What is yohimbine
Yohimbine is an indole alkaloid derived from the bark of the Central African Pausinystalia yohimbe tree with alpha-2-adrenergic blocking activity that is widely used as therapy for erectile dysfunction and as a mydriatic (induces dilation of the pupil). The Pausinystalia yohimbe tree bark is used to make extracts, tablets, and capsules. Yohimbe supplement, though banned in many countries, are sold in hundreds of dietary supplements in the USA. In the USA, dietary supplements are regulated as food rather than drugs 1. In parts of Africa, tea made from yohimbe bark has been used as an aphrodisiac (to increase sexual desire). However, there are no human clinical studies of Pausinystalia yohimbe extract for low libido or erectile dysfunction 2. As a leading P. johimbe expert has succinctly summarized, ‘Any discussion of the use of the Pausinystalia yohimbe tree bark for sexual enhancement begins and ends with folklore 2. In African folk medicine, yohimbe is used for multiple conditions including cough, fever, leprosy, heart disease, impotence, athletic performance, weight loss, chest pain, high blood pressure, diabetic neuropathy and as an anesthetic, hallucinogen and aphrodisiac.
Yohimbine is promoted for erectile dysfunction, athletic performance, weight loss, angina (chest pain caused by not enough blood flow to the heart), high blood pressure, diabetic neuropathy, and more. However, there is very little research in people on the effects of yohimbe as a dietary supplement and there are no human clinical studies of Pausinystalia yohimbe extract for low libido or erectile dysfunction 2. As a leading Pausinystalia yohimbe expert has succinctly summarized, “Any discussion of the use of the Pausinystalia yohimbe bark for sexual enhancement begins and ends with folklore” 2. But studies have documented the risks of taking yohimbe supplements. The amount of yohimbine in dietary supplements may vary; some yohimbe products contain very little yohimbine. Yohimbe sold as a dietary supplement may not work like the prescription medication that contains yohimbine. In an analysis 49 brands of Yohimbe supplements labelled as containing yohimbe or yohimbine available for sale from seven major retailers in the USA 3. The quantity of the most active alkaloid, yohimbine, per recommended serving ranged from none detected to 12.1 mg 3. Thirty‐nine percent of the supplements (19/49) did not contain rauwolscine and corynanthine suggesting that the yohimbine was either from highly processed plant extract or synthetic in origin 3. Only 11 supplement brands (22%, 11/49) listed a specific quantity of yohimbine on the label 3. Most of these were inaccurately labelled (actual content ranged from 23% to 147% of the content on the label) 3. Eighteen percent (9/49) of the supplements’ labels did not provide any information about yohimbine’s adverse effects. Of the 49 yohimbine supplement brands sold at seven major retail chains in the USA, only 4.1% (2/49) provided consumers with both accurate information about the quantity of yohimbine as well as information about yohimbine’s known adverse effects 3.
Yohimbine hydrochloride is a standardized form of yohimbine, is available in the United States as a prescription drug for erectile dysfunction. This is a different product than dietary supplements made from the bark of the yohimbe tree. The amount of yohimbine in dietary supplements may vary; some yohimbe products contain very little yohimbine. Yohimbe sold as a dietary supplement may not work like the prescription medication that contains yohimbine. It is illegal in the United States to market an over-the-counter product containing yohimbine as a treatment for erectile dysfunction without getting approval from the U.S. Food and Drug Administration to do so. There is not enough research to say whether yohimbe as a dietary supplement is helpful for any condition, including erectile dysfunction, athletic performance, or weight loss.
- Yohimbe has been associated with heart attacks and seizures.
- Yohimbe caused stomach problems, tachycardia (a rapid heartbeat), anxiety, and high blood pressure, according to a study comparing calls about yohimbe and other substances made to the California Poison Control System between 2000 and 2006 4. People calling about yohimbe were generally more likely to need medical care than other callers.
- Most yohimbe products don’t say how much yohimbine they contain. The amount may vary a lot among products, according to a recent analysis of 49 brands of supplements labeled as containing yohimbe or yohimbine for sale in the United States. Some of the yohimbine was either synthetic or from highly processed plant extract.
