Vitamin D deficiency
Vitamin D deficiency also known as hypovitaminosis D can lead to bone diseases such as osteoporosis, rickets and osteomalacia 1, 2, 3, 4, 5, 6. Osteoporosis is a condition in which bones become weak and brittle, increasing the chance they may break and increases the risk of falling. Rickets is a condition in children in which bones become soft and deformed because they don’t have enough calcium and phosphorus with vitamin D deficiency. Rickets is caused by not having enough vitamin D in the diet or by not getting enough sunlight. In adults, this condition is called osteomalacia. Osteomalacia is a condition in adults in which bones become soft and deformed because they don’t have enough calcium and phosphorus. It is usually caused by not having enough vitamin D in the diet, not getting enough sunlight, or a problem with the way the body uses vitamin D. These conditions are characterized by reduced bone density, an increased risk of fractures, and muscle weakness and pain 5, 7, 6, 8. Whereas osteoporosis is not associated with bone pain, osteomalacia has been associated with isolated or generalized bone pain 9, 10. The cause is thought to be hydration of the demineralized gelatin matrix beneath the periosteum; the hydrated matrix pushes outward on the periosteum, causing throbbing, aching pain 11. Osteomalacia can often be diagnosed by using moderate force to press the thumb on the sternum or anterior tibia, which can elicit bone pain 11, 10. One study showed that 93% of persons 10 to 65 years of age who were admitted to a hospital emergency department with muscle aches and bone pain and who had a wide variety of diagnoses, including fibromyalgia, chronic fatigue syndrome, and depression, were deficient in vitamin D 6. Vitamin D deficiency can also cause muscle weakness and pain in children and adults. Muscle pain and weakness were prominent symptoms of vitamin D deficiency in a study of Arab and Danish Muslim women living in Denmark 12. In a cross-sectional study of 150 consecutive patients referred to a clinic in Minnesota for the evaluation of persistent, nonspecific musculoskeletal pain, 93% had serum 25-hydroxyvitamin D concentrations equal to or below 20 ng/mL, with a mean concentration of 12.1 ng/mL, which is indicative of vitamin D insufficiency 6.
Loss of muscle strength greatly contributes to increased risk of falling and bone fractures, especially in older people 13. In addition, long-term vitamin D deficiency may be a contributing factor to osteoporosis in the elderly 14, 15. A meta-analysis of 18 randomized controlled trials including over 57,000 subjects found that intake of daily doses of vitamin D supplements decreased total mortality rates from any cause 16. Vitamin D deficiency is associated with an increased risk of several chronic diseases, including osteoporosis, type 2 diabetes, autoimmune diseases, and some types of cancer 5, 17, 18, 19. In the Women’s Health Initiative, calcium and vitamin D supplementation decreased the risk of total cancer, breast cancer, and colorectal cancer while not changing total mortality 20. One randomized controlled trial showed that calcium plus vitamin D substantially reduced all cancer risk in postmenopausal women 21. In a meta-analysis study from three randomized controlled trials, vitamin D supplementation was found to reduce the rate of chronic obstructive pulmonary disease (COPD) worsening in patients with vitamin D levels below 25 nmol/L 22.
The main function of vitamin D also referred to as “calciferol” is to help your body absorb calcium from the gut and maintains adequate serum calcium and phosphate concentrations. Vitamin D also helps maintain proper levels of calcium, phosphate, and parathyroid hormone (PTH) in your blood. Calcium is one of the main building blocks of bones and teeth. Without vitamin D, only 10 to 15% of dietary calcium and about 60% of phosphorus is absorbed 23, 24, 25. In the musculoskeletal system, vitamin D helps maintain the balance between bone formation and resorption, which is why maintaining adequate levels of vitamin D supports healthy bone density and strength 5, 7. In addition, vitamin D has other roles in the body, including anti-inflammatory and other properties that play a role in maintaining normal muscle and improving muscle strength and function, immune, and nervous system functions and glucose metabolism, particularly in older adults 26, 27, 28. Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D 29.
In vitamin D deficiency, calcium absorption cannot be increased enough to satisfy the body’s calcium needs 30. The interaction of 1,25-dihydroxyvitamin D with the vitamin D receptor increases the efficiency of intestinal calcium absorption to 30 to 40% and phosphorus absorption to approximately 80% 23, 24, 25, 31. Consequently, the parathyroid hormone (PTH) production by the parathyroid glands is increased and calcium is mobilized from the skeleton to maintain normal serum calcium concentrations — a condition known as secondary hyperparathyroidism. Although it has long been known that severe vitamin D deficiency has serious consequences for bone health, research suggests that less obvious states of vitamin D deficiency are common and increase the risk of osteoporosis and various other health problems.
The major source of vitamin D is sunlight (exposure to ultraviolet B radiation). Vitamin D deficiency is typically due to limited sunlight exposure. However, too much sun exposure can lead to skin aging and skin cancer. So many people try to get their vitamin D from other sources. Vitamin D-rich foods include egg yolks, saltwater fish, and liver. Some other foods, like milk and cereal, often have added vitamin D. You can also take vitamin D supplements. Check with your health care provider to see how much vitamin D you should take.
People can become deficient in vitamin D because they don’t consume enough or absorb enough from food, their exposure to sunlight is limited, or their kidneys cannot convert vitamin D to its active form in the body. In children, vitamin D deficiency causes rickets, a disease where the bones become soft and bend due to a failure of bone tissue to properly mineralize 32. The fortification of milk and other staples, such as breakfast cereals and margarine, with vitamin D beginning in the 1930s has made rickets a rare disease in the United States, although it is still reported periodically, particularly among African American infants and children, immigrants from African, Middle-Eastern, and Asian countries 33, 34, 35. Rickets is also more prevalent among immigrants from Asia, Africa, and the Middle East, possibly because of genetic differences in vitamin D metabolism, dietary preferences, and behavioral differences that lead to less sun exposure 36.
Prolonged exclusive breastfeeding without the American Academy of Pediatrics-recommended vitamin D supplementation is a significant cause of rickets, particularly in dark-skinned infants breastfed by mothers who are not vitamin D replete 37. Additional causes of rickets include extensive use of sunscreens and placement of children in daycare programs, where they often have less outdoor activity and sun exposure 32, 38.
In adults and adolescents, vitamin D deficiency can lead to osteomalacia, in which existing bone is incompletely or defectively mineralized during the remodeling process, resulting in weak bones causing bone pain and muscle weakness 39. Signs and symptoms of osteomalacia are similar to those of rickets and include bone deformities and pain, hypocalcemic seizures, tetanic spasms, and dental abnormalities 40.
A lack of vitamin D has been associated with:
- An impairment in memory and thinking skills in older adults
- Bone, back, or muscle pain
- Cancer (particularly colon cancer)
- Cardiovascular disease, and an increased risk of dying from a stroke or a heart attack
- Constant fatigue and tiredness
- Frequent infections (such as colds and flu)
- Hair loss
- Kidney disease
- Low mood or depression
- Osteomalacia
- Osteoporosis
- Poor dental health
- Rickets
- Severe asthma in children
- Skin wounds that take a long time to heal.
Research also suggests low vitamin D may be a factor in several other conditions such as type 2 diabetes, high blood pressure, and multiple sclerosis.
People who might need extra vitamin D include 41, 42, 43:
- Older adults
- Exclusively breastfed infants
- People with dark skin
- People with certain conditions, such as liver diseases, cystic fibrosis and Crohn’s disease
- People who have obesity or have had gastric bypass surgery
- People with habitually limited sun exposure
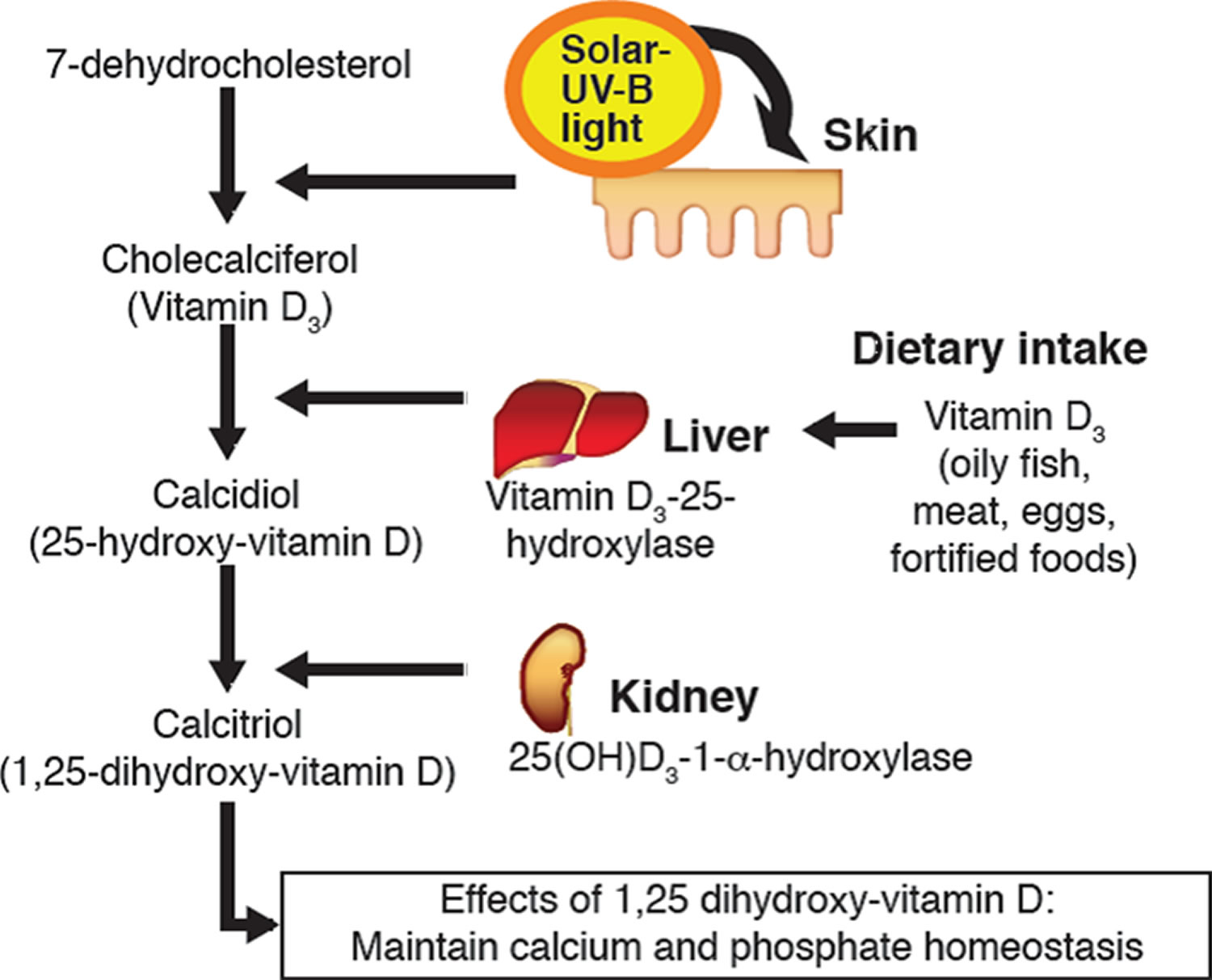
Vitamin D enters the circulation and is transported to the liver, where it is hydroxylated to form 25-hydroxyvitamin D (calcidiol; the major circulating form of vitamin D). In the kidneys, the 1-alpha-hydroxylase enzyme catalyzes a second hydroxylation of 25-hydroxyvitamin D, resulting in the formation of 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D] — the active form of vitamin D 44. Most of the physiological effects of vitamin D in the body are related to the activity of 1,25-dihydroxyvitamin D (calcitriol or 1,25(OH)2D).
Most of the time, vitamin D levels will be tested by measuring blood levels of 25-hydroxyvitamin D [25(OH)D or calcidiol]. Testing 25-hydroxyvitamin D [25(OH)D or calcidiol] is considered the most accurate way to measure how much vitamin D is in your body because 25-hydroxyvitamin D [25(OH)D or calcidiol] is the major form of vitamin D circulating in your bloodstream. Sometimes, doctors may check your blood level of 1,25 dihydroxyvitamin D (active vitamin D), which is also called calcitriol. However, 1,25 dihydroxyvitamin D (calcitriol) is generally not used to detect inadequate vitamin D levels, but it may be measured in patients with abnormal calcium levels or kidney problems 45.
Vitamin D blood testing is used to diagnose vitamin D deficiencies or to monitor treatment for a known vitamin D deficiency. Less commonly, vitamin D testing may be used to detect vitamin D toxicity, a condition in which there is an excess of vitamin D in the body.
There is a bit of controversy regarding what is considered a low vitamin D level between different expert organizations. A vitamin D level measures levels of 25-hydroxyvitamin D (25(OH)D) also known as calcidiol, in the blood.
Most experts recommend:
- Levels of 20-50 nanograms/milliliter (ng/ml) of 25-hydroxyvitamin D (calcidiol): Sufficient (good)
- Levels of 12-19 ng/ml of 25-hydroxyvitamin D (calcidiol): Borderline
- Levels of less than 12 ng/ml of 25-hydroxyvitamin D (calcidiol): Deficient (low)
- Levels of less than 5 ng/ml of 25-hydroxyvitamin D (calcidiol): Severe deficiency
However, not everybody agrees, and some organizations suggest different cut-off values.
The Institute of Medicine states:
- Levels above 20 ng/ml of 25-hydroxyvitamin D (calcidiol): Sufficient
- Levels below 20 ng/ml of 25-hydroxyvitamin D (calcidiol): Deficient
Note that several members of the Institute of Medicine committee publicly stated that over screening for vitamin D deficiency was a problem which typically resulted in unnecessary treatment. They were not in agreement with a cut-off level of 20 ng/ml for deficiency and recommended a lower level of 12.5 ng/ml.
The Endocrine Society states:
- Levels above 30 ng/ml of 25-hydroxyvitamin D (calcidiol): Sufficient; however, some assays are inaccurate and levels of 40-60 ng/ml better guarantee sufficiency
- Levels of 21-29 ng/ml of 25-hydroxyvitamin D (calcidiol): Insufficient
- Levels below 20 ng/ml pf 25-hydroxyvitamin D (calcidiol): Deficient
Other medical institution states 46:
- Levels below 20 ng/mL of 25-hydroxyvitamin D (calcidiol): Mild deficiency
- Levels below 10 ng/mL of 25-hydroxyvitamin D (calcidiol): Moderate deficiency
- Levels below 5 ng/mL of 25-hydroxyvitamin D (calcidiol): Severe deficiency
Talk to your doctor about what he/she considers to be a low vitamin D level. Abnormal levels of vitamin D can indicate bone disorders, nutrition problems, organ damage, or other medical conditions.
Although there is no consensus on optimal levels of 25-hydroxyvitamin D as measured in serum, most experts define vitamin D deficiency as a 25-hydroxyvitamin D (calcidiol) level of less than 20 ng per milliliter (50 nmol per liter) 11, 47, 48, 49. 25-Hydroxyvitamin D (calcidiol) levels are inversely associated with parathyroid hormone (PTH) levels until 25-hydroxyvitamin D (calcidiol) reach 30 to 40 ng per milliliter (75 to 100 nmol per liter), at which point parathyroid hormone (PTH) levels begin to level off (at their lowest point) 49, 50, 51. Furthermore, intestinal calcium transport increased by 45 to 65% in women when 25-hydroxyvitamin D levels were increased from an average of 20 to 32 ng per milliliter (50 to 80 nmol per liter) 31. Given such data, a level of 25-hydroxyvitamin D of 21 to 29 ng per milliliter (52 to 72 nmol per liter) can be considered to indicate a relative insufficiency of vitamin D, and a level of 30 ng per milliliter or greater can be considered to indicate sufficient vitamin D 52. Vitamin D intoxication is observed when serum levels of 25-hydroxyvitamin D are greater than 150 ng per milliliter (374 nmol per liter) 5.
With the use of such definitions, it has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency 5. According to several studies, 40 to 100% of U.S. and European elderly men and women still living in the community (not in nursing homes) are deficient in vitamin D , 53, 14, 54, 55, 56, 15, 57, 58. More than 50% of postmenopausal women taking medication for osteoporosis had suboptimal levels of 25-hydroxyvitamin D — below 30 ng per milliliter (75 nmol per liter) 51, 58.
Children and young adults are also potentially at high risk for vitamin D deficiency. For example, 52% of Hispanic and black adolescents in a study in Boston 59 and 48% of white preadolescent girls in a study in Maine 60 had 25-hydroxyvitamin D levels below 20 ng per milliliter. In other studies, at the end of the winter, 42% of 15- to 49-year-old black girls and women throughout the United States had 25-hydroxyvitamin D levels below 20 ng per milliliter 61 and 32% of healthy students, physicians, and residents at a Boston hospital were found to be vitamin D–deficient, despite drinking a glass of milk and taking a multivitamin daily and eating salmon at least once a week 62.
In Europe, where very few foods are fortified with vitamin D, children and adults would appear to be at especially high risk 63, 11, 50, 14, 54, 55, 56, 15, 57, 58. People living near the equator who are exposed to sunlight without sun protection have good levels of 25-hydroxyvitamin D — above 30 ng per milliliter 64, 65. However, even in the sunniest areas, vitamin D deficiency is common when most of the skin is shielded from the sun. In studies in Saudi Arabia, the United Arab Emirates, Australia, Turkey, India, and Lebanon, 30 to 50% of children and adults had 25-hydroxyvitamin D levels under 20 ng per milliliter 66, 67, 68, 69. Also at risk were pregnant and lactating women who were thought to be immune to vitamin D deficiency since they took a daily prenatal multivitamin containing 400 IU of vitamin D (70% took a prenatal vitamin, 90% ate fish, and 93% drank approximately 2.3 glasses of milk per day) 70, 71, 72; 73% of the women and 80% of their infants were vitamin D–deficient (25-hydroxyvitamin D level, <20 ng per milliliter) at the time of birth 71.
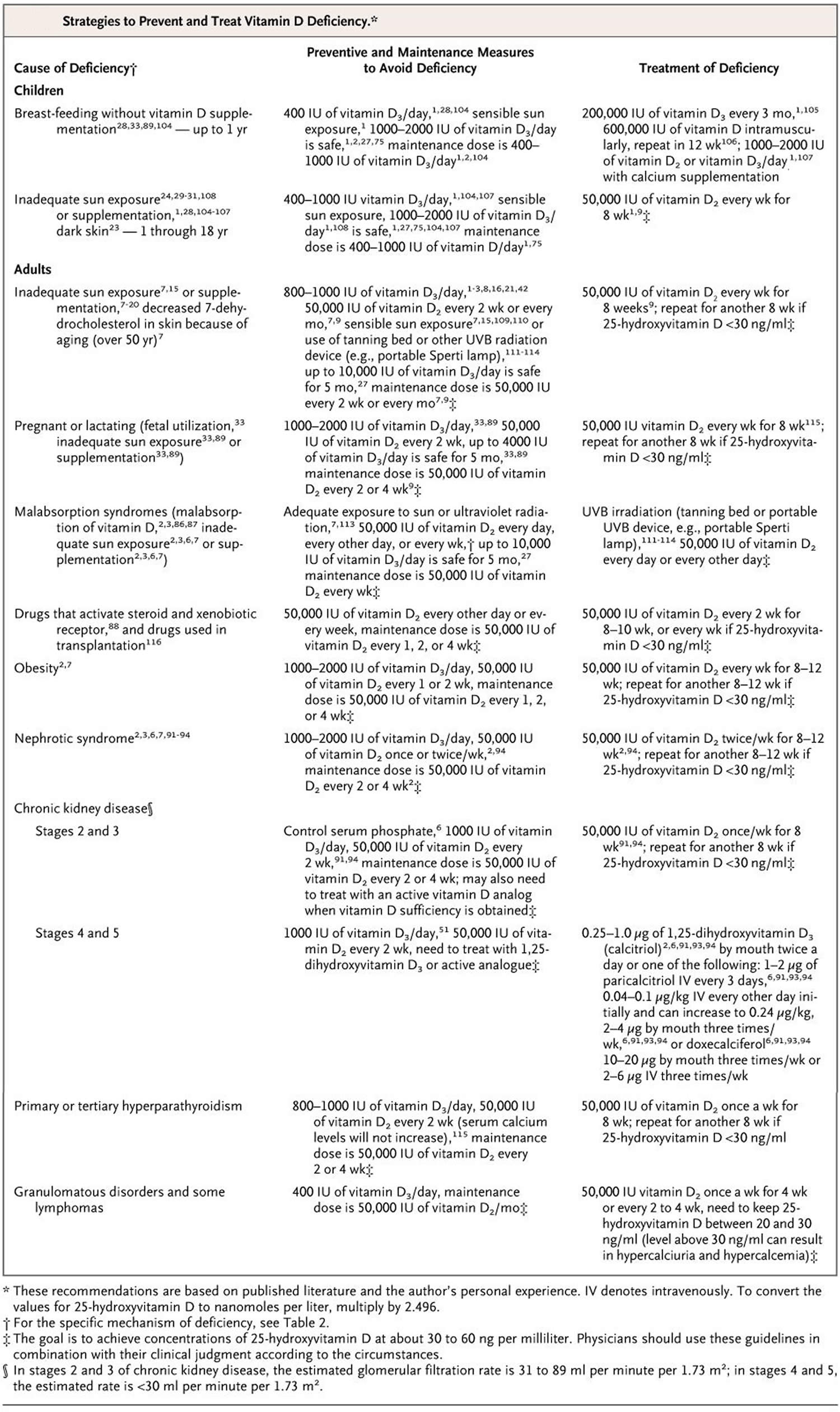
In terms of vitamin D deficiency treatment several preparations of vitamin D are available. Vitamin D3 (cholecalciferol), when compared with vitamin D2 (ergocalciferol), has been shown to be more efficacious in achieving optimal 25-hydroxyvitamin D levels, thus favoring vitamin D3 as a treatment of choice 73. The amount of vitamin D required to treat vitamin D deficiency depends largely on the degree of the vitamin D deficiency and underlying risk factors 74:
- Initial supplementation for 8 weeks with Vitamin D3 either 6,000 IU daily or 50,000 IU weekly can be considered 75. Once the serum 25-hydroxyvitamin D level exceeds 30 ng/mL, a daily maintenance dose of 1,000 to 2,000 IU is recommended.
- A higher-dose initial supplementation with vitamin D3 at 10,000 IU daily may be needed in high-risk adults who are vitamin D deficient (African Americans, Hispanics, obese, taking certain medications, malabsorption syndrome). Once serum 25-hydroxyvitamin D level exceeds 30ng/mL, 3000 to 6000 IU/day maintenance dose is recommended.
- Children who are vitamin D deficient require 2000 IU/day of vitamin D3 or 50,000 IU of vitamin D3 once weekly for 6 weeks. Once the serum 25-hydroxyvitamin D level exceeds 30 ng/mL, 1000 IU/day maintenance treatment is recommended. According to the American Academy of Pediatrics, infants who are breastfed and children who consume less than 1 L of vitamin D-fortified milk need 400 IU of vitamin D supplementation.
- Calcitriol (1,25-dihydroxyvitamin D [1,25(OH)2D]) can be considered where vitamin D deficiency persists despite treatment with vitamin D2 and/or vitamin D3. The serum calcium level shall be closely monitored in these individuals due to an increased risk of hypercalcemia secondary to calcitriol.
- Calcidiol (25-hydroxyvitamin D [25(OH)D]) can be considered in patients with fat malabsorption or severe liver disease.
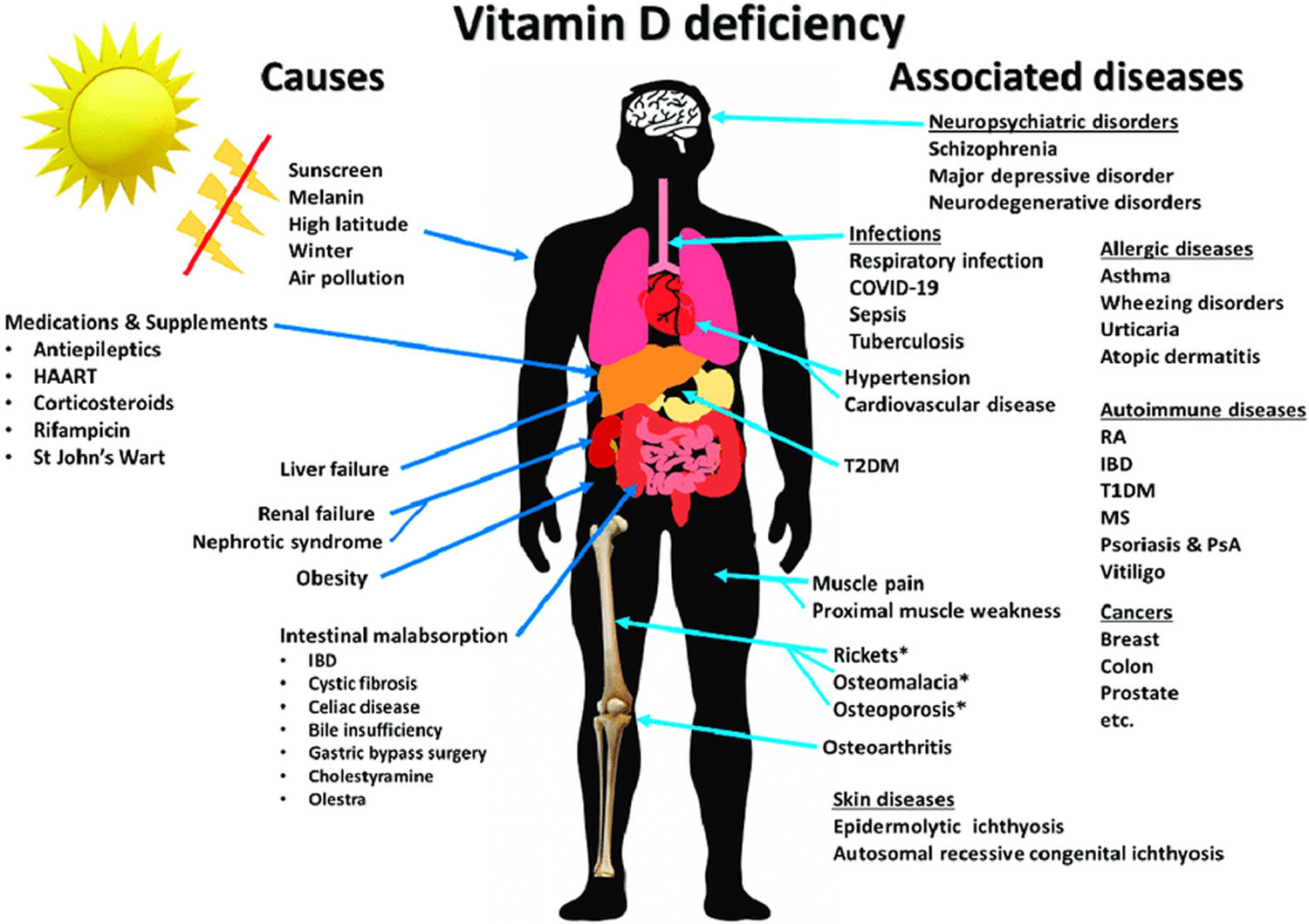
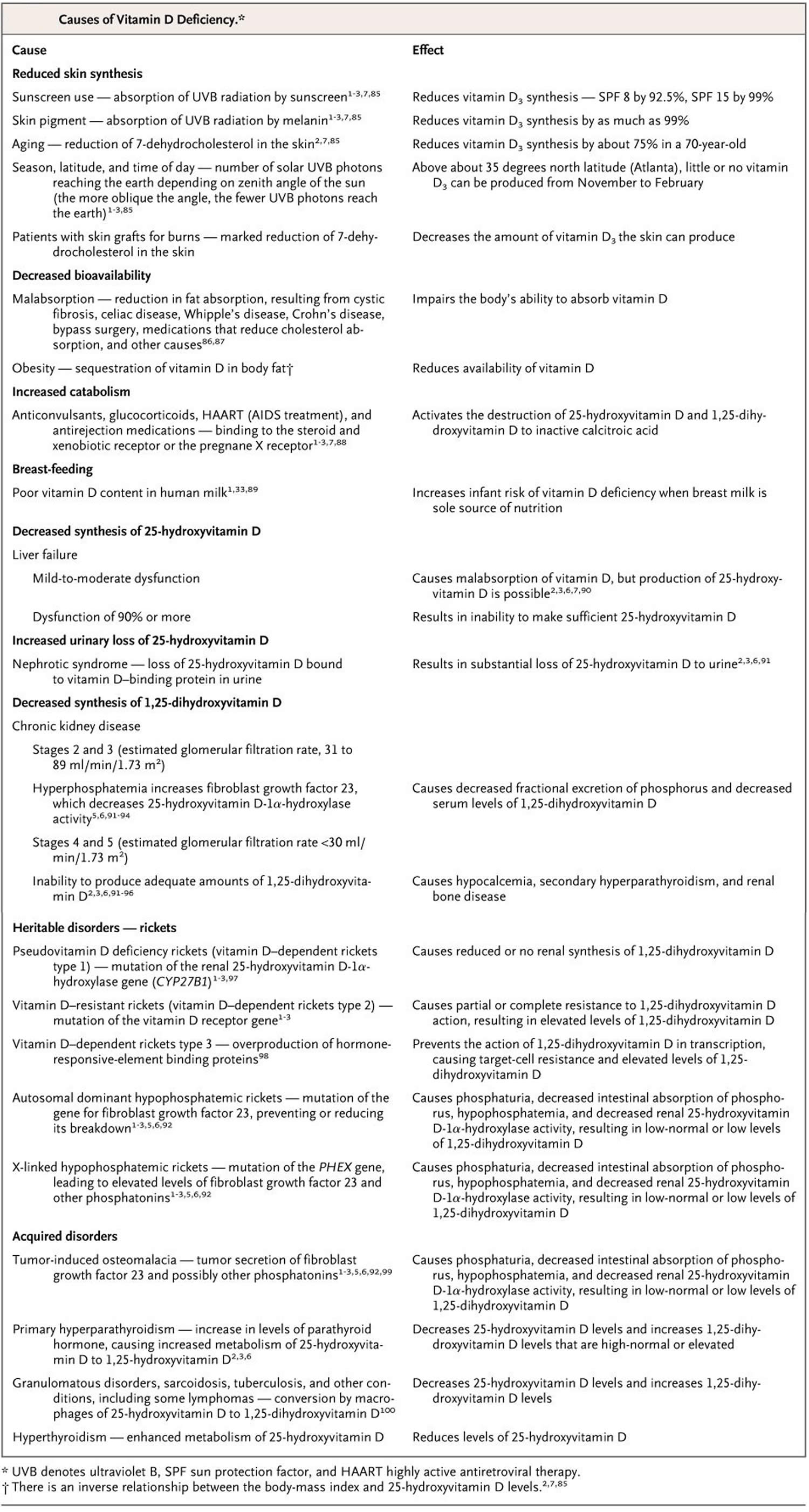
Figure 1. Vitamin D deficiency causes and symptoms
Footnote: Summary of causes of vitamin D deficiency and diseases and disorders associated with vitamin D deficiency. * Denotes diseases that are direct consequences of vitamin D deficiency.
