The 5:2 Fast Diet
Fasting has been practiced for millennia, but, only recently, studies have shed light on its role in adaptive cellular responses that reduce oxidative damage and inflammation, optimize energy metabolism, and bolster cellular protection. In lower fungi, plants, and animals, chronic fasting extends longevity, in part, by reprogramming metabolic and stress resistance pathways. In rodents intermittent or periodic fasting protects against diabetes, cancers, heart disease, and neurodegeneration, while in humans it helps reduce obesity, hypertension, asthma, and rheumatoid arthritis. Thus, fasting has the potential to delay aging and help prevent and treat diseases while minimizing the side effects caused by chronic dietary interventions 1.
In humans, fasting is achieved by ingesting no or minimal amounts of food and caloric beverages for periods that typically range from 12 hours to three weeks 1. In many clinics, patients are now monitored by physicians while undergoing water only or very low calorie (less than 200 kcal/day) fasting periods lasting from 1 week or longer for weight management, and for disease prevention and treatment 1. Fasting is distinct from caloric restriction in which the daily caloric intake is reduced chronically by 20–40%, but meal frequency is maintained.
Starvation is instead a chronic nutritional insufficiency that is commonly used as a substitute for the word fasting, particularly in lower eukaryotes (eukaryote is an organism with complex cells, or a single cell with a complex structure. In these cells the genetic material is organized into chromosomes in the cell nucleus. Animals, plants, algae and fungi are all eukaryotes), but that is also used to define extreme forms of fasting, which can result in degeneration and death.
Scientists now know that fasting results in ketogenesis, promotes potent changes in metabolic pathways and cellular processes such as stress resistance, lipolysis and autophagy, and can have medical applications that in some cases are as effective as those of approved drugs such as the dampening of seizures and seizure-associated brain damage and the amelioration of rheumatoid arthritis 2, 3, 4. Findings from well-controlled investigations in experimental animals, and emerging findings from human studies, indicate that different forms of fasting may provide effective strategies to reduce weight, delay aging, and optimize health.
The Fast Diet also known as the 5:2 Fast Diet is a diet created by the BBC journalist, doctor and author of the best-selling 5:2 diet, Dr. Michael Mosley. The 5:2 diet first reached the mainstream via a BBC Horizon documentary called Eat, Fast and Live Longer, broadcast in August 2012. The diet, which Mosley insists he was initially skeptical about, is not the only interesting discovery he has made through his research. Because of his family history of diabetes and his recent diagnosis for the metabolic syndrome, Dr. Mosley, instead of resorting to medication, decided to get drastic with his diet and see whether he could effect any change.