The German Commission E (the German equivalent of the Food and Drug Administration [FDA]) 5, 6 has assessed yohimbehe cortex (yohimbe bark consisting of dried bark from the trunk and stems of Pausinystalia yohimbe and preparations made from them) as a herbal medicine. Its therapeutic use to treat sexual dysfunction, as an aphrodisiac and for fatigue and exhaustion, has been rejected because of inadequate proof of its efficacy, and the inability to carry out a risk-benefit assessment 7. The risks in the therapeutic use of the main alkaloid yohimbine are excitation, tremor, insomnia, fear, hypertension, tachycardia, nausea and vomiting. There were also interactions with psychotropic pharmaceuticals 7.
What is yohimbe vs yohimbine?
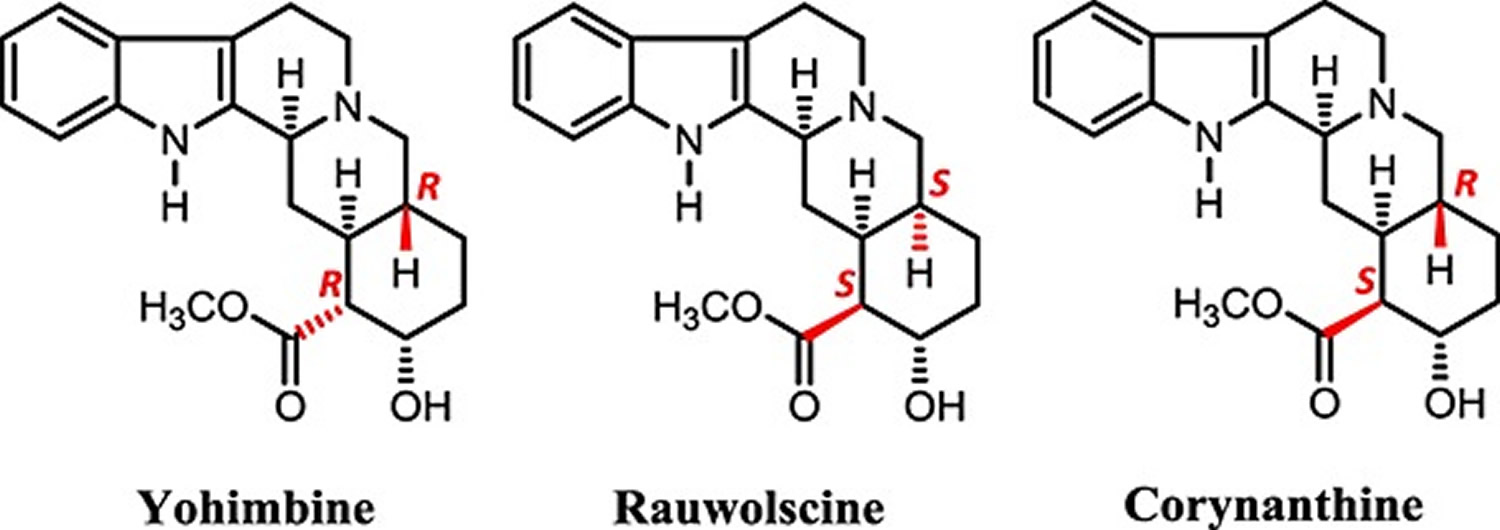
Yohimbe is an evergreen tree native to Central Africa. The Central African Pausinystalia yohimbe tree bark has three alkaloids – yohimbine (10–15% of total content), rauwolscine (alpha-Yohimbine) and corynanthine 8. alpha-Yohimbine (α-Yohimbine) is also called rauwolscine. Other alkaloids, including corynanthine, ajmalicine, and other yohimbine isomers, are also found in Pausinystalia yohimbe tree bark 9. But the focus of most interest has been yohimbine, an indole alkaloid which has been shown to be an alpha 2 adrenergic receptor antagonist. In animal models, yohimbine increases sexual activity and is likely to act by engagement and inhibition of the alpha 2 adrenergic receptors in the corpus cavernosum (part of the penis), causing sustained engorgement of the corporeal tissue of the penis (penile erection). Yohimbine has been chemically synthesized and is the synthetic form is currently marketed in the United States. The herbal bark extract may have other active components and is purported to be more potent and have more side effects. In clinical trials, synthetic yohimbine has had a consistent, although limited effect on erective dysfunction. Yohimbine effect on sexual desire is less well defined. The usual recommended dose of purified yohimbine is 5 to 10 mg three times a day. Drug tolerance or tachyphylaxis may occur. Side effects are usually mild and transient and are typical of alpha 2 adrenergic inhibition, including insomnia, anxiety, palpitations, chest pain, sweating, blurred vision and hypertension. Overdose can cause hypotension, tachycardia, seizures, paralysis and coma; deaths from overdose have been described.