Abbreviations: HARRT = highly active antiretroviral therapy; IBD = inflammatory bowel diseases; MS = multiple sclerosis; PsA = psoriatic arthritis; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus; RA = rheumatoid arthritis.
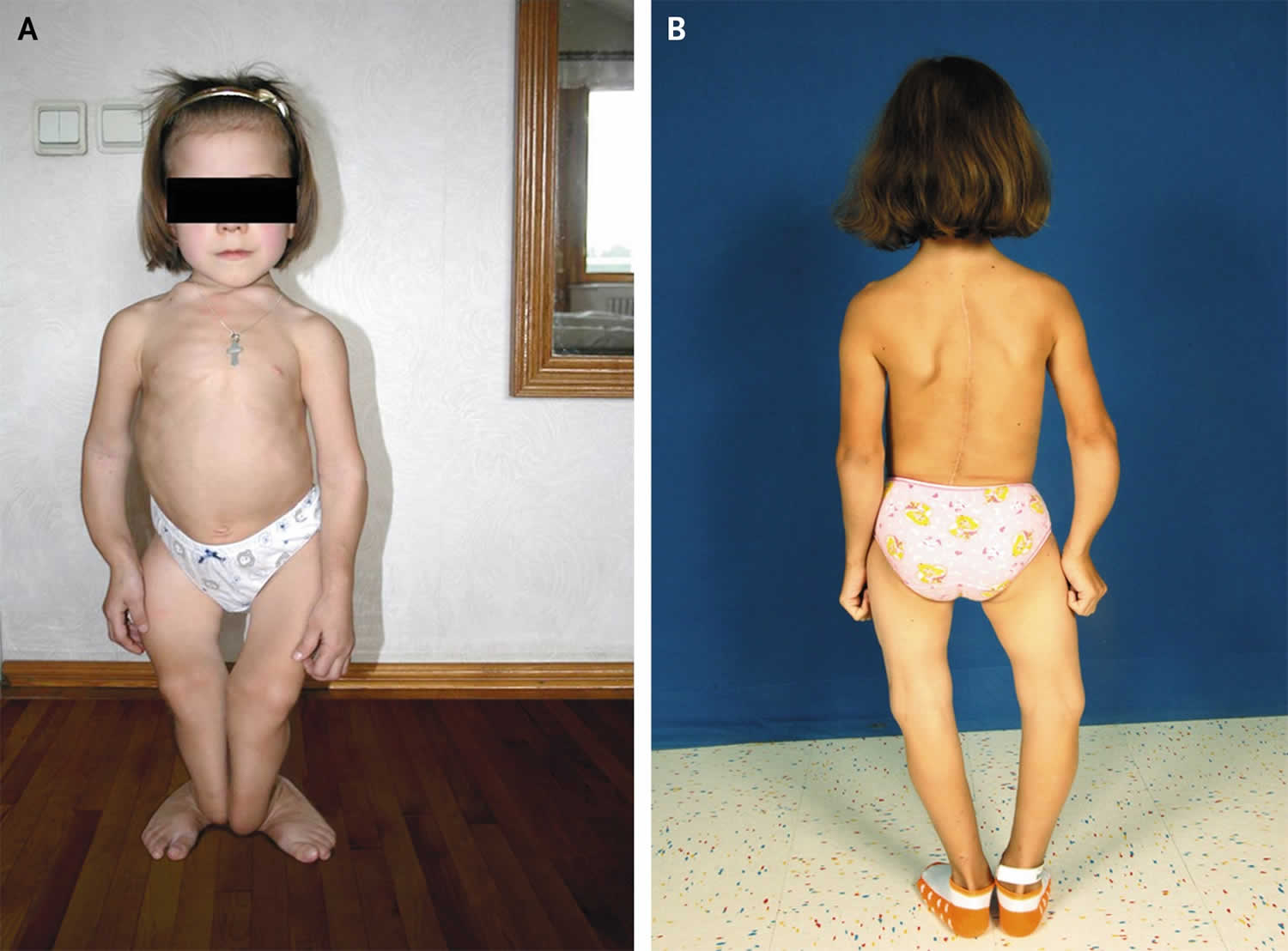
[Source 76 ]Figure 2. Rickets
Footnotes: A 12-year-old girl from Ukraine was hospitalized for evaluation of a history of long-bone fractures and failure to thrive. On initial presentation, she had hypocalcemia (calcium level, 6.7 mg per deciliter [1.7 mmol per liter]), a low 25-hydroxyvitamin D level (5 ng per milliliter [12 nmol per liter]), an elevated parathyroid hormone level (435 pg per milliliter), an elevated alkaline phosphatase level (546 U per liter), and a normal phosphorus level (4.1 mg per deciliter [1.3 mmol per liter]). These findings were consistent with vitamin D–deficient rickets. She also showed multiple sequelae of long-standing rickets, including costochondral swelling (rachitic rosary), severe thoracic scoliosis, and bilateral tibial–fibular valgus deformities (Panel A). In addition to a diet poor in vitamin D and calcium, the patient had a history of biliary dyskinesia, which may have contributed to poor absorption of fat-soluble vitamins, including vitamin D. The patient received nutritional counseling and was started on calcium and vitamin D supplementation. She underwent spinal fusion and bilateral tibial–fibular osteotomies with considerable improvement (Panel B).
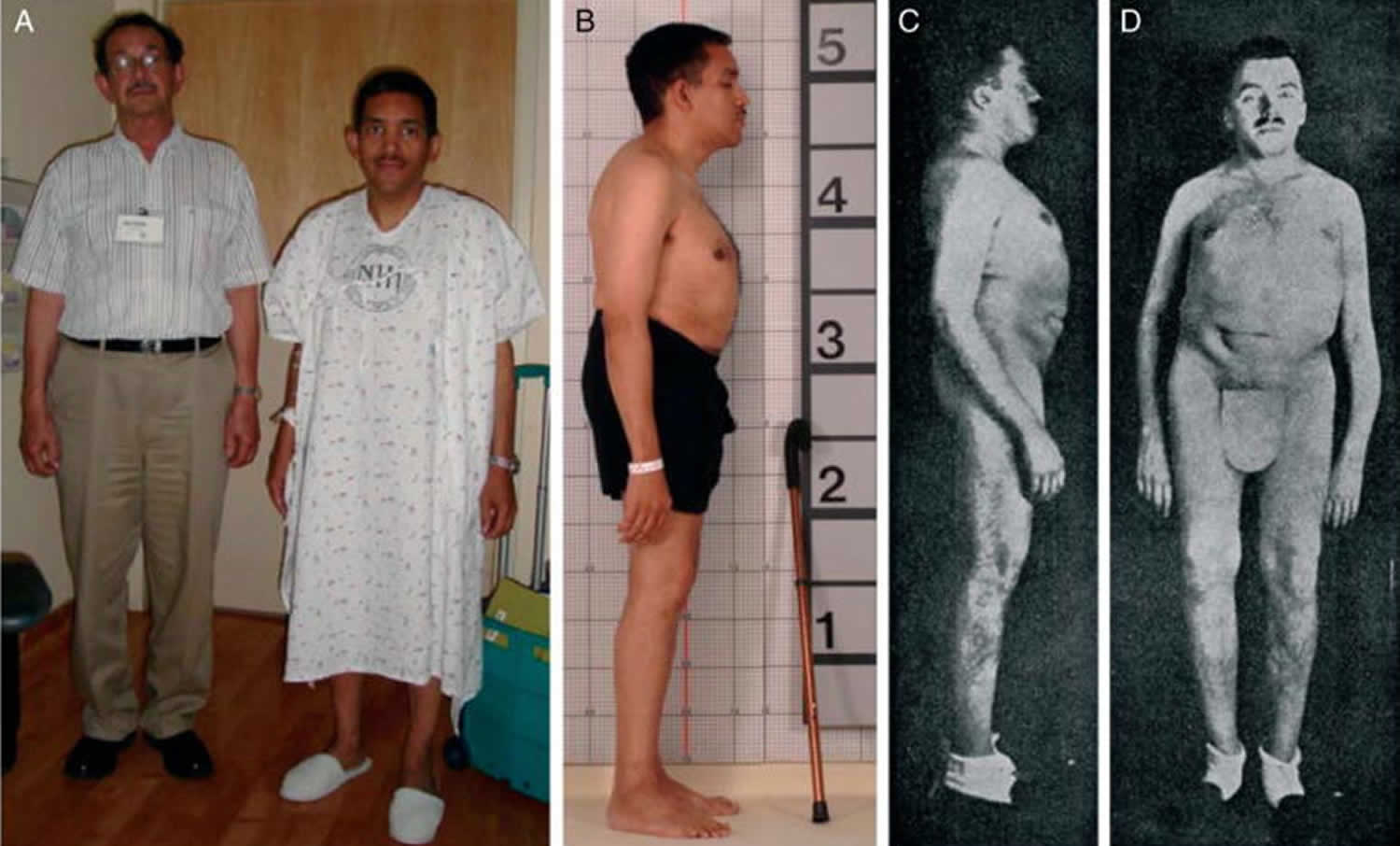
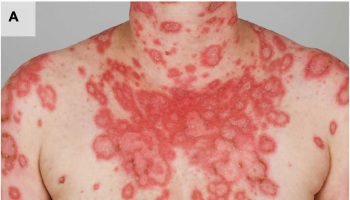
[Source 77 ]Figure 3. Osteomalacia (tumor-induced osteomalacia)
Footnotes: Tumor-induced osteomalacia. The patient in the gown in panel A is depicted standing next to his father. The patient was previously taller than his father, but this is no longer the case. Panel B demonstrates kyphosis and pectus carinatum, which resulted from multiple compression fractures due to osteomalacia. While these findings are the result of advanced osteomalacia, they are strikingly similar to those seen in advanced hyperparathyroidism, as demonstrated by the famous patient reported by Fuller Albright, Captain Martell, shown in panels C and D, who suffered from years of untreated hyperparathyroidism.
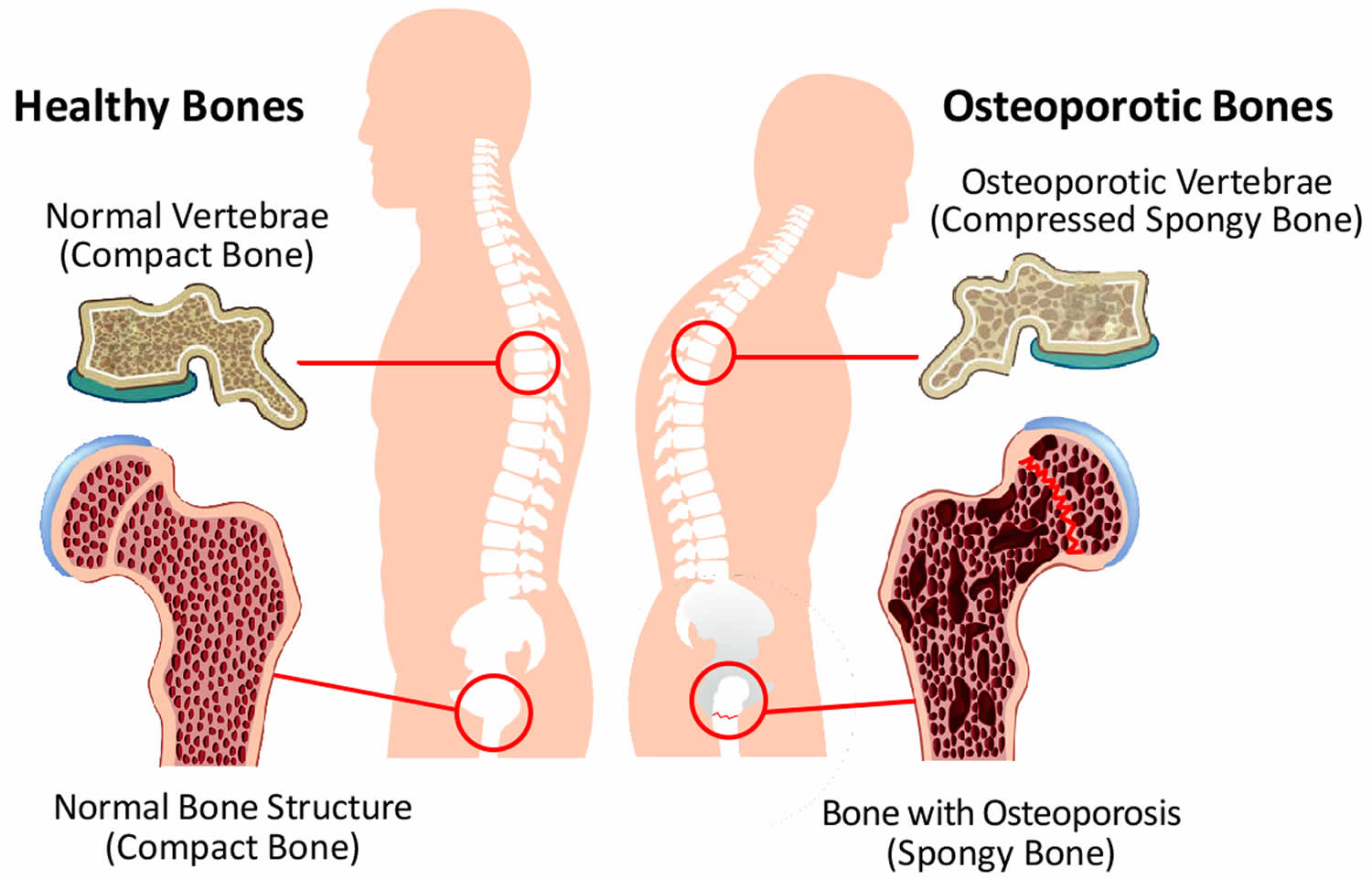
[Source 78 ]Figure 4. Osteoporosis
What is vitamin D?
Vitamin D also called calciferol, is a fat-soluble vitamin that is naturally present in very few foods, added to others, and available as a dietary supplement. In foods and dietary supplements, vitamin D has two main forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), that differ chemically only in their side-chain structures. Vitamin D2 (ergocalciferol) is synthesized from ergosterol and found in yeast, sun dried and ultraviolet irradiated mushrooms, and plants 5. Vitamin D3 (cholecalciferol) is synthesized endogenously from 7-dehydrocholesterol in the skin and found naturally in cod liver oil and oily fish 5. Both forms are well absorbed in the small intestine and raise serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels, and they seem to have equivalent ability to cure rickets 79. However, most evidence indicates that vitamin D3 (cholecalciferol) increases serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels to a greater extent and maintains these higher levels longer than vitamin D2 (ergocalciferol), even though both forms are well absorbed in the gut 80.
- Vitamin D2 or ergocalciferol, is found naturally in mushrooms that have been exposed to the sun. Mushrooms contain a yeast compound called ergosterol, which is converted to ergocalciferol on exposure to UV light. Maitake mushrooms are one of the best sources of vitamin D2 at 786 IU per cup, followed closely by portobello mushrooms (634 IU/cup). Chanterelle mushrooms contain a lot less D2 (114 IU/cup). Vitamin D2 derived from mushrooms is vegetarian/vegan-friendly. Vitamin D2 supplements can also be made synthetically by irradiating fungus and plant matter that naturally contain ergosterol. Drisdol is another name for supplemental vitamin D2. Supplemental D2 is cheaper to produce than supplemental D3; however, it is not as effective at raising levels of vitamin D in the blood nor as stable as synthetic vitamin D3. Vitamin D2 still requires conversion in the body to become vitamin D3 active.
- Vitamin D3 or cholecalciferol, is made when cholesterol in your skin is exposed to sunlight and it is also contained in small amounts in some animal-sourced foods. Your skin stores a specific type of cholesterol, called 7-dehydrocholesterol, which is converted to previtamin D3 on exposure to UVB (wavelengths of 290 to 315 nanometers). Another process changes this into cholecalciferol before it undergoes activation in the liver and kidneys to become active vitamin D. Active vitamin D is called 1,25 dihydroxyvitamin D3 (1,25(OH)D) or calcitriol. The process of converting 7-dehydrocholesterol into active vitamin D3, although complex, is reasonably efficient and it has been estimated that only 10 minutes of summer sun on your hands and face is required to generate our daily requirement of 10 micrograms of vitamin D3. Foods that naturally contain vitamin D3 include beef liver, cheese, cod liver oil, egg yolks, and fatty fish (such as mackerel, tuna, and salmon). Supplements of vitamin D3 can be made by extracting cholesterol from lanolin derived from sheep wool, then subjecting it to a series of chemical reactions to yield 7-dehydrocholesterol. This is then irradiated to produce D3 (cholecalciferol). Supplements obtained from lanolin are not vegan-friendly; however, a D3 supplement extracted from lichen is vegan and vegetarian-friendly.
The main function of vitamin D is to help your body absorb calcium from the gut and maintains adequate serum calcium and phosphate concentrations to enable normal mineralization of bone and to prevent hypocalcemic tetany. Vitamin D also helps maintain proper levels of calcium, phosphate, and parathyroid hormone in your blood. Calcium is one of the main building blocks of bones and teeth. Vitamin D is needed for bone growth and bone remodeling by osteoblasts and osteoclasts 81. Maintaining adequate levels of vitamin D supports healthy bones. Vitamin D deficiency can lead to bone diseases such as osteoporosis, rickets and osteomalacia 82. In addition, vitamin D has other roles in the body, including anti-inflammatory and other properties that play a role in maintaining normal muscle, immune, and nervous system functions and glucose metabolism 26. Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D 29. The major source of vitamin D is sunlight (exposure to ultraviolet B radiation). Vitamin D deficiency is typically due to limited sunlight exposure. However, too much sun exposure can lead to skin aging and skin cancer. So many people try to get their vitamin D from other sources. Vitamin D-rich foods include egg yolks, saltwater fish, and liver. Some other foods, like milk and cereal, often have added vitamin D. You can also take vitamin D supplements. Check with your health care provider to see how much vitamin D you should take.
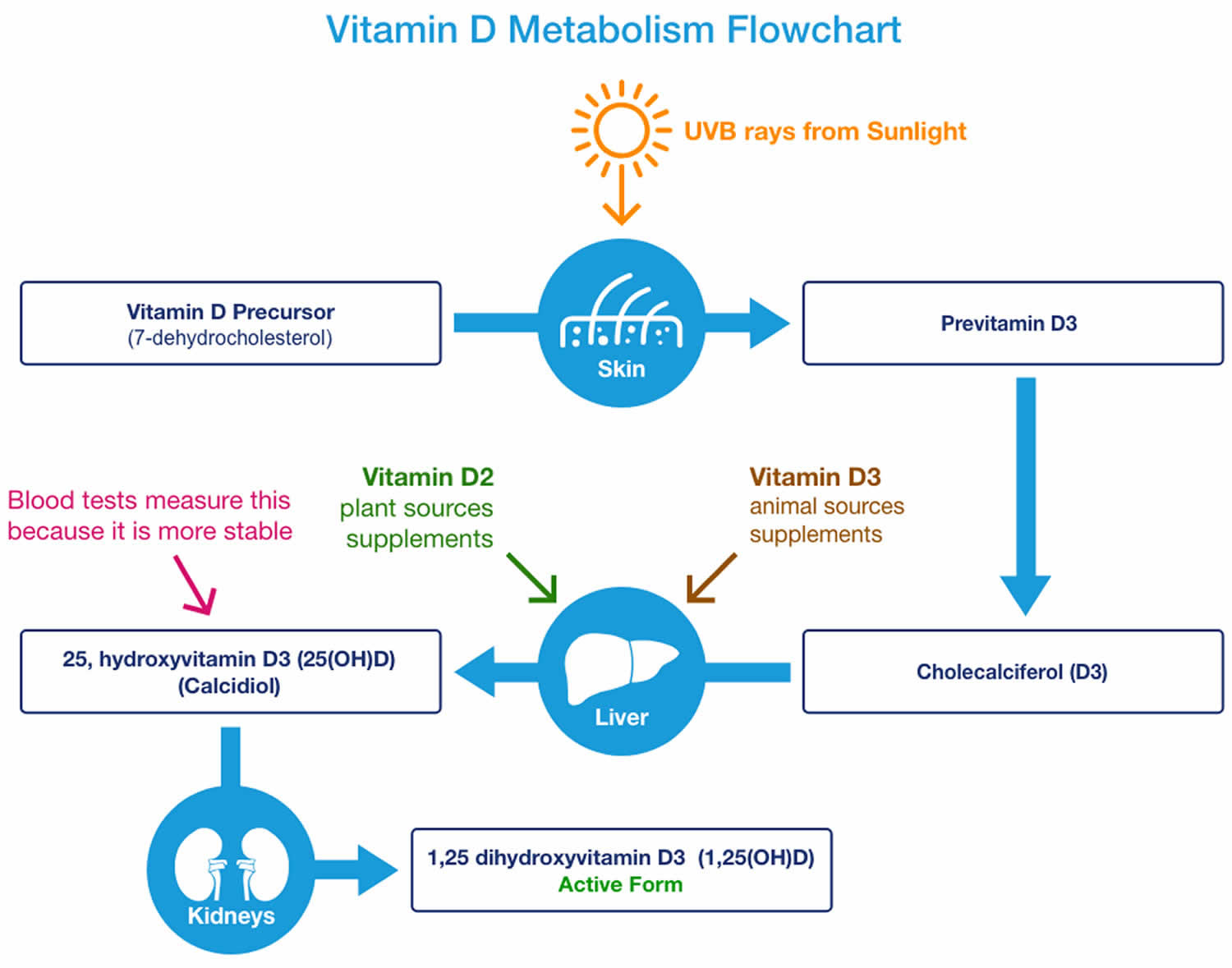
You can get vitamin D in three ways: through your skin, from your diet, and from supplements. Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylations in the body for activation before being able to be used by your body (see Figure 5 below) 83. Both vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) need to go through chemical changes in your liver and kidneys before being able to be used by your body. The first occurs in your liver where vitamin D is converted by vitamin D-25-hydroxylase (CYP2R1) enzyme into measurable substance called 25-hydroxyvitamin D [25(OH)D], also known as “calcidiol” 84. The second hydroxylation occurs primarily in your kidneys where the enzyme 25-hydroxyvitamin D-1-alpha-hydroxylase (CYP27B1) convert 25-hydroxyvitamin D [25(OH)D] into a hormone called active vitamin D or 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as “calcitriol” (active vitamin D) 85. The enzyme 25-hydroxyvitamin D-1-alpha-hydroxylase (CYP27B1) is also expressed by many other tissues including activated macrophages, parathyroid glands, microglia, breast, colon, and keratinocytes where 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D] is produced and exerts its autocrine and paracrine functions 86. 1,25-dihydroxyvitamin D [1,25(OH)2D or calcitriol] exerts its physiologic functions in the target tissue by binding to the vitamin D receptor in the nucleus where it leads to up- or down-regulation of a multitude of genes 87. A manufactured calcitriol (1,25-dihydroxyvitamin D3) is used to treat kidney disease with low blood calcium, hyperparathyroidism due to kidney disease, low blood calcium due to hypoparathyroidism, osteoporosis, osteomalacia, and familial hypophosphatemia. It is taken by mouth or by injection into a vein.
Vitamin D absorption occurs by simple passive diffusion and by a mechanism that involves intestinal membrane carrier proteins 79. The concurrent presence of fat in the gut enhances vitamin D absorption, but some vitamin D is absorbed even without dietary fat. Neither aging nor obesity alters vitamin D absorption from the gut 79.
Vitamin D (calciferol) is also produced in your body when ultraviolet (UV) rays from sunlight strike your skin and trigger vitamin D synthesis (see Figure 5). Sunlight exposure is the primary source of vitamin D for most people. Solar ultraviolet-B radiation (UVB; wavelengths of 290 to 315 nanometers) stimulates the production of vitamin D3 (cholecalciferol) from 7-dehydrocholesterol in the epidermis of your skin. Hence, vitamin D is actually more like a hormone than a vitamin, a substance that is required from the diet.
Vitamin D enters the circulation and is transported to the liver, where it is hydroxylated to form 25-hydroxyvitamin D (calcidiol; the major circulating form of vitamin D). In the kidneys, the 1-alpha-hydroxylase enzyme catalyzes a second hydroxylation of 25-hydroxyvitamin D, resulting in the formation of 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D] — the most potent form of vitamin D 44. Most of the physiological effects of vitamin D in the body are related to the activity of 1,25-dihydroxyvitamin D (calcitriol or 1,25(OH)2D).
Most of the time, vitamin D levels will be tested by measuring blood levels of 25-hydroxyvitamin D [25(OH)D or calcidiol]. Testing 25-hydroxyvitamin D [25(OH)D or calcidiol] is considered the most accurate way to measure how much vitamin D is in your body because 25-hydroxyvitamin D [25(OH)D or calcidiol] is the major form of vitamin D circulating in your bloodstream. Sometimes, doctors may check your blood level of 1,25 dihydroxyvitamin D (active vitamin D), which is also called calcitriol. However, 1,25 dihydroxyvitamin D (calcitriol) is generally not used to detect inadequate vitamin D levels, but it may be measured in patients with abnormal calcium levels or kidney problems 45.
Vitamin D testing measures the level of this essential substance in your blood. Vitamin D blood testing is used to diagnose vitamin D deficiencies or to monitor treatment for a known vitamin D deficiency. Less commonly, vitamin D testing may be used to detect vitamin D toxicity, a condition in which there is an excess of vitamin D in the body.
There is a bit of controversy regarding what is considered a low vitamin D level between different expert organizations. A vitamin D level measures levels of 25-hydroxyvitamin D (25(OH)D) also known as calcidiol, in the blood.
Most experts recommend:
- Levels of 20-50 nanograms/milliliter (ng/ml) of 25-hydroxyvitamin D (calcidiol): Sufficient (good)
- Levels of 12-19 ng/ml of 25-hydroxyvitamin D (calcidiol): Borderline
- Levels of less than 12 ng/ml of 25-hydroxyvitamin D (calcidiol): Deficient (low)
However, not everybody agrees, and some organizations suggest different cut-off values.
The Institute of Medicine states:
- Levels above 20 ng/ml of 25-hydroxyvitamin D (calcidiol): Sufficient
- Levels below 20 ng/ml of 25-hydroxyvitamin D (calcidiol): Deficient
Note that several members of the Institute of Medicine committee publicly stated that over screening for vitamin D deficiency was a problem which typically resulted in unnecessary treatment. They were not in agreement with a cut-off level of 20 ng/ml for deficiency and recommended a lower level of 12.5 ng/ml.
The Endocrine Society states:
- Levels above 30 ng/ml of 25-hydroxyvitamin D (calcidiol): Sufficient; however, some assays are inaccurate and levels of 40-60 ng/ml better guarantee sufficiency
- Levels of 21-29 ng/ml of 25-hydroxyvitamin D (calcidiol): Insufficient
- Levels below 20 ng/ml pf 25-hydroxyvitamin D (calcidiol): Deficient
Other medical institution states 46:
- Levels below 20 ng/mL of 25-hydroxyvitamin D (calcidiol): Mild deficiency
- Levels below 10 ng/mL of 25-hydroxyvitamin D (calcidiol): Moderate deficiency
- Levels below 5 ng/mL of 25-hydroxyvitamin D (calcidiol): Severe deficiency
Talk to your doctor about what he/she considers to be a low vitamin D level. Abnormal levels of vitamin D can indicate bone disorders, nutrition problems, organ damage, or other medical conditions.
Although there is no consensus on optimal levels of 25-hydroxyvitamin D as measured in serum, most experts define vitamin D deficiency as a 25-hydroxyvitamin D (calcidiol) level of less than 20 ng per milliliter (50 nmol per liter) 11, 47, 48, 49. 25-Hydroxyvitamin D (calcidiol) levels are inversely associated with parathyroid hormone (PTH) levels until 25-hydroxyvitamin D (calcidiol) reach 30 to 40 ng per milliliter (75 to 100 nmol per liter), at which point parathyroid hormone (PTH) levels begin to level off (at their lowest point) 49, 50, 51. Furthermore, intestinal calcium transport increased by 45 to 65% in women when 25-hydroxyvitamin D levels were increased from an average of 20 to 32 ng per milliliter (50 to 80 nmol per liter) 31. Given such data, a level of 25-hydroxyvitamin D of 21 to 29 ng per milliliter (52 to 72 nmol per liter) can be considered to indicate a relative insufficiency of vitamin D, and a level of 30 ng per milliliter or greater can be considered to indicate sufficient vitamin D 52. Vitamin D intoxication is observed when serum levels of 25-hydroxyvitamin D are greater than 150 ng per milliliter (374 nmol per liter) 5.