He ended up testing all sorts of different forms of fasting, including alternate-day fasting. Eventually, Dr. Mosley came up with something that he called the 5:2 Diet, where you eat normally five days a week and eat less calories on the other two days, which is really counting calories two days a week and eating normally the other five days. He stuck to that for about three months. During that period, he lost about 20 pounds of fat, his body fat went down from 28 percent to 20 percent, and his blood glucose went back to normal. However the body of evidence about 5:2 diet and intermittent fasting is limited when compared to other types of weight loss techniques.
The basic concept of intermittent fasting, where for two days of the week you restrict your calorie intake to about 2500 kilojoules (598 kcal) a day, giving your body a break from processing food and a period where your blood is not filled with glucose.
There’s exciting research indicating that intermittent fasting can have a very beneficial impact on your brain function, too. It may even hold the key to preventing Alzheimer’s disease. Dr. Mark Mattson, on his research with genetically engineered mice. They’ve been genetically engineered so they will develop Alzheimer’s or dementia around 1 year which equivalent of about 40 years or 50 years in humans. When he put them on a junk diet, a junk food diet, they developed it at about nine months. But when he put them on an intermittent fasting diet – alternate-day fasting diet in fact – they developed it at around two years, which is equivalent to being 90 years of age.
When he looked into their brains, he discovered that the ones who had been on intermittent fasting diet have grown 40 percent new brain cells particularly in the area associated with memory. He identified this thing called brain-derived neurotrophic factor (BNF), which seems to be driving those changes and also protecting the brains. He’s doing this big study in humans at the moment to see if the same thing happens with fasting humans.
Adaptive responses to fasting in humans
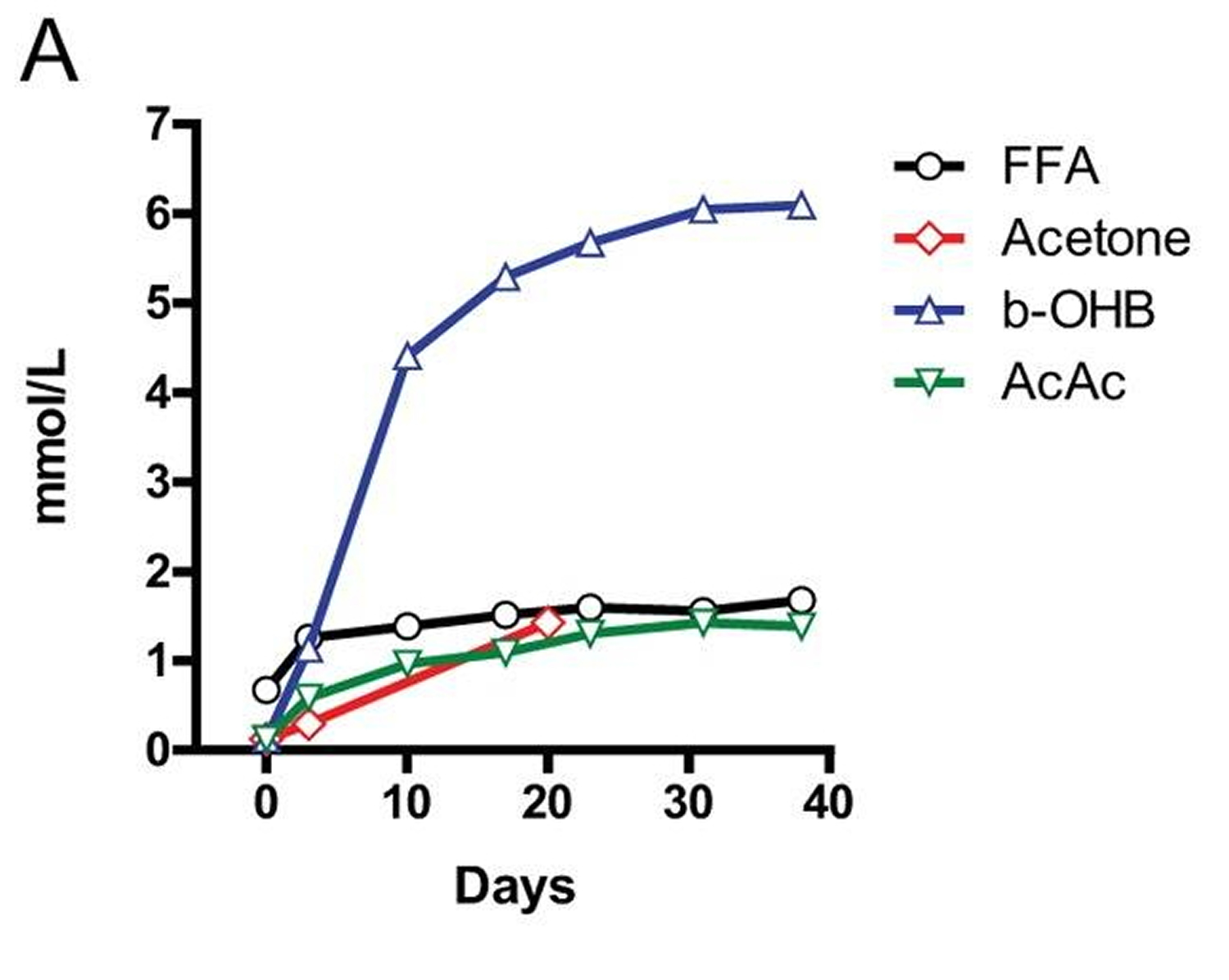
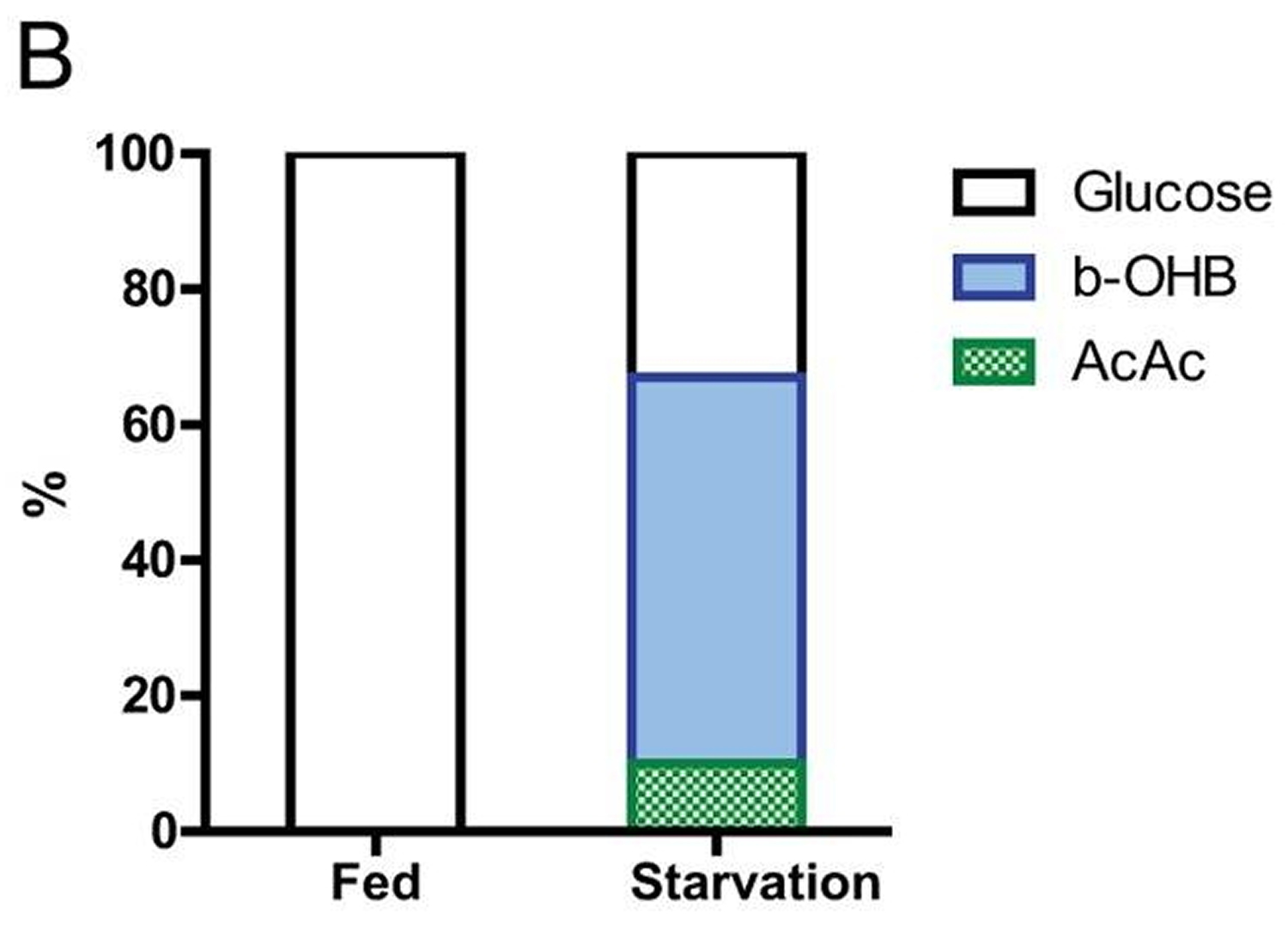
In most mammals, the liver serves as the main reservoir of glucose, which is stored in the form of glycogen. In humans, depending upon their level of physical activity, 12 to 24 hours of fasting typically results in a 20% or greater decrease in serum glucose and depletion of the hepatic glycogen, accompanied by a switch to a metabolic mode in which non-hepatic glucose, fat-derived ketone bodies and free fatty acids are used as energy sources (Figures C and A). Whereas most tissues can utilize fatty acids for energy, during prolonged periods of fasting, the brain relies on the ketone bodies β-hydroxybutyrate and acetoacetate in addition to glucose for energy consumption (Figure B). Ketone bodies are produced in hepatocytes from the acetyl-CoA generated from β oxidation of fatty acids released into the bloodstream by adipocytes, and also by the conversion of ketogenic amino acids. After hepatic glycogen depletion, ketone bodies, fat-derived glycerol, and amino acids account for the gluconeogenesis-dependent generation of approximately 80 grams/day of glucose, which is mostly utilized by the brain. Depending on body weight and composition, the ketone bodies, free fatty acids and gluconeogenesis allow the majority of human beings to survive 30 or more days in the absence of any food and allow certain species, such as king penguins, to survive for over 5 months without food 5.
