What is blood plasma
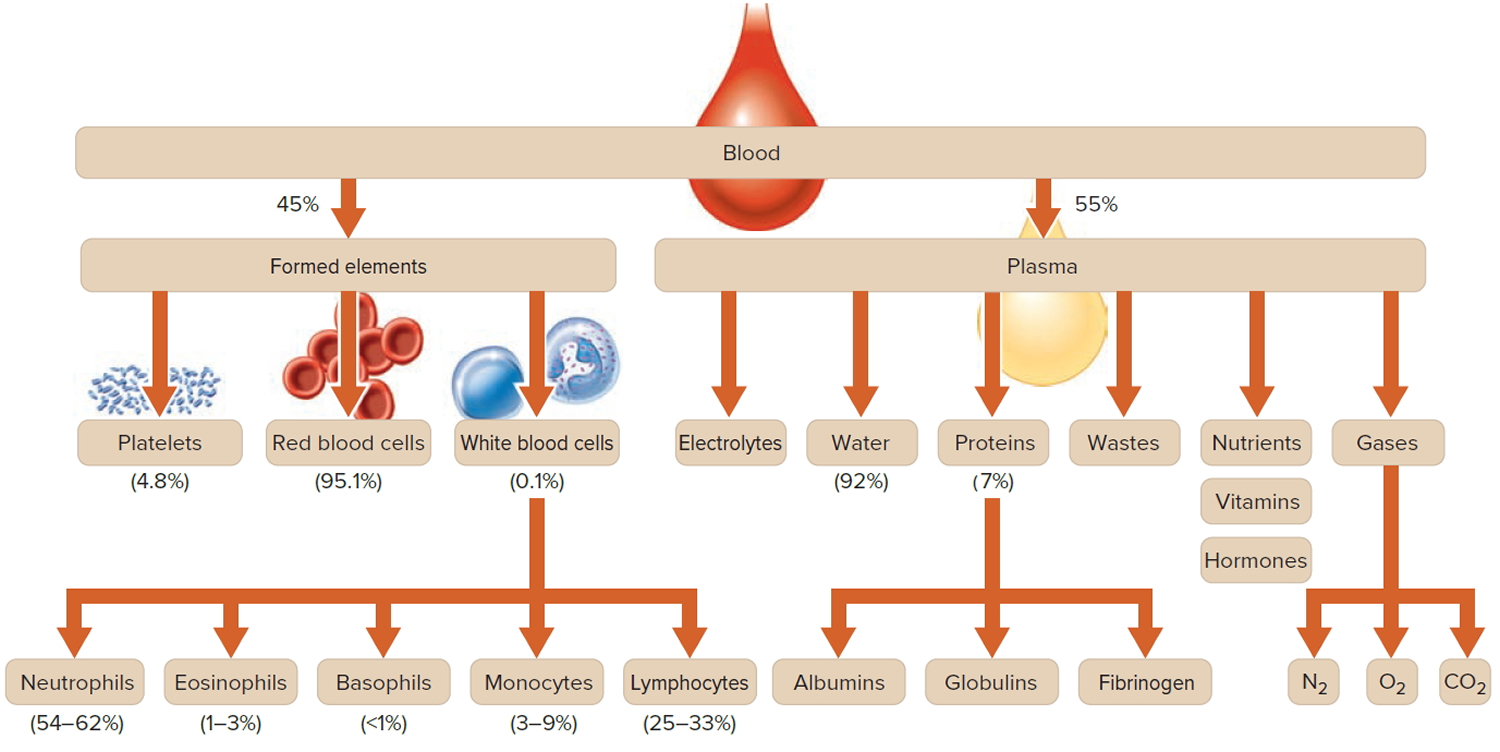
How much blood you have depends mostly on your size and weight. A man who weighs about 70 kg (about 154 pounds) has about 5 to 6 liters of blood in his body. Blood is 55% blood plasma and about 45% different types of blood cells. Blood is composed of solid particles (red blood cells, white blood cells, and cell fragments called platelets) suspended in a fluid extracellular matrix called blood plasma. Over 99% of the solid particles present in blood are cells that are called red blood cells (erythrocytes) due to their red color. The rest are pale or colorless white blood cells (leukocytes) and platelets (thrombocytes).
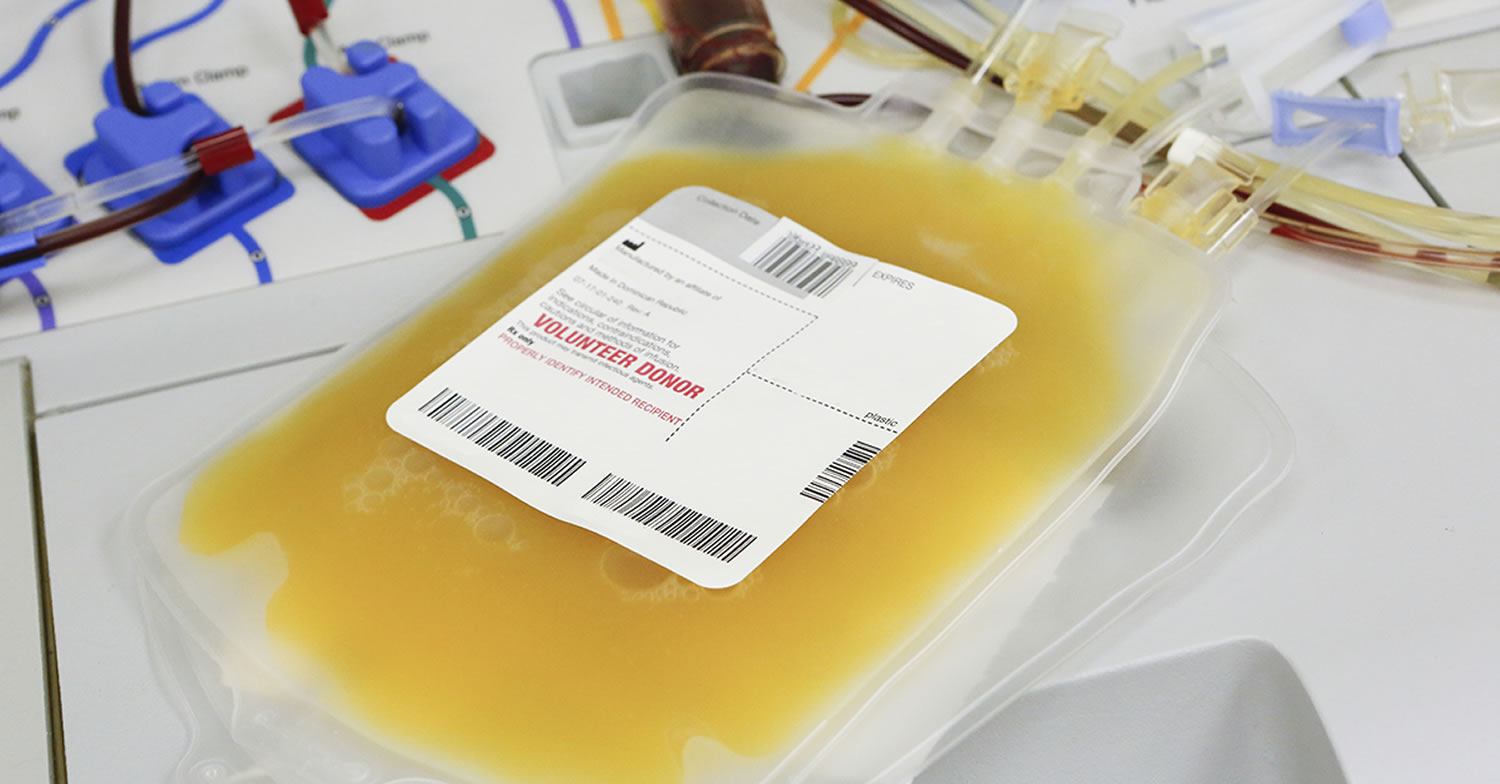
The blood plasma is the clear, straw-colored, liquid portion of the blood in which the cells (red blood cells, white blood cells) and platelets are suspended. It is
approximately 92% water and less than 8% is dissolved substances, mostly proteins, a complex mixture of organic and inorganic biochemicals. Functions of plasma include transporting gases, vitamins, and other nutrients; helping to regulate fluid and electrolyte balance; and maintaining a favorable pH. Blood plasma also contain antibodies to fight infections (part of the immune system), glucose, amino acids and the proteins that form blood clots (part of the hemostatic system)..
Blood plasma contains electrolytes that are absorbed from the intestine or released as by-products of cellular metabolism. They include sodium, potassium, calcium, magnesium, chloride, bicarbonate, phosphate, and sulfate ions. Sodium and chloride ions are the most abundant. Bicarbonate ions are important in maintaining the pH of plasma. Like other plasma constituents, bicarbonate ions are regulated so that their blood concentrations remain relatively stable.