Screening for vitamin D status is becoming a more common part of the routine laboratory bloodwork ordered by primary-care physicians, irrespective of any indications for this practice 88. No studies have examined whether such screening for vitamin D deficiency results in improved health outcomes 89. The U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to assess the benefits and harms of screening for vitamin D deficiency in asymptomatic adults 90. It added that no national professional organization recommends population screening for vitamin D deficiency.
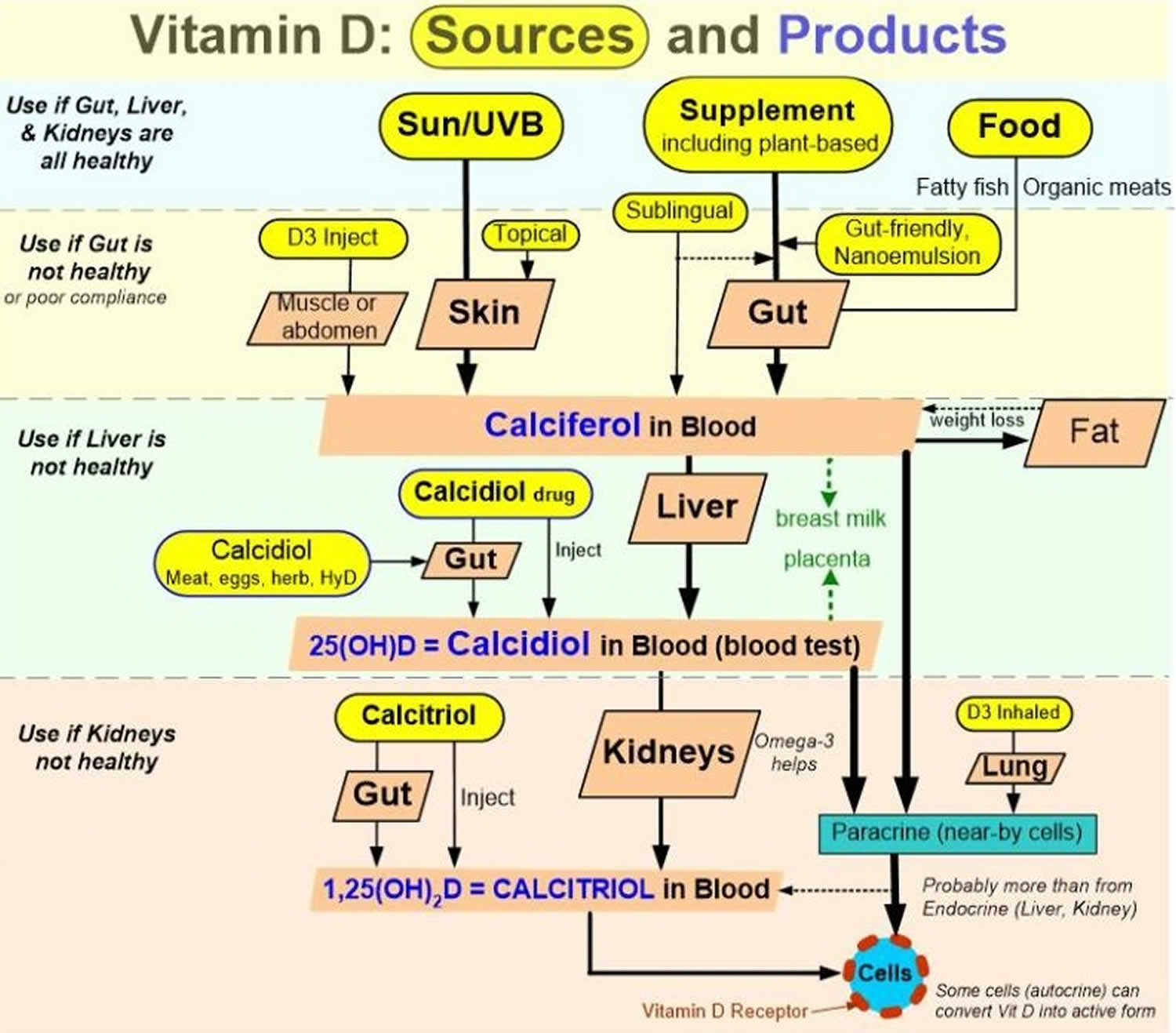
Figure 5. Vitamin D physiology
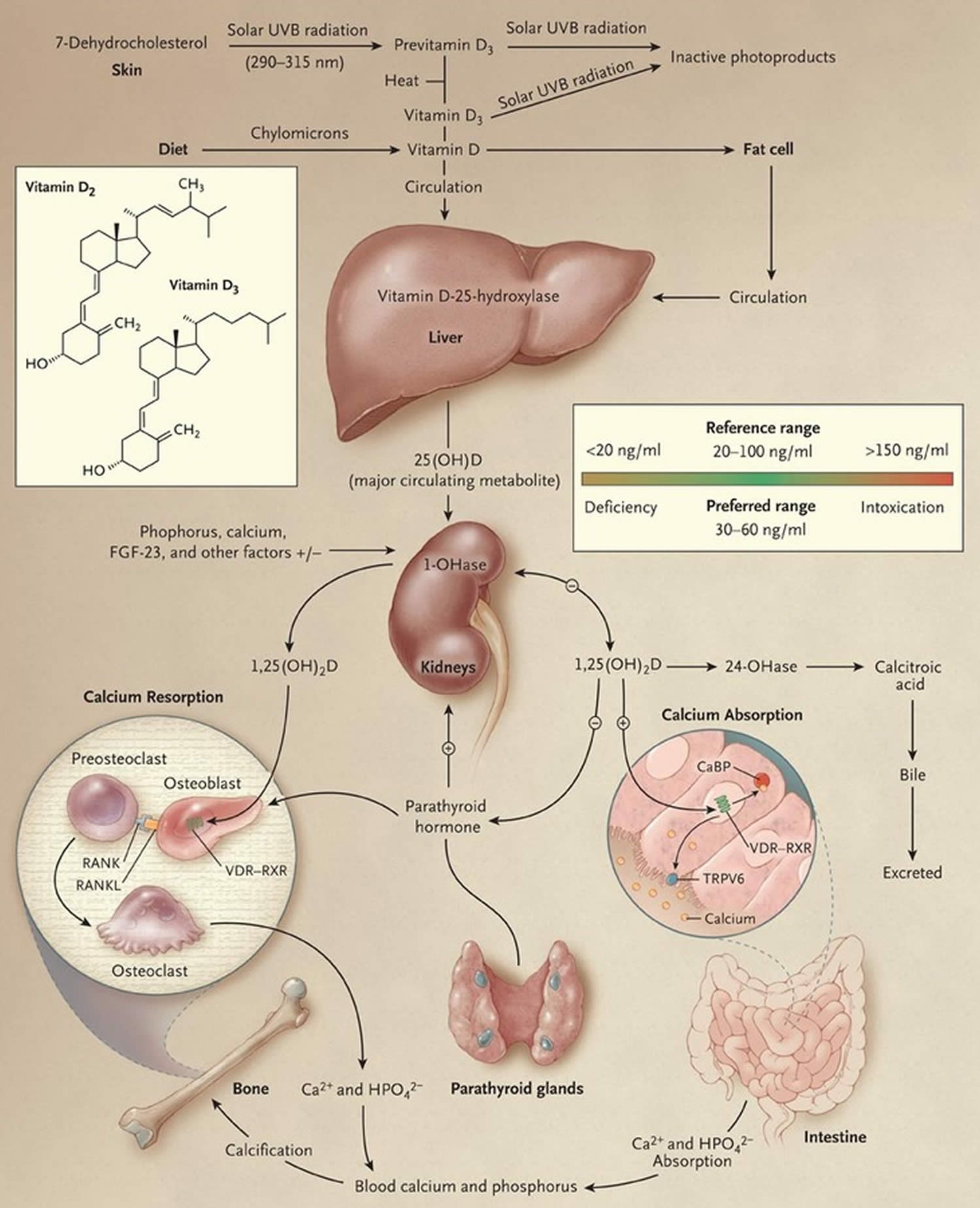
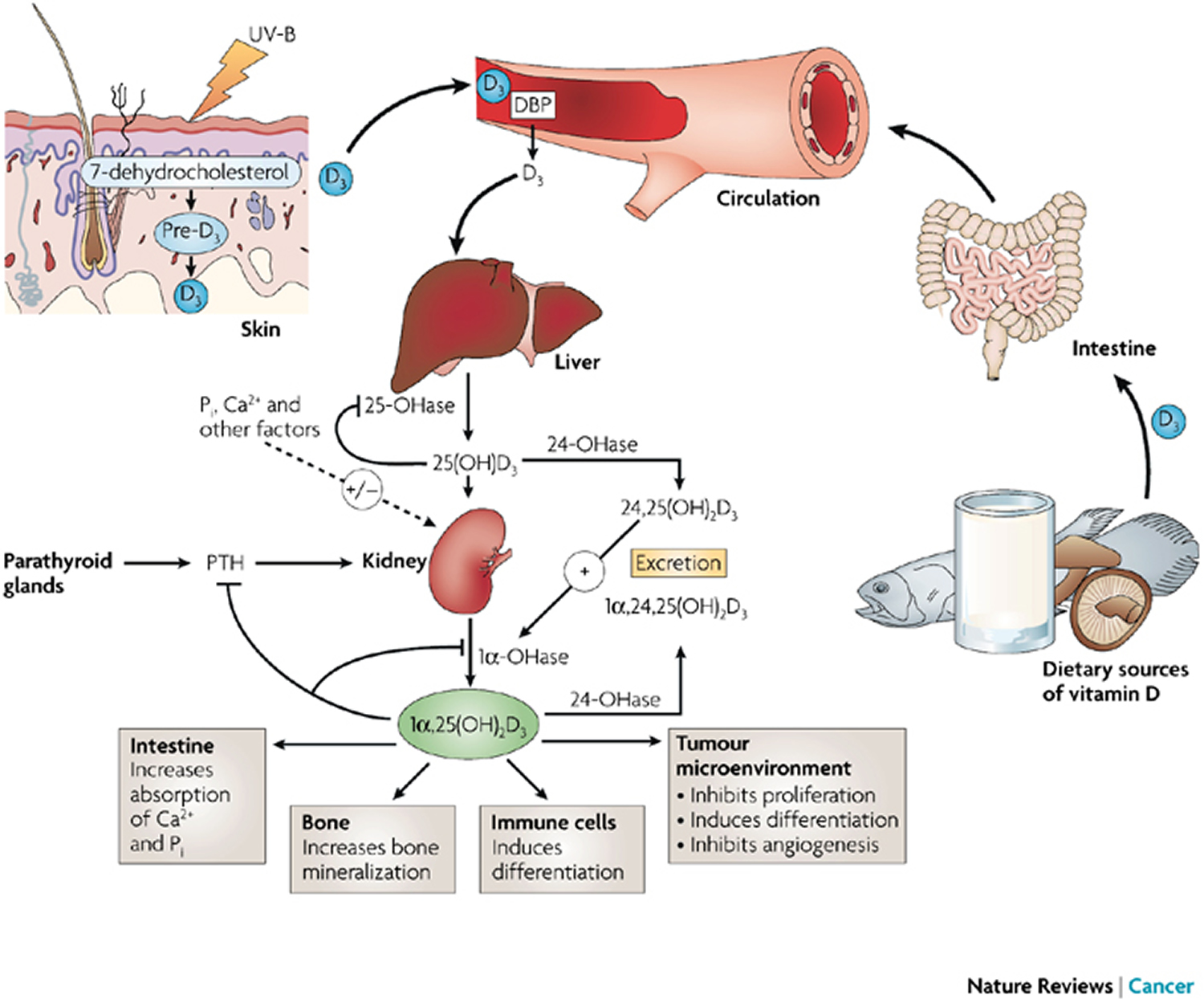
Figure 6. Vitamin D Synthesis and Metabolism in the Regulation of Calcium, Phosphorus, and Bone Metabolism.
Footnotes: During exposure to solar ultraviolet B (UVB) radiation, 7-dehydrocholesterol in the skin is converted to previtamin D3, which is immediately converted to vitamin D3 in a heat-dependent process. Excessive exposure to sunlight degrades previtamin D3 and vitamin D3 into inactive photoproducts. Vitamin D2 and vitamin D3 from dietary sources are incorporated into chylomicrons and transported by the lymphatic system into the venous circulation. Vitamin D (hereafter “D” represents D2 or D3) made in the skin or ingested in the diet can be stored in and then released from fat cells. Vitamin D in the circulation is bound to the vitamin D–binding protein, which transports it to the liver, where vitamin D is converted by vitamin D-25-hydroxylase to 25-hydroxyvitamin D [25(OH)D]. This is the major circulating form of vitamin D that is used by clinicians to determine vitamin D status. (Although most laboratories report the normal range to be 20 to 100 ng per milliliter [50 to 250 nmol per liter], the preferred range is 30 to 60 ng per milliliter [75 to 150 nmol per liter].) This form of vitamin D is biologically inactive and must be converted in the kidneys by 25-hydroxyvitamin D-1α-hydroxylase (1-OHase) to the biologically active form — 1,25-dihydroxyvitamin D [1,25(OH)2D]. Serum phosphorus, calcium, fibroblast growth factor 23 (FGF-23), and other factors can either increase (+) or decrease (–) the renal production of 1,25(OH)2D. 1,25(OH)2D decreases its own synthesis through negative feedback and decreases the synthesis and secretion of parathyroid hormone by the parathyroid glands. 1,25(OH)2D increases the expression of 25-hydroxyvitamin D-24-hydroxylase (24-OHase) to catabolize 1,25(OH)2D to the water-soluble, biologically inactive calcitroic acid, which is excreted in the bile. 1,25(OH)2D enhances intestinal calcium absorption in the small intestine by interacting with the vitamin D receptor–retinoic acid x-receptor complex (VDR-RXR) to enhance the expression of the epithelial calcium channel (transient receptor potential cation channel, subfamily V, member 6 [TRPV6]) and calbindin 9K, a calcium-binding protein (CaBP). 1,25(OH)2D is recognized by its receptor in osteoblasts, causing an increase in the expression of the receptor activator of nuclear factor-κB ligand (RANKL). RANK, the receptor for RANKL on preosteoclasts, binds RANKL, which induces preosteoclasts to become mature osteoclasts. Mature osteoclasts remove calcium and phosphorus from the bone, maintaining calcium and phosphorus levels in the blood. Adequate calcium (Ca2+) and phosphorus (HPO4 2−) levels promote the mineralization of the skeleton.
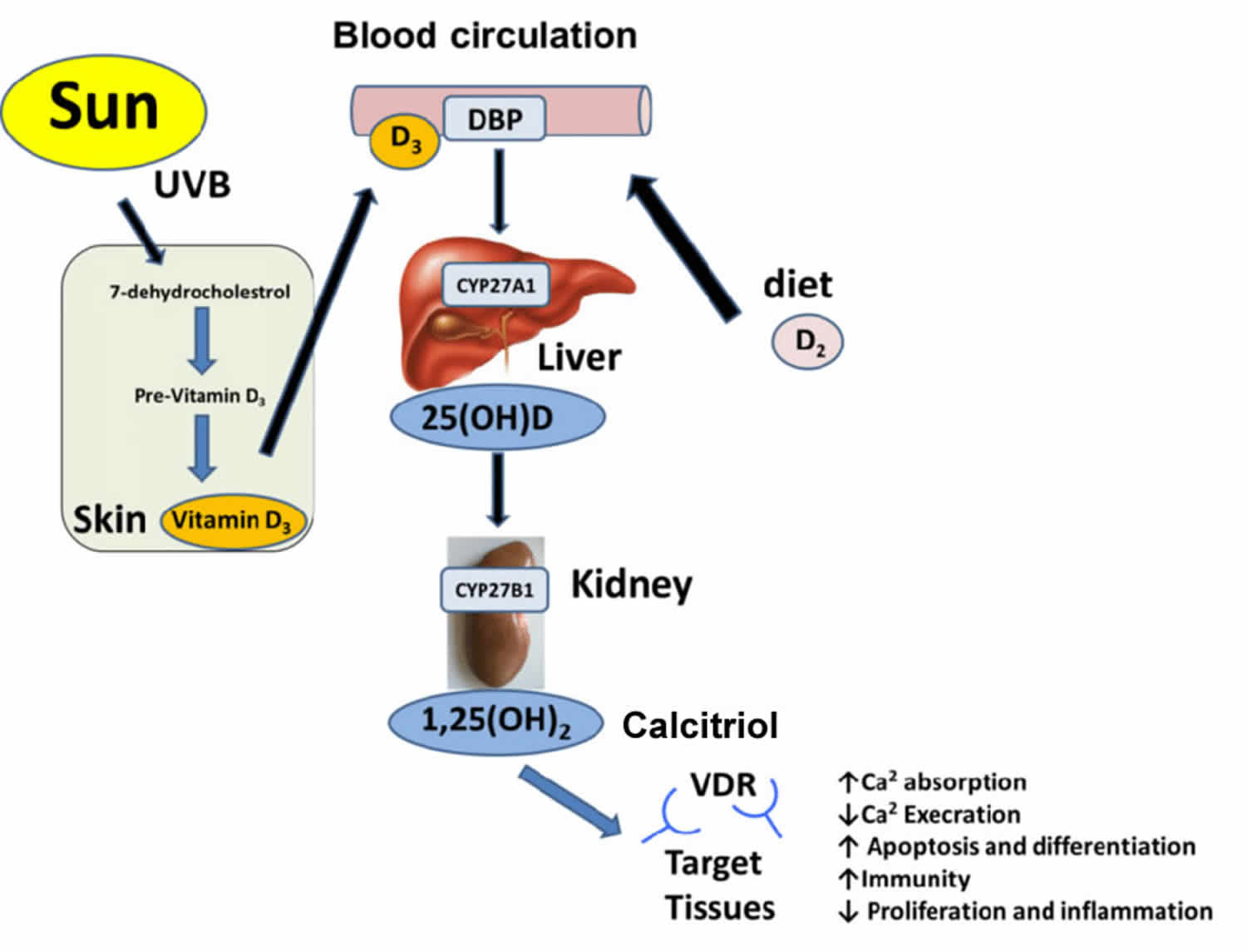
[Source 5 ]Figure 7. Production of vitamin D3 in the skin
Figure 8. Vitamin D metabolism
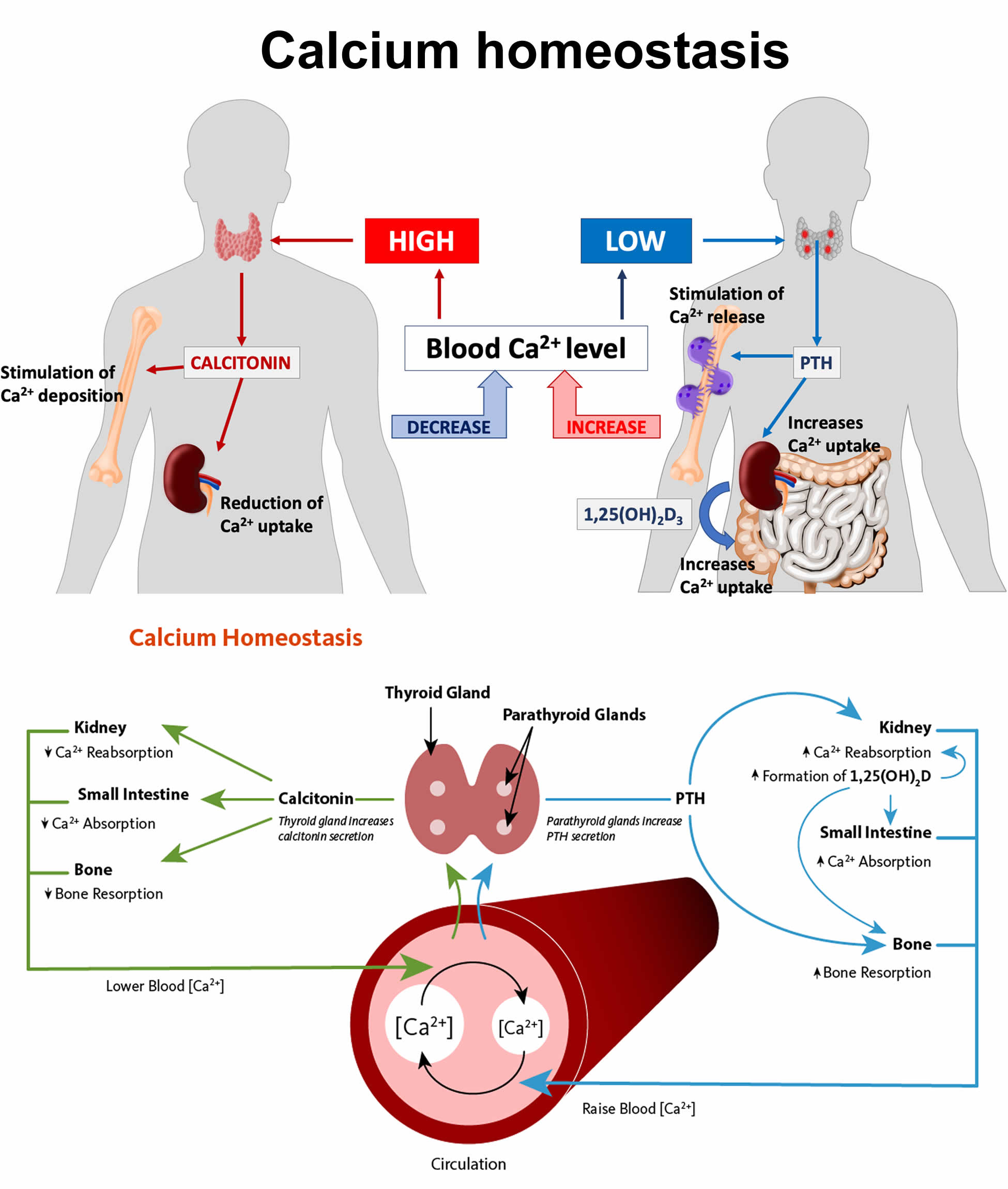
Figure 9. Calcium homeostasis (regulation of serum calcium)
Normal vitamin D level
Serum concentration of 25-hydroxyvitamin D (25(OH)D or calcidiol) is the best indicator of vitamin D status. 25-hydroxyvitamin D (25(OH)D or calcidiol) reflects vitamin D produced cutaneously and that obtained from food and supplements 82 and has a fairly long circulating half-life of 15 days 91. 25-hydroxyvitamin D (25(OH)D or calcidiol) functions as a biomarker of exposure, but it is not clear to what extent 25-hydroxyvitamin D (25(OH)D or calcidiol) levels also serve as a biomarker of effect (i.e., relating to health status or outcomes) 82. Serum 25-hydroxyvitamin D (25(OH)D or calcidiol) levels do not indicate the amount of vitamin D stored in body tissues.
In contrast to 25-hydroxyvitamin D [25(OH)D or calcidiol], circulating Calcitriol [1,25-dihydroxyvitamin D or 1,25(OH)2D] is generally not a good indicator of vitamin D status because it has a short half-life of 15 hours and serum concentrations are closely regulated by parathyroid hormone, calcium, and phosphate 91. Levels of 1,25-dihydroxyvitamin D (1,25(OH)2D or calcitriol) do not typically decrease until vitamin D deficiency is severe 81, 5.
Researchers have not definitively identified serum concentrations of 25-hydroxyvitamin D [25(OH)D] associated with vitamin D deficiency (e.g., rickets), adequacy for bone health, and overall health. After reviewing data on vitamin D needs, an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine concluded that people are at risk of vitamin D deficiency at serum 25-hydroxyvitamin D [25(OH)D] concentrations less than 30 nmol/L (12 ng/mL; see Table 1 below for definitions of “deficiency” and “inadequacy”) 85. Some people are potentially at risk of vitamin D inadequacy at 30 to 50 nmol/L (12–20 ng/mL). Levels of 25-hydroxyvitamin D [25(OH)D] of 50 nmol/L (20 ng/mL) or more are sufficient for most people. In contrast, the Endocrine Society stated that, for clinical practice, a serum 25-hydroxyvitamin D [25(OH)D] concentration of more than 75 nmol/L (30 ng/mL) is necessary to maximize the effect of vitamin D on calcium, bone, and muscle metabolism 75. The Food and Nutrition Board committee also noted that serum concentrations greater than 125 nmol/L (50 ng/mL) can be associated with adverse effects (Table 1).
Optimal serum concentrations of 25-hydroxyvitamin D [25(OH)D] for bone and general health have not been established because they are likely to vary by stage of life, by race and ethnicity, and with each physiological measure used 92. In addition, although 25-hydroxyvitamin D [25(OH)D] levels rise in response to increased vitamin D intake, the relationship is nonlinear 82. The amount of increase varies, for example, by baseline serum levels and duration of supplementation.
An additional complication in assessing vitamin D status is in the actual measurement of 25-hydroxyvitamin D (25(OH)D or calcidiol) concentrations. Considerable variability exists among the various assays available (the two most common methods being antibody based and liquid chromatography based) and among laboratories that conduct the analyses 82, 93, 94. This means that compared with the actual concentration of 25-hydroxyvitamin D (25(OH)D or calcidiol) in a sample of blood serum, a falsely low or falsely high value may be obtained depending on the assay or laboratory used 95. A standard reference material for 25-hydroxyvitamin D (25(OH)D or calcidiol) became available in July 2009 that permits standardization of values across laboratories and may improve method-related variability 82, 96.
Table 1. Serum 25-Hydroxyvitamin D Concentrations and Health
| nmol/L** | ng/mL* | Health status |
|---|---|---|
| <30 | <12 | Associated with vitamin D deficiency, leading to rickets in infants and children and osteomalacia in adults |
| 30 to <50 | 12 to <20 | Generally considered inadequate for bone and overall health in healthy individuals |
| ≥50 | ≥20 | Generally considered adequate for bone and overall health in healthy individuals |
| >125 | >50 | Emerging evidence links potential adverse effects to such high levels, particularly >150 nmol/L (>60 ng/mL) |
Footnotes:
* Serum concentrations of 25(OH)D are reported in both nanomoles per liter (nmol/L) and nanograms per milliliter (ng/mL).
** 1 nmol/L = 0.4 ng/mL and 1 ng/mL = 2.5 nmol/L.
How much vitamin D do I need?
The amount of vitamin D you need each day depends on your age. Average daily recommended amounts are listed below in micrograms (mcg) and International Units (IU). Intake reference values for vitamin D and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of The National Academies (formerly National Academy of Sciences) 82. Dietary Reference Intake (DRI) is the general term for a set of reference values used to plan and assess nutrient intakes of healthy people. These values, which vary by age and gender, include:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy people.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects 82.
The Food and Nutrition Board (FNB) established an RDA for vitamin D representing a daily intake that is sufficient to maintain bone health and normal calcium metabolism in healthy people. RDAs for vitamin D are listed in both International Units (IUs) and micrograms (mcg); the biological activity of 40 IU is equal to 1 mcg (Table 2). Even though sunlight may be a major source of vitamin D for some, the vitamin D RDAs are set on the basis of minimal sun exposure 82.
Table 2. Recommended Dietary Allowances (RDAs) for Vitamin D
| Life Stage | Recommended Amount |
|---|---|
| Birth to 12 months | 400 IU (10 mcg) |
| Children 1–13 years | 600 IU (15 mcg) |
| Teens 14–18 years | 600 IU (15 mcg) |
| Adults 19–70 years | 600 IU (15 mcg) |
| Adults 71 years and older | 800 IU (20 mcg) |
| Pregnant and breastfeeding women | 600 IU (15 mcg) |
Footnote: The amount of vitamin D contained in supplements is sometimes expressed in international units (IU) where 40 IU is equal to one microgram (1 mcg) of vitamin D.
[Source 97 ]What foods provide vitamin D?
Very few foods naturally have vitamin D. The flesh of fatty fish (such as salmon, tuna, and mackerel) and fish liver oils are among the best sources of vitamin D 82, 98. An animal’s diet affects the amount of vitamin D in its tissues. Small amounts of vitamin D are found in beef liver, cheese, and egg yolks. Vitamin D in these foods is primarily in the form of vitamin D3 and its metabolite 25(OH)D3 99. Mushrooms provide variable amounts of vitamin D2 100. Some mushrooms available on the market have been treated with UV light to increase their levels of vitamin D2. In addition, the Food and Drug Administration (FDA) has approved UV-treated mushroom powder as a food additive for use as a source of vitamin D2 in food products 101. Very limited evidence suggests no substantial differences in the bioavailability of vitamin D from various foods 102.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin D arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminD-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminD-Food.pdf). However, FoodData Central does not include the amounts of 25(OH)D in foods. A variety of foods and their vitamin D levels per serving are listed in Table 3.
Animal-based foods typically provide some vitamin D in the form of 25-hydroxyvitamin D (25(OH)D or calcidiol) in addition to vitamin D3 (cholecalciferol). The impact of this form on vitamin D status is an emerging area of research. Studies show that 25-hydroxyvitamin D (25(OH)D or calcidiol) appears to be approximately five times more potent than the parent vitamin D for raising serum 25(OH)D concentrations 100. One study found that when the 25-hydroxyvitamin D (25(OH)D or calcidiol) content of beef, pork, chicken, turkey, and eggs is taken into account, the total amount of vitamin D in the food is 2 to 18 times higher than the amount in the parent vitamin D alone, depending on the food 103.