Figure 1. Yohimbe tree bark alkaloids – yohimbine, rauwolscine (alpha-yohimbine) and corynanthine
Footnote: Structures of yohimbine, rauwolscine and corynanthine.
[Source 10 ]Yohimbine effects
Yohimbine is marketed as a pharmaceutical prescribed for the treatment of erectile impotence and has been used in multiple clinical trials as a probe to identify abnormal physiological and affective responses to increased noradrenergic signaling, especially in patients with panic disorders 10. While Pausinystalia yohimbe bark extract has not been studied in human clinical trials, the most active alkaloid has been developed as a pharmaceutical drug, yohimbine hydrochloride (HCl), and studied in multiple clinical trials for treating sexual dysfunction 2. Yohimbine is a potent α‐2 antagonist (alpha-2 adrenoceptors blocker), a moderate α‐1 antagonist (alpha-1 adrenoceptors blocker) and low affinity to some of the serotonin and dopamine receptors which likely has both peripheral and central nervous system effects 2. Alpha-2 adrenoceptors mediate erection-inhibiting impulses in the central nervous system. Yohimbine is generally believed to enhance central sexual impulse by blocking the alpha-2 adrenoceptors in the locus coeruleus in the brain 11. In the periphery, yohimbine has been suggested to inhibit alpha-1 and alpha-2 adrenoceptors as well as enhancing the release of nitric oxide (NO) from cavernosal endothelial cells, producing a relaxation of smooth muscle cells and consequent erection, increasing sexual potency 12. In conclusion, the mechanism of action of yohimbine in enhancing sexual function is currently unclear.
Yohimbine hydrochloride is rapidly absorbed and the maximum plasma concentration is generally achieved in less than one hour after oral administration 13. The mean bioavailability is low and is subject to very high variation from one individual to another 14. The plasma concentration of yohimbine does not appear to correlate with the dose of the compound administered 7.
In humans, yohimbine has limited efficacy for sexual dysfunction and significant adverse effects including headaches, hypertension, and panic attacks 15. More effective and safer treatments for erectile dysfunction, such as sildenafil (Viagra), have therefore completely replaced yohimbine in the modern physician’s armamentarium 16. Prescriptions of yohimbine, previously available at dosages from 5 to 10 mg, have become so rare that most US pharmaceutical manufacturers have discontinued production of prescription capsules and tablets, and yohimbine has not appeared in the Physician’s Desk Reference since 2005 17.
While prescription capsules and tablets of yohimbine are no longer prescribed by mainstream US physicians, yohimbine remains an ingredient in hundreds of dietary supplements, many marketed for sexual and sports enhancement. These yohimbine supplements can confer significant risks to consumers: researchers analyzing data from Poison Control Centers found that in California alone, yohimbine supplements were linked to more than 130 hospitalizations between 2000 and 2006 18. Because of these risks, many countries have banned extracts of Pausinystalia yohimbe from supplements and foods 15. Yohimbine supplements are banned in Canada, Australia, the Netherlands, and the United Kingdom 15. In contrast, more than 550 brands of yohimbine supplements are available for sale as dietary supplements in the USA 3.
It is not known if supplements containing yohimbine available for sale in the USA at major retailers’ stores are labelled such that consumers are informed of the quantity and adverse effects of yohimbine.