In humans, during prolonged fasting, the plasma levels of 3-β-hydroxybutyrate (b-OHB) are about 5 times those of free fatty acids and acetoacetic acid (AcAc) (Figure A and B). The brain and other organs utilize ketone bodies in a process termed ketolysis, in which acetoacetic acid and 3-β-hydroxybutyrate are converted into acetoacetyl-CoA and then acetyl-CoA. These metabolic adaptations to fasting in mammals are reminiscent of those described earlier for E. coli and yeast, in which acetic acid accumulates in response to food deprivation 6, 7. It will be important to understand how the different carbon sources generated during fasting affect cellular protection and aging and to determine whether glycerol, specific ketone bodies or fatty acids can provide nourishment while reducing cellular aging in mammals, a possibility suggested by beneficial effects of a dietary ketone precursor in a mouse model of Alzheimer’s disease 8. It will also be important to study, in various model organisms and humans, how high intake of specific types of fats (medium- vs. long-chain fatty acids, etc.) in substitution of carbohydrates and proteins influences gluconeogenesis and glucose levels as well as aging and diseases.
Figure A: Concentrations of ketone bodies (acetone, β-hydroxybutyric acid, acetoacetic acid (AcAc)) and plasma free fatty acids (FFA) during 40 days of fasting in humans. Note the more than three orders of magnitude change in β-hydroxybutyrate (b-OHB) and the doubling of free fatty acids (FFA) 9.
Fasting and the brain
In mammals, severe caloric restriction/food deprivation results in a decrease in the size of most organs except the brain, and the testicles in male mice 10. From an evolutionary perspective this implies that maintenance of a high level of cognitive function under conditions of food scarcity is of preeminent importance. Indeed, a highly conserved behavioral trait of all mammals is to be active when hungry and sedentary when satiated. In rodents, alternating days of normal feeding and fasting (intermittent fasting) can enhance brain function as indicated by improvements in performance on behavioral tests of sensory and motor function 11 and learning and memory 12. The behavioral responses to intermittent fasting are associated with increased synaptic plasticity and increased production of new neurons from neural stem cells 13.
Particularly interesting with regards to adaptive responses of the brain to limited food availability during human evolution is brain-derived neurotrophic factor (BDNF). The genes encoding brain-derived neurotrophic factor (BDNF) and its receptor TrkB appeared in genomes relatively recently as they are present in vertebrates, but absent from worms, flies and lower species 14. The prominent roles of BDNF in the regulation of energy intake and expenditure in mammals is highlighted by the fact that the receptors for both BDNF and insulin are coupled to the highly conserved PI3 kinase – Akt, and MAP kinase signaling pathways. Studies of rats and mice have shown that running wheel exercise and intermittent fasting increase BDNF expression in several regions of the brain, and that brain-derived neurotrophic factor in part mediates exercise- and intermittent fasting-induced enhancement of synaptic plasticity, neurogenesis and neuronal resistance to injury and disease. Brain-derived neurotrophic factor signaling in the brain may also mediate behavioral and metabolic responses to fasting and exercise including regulation of appetite, activity levels, peripheral glucose metabolism and autonomic control of the cardiovascular and gastrointestinal systems 15, 16.
Hunger is an adaptive response to food deprivation that involves sensory, cognitive and neuroendocrine changes which motivate and enable food seeking behaviors. It has been proposed that hunger-related neuronal networks, neuropeptides and hormones play pivotal roles in the beneficial effects of energy restriction on aging and disease susceptibility. As evidence, when mice in which the hypothalamic ‘hunger peptide’ NPY is selectively ablated are maintained on a CR diet, the ability of CR to suppress tumor growth is abolished 17. The latter study further showed that the ability of caloric restriction to elevate circulating adiponectin levels was also compromised in NPY-deficient mice, suggesting a key role for the central hunger response in peripheral endocrine adaptations to energy restriction. Adiponectin levels increase dramatically in response to fasting; and data suggest roles for adiponectin in the beneficial effects of IF on the cardiovascular system 18. The hunger response may also improve immune function during aging as ghrelin-deficient mice exhibit accelerated thymic involution during aging, and treatment of middle age mice with ghrelin increases thymocyte numbers and improves the functional diversity of peripheral T cell subsets 19. In addition to its actions on the hypothalamus and peripheral endocrine cells, fasting may increase neuronal network activity in brain regions involved in cognition, resulting in the production of BDNF, enhanced synaptic plasticity and improved stress tolerance 20. Thus, hunger may be a critical factor involved in widespread central and peripheral adaptive responses to the challenge of food deprivation for extended time periods.
Our current understanding of the impact of intermittent fasting on the nervous system and cognitive functions is largely inferred from animal studies (see above). Interventional studies to determine the impact of fasting on brain function and neurodegenerative disease processes are lacking After 3–4 month, caloric restriction improved cognitive function (verbal memory) in overweight women 21 and in elderly subjects 22. Similarly, when subjects with mild cognitive impairment were maintained for 1 month on a low glycemic diet, they exhibited improved delayed visual memory, cerebrospinal fluid biomarkers of Aβ metabolism and brain bioenergetics 23. Studies in which cognitive function, regional brain volumes, neural network activity, and biochemical analyses of cerebrospinal fluid are measured in human subjects before and during an extended period of intermittent fasting should clarify the impact of intermittent fasting on human brain structure and function.
Figure B: Brain substrate utilization in three fasting obese volunteers after several weeks of food deprivation. Many studies suggest that human brain cells can survive with little to no glucose, but this has not been clearly demonstrated 24.