Fortified foods provide most of the vitamin D in the American diet 82, 104. For example, almost all of the U.S. milk supply is voluntarily fortified with about 3 mcg/cup (120 IU), usually in the form of vitamin D3 105. In the 1930s, a milk fortification program was implemented in the United States to combat rickets, then a major public health problem 82. In Canada, milk must be fortified with 0.88–1.0 mcg/100 mL (35–40 IU), and the required amount for margarine is at least 13.25 mcg/100 g (530 IU). Other dairy products made from milk, such as cheese and ice cream, are not usually fortified in the United States or Canada. Plant milk alternatives (such as beverages made from soy, almond, or oats) are often fortified with similar amounts of vitamin D to those in fortified cow’s milk (about 3 mcg [120 IU]/cup); the Nutrition Facts label lists the actual amount 106. Ready-to-eat breakfast cereals often contain added vitamin D, as do some brands of orange juice, yogurt, margarine, and other food products.
Both the United States and Canada mandate the fortification of infant formula with vitamin D: 1–2.5 mcg/100 kcal (40–100 IU) vitamin D in the United States and 1–2 mcg/100 kcal (40–80 IU) in Canada 82.
Fortified foods provide most of the vitamin D in American diets 82:
- Fatty fish such as salmon, tuna, and mackerel are among the best sources.
- Beef liver, cheese, and egg yolks provide small amounts.
- Mushrooms provide some vitamin D. In some mushrooms that are newly available in stores, the vitamin D content is being boosted by exposing these mushrooms to ultraviolet light.
- Almost all of the U.S. milk supply is fortified with 400 IU of vitamin D per quart. But foods made from milk, like cheese and ice cream, are usually not fortified.
- Vitamin D is added to many breakfast cereals and to some brands of orange juice, yogurt, margarine, and soy beverages; check the labels.
Table 3. Vitamin D content of selected foods
| Food | Micrograms (mcg) per serving | International Units (IU) per serving | Percent DV* |
|---|---|---|---|
| Cod liver oil, 1 tablespoon | 34 | 1360 | 170 |
| Trout (rainbow), farmed, cooked, 3 ounces | 16.2 | 645 | 81 |
| Salmon (sockeye), cooked, 3 ounces | 14.2 | 570 | 71 |
| Mushrooms, white, raw, sliced, exposed to UV light, ½ cup | 9.2 | 366 | 46 |
| Milk, 2% milkfat, vitamin D fortified, 1 cup | 2.9 | 120 | 15 |
| Soy, almond, and oat milks, vitamin D fortified, various brands, 1 cup | 2.5-3.6 | 100-144 | 13-18 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 1 serving | 2 | 80 | 10 |
| Sardines (Atlantic), canned in oil, drained, 2 sardines | 1.2 | 46 | 6 |
| Egg, 1 large, scrambled** | 1.1 | 44 | 6 |
| Liver, beef, braised, 3 ounces | 1 | 42 | 5 |
| Tuna fish (light), canned in water, drained, 3 ounces | 1 | 40 | 5 |
| Cheese, cheddar, 1.5 ounce | 0.4 | 17 | 2 |
| Mushrooms, portabella, raw, diced, ½ cup | 0.1 | 4 | 1 |
| Chicken breast, roasted, 3 ounces | 0.1 | 4 | 1 |
| Beef, ground, 90% lean, broiled, 3 ounces | 0 | 1.7 | 0 |
| Broccoli, raw, chopped, ½ cup | 0 | 0 | 0 |
| Carrots, raw, chopped, ½ cup | 0 | 0 | 0 |
| Almonds, dry roasted, 1 ounce | 0 | 0 | 0 |
| Apple, large | 0 | 0 | 0 |
| Banana, large | 0 | 0 | 0 |
| Rice, brown, long-grain, cooked, 1 cup | 0 | 0 | 0 |
| Whole wheat bread, 1 slice | 0 | 0 | 0 |
| Lentils, boiled, ½ cup | 0 | 0 | 0 |
| Sunflower seeds, roasted, ½ cup | 0 | 0 | 0 |
| Edamame, shelled, cooked, ½ cup | 0 | 0 | 0 |
Footnotes:
* DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin D is 20 mcg (800 IU) for adults and children aged 4 years and older 107. The labels must list vitamin D content in mcg per serving and have the option of also listing the amount in IUs in parentheses. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
** Vitamin D is in the yolk.
[Source 108 ]Can Excessive Vitamin D be harmful?
Yes, excess amounts of vitamin D are toxic. Because vitamin D increases calcium absorption in the gastrointestinal tract, vitamin D toxicity results in marked hypercalcemia (total calcium greater than 11.1 mg/dL, beyond the normal range of 8.4 to 10.2 mg/dL), hypercalciuria, and high serum 25(OH)D levels (typically greater than 375 nmol/l [150 ng/mL]) 109. Hypercalcemia (high blood calcium), in turn, can lead to nausea, poor appetite, vomiting, constipation, muscle weakness, weight loss, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones. And by raising blood levels of calcium, too much vitamin D can cause confusion, disorientation, and problems with heart rhythm. Excess vitamin D can also damage the kidneys.
In extreme cases, vitamin D toxicity causes renal failure, calcification of soft tissues throughout the body (including in coronary vessels and heart valves), cardiac arrhythmias, and even death. Vitamin D toxicity has been caused by consumption of dietary supplements that contained excessive vitamin D amounts because of manufacturing errors, that were taken inappropriately or in excessive amounts, or that were incorrectly prescribed by physicians 110.
Vitamin D toxicity can cause non-specific symptoms such as anorexia, weight loss, polyuria, and heart arrhythmias. More seriously, it can also raise blood levels of calcium which leads to vascular and tissue calcification, with subsequent damage to the heart, blood vessels, and kidneys 85. The use of supplements of both calcium (1,000 mg/day) and vitamin D (10 mcg (400 IU)/day vitamin D) by postmenopausal women was associated with a 17% increase in the risk of kidney stones over 7 years in the Women’s Health Initiative 111. A serum 25(OH)D concentration consistently >500 nmol/L (>200 ng/mL) is considered to be potentially toxic 112. However, other, shorter (from 24 weeks to 5 years) clinical trials of vitamin D supplementation alone or with calcium in adults found greater risks of hypercalcemia and hypercalciuria, but not of kidney stones 113.
Experts do not believe that excessive sun exposure does not result in vitamin D toxicity because the sustained heat on the skin is thought to photodegrade previtamin D3 and vitamin D3 as it is formed 114. In addition, thermal activation of previtamin D3 in the skin gives rise to various non-vitamin D forms that limit formation of vitamin D3 itself. Some vitamin D3 is also converted to nonactive forms 85. Intakes of vitamin D from food that are high enough to cause toxicity are very unlikely. Toxicity is much more likely to occur from high intakes of dietary supplements containing vitamin D. However, frequent use of tanning beds, which provide artificial UV radiation, can lead to 25(OH)D levels well above 375–500 nmol/L (150–200 ng/mL) 115.
Long-term intakes above the upper limit (UL) increase the risk of adverse health effects 85 (Table 6). Most reports suggest a toxicity threshold for vitamin D of 10,000 to 40,000 IU/day and serum 25(OH)D levels of 500–600 nmol/L (200–240 ng/mL). While symptoms of toxicity are unlikely at daily intakes below 10,000 IU/day, the Food and Nutrition Board (FNB) pointed to emerging science from national survey data, observational studies, and clinical trials suggesting that even lower vitamin D intakes and serum 25(OH)D levels might have adverse health effects over time. The Food and Nutrition Board (FNB) concluded that serum 25(OH)D levels above approximately 125–150 nmol/L (50–60 ng/mL) should be avoided, as even lower serum levels (approximately 75–120 nmol/L or 30–48 ng/mL) are associated with increases in all-cause mortality, greater risk of cancer at some sites like the pancreas, greater risk of cardiovascular events, and more falls and fractures among the elderly. The FNB committee cited research which found that vitamin D intakes of 5,000 IU/day achieved serum 25(OH)D concentrations between 100–150 nmol/L (40–60 ng/mL), but no greater. Applying an uncertainty factor of 20% to this intake value gave a UL of 4,000 IU which the FNB applied to children aged 9 and older and adults, with corresponding lower amounts for younger children.
The upper limit for vitamin D is 1,000 to 1,500 IU/day for infants, 2,500 to 3,000 IU/day for children 1-8 years, and 4,000 IU/day for children 9 years and older, adults, and pregnant and lactating teens and women. Vitamin D toxicity almost always occurs from overuse of supplements. Excessive sun exposure doesn’t cause vitamin D poisoning because the body limits the amount of this vitamin it produces.
Table 4. Tolerable Upper Intake Levels (ULs) for Vitamin D
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| 0–6 months | 1,000 IU (25 mcg) | 1,000 IU (25 mcg) | ||
| 7–12 months | 1,500 IU (38 mcg) | 1,500 IU (38 mcg) | ||
| 1–3 years | 2,500 IU (63 mcg) | 2,500 IU (63 mcg) | ||
| 4–8 years | 3,000 IU (75 mcg) | 3,000 IU (75 mcg) | ||
| 9–18 years | 4,000 IU (100 mcg) | 4,000 IU (100 mcg) | 4,000 IU (100 mcg) | 4,000 IU (100 mcg) |
| 19+ years | 4,000 IU (100 mcg) | 4,000 IU (100 mcg) | 4,000 IU (100 mcg) | 4,000 IU (100 mcg) |
Vitamin D side effects and warnings
Vitamin D is a fat-soluble vitamin, hence, toxicity is possible, although rarely noted. Hypervitaminosis D results from excess oral intake and not due to excessive sunlight exposure. Toxicity has been reported at a serum 25-hydroxyvitamin D level of more than 88 ng/mL. Acute intoxication can lead to acute hypercalcemia that can cause confusion, anorexia, vomiting, polyuria, polydipsia, and muscle weakness. Chronic intoxication can lead to nephrocalcinosis and bone pain.
Vitamin D is likely safe when taken by mouth in doses of 100 micrograms of vitamin D3 daily (4,000 IU) and when applied to the skin alone or in combination with corticosteroids for up to three months 116.
Vitamin D is possibly safe when taken by mouth or injected into the muscle in doses of 300,000 IU three times a year for vitamin D deficiency.
Vitamin D may cause allergic skin reactions (inflammation, irritation, rash, and thinning), build-up of calcium in the arteries, changes in cholesterol levels, daytime sleepiness, excessive vitamin D levels, hardening of the arteries, headaches, increased calcium excretion or levels, increased risk of falls and fractures, increased risk of heart attack and stroke, increased risk of high blood pressure during pregnancy, increased risk of urinary tract infection, kidney or urinary stones, muscle pain, respiratory tract infection, and stomach problems (constipation, cramps, diarrhea, upset stomach, and vomiting).
Children age 9 years and older, adults, and pregnant and breastfeeding women who take more than 4,000 IU a day of vitamin D might experience:
- Nausea and vomiting
- Poor appetite and weight loss
- Constipation
- Weakness
- Confusion and disorientation
- Heart rhythm problems
- Kidney stones and kidney damage
Vitamin D may affect blood sugar levels. Caution is advised in people with diabetes or low blood sugar, and in those taking drugs, herbs, or supplements that affect blood sugar. Blood sugar levels may need to be monitored by a qualified healthcare professional, including a pharmacist, and medication adjustments may be necessary.
Vitamin D may affect blood pressure. Caution is advised in people with blood pressure disorders or those taking drugs or herbs and supplements that affect blood pressure.
Use cautiously in people with headaches, heart disease, immune disorders (including lymph cancer and tuberculosis), kidney disease, liver disease, lung disorders, musculoskeletal disorders, skin disorders, stomach disorders, and thyroid disorders.
Use cautiously in pregnant women at risk of high blood pressure associated with pregnancy.
Use cautiously in breastfeeding women.
Avoid in people with known allergy or sensitivity to vitamin D, any similar compounds, or any part of the formula.
Avoid in people with abnormal calcium excretion or levels.
Pregnancy and Breastfeeding
Use cautiously in pregnant women at risk of high blood pressure associated with pregnancy. The recommended adequate intake for pregnant women is the same as for non-pregnant adults. Most prenatal vitamins provide 400 IU of vitamin D daily as cholecalciferol, while high-risk populations may benefit from higher amounts (2,000-4,000 IU daily).
Use cautiously in breastfeeding women. The daily recommended intake for vitamin D during breastfeeding is 400 IU (10 micrograms) daily. Vitamin D2 in doses of 2,000 IU daily or 60,000 IU monthly for three months has been found to be safe and effective. Exclusively breastfed babies may be supplemented with 400-2,000 IU daily.
What is a healthy vitamin D level?
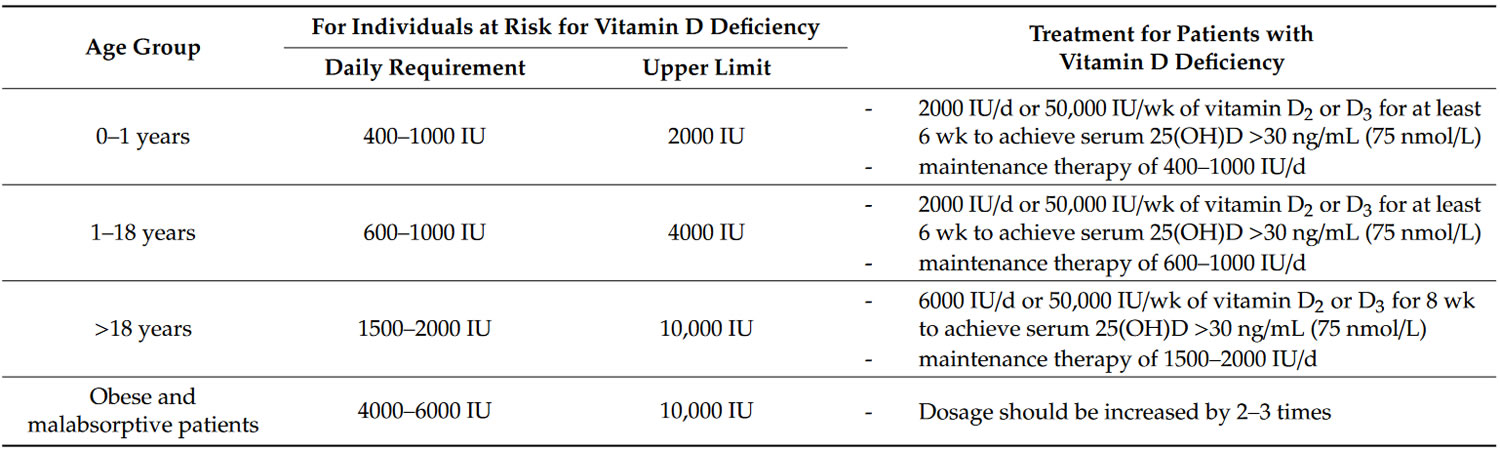
It is still controversial how much vitamin D is needed, how it should be given, i.e., daily versus weekly or monthly (bolus doses), and what level of serum 25-hydroxyvitamin D [25(OH)D or calcidiol] is optimal for immune health and overall health benefits 117. It is also unknown whether maintenance of serum vitamin D itself has its own effect on modulating immune function. However, historical evidence suggests that our hunter gatherer forefathers maintained their circulating vitamin D levels in the range of 10–50 ng/mL (25–125 nmol/L). Indigenous populations such as Maasai herders and Hadza tribesmen were found to have serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels in the range of 40–60 ng/mL (100–150 nmol/L) 118. These levels are consistent with those reported in populational studies to be associated with the lowest risk of several types of cancers, cardiovascular diseases, autoimmune diseases, and all-cause mortality 119. To maintain these blood levels with minimal sunlight exposure, a person would require ingestion of 4000–6000 IUs of vitamin D daily, which would maintain serum vitamin D levels in the range of 20–40 ng/mL (50–100 nmol/L) and serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels in the range of 40–60 ng/mL (50–100 nmol/L) 75. The recommended dosage for vitamin D intake by the Endocrine Society Guidelines on Vitamin D for treatment and prevention for vitamin D deficiency is shown in Table 5.
Table 5. The recommended dosage for vitamin D intake in individuals who are at risk for vitamin D deficiency and dosage of vitamin therapy treatment for patients with vitamin D deficiency.
[Source 75 ]Can you get vitamin D from the sun?
Most people meet at least some of their vitamin D needs through exposure to sunlight 85, 81. Ultraviolet (UV) B radiation with a wavelength of 290–320 nanometers penetrates uncovered skin and converts cutaneous 7-dehydrocholesterol to previtamin D3, which in turn becomes vitamin D3 85. Season, time of day, length of day, cloud cover, smog, skin melanin content, and sunscreen are among the factors that affect UV radiation exposure and vitamin D synthesis 85. Perhaps surprisingly, geographic latitude does not consistently predict average serum 25(OH)D levels in a population. Ample opportunities exist to form vitamin D (and store it in the liver and fat) from exposure to sunlight during the spring, summer, and fall months even in the far north latitudes 85. Older people and people with dark skin are less able to produce vitamin D from sunlight 85.
Complete cloud cover reduces UV energy by 50%; shade (including that produced by severe pollution) reduces it by 60% 32. UVB radiation does not penetrate glass, so exposure to sunshine indoors through a window does not produce vitamin D 120. Sunscreens with a sun protection factor (SPF) of 8 or more appear to block vitamin D-producing UV rays, although in practice people generally do not apply sufficient amounts, cover all sun-exposed skin, or reapply sunscreen regularly 85, 121. Therefore, skin likely synthesizes some vitamin D even when it is protected by sunscreen as typically applied.
The factors that affect UV radiation exposure and research to date on the amount of sun exposure needed to maintain adequate vitamin D levels make it difficult to provide general guidelines. It has been suggested by some vitamin D researchers, for example, that approximately 5–30 minutes of sun exposure between 10 AM and 3 PM at least twice a week to the face, arms, legs, or back without sunscreen usually lead to sufficient vitamin D synthesis and that the moderate use of commercial tanning beds that emit 2%–6% UVB radiation is also effective 5, 122. Individuals with limited sun exposure need to include good sources of vitamin D in their diet or take a supplement to achieve recommended levels of intake.
Despite the importance of the sun for vitamin D synthesis, it is prudent to limit exposure of skin to sunlight 121 and UV radiation from tanning beds 123. UV radiation is a carcinogen responsible for most of the estimated 1.5 million skin cancers and the 8,000 deaths due to metastatic melanoma that occur annually in the United States 121. Lifetime cumulative UV damage to skin is also largely responsible for some age-associated dryness and other cosmetic changes. The American Academy of Dermatology advises that photoprotective measures be taken, including the use of sunscreen, whenever one is exposed to the sun 35. Assessment of vitamin D requirements cannot address the level of sun exposure because of these public health concerns about skin cancer, and there are no studies to determine whether UVB-induced synthesis of vitamin D can occur without increased risk of skin cancer 85.
People who avoid the sun or who cover their bodies with sunscreen or clothing should include good sources of vitamin D in their diets or take a supplement. Recommended intakes of vitamin D are set on the assumption of little sun exposure.
How long should you spend in the sun?
Most people can make enough vitamin D from being out in the sun daily for short periods with their forearms, hands or lower legs uncovered and without sunscreen from late March or early April to the end of September, especially from 11am to 3pm.
It’s not known exactly how much time is needed in the sun to make enough vitamin D to meet your body’s requirements. This is because there are a number of factors that can affect how vitamin D is made, such as your skin color or how much skin you have exposed. But you should be careful not to burn in the sun, so take care to cover up, or protect your skin with sunscreen, before your skin starts to turn red or burn.
Your risk of sunburn depends on 2 things. How sun-sensitive your skin is, and how strong the UV rays are you’re exposed to. Different people will have a different risk of sunburn on the same day, so it’s a good idea to know when your risk is high, so you can protect your skin.
In general people who have one or more of the following are at more risk:
- skin that burns easily
- light or fair colored skin, hair, or eyes
- lots of moles or freckles
- a history of sunburn
- a personal or family history of skin cancer
People with dark skin, such as those of African, African-Caribbean or south Asian origin, will need to spend longer in the sun to produce the same amount of vitamin D as someone with lighter skin.
- Children aged under six months should be kept out of direct strong sunlight. To ensure they get enough vitamin D, babies and children aged under five years should be given vitamin D supplements even if they do get out in the sun.
How long it takes for your skin to go red or burn varies from person to person. You’re the best person to know how your skin reacts in the sun. The more easily you get sunburnt, the more careful you need to be. Remember, you don’t need to peel – if your skin’s gone red or pink in the sun, that’s sunburn, and it’s dangerous. For people with darker skin it may feel irritated, tender or itchy. The longer you stay in the sun, especially for prolonged periods without sun protection, the greater your risk of skin cancer. Using sunbeds is not a recommended way of making vitamin D.
Other things that affect the strength of UV rays are the:
- Time of year – the highest risk months in the US are April to September. Near the equator, there are strong UV rays all year round.
- Altitude – UV rays are stronger the higher you go. So skiers and mountaineers can easily get caught out.
- Cloud cover – over 90% of UV can pass through light cloud.
- Reflection – up to 80% of UV rays are reflected back from snow, 15% from sand, 10% from concrete and up to 30% from water (depending on how choppy it is).
What is pseudovitamin D deficiency and vitamin D dependency?
The term pseudovitamin D deficiency refers to a state with biochemical and tissue features of vitamin D deficiency (calcium deficiency, secondary hyperparathyroidism, impaired bone matrix mineralization) with no history of vitamin D or calcium deficiency or low serum levels of 25-hydroxyvitamin D [25(OH)D] 124. This is an ambiguous term as it includes two different diseases: 1,25-dihydroxyvitamin D [1,25(OH)2D] deficiency and resistance to 1,25-dihydroxyvitamin D [1,25(OH)2D], the so-called pseudovitamin D deficiency type 1 and 2 respectively, and does not include the known etiology and pathogenesis of these disturbances 124.
The term vitamin D dependency has been used interchangeably with pseudovitamin D deficiency 124. Vitamin D dependency meant to describe patients capable or responding to, and thus dependent on, supraphysiological doses of vitamin D. This is the situation in patients with simple hereditary 1,25-dihydroxyvitamin D [1,25(OH)2D] deficiency due to defects in the kidney enzyme 25(OH)vitamin D 1-alpha-hydroxylase. Patients with this disease have a complete clinical remission on physiological replacement doses of calcitriol (1,25-dihydroxyvitamin D [1,25(OH)2D]). The term vitamin D dependency type 2 was applied to describe patients with simple hereditary resistance to 1,25(OH)2D, the majority of whom are unresponsive to any dose of vitamin D or its active metabolites, and therefore are not dependent on vitamin D.
When should I get a vitamin D test?
Vitamin D testing is ordered to determine if a deficiency, insufficiency, or toxic level of vitamin D is present or to monitor treatment for a previously diagnosed deficiency.
Your health care provider may order a vitamin D blood test for you if you are experiencing symptoms of a vitamin D deficiency, such as:
- Weakening of the bones
- Abnormal bone development
- Bone deformity
- Bone pain
- Muscle weakness or cramps
- Seizures
- Dental abnormalities
Sometimes vitamin D tests are used as screening tests for individuals at increased risk of a vitamin D deficiency. Screening tests are conducted before any symptoms occur. The following are factors that may increase your risk of developing a vitamin D insufficiency or deficiency:
- Age over 65
- Obesity
- History of weight loss surgery
- Osteoporosis
- Reduced ability to make vitamin D in the skin due to limited sun exposure, sunscreen use, or dark skin pigmentation
- Digestive diseases that make it difficult to absorb nutrients from food, including celiac disease and Crohn’s disease
- Kidney and liver disease
- Use of certain medications
Vitamin D tests may also be ordered if your health care provider suspects that you may have abnormally high vitamin D levels, known as vitamin D toxicity. This occurs as a result of taking too much vitamin D in supplements rather than from too much sun exposure or dietary intake.
Excess vitamin D in supplement form may cause your body to absorb more calcium from food and to reabsorb calcium from the bones into the blood. This results in excess calcium in the blood, also known as hypercalcemia, which can lead to symptoms like fatigue, confusion, bone pain, nausea and vomiting, frequent urination, and kidney problems.
A health care provider who is familiar with your medical history is in the best position to determine whether you might benefit from vitamin D testing.
How to interpret vitamin D test results
Your vitamin D test report will include information about your level of 25-hydroxyvitamin D (25(OH)D or calcidiol) and the reference range used to interpret your result. Reference ranges are the test result values that are considered optimal for health. Results that fall outside the reference range may indicate a health issue. Reference ranges can vary by laboratory. Additionally, some labs may break down your levels of vitamin D2 and D3, while other laboratories report the combined total.
Medical experts and organizations have differing opinions on the ideal levels of vitamin D. Generally, test results can be used to distinguish between an insufficiency, in which vitamin D amounts are only slightly outside of the ideal range, and a deficiency, which can cause more serious problems.
The table below summarizes the test results, interpretations, and potential medical outcomes that experts consider when looking at a patient’s 25-hydroxyvitamin D level. Your health care provider can determine what your vitamin D test result means for your health.
Table 6. Vitamin D (25-hydroxyvitamin D) test results
| Vitamin D (25-hydroxyvitamin D) Test Results | ||
|---|---|---|
| Test Result | Interpretation | Potential Medical Outcome |
| Deficient | Vitamin D levels too low to support healthy bodily functions | There is not enough calcium in the blood. Weakening of bones or painful bone problems may occur. |
| Insufficient | Vitamin D levels lower than the ideal reference range | Most people are asymptomatic. There may be accelerated bone loss or increased risk of fracture. |
| Sufficient | Vitamin D levels fall within reference range | There is an adequate amount of vitamin D and no known risks for health problems. |
| Risk of toxicity | Excessive amount of vitamin D in the body | This can cause too much calcium to circulate in the blood, which can lead to confusion, loss of appetite, vomiting, and muscle weakness. |
Are vitamin D test results accurate?
Testing 25-hydroxyvitamin D (25(OH)D or calcidiol) is considered the most accurate way to measure whether your intake of vitamin D, both through sun exposure and diet, is adequate. However, a number of factors are taken into account when interpreting your vitamin D test results, including differences in tests and laboratory procedures, your weight and skin pigmentation, and medical conditions like kidney failure which can impact the body’s ability to use vitamin D. You can talk with your health care provider to learn more about the accuracy and significance of vitamin D testing in your specific case.
Do I need follow-up vitamin D tests?
Not all people who have abnormal levels of vitamin D require follow-up testing. If your vitamin D test shows an abnormal result, your health care provider will determine whether you need additional testing based on several factors. These include your symptoms, the results of a physical examination, and how far outside the reference range your vitamin D level falls.
When vitamin D levels are very low, more tests may be ordered. These may include repeat vitamin D testing as well as tests of other substances, such as:
- Calcium
- Phosphorus
- Alkaline phosphatase (ALP)
- Parathyroid hormone (PTH)
- Electrolytes
- Blood urea nitrogen (BUN)
- Creatinine
- Tissue transglutaminase antibodies
If your vitamin D level is abnormally high, you may require additional tests, including:
- Calcium
- Parathyroid hormone (PTH)
- Basic metabolic panel (BMP)
X-rays or other imaging tests may be ordered for some patients with vitamin D levels outside the reference range. Imaging tests are helpful in assessing how abnormal vitamin D levels have impacted the bones.
If you are given treatment to bring your vitamin D levels into the normal range, repeat vitamin D testing and calcium levels may be used to monitor treatment response.
What kinds of vitamin D supplements are available?
Vitamin D is found in supplements (and fortified foods) in two different forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Both increase vitamin D in the blood.
In supplements and fortified foods, vitamin D is available in two forms, D2 (ergocalciferol) and D3 (cholecalciferol) that differ chemically only in their side-chain structure. Vitamin D2 is manufactured by the UV irradiation of ergosterol in yeast, and vitamin D3 is manufactured by the irradiation of 7-dehydrocholesterol from lanolin and the chemical conversion of cholesterol 5. The two forms of vitamin D (vitamins D2 or vitamin D3) have traditionally been regarded as equivalent based on their ability to cure rickets 79. In addition, most steps in the metabolism and actions of vitamins D2 and D3 are identical 79. Both forms (as well as vitamin D in foods and from cutaneous synthesis) effectively raise serum Calcidiol [25-hydroxyvitamin D or 25(OH)D] levels 125. However, most evidence indicates that vitamin D3 (cholecalciferol) increases serum hydroxyvitamin D or 25(OH)D] levels to a greater extent and maintains these higher levels longer than vitamin D2 (ergocalciferol), even though both forms are well absorbed in the gut 80.
Some studies have used dietary supplements containing the 25-hydroxyvitamin D3 [25(OH)D3 or 25-hydroxycholecalciferol] form of vitamin D. Per equivalent microgram dose, 25-hydroxyvitamin D3 [25(OH)D3 or 25-hydroxycholecalciferol] is three to five times as potent as vitamin D3 126. However, no 25-hydroxyvitamin D3 [25(OH)D3 or 25-hydroxycholecalciferol] dietary supplements appear to be available to consumers on the U.S. market at this time 97.
- Vitamin D3 (cholecalciferol) is available in 400, 800, 1000, 2000, 5000, 10,000, and 60,000 IU capsules. It is available in some countries as an intramuscular injection (Arachital 600,000 IU, which maintains vitamin D levels for 1 year). However, it can be extremely painful 127.
- Vitamin D2 (ergocalciferol) is available for oral use in 400 and 50,000 unit capsules or in a liquid form (8000 IU/mL) 127.