Yohimbine uses
A meta-analysis in 1998 of seven clinical trials with yohimbine has shown a superiority of the compound over placebo in treatment of erectile dysfunction 19. Yohimbine has also been reported to be effective in treatment of orgasmic disorders such as delayed ejaculation 20. There have been no clinical studies testing Yohimbe bark. The Clinical Guidelines Panel on Erectile Dysfunction of the American Urological Association has concluded that the data available on Yohimbine do not allow it to be recommended as standard treatment in erectile dysfunction particularly not in organic cause 21. International Society for Sexual Medicine Standards Committee has recently stated “if yohimbine has any potential indications for use in ED management, it would be among nonorganic ED; apart from ED, yohimbine has shown a limited efficacy in the treatment of premature ejaculation” 22.
The idea to use α2-adrenoceptor antagonists, such as yohimbine, in the treatment of obesity has been considered by many researchers 23. However, their side effects were unacceptable and their effectiveness was insufficient. Yohimbine, a well-known α2-adrenoceptor antagonist, has an undesirable effect on blood pressure and heart rate 23, due to its effect on both central and peripheral adrenoceptors. Moreover, the higher doses of this drug, which were used for weight reduction, affected α1-adrenoreceptors as well. Since yohimbine at such doses can block both postsynaptic α1– and α2-adrenoceptors present in arterial vessels, there is a risk of a very significant drop in blood pressure. Yohimbine at the dose acting anorexically (reduces appetite) at 5 mg/kg body weight showed a hypotensive effect. This correlates with the findings that the drug blocks α2-adrenoreceptors, and in large doses also α1-adrenoreceptors. Non-selective α2-adrenoreceptor antagonists possess hypotensive properties, because they cause vasodilation by blocking α2B-adrenoreceptors and prevent vasospasm by blocking α2C-adrenoreceptors 24. Recent studies using the reinstatement model showed that the α-2 adrenoceptor antagonist yohimbine reinstates cocaine seeking in monkeys 25 as well as alcohol and methamphetamine seeking in rats 26.
Yohimbine is also frequently used as a pharmacological agent to study acute stress effects, since the compound can be studied across species and provides the opportunity to titrate stress with varying doses 27. In humans, it has been shown that the administration of yohimbine leads to panic and anxious feelings, increased heart rate, and increased cortisol measures and noradrenaline metabolite levels 28. The remarkable similarity between yohimbine and psychostimulants can be explained by the fact that psychostimulants have been shown to target both dopaminergic and noradrenergic signaling 29, which is suggested to contribute to their effects on impulsive behavior 30. Similarly, yohimbine not only elevates noradrenaline signaling but also increases dopamine release 31. This is in line with the observation that stress leads to increased striatal dopaminergic signaling 32, resulting in sensitization of dopaminergic motivation systems, which are involved in impulsive behavior and other psychiatric disorders related to stress such as substance abuse. This in turn may contribute to cross sensitization of stress and psychostimulants or other drugs of abuse, which is hypothesized to underlie the relation between stress and increased risk of substance abuse and relapse 33. This increase in sensitivity of noradrenergic systems was revealed by administering yohimbine, which mimics the stress response by antagonizing α2-adrenergic receptors, to people with cocaine 34, opioid 35, or alcohol use disorder 36. Yohimbine increased 3-methoxy-4-hydroxyphenylglycol, the main metabolite of norepinephrine, and induced panic attacks in people with cocaine use disorder with 1–2 days of abstinence, but not in the same people when they had 2 weeks of abstinence 34. This suggests increased sensitivity of noradrenergic systems could diminish in 2 weeks of abstinence. However, although this may diminish effects on emotional systems, norepinephrine seems to remain sensitized and affect other systems suggested by yohimbine-induced increased startle in people with opioid dependence on methadone maintenance compared to healthy controls 37, and increased startle in people with alcohol use disorder who had 2–4 weeks of abstinence who had more quit attempts 36.
Yohimbine side effects
The side effects of yohimbine include high blood pressure, increased heart rate, manic reactions, bronchospasm, palpitations, insomnia, anxiety, irritability, shivering, sweating, nausea, flushing, chest pain, atrial fibrillation and headaches which all can be attributed to its central adrenergic activity 7.
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the following side effects continue or are bothersome or if you have any questions about them:
Less common
- dizziness
- headache
- irritability
- nervousness or restlessness
Rare
- nausea and vomiting
- skin flushing
- sweating
- tremor
Other side effects not listed may also occur in some patients. If you notice any other effects, check with your healthcare professional.
Call your doctor for medical advice about side effects.
Nervous system
- Nervous system side effects have included excitation, increased motor activity, tremor, and dizziness.
Cardiovascular
- Cardiovascular side effects have included elevation in blood pressure and increase in heart rate.
Gastrointestinal
- Gastrointestinal side effects have included nausea and vomiting, commonly reported after parenteral administration of yohimbine.
Psychiatric
- Psychiatric side effects have included irritability and generalized anxiety.
General
- General side effects have included headache and increase in sweating.
Dermatologic
- Dermatologic side effects have included skin flushing reported with oral administration.
Other
A severe case of Raynaud’s phenomenon has been reported in a 65-year-old man diagnosed with CREST syndrome (calcinosis, Raynaud’s phenomenon, esophageal dysfunction, sclerodactyly, and telangiectasia). Painful episodes which affected all toes and fingers and his penis lasted 10 to 15 minutes and were relieved with heat. Symptoms resolved slowly over time upon discontinuation of yohimbine. Upon rechallenge with yohimbine, the patient developed more severe Raynaud’s phenomenon.
Case reports concerning safety of yohimbine
Adverse events mostly due to self-administration of yohimbine have been reported since 1980s. In 1993, Sandler and Aronson have described the development of progressive renal failure, cutaneous eruption, and a lupus-like syndrome in a 42-year-old Afro-American man following yohimbine use 38. Myers has reported a case of refractory priapism in a 42-year-old HIV infected and depressed man who self-administered a product containing yohimbine to treat his erectile dysfunction and was subsequently admitted to emergency department with a 20-hour lasting erection which required insertion of a proximal corpus cavernosum to spongiosum shunt (Quackles shunt) 39. According to California Poison Control System, 238 cases of adverse reactions after yohimbine consumption have been identified within a 7-year period (2000–2006): most commonly reported adverse events have been gastrointestinal distress, tachycardia, anxiety, agitation, and hypertension 40. US National Institute of Health also warns consumers about taking yohimbine supplements if they suffer from schizophrenia, anxiety, depression, or posttraumatic stress disorder 41. Additional situation of emergency has been reported in Amman where the public corporation of the food and the medicine warned the citizens about the use of some unlicensed sexual stimulants, such as yohimbine, for its inclusion of poisonous substance strychnine that caused convulsions, hallucinations, and heart and kidney failure 42. More recently, Yohimbine was detected in a product labelled as OxyELITE Pro and sold online as slimming pills. This caused the hospitalisation of two female patients aged 34 and 21 in Hong Kong for acute hepatitis symptoms. As a result, the Department of Health opened an investigation and appealed to members of the public not to buy or consume the product 43.
Psychological side effects
As mentioned above, anxiety and agitation are among the most common side effects of yohimbine or yohimbine-containing products 44. US National Institute of Health warns consumers about taking yohimbine or Yohimbe supplements with some of the antidepressants and antipsychosis drugs: “People should not combine Yohimbe with monoamine oxidase (MAO) inhibitors as effects may be additive. Yohimbe should be used with caution when taken with medicines for high blood pressure, tricyclic antidepressants, or phenothiazines (a group of medicines used mostly for mental health conditions such as schizophrenia). People with kidney problems and people with psychiatric conditions should not use Yohimbe” 41. In 1985, Linden et al. 45 have reported a case of a 16-year-old girl who experienced an acute dissociative reaction accompanied by weakness, paraesthesia, incoordination, anxiety, headache, and chest pain after the ingestion of an aphrodisiac called “yo-yo,” which has been later identified as yohimbine. Acute neurotoxic effects related to the ingestion of yohimbine-containing products have been reported by Giampreti et al. 46, who has reported the case of a 37-year-old bodybuilder presented with malaise, vomiting, loss of consciousness, and repeated seizures after ingestion of 5 g of yohimbine during a competition.
Yohimbine overdose
In case of overdose, weakness, generalized paresthesia, loss of coordination and memory problems, as well as severe headaches combined with dizziness, tremor,
palpitations and fear occur after 20-30 mins 7. After 4 hour severe chest pain can occur, lasting for several hours. Other side effects include headache, increased blood pressure, tachycardia lasting several hours, nausea, vomiting, mydriasis, increased saliva and tear flow and perspiration. Greatly increased norepinephrine values have been shown. Side effects may persist for 1-2 days. Instances of intoxication have been described at doses above 200 mg yohimbine HCl 7.
In one 16-year-old girl, 250 mg yohimbine brought about an overdose with dissociative reactions, weakness, paranoia, headache, nausea, chest pains, shivering, increased respiration rate, high blood pressure and tachycardia. The serum epinephrine and norepinephrine concentrations were increased. These symptoms persisted for 36 hours 45. A dose of 1.8 g is reputed to have resulted in several hours’ loss of consciousness 7. In a 37-year-old body builder, 5 g of yohimbine resulted in neurotoxic effects after 2 hours. These expressed themselves in nausea, vomiting and repeated episodes, hypertension (259/107 mmHg) and tachycardia. The plasma levels measured 3, 6, 14 and 22 hours after intake were 5240, 2250, 1530 and 865 ng/ml 46.
Acute toxicity (oral LD50 or lethal dose 50% where 50% of the test subjects die) in mice arises at less than 50 mg/kg body weight. The LD50 for humans has been calculated as 5 mg/kg body weight (= 350 mg/70 kg body weight). The lethal dose is ten times higher (50 mg/kg). There are no data available for repeated or chronic use 7.
Interactions with Medicines
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking yohimbine, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using yohimbine with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Clomipramine
- Clonidine
- Guanabenz
- Guanadrel
- Guanethidine
- Guanfacine
- Lithium
- Morphine
- Morphine Sulfate Liposome
- Naloxone
- Naltrexone
- Reserpine
Interactions with Food/Tobacco/Alcohol
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using yohimbine with any of the following may cause an increased risk of certain side effects but may be unavoidable in some cases. If used together, your doctor may change the dose or how often you use yohimbine, or give you special instructions about the use of food, alcohol, or tobacco.
- Ethanol
Other Medical Problems
The presence of other medical problems may affect the use of yohimbine. Make sure you tell your doctor if you have any other medical problems, especially:
- Angina pectoris or
- Depression or
- Other psychiatric illness or
- Heart disease or
- High blood pressure or
- Kidney disease—Yohimbine may make these conditions worse
Liver disease—Effects of yohimbine may be increased because of slower removal from the body.
References- Dietary Supplement Health and Education Act of 1994. Pub L No.103–417, 1994. 103rd Congress, 2nd sess., S784
- J. M. Betz Yohimbe. In: P. M. Coates, J. M. Betz, M. R. Blackman, G. M. Craig, M. Levine, J. Moss, J. D. White. Encyclopedia of dietary supplements, 2nd edition, Informa, London, UK, 2010, p. 861.
- Pharmaceutical quantities of yohimbine found in dietary supplements in the USA. Drug Testing and Analysis March-April 2016, Volume8, Issue3-4, Pages 357-369. https://onlinelibrary.wiley.com/doi/full/10.1002/dta.1849
- Kearney T, Tu N, Haller C. Adverse drug events associated with yohimbine-containing products: a retrospective review of the California Poison Control System reported cases. Annals of Pharmacotherapy. 2010;44(6):1022-1029. http://journals.sagepub.com/doi/abs/10.1345/aph.1P060
- Kommission E (2.1.1990). Yohimbehe cortex. Bundesanzeiger 22a.
- Kommission E (10.15.1987). Yohimbehe cortex. Bundesanzeiger 193a.
- Scientific assessment of yohimbe (Pausinystalia yohimbe), Federal Institute for Risk Assessment. https://ec.europa.eu/food/sites/food/files/safety/docs/labelling_nutrition-vitamins_minerals-sa_yohimbe_en.pdf
- Raman V, Avula B, Galal AM, Wang YH, Khan IA. Microscopic and UPLC-UV-MS analyses of authentic and commercial yohimbe (Pausinystalia johimbe) bark samples. J Nat Med. 2013 Jan;67(1):42-50. doi: 10.1007/s11418-012-0642-2
- D. Lucas, J. Neal‐Kababick, J. Zweigenbaum. Characterization and quantitation of yohimbine and its analogs in botanicals and dietary supplements using LC/QTOF‐MS and LC/QQQ‐MS for determination of the presence of bark extract and yohimbine adulteration. J. AOAC Intern. 2015, 98, 330.
- Cohen PA, Wang YH, Maller G, DeSouza R, Khan IA. Pharmaceutical quantities of yohimbine found in dietary supplements in the USA. Drug Test Anal. 2016 Mar-Apr;8(3-4):357-69. doi: 10.1002/dta.1849
- Involvement of noradrenergic innervation from locus coeruleus to hippocampal formation in negative feedback regulation of penile erection in the rat. Chang AY, Huang CM, Chan JY, Chan SH. Hippocampus. 2001; 11(6):783-92. https://www.ncbi.nlm.nih.gov/pubmed/11811673/
- SOP conservative (medical and mechanical) treatment of erectile dysfunction. Porst H, Burnett A, Brock G, Ghanem H, Giuliano F, Glina S, Hellstrom W, Martin-Morales A, Salonia A, Sharlip I, ISSM Standards Committee for Sexual Medicine. J Sex Med. 2013 Jan; 10(1):130-71. https://www.ncbi.nlm.nih.gov/pubmed/23343170/
- Yohimbine: a clinical review. Tam SW, Worcel M, Wyllie M. Pharmacol Ther. 2001 Sep; 91(3):215-43. https://doi.org/10.1016/S0163-7258(01)00156-5
- Yohimbine bioavailability in humans. Guthrie SK, Hariharan M, Grunhaus LJ. Eur J Clin Pharmacol. 1990; 39(4):409-11. https://www.ncbi.nlm.nih.gov/pubmed/2076728/
- F. Aguilar, R. Crebelli, B. Dusemund, P. Galtier, D. Gott, U. Gundert‐Remy, J. König, C. Lambré, J‐C. Leblanc, P. Mosesso, A. Mortensen, A. Oskarsson, D. Parent‐Massin, M. Rose, I. Stankovic, P. Tobback, I. Waalkens‐Berendsen, R. Woutersen, M. Wright. Scientific opinion on the evaluation of the safety in use of (Pausinystalia yohimbe (K. Schum.) Pierre ex Beille). EFSA J. 2013, 11, 3302.
- K. T. McVary. Erectile dysfunction. N. Engl. J. Med. 2007, 357, 2472. https://www.nejm.org/doi/full/10.1056/NEJMcp067261
- Physicians’ Desk Reference, 59th Edn, Medical Economics Company, Montvale, NJ, 2005.
- T. Kearney, N. Tu, C. Haller. Adverse drug events associated with yohimbine‐containing products: a retrospective review of the California Poison Control system reported cases. Annal Pharm. 2010, 44, 1022.
- Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. Journal of Urology. 1998;159(2):433–436. https://www.ncbi.nlm.nih.gov/pubmed/9649257
- Adeniyi AA, Brindley GS, Pryor JP, Ralph DJ. Yohimbine in the treatment of orgasmic dysfunction. Asian Journal of Andrology. 2007;9(3):403–407. http://www.asiaandro.com/archive/1008-682X/9/403.htm
- Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: the management of erectile dysfunction: an AUA update. The Journal of Urology. 2005;174(1):230–239 https://www.jurology.com/article/S0022-5347(05)60085-7/pdf
- Porst H, Burnett A, Brock G, et al. SOP conservative (Medical and Mechanical) treatment of erectile dysfunction. Journal of Sexual Medicine. 2013;10(1):130–171 https://www.ncbi.nlm.nih.gov/pubmed/23343170
- Galitzky J, Vermorel M, Lafontan M, Montastruc P, Berlan M. Thermogenetic and lipolytic effect of yohimbine in the dog. Br J Pharmacol. 1991;104:514–518 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1908559/pdf/brjpharm00230-0234.pdf
- Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Philipp M, Brede M, Hein L. Am J Physiol Regul Integr Comp Physiol. 2002 Aug; 283(2):R287-95.
- Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Lee B, Tiefenbacher S, Platt DM, Spealman RD. Neuropsychopharmacology. 2004 Apr; 29(4):686-93. https://www.ncbi.nlm.nih.gov/pubmed/14872205/
- The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Shepard JD, Bossert JM, Liu SY, Shaham Y. Biol Psychiatry. 2004 Jun 1; 55(11):1082-9. https://www.ncbi.nlm.nih.gov/pubmed/15158427/
- Schippers MC, Schetters D, De Vries TJ, Pattij T. Differential effects of the pharmacological stressor yohimbine on impulsive decision making and response inhibition. Psychopharmacology. 2016;233:2775-2785. doi:10.1007/s00213-016-4337-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4917594/
- Swann AC, Birnbaum D, Jagar AA, Dougherty DM, Moeller FG. Acute yohimbine increases laboratory-measured impulsivity in normal subjects. Biol Psychiatry. 2005;57:1209–1211. doi: 10.1016/j.biopsych.2005.02.007
- Catecholamine mapping within nucleus accumbens: differences in basal and amphetamine-stimulated efflux of norepinephrine and dopamine in shell and core. McKittrick CR, Abercrombie ED. J Neurochem. 2007 Mar; 100(5):1247-56. https://www.ncbi.nlm.nih.gov/pubmed/17241132/
- The neuropharmacology of impulsive behaviour. Pattij T, Vanderschuren LJ. Trends Pharmacol Sci. 2008 Apr; 29(4):192-9. https://www.ncbi.nlm.nih.gov/pubmed/18304658/
- Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Tanda G, Bassareo V, Di Chiara G. Psychopharmacology (Berl). 1996 Jan; 123(2):127-30. https://www.ncbi.nlm.nih.gov/pubmed/8741935/
- Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. J Neurosci. 2005 Jun 1; 25(22):5389-96. http://www.jneurosci.org/content/25/22/5389.long
- Stress modulates illness-course of substance use disorders: a translational review. Lijffijt M, Hu K, Swann AC. Front Psychiatry. 2014; 5():83. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4101973/
- McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, et al. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry (1994) 51:713–910.1001/archpsyc.1994.03950090045007
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry (2002) 51:642–5110.1016/S0006-3223(01)01292-6
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, III, et al. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP). Psychopharmacology (Berl) (1997) 131:207–1510.1007/s002130050285
- Stine SM, Grillon CG, Morgan CA, III, Kosten TR, Charney DS, Krystal JH. Methadone patients exhibit increased startle and cortisol response after intravenous yohimbine. Psychopharmacology (Berl) (2001) 154:274–8110.1007/s002130000644
- Sandler B, Aronson P. Yohimbine-induced cutaneous drug eruption, progressive renal failure, and lupus-like syndrome. Urology. 1993;41(4):343–345.
- Myers A, Barrueto F., Jr. Refractory priapism associated with ingestion of yohimbe extract. Journal of Medical Toxicology. 2009;5(4):223–225. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3550410/
- Kearney T, Tu N, Haller C. Adverse drug events associated with yohimbine-containing products: a retrospective review of the California poison control system reported cases. Annals of Pharmacotherapy. 2010;44(6):1022–1029.
- Yohimbe. https://nccih.nih.gov/health/yohimbe
- GPHIN. Warning on of the use of sexual stimulants that contains a poisonous substance, communication posted on 2008/07/29 from Jordan (in Arabic), 2008.
- GPHIN. Public urged not to buy or use slimming products with undeclared and banned drug ingredients, communication posted on 2013/06/05 from Hong Kong Government, 2013.
- Kearney T, Tu N, Haller C. Adverse drug events associated with yohimbine-containing products: a retrospective review of the California poison control system reported cases. Annals of Pharmacotherapy. 2010;44(6):1022–1029
- Linden CH, Vellman WP, Rumack B. Yohimbine: a new street drug. Annals of Emergency Medicine. 1985;14(10):1002–1004. https://www.ncbi.nlm.nih.gov/pubmed/4037464
- Giampreti A, Lonati D, Locatelli C, Rocchi L, Campailla MT. Acute neurotoxicity after yohimbine ingestion by a body builder. Clinical Toxicology. 2009;47(8):827–829. https://www.ncbi.nlm.nih.gov/pubmed/19640235