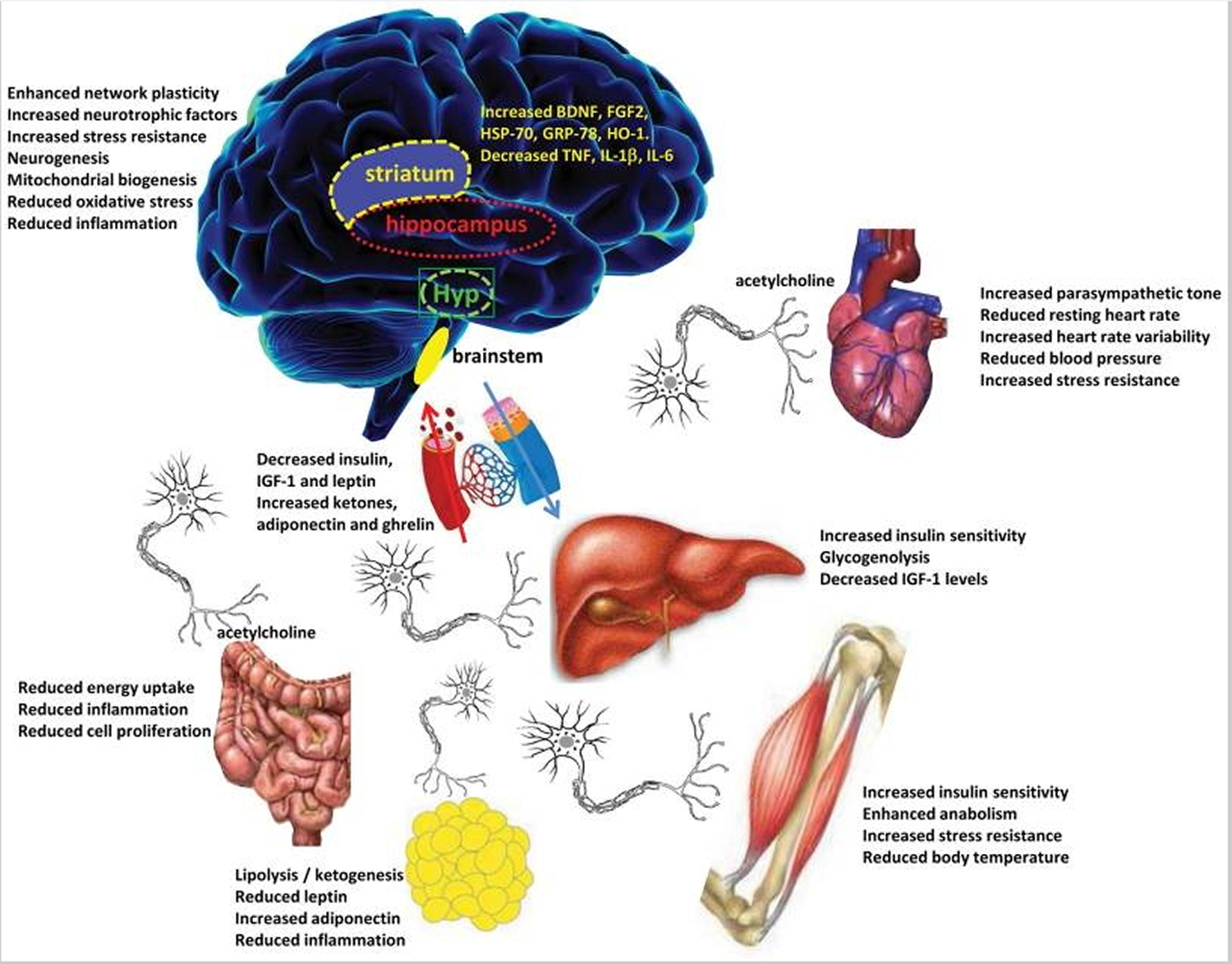
Figure C: Intermittent Fasting modifies brain neurochemistry and neuronal network activity in ways that optimize brain function and peripheral energy metabolism. Four brain regions that are particularly important in adaptive responses to Intermittent Fasting include the hippocampus (cognitive processing), striatum (control of body movements), hypothalamus (Hyp, control of food intake and body temperature) and brainstem (control of cardiovascular and digestive systems). The brain communicates with all of the peripheral organs involved in energy metabolism. Intermittent Fasting enhances parasympathetic activity (mediated by the neurotransmitter acetylcholine) in the autonomic neurons that innervate the gut, heart and arteries, resulting in improved gut motility and reduced heart rate and blood pressure. By depleting glycogen from liver cells, fasting results in lipolysis and the generation of ketone bodies resulting in a reduction in body fat. Intermittent Fasting enhances insulin sensitivity of muscle and liver cells, and reduces IGF-1 production. Levels of oxidative stress and inflammation are reduced throughout the body and brain in response to Intermittent Fasting 9.
Fasting and Aging
Clinical and epidemiological data are consistent wit h an ability of fasting to retard the aging process and associated diseases 9. Among the major effects of fasting relevant to aging and diseases are changes in the levels of IGF-1, IGFBP1, glucose, and insulin. Fasting for 3 or more days causes a 30% or more decrease in circulating insulin and glucose, as well as rapid decline in the levels of insulin-like growth factor 1 (IGF-1), the major growth factor in mammals, which together with insulin is associated with accelerated aging and cancer 25. In humans, five days of fasting causes an over 60% decrease in IGF-1and a 5-fold or higher increase in one of the principal IGF-1-inhibiting proteins: IGFBP1 26. This effect of fasting on IGF-1 is mostly due to protein restriction and particularly to the restriction of essential amino acids, but is also supported by calorie restriction since the decrease in insulin levels during fasting promotes reduction in IGF-1 26. Notably, in humans, chronic calorie restriction does not lead to a decrease in IGF-1 unless combined with protein restriction 27. Although extreme dietary interventions during old age may continue to protect from age-related diseases, they could have detrimental effects on the immune system and the ability to respond to certain infectious diseases, wounds and other challenges 28, 29.
Fasting and Cancer
Fasting has the potential for applications in both cancer prevention and treatment. Although no human data are available on the effect of intermittent fasting or periodic fasting in cancer prevention, their effect on reducing IGF-1, insulin and glucose levels, and increasing IGFBP1 and ketone body levels could generate a protective environment that reduces DNA damage and carcinogenesis, while at the same time creating hostile conditions for tumor and pre-cancerous cells. In fact, elevated circulating IGF-1 is associated with increased risk of developing certain cancers 30, 31 and individuals with severe IGF-1deficiency caused by growth hormone receptor deficiency, rarely develop cancer 32, 33, 34.
In a preliminary study of 10 subjects with a variety of malignancies, the combination of chemotherapy with fasting resulted in a decrease in a range of self-reported common side effects caused by chemotherapy compared to the same subjects receiving chemotherapy while on a standard diet 35. The effect of fasting on chemotherapy toxicity and cancer progression is now being tested in clinical trials in both Europe and the US.
Fasting, inflammation and hypertension
In humans, one of the best demonstrations of the beneficial effects of long-term fasting lasting one to 3 weeks is in the treatment of rheumatoid arthritis. In agreement with the results in rodents, there is little doubt that during the period of fasting both inflammation and pain are reduced in rheumatoid arthritis patients 4. However, after the normal diet is resumed, inflammation returns unless the fasting period is followed by a vegetarian diet 36, a combination therapy that has beneficial effects lasting for two years or longer 37. The validity of this approach is supported by four differently controlled studies, including two randomized trials 4. Therefore, fasting combined with a vegetarian diet and possibly with other modified diets provides beneficial effects in the treatment of rheumatoid arthritis. Alternate day intermittent fasting also resulted in significant reductions in serum TNFα and ceramides in asthma patients during a 2 month period 38. The latter study further showed that markers of oxidative stress often associated with inflammation (protein and lipid oxidation) were significantly reduced in response to intermittent fasting. Thus, for many patients able and willing to endure long-term fasting and to permanently modify their diet, fasting cycles would have the potential to not only augment but also replace existing medical treatments.
Water only and other forms of long-term fasting have also been documented to have potent effects on hypertension. An average of 13 days of water only fasting resulted in the achievement of a systolic blood pressure (BP) below 120 in 82% of subjects with borderline hypertension with a mean 20 mm Hg reduction in BP 39. BP remained significantly lower compared to baseline even after subjects resumed the normal diet for an average of 6 days 39. A small pilot study of patients with hypertension (140 mm and above systolic BP) also showed that 10–11 days of fasting caused a 37–60 mm decrease in systolic BP 40. These preliminary studies are promising but underscore the need for larger controlled and randomized clinical studies that focus on periodic fasting strategies that are feasible for a larger portion of the population.
Fasting and the metabolic syndrome
Periodic fasting can reverse multiple features of the metabolic syndrome in humans: it enhances insulin sensitivity, stimulates lipolysis and reduces blood pressure. Body fat and blood pressure were reduced and glucose metabolism improved in obese subjects in response to an alternate day modified fast 41, 42. Overweight subjects maintained for 6 months on a twice weekly intermittent fasting diet in which they consumed only 500–600 calories on the fasting days, lost abdominal fat, displayed improved insulin sensitivity and reduced blood pressure 43. Three weeks of alternate day fasting resulted in reductions in body fat and insulin levels in normal weight men and women 44 and Ramadan fasting (2 meals/day separated by approximately 12 hours) in subjects with MS resulted in decreased daily energy intake, decreased plasma glucose levels and increased insulin sensitivity 45. Subjects undergoing coronary angiography who reported that they fasted regularly exhibited a lower prevalence of diabetes compared to non-fasters 46. Anti-metabolic syndrome effects of intermittent fasting were also observed in healthy young men (BMI of 25) after 15 days of alternate day fasting: their whole-body glucose uptake rates increased significantly, levels of plasma ketone bodies and adiponectin were elevated, all of which occurred without a significant decrease in body weight 47. The latter findings are similar to data from animal studies showing that IF can improve glucose metabolism even with little or no weight change 48. It will be important to determine if longer fasting periods which promote a robust switch to a fat breakdown and ketone body-based metabolism, can cause longer lasting and more potent effects.
How does the Fast Diet work ?
On the 5:2 plan, you cut your food down to one-fourth of your normal daily calories on fasting days (about 600 calories for men and about 500 for women), along with plenty of water and tea. On the other five days of the week, you can eat normally.
If we were to distill the Fast Diet into a single sound-bite, it would all come down to 5:2. That’s five days of normal eating, with little thought to calorie control and a slice of pie for pudding if that’s what you want. Then, on the other two days, you reduce your calorie intake to 500 calories for women and 600 calories for men.
- Day 1 Normal
- Day 2 Normal
- Day 3 FASTING (reduce your calorie intake to 500 calories for women and 600 calories for men)
- Day 4 Normal
- Day 5 FASTING (reduce your calorie intake to 500 calories for women and 600 calories for men)
- Day 6 Normal
- Day 7 Normal
Since you are only fasting for two days of your choice each week, and eating normally on the other five days, there is always something new and tasty on the near horizon. In short, it’s easy to comply with a regime that only asks you to restrict your calorie intake occasionally. It recalibrates the diet equation, and stacks the odds in your favour.
It is important to note that this is not a permanent eating program and once your insulin resistance improves and you are normal weight, you can start eating more food as you will have reestablished your body’s ability to burn fat for fuel.
One of the arguments for intermittent fasting is that it mimics the way our ancestors ate. They didn’t have access to food 24/7, and underwent alternating intervals of “feast and famine.” The human body is adapted to this, and research shows that abstaining from food now and then actually optimizes biological function all-around.
Perhaps best of all, intermittent fasting is not something you have to do non-stop for the rest of your life. Most who are insulin/leptin resistant would benefit from doing it continuously until the resistance resolves. However, once your weight is ideal and you have no high blood pressure, abnormal cholesterol ratios, or diabetes, then you can have more meals until or unless the insulin/leptin resistance returns.

How many calories on a non-Fast Day ?
You may have wondered how he came up with the recommendation that women have 500 calories and men have 600 calories on a Fast Day. Dr. Mosley used the rule of thumb that women need 2000 calories and men need 2400 calories per day and on a Fast Day you should eat a quarter of a normal day’s recommended calories. Some of you have also wondered exactly how many calories you should be eating on days when you’re not fasting.
Intermittent Fasting Actually curbs Your Hunger
Many are hesitant to try fasting as they fear they’ll be ravenously hungry all the time. But one of the most incredible side effects of intermittent fasting that we’ve found is the disappearance of hunger and sugar cravings.
Dr. Mosley had the same experience once he began fasting. Others have also contacted him saying they’re astonished to realize that hunger no longer dominates their lives; they’re back in control. Now, you get hungry because your body needs fuel. But the vast majority of people in the world, certainly in the developed world, are eating foods that severely inhibit their ability to produce lipase and use fat as an energy source. Lipase is inhibited because of high insulin levels, and your insulin rises in response to eating foods high in carbohydrates.
If you struggle with food cravings, especially sugar, know that once you make this shift to burning fat instead of sugar as your body’s primary fuel, your hunger for unhealthy foods will vanish, and you will not have to exert enormous amounts of self-discipline to resist unhealthy foods any longer. You will be back in control!
What can you eat on the 5:2 Fast Diet
- High in healthy fats. Many will benefit from 50-85 percent of their daily calories in the form of healthy fat from avocados, organic grass-fed butter, pastured egg yolks, coconut oil, and raw nuts such as macadamia, pecans, and pine nuts.
- Moderate amounts of high-quality protein from organically raised, grass-fed or pastured animals. Most will likely not need more than 40 to 70 grams of protein per day.
- Unrestricted amounts of fresh vegetables.
Intermittent Exercise
Dr. Mosley is also a proponent of high intensity interval training (HIIT), and recently finished a new book called Fast Exercise.
- Sedentary : Little or no exercise. This level is for someone who does not or cannot incorporate exercise into their daily life (eg drives rather than walks, takes the lift rather than the stairs, has a desk job or restricted mobility).
- Lightly active : Light exercise or sports 1-3 days per week. This level would include people who incorporate walking and activity into their day to day activities but do not have an exercise regime at such or exercise or play sports fewer than three times a week.
- Moderately active : Moderate exercise or sports 3-5 days per week. This level is for people who exercise or play very active sports at least 30 minutes non-stop at a time at least three times a week, every week. This is the level for people who keep up a good fitness regime that fits into their daily life.
- Very active : Hard exercise or sports 6-7 days per week. This level would include serious non-professional athletes actively training for, eg, a triathlon that requires near daily hard exercise for at least an hour at a time.
- Extremely active : Very hard exercise or sports more than once every day and a physical job. This level is for people doing exercise multiple times per day, at least an hour at a time and with the type of physical job that requires top fitness. This level is not common – most non-professional athletes in serious training will be in the “Very active” level at most.
Optimizing your brain function is yet another amazing benefit of applying these two powerful approaches – intermittent fasting and intermittent exercise. You’re actually able to think clearer, get more done, and be far more efficient. It’s a phenomenal side effect of following this type of program.
Health Effects of a Intermittent Fasting Diet
Dietary restriction has been shown in a variety of animal models to have many health benefits. Fasting, in which food isn’t consumed (but water is), represents the extreme form of restriction. Previous studies in animals and people suggested that periodic cycles of fasting may improve certain metabolic and immune functions. Fasting for 2 or more days, like the 5:2 Diet, however, is difficult for many people, and can have adverse health effects.
Here we review the fascinating and potent effects of different forms of fasting including intermittent fasting (including alternate day fasting, or twice weekly fasting, the 5:2 Diet) and periodic fasting lasting several days or longer every 2 or more weeks.
Fasting, the most extreme form of Dietary Restriction, which entails the abstinence from all food but not water, can be applied in a chronic manner as intermittent fasting or periodically as cycles of prolonged fasting lasting 2 or more days 1. In rodents, intermittent fasting promotes protection against diabetes, cancer, heart disease and neuro-degeneration (Longo and Mattson, 2014). In humans, IF and less severe regimens (e.g. consumption of approximately 500 kcal/day for 2 days a week), have beneficial effects on insulin, glucose, C-reactive protein, and blood pressure (Harvie et al., 2011).
A team led by Dr. Valter Longo at the University of Southern California 49 studied diets designed to mimic the beneficial effects of fasting while minimizing the risks and difficulty associated with complete food restriction. The research was funded in part by National Institutes of Health’s National Institute on Aging. Results were published in Cell Metabolism on July 7, 2015 49.
The team first tested cycles of prolonged fasting in yeast, a single-celled organism. Yeast that were switched back and forth from a nutrient-rich environment to water for several cycles had a longer lifespan and were better able to survive toxin exposure—a marker of increased stress resistance—than yeast not exposed to periodic starvation.
The team next tested a very low-calorie, low-protein diet in mice. The diet was designed to mimic some of the beneficial effects of fasting, including improving markers of longevity and metabolism. Middle-aged mice (16 months old) were fed the diet for 4 consecutive days, followed by 10 days of unlimited access to food. The mice overate during these phases so that their overall calorie intake was similar to mice continuously fed a regular diet.
Mice fed the diet twice a month for several months had various metabolic changes, including lower blood glucose and insulin levels, than mice fed a control diet. These metabolic markers all returned to normal levels during periods of re-feeding. Mice fed the diet had less fat around their organs (known as deep or visceral fat) at 28 months of age. They also had greater bone density at old age and increased nerve cell development in the brain. At the end of life, mice on the diet had fewer tumors and skin lesions than control mice.
The team next conducted a pilot study in a small group of people. Nineteen healthy adults consumed a proprietary plant-based diet that provided between 34% and 54% of the normal caloric intake with at least 9–10% protein, 34–47% carbohydrate, and 44–56% fat. Participants consumed the diet 5 days a month for 3 months (3 cycles), resuming their normal diet at the end of each diet period. A control group of 19 adults ate a normal diet.
People on the diet had improvements in blood glucose and decreased body weight compared to the control group. Those with initially elevated C-reactive protein levels (a marker of heart disease risk) had lower levels, while those with normal levels had no change. Reports of side effects were low and included fatigue, weakness, and headache.
“Strict fasting is hard for people to stick to, and it can also be dangerous, so we developed a complex diet that triggers the same effects in the body,” Longo says. “It’s not a typical diet because it isn’t something you need to stay on.”
More research will be needed to determine the long-term impact of the diet on human health and provide information on when and how such a diet might be applied.
Are there any side effects from intermittent fasting ?
Little is known about possible side effects as no systematic attempt has been made to study this issue. Anecdotal reports of effects include:
- difficulties sleeping
- bad breath (a known problem with low carbohydrate diets)
- irritability
- anxiety
- dehydration
- daytime sleepiness
However, more research would be needed to confirm these side effects and their severity.
If you are fasting, you may want to think about how fasting will impact on your life during your fasting days. You are likely to be very hungry and have less energy and this could affect your ability to function (such as at work), in particular it may affect your ability to exercise which is an important part of maintaining a healthy weight.
Also, intermittent fasting may not be suitable for pregnant women and people with specific health conditions, such as diabetes, or a history of eating disorders.
Because it is a fairly radical approach to weight loss, if you are considering trying IF for yourself, it is wise to speak to your GP first to see if it is safe to do so.
Summary
Based on the existing evidence from animal and human studies described, there is great potential for lifestyles that incorporate periodic fasting (Intermittent Fasting) during adult life to promote optimal health and reduce the risk of many chronic diseases, particularly for those who are overweight and sedentary. Fasting periods lasting longer than 24 hours and particularly those lasting 3 or more days should be done under the supervision of a physician and preferably in a clinic.
Despite 5:2 diet increasing popularity, there is a great deal of uncertainty about the 5:2 diet with significant gaps in the evidence.
For example, it is unclear:
- What pattern of intermittent fasting is the most effective in improving health outcomes – 5:2, alternative day fasting, or something else entirely different
- What is the optimal calorie consumption during the fasting days – the 5:2 diet recommends 500 calories for women and 600 for men, but these recommendations seem arbitrary without clear evidence to support them.
- How sustainable is intermittent fasting in the long-term – would most people be willing to stick with the plan for the rest of their lives ?
- Fasting: molecular mechanisms and clinical applications. Longo VD, Mattson MP. Cell Metab. 2014 Feb 4; 19(2):181-92. https://www.ncbi.nlm.nih.gov/pubmed/24440038/
- Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Ann Neurol. 1999 Jan; 45(1):8-15.
- Intermittent fasting: a “new” historical strategy for controlling seizures ? Hartman AL, Rubenstein JE, Kossoff EH. Epilepsy Res. 2013 May; 104(3):275-9. https://www.ncbi.nlm.nih.gov/pubmed/23206889/
- Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Müller H, de Toledo FW, Resch KL. Scand J Rheumatol. 2001; 30(1):1-10. https://www.ncbi.nlm.nih.gov/pubmed/11252685/
- Heterothermy in growing king penguins. Eichhorn G, Groscolas R, Le Glaunec G, Parisel C, Arnold L, Medina P, Handrich Y. Nat Commun. 2011 Aug 16; 2():435. https://www.ncbi.nlm.nih.gov/pubmed/21847109/
- Genome-wide screen identifies Escherichia coli TCA-cycle-related mutants with extended chronological lifespan dependent on acetate metabolism and the hypoxia-inducible transcription factor ArcA. Gonidakis S, Finkel SE, Longo VD. Aging Cell. 2010 Oct; 9(5):868-81. https://www.ncbi.nlm.nih.gov/pubmed/20707865/
- Replicative and chronological aging in Saccharomyces cerevisiae. Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Cell Metab. 2012 Jul 3; 16(1):18-31. https://www.ncbi.nlm.nih.gov/pubmed/22768836/
- A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. Neurobiol Aging. 2013 Jun; 34(6):1530-9. https://www.ncbi.nlm.nih.gov/pubmed/23276384/
- Fasting: molecular mechanisms and clinical applications. Longo VD, Mattson MP. Cell Metab. 2014 Feb 4; 19(2):181-92. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3946160/
- Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. Weindruch R, Sohal RS. N Engl J Med. 1997 Oct 2; 337(14):986-94. https://www.ncbi.nlm.nih.gov/pubmed/9309105/
- Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G. Age (Dordr). 2012 Aug; 34(4):917-33. https://www.ncbi.nlm.nih.gov/pubmed/21861096/
- Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. Fontán-Lozano A, Sáez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Domínguez SA, López-Lluch G, Delgado-García JM, Carrión AM. J Neurosci. 2007 Sep 19; 27(38):10185-95. https://www.ncbi.nlm.nih.gov/pubmed/17881524/
- Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. Lee J, Seroogy KB, Mattson MP. J Neurochem. 2002 Feb; 80(3):539-47. https://www.ncbi.nlm.nih.gov/pubmed/11905999/
- Trophic factors: An evolutionary cul-de-sac or door into higher neuronal function ? Chao MV. J Neurosci Res. 2000 Feb 1; 59(3):353-5. https://www.ncbi.nlm.nih.gov/pubmed/10679770/
- Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Mattson MP. Cell Metab. 2012 Dec 5; 16(6):706-22. https://www.ncbi.nlm.nih.gov/pubmed/23168220/
- Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Rothman SM, Griffioen KJ, Wan R, Mattson MP. Ann N Y Acad Sci. 2012 Aug; 12, 64:49-63. https://www.ncbi.nlm.nih.gov/pubmed/22548651/
- Starvation-induced activation of ATM/Chk2/p53 signaling sensitizes cancer cells to cisplatin. Shi Y, Felley-Bosco E, Marti TM, Orlowski K, Pruschy M, Stahel RA. BMC Cancer. 2012 Dec 4; 12:571. https://www.ncbi.nlm.nih.gov/pubmed/23211021/
- Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, Mattson MP. J Nutr Biochem. 2010 May; 21(5):413-7. https://www.ncbi.nlm.nih.gov/pubmed/19423320/
- Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Sci Transl Med. 2012 Jan 25; 4(118):118ra11. https://www.ncbi.nlm.nih.gov/pubmed/22277968/
- Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Rothman SM, Griffioen KJ, Wan R, Mattson MP. Ann N Y Acad Sci. 2012 Aug; 1264():49-63. https://www.ncbi.nlm.nih.gov/pubmed/22548651/
- Cognitive effects of a long-term weight reducing diet. Kretsch MJ, Green MW, Fong AK, Elliman NA, Johnson HL. Int J Obes Relat Metab Disord. 1997 Jan; 21(1):14-21. https://www.ncbi.nlm.nih.gov/pubmed/9023595/
- Caloric restriction improves memory in elderly humans. Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Proc Natl Acad Sci U S A. 2009 Jan 27; 106(4):1255-60. https://www.ncbi.nlm.nih.gov/pubmed/19171901/
- Diet intervention and cerebrospinal fluid biomarkers in amnestic mild cognitive impairment. Bayer-Carter JL, Green PS, Montine TJ, VanFossen B, Baker LD, Watson GS, Bonner LM, Callaghan M, Leverenz JB, Walter BK, Tsai E, Plymate SR, Postupna N, Wilkinson CW, Zhang J, Lampe J, Kahn SE, Craft S. Arch Neurol. 2011 Jun; 68(6):743-52. https://www.ncbi.nlm.nih.gov/pubmed/21670398/
- Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. https://www.ncbi.nlm.nih.gov/pubmed/16848698
- Extending healthy life span–from yeast to humans. Fontana L, Partridge L, Longo VD. Science. 2010 Apr 16; 328(5976):321-6. https://www.ncbi.nlm.nih.gov/pubmed/20395504/
- Nutritional regulation of the insulin-like growth factors. Thissen JP, Ketelslegers JM, Underwood LE. Endocr Rev. 1994 Feb; 15(1):80-101. https://www.ncbi.nlm.nih.gov/pubmed/8156941/
- Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Aging Cell. 2008 Oct; 7(5):681-7. https://www.ncbi.nlm.nih.gov/pubmed/18843793/
- Calorie restriction and susceptibility to intact pathogens. Kristan DM. Age (Dordr). 2008 Sep; 30(2-3):147-56. https://www.ncbi.nlm.nih.gov/pubmed/19424864/
- Enhanced cell proliferation and biosynthesis mediate improved wound repair in refed, caloric-restricted mice. Reed MJ, Penn PE, Li Y, Birnbaum R, Vernon RB, Johnson TS, Pendergrass WR, Sage EH, Abrass IB, Wolf NS. Mech Ageing Dev. 1996 Jul 31; 89(1):21-43. https://www.ncbi.nlm.nih.gov/pubmed/8819104/
- Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and prostate cancer risk: epidemiological studies. Chan JM, Stampfer MJ, Giovannucci E, Ma J, Pollak M. Growth Horm IGF Res. 2000 Apr; 10 Suppl A:S32-3. https://www.ncbi.nlm.nih.gov/pubmed/10984284/
- Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and the risk of colorectal adenoma and cancer in the Nurses’ Health Study. Giovannucci E, Pollak M, Platz EA, Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE. Growth Horm IGF Res. 2000 Apr; 10 Suppl A():S30-1. https://www.ncbi.nlm.nih.gov/pubmed/10984283/
- Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Sci Transl Med. 2011 Feb 16; 3(70):70ra13. https://www.ncbi.nlm.nih.gov/pubmed/21325617/
- Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Shevah O, Laron Z. Growth Horm IGF Res. 2007 Feb; 17(1):54-7. https://www.ncbi.nlm.nih.gov/pubmed/17166755/
- Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Steuerman R, Shevah O, Laron Z. Eur J Endocrinol. 2011 Apr; 164(4):485-9. https://www.ncbi.nlm.nih.gov/pubmed/21292919/
- Fasting and cancer treatment in humans: A case series report. Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, Lee C, Cohen P, Longo VD. Aging (Albany NY). 2009 Dec 31; 1(12):988-1007. https://www.ncbi.nlm.nih.gov/pubmed/20157582/
- Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Laerum E, Eek M, Mowinkel P, Hovi K, Førre O. Lancet. 1991 Oct 12; 338(8772):899-902. https://www.ncbi.nlm.nih.gov/pubmed/1681264/
- Vegetarian diet for patients with rheumatoid arthritis–status: two years after introduction of the diet. Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Førre O. Clin Rheumatol. 1994 Sep; 13(3):475-82. https://www.ncbi.nlm.nih.gov/pubmed/7835013/
- Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Free Radic Biol Med. 2007 Mar 1; 42(5):665-74. https://www.ncbi.nlm.nih.gov/pubmed/17291990/
- Medically supervised water-only fasting in the treatment of borderline hypertension. Goldhamer AC, Lisle DJ, Sultana P, Anderson SV, Parpia B, Hughes B, Campbell TC. J Altern Complement Med. 2002 Oct; 8(5):643-50. https://www.ncbi.nlm.nih.gov/pubmed/12470446/
- Medically supervised water-only fasting in the treatment of hypertension. Goldhamer A, Lisle D, Parpia B, Anderson SV, Campbell TC. J Manipulative Physiol Ther. 2001 Jun; 24(5):335-9. https://www.ncbi.nlm.nih.gov/pubmed/11416824/
- Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Klempel MC, Kroeger CM, Varady KA. Metabolism. 2013 Jan; 62(1):137-43. https://www.ncbi.nlm.nih.gov/pubmed/22889512/
- Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Varady KA, Bhutani S, Church EC, Klempel MC. Am J Clin Nutr. 2009 Nov; 90(5):1138-43. https://www.ncbi.nlm.nih.gov/pubmed/19793855/
- The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. Int J Obes (Lond). 2011 May; 35(5):714-27. https://www.ncbi.nlm.nih.gov/pubmed/20921964/
- Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Am J Clin Nutr. 2005 Jan; 81(1):69-73. https://www.ncbi.nlm.nih.gov/pubmed/15640462/
- Effect of Ramadan fasting on some indices of insulin resistance and components of the metabolic syndrome in healthy male adults. Shariatpanahi ZV, Shariatpanahi MV, Shahbazi S, Hossaini A, Abadi A. Br J Nutr. 2008 Jul; 100(1):147-51. https://www.ncbi.nlm.nih.gov/pubmed/18053308/
- Relation of routine, periodic fasting to risk of diabetes mellitus, and coronary artery disease in patients undergoing coronary angiography. Horne BD, Muhlestein JB, May HT, Carlquist JF, Lappé DL, Bair TL, Anderson JL, Intermountain Heart Collaborative Study Group. Am J Cardiol. 2012 Jun 1; 109(11):1558-62. https://www.ncbi.nlm.nih.gov/pubmed/22425331/
- Effect of intermittent fasting and refeeding on insulin action in healthy men. Halberg N, Henriksen M, Söderhamn N, Stallknecht B, Ploug T, Schjerling P, Dela F. J Appl Physiol (1985). 2005 Dec; 99(6):2128-36. https://www.ncbi.nlm.nih.gov/pubmed/16051710/
- Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Proc Natl Acad Sci U S A. 2003 May 13; 100(10):6216-20. https://www.ncbi.nlm.nih.gov/pubmed/12724520/
- A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB, Longo VD. Cell Metab. 2015 Jul 7;22(1):86-99. doi: 10.1016/j.cmet.2015.05.012. Epub 2015 Jun 18. PMID: 26094889. https://www.ncbi.nlm.nih.gov/pubmed/26094889