The American Academy of Pediatrics (AAP) recommends that exclusively and partially breastfed infants receive supplements of 400 IU/day of vitamin D shortly after birth and continue to receive these supplements until they are weaned and consume ≥1,000 mL/day of vitamin D-fortified formula or whole milk 128. Similarly, all non-breastfed infants ingesting <1,000 mL/day of vitamin D-fortified formula or milk should receive a vitamin D supplement of 400 IU/day 128. The American Academy of Pediatrics also recommends that older children and adolescents who do not obtain 400 IU/day through vitamin D-fortified milk and foods should take a 400 IU vitamin D supplement daily. However, this latter recommendation (issued November 2008) needs to be reevaluated in light of the Food and Nutrition Board’s vitamin D RDA of 600 IU/day for children and adolescents (issued November 2010 and which previously was an AI of 200 IU/day).
Vitamin D from the sun
Most people meet at least some of their vitamin D needs through exposure to sunlight 85, 81. Ultraviolet (UV) B radiation with a wavelength of 290–320 nanometers penetrates uncovered skin and converts cutaneous 7-dehydrocholesterol to previtamin D3, which in turn becomes vitamin D3 85. Season, time of day, length of day, cloud cover, smog, skin melanin content, and sunscreen are among the factors that affect UV radiation exposure and vitamin D synthesis 85. Perhaps surprisingly, geographic latitude does not consistently predict average serum 25(OH)D levels in a population. Ample opportunities exist to form vitamin D (and store it in the liver and fat) from exposure to sunlight during the spring, summer, and fall months even in the far north latitudes 85. Older people and people with dark skin are less able to produce vitamin D from sunlight 85.
Complete cloud cover reduces UV energy by 50%; shade (including that produced by severe pollution) reduces it by 60% 32. UVB radiation does not penetrate glass, so exposure to sunshine indoors through a window does not produce vitamin D 120. Sunscreens with a sun protection factor (SPF) of 8 or more appear to block vitamin D-producing UV rays, although in practice people generally do not apply sufficient amounts, cover all sun-exposed skin, or reapply sunscreen regularly 85, 121. Therefore, skin likely synthesizes some vitamin D even when it is protected by sunscreen as typically applied.
The factors that affect UV radiation exposure and research to date on the amount of sun exposure needed to maintain adequate vitamin D levels make it difficult to provide general guidelines. It has been suggested by some vitamin D researchers, for example, that approximately 5–30 minutes of sun exposure between 10 AM and 3 PM at least twice a week to the face, arms, legs, or back without sunscreen usually lead to sufficient vitamin D synthesis and that the moderate use of commercial tanning beds that emit 2%–6% UVB radiation is also effective 5, 122. Individuals with limited sun exposure need to include good sources of vitamin D in their diet or take a supplement to achieve recommended levels of intake.
Despite the importance of the sun for vitamin D synthesis, it is prudent to limit exposure of skin to sunlight 121 and UV radiation from tanning beds 123. UV radiation is a carcinogen responsible for most of the estimated 1.5 million skin cancers and the 8,000 deaths due to metastatic melanoma that occur annually in the United States 121. Lifetime cumulative UV damage to skin is also largely responsible for some age-associated dryness and other cosmetic changes. The American Academy of Dermatology advises that photoprotective measures be taken, including the use of sunscreen, whenever one is exposed to the sun 35. Assessment of vitamin D requirements cannot address the level of sun exposure because of these public health concerns about skin cancer, and there are no studies to determine whether UVB-induced synthesis of vitamin D can occur without increased risk of skin cancer 85.
People who avoid the sun or who cover their bodies with sunscreen or clothing should include good sources of vitamin D in their diets or take a supplement. Recommended intakes of vitamin D are set on the assumption of little sun exposure.
Never use a solarium to boost vitamin D levels because they emit dangerous levels of ultraviolet (UV) radiation that increase your risk of skin cancer.
How long should you spend in the sun?
Most people can make enough vitamin D from being out in the sun daily for short periods with their forearms, hands or lower legs uncovered and without sunscreen from late March or early April to the end of September, especially from 11am to 3pm.
It’s not known exactly how much time is needed in the sun to make enough vitamin D to meet your body’s requirements. This is because there are a number of factors that can affect how vitamin D is made, such as your skin color or how much skin you have exposed. But you should be careful not to burn in the sun, so take care to cover up, or protect your skin with sunscreen, before your skin starts to turn red or burn.
Your risk of sunburn depends on 2 things. How sun-sensitive your skin is, and how strong the UV rays are you’re exposed to. Different people will have a different risk of sunburn on the same day, so it’s a good idea to know when your risk is high, so you can protect your skin.
In general people who have one or more of the following are at more risk:
- skin that burns easily
- light or fair colored skin, hair, or eyes
- lots of moles or freckles
- a history of sunburn
- a personal or family history of skin cancer
People with dark skin, such as those of African, African-Caribbean or south Asian origin, will need to spend longer in the sun to produce the same amount of vitamin D as someone with lighter skin.
- Children aged under six months should be kept out of direct strong sunlight. To ensure they get enough vitamin D, babies and children aged under five years should be given vitamin D supplements even if they do get out in the sun.
How long it takes for your skin to go red or burn varies from person to person. You’re the best person to know how your skin reacts in the sun. The more easily you get sunburnt, the more careful you need to be. Remember, you don’t need to peel – if your skin’s gone red or pink in the sun, that’s sunburn, and it’s dangerous. For people with darker skin it may feel irritated, tender or itchy. The longer you stay in the sun, especially for prolonged periods without sun protection, the greater your risk of skin cancer. Using sunbeds is not a recommended way of making vitamin D.
Other things that affect the strength of UV rays are the:
- Time of year – the highest risk months in the US are April to September. Near the equator, there are strong UV rays all year round.
- Altitude – UV rays are stronger the higher you go. So skiers and mountaineers can easily get caught out.
- Cloud cover – over 90% of UV can pass through light cloud.
- Reflection – up to 80% of UV rays are reflected back from snow, 15% from sand, 10% from concrete and up to 30% from water (depending on how choppy it is).
Effects of vitamin D on health
Vitamin D is being studied for its possible connections to several diseases and medical problems, including type 2 diabetes, depression, high blood pressure, cancer, and autoimmune conditions such as multiple sclerosis.
Vitamin D on bone health and osteoporosis
Bone is constantly being remodeled. However, as people age—and particularly in women during menopause—bone breakdown rates overtake rates of bone building. Over time, bone density can decline and osteoporosis can eventually develop 129. More than 53 million adults in the United States have or are at risk of developing osteoporosis, which is characterized by low bone mass and structural deterioration of bone tissue that increases bone fragility and the risk of bone fractures 130. About 2.3 million osteoporotic fractures occurred in the United States in 2015 131. Osteoporosis is, in part, a long-term effect of calcium and/or vitamin D insufficiency, in contrast to rickets and osteomalacia, which result from vitamin D deficiency. Osteoporosis is most often associated with inadequate calcium intakes, but insufficient vitamin D intakes contribute to osteoporosis by reducing calcium absorption 85. All adults should consume recommended amounts of vitamin D and calcium from foods and supplements if needed. Older women and men should consult their healthcare providers about their needs for both nutrients as part of an overall plan to maintain bone health and to prevent or treat osteoporosis.
Bone health also depends on support from the surrounding muscles to assist with balance and postural sway and thereby reduce the risk of falling. Vitamin D is also needed for the normal development and growth of muscle fibers. In addition, inadequate vitamin D levels can adversely affect muscle strength and lead to muscle weakness and pain (myopathy) 85.
Bone mineral density, bone mass, and fracture risk are correlated with serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels in White Americans and Mexican Americans, but not in Black Americans 132. Factors such as adiposity, skin pigmentation, vitamin D binding protein polymorphisms, and genetics contribute to differences in 25-hydroxyvitamin D [25(OH)D or calcidiol] levels between Black and White Americans 132.
Most trials of the effects of vitamin D supplements on bone health also included calcium supplements, so isolating the effects of each nutrient is difficult. In addition, studies provided different amounts of nutrients and used different dosing schedules.
Among postmenopausal women and older men, many clinical trials have shown that supplements of both vitamin D and calcium result in small increases in bone mineral density throughout the skeleton 133. They also help reduce fracture rates in institutionalized older people. However, the evidence on the impact of vitamin D and calcium supplements on fractures in community-dwelling individuals is inconsistent.
The United States Preventive Services Task Force evaluated 11 randomized clinical trials of vitamin D and/or calcium supplementation in a total of 51,419 healthy, community-dwelling adults aged 50 years and older who did not have osteoporosis, vitamin D deficiency, or prior fractures 134. It concluded that the current evidence was insufficient to evaluate the benefits and harms of vitamin D and/or calcium supplementation to prevent fractures. In addition, the United States Preventive Services Task Force recommended against supplementation with 10 mcg (400 IU) or less of vitamin D and 1,000 mg or less of calcium to prevent fractures in this population, but it could not determine the balance of benefits and harms from higher doses.
The United States Preventive Services Task Force also reviewed the seven published studies on the effects of vitamin D supplementation (two of them also included calcium supplementation) on the risk of falls in community-dwelling adults aged 65 years or older who did not have osteoporosis or vitamin D deficiency. It concluded “with moderate certainty” that vitamin D supplementation does not reduce the numbers of falls or injuries, such as fractures, resulting from falls 135. Another recent systematic review also found that vitamin D and calcium supplements had no beneficial effects on fractures, falls, or bone mineral density 136. In contrast, a meta-analysis of 6 trials in 49,282 older adults found that daily vitamin D (10 or 20 mcg [400 IU or 800 IU]/day) and calcium (800 or 1,200 mg/day) supplementation for a mean of 5.9 years reduced the risk of any fracture by 6% and of hip fracture by 16% 137.
One systematic review and meta-analysis of 11 randomized, controlled trials published through 2018 of vitamin D supplementation alone (10–20 mcg [400–800 IU]/day or more at least every week or as rarely as once a year) for 9 months to 5 years found that the supplements provided no protection from fractures in 34,243 older adults 137.
One clinical trial randomized 260 Black women aged 60 years and older (mean age 68.2 years) to receive 60 to 120 mcg (2,400 to 4,800 IU) per day vitamin D3 supplementation to maintain serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels above 75 nmol/L (30 ng/mL) for 3 years 138. The results showed no association between 25-hydroxyvitamin D [25(OH)D or calcidiol] levels or vitamin D dose and the risk of falling in the 184 participants who completed the study. In fact, Black Americans might have a greater risk than White Americans of falls and fractures with daily vitamin D intakes of 50 mcg (2,000 IU) or more 132. Furthermore, the bone health of older Black American women does not appear to benefit from raising serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels beyond 50 nmol/L (20 ng/mL) 138.
Studies examining the effects of supplemental vitamin D on muscle strength and on rate of decline in muscle function have had inconsistent results 89. One recent clinical trial, for example, randomized 78 frail and near-frail adults aged 65 years and older to receive 20 mcg (800 IU) vitamin D3, 10 mcg 25-hydroxyvitamin D [25(OH)D or calcidiol], or placebo daily for 6 months. The groups showed no significant differences in measures of muscle strength or performance 139. Another study randomized 100 community-dwelling men and women aged 60 years and older (most were White) with serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels of 50 nmol/L (20 ng/ml) or less to 800 IU vitamin D3 or placebo for 1 year 140. Participants in the treatment group whose serum 25-hydroxyvitamin D [25(OH)D or calcidiol] level was less than 70 nmol/L (28 ng/ml) after 4 months received an additional 800 IU/day vitamin D3. Despite increasing serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels to an average of more than 80 nmol/L (32 ng/ml), vitamin D supplementation did not affect lower-extremity power, strength, or lean mass.
Alzheimer’s disease and Dementia
People with lower vitamin D in their blood, a condition known as vitamin D deficiency, appear to have a higher risk of age-related diseases, including cognitive decline, Alzheimer’s disease and other forms of dementia. Multiple meta-analyses and systematic reviews of observational research have examined the relationship between vitamin D levels and cognitive function. While a few small studies suggest that vitamin D supplementation may improve some aspects of cognitive functions, no randomized controlled trials have confirmed that it can protect against dementia.
For example, people with low levels or low dietary intake of vitamin D appear to be more likely to develop mild cognitive impairment (MCI) or dementia 141, but no clinical research has yet tested whether treatment with vitamin D can protect from this risk. In a small non-randomized clinical trial, elderly people receiving vitamin D3 supplements had better cognitive function compared to untreated people, with particular improvement in executive function 142, but the study was not controlled or designed to look at the risk of cognitive decline.
Clinical trials are underway to examine the effects of vitamin D on cognitive function in older adults who have low vitamin D levels 143, memory complaints 144, mild cognitive impairment (MCI) 145 and type 2 diabetes 146, as well as those in good health 147. These studies are scheduled to be completed in late 2016 to mid-2019. Another trial is testing whether vitamin D can reduce the risk of cancer, heart disease, and stroke in 20,000 men and women 148, with a subgroup undergoing testing for cognitive decline and dementia (scheduled to complete by late 2017) 149.
Research on the benefits of vitamin D for dementia patients is very limited and has produced mixed results. In a small, six-month pilot study, Alzheimer’s patients who were treated with memantine plus vitamin D improved their cognitive scores, whereas those taking memantine alone or vitamin D alone remained the same 150. A larger trial testing the effects of vitamin D in combination with memantine was scheduled to be completed in 2013 151, but the results have not been published. A small randomized trial of Parkinson’s disease patients suggested that vitamin D supplementation stabilized the disease, possibly by improving strength and balance 152.
At this point, the association between vitamin D deficiency and dementia risk is only observational. More research is needed to show cause and effect.
Vitamin D is vital to bone metabolism, calcium absorption and other metabolic processes in the body. Its role in brain function, cognition and the aging process is still unclear. Some studies suggest that vitamin D may be involved in a variety of processes related to cognition, but more research is needed to better understand this relationship.
It’s too early to recommend increasing your daily dose of vitamin D in hopes of preventing dementia or Alzheimer’s disease. But maintaining healthy vitamin D levels can’t hurt and may pay off in other ways, such as reducing the risk of osteoporosis. According to the National Institutes of Health, adults age 70 and younger need 600 international units (IU) of vitamin D daily, and adults over age 70 need 800 IU daily.
More studies are needed to determine if vitamin D deficiency is indeed a risk factor for Alzheimer’s disease and dementia and if the use of vitamin D supplements or sun exposure can prevent or treat these conditions.
Multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system that damages the myelin sheath surrounding and protecting nerve cells in the brain and spinal cord. This damage hinders or blocks messages between the brain and body, leading to clinical features, such as vision loss, motor weakness, spasticity, ataxia, tremor, sensory loss, and cognitive impairment 153. Some people with multiple sclerosis eventually lose the ability to write, speak, or walk.
The geographical distribution of multiple sclerosis around the world is unequal. Few people near the equator develop the disease, whereas the prevalence is higher further north and south. This uneven distribution has led to speculation that lower vitamin D levels in people who have less sunlight exposure might predispose them to the disease 153.
Many epidemiological and genetic studies have shown an association between multiple sclerosis and low 25-hydroxyvitamin D [25(OH)D or calcidiol] levels before and after the disease begins 153. Observational studies suggest that adequate vitamin D levels might reduce the risk of contracting multiple sclerosis and, once multiple sclerosis is present, decrease the risk of relapse and slow the disease’s progression 154. One study, for example, tested 25(OH)D levels in 1,092 women in Finland an average of 9 years before their multiple sclerosis diagnosis and compared their outcomes with those of 2,123 similar women who did not develop multiple sclerosis 155. More than half the women who developed multiple sclerosis had deficient or insufficient vitamin D levels. Women with 25(OH)D levels of less than 30 nmol/L (12 ng/mL) had a 43% higher multiple sclerosis risk than women with levels of 50 nmol/L (20 ng/mL) or higher. Among the women with two or more serum 25(OH)D samples taken before diagnosis (which reduced random measurement variation), a 50 nmol/L increase in 25(OH)D was associated with a 41% reduced risk of multiple sclerosis, and 25(OH)D levels less than 30 nmol/L were associated with an multiple sclerosis risk that was twice as high as levels of 50 nmol/L or higher.
Two earlier prospective studies of similar design—one in the United States with 444 non-Hispanic White individuals 156 and the other with 576 individuals in northern Sweden 157—found that levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] greater than 99.1 nmol/L (39.6 ng/mL) and at least 75 nmol/L (30 ng/mL), respectively, were associated with a 61–62% lower risk of multiple sclerosis.
No clinical trials have examined whether vitamin D supplementation can prevent the onset of multiple sclerosis, but several have investigated whether supplemental vitamin D can help manage the disease. A 2018 Cochrane review analyzed 12 such trials that had a total of 933 participants with multiple sclerosis; the reviewers judged all of these trials to be of low quality 153. Overall, vitamin D supplementation, when compared with placebo administration, had no effect on relevant clinical outcomes, such as recurrent relapse or worsened disability.
Experts have reached no firm consensus on whether vitamin D can help prevent multiple sclerosis given the lack of clinical trial evidence 158. In addition, studies have not consistently shown that vitamin D supplementation tempers the signs and symptoms of active multiple sclerosis or reduces rates of relapse.
Type 2 diabetes
Vitamin D plays a role in glucose metabolism. It stimulates insulin secretion via the vitamin D receptor on pancreatic beta cells and reduces peripheral insulin resistance through vitamin D receptors in the muscles and liver 159. Vitamin D might be involved in the pathophysiology of type 2 diabetes through its effects on glucose metabolism and insulin signaling as well as its ability to reduce inflammation and improve pancreatic beta-cell function 160.
Observational studies have linked lower serum 25(OH)D levels to an increased risk of diabetes, but their results might have been confounded by the fact that many participants were overweight or obese and were therefore more predisposed to developing diabetes and having lower 25(OH)D levels 85. A review of 71 observational studies in adults with and without type 2 diabetes from 16 countries found a significant inverse relationship between vitamin D status and blood sugar levels in participants who did and did not have diabetes 161.
In contrast to observational studies, clinical trials provide little support for the benefits of vitamin D supplementation for glucose homeostasis. One trial included 65 overweight or obese adult men and women (mean age 32 years) who were otherwise healthy, did not have diabetes, and had low serum vitamin D levels (at or below 50 nmol/L [20 ng/mL]) 162. The investigators randomly assigned participants to receive either a bolus oral dose of 2,500 mcg (100,000 IU) vitamin D3 followed by 100 mcg (4,000 IU)/day or a placebo for 16 weeks. In the 54 participants who completed the study, vitamin D supplementation did not improve insulin sensitivity or insulin secretion in comparison with placebo.
One systematic review and meta-analysis evaluated 35 clinical trials that included 43,407 adults with normal glucose tolerance, prediabetes, or type 2 diabetes who received a median of 83 mcg (3,332 IU)/day vitamin D supplements or placebo for a median of 16 weeks 163. Vitamin D had no significant effects on glucose homeostasis, insulin secretion or resistance, or hemoglobin A1c levels (HbA1c, a measure of average blood sugar levels over the previous 2–3 months), irrespective of the study population, vitamin D dose, or trial quality.
Several trials have investigated whether vitamin D supplementation can prevent the transition from prediabetes to diabetes in patients with adequate 25(OH)D levels, and all have had negative results. In a trial in Norway, 511 men and women aged 25–80 years (mean age 62 years) with prediabetes received 500 mcg (20,000 IU) vitamin D3 or a placebo each week for 5 years 164. The results showed no significant differences in rates of progression to type 2 diabetes; in serum glucose, insulin, or hemoglobin A1c levels; or in measures of insulin resistance. At baseline, participants had an adequate mean serum 25(OH)D level of 60 nmol/L (24 ng/mL).
The largest trial to date of vitamin D supplements for diabetes prevention randomized 2,423 men and women aged 25 years and older (mean age 60 years) with prediabetes who were overweight or obese (mean BMI of 32.1) to 100 mcg (4,000 IU)/day vitamin D3 or placebo for a median of 2.5 years 160. Most participants (78%) had adequate serum levels of vitamin D at baseline (at least 50 nmol/L [20 ng/mL]). Vitamin D did not significantly prevent the development of diabetes in comparison with placebo. However, a post hoc analysis showed a 62% lower incidence of diabetes among participants with low baseline serum 25(OH)D levels (less than 30 nmol/L [12 ng/mL]) who took the vitamin D supplement than among those who took the placebo 165.
Studies have also assessed the value of vitamin D supplementation for managing diabetes, and they have found that the vitamin offers limited benefits. One meta-analysis of 20 clinical trials compared the effects of 0.5 mcg (20 IU)/day to 1,250 mcg (50,000 IU)/week vitamin D supplementation for 2–6 months with those of placebo on glycemic control in 2,703 adults from around the world who had diabetes 159. The vitamin D reduced insulin resistance to a small but significant degree, especially in people taking more than 50 mcg (2,000 IU)/day who were vitamin D deficient at baseline, had good glycemic control, were not obese, and were of Middle Eastern ethnicity. However, the supplementation had no significant effects on fasting blood glucose, hemoglobin A1c (HbA1c) or fasting insulin levels.
Clinical trials to date provide little evidence that vitamin D supplementation helps maintain glucose homeostasis, reduces the risk of progression from prediabetes to type 2 diabetes, or helps manage the disease, particularly in vitamin D-replete individuals.
Cancer
Early epidemiologic research showed that incidence and death rates for certain cancers were lower among individuals living in southern latitudes, where levels of sunlight exposure are relatively high, than among those living at northern latitudes. Because exposure to ultraviolet light from sunlight leads to the production of vitamin D, researchers hypothesized that variation in vitamin D levels might account for this association. However, additional research based on stronger study designs is required to determine whether higher vitamin D levels are related to lower cancer incidence or death rates. Experimental evidence has also suggested a possible association between vitamin D and cancer risk. In studies of cancer cells and of tumors in mice, vitamin D has been found to have several activities that might slow or prevent the development of cancer, including promoting cellular differentiation, decreasing cancer cell growth, stimulating cell death (apoptosis), and reducing tumor blood vessel formation (angiogenesis) 166.
A number of epidemiologic studies have investigated whether people with higher vitamin D intakes or higher blood levels of vitamin D have lower risks of specific cancers. The results of these studies have been inconsistent, possibly because of the challenges in carrying out such studies. For example, dietary studies do not account for vitamin D made in the skin from sunlight exposure, and the level of vitamin D measured in the blood at a single point in time (as in most studies) may not reflect a person’s true vitamin D status. Also, it is possible that people with higher vitamin D intakes or blood levels are more likely to have other healthy behaviors. It may be one of these other behaviors, rather than vitamin D intake, that influences cancer risk.
Several randomized trials of vitamin D intake have been carried out, but these were designed to assess bone health or other non-cancer outcomes. Although some of these trials have yielded information on cancer incidence and mortality, the results need to be confirmed by additional research because the trials were not designed to study cancer specifically.
The cancers for which the most human data are available are colorectal, breast, prostate, and pancreatic cancer. In a meta-analysis of 16 prospective cohort studies in a total of 137,567 participants who had 8,345 diagnoses of cancer, 5,755 participants died from cancer 167. A 50 nmol/L (20 ng/mL) increase in 25-hydroxyvitamin D [25(OH)D or calcidiol] levels was associated with an 11% reduction in total cancer incidence rates and, in women but not men, a 24% reduction in cancer mortality rates. A meta-analysis of prospective studies that evaluated the association between serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and cancer incidence (8 studies) or cancer mortality (16 studies) found that cancer risk decreased by 7% and cancer mortality rates decreased by 2% with each 20 nmol/L (8 ng/mL) increase in serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels 168. Importantly, not all observational studies found higher vitamin D status to be beneficial, and the studies varied considerably in study populations, baseline comorbidities, and measurement of vitamin D levels.
Clinical trial evidence provides some support for the observational findings. For example, three meta-analyses of clinical trial evidence found that vitamin D supplementation does not affect cancer incidence but does significantly reduce total cancer mortality rates by 12–13% 169. In the most recent meta-analysis, 10 randomized clinical trials (including the Vitamin D and Omega-3 Trial [VITAL] trial described below) that included 6,537 cancer cases provided 10 to 50 mcg (400 to 2,000 IU) vitamin D3 daily (six trials) or 500 mcg (20,000 IU)/week to 12,500 mcg (500,000 IU)/year boluses of vitamin D3 (four trials) 169. The study reports included 3–10 years of followup data. The vitamin D supplements were associated with serum 25(OH)D levels of 54 to 135 nmol/L (21.6 to 54 ng/mL). Vitamin D supplementation reduced cancer mortality rates by 13%, and most of the benefit occurred with daily supplementation.
The largest clinical trial, Vitamin D and Omega-3 Trial [VITAL], to investigate the effects of vitamin D supplementation on the primary prevention of cancer in the general population gave 50 mcg (2,000 IU)/day vitamin D3 supplements with or without 1,000 mg/day marine omega-3 fatty acids or a placebo for a median of 5.3 years 170. The study included 25,871 men aged 50 years and older and women aged 55 years and older who had no history of cancer, and most had adequate serum 25(OH)D levels at baseline. Rates of breast, prostate, and colorectal cancer did not differ significantly between the vitamin D and placebo groups. However, normal-weight participants had greater reductions in cancer incidence and mortality rates than those who were overweight or obese.
Numerous epidemiologic studies have shown that higher intake or blood levels of vitamin D are associated with a reduced risk of colorectal cancer 171, 172. In contrast, the Women’s Health Initiative randomized trial found that healthy women who took vitamin D and calcium supplements for an average of 7 years did not have a reduced incidence of colorectal cancer 173. Some scientists have pointed out that the relatively low level of vitamin D supplementation (10 mcg or 400 IU, once a day), the ability of participants to take additional vitamin D on their own, and the short duration of participant follow-up in this trial might explain why no reduction in colorectal cancer risk was found. Evidence on the association between vitamin D and the risks of all other malignancies studied is inconclusive.
Taken together, the available data are not comprehensive enough to establish whether taking vitamin D can prevent cancer 174. To fully understand the effects of vitamin D on cancer and other health outcomes, new randomized trials need to be conducted 175. However, the appropriate dose of vitamin D to use in such trials is still not clear 176. Other remaining questions include when to start taking vitamin D, and for how long, to potentially see a benefit.
The United States Preventive Services Task Force stated that, due to insufficient evidence, it was unable to assess the balance of benefits and harms of supplemental vitamin D to prevent cancer 177. Taken together, studies to date do not indicate that vitamin D with or without calcium supplementation reduces the incidence of cancer, but adequate or higher 25-hydroxyvitamin D [25(OH)D or calcidiol] levels might reduce cancer mortality rates. Further research is needed to determine whether vitamin D inadequacy increases cancer risk, whether greater exposure to the nutrient can prevent cancer, and whether some individuals could have an increased risk of cancer because of their vitamin D status over time.
Breast cancer
Some observational studies support an inverse association between 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and breast cancer risk and mortality, but others do not 178. The Women’s Health Initiative clinical trial randomized 36,282 postmenopausal women to receive 400 IU vitamin D3 plus 1,000 mg calcium daily or a placebo for a mean of 7 years 173. The vitamin D3 and calcium supplements did not reduce breast cancer incidence, and 25-hydroxyvitamin D [25(OH)D or calcidiol] levels at the start of the study were not associated with breast cancer risk 179.
In a subsequent investigation for 4.9 years after the study’s end, women who had taken the vitamin D and calcium supplements (many of whom continued to take them) had an 18% lower risk of in situ (noninvasive) breast cancer 180. However, women with vitamin D intakes higher than 15 mcg (600 IU)/day at the start of the trial and who received the supplements experienced a 28% increased risk of invasive (but not in situ) breast cancer.
Colorectal cancer
A large case-control study included 5,706 individuals who developed colorectal cancer and whose 25-hydroxyvitamin D [25(OH)D or calcidiol] levels were assessed a median of 5.5 years from blood draw to cancer diagnosis and 7,105 matched controls 181. The results showed an association between 25-hydroxyvitamin D [25(OH)D or calcidiol] levels lower than 30 nmol/L (12 ng/mL) and a 31% higher colorectal cancer risk. Levels of 75 to less than 87.5 nmol/L (30 to less than 35 ng/mL) and 87.5 to less than 100 nmol/L (35 to less than 40 ng/mL) were associated with a 19% and 27% lower risk, respectively. The association was substantially stronger in women.
In the Women’s Health Initiative clinical trial, vitamin D3 and calcium supplements had no effect on rates of colorectal cancer. In a subsequent investigation for 4.9 years after the study’s end, women who had taken the vitamin D and calcium supplements (many of whom continued to take them) still had the same colorectal cancer risk as those who received placebo 179.
Another study included 2,259 healthy individuals aged 45 to 75 years who had had one or more serrated polyps (precursor lesions to colorectal cancer) that had been removed 182. These participants were randomized to take 25 mcg (1,000 IU) vitamin D3, 1,200 mg calcium, both supplements, or a placebo daily for 3–5 years, followed by an additional 3–5 years of observation after participants stopped the treatment. Vitamin D alone did not significantly affect the development of new serrated polyps, but the combination of vitamin D with calcium increased the risk almost fourfold. The Vitamin D and Omega-3 Trial (VITAL) trial found no association between vitamin D supplementation and the risk of colorectal adenomas or serrated polyps 182.
Lung cancer
A study of cohorts that included 5,313 participants who developed lung cancer and 5,313 matched controls found no association between serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and risk of subsequent lung cancer, even when the investigators analyzed the data by sex, age, race and ethnicity, and smoking status 183.
Pancreatic cancer
One study comparing 738 men who developed pancreatic cancer to 738 matched controls found no relationship between serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and risk of pancreatic cancer 184. Another study that compared 200 male smokers in Finland with pancreatic cancer to 400 matched controls found that participants in the highest quintile of 25-hydroxyvitamin D [25(OH)D or calcidiol] levels (more than 65.5 nmol/L [26.2 ng/mL]) had a threefold greater risk of developing pancreatic cancer over 16.7 years than those in the lowest quintile (less than 32 nmol/L [12.8 ng/mL]) 185. An investigation that pooled data from 10 studies of cancer in 12,205 men and women found that concentrations of 25-hydroxyvitamin D [25(OH)D or calcidiol] greater than 75 nmol/L (30 ng/mL) but less than 100 nmol/L (40 ng/mL) did not reduce the risk of pancreatic cancer. However, the results did show an increased risk of pancreatic cancer with 25-hydroxyvitamin D [25(OH)D or calcidiol] levels of 100 nmol/L (40 ng/mL) or above 186.
Prostate cancer
Research to date provides mixed evidence on whether levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] are associated with the development of prostate cancer. Several studies published in 2014 suggested that high levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] might increase the risk of prostate cancer. For example, a meta-analysis of 21 studies that included 11,941 men with prostate cancer and 13,870 controls found a 17% higher risk of prostate cancer for participants with higher levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] 187. What constituted a “higher” level varied by study but was typically at least 75 nmol/L (30 ng/mL). In a cohort of 4,733 men, of which 1,731 had prostate cancer, those with 25-hydroxyvitamin D [25(OH)D or calcidiol] levels of 45–70 nmol/L (18–28 ng/mL) had a significantly lower risk of the disease than men with either lower or higher values 188. This U-shaped association was most pronounced for men with the most aggressive forms of prostate cancer. A case-control analysis of 1,695 cases of prostate cancer and 1,682 controls found no associations between 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and prostate cancer risk 189. However, higher serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels (at a cut point of 75 nmol/L [30 ng/mL]) were linked to a modestly higher risk of slow-growth prostate cancer and a more substantial lower risk of aggressive disease.
Since 2014, however, several published studies and meta-analyses have found no relationship between 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and prostate cancer risk 190, 191. For example, an analysis was conducted of 19 prospective studies that provided data on prediagnostic levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] for 13,462 men who developed prostate cancer and 20,261 control participants 192. Vitamin D deficiency or insufficiency did not increase the risk of prostate cancer, and higher 25-hydroxyvitamin D [25(OH)D or calcidiol] concentrations were not associated with a lower risk.
Several studies have examined whether levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] in men with prostate cancer are associated with a lower risk of death from the disease or from any cause. One study included 1,119 men treated for prostate cancer whose plasma 25-hydroxyvitamin D [25(OH)D or calcidiol] levels were measured 4.9 to 8.6 years after their diagnosis. Among the 198 participants who died (41 deaths were due to prostate cancer), 25-hydroxyvitamin D [25(OH)D or calcidiol] levels were not associated with risk of death from prostate cancer or any cause 193. However, a meta-analysis of 7 cohort studies that included 7,808 men with prostate cancer found higher 25-hydroxyvitamin D [25(OH)D or calcidiol] levels to be significantly associated with lower mortality rates from prostate cancer or any other cause 194. A dose-response analysis found that each 20 nmol/L [8 ng/mL] increase in 25-hydroxyvitamin D [25(OH)D or calcidiol] was associated with a 9% lower risk of both all-cause and prostate cancer-specific mortality.
For men with prostate cancer, whether vitamin D supplementation lengthens cancer-related survival is not clear. A meta-analysis of 3 randomized controlled trials in 1,273 men with prostate cancer found no significant differences in total mortality rates between those receiving vitamin D supplementation (from 10 mcg [400 IU]/day for 28 days to 45 mcg [1,800 IU] given in three doses total at 2-week intervals) and those receiving a placebo 195.
Depression
Vitamin D is involved in various brain processes, and vitamin D receptors are present on neurons and glia in areas of the brain thought to be involved in the pathophysiology of depression 196. A systematic review and meta-analysis of 14 observational studies that included a total of 31,424 adults (mean age ranging from 27.5 to 77 years) found an association between deficient or low levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] and depression 196. Clinical trials, however, do not support these findings. For example, a meta-analysis of 9 trials with a total of 4,923 adult participants diagnosed with depression or depressive symptoms found no significant reduction in symptoms after supplementation with vitamin D 197. The trials administered different amounts of vitamin D (ranging from 10 mcg [400 IU]/day to 1,000 mcg [40,000 IU]/week). They also had different study durations (5 days to 5 years), mean participant ages (range, 22 years to 75 years), and baseline 25-hydroxyvitamin D [25(OH)D or calcidiol] levels; furthermore, some but not all studies administered concurrent antidepressant medications.
Three trials conducted since that meta-analysis also found no effect of vitamin D supplementation on depressive symptoms 198, 199, 200. One trial included 206 adults (mean age 52 years) who were randomized to take a bolus dose of 2,500 mcg (100,000 IU) vitamin D3 followed by 500 mcg (20,000 IU)/week or a placebo for 4 months 198. Most participants had minimal or mild depression, had a low mean baseline 25(OH) level of 33.8 nmol/L (13.5 ng/mL), and were not taking antidepressants. The second trial included 155 adults aged 60–80 years who had clinically relevant depressive symptoms, no major depressive disorder, and serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels less than 50 to 70 nmol/L (20 to 28 ng/mL) depending on the season; in addition, they were not taking antidepressants 199. Participants were randomized to take either 30 mcg (1,200 IU)/day vitamin D3 or a placebo for 1 year. In the VITAL trial described above, 16,657 men and women 50 years of age and older with no history of depression and 1,696 with an increased risk of recurrent depression (that had not been medically treated for the past 2 years) were randomized to take 50 mcg (2,000 IU)/day vitamin D3 (with or without fish oil) or a placebo for a median of 5.3 years 201. The groups showed no significant differences in the incidence and recurrent rates of depression, clinically relevant depressive symptoms, or changes in mood scores.
Overall, clinical trials did not find that vitamin D supplements helped prevent or treat depressive symptoms or mild depression, especially in middle-aged to older adults who were not taking prescription antidepressants. No studies have evaluated whether vitamin D supplements may benefit individuals under medical care for clinical depression who have low or deficient 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and are taking antidepressant medication.
Cardiovascular disease
Vitamin D helps regulate the renin-angiotensin-aldosterone system (and thereby blood pressure), vascular cell growth, and inflammatory and fibrotic pathways 202. Vitamin D deficiency is associated with vascular dysfunction, arterial stiffening, left ventricular hypertrophy, and hyperlipidemia 203. For these reasons, vitamin D has been linked to heart health and risk of cardiovascular disease.
Observational studies support an association between higher serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels and a lower risk of cardiovascular disease incidence and mortality. For example, a meta-analysis included 34 observational studies that followed 180,667 participants (mean age greater than 50 years) for 1.3 to more than 32 years. The results showed that baseline serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels were inversely associated with total number of cardiovascular disease events (including myocardial infarction, ischemic heart disease, heart failure, and stroke) and mortality risk 204. Overall, the risk of cardiovascular disease events was 10% lower for each 25 nmol/L (10 ng/mL) increase in serum 25-hydroxyvitamin D [25(OH)D or calcidiol].
Another large observational study that followed 247,574 adults from Denmark for 0–7 years found that levels of 25-hydroxyvitamin D [25(OH)D or calcidiol] that were low (about 12.5 nmol/L [5 ng/mL]) and high (about 125 nmol/L [50 ng/mL]) were associated with a greater risk of mortality from cardiovascular disease, stroke, and acute myocardial infarction 205. Other meta-analyses of prospective studies have found associations between lower vitamin D status measured by serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels or vitamin D intakes and an increased risk of ischemic stroke, ischemic heart disease, myocardial infarction, and early death 206, 207.
In contrast to the observational studies, clinical trials have provided little support for the hypothesis that supplemental vitamin D reduces the risk of cardiovascular disease or cardiovascular disease mortality. For example, a 3-year trial in New Zealand randomized 5,110 adults (mean age 65.9 years) to a single dose of 5,000 mcg (200,000 IU) vitamin D3 followed by 2,500 mcg (100,000 IU) each month or a placebo for a median of 3.3 years 208. Vitamin D supplementation had no effect on the incidence rate of myocardial infarction, angina, heart failure, arrhythmia, arteriosclerosis, stroke, venous thrombosis, or death from cardiovascular disease. Similarly, the VITamin D and Omega-3 TriaL (VITAL) clinical trial described above found that vitamin D supplements did not significantly decrease rates of heart attacks, strokes, coronary revascularization, or deaths from cardiovascular causes 170. Moreover, the effects did not vary by baseline serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels or whether participants took the trial’s omega-3 supplement in addition to vitamin D.
However, another clinical trial designed to investigate bone fracture risk found that 800 IU/day vitamin D3 (with or without calcium) or a placebo in 5,292 adults aged 70 years and older for a median of 6.2 years offered protection from cardiac failure, but not myocardial infarction or stroke 209.
High serum cholesterol levels and hypertension are two of the main risk factors for cardiovascular disease. The data on supplemental vitamin D and cholesterol levels are mixed, as shown in one meta-analysis of 41 clinical trials in a total of 3,434 participants (mean age 55 years). The results of this analysis showed that 0.5 mcg (20 IU) to 214 mcg (8,570 IU)/day vitamin D supplementation (mean of 2,795 IU) for 6 weeks to 3 years reduced serum total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels, but not high-density lipoprotein cholesterol levels 210.
Studies of the effects of vitamin D supplements on hypertension have also had mixed findings. In one meta-analysis of 46 clinical trials that included 4,541 participants, vitamin D supplements (typically 40 mcg [1,600 IU]/day or less) for a minimum of 4 weeks had no significant effects on systolic or diastolic blood pressure 211. In contrast, another meta-analysis of 30 clinical trials in 4,744 participants (mean age 54.5 years) that administered 5 mcg (200 IU) to 300 mcg (12,000 IU)/day vitamin D3 for a mean of 5.6 months showed that more than 20 mcg (800 IU)/day significantly reduced systolic and diastolic blood pressure in normal-weight participants who had hypertension 212. However, more than 20 mcg (800 IU)/day vitamin D3, when taken with calcium supplements, significantly increased blood pressure in overweight and obese participants. Another meta-analysis of genetic studies in 146,581 participants (primarily adults) found that a low vitamin D status increased blood pressure and hypertension risk in people with genetic variants associated with low endogenous production of 25-hydroxyvitamin D [25(OH)D or calcidiol] 213.
Overall, clinical trials show that vitamin D supplementation does not reduce cardiovascular disease risk, even for people with low 25-hydroxyvitamin D [25(OH)D or calcidiol] status (below 20 nmol/L [12 ng/mL]) at baseline 170, 208.
Weight loss
Observational studies indicate that greater body weights are associated with lower vitamin D status, and obese individuals frequently have marginal or deficient circulating 25-hydroxyvitamin D [25(OH)D or calcidiol] levels 214. However, clinical trials do not support a cause-and-effect relationship between vitamin D and weight loss.
A systematic review and meta-analysis of 15 weight-loss intervention studies that used caloric restriction, exercise, or both, but not necessarily vitamin D supplementation or other treatments, found that people who lost weight had significantly greater increases in serum 25-hydroxyvitamin D [25(OH)D or calcidiol] levels than those who maintained their weight 215. In another study, 10 mcg (400 IU)/day vitamin D and 1,000 mg/day calcium supplementation slightly, but significantly, reduced weight gain amounts in comparison with placebo in postmenopausal women, especially those with a baseline total calcium intake of less than 1,200 mg/day 216. However, a meta-analysis of 12 vitamin D supplementation trials (including 5 in which body composition measurements were primary outcomes) found that vitamin D supplements without calorie restriction did not affect body weight or fat mass when the results were compared with those of placebo 217.
Overall, the available research suggests that consuming higher amounts of vitamin D or taking vitamin D supplements does not promote weight loss.
Vitamin D deficiency causes
People can develop vitamin D deficiency when usual intakes are lower over time than recommended levels, exposure to sunlight is limited, the kidneys cannot convert 25-hydroxyvitamin D [25(OH)D] to its active form 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D] or absorption of vitamin D from the digestive tract is inadequate.
Vitamin D deficiency can result from several causes 218:
- Decreased dietary intake and/or absorption. Diets low in vitamin D are more common in people who have milk allergy or lactose intolerance and those who consume an ovo-vegetarian or vegan diet 85. Certain malabsorption syndromes such as celiac disease, short bowel syndrome, gastric bypass, inflammatory bowel disease, chronic pancreatic insufficiency, and cystic fibrosis may lead to vitamin D deficiency. Lower vitamin D intake orally is more prevalent in the elderly population 219.
- Decreased sun exposure. About 50% to 90% of vitamin D is absorbed through the skin via sunlight while the rest comes from the diet. Twenty minutes of sunshine daily with over 40% of skin exposed is required to prevent vitamin D deficiency 220. Cutaneous synthesis of vitamin D declines with aging. Dark-skinned people have less cutaneous vitamin D synthesis. Decreased exposure to the sun as seen in individuals who are institutionalized, or have prolonged hospitalizations can also lead to vitamin D deficiency 49. Effective sun exposure is decreased in individuals who use sunscreens consistently.
- Decreased endogenous synthesis. Individuals with chronic liver disease such as cirrhosis can have defective 25-hydroxylation leading to deficiency of 25-hydroxyvitamin D [25(OH)D]. Defect in 1-alpha 25-hydroxylation leading to deficiency of 1,25-dihydroxyvitamin D [calcitriol or 1,25(OH)2D] can be seen in hyperparathyroidism, renal failure and 1-alpha hydroxylase deficiency.
- Increased hepatic catabolism. Medications such as phenobarbital, carbamazepine, dexamethasone, nifedipine, spironolactone, clotrimazole, and rifampin induce hepatic p450 enzymes which activate degradation of vitamin D 221.
- End organ resistance. End organ resistance to vitamin D can be seen in hereditary vitamin D resistant rickets.
Prolonged exclusive breastfeeding without vitamin D supplementation can cause rickets in infants, and, in the United States, rickets is most common among breastfed Black infants and children 222. In one Minnesota county, the incidence rate of rickets in children younger than 3 years in the decade beginning in 2000 was 24.1 per 100,000 223. Rickets occurred mainly in Black children who were breastfed longer, were born with low birthweight, weighed less, and were shorter than other children. The incidence rate of rickets in the infants and children (younger than 7) seen by 2,325 pediatricians throughout Canada was 2.9 per 100,000 in 2002–2004, and almost all patients with rickets had been breastfed 224.
[Source 5 ]Risk factors for developing vitamin D deficiency
Common risk factors for vitamin D deficiency:
- Old age 225. The elderly have reduced capacity to synthesize vitamin D in skin when exposed to UVB radiation and are more likely to stay indoors or use sunscreen, which prevents vitamin D synthesis. It has been estimated that across Canada, the US, and Europe, the prevalence of vitamin D deficiency ranges between 20%-100% in free-living elderly 75. Moreover, institutionalized adults who are not supplemented with vitamin D are at extremely high risk of vitamin D deficiency 226, 227..
- Immobility and reduced kidney function 228
- Chronic kidney disease (CKD) 229. Vitamin D deficiency in patients with impaired renal function is due to a reduced synthesis of 1α,25-dihydroxyvitamin D and an increased loss of 25-hydroxyvitamin D in urine 230.
- Stay mostly indoors for health, work or other reasons
- Have naturally dark skin 231. People with a dark complexion synthesize less vitamin D on exposure to sunlight than those with light-colored skin 232. A national US survey reported average serum 25-hydroxyvitamin D concentrations of 28.1 ng/mL, 21.6 ng/mL, and 16.9 ng/mL in Caucasian, Mexican American, and African American adults aged ≥20 years old, respectively 233.
- Cover your body for religious or cultural reasons
- Avoid the sun for skin protection or due to medical reasons
- Are obese 234. Obesity (body mass index ≥30 kg/m²) increases the risk of vitamin D deficiency 235. Once vitamin D is synthesized in the skin or ingested, it can be sequestered in body fat stores, making it less bioavailable to people with higher body fat mass. Moreover, vitamin D supplementation trials have shown that obese people reached much lower serum 25-hydroxyvitamin D concentrations compared to normal weight (BMI <25 kg/m²) participants with equivalent oral dosages 236.
- Have a health condition that affects vitamin D absorption from your diet (e.g., lactose intolerance, dietary restrictions, vegans, etc.)
- Inflammatory bowel disease: People with inflammatory bowel disease like Crohn’s disease appear to be at increased risk of vitamin D deficiency, especially those who have had small bowel resections 237.
- Have intestinal malabsorption syndromes or other health conditions (e.g., small bowel syndrome, pancreatitis, amyloidosis, Crohn’s disease, cystic fibrosis, severe liver disease, celiac disease, malabsorptive bariatric surgical procedures) 238
- Fat malabsorption syndromes: Vitamin D deficiency is common among people with cystic fibrosis and both cholestatic and non-cholestatic liver diseases due to decreased absorption of dietary vitamin D and impaired conversion of vitamin D to 25-hydroxyvitamin D 239.
- Take medicines that cause vitamin D to break down (e.g., anticonvulsants, rifampicin, cimetidine, thiazides, corticosteroids) 240
- Take medicines that decrease the absorption of vitamin D (e.g., mineral oil, laxatives, orlistat, cholestyramine) 241
- Infants born to a vitamin D deficient mother
- Exclusively breastfed infants 242
- Genetic variations: An international, multicenter, genome-wide association study (GWAS) of 15 cohorts, including ~30,000 participants of European descent — known as the SUNLIGHT [Study of Underlying Genetic Determinants of Vitamin D and Highly Related Traits] consortium — identified common variations called polymorphisms in genes involved in cholesterol synthesis, hydroxylation, and vitamin D transport that influence vitamin D status 243. While genetic determinants of low vitamin D status are being identified in populations of European 244, 245 and Asian descent 246, 247, genome-wide association studies are needed in populations of African descent.
- Magnesium deficiency: Recent findings suggest that high magnesium intakes may reduce the risk of vitamin D insufficiency. Magnesium regulates the activity of critical enzymes in vitamin D metabolism, which would explain how magnesium deficiency negatively affects vitamin D status 248.
Older adults
Older adults are at increased risk of developing vitamin D insufficiency, partly because the skin’s ability to synthesize vitamin D declines with age 249. In addition, older adults are likely to spend more time than younger people indoors, and they might have inadequate dietary intakes of the vitamin 85.
Vitamin D deficiency is common among elderly patients in temperate countries, especially in early spring due to reduced cutaneous synthesis during the winter months 250 and in housebound individuals 54 and medical inpatients 49. The prevalence of vitamin D deficiency is even higher in elderly patients with fragility fractures, ranging from 55%–91.6% 251. A recent study in Singapore showed a vitamin D deficiency prevalence of 57.5% and vitamin D insufficiency of 34.5% in elderly patients admitted to hospital with hip fractures 252.
Breastfed infants
Consumption of human milk alone does not ordinarily enable infants to meet vitamin D requirements, because it provides less than 0.6 to 2.0 mcg/L (25 to 78 IU/L) 42. The vitamin D content of human milk is related to the mother’s vitamin D status; studies suggest that the breastmilk of mothers who take daily supplements containing at least 50 mcg (2,000 IU) vitamin D3 have higher levels of the nutrient 253.
Although UVB exposure can produce vitamin D in infants, the American Academy of Pediatrics advises parents to keep infants younger than 6 months out of direct sunlight, dress them in protective clothing and hats, and apply sunscreen on small areas of exposed skin when sun exposure is unavoidable 254. The American Academy of Pediatrics recommends 10 mcg (400 IU)/day vitamin D supplements for exclusively and partially breastfed infants starting shortly after birth and lasting until they are weaned and consume at least 1,000 mL/day vitamin D-fortified formula or whole milk 42. The American Academy of Pediatrics also recommends 10 mcg (400 IU)/day supplemental vitamin D for all infants who are not breastfed and ingest less than 1,000 mL/day vitamin D-fortified formula or milk. An analysis of NHANES 2009–2016 data found that only 20.5% of breastfed infants and 31.1% of infants who were not breastfed ingested these recommended amounts of supplements 255.
People with limited sun exposure
Homebound individuals, women who wear long robes and head coverings for religious reasons, and people with occupations that limit sun exposure are unlikely to obtain adequate vitamin D from sunlight 256. The use of sunscreen also limits vitamin D synthesis from sunlight. However, because the extent and frequency of sunscreen use are unknown, the role that sunscreen may play in reducing vitamin D synthesis is unclear 85. Ingesting RDA levels of vitamin D from foods and/or supplements will provide these individuals with adequate amounts of this nutrient.
People with dark skin
Greater amounts of the pigment melanin in the epidermal layer result in darker skin and reduce the skin’s ability to produce vitamin D from sunlight 85. Various reports consistently show lower serum 25(OH)D levels in persons identified as black compared with those identified as white. However, it is not clear that lower levels of 25(OH)D for persons with dark skin have significant health consequences. Those of African American ancestry, for example, have reduced rates of fracture and osteoporosis compared with Caucasians 92. Ingesting RDA levels of vitamin D from foods and/or supplements will provide these individuals with adequate amounts of this nutrient.
People with conditions that limit fat absorption
Because vitamin D is a fat-soluble vitamin, its absorption depends on the gut’s ability to absorb dietary fat 79. Individuals who have a reduced ability to absorb dietary fat might require vitamin D supplementation 239. Fat malabsorption is associated with a variety of medical conditions, including some forms of liver disease, cystic fibrosis, celiac disease, and Crohn’s disease, as well as ulcerative colitis when the terminal ileum is inflamed 85, 33, 239. In addition, people with some of these conditions might have lower intakes of certain foods, such as dairy products (many of which are fortified with vitamin D), or eat only small amounts of these foods. Individuals who have difficulty absorbing dietary fat might therefore require vitamin D supplementation 239.
People who are obese or have undergone gastric bypass surgery
Individuals with a body mass index (BMI) of 30 or more have lower serum 25(OH)D levels than nonobese individuals. Obesity does not affect the skin’s capacity to synthesize vitamin D. However, greater amounts of subcutaneous fat sequester more of the vitamin 85. Obese people might need greater intakes of vitamin D to achieve 25(OH)D levels similar to those of people with normal weight 257.
Obese individuals who have undergone gastric bypass surgery can also become vitamin D deficient. In this procedure, part of the upper small intestine, where vitamin D is absorbed, is bypassed, and vitamin D that is mobilized into the bloodstream from fat stores might not raise 25(OH)D to adequate levels over time 258. Various expert groups—including the American Association of Metabolic and Bariatric Surgery, The Obesity Society, and the British Obesity and Metabolic Surgery Society—have developed guidelines on vitamin D screening, monitoring, and replacement before and after bariatric surgery 259.
Vitamin D deficiency prevention
Adults less than 65 years of age who do not have year-round effective sun exposure shall consume 600 to 800 international units (IU) of vitamin D3 daily to prevent deficiency. Older adults 65 years of age or more shall consume 800 to 1000 international units (IU) of vitamin D3 daily to prevent deficiency and to reduce the risk of fractures and falls.
[Source 5 ]Vitamin D deficiency symptoms
The majority of people with vitamin D deficiency are asymptomatic 218. However, even mild chronic vitamin D deficiency can lead to chronic hypocalcemia and hyperparathyroidism which can contribute risk of osteoporosis, falls and fractures especially in the elderly population. Patients with a prolonged and severe vitamin D deficiency can experience symptoms associated with secondary hyperparathyroidism including bone pain, arthralgias, myalgias, fatigue, muscle twitching (fasciculations), and weakness. Fragility fractures may result from chronic vitamin D deficiency leading to osteoporosis. In children, irritability, lethargy, developmental delay, bone changes, or fractures can be symptoms of vitamin D deficiency.
In children, vitamin D deficiency is manifested as rickets, a disease characterized by a failure of bone tissue to become properly mineralized, resulting in soft bones and skeletal deformities 260. In addition to bone deformities and pain, severe rickets can cause failure to thrive, developmental delay, hypocalcemic seizures, tetanic spasms, cardiomyopathy, and dental abnormalities 261.
In adults and adolescents, vitamin D deficiency can lead to osteomalacia, in which existing bone is incompletely or defectively mineralized during the remodeling process, resulting in weak bones 261. Signs and symptoms of osteomalacia are similar to those of rickets and include bone deformities and pain, hypocalcemic seizures, tetanic spasms, and dental abnormalities 262.
People with very low levels of vitamin D (moderate to severe deficiency) are the most at risk of developing health problems.
A number of diseases have been linked to low vitamin D levels such as increased risk of death from cardiovascular disease, cognitive impairment in older adults, severe asthma in children and cancer. Research suggests that vitamin D could play a role in the prevention and treatment of a number of different conditions, including type1 and type 2 diabetes, hypertension, glucose intolerance and multiple sclerosis. However, the U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to assess the benefits and harms of screening for vitamin D deficiency in asymptomatic adults 90. It added that no national professional organization recommends population screening for vitamin D deficiency unless you are considered specifically at risk.
Vitamin D deficiency diagnosis
In clinical practice the diagnosis of vitamin D deficiency states is usually established by measurements of serum 25-hydroxyvitamin D [25(OH)D] levels. Circulating levels of 25-hydroxyvitamin D [25(OH)D] are a good and reliable measure of vitamin D status in almost all clinical relevant situations. However, optimal serum levels of 25-hydroxyvitamin D [25(OH)D] is still a matter of controversy. There are substantial differences in mineral metabolism amongst different races. African Americans, for example, have higher bone density and low fracture risk compared to other races. Furthermore, the effects of calcium and vitamin-D supplementation in the non-Caucasian population have not yet been completely evaluated or reported. The International Society for Clinical Densitometry and International Osteoporosis Foundation recommend minimum serum levels of 25-hydroxyvitamin D [25(OH)D] of 30 ng/mL to minimize the risk of fall and fractures in older individuals 263. There is insufficient data about the maximum safe upper level of serum 25-hydroxyvitamin D, however, at high levels such as above 100 ng/mL, there is a potential risk of toxicity due to the secondary hypercalcemia. In patients where vitamin-D deficiency has been diagnosed, it is important to evaluate for secondary hyperparathyroidism and levels of parathyroid hormone and serum calcium shall be checked.
All additional biochemical parameters as well as clinical signs and symptoms reflect the primary and secondary perturbations in mineral and bone metabolism caused by vitamin D deficiency and are common to all disorders in vitamin D action and calcium deficiency (Tables 7 and 8). Those parameters include low to low normal serum calcium levels, hypocalciuria, secondary hyperparathyroidism, hypophosphatemia, increased levels of biochemical markers of bone turnover, rickets and/or osteomalacia. Therefore, all these measures can be used to support but not to establish the diagnosis, and mainly to assess the relative severity of the vitamin D deficiency and the response to treatment. It is important to note that in vitamins D deficiency, circulating levels of 1,25-dihydroxyvitamin D [1,25(OH)2D] could vary from low to elevated (Table 7) and thus are useless for the diagnosis.
Table 7. Serum levels of vitamin D metabolites in patients with disorders in vitamin D action, by etiology
| Serum levels | ||
|---|---|---|
| Etiology | 25(OH)D | 1,25(OH)2D |
| Vitamin D deficiency | Low | Low to normal |
| 1,25(OH)2D deficiency | Normal to elevated | Very low |
| Resistance to 1,25(OH)2D | Normal to elevated | Markedly elevated |
Table 8. Biochemical parameters of mineral and bone metabolism in patients with rickets and/or osteomalacia, by cause
| Serum levels | 24 hour urinary calcium excretion | ||||
|---|---|---|---|---|---|
| Cause | Calcium | Phosphorous | iPTH | Bone specific alkaline phosphatase activity | |
| Hypocalcemic e.g. vitamin D deficiency | Low to low normal | Low | Elevated | Elevated | Low |
| Hypophosphatemice.g. X-linked hypophosphatemia | Normal | Low | Normal to low normal | Elevated | Low to elevated |
| No abnormality in mineral homeostasis e.g. hypophosphatasia | Normal | Normal | Normal | Low | Normal |
Footnote: iPTH = immunoreactive parathyroid hormone
[Source 124 ]Vitamin D deficiency treatment
If you have a mild vitamin D deficiency then your doctor may recommend a few simple things such as:
- Increasing your sun exposure
- Increasing calcium in your diet
- Increasing physical activity
- Taking a vitamin D supplement
If you have a moderate to severe vitamin D deficiency then you might need to take a high-dose vitamin D supplement and repeat the blood test in 3 months’ time. The amount of vitamin D required to treat the deficiency depends largely on the degree of the deficiency and underlying risk factors. Your doctor will discuss this course of treatment with you.
Some children and teenagers may need to be tested every year if they are identified as having a high risk of vitamin D deficiency.
Management of Vitamin D deficiency
Several preparations of vitamin D are available. Vitamin D3 (cholecalciferol), when compared with vitamin D2 (ergocalciferol), has been shown to be more efficacious in achieving optimal 25-hydroxyvitamin D levels, thus favoring vitamin D3 as a treatment of choice 264.
- Initial vitamin D supplementation for 8 weeks with Vitamin D3 either 6,000 IU daily or 50,000 IU weekly can be considered 75. Once the serum 25-hydroxyvitamin D level exceeds 30 ng/mL, a daily maintenance dose of 1,000 to 2,000 IU is recommended.
- A higher-dose initial supplementation with vitamin D3 at 10,000 IU daily may be needed in high-risk adults who are vitamin D deficient (African Americans, Hispanics, obese, taking certain medications, malabsorption syndrome). Once serum 25-hydroxyvitamin D level exceeds 30ng/mL, 3000 to 6000 IU/day maintenance dose is recommended.
- Children who are vitamin D deficient require 2000 IU/day of vitamin D3 or 50,000 IU of vitamin D3 once weekly for 6 weeks. Once the serum 25(OH)D level exceeds 30 ng/mL, 1000 IU/day maintenance treatment is recommended. According to the American Academy of Pediatrics, infants who are breastfed and children who consume less than 1 L of vitamin D-fortified milk need 400 IU of vitamin D supplementation.
- Calcitriol or 1,25-dihydroxyvitamin D [1,25(OH)2D] can be considered where the deficiency persists despite treatment with vitamin D2 and/or vitamin D3. The serum calcium level shall be closely monitored in these individuals due to an increased risk of hypercalcemia secondary to calcitriol.
- Calcidiol or 25-hydroxyvitamin D [25(OH)D] can be considered in patients with fat malabsorption or severe liver disease.
All patients should maintain a daily calcium intake of 1,300 mg (particularly older people in institutions), since the combination of calcium and vitamin D prevents fractures.
The response to treatment with vitamin D will depend on the degree and severity of vitamin D deficiency and the secondary changes in mineral and bone metabolism. In severe vitamin D deficiency with osteomalacia, a dramatic response in the signs, symptoms and laboratory parameters will be observed. Bone pain and muscle weakness will improve quickly, pseudofractures will show signs of healing on x-ray, and serum calcium, parathyroid hormone (PTH) and biochemical markers of bone turnover will return towards the normal range. In moderate or mild vitamin D deficiency or insufficiency, the response to treatment is more subtle. Muscle weakness and bone pain may improve, serum 25(OH)D levels will increase towards the normal, serum PTH (parathyroid hormone) and biochemical markers of bone turnover will return towards normal (this will be a function of the severity of the initial vitamin D deficiency). In the long run, bone mineral density may increase somewhat and the incidence of fractures may decrease. These results are based on responses of groups of patients in clinical trials and not individuals 265.
Breast-Fed Infants and Children
Human milk contains little vitamin D (approximately 20 IU per liter), and women who are vitamin D–deficient provide even less to their breast-fed infants 70, 266. Lactating women given 4000 IU of vitamin D3 per day not only had an increase in the level of 25-hydroxyvitamin D to more than 30 ng per milliliter but were also able to transfer enough vitamin D3 into their milk to satisfy an infant’s requirement 266.
In Canada, to prevent vitamin D deficiency, current guidelines recommend that all infants and children receive 400 IU of vitamin D3 per day 104.
People with Chronic Kidney Disease
In patients with any stage of chronic kidney disease, 25-hydroxyvitamin D should be measured annually, and the level should be maintained at 30 ng per milliliter or higher, as recommended in the Kidney Disease Outcomes Quality Initiative guidelines from the National Kidney Foundation 267, 268, 269, 270. It is a misconception to assume that patients taking an active vitamin D analogue have sufficient vitamin D; many do not. Levels of 25-hydroxyvitamin D are inversely associated with parathyroid hormone levels, regardless of the degree of chronic renal failure 23, 267, 269, 270, 271, 272. Parathyroid glands convert 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D, which directly inhibits parathyroid hormone expression 267, 269, 270, 271, 272, 273. Patients with stage 4 or 5 chronic kidney disease and an estimated glomerular filtration rate of less than 30 ml per minute per 1.73 m² of body-surface area, as well as those requiring dialysis, are unable to make enough 1,25-dihydroxyvitamin D and need to take 1,25-dihydroxyvitamin D3 or one of its less calcemic analogues to maintain calcium metabolism and to decrease parathyroid hormone levels and the risk of renal bone disease 267, 268, 269, 270.
Patients with intestinal malabsorption and medication
Patients with mild or moderate hepatic failure or intestinal fat-malabsorption syndromes, as well as patients who are taking anticonvulsant medications, glucocorticoids, or other drugs that activate steroid and xenobiotic receptor, require higher doses of vitamin D 11, 274. Exposure to sunlight or ultraviolet B radiation from a tanning bed or other ultraviolet B–emitting device is also effective 11, 275, 276.
References- Siddiqee MH, Bhattacharjee B, Siddiqi UR, Rahman MM. High burden of hypovitaminosis D among the children and adolescents in South Asia: a systematic review and meta-analysis. J Health Popul Nutr. 2022 Mar 17;41(1):10. doi: 10.1186/s41043-022-00287-w
- Fiscaletti M, Stewart P, Munns CF. The importance of vitamin D in maternal and child health: a global perspective. Public Health Rev. 2017;38(1):1–7. doi: 10.1186/s40985-017-0066-3
- Nair R, Maseeh A. Vitamin D: the sunshine vitamin. J Pharmacol Pharmacother. 2012;3(2):118–126. doi: 10.4103/0976-500X.95506
- Huh SY, Gordon CM. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord. 2008;9(2):161–170. doi: 10.1007/s11154-007-9072-y
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266-81. doi: 10.1056/NEJMra070553
- Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003 Dec;78(12):1463-70. doi: 10.4065/78.12.1463
- Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006 Sep;92(1):4-8. doi: 10.1016/j.pbiomolbio.2006.02.016
- Wimalawansa SJ. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol. 2018 Jan;175:60-81. doi: 10.1016/j.jsbmb.2016.09.016
- Gloth FM 3rd, Lindsay JM, Zelesnick LB, Greenough WB 3rd. Can vitamin D deficiency produce an unusual pain syndrome? Arch Intern Med. 1991 Aug;151(8):1662-4.
- Malabanan AO, Turner AK, Holick MF. Severe generalized bone pain and osteoporosis in a premenopausal black female: effect of vitamin D replacement. J Clin Densitometr 1998;1:201-204
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006 Mar;81(3):353-73. doi: 10.4065/81.3.353
- Bringhurst FR, Demay MB, Kronenberg HM. Mineral Metabolism. In: Larson PR, Kronenberg HM, Melmed S, Polonsky KS, eds. Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders Book Company; 2003:1317-1320.
- Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010 Sep;21(5):658-68. doi: 10.1097/EDE.0b013e3181e89905
- Boonen S, Bischoff-Ferrari HA, Cooper C, Lips P, Ljunggren O, Meunier PJ, Reginster JY. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcif Tissue Int. 2006 May;78(5):257-70. doi: 10.1007/s00223-005-0009-8
- Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004 Mar;19(3):370-8. doi: 10.1359/JBMR.0301240
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007 Sep 10;167(16):1730-7. doi: 10.1001/archinte.167.16.1730
- Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012 Jun;33(3):456-92. doi: 10.1210/er.2012-1000
- Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ, Kienreich K, Soni M. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013 Aug;12(10):976-89. doi: 10.1016/j.autrev.2013.02.004
- Garland CF, Mohr SB, Gorham ED, Grant WB, Garland FC. Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am J Prev Med. 2006 Dec;31(6):512-4. doi: 10.1016/j.amepre.2006.08.018
- Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011 Oct;94(4):1144-9. doi: 10.3945/ajcn.111.015032
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007 Jun;85(6):1586-91. doi: 10.1093/ajcn/85.6.1586. Erratum in: Am J Clin Nutr. 2008 Mar;87(3):794.
- Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, Camargo CA, Griffiths CJ, Janssens W, Martineau AR. Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax. 2019 Apr;74(4):337-345. doi: 10.1136/thoraxjnl-2018-212092
- Holick MF, Garabedian M. Vitamin D: photobiology, metabolism, mechanism of action, and clinical applications. In: Favus MJ, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed. Washington, DC: American Society for Bone and Mineral Research, 2006:129-37.
- Bouillon R. Vitamin D: from photosynthesis, metabolism, and action to clinical applications. In: DeGroot LJ, Jameson JL, eds. Endocrinology. Philadelphia: W.B. Saunders, 2001:1009-28.
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1689S-96S. doi: 10.1093/ajcn/80.6.1689S
- Jones G. Vitamin D. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease, 11th ed. Philadelphia: Lippincott Williams & Wilkins, 2014.
- Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002 Apr;75(4):611-5. doi: 10.1093/ajcn/75.4.611
- Ceglia L, Harris SS. Vitamin D and its role in skeletal muscle. Calcif Tissue Int. 2013 Feb;92(2):151-62. doi: 10.1007/s00223-012-9645-y
- Norman AW, Henry HH. Vitamin D. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition, 10th ed. Washington DC: Wiley-Blackwell, 2012.
- Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003 Feb 1;88(2):296-307. doi: 10.1002/jcb.10338
- Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003 Apr;22(2):142-6. doi: 10.1080/07315724.2003.10719287
- Wharton B, Bishop N. Rickets. Lancet. 2003 Oct 25;362(9393):1389-400. doi: 10.1016/S0140-6736(03)14636-3
- Holick MF. Vitamin D. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease, 10th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Wharton B, Bishop N. Rickets. Lancet 2003;362:1389-400. https://www.ncbi.nlm.nih.gov/pubmed/14585642
- American Academy of Dermatology. Position statement on vitamin D. November 1, 2008. https://www.aad.org/Forms/Policies/Uploads/PS/PS-Vitamin%20D.pdf
- Uday S, Högler W. Erratum to: Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr Osteoporos Rep. 2017;15(5):507. doi:10.1007/s11914-017-0395-7
- Goldring SR, Krane S, Avioli LV. Disorders of calcification: osteomalacia and rickets. In: DeGroot LJ, Besser M, Burger HG, Jameson JL, Loriaux DL, Marshall JC, et al., eds. Endocrinology. 3rd ed. Philadelphia: WB Saunders, 1995:1204-27.
- Chesney RW. Rickets: an old form for a new century. Pediatr Int. 2003 Oct;45(5):509-11. doi: 10.1046/j.1442-200x.2003.01783.x
- Uday S, Högler W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr Osteoporos Rep. 2017 Aug;15(4):293-302. doi: 10.1007/s11914-017-0383-y. Erratum in: Curr Osteoporos Rep. 2017 Aug 14
- Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016 Feb;101(2):394-415. doi: 10.1210/jc.2015-2175
- Creo AL, Thacher TD, Pettifor JM, Strand MA, Fischer PR. Nutritional rickets around the world: an update. Paediatr Int Child Health. 2017 May;37(2):84-98. doi: 10.1080/20469047.2016.1248170
- Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008 Nov;122(5):1142-52. doi: 10.1542/peds.2008-1862. Erratum in: Pediatrics. 2009 Jan;123(1):197.
- Lips P. Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol. 2010 Jul;121(1-2):297-300. doi: 10.1016/j.jsbmb.2010.02.021
- Brunette MG, Chan M, Ferriere C, Roberts KD. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978 Nov 16;276(5685):287-9. doi: 10.1038/276287a0
- Vitamin D Tests. https://www.testing.com/tests/vitamin-d-tests
- Gani, L. U., & How, C. H. (2015). PILL Series. Vitamin D deficiency. Singapore medical journal, 56(8), 433–437. https://doi.org/10.11622/smedj.2015119
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006 Jul;84(1):18-28. doi: 10.1093/ajcn/84.1.18. Erratum in: Am J Clin Nutr. 2006 Nov;84(5):1253. Dosage error in published abstract; MEDLINE/PubMed abstract corrected. Erratum in: Am J Clin Nutr. 2007 Sep;86(3):809.
- Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998 Mar 14;351(9105):805-6. doi: 10.1016/s0140-6736(05)78933-9
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998 Mar 19;338(12):777-83. doi: 10.1056/NEJM199803193381201
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439-43. doi: 10.1007/s001980050030
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005 Jun;90(6):3215-24. doi: 10.1210/jc.2004-2364
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005 Jul;16(7):713-6. doi: 10.1007/s00198-005-1867-7
- Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, Charles P, Eriksen EF. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000 Feb;247(2):260-8. doi: 10.1046/j.1365-2796.2000.00595.x
- Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001 Aug;22(4):477-501. doi: 10.1210/edrv.22.4.0437
- Bakhtiyarova S, Lesnyak O, Kyznesova N, Blankenstein MA, Lips P. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos Int. 2006;17(3):441-6. doi: 10.1007/s00198-005-0006-9
- McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992 Jul;93(1):69-77. doi: 10.1016/0002-9343(92)90682-2
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med. 1992 Dec 3;327(23):1637-42. doi: 10.1056/NEJM199212033272305
- Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006 Sep;260(3):245-54. doi: 10.1111/j.1365-2796.2006.01685.x. Erratum in: J Intern Med. 2007 Apr;261(4):408.
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004 Jun;158(6):531-7. doi: 10.1001/archpedi.158.6.531
- Sullivan SS, Rosen CJ, Halteman WA, Chen TC, Holick MF. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005 Jun;105(6):971-4. doi: 10.1016/j.jada.2005.03.002
- Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002 Jul;76(1):187-92. doi: 10.1093/ajcn/76.1.187
- Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002 Jun 1;112(8):659-62. doi: 10.1016/s0002-9343(02)01091-4
- Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006 Aug;116(8):2062-72. doi: 10.1172/JCI29449
- Vieth R. Why the optimal requirement for Vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004 May;89-90(1-5):575-9. doi: 10.1016/j.jsbmb.2004.03.038
- Pettifor JM. Vitamin D deficiency and nutritional rickets in children in vitamin D. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. 2nd ed. Boston: Elsevier Academic Press, 2005:1065-84.
- Sedrani SH. Low 25-hydroxyvitamin D and normal serum calcium concentrations in Saudi Arabia: Riyadh region. Ann Nutr Metab. 1984;28(3):181-5. doi: 10.1159/000176801
- Marwaha RK, Tandon N, Reddy DR, Aggarwal R, Singh R, Sawhney RC, Saluja B, Ganie MA, Singh S. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005 Aug;82(2):477-82. doi: 10.1093/ajcn.82.2.477
- El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, Tannous R. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001 Apr;107(4):E53. doi: 10.1542/peds.107.4.e53
- McGrath JJ, Kimlin MG, Saha S, Eyles DW, Parisi AV. Vitamin D insufficiency in south-east Queensland. Med J Aust. 2001 Feb 5;174(3):150-1. doi: 10.5694/j.1326-5377.2001.tb143195.x
- Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004 May;79(5):717-26. doi: 10.1093/ajcn/79.5.717
- Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila). 2007 Jan;46(1):42-4. doi: 10.1177/0009922806289311
- Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007 Feb;137(2):447-52. doi: 10.1093/jn/137.2.447
- Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S, Chope G, Hyppönen E, Berry J, Vieth R, Lanham-New S. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr. 2012 Jun;95(6):1357-64. doi: 10.3945/ajcn.111.03107
- Sizar O, Khare S, Goyal A, et al. Vitamin D Deficiency. [Updated 2023 Feb 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532266
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911-30. doi: 10.1210/jc.2011-0385. Epub 2011 Jun 6. Erratum in: J Clin Endocrinol Metab. 2011 Dec;96(12):3908.
- Charoenngam, Nipith & Holick, Michael. (2020). Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 12. 2097. https://www.researchgate.net/publication/342970598_Immunologic_Effects_of_Vitamin_D_on_Human_Health_and_Disease
- Caruso TJ, Fuzaylov G. Images in clinical medicine. Severe vitamin D deficiency–rickets. N Engl J Med. 2013 Aug 29;369(9):e11. doi: 10.1056/NEJMicm1205540
- Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011 Jun 8;18(3):R53-77. doi: 10.1530/ERC-11-0006
- Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: a systematic review. Nutr Rev. 2018 Jan 1;76(1):60-76. doi: 10.1093/nutrit/nux034
- Tripkovic L, Wilson LR, Hart K, Johnsen S, de Lusignan S, Smith CP, Bucca G, Penson S, Chope G, Elliott R, Hyppönen E, Berry JL, Lanham-New SA. Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: a 12-wk randomized, placebo-controlled food-fortification trial. Am J Clin Nutr. 2017 Aug;106(2):481-490. doi: 10.3945/ajcn.116.138693
- Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep). 2007 Aug;(158):1-235.
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010
- National Institute of Health. Vitamin D. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019,10, 1082–1093.
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014,144, 22–27.
- Haussler MR, Haussler CA, Jurutka PW, Thompson PD, Hsieh JC, Remus LS, Selznick SH, Whitfield GK. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol. 1997 Sep;154 Suppl:S57-73.
- Taylor CL, Rosen CJ, Dwyer JT. Considerations in Dietetic Counseling for Vitamin D. J Acad Nutr Diet. 2019 Jun;119(6):901-909. doi: 10.1016/j.jand.2018.04.013
- Agency for Healthcare Research and Quality. Screening for vitamin D deficiency: Systematic review for the U.S. Preventive Services Task Force recommendation. Evidence Synthesis Number 118. AHRQ-Pub No. 13-05183-EF-1. June 2014.
- LeFevre ML; U.S. Preventive Services Task Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015 Jan 20;162(2):133-40. doi: 10.7326/M14-2450
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008 Aug;88(2):582S-586S. doi: 10.1093/ajcn/88.2.582S
- Brown LL, Cohen B, Tabor D, Zappalà G, Maruvada P, Coates PM. The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health – expert panel meeting report. BMC Proc. 2018 May 9;12(Suppl 6):6. doi: 10.1186/s12919-018-0102-4
- Carter GD. 25-hydroxyvitamin D assays: the quest for accuracy. Clin Chem 2009;55:1300-02.
- Hollis BW. Editorial: the determination of circulating 25-hydroxyvitamin D: no easy task. J. Clin Endocrinol Metab 2004;89:3149-3151.
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004 Jul;89(7):3152-7. doi: 10.1210/jc.2003-031979
- National Institute of Standards and Technology. NIST releases vitamin D standard reference material, 2009. https://www.nist.gov/news-events/news/2009/07/nist-releases-vitamin-d-standard-reference-material
- Vitamin D. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional
- U.S. Department of Agriculture, Agricultural Research Service. 2011. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/
- Ovesen L, Brot C, Jakobsen J. Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab. 2003;47(3-4):107-13. doi: 10.1159/000070031
- Roseland JM, Phillips KM, Patterson KY, Pehrsson PR, Taylor CL. Vitamin D in foods: An evolution of knowledge. Pages 41-78 in Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D, Volume 2: Health, Disease and Therapeutics, Fourth Edition. Elsevier, 2018.
- U.S. Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; vitamin D2 mushroom powder. Federal Register 2020;85:41916-20.
- Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55(9):1193-205. doi: 10.1080/10408398.2012.688897
- Taylor, C. L., Patterson, K. Y., Roseland, J. M., Wise, S. A., Merkel, J. M., Pehrsson, P. R., & Yetley, E. A. (2014). Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. The Journal of nutrition, 144(5), 654–659. https://doi.org/10.3945/jn.113.189811
- Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1710S-6S. doi: 10.1093/ajcn/80.6.1710S
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008 Aug;88(2):558S-564S. doi: 10.1093/ajcn/88.2.558S
- Vitamin D for Milk and Milk Alternatives. https://www.fda.gov/food/food-additives-petitions/vitamin-d-milk-and-milk-alternatives
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov/index.html
- Galior K, Grebe S, Singh R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients. 2018 Jul 24;10(8):953. doi: 10.3390/nu10080953
- Auguste BL, Avila-Casado C, Bargman JM. Use of vitamin D drops leading to kidney failure in a 54-year-old man. CMAJ. 2019 Apr 8;191(14):E390-E394. doi: 10.1503/cmaj.180465
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669-83. DOI: 10.1056/NEJMoa055218
- Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008;88:582S-6S. https://www.ncbi.nlm.nih.gov/pubmed/18689406
- Malihi Z, Lawes CMM, Wu Z, Huang Y, Waayer D, Toop L, Khaw KT, Camargo CA, Scragg R. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: results from a randomized controlled trial. Am J Clin Nutr. 2019 Jun 1;109(6):1578-1587. doi: 10.1093/ajcn/nqy378
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. https://www.ncbi.nlm.nih.gov/pubmed/17634462?dopt=Abstract
- Laurent MR, Gielen E, Pauwels S, Vanderschueren D, Bouillon R. Hypervitaminosis D Associated With Tanning Bed Use: A Case Report. Ann Intern Med. 2017 Jan 17;166(2):155-156. doi: 10.7326/L16-0138
- Mayo Foundation for Medical Education and Research. Vitamin D Safety. http://www.mayoclinic.org/drugs-supplements/vitamin-d/safety/hrb-20060400
- Charoenngam N, Shirvani A, Holick MF. The ongoing D-lemma of vitamin D supplementation for nonskeletal health and bone health. Curr Opin Endocrinol Diabetes Obes. 2019 Dec;26(6):301-305. doi: 10.1097/MED.0000000000000508
- Holick, M.F. The Death D-fying Vitamin. Mayo Clin. Proc. 2018,93, 679–681.
- Dudenkov, D.V.; Mara, K.C.; Petterson, T.M.; Maxson, J.A.; Thacher, T.D. Serum 25-Hydroxyvitamin D Values and Risk of All-Cause and Cause-Specific Mortality: A Population-Based Cohort Study. Mayo Clin. Proc. 2018,93, 721–730.
- Hossein-nezhad, A., & Holick, M. F. (2013). Vitamin D for health: a global perspective. Mayo Clinic proceedings, 88(7), 720–755. https://doi.org/10.1016/j.mayocp.2013.05.011
- Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006 Feb;54(2):301-17. doi: 10.1016/j.jaad.2005.11.1057
- Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 2002;9:87-98.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007 Mar 1;120(5):1116-22. doi: 10.1002/ijc.22453. Erratum in: Int J Cancer. 2007 Jun 1;120(11):2526
- Liberman UA. Disorders in Vitamin D Action. [Updated 2014 Jan 1]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279150
- Cranney C, Horsely T, O’Donnell S, Weiler H, Ooi D, Atkinson S, et al. Effectiveness and safety of vitamin D. Evidence Report/Technology Assessment No. 158 prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-02.0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality, 2007. https://www.ncbi.nlm.nih.gov/pubmed/18088161
- Graeff-Armas LA, Bendik I, Kunz I, Schoop R, Hull S, Beck M. Supplemental 25-Hydroxycholecalciferol Is More Effective than Cholecalciferol in Raising Serum 25-Hydroxyvitamin D Concentrations in Older Adults. J Nutr. 2020 Jan 1;150(1):73-81. doi: 10.1093/jn/nxz209
- Sahay M, Sahay R. Rickets–vitamin D deficiency and dependency. Indian Journal of Endocrinology and Metabolism. 2012;16(2):164-176. doi:10.4103/2230-8210.93732. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3313732/
- Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142-1152. https://www.ncbi.nlm.nih.gov/pubmed/18977996?dopt=Abstract
- Jin J. Vitamin D and Calcium Supplements for Preventing Fractures. JAMA. 2018 Apr 17;319(15):1630. doi: 10.1001/jama.2018.3892
- Osteoporosis Overview. https://www.bones.nih.gov/health-info/bone/osteoporosis/overview
- Medicare cost of osteoporotic fractures. Milliman research report, August 2019. https://static1.squarespace.com/static/5c0860aff793924efe2230f3/t/5d76b949deb7e9086ee3d7dd/1568061771769/Medicare+Cost+of+Osteoporotic+Fractures+20190827.pdf
- Brown, L. L., Cohen, B., Tabor, D., Zappalà, G., Maruvada, P., & Coates, P. M. (2018). The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health – expert panel meeting report. BMC proceedings, 12(Suppl 6), 6. https://doi.org/10.1186/s12919-018-0102-4
- Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Vitamin D and calcium: A systematic review of health outcomes. Evidence Report/Technology Assessment No. 183 prepared by the Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I. AHRQ Publication No. 09-E015. Rockville, MD: Agency for Healthcare Research and Quality, 2009.
- US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Kemper AR, Krist AH, Kubik M, Landefeld S, Mangione CM, Silverstein M, Simon MA, Tseng CW. Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018 Apr 17;319(15):1592-1599. doi: 10.1001/jama.2018.3185
- Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to Prevent Falls in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018 Apr 24;319(16):1705-1716. doi: 10.1001/jama.2017.21962
- Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018 Nov;6(11):847-858. doi: 10.1016/S2213-8587(18)30265-1
- Yao P, Bennett D, Mafham M, Lin X, Chen Z, Armitage J, Clarke R. Vitamin D and calcium for the prevention of fracture: A systematic review and meta-analysis. JAMA Network Open 2019;2(12):e1917789. doi: 10.1001/jamanetworkopen.2019.17789
- Aloia JF, Rubinova R, Fazzari M, Islam S, Mikhail M, Ragolia L. Vitamin D and Falls in Older African American Women: The PODA Randomized Clinical Trial. J Am Geriatr Soc. 2019 May;67(5):1043-1049. doi: 10.1111/jgs.15760. Epub 2019 Jan 30. Erratum in: J Am Geriatr Soc. 2019 Jul;67(7):1538.
- Vaes AMM, Tieland M, Toussaint N, Nilwik R, Verdijk LB, van Loon LJC, de Groot LCPGM. Cholecalciferol or 25-Hydroxycholecalciferol Supplementation Does Not Affect Muscle Strength and Physical Performance in Prefrail and Frail Older Adults. J Nutr. 2018 May 1;148(5):712-720. doi: 10.1093/jn/nxy024
- Shea MK, Fielding RA, Dawson-Hughes B. The effect of vitamin D supplementation on lower-extremity power and function in older adults: a randomized controlled trial. Am J Clin Nutr. 2019 Feb 1;109(2):369-379. doi: 10.1093/ajcn/nqy290
- Littlejohns, T. J., Henley, W. E., Lang, I. A., Annweiler, C., Beauchet, O., Chaves, P. H., Fried, L., Kestenbaum, B. R., Kuller, L. H., Langa, K. M., Lopez, O. L., Kos, K., Soni, M., & Llewellyn, D. J. (2014). Vitamin D and the risk of dementia and Alzheimer disease. Neurology, 83(10), 920–928. https://doi.org/10.1212/WNL.0000000000000755
- Annweiler C, Fantino B, Gautier J, Beaudenon M, Thiery S, Beauchet O. Cognitive effects of vitamin D supplementation in older outpatients visiting a memory clinic: a pre-post study. J Am Geriatr Soc. 2012 Apr;60(4):793-5. doi: 10.1111/j.1532-5415.2011.03877.x
- An Evaluation of the Effect of Vitamin D Supplementation on the Cognitive Function of Older Subjects With Low Levels of This Vitamin. https://clinicaltrials.gov/ct2/show/study/NCT02381600
- Effect of Vitamin D on Cognitive Decline of Patients With Memory Complaint (D-COG). https://clinicaltrials.gov/ct2/show/study/NCT02185222
- Lifestyle Intervention Program for Cognitive Impairment. https://clinicaltrials.gov/ct2/show/study/NCT02741804
- Can Vitamin D3 Improve Cognitive Function in Individuals With Type 2 Diabetes? (THINK-D) (THINK-D). https://clinicaltrials.gov/ct2/show/study/NCT02416193
- DO-HEALTH / Vitamin D3 – Omega3 – Home Exercise – Healthy Ageing and Longevity Trial (DO-HEALTH). https://clinicaltrials.gov/ct2/show/NCT01745263
- Vitamin D and Omega-3 Trial (VITAL) (VITAL). https://clinicaltrials.gov/ct2/show/NCT01169259
- A Large Randomized Trial of Vitamin D, Omega-3 Fatty Acids and Cognitive Decline (VITAL-Cog). https://clinicaltrials.gov/ct2/show/NCT01669915
- Annweiler C, Herrmann FR, Fantino B, Brugg B, Beauchet O. Effectiveness of the combination of memantine plus vitamin D on cognition in patients with Alzheimer disease: a pre-post pilot study. Cogn Behav Neurol. 2012 Sep;25(3):121-7. doi: 10.1097/WNN.0b013e31826df647
- Annweiler, C., Fantino, B., Parot-Schinkel, E., Thiery, S., Gautier, J., & Beauchet, O. (2011). Alzheimer’s disease–input of vitamin D with mEmantine assay (AD-IDEA trial): study protocol for a randomized controlled trial. Trials, 12, 230. https://doi.org/10.1186/1745-6215-12-230
- Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D, Urashima M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013 May;97(5):1004-13. doi: 10.3945/ajcn.112.051664
- Jagannath VA, Filippini G, Di Pietrantonj C, Asokan GV, Robak EW, Whamond L, Robinson SA. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD008422. doi: 10.1002/14651858.CD008422.pub3
- Sintzel MB, Rametta M, Reder AT. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol Ther. 2018 Jun;7(1):59-85. doi: 10.1007/s40120-017-0086-4
- Munger KL, Hongell K, Åivo J, Soilu-Hänninen M, Surcel HM, Ascherio A. 25-Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology. 2017 Oct 10;89(15):1578-1583. doi: 10.1212/WNL.0000000000004489
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006 Dec 20;296(23):2832-8. doi: 10.1001/jama.296.23.2832
- Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012 Nov 20;79(21):2140-5. doi: 10.1212/WNL.0b013e3182752ea8
- Marrie RA, Beck CA. Preventing multiple sclerosis: To (take) vitamin D or not to (take) vitamin D? Neurology. 2017 Oct 10;89(15):1538-1539. doi: 10.1212/WNL.0000000000004506
- Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients. 2018 Mar 19;10(3):375. doi: 10.3390/nu10030375
- Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O’Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M; D2d Research Group. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med. 2019 Aug 8;381(6):520-530. doi: 10.1056/NEJMoa1900906
- Rafiq S, Jeppesen PB. Is Hypovitaminosis D Related to Incidence of Type 2 Diabetes and High Fasting Glucose Level in Healthy Subjects: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2018 Jan 10;10(1):59. doi: 10.3390/nu10010059
- Mousa A, Naderpoor N, de Courten MP, Teede H, Kellow N, Walker K, Scragg R, de Courten B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: a randomized placebo-controlled trial. Am J Clin Nutr. 2017 Jun;105(6):1372-1381. doi: 10.3945/ajcn.117.152736
- Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014 Oct;99(10):3551-60. doi: 10.1210/jc.2014-2136. Epub 2014 Jul 25. Erratum in: J Clin Endocrinol Metab. 2015 Aug;100(8):3219.
- Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y, Hutchinson MY. Vitamin D 20,000 IU per Week for Five Years Does Not Prevent Progression From Prediabetes to Diabetes. J Clin Endocrinol Metab. 2016 Apr;101(4):1647-55. doi: 10.1210/jc.2015-4013
- Pittas A, Dawson-Hughes B, Staten M. Vitamin D Supplementation and Prevention of Type 2 Diabetes. Reply. N Engl J Med. 2019 Oct 31;381(18):1785-1786. doi: 10.1056/NEJMc1912185
- Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008 May;67(2):115-27. doi: 10.1017/S0029665108006964
- Yin L, Ordóñez-Mena JM, Chen T, Schöttker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: a systematic review and meta-analysis. Prev Med. 2013 Dec;57(6):753-64. doi: 10.1016/j.ypmed.2013.08.026
- Han, J., Guo, X., Yu, X., Liu, S., Cui, X., Zhang, B., & Liang, H. (2019). 25-Hydroxyvitamin D and Total Cancer Incidence and Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutrients, 11(10), 2295. https://doi.org/10.3390/nu11102295
- Keum, N., Lee, D. H., Greenwood, D. C., Manson, J. E., & Giovannucci, E. (2019). Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Annals of oncology : official journal of the European Society for Medical Oncology, 30(5), 733–743. https://doi.org/10.1093/annonc/mdz059
- Manson, J. E., Cook, N. R., Lee, I. M., Christen, W., Bassuk, S. S., Mora, S., Gibson, H., Gordon, D., Copeland, T., D’Agostino, D., Friedenberg, G., Ridge, C., Bubes, V., Giovannucci, E. L., Willett, W. C., Buring, J. E., & VITAL Research Group (2019). Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. The New England journal of medicine, 380(1), 33–44. https://doi.org/10.1056/NEJMoa1809944
- Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011 Oct 1;29(28):3775-82. doi: 10.1200/JCO.2011.35.7566
- Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011 Mar 15;128(6):1414-24. doi: 10.1002/ijc.25439
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006 Feb 16;354(7):684-96. doi: 10.1056/NEJMoa055222. Erratum in: N Engl J Med. 2006 Mar 9;354(10):1102.
- Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011 Dec 20;155(12):827-38. doi: 10.7326/0003-4819-155-12-201112200-00005. Erratum in: Ann Intern Med. 2014 Oct 21;161(8):615-6.
- IARC Working Group on Vitamin D. Vitamin D and cancer: A report of the IARC Working Group on Vitamin D. IARC Working Group Reports. Lyon, France: International Agency for Research on Cancer, 2008.
- Yetley, E. A., Brulé, D., Cheney, M. C., Davis, C. D., Esslinger, K. A., Fischer, P. W., Friedl, K. E., Greene-Finestone, L. S., Guenther, P. M., Klurfeld, D. M., L’Abbe, M. R., McMurry, K. Y., Starke-Reed, P. E., & Trumbo, P. R. (2009). Dietary reference intakes for vitamin D: justification for a review of the 1997 values. The American journal of clinical nutrition, 89(3), 719–727. https://doi.org/10.3945/ajcn.2008.26903
- Moyer VA; U.S. Preventive Services Task Force. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive services Task Force recommendation statement. Ann Intern Med. 2014 Apr 15;160(8):558-64. doi: 10.7326/M14-0198
- McNamara M, Rosenberger KD. The Significance of Vitamin D Status in Breast Cancer: A State of the Science Review. J Midwifery Womens Health. 2019 May;64(3):276-288. doi: 10.1111/jmwh.12968
- Chlebowski, R. T., Johnson, K. C., Kooperberg, C., Pettinger, M., Wactawski-Wende, J., Rohan, T., Rossouw, J., Lane, D., O’Sullivan, M. J., Yasmeen, S., Hiatt, R. A., Shikany, J. M., Vitolins, M., Khandekar, J., Hubbell, F. A., & Women’s Health Initiative Investigators (2008). Calcium plus vitamin D supplementation and the risk of breast cancer. Journal of the National Cancer Institute, 100(22), 1581–1591. https://doi.org/10.1093/jnci/djn360
- Cauley, J. A., Chlebowski, R. T., Wactawski-Wende, J., Robbins, J. A., Rodabough, R. J., Chen, Z., Johnson, K. C., O’Sullivan, M. J., Jackson, R. D., & Manson, J. E. (2013). Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. Journal of women’s health (2002), 22(11), 915–929. https://doi.org/10.1089/jwh.2013.4270
- McCullough, M. L., Zoltick, E. S., Weinstein, S. J., Fedirko, V., Wang, M., Cook, N. R., Eliassen, A. H., Zeleniuch-Jacquotte, A., Agnoli, C., Albanes, D., Barnett, M. J., Buring, J. E., Campbell, P. T., Clendenen, T. V., Freedman, N. D., Gapstur, S. M., Giovannucci, E. L., Goodman, G. G., Haiman, C. A., Ho, G., … Smith-Warner, S. A. (2019). Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. Journal of the National Cancer Institute, 111(2), 158–169. https://doi.org/10.1093/jnci/djy087
- Song, M., Lee, I. M., Manson, J. E., Buring, J. E., Dushkes, R., Gordon, D., Walter, J., Wu, K., Chan, A. T., Ogino, S., Fuchs, C. S., Meyerhardt, J. A., & Giovannucci, E. L. (2021). No Association Between Vitamin D Supplementation and Risk of Colorectal Adenomas or Serrated Polyps in a Randomized Trial. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 19(1), 128–135.e6. https://doi.org/10.1016/j.cgh.2020.02.013
- Muller, D. C., Hodge, A. M., Fanidi, A., Albanes, D., Mai, X. M., Shu, X. O., Weinstein, S. J., Larose, T. L., Zhang, X., Han, J., Stampfer, M. J., Smith-Warner, S. A., Ma, J., Gaziano, J. M., Sesso, H. D., Stevens, V. L., McCullough, M. L., Layne, T. M., Prentice, R., Pettinger, M., … Brennan, P. (2018). No association between circulating concentrations of vitamin D and risk of lung cancer: an analysis in 20 prospective studies in the Lung Cancer Cohort Consortium (LC3). Annals of oncology : official journal of the European Society for Medical Oncology, 29(6), 1468–1475. https://doi.org/10.1093/annonc/mdy104
- van Duijnhoven, F., Jenab, M., Hveem, K., Siersema, P. D., Fedirko, V., Duell, E. J., Kampman, E., Halfweeg, A., van Kranen, H. J., van den Ouweland, J., Weiderpass, E., Murphy, N., Langhammer, A., Ness-Jensen, E., Olsen, A., Tjønneland, A., Overvad, K., Cadeau, C., Kvaskoff, M., Boutron-Ruault, M. C., … Bueno-de-Mesquita, H. (2018). Circulating concentrations of vitamin D in relation to pancreatic cancer risk in European populations. International journal of cancer, 142(6), 1189–1201. https://doi.org/10.1002/ijc.31146
- Stolzenberg-Solomon RZ, Vieth R, Azad A, Pietinen P, Taylor PR, Virtamo J, Albanes D. A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res. 2006 Oct 15;66(20):10213-9. doi: 10.1158/0008-5472.CAN-06-1876
- Helzlsouer, K. J., & VDPP Steering Committee (2010). Overview of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. American journal of epidemiology, 172(1), 4–9. https://doi.org/10.1093/aje/kwq119
- Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, Lin Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. J Cancer Res Clin Oncol. 2014 Sep;140(9):1465-77. doi: 10.1007/s00432-014-1706-3
- Kristal, A. R., Till, C., Song, X., Tangen, C. M., Goodman, P. J., Neuhauser, M. L., Schenk, J. M., Thompson, I. M., Meyskens, F. L., Jr, Goodman, G. E., Minasian, L. M., Parnes, H. L., & Klein, E. A. (2014). Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 23(8), 1494–1504. https://doi.org/10.1158/1055-9965.EPI-14-0115
- Schenk, J. M., Till, C. A., Tangen, C. M., Goodman, P. J., Song, X., Torkko, K. C., Kristal, A. R., Peters, U., & Neuhouser, M. L. (2014). Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 23(8), 1484–1493. https://doi.org/10.1158/1055-9965.EPI-13-1340
- Heath AK, Hodge AM, Ebeling PR, Eyles DW, Kvaskoff D, Buchanan DD, Giles GG, Williamson EJ, English DR. Circulating 25-Hydroxyvitamin D Concentration and Risk of Breast, Prostate, and Colorectal Cancers: The Melbourne Collaborative Cohort Study. Cancer Epidemiol Biomarkers Prev. 2019 May;28(5):900-908. doi: 10.1158/1055-9965.EPI-18-1155
- Jiang, X., Dimou, N. L., Al-Dabhani, K., Lewis, S. J., Martin, R. M., Haycock, P. C., Gunter, M. J., Key, T. J., Eeles, R. A., Muir, K., Neal, D., Giles, G. G., Giovannucci, E. L., Stampfer, M., Pierce, B. L., Schildkraut, J. M., Warren Andersen, S., Thompson, D., Zheng, W., Kraft, P., … PRACTICAL, CRUK, BPC3, CAPS and PEGASUS consortia (2019). Circulating vitamin D concentrations and risk of breast and prostate cancer: a Mendelian randomization study. International journal of epidemiology, 48(5), 1416–1424. https://doi.org/10.1093/ije/dyy284
- Travis, R. C., Perez-Cornago, A., Appleby, P. N., Albanes, D., Joshu, C. E., Lutsey, P. L., Mondul, A. M., Platz, E. A., Weinstein, S. J., Layne, T. M., Helzlsouer, K. J., Visvanathan, K., Palli, D., Peeters, P. H., Bueno-de-Mesquita, B., Trichopoulou, A., Gunter, M. J., Tsilidis, K. K., Sánchez, M. J., Olsen, A., … Allen, N. E. (2019). A Collaborative Analysis of Individual Participant Data from 19 Prospective Studies Assesses Circulating Vitamin D and Prostate Cancer Risk. Cancer research, 79(1), 274–285. https://doi.org/10.1158/0008-5472.CAN-18-2318
- Nair-Shalliker, V., Bang, A., Egger, S., Clements, M., Gardiner, R. A., Kricker, A., Seibel, M. J., Chambers, S. K., Kimlin, M. G., Armstrong, B. K., & Smith, D. P. (2020). Post-treatment levels of plasma 25- and 1,25-dihydroxy vitamin D and mortality in men with aggressive prostate cancer. Scientific reports, 10(1), 7736. https://doi.org/10.1038/s41598-020-62182-w
- Song, Z. Y., Yao, Q., Zhuo, Z., Ma, Z., & Chen, G. (2018). Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocrine connections, 7(12), R294–R303. https://doi.org/10.1530/EC-18-0283
- Shahvazi S, Soltani S, Ahmadi SM, de Souza RJ, Salehi-Abargouei A. The Effect of Vitamin D Supplementation on Prostate Cancer: A Systematic Review and Meta-Analysis of Clinical Trials. Horm Metab Res. 2019 Jan;51(1):11-21. doi: 10.1055/a-0774-8809
- Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013 Feb;202:100-7. doi: 10.1192/bjp.bp.111.106666
- Gowda U, Mutowo MP, Smith BJ, Wluka AE, Renzaho AM. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015 Mar;31(3):421-9. doi: 10.1016/j.nut.2014.06.017
- Jorde, R., & Kubiak, J. (2018). No improvement in depressive symptoms by vitamin D supplementation: results from a randomised controlled trial. Journal of nutritional science, 7, e30. https://doi.org/10.1017/jns.2018.19
- de Koning, E. J., Lips, P., Penninx, B., Elders, P., Heijboer, A. C., den Heijer, M., Bet, P. M., van Marwijk, H., & van Schoor, N. M. (2019). Vitamin D supplementation for the prevention of depression and poor physical function in older persons: the D-Vitaal study, a randomized clinical trial. The American journal of clinical nutrition, 110(5), 1119–1130. https://doi.org/10.1093/ajcn/nqz141
- Jorde R, Grimnes G. Vitamin D: no cure for depression. Am J Clin Nutr. 2019 Nov 1;110(5):1043-1044. doi: 10.1093/ajcn/nqz186
- Okereke, O. I., Reynolds, C. F., 3rd, Mischoulon, D., Chang, G., Vyas, C. M., Cook, N. R., Weinberg, A., Bubes, V., Copeland, T., Friedenberg, G., Lee, I. M., Buring, J. E., & Manson, J. E. (2020). Effect of Long-term Vitamin D3 Supplementation vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA, 324(5), 471–480. https://doi.org/10.1001/jama.2020.10224
- Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG. Role of vitamin D in atherosclerosis. Circulation. 2013 Dec 3;128(23):2517-31. doi: 10.1161/CIRCULATIONAHA.113.002654
- Al Mheid I, Quyyumi AA. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J Am Coll Cardiol. 2017 Jul 4;70(1):89-100. doi: 10.1016/j.jacc.2017.05.031
- Zhang R, Li B, Gao X, Tian R, Pan Y, Jiang Y, Gu H, Wang Y, Wang Y, Liu G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2017 Apr;105(4):810-819. doi: 10.3945/ajcn.116.140392
- Durup D, Jørgensen HL, Christensen J, Tjønneland A, Olsen A, Halkjær J, Lind B, Heegaard AM, Schwarz P. A Reverse J-Shaped Association Between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J Clin Endocrinol Metab. 2015 Jun;100(6):2339-46. doi: 10.1210/jc.2014-4551
- Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012 Nov;32(11):2794-802. doi: 10.1161/ATVBAHA.112.248039
- Zhou, R., Wang, M., Huang, H., Li, W., Hu, Y., & Wu, T. (2018). Lower Vitamin D Status Is Associated with an Increased Risk of Ischemic Stroke: A Systematic Review and Meta-Analysis. Nutrients, 10(3), 277. https://doi.org/10.3390/nu10030277
- Scragg, R., Stewart, A. W., Waayer, D., Lawes, C., Toop, L., Sluyter, J., Murphy, J., Khaw, K. T., & Camargo, C. A., Jr (2017). Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study : A Randomized Clinical Trial. JAMA cardiology, 2(6), 608–616. https://doi.org/10.1001/jamacardio.2017.0175
- Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M; RECORD Trial Group. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014 Sep;100(3):746-55. doi: 10.3945/ajcn.113.082602
- Dibaba DT. Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr Rev. 2019 Dec 1;77(12):890-902. doi: 10.1093/nutrit/nuz037
- Beveridge, L. A., Struthers, A. D., Khan, F., Jorde, R., Scragg, R., Macdonald, H. M., Alvarez, J. A., Boxer, R. S., Dalbeni, A., Gepner, A. D., Isbel, N. M., Larsen, T., Nagpal, J., Petchey, W. G., Stricker, H., Strobel, F., Tangpricha, V., Toxqui, L., Vaquero, M. P., Wamberg, L., … D-PRESSURE Collaboration (2015). Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA internal medicine, 175(5), 745–754. https://doi.org/10.1001/jamainternmed.2015.0237
- Golzarand M, Shab-Bidar S, Koochakpoor G, Speakman J R, Djafarian K. Effect of vitamin D3 supplementation on blood pressure in adults: An updated meta-analysis. Nutr Metab Cardiovasc Dis. 2016 Aug;26(8):663-73. doi: 10.1016/j.numecd.2016.04.011
- Vimaleswaran, K. S., Cavadino, A., Berry, D. J., LifeLines Cohort Study investigators, Jorde, R., Dieffenbach, A. K., Lu, C., Alves, A. C., Heerspink, H. J., Tikkanen, E., Eriksson, J., Wong, A., Mangino, M., Jablonski, K. A., Nolte, I. M., Houston, D. K., Ahluwalia, T. S., van der Most, P. J., Pasko, D., Zgaga, L., … Hyppönen, E. (2014). Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. The lancet. Diabetes & endocrinology, 2(9), 719–729. https://doi.org/10.1016/S2213-8587(14)70113-5
- Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond). 2012 Mar;36(3):387-96. doi: 10.1038/ijo.2011.119
- Mallard SR, Howe AS, Houghton LA. Vitamin D status and weight loss: a systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. Am J Clin Nutr. 2016 Oct;104(4):1151-1159. doi: 10.3945/ajcn.116.136879
- Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, LeBoff MS, Margolis KL, Powell L, Uwaifo G, Whitlock E, Wylie-Rosett J, LaCroix A. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern Med. 2007 May 14;167(9):893-902. doi: 10.1001/archinte.167.9.893
- Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J. Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2014 Jun;15(6):528-37. doi: 10.1111/obr.12162
- Sizar O, Khare S, Goyal A, et al. Vitamin D Deficiency. [Updated 2021 Jul 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532266
- Czernichow S, Fan T, Nocea G, Sen SS. Calcium and vitamin D intake by postmenopausal women with osteoporosis in France. Curr Med Res Opin. 2010 Jul;26(7):1667-74. doi: 10.1185/03007995.2010.483658
- Naeem Z. (2010). Vitamin d deficiency- an ignored epidemic. International journal of health sciences, 4(1), V–VI. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3068797
- Gröber, U., & Kisters, K. (2012). Influence of drugs on vitamin D and calcium metabolism. Dermato-endocrinology, 4(2), 158–166. https://doi.org/10.4161/derm.20731
- Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1697S-705S. doi: 10.1093/ajcn/80.6.1697S
- Thacher, T. D., Fischer, P. R., Tebben, P. J., Singh, R. J., Cha, S. S., Maxson, J. A., & Yawn, B. P. (2013). Increasing incidence of nutritional rickets: a population-based study in Olmsted County, Minnesota. Mayo Clinic proceedings, 88(2), 176–183. https://doi.org/10.1016/j.mayocp.2012.10.018
- Ward, L. M., Gaboury, I., Ladhani, M., & Zlotkin, S. (2007). Vitamin D-deficiency rickets among children in Canada. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne, 177(2), 161–166. https://doi.org/10.1503/cmaj.061377
- Durvasula S, Kok C, Sambrook PN, Cumming RG, Lord SR, March LM, Mason RS, Seibel MJ, Simpson JM, Cameron ID. Sunlight and health: attitudes of older people living in intermediate care facilities in southern Australia. Arch Gerontol Geriatr. 2010 Nov-Dec;51(3):e94-9. doi: 10.1016/j.archger.2010.01.008
- Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000 Nov;85(11):4125-30. doi: 10.1210/jcem.85.11.6962
- Allain TJ, Dhesi J. Hypovitaminosis D in older adults. Gerontology. 2003 Sep-Oct;49(5):273-8. doi: 10.1159/000071707. Erratum in: Gerontology. 2004:50(2): 81.
- Oudshoorn C, van der Cammen TJ, McMurdo ME, van Leeuwen JP, Colin EM. Ageing and vitamin D deficiency: effects on calcium homeostasis and considerations for vitamin D supplementation. Br J Nutr. 2009 Jun;101(11):1597-606. doi: 10.1017/S0007114509338842
- Patel S, Barron JL, Mirzazedeh M, Gallagher H, Hyer S, Cantor T, Fraser WD. Changes in bone mineral parameters, vitamin D metabolites, and PTH measurements with varying chronic kidney disease stages. J Bone Miner Metab. 2011 Jan;29(1):71-9. doi: 10.1007/s00774-010-0192-1
- Doorenbos CR, van den Born J, Navis G, de Borst MH. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol. 2009 Dec;5(12):691-700. doi: 10.1038/nrneph.2009.185
- Springbett P, Buglass S, Young AR. Photoprotection and vitamin D status. J Photochem Photobiol B. 2010 Nov 3;101(2):160-8. doi:10.1016/j.jphotobiol.2010.03.006
- Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Kohn N, Martinello S, Berkowitz R, Holick MF. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007 Apr 15;460(2):213-7. doi: 10.1016/j.abb.2006.12.017
- Al-Khalidi B, Kimball SM, Rotondi MA, Ardern CI. Standardized serum 25-hydroxyvitamin D concentrations are inversely associated with cardiometabolic disease in U.S. adults: a cross-sectional analysis of NHANES, 2001-2010. Nutr J. 2017 Feb 28;16(1):16. doi: 10.1186/s12937-017-0237-6. Erratum in: Nutr J. 2017 May 22;16(1):32.
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000 Sep;72(3):690-3. doi: 10.1093/ajcn/72.3.690. Erratum in: Am J Clin Nutr. 2003 May;77(5):1342.
- Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003 Jan;88(1):157-61. doi: 10.1210/jc.2002-020978
- Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J Steroid Biochem Mol Biol. 2013 Jul;136:195-200. doi: 10.1016/j.jsbmb.2012.12.003
- Jahnsen J, Falch JA, Mowinckel P, Aadland E. Vitamin D status, parathyroid hormone and bone mineral density in patients with inflammatory bowel disease. Scand J Gastroenterol. 2002 Feb;37(2):192-9. doi: 10.1080/003655202753416876
- Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985 Oct;42(4):644-9. doi: 10.1093/ajcn/42.4.644
- Pappa HM, Bern E, Kamin D, Grand RJ. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008 Mar;24(2):176-83. doi: 10.1097/MOG.0b013e3282f4d2f3
- Kennel, K. A., Drake, M. T., & Hurley, D. L. (2010). Vitamin D deficiency in adults: when to test and how to treat. Mayo Clinic proceedings, 85(8), 752–758. https://doi.org/10.4065/mcp.2010.0138
- Sohl E, van Schoor NM, de Jongh RT, de Vries OJ, Lips P. The impact of medication on vitamin D status in older individuals. Eur J Endocrinol. 2012 Mar;166(3):477-85. doi: 10.1530/EJE-11-0917
- Thandrayen K, Pettifor JM. Maternal vitamin D status: implications for the development of infantile nutritional rickets. Endocrinol Metab Clin North Am. 2010 Jun;39(2):303-20, table of contents. doi: 10.1016/j.ecl.2010.02.006
- Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010 Jul 17;376(9736):180-8. doi: 10.1016/S0140-6736(10)60588-0
- Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010 Jul 1;19(13):2739-45. doi: 10.1093/hmg/ddq155
- Wang W, Ingles SA, Torres-Mejía G, Stern MC, Stanczyk FZ, Schwartz GG, Nelson DO, Fejerman L, Wolff RK, Slattery ML, John EM. Genetic variants and non-genetic factors predict circulating vitamin D levels in Hispanic and non-Hispanic White women: the Breast Cancer Health Disparities Study. Int J Mol Epidemiol Genet. 2014 Feb 17;5(1):31-46. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3939005
- Elkum N, Alkayal F, Noronha F, Ali MM, Melhem M, Al-Arouj M, Bennakhi A, Behbehani K, Alsmadi O, Abubaker J. Vitamin D insufficiency in Arabs and South Asians positively associates with polymorphisms in GC and CYP2R1 genes. PLoS One. 2014 Nov 18;9(11):e113102. doi: 10.1371/journal.pone.0113102
- Zhang Y, Yang S, Liu Y, Ren L. Relationship between polymorphisms in vitamin D metabolism-related genes and the risk of rickets in Han Chinese children. BMC Med Genet. 2013 Sep 30;14:101. doi: 10.1186/1471-2350-14-101
- Deng X, Song Y, Manson JE, Signorello LB, Zhang SM, Shrubsole MJ, Ness RM, Seidner DL, Dai Q. Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013 Aug 27;11:187. doi: 10.1186/1741-7015-11-187
- Chalcraft, J. R., Cardinal, L. M., Wechsler, P. J., Hollis, B. W., Gerow, K. G., Alexander, B. M., Keith, J. F., & Larson-Meyer, D. E. (2020). Vitamin D Synthesis Following a Single Bout of Sun Exposure in Older and Younger Men and Women. Nutrients, 12(8), 2237. https://doi.org/10.3390/nu12082237
- Nurmi I, Kaukonen JP, Lüthje P, Naboulsi H, Tanninen S, Kataja M, Kallio ML, Leppilampi M. Half of the patients with an acute hip fracture suffer from hypovitaminosis D: a prospective study in southeastern Finland. Osteoporos Int. 2005 Dec;16(12):2018-24. doi: 10.1007/s00198-005-1987-0
- Beringer T, Heyburn G, Finch M, McNally C, McQuilken M, Duncan M, Dixon T. Prevalence of vitamin D inadequacy in Belfast following fragility fracture. Curr Med Res Opin. 2006 Jan;22(1):101-5. doi: 10.1185/030079906X80332
- Ramason, R., Selvaganapathi, N., Ismail, N. H., Wong, W. C., Rajamoney, G. N., & Chong, M. S. (2014). Prevalence of vitamin d deficiency in patients with hip fracture seen in an orthogeriatric service in sunny singapore. Geriatric orthopaedic surgery & rehabilitation, 5(2), 82–86. https://doi.org/10.1177/2151458514528952
- Dawodu A, Tsang RC. Maternal vitamin D status: effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv Nutr. 2012 May 1;3(3):353-61. doi: 10.3945/an.111.000950
- Davis CD, Dwyer JT. The “sunshine vitamin”: benefits beyond bone? J Natl Cancer Inst. 2007 Nov 7;99(21):1563-5. doi: 10.1093/jnci/djm211
- Simon AE, Ahrens KA. Adherence to Vitamin D Intake Guidelines in the United States. Pediatrics. 2020 Jun;145(6):e20193574. doi: 10.1542/peds.2019-3574
- Sowah D, Fan X, Dennett L, Hagtvedt R, Straube S. Vitamin D levels and deficiency with different occupations: a systematic review. BMC Public Health. 2017 Jun 22;17(1):519. doi: 10.1186/s12889-017-4436-z
- Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014 Nov 5;9(11):e111265. doi: 10.1371/journal.pone.0111265
- Chakhtoura M, Rahme M, El-Hajj Fuleihan G. Vitamin D Metabolism in Bariatric Surgery. Endocrinol Metab Clin North Am. 2017 Dec;46(4):947-982. doi: 10.1016/j.ecl.2017.07.006
- Chakhtoura MT, Nakhoul N, Akl EA, Mantzoros CS, El Hajj Fuleihan GA. Guidelines on vitamin D replacement in bariatric surgery: Identification and systematic appraisal. Metabolism. 2016 Apr;65(4):586-97. doi: 10.1016/j.metabol.2015.12.013
- Elder CJ, Bishop NJ. Rickets. Lancet. 2014 May 10;383(9929):1665-1676. doi: 10.1016/S0140-6736(13)61650-5
- Uday, S., & Högler, W. (2017). Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Current osteoporosis reports, 15(4), 293–302. https://doi.org/10.1007/s11914-017-0383-y
- Munns, C. F., Shaw, N., Kiely, M., Specker, B. L., Thacher, T. D., Ozono, K., Michigami, T., Tiosano, D., Mughal, M. Z., Mäkitie, O., Ramos-Abad, L., Ward, L., DiMeglio, L. A., Atapattu, N., Cassinelli, H., Braegger, C., Pettifor, J. M., Seth, A., Idris, H. W., Bhatia, V., … Högler, W. (2016). Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. The Journal of clinical endocrinology and metabolism, 101(2), 394–415. https://doi.org/10.1210/jc.2015-2175
- Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GE, Josse RG, Lips P, Morales-Torres J, Yoshimura N. IOF position statement: vitamin D recommendations for older adults. Osteoporos Int. 2010 Jul;21(7):1151-4. doi: 10.1007/s00198-010-1285-3
- Tripkovic, L., Lambert, H., Hart, K., Smith, C. P., Bucca, G., Penson, S., Chope, G., Hyppönen, E., Berry, J., Vieth, R., & Lanham-New, S. (2012). Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. The American journal of clinical nutrition, 95(6), 1357–1364. https://doi.org/10.3945/ajcn.111.031070
- Lips, P. (2001). Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 22, 477-501.
- Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1752S-8S. doi: 10.1093/ajcn/80.6.1752S
- Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005 Jul;289(1):F8-28. doi: 10.1152/ajprenal.00336.2004
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 Oct;42(4 Suppl 3):S1-201. https://doi.org/10.1016/S0272-6386(03)00905-3
- Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001 Nov;38(5 Suppl 5):S3-S19. doi: 10.1053/ajkd.2001.28111
- Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial. 2005 Jul-Aug;18(4):266-75. doi: 10.1111/j.1525-139X.2005.18402.x
- Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006 Aug;70(4):654-9. doi: 10.1038/sj.ki.5000394. Erratum in: Kidney Int. 2006 Sep;70(6):1190.
- Dusso AS, Sato T, Arcidiacono MV, Alvarez-Hernandez D, Yang J, Gonzalez-Suarez I, Tominaga Y, Slatopolsky E. Pathogenic mechanisms for parathyroid hyperplasia. Kidney Int Suppl. 2006 Jul;(102):S8-11. doi: 10.1038/sj.ki.5001595
- Correa P, Segersten U, Hellman P, Akerstrom G, Westin G. Increased 25-hydroxyvitamin D3 1alpha-hydroxylase and reduced 25-hydroxyvitamin D3 24-hydroxylase expression in parathyroid tumors–new prospects for treatment of hyperparathyroidism with vitamin d. J Clin Endocrinol Metab. 2002 Dec;87(12):5826-9. doi: 10.1210/jc.2002-021356
- Zhou C, Assem M, Tay JC, Watkins PB, Blumberg B, Schuetz EG, Thummel KE. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006 Jun;116(6):1703-12. doi: 10.1172/JCI27793
- Koutkia P, Lu Z, Chen TC, Holick MF. Treatment of vitamin D deficiency due to Crohn’s disease with tanning bed ultraviolet B radiation. Gastroenterology. 2001 Dec;121(6):1485-8. doi: 10.1053/gast.2001.29686
- Holick EA, Lu Z, Holick MT, Chen TC, Sheperd J, Holick MF. Production of previtamin D3 by a mercury arc lamp and a hybrid incandescent/mercury arc lamp. In: Holick MF, ed. Biologic effects of light 2001: proceedings of a symposium. Boston: Kluwer Academic, 2002:205-12.