What is Zinc
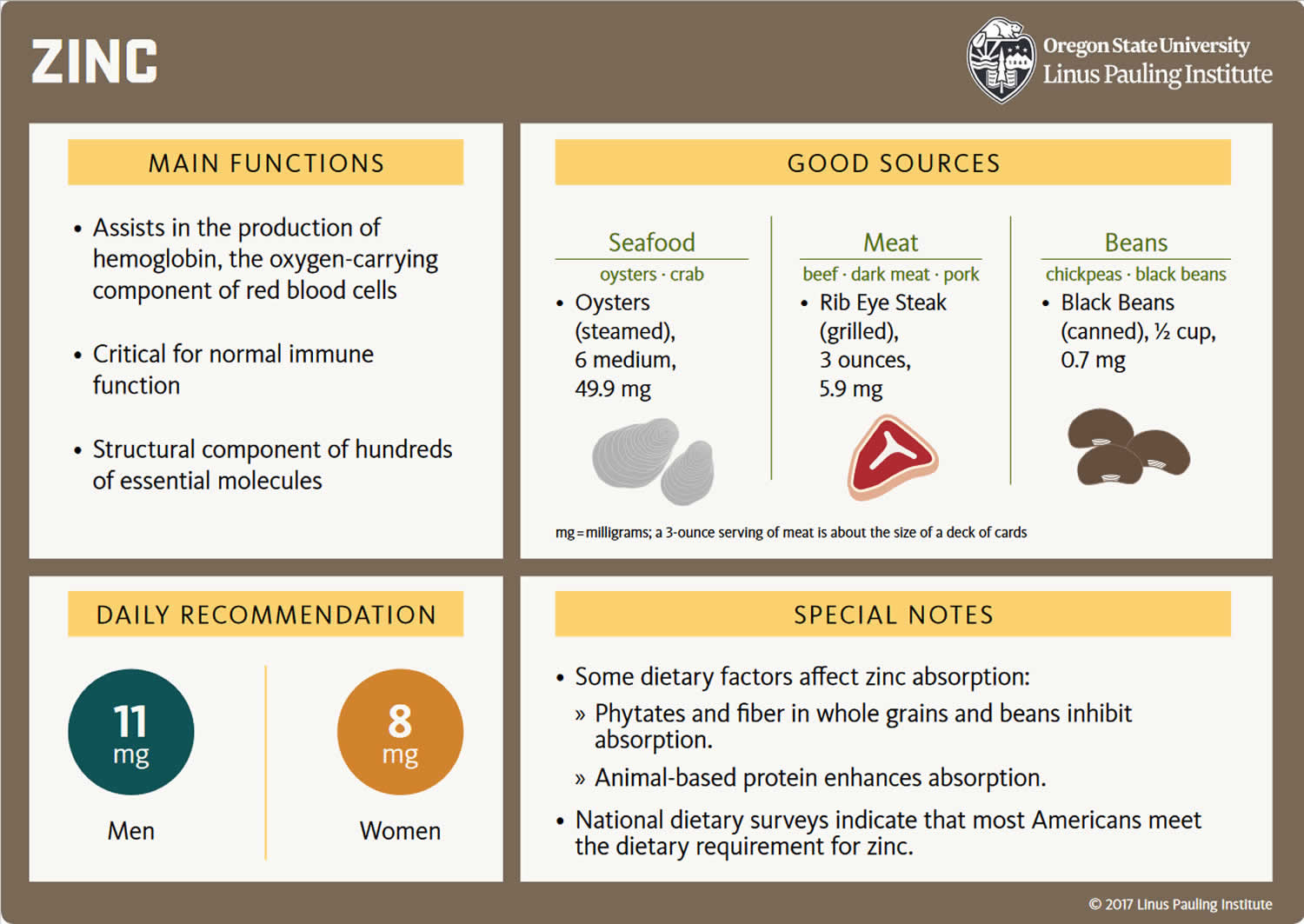
Zinc is an essential mineral that is naturally present in some foods, added to others, and available as a dietary supplement. Zinc is also found in many cold lozenges and some over-the-counter drugs sold as cold remedies. Zinc is a nutrient that people need to stay healthy. A daily intake of zinc is required to maintain a steady state because the body has no specialized zinc storage system 1. The recommended dietary allowance (RDA) for zinc in adult men is 11 mg/day and women 8 mg/day 2, 3. The total amount of zinc in the body is approximately 1.5 g in women and 2.5 g in men 4. Most of this zinc is stored in skeletal muscle and bone 2, 4, 5. The processes that maintain zinc homeostasis are absorption of zinc from the diet, excretion into the gastrointestinal tract, and reabsorption in the gastrointestinal lumen 4, 5. In general, as zinc intakes rise, the amount of zinc absorbed also increases, but its fractional absorption drops 4, 5.
Zinc is found in cells throughout the body, found mainly in bones, teeth, hair, skin, liver, muscle, leukocytes, and testes 6. Zinc helps the immune system fight off invading bacteria and viruses. The body also needs zinc to make proteins and DNA, the genetic material in all cells 7, 2, 4, 5, 8. During pregnancy, infancy, and childhood, the body needs zinc to grow and develop properly. Zinc also helps wounds heal and is important for proper senses of taste and smell 4, 5, 9.
Zinc is involved in numerous aspects of cellular metabolism. It is required for the catalytic activity of approximately 100 enzymes 10 and it plays a role in immune function 11, protein synthesis 11, wound healing 12, DNA synthesis 10, and cell division 11. Zinc also supports normal growth and development during pregnancy, childhood, and adolescence 13 and is required for proper sense of taste and smell 14.
Most Americans get enough zinc from the foods they eat.
However, certain groups of people are more likely than others to have trouble getting enough zinc:
- People who have had gastrointestinal surgery, such as weight loss surgery, or who have digestive disorders, such as ulcerative colitis or Crohn’s disease. These conditions can both decrease the amount of zinc that the body absorbs and increase the amount lost in the urine.
- Vegetarians or vegans because they do not eat meat, which is a good source of zinc. Also, the beans and grains they typically eat have compounds that keep zinc from being fully absorbed by the body. For this reason, vegetarians might need to eat as much as 50% more zinc than the recommended amounts.
- Women who are pregnant or breastfeeding because they need more zinc for their growing baby and to make breast milk.
- Older infants who are breastfed because breast milk does not have enough zinc for infants over 6 months of age. Older infants who do not take formula should be given foods that have zinc such as pureed meats. Formula-fed infants get enough zinc from infant formula.
- Alcoholics because alcoholic beverages decrease the amount of zinc that the body absorbs and increase the amount lost in the urine. Also, many alcoholics eat a limited amount and variety of food, so they may not get enough zinc.
- Children and individuals who have sickle cell disease, possibly because the medications they take can cause low levels of zinc. These people might benefit from taking zinc supplements.
Clinical zinc deficiency in humans was first described in 1961, when the consumption of diets with low zinc bioavailability due to high phytate content was associated with “adolescent nutritional dwarfism” in the Middle East 15. Since then, zinc insufficiency has been recognized by a number of experts as an important public health issue, especially in low-resource countries 16. Severe zinc deficiency is rare and caused by an inherited condition called acrodermatitis enteropathica. Acquired zinc deficiency is primarily due to malabsorption syndromes and chronic alcoholism.
Serum or plasma zinc levels are typically used in clinical practice to assess zinc status 17. In healthy people, the amount of zinc in serum or plasma is 80 to 120 microgram (mcg)/dL (12 to 18 micromol/L) 4. Serum zinc levels below 70 mcg/dL in women and 74 mcg/dL in men indicate zinc deficiency 17. However, both serum and plasma measures have important limitations. Zinc concentrations in serum are associated with the patient’s sex and age as well as the time of the blood draw (morning vs. evening) and do not always correlate with dietary or supplemental zinc intakes 18. Zinc levels also fluctuate in response to other factors, including infections, changes in steroid hormones, stress and muscle catabolism during weight loss or illness 19, 20, 2, 5. Doctors consider risk factors (such as inadequate caloric intake, chronic alcohol use, and malabsorptive digestive diseases) and signs of zinc deficiency (such as impaired growth in infants and children) when they assess a patient’s zinc status 2.
Zinc key points
- Zinc is a nutritionally essential mineral needed for catalytic, structural, and regulatory functions in the body.
- Severe zinc deficiency is rare and caused by an inherited condition called acrodermatitis enteropathica. Acquired zinc deficiency is primarily due to malabsorption syndromes and chronic alcoholism.
- Dietary zinc deficiency is quite common in the developing world, affecting an estimated 2 billion people. Consumption of diets high in phytate and lacking foods from animal origin drive zinc deficiency in these populations.
- The recommended dietary allowance (RDA) for adult men and women is 11 mg/day and 8 mg/day of zinc, respectively.
- Long-term consumption of zinc in excess of the tolerable upper intake level (UL) is 40 mg/day for adults can result in copper deficiency 2. Copper deficiency has also been reported following chronic use of excessive amounts of zinc-containing denture creams (≥2 tubes per week containing 17-34 mg/g of zinc) 21.
- Dietary zinc deficiency has been associated with impaired growth and development in children, pregnancy complications, and immune dysfunction with increased susceptibility to infections.
- Supplementation with doses of zinc in excess of the upper intake level (40 mg/day for adults) is effective to reduce the duration of common cold symptoms.
- Milder gastrointestinal distress has been reported at doses of 50 to 150 mg/day of supplemental zinc. Single doses of 225 to 450 mg of zinc usually induce vomiting.
- Current evidence suggests that supplemental zinc may be useful in the management of chronic conditions, such as age-related macular degeneration, diabetes mellitus, Wilson’s disease, and HIV/AIDS.
- Zinc bioavailability is relatively high in meat, eggs, and seafood; zinc is less bioavailable from whole grains and legumes due to their high content in phytate that inhibits zinc absorption.
What is the function of Zinc?
Zinc is a nutritionally essential mineral needed for catalytic, structural, and regulatory functions in your body 5. It has been estimated that over 3,000 proteins in humans have functional zinc-binding sites 22. Zinc plays important roles in growth and development, immune function, neurotransmission, vision, reproduction, and intestinal ion transport 23. Zinc is required for the catalytic activity of approximately 100 enzymes, including many nicotinamide adenine dinucleotide (NADH) dehydrogenases, RNA and DNA polymerases, and DNA transcription factors as well as alkaline phosphatase, superoxide dismutase, and carbonic anhydrase 24, 22, 25, 26, 27 and it plays a role in immune function 28, 11, protein synthesis 11, wound healing 12, DNA synthesis for cell growth 10, and cell division 11 and the breakdown of carbohydrates. Zinc also supports normal growth and development during pregnancy, infancy, childhood, and adolescence 13 and is required for proper sense of taste and smell 14. Zinc also enhances the action of insulin. A daily intake of zinc is required to maintain a steady state because the body has no specialized zinc storage system 1.
Information from an expert review on zinc supplements showed that 29, 30, 31:
- When taken for at least 5 months, zinc may reduce your risk of becoming sick with the common cold. Starting to take zinc supplements within 24 hours after cold symptoms begin may reduce how long the symptoms last and make the symptoms less severe. However, supplementation beyond the recommended dietary allowance (RDA) and adequate intake (AI) is not recommended at this time.
People commonly use zinc for zinc deficiency, diarrhea, and Wilson disease. Zinc is also used for acne, diabetes, anorexia, burns, and many other purposes. There is some scientific evidence to support its use for some of these conditions. But for most, there is no good scientific evidence to support its use 32. There is also no good evidence to support using zinc for COVID-19.
Zinc supplements taken in large amounts may cause diarrhea, abdominal cramps, and vomiting. These symptoms most often appear within 3 to 10 hours of swallowing the supplements. The symptoms go away within a short period of time after stopping the supplements. An excess intake of zinc can lead to copper or iron deficiency.
People who use nasal sprays and gels that contain zinc may have side effects, such as losing their sense of smell.
Zinc catalytic function
Over 50 different enzymes depend on zinc for their ability to catalyze vital chemical reactions 33. Zinc-dependent enzymes can be found in all six classes of enzymes 34, as defined by the International Union of Biochemistry and Molecular Biology 35. During enzymatic reactions, zinc may have either a direct catalytic role or a structural role (i.e., stabilizing the structure of catalytic enzymes).
Zinc structural function
Zinc plays an essential role in the folding of some proteins. A finger-like structure, known as a zinc finger motif, stabilizes the structure several proteins. Examples of zinc finger proteins include the superfamily of nuclear receptors that bind and respond to steroids and other molecules, such as estrogens, thyroid hormones, vitamin D, and vitamin A 36. Zinc finger motifs in the structure of nuclear receptors allow them to bind to DNA and act as transcription factors to regulate gene expression (see zinc regulatory function). Zinc finger motifs are also involved in interactions of proteins with other proteins, ribonucleotides, and lipids 5. Removal of zinc from zinc-containing proteins results in protein misfolding and loss of function.
Metallothioneins are examples of proteins with a zinc-binding motif. Metallothioneins are small metal-binding cysteine-rich proteins with a high affinity for zinc. They work in concert with zinc transporters, regulating free zinc concentrations in the cytosol 37. Metallothioneins are also involved in the regulation of metal ion homeostasis, cellular defense against oxidative stress, and detoxification of heavy metals 37, 38.
The antioxidant enzyme, copper-zinc superoxide dismutase 1 (SOD 1), is made of two identical dimers, each including an active site with a catalytic copper ion and a structural zinc ion. Demetalation of SOD1 has been implicated in the formation of amyloid aggregates in some forms of inherited amyotrophic lateral sclerosis (ALS) — a motor neuron disease leading to muscle atrophy and paralysis 39.
Zinc regulatory function
Zinc finger proteins have been found to regulate gene expression by acting as transcription factors (see above). Zinc also plays a role in cell signaling via the metal-response element-binding transcription factor 1 (MTF1); MTF1 has a zinc finger domain that allows its binding to metal-response element sequences in the promoter of target genes and the subsequent expression of zinc-responsive genes (6). Zinc may also have a direct regulatory function, modulating the activity of cell-signaling enzymes and transcription factors 5. Extracellular zinc can also stimulate a zinc-sensing receptor that triggers the release of intracellular calcium, a second messenger in signaling pathways 40. Zinc has been found to influence hormone release (e.g., type 2 diabetes mellitus) 41 and nerve impulse transmission 42.
Immune system function
Adequate zinc intake is essential in maintaining the integrity of your immune system 43. Severe zinc deficiency depresses immune function 44, and even mild to moderate degrees of zinc deficiency can impair macrophage and neutrophil functions, natural killer cell activity, and complement activity 45. The body requires zinc for normal development and function of cells that mediate both innate (neutrophils, macrophages, and natural killer cells) and adaptive (B-lymphocytes and T-lymphocytes) immune responses 46, 27, 47. Individuals with low zinc levels have shown reduced lymphocyte proliferation response to mitogens and other adverse alterations in immunity that can be corrected by zinc supplementation 45, 48. These alterations in immune function might explain why low zinc status has been associated with increased susceptibility to pneumonia and other infections in children in developing countries and the elderly 49, 50, 51, 52.
Because bacteria and viruses also require zinc to thrive and invade, a well-established antimicrobial defense mechanism in your body sequesters free zinc away from microbes 53. Another opposite mechanism consists in intoxicating intracellular microbes within macrophages with excess zinc 54. Through weakening innate and adaptive immune responses, zinc deficiency diminishes the capacity of the body to combat pathogens 53, 55. As a consequence, zinc-deficient individuals experience an increased risk to a variety of infectious agents 56.
Zinc supplements
Supplements contain several forms of zinc, including zinc gluconate, zinc sulfate, and zinc acetate 17. The percentage of elemental zinc varies by form. Zinc oxide, zinc acetate, zinc sulfate, and zinc gluconate, contain elemental zinc percentages of 80%, 30%, 23%, and 14.3%, respectively 57. For example, approximately 23% of zinc sulfate consists of elemental zinc; thus, 220 mg of zinc sulfate contains 50 mg of elemental zinc. The elemental zinc content appears in the Supplement Facts panel on the supplement container. Research has not determined whether differences exist among forms of zinc in absorption, bioavailability, or tolerability 58.
In addition to standard tablets and capsules, some zinc-containing cold lozenges are labeled as dietary supplements.
Are there any interactions with zinc that I should know about?
Yes. Zinc dietary supplements can interact or interfere with certain medications that you take and, in some cases, medicines can lower zinc levels in the body. Here are several examples:
- Taking a zinc dietary supplement along with quinolone (such as Cipro®) or tetracycline antibiotics (such as Achromycin® and Sumycin®) might interact with zinc in the gastrointestinal tract, which could inhibit the absorption of both zinc and the antibiotic if they are taken at the same time 59, 60. Taking the antibiotic at least 2 hours before or 4–6 hours after the zinc supplement minimizes this interaction 59.
- Zinc can reduce the absorption and action of penicillamine, a drug used to treat rheumatoid arthritis and Wilson disease 61. To minimize this interaction, people should take zinc supplements and penicillamine at least 1 hour apart.
- Thiazide diuretics, such as chlorthalidone (brand name Hygroton® and Thalitone®) and hydrochlorothiazide (brand names Esidrix® and HydroDIURIL®) increase the amount of zinc lost in the urine. Taking thiazide diuretics for a long time could decrease the amount of zinc in the body 62.
Tell your doctor, pharmacist, and other healthcare providers about any dietary supplements and medicines you take. They can tell you if those dietary supplements might interact or interfere with your prescription or over-the-counter medicines or if the medicines might interfere with how your body absorbs, uses, or breaks down nutrients.
Zinc interaction with Copper
Taking large quantities of zinc (50 mg/day or more) over a period of weeks can inhibit copper absorption, sometimes producing copper deficiency and associated anemia 63, 64. High intake of zinc induces the intestinal synthesis of a copper-binding protein called metallothionein. Metallothionein traps copper within intestinal cells and prevents its systemic absorption. For this reason, dietary supplement formulations containing high levels of zinc, such as the one used in the Age-Related Eye Disease Study Research Group (AREDS) study for age-related macular degeneration (AMD) and vision loss, sometimes contain copper 65. However, typical intakes of zinc do not affect copper absorption, and high copper intakes do not affect zinc absorption 66.
Zinc interaction with Iron
Iron and zinc compete for absorptive pathways 67. Supplemental iron (38-65 mg/day of elemental iron) but not dietary levels of iron may decrease zinc absorption 67, 68. This interaction is of concern in the management of iron supplementation during pregnancy and lactation and has led some experts to recommend zinc supplementation for pregnant and lactating women taking iron supplements 69, 70. Food fortification with iron has not been shown to negatively affect zinc absorption 71.
Iron-deficiency anemia is a serious world-wide public health problem. Iron fortification programs have been credited with improving the iron status of millions of women, infants, and children. Fortification of foods with iron does not significantly affect zinc absorption. However, large amounts of supplemental iron (greater than 25 mg) might decrease zinc absorption 27. In a placebo-controlled study, supplementation with zinc (10 mg/day) for three months in children aged eight to nine years significantly decreased serum iron concentrations, yet not to the extent of causing iron-deficiency anemia 72. Additional randomized controlled studies have reported a worsening of nutritional iron status with chronic zinc supplementation 73, 74. Taking iron supplements between meals helps decrease its effect on zinc absorption 75.
Zinc interaction with Calcium
High levels of dietary calcium impair zinc absorption in animals, but it is uncertain whether this occur in humans 66. One study showed that increasing the calcium intake of postmenopausal women by 890 mg/day in the form of milk or calcium phosphate (total calcium intake, 1,360 mg/day) reduced zinc absorption and zinc balance in postmenopausal women 76. However, another study found that increasing the calcium intake of adolescent girls by 1,000 mg/day in the form of calcium citrate malate (total calcium intake, 1,667 mg/day) did not affect zinc absorption or balance 77. Calcium in combination with phytate might affect zinc absorption, which would be particularly relevant to individuals who very frequently consume tortillas made with lime (i.e., calcium oxide). A study in 10 healthy women (age range, 21-47 years) found that high intake of dietary calcium (~1,800 mg/day) did not impair zinc absorption regardless of the phytate content of the diet 78.
Zinc interaction with Folate
The bioavailability of dietary folate (vitamin B9) is increased by the action of a zinc-dependent enzyme. Accordingly, some studies found low zinc intake decreased folate absorption. It was also suggested that supplementation with folic acid — the synthetic form of folate — might impair zinc utilization in individuals with marginal zinc status 66, 2. However, one study reported that supplementation with a relatively high dose of folic acid (800 µg/day) for 25 days did not alter zinc absorption or status in a group of students being fed a low-zinc diet (3.5 mg/day) 79.
Zince interaction with Vitamin A
Zinc and vitamin A interact in several ways. Zinc is a component of retinol-binding protein, a protein necessary for transporting vitamin A in the blood. Zinc is also required for the enzyme that converts retinol (vitamin A) to retinal. This latter form of vitamin A is necessary for the synthesis of rhodopsin, a protein in the eye that absorbs light and thus is involved in dark adaptation. Zinc deficiency has been associated with a decreased release of vitamin A from the liver, which may contribute to symptoms of night blindness that are seen with zinc deficiency 80, 81.
Other Sources of Zinc
Zinc is present in several products, including some labeled as homeopathic medications, sold over the counter for the treatment and prevention of colds. Numerous case reports of anosmia (loss of the sense of smell), in some cases long-lasting or permanent, have been associated with the use of zinc-containing nasal gels or sprays 82, 83. In June 2009, the FDA warned consumers to stop using three zinc-containing intranasal products because they might cause anosmia 84. The manufacturer recalled these products from the marketplace. Currently, these safety concerns have not been found to be associated with cold lozenges containing zinc.
Zinc is also present in some denture adhesive creams at levels ranging from 17–34 mg/g 21. While use of these products as directed (0.5–1.5 g/day) is not of concern, chronic, excessive use can lead to zinc toxicity, resulting in copper deficiency and neurologic disease. Such toxicity has been reported in individuals who used 2 or more standard 2.4 oz tubes of denture cream per week 21, 85. Many denture creams have now been reformulated to eliminate zinc.

Zinc Health Benefits
Common cold
The common cold is often caused by the rhinovirus (other respiratory viruses include adenoviruses, influenza, and RSV) 86. There are many different rhinovirus types. Most rhinovirus infections are mild, but they can cause severe illness, especially in people with weakened immune systems, asthma, or other underlying medical conditions. Common cold is one of the most widespread illnesses and is a leading cause of visits to the doctor and absenteeism from school and work. Complications of the common cold include otitis media (middle ear infection), sinusitis and exacerbations of reactive airway diseases 87. Treatments with proven effectiveness for cold symptoms in adults include over-the-counter analgesics, zinc, nasal decongestants with or without antihistamines, and ipratropium for cough. Lower-quality evidence suggests that Lactobacillus casei may be beneficial in older adults. The only established safe and effective treatments for children are acetylcysteine, honey (for children one year and older), nasal saline irrigation, intranasal ipratropium, and topical application of ointment containing camphor, menthol, and eucalyptus oils. Over-the-counter cold medications should not be used in children younger than four years. 86, 88, 89.
Zinc, which can inhibit rhinovirus replication in test tube studies and has activity against other respiratory viruses such as respiratory syncytial virus 90. The exact mechanism of zinc’s activity on viruses remains uncertain. Zinc may also reduce the severity of cold symptoms by acting as an astringent on the trigeminal nerve 91.
Researchers have hypothesized that zinc could reduce the severity and duration of cold signs and symptoms by directly inhibiting rhinovirus binding and replication in the nasal mucosa and suppressing inflammation 92, 93. In studies examining the effects of zinc supplements on the common cold, zinc is usually administered in a lozenge or syrup that temporarily “sticks” to the mouth and throat, placing the zinc in contact with the rhinovirus in those areas. The use of zinc lozenges within 24 hours of the onset of cold symptoms, and continued intake every two to three hours while awake until symptoms resolve, have been advocated for reducing the duration of the common cold 94.
The results from clinical trials that have examined the effects of zinc supplements on the common cold have been inconsistent. But overall, supplemental zinc in lozenge or syrup form appears to reduce the common cold signs and symptoms duration, but not the severity of the common cold when taken shortly after a person develops a cold 95, 96, 97, 98.
In one clinical trial, 50 adults took a zinc acetate lozenge (13.3 mg zinc) or placebo every 2 to 3 wakeful hours within 24 hours of developing the common cold for as long as they had cold symptoms. In comparison with placebo, the zinc lozenges reduced the duration of colds by 3 days and the severity of cold symptoms (cough, nasal discharge, and muscle aches) 99. In another clinical trial, 273 adults with experimentally induced colds were randomly assigned to take zinc gluconate (13.3 mg zinc) or zinc acetate (5.0 mg or 11.5 mg) lozenges every 2 to 3 hours while awake, for a total of 6 lozenges per day, or placebo, for up to 14 days 100. Duration of illness was 1 day less with the zinc gluconate supplements than with the placebo, but the lozenges had no effect on symptom severity. Furthermore, the 5.0 and 11.5 mg zinc acetate lozenges had no effect on either cold duration or severity. In a second trial described in the same report, neither zinc gluconate nor zinc acetate lozenges affected the duration or severity of cold symptoms in comparison with placebo in 281 adults with colds 100.
A 2021 systematic review and meta-analysis included 28 randomized controlled trials (including the three described above) with a total of 5,446 participants (mostly adults younger than 65 years) who had a community-acquired viral respiratory tract infection or were inoculated with a rhinovirus 95. Most trials provided zinc in the form of zinc acetate or gluconate lozenges at total daily doses of 45 mg to 300 mg for up to 2 weeks, but some trials used nasal sprays or gels. In participants who used products containing zinc, symptoms resolved an average of 2 days earlier than in those who received a placebo. However, average daily symptom severity did not differ between those who were and were not treated with zinc. The author of an earlier systematic review concluded that the use of zinc lozenges at doses of over 75 mg/day reduced the duration of the common cold, whereas lower doses did not 97.
Additional research is needed to determine the optimal dosage, formulation, and administration schedule before a general recommendation can be made regarding the use of zinc lozenges, gels, and sprays to reduce the severity and duration of symptoms of the common cold 96, 97, 98.
Finally, although taking zinc lozenges for a cold every 2 to 3 hours while awake will result in daily zinc intakes well above the tolerable upper intake level (UL) of 40 mg/day for adults 2, the use of zinc at daily doses of 50 to 180 mg for one to two weeks has not resulted in serious side effects 98. Bad taste and nausea were the most frequent adverse effects reported in therapeutic trials 98. Use of zinc lozenges for prolonged periods (e.g., 6-8 weeks) is likely to result in copper deficiency.
Wilson’s disease
The protein, ATP7B, is responsible for the excretion of hepatic copper into the biliary tract, and its impairment in Wilson’s disease results in an increased concentration of ‘free’ copper (i.e., not bound to the copper-carrying protein, ceruloplasmin) in blood, an increased excretion of copper in the urine (hypercupriuria), the deposition of copper in part of the cornea (forming Kayser-Fleischer rings), and the accumulation of copper in the liver and brain 101. This inherited condition is progressive and fatal if untreated. The standard-of-care for symptomatic Wilson’s disease patients usually includes an initial phase (around 2-6 months) of copper chelation with agents, such as penicillamine or trientine (triethylenetetramine), followed by lifelong maintenance therapy with penicillamine and/or trientine and/or zinc salts (107). Patients presenting without symptoms can be treated with maintenance therapeutic doses of a chelating agent or with zinc 102. Zinc-induced metallothionein in the intestinal mucosa binds copper and prevents its absorption. There is growing evidence to suggest that zinc salts are a safer, much cheaper, and efficacious alternative to metal-chelating agents — which have been associated with a worsening of symptoms during the initial phase of treatment in some patients 103. The use of zinc is advocated as safe and efficacious in both children 104, 105 and adult patients with Wilson’s disease 106, 103, 107.
Acute diarrhea in children
Zinc promotes mucosal resistance to infections by supporting the activity of immune cells and the production of antibodies against invading pathogens 55, 53, 44. Therefore, zinc deficiency increases the susceptibility to intestinal infections and constitutes a major contributor to diarrheal diseases in children (66). In turn, persistent diarrhea contributes to zinc deficiency and malnutrition 56. Research indicates that zinc deficiency may also potentiate the effects of toxins produced by diarrhea-causing bacteria like E. coli 108. Acute diarrhea is associated with high mortality rates among children in low-income countries, where it causes about 525,000 deaths annually 109, 110. Zinc is used to treat acute diarrhea in children, especially in low-income countries where zinc deficiency is common. Scientists believe that zinc’s beneficial effects stem from its role in supporting adaptive immunity and maintaining the mucosal integrity of the gastrointestinal system 109.
Zinc supplementation in combination with oral rehydration therapy has been shown to significantly reduce the duration and severity of acute and persistent childhood diarrhea and to increase survival in low-income countries in a number of randomized controlled trials 111. A 2016 Cochrane review of 33 randomized controlled trials that compared the effects of zinc supplementation with placebo in 10,841 children aged 1 month to 5 years with acute or persistent diarrhea found that zinc supplementation reduced the duration of acute diarrhea by one day in children aged older than 6 months who presented signs of malnutrition (5 trials; 419 children) 112. Most studies were conducted in Asian countries with high rates of zinc deficiency. The investigators administered zinc in the form of zinc acetate, zinc gluconate, or zinc sulfate 112. The most common zinc dose was 20 mg/day, and about half the studies administered zinc for 2 weeks. The authors concluded, on the basis of evidence of low to moderate certainty, that zinc supplementation shortens the duration of diarrhea by about half a day in children older than 6 months and reduces the likelihood that diarrhea will persist for at least 7 days by 27% 112. In children younger than 6 months, however, zinc supplementation did not affect mean duration of diarrhea or persistence of diarrhea for 7 days. In addition, evidence that the authors deemed to have high certainty showed that zinc supplementation reduces the duration of diarrhea in children with signs of malnutrition by about a day. However, there was little evidence to suggest that zinc could be as efficacious to reduce the duration of acute diarrheal episodes in children aged younger than 6 months and in well-nourished children aged older than 6 months 112.
A 2018 systematic review and meta-analysis had similar findings 113. It examined the use of zinc alone or in combination with other treatments for acute diarrhea and gastroenteritis in 174 studies in 32,430 children, mostly from low- and middle-income countries 113. The authors concluded that zinc was one of the most effective interventions of those examined, especially when it was combined with Saccharomyces boulardii (a probiotic) or smectite (a natural clay that contains minerals), for reducing the duration of acute diarrhea and gastroenteritis in children 113. Analyses showed that zinc alone or in combination reduced the duration of diarrhea by about ¾ to 1½ days. Zinc supplements might have only a marginal effect on diarrhea duration in well-nourished children.
The World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) currently recommend short-term zinc supplementation—20 mg zinc per day, or 10 mg for infants under 6 months, for 10 to 14 days—to treat acute childhood diarrhea 114. Most trials of zinc supplementation for diarrhea have been conducted in low-income countries 109.
Pneumonia in children
Pneumonia is caused by lower respiratory tract viral or bacterial infections (LRTIs) — accounts for nearly 1 million deaths among children annually, primarily in low-and middle-income countries 115, 116. In low-income countries, pneumonia is responsible for 15% of all deaths in children younger than 5 years and for 19% of all childhood deaths 117. Poor zinc status is associated with greater susceptibility to pneumonia, more severe disease, and higher mortality risk in children 118, 119, 52, 120.
Vaccinations against Haemophilus influenzae type B (Hib), pneumococcus, pertussis (whooping cough), and measles can help prevent pneumonia 116. According to a 2009 WHO report on disease risk factors, zinc deficiency may be responsible for 13% of all lower respiratory tract viral or bacterial infection (LRTI) cases, primarily pneumonia and flu cases, in children younger than 5 years 121. Accordingly, a 2016 Cochrane review of 6 trials in low-income countries found that zinc supplementation with 10–20 mg zinc for up to 20 months in a total of 5,193 children aged 2 to 59 months resulted in lower incidence and prevalence of pneumonia than placebo 117. However, it remains unclear whether supplemental zinc, in conjunction with antibiotic therapy, is beneficial in the treatment of pneumonia. A recent randomized, placebo-controlled trial conducted in Gambian children who were not zinc deficient failed to show any benefit of zinc supplementation (10 mg/day or 20 mg/day [depending on child’s age] for 7 days) given alongside antibiotics in the treatment of severe pneumonia 122. A 2018 meta-analysis of five trials (1,822 participants) found no improvement when zinc was used as an adjunct to antibiotic treatment in children with pneumonia 123. There was, however, evidence that supplemental zinc reduced the risk of pneumonia-related mortality (3 trials; 1,318 participants) 123.
A 2020 systematic review and meta-analysis included 11 clinical trials in children aged 2 to 60 months with mostly severe pneumonia in low- and middle-income countries 124. Mortality rates from pneumonia and time to recovery from severe pneumonia did not differ between children treated with 10–20 mg/day supplemental zinc and those treated with placebo for 7–14 days or until discharge (Brown N, Kukka AJ, Mårtensson A. Efficacy of zinc as adjunctive pneumonia treatment in children aged 2 to 60 months in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Paediatr Open. 2020 Jul 12;4(1):e000662. doi: 10.1136/bmjpo-2020-000662)). Another meta-analysis of 6 placebo-controlled trials that included 2,216 children aged 2 to 60 months found that zinc supplementation reduced mortality rates from severe pneumonia but not rates of treatment failure or of changes in antibiotic therapy 123.
HIV in children and adults
Sufficient zinc is essential to maintain immune system function, and human immunodeficiency virus (HIV)-infected individuals are particularly susceptible to zinc deficiency. Human immunodeficiency virus (HIV) infection reduces the absorption and metabolism of zinc from foods 5. In addition, people with HIV often have diarrhea, which can result in excessive losses of zinc. For these reasons, people with HIV often have low plasma or serum zinc levels.
In HIV-infected patients, low serum zinc concentrations have been associated with disease progression and increased mortality 125, 126. Several clinical trials have found some beneficial effects of zinc supplementation to manage the morbidity and mortality associated with HIV infection.
In one study conducted in AIDS patients, 45 mg/day of zinc for one month resulted in a decreased incidence of opportunistic infections compared to placebo 127. A placebo-controlled trial in 231 HIV-positive adults with low zinc status (plasma zinc levels lower than 75 mcg/dL) found that zinc supplementation (12 mg/day for women and 15 mg/day for men) or placebo for 18 months reduced the incidence of immunological failure (defined by a CD4+ count less than 200 cells/mm³) by 76% and the rate of diarrhea by 60%, but had no effect on mortality 128. A 2011 systematic review that identified three randomized controlled trials in primarily resource-poor settings concluded that zinc supplementation was safe and efficacious in reducing opportunistic infections in HIV-positive adults 129. In 2020 trial in Iran that randomized 146 adults with HIV to 50 mg/day zinc, 200 mcg/day selenium, or placebo for 6 months and then followed participants for another 3 months, the zinc supplements decreased rates of opportunistic infections but did not improve CD4+ T-cell counts 130.
However, findings were less positive in two Cochrane reviews and another trial (not included in either Cochrane review) that assessed the potential benefits of supplementation with micronutrients, including zinc, or placebo in various populations with HIV. The first Cochrane review, which focused on micronutrient supplementation for children with HIV, included two trials that administered 10 mg/day zinc with or without vitamin A for up to 15 months in a total of 128 children with HIV in South Africa 131. One of these trials found that zinc supplementation had no beneficial effects in comparison with vitamin A, whereas the other found that the risk of watery diarrhea was 49% lower with zinc supplements than with placebo.
A subsequent Cochrane review that evaluated micronutrient supplements for adults with HIV included six placebo-controlled trials of zinc supplements (12–50 mg/day for 14 days to 18 months or 1 weekly 90 mg dose for 6 months) in a total of 826 participants 132. The authors concluded that although zinc supplements might improve zinc status, the supplements appeared to have little if any effect on CD4+ T-cell counts or viral load and inconclusive effects on mortality and diarrhea frequency.
Evidence of benefits of zinc supplementation in HIV-positive pregnant women and children is very limited. In a double-blind, randomized, placebo-controlled trial in Tanzania, the administration of zinc (25 mg/day) to 400 pregnant women between 12 and 27 weeks’ gestation until 6 weeks after delivery did not result in any differences in birth weight, duration of gestation, or rates of fetal mortality or early mother-to-child transmission of HIV in comparison with placebo 133, 134. In addition, zinc supplementation did not affect maternal viral load or CD4+, CD8+, or CD3+ T-cell counts. However, zinc supplements blunted the rise in hemoglobin concentrations between baseline and 6 weeks postpartum.
A randomized placebo-controlled trial of zinc supplementation (10 mg/day for 6 months) in 96 HIV-positive children (6 months to 5 years old) in South Africa showed no effect on CD4+ count and viral load 135. There was evidence showing a reduction in the incidence of watery diarrhea in zinc-supplemented children compared to those taking a placebo, yet no differences in the incidence of pneumonia, ear infection, or upper respiratory tract infection 135. Another trial in Uganda showed that supplemental zinc in children with severe pneumonia effectively reduced case fatality regardless of children’s HIV status 136. While zinc supplementation during pregnancy and infancy is recommended in populations likely to be zinc deficient 112, 117, 137, more evidence is required to determine whether zinc supplements might be helpful for people with HIV 138.
Neonatal sepsis
Sepsis is a life-threatening condition that causes organ dysfunction as a consequence of a dysregulated host’s response to infection 139. Sepsis is accompanied by changes in zinc homeostasis characterized in particular by a decrease in serum zinc concentration and an increase in liver zinc concentration 140. These changes in zinc distribution are thought to be part of a host’s defense mechanism whereby the host can limit zinc availability to pathogens, as well as stimulate the immune system. Such a mechanism has been described for other transition metals, including iron and manganese 139. However, lower serum zinc concentrations in critically ill patients at high risk of organ failure have been associated with recurrent sepsis episodes and poorer outcomes 141, 118. A 2018 systematic review identified four trials that examined the effect of zinc supplementation in newborns with sepsis 140. Zinc supplementation was found to result in decreased inflammation 142 and better neurological development 143, 144. Three out of four trials that examined the rate of mortality showed no effect of zinc supplementation 142, 144, 145.
Malaria
Early studies have indicated that zinc supplementation may reduce the incidence of clinical attacks of malaria in children 146. A placebo-controlled trial in preschool-aged children in Papua New Guinea found that zinc supplementation reduced the frequency of health center attendance due to Plasmodium falciparum malaria by 38% 147. Additionally, the number of malaria episodes accompanied by high circulating parasite concentrations was reduced by 68%, suggesting that zinc supplementation may be of benefit in preventing more severe episodes of malaria. However, a six-month trial in more than 700 West African children did not find any difference in the frequency or severity of malaria episodes between children supplemented with zinc and those given a placebo 148. Another randomized controlled trial reported that zinc supplementation did not benefit preschool-aged children with acute, uncomplicated malaria 149. There is also little evidence to suggest that zinc supplementation could reduce the risk of malaria-related mortality in children 150. At present, there is not enough evidence to suggest a prophylactic and/or therapeutic role for supplemental zinc in the management of childhood malaria 151. A recent randomized, placebo-controlled trial did not provide clear-cut evidence of a protective effect of zinc (25 mg/day) administered to Tanzanian women during their first gestational trimester until delivery on the risk of placental malaria infection 152.
Inadequate zinc status in elderly subjects is not uncommon and is thought to worsen the age-related decline in immune function 153. In one study, low serum zinc concentrations in nursing home residents were associated with higher risks of pneumonia and pneumonia-related and all-cause mortality 51. Trials examining the effects of zinc supplementation on immune function in middle-aged and elderly adults have given mixed results 154. Some studies showed mixed or no effects of zinc supplementation on parameters of immune function 155, 156, 157. However, zinc supplementation was found to have a positive impact on certain aspects of immune function that are affected by zinc deficiency, such as the decline in T-cell (a type of lymphocyte) function 158. For example, a randomized, placebo-controlled study in adults over 65 years of age found that zinc supplementation (25 mg/day) for three months increased blood concentrations of helper T-cells and cytotoxic T-cells 159. Additionally, a randomized, double-blind, placebo-controlled trial in 101 older adults (aged 50-70 years) with normal blood zinc concentrations showed that zinc supplementation at 15 mg/day for six months improved the helper T-cells/cytotoxic T-cells ratio, which tends to decline with age and is a predictor of survival 153. However, the study also suggested that a dose of 30 mg/day of zinc might reduce the number of B-lymphocytes, which play a central role in humoral immunity. Furthermore, zinc supplementation had no effect on various immune parameters, including markers of inflammation, measures of granulocyte and monocyte phagocytic capacity, or cytokine production by activated monocytes 153.
A more recent trial examined the effect of daily supplementation with a multiple micronutrients, including 5 mg or 30 mg of zinc for three months, on zinc status and markers of immune function in institutionalized elderly participants (mean age, >80 years) with low serum zinc concentrations 160. Zinc status was improved with the 30 mg/day dose — but not with 5 mg/day — yet the most zinc-deficient individuals failed to achieve normal serum zinc concentrations within the intervention period. The number of circulating T-cells was also significantly increased in those who took the micronutrient supplement with the higher versus low dose of zinc 160.
More research is warranted before zinc supplementation could be recommended to older adults, especially those with no symptoms of declining immunity. Nonetheless, the high prevalence of zinc deficiency among institutionalized elderly adults should be addressed and would likely improve the performance of their immune systems 154.
Wound healing
Zinc helps maintain the integrity of skin and mucosal membranes 161. Patients with chronic leg ulcers have abnormal zinc metabolism and low serum zinc levels [56], and clinicians frequently treat skin ulcers with zinc supplements 162. The authors of a systematic review concluded that zinc sulfate might be effective for treating leg ulcers in some patients who have low serum zinc levels 163, 164. However, research has not shown that the general use of zinc sulfate in patients with chronic leg ulcers or arterial or venous ulcers is effective 165.
Age-related macular degeneration (AMD) is a degenerative eye disease that affects the macula and a leading cause of blindness in people aged over 65 years in America 166. The macula is the portion of the retina in the back of the eye involved with central vision. Zinc is hypothesized to play a role in the development of age-related macular degeneration (AMD) for several reasons: (1) zinc is found at high concentrations in the part of the retina affected by age-related macular degeneration (AMD), (2) retinal zinc content has been shown to decline with age, and (3) the activities of some zinc-dependent retinal enzymes have been shown to decline with age. To date, prospective cohort studies have shown limited evidence suggesting an association between dietary zinc intake and the incidence of AMD 167, 168, 169.
Researchers have suggested that both zinc and antioxidants delay the progression of age-related macular degeneration (AMD) and vision loss, possibly by preventing cellular damage in the retina 170, 65. A large randomized, placebo-controlled trial — the Age-Related Eye Disease Study (AREDS) — of daily supplementation with antioxidants (500 mg of vitamin C, 400 IU of vitamin E, and 15 mg of beta-carotene) and high-dose zinc (80 mg of zinc as zinc oxide and 2 mg of copper as cupric oxide) found that administration of high-dose zinc alone or with the antioxidant combination to individuals with signs of moderate-to-severe macular degeneration significantly reduced the risk of developing advanced macular degeneration over a mean follow-up of 6.3 years 65. A follow-up analysis conducted four years after the cessation of the trial in 2001, including nearly 85% of the surviving participants, found that the benefit of the AREDS (combined antioxidants plus zinc) formulation had persisted 171. The odds of developing late AMD, especially neovascular AMD, was lower in both participants with a low risk of developing AMD and those who were at risk and recommended to continue taking the AREDS formulation after the trial ended. There was, however, no effect of AREDS formulation on the risk of developing central geographic atrophy 171. Another trial, AREDS2, examined the effect of an AREDS formulation without beta-carotene and/or containing 25 mg instead of 80 mg of zinc 172. The trial showed no apparent difference in the risk of developing advanced AMD with the use of AREDS formulations containing either 25 mg or 80 mg of zinc and/or beta-carotene 173. A 2017 Cochrane review of five trials (including the original AREDS study) confirmed the protective effect of supplemental zinc against neovascular and advanced AMD 174.
In a population-based cohort study in the Netherlands that included 4,170 adults aged 55 or older, dietary zinc intake as well as beta carotene, vitamin C, and vitamin E was inversely associated with the risk of AMD over a mean follow-up period of 8 years 168. Similarly, a study of 2,464 adults aged 49 or older in Australia found that at 5-year and 10-year follow-up, participants with intakes of zinc from food and supplements in the top decile (at least 15.8 mg/day) had a 44% lower risk of any AMD and a 46% lower risk of early AMD than participants in all other deciles 172. However, the authors of a systematic review and meta-analysis published in 2007 concluded that zinc is not effective for the primary prevention of early AMD, although zinc might reduce the risk of progression to advanced AMD 175.
In conclusion, the Age-Related Eye Disease Study (AREDS) formulation combining antioxidants and zinc (25 mg or 80 mg) may delay the progression of the disease in patients with AMD. Patients, especially smokers and those with vascular disease, are advised to discuss with their physician the benefits versus potential harms that could be associated with the long-term use of high-dose antioxidant vitamins and carotenoids 174.
Type 2 diabetes
Zinc concentrations are often low in people with type 2 diabetes 4, 5. Researchers therefore hypothesize that zinc depletion might play a role in diabetes progression.
Several observational studies have found an association between zinc intakes and risk of type 2 diabetes. In a systematic review and meta-analysis that included 16 observational studies conducted in the United States, Australia, Sweden, India, and Japan with a total of 146,027 men and women aged 18 to 84 years, the risk of type 2 diabetes was 13% lower in participants with the highest zinc intakes than in those with the lowest intakes 176. When the researchers analyzed the influence of area of residence on the results, the associations between zinc intakes and type 2 diabetes risk were significant only for studies conducted in rural areas, and not those conducted in urban areas.
Clinical trials have assessed the utility of zinc supplements to reduce the risk of type 2 diabetes or to manage its complications. In a 2015 Cochrane review of the clinical trial evidence on zinc supplementation for type 2 diabetes prevention, only 3 trials with a total of 128 participants met the inclusion criteria 177. These studies administered 30 mg to 100 mg zinc (in the form of zinc sulfate or zinc amino chelate) per day for 4 to 12 weeks, but the quality of these studies could not be assessed because of the lack of relevant information in the study reports. The Cochrane review authors concluded that evidence is lacking on which to base conclusions about the use of zinc supplementation to prevent type 2 diabetes 177.
However, studies published since the Cochrane review have had more positive findings. A systematic review and meta-analysis included 9 placebo-controlled trials assessing the effects of zinc supplementation (7 mg/day to 150 mg/day) for 6 to 52 weeks on lipid profiles in a total of 424 people with type 2 diabetes 178. The supplements had beneficial effects on serum levels of triglycerides and total cholesterol but not on serum low-density lipoprotein (LDL) cholesterol or high-density lipoprotein (HDL) cholesterol levels.
A second systematic review and meta-analysis compared the effects of low-dose zinc supplements (less than 25 mg/day) with those of high-dose supplements (25 to 75 mg/day) on risk factors for type 2 diabetes and cardiovascular disease in 27 clinical trials (including two from the 2015 Cochrane review) 179. Zinc supplements were administered for 4 weeks to 12 months (mean 11 weeks) to a total of 1,042 participants aged 6 to 106 years (but most studies included adults aged 20 to 70 years) and placebo to 974 participants. Although low-dose and high-dose zinc supplements had beneficial effects on several outcomes assessed, low doses of zinc and longer administration improved a larger number of risk factors. For example, studies that administered zinc supplements for less than 12 weeks had beneficial effects on fasting blood glucose, insulin resistance, and triglyceride levels, whereas studies that administered the supplements for longer had positive effects on fasting blood glucose, hemoglobin A1c (HbA1c), triglyceride, total cholesterol, and LDL cholesterol levels. Sensitivity analyses showed no significant effect of zinc dosage on these outcomes.
A third systematic review and meta-analysis evaluated the effects of zinc supplements on type 2 diabetes prevention and management in 32 trials that administered zinc supplements (4 to 240 mg/day zinc, mean 35 mg/day) or placebo for 1 to 12 months to a total of 1,700 adults aged 18 and older with type 2 diabetes (except that one included study was in children aged 6 to 10 years) 180. The supplements reduced fasting glucose, 2-hour postprandial glucose, fasting insulin, insulin resistance, glycated hemoglobin, and C-reactive protein levels. Glycemic indicators did not vary by zinc dosage (less than 30 mg/day or at least 30 mg/day) or duration of supplementation (1 month or more than 1 month).
Research has also explored the potential benefits of zinc supplementation in people with gestational diabetes. A systematic review and meta-analysis of 5 placebo-controlled trials that included 263 people with gestational diabetes evaluated the effects of zinc supplementation (4 to 30 mg/day zinc with or without vitamin E, magnesium, calcium, vitamin D, or a combination) on metabolic status 181. The supplements had beneficial effects on fasting plasma glucose, insulin, and insulin resistance, but did not affect LDL or total cholesterol levels. However, because this analysis included studies that administered zinc in combination with other nutrients, the potential contribution of zinc by itself cannot be assessed.
Up to one quarter of people with type 2 diabetes develop diabetic foot ulcers, which can result in amputation 182. A Cochrane review of nutritional interventions to treat diabetic foot ulcers included one trial in 60 participants of 50 mg zinc (in the form of zinc sulfate) or placebo for 12 weeks 183. The authors concluded that whether zinc supplements affect diabetic foot ulcers over time is uncertain.
Overall, the evidence to date is insufficient to support any conclusions about the impact of zinc supplementation on the prevalence or severity of type 2 diabetes, gestational diabetes, or diabetic foot ulcers.
Gestational diabetes
Gestational diabetes mellitus is defined as hyperglycemia that is first diagnosed during pregnancy. The condition is associated with an increased risk for adverse pregnancy outcomes 184. A group of investigators in Iran conducted two small randomized, placebo-controlled trials to examine the effect of zinc supplementation in pregnant women with gestational diabetes. Supplemental zinc (30 mg/day) for six weeks during pregnancy improved zinc status, reduced fasting blood glucose, and improved insulin sensitivity in women with gestational diabetes but had no impact on pregnancy outcomes, including the need for cesarean section, need for insulin therapy, newborn’s birth size and Apgar scores, or incidence of hyperbilirubinemia 185, 186. Similar improvements of markers of glycemic control were reported in another placebo-controlled trial that randomized pregnant women with gestational diabetes to receive zinc (4 mg) together with magnesium (100 mg), calcium (400 mg), and vitamin D (200 IU) twice a day for six weeks 187. There was also some evidence suggesting that supplemental zinc might help correct other metabolic disorders (e.g., abnormal blood lipid profile) associated with gestational diabetes 187, 188.
Alzheimer’s disease
Abnormal homeostasis of trace metals, in particular copper and zinc, has been reported in individuals affected by Alzheimer’s disease — the most common form of dementia. Specifically, results from case-control studies have shown higher serum copper concentrations and lower serum zinc concentrations in people with Alzheimer’s disease compared to cognitively healthy controls 189, 190, 191. Based on the utilization of zinc salts in Wilson’s disease, it has been proposed that zinc supplementation could improve zinc and copper status and limit further cognitive deterioration in individuals with Alzheimer’s disease. The use of slow-release zinc acetate (150 mg/day for six months) in a randomized, placebo-controlled study of 60 patients with mild-to-moderate Alzheimer’s disease corrected low zinc status and decreased serum ‘free’ copper (i.e., unbound to ceruloplasmin) 192. Moreover, when a post-hoc analysis was restricted to participants over 70 years of age (N=29), it was found that zinc supplementation prevented the deterioration of cognition scores over the trial period 192. Additional evidence is needed to confirm whether zinc supplementation could play a role in stabilizing cognitive deficits in older adults with dementia.
Depression
A data analysis of the Boston Area Community Health (BACH) survey, including 3,708 participants (ages, 30-79 years), reported higher odds of depression symptoms in women (but not in men) in the lowest versus highest quartiles of total (median values, 8.7 mg/day versus 26.8 mg/day) and dietary (median values, 7.6 mg/day versus 13.1 mg/day) zinc intakes 193. The possibility that zinc could play a role in preventing or alleviating depression has been explored in two trials conducted by one research group. The data from these trials were analyzed following a per-protocol approach (i.e., restricted to the participants who completed the studies). A preliminary randomized, double-blind, placebo-controlled trial in 20 subjects (mean age, 43 years) treated for major depression showed that supplementation with 25 mg/day of zinc reduced depression symptoms at 6 and 12 weeks as assessed by the Hamilton Depression Rating Scale (HDRS) and Beck Depression Inventory (BDI) scores 194. A second placebo-controlled trial in 60 participants treated with the antidepressant imipramine (Tofranil; 100-200 mg/day) assessed the therapeutic response to supplemental zinc (25 mg/day) using HDRS, BDI, Clinical Global Impression scale (CGI), and Montgomery-Åsberg Depression Rating Scale (MADRS) scores 195. Zinc supplementation improved score-based measures of therapeutic response and remission after six weeks but only when the analysis was restricted to participants resistant to imipramine. There was, however, no evidence of an effect of zinc after 12 weeks 195.
How much zinc do you need?
Intake recommendations for zinc and other nutrients are provided in the Dietary Reference Intakes (DRIs) developed by the Food and Nutrition Board (FNB) at the Institute of Medicine of the National Academies 27. Dietary Reference Intake is the general term for a set of reference values used for planning and assessing nutrient intakes of healthy people. These values, which vary by age and gender 27, include the following:
- Recommended Dietary Allowance (RDA): average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals.
- Adequate Intake (AI): established when evidence is insufficient to develop an RDA and is set at a level assumed to ensure nutritional adequacy.
- Tolerable Upper Intake Level (UL): maximum daily intake unlikely to cause adverse health effects 27.
The current RDAs for zinc are listed in Table 1 27. For infants aged 0 to 6 months, the FNB established an AI for zinc that is equivalent to the mean intake of zinc in healthy, breastfed infants.
Most infants (especially those who are formula fed), children, and adults in the United States consume recommended amounts of zinc according to two national surveys, the 1988–1991 National Health and Nutrition Examination Survey 196 and the 1994 Continuing Survey of Food Intakes of Individuals 197.
However, some evidence suggests that zinc intakes among older adults might be marginal. An analysis of National Health and Nutrition Examination Survey data found that 35%–45% of adults aged 60 years or older had zinc intakes below the estimated average requirement of 6.8 mg/day for elderly females and 9.4 mg/day for elderly males. When the investigators considered intakes from both food and dietary supplements, they found that 20%–25% of older adults still had inadequate zinc intakes 198.
Zinc intakes might also be low in older adults from the 2%–4% of U.S. households that are food insufficient (sometimes or often not having enough food) 199. Data from National Health and Nutrition Examination Survey indicate that adults aged 60 years or older from food-insufficient families had lower intakes of zinc and several other nutrients and were more likely to have zinc intakes below 50% of the RDA on a given day than those from food-sufficient families 200.
Table 1. Recommended Dietary Allowances for Zinc
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 2 mg |
| Infants 7–12 months | 3 mg |
| Children 1–3 years | 3 mg |
| Children 4–8 years | 5 mg |
| Children 9–13 years | 8 mg |
| Teens 14–18 years (boys) | 11 mg |
| Teens 14–18 years (girls) | 9 mg |
| Adults (men) | 11 mg |
| Adults (women) | 8 mg |
| Pregnant teens | 12 mg |
| Pregnant women | 11 mg |
| Breastfeeding teens | 13 mg |
| Breastfeeding women | 12 mg |

What foods provide zinc?
Zinc is found in a wide variety of foods. You can get recommended amounts of zinc by eating a variety of foods including the following:
- Oysters, which are the best source of zinc. Oysters contain more zinc per serving than any other food.
- Red meat, poultry, seafood such as crab and lobsters, and fortified breakfast cereals, which are also good sources of zinc. They provide the majority of zinc in the American diet.
- Beans, nuts, whole grains, and dairy products, which provide some zinc.
Phytates (is the principal storage form of phosphorus in many plant tissues, especially bran and seeds), which are present in whole-grain breads, cereals, legumes, and other foods—bind zinc and inhibit its absorption 27, 202, 203. Thus, the bioavailability of zinc from grains and plant foods is lower than that from animal foods, although many grain- and plant-based foods are still good sources of zinc 27.
The U.S. Department of Agriculture’s (USDA’s) Nutrient Database website (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing zinc arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Zinc-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Zinc-Food.pdf).
Most Americans get enough zinc from the foods they eat.
However, certain groups of people are more likely than others to have trouble getting enough zinc:
- People who have had gastrointestinal surgery, such as weight loss surgery, or who have digestive disorders, such as ulcerative colitis or Crohn’s disease. These conditions can both decrease the amount of zinc that the body absorbs and increase the amount lost in the urine.
- Vegetarians because they do not eat meat, which is a good source of zinc. Also, the beans and grains they typically eat have compounds that keep zinc from being fully absorbed by the body. For this reason, vegetarians might need to eat as much as 50% more zinc than the recommended amounts.
- Older infants who are breastfed because breast milk does not have enough zinc for infants over 6 months of age. Older infants who do not take formula should be given foods that have zinc such as pureed meats. Formula-fed infants get enough zinc from infant formula.
- Alcoholics because alcoholic beverages decrease the amount of zinc that the body absorbs and increase the amount lost in the urine. Also, many alcoholics eat a limited amount and variety of food, so they may not get enough zinc.
- People with sickle cell disease because they might need more zinc.
Table 2. Food Sources of Zinc
| Food | Milligrams (mg) per serving | Percent DV* |
|---|---|---|
| Oysters, cooked, breaded and fried, 3 ounces | 74.0 | 493 |
| Beef chuck roast, braised, 3 ounces | 7.0 | 47 |
| Crab, Alaska king, cooked, 3 ounces | 6.5 | 43 |
| Beef patty, broiled, 3 ounces | 5.3 | 35 |
| Breakfast cereal, fortified with 25% of the DV for zinc, ¾ cup serving | 3.8 | 25 |
| Lobster, cooked, 3 ounces | 3.4 | 23 |
| Pork chop, loin, cooked, 3 ounces | 2.9 | 19 |
| Baked beans, canned, plain or vegetarian, ½ cup | 2.9 | 19 |
| Chicken, dark meat, cooked, 3 ounces | 2.4 | 16 |
| Yogurt, fruit, low fat, 8 ounces | 1.7 | 11 |
| Cashews, dry roasted, 1 ounce | 1.6 | 11 |
| Chickpeas, cooked, ½ cup | 1.3 | 9 |
| Cheese, Swiss, 1 ounce | 1.2 | 8 |
| Oatmeal, instant, plain, prepared with water, 1 packet | 1.1 | 7 |
| Milk, low-fat or non fat, 1 cup | 1.0 | 7 |
| Almonds, dry roasted, 1 ounce | 0.9 | 6 |
| Kidney beans, cooked, ½ cup | 0.9 | 6 |
| Chicken breast, roasted, skin removed, ½ breast | 0.9 | 6 |
| Cheese, cheddar or mozzarella, 1 ounce | 0.9 | 6 |
| Peas, green, frozen, cooked, ½ cup | 0.5 | 3 |
| Flounder or sole, cooked, 3 ounces | 0.3 | 2 |
Footnote: * DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration to help consumers compare the nutrient contents of products within the context of a total diet. The DV for zinc is 15 mg for adults and children age 4 and older. Food labels, however, are not required to list zinc content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 204]Zinc and healthy eating
People should get most of their nutrients from food because foods contain vitamins, minerals, dietary fiber and other substances that benefit health. In some cases, fortified foods and dietary supplements may provide nutrients that otherwise may be consumed in less-than-recommended amounts.
Groups at risk of zinc deficiency
In North America, overt zinc deficiency is uncommon 27. When zinc deficiency does occur, it is usually due to inadequate zinc intake or absorption, increased losses of zinc from the body, or increased requirements for zinc 205, 206, 207. People at risk of zinc deficiency or inadequacy need to include good sources of zinc in their daily diets. Supplemental zinc might also be appropriate in certain situations.
The following groups are among those most likely to have inadequate zinc status.
People with gastrointestinal disorders or who have had weight loss surgery (bariatric surgery)
Zinc deficiency is common in people with inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn’s disease or have had weight loss surgery (bariatric surgery) involving resection of the gastrointestinal tract because of poor dietary intakes, decreased absorption, or increased urinary excretion as a result of inflammation 208, 209. Approximately 15% to 40% of people with inflammatory bowel disease (ulcerative colitis and Crohn’s disease) have zinc deficiency during active disease states and while in remission 208, 209. In patients with zinc deficiency, the risk of inflammatory bowel disease-related symptoms (including anemia, hemorrhage, and abdominal or perianal fistula) increases, and these patients are more likely to need hospitalization or surgery. Zinc supplementation might reduce these risks 208.
Approximately 50% of people with newly diagnosed celiac disease have a high risk of zinc inadequacy or deficiency; potential contributors to this risk might include zinc malabsorption and mucosal inflammation 210, 211. Zinc deficiency sometimes persist even when people with celiac disease avoid foods containing gluten 211.
Vegetarians (especially vegans)
The bioavailability of zinc from vegetarian diets is lower than from non-vegetarian diets because vegetarians typically eat large amounts of legumes and whole grains, which contain phytates that bind zinc and inhibit its absorption 4. In addition, meat is high in bioavailable zinc 212. As a result, vegetarians and vegans usually have lower dietary intakes of zinc and lower serum zinc levels than non-vegetarians 213.
Vegetarians and vegans might benefit from using certain food preparation techniques that reduce the binding of zinc by phytates and increase its bioavailability, such as soaking beans, grains, and seeds in water for several hours before cooking them 214. In addition, organic acids in fermented foods might increase zinc absorption 214. Vegetarians and vegans might also benefit from zinc supplements 215.
Women who are pregnant or lactating
During pregnancy, the amount of zinc needed increases to accommodate fetal growth, and the Food and Nutrition Board therefore recommends that pregnant people consume 3 mg/day more zinc than nonpregnant people in the same age group 2, 5. Similarly, the zinc requirement increases by 4 mg/day during lactation.
The National Health and Nutrition Examination Survey (NHANES) data from 2001–2014 show that 11% of pregnant women in the United States have total zinc intakes from foods and supplements that are below the Estimated Average Requirement (the average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals) 216. Low serum zinc concentrations during pregnancy might increase the risk of preeclampsia and low-birthweight infants 217, 218. Routine zinc supplementation during pregnancy does not appear to reduce the risk of low birthweight, stillbirth, or neonatal death, but it might lower the risk of preterm birth 219.
During lactation, some but not all studies show that adequate intakes of foods rich in zinc increase concentrations of the mineral in breast milk 220, 221, 222. Evidence is also conflicting on whether zinc supplementation during lactation increases the zinc content of breast milk 223, 224.
Older infants who are exclusively breastfed
Zinc concentrations in breast milk peak during the first month after birth and then decline by approximately 75% by the ninth month 5. Because of this sharp drop, human breast milk alone is not sufficient to meet the infant’s zinc requirement after age 6 months 5, 225. The Food and Nutrition Board recommends that in addition to breast milk, infants aged 7–12 months consume age-appropriate foods or formula containing zinc 2.
Children with sickle cell disease
Children with sickle cell disease have a high risk of zinc insufficiency or deficiency, possibly as a result of the chelation therapy used to treat iron overload 5, 226. Children with sickle cell disease and low zinc status often are shorter and weigh less than age-matched peers, and they also have a higher risk of maturation delays, vaso-occlusive pain crises (blockages of blood flow to an area of the body), and associated hospitalizations 226. Supplemental zinc might enhance growth in children with sickle cell disease and decrease the risk of bacterial infections, hospitalizations, and vaso-occlusive pain crises 227, 5, 226.
People with alcohol use disorder
Low zinc status has been observed in 30% to 50% of people with alcohol use disorder 2, 228. Ethanol consumption decreases intestinal absorption of zinc and increases urinary zinc excretion 2, 228, 229, 230. In addition, the variety and amount of food consumed by many people with alcohol use disorder is limited, leading to inadequate zinc intake 231, 232.
What happens if you don’t get enough zinc?
Zinc deficiency is rare in North America. It causes slow growth in infants and children, delayed sexual development in adolescents and impotence in men. Zinc deficiency also causes hair loss, diarrhea, eye and skin sores and loss of appetite. Weight loss, problems with wound healing, decreased ability to taste food, and lower alertness levels can also occur.
Many of these symptoms can be signs of problems other than zinc deficiency. If you have these symptoms, your doctor can help determine whether you might have a zinc deficiency.
Zinc deficiency
Zinc deficiency is a lack of sufficient zinc to maintain optimal health. Zinc deficiency can be caused by acquired zinc deficiency or a result of an inherited zinc transporter defect (e.g., acrodermatitis enteropathica caused by mutations in the SLC39A4 gene) 233, 234. Acquired zinc deficiency is typically caused by inadequate intake, increased demand, malabsorption disorders, or excessive losses (e.g., celiac and Crohn’s disease, premature, low birth weight, and those who received exclusive parenteral low zinc breast milk feeding). Acrodermatitis enteropathica caused is an autosomal recessive disorder caused by a mutation in the SLC39A4 gene that encodes the zinc-iron-regulated transporter-like protein 4 that reduces the uptake and transport of zinc, particularly across the small intestinal mucosa 235, 236. Human milk usually contains enough zinc to maintain adequate levels despite zinc-iron transporter defects; therefore, acrodermatitis enteropathica tends to present when weaning from breast milk 237.
Zinc is a key micronutrient important in growth and development, immune function, taste, smell, wound healing, protein synthesis, and maintenance of skin and hair. Because zinc has many functions throughout the body, zinc deficiency affects many different tissues and organs 238. Zinc deficiency can affect, for example, skin; bones; and the digestive, reproductive, central nervous, and immune systems 238. The signs and symptoms of zinc deficiency vary by age 238. In infants and children, diarrhea is a common sign. In older children, hair loss (alopecia), delayed growth, and frequent infections become more prevalent. In both infants and children, zinc deficiency can impair growth and lead to a loss of appetite and reproductive problems when they reach adulthood 4, 5, 239, 19. In populations with low intakes of absorbable zinc (e.g., from meat and fish), including many low-income and middle-income countries, zinc deficiency affects the health of pregnant women and their infants by increasing the risk of child morbidity (including premature birth and low birthweight) and mortality, maternal morbidity, and adverse birth outcomes 239. In addition, zinc deficiency can interfere with the senses of taste and smell 9. Zinc deficiency in older adults can cause delays in wound healing and changes in cognitive and psychological function 238.
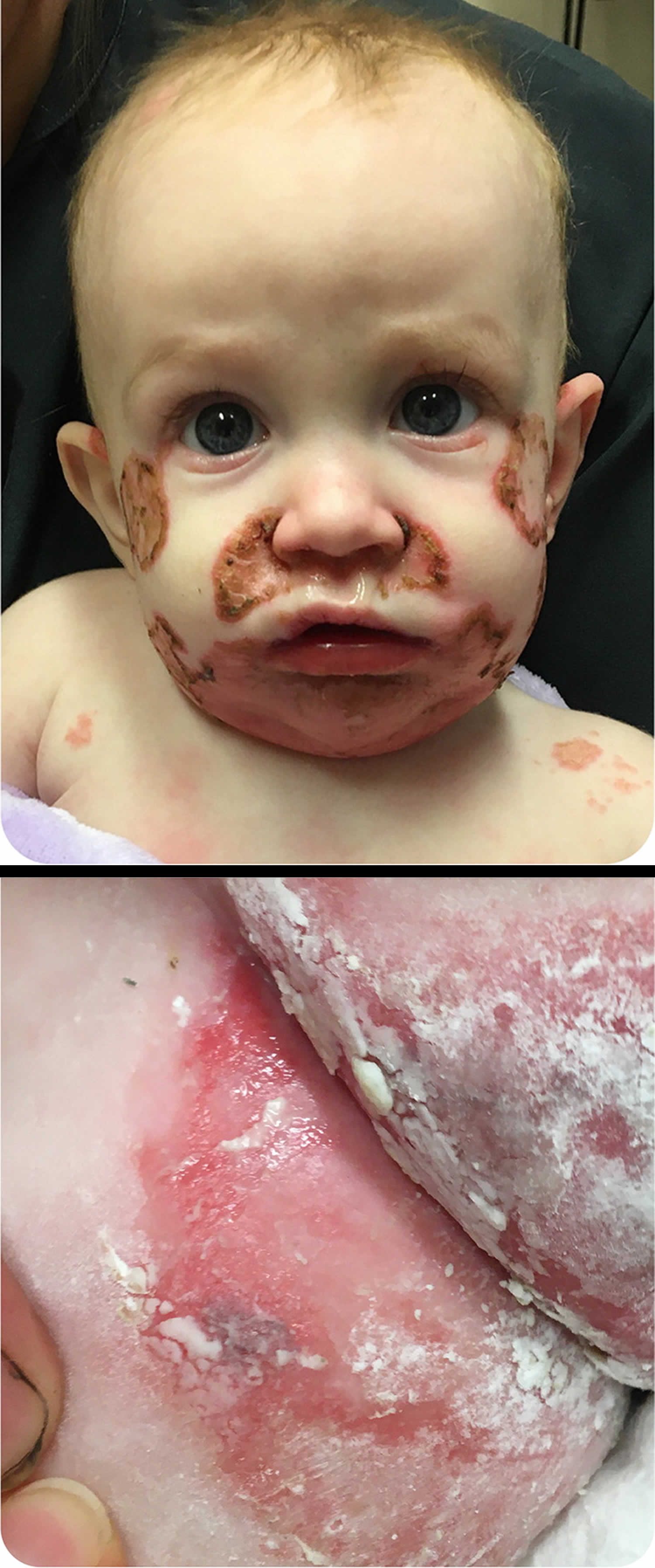
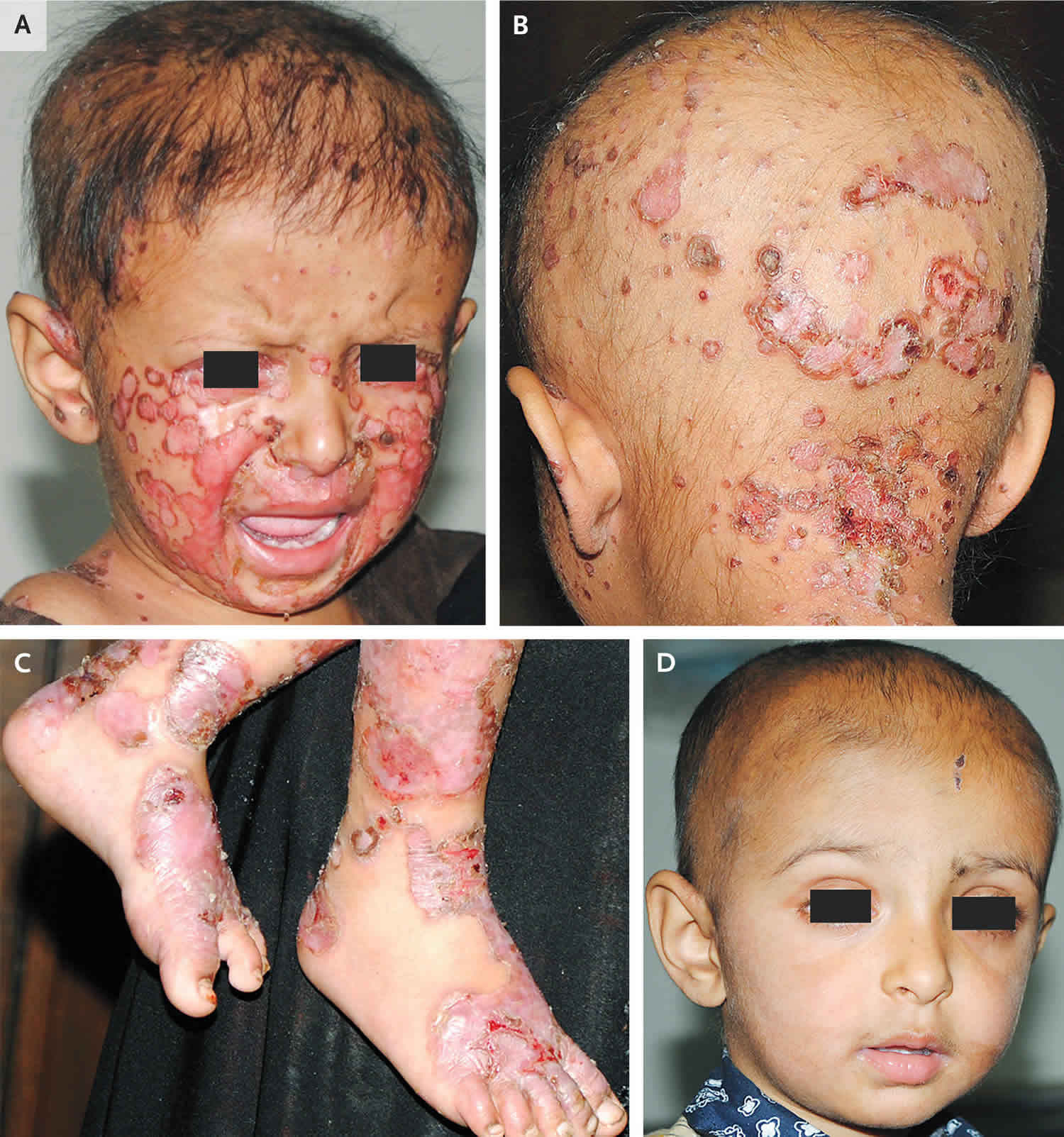
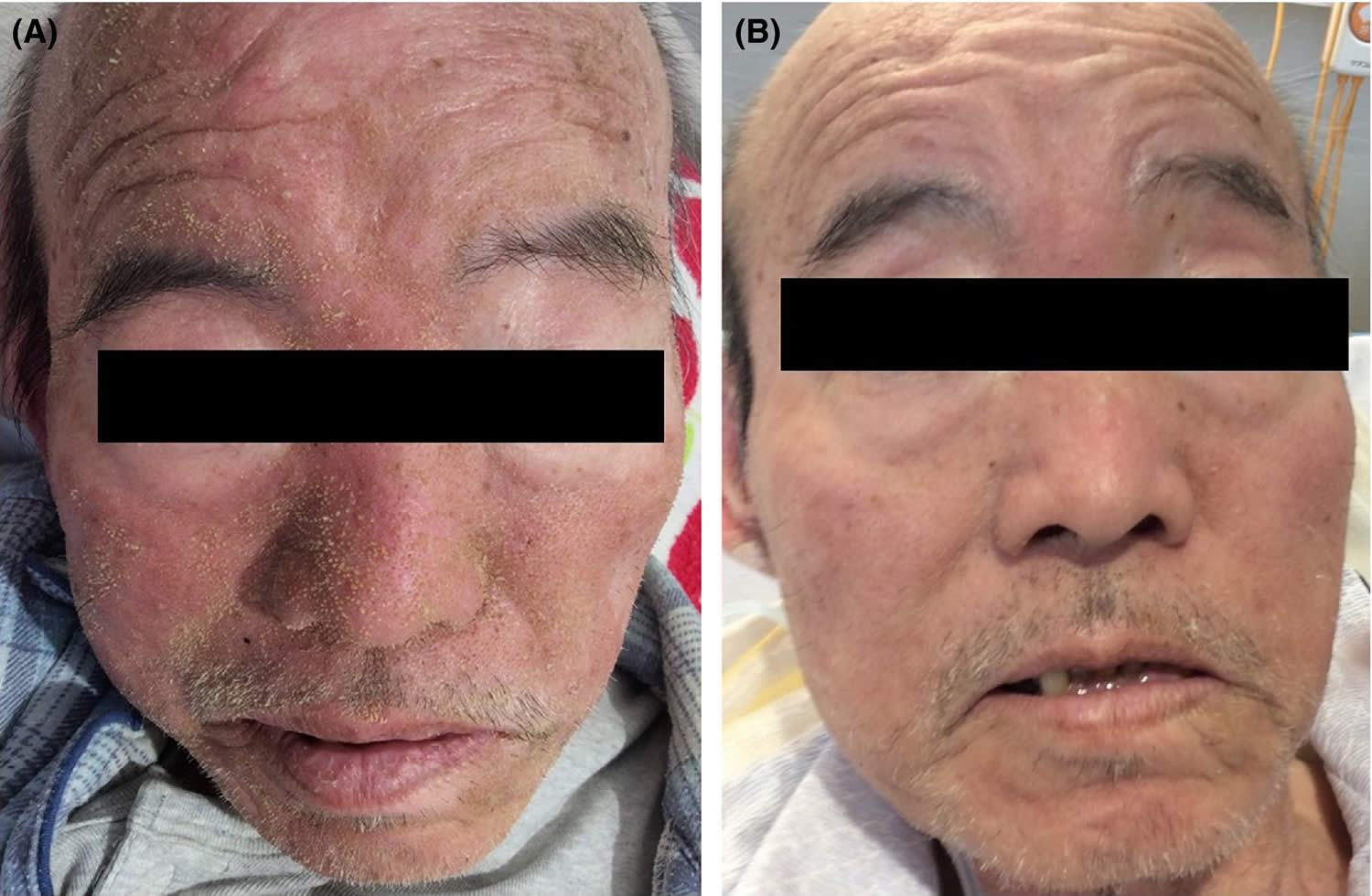
In general, individuals with zinc deficiency have compromised skin integrity because of decreased keratinocyte proliferation, differentiation, and survival 26. Zinc deficiency classically presents with a perioral facial rash in a U-shaped pattern, with cheeks to chin involvement and sparing of the upper lip (see Figures 1 to 3). There is sharp demarcation between the affected area and normal skin. A symmetrical rash with excoriation can also be prominent in the perineal, gluteal, and perianal areas 240. Zinc deficiency is often associated with hair loss (alopecia), diarrhea, and nails that tend to be soft with bridging, dystrophy, and paronychia (infection of the tissue folds around the nails). Other symptoms of zinc deficiency may include conjunctivitis (pink eye or inflammation or infection of the outer membrane of the eyeball and the inner eyelid) and sensitivity to light, loss of appetite, irritability, and impeded growth 241, 242, 243.
Endemic zinc deficiency is common in up to one-third of the population in various parts of the world, primarily in Southeast Asia and sub-Saharan Africa 244, 245. It is estimated that up to 17% of the global population is at risk for inadequate zinc intake, while in South Asia, up to 30% of the population may be deficient. Other areas at risk include sub-Saharan Africa and Central America 244. Zinc deficiency is also prevalent in Iran, Egypt, and Turkey, secondary to high phytate intake.
Clay eating or ‘pica’ is commonly seen in children of certain communities and regions. Clay effectively binds zinc, causing a dramatic decrease in the bioavailability of zinc. Approximately two billion people in developing regions are deficient in zinc to some extent. The at-risk population is comprised of children and elderly adults 246, 56, 247.
Worldwide trends and prevalence of zinc deficiency have largely been stable; however, notable reductions have been seen exemplified by countries like China with a decrease of prevalence from 17% to 8% recorded in 2005 248, 249.
The treatment for zinc deficiency is supplementation with elemental zinc (0.5 to 1 mg per kg per day) until the symptoms resolve 250. Treatment for acrodermatitis enteropathica is lifelong supplementation with elemental zinc (3 mg per kg per day); a higher dosage is needed to overcome the zinc transporter defect 241. The only known adverse effect of zinc supplementation is copper deficiency. High zinc levels inhibit copper absorption by competitively inhibiting a common cationic transporter; therefore, copper levels should be monitored during treatment of zinc deficiency.
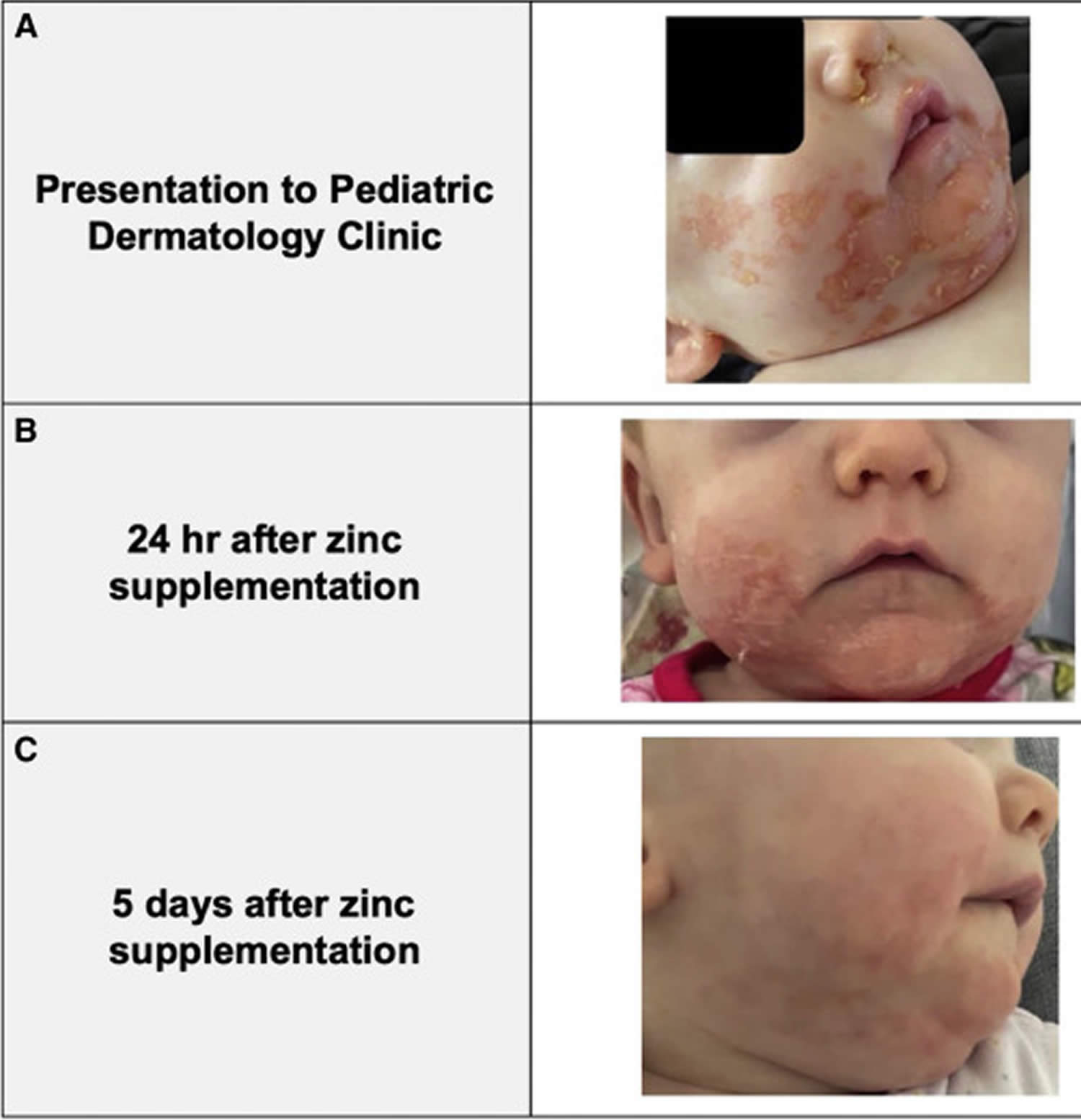
Figure 1. Zinc deficiency in a preterm baby
Footnotes: (A) 6-month-old female, born at 27 weeks of gestational age presented with sharply demarcated, eczematous, pinkish-orange, hyperkeratotic papules with “scald-like” in appearance, and plaques in a U-shaped distribution, extending from her cheeks to her chin and notably sparing the lips and eyebrow hair (rash also found on her fingers, legs, and perianal area). Fingernails were slightly ridged and soft. Mouth, tongue, and muscle tone were normal. Baby’s clinical course from initial presentation to pediatric dermatology to 5 days post-zinc supplementation. (B) Baby’s rapid response to 24 hours of oral zinc supplementation. (C) Near-total clinical resolution after only 5 days of oral zinc supplementation.
[Source 251 ]Figure 2. Zinc deficiency in a 5 month old baby
[Source 240 ]Figure 3. Dietary zinc deficiency
Footnotes: A 2-year-old boy was referred to the dermatology clinic with an 8-month history of rash, hair loss, and watery diarrhea. The child had been exclusively breast-fed until 6 months of age, at which time a vegetarian solid-food diet of produce and natural grains had been introduced. When the child was weaned from breast milk to cow’s milk at 16 months of age, the symptoms had started. On examination, the child was irritable; he had angular cheilitis, alopecia, and erosions with crusted borders across the face and scalp and around the mouth, eyes, and ears (Panels A and B). The rash also involved the perianal region, trunk, and all four limbs (Panel C). Laboratory testing showed a serum zinc level of 0.32 mcg per milliliter (reference range, 0.65 to 1.10) and a low alkaline phosphatase level. Zinc is a cofactor for alkaline phosphatase activity, and therefore a low alkaline phosphatase level may be seen in a patient with zinc deficiency. A diagnosis of dietary zinc deficiency–associated dermatitis was made. Oral zinc supplementation and dietary changes were recommended. At a 3-week follow-up visit, the alopecia and dermatitis had abated (Panel D). When zinc supplementation was stopped 3 months later, the symptoms did not recur.
[Source 252 ]Figure 4. Acquired zinc deficiency
Footnotes: A 66-year-old man with a history of diabetes mellitus experienced anorexia six months before admission. He had lost weight (52 kg → 42 kg), and his energy levels had fallen. His vital signs were unremarkable. On physical examination, he had redness with many wet yellowish scales over the entire face, except the eyelids (A), and slow healing decubitus. Alopecia, diarrhea, aphthous ulcer, or taste disorder was not observed. Laboratory testing revealed a zinc level of 35 (80-130) mcg/dL. Based on clinical and serological findings, the patient was diagnosed with acquired zinc deficiency. The patient was administered 100 mg/day of zinc acetate dihydrate, and his appetite and facial rash improved immediately (B). After 14 days, the patient’s zinc level increased to 92 mcg/dL. The patient’s nutritional status improved, and he was discharged. The patient’s zinc intake has remained satisfactory. His vitality improved.
[Source 253 ]Zinc deficiency causes
Zinc deficiency can be caused by acquired zinc deficiency or a result of an inherited zinc transporter defect (e.g., acrodermatitis enteropathica caused by mutations in the SLC39A4 gene) 233, 234, 254. Acquired zinc deficiency is typically caused by inadequate intake, increased demand, malabsorption disorders, or excessive losses (e.g., celiac and Crohn’s disease, premature, low birth weight, and those who received exclusive parenteral low zinc breast milk feeding) 5. Inadequate dietary intake of absorbable zinc is the primary cause of zinc deficiency in most situations 255. This may result from low dietary intake or heavy reliance on foods with little or poorly absorbable zinc. Inadequate dietary zinc intake is common in many parts of the world. It is often exacerbated by physiologic conditions associated with elevated zinc requirements 255, 256.
Malabsorption of zinc may occur in a number of situations for example, acrodermatitis enteropathica 257. Malabsorption syndromes and inflammatory diseases of the bowel, resulting in poor absorption and loss of zinc, may lead to secondary zinc deficiency particularly in the presence of marginal dietary intakes 5. Utilization of zinc is impaired in the presence of infection as decreased circulation of zinc reduces the availability of zinc to the tissues.
Acrodermatitis enteropathica caused is an autosomal recessive disorder caused by a mutation in the SLC39A4 gene that encodes the zinc-iron-regulated transporter-like protein 4 that reduces the uptake and transport of zinc, particularly across the small intestinal mucosa 235, 236. Human milk usually contains enough zinc to maintain adequate levels despite zinc-iron transporter defects; therefore, acrodermatitis enteropathica tends to present when weaning from breast milk 237.
Conditions of impaired intestinal integrity not only reduce absorption, but also result in increased endogenous losses of zinc. Fecal excretion of zinc is increased during acute diarrhea 258. It is not clear to what extent this represents unabsorbed zinc or zinc of endogenous origin 259. Diarrheal diseases are common in many low-income countries. The fact that zinc deficiency increases the susceptibility to childhood diarrhea while increased losses of endogenous zinc associated with diarrhea further deplete body zinc, results in a vicious cycle that merits further study 260.
Inherited zinc deficiency
Much of what is known about severe zinc deficiency was derived from the study of individuals born with acrodermatitis enteropathica, a rare genetic disorder resulting from the impaired uptake and transport of zinc 261, 257. The symptoms of severe zinc deficiency include the slowing or cessation of growth and development, delayed sexual maturation, characteristic skin rashes, chronic and severe diarrhea, immune system deficiencies, impaired wound healing, diminished appetite, impaired taste sensation, night blindness, swelling and clouding of the cornea, and behavioral disturbances. Before the cause of acrodermatitis enteropathica was known, patients typically died in infancy. Oral zinc therapy results in the complete remission of symptoms, though it must be maintained indefinitely in individuals with the genetic disorder 261, 254.
Acquired zinc deficiency
In modern society, acquired zinc deficiency is common, occurring in approximately 17% of the world population 262; vegetarians, alcoholics, malnourished individuals, and premature infants are most at risk 241.
It is now recognized that milder zinc deficiency contributes to a number of health problems, especially common in children who live in low-resource countries. An estimated 2 billion people worldwide are affected by dietary zinc deficiency 16. The lack of a sensitive and specific indicator of marginal zinc deficiency hinders the scientific study of its health implications 34. However, controlled trials of moderate zinc supplementation have demonstrated that marginal zinc deficiency contributes to impaired physical and neuropsychological development and increased susceptibility to life-threatening infections in young children 254. In fact, zinc deficiency has been estimated to cause more than 450,000 deaths annually in children under five years of age, comprising 4.4% of global childhood deaths 263.
In industrialized countries, dietary zinc deficiency is unlikely to cause severe zinc deficiency in individuals without a genetic disorder, zinc malabsorption or conditions of increased zinc loss, such as severe burns or prolonged diarrhea. Severe zinc deficiency has also been reported in individuals undergoing total parenteral nutrition without zinc, in those who abuse alcohol, and in those who are taking certain medications like penicillamine 264.
Individuals at risk of zinc deficiency 5, 264, 259, 19:
- Premature and low-birth-weight infants
- Older breast-fed infants and toddlers with inadequate intake of zinc-rich complementary foods
- Children and adolescents
- Pregnant and lactating (breast-feeding) women, especially adolescents
- Patients receiving total parenteral nutrition (intravenous feedings)
- Malnourished individuals, including those with protein-energy malnutrition and anorexia nervosa
- Individuals with severe or persistent diarrhea
- Individuals with malabsorption syndromes, including celiac disease and short bowel syndrome
- Individuals with inflammatory bowel disease, including Crohn’s disease and ulcerative colitis
- Alcoholics and those with alcoholic liver disease who have increased urinary zinc excretion and low liver zinc levels
- Individuals with chronic renal disease
- Individuals with sickle cell anemia
- Individuals who use medications that decrease intestinal zinc absorption, increase zinc excretion, or impair zinc utilization
- Older adults (65 years and older)
- Vegetarians: The requirement for dietary zinc may be as much as 50% greater for vegetarians whose major food staples are grains and legumes, because high levels of phytate in these foods reduce zinc absorption 2.
Groups at risk of zinc deficiency
In North America, overt zinc deficiency is uncommon 27. When zinc deficiency does occur, it is usually due to inadequate zinc intake or absorption, increased losses of zinc from the body, or increased requirements for zinc 205, 206, 264. People at risk of zinc deficiency or inadequacy need to include good sources of zinc in their daily diets. Supplemental zinc might also be appropriate in certain situations.
The following groups are among those most likely to have inadequate zinc status.
People with gastrointestinal disorