Vitamin A deficiency
Vitamin A deficiency usually results from inadequate intakes of vitamin A from animal products (as preformed vitamin A found in fish, liver, dairy products, and eggs) and fruits, vegetables, and other plant-based products (as provitamin A carotenoids that are turned into vitamin A by your body). Vitamin A is important for normal vision, the immune system, male and female reproduction, and growth and development 1, 2, 3. Vitamin A also helps your heart, lungs, eyes, and other organs to work properly 1, 2. Vitamin A is also critical for vision as an essential component of rhodopsin, the light-sensitive protein in the retina that responds to light entering the eye, and because it supports the normal differentiation and functioning of the conjunctival membranes and cornea 4, 2. Vitamin A deficiency is rare in the United States, except in individuals with poor absorption of fats due to cystic fibrosis, ulcerative colitis, Crohn’s disease, weight loss surgery, short bowel syndrome, celiac disease, and, liver disease 5, 6, 7. However, vitamin A deficiency is common in many developing countries, often because residents have limited access to foods containing preformed vitamin A from animal-based food sources and they do not commonly consume available foods containing beta-carotene due to poverty or traditional diets 8, 9. A pooled analysis of population-based surveys from 138 low-income and middle-income countries found that 29% of children aged 6 months to 5 years had vitamin A deficiency in 2013 10. Vitamin A deficiency rates were highest in sub-Saharan Africa (48%) and South Asia (44%) 10. In addition, approximately 10% to 20% of pregnant people in low-income countries have vitamin A deficiency 11. Most vitamin A is stored in the liver, so measuring vitamin A levels in the liver is the best way to assess vitamin A adequacy 1. In clinical practice, plasma retinol levels alone can be used to document significant vitamin A deficiency 12. A serum or plasma retinol concentration of 0.70 μmol/L (20 mcg/dL) or less frequently reflects subclinical vitamin A deficiency, and a serum or plasma retinol level of 0.35 μmol/L (10 mcg/dL) or less is considered an indicator of severe vitamin A deficiency, where vitamin A body stores are depleted 1. Of note, the World Health Organization (WHO) considers vitamin A deficiency a public health problem when the prevalence of low serum retinol (<0.70 μmol/L) reaches 15% or more of a defined population 13. Long‐term or severe vitamin A deficiency may lead to eye lesions such as xerophthalmia (inability to see in low light) and keratomalacia (an eye disorder that involves drying and clouding of the cornea), eventually resulting in visual impairment, blindness, skin disease and growth retardation in children 14. The most common clinical sign of vitamin A deficiency is xerophthalmia, which develops after plasma retinol has been low and the eye’s vitamin A reserves have become depleted. The first sign is night blindness, or the inability to see in low light or darkness as a result of low rhodopsin levels in the retina 1, 10, 11. Xerophthalmia also affects the cornea and can eventually lead to permanent blindness; vitamin A deficiency is one of the top causes of preventable blindness in children 11.
In developing countries, vitamin A deficiency and associated disorders predominantly affect children and women of reproductive age. According to the World Health Organization (WHO), 190 million preschool-aged children and 19.1 million pregnant women around the world have a serum retinol concentration below 0.70 micromoles/L 15. In these countries, low vitamin A intake is most strongly associated with health consequences during periods of high nutritional demand, such as during infancy, childhood, pregnancy, and lactation.
Vitamin A deficiency is the leading cause of preventable blindness in children worldwide:
- Impaired dark adaptation (night blindness) due to lack of the photoreceptor pigment rhodopsin.
- Xerophthalmia: dry, thickened conjunctiva and cornea
- Bitot spots: keratinized growths (metaplasia) on the conjunctivae causing hazy vision
- Keratomalacia: corneal erosions and ulceration
Vitamin A deficiency can also be recognized by its keratinizing effect on the skin and mucous membranes:
- Dry, scaly, thickened skin with prominent follicular scale (phrynoderma or follicular hyperkeratosis)
- Dry lips and thickened tongue
- Keratinisation of the urinary, gastrointestinal and respiratory tracts
Other symptoms and signs of vitamin A deficiency are:
- Impaired immunity leading to gastrointestinal and respiratory tract infections
- Growth retardation in children
Chronic vitamin A deficiency has also been associated with abnormal lung development, respiratory diseases (such as pneumonia), and an increased risk of anemia and death 16, 10, 17.
Another effect of chronic vitamin A deficiency is increased severity and mortality risk of infections (particularly measles and infection-associated diarrhea) 16. In 2013, 94,500 children in low-income and middle-income countries died of diarrhea and 11,200 died of measles as a result of vitamin A deficiency 10. More than 95% of deaths attributable to vitamin A deficiency occurred in sub-Saharan Africa and Asia, where vitamin A deficiency was responsible for 2% of all deaths in children younger than 5 years 10.
In developing countries, vitamin A deficiency typically begins during infancy, when infants do not receive adequate supplies of colostrum or breast milk 15. Chronic diarrhea also leads to excessive loss of vitamin A in young children, and vitamin A deficiency increases the risk of diarrhea 18. The most common symptom of vitamin A deficiency in young children and pregnant women is xerophthalmia. One of the early signs of xerophthalmia is night blindness, or the inability to see in low light or darkness 9. Vitamin A deficiency is one of the top causes of preventable blindness in children 15. People with vitamin A deficiency (and, often, xerophthalmia with its characteristic Bitot’s spots) tend to have low iron status, which can lead to anemia 15. Vitamin A deficiency also increases the severity and mortality risk of infections (particularly diarrhea and measles) even before the onset of xerophthalmia 15.
During pregnancy, vitamin A is essential for fetal organ and skeletal growth and maturation, maintenance of the maternal immune system, development of vision in the fetus, and maintenance of maternal eye health and night vision 19. Although pregnant women are susceptible to vitamin A deficiency throughout gestation, deficiency is most common in the third trimester. It is unclear whether this is due to increased demands during pregnancy from accelerated fetal development and the physiological increase in blood volume during this period 20, or to lowered serum retinol concentration due to an increase in plasma volume. In a pregnant woman with moderate vitamin A deficiency, the fetus can still obtain sufficient vitamin A to develop appropriately but at the expense of the maternal vitamin A stores 21.
Figure 1. Eye signs of vitamin A deficiency
Footnote: A 24-year-old, 26 weeks pregnant woman with a 7-week history of progressive loss of vision, most notable at night. The woman had anorexia nervosa and had limited her diet to white onions, white potatoes, and red meat for the past 7 years. Numerous Bitot spots (figure A) and keratitis (figure B) were seen on examination of her eyes; however, her retina was grossly normal (figure C, D). Electroretinography showed a generalized rod-cone dysfunction, and fundus autofluorescence showed faint retinal vasculature in her right eye (figure E) and a black image in the left eye. Autofluorescence from the left eye of an age-match control is shown (figure F). Acquired vitamin A deficiency was confirmed by a serum concentration of <0·002 μmol/L (normal range 0·70–2·8 μmol/L). Her vision and the appearance of her anterior segment returned to normal with vitamin A supplementation.
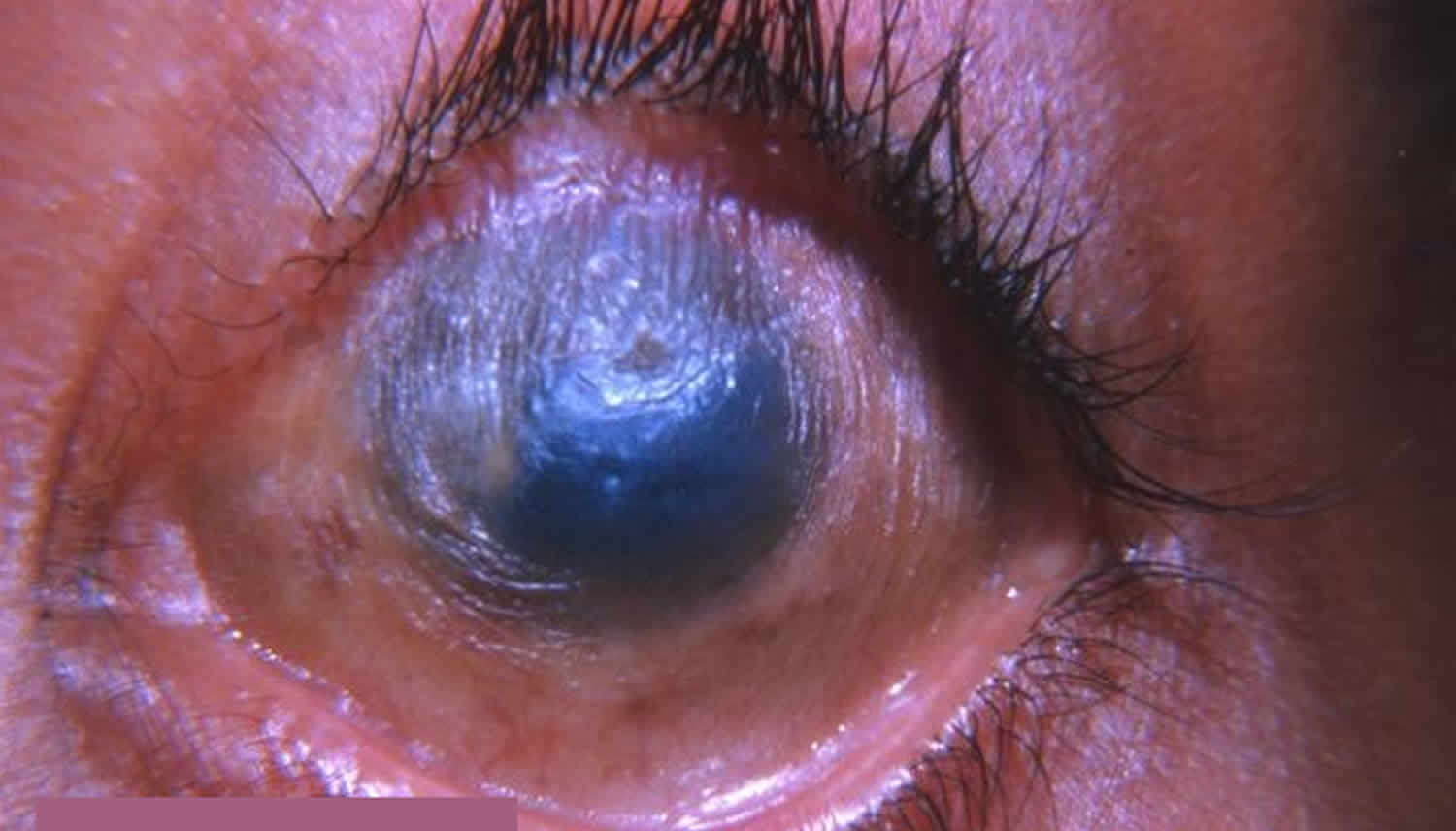
[Source 22]Figure 2. Bitot spots
Footnote: Left eye of an eight-year-old boy, with a large, superficial, triangular, foamy, keratinized patch in the interpalpebral region over the bulbar conjunctiva, adjacent to the temporal limbus (black arrow), suggestive of Bitot’s spots.
[Source 23 ]Figure 3. Conjunctival xerosis (dryness of the conjunctiva)
Footnote: Conjunctival xerosis (dryness of the conjunctiva) is another sign of long-standing vitamin A deficiency. Note the slight wrinkling of the temporal conjunctiva. Conjunctival xerosis can be quite difficult to detect and is therefore not a very reliable sign.
[Source 24 ]Figure 4. Corneal xerosis (drying of the cornea)
Footnote: Corneal xerosis (drying of the cornea) is a sign of sudden, acute vitamin A deficiency. The cornea becomes dry because glands in the conjunctiva no longer function normally. This leads to loss of tears and also loss of mucous, which acts as a ‘wetting agent’. The lack of mucus together with lack of tears not only leads to the dry appearance but also increases the risk of eye infection.
[Source 24 ]Figure 5. Corneal ulcer due to vitamin A deficiency
Footnote: If the acute vitamin A deficiency is not reversed as a matter of urgency, the cornea can become ulcerated and melt away. The corneal ulcer may have the appearance of a small, punched-out area in the cornea (top image), or the ulcer may have a more fluffy appearance (lower picture). In the absence of secondary infection, the eye can look surprisingly white, as in both images; however, secondary infection of the corneal ulcer is common, leading to an acutely inflamed eye.
[Source 24 ]Figure 6. Corneal ulcer due to vitamin A deficiency
Footnote: Right eye showing corneal ulcer measuring 2 mm X 10 mm (red arrow) in the setting of chronic alcoholism.
[Source 25 ]Figure 7. Keratomalacia (corneal ulcer covering at least 1/3 of the cornea)
Footnote: The most severe form of vitamin A deficiency xerophthalmia is keratomalacia, in which more than one-third of the cornea is affected. The cornea may become edematous and thickened, and then melt away. This occurs because the structure of the collagen in the cornea is affected by a process known as necrosis. The cornea can be destroyed in just a few days. Children with keratomalacia are often malnourished, but children who previously appeared relatively healthy can also develop keratomalacia following measles infection or episodes of diarrhea; this is usually because they were vitamin A deficient and the measles infection resulted in depletion of their vitamin A stores. If you are not sure whether the child you are seeing has keratomalacia, ask about recent illness, particularly measles.
[Source 26 ]Figure 8. Corneal scarring
Footnote: The end result of corneal ulceration and keratomalacia is corneal scarring, staphylomas (forward bulging of a badly damaged cornea) or phthisis bulbi (an eye that has shrivelled up), depending on the extent of the pathology in the cornea. Most of the eye signs of vitamin A deficiency are symmetrical and bilateral, and so can lead to blindness.
[Source 24 ]Vitamin A deficiency can result from inadequate intake, fat malabsorption, or liver disorders. Vitamin A deficiency impairs immunity and formation of red blood cells (hematopoiesis) and causes skin rashes and typical eye signs and symptoms (e.g., Bitot spots, conjunctival xerosis, corneal xerosis, xerophthalmia, night blindness). Vegetarians, young children, and alcoholics may need extra vitamin A. You might also need more if you have certain conditions, such as liver diseases, cystic fibrosis, Crohn’s disease and ulcerative colitis 27.
Vitamin A deficiency diagnosis is based on typical ocular findings and low vitamin A levels. Treatment consists of vitamin A given orally or if symptoms are severe or malabsorption is the cause, parenterally.
The most specific clinical effect of inadequate vitamin A intake is xerophthalmia 28. It is estimated that 3 to 10 million children, mostly in developing countries, become xerophthalmic, and 250,000 to 500,000 go blind annually 29, 30. The World Health Organization 31 classified various stages of xerophthalmia to include night blindness (impaired dark adaptation due to slowed regeneration of rhodopsin), conjunctival xerosis, Bitot’s spots, corneal xerosis, corneal ulceration, and scarring, all related to vitamin A deficiency. Night blindness is the first ocular symptom to be observed with vitamin A deficiency 32, and it responds rapidly to treatment with vitamin A 33. High-dose (60 mg) vitamin A supplementation reduced the incidence of night blindness by 63 percent in Nepalese children 34. Similarly, night blindness was reduced by 50 percent in women after weekly supplementation with either 7,500 μg RE of vitamin A or β-carotene 35.
Because of the role of vitamin A in maintaining the structural integrity of epithelial cells, follicular hyperkeratosis has been observed with inadequate vitamin A intake 36, 37. Men who were made vitamin A deficient under controlled conditions were then supplemented with either retinol or β-carotene, which caused the hyperkeratosis to gradually clear 37.
Vitamin A deficiency has been associated with a reduction in lymphocyte numbers, natural killer cells, and antigen-specific immunoglobulin responses 38, 39. A decrease in leukocytes and lymphoid organ weights, impaired T cell function, and decreased resistance to immunogenic tumors have been observed with inadequate vitamin A intake 40, 41. A generalized dysfunction of humoral and cell-mediated immunity is common in experimental animals and is likely to exist in humans.
In addition to xerophthalmia, vitamin A deficiency has been associated with increased risk of infectious morbidity and mortality in experimental animals and humans, especially in developing countries. A higher risk of respiratory infection and diarrhea has been reported among children with mild to moderate vitamin A deficiency 42. Mortality rates were about four times greater among children with mild xerophthalmia than those without it 43. The risk of severe morbidity and mortality decreases with vitamin A repletion. In children hospitalized with measles, case fatality 44, 45 and the severity of complications on admission were reduced when they received high doses (60 to 120 mg) of vitamin A 46, 45. In some studies, vitamin A supplementation (30 to 60 mg) has been shown to reduce the severity of diarrhea 47, 48 and Plasmodium falciparum malaria 49 in young children, but vitamin A supplementation has had little effect on the risk or severity of respiratory infections, except when associated with measles 50.
In developing countries, vitamin A supplementation has been shown to reduce the risk of mortality among young children 51, 52, 53, 54, 55, infants 50, and pregnant and postpartum women 56. Meta-analyses of the results from these and other community-based trials are consistent with a 23 to 30 percent reduction in mortality of young children beyond 6 months of age after vitamin A supplementation 57, 58, 59. The World Health Organization recommends broad-based prophylaxis in vitamin A-deficient populations. It also recommends treating children who suffer from xerophthalmia, measles, prolonged diarrhea, wasting malnutrition, and other acute infections with vitamin A 60. Furthermore, the American Academy of Pediatrics 61 recommends vitamin A supplementation for children in the United States who are hospitalized with measles.
Who is at risk of vitamin A deficiency?
The following groups are among those most likely to have inadequate intakes of vitamin A.
Premature infants
Preterm infants have low liver stores of vitamin A at birth, and their plasma concentrations of retinol often remain low throughout the first year of life 62, 63. Preterm infants with vitamin A deficiency have a higher risk of eye and chronic lung diseases 64, 65. However, in high-income countries, clinical vitamin A deficiency is rare in infants and occurs only in those with malabsorption disorders 66.
Infants, children, and pregnant and lactating women in low-income and middle-income countries
Pregnant women need extra vitamin A for fetal growth and tissue maintenance and to support their own metabolism 67, 68, 69. The breast milk of lactating women with adequate vitamin A intakes contains sufficient amounts of vitamin A to meet infants’ needs for the first 6 months of life70. But in people with vitamin A deficiency, the vitamin A content of breast milk is not sufficient to maintain adequate vitamin A stores in infants who are exclusively breastfed 70.
About 190 million preschool-aged children (one third of all children in this age group), mostly in Africa and Southeast Asia, have vitamin A deficiency, according to the World Health Organization 71, 10. They have a higher risk of visual impairment and of illness and death from childhood infections, such as measles and infections that cause diarrheal diseases 71, 1.
The World Health Organization estimates that 9.8 million pregnant women (15% of all pregnant women) around the world, mostly in low-income and middle-income countries, have xerophthalmia as a result of vitamin A deficiency 72.
People with cystic fibrosis
Up to 90% of people with cystic fibrosis have pancreatic insufficiency, which increases their risk of vitamin A deficiency due to difficulty absorbing fat 1, 73. Studies in Australia and the Netherlands indicate that 2% to 13% of children and adolescents with cystic fibrosis have vitamin A deficiency 74, 75. As a result, standard care for cystic fibrosis includes lifelong treatment with vitamin A (daily amounts of 750 mcg RAE to 3,000 mcg RAE, depending on age, are recommended in the United States and Australia), other fat-soluble vitamins, and pancreatic enzymes 73, 75.
Individuals with gastrointestinal disorders
Approximately one quarter of children with Crohn’s disease and ulcerative colitis have vitamin A deficiency; adults with these disorders, especially those who have had the disorder for several years, also have a higher risk of vitamin A deficiency 76, 77. Although some evidence supports the use of vitamin A supplements in people with these disorders 78, other research has found that supplementation offers no benefit 79. Some children and adults with newly diagnosed celiac disease also have vitamin A deficiency; a gluten-free diet can, but does not always, eliminate vitamin A deficiency 80, 81, 82.
What is vitamin A
Vitamin A is the name of a group of fat-soluble retinoids, including retinol, retinal, and retinyl esters 83. Vitamin A is involved in immune function, vision, reproduction, and cellular communication 83. Vitamin A is critical for vision as an essential component of rhodopsin, a protein that absorbs light in the retinal receptors, and because it supports the normal differentiation and functioning of the conjunctival membranes and cornea 9. Vitamin A also supports cell growth and differentiation, playing a critical role in the normal formation and maintenance of the heart, lungs, kidneys, and other organs 9.
Two forms of vitamin A are available in the human diet 9:
- Preformed vitamin A (retinol and its esterified form, retinyl ester). Preformed vitamin A is found in foods from animal sources, such as fish, organ meats (such as liver), dairy products, and eggs.
- Provitamin A carotenoids. Provitamin A carotenoids are turned into vitamin A by your body. Provitamin A carotenoids are found in fruits, vegetables, and other plant-based products. The most common provitamin A carotenoid in foods and dietary supplements is beta-carotene; other provitamin A carotenoids are alpha-carotene and beta-cryptoxanthin.
Both provitamin A and preformed vitamin A must be metabolized inside the cell to retinal and retinoic acid, the active forms of vitamin A, to support the vitamin’s important biological functions 9. Retinol can be converted by the body to retinal, which can be in turn be oxidized to retinoic acid, the form of vitamin A known to regulate gene transcription 84. Retinol, retinal, retinoic acid, and related compounds are known as retinoids. Beta-carotene and other food carotenoids that can be converted by the body into retinol are referred to as provitamin A carotenoids. Hundreds of different carotenoids are synthesized by plants, but only about 10% of them are capable of being converted to retinol 85. Other carotenoids found in food, such as lycopene, lutein, and zeaxanthin, are not converted into vitamin A.
The various forms of vitamin A are solubilized into micelles in the intestinal lumen and absorbed by duodenal mucosal cells 86. Both retinyl esters and provitamin A carotenoids are converted to retinol, which is oxidized to retinal and then to retinoic acid 9. Most of the body’s vitamin A is stored in the liver in the form of retinyl esters.
Retinol and carotenoid levels are typically measured in plasma, and plasma retinol levels are useful for assessing vitamin A inadequacy. However, their value for assessing marginal vitamin A status is limited because they do not decline until vitamin A levels in the liver are almost depleted 87. Liver vitamin A reserves can be measured indirectly through the relative dose-response test, in which plasma retinol levels are measured before and after the administration of a small amount of vitamin A 86. A plasma retinol level increase of at least 20% indicates an inadequate vitamin A level 87. For clinical practice purposes, plasma retinol levels alone are sufficient for documenting significant deficiency.
A plasma retinol concentration lower than 0.70 micromoles/L (or 20 micrograms [mcg]/dL) reflects vitamin A inadequacy in a population, and concentrations of 0.70–1.05 micromoles/L could be marginal in some people 86. In some studies, high plasma or serum concentrations of some provitamin A carotenoids have been associated with a lower risk of various health outcomes, but these studies have not definitively demonstrated that this relationship is causal.
Overconsumption of preformed vitamin A (vitamin A in the form retinol and retinyl ester, but not carotenoids) can be highly toxic and is also called hypervitaminosis A, is especially contraindicated prior to and during pregnancy as it can result in severe birth defects 5, 88, 84. These birth defects can include malformations of the eye, skull, lungs, and heart 89. Experts advise people who are or might be pregnant and those who are lactating not to take high doses (more than 3,000 mcg RAE [10,000 IU] daily) of vitamin A supplements 1.
Acute vitamin A toxicity is relatively rare and symptoms include nausea, headache, fatigue, loss of appetite, dizziness, dry skin, desquamation, and cerebral edema 84. Signs of chronic vitamin A toxicity include dry itchy skin, desquamation, anorexia, weight loss, headache, cerebral edema, enlarged liver, enlarged spleen, anemia, and bone and joint pain 84. Also, symptoms of vitamin A toxicity in infants include bulging fontanels 84. Severe cases of hypervitaminosis A (overconsumption of preformed vitamin A) may result in liver damage, hemorrhage, and coma. Generally, signs of vitamin A toxicity are associated with long-term consumption of vitamin A in excess of 10 times the RDA (8,000-10,000 mcg RAE/day or 25,000-33,000 IU/day) 84. However, more research is necessary to determine if subclinical vitamin A toxicity is a concern in certain populations 90. There is evidence that some populations may be more susceptible to vitamin A toxicity at lower doses, including the elderly, chronic alcohol users, and some people with a genetic predisposition to high cholesterol 91. In January 2001, the Food and Nutrition Board of the US Institute of Medicine set the tolerable upper intake level (UL) of vitamin A intake for adults at 3,000 mcg RAE (10,000 IU)/day of preformed vitamin A 88. The tolerable upper intake level (UL) does not apply to vitamin A derived from carotenoids. Retinol activity equivalents (RAE) were developed because provitamin A carotenoids have less vitamin A activity than preformed vitamin A; 1 mcg retinol = 3.33 IU.
Figure 9. Vitamin A chemical structures
Footnote: Structures of vitamin A and retinoids
[Source 4 ]Recommended vitamin A intakes
The amount of vitamin A you need depends on your age and sex. Average daily recommended amounts of preformed vitamin A and provitamin A carotenoids are listed below in micrograms (mcg) of retinol activity equivalents (RAE).
Intake recommendations for vitamin A and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies (formerly National Academy of Sciences) 86. Dietary Reference Intake (DRI) is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and gender, include:
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
- Estimated Average Requirement (EAR): Average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals.
- Tolerable Upper Intake Level (UL): Maximum daily intake unlikely to cause adverse health effects.
Recommended Dietary Allowances (RDAs) for vitamin A are given as mcg of retinol activity equivalents (RAE) to account for the different bioactivities of retinol and provitamin A carotenoids (see Table 1). Because the body converts all dietary sources of vitamin A into retinol, 1 mcg of physiologically available retinol is equivalent to the following amounts from dietary sources: 1 mcg of retinol, 12 mcg of beta-carotene, and 24 mcg of alpha-carotene or beta-cryptoxanthin. From dietary supplements, the body converts 2 mcg of beta-carotene to 1 mcg of retinol.
Retinol activity equivalents (RAE) were developed because provitamin A carotenoids have less vitamin A activity than preformed vitamin A; 1 mcg retinol = 3.33 IU.
Currently, vitamin A is listed on food and supplement labels in international units (IUs) even though nutrition scientists rarely use this measure. Conversion rates between mcg RAE and IU are as follows 92:
- 1 IU retinol = 0.3 mcg RAE (retinol activity equivalents)
- 1 IU beta-carotene from dietary supplements = 0.15 mcg RAE
- 1 IU beta-carotene from food = 0.05 mcg RAE
- 1 IU alpha-carotene or beta-cryptoxanthin = 0.025 mcg RAE
However, under FDA’s new labeling regulations for foods and dietary supplements that take effect by January 1, 2020 (for companies with annual sales of $10 million or more) or January 1, 2021 (for smaller companies), vitamin A will be listed only in mcg RAE and not IUs 93.
An RAE cannot be directly converted into an IU without knowing the source(s) of vitamin A. For example, the RDA of 900 mcg RAE for adolescent and adult men is equivalent to 3,000 IU if the food or supplement source is preformed vitamin A (retinol). However, this RDA is also equivalent to 6,000 IU of beta-carotene from supplements, 18,000 IU of beta-carotene from food, or 36,000 IU of alpha-carotene or beta-cryptoxanthin from food. So a mixed diet containing 900 mcg RAE provides between 3,000 and 36,000 IU of vitamin A, depending on the foods consumed.
Table 1. Recommended Dietary Allowances (RDAs) for Vitamin A
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months* | 400 mcg RAE |
| Infants 7–12 months* | 500 mcg RAE |
| Children 1–3 years | 300 mcg RAE |
| Children 4–8 years | 400 mcg RAE |
| Children 9–13 years | 600 mcg RAE |
| Teen males 14–18 years | 900 mcg RAE |
| Teen females 14–18 years | 700 mcg RAE |
| Adult males | 900 mcg RAE |
| Adult females | 700 mcg RAE |
| Pregnant teens | 750 mcg RAE |
| Pregnant adults | 770 mcg RAE |
| Breastfeeding teens | 1,200 mcg RAE |
| Breastfeeding adults | 1,300 mcg RAE |
Footnote: * Adequate Intake (AI), equivalent to the mean intake of vitamin A in healthy, breastfed infants.
[Source 86 ]Sources of Vitamin A
Vitamin A is found naturally in many foods and is added to some foods, such as milk and cereal. You can get recommended amounts of vitamin A by eating a variety of foods, including the following:
- Some types of fish, such as herring and salmon
- Beef liver and other organ meats (which are also high in cholesterol, so limit the amount you eat)
- Green leafy vegetables and other green, orange, and yellow vegetables, such as spinach, sweet potatoes, carrots, broccoli, and winter squash
- Fruits, including cantaloupe, mangos, and apricots
- Dairy products, such as milk and cheese
- Fortified breakfast cereals
- Eggs
Free retinol is not generally found in food. Retinyl esters (including retinyl palmitate) are the storage form of retinol in animals and thus the main precursors of retinol in food from animals. Plants contain carotenoids, some of which are precursors for vitamin A (e.g., α-carotene, β-carotene, and β-cryptoxanthin). Yellow- and orange-colored vegetables contain significant quantities of carotenoids. Green vegetables also contain carotenoids, though yellow-to-red pigments are masked by the green pigment of chlorophyll 85.
Concentrations of preformed vitamin A are highest in liver, fish, eggs, and dairy products 1. Other sources of preformed vitamin A are milk and eggs, which also include some provitamin A 2. Most dietary provitamin A in the U.S. diet comes from leafy green vegetables, orange and yellow vegetables, tomato products, fruits, and some vegetable oils 1, 89, 94. Vitamin A is routinely added to some foods, including milk and margarine 1. Some ready-to-eat cereals are also fortified with vitamin A.
Table 2 below lists a number of good food sources of vitamin A. The foods from animal sources in Table 2 contain primarily preformed vitamin A, the plant-based foods have provitamin A, and the foods with a mixture of ingredients from animals and plants contain both preformed vitamin A and provitamin A.
Table 2. Selected Food Sources of Vitamin A
| Food | mcg RAE per serving | Percent DV* |
|---|---|---|
| Beef liver, pan fried, 3 ounces | 6582 | 731 |
| Sweet potato, baked in skin, 1 whole | 1403 | 156 |
| Spinach, frozen, boiled, ½ cup | 573 | 64 |
| Pumpkin pie, commercially prepared, 1 piece | 488 | 54 |
| Carrots, raw, ½ cup | 459 | 51 |
| Herring, Atlantic, pickled, 3 ounces | 219 | 24 |
| Ice cream, French vanilla, soft serve, ⅔ cup | 185 | 21 |
| Milk, skim, with added vitamin A and vitamin D, 1 cup | 149 | 17 |
| Cantaloupe, raw, ½ cup | 135 | 15 |
| Cheese, ricotta, part skim, ½ cup | 133 | 15 |
| Peppers, sweet, red, raw, ½ cup | 117 | 13 |
| Mangos, raw, 1 whole | 112 | 12 |
| Breakfast cereals, fortified with 10% of the DV for vitamin A, 1 serving | 90 | 10 |
| Egg, hard boiled, 1 large | 75 | 8 |
| Black-eyed peas (cowpeas), boiled, 1 cup | 66 | 7 |
| Apricots, dried, sulfured, 5 apricots | 63 | 7 |
| Broccoli, boiled, ½ cup | 60 | 7 |
| Salmon, sockeye, cooked, 3 ounces | 59 | 7 |
| Tomato juice, canned, ¾ cup | 42 | 5 |
| Yogurt, plain, low fat, 1 cup | 32 | 4 |
| Tuna, light, canned in oil, drained solids, 3 ounces | 20 | 2 |
| Baked beans, canned, plain or vegetarian, 1 cup | 13 | 1 |
| Summer squash, all varieties, boiled, ½ cup | 10 | 1 |
| Chicken, breast meat and skin, roasted, ½ breast | 5 | 1 |
| Pistachio nuts, dry roasted, 1 ounce | 4 | 0 |
Footnote: *DV = Daily Value. U.S. Food and Drug Administration (FDA) developed Daily Values (DVs) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The Daily Value (DV) for vitamin A is 900 mcg RAE for adults and children age 4 years and older, where 1 mcg RAE = 1 mcg retinol, 2 mcg beta-carotene from supplements, 12 mcg beta-carotene from foods, 24 mcg alpha-carotene, or 24 mcg beta-cryptoxanthin 93. FDA does not require food labels to list vitamin A content unless vitamin A has been added to the food. Foods providing 20% or more of the Daily Value (DV) are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin A in IUs arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminA-Food.pdf), and foods containing beta-carotene in mcg arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Food.pdf).
What kinds of vitamin A dietary supplements are available?
Vitamin A is available in dietary supplements, usually in the form of retinyl acetate or retinyl palmitate (preformed vitamin A), beta-carotene (provitamin A), or a combination of preformed and provitamin A 1. Most multivitamin-mineral supplements contain vitamin A. Dietary supplements that contain only vitamin A are also available.
The amounts of vitamin A in supplements vary widely, but 3,000 mcg RAE (333% of the DV) is common. This is due to the fact that the Daily Values (DV) used by the US Food and Drug Administration (FDA) for supplement labeling are based on the RDA established in 1968 rather than the most recent RDA, and multivitamin supplements typically provide 100% of the DV for most nutrients.
Multivitamins commonly have somewhat lower vitamin A amounts, often 750 to 1,050 mcg RAE (83% to 117% of the DV). Because retinol intakes of 5,000 IU/day (1,500 mcg RAE) may be associated with an increased risk of osteoporosis in older adults, some companies have reduced the retinol content in their multivitamin supplements to 2,500 IU (750 mcg RAE).
The absorption of preformed vitamin A esters from dietary supplements is 70–90%, and that of beta-carotene ranges from 8.7% to 65% 95, 96.
What is the tolerable upper intake level of vitamin A?
Because vitamin A is fat soluble, the body stores excess vitamin A primarily in the liver, and these vitamin A levels can accumulate. Acute vitamin A toxicity also referred to as hypervitaminosis A, occurs within days to weeks after someone ingests one or a few very high doses (typically more than 100 times the Recommended Dietary Allowance [RDA]) of preformed vitamin A (retinol and retinyl ester but not carotenoids) 97. Resulting signs and symptoms typically include severe headache, blurred vision, nausea, dizziness, aching muscles, and coordination problems. In severe cases, cerebral spinal fluid pressure can increase, leading to drowsiness and, eventually, coma and even death 97. In January 2001, the Food and Nutrition Board of the US Institute of Medicine set the tolerable upper intake level (UL) of vitamin A intake for adults at 3,000 mcg RAE (10,000 IU)/day of preformed vitamin A 88. The tolerable upper intake level (UL) does not apply to vitamin A derived from carotenoids (vitamin A in fruits and vegetables) (see Table 3).
- Recommended Dietary Allowance (RDA) is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Retinol activity equivalents (RAE) were developed because provitamin A carotenoids have less vitamin A activity than preformed vitamin A; 1 mcg retinol = 3.33 IU.
Chronic hypervitaminosis A (regular consumption of high doses of vitamin A) can cause dry itchy skin, painful muscles and joints, fatigue, depression, weight loss, headache, cerebral edema, enlarged liver, enlarged spleen, anemia and abnormal liver test results 97, 84. Also, symptoms of vitamin A toxicity in infants include bulging fontanels 84. Severe cases of hypervitaminosis A (overconsumption of preformed vitamin A) may result in liver damage, hemorrhage, and coma. Generally, signs of vitamin A toxicity are associated with long-term consumption of vitamin A in excess of 10 times the RDA (8,000-10,000 mcg RAE/day or 25,000-33,000 IU/day) 84. However, more research is necessary to determine if subclinical vitamin A toxicity is a concern in certain populations 90. There is evidence that some populations may be more susceptible to vitamin A toxicity at lower doses, including the elderly, chronic alcohol users, and some people with a genetic predisposition to high cholesterol 91.
Total intakes of preformed vitamin A that exceed the tolerable upper intake level (UL), as well as some retinoid medications used as topical therapies (such as isotretinoin, used to treat severe acne, and etretinate, a treatment for severe psoriasis) can cause congenital birth defects 1. These birth defects can include malformations of the eye, skull, lungs, and heart 89. Experts advise people who are or might be pregnant and those who are lactating not to take high doses (more than 3,000 mcg RAE [10,000 IU] daily) of vitamin A supplements 1.
Unlike preformed vitamin A, beta-carotene (provitamin A) is not known to be teratogenic or lead to reproductive toxicity 1. The most common effect of long-term, excess beta-carotene is carotenodermia, a harmless condition in which the skin becomes yellow-orange 98. Carotenodermia or excess beta-carotene can be reversed by discontinuing beta-carotene ingestion. However, the ATBC trial found that supplementation with a large amount of beta-carotene (20 mg/day), with or without 50 mg/day vitamin E, for 5–8 years increased the risk of lung cancer and mortality (mainly from lung cancer and ischemic heart disease) in male smokers 99. The CARET trial also showed that supplementation with a large amount of beta-carotene (30 mg/day) plus 7,500 mcg RAE (25,000 IU)/day retinyl palmitate for 4–8 years in current and former smokers, as well as some men occupationally exposed to asbestos, increased the risk of lung cancer and death from lung cancer 100.
The Food and Nutrition Board of the US Institute of Medicine has not established tolerable upper intake levels (ULs) for beta-carotene and other provitamin A carotenoids 98. However, the Food and Nutrition Board advises against the use of beta-carotene supplements for the general population, except as a provitamin A source to prevent vitamin A deficiency.
Table 3. Tolerable Upper Intake Levels (ULs) for Preformed Vitamin A
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 12 months | 600 mcg | 600 mcg | ||
| 1–3 years | 600 mcg | 600 mcg | ||
| 4–8 years | 900 mcg | 900 mcg | ||
| 9–13 years | 1,700 mcg | 1,700 mcg | ||
| 14–18 years | 2,800 mcg | 2,800 mcg | 2,800 mcg | 2,800 mcg |
| 19+ years | 3,000 mcg | 3,000 mcg | 3,000 mcg | 3,000 mcg |
Footnotes: These tolerable upper intake levels (ULs) apply only to products from animal sources and supplements whose vitamin A comes entirely from retinol or its ester forms, such as retinyl palmitate. However, many dietary supplements (such as multivitamins) do not provide all of their vitamin A in retinol or its ester forms. For example, the vitamin A in some supplements consists partly or entirely of beta-carotene. In such cases, the percentage of retinol or retinyl ester in the supplement should be used to determine whether an individual’s vitamin A intake exceeds the UL. For example, a supplement whose label indicates that the product contains 3,000 mcg RAE vitamin A and that 60% of this vitamin A comes from beta-carotene (and therefore 40% comes from retinol or retinyl ester) provides 1,200 mcg RAE of preformed vitamin A. That amount is above the UL for children from birth to 8 years but below the UL for older children and adults.
[Source 94 ]Benefits of Vitamin A
Vitamin A is an antioxidant. It can come from plant or animal sources. Plant sources include colorful fruits and vegetables. Animal sources include liver and whole milk. Vitamin A is also added to foods like cereals.
Vitamin A plays a role in your:
- Vision
- Bone growth
- Reproduction 101. The vitamin A metabolite, trans retinoic acid, is essential for reproduction in both the male and female, as well as for many events in the developing embryo.
- Cell functions
- Immune system 102
Scientists are studying vitamin A to understand how it affects health. Here are some examples of what this research has shown.
Cancer
Because of the role vitamin A plays in regulating cell growth and differentiation, several studies have examined the association between vitamin A and various types of cancer. However, the relationship between serum vitamin A levels or vitamin A supplementation and cancer risk or cancer-related death is unclear. However, this does not include studies of all-trans retinoic acid, a vitamin A metabolite that is used as a drug in high doses to treat a form of leukemia 103, 104. Furthermore, in addition to tretinoin and alitretinoin, other generation retinoids are already being used clinically as anti-cancer agents 105, 106.
Several systematic reviews and meta-analyses of observational studies have shown that higher dietary intakes of retinol, carotenoids, fruits and vegetables, or a combination are associated with a lower risk of lung cancer 107, non-Hodgkin lymphoma 108, pancreatic cancer 109, oral cavity cancer 110, laryngeal cancer 110, esophageal cancer 111, ovarian cancer 112, 113, glioma 114, and bladder cancer 115. However, other observational studies have found no association between intakes of different forms of vitamin A and risk of liver cancer 116, non-Hodgkin lymphoma 117, colorectal cancer 118, prostate cancer 118 or all cancers 119.
Some clinical trial evidence suggests that supplemental vitamin A might reduce the risk of certain cancers but increase the risk of other forms of cancer, cardiovascular disease morbidity and mortality, and all-cause mortality. Examples are provided below.
The Carotene and Retinol Efficacy Trial (CARET) included 18,314 male and female current and former smokers (with at least a 20 pack-year history [equivalent to smoking 1 pack per day for 20 years or 2 packs per day for 10 years, for example] of cigarette smoking), as well as some men occupationally exposed to asbestos (who also have a higher risk of lung cancer), all aged 45–74 years. The study randomized participants to take supplements containing 30 mg beta-carotene plus 25,000 IU (7,500 mcg RAE) retinyl palmitate or a placebo daily for about 6 years to evaluate the potential effects on lung cancer risk 100. The trial was ended prematurely after a mean of 4 years, partly because the supplements were unexpectedly found to have increased lung cancer risk by 28% and death from lung cancer by 46%; the supplements (30 mg beta-carotene plus 25,000 IU (7,500 mcg RAE) retinyl palmitate) also increased the risk of all-cause mortality by 17% 100.
A subsequent study followed CARET participants for an additional 6 years after they stopped taking the study supplements 120. During this time, the differences in lung cancer risk between the intervention and placebo groups were no longer statistically significant, with one exception: women in the intervention group had a 33% higher risk of lung cancer. In a separate analysis of CARET study data, men who took the two supplements had a 35% lower risk of nonaggressive prostate cancer during the 4-year active trial, but not during the 6-year postintervention period 121. In contrast, men who took these two supplements in addition to another self-prescribed supplement (typically a multivitamin) had a 52% higher risk of aggressive prostate cancer during the active trial, but not during the postintervention period 121.
The Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study also found that beta-carotene supplements increased the risk of lung cancer in smokers 99. In this study, 29,133 male smokers aged 50–69 years who smoked an average of 20.4 cigarettes a day for an average of 35.9 years took a supplement containing 50 mg/day alpha-tocopherol, 20 mg/day beta-carotene, both alpha-tocopherol and beta-carotene, or a placebo for 5–8 years 99. The beta-carotene supplements increased the risk of lung cancer by 18%, although they had little to no effect on the incidence of other cancers 99. The overall rate of death, primarily from lung cancer and ischemic heart disease, was 8% higher in participants who took beta-carotene. A subsequent study followed 25,563 of these participants for an additional 18 years 122. During this period, participants were no longer taking the supplements, but most continued to smoke. Participants who had taken beta-carotene in the original trial did not have a higher risk of lung cancer, but they had a 20% higher risk of death due to prostate cancer 122.
The Age-Related Eye Disease Study 2 (AREDS2) was a 5-year randomized clinical trial with 4,203 participants aged 50–85 years examining the effects on age-related macular degeneration (AMD) of a dietary supplement containing several ingredients with or without beta-carotene (15 mg [7,500 mcg RAE]) 123. No current smokers received the supplements containing beta-carotene. At the end of the trial, more lung cancers were discovered in the beta-carotene group than in the no beta-carotene group (23 vs 11 cases), and 31 of the 34 affected were former smokers. In a follow-up analysis of 3,882 of the participants 5 years after the end of AREDS2 (during which they took the AREDS2 formulation containing lutein and zeaxanthin instead of beta-carotene), the increased lung cancer risk persisted, with an 82% higher risk among participants who took the supplement containing beta-carotene during the 5-year AREDS2 trial 124.
Three other clinical trials have found no relationship between taking vitamin A or beta-carotene supplements and lung cancer incidence or mortality 125. One trial randomized 22,071 male physicians aged 40–84 years to take 50 mg beta carotene on alternate days or a placebo for 12 years 126. Eleven percent of the physicians were current smokers, and 38% were former smokers at the start of the study. The results showed no differences between the groups in number of cases of lung cancer or any malignant neoplasms or number of deaths from cancer. Another trial randomized 7,627 women (mean age 60.4 years) to take 50 mg beta-carotene on alternate days, 600 IU vitamin E on alternate days, 500 mg vitamin C daily, or a placebo for a mean of 9.4 years 127. Fifteen percent of the women were current smokers, and 41% were former smokers at the start of the study. None of the supplements had any significant effect on total cancer incidence or cancer mortality, including from lung cancer. A third trial included 29,584 healthy men and women aged 40–69 years who were living in Linxian, China, where micronutrient deficiencies are common 128. The study randomized participants to take either a placebo or one of four vitamin and mineral combinations (including one providing retinol and zinc and another providing beta carotene, vitamin E, and selenium) for 5.25 years. The investigators followed participants for an additional 10 years after they stopped taking the supplements. The nutrient doses in the supplements were equivalent to or twice as high as U.S. recommended intakes, but the study report did not provide the exact doses. During both the intervention and follow-up periods, lung cancer death rates did not differ among the five groups, even when the investigators further analyzed the results for differences by age, sex, and smoking status 128.
The Carotene and Retinol Efficacy Trial (CARET) and Alpha-Tocopherol, Beta-Carotene (ATBC) study results suggest that large supplemental doses of beta-carotene with or without retinyl palmitate have detrimental effects in current or former smokers and workers exposed to asbestos. However, the other studies described above that used similar vitamin A doses but had smaller proportions of current or former smokers do not raise this concern. Among nonsmokers, beta-carotene and vitamin A supplements do not appear to affect the risk of cancer.
Age-Related Macular Degeneration
Age-related macular degeneration (AMD), or the loss of central vision as people age, is one of the most common causes of vision loss in older people 129. Age-related macular degeneration’s causes is usually unknown, but may involve complex interactions among genetic susceptibility, environmental factors (including exposure to oxidative stress), and normal aging 129. Because of the role of oxidative stress in age-related macular degeneration (AMD) pathophysiology, supplements containing carotenoids with antioxidant functions, such as beta-carotene, lutein, and zeaxanthin, might be useful for preventing or treating this condition. Lutein and zeaxanthin (which are not precursors of vitamin A), in particular, accumulate in the retina, the tissue in the eye that is damaged by age-related macular degeneration (AMD).
The Age-Related Eye Disease Study (AREDS) trial found that participants with a high risk of developing advanced age-related macular degeneration (AMD) (i.e., those who had intermediate AMD or who had advanced AMD in one eye) had a 25% lower risk of developing advanced AMD after they took a daily supplement containing beta-carotene (15 mg [7,500 mcg RAE]), vitamin E (180 mg [400 IU] dl-alpha-tocopheryl acetate), vitamin C (500 mg), zinc (80 mg), and copper (2 mg) for 5 years than participants taking a placebo 130.
The follow-up AREDS2 study confirmed the value of this supplement in reducing the progression of AMD over a median follow-up period of 5 years 123. However, this follow-up study showed that adding lutein (10 mg) and zeaxanthin (2 mg) or omega-3 fatty acids to the formulation produced no additional benefits 123. Importantly, the follow-up study also revealed that beta-carotene was not a required ingredient; the original AREDS formulation without beta-carotene provided the same protective effect against developing advanced AMD.
In a more detailed analysis, participants with the lowest dietary intakes of lutein and zeaxanthin had a 26% lower risk of advanced AMD when they took a supplement containing these two carotenoids than those who did not take a supplement with these carotenoids 123. The risk of advanced AMD was also 18% lower in participants who took the modified AREDS supplement containing lutein and zeaxanthin but not beta-carotene than in participants who took the formulation with beta-carotene but not lutein or zeaxanthin.
A subsequent study monitored dietary intakes of several nutrients in 4,504 AREDS participants and 3,738 AREDS2 participants (mean age 71 years) for a median of 10.2 years 131. Participants in the two highest quintiles of intakes for vitamin A, beta-carotene, or lutein and zeaxanthin had a lower risk of progression to late AMD 131. For example, the risk of late AMD was 18% lower among those in the fifth quintile for vitamin A intake and 20% lower among those in the fourth quintile than among those in the first quintile 131.
At the end of the 5-year AREDS2 trial, participants were all offered the final AREDS2 formulation that included lutein and zeaxanthin in place of beta-carotene. Researchers followed up with 3,882 of these participants for an additional 5 years 124. After 10 years, participants who had taken the AREDS2 supplement with lutein and zeaxanthin had an additional 20% reduced risk of progression to late AMD compared with those who took the supplement containing beta-carotene 124. This finding confirmed the benefit of replacing beta-carotene with lutein and zeaxanthin.
Individuals who have or are developing age-related macular degeneration (AMD) should talk to their health care provider about their vitamin A intakes and the supplement formulations used in the AREDS studies.
Measles
When children with vitamin A deficiency (which is rare in North America) get measles, the disease tends to be more severe. In these children, taking supplements with high doses of vitamin A can shorten the fever and diarrhea caused by measles. These supplements can also lower the risk of death in children with measles who live in developing countries where vitamin A deficiency is common.
Measles is a major cause of morbidity and mortality in children in developing countries. In 2019, measles was responsible for more than 207,500 deaths around the world, mostly in young children in low-income countries 132. About half of all measles deaths happen in Africa, but the disease is not limited to low-income countries. Vitamin A deficiency is a known risk factor for severe measles. In 2013, 11,200 deaths from measles were associated with vitamin A deficiency, and more than 95% of these deaths occurred in sub-Saharan Africa and south Asia. In a pooled analysis of randomized controlled trials within this study, vitamin A supplementation was associated with a 26% lower risk of dying from measles.
The World Health Organization recommends high oral doses (200,000 IU) of vitamin A for two days for children over age 1 with measles who live in areas with a high prevalence of vitamin A deficiency 133, 134. Recommended doses are 30,000 mcg RAE (100,000 IU) of vitamin A once for infants ages 6–11 months and 60,000 mcg RAE (200,000 IU) every 4–6 months for ages 1–5 years 133.
However, a Cochrane review that included 6 randomized controlled trials of vitamin A supplementation (15,000 mcg RAE [50,000 IU] to 60,000 mcg RAE [200,000 IU], depending on age) found that the supplementation did not affect risk of death due to measles, although it did help prevent new cases of measles 135. These randomized controlled trials assessed the value of supplementation to prevent morbidity and mortality due to measles in a total of 19,566 children aged 6 months to 5 years. The vitamin A doses used in these studies are much higher than the UL. The effectiveness of vitamin A supplementation to treat measles in countries, such as the United States, where vitamin A intakes are usually adequate is uncertain.
The body needs vitamin A to maintain the corneas and other epithelial surfaces, so the lower serum concentrations of vitamin A associated with measles, especially in people with protein-calorie malnutrition, can lead to blindness. None of the studies evaluated in a Cochrane review evaluated blindness as a primary outcome 136. However, a careful clinical investigation of 130 African children with measles revealed that half of all corneal ulcers in these children, and nearly all bilateral blindness, occurred in those with vitamin A deficiency 137.
Vitamin A deficiency signs and symptoms
Subclinical forms of vitamin A deficiency may not cause any symptoms, but the risk of developing respiratory and diarrheal infections is increased, the growth rate is decreased, and bone development is slowed 138. Patients may have a recent history of increased infections, infertility secondary to impaired spermatogenesis, or recent spontaneous abortion secondary to impaired embryonic development 138. The patient may also report increased fatigue, as a manifestation of vitamin A deficiency anemia.
Signs and symptoms of vitamin A deficiency include the following 139:
- Bitot spots – Areas of abnormal squamous cell proliferation and keratinization of the conjunctiva can be seen in young children with vitamin A deficiency.
- Blindness – Vitamin A has a major role in phototransduction. The cone cells are responsible for the absorption of light and for color vision in bright light. The rod cells detect motion and are responsible for night vision. In the rod cells of the retina, all-trans-retinol is converted into 11-cis -retinol, which then combines with a membrane-bound protein called opsin to yield rhodopsin. [21] A similar type of reaction occurs in the cone cells of the retina to produce iodopsin. The visual pigments absorb light at different wavelengths, according to the type of cone cell they occupy. Vitamin A deficiency leads to a lack of visual pigments; this reduces the absorption of various wavelengths of light, resulting in blindness.
- Poor adaptation to darkness (nyctalopia)
- Dry skin
- Follicular hyperkeratosis (phrynoderma) secondary to blockage of hair follicles with plugs of keratin.
- Dry hair
- Itchy skin (prutitus)
- Broken fingernails
- Conjunctival xerosis
- Corneal xerosis
- Corneal ulcer
- Keratomalacia
- Corneal perforation
- Corneal scarring
- Blindness
- Other signs of vitamin A deficiency include excessive deposition of periosteal bone secondary to reduced osteoclastic activity, anemia, keratinization of mucous membranes, and impairment of the humoral and cell-mediated immune system.
In the eye, vitamin A is essential for maintenance of conjunctival and corneal epithelia as well as night vision. Vitamin A deficiency causes metaplasia and keratinization of mucus-secreting epithelium, which can cause conjunctival and corneal xerosis, corneal ulcers, keratomalacia, and corneal scarring. Rods are the retinal photoreceptor that is responsible for night vision. Rods have a singular photopigment, rhodopsin. Retinol is a vitamin A-derived cofactor that is required for the formation of rhodopsin; thus, vitamin A deficiency leads to impairment of rod function and causes nyctalopia, or night blindness due to the eye’s inability to adapt from light to dark 140.
The most common sign of severe vitamin A deficiency is an eye condition called xerophthalmia (dry eye). Xerophthalmia refers to the spectrum of eye disease that affect the conjunctiva, cornea, and retina caused by severe vitamin A deficiency. Xerophthalmia can impair your ability to see in low light (dark adaptation of the eyes), which can lead to night blindness and is an early symptom of severe vitamin A deficiency.
Xerophthalmia (dry eye) is the most common sign of vitamin A deficiency (which is nearly characteristic) results from keratinization of the eyes. It involves drying (xerosis) and thickening of the conjunctivae (conjunctival xerosis) and corneas (corneal xerosis). Superficial foamy patches composed of epithelial debris and secretions on the exposed bulbar conjunctiva (Bitot spots) develop. In advanced vitamin A deficiency, the cornea becomes hazy and can develop erosions (corneal ulcers), which can lead to its destruction (keratomalacia). Xerophthalmia can lead to blindness if it isn’t treated.
Keratinization of the skin and of the mucous membranes in the respiratory, gastrointestinal, and urinary tracts can occur. Drying, scaling, and follicular thickening of the skin and respiratory infections can result.
Immunity is generally impaired. Infection with the measles virus was found to precipitate conjunctival and corneal damage, leading to blindness in children with poor vitamin A status 141. Conversely, vitamin A deficiency can be considered a nutritionally acquired immunodeficiency disease 142. Even children who are only mildly deficient in vitamin A have a higher incidence of respiratory complications and diarrhea, as well as a higher rate of mortality from measles infection compared to children consuming sufficient vitamin A 143. Because vitamin A supplementation may decrease both the severity and incidence of measles complications in developing countries, WHO recommends that children aged at least one year receive 200,000 IU of vitamin A (60,000 mcg RAE) for two consecutive days in addition to standard treatment when children are infected with measles virus and live in areas of vitamin A deficiency 133.
The younger the patient, the more severe are the effects of vitamin A deficiency. Growth retardation and infections are common among children. Mortality rate can exceed 50% in children with severe vitamin A deficiency. Routine vitamin A supplementation with retinol capsule of 200 000 IU retinyl acetate in oil to children in endemic areas leads to a decrease of childhood mortality of 5-15% 144.
A long-term vitamin A deficiency can also lead to a higher risk of respiratory diseases (such as pneumonia) and infections (such as measles and diarrhea). It can also cause anemia (a condition in which the red blood cells do not supply enough oxygen to the body). In severe cases, not getting enough vitamin A can increase your chances of dying. A study from Bangladesh showed that almost two-thirds of children with the most severe form of xerophthalmia – known as keratomalacia (a corneal ulcer affecting more than a third of the cornea) – had died within a few months 145.
Table 4. World Health Organization (WHO) classification of vitamin A deficiency and the age groups most affected
| Grade of xerophthalmia | Peak age group (years) | Type of deficiency | Risk of death | |
|---|---|---|---|---|
| XN | Night blindness | 2–6; adult women | Longstanding. Not blinding | + |
| X1A | Conjunctival xerosis | 3–6 | Longstanding. Not blinding | + |
| X1B | Bitot’s spot | 3–6 | Longstanding. Not blinding | + |
| X2 | Corneal xerosis | 1–4 | Acute deficiency. Can be blinding | ++ |
| X3A | Corneal ulcer/ <1/3 cornea | 1–4 | Severe acute deficiency. Blinding | +++ |
| X3B | Corneal ulcer/keratomalacia ≥1/3 | 1–4 | Severe acute deficiency. Blinding | ++++ |
| XS | Corneal scarring (from X3) | >2 | Consequence of corneal ulceration | +/– |
| XF | Xerophthalmic fundus | Adults | Longstanding. Not blinding. Rare | – |
Night blindness
Night blindness or defective vision in dim light is one of the most common manifestations of vitamin A deficiency, especially in children age 2-6 or pregnant or lactating women. Although it is considered one of the earliest manifestations, children with vitamin A deficiency may develop one of the more severe signs, such as corneal ulcers, after infection or diarrhea without any of the classically early signs. Children may not be able to verbalize their symptoms, and parents need to be asked if they have noticed their children behaving differently in the dark, e.g. becoming less active or fearful. Night blindness is considered to be both a sensitive and specific indicator for serum retinol levels.
Conjunctival xerosis
Conjunctival xerosis is characterized by a dull and dry appearance of the conjunctiva with slight wrinkling. It is caused by the loss of goblet cells and insufficient mucin secretion, and it can be subtle and difficult to detect clinically.
Bitot spots
Bitot spots are collections of desquamated, keratinized epithelial cells mixed with the gas-forming bacteria Corynebacterium xerosis. They appear as triangular patches of foamy, whitish, opaque deposits, typically located on the bulbar conjunctiva near the limbus at the 3 and 9 o’clock positions. They are more common temporally.
Corneal xerosis
Corneal xerosis is characterized by a dull and hazy appearance of the cornea that is caused by drying of the cornea secondary to conjunctival gland dysfunction. It may initially present with bilateral punctate corneal epithelial erosions, and it can quickly progress to the stage of corneal ulceration. Up to this stage, high-dose Vitamin A supplementation can result in the full preservation of vision.
Corneal ulceration
Corneal xerosis can lead corneal ulceration and melting if not treated urgently. Keratomalacia, the melting away of the cornea by liquefactive necrosis, is the most severe form of xerophthalmia. It can perforate and destroy the cornea in just a matter of days. A child who appears relatively healthy but develops keratomalacia should be questioned about a recent history of measles or diarrhea, which could rapidly deplete already deficient vitamin A stores.
Corneal scar
Corneal scarring due to vitamin A deficiency is often symmetric and bilateral. Other causes of corneal scarring must be ruled out.
Xerophthalmic fundus
Prolonged vitamin A deficiency can lead to structural changes in the retina. Xerophthalmic fundus changes appear as small, white, deep retinal lesions scattered throughout the posterior pole.
Vitamin A deficiency causes
The major underlying causes of vitamin A deficiency may be summarized as follows 146, 147:
- Reduced intake of vitamin A
- Inadequate food supply. Vitamin A deficiency is endemic in areas such as southern and eastern Asia, where rice, devoid of beta-carotene, is the staple food. Xerophthalmia due to primary vitamin A deficiency is a common cause of blindness among young children in developing countries.
- Alcoholism
- Mental illness
- Dysphagia
- Vitamin A deficiency is common in prolonged protein-energy undernutrition not only because the diet is deficient but also because vitamin A storage and transport is defective.
- Impaired absorption of vitamin A
- Crohn’s disease
- Ulcerative colitis
- Celiac disease
- Pancreatic insufficiency
- Cystic fibrosis
- Bile duct obstruction
- Duodenal bypass
- Short bowel syndrome
- Chronic diarrhea
- Giardiasis
- Disordered transport of vitamin A
- Abetalipoproteinemia
- Reduced storage of vitamin A
- Liver disease and cirrhosis
- Cystic fibrosis
- Zinc deficiency
- Zinc deficiency may also be involved with the pathogenesis of secondary vitamin A deficiency. Inadequate zinc can depress the hepatic synthesis of retinol-binding protein (RBP), which is required for mobilization of retinol from the liver. In addition, zinc may play a role in the conversion of beta-carotene to retinol via the enzyme 15-15 dioxygenase 148.
Groups at Risk of Vitamin A Deficiency
The following groups are among those most likely to have inadequate intakes of vitamin A.
Premature infants
Preterm infants have low liver stores of vitamin A at birth, and their plasma concentrations of retinol often remain low throughout the first year of life 62, 63. Preterm infants with vitamin A deficiency have a higher risk of eye and chronic lung diseases 64, 65. However, in high-income countries, clinical vitamin A deficiency is rare in infants and occurs only in those with malabsorption disorders 66.
Infants, children, and pregnant and lactating women in low-income and middle-income countries
Pregnant women need extra vitamin A for fetal growth and tissue maintenance and to support their own metabolism 67, 68, 69. The breast milk of lactating women with adequate vitamin A intakes contains sufficient amounts of vitamin A to meet infants’ needs for the first 6 months of life70. But in people with vitamin A deficiency, the vitamin A content of breast milk is not sufficient to maintain adequate vitamin A stores in infants who are exclusively breastfed 70.
About 190 million preschool-aged children (one third of all children in this age group), mostly in Africa and Southeast Asia, have vitamin A deficiency, according to the World Health Organization 71, 10. They have a higher risk of visual impairment and of illness and death from childhood infections, such as measles and infections that cause diarrheal diseases 71, 1.
The World Health Organization estimates that 9.8 million pregnant women (15% of all pregnant women) around the world, mostly in low-income and middle-income countries, have xerophthalmia as a result of vitamin A deficiency 72.
People with cystic fibrosis
Up to 90% of people with cystic fibrosis have pancreatic insufficiency, which increases their risk of vitamin A deficiency due to difficulty absorbing fat 1, 73. Studies in Australia and the Netherlands indicate that 2% to 13% of children and adolescents with cystic fibrosis have vitamin A deficiency 74, 75. As a result, standard care for cystic fibrosis includes lifelong treatment with vitamin A (daily amounts of 750 mcg RAE to 3,000 mcg RAE, depending on age, are recommended in the United States and Australia), other fat-soluble vitamins, and pancreatic enzymes 73, 75.
Individuals with gastrointestinal disorders
Approximately one quarter of children with Crohn’s disease and ulcerative colitis have vitamin A deficiency; adults with these disorders, especially those who have had the disorder for several years, also have a higher risk of vitamin A deficiency 76, 77. Although some evidence supports the use of vitamin A supplements in people with these disorders 78, other research has found that supplementation offers no benefit 79. Some children and adults with newly diagnosed celiac disease also have vitamin A deficiency; a gluten-free diet can, but does not always, eliminate vitamin A deficiency 80, 81, 82.
Vitamin A deficiency prevention
The diet should include dark green leafy vegetables, deep- or bright-colored fruits (eg, papayas, oranges), carrots, and yellow vegetables (eg, squash, pumpkin). Vitamin A–fortified milk and cereals, liver, egg yolks, and fish liver oils are helpful. Carotenoids are absorbed better when consumed with some dietary fat. If milk allergy is suspected in infants, they should be given adequate vitamin A in formula feedings.
In developing countries, prophylactic supplements of vitamin A palmitate in oil 200,000 IU (60,000 RAE) orally every 6 months are advised for all children between 1 and 5 yr of age; infants < 6 months can be given a one-time dose of 50,000 IU (15,000 RAE), and those aged 6 to 12 months can be given a one-time dose of 100,000 IU (30,000 RAE).
Vitamin A deficiency diagnosis
Patients presenting with clinical signs of xerophthalmia (or, in the case of a children, their parents) must be questioned on dietary, medical, and social history, including alcohol intake. Be sure to ask about any malabsorptive process, as described above, or risk factors such as living in a resource-poor country or current pregnancy or lactation. Because xerophthalmia is a manifestation of moderate-to-severe vitamin A deficiency, it is important to ask about the systemic signs of milder vitamin A deficiency, including frequent gastrointestinal and respiratory tract infections, anemia, iron deficiency, and development of xeroderma and phrynoderma (follicular hyperkeratosis often found on extensor surfaces, shoulders, and buttocks) 149.
Serum retinol levels, clinical evaluation, and response to vitamin A help diagnose vitamin A deficiency in people with symptoms, such as night blindness, or in people with diseases that impair intestinal absorption of nutrients and who are at risk of vitamin A deficiency.
Ocular findings suggest vitamin A deficiency. Dark adaptation can be impaired in other disorders (eg, zinc deficiency, retinitis pigmentosa, severe refractive errors, cataracts, diabetic retinopathy). If dark adaptation is impaired, rod scotometry and electroretinography are done to determine whether vitamin A deficiency is the cause.
Serum levels of retinol are measured. Normal range of vitamin A/retinol is 28 to 86 mcg/dL (1 to 3 mcmol/L). Vitamin A deficiency is defined as serum retinol levels of below 28 μg/dL. However, levels decrease only after the deficiency is advanced because the liver contains large stores of vitamin A. Also, decreased levels may result from acute infection, which causes retinol-binding protein and transthyretin (also called prealbumin) levels to decrease transiently.
A therapeutic trial of vitamin A may help confirm the diagnosis.
Physical exam
Aside from the ocular exam, the physical exam should include assessments for weight/body habitus, jaundice, and abdominal exam for hepatomegaly.
Blood tests
The following laboratory studies may be considered in the workup 150, 151:
- Serum vitamin A or retinol (reference range: 20-60 mcg/dL). These levels can be normal due to maintenance of circulating retinol levels by hepatic stores. A serum retinol concentration of 0.70 μmol/L (20 mcg/dL) or less frequently reflects subclinical vitamin A deficiency. A value of less than 0.70 μmol/L (20 mcg/dL) in children younger than 12 years is considered low 152. Vitamin A deficiency-related ocular symptoms have been shown to develop at serum retinol concentration less than 0.35 μmol/L (10 mcg/dL), and is considered an indicator of severe vitamin A deficiency, where vitamin A body stores are depleted 1
- Serum retinol-binding protein (RBP) (reference range: 30-75 ug/ml). A serum retinol-binding protein (RBP) study is easier to perform and less expensive than a serum retinol study, because retinol-binding protein (RBP) is a protein and can be detected by an immunologic assay. Retinol-binding protein (RBP) is also a more stable compound than retinol with respect to light and temperature. However, retinol-binding protein (RBP) levels are less accurate, because they are affected by serum protein concentrations and because types of retinol-binding protein (RBP) cannot be differentiated 153, 154, 155. The serum retinol level may be low during infection because of a transient decrease in the retinol-binding protein (RBP).
- Serum zinc (reference range: 75-120 mcg/dL). A zinc level is useful because zinc deficiency interferes with retinol-binding protein (RBP) production.
- An iron panel is useful because iron deficiency can affect the metabolism of vitamin A.
- Albumin levels are indirect measures of vitamin A levels.
- Obtain a complete blood count (CBC) with differential if anemia, infection, or sepsis is a possibility.
- An electrolyte evaluation and liver function studies should be performed to evaluate for nutritional and volume status.
Other testing
- Dark adatopmetry and night vision threshold tests 91
- Electroretinogram (ERG): Retinopathy from vitamin A deficiency is associated with decreased amplitude
- Impression cytology: conjunctival specimens can be viewed for the presence of goblet cells. A decrease in normal amount is considered an effective measurement of vitamin A deficiency 156.
- In children, radiographic films of the long bones may be useful when an evaluation is being made for bone growth and for excessive deposition of periosteal bone.
- Liver biopsy: considered the gold standard for evaluating total body vitamin A, although it is not routinely used outside of the research setting due to procedural risks.
Vitamin A deficiency treatment
The recommended vitamin A deficiency treatment regimens are described in table 5 below. Dietary deficiency of vitamin A is traditionally treated with vitamin A palmitate in oil 60,000 IU oral once/day for 2 days, followed by 4500 IU oral once/day. If vomiting or malabsorption is present or xerophthalmia is probable, a dose of 50,000 IU for infants less than 6 months of age, 100,000 IU for infants 6 to 12 months of age, or 200,000 IU for children greater than 12 months of age and adults should be given for 2 days, with a third dose at least 2 weeks later. Vitamin A deficiency is a risk factor for severe measles; treatment with vitamin A can shorten the duration of the measles and may reduce the severity of symptoms and risk of death. The World Health Organization (WHO) recommends that all children with measles in developing countries should receive 2 doses of vitamin A, (100,000 IU for children < 12 months and 200,000 IU for those >12 months) given 24 hour apart 157.
Vitamin A deficiency associated with malabsorptive or other processes is treated based on the severity of vitamin A deficiency and at the doctor’s discretion, usually involving daily treatment.
Infants born of HIV-positive mothers should receive 50,000 IU (15,000 RAE) within 48 hours of birth. Prolonged daily administration of large doses, especially to infants, must be avoided because toxicity may result.
Patients with concomitant zinc deficiency should also undergo zinc supplementation 158. If the patient’s vitamin A deficiency is from malabsorption, doctors should consider intramuscular vitamin A supplementation formulations.
For pregnant or breastfeeding women, prophylactic or therapeutic doses should not exceed 10,000 IU (3000 RAE)/day to avoid possible damage to the fetus or infant.
In regions with a high prevalence of vitamin A deficiency, the World Health Organization (WHO) recommends universal vitamin A supplementation of select populations 159. The World Health Organization (WHO) recommends a one-time dose of 100,000 IU in children 6 to 11 months of age followed by doses of 200,000 IU every 4 to 6 months up to 5 years of age 71. At-risk pregnant women should receive vitamin A supplementation at lower doses due to concern for fetotoxicity; the recommended dosing is 10,000 IU daily or 25,000 IU weekly for 12 weeks 160. The WHO no longer recommends universal supplementation for children less than 6 months of age or postpartum women 161, 162, 163.
International guidelines do not clearly outline dosing of vitamin A supplementation for asymptomatic vitamin A deficiency in resource-rich regions 159. Treatment for subclinical vitamin A deficiency includes the consumption of vitamin A rich foods, such as liver, beef, chicken, eggs, fortified milk, carrots, mangoes, sweet potatoes, and leafy green vegetables 164.
In resource-rich countries, post-weight loss surgery patients and neonates have particular dosing recommendations. Post-weight loss surgery patients are recommended to take 10,000 IU vitamin A supplementation daily and adjust as needed based on regular serum retinol level monitoring. Some weight loss surgery patients have been known to need up to 100,000 IU vitamin A supplementation daily 165. For premature infants, guidelines do not exist yet for vitamin A supplementation. Still, recent studies have shown that vitamin A supplementation of 10,000 IU every other day in very low birth weight neonates for 4 weeks has significant results, decreasing all-cause mortality by 56% and decreasing rates of oxygen requirement, sepsis, patent ductus arteriosus, and length of hospital stay 166. Vitamin A supplementation of 1,500 IU daily in extremely premature infants had a significant decrease in retinopathy of prematurity (1.6% vs. 6.9%) and a nearly 50% decrease in bronchopulmonary dysplasia 65. Vitamin A deficiency associated with other malabsorptive processes is treated on a case-by-case basis.
Treatment can be adjusted as needed based on regular serum retinol level monitoring.
Localized ocular treatment includes intense lubrication, topical retinoid acid, and management of perforation.
Table 5. Vitamin A deficiency treatment regimens
| Vitamin A dosage (IU) | |
|---|---|
| Young infants 0-5 months 1 | 50,000 IU |
| Older infants 6-11 months 1 | 100,000 IU |
| Children (males: 12 mo or more; females 12 mo to 12 y and 50 y or more) 1 | 200,000 IU |
| Women (13-49 years of age) with night blindness and/or Bitot’s spots | 10,000 every day or 25,000 every week for at least 3 months |
| Women (13-49 years of age) with active corneal lesions | 200,000 on days 1, 2, and 14 |
Footnote: 1 Schedule: severe malnutrition, day 1; measles, days 1 and 2; xerophthalmia, days 1, 2, and 14.
[Source 167 ]Vitamin A deficiency prognosis
The prognosis of vitamin A deficiency depends on the severity of the disease at treatment initiation 24. If treated promptly, patients with subclinical vitamin A deficiency have a very good prognosis without long term consequences. Treatment at any stage of severity can show improvement within a week 168.
The early ophthalmologic signs, such as night blindness, conjunctival xerosis, and Bitot spots, will resolve completely within about 2 months of vitamin A supplementation, while corneal xerosis and ulceration results in scarring that may lead to permanent vision loss despite treatment 24, 169. Irreversible conditions include punctate keratopathy, keratomalacia, and corneal perforation. At the onset of vitamin A deficiency eye signs and symptoms, patients develop an increased susceptibility to infection. In preschool children with vitamin A deficiency, the presence of ophthalmologic signs indicates increased overall mortality from gastrointestinal, pulmonary, and other vitamin A deficiency-related mucosal infections 24.
Xerophthalmia signifies a severity of vitamin A deficiency that can cause mortality from malnutrition and increased susceptibility to mucosal infections. The death rate (mortality) in children with night blindness is triple the mortality found in children with subclinical vitamin A deficiency 159. Children with both Bitot’s spots and night blindness have death rate nine times that of children with subclinical vitamin A deficiency. Nearly two-thirds of children with keratomalacia die within months 24.
References- Blaner WS. Vitamin A and Provitamin A Carotenoids. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Cambridge, Massachusetts: Wiley-Blackwell; 2020:73-91.
- Ross A. Vitamin A. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:260-77.
- Vitamin A and Carotenoids. https://ods.od.nih.gov/factsheets/VitaminA-Consumer
- Carazo A, Macáková K, Matoušová K, Krčmová LK, Protti M, Mladěnka P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients. 2021 May 18;13(5):1703. doi: 10.3390/nu13051703
- Ross AC. Vitamin A. In: Ross A, Caballero B, Cousins R, Tucker K, Ziegler T, eds. Modern Nutrition in Health and Disease. 11th ed: Lippincott Williams & Wilkins; 2014:260-277.
- Starck T. Advances in Corneal Research. Boston, MA: Springer; 1997. Severe corneal ulcerations and vitamin A deficiency; pp. 557–567.
- Lange AP, Moloney G, Sheldon CA, Sasaki S, Holland SP. Bilateral corneal ulceration caused by vitamin a deficiency in eosinophilic gastroenteropathy. Case Rep Ophthalmol. 2011 Sep;2(3):302-6. doi: 10.1159/000331886
- Wiseman EM, Bar-El Dadon S, Reifen R. The vicious cycle of vitamin a deficiency: A review. Crit Rev Food Sci Nutr. 2017 Nov 22;57(17):3703-3714. doi: 10.1080/10408398.2016.1160362
- Ross CA. Vitamin A. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:778-91.
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De-Regil LM, Rogers L, Danaei G, Li G, White RA, Flaxman SR, Oehrle SP, Finucane MM, Guerrero R, Bhutta ZA, Then-Paulino A, Fawzi W, Black RE, Ezzati M. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health. 2015 Sep;3(9):e528-36. doi: 10.1016/S2214-109X(15)00039-X
- Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66 Suppl 2:22-33. doi: 10.1159/000371618
- Vitamin A and Carotenoids. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional
- World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva: WHO, 2011.
- West KP. Dietary vitamin A deficiency: effects on growth, infection and mortality. Food and Nutrition Bulletin 1991;13(2):77.
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. http://apps.who.int/iris/bitstream/handle/10665/44110/9789241598019_eng.pdf?sequence=1
- Boomsma DI, Martin NG, Molenaar PC. Factor and simplex models for repeated measures: application to two psychomotor measures of alcohol sensitivity in twins. Behav Genet. 1989 Jan;19(1):79-96. doi: 10.1007/BF01065885
- Timoneda J, Rodríguez-Fernández L, Zaragozá R, Marín MP, Cabezuelo MT, Torres L, Viña JR, Barber T. Vitamin A Deficiency and the Lung. Nutrients. 2018 Aug 21;10(9):1132. doi: 10.3390/nu10091132
- Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094
- Institute of Medicine, Food, Nutrition Board. Vitamin A. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2001:82–146.
- Mills JP, Terasawa E, Tanumihardjo SA. Ingestion of excessive preformed vitamin A by mothers amplifies storage of retinyl esters in early fetal livers of captive Old World monkeys. Comp Med. 2007 Oct;57(5):505-11.
- Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005 Oct;146(10):4479-90. doi: 10.1210/en.2005-0158
- Braunstein A, Trief D, Wang N-K, Chang S, Tsang SH. Vitamin A deficiency in New York City. Lancet. 2010;376(9737):267. doi:10.1016/S0140-6736(09)61874-2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2929009/
- Bitot spot: early marker for avoidable blindness. Siddharth Madan, Sarita Beri. CMAJ Oct 2017, 189 (40) E1264; DOI: 10.1503/cmaj.170792
- Gilbert C. The eye signs of vitamin A deficiency. Community Eye Health. 2013;26(84):66-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3936686
- Sohal RJ, Aung TT, Sohal S, Harish A. A Rare Cause of Bilateral Corneal Ulcers: Vitamin A Deficiency in the Setting of Chronic Alcoholism. Cureus. 2020 May 6;12(5):e7991. doi: 10.7759/cureus.7991
- Soni, D., Sharma, B., Singh, P., Kubrey, S. (2022). Keratomalacia. In: Sharma, B., Titiyal, J.S. (eds) Corneal Emergencies. Springer, Singapore. https://doi.org/10.1007/978-981-16-5876-1_19
- U.S. National Library of Medicine, MedlinePlus. Vitamin A. https://medlineplus.gov/vitamina.html
- Ross AC, Tan L. Vitamin A deficiencies and excess. In: Kliegman RM, Stanton BF, St Geme JW, Schor NF, eds. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016:chap 48.
- Sommer A, West KP Jr. 1996. Vitamin A Deficiency: Health, Survival, and Vision . New York: Oxford University Press.
- WHO. 1995. Global Prevalence of Vitamin A Deficiency . Micronutrient Deficiency Information System Working Paper, No. 2. Geneva: WHO.
- WHO. 1982. Control of Vitamin A Deficiency and Xerophthalmia . Technical Report Series No. 672. Geneva: WHO. https://www.ncbi.nlm.nih.gov/pubmed/6803444
- Dowling JE, Gibbons IR. 1961. In: Smelser GK, ed, editor. . The Structure of the Eye . New York: Academic Press.
- Sommer A. 1982. Nutritional Blindness. Xerophthalmia and Keratomalacia . New York: Oxford University Press.
- Katz J, West KP Jr, Khatry SK, Thapa MD, LeClerq SC, Pradhan EK, Pokhrel RP, Sommer A. 1995. Impact of vitamin A supplementation on prevalence and incidence of xerophthalmia in Nepal. Invest Ophthalmol Vis Sci 36:2577–2583. https://www.ncbi.nlm.nih.gov/pubmed/7499080
- Christian P, West KP Jr, Khatry SK, Katz J, LeClerq S, Pradhan EK, Shrestha SR. 1998. b. Vitamin A or beta-carotene supplementation reduces but does not eliminate maternal night blindness in Nepal. J Nutr 128:1458–1463. https://www.ncbi.nlm.nih.gov/pubmed/9732305
- Chase HP, Kumar V, Dodds JM, Sauberlich HE, Hunter RM, Burton RS, Spalding V. 1971. Nutritional status of preschool Mexican-American migrant farm children. Am J Dis Child 122:316–324. https://www.ncbi.nlm.nih.gov/pubmed/5115528
- Sauberlich HE, Hodges HE, Wallace DL, Kolder H, Canham JE, Hood J, Raica N, Lowry LK. 1974. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 32:251–275. https://www.ncbi.nlm.nih.gov/pubmed/4617402
- Cantorna MT, Nashold FE, Hayes CE. 1995. Vitamin A deficiency results in a priming environment conducive for TH1 cell development. Eur J Immunol 25:1673–1679. https://www.ncbi.nlm.nih.gov/pubmed/7614995
- Nauss KM, Newberne PM. 1985. Local and regional immune function of vitamin A-deficient rats with ocular herpes simplex virus (HSV) infections. J Nutr 115:1316–1324. https://www.ncbi.nlm.nih.gov/pubmed/3876416
- Dawson HD, Ross AC. 1999. Chronic marginal vitamin A status effects the distribution and function of T cells and natural T cells in aging Lewis rats. J Nutr 129:1782–1790. https://www.ncbi.nlm.nih.gov/pubmed/10498748
- Wiedermann U, Hanson LA, Kahu H, Dahlgren UI. 1993. Aberrant T-cell function in vitro and impaired T-cell dependent antibody response in vivo in vitamin A-deficient rats. Immunology 80:581–586. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1422259/
- Sommer A, Katz J, Tarwotjo I. 1984. Increased risk of respiratory disease and diarrhea in children with pre-existing mild vitamin A deficiency. Am J Clin Nutr 40:1090–1095. https://www.ncbi.nlm.nih.gov/pubmed/6496388
- Sommer A, Tarwotjo I, Hussaini G, Susanto D. 1983. Increased mortality in children with mild vitamin A deficiency. Lancet 2:585–588. https://www.ncbi.nlm.nih.gov/pubmed/6136744
- Barclay AJ, Foster A, Sommer A. 1987. Vitamin A supplements and mortality related to measles: A randomised clinical trial. Br Med J 294:294–296. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1245303/
- Hussey GD, Klein M. 1990. A randomized, controlled trial of vitamin A in children with severe measles. N Engl J Med 323:160–164. https://www.ncbi.nlm.nih.gov/pubmed/2194128
- Coutsoudis A, Broughton M, Coovadia HM. 1991. Vitamin A supplementation reduces measles morbidity in young African children: A randomized, placebo-controlled, double-blind trial. Am J Clin Nutr 54:890–895. https://www.ncbi.nlm.nih.gov/pubmed/1951162
- Barreto ML, Santos LM, Assis AM, Araujo MP, Farenzena GG, Santos PA, Fiaccone RL. 1994. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet 344:228–231. https://www.ncbi.nlm.nih.gov/pubmed/7913157
- Donnen P, Dramaix M, Brasseur D, Bitwe R, Vertongen F, Hennart P. 1998. Randomized placebo-controlled clinical trial of the effect of a single high dose or daily low doses of vitamin A on the morbidity of hospitalized, malnourished children. Am J Clin Nutr 68:1254–1260. https://www.ncbi.nlm.nih.gov/pubmed/9846855
- Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, Alpers MP, West KP Jr. 1999. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua, New Guinea: A randomised trial. Lancet 354:203–209. https://www.ncbi.nlm.nih.gov/pubmed/10421302
- Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, Hidayat S, Tielsch J, West KP Jr, Sommer A. 1996. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 128:489–496. https://www.ncbi.nlm.nih.gov/pubmed/8618182
- Ghana VAST Study Team. 1993. Vitamin A supplementation in northern Ghana: Effects on clinic attendances, hospital admissions, and child mortality. Lancet 342:7–12. https://www.ncbi.nlm.nih.gov/pubmed/8100345
- Muhilal, Permeisih D, Idjradinata YR, Muherdiyantiningsih, Karyadi D. 1988. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: A controlled field trial. Am J Clin Nutr 48:1271–1276. https://www.ncbi.nlm.nih.gov/pubmed/3189216
- Rahmathullah L, Underwood BA, Thulasiraj RD, Milton RC, Ramaswamy K, Rahmathullah R, Babu G. 1990. Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. N Engl J Med 323:929–935. https://www.ncbi.nlm.nih.gov/pubmed/2205798
- Sommer A, Tarwotjo I, Djunaedi E, West KP Jr, Loeden AA, Tilden R, Mele L. 1986. Impact of vitamin A supplementation on childhood mortality: A randomized controlled community trial. Lancet 1:1169–1173. https://www.ncbi.nlm.nih.gov/pubmed/2871418
- West KP Jr, Pokhrel RP, Katz J, LeClerq SC, Khatry SK, Shrestha SR, Pradhan EK, Tielsch JM, Pandey MR, Sommer A. 1991. Efficacy of vitamin A in reducing preschool child mortality in Nepal. Lancet 338:67–71. https://www.ncbi.nlm.nih.gov/pubmed/1676467
- West KP Jr, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Conner PB, Dali SM, Christian P, Pokhrel RP, Sommer A. 1999. Double blind, cluster randomized trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. Br Med J 318:570–575. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC27760/
- Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, Harvey B. 1993. Effectiveness of Vitamin A Supplementation in the Control of Young Child Morbidity and Mortality in Developing Countries . Geneva: Subcommittee on Nutrition, Administrative Committee on Coordination, World Health Organization.
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. 1993. Vitamin A supplementation and child mortality. A meta-analysis. J Am Med Assoc 269:898–903. https://www.ncbi.nlm.nih.gov/pubmed/8426449
- Glasziou PP, Mackerras DE. 1993. Vitamin A supplementation and infectious disease: A meta-analysis. Br Med J 306:366–370. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1676417/
- WHO. 1997. Vitamin A Supplements: A Guide to Their Use in the Treatment of Vitamin A Deficiency and Xerophthalmia . Geneva: WHO.
- AAP (American Academy of Pediatrics Committee on Infectious Diseases). 1993. Vitamin A treatment of measles. Pediatrics 91:1014–1015. https://www.ncbi.nlm.nih.gov/pubmed/8474793
- Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD000501. doi: 10.1002/14651858.CD000501.pub3. Update in: Cochrane Database Syst Rev. 2016;8:CD000501
- Schwartz, E., Zelig, R., Parker, A. and Johnson, S. (2017), Vitamin A Supplementation for the Prevention of Bronchopulmonary Dysplasia in Preterm Infants: An Update. Nutrition in Clinical Practice, 32: 346-353. https://doi.org/10.1177/0884533616673613
- Rakshasbhuvankar A, Patole S, Simmer K, Pillow JJ. Enteral vitamin A for reducing severity of bronchopulmonary dysplasia in extremely preterm infants: a randomised controlled trial. BMC Pediatr. 2017 Dec 16;17(1):204. doi: 10.1186/s12887-017-0958-x
- Sun H, Cheng R, Wang Z. Early vitamin A supplementation improves the outcome of retinopathy of prematurity in extermely premature infants. Retina. 2020 Jun;40(6):1176-1184. doi: 10.1097/IAE.0000000000002543
- Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed. 2005 Mar;90(2):F103-8. doi: 10.1136/adc.2004.057547
- McCauley ME, van den Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2015 Oct 27;2015(10):CD008666. doi: 10.1002/14651858.CD008666.pub3
- Haider BA, Sharma R, Bhutta ZA. Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database Syst Rev. 2017 Feb 24;2(2):CD006980. doi: 10.1002/14651858.CD006980.pub3
- Ota E, da Silva Lopes K, Middleton P, Flenady V, Wariki WM, Rahman MO, Tobe-Gai R, Mori R. Antenatal interventions for preventing stillbirth, fetal loss and perinatal death: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2020 Dec 18;12(12):CD009599. doi: 10.1002/14651858.CD009599.pub2
- Oliveira JM, Allert R, East CE. Vitamin A supplementation for postpartum women. Cochrane Database Syst Rev. 2016 Mar 25;3(3):CD005944. doi: 10.1002/14651858.CD005944.pub3
- World Health Organization. Guideline: vitamin A supplementation in infants and children 6-59 months of age. 25 July 2011. https://www.who.int/publications/i/item/9789241501767
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Gobal Database on Vitamin A Deficiency. Geneva: World Health Organization; 2009.
- de Vries JJ, Chang AB, Bonifant CM, Shevill E, Marchant JM. Vitamin A and beta (β)-carotene supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2018 Aug 9;8(8):CD006751. doi: 10.1002/14651858.CD006751.pub5
- Rana M, Wong-See D, Katz T, Gaskin K, Whitehead B, Jaffe A, Coakley J, Lochhead A. Fat-soluble vitamin deficiency in children and adolescents with cystic fibrosis. J Clin Pathol. 2014 Jul;67(7):605-8. doi: 10.1136/jclinpath-2013-201787
- Woestenenk JW, Broos N, Stellato RK, Arets HG, van der Ent CK, Houwen RH. Vitamin A intake and serum retinol levels in children and adolescents with cystic fibrosis. Clin Nutr. 2016 Jun;35(3):654-9. doi: 10.1016/j.clnu.2015.04.010
- Fabisiak N, Fabisiak A, Watala C, Fichna J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2017 Nov/Dec;51(10):878-889. doi: 10.1097/MCG.0000000000000911
- Rempel J, Grover K, El-Matary W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients. 2021 Jan 15;13(1):236. doi: 10.3390/nu13010236
- Santucci NR, Alkhouri RH, Baker RD, Baker SS. Vitamin and zinc status pretreatment and posttreatment in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014 Oct;59(4):455-7. doi: 10.1097/MPG.0000000000000477
- Wright JP, Mee AS, Parfitt A, Marks IN, Burns DG, Sherman M, Tigler-Wybrandi N, Isaacs S. Vitamin A therapy in patients with Crohn’s disease. Gastroenterology. 1985 Feb;88(2):512-4. doi: 10.1016/0016-5085(85)90514-1
- McGrogan L, Mackinder M, Stefanowicz F, Aroutiounova M, Catchpole A, Wadsworth J, Buchanan E, Cardigan T, Duncan H, Hansen R, Russell RK, Edwards CA, Talwar D, McGrogan P, Gerasimidis K. Micronutrient deficiencies in children with coeliac disease; a double-edged sword of both untreated disease and treatment with gluten-free diet. Clin Nutr. 2021 May;40(5):2784-2790. doi: 10.1016/j.clnu.2021.03.006
- Shepherd, S.J. & Gibson, P.R (2012) Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J Hum Nutr Diet. 26, 349–358 doi:10.1111/jhn.12018
- Tokgöz Y, Terlemez S, Karul A. Fat soluble vitamin levels in children with newly diagnosed celiac disease, a case control study. BMC Pediatr. 2018 Apr 9;18(1):130. doi: 10.1186/s12887-018-1107-x
- Johnson EJ, Russell RM. Beta-Carotene. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:115-20.
- Vitamin A. https://lpi.oregonstate.edu/mic/vitamins/vitamin-A
- Groff JL. Advanced Nutrition and Human Metabolism. 2nd ed. St. Paul: West Publishing; 1995.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2001.
- Ross A. Vitamin A and Carotenoids. In: Shils M, Shike M, Ross A, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006:351-75
- Food and Nutrition Board, Institute of Medicine. Vitamin A. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, D.C.: National Academy Press; 2001:65-126. https://nap.nationalacademies.org/read/10026/chapter/6
- Solomons NW. Vitamin A. In: Bowman B, Russell R, eds. Present Knowledge in Nutrition. 9th ed. Washington, DC: International Life Sciences Institute; 2006:157-83.
- Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006 Feb;83(2):191-201. doi: 10.1093/ajcn/83.2.191
- Russell RM. The vitamin A spectrum: from deficiency to toxicity. Am J Clin Nutr. 2000 Apr;71(4):878-84. doi: 10.1093/ajcn/71.4.878
- Otten JJ, Hellwig JP, Meyers LD, eds. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press; 2006.
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. https://www.federalregister.gov/documents/2017/10/02/2017-21019/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-and-serving-sizes-of-foods-that
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2001. https://nap.nationalacademies.org/read/10026/chapter/1
- Haskell MJ. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion–evidence in humans. Am J Clin Nutr. 2012 Nov;96(5):1193S-203S. doi: 10.3945/ajcn.112.034850
- Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)-Vitamin A Review. J Nutr. 2016 Sep;146(9):1816S-48S. doi: 10.3945/jn.115.229708
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Vitamin A. [Updated 2020 Nov 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548165
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000. https://nap.nationalacademies.org/read/9810/chapter/1
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994 Apr 14;330(15):1029-35. doi: 10.1056/NEJM199404143301501
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996 May 2;334(18):1150-5. doi: 10.1056/NEJM199605023341802
- Nutrients. 2011 Apr; 3(4): 385–428. Published online 2011 Mar 29. doi: 10.3390/nu3040385. Vitamin A in Reproduction and Development. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3257687/
- Nat Rev Immunol. 2008 Sep; 8(9): 685–698. doi: 10.1038/nri2378. Vitamin effects on the immune system: vitamins A and D take centre stage. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2906676/
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008 Mar 1;111(5):2505-15. doi: 10.1182/blood-2007-07-102798
- Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E, Specchia G, Sica S, Divona M, Levis A, Fiedler W, Cerqui E, Breccia M, Fioritoni G, Salih HR, Cazzola M, Melillo L, Carella AM, Brandts CH, Morra E, von Lilienfeld-Toal M, Hertenstein B, Wattad M, Lübbert M, Hänel M, Schmitz N, Link H, Kropp MG, Rambaldi A, La Nasa G, Luppi M, Ciceri F, Finizio O, Venditti A, Fabbiano F, Döhner K, Sauer M, Ganser A, Amadori S, Mandelli F, Döhner H, Ehninger G, Schlenk RF, Platzbecker U; Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013 Jul 11;369(2):111-21. doi: 10.1056/NEJMoa1300874
- Dragnev K.H., Petty W.J., Shah S.J., Lewis L.D., Black C.C., Memoli V., Nugent W.C., Hermann T., Negro-Vilar A., Rigas J.R., et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin. Cancer Res. 2007;13:1794–1800. doi: 10.1158/1078-0432.CCR-06-1836
- Esteva F.J., Glaspy J., Baidas S., Laufman L., Hutchins L., Dickler M., Tripathy D., Cohen R., DeMichele A., Yocum R.C., et al. Multicenter phase II study of oral bexarotene for patients with metastatic breast cancer. J. Clin. Oncol. 2003;21:999–1006. doi: 10.1200/JCO.2003.05.068
- Rowles JL 3rd, Ranard KM, Smith JW, An R, Erdman JW Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017 Dec;20(4):361-377. doi: 10.1038/pcan.2017.25
- Chen F, Hu J, Liu P, Li J, Wei Z, Liu P. Carotenoid intake and risk of non-Hodgkin lymphoma: a systematic review and dose-response meta-analysis of observational studies. Ann Hematol. 2017 Jun;96(6):957-965. doi: 10.1007/s00277-016-2898-1
- Chen J, Jiang W, Shao L, Zhong D, Wu Y, Cai J. Association between intake of antioxidants and pancreatic cancer risk: a meta-analysis. Int J Food Sci Nutr. 2016 Nov;67(7):744-53. doi: 10.1080/09637486.2016.1197892
- Leoncini E, Nedovic D, Panic N, Pastorino R, Edefonti V, Boccia S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer Epidemiol Biomarkers Prev. 2015 Jul;24(7):1003-11. doi: 10.1158/1055-9965.EPI-15-0053
- Li H, He P, Lin T, Guo H, Li Y, Song Y, Wang B, Liu C, Liu L, Li J, Zhang Y, Huo Y, Zhou H, Yang Y, Ling W, Wang X, Zhang H, Xu X, Qin X. Association between plasma retinol levels and the risk of all-cause mortality in general hypertensive patients: A nested case-control study. J Clin Hypertens (Greenwich). 2020 May;22(5):906-913. doi: 10.1111/jch.13866
- Li X, Xu J. Meta-analysis of the association between dietary lycopene intake and ovarian cancer risk in postmenopausal women. Sci Rep. 2014 May 9;4:4885. doi: 10.1038/srep04885
- Wang Q, He C. Dietary vitamin A intake and the risk of ovarian cancer: a meta-analysis. Biosci Rep. 2020 Apr 30;40(4):BSR20193979. doi: 10.1042/BSR20193979
- Lv W, Zhong X, Xu L, Han W. Association between Dietary Vitamin A Intake and the Risk of Glioma: Evidence from a Meta-analysis. Nutrients. 2015 Oct 28;7(11):8897-904. doi: 10.3390/nu7115438
- Tang JE, Wang RJ, Zhong H, Yu B, Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. World J Surg Oncol. 2014 Apr 29;12:130. doi: 10.1186/1477-7819-12-130
- Leelakanok N, D’Cunha RR, Sutamtewagul G, Schweizer ML. A systematic review and meta-analysis of the association between vitamin A intake, serum vitamin A, and risk of liver cancer. Nutr Health. 2018 Jun;24(2):121-131. doi: 10.1177/0260106018777170
- Psaltopoulou T, Ntanasis-Stathopoulos I, Tsilimigras DI, Tzanninis IG, Gavriatopoulou M, Sergentanis TN. Micronutrient Intake and Risk of Hematological Malignancies in Adults: A Systematic Review and Meta-analysis of Cohort Studies. Nutr Cancer. 2018 Aug-Sep;70(6):821-839. doi: 10.1080/01635581.2018.1490444
- Wang X, Yang HH, Liu Y, Zhou Q, Chen ZH. Lycopene Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Nutr Cancer. 2016 Oct;68(7):1083-96. doi: 10.1080/01635581.2016.1206579
- Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2018 Nov 1;108(5):1069-1091. doi: 10.1093/ajcn/nqy097
- Gary E. Goodman, Mark D. Thornquist, John Balmes, Mark R. Cullen, Frank L. Meyskens, Jr., Gilbert S. Omenn, Barbara Valanis, James H. Williams, Jr., The Beta-Carotene and Retinol Efficacy Trial: Incidence of Lung Cancer and Cardiovascular Disease Mortality During 6-Year Follow-up After Stopping β-Carotene and Retinol Supplements, JNCI: Journal of the National Cancer Institute, Volume 96, Issue 23, 1 December 2004, Pages 1743–1750, https://doi.org/10.1093/jnci/djh320
- Neuhouser ML, Barnett MJ, Kristal AR, Ambrosone CB, King IB, Thornquist M, Goodman GG. Dietary supplement use and prostate cancer risk in the Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2009 Aug;18(8):2202-6. doi: 10.1158/1055-9965.EPI-09-0013
- Virtamo J, Taylor PR, Kontto J, Männistö S, Utriainen M, Weinstein SJ, Huttunen J, Albanes D. Effects of α-tocopherol and β-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the Alpha-tocopherol, Beta-carotene Cancer Prevention Study. Int J Cancer. 2014 Jul 1;135(1):178-85. doi: 10.1002/ijc.28641
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013 May 15;309(19):2005-15. doi: 10.1001/jama.2013.4997. Erratum in: JAMA. 2013 Jul 10;310(2):208.
- Chew EY, Clemons TE, Agrón E, Domalpally A, Keenan TDL, Vitale S, Weber C, Smith DC, Christen W; AREDS2 Research Group. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022 Jul 1;140(7):692-698. doi: 10.1001/jamaophthalmol.2022.1640
- Cortés-Jofré M, Rueda JR, Asenjo-Lobos C, Madrid E, Bonfill Cosp X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020 Mar 4;3(3):CD002141. doi: 10.1002/14651858.CD002141.pub3
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996 May 2;334(18):1145-9. doi: 10.1056/NEJM199605023341801
- Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009 Jan 7;101(1):14-23. doi: 10.1093/jnci/djn438
- Kamangar F, Qiao YL, Yu B, Sun XD, Abnet CC, Fan JH, Mark SD, Zhao P, Dawsey SM, Taylor PR. Lung cancer chemoprevention: a randomized, double-blind trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2006 Aug;15(8):1562-4. doi: 10.1158/1055-9965.EPI-06-0316
- Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY. Age-related macular degeneration. Nat Rev Dis Primers. 2021 May 6;7(1):31. doi: 10.1038/s41572-021-00265-2
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36. doi: 10.1001/archopht.119.10.1417. Erratum in: Arch Ophthalmol. 2008 Sep;126(9):1251.
- Agrón E, Mares J, Clemons TE, Swaroop A, Chew EY, Keenan TDL; AREDS and AREDS2 Research Groups. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology. 2021 Mar;128(3):425-442. doi: 10.1016/j.ophtha.2020.08.018
- Patel M, Lee AD, Redd SB, Clemmons NS, McNall RJ, Cohn AC, Gastañaduy PA. Increase in Measles Cases – United States, January 1-April 26, 2019. MMWR Morb Mortal Wkly Rep. 2019 May 3;68(17):402-404. doi: 10.15585/mmwr.mm6817e1
- World Health Organization. Guideline: Vitamin A Supplementation in Infants and Children 6-59 Months of Age. 2011. https://www.who.int/publications/i/item/9789241501767
- Yang HM, Mao M, Wan C. Vitamin A for treating measles in children. Cochrane Database Syst Rev 2011;2005. http://www.cochrane.org/CD001479/ARI_vitamin-a-for-measles-in-children
- Imdad A, Mayo-Wilson E, Herzer K, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev. 2017 Mar 11;3(3):CD008524. doi: 10.1002/14651858.CD008524.pub3. Update in: Cochrane Database Syst Rev. 2022 Mar 16;3:CD008524.
- Bello S, Meremikwu MM, Ejemot-Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database Syst Rev. 2011 Apr 13;(4):CD007719. doi: 10.1002/14651858.CD007719.pub2. Update in: Cochrane Database Syst Rev. 2014;1:CD007719.
- Foster A, Sommer A. Corneal ulceration, measles, and childhood blindness in Tanzania. Br J Ophthalmol. 1987 May;71(5):331-43. doi: 10.1136/bjo.71.5.331
- Vitamin A Deficiency Clinical Presentation. https://emedicine.medscape.com/article/126004-clinical
- Vitamin A Deficiency Clinical Presentation. https://emedicine.medscape.com/article/126004-clinical#b2
- Mehra D, Le PH. Physiology, Night Vision. [Updated 2022 Sep 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545246
- Gilbert C, Awan H. Blindness in children. BMJ. 2003 Oct 4;327(7418):760-1. doi: 10.1136/bmj.327.7418.760
- Semba RD. Vitamin A and human immunodeficiency virus infection. Proc Nutr Soc. 1997 Mar;56(1B):459-69. doi: 10.1079/pns19970046
- Field CJ, Johnson IR, Schley PD. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002 Jan;71(1):16-32.
- Awasthi S, Peto R, Read S, Clark S, Pande V, Bundy D; DEVTA (Deworming and Enhanced Vitamin A) team. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet. 2013 Apr 27;381(9876):1469-77. doi: 10.1016/S0140-6736(12)62125-4
- Cohen N, Rahman H, Sprague J, Jalil MA, Leemhuis de Regt E, Mitra M. Prevalence and determinants of nutritional blindness in Bangladeshi children. World Health Stat Q. 1985;38(3):317-30. English, French.
- Rubino P, Mora P, Ungaro N, Gandolfi SA, Orsoni JG. Anterior Segment Findings in Vitamin A Deficiency: A Case Series. Case Rep Ophthalmol Med. 2015;2015:181267. doi: 10.1155/2015/181267
- Fernando-Langit A, Ilsen PF. Ocular manifestations of vitamin A deficiency. Clin Refract Optom. 2008;19:86–93.
- Tinley CG, Withers NJ, Sheldon CD, Quinn AG, Jackson AA. Zinc therapy for night blindness in cystic fibrosis. J Cyst Fibros. 2008 Jul;7(4):333-335. doi: 10.1016/j.jcf.2007.11.00
- Chiu, M., Dillon, A., and Watson, S. (2016) Vitamin A deficiency and xerophthalmia in children of a developed country. Journal of Paediatrics and Child Health, 52: 699– 703. doi: 10.1111/jpc.13243
- Management of Bitot’s Spots. https://www.aao.org/eyenet/article/management-of-bitot-s-spots#chart
- Miller M, Humphrey J, Johnson E, Marinda E, Brookmeyer R, Katz J. Why do children become vitamin A deficient? J Nutr. 2002 Sep;132(9 Suppl):2867S-2880S. doi: 10.1093/jn/132.9.2867S
- Gomes MM, Saunders C, Ramalho A. Placenta: a possible predictor of vitamin A deficiency. Br J Nutr. 2010 May;103(9):1340-4. doi: 10.1017/S0007114509993072
- de Pee S, Dary O. Biochemical indicators of vitamin A deficiency: serum retinol and serum retinol binding protein. J Nutr. 2002 Sep;132(9 Suppl):2895S-2901S. doi: 10.1093/jn/132.9.2895S
- Weinman AR, Jorge SM, Martins AR, de Assis Md, Martinez FE, Camelo JS Jr. Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition. 2007 Jun;23(6):454-60. doi: 10.1016/j.nut.2007.04.003
- Gorstein JL, Dary O, Pongtorn, Shell-Duncan B, Quick T, Wasanwisut E. Feasibility of using retinol-binding protein from capillary blood specimens to estimate serum retinol concentrations and the prevalence of vitamin A deficiency in low-resource settings. Public Health Nutr. 2008 May;11(5):513-20. doi: 10.1017/S1368980007000821
- Lietman TM, Dhital SP, Dean D. Conjunctival impression cytology for vitamin A deficiency in the presence of infectious trachoma. Br J Ophthalmol. 1998 Oct;82(10):1139-42. doi: 10.1136/bjo.82.10.1139
- Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009 Aug 28;84(35):349-60. English, French.
- Rahman MM, Wahed MA, Fuchs GJ, Baqui AH, Alvarez JO. Synergistic effect of zinc and vitamin A on the biochemical indexes of vitamin A nutrition in children. Am J Clin Nutr. 2002 Jan;75(1):92-8. doi: 10.1093/ajcn/75.1.92
- Hodge C, Taylor C. Vitamin A Deficiency. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567744
- World Health Organization. (2011). Guideline : vitamin A supplementation in pregnant women. World Health Organization. https://apps.who.int/iris/handle/10665/44625
- World Health Organization. (2011). Guideline : vitamin A supplementation in infants 1-5 months of age. World Health Organization. https://apps.who.int/iris/handle/10665/44628
- World Health Organization. (2011). Guideline : neonatal vitamin A supplementation. World Health Organization. https://apps.who.int/iris/handle/10665/44626
- World Health Organization. (2011). Guideline : vitamin A supplementation in postpartum women. World Health Organization. https://apps.who.int/iris/handle/10665/44623
- Vitamin A Deficiency Treatment & Management. https://emedicine.medscape.com/article/126004-treatment
- Via MA, Mechanick JI. Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr Obes Rep. 2017 Sep;6(3):286-296. doi: 10.1007/s13679-017-0271-x
- Basu, S., Khanna, P., Srivastava, R. et al. Oral vitamin A supplementation in very low birth weight neonates: a randomized controlled trial. Eur J Pediatr 178, 1255–1265 (2019). https://doi.org/10.1007/s00431-019-03412-w
- Ross DA. Recommendations for Vitamin A Supplementation. The Journal of Nutrition. Volume 132, Issue 9, September 2002, Pages 2902S-2906S. https://doi.org/10.1093/jn/132.9.2902S
- Bors F, Fells P. Reversal of the complications of self-induced vitamin A deficiency. Br J Ophthalmol. 1971 Mar;55(3):210-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1208224/pdf/brjopthal00303-0070.pdf
- Crum AR, Srikumaran D, Woreta F. Bitot’s Spots following Bariatric Surgery: An Ocular Manifestation of a Systemic Disease. Case Rep Ophthalmol. 2017 Dec 21;8(3):581-589. doi: 10.1159/000485235