Blood transports a variety of materials between interior body cells and those that exchange substances with the external environment. In this way, blood helps maintain stable internal environmental conditions.
Hemostasis refers to the process that stops bleeding, which is vitally important when blood vessels are damaged. Following an injury to the blood vessels, several actions may help to limit or prevent blood loss. These include vascular spasm, platelet plug formation, and blood coagulation.
Platelets adhere to any rough surface, particularly to the collagen in connective tissue. When a blood vessel breaks, platelets adhere to the collagen underlying the endothelium lining blood vessels. Platelets also adhere to each other, forming a platelet plug in the vascular break. A plug may control blood loss from a small break, but a larger break may require a blood clot to halt bleeding.
Coagulation, the most effective hemostatic mechanism, forms a blood clot in a series of reactions, each one activating the next. Blood coagulation is complex and utilizes many biochemicals called clotting factors. Some of these factors promote coagulation, and others inhibit it. Whether or not blood coagulates depends on the balance between these two groups of factors. Normally, anticoagulants prevail, and the blood does not clot. However, as a result of injury (trauma), biochemicals that favor coagulation may increase in concentration, and the blood may coagulate. The resulting mass is a blood clot, which may block a vascular break and prevent further blood loss. The clear, yellow liquid that remains after the clot forms is called serum. Serum is plasma minus the clotting factors.
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
Figure 1. Blood composition
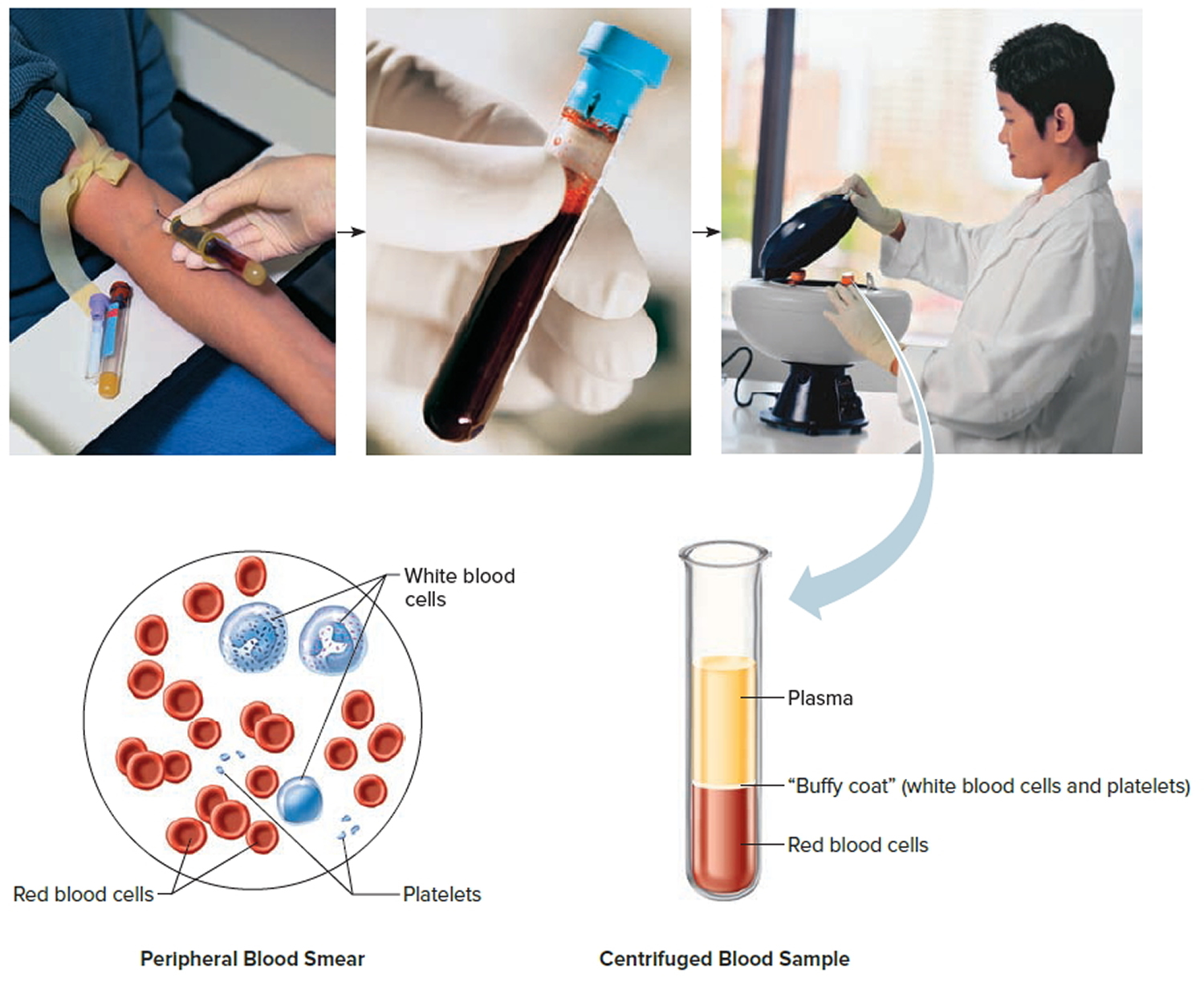
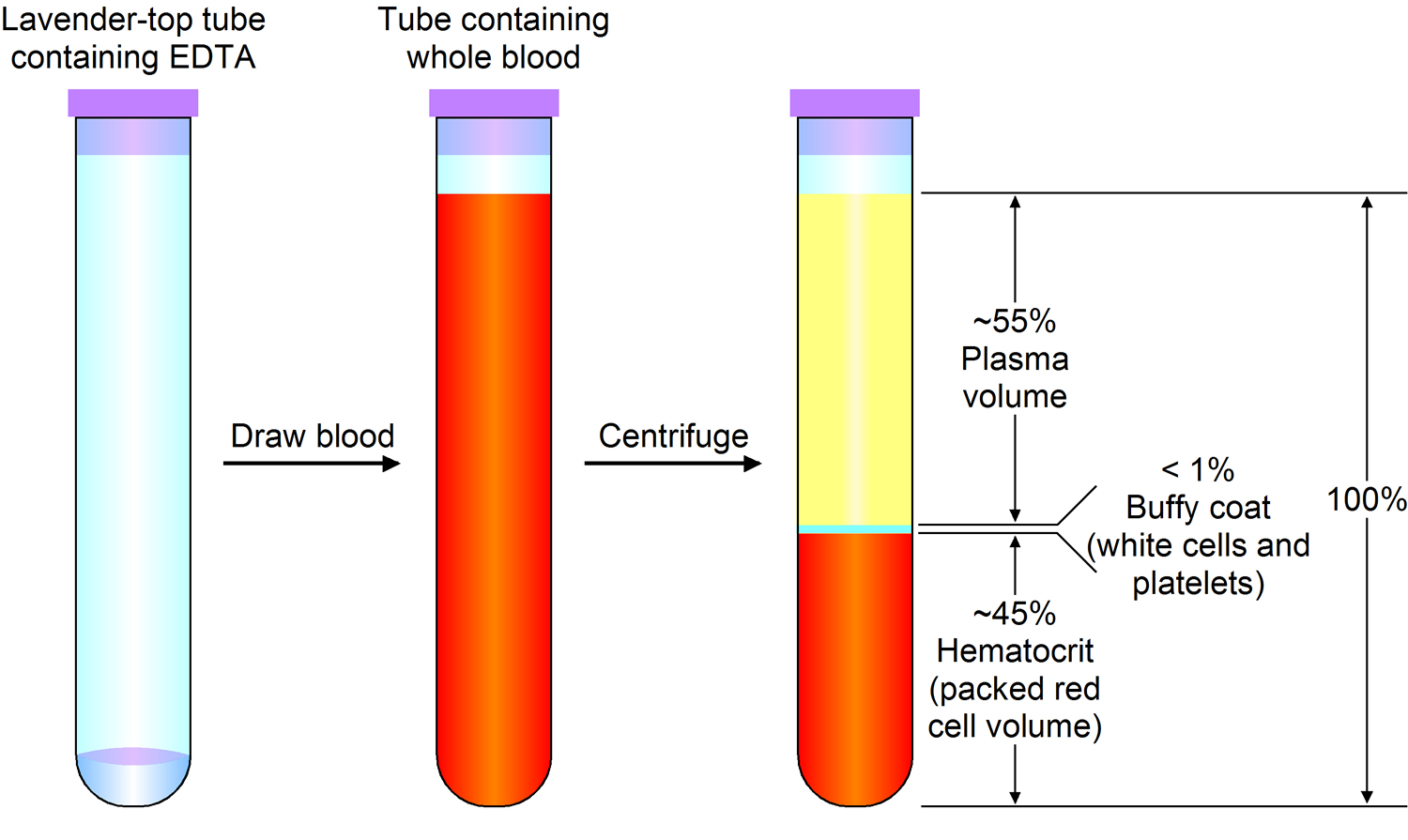
Note: Blood consists of a liquid portion called plasma and a solid portion (the formed elements) that includes red blood cells, white blood cells, and platelets. When blood components are separated by centrifugation, the white blood cells and platelets form a thin layer, called the “buffy coat,” between the plasma and the red blood cells, which accounts for about 1% of the total blood volume. Blood cells and platelets can be seen under a light microscope when a blood sample is smeared onto a glass slide.
Blood Plasma Proteins
Blood plasma proteins are the most abundant of the dissolved substances (solutes) in plasma. These proteins remain in the blood and interstitial fluids, and ordinarily are not used as energy sources. The three main types of blood plasma proteins—albumins, globulins, and fibrinogen—differ in composition and function.
Albumins are the smallest plasma proteins, yet account for about 60% of them by weight. Albumins are synthesized in the liver.
Plasma proteins are too large to pass through the capillary walls, so they are impermeant. They create an osmotic pressure that holds water in the capillaries, despite blood pressure forcing water out of capillaries by filtration. The term colloid osmotic pressure is used to describe this osmotic effect due to the plasma proteins. Because albumins are so plentiful, they are an important determinant of the colloid osmotic pressure of the plasma.
By maintaining the colloid osmotic pressure of plasma, albumins and other plasma proteins help regulate water movement between the blood and the tissues. In doing so, the blood plasma proteins help control blood volume, which, in turn, directly affects blood pressure.
If the concentration of plasma proteins falls, tissues swell. This condition is called edema. As the concentration of plasma proteins drops, so does the colloid osmotic pressure. Water leaves the blood vessels and accumulates in the interstitial spaces, causing swelling. A low plasma protein concentration may result from starvation, a protein-deficient diet, or an impaired liver that cannot synthesize plasma proteins.
Globulins make up about 36% of the plasma proteins. They can be further subdivided into alpha, beta, and gamma globulins. The liver synthesizes alpha and
beta globulins, which have a variety of functions. The globulins transport lipids and fat-soluble vitamins. Lymphatic tissues produce the gamma globulins, which are a type of antibody.
Fibrinogen constitutes about 4% of the plasma proteins, and functions in blood coagulation (clotting). Fibrinogen is synthesized in the liver, fibrinogen is the largest of the plasma proteins.
Plasma products and their uses
Plasma products can be grouped into three main types:
- clotting or coagulation factors
- albumin solutions
- immunoglobulins
Coagulation or clotting factors
Coagulation is the name for the complex process of blood clotting. Clotting factors are proteins that work together with platelets to clot blood.
People need clotting factors for their blood to successfully clot. Missing one or more of these factors leads to blood clotting disorders such as haemophilia and Von Willebrand disease.
In the US, hemophilia is commonly treated with ‘recombinant factors’ that are manufactured in a laboratory and do not come from donated plasma.
Other blood clotting disorders that are treated with coagulation factors made from donated plasma.
Albumin
Albumin is the most common protein in blood plasma. It helps to:
- carry substances around the body
- maintain the right amount of fluid circulating in the body
If the circulation is working properly, vital hormones, cells and enzymes are transported to the right parts of the body to do their job.
If it’s not working properly, the circulatory system starts to break down, with serious consequences such as fluids being retained in the cells.
This can be treated by using human albumin solution which makes sure that the right amount of fluid is circulating in the blood stream.
Albumin can also be used to treat people with some types of liver or kidney disease and patients who have suffered burns.
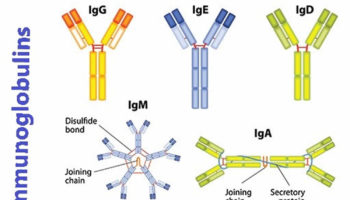
Immunoglobulins
Immunoglobulins are protective antibodies which are produced by the body to fight against invading viruses or bacteria. There are two different types of immunoglobulins, specific and non-specific.
Specific immunoglobulins
Specific immunoglobulins contain high levels of antibody to a particular illness. These are given to people who have been exposed to a specific infection.
Antidotes to tetanus, rabies, chickenpox and hepatitis are all examples of specific immunoglobulins.
For example, a donor who has had chicken pox will have high levels of chicken pox antibodies. So their plasma would be ideal to treat a child with leukaemia who has been exposed to chicken pox.
Non-specific immunoglobulins
Non-specific immunoglobulins contain a wide variety of antibodies. These are given to people:
- who make faulty antibodies, or can’t make their own antibodies
- who are having treatments for diseases like cancer, where the treatment harms their ability to make antibodies
People with a faulty immune system need these products to live. Over 1,000 donations of plasma contribute to a single dose of an immunoglobulin product that contains all the necessary antibodies.
Table 1 summarizes the characteristics of the blood plasma proteins.
Table 1. Blood plasma proteins
Protein | Percentage of Total | Origin | Function |
Albumins | 60% | Liver | Help maintain colloid osmotic pressure |
Globulins | 36% | ||
Alpha globulins | Liver | Transport lipids and fat-soluble vitamins | |
Beta globulins | Liver | Transport lipids and fat-soluble vitamins | |
Gamma globulins | Lymphatic tissues | Constitute the antibodies of immunity | |
Fibrinogen | 4% | Liver | Plays a key role in blood coagulation |
Separating plasma products
Plasma is first tested to be sure it’s safe to use, in the same way that whole blood is tested.
Many chemical and physical processes (e.g., spinning and heat treatments) are then carried out to separate the individual proteins. This is known as the fractionation process.
This process is fully automated and takes up to five days to complete.
Solvent or detergent treatment, dry heat treatment, filtration and pasteurization are also used to kill or remove any viruses that may be present.
The finished products are then tested again to make sure they contain the right biological make-up.
Once processing and testing is complete, the products are labeled, coded and packed, ready to be used by hospitals, clinics and doctors’ surgeries.
The total number of products that can be made with plasma fractionation runs into hundreds.
Blood Plasma Gases and Nutrients
The most important blood gases are oxygen (O2) and carbon dioxide (CO2). Blood plasma also contains a considerable amount of dissolved nitrogen, which ordinarily has no physiological function.
The plasma nutrients include amino acids, simple sugars, nucleotides, and lipids, all absorbed from the digestive tract. For example, blood plasma transports glucose from the small intestine to the liver, where it may be stored as glycogen or converted to fat. If blood glucose concentration drops below the normal range, glycogen may be broken down into glucose.
Blood plasma also carries recently absorbed amino acids to the liver, where they may be used to manufacture proteins, or deaminated and used as an energy source.
Blood plasma lipids include fats (triglycerides), phospholipids, and cholesterol. Because lipids are not water-soluble and plasma is almost 92% water, these lipids are carried in the plasma attached to proteins.
Blood Plasma Nonprotein Nitrogenous Substances
Molecules that contain nitrogen atoms but are not proteins comprise a group called nonprotein nitrogenous substances. In plasma, this group includes amino acids, urea, uric acid, creatine and creatinine. Amino acids come from protein digestion and amino acid absorption. Urea and uric acid are products of protein and nucleic acid catabolism, respectively. Creatinine results from the metabolism of creatine. In the skeletal muscle creatine is part of creatine phosphate in muscle tissue, where it stores energy in phosphate bonds.
Blood Plasma Electrolytes
Blood plasma contains electrolytes that are absorbed from the intestine or released as by-products of cellular metabolism. They include sodium, potassium, calcium, magnesium, chloride, bicarbonate, phosphate, and sulfate ions. Sodium and chloride ions are the most abundant. Bicarbonate ions are important in maintaining the pH of plasma. Like other plasma constituents, bicarbonate ions are regulated so that their blood concentrations remain relatively stable.
Blood plasma donation
Plasmapheresis is the standard procedure by which blood plasma is separated from whole blood and collected 1. Blood flows through a single needle placed in an arm vein, into a machine that contains a sterile, disposable plastic kit. The plasma is isolated and channeled out into a special bag, and red blood cells and other parts of the blood are returned to you through the same needle.
Is Plasmapheresis Safe ?
Absolutely. The machine and the procedure have been evaluated and approved by the Food and Drug Administration (FDA), and all plastics and needles coming into contact with you are used once and discarded 1. At no time during the procedure is the blood being returned to you detached from the needle in your arm, so there is no risk of returning the wrong blood to you.
Who Is Eligible to Participate in the AB Plasma Program ?
Donors must have blood group AB and must be male, because men lack plasma proteins (antibodies) directed against blood cell elements 1. Otherwise, eligibility for plasmapheresis procedures is the same as that for whole-blood donation. The interval between consecutive group AB plasmapheresis donations at National Institutes of Health Blood Bank is 1 month.
How Long Does Plasmapheresis Take ?
Plasmapheresis procedures take about 40 minutes, but you should allow another 20 minutes for staff to obtain your medical history 1.
What is blood plasma used for
Blood plasma is the pale yellow liquid part of whole blood. It is enriched in proteins that help fight infection (part of the immune system) and aid the blood in clotting (part of the hemostatic system). AB plasma is plasma collected from blood group AB donors. It is considered “universal donor” plasma because it is suitable for all recipients, regardless of blood group. Due to its value as a transfusion component, it is sometimes referred to as “liquid gold.”
Blood plasma products available in the United States include fresh frozen plasma and thawed plasma that may be stored at 33.8 to 42.8°F (1 to 6°C) for up to five days 2. Blood plasma contains all of the coagulation factors. Fresh frozen plasma infusion can be used for reversal of anticoagulant effects. Thawed plasma has lower levels of factors V and VIII and is not indicated in patients with consumption coagulopathy (diffuse intravascular coagulation) 3.
Blood plasma transfusion is recommended in patients with active bleeding and an International Normalized Ratio (INR) greater than 1.6, or before an invasive procedure or surgery if a patient has been anticoagulated 4, 5. Blood plasma is often inappropriately transfused for correction of a high INR when there is no bleeding. Supportive care can decrease high-normal to slightly elevated INRs (1.3 to 1.6) without transfusion of plasma. Table 2 gives indications for plasma transfusion 4, 5, 6.
Table 2. Indications for Transfusion of Blood Plasma Products
| Indication | Associated condition/additional information |
|---|---|
International Normalized Ratio > 1.6 | Inherited deficiency of single clotting factors with no virus-safe or recombinant factor available—anticoagulant factors II, V, X, or XI |
Prevent active bleeding in patient on anticoagulant therapy before a procedure | |
Active bleeding | |
Emergency reversal of warfarin (Coumadin) | Major or intracranial hemorrhage |
Prophylactic transfusion in a surgical procedure that cannot be delayed | |
Acute disseminated intravascular coagulopathy | With active bleeding and correction of underlying condition |
Microvascular bleeding during massive transfusion | ≥ 1 blood volume (replacing approximately 5,000 mL in an adult who weighs 155.56 lb [70 kg]) |
Replacement fluid for apheresis in thrombotic microangiopathies | Thrombotic thrombocytopenic purpura; hemolytic uremic syndrome |
Hereditary angioedema | When C1 esterase inhibitor is unavailable |
Cryoprecipitate
Cryoprecipitate is prepared by thawing fresh frozen plasma and collecting the precipitate. Cryoprecipitate contains high concentrations of factor VIII and fibrinogen 2. Cryoprecipitate is used in cases of hypofibrinogenemia, which most often occurs in the setting of massive hemorrhage or consumptive coagulopathy. Indications for cryoprecipitate transfusion are listed in Table 3 7, 8. Each unit will raise the fibrinogen level by 5 to 10 mg per dL (0.15 to 0.29 μmol per L), with the goal of maintaining a fibrinogen level of at least 100 mg per dL (2.94 μmol per L) 8. The usual dose in adults is 10 units of pooled cryoprecipitate 3. Recommendations for dosing regimens in neonates vary, ranging from 2 mL of cryoprecipitate per kg to 1 unit of cryoprecipitate (15 to 20 mL) per 7 kg 7.
Table 3. Indications for Transfusion of Cryoprecipitate
Adults |
Hemorrhage after cardiac surgery |
Massive hemorrhage or transfusion |
Surgical bleeding |
Neonates |
Anticoagulant factor VIII deficiency* |
Anticoagulant factor XIII deficiency |
Congenital dysfibrinogenemia |
Congenital fibrinogen deficiency |
von Willebrand disease* |
*—Use when recombinant factors are not available.
Blood plasma for patients undergoing surgery on the heart or blood vessels
Cardiovascular surgery includes many types of major surgery on the heart and major blood vessels, including procedures such as: heart valve replacements, coronary artery bypass grafts, aortic aneurysm repairs and corrections or congenital abnormalities of the heart. Cardiovascular surgery is associated with a significant risk of bleeding, with 8% of patients losing more than 2 ml/kg/hour of blood postoperatively 9. A number of features make patients undergoing cardiovascular surgery more likely to bleed 10, 11:
- These patients may be taking drugs that predispose towards bleeding, such as aspirin or clopidogrel.
- Patients undergoing major heart surgery will often require a cardiopulmonary bypass, where a circuit is formed by removing the heart from the circulation by passing a catheter into the aorta and the pulmonary artery while a cardiopulmonary bypass machine circulates blood round the body and ensures that it is adequately oxygenated. Heparin is used to prevent the cardiopulmonary bypass circuit from clotting. Heparin is an anticoagulant and can predispose patients to bleeding. When cardiopulmonary bypass is complete, heparin is neutralised with protamine.
- Hypothermia and acidosis during the procedure may also contribute towards excess bleeding.
- Dilution of clotting factors with administration of intravenous fluid; this is a particular problem in the paediatric setting.
- When acute bleeding develops, clotting factors are consumed, resulting in a coagulopathy and predisposing the patient towards further bleeding.
In some cases these patients will have a clearly defined bleeding risk. They may already be haemorrhaging and, if this is the case, treatments to reduce bleeding would be considered therapeutic. Alternatively they may have abnormal blood results, such as a prolonged prothrombin time, suggesting that clotting factors may be deficient. Lastly, in some cases it may be presumed that a coagulopathy may develop and that prophylactic treatment before this event would reduce the risk of bleeding.
Treatment strategies to reduce bleeding include optimising surgical technique to minimise blood loss; antifibrinolytic agents such as tranexamic acid; careful monitoring and neutralisation of heparin; optimising the management of anticoagulant and antiplatelet drugs; and blood components such as fresh frozen plasma 12.
Fresh frozen plasma obtained from whole blood from blood donors, is a source of procoagulant factors, including fibrinogen and is used for either the treatment or prophylaxis of bleeding 13. Many audits indicate that patients undergoing major cardiac and vascular surgery receive a significant proportion of all clinical plasma transfusions. Some studies have reported wide variation in the use of clinical plasma for cardiac surgery and in critical care among centres within the same country 14.
Fresh frozen plasma contains a number of factors that help blood to clot. The risk of bleeding in open heart surgery or surgery on the main blood vessels in the body is high. Fresh frozen plasma is sometimes administered to these patients to reduce bleeding. It can be administered prophylactically (to prevent bleeding) or therapeutically (to treat bleeding). However, there are risks of side effects from fresh frozen plasma, such as severe allergic reactions or breathing problems 15.
However a 2015 Cochrane Review found no evidence for the efficacy of fresh frozen plasma for the prevention of bleeding in heart surgery and it found some evidence of an increased overall need for red cell transfusion in those treated with fresh frozen plasma 15. There were no reported adverse events due to fresh frozen plasma transfusion. Overall the evidence for the safety and efficacy of prophylactic fresh frozen plasma for cardiac surgery is insufficient. The trials focused on prevention of bleeding and did not address prevention of bleeding for patients with abnormal blood clotting or for the treatment of bleeding patients.
Blood plasma for critically ill patients
Plasma transfusions are a frequently used treatment for critically ill patients, and they are usually prescribed to correct abnormal coagulation tests and to prevent or stop bleeding 16. Plasma transfusions have been used since 1941 17. In 2008, 4,484,000 plasma units were transfused in patients in the United States 18. More than 10% of critically ill patients, both adults and children, receive a plasma transfusion during their hospital stay, making plasma transfusion a frequently used treatment modality 19, 20, 21. In current practice, plasma transfusions are widely used in critical care; they are administered most often to correct abnormal coagulation tests or to prevent bleeding 22.
In situations in which active bleeding is attributable to a coagulation factor deficiency, plasma transfusions can constitute a life-saving intervention by improving coagulation factor deficit 23, especially in patients requiring massive transfusion 24. Although plasma transfusions are frequently prescribed for critically ill patients, some of the reasons for their use are not supported by evidence from medical research. Some research has found an association of plasma transfusions with worse outcomes, and other studies have suggested that plasma transfusions do not help to return blood to its normal thickness 25.
Blood plasma for chronic inflammatory demyelinating polyradiculoneuropathy
Chronic inflammatory demyelinating polyradiculoneuropathy is a disease that causes progressive or relapsing and remitting weakness and numbness. At least one or two cases per 100,000 of the population and may be as high as 8.9 per 100,000 26. It is probably caused by an autoimmune process. Chronic inflammatory demyelinating polyradiculoneuropathy is an uncommon disease that causes weakness and numbness of the arms and legs. The condition may progress steadily or have periods of worsening and improvement. Although not proven, chronic inflammatory demyelinating polyradiculoneuropathy is generally considered to be an autoimmune disease caused by either humoral or cell-mediated immunity directed against myelin around individual nerve fibres or Schwann cell antigens which have not been identified 27. In severe cases, the disease affects the actual nerve fibres themselves. There has been debate as to whether people with the clinical features of an acquired demyelinating neuropathy and a systemic disease, such as cancer, diabetes mellitus, systemic lupus erythematosus, and other connective tissue diseases should be categorised as having chronic inflammatory demyelinating polyradiculoneuropathy 28.
Immunosuppressive or immunomodulatory drugs would be expected to be beneficial. According to Cochrane systematic reviews, three immune system treatments are known to help. These are corticosteroids (‘steroids’), plasma exchange (which removes and replaces blood plasma), and intravenous immunoglobulin (infusions into a vein of human antibodies). Moderate- to high-quality evidence from two small trials shows that plasma exchange provides significant short-term improvement in disability, clinical impairment, and motor nerve conduction velocity in chronic inflammatory demyelinating polyradiculoneuropathy but rapid deterioration may occur afterwards 29. Adverse events related to difficulty with venous access, use of citrate, and haemodynamic changes are not uncommon.
Blood plasma for generalised myasthenia gravis
Myasthenia gravis is an autoimmune disease caused by antibodies in the blood which attack the junctions (against the nicotinic acetylcholine receptor) between nerves and muscles they stimulate. Less than five per cent of patients have auto-antibodies to a muscle tyrosine kinase. Myasthenia gravis is characterised by weakness and fatigability of voluntary muscle, which changes over time. Acute exacerbations are life-threatening because they can cause swallowing difficulties or respiratory failure. Historically, with treatment – including thymectomy, steroids, and immunosuppressive drugs – after one to 21 (mean 12) years, 6% of patients went into remission, 36% improved, 42% were unchanged, and 2% were worse 30. In recent years, expert opinion has highlighted the greater efficacy of combined immunosuppressive treatments 31. A new subtype of myasthenia is associated with autoantibodies to a muscle specific kinase and these antibodies are also pathogenic on passive transfer 32.
Plasma exchange was introduced in 1976 as a short-term therapy for acute exacerbations of myasthenia gravis 33. It is thought to work because the exchange removes circulating anti-AChR (anti-acetylcholine receptor) antibodies. However, improvement has also been reported in so-called seronegative myasthenia gravis (where no anti-AChR antibodies can be detected) following plasma exchange 34. A symposium held in 1978 35, and numerous papers have recognised the short-term benefit of plasma exchanges 36. The use of repeated plasma exchange over a long period in refractory myasthenia gravis has also been reported 37. Plasma exchange is used worldwide for the treatment of myasthenia gravis but despite the published case series and the conferences of experts many questions remains unanswered concerning its efficacy for the treatment of chronic, more or less severe, myasthenia gravis as well as of myasthenic exacerbation or crisis and its efficacy in comparison with other treatments. Few randomised controlled trials have been published 38, 39. Plasma exchange removes these circulating auto-antibodies. Many case series suggest that plasma exchange helps to treat myasthenia gravis. However, two Cochrane Reviews 2002 40 and 2003 41, both found no adequate randomised controlled trials have been performed to determine whether plasma exchange improves the short- or long-term outcome for chronic myasthenia gravis or myasthenia gravis exacerbation. However, many case series studies convincingly report short-term benefit from plasma exchange in myasthenia gravis, especially in myasthenic exacerbation or crisis. In severe exacerbations of myasthenia gravis one randomised controlled trial did not show a significant difference between plasma exchange and intravenous immunoglobulin. Further research is need to compare plasma exchange with alternative short-term treatments for myasthenic crisis or before thymectomy and to determine the value of long-term plasma exchange for treating myasthenia gravis.
Blood plasma for the prevention of ovarian hyperstimulation syndrome
Ovarian hyperstimulation syndrome is an iatrogenic, serious and potentially fatal complication of ovarian stimulation which affects 1% to 14% of all in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) cycles 42. Ovarian hyperstimulation syndrome may be associated with massive ovarian enlargement, extracellular exudate accumulation combined with profound intravascular volume depletion, ascites, hydrothorax, haemoconcentration, liver dysfunction and renal failure 43. It can lead to cancellation of an IVF cycle and prolonged bed rest or hospitalisation, which may have significant emotional, social, and economic impacts 44. Ovarian hyperstimulation syndrome can be classified into an early form that is related to the ovarian response and exogenous human chorionic gonadotrophin (hCG) administration, and is detected three to nine days after hCG administration. A late form of ovarian hyperstimulation syndrome, diagnosed 10 to 17 days later, is due to endogenous hCG 45 and is categorised as mild, moderate, severe or life-threatening. The aetiology of ovarian hyperstimulation syndrome is not completely clear at this moment; however the syndrome is strongly associated with serum hCG and certain vasoactive substances 46 are not elevated during gonadotropin stimulation in in vitro fertilization (IVF) patients developing ovarian hyperstimulation syndrome (OHSS): results of a prospective cohort study with matched controls. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;96:196-201. https://www.ncbi.nlm.nih.gov/pubmed/11384807)).
Blood plasma albumin has both osmotic and transport functions. It contributes about 75% of the plasma oncotic pressure and administration of 50 g human albumin solution will draw more than 800 mL of extracellular fluid into the circulation within 15 minutes 47. It has been suggested that the binding and transport properties of human albumin play a major role in the prevention of severe ovarian hyperstimulation syndrome, as albumin may result in binding and inactivation of the vasoactive intermediates responsible for the pathogenesis of ovarian hyperstimulation syndrome. The osmotic function is responsible for maintaining the intra-vascular volume in the event of capillary leakage, thus preventing the sequelae of hypovolaemia, ascites and haemoconcentration 48. A 2016 Cochrane Review concluded that there is some eEvidence suggesting that the plasma expanders assessed in this review (human albumin, hydroxyethyl starch and mannitol) reduce rates of moderate and severe ovarian hyperstimulation syndrome in women at high risk. Adverse events appear to be uncommon, but were too poorly reported to reach any firm conclusions 48.
References- AB Plasma Donor Program. National Institutes of Health Blood Bank. https://clinicalcenter.nih.gov/blooddonor/donationtypes/ab_plasma.html
- Transfusion of Blood and Blood Products: Indications and Complications. Am Fam Physician. 2011 Mar 15;83(6):719-724. http://www.aafp.org/afp/2011/0315/p719.html
- King KE, Bandarenko N. Blood Transfusion Therapy: A Physician’s Handbook. 9th ed. Bethesda, Md.: American Association of Blood Banks; 2008:236.
- Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA. 1994;271(10):777–781.
- Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion: the effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126(1):133–139.
- Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G; Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Work Group. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009;7(2):132–150.
- Poterjoy BS, Josephson CD. Platelets, frozen plasma, and cryoprecipitate: what is the clinical evidence for their use in the neonatal intensive care unit? Semin Perinatol. 2009;33(1):66–74.
- Callum JL, Karkouti K, Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev. 2009;23(3):177–188.
- Vuylsteke A, Pagel C, Gerrard C, Reddy B, Nashef S, Aldam P, et al. The Papworth Bleeding Risk Score: a stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. European Journal of Cardiothoracic Surgery 2011;39(6):924-30. https://www.ncbi.nlm.nih.gov/pubmed/21094051
- Bevan DH. Cardiac bypass haemostasis: putting blood through the mill. British Journal of Haematology 1999;104(2):208-19. https://www.ncbi.nlm.nih.gov/pubmed/10050700
- Hartmann M, Sucker C, Boehm O, Koch A, Loer S, Zacharowski K. Effects of cardiac surgery on hemostasis. Transfusion Medicine Reviews 2006;20(3):230-41. https://www.ncbi.nlm.nih.gov/pubmed/16787830
- Davidson S. State of the art: how I manage coagulopathy in cardiac surgery patients. British Journal of Haematology 2014;164(6):779-89. https://www.ncbi.nlm.nih.gov/pubmed/24450971
- Desborough M, Stanworth S. Plasma transfusion for bedside, radiologically guided, and operating room invasive procedures. Transfusion 2012;52(Suppl 1):20S-9S. https://www.ncbi.nlm.nih.gov/pubmed/22578367
- Stanworth SJ, Grant-Casey J, Lowe D, Laffan M, New H, Murphy MF, et al. The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion 2011;51(1):62-70. https://www.ncbi.nlm.nih.gov/pubmed/20804532
- Desborough M, Sandu R, Brunskill SJ, Doree C, Trivella M, Montedori A, Abraha I, Stanworth S. Fresh frozen plasma for cardiovascular surgery. Cochrane Database of Systematic Reviews 2015, Issue 7. Art. No.: CD007614. DOI: 10.1002/14651858.CD007614.pub2. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007614.pub2/full
- Plasma transfusion strategies for critically ill patients. National Center for Biotechnology Information, U.S. National Library of Medicine. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0061586/
- Schmidt PJ. The plasma wars: a history. Transfusion 2012;52(S1):2S-4S.
- Office of the Assistant Secretary for Health. The 2009 National Blood Collection and Utilization Survey Report. Washington, DC: US Department of Health and Human Services, 2011.
- Luk C, Eckert KM, Barr RM, Chin-Yee IH. Prospective audit of the use of fresh-frozen plasma, based on Canadian Medical Association transfusion guidelines. Canadian Medical Association Journal 2002;166(12):1539-40. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC113799/
- Puetz J, Witmer C, Huang Y, Raffini L. Widespread use of fresh frozen plasma in US children’s hospitals despite limited evidence demonstrating a beneficial effect. Journal of Pediatrics 2012;160(2):210-5.
- Stanworth SJ, Walsh TS, Prescott RJ, Lee RJ, Watson DM, Wyncoll D. A national study of plasma use in critical care: clinical indications, dose and effect on prothrombin time. Critical Care 2011;15(2):R108.
- Vlaar AP, in der Maur AL, Binnekade JM, Schultz MJ, Juffermans NP. A survey of physicians’ reasons to transfuse plasma and platelets in the critically ill: a prospective single-centre cohort study. Transfusion Medicine 2009;19(4):207-12.
- Stanworth SJ, Hyde CJ, Murphy MF. Evidence for indications of fresh frozen plasma. Transfusion Clinique et Biologique 2007;14(6):551-6. https://www.ncbi.nlm.nih.gov/pubmed/18430602
- Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. American Journal of Surgery 2009;197(5):565-70. https://www.ncbi.nlm.nih.gov/pubmed/19393349
- Karam O, Tucci M, Combescure C, Lacroix J, Rimensberger PC. Plasma transfusion strategies for critically ill patients. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No.: CD010654. DOI: 10.1002/14651858.CD010654.pub2. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010654.pub2/full
- Mahdi-Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. European Journal of Neurology 2014;21(1):28-33. https://www.ncbi.nlm.nih.gov/pubmed/23679015
- Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. New England Journal of Medicine 2005;352(13):1343-56. https://www.ncbi.nlm.nih.gov/pubmed/15800230
- Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies, Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society – first revision. Journal of the Peripheral Nervous System : JPNS 2010; Vol. 17, issue 3:356-63.
- Mehndiratta MM, Hughes RAC, Pritchard J. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No.: CD003906. DOI: 10.1002/14651858.CD003906.pub4. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003906.pub4/full
- Grob D, Brunner NG, Namba T. The natural course of myasthenia gravis and effect of therapeutic measures. Annals of the New York Academy of Science 1981;377(1):652-69. https://www.ncbi.nlm.nih.gov/pubmed/6951490
- Oosterhuis HJGH. Myasthenia gravis. Groningen: Groningen Neurological Press, 1997.
- Cole RN, Reddel SW, Gervasio OL, Phillips WD. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction.. Ann Neurol 2008;63(6):782-9. https://www.ncbi.nlm.nih.gov/pubmed/18384168
- Pinching AS, Peters DK. Remission of myasthenia gravis following plasma exchange. Lancet 1976;2(8000):1373-6. https://www.ncbi.nlm.nih.gov/pubmed/63848
- Miller RG, Milner-Brown HS, Dau PC. Antibody-negative acquired myasthenia gravis. Successful therapy with plasma exchange (letter). Muscle and Nerve 1981;4(3):255. https://www.ncbi.nlm.nih.gov/pubmed/7242562
- Dau PC. Plasmapheresis and the immunology of myasthenia gravis. Boston: Hougton-Mifflin, 1979.
- NIH Consensus Conference. The utility of therapeutic plasmapheresis for neurological disorders. NIH Consensus Development.. Journal of the American Medical Association 1986;256(10):1333-7. https://www.ncbi.nlm.nih.gov/pubmed/3747048
- Rodnitzky RL, Bosch EP. Chronic long-interval plasma exchange in myasthenia gravis. Archives of Neurology 1984;41(7):715-7. https://www.ncbi.nlm.nih.gov/pubmed/6743060
- Rønager J, Ravnborg M, Hermansen I, Vorstrup S. Immunoglobulin treatment versus plasma exchange in patients with chronic moderate to severe myasthenia gravis. Artificial Organs 2001;25(12):967-73. https://www.ncbi.nlm.nih.gov/pubmed/11843764
- Kamel A, Essa M. Effectiveness of prethymecthomy plasmapheresis on short-term outcome of non-thymomatous generalized myasthenia gravis. Egyptian Journal Neurology, Psychiatry and Neurosurgurgery 2009;46(1):161-8.
- Gajdos P, Chevret S, Toyka KV. Plasma exchange for generalised myasthenia gravis. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No.: CD002275. DOI: 10.1002/14651858.CD002275. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002275/full
- Gajdos P, Chevret S, Toyka K. Intravenous immunoglobulin for myasthenia gravis. The Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No.: CD002277. DOI: 10.1002/14651858.CD002277. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002277
- Garcia-Velasco JA, Pellicer A. New concepts in the understanding of the ovarian hyperstimulation syndrome. Current Opinion in Obstetrics and Gynecology 2003;15(3):251-6. https://www.ncbi.nlm.nih.gov/pubmed/12858114
- Vloeberghs V, Peeraer K, Pexsters A, D’Hooghe T. Ovarianhyperstimulation syndrome and complications of ART. Best Practice & Research. Clinical Obstetrics & Gynaecology 2009;23(5):691-709. https://www.ncbi.nlm.nih.gov/pubmed/19632900
- Engmann L, DiLugie A, Schmidt D, Nulsen J, Maier D, Benavida C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomised controlled study. Fertility and Sterility 2008;89(1):84-91. https://www.ncbi.nlm.nih.gov/pubmed/17462639
- Mathur RS, Jenkins JM. Is ovarian hyperstimulation syndrome associated with a poor obstetric outcome?. BJOG 2000;107:943-6. https://www.ncbi.nlm.nih.gov/pubmed/10955422
- Enskog A, Nilsson L, Brännström M. Plasma levels of free vascular endothelial growth factor(165) (VEGF(165
- McClelland DB. Human albumin solutions. BMJ 1990;300:35-57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1661866/
- Shalev E, Giladi Y, Matilsky M, Ben-Ami M. Decreased incidence of severe ovarian hyperstimulation syndrome in high risk in-vitro fertilisation patients receiving intravenous albumin: a prospective study. Human Reproduction 1995;10:1373-6. https://www.ncbi.nlm.nih.gov/pubmed/7593499