recommend having a gynecologic oncologist be primary surgeon, or be available for surgical staging if needed based on frozen section of uterine specimen 57, 47. Patients should be counseled and consented for washings, hysterectomy, possible bilateral-salpingo-oophorectomy and lymph node dissection depending on intra-op exam or frozen pathology findings. If a D&C with hysteroscopy had been performed to rule out concurrent carcinoma more definitively, an oncologic surgeon is likely not needed. Due to the risk of cancer, supracervical, morcellation, or endometrial ablation is not recommended. While a simple hysterectomy is adequate for definitive treatment of hyperplasia, one can consider bilateral salpingo-oophorectomy in perimenopausal or postmenopausal women due to possibility of cancer on permanent section. Ovaries should only be removed if cancer is diagnosed in premenopausal women. They should be counseled a second surgery may be required to remove ovaries and perform lymph node staging if cancer if found on final pathology.
The need for hysterectomy to exclude concurrent myoinvasive endometrioid adenocarcinoma presents a barrier to nonsurgical management of endometrial hyperplasia. A Gynecologic Oncology Group study examined the histomorphometric 4-class rule (4C), which measures epithelial abundance, thickness, and nuclear variation as applied to diagnostic biopsies to predict myoinvasive cancer outcomes at hysterectomy 58. Women with endometrial biopsies or curettages with a community diagnosis of atypical endometrial hyperplasia were enrolled in a clinical trial in which subsequent hysterectomy was scored for endometrial adenocarcinoma, and 4C rule ability to predict cancer outcomes was measured. Qualifying biopsies were stratified into high-risk and low-risk histomorphometric subgroups 58. Assignment to a high-risk category by 4C rule was highly sensitive in predicting any (71%) or deeply (92%) myoinvasive adenocarcinoma at hysterectomy, and assignment to a low-risk group had a high negative predictive value for absence of any (90%) or deeply (99%) myoinvasive disease 58. At present, this use of histomorphometry is most suited to a centralized reference laboratory performing histomorphometry for a variety of diagnostic applications. However, in the future, formal histomorphometry of endometrial biopsies using the 4C rule may become a more common method to identify a subset of women with premalignant disease who are unlikely to have concurrent myoinvasive adenocarcinoma and therefore may qualify for nonsurgical therapy.
Endometrial hyperplasia prognosis
Several studies have shown that progestogen (synthetic forms of progesterone) therapy leads to a high rate of regression in endometrial hyperplasia without atypia (89% to 96%) 53, 49, 50. However, in the presence of endometrial intraepithelial neoplasia (EIN), there is a reduction in the success rate of progestogen therapy 16. The presence of atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) has a higher risk of progression to invasive malignancy – as high as 27.5% if not treated 59. Also, atypical endometrial hyperplasia or endometrial intraepithelial neoplasia (EIN) has a possibility of coexistent endometrial malignancy in 43% of cases 16.
Types of uterine cancer
Uterine cancers can be of two types: endometrial cancer (common) and uterine sarcoma (rare).
Endometrial cancer
Endometrial cancer (endometrial carcinoma) is cancer that begins in the layer of cells that form the lining (the endometrium) of the uterus (see Figures 1 and 3). Endometrial cancer makes up the majority of uterine cancers. Endometrial cancer is the fourth most common cancer in women in the United States after breast, lung, and colorectal cancers 1. Cancer of the endometrium is the most common gynecologic malignancy in the United States and accounts for 7% of all cancers in women. Endometrial cancer is often detected at an early stage, because it frequently produces abnormal vaginal bleeding such as vaginal bleeding after menopause or bleeding between periods, which prompts women to see their health care providers. If endometrial cancer is discovered early, removing the uterus surgically often cures endometrial cancer. The majority of endometrial cancer cases are diagnosed at an early stage and are amenable to treatment with surgery alone 2.
Endometrial cancer types
Endometrial cancer also called endometrial carcinoma, starts in the cells of the inner lining of the uterus (the endometrium). This is the most common type of cancer in the uterus 60.
Endometrial carcinomas can be divided into different types based on how the cells look under the microscope. These are called histologic types. They include:
- Adenocarcinoma (most endometrial cancers are a type of adenocarcinoma called endometrioid cancer or endometrial adenocarcinoma)
- Uterine carcinosarcoma
- Squamous cell carcinoma
- Small cell carcinoma
- Transitional carcinoma
- Serous carcinoma
Clear-cell carcinoma, mucinous adenocarcinoma, undifferentiated carcinoma, dedifferentiated carcinoma, and serous adenocarcinoma are less common types of endometrial adenocarcinomas. They tend to grow and spread faster than most types of endometrial cancer. They often have spread outside the uterus by the time they’re diagnosed.
Endometrioid adenocarcinoma
Endometrioid adenocarcinoma also called endometrial adenocarcinoma or endometrioid cancer, is the most common type of adenocarcinoma, by far. Endometrioid cancers start in gland cells and look a lot like the normal uterine lining (endometrium). Some of these cancers have squamous cells (squamous cells are flat, thin cells), as well as glandular cells.
There are many variants (or sub-types) of endometrioid cancers including:
- Adenocarcinoma, (with squamous differentiation)
- Adenoacanthoma
- Adenosquamous (or mixed cell)
- Secretory carcinoma
- Ciliated carcinoma
- Villoglandular adenocarcinoma
Endometrioid cancers are often diagnosed at an early stage and so are usually treated successfully.
Uterine carcinosarcoma
Uterine carcinosarcoma tumors are also known as malignant mixed mesodermal tumors or malignant mixed mullerian tumors. They make up about 3% of uterine cancers. Uterine carcinosarcoma starts in the endometrium and has features of both endometrial carcinoma and sarcoma. Sarcoma is cancer that starts in muscle cells of the uterus. In the past, uterine carcinosarcoma was considered a different type of uterine cancer called uterine sarcoma, but doctors now believe that uterine carcinosarcoma is an endometrial carcinoma that’s so abnormal it no longer looks much like the cells it came from (it’s poorly differentiated).
Uterine carcinosarcoma is a type 2 endometrial carcinoma.
Type 1 and type 2 endometrial cancer
Doctors sometimes divide endometrial cancers into 2 types 61.
- Type 1 endometrial cancers are the most common type representing more than 70% of cases. They are usually endometrioid adenocarcinomas and are associated with unopposed estrogen stimulation or due to excess estrogen in the body. They are generally low grade, slow growing and less likely to spread 62.
- Type 2 endometrial cancers are not linked to excess estrogen. Type 2 endometrial cancer accounts for only 10% of endometrial cancers, but it is associated with 40% of related deaths 62. Type 2 tumors are more likely to be high grade and of papillary serous or clear cell histologic type. They are generally faster growing, carry a poor prognosis and have a high risk of relapse and more likely to spread. They include uterine serous carcinomas and clear cell carcinomas.
Endometrial cancer grades
The grade of an endometrial cancer is based on how much the cancer cells are organized into glands that look like the glands found in a normal, healthy endometrium. The grade of an endometrial cancer gives your doctor an idea of how quickly or slowly the cancer might grow and whether it is likely to spread. In lower-grade cancers (grades 1 and 2), more of the cancer cells form glands. In higher-grade cancers (grade 3), more of the cancer cells are disorganized and do not form glands.
Grading is a way of dividing cancer cells into groups depending on how much the cells look like normal cells.
- Grade 1 endometrial cancers also called low grade or well differentiated, have 95% or more of the cancer tissue forming glands. They tend to be slow growing and are less likely to spread than higher grade cancer cells.
- Grade 2 endometrial cancers also called moderately differentiated or moderate grade, have between 50% and 94% of the cancer tissue forming glands.
- Grade 3 endometrial cancers also called poorly differentiated or high grade, have less than half of the cancer tissue forming glands. Grade 3 cancers tend to be aggressive (they grow and spread fast) and have a worse outlook than lower-grade cancers.
Grades 1 and 2 endometrioid cancers are type 1 endometrial cancers. Type 1 cancers are usually not very aggressive and they don’t spread to other tissues quickly. Type 1 endometrial cancers are thought to be caused by too much estrogen. They sometimes develop from atypical hyperplasia, an abnormal overgrowth of cells in the endometrium.
A small number of endometrial cancers are type 2 endometrial cancer. Type 2 cancers are more likely to grow and spread outside the uterus, they have a poorer outlook (than type 1 cancers). Doctors tend to treat these cancers more aggressively. They don’t seem to be caused by too much estrogen. Type 2 cancers include all endometrial carcinomas that aren’t type 1, such as papillary serous carcinoma, clear-cell carcinoma, undifferentiated carcinoma, and grade 3 endometrioid carcinoma. These cancers don’t look at all like normal endometrium and so are called poorly differentiated or high-grade.
Endometrial cancer causes
Scientists do not yet know exactly what causes most cases of endometrial cancer, but they do know there are risk factors, like obesity and hormone imbalance, that are strongly linked to endometrial cancer. A great deal of research is going on to learn more about endometrial cancer.
Scientists know that most endometrial cancer cells contain estrogen and/or progesterone receptors on their surfaces. Somehow, interaction of these receptors with their hormones leads to increased growth of the endometrium. This can mark the beginning of cancer. The increased growth can become more and more abnormal until it develops into a cancer.
As noted in the risk factors section below, many of the known endometrial cancer risk factors affect the balance between estrogen and progesterone in the body.
Scientists are learning more about changes (mutations) in the DNA of certain genes that occur when normal endometrial cells become cancerous. The mutation turns normal, healthy cells into abnormal cells. Healthy cells grow and multiply at a set rate, eventually dying at a set time. Abnormal cells grow and multiply out of control, and they don’t die at a set time. The accumulating abnormal cells form a mass (tumor). Cancer cells invade nearby tissues and can separate from an initial tumor to spread elsewhere in the body (metastasize).
Endometrial cancer risk factors
A risk factor is anything that affects your chance of getting a disease such as cancer. Different cancers have different risk factors. Some risk factors, like smoking, can be changed. Others, like a person’s age or family history, can’t be changed.
Although certain factors increase a woman’s risk for developing endometrial cancer, they do not always cause the disease. Many women with one or more risk factors never develop endometrial cancer.
Some women with endometrial cancer do not have any known risk factors. Even if a woman with endometrial cancer has one or more risk factors, there is no way to know which, if any, of these factors was responsible for her cancer.
Many factors affect the risk of developing endometrial cancer, including:
- Things that affect hormone levels, like taking estrogen after menopause, birth control pills, or tamoxifen; the number of menstrual cycles (over a lifetime), pregnancy, obesity, certain ovarian tumors, and polycystic ovarian syndrome (PCOS)
- Use of an intrauterine device (IUD)
- Never having been pregnant
- Age
- Obesity
- Diet and exercise
- Type 2 diabetes
- Family history (having close relatives with endometrial or colorectal cancer)
- Having been diagnosed with breast or ovarian cancer in the past
- Having been diagnosed with endometrial hyperplasia in the past
- Treatment with radiation therapy to the pelvis to treat another cancer
Some of these, like pregnancy, birth control pills, and the use of an intrauterine device are linked to a lower risk of endometrial cancer, while many are linked to a higher risk. These factors and how they affect endometrial cancer risk are discussed in more detail below.
Changes in the balance of female hormones in the body
A woman’s hormone balance plays a part in the development of most endometrial cancers. Many of the risk factors for endometrial cancer affect estrogen levels. Before menopause, the ovaries are the major source of the 2 main types of female hormones — estrogen and progesterone. The balance between these hormones changes each month during a woman’s menstrual cycle. This produces a woman’s monthly periods and keeps the endometrium healthy. A shift in the balance of these hormones toward more estrogen increases a woman’s risk for endometrial cancer. A disease or condition that increases the amount of estrogen, but not the level of progesterone, in your body can increase your risk of endometrial cancer. Examples include irregular ovulation patterns, which might happen in polycystic ovary syndrome, obesity and diabetes. A rare type of ovarian tumor that secretes estrogen also can increase the risk of endometrial cancer.
After menopause, the ovaries stop making these hormones, but a small amount of estrogen is still made naturally in fat tissue. Estrogen from fat tissue has a bigger impact after menopause than it does before menopause. Also taking hormones after menopause that contain estrogen but not progesterone increases the risk of endometrial cancer.
Estrogen therapy
Treating the symptoms of menopause with hormones is known as menopausal hormone therapy (or sometimes hormone replacement therapy [HRT]). Estrogen is the major part of this treatment. Estrogen treatment can help reduce hot flashes, improve vaginal dryness, and help prevent the weakening of the bones (osteoporosis) that can occur with menopause.
But using estrogen alone (without progesterone) can lead to endometrial cancer in women who still have a uterus. To lower that risk, a progestin (progesterone or a drug like it) must be given along with estrogen. This is called combination hormone therapy.
Women who take progesterone along with estrogen to treat menopausal symptoms do not have an increased risk of endometrial cancer. Still, taking this combination increases a woman’s chance of developing breast cancer and also increases the risk of serious blood clots.
If you are taking (or plan to take) hormones after menopause, it’s important to discuss the possible risks (including cancer, blood clots, heart attacks, and stroke) with your doctor.
Like any other medicine, hormones should be used at the lowest dose needed and for the shortest possible time to control symptoms. As with any other medicine you take for a long time, you’ll need to see your doctor regularly. Experts recommend yearly follow-up pelvic exams. If you have any abnormal bleeding or discharge from your vagina you should see a health care provider right away. Do not wait until your next check-up.
Birth control pills
Using birth control pills (oral contraceptives) lowers the risk of endometrial cancer. The risk is lowest in women who take the pill for a long time, and this protection lasts for at least 10 years after a woman stops taking the pill. But it’s important to look at all of the risks and benefits when choosing a contraceptive method; endometrial cancer risk is only one factor to consider. It’s a good idea to discuss the pros and cons of different types of birth control with your doctor.
Total number of menstrual cycles
Having more menstrual cycles during a woman’s lifetime raises her risk of endometrial cancer. Starting first menstrual periods (menarche) before age 12 and/or going through menopause later in life raises the risk. Starting periods early is less a risk factor for women with early menopause. Likewise, late menopause may not lead to a higher risk in women whose periods began later in their teens.
Never having been pregnant
The hormonal balance shifts toward more progesterone during pregnancy. So having many pregnancies helps protect against endometrial cancer. Women who have never been pregnant have a higher risk, especially if they were also infertile (unable to become pregnant).
Obesity
Obesity is a strong risk factor for endometrial cancer and linked to hormone changes. A woman’s ovaries produce most of her estrogen before menopause. But fat tissue can change some other hormones (called androgens) into estrogens. This can impact estrogen levels, especially after menopause. Having more fat tissue can increase a woman’s estrogen levels, which increases her endometrial cancer risk.
In comparison with women who stay at a healthy weight, endometrial cancer is twice as common in overweight women (BMI 25 to 29.9), and more than 3 times as common in obese women (BMI > 30).
Gaining weight as you get older age and weight cycling (gaining and losing a lot of weight many times in your life) have also been linked to a higher risk of endometrial cancer after menopause.
Tamoxifen
Tamoxifen is a drug that is used to help prevent and treat breast cancer. Tamoxifen acts as an anti-estrogen in breast tissue, but it acts like an estrogen in the uterus. In women who have gone through menopause, it can cause the uterine lining to grow, which increases the risk of endometrial cancer.
The risk of developing endometrial cancer from tamoxifen is low (less than 1% per year). Women taking tamoxifen must balance this risk against the benefits of this drug in treating and preventing breast cancer. This is an issue women should discuss with their providers. If you are taking tamoxifen, you should have yearly gynecologic exams and should be sure to report any abnormal bleeding, as this could be a sign of endometrial cancer.
Ovarian tumors
A certain type of ovarian tumor, the granulosa cell tumor, often makes estrogen. Estrogen made by one of these tumors isn’t controlled the way hormone release from the ovaries is, and it can sometimes lead to high estrogen levels. The resulting hormone imbalance can stimulate the endometrium and even lead to endometrial cancer. In fact, sometimes vaginal bleeding from endometrial cancer is the first symptom of one of these tumors.
Polycystic ovarian syndrome (PCOS)
Women with a condition called polycystic ovarian syndrome (PCOS) have abnormal hormone levels, such as higher androgen (male hormones) and estrogen levels and lower levels of progesterone. The increase in estrogen relative to progesterone can increase a woman’s chance of getting endometrial cancer. PCOS is also a leading cause of infertility in women.
Using an intrauterine device (IUD)
Women who used an intrauterine device (IUD) for birth control seem to have a lower risk of getting endometrial cancer. Information about this protective effect is limited to IUDs that do not contain hormones. Researchers have not yet studied whether newer types of IUDs that release progesterone have any effect on endometrial cancer risk. But these IUDs are sometimes used to treat pre-cancers and early endometrial cancers in women who wish to be able to get pregnant in the future.
Age
As you get older, your risk of endometrial cancer increases. Endometrial cancer occurs most often after menopause.
Diet and exercise
A high-fat diet can increase the risk of many cancers, including endometrial cancer. Because fatty foods are also high-calorie foods, a high-fat diet can lead to obesity, which is a well-known endometrial cancer risk factor. Many scientists think this is the main way in which a high-fat diet raises endometrial cancer risk. Some scientists think that fatty foods may also have a direct effect on how the body uses estrogen, which increases endometrial cancer risk.
Physical activity lowers the risk of endometrial cancer. Many studies have found that women who exercise more have a lower risk of endometrial cancer, while others suggest that women who spent more time sitting have a higher risk.
Diabetes
Endometrial cancer may be about twice as common in women with type 2 diabetes. But diabetes is more common in people who are overweight and less active, which are also risk factors for endometrial cancer. This makes it hard to find a clear link.
Family history
Endometrial cancer tends to run in some families. Some of these families also have a higher risk for colon cancer. This disorder is called hereditary nonpolyposis colon cancer (HNPCC) or Lynch syndrome. In most cases, hereditary nonpolyposis colon cancer (HNPCC) is caused by a defect in either the mismatch repair gene MLH1 or the gene MSH2. But at least 5 other genes can cause HNPCC: MLH3, MSH6, TGBR2, PMS1, and PMS2. An abnormal copy of any one of these genes reduces the body’s ability to repair damage to its DNA or control cell growth. This results in a very high risk of colon cancer, as well as a high risk of endometrial cancer. Women with this syndrome have a up to a 70% risk of developing endometrial cancer at some point. The risk for women in general is about 3%. The risk of ovarian cancer is also increased.
Some families have a higher rate of only endometrial cancer. These families may have a different genetic disorder that hasn’t been found yet.
Breast or ovarian cancer
Women who have had breast cancer or ovarian cancer may have an increased risk of endometrial cancer, too. Some of the dietary, hormone, and reproductive risk factors for breast and ovarian cancer also increase endometrial cancer risk.
Endometrial hyperplasia
Endometrial hyperplasia is an increased growth of the endometrium. Mild or simple hyperplasia, the most common type, has a very small risk of becoming cancer. It may go away on its own or after treatment with hormone therapy. If the hyperplasia is called “atypical,” it has a higher chance of becoming a cancer. Simple atypical hyperplasia turns into cancer in about 8% of cases if it’s not treated. Complex atypical hyperplasia has a risk of becoming cancer in up to 29% of cases if it’s not treated, and the risk of having an undetected endometrial cancer is even higher. For this reason, complex atypical hyperplasia is usually treated.
Prior pelvic radiation therapy
Radiation used to treat some other cancers can damage the DNA of cells, sometimes increasing the risk of a second type of cancer such as endometrial cancer.
Endometrial cancer prevention
Most cases of endometrial cancer cannot be prevented, but there are some things that may lower your risk of developing this disease.
To reduce your risk of endometrial cancer, you may wish to:
- Get to and stay at a healthy weight. One way to lower endometrial cancer risk is to do what you can to change your risk factors whenever possible. For example, women who are overweight or obese have up to 3½ times the risk of getting endometrial cancer compared with women at a healthy weight. Getting to and maintaining a healthy weight is one way to lower the risk of this cancer.
- Be physically active. Studies have also linked higher levels of physical activity to lower risks of endometrial cancer, so engaging in regular physical activity (exercise) may also be a way to help lower endometrial cancer risk. An active lifestyle can help you stay at a healthy weight, as well as lower the risk of high blood pressure and diabetes (other risk factors for endometrial cancer.
- Discuss pros and cons of hormone therapy with your doctor. Estrogen to treat the symptoms of menopause is available in many different forms like pills, skin patches, shots, creams, and vaginal rings. If you are thinking about using estrogen for menopausal symptoms, ask your doctor about how it will affect your risk of endometrial cancer. Progestins (progesterone-like drugs) can reduce the risk of endometrial cancer in women taking estrogen therapy, but this combination increases the risk of breast cancer. If you still have your uterus and are taking estrogen therapy, discuss this issue with your doctor.
- Consider taking birth control pills. Using oral contraceptives for at least one year may reduce endometrial cancer risk. The risk reduction is thought to last for several years after you stop taking oral contraceptives. Oral contraceptives have side effects, though, so discuss the benefits and risks with your doctor.
- Get treated for endometrial problems. Getting proper treatment of pre-cancerous disorders of the endometrium is another way to lower the risk of endometrial cancer. Most endometrial cancers develop over a period of years. Many are known to follow and possibly start from less serious abnormalities of the endometrium called endometrial hyperplasia. Some cases of hyperplasia will go away without treatment, but it sometimes needs to be treated with hormones or even surgery. Treatment with progestins and a dilation and curettage (D&C) or hysterectomy can prevent hyperplasia from becoming cancerous. Abnormal vaginal bleeding is the most common symptom of endometrial pre-cancers and cancers, and it needs to be reported and evaluated right away.
- Hereditary nonpolyposis colon cancer (HNPCC). Women with hereditary nonpolyposis colon cancer (HNPCC or Lynch syndrome) have a very high risk of endometrial cancer. Most experts recommend that a woman with HNPCC have her uterus, ovaries, and fallopian tubes removed (a hysterectomy and bilateral salpingo-oophorectomy) after she’s finished having children to prevent endometrial cancer.
Endometrial cancer signs and symptoms
There are a few symptoms that may point to endometrial cancer, but some are more common as this cancer becomes advanced.
Unusual vaginal bleeding, spotting, or other discharge
About 90% of women diagnosed with endometrial cancer have abnormal vaginal bleeding, such as a change in their periods or bleeding between periods or after menopause. This symptom can also occur with some non-cancerous conditions, but it is important to have a doctor look into any irregular bleeding right away. If you have gone through menopause already, it’s especially important to report any vaginal bleeding, spotting, or abnormal discharge to your doctor.
Non-bloody vaginal discharge may also be a sign of endometrial cancer. Even if you cannot see blood in the discharge, it does not mean there is no cancer. In about 10% of cases, the discharge associated with endometrial cancer is not bloody. Any abnormal discharge should be checked out by your doctor.
Pelvic pain, a mass, and weight loss
Pain in the pelvis, feeling a mass (tumor), and losing weight without trying can also be symptoms of endometrial cancer. These symptoms are more common in later stages of the disease. Still, any delay in seeking medical help may allow the disease to progress even further. This lowers the odds of treatment being successful.
Although any of these symptoms can be caused by things other than cancer, it’s important to have them checked out by a doctor.
Can endometrial cancer be found early?
In most cases, noticing any signs and symptoms of endometrial cancer, such as abnormal vaginal bleeding or discharge (that is increasing in amount, occurring between periods, or occurring after menopause), and reporting them right away to your doctor allows the disease to be diagnosed at an early stage. Early detection improves the chances that your cancer will be treated successfully. But some endometrial cancers may reach an advanced stage before signs and symptoms can be noticed.
Early detection tests (Screening)
Early detection (also called screening) refers to the use of tests to find a disease such as cancer in people who do not have symptoms of that disease.
Women at average endometrial cancer risk
At this time, there are no screening tests or exams to find endometrial cancer early in women who are at average endometrial cancer risk and have no symptoms.
The American Cancer Society recommends that, at menopause, all women should be told about the risks and symptoms of endometrial cancer and strongly encouraged to report any vaginal bleeding, discharge, or spotting to their doctor.
Women should talk to their doctors about getting regular pelvic exams. A pelvic exam can find some cancers, including some advanced uterine cancers, but it is not very effective in finding early endometrial cancers.
The Pap test, which screens women for cervical cancer, can occasionally find some early endometrial cancers, but it’s not a good test for this type of cancer.
Women at increased endometrial cancer risk
The American Cancer Society recommends that most women at increased risk should be informed of their risk and be advised to see their doctor whenever they have any abnormal vaginal bleeding. This includes women whose risk of endometrial cancer is increased due to increasing age, late menopause, never giving birth, infertility, obesity, diabetes, high blood pressure, estrogen treatment, or tamoxifen therapy.
Women who have (or may have) hereditary non-polyposis colon cancer (HNPCC, sometimes called Lynch syndrome) have a very high risk of endometrial cancer. If several family members have had colon or endometrial cancer, you might want to think about having genetic counseling to learn about your family’s risk of having HNPCC.
If you (or a close relative) have genetic testing and are found to have a mutation in one of the genes for HNPCC, you are at high risk of getting endometrial cancer.
The American Cancer Society recommends that women who have (or may have) HNPCC be offered yearly testing for endometrial cancer with endometrial biopsy beginning at age 35. Their doctors should discuss this test with them, including its risks, benefits, and limitations. This applies to women known to carry HNPCC-linked gene mutations, women who are likely to carry such a mutation (those with a mutation known to be present in the family), and women from families with a tendency to get colon cancer where genetic testing has not been done.
Another option for a woman who has (or may have) HNPCC would be to have a hysterectomy once she is done having children.
Endometrial cancer diagnosis
If you have any of the symptoms of endometrial cancer, you should see a doctor right away. Your doctor will ask you about your symptoms including what they are, when you get them and whether anything you do makes them better or worse. Your doctor will also do a physical exam and a pelvic exam.
Your doctor decide about whether to refer you for tests or to a specialist. If you are diagnosed with endometrial cancer you will have more tests to find out how big it is and whether it has spread. This is called staging.
Tests and procedures used to diagnose endometrial cancer include:
Pelvic examination
During a pelvic exam, your doctor carefully inspects the outer portion of your genitals (vulva), and then inserts two fingers of one hand into your vagina and simultaneously presses the other hand on your abdomen to feel your uterus and ovaries. He or she also inserts a device called a speculum into your vagina. The speculum opens your vagina so that your doctor can view your vagina and cervix for abnormalities.
Ultrasound
Ultrasound is often one of the first tests used to look at the uterus, ovaries, and fallopian tubes in women with a possible gynecologic problem. Ultrasound tests use sound waves to take pictures of parts of the body. A small instrument called a transducer or probe gives off sound waves and picks up the echoes as they bounce off the organs. A computer translates the echoes into pictures.
For a pelvic ultrasound, the transducer is placed on the skin of the lower part of the abdomen. Often, to get good pictures of the uterus, ovaries, and fallopian tubes, the bladder needs be full. That is why women getting a pelvic ultrasound are asked to drink lots of water before the exam.
A transvaginal ultrasound (TVUS) is often preferred for looking at the uterus. For this test, the TVUS probe (that works the same way as the ultrasound transducer) is put into the vagina. Images from the TVUS can be used to see if the uterus contains a mass (tumor), or if the endometrium is thicker than usual, which can be a sign of endometrial cancer. It may also help see if a cancer is growing into the muscle layer of the uterus (myometrium).
A small tube may be used to put salt water (saline) into the uterus before the ultrasound. This helps the doctor see the uterine lining more clearly. This procedure is called a saline infusion sonogram or hysterosonogram. Sonogram is another term for ultrasound.
Ultrasound can be used to see endometrial polyps (growths) , measure how thick the endometrium is, and can help doctors pinpoint the area they want to biopsy.
Endometrial tissue sampling
To find out exactly what kind of endometrial change is present, the doctor must take out some tissue so that it can be tested and looked at with a microscope. Endometrial tissue can be removed by endometrial biopsy or by dilation and curettage (D&C) with or without a hysteroscopy. A gynecologist usually does these procedures.
Endometrial biopsy
An endometrial biopsy is the most commonly performed test for endometrial cancer and is very accurate in postmenopausal women. It can be done in the doctor’s office. In this procedure a very thin flexible tube is inserted into the uterus through the cervix. Then, using suction, a small amount of endometrium is removed through the tube. The suctioning takes about a minute or less. The discomfort is similar to menstrual cramps and can be helped by taking a nonsteroidal anti-inflammatory drug such as ibuprofen before the procedure. Sometimes numbing medicine (local anesthetic) is injected into the cervix just before the procedure to help reduce the pain.
Hysteroscopy
For this technique doctors insert a tiny telescope (about 1/6 inch in diameter) into the uterus through the cervix. To get a better view of the inside of the uterus, the uterus is filled with salt water (saline). This lets the doctor see and biopsy anything abnormal, such as a cancer or a polyp. This is usually done using a local anesthesia (numbing medicine) with the patient awake.
Dilation and curettage (D&C)
If the endometrial biopsy sample doesn’t provide enough tissue, or if the biopsy suggests cancer but the results are uncertain, a D&C must be done. In this outpatient procedure, the opening of the cervix is enlarged (dilated) and a special instrument is used to scrape tissue from inside the uterus. This may be done with or without a hysteroscopy.
This procedure takes about an hour and may require general anesthesia (where you are asleep) or conscious sedation (given medicine into a vein to make you drowsy) either with local anesthesia injected into the cervix or a spinal (or epidural). A D&C is usually done in an outpatient surgery area of a clinic or hospital. Most women have little discomfort after this procedure.
Testing endometrial tissue samples
Endometrial tissue samples removed by biopsy or D&C are looked at with a microscope to see whether cancer is present. If cancer is found, the lab report will state what type of endometrial cancer it is (like endometrioid or clear cell) and what grade it is.
Endometrial cancer is graded on a scale of 1 to 3 based on how much it looks like normal endometrium. Women with lower grade cancers are less likely to have advanced disease or recurrences.
Testing for gene and protein changes in the cancer cells
If the doctor suspects hereditary non-polyposis colon cancer (HNPCC) as an underlying cause of the endometrial cancer, the tumor cells can be tested for protein and gene changes. Examples of HNPCC-related changes include:
- Having fewer mismatch repair (MMR) proteins
- Defects in mismatch repair genes (dMMR)
- DNA changes (high levels of microsatellite instability, or MSI-H) that can happen when one of the genes that causes HNPCC is faulty
If these protein or DNA changes are present, the doctor may suggest genetic testing for the genes that cause HNPCC.
Testing the cancer cells for dMMR, MSI-H, and/or a high tumor mutational burden (TMB-H) might also be done to see if treatment with immunotherapy might be an option, especially for more advanced endometrial cancers.
Tests to look for cancer spread
If the doctor suspects that your cancer is advanced, you will probably have to have other tests to look for cancer spread.
Chest x-ray
A plain x-ray of your chest may be done to see if cancer has spread to your lungs.
Computed tomography (CT)
The CT scan is an x-ray procedure that creates detailed, cross-sectional images of your body. For a CT scan, you lie on a table while an X-ray takes pictures. Instead of taking one picture, like a standard x-ray, a CT scanner takes many pictures as the camera rotates around you. A computer then combines these pictures into an image of a slice of your body. The machine will take pictures of many slices of the part of your body that is being studied.
CT scans are not used to diagnose endometrial cancer. However, they may be helpful to see whether the cancer has spread to other organs and to see if the cancer has come back after treatment.
Magnetic resonance imaging (MRI)
MRI scans use radio waves and strong magnets instead of x-rays. The energy from the radio waves is absorbed and then released in a pattern formed by the type of tissue and by certain diseases. A computer translates the pattern of radio waves given off by the tissues into a very detailed image of parts of the body. This creates cross sectional slices of the body like a CT scanner and it also produces slices that are parallel with the length of your body.
MRI scans are particularly helpful in looking at the brain and spinal cord. Some doctors also think MRI is a good way to tell whether, and how far, the endometrial cancer has grown into the body of the uterus. MRI scans may also help find enlarged lymph nodes with a special technique that uses very tiny particles of iron oxide. These are given into a vein and settle into lymph nodes where they can be spotted by MRI.
Positron emission tomography (PET)
In this test radioactive glucose (sugar) is given to look for cancer cells. Because cancers use glucose (sugar) at a higher rate than normal tissues, the radioactivity will tend to concentrate in the cancer. A scanner can spot the radioactive deposits. This test can be helpful for spotting small collections of cancer cells. Special scanners combine a PET scan with a CT to more precisely locate areas of cancer spread. PET scans are not a routine part of the work-up of early endometrial cancer, but may be used for more advanced cases.
Cystoscopy and proctoscopy
If a woman has problems that suggest the cancer has spread to the bladder or rectum, the inside of these organs will probably be looked at through a lighted tube. In cystoscopy the tube is placed into the bladder through the urethra. In proctoscopy the tube is placed in the rectum. These exams allow the doctor to look for possible cancers. Small tissue samples can also be removed during these procedures for pathologic (microscopic) testing. They can be done using a local anesthetic but some patients may require general anesthesia. Your doctor will let you know what to expect before and after the procedure. These procedures were used more often in the past, but now are rarely part of the work up for endometrial cancer.
Blood tests
Complete blood count
The complete blood count (CBC) is a test that measures the different cells in the blood, such as the red blood cells, the white blood cells, and the platelets. Endometrial cancer can cause bleeding, which can lead to low red blood cell counts ( anemia).
CA-125 blood test
Cancer antigen 125 (CA-125) is a protein that may be found at higher levels in people with certain types of cancer and other health conditions. The CA-125 test checks the levels of this protein in the blood. CA-125 is a substance released into the bloodstream by many, but not all, endometrial and ovarian cancers. If a woman has endometrial cancer, a very high blood CA-125 level suggests that the cancer has probably spread beyond the uterus. Some doctors check CA-125 levels before surgery or other treatment. If they are elevated, they can be checked again to find out how well the treatment is working (for example, levels will drop after surgery if all the cancer is removed).
CA-125 levels are not needed to diagnose endometrial cancer, and so this test isn’t ordered on all patients.
Endometrial cancer staging
After a woman is diagnosed with endometrial cancer, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes the amount of cancer in the body. It helps determine how serious the cancer is and how best to treat it. The stage is one of the most important factors in deciding how to treat the cancer and determining how successful the treatment might be.
Endometrial cancer stages range from stage I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV (4), means cancer has spread more. And within a stage, an earlier letter means a lower stage. Although each person’s cancer experience is unique, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
How is the stage determined?
The 2 systems used for staging endometrial cancer, the FIGO (International Federation of Gynecology and Obstetrics) system and the American Joint Committee on Cancer (AJCC) TNM staging system are basically the same.
They both stage (classify) this cancer based on 3 factors:
- The extent (size) of the tumor (T): How far has the cancer grown into the uterus? Has the cancer reached nearby structures or organs?
- The spread to nearby lymph nodes (N): Has the cancer spread to the lymph nodes in the pelvis or around the aorta (the main artery that runs from the heart down along the back of the abdomen and pelvis). Also called para-aortic lymph nodes.
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant lymph nodes or distant organs?
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
The staging system in the table below uses the pathologic stage (also called the surgical stage). It is determined by examining tissue removed during an operation. This is also known as surgical staging. Sometimes, if surgery is not possible right away, the cancer will be given a clinical stage instead. This is based on the results of a physical exam, biopsy, and imaging tests done before surgery. For more information see Cancer Staging.
The system described below is the most recent AJCC system. It went into effect January 2018.
Endometrial cancer staging can be complex, so ask your doctor to explain it to you in a way you understand.
Table 2. Endometrial cancer staging
| TNM Stage | Stage grouping | FIGO Stage | Stage description* |
|---|---|---|---|
| 1 | T1 N0 M0 | 1 | The cancer is growing inside the uterus. It may also be growing into the glands of the cervix, but not into the supporting connective tissue of the cervix (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 1A | T1a N0 M0 | 1A | The cancer is in the endometrium (inner lining of the uterus) and may have grown less than halfway through the underlying muscle layer of the uterus (the myometrium) (T1a). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 1B | T1b N0 M0 | 1B | The cancer has grown from the endometrium into the myometrium. It has grown more than halfway through the myometrium, but has not spread beyond the body of the uterus (T1b). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 2 | T2 N0 M0 | 2 | The cancer has spread from the body of the uterus and is growing into the supporting connective tissue of the cervix (called the cervical stroma). But it has not spread outside the uterus (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3 | T3 N0 M0 | 3 | The cancer has spread outside the uterus, but has not spread to the inner lining of the rectum or urinary bladder (T3). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3A | T3a N0 M0 | 3A | The cancer has spread to the outer surface of the uterus (called the serosa) and/or to the fallopian tubes or ovaries (the adnexa) (T3a). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3B | T3b N0 M0 | 3B | The cancer has spread to the vagina or to the tissues around the uterus (the parametrium) (T3b). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3C1 | T1-T3 N1, N1mi or N1a M0 | 3C1 | The cancer is growing in the body of the uterus. It may have spread to some nearby tissues, but is not growing into the inside of the bladder or rectum (T1 to T3). It has also spread to pelvic lymph nodes (N1, N1mi, or N1a), but not to lymph nodes around the aorta or distant sites (M0). |
| 3C2 | T1-T3 N2, N2mi or N2a M0 | 3C2 | The cancer is growing in the body of the uterus. It may have spread to some nearby tissues, but is not growing into the inside of the bladder or rectum (T1 to T3). It has also spread to lymph nodes around the aorta (para-aortic lymph nodes) (N2, N2mi, or N2a), but not to distant sites (M0). |
| 4A | T4 Any N M0 | The cancer has spread to the inner lining of the rectum or urinary bladder (called the mucosa) (T4). It may or may not have spread to nearby lymph nodes (Any N), but has not spread to distant sites (M0). | |
| 4B | Any T Any N M1 | 4B | The cancer has spread to inguinal (groin) lymph nodes, the upper abdomen, the omentum, or to organs away from the uterus, such as the lungs, liver, or bones (M1). The cancer can be any size (Any T) and it might or might not have spread to other lymph nodes (Any N). |
Footnotes: *The following additional categories are not listed on the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Stage 1 endometrial cancer
Stage 1 endometrial cancers are early cancers and the easiest to treat. The cancer is within the uterus. Stage 1 is divided into stage 1A and 1B.
- Stage 1A endometrial cancer means that the cancer may have grown into the muscle wall (myometrium) of the womb, but no more than halfway
- Stage 1B endometrial cancer means the cancer has grown halfway or more into the muscle wall of the uterus
Stage 2 endometrial cancer
Stage 2 endometrial cancer means the cancer has grown into the cervix.
[Source 65 ]Stage 3 endometrial cancer
Stage 3 endometrial cancer means the cancer has spread outside the womb, but is still within the pelvis. Your doctor may call this locally advanced womb cancer. There are 3 categories of stage 3 endometrial cancer:
- Stage 3A endometrial cancer means the cancer has grown into the outer covering of the uterus (the serosa) and/or to the ovaries, fallopian tubes or ligaments of the uterus.
- Stage 3B endometrial cancer means the cancer has grown into the vagina or to the connective tissue and fat around the uterus and cervix (the parametrium)
- Stage 3C endometrial cancer means the cancer has spread to nearby lymph nodes (glands)
Stage 4 metastatic endometrial cancer
Stage 4 endometrial cancer means the cancer has spread to another area of the body. There are 2 categories of stage 4 endometrial cancer:
- Stage 4A endometrial cancer means the cancer has grown into the bowel or bladder
- Stage 4B endometrial cancer means the cancer has spread to lymph nodes further away or to other parts of the body, such as the lungs, liver, bones or brain (secondary cancers or metastases)
Endometrial cancer survival rates
Survival rates tell you what percentage of people with the same type and stage of cancer are still alive a certain length of time (usually 5 years) after they were diagnosed. These numbers can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful.
Statistics on the outlook for a certain type and stage of cancer are often given as 5-year survival rates, but many people live longer – often much longer – than 5 years. The 5-year survival rate is the percentage of people who live at least 5 years after being diagnosed with cancer.
Survival rates are estimates – your outlook can vary based on a number of factors specific to you.
Survival rates don’t tell the whole story
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. Your doctor can tell you how the numbers below may apply to you, as he or she is familiar with the aspects of your particular situation.
The survival rates below are based on the stage of the cancer at the time it was diagnosed. These rates do not apply to cancers that have come back after treatment or have spread after treatment starts.
The numbers below come from the National Cancer Data Base as published in the AJCC Staging Manual in 2017, and are based on people diagnosed between 2000 and 2002.
Endometrial cancer survival rates
- The 5-year survival rate for women with stage 0 endometrial cancer is 90%*
- The 5-year survival rate for women with stage 1A endometrial cancer is 88%
- The 5-year survival rate for women with stage 1B endometrial cancer is 75%
- The 5-year survival rate for women with stage 2 endometrial cancer is 69%
- The 5-year survival rate for women with stage 3A endometrial cancer is 58%
- The 5-year survival rate for women with stage 3B endometrial cancer is 50%
- The 5-year survival rate for women with stage 3C endometrial cancer is 47%
- The 5-year survival rate for women with stage 4A endometrial cancer is 17%
- The 5-year survival rate for women with stage 4B endometrial cancer is 15%
*The new staging system that went into effect January 2018 no longer includes Stage 0 cancers.
Endometrial cancer Survival by stage
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database tracks 5-year relative survival rates for endometrial cancer in the United States, based on how far the cancer has spread 4. The SEER database, however, does not group cancers by American Joint Committee on Cancer (AJCC) TNM Staging System stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized (sometimes referred to as stage 1): There is no sign the cancer has spread outside of the uterus.
- Regional: The cancer has spread outside the uterus to nearby structures or lymph nodes.
- Distant: The cancer has spread to distant parts of the body such as the lungs, liver, or bones.
Table 3. 5-year relative survival rates for endometrial cancer
| SEER stage | 5-year relative survival rate |
|---|---|
| Localized | 94.9% |
| Regional | 69.8% |
| Distant | 18.4% |
| All SEER stages combined | 52.2% |
Footnotes:
- SEER = Surveillance, Epidemiology, and End Results. The SEER database is maintained by the National Cancer Institute (NCI), to provide survival statistics for different types of cancer.
- These numbers are based on people diagnosed with endometrial cancer between 2012–2018
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, how well the cancer responds to treatment, and other factors can also affect your outlook.
- People now being diagnosed with endometrial cancer may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Keep in mind that survival rates are estimates and are often based on previous outcomes of large numbers of people who had a specific cancer, but they can’t predict what will happen in any particular person’s case. These statistics can be confusing and may lead you to have more questions. Your doctor knows your situation, so ask them how these numbers might apply to you.
[Source 4 ]Generally for women with endometrial cancer in England between 2013 and 2017 66:
- 90 out of every 100 (90%) survive their cancer for 1 year or more after they are diagnosed
- around 75 out of every 100 (around 75%) will survive their cancer for 5 years or more
- more than 70 out of every 100 (more than 70%) will survive their cancer for 10 years or more after diagnosis
Endometrial cancer treatment
Treatment for endometrial cancer will be determined by your doctor(s) based on:
- Your overall health and medical history
- Extent of the disease (the stage)
- Your tolerance for specific medications, procedures or therapies
- Expectations for the course of the disease
- Your opinion or preference
The choice of treatment for endometrial cancer depends on the stage of cancer — whether it is only in the endometrium, or if it has spread to other parts of the uterus or body. Most people will be treated with surgery first to remove the uterus, fallopian tubes and ovaries. Some may need additional therapy. Another option is radiation therapy with energy beams, such as X-rays and protons, to kill cancer cells. Drug treatments for endometrial cancer include chemotherapy with powerful drugs and hormone therapy to block hormones that cancer cells rely on. Other options might be targeted therapy with drugs that attack specific weaknesses in the cancer cells and immunotherapy to help your immune system fight cancer.
Generally, treatment for people with cancer of the endometrium includes one or more of the following.
- Surgery. Surgery to remove the uterus (hysterectomy) is recommended for most women with endometrial cancer. Most women with endometrial cancer undergo a procedure to remove the uterus (hysterectomy), as well as to remove the fallopian tubes and ovaries (salpingo-oophorectomy). A hysterectomy makes it impossible for you to have children in the future. Also, once your ovaries are removed, you’ll experience menopause, if you haven’t already. During surgery, your surgeon will also inspect the areas around your uterus to look for signs that cancer has spread. Your surgeon may also remove lymph nodes for testing. This helps determine your cancer’s stage. The surgeon who does the surgery is usually a specialist surgeon called a gynecological oncologist.
- Radiation therapy. Radiation therapy uses powerful energy beams, such as X-rays and protons, to kill cancer cells. In some instances, your doctor may recommend radiation to reduce your risk of a cancer recurrence after surgery. In certain situations, radiation therapy may also be recommended before surgery, to shrink a tumor and make it easier to remove. If you aren’t healthy enough to undergo surgery, you may opt for radiation therapy only. Radiation therapy can involve:
- Radiation from a machine outside your body. During external beam radiation, you lie on a table while a machine directs radiation to specific points on your body.
- Radiation placed inside your body. Internal radiation (brachytherapy) involves placing a radiation-filled device, such as small seeds, wires or a cylinder, inside your vagina for a short period of time.
- Chemotherapy. Chemotherapy uses chemicals to kill cancer cells. You may receive one chemotherapy drug, or two or more drugs can be used in combination. You may receive chemotherapy drugs by pill (orally) or through your veins (intravenously). These drugs enter your bloodstream and then travel through your body, killing cancer cells. Chemotherapy is sometimes recommended after surgery if there’s an increased risk that the cancer might return. It can also be used before surgery to shrink the cancer so that it’s more likely to be removed completely during surgery. Chemotherapy may be recommended for treating advanced or recurrent endometrial cancer that has spread beyond the uterus.
- Hormone therapy. Hormone therapy involves taking medications to lower the hormone levels in the body. In response, cancer cells that rely on hormones to help them grow might die. Hormone therapy may be an option if you have advanced endometrial cancer that has spread beyond the uterus.
- Targeted drug therapy. Targeted drug treatments focus on specific weaknesses present within cancer cells. By blocking these weaknesses, targeted drug treatments can cause cancer cells to die. Targeted drug therapy is usually combined with chemotherapy for treating advanced endometrial cancer.
- Immunotherapy. Immunotherapy is a drug treatment that helps your immune system to fight cancer. Your body’s disease-fighting immune system might not attack cancer because the cancer cells produce proteins that blind the immune system cells. Immunotherapy works by interfering with that process. For endometrial cancer, immunotherapy might be considered if the cancer is advanced and other treatments haven’t helped.
- Supportive (palliative) care. Palliative care is specialized medical care that focuses on providing relief from pain and other symptoms of a serious illness. Palliative care specialists work with you, your family and your other doctors to provide an extra layer of support that complements your ongoing care. Palliative care can be used while undergoing other aggressive treatments, such as surgery, chemotherapy or radiation therapy. When palliative care is used along with all of the other appropriate treatments, people with cancer may feel better and live longer. Palliative care is provided by a team of doctors, nurses and other specially trained professionals. Palliative care teams aim to improve the quality of life for people with cancer and their families. This form of care is offered alongside curative or other treatments you may be receiving.
Uterine sarcoma
Uterine sarcoma is a rarer type of uterine cancer that develops in the muscles of the uterus (the myometrium) or other tissues that support the uterus such as fat, bone, and fibrous tissue (the material that forms tendons and ligaments). Sarcoma accounts for about 2% to 4% of uterine cancers. Subtypes of endometrial sarcoma include uterine leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated sarcoma.
Uterine sarcoma types
Most uterine sarcomas are put into categories, based on the type of cell they start in. Subtypes of endometrial sarcoma include uterine leiomyosarcoma, endometrial stromal sarcoma, and undifferentiated sarcoma.
Uterine leiomyosarcoma
Uterine leiomyosarcoma (LMS) starts in the muscular wall of the uterus (the myometrium). Uterine leiomyosarcoma is by far the most common type of uterine sarcoma. These tumors can grow and spread quickly.
Endometrial stromal sarcoma
Endometrial stromal sarcoma (ESS) tumors start in the supporting connective tissue (stroma) of the lining of the uterus (the endometrium). These cancers are rare.
If the tumor is low grade, the cancer cells do not look very different from normal cells and the tumor tends to grow slowly. Women with low-grade endometrial stromal sarcoma (ESS) have a better outlook (prognosis) than women with other kinds of uterine sarcomas.
High-grade endometrial stromal sarcoma (ESS) means the cancer cells look very different from normal cells, and the tumor is growing quickly. This type of endometrial stromal sarcoma (ESS) is most often found when the tumor is already large and/or has spread. These tumors are hard to treat.
Undifferentiated sarcoma
Undifferentiated sarcomas may start in the endometrium or the myometrium. They grow and spread quickly and tend to have a poor outlook.
Uterine sarcoma signs and symptoms
About 85% of patients diagnosed with uterine sarcomas have abnormal vaginal bleeding, such as a change in their periods or bleeding between periods or after menopause. This symptom is more often caused by something other than cancer, but it is important to have a doctor look into any irregular bleeding right away. If you have gone through menopause already, it’s especially important to report any vaginal bleeding, spotting, or abnormal discharge to your doctor.
Vaginal discharge
About 10% of women with uterine sarcomas have a vaginal discharge that does not have any visible blood. A discharge is most often a sign of infection or another non-cancer condition, but it also can be a sign of cancer. Any abnormal discharge should be checked by a health care professional.
Pelvic pain and/or pelvic a mass
When they’re first diagnosed, about 10% of women with uterine sarcomas have pelvic pain and/or a mass (tumor) that can be felt. You or your doctor may be able to feel the mass in your uterus, or you might have a feeling of fullness in your belly and/or pelvis.
Can uterine sarcoma be found early?
In some cases, knowing the signs and symptoms of uterine sarcoma and seeing a health care professional right away can help find it at an early stage (when it’s small and hasn’t spread). But many uterine sarcomas reach an advanced stage before signs and symptoms are present. The signs and symptoms for the main types of uterine sarcoma are different.
Early detection tests (Screening)
Screening refers to testing to find a disease such as cancer in people who don’t have symptoms of the disease. At this time, there are no tests or exams to detect uterine sarcomas in women without symptoms (asymptomatic women). Screening tests used for cervical cancer, such as a Pap test or HPV (human papillomavirus) test aren’t effective tests for uterine sarcomas. The Pap test, which screens for cervical cancer, can sometimes find early uterine sarcomas, but it’s not a good test for this type of cancer. Still, the Pap test is very good at finding early carcinomas of the cervix (the lower part of the uterus).
Uterine sarcoma causes
Doctors don’t know exactly what causes most uterine sarcomas, but certain risk factors have been identified. Research is helping to learn more about this rare disease.
Uterine sarcoma risk factors
A risk factor is anything that affects your chance of getting a disease such as cancer. Different cancers have different risk factors.
The following risk factors are known to change a woman’s risk of developing a uterine sarcoma 67:
- Pelvic radiation therapy. High-energy (ionizing) radiation used to treat some cancers can damage cells’ DNA, sometimes increasing the risk of developing a second type of cancer. If you’ve had pelvic radiation, your risk for developing uterine sarcoma is increased. These cancers usually are diagnosed 5 to 25 years after you’ve been exposed to the radiation.
- Race. Uterine sarcomas are about twice as common in African American women as they are in white or Asian women. The reason for this is unknown.
- Retinoblastoma (RB) gene changes. Women who have had a type of eye cancer called retinoblastoma that was caused by being born with an abnormal copy of the RB gene have an increased risk of uterine leiomyosarcomas. The RB gene provides instructions for making a protein called pRB 68. This protein acts as a tumor suppressor, which means that it regulates cell growth and keeps cells from dividing too fast or in an uncontrolled way. Under certain conditions, pRB stops other proteins from triggering DNA replication, the process by which DNA makes a copy of itself. Because DNA replication must occur before a cell can divide, tight regulation of this process controls cell division and helps prevent the growth of tumors. Additionally, pRB interacts with other proteins to influence cell survival, the self-destruction of cells (apoptosis), and the process by which cells mature to carry out special functions (differentiation).
Remember, that these factors increase the risk for developing some uterine sarcomas, but they may not always cause the disease.
Uterine sarcoma prevention
Most cases of uterine sarcoma cannot be prevented. Although pelvic radiation increases the risk of developing a uterine sarcoma, the benefit of pelvic radiation in treating other cancers far outweighs the risk of developing a rare cancer such as uterine sarcoma many years later.
Uterine sarcoma diagnosis
Many uterine sarcomas are diagnosed during or after surgery for what’s thought to be benign fibroid tumors 69. Some are diagnosed because of symptoms. If you have symptoms of uterine cancer, the first step is to see your doctor.
Consultation, medical history, and physical exam
Your doctor will ask you about your personal and family medical history, examine you, and might order some tests. You also will be asked about any symptoms, risk factors, and other health problems. A general physical and a pelvic exam will be done. An ultrasound may be used to look at the inside of your uterus.
If your doctor suspects cancer, you may be referred to a gynecologist or a doctor specializing in cancers of the female reproductive system (called a gynecologic oncologist).
Sampling and testing endometrial tissue
To find the cause of abnormal uterine bleeding, a small piece of tissue (a sample) will be taken from the lining of the uterus and looked at with a microscope. The tissue can be removed by endometrial biopsy or by dilation and curettage (D&C). Often a hysteroscopy is done with the D&C..
These procedures let the doctor see if the bleeding is caused by a endometrial overgrowth that’s not cancer (hyperplasia), endometrial carcinoma, uterine sarcoma, or some other problem. The tests will find many endometrial stromal sarcomas and undifferentiated sarcomas, but less than half of leiomyosarcomas (abbreviated LMSs). These tests don’t find all leiomyosarcomas because these cancers start in the muscle layer of the wall of the uterus. To be found by an endometrial biopsy or D&C, they need to have spread from the middle (muscle) layer to the inner lining of the uterus. In most cases, the only way to diagnose a leiomyosarcomas by removing it with surgery.
Endometrial biopsy
In this procedure, a very thin, flexible tube is put into the uterus through the cervix. Then, using suction, a small amount of the uterine lining (endometrium) is taken out through the tube. Suctioning takes about a minute or less. The discomfort is a lot like severe menstrual cramps and can be helped by taking a nonsteroidal anti-inflammatory drug like ibuprofen an hour before the biopsy. This procedure is usually done in the doctor’s office.
Hysteroscopy
This procedure allows doctors to look inside the uterus. A tiny telescope is put into the uterus through the cervix. To get a better view, the uterus is then expanded by filling it with salt water (saline). This lets the doctor see and take out anything abnormal, such as a cancer or a polyp. This procedure is usually done with the patient awake, using local anesthesia (numbing medicine). But if a polyp or mass has to be removed, general or regional anesthesia is sometimes used. (General anesthesia means you are given drugs that put you into a deep sleep and keep you from feeling pain. Regional anesthesia is a nerve block that numbs a larger area of the body).
Dilation and curettage
If the results of the endometrial biopsy are not clear (meaning they can’t tell for sure if cancer is present), a procedure called dilation and curettage (D&C) must be done. A D&C is usually done in the outpatient surgery area of a clinic or hospital. It’s done while the woman is under general or regional anesthesia or conscious sedation (medicine is given into a vein to make her drowsy). It takes about an hour. In a D&C, the cervix is dilated (opened) and a special surgical tool is used to scrape the endometrial tissue from inside the uterus. A hysteroscopy may be done as well. Some women have mild to moderate cramping and discomfort after this procedure.
Testing endometrial tissue
Any tissue samples taken out are looked at under a microscope to see if cancer is present. If cancer is found, the lab report will say if it’s a carcinoma or sarcoma, what type it is, and its grade.
A tumor’s grade is based on how much it looks like normal tissue under the microscope. If the tumor looks a lot like normal tissue, it’s called low grade. If it doesn’t at all look like normal tissue, it’s high grade. The rate at which the cancer cells appear to be growing is another important factor in grading a uterine sarcoma. High-grade sarcomas tend to grow and spread faster than low-grade sarcomas.
The tissue may also be tested to see if the cancer cells have estrogen receptors and progesterone receptors. These hormone receptors are found on many endometrial stromal sarcomas. Cancers with estrogen receptors on the cells are more likely to grow in response to estrogen, while those with progesterone receptors often have their growth decreased by progesterone. These cancers may stop growing (or even shrink) when treated with certain hormone drugs. Checking for these receptors helps predict which patients will benefit from treatment with these drugs.
Cystoscopy and proctoscopy
If a woman has signs or symptoms that suggest uterine sarcoma has spread to the bladder or rectum, the inside of these organs can be looked at through a lighted tube. These exams are called cystoscopy and proctoscopy, respectively. They are rarely done in the diagnosis and work-up of patients with uterine sarcoma.
Imaging tests
- Transvaginal ultrasound. Ultrasound tests use sound waves to take pictures of parts of the body. For a transvaginal ultrasound, a probe that gives off sound waves is put into the vagina. The sound waves are used to create images of the uterus and other pelvic organs. These images can often show if there’s a tumor and if it affects the myometrium (muscular layer of the uterus). For an ultrahysterosonogram or saline infusion sonogram, salt water (saline) is put into the uterus through a small tube before the transvaginal sonogram. This allows the doctor to see changes in the uterine lining more clearly.
- Computed tomography (CT). The CT scan is an x-ray test that produces detailed cross-sectional images of your body. Instead of taking one picture, like a standard x-ray, a CT scanner takes many pictures as it rotates around you. A computer then combines these pictures into an image of a slice of your body. CT scans are rarely used to diagnose uterine cancer, but they may be helpful in seeing if the cancer has spread to other organs.
- CT-guided needle biopsy: CT scans can also be used to guide a biopsy needle precisely into a suspected tumor. For this procedure, the patient remains on the CT scanning table while the doctor moves a biopsy needle through the skin and toward the tumor. CT scans are repeated until the needle is within the tumor. A fine needle biopsy sample or a larger core needle biopsy sample is then removed to be looked at with a microscope. This isn’t done to biopsy tumors in the uterus, but might be used to biopsy areas that look like metastasis (cancer spread).
- Magnetic resonance imaging (MRI). MRI scans use radio waves and strong magnets instead of x-rays. The energy from the radio waves is absorbed and then released in a pattern formed by the type of tissue and by certain diseases. A computer translates the pattern of radio waves given off by the tissues into a very detailed image of parts of the body. MRI scans can help tell if a uterine tumor looks like cancer, but a biopsy is still needed to tell for sure. MRI scans are also very helpful in looking for cancer that has spread to the brain and spinal cord.
- Positron emission tomography (PET) scan. In a PET scan, radioactive glucose (sugar) is injected into the patient’s vein. Because many cancers use glucose much faster than normal tissues, the radioactivity tends to collect in the cancer. A scanner can then spot the radioactive deposits. This test can be helpful for spotting small collections of cancer cells that have spread beyond the uterus (metastasized).
- Chest x-ray. A regular (plain) x-ray of the chest may be done to see if a uterine sarcoma has spread to the lungs and as part of the testing before surgery.
Uterine sarcoma stages
After a woman is diagnosed with uterine sarcoma, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes the amount of cancer in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about survival statistics.
Uterine sarcoma stages range from stage I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV, means cancer has spread more. And within a stage, an earlier letter means a lower stage. Although each person’s cancer experience is unique, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
The 2 systems used for staging uterine sarcoma are the FIGO (International Federation of Gynecology and Obstetrics) system and the American Joint Committee on Cancer (AJCC) TNM staging system, which are basically the same.
They both stage (classify) uterine sarcoma based on 3 factors:
- The extent (size) of the tumor (T): How large is the cancer? Has the cancer grown out of the uterus into the pelvis or organs such as the bladder or rectum?
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant lymph nodes or organs?
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
The staging system in the table below uses the pathologic stage also called the surgical stage. It is determined by examining tissue removed during an operation. Sometimes, if surgery is not possible right away, the cancer will be given a clinical stage instead. This is based on the results of a physical exam, biopsy, and imaging tests done before surgery.
The system described below is the most recent American Joint Committee on Cancer (AJCC) TNM system. It went into effect January 2018. It is specific for staging two types of uterine sarcomas: leiomyosarcoma and endometrial stromal sarcoma.
Uterine sarcoma staging can be complex, so ask your doctor to explain it to you in a way you understand.
Table 4. Uterine cancer staging
| TNM Stage | Stage grouping | FIGO Stage | Stage description* |
|---|---|---|---|
| 1 | T1 N0 M0 | 1 | The cancer is growing in the uterus, but has not started growing outside the uterus. It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 1A | T1a N0 M0 | 1A | The cancer is only in the uterus and is no larger than 5 cm across (about 2 inches) (T1a). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 1B | T1b N0 M0 | 1B | The cancer is only in the uterus and is larger than 5 cm across (about 2 inches). (T1b). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 2 | T2 N0 M0 | 2 | The cancer is growing outside the uterus but is not growing outside of the pelvis (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3A | T3a N0 M0 | 3A | The cancer is growing into tissues of the abdomen in one place only (T3a). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3B | T3b N0 M0 | 3B | The cancer is growing into tissues of the abdomen in 2 or more places (T3b). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3C | T1-T3 N1 M0 | 3C | The cancer is growing in the body of the uterus and it might have spread into tissues of the abdomen, but is not growing into the bladder or rectum (T1 to T3). The cancer has spread to nearby lymph nodes (N1), but not to distant sites (M0). |
| 4A | T4 Any N M0 | 4A | The cancer has spread to the rectum or urinary bladder (T4). It might or might not have spread to nearby lymph nodes (Any N) but has not spread to distant sites (M0). |
| 4B | Any T Any N M1 | 4B | The cancer has spread to distant sites such as the lungs, bones, or liver (M1). The cancer in the uterus can be any size and may or may not have grown into tissues in the pelvis and/or abdomen (including the bladder or rectum) (any T) and it might or might not have spread to nearby lymph nodes (Any N). |
Footnotes:* The following additional categories are not listed on the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Uterine sarcoma treatment
Treatment for uterine sarcoma will be determined by your doctor(s) based on:
- Your overall health and medical history
- Type and grade of the cancer
- Extent of the disease (the stage)
- Your tolerance for specific medications, procedures or therapies
- Expectations for the course of the disease
- Your opinion or preference
The choice of treatment for uterine sarcoma depends on the stage of cancer — whether it is only in the uterus, or if it has spread to other parts of the uterus or body. Surgery is the main treatment for uterine sarcoma. The goal of surgery is to remove all of the cancer as one piece. This usually means removing the entire uterus (hysterectomy). In some cases the fallopian tubes and ovaries (salpingo-oophorectomy) and part of the vagina may also need to be removed. Some lymph nodes or other tissue may be taken out as well to see if the cancer has spread outside the uterus.
In some cases, tests done before surgery let the doctor plan the operation in detail ahead of time. These tests include imaging studies, like ultrasound, as well as a pelvic exam, endometrial biopsy, and/or D&C. In other cases, the surgeon has to decide what needs to be done based on what’s found during surgery. For example, sometimes there’s no way to know for certain that a tumor is cancer until it’s removed during surgery.
Generally, treatment for people with uterine sarcoma includes one or more of the following.
- Surgery. Surgery to remove the uterus (hysterectomy) is recommended for most women with uterine sarcoma. Most women with uterine sarcoma undergo a procedure to remove the entire uterus (hysterectomy). In some cases the fallopian tubes and ovaries (salpingo-oophorectomy) and part of the vagina may also need to be removed. A hysterectomy makes it impossible for you to have children in the future. Also, once your ovaries are removed, you’ll experience menopause, if you haven’t already. During surgery, your surgeon will also inspect the areas around your uterus to look for signs that cancer has spread. Your surgeon may also remove lymph nodes for testing. This helps determine your cancer’s stage. The surgeon who does the surgery is usually a specialist surgeon called a gynecological oncologist.
- Radiation therapy. Radiation therapy uses high-energy radiation (such as x-rays) to kill cancer cells. Two types of radiation treatments may used for uterine sarcoma:
- External beam radiation therapy
- Internal radiation therapy or brachytherapy.
- Sometimes both brachytherapy and external beam radiation therapy are used. How much of the pelvis needs to be exposed to radiation therapy and the type(s) of radiation used depend on the extent of the disease.
- Radiation may be used to treat uterine sarcoma in these ways:
- When the tumor can be seen growing through the cervix, radiation therapy might be used before surgery to make it easier to remove all the cancer.
- After surgery it may help lower the chance of the cancer coming back in the pelvis. This is called adjuvant radiation. It may be done for cancers that are high grade or when cancer cells are found in the lymph nodes. In these cases, the entire pelvis may be treated with external beam radiation therapy. Sometimes the radiation field will also include an area of the abdomen (belly) called the para-aortic field. This is the area around the aorta (the main artery).
- It may be the main treatment in a woman who can’t have surgery because of other health problems.
- It may be used to treat problems caused by tumor growth, but is not intended to treat the cancer. For instance, radiation can be used to shrink a tumor that’s causing pain and swelling by pressing on nerves and blood vessels. This is called supportive or palliative care.
- Chemotherapy. Chemotherapy (chemo) is the use of drugs to treat cancer. The drugs can be swallowed as pills or they can be injected by needle into a vein or muscle. When chemo is given to shrink the cancer before surgery, it’s called neoadjuvant treatment. If it’s given after the cancer has been removed with surgery, it’s called adjuvant therapy. Here are some ways chemo may be used for uterine sarcoma:
- Adjuvant chemo is often used to help keep the cancer from coming back later.
- Chemo can also be used as the main therapy to treat the cancer if a woman is unable to have surgery.
- Sometimes chemo is used to control uterine sarcoma that has spread to other parts of the body or came back after surgery. In this case, the goal may be to ease symptoms and try to keep the tumor from growing.
- Chemo may not work for certain types of uterine sarcoma. Better results seem to be seen with earlier stages of this cancer, and types that are more likely to come back after surgery. And some types of uterine sarcoma have been found to respond better to certain drugs and drug combinations. The role of chemo, as well as the best chemo drugs to use are not clear. Still, there are a lot of clinical trials looking at this, and some studies have shown that chemo can help some women live longer after surgery.
- Some of the drugs commonly used to treat uterine sarcomas include:
- Dacarbazine (DTIC)
- Docetaxel (Taxotere®)
- Doxorubicin (Adriamycin®)
- Liposomal doxorubicin (Doxil®)
- Epirubicin (Ellence®)
- Gemcitabine (Gemzar®)
- Ifosfamide (Ifex®)
- Paclitaxel (Taxol®)
- Temozolomide (Temodar®)
- Trabectedin (Yondelis®)
- Vinorelbine (Navelbine®)
- In most cases, more than one drug is used. For example, gemcitabine and docetaxel are often used together to treat leiomyosarcoma.
- Some side effects from chemotherapy can last a long time. For example, the drug doxorubicin can damage the heart muscle over time. The chance of heart damage goes up as the total dose of the drug goes up, so doctors limit how much doxorubicin can be given. Cisplatin can cause kidney damage. Giving large amounts of fluid before and after chemo can help protect the kidneys. Both cisplatin and paclitaxel can cause nerve damage (called neuropathy). This can cause numbness, tingling, or even pain in the hands and feet.
- Hormone therapy. Hormone therapy is the use of hormones or hormone-blocking drugs to fight cancer. Part of diagnosing uterine sarcoma includes lab tests that check the cancer cells to see if they have receptors where hormones can attach. If they do, they may respond to hormone treatment. Hormone therapy is mainly used to treat women with endometrial stromal sarcomas (ESS) and is rarely used for the other types of uterine sarcomas.
- Progestins. Progestins are drugs that act like the hormone progesterone. The progestins used most often to treat uterine sarcomas are megestrol (Megace®) and medroxyprogesterone (Provera®). Both of these drugs are pills you take every day. Side effects can include increased blood sugar levels in patients with diabetes. Hot flashes, night sweats, and weight gain (from fluid retention and an increased appetite) also occur. Rarely, serious blood clots are seen in patients taking progestins.
- Gonadotropin-releasing hormone (GNRH) agonists. Gonadotropin-releasing hormone (GNRH) agonists keep the ovaries from making estrogen. These drugs are used to lower estrogen levels in women who are premenopausal. Before menopause, almost all a woman’s estrogen is made by the ovaries. Examples of GNRH agonists include goserelin (Zoladex®) and leuprolide (Lupron®). These drugs are given as a shot every 1 to 3 months. Side effects can include any of the symptoms of menopause, such as hot flashes and vaginal dryness. If they are taken for a long time, these drugs can weaken bones, sometimes leading to osteoporosis.
- Aromatase inhibitors. After the ovaries are removed, or aren’t working (after menopause), estrogen is still made in fat tissue. This becomes the body’s main source of estrogen. Drugs called aromatase inhibitors can stop this estrogen from being made. Examples of aromatase inhibitors include letrozole (Femara®), anastrozole (Arimidex®), and exemestane (Aromasin®). These drugs are most often used to treat breast cancer, but they also might be helpful in treating endometrial stromal sarcoma. Because they don’t affect estrogen made by the ovaries, they are only useful in women whose ovaries have been removed or no longer work (like after menopause). Side effects can include any of the symptoms of menopause, such as hot flashes and vaginal dryness, as well as joint/muscle pain. If they are taken for a long time (years), these drugs can weaken bones, sometimes leading to osteoporosis.
- Targeted drug therapy. Targeted therapy is treatment with drugs that are made to target changes in the cancer cells. Some people group them with chemotherapy, but they aren’t the same. Targeted therapies leave most healthy cells alone. Targeted therapy often cause fewer and different side effects than chemo. They are very new in the treatment of certain types of uterine sarcoma. As doctors learn more about these cancer cells it could become an important part of treatment.
- Pazopanib (Votrient) is a targeted therapy that may be used to treat leiomyosarcoma that has spread or come back after treatment. Side effects include things like high blood pressure, headache, and skin changes.
- Supportive (palliative) care. Palliative care is specialized medical care that focuses on providing relief from pain and other symptoms of a serious illness. Palliative care specialists work with you, your family and your other doctors to provide an extra layer of support that complements your ongoing care. Palliative care can be used while undergoing other aggressive treatments, such as surgery, chemotherapy or radiation therapy. When palliative care is used along with all of the other appropriate treatments, people with cancer may feel better and live longer. Palliative care is provided by a team of doctors, nurses and other specially trained professionals. Palliative care teams aim to improve the quality of life for people with cancer and their families. This form of care is offered alongside curative or other treatments you may be receiving.
Stages 1 and 2 leiomyosarcoma and undifferentiated sarcoma treatment
Most women have surgery to remove the uterus (hysterectomy), as well as the fallopian tubes and ovaries (bilateral salpingo-oophorectomy). Pelvic and para-aortic lymph node dissection or laparoscopic lymph node sampling may be done if swollen nodes are seen on imaging tests. During surgery, organs near the uterus and the thin membrane that lines the pelvic and abdominal cavities (called the peritoneum) are closely checked to see if the cancer has spread beyond the uterus.
Very rarely, young women with low-grade leiomyosarcomas (LMS) that have not spread beyond the uterus may be able to have just the tumor removed, leaving the uterus, fallopian tubes, and ovaries in place. This is not standard treatment, little is known about long-term outcomes , and it’s not often offered. Still, it may be a choice for some women who want to be able to have children after cancer treatment. This option has risks, so women thinking about this surgery need to talk about the pros and cons with their treatment team before making a decision. It may also be possible to leave a young woman’s ovaries in place (but remove the uterus and fallopian tubes), since it isn’t clear that this will lead to worse outcomes. Again, this is not a standard treatment, and you should discuss the risks and benefits with your doctor. In either case, close follow-up is important, and more surgery may be needed if the cancer comes back.
Women with stage 1 cancers may not need more treatment and are watched closely after surgery. In other cases, treatment with radiation, with or without chemo, may be needed after surgery if there’s a high chance of the cancer coming back in the pelvis. This is called adjuvant treatment. The goal of surgery is to take out all of the cancer, but the surgeon can only remove what can be seen. Tiny clumps of cancer cells that are too small to be seen can be left behind. Treatments given after surgery are meant to kill those cancer cells so that they don’t get the chance to grow into larger tumors. For low-grade leiomyosarcomas of the uterus, adjuvant radiation may lower the chance of the cancer growing back in the pelvis (called local recurrence), but it doesn’t seem to help women live longer.
Since the cancer can still come back in the lungs or other distant organs, some experts recommend giving chemo after surgery (adjuvant chemotherapy) for stage 2 cancers. Chemo is sometimes recommended for stage 1 low-grade leiomyosarcomas as well, but it’s less clear that it’s really helpful. So far, results from studies of adjuvant chemo have been promising in early stage low-grade leiomyosarcomas, but long-term follow-up is still needed to see if this treatment really helps women live longer. Studies of adjuvant therapy are in progress.
Stage 3 leiomyosarcoma and undifferentiated sarcoma treatment
Surgery is done to remove all of the cancer. This includes removing the uterus (a hysterectomy), removing both fallopian tubes and ovaries (bilateral salpingo-oophorectomy), and lymph node dissection or sampling. If the tumor has spread to the vagina, part (or even all) of the vagina will need to be removed as well.
After surgery, treatment with radiation (with or without chemo) may be offered to lower the chance that the cancer will come back.
Women who are too sick (from other medical problems) to have surgery may be treated with radiation and/or chemo.
Stage 4 leiomyosarcoma and undifferentiated sarcoma treatment
This is divided into stage 4A and stage 4b.
Stage 4A cancers have spread to nearby organs and tissues, such as the bladder or rectum, and maybe to nearby lymph nodes. These cancers might be able to be completely removed with surgery, and this is usually done if possible. If the cancer cannot be removed completely, radiation may be given, either alone or with chemo.
Stage 4B cancers have spread outside the pelvis, most often to the lungs, liver, or bone. There’s no standard treatment for these cancers. Chemo may be able to shrink the tumors for a time, but is not thought to be able to cure the cancer. Radiation therapy, given along with chemo, may also be an option.
These cancers might also be treated with targeted therapy when other treatments don’t work. They’re often given along with chemo.
Stages 1 and 2 endometrial stromal sarcoma treatment
Early stage endometrial stromal sarcoma is treated with surgery: hysterectomy and bilateral salpingo-oophorectomy. This means removal of the uterus, both fallopian tubes. and both ovaries. Some young women may be given the option of keeping their ovaries, but this is not the standard treatment. Pelvic lymph nodes may be removed if they look swollen on imaging tests.
After surgery, most women don’t need more treatment. These women are watched closely for signs that the cancer has returned. Others may be treated with hormone therapy and sometimes radiation to the pelvis. These can lower the chances of the cancer coming back, but they have not been shown to help patients live longer. This type of uterine sarcoma does not respond well to chemo, and it’s not often used at these early stages.
Women who are too sick (from other medical conditions) to have surgery may be treated with radiation and/or hormone therapy.
Stage 3 endometrial stromal sarcoma treatment
Surgery is done to remove all of the cancer. This includes removing the uterus (a hysterectomy), as well as removing both fallopian tubes and ovaries (bilateral salpingo-oophorectomy). Lymph nodes may checked if they look swollen. If the tumor has spread to the vagina, part (or even all) of the vagina will need to be removed too.
Women with endometrial stromal sarcomas might get radiation, hormone therapy, or both after surgery. Chemo may be used if other treatments don’t work.
Women who are too sick (from other medical conditions) to have surgery may be treated with radiation, chemo, and/or hormone therapy.
Stage 4 endometrial stromal sarcoma treatment
This stage is divided into stage 4A and stage 4B.
Stage 4A cancers have spread to nearby organs and tissues, such as the bladder or rectum. These cancers may be able to be completely removed with surgery, and this is usually done if possible. If the cancer cannot be removed completely, radiation may be given, either alone or with chemo. Hormone therapy is also an option.
Stage 4B cancers have spread outside of the pelvis, most often to the lungs, liver, or bone. Hormone therapy can help for a time. Chemo and radiation are also options to help ease symptoms.
Recurrent uterine sarcoma treatment
If a cancer comes back after treatment, it’s called recurrent cancer. If it comes back in the same place as it was before, it’s called a local recurrence. For uterine sarcoma, the cancer growing back as a tumor in the pelvis would be a local recurrence. If it comes back in another part of the body, like the liver or lungs, it’s called a distant recurrence.
Uterine sarcoma often comes back in the first few years after treatment. Treatment options for recurrent uterine sarcoma are the same as those for stage 4. If the cancer can be removed, surgery may be done. If not already given, radiation may be used to reduce the size of the tumor and relieve the symptoms of large pelvic tumors. Easing symptoms caused by cancer is called palliative or supportive care.
Sarcoma often comes back in the lungs. If there are only 1 or 2 small tumors, these may be able to be removed with surgery. Chemo and/or radiation are options after surgery. They may also be used for distant recurrence that can’t be taken out with surgery.
Women with recurrent uterine sarcomas might want to take part in clinical trials (scientific studies of promising treatments) testing new chemo or other treatments.
Uterine sarcoma survival rates
Survival rates can give you an idea of what percentage of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell you how long you will live, but they may help give you a better understanding of how likely it is that your treatment will be successful. Keep in mind that survival rates are estimates and are often based on previous outcomes of large numbers of people who had a specific cancer, but they can’t predict what will happen in any particular person’s case. These statistics can be confusing and may lead you to have more questions. Your doctor is familiar with your situation; ask how these numbers may apply to you.
A relative survival rate compares people with the same type and stage of uterine sarcoma to people in the overall population. For example, if the 5-year relative survival rate for a specific stage of uterine sarcoma is 62%, it means that people who have that cancer are, on average, about 62% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed.
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database tracks 5-year relative survival rates for uterine sarcoma in the United States, based on how far the cancer has spread. The SEER database, however, does not group cancers by FIGO or AJCC TNM stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized: There is no sign the cancer has spread outside of the uterus.
- Regional: The cancer has spread outside the uterus to nearby structures or lymph nodes.
- Distant: The cancer has spread to distant parts of the body such as the lungs, liver, or bones.
Table 5. 5-year relative survival rates for Uterine Leiomyosarcoma
| SEER Stage | 5-Year Relative Survival Rate |
|---|---|
| Localized | 62% |
| Regional | 34% |
| Distant | 13% |
| All SEER stages combined | 39% |
Footnotes: *SEER= Surveillance, Epidemiology, and End Results
- These numbers are based on women diagnosed with uterine sarcoma between 2011 and 2017.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, tumor grade, tumor type, how well the cancer responds to treatment, and other factors can also affect your outlook.
- People now being diagnosed with uterine sarcoma may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Table 6. 5-year relative survival rates for Undifferentiated Uterine Sarcoma
| SEER Stage | 5-Year Relative Survival Rate |
|---|---|
| Localized | 68% |
| Regional | 36% |
| Distant | 21% |
| All SEER stages combined | 43% |
Footnotes: *SEER= Surveillance, Epidemiology, and End Results
- These numbers are based on women diagnosed with uterine sarcoma between 2011 and 2017.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, tumor grade, tumor type, how well the cancer responds to treatment, and other factors can also affect your outlook.
- People now being diagnosed with uterine sarcoma may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Table 7. 5-year relative survival rates for Endometrial Stromal Sarcoma – low grade
| SEER Stage | 5-Year Relative Survival Rate |
|---|---|
| Localized | 98% |
| Regional | 95% |
| Distant | 78% |
| All SEER stages combined | 95% |
Footnotes: *SEER= Surveillance, Epidemiology, and End Results
- The outlook for high-grade endometrial stromal sarcoma (ESS) tends to be worse than for low-grade endometrial stromal sarcoma (ESS), and is more likely to be similar to that for undifferentiated sarcoma.
- These numbers are based on women diagnosed with uterine sarcoma between 2011 and 2017.
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, tumor grade, tumor type, how well the cancer responds to treatment, and other factors can also affect your outlook.
- People now being diagnosed with uterine sarcoma may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Uterine cancer signs and symptoms
Main symptoms of uterine cancer can include:
- bleeding or spotting from the vagina after the menopause
- heavy periods from your vagina that is unusual for you
- vaginal bleeding between your periods
- a change to your vaginal discharge
Other symptoms of uterine cancer can include:
- a lump or swelling in your abdomen or between your hip bones (pelvis)
- pain in your lower back or between your hip bones (pelvis)
- pain during sex
- blood in your urine
The most common symptom of endometrial cancer is abnormal vaginal bleeding, ranging from a watery and blood-streaked flow to a flow that contains more blood. Vaginal bleeding during or after menopause is often a sign of a problem.
If you are concerned about any changes you experience, please talk with your doctor. Do not wait to contact a doctor. This is because if your symptoms are caused by cancer, finding it early can mean it’s easier to treat.
Uterine cancer causes
Anyone with a uterus can get uterine cancer. This includes women, trans men, non-binary people and intersex people with a uterus.
You cannot get uterine cancer if you’ve had surgery to remove your uterus (hysterectomy).
Having a high level of a hormone called estrogen is one of the main factors that can increase your chance of getting uterus cancer.
You may have high levels of estrogen if you:
- are overweight
- take some types of hormone replacement therapy (HRT)
- have never given birth
- have polycystic ovary syndrome (PCOS)
- went through the menopause after the age of 55
You might also be more likely to get uterus cancer if you have:
- type 2 diabetes
- a family history of bowel, ovarian or uterus cancer
- inherited a rare gene that causes hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome
- taken medicines to treat like Tamoxifen (used to treat breast cancer)
- had radiation therapy on your pelvis
Uterine cancer risk factors
A risk factor is anything that increases a person’s chance of developing cancer. Although risk factors often influence the development of cancer, most do not directly cause cancer. Some people with several risk factors never develop cancer, while others with no known risk factors do. Knowing your risk factors and talking about them with your doctor may help you make more informed lifestyle and health care choices that may help you minimize your cancer risk.
The following factors may raise the risk of developing uterine cancer:
- Age. Uterine cancer most often occurs after age 50. The average age at diagnosis is 60. Uterine cancer is not common in people younger than 45.
- Obesity. Fatty tissue in people who are overweight produces additional estrogen, a sex hormone that can increase the risk of uterine cancer. This risk increases with an increase in body mass index (BMI), which is the ratio of a person’s weight to height. About 70% of uterine cancer cases are linked to obesity.
- Race. White women are more likely to develop uterine cancer than women of other races/ethnicities. However, Black women have a higher chance of being diagnosed with advanced uterine cancer. Black women and Hispanic women also have a higher risk of developing aggressive tumors.
- Genetics. Uterine cancer may run in families where colon cancer is hereditary. As explained in the Introduction, people in families with Lynch syndrome, also called hereditary non-polyposis colorectal cancer (HNPCC), have a higher risk for uterine cancer. It is recommended that all women with endometrial cancer should have their tumor tested for Lynch syndrome, even if they have no family history of colon cancer or other cancers. The presence of Lynch syndrome has important implications for women and their family members. About 2% to 5% of women with endometrial cancer have Lynch syndrome. In the United States, about 1,000 to 2,500 women diagnosed with endometrial cancer each year may have this genetic condition.
- Type 2 diabetes. A person may have an increased risk of uterine cancer if they have type 2 diabetes, which is often associated with obesity.
- Other cancers. People who have had breast cancer, colon cancer, or ovarian cancer may have an increased risk of uterine cancer.
- Tamoxifen. People taking the drug tamoxifen (Nolvadex) to reduce the risk of developing breast cancer or a breast cancer recurrence have an increased risk of developing uterine cancer. The benefits of tamoxifen usually outweigh the risk of developing uterine cancer, but anyone who is prescribed tamoxifen should talk with their doctor about their personal benefits and risks.
- Radiation therapy. People who have had previous radiation therapy for another cancer in the pelvic area, which is the lower part of the abdomen between the hip bones, have an increased risk of uterine cancer.
- Diet and nutrition. People who eat foods high in animal fat may have an increased risk of uterine cancer.
- Estrogen. Extended exposure to estrogen and/or an imbalance of estrogen is related to many of the following risk factors:
- Starting their first menstrual periods before age 12 and/or going through menopause later in life.
- Taking hormone replacement therapy (HRT) after menopause, especially if they are taking estrogen alone. The risk is lower when estrogen is taken with progesterone, which is another sex hormone.
- Never having been pregnant.
Uterine cancer prevention
Scientists continue to investigate what factors cause uterine cancer, including ways to prevent it. Although there is no proven way to completely prevent uterine cancer, you may be able to lower your risk. Talk with your health care team for more information about your personal risk of cancer.
Research has shown that certain factors can lower the risk of uterine cancer:
- Taking birth control pills. Birth control pills have a combination of estrogen and progesterone that are taken cyclically to produce a monthly menstrual period. This reduces the risk of an overgrowth of the uterine lining, especially when taken over a long period of time.
- Using a progestin-secreting intrauterine device (IUD), which is a form of birth control.
- Considering the risk of uterine cancer before starting hormone replacement therapy (HRT), especially estrogen replacement therapy alone, which is associated with an increased risk. Using a combination of estrogen and progesterone for HRT may help lower risk. However, combined HRT is associated with breast cancer risk.
- Maintaining a healthy weight, ideally a body mass index (BMI) less than 25. You can maintain a healthy weight by staying active and doing regular exercise. And by having a healthy diet and cutting down on alcohol
- If you have diabetes, careful disease management, such as regularly monitoring blood glucose levels, can help lower your risk.
Uterine cancer diagnosis
You will be asked some questions about your health, family medical history, medical conditions and your symptoms. Tell your doctor if you or your family have any history of cancer or Lynch syndrome.
In addition to a physical examination, the following tests may be used to diagnose uterine cancer:
- Pelvic examination. The doctor feels the uterus, vagina, ovaries, and rectum to check for any unusual findings. A Pap test, often done with a pelvic examination, is primarily used to check for cervical cancer. Sometimes, a Pap test may find abnormal glandular cells, which are caused by uterine cancer.
- Endometrial biopsy. A biopsy is the removal of a small amount of tissue for examination under a microscope. For an endometrial biopsy, the doctor removes a small sample of tissue with a very thin tube. The tube is inserted into the vagina to reach the uterus through the cervix, and the tissue is removed with suction. This process takes a few minutes. Afterward, you may have cramps and vaginal bleeding. These symptoms should go away soon and can be reduced by taking a nonsteroidal anti-inflammatory drug (NSAID) as directed by the doctor. Endometrial biopsy is often a very accurate way to diagnose uterine cancer. People who have abnormal vaginal bleeding before the test may still need a dilation and curettage (D&C; see below), even if no abnormal cells are found during the biopsy. A pathologist analyzes the sample(s). A pathologist is a doctor who specializes in interpreting laboratory tests and evaluating cells and tissue samples to diagnose disease.
- Dilation and curettage (D&C). A D&C is a procedure to remove tissue samples from the uterus. The patient is given anesthesia during the procedure to block the awareness of pain. A D&C is often done in combination with a hysteroscopy so the doctor can view the lining of the uterus during the procedure. During a hysteroscopy, the doctor inserts a thin, flexible tube with a light on it through the cervix into the vagina and uterus. After endometrial tissue has been removed, during a biopsy or D&C, the sample is checked by a pathologist for cancer cells, endometrial hyperplasia, and other conditions.
- Transvaginal ultrasound. An ultrasound uses sound waves to create a picture of internal organs. In a transvaginal ultrasound, an ultrasound wand is inserted into the vagina and aimed at the uterus to take pictures. If the endometrium looks too thick, the doctor may decide to perform a biopsy.
- Computed tomography (CT or CAT) scan. A CT scan takes pictures of the inside of the body using x-rays taken from different angles. A computer combines these pictures into a detailed, 3-dimensional image that shows any abnormalities or tumors. A CT scan can be used to measure the tumor’s size. Sometimes, a special dye called a contrast medium is given before the scan to provide better detail on the image. This dye is most commonly injected into a patient’s vein, but it can also be given as a pill or liquid to swallow.
- Magnetic resonance imaging (MRI). An MRI uses magnetic fields, not x-rays, to produce detailed images of the body. MRI can be used to measure the tumor’s size. Like with a CT scan, a special dye called a contrast medium can be given intravenously or orally before the scan to create a clearer picture. MRI is very useful for getting detailed images if the treatment plan will include hormone management. MRI is often used in people with low-grade uterine cancer (see Stages and Grades) to see how far the cancer has grown into the wall of the uterus.
- Biomarker testing of the tumor. Your doctor may recommend running laboratory tests on a tumor sample to identify specific genes, proteins, and other factors unique to the tumor. This may also be called molecular testing of the tumor. Results of these tests can help determine your treatment options.
After diagnostic tests are done, your doctor will review the results with you. If the diagnosis is cancer, additional testing will be performed to discover how far the disease has grown. This helps to categorize the disease by stage and grade and directs the type of treatment that will be needed.
Uterine cancer stages
After a woman is diagnosed with uterine cancer, doctors will try to figure out if it has spread, and if so, how far. This process is called staging. The stage of a cancer describes the amount of cancer in the body. It helps determine how serious the cancer is and how best to treat it. Doctors also use a cancer’s stage when talking about survival statistics.
The 2 systems used for staging uterine cancer, the FIGO (International Federation of Gynecology and Obstetrics) system and the American Joint Committee on Cancer (AJCC) TNM staging system are basically the same.
They both stage (classify) uterine cancer based on 3 factors:
- The extent (size) of the tumor (T): How large is the cancer? Has the cancer grown out of the uterus into the pelvis or organs such as the bladder or rectum?
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant lymph nodes or organs?
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
The staging system in the table below uses the pathologic stage also called the surgical stage. It is determined by examining tissue removed during an operation. Sometimes, if surgery is not possible right away, the cancer will be given a clinical stage instead. This is based on the results of a physical exam, biopsy, and imaging tests done before surgery.
The system described below is the most recent AJCC TNM system. It went into effect January 2018. It is specific for staging uterine cancer.
Uterine cancer staging can be complex, so ask your doctor to explain it to you in a way you understand.
Table 8. Uterine cancer staging
| TNM Stage | Stage grouping | FIGO Stage | Stage description* |
|---|---|---|---|
| 1 | T1 N0 M0 | 1 | The cancer is growing inside the uterus. It may also be growing into the glands of the cervix, but not into the supporting connective tissue of the cervix (T1). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 1A | T1a N0 M0 | 1A | The cancer is in the endometrium (inner lining of the uterus) and may have grown less than halfway through the underlying muscle layer of the uterus (the myometrium) (T1a). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 1B | T1b N0 M0 | 1B | The cancer has grown from the endometrium into the myometrium. It has grown more than halfway through the myometrium, but has not spread beyond the body of the uterus (T1b). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 2 | T2 N0 M0 | 2 | The cancer has spread from the body of the uterus and is growing into the supporting connective tissue of the cervix (called the cervical stroma). But it has not spread outside the uterus (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3 | T3 N0 M0 | 3 | The cancer has spread outside the uterus, but has not spread to the inner lining of the rectum or urinary bladder (T3). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3A | T3a N0 M0 | 3A | The cancer has spread to the outer surface of the uterus (called the serosa) and/or to the fallopian tubes or ovaries (the adnexa) (T3a). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3B | T3b N0 M0 | 3B | The cancer has spread to the vagina or to the tissues around the uterus (the parametrium) (T3b). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3C1 | T1-T3 N1, N1mi or N1a M0 | 3C1 | The cancer is growing in the body of the uterus. It may have spread to some nearby tissues, but is not growing into the inside of the bladder or rectum (T1 to T3). It has also spread to pelvic lymph nodes (N1, N1mi, or N1a), but not to lymph nodes around the aorta or distant sites (M0). |
| 3C2 | T1-T3 N2, N2mi or N2a M0 | 3C2 | The cancer is growing in the body of the uterus. It may have spread to some nearby tissues, but is not growing into the inside of the bladder or rectum (T1 to T3). It has also spread to lymph nodes around the aorta (para-aortic lymph nodes) (N2, N2mi, or N2a), but not to distant sites (M0). |
| 4A | T4 Any N M0 | The cancer has spread to the inner lining of the rectum or urinary bladder (called the mucosa) (T4). It may or may not have spread to nearby lymph nodes (Any N), but has not spread to distant sites (M0). | |
| 4B | Any T Any N M1 | 4B | The cancer has spread to inguinal (groin) lymph nodes, the upper abdomen, the omentum, or to organs away from the uterus, such as the lungs, liver, or bones (M1). The cancer can be any size (Any T) and it might or might not have spread to other lymph nodes (Any N). |
Footnotes: *The following additional categories are not listed on the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Stage 1 uterine cancer
Stage 1 uterine cancers are early cancers and the easiest to treat. Stage 1 uterine cancer means the cancer is found only in the uterus or womb, and it has not spread to other parts of the body.
- Stage 1A uterine cancer: The cancer is found only in the endometrium or less than one-half of the muscle wall (myometrium) of the uterus.
- Stage 1B uterine cancer: The cancer has grown halfway or more into the muscle wall (myometrium) of the uterus.
Stage 2 uterine cancer
Stage 2 uterine cancer, the cancer has grown from the uterus into the cervix but not to other parts of the body.
Stage 3 uterine cancer
Stage 3 uterine cancer means the cancer has spread outside the uterus, but is still within the pelvis. Your doctor may call this locally advanced uterine cancer. There are 3 categories of stage 3 uterine cancer:
- Stage 3A uterine cancer means the cancer has grown into the outer covering of the uterus (the serosa) and/or to the ovaries, fallopian tubes or ligaments of the uterus.
- Stage 3B uterine cancer means the cancer has grown into the vagina or to the connective tissue and fat around the uterus and cervix (the parametrium)
- Stage 3C uterine cancer means the cancer has spread to nearby lymph nodes (glands)
Stage 4 uterine cancer
Stage 4 uterine cancer means the cancer has metastasized to the rectum, bladder, and/or distant organs. There are 2 categories of stage 4 uterine cancer:
- Stage 4A uterine cancer: The cancer has spread to the mucosa of the rectum or bladder.
- Stage 4B uterine cancer: The cancer has spread to lymph nodes in the groin area, and/or it has spread to distant organs, such as the bones or lungs.
Uterine cancer grades
Doctors also describe uterine cancer by its grade. The grade describes how much cancer cells resemble healthy cells when viewed under a microscope. The doctor compares the cancerous tissue with healthy tissue. Healthy tissue usually contains many different types of cells grouped together. If the cancer appears similar to healthy tissue and has different cell groupings, it is called “differentiated” or a “low-grade tumor.” If the cancerous tissue looks very different from healthy tissue, it is called “poorly differentiated” or a “high-grade tumor.” The cancer’s grade may help the doctor predict how quickly the cancer will grow. In general, the slower the growth, the better the prognosis.
The letter “G” is used to define a grade for uterine cancer.
- GX: The grade cannot be evaluated.
- G1: The cells are well differentiated.
- G2: The cells are moderately differentiated.
- G3: The cells are poorly differentiated
Uterine cancer treatment
Uterine cancer is usually treatable when it’s found early. The treatment you have for uterine cancer will depend on:
- the size of the cancer
- the type and grade of the cancer
- where it is
- if it has spread
- your general health
- whether the cancer has just been diagnosed or has recurred (come back).
Uterine cancer treatment will usually include surgery, chemotherapy or radiotherapy. It may also include treatment with targeted medicines to treat the cancer.
Your specialist care team looking after you will:
- explain the treatments, benefits and side effects
- work with you to create a treatment plan that’s best for you
- help you manage any side effects, including any changes to your diet
- talk to you about the impact your treatment may have on your fertility
You’ll have regular check-ups during and after any treatments. You may also have more tests and scans.
If you have any symptoms or side effects that you are worried about, talk to your specialists. You do not need to wait for your next check-up.
Surgery for uterine cancer
Surgery is the removal of the tumor and some surrounding healthy tissue, called a margin, during an operation. It is typically the first treatment used for uterine cancer. A gynecologic oncologist is a doctor who specializes in treating gynecologic cancer using surgery. Before surgery, talk with your health care team about the possible side effects from the specific surgery you will have
Common surgical procedures for uterine cancer include:
- Hysterectomy. Depending on the extent of the cancer, the surgeon will perform either a simple hysterectomy (removal of the uterus and cervix) or a radical hysterectomy (removal of the uterus, cervix, the upper part of the vagina, and nearby tissues). For patients who have been through menopause, the surgeon will typically also perform a bilateral salpingo-oophorectomy, which is the removal of both fallopian tubes and ovaries. A hysterectomy may be performed either by abdominal incision, by laparoscopy or robotically, which uses several small incisions, or vaginally. A hysterectomy is usually performed by a gynecologic surgeon, which is a surgeon who specializes in surgery of the female reproductive system. In robotic-assisted surgery, a camera and instruments are inserted through small, keyhole-sized incisions. The surgeon directs the robotic instruments to remove the uterus, cervix, and surrounding tissue. If the patient has no cancer remaining in the tissue removed during a hysterectomy, additional treatment may not be needed. However, regular screening and testing to check for a return of the cancer is recommended.
- Lymph node removal. At the same time as a hysterectomy, the surgeon may remove lymph nodes near the tumor to determine if the cancer has spread beyond the uterus. This may be done through a procedure called a sentinel lymph node biopsy or lymphadenectomy. A sentinel lymph node biopsy might involve an injection of dye into the uterus during the hysterectomy and removal of the few lymph nodes where dye collects. This procedure has become more common in uterine cancer than lymphadenectomy. A lymphadenectomy, or lymph node dissection, is a surgical procedure in which a group of lymph nodes is removed.
Talk with your doctor about the risks and benefits of the different surgical approaches and which approach might be best for you.
Side effects of surgery
After surgery, the most common short-term side effects include pain and tiredness. If a patient is experiencing pain, their doctor will prescribe medications to relieve the pain. Other immediate side effects may include nausea and vomiting as well as difficulty emptying the bladder and having bowel movements. After surgery, the patient’s diet may be restricted to liquids, followed by a gradual return to solid foods.
If the ovaries are removed, this ends the body’s production of sex hormones, resulting in early menopause (if the patient has not already gone through menopause). While removal of the ovaries substantially reduces the sex hormones that are produced by the body, the adrenal glands and fat tissues will still provide some hormones. Soon after removing the ovaries, the patient is likely to experience menopausal symptoms, including hot flashes and vaginal dryness. Talk with your doctor about ways to relieve and manage these menopausal symptoms.
If a lymphadenectomy is done, some people may experience swelling in their legs, which is a side effect called lymphedema.
After a hysterectomy, pregnancy is no longer possible. For this reason, premenopausal patients who wish to preserve their fertility and have children in the future should talk with their doctor about all their options before any treatment begins. Sometimes, fertility preservation is possible and might include less extensive surgery followed by hormone therapy (see below). Your doctor can talk with you about the potential risks and benefits of this approach and can provide information to help you make an informed decision.
Before any operation for uterine cancer, you are also encouraged to talk with your doctor about sexual and emotional side effects, including ways to address these issues before and after cancer treatment.
The treatment options after surgery for endometrial cancer depend on the stage and grade of the cancer. For people who have had surgery and have grade 1 or 2 cancer that either has not spread to the myometrium or more than halfway through the myometrium, additional treatment may be avoided.
When considering your options for treatment after surgery, talk with your doctor about how each treatment will affect you. It’s important to weigh the benefits of treatment to possibly keep the cancer from returning with the risks of the treatment. The risks of treatment may include the short- and long-term side effects and a possible decrease in your quality of life. What you consider a decrease in quality of life is very personal. This is why it is important to talk with your doctor about possible side effects, how long they will last, and how they might affect you now and in the future.
Radiation therapy for uterine cancer
Radiation therapy is the use of high-energy x-rays or other particles to destroy cancer cells. A doctor who specializes in giving radiation therapy to treat cancer is called a radiation oncologist. A radiation therapy regimen, or schedule, usually consists of a specific number of treatments given over a set period of time. Radiation therapy can be delivered externally or internally. External-beam radiation therapy uses a machine outside the body to deliver radiation to the pelvic region or the area designated by your radiation oncologist. Radiation can also be delivered internally. This form of radiation is called brachytherapy. External-beam radiation therapy can be given alone or in combination with brachytherapy. For some people, brachytherapy alone will be recommended. The most common type of radiation treatment is external-beam radiation therapy.
Some people with uterine cancer need surgery and radiation therapy. The radiation therapy is most often given after surgery to destroy any remaining cancer cells. Radiation therapy is occasionally given before surgery to shrink the tumor. If a person cannot have surgery, the doctor may recommend radiation therapy instead.
Options for giving radiation therapy to treat uterine cancer may include radiation therapy directed towards the whole pelvis and/or applied only to the vaginal cavity, often called vaginal brachytherapy. A number of factors play into decisions about what type of radiation is best, but some patients with low-risk disease may be able to undergo vaginal brachytherapy instead of radiation to the pelvis.
For some patients, radiation therapy to the pelvis may be the best option to help prevent a return of the cancer. These patients include those with a grade 3 cancer that has spread through half or more of the myometrium; those with a cancer of any grade that has spread to tissue in the cervix; and those with a cancer that has spread outside the uterus to nearby tissue or organs. In these situations, the patient may need only radiation therapy after surgery, only chemotherapy, or a combination of radiation therapy and chemotherapy. Your doctor will be able to help you figure out which of these options is right for you.
Radiation therapy to the pelvis may also be considered for some people with grade 1 or 2 cancer that has spread through half or more of the thickness of the myometrium, depending on factors such as age and whether the cancer has spread to blood or lymphatic vessels.
Side effects from radiation therapy will depend on the extent of radiation therapy given and may include fatigue, skin reactions, changes in urinary frequency, and loose bowel movements. Most side effects go away soon after treatment is finished, but long-term side effects are possible. Talk with your radiation oncologist about what you can expect and how side effects will be managed.
Researchers are always looking for new ways to improve radiation therapy to reduce its side effects and improve its effectiveness.
Chemotherapy for uterine cancer
Chemotherapy is the use of drugs to destroy cancer cells, usually by keeping the cancer cells from growing, dividing, and making more cells. When recommended for endometrial cancer, chemotherapy usually is given after surgery. Chemotherapy is also considered if the endometrial cancer returns after the person’s initial treatment.
A chemotherapy regimen, or schedule, usually consists of a specific number of cycles given over a set period of time. A patient may receive 1 drug at a time or a combination of different drugs given at the same time.
The goal of chemotherapy is to destroy cancer remaining after surgery or to shrink the cancer and slow the tumor’s growth if it comes back or has spread to other parts of the body. Although chemotherapy can be given orally, most drugs used to treat uterine cancer are given by IV. IV chemotherapy is either injected directly into a vein or through a catheter, which is a thin tube inserted into a vein.
The side effects of chemotherapy depend on the individual, the type of chemotherapy, and the dose used, but they can include fatigue, risk of infection, nausea and vomiting, hair loss, loss of appetite, and diarrhea. These side effects usually go away after treatment is finished. There have also been advances in chemotherapy during the last 10 years that include the development of new drugs for the prevention and treatment of side effects, such as antiemetics for nausea and vomiting and growth factors to prevent low white blood cell counts and reduce the risk of infection.
Other potential side effects of chemotherapy for uterine cancer include the inability to become pregnant in the future (infertility) and experiencing early menopause, if the patient has not already had a hysterectomy. Talk with your doctor before treatment starts if you want to preserve your fertility. Rarely, some drugs cause some hearing loss. Others may cause kidney damage. Patients may be given extra fluid intravenously to protect their kidneys. Talk with your doctor about what side effects you may experience with chemotherapy and how they can be prevented or managed.
Hormone therapy for uterine cancer
Hormone therapy is used to slow the growth of certain types of uterine cancer cells that have receptors to the hormones on them. These tumors are generally adenocarcinoma and are grade 1 or 2.
Hormone therapy for uterine cancer often involves a high dose of the sex hormone progesterone given in pill form. Other hormone therapies include hormone-expressing intrauterine devices (IUDs) and aromatase inhibitors (AIs), such as anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin), which are often used for the treatment of breast cancer. An aromatase inhibitor is a drug that reduces the amount of the hormone estrogen in a person’s body by stopping tissues and organs other than the ovaries from producing it. Hormone therapy may also be used for people who cannot have surgery or radiation therapy, or it can be used in combination with other types of treatment.
Side effects of hormone therapy may include fluid retention, increase in appetite, insomnia, muscle aches, and weight gain. Most side effects are manageable with the help of your health care team. Talk with your doctor about what you can expect.
Targeted therapy for uterine cancer
Targeted therapy is a treatment that targets the cancer’s specific genes, proteins, or the tissue environment that contributes to cancer growth and survival. This type of treatment blocks the growth and spread of cancer cells and limits damage to healthy cells.
Not all tumors have the same targets. To find the most effective treatment, your doctor may run tests to identify the genes, proteins, and other factors in your tumor. This helps doctors better match each patient with the most effective treatment whenever possible. In addition, research studies continue to find out more about specific molecular targets and new treatments directed at them.
Targeted therapy for uterine cancer is available in clinical trials and, in some instances, as part of standard-of-care treatment plans. Targeted therapy for uterine cancer includes:
- Anti-angiogenesis therapy. Anti-angiogenesis therapy is focused on stopping angiogenesis, which is the process of making new blood vessels. Because a tumor needs the nutrients delivered by blood vessels to grow and spread, the goal of anti-angiogenesis therapies is to “starve” the tumor. Bevacizumab (Avastin) is a type of anti-angiogenesis therapy used to treat uterine cancer.
- Mammalian target of rapamycin (mTOR) inhibitors. In endometrial cancer, mutations in a pathway called mTOR are commonly found. People with advanced or recurrent uterine cancer may be treated with a drug that blocks this pathway, such as everolimus (Afinitor). Other drugs that target this pathway are being studied, such as ridaforolimus and temsirolimus (Torisel), a targeted therapy approved to treat a type of kidney cancer called renal cell carcinoma.
- Targeted therapy to treat a rare type of uterine cancer. Uterine serous carcinoma is a rare but aggressive type of endometrial cancer. About 30% of these tumors express the HER2 gene. In a phase II clinical trial, researchers found that trastuzumab (Herceptin) combined with a combination of chemotherapy was effective in treating these kinds of tumors. Trastuzumab is a HER2 targeted therapy mostly used to treat HER2-positive breast cancer.
Different targeted therapies have different side effects. Talk with your doctor about these possible side effects and how they can be managed.
Immunotherapy for uterine cancer
Immunotherapy uses the body’s natural defenses to fight cancer by improving your immune system’s ability to attack cancer cells.
Uterine cancers with mismatch repair defects (dMMR) are more sensitive to immunotherapy. The immunotherapy drug pembrolizumab (Keytruda) is approved to treat tumors that have either high microsatellite instability (MSI-high) or dMMR, regardless of the tumor’s location in the body. Pembrolizumab can be used to treat uterine tumors with dMMR if other previous treatments have not worked.
A combination of lenvatinib (Lenvima), a targeted therapy drug, and pembrolizumab is also approved to treat advanced endometrial cancer. This combination can be used to treat disease that is not MSI-high or dMMR, has not been controlled by systemic therapy, and cannot be cured with surgery or radiation therapy. Lenvatinib may cause high blood pressure.
In April 2021, the FDA approved the immunotherapy drug dostarlimab (Jemperli) to treat recurrent or advanced endometrial cancer with dMMR that has progressed either while on or after completing platinum-containing chemotherapy. Dostarlimab is given by vein every 3 weeks. Its most common side effects are fatigue, nausea, diarrhea, and constipation.
Different types of immunotherapy can cause different side effects. Common side effects include skin reactions, flu-like symptoms, diarrhea, and weight changes. Talk with your doctor about possible side effects for the immunotherapy recommended for you.
Stage 1 uterine cancer treatment
Stage 1 uterine cancer is only in the uterus. It has not spread to lymph nodes or distant sites.
Surgery is the main treatment for stage 1 endometrial cancer. Your surgeon removes your uterus and cervix. This is a hysterectomy. They usually also remove both of your fallopian tubes and ovaries. They may also remove lymph nodes in your pelvis to check for cancer cells. Sometimes this is the only treatment needed. The patient is then closely watched for signs that the cancer has come back (recurred).
For women with higher grade tumors, radiation will likely be recommended after surgery. Vaginal brachytherapy, pelvic radiation, or both can be used.
Women who have early endometrial cancer, have not been through the menopause and would like to have children may be able to have treatment that preserves their fertility (have surgery to remove their uterus without removing the ovaries). This prevents menopause and the issues that can come with it. This also increases the chance that the cancer will come back, but it doesn’t make it more likely that you will die from the cancer. It is not standard treatment. So talk to your specialist about your options and the possible risks. You will need to have treatment in a specialist center. This might not be your nearest hospital.
Women who cannot have surgery because of other medical problems or who are frail due to age are often treated with just radiation (external radiation and/or vaginal brachytherapy).
For young women who still want to have children, surgery may be postponed while progestin therapy is used to treat the cancer. Progestin treatment can cause the cancer to shrink or even go away for some time, giving the woman a chance to get pregnant. Still, this is experimental and can be risky if the patient isn’t watched closely. An endometrial biopsy or a D&C should be done every 3 to 6 months. If there’s still no cancer after 6 months, the woman can try to become pregnant. She will continue to be checked for cancer every 6 months. Because the cancer often comes back again, doctors recommend total hysterectomy bilateral salpingo-oophorectomy (TH/BSO) after childbearing is complete.
Many times, progestin treatment doesn’t work and the cancer doesn’t get better or keeps growing. Putting off surgery can give the cancer time to spread outside the uterus. If it doesn’t go away in 6 to 12 months , surgery to remove and stage the cancer is recommended (hysterectomy and removal of both fallopian tubes and ovaries).
A second opinion from a gynecologic oncologist and pathologist (to confirm the grade of the cancer) before starting progestin therapy is important. Seeing a fertility expert is also a good idea. It’s important to understand that this isn’t a standard treatment and may increase risk of cancer growth and spread.
Stage 2 uterine cancer treatment
When a uterine cancer is stage 2, it has spread to the connective tissue of the cervix. But it still hasn’t grown outside the uterus.
One treatment option is to have surgery first, followed by radiation therapy. The surgery includes a radical hysterectomy (the entire uterus, the tissues next to the uterus, and the upper part of the vagina are removed), removal of both fallopian tubes and ovaries (BSO), and pelvic and para-aortic lymph node dissection or sampling. Radiation therapy, often both vaginal brachytherapy and external pelvic radiation, may be given after the patient has recovered from surgery. Another option is to give the radiation therapy first, and then do a simple hysterectomy, bilateral salpingo-oophorectomy (BSO) and possible lymph node dissection or lymph node sampling.
The lymph nodes that have been removed are checked for cancer cells. If there’s cancer in them, the cancer isn’t really a stage 2 – it’s a stage 3C endometrial cancer.
In some cases, a woman with early stage endometrial cancer might be too frail or ill from other diseases to safely have surgery. These women are treated with external radiation and brachytherapy.
For women with high-grade cancers, like papillary serous carcinoma or clear cell carcinoma, the surgery may include omentectomy and peritoneal biopsies along with the total hysterectomy, removal of both fallopian tubes and ovaries, pelvic and para-aortic lymph node dissections, and pelvic washings. After surgery, radiation therapy, chemo, or both may be given to help keep the cancer from coming back. The chemo usually includes the drugs carboplatin and paclitaxel or possibly cisplatin and doxorubicin.
Someone with a stage 2 uterine carcinosarcoma often has the same type of surgery that’s used for a high-grade cancer. After surgery, radiation, chemo, or both may be used. The chemo often includes paclitaxel and carboplatin but may instead include ifosfamide, along with paclitaxel or cisplatin.
Stage 3 uterine cancer treatment
Stage 3 uterine cancers have spread outside of the uterus.
If the surgeon thinks that all visible cancer can be removed, a hysterectomy is done and both ovaries and fallopian tubes are removed. Sometimes women with stage 3 cancers need a radical hysterectomy. A pelvic and para-aortic lymph node dissection may also be done. Pelvic washings will be done and the omentum may be removed. Some doctors will try to remove any remaining cancer (called debulking), but it isn’t clear that this helps patients live longer.
If tests done before surgery show that the cancer has spread too far to be removed completely, in rare cases, radiation therapy may be given before any surgery. It might shrink the tumor enough to make surgery an option. For advanced endometrial cancers that cannot be treated with surgery or radiation, treatment with the immunotherapy drug, pembrolizumab might be an option.
For women with high-grade cancers, such as papillary serous carcinoma or clear cell carcinoma, the surgery may include omentectomy and peritoneal biopsies along with the total hysterectomy, removal of both ovaries and fallopian tubes, pelvic and para-aortic lymph node dissections, and pelvic washings. After surgery, chemo, radiation therapy, or both may be given to help keep the cancer from coming back. The chemo usually includes the drugs carboplatin and paclitaxel or cisplatin and doxorubicin.
Women with stage 3 uterine carcinosarcoma often have the same type of surgery that’s used for a high-grade cancer. After surgery, radiation, chemo, or both may be used. The chemo often includes the drug paclitaxel and carboplatin, but ifosfamide, along with paclitaxel or cisplatin may be used. Targeted and/or immunotherapy may also be options for some women.
Stage 4 uterine cancer treatment
If cancer spreads to a part of the body that is different from where it started, doctors call it metastatic cancer. If this happens, it is a good idea to talk with doctors who have experience in treating it. Doctors can have different opinions about the best standard treatment plan. Clinical trials might also be an option.
Your treatment plan may include radiation therapy, especially for recurrent cancer in the pelvis, or surgery. Hormone therapy may be used for cancer that has spread to distant parts of the body. A cancer that is high grade or that does not respond to hormone therapy is treated with chemotherapy and/or targeted therapy. People with stage 4 uterine cancer have many standard-of-care treatment options. They are also encouraged to consider participating in clinical trials. Palliative care will be important to help relieve symptoms and side effects.
Supportive (palliative) care
Palliative care is specialized medical care that focuses on providing relief from pain and other symptoms of a serious illness. Palliative care specialists work with you, your family and your other doctors to provide an extra layer of support that complements your ongoing care. Palliative care can be used while undergoing other aggressive treatments, such as surgery, chemotherapy or radiation therapy.
When palliative care is used along with all of the other appropriate treatments, people with cancer may feel better and live longer.
Palliative care is provided by a team of doctors, nurses and other specially trained professionals. Palliative care teams aim to improve the quality of life for people with cancer and their families. This form of care is offered alongside curative or other treatments you may be receiving.
Coping and support
Coping with the shock, fear and sadness that come with a cancer diagnosis can take time. You may feel overwhelmed just when you need to make crucial decisions. With time, each person finds a way of coping and coming to terms with the diagnosis.
Until you find what brings you the most comfort, consider trying to:
- Find out enough about endometrial cancer to make decisions about your care. Ask your doctor for the specifics about your cancer, such as its type and stage. And ask for recommended sources of information where you can learn more about your treatment options. The National Cancer Institute 72 and the American Cancer Society 73 are good places to start.
- Stay connected to friends and family. Your friends and family can provide a crucial support network for you during your cancer treatment. As you begin telling people about your endometrial cancer diagnosis, you’ll likely get offers for help. Think ahead about things you may like help with, whether it’s having someone to talk to if you’re feeling low or getting help preparing meals.
- Find someone to talk to. You might have a close friend or family member who’s a good listener. Or talk to a counselor, medical social worker, or pastoral or religious counselor.
Consider joining a support group for people with cancer. You may find strength and encouragement in being with people who are facing the same challenges you are. Ask your doctor, nurse or social worker about groups in your area. Or try online message boards, such as those available through the American Cancer Society 73.
Uterine cancer survival rates
Survival rates tell you what percentage of people with the same type and stage of cancer are still alive a certain length of time (usually 5 years) after they were diagnosed. These numbers can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful.
Statistics on the outlook for a certain type and stage of cancer are often given as 5-year survival rates, but many people live longer – often much longer – than 5 years. The 5-year survival rate is the percentage of people who live at least 5 years after being diagnosed with cancer.
Survival rates are estimates – your outlook can vary based on a number of factors specific to you.
Survival rates don’t tell the whole story
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. Your doctor can tell you how the numbers below may apply to you, as he or she is familiar with the aspects of your particular situation.
The survival rates below are based on the stage of the cancer at the time it was diagnosed. These rates do not apply to cancers that have come back after treatment or have spread after treatment starts.
The numbers below come from the National Cancer Data Base as published in the AJCC Staging Manual in 2017, and are based on people diagnosed between 2000 and 2002.
Endometrial cancer survival rates
- The 5-year survival rate for women with stage 0 endometrial cancer is 90%*
- The 5-year survival rate for women with stage 1A endometrial cancer is 88%
- The 5-year survival rate for women with stage 1B endometrial cancer is 75%
- The 5-year survival rate for women with stage 2 endometrial cancer is 69%
- The 5-year survival rate for women with stage 3A endometrial cancer is 58%
- The 5-year survival rate for women with stage 3B endometrial cancer is 50%
- The 5-year survival rate for women with stage 3C endometrial cancer is 47%
- The 5-year survival rate for women with stage 4A endometrial cancer is 17%
- The 5-year survival rate for women with stage 4B endometrial cancer is 15%
*The new staging system that went into effect January 2018 no longer includes Stage 0 cancers.
Uterine cancer survival by stage
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database tracks 5-year relative survival rates for uterine cancer in the United States, based on how far the cancer has spread 4. The SEER database, however, does not group cancers by American Joint Committee on Cancer (AJCC) TNM Staging System stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages:
- Localized (sometimes referred to as stage 1): There is no sign the cancer has spread outside of the uterus.
- Regional: The cancer has spread outside the uterus to nearby structures or lymph nodes.
- Distant: The cancer has spread to distant parts of the body such as the lungs, liver, or bones.
Table 9. 5-year relative survival rates for uterine cancer
| SEER stage | 5-year relative survival rate |
|---|---|
| Localized | 94.9% |
| Regional | 69.8% |
| Distant | 18.4% |
| All SEER stages combined | 52.2% |
Footnotes:
- SEER = Surveillance, Epidemiology, and End Results. The SEER database is maintained by the National Cancer Institute (NCI), to provide survival statistics for different types of cancer.
- These numbers are based on people diagnosed with endometrial cancer between 2012–2018
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, how well the cancer responds to treatment, and other factors can also affect your outlook.
- People now being diagnosed with endometrial cancer may have a better outlook than these numbers show. Treatments have improved over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
Keep in mind that survival rates are estimates and are often based on previous outcomes of large numbers of people who had a specific cancer, but they can’t predict what will happen in any particular person’s case. These statistics can be confusing and may lead you to have more questions. Your doctor knows your situation, so ask them how these numbers might apply to you.
[Source 4 ]Generally for women with endometrial cancer in England between 2013 and 2017 66:
- 90 out of every 100 (90%) survive their cancer for 1 year or more after they are diagnosed
- around 75 out of every 100 (around 75%) will survive their cancer for 5 years or more
- more than 70 out of every 100 (more than 70%) will survive their cancer for 10 years or more after diagnosis
Uterine cancer prognosis
Based on the data available from 2009 to 2015, women diagnosed with localized, regionally extended, and distantly metastasized endometrial carcinoma had a 5-year survival rate of 95%, 69%, and 17%, respectively 74. For all stages taken together, the overall 5-year survival is around 80% 75. Some biochemical markers such as cancer antigen 125 (CA-125), estrogen and progesterone receptor expression, presence of mutations (e.g., PTEN, TP53), E-cadherin (CDH1), and neural cell adhesion molecule L1 (L1CAM) are useful prognostic factors 76.
References- Diagnosis and Management of Endometrial Cancer. Am Fam Physician. 2016 Mar 15;93(6):468-474. https://www.aafp.org/afp/2016/0315/p468.html
- American Cancer Society: Cancer Facts and Figures 2022. American Cancer Society, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf
- Key Statistics for Endometrial Cancer. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html
- Uterine Cancer — Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/corp.html
- Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004 May;59(5):368-78. doi: 10.1097/00006254-200405000-00025
- Singh G, Puckett Y. Endometrial Hyperplasia. [Updated 2022 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560693
- van der Meer, A.C.L. and Hanna, L.S. (2017), Development of endometrioid adenocarcinoma despite Levonorgestrel-releasing intrauterine system: a case report with discussion and review of the RCOG/BSGE Guideline on the Management of Endometrial Hyperplasia. Clin Obes, 7: 54-57. https://doi.org/10.1111/cob.12168
- The American College of Obstetricians and Gynecologists Committee Opinion no. 631. Endometrial intraepithelial neoplasia. Obstet Gynecol. 2015 May;125(5):1272-1278. doi: 10.1097/01.AOG.0000465189.50026.20
- Palmer, J.E., Perunovic, B. and Tidy, J.A. (2008), Endometrial hyperplasia. The Obstetrician & Gynaecologist, 10: 211-216. https://doi.org/10.1576/toag.10.4.211.27436
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a1
- Endometrial Hyperplasia. https://www.acog.org/womens-health/faqs/endometrial-hyperplasia
- Chen, Y. L., Wang, K. L., Chen, M. Y., Yu, M. H., Wu, C. H., Ke, Y. M., Chen, Y. J., Chang, Y. Y., Hsu, K. F., & Yen, M. S. (2013). Risk factor analysis of coexisting endometrial carcinoma in patients with endometrial hyperplasia: a retrospective observational study of Taiwanese Gynecologic Oncology Group. Journal of gynecologic oncology, 24(1), 14–20. https://doi.org/10.3802/jgo.2013.24.1.14
- Endometrial hyperplasia. https://radiopaedia.org/cases/endometrial-hyperplasia?lang=us
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985 Jul 15;56(2):403-12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x
- Rakha E, Wong SC, Soomro I, Chaudry Z, Sharma A, Deen S, Chan S, Abu J, Nunns D, Williamson K, McGregor A, Hammond R, Brown L. Clinical outcome of atypical endometrial hyperplasia diagnosed on an endometrial biopsy: institutional experience and review of literature. Am J Surg Pathol. 2012 Nov;36(11):1683-90. doi: 10.1097/PAS.0b013e31825dd4ff
- Trimble, C.L., Kauderer, J., Zaino, R., Silverberg, S., Lim, P.C., Burke, J.J., II, Alberts, D. and Curtin, J. (2006), Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia. Cancer, 106: 812-819. https://doi.org/10.1002/cncr.21650
- Zaino, R.J., Kauderer, J., Trimble, C.L., Silverberg, S.G., Curtin, J.P., Lim, P.C. and Gallup, D.G. (2006), Reproducibility of the diagnosis of atypical endometrial hyperplasia. Cancer, 106: 804-811. https://doi.org/10.1002/cncr.21649
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a2
- Salman, M. C., Usubutun, A., Boynukalin, K., & Yuce, K. (2010). Comparison of WHO and endometrial intraepithelial neoplasia classifications in predicting the presence of coexistent malignancy in endometrial hyperplasia. Journal of gynecologic oncology, 21(2), 97–101. https://doi.org/10.3802/jgo.2010.21.2.97
- Zaino R, Carinelli S G, Ellenson L H, Lyon: WHO Press; 2014. Tumours of the uterine Corpus: epithelial Tumours and Precursors; pp. 125–126.
- Cancer Genome Atlas Research Network, Kandoth, C., Schultz, N., Cherniack, A. D., Akbani, R., Liu, Y., Shen, H., Robertson, A. G., Pashtan, I., Shen, R., Benz, C. C., Yau, C., Laird, P. W., Ding, L., Zhang, W., Mills, G. B., Kucherlapati, R., Mardis, E. R., & Levine, D. A. (2013). Integrated genomic characterization of endometrial carcinoma. Nature, 497(7447), 67–73. https://doi.org/10.1038/nature12113
- Baak, J. P., Mutter, G. L., Robboy, S., van Diest, P. J., Uyterlinde, A. M., Orbo, A., Palazzo, J., Fiane, B., Løvslett, K., Burger, C., Voorhorst, F., & Verheijen, R. H. (2005). The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer, 103(11), 2304–2312. https://doi.org/10.1002/cncr.21058
- Emons, G., Beckmann, M. W., Schmidt, D., Mallmann, P., & Uterus commission of the Gynecological Oncology Working Group (AGO) (2015). New WHO Classification of Endometrial Hyperplasias. Geburtshilfe und Frauenheilkunde, 75(2), 135–136. https://doi.org/10.1055/s-0034-1396256
- Trimble, C. L., Method, M., Leitao, M., Lu, K., Ioffe, O., Hampton, M., Higgins, R., Zaino, R., Mutter, G. L., & Society of Gynecologic Oncology Clinical Practice Committee (2012). Management of endometrial precancers. Obstetrics and gynecology, 120(5), 1160–1175. https://doi.org/10.1097/aog.0b013e31826bb121
- https://www.awmf.org/leitlinien/detail/ll/032-034OL.html
- Antonsen SL, Ulrich L, Høgdall C. Patients with atypical hyperplasia of the endometrium should be treated in oncological centers. Gynecol Oncol. 2012 Apr;125(1):124-8. doi: 10.1016/j.ygyno.2011.12.436
- Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015 Jul;76(7):e857-61. doi: 10.4088/JCP.15f10150
- Parkash V, Fadare O, Tornos C, McCluggage WG. Committee Opinion No. 631: Endometrial Intraepithelial Neoplasia. Obstet Gynecol. 2015 Oct;126(4):897. doi: 10.1097/AOG.0000000000001071
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a3
- Lethaby A, Suckling J, Barlow D, Farquhar CM, Jepson RG, Roberts H. Hormone replacement therapy in postmenopausal women: endometrial hyperplasia and irregular bleeding. Cochrane Database Syst Rev. 2004;(3):CD000402. doi: 10.1002/14651858.CD000402.pub2. Update in: Cochrane Database Syst Rev. 2009;(2):CD000402
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996 Feb 7;275(5):370-5. doi: 10.1001/jama.1996.03530290040035
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SA, Pettinger M, Liu J, McNeeley SG, Lopez AM; Women’s Health Initiative Investigators. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women’s Health Initiative randomized trial. JAMA. 2003 Oct 1;290(13):1739-48. doi: 10.1001/jama.290.13.1739
- Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004 Aug;94(2):256-66. doi: 10.1016/j.ygyno.2004.03.048
- Lancaster JM, Powell CB, Chen LM, Richardson DL; SGO Clinical Practice Committee. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015 Jan;136(1):3-7. doi: 10.1016/j.ygyno.2014.09.009. Epub 2014 Sep 17. Erratum in: Gynecol Oncol. 2015 Sep;138(3):765.
- Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000 Mar;13(3):295-308. doi: 10.1038/modpathol.3880051
- Furness, S., Roberts, H., Marjoribanks, J., & Lethaby, A. (2012). Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. The Cochrane database of systematic reviews, 2012(8), CD000402. https://doi.org/10.1002/14651858.CD000402.pub4
- Kaminski P, Bobrowska K, Pietrzak B, Bablok L, Wielgos M. Gynecological issues after organ transplantation. Neuro Endocrinol Lett. 2008 Dec;29(6):852-6.
- Niskakoski A, Pasanen A, Porkka N, Eldfors S, Lassus H, Renkonen-Sinisalo L, Kaur S, Mecklin JP, Bützow R, Peltomäki P. Converging endometrial and ovarian tumorigenesis in Lynch syndrome: Shared origin of synchronous carcinomas. Gynecol Oncol. 2018 Jul;150(1):92-98. doi: 10.1016/j.ygyno.2018.04.566
- Endometrial hyperplasia. https://radiopaedia.org/articles/endometrial-hyperplasia-1?lang=us
- Park, Y. R., Lee, S. W., Kim, Y., Bae, I. Y., Kim, H. K., Choe, J., & Kim, Y. M. (2019). Endometrial thickness cut-off value by transvaginal ultrasonography for screening of endometrial pathology in premenopausal and postmenopausal women. Obstetrics & gynecology science, 62(6), 445–453. https://doi.org/10.5468/ogs.2019.62.6.445
- Jorizzo JR, Chen MY, Martin D, Dyer RB, Weber TM. Spectrum of endometrial hyperplasia and its mimics on saline hysterosonography. AJR Am J Roentgenol. 2002 Aug;179(2):385-9. doi: 10.2214/ajr.179.2.1790385
- Gupta A, Desai A, Bhatt S. Imaging of the Endometrium: Physiologic Changes and Diseases: Women’s Imaging. Radiographics. 2017 Nov-Dec;37(7):2206-2207. doi: 10.1148/rg.2017170008
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a7
- Siebers AG, Verbeek AL, Massuger LF, Grefte JM, Bulten J. Normal appearing endometrial cells in cervical smears of asymptomatic postmenopausal women have predictive value for significant endometrial pathology. Int J Gynecol Cancer. 2006 May-Jun;16(3):1069-74. doi: 10.1111/j.1525-1438.2006.00578.x
- ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Bleeding. Obstet Gynecol. 2018 May;131(5):e124-e129. doi: 10.1097/AOG.0000000000002631
- Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, Holschneider CH. Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol. 2006 Apr;101(1):120-5. doi: 10.1016/j.ygyno.2005.09.042
- Suh-Burgmann E, Hung YY, Armstrong MA. Complex atypical endometrial hyperplasia: the risk of unrecognized adenocarcinoma and value of preoperative dilation and curettage. Obstet Gynecol. 2009 Sep;114(3):523-529. doi: 10.1097/AOG.0b013e3181b190d5
- Premalignant Lesions of the Endometrium. https://emedicine.medscape.com/article/269919-overview#a8
- BUTTINI, M.J., JORDAN, S.J. and WEBB, P.M. (2009), The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: An Australian study and systematic review. Australian and New Zealand Journal of Obstetrics and Gynaecology, 49: 316-322. https://doi.org/10.1111/j.1479-828X.2009.00981.x
- Gambrell RD Jr. Progestogens in estrogen-replacement therapy. Clin Obstet Gynecol. 1995 Dec;38(4):890-901. doi: 10.1097/00003081-199538040-00023
- Orbo, A., Vereide, A., Arnes, M., Pettersen, I., & Straume, B. (2014). Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG : an international journal of obstetrics and gynaecology, 121(4), 477–486. https://doi.org/10.1111/1471-0528.12499
- Scarselli G, Bargelli G, Taddei GL, Marchionni M, Peruzzi E, Pieralli A, Mattei A, Buccoliero AM, Fambrini M. Levonorgestrel-releasing intrauterine system (LNG-IUS) as an effective treatment option for endometrial hyperplasia: a 15-year follow-up study. Fertil Steril. 2011 Jan;95(1):420-2. doi: 10.1016/j.fertnstert.2010.07.1044
- Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010 Dec;203(6):547.e1-10. doi: 10.1016/j.ajog.2010.07.037
- Reed, S. D., Voigt, L. F., Newton, K. M., Garcia, R. H., Allison, H. K., Epplein, M., Jordan, D., Swisher, E., & Weiss, N. S. (2009). Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstetrics and gynecology, 113(3), 655–662. https://doi.org/10.1097/AOG.0b013e318198a10a
- Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012 Oct;207(4):266.e1-12. doi: 10.1016/j.ajog.2012.08.011
- Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012 May;125(2):477-82. doi: 10.1016/j.ygyno.2012.01.003
- Morotti M, Menada MV, Moioli M, Sala P, Maffeo I, Abete L, Fulcheri E, Menoni S, Venturini P, Papadia A. Frozen section pathology at time of hysterectomy accurately predicts endometrial cancer in patients with preoperative diagnosis of atypical endometrial hyperplasia. Gynecol Oncol. 2012 Jun;125(3):536-40. doi: 10.1016/j.ygyno.2012.02.011
- Mutter, G. L., Kauderer, J., Baak, J. P., Alberts, D., & Gynecologic Oncology Group (2008). Biopsy histomorphometry predicts uterine myoinvasion by endometrial carcinoma: a Gynecologic Oncology Group study. Human pathology, 39(6), 866–874. https://doi.org/10.1016/j.humpath.2007.09.023
- Lacey, J. V., Jr, Sherman, M. E., Rush, B. B., Ronnett, B. M., Ioffe, O. B., Duggan, M. A., Glass, A. G., Richesson, D. A., Chatterjee, N., & Langholz, B. (2010). Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 28(5), 788–792. https://doi.org/10.1200/JCO.2009.24.1315
- What Is Endometrial Cancer? https://www.cancer.org/cancer/endometrial-cancer/about/what-is-endometrial-cancer.html
- Sorosky JI. Endometrial cancer. Obstetrics and Gynecology. 2012 Aug;120(2 Pt 1):383-397. DOI: 10.1097/aog.0b013e3182605bf1
- Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol. 2015 Apr;125(4):1006-1026. doi: 10.1097/01.AOG.0000462977.61229.de
- Endometrial Cancer Stages. https://www.cancer.org/cancer/endometrial-cancer/detection-diagnosis-staging/staging.html
- PDQ Adult Treatment Editorial Board. Endometrial Cancer Treatment (PDQ®): Patient Version. 2020 Nov 13. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [Figure, Stage IA and stage IB…] Available from: https://www.ncbi.nlm.nih.gov/books/NBK65896/figure/CDR0000062964__224
- PDQ Adult Treatment Editorial Board. Endometrial Cancer Treatment (PDQ®): Health Professional Version. 2022 Feb 24. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65829
- Womb cancer survival. https://www.cancerresearchuk.org/about-cancer/womb-cancer/survival
- Risk Factors for Uterine Sarcoma. https://www.cancer.org/cancer/uterine-sarcoma/causes-risks-prevention/risk-factors.html
- RB1 gene. https://medlineplus.gov/genetics/gene/rb1
- How Is Uterine Sarcoma Diagnosed? https://www.cancer.org/cancer/uterine-sarcoma/detection-diagnosis-staging/how-diagnosed.html
- Uterine Sarcoma Stages. https://www.cancer.org/cancer/uterine-sarcoma/detection-diagnosis-staging/staging.html
- Survival Rates for Uterine Sarcoma. https://www.cancer.org/cancer/uterine-sarcoma/detection-diagnosis-staging/survival-rates.html
- National Cancer Institute. https://www.cancer.gov/
- American Cancer Society. https://www.cancer.org/
- Faizan U, Muppidi V. Uterine Cancer. [Updated 2022 May 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562313
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005 Aug 6-12;366(9484):491-505. doi: 10.1016/S0140-6736(05)67063-8
- Coll-de la Rubia, E., Martinez-Garcia, E., Dittmar, G., Gil-Moreno, A., Cabrera, S., & Colas, E. (2020). Prognostic Biomarkers in Endometrial Cancer: A Systematic Review and Meta-Analysis. Journal of clinical medicine, 9(6), 1900. https://doi.org/10.3390/jcm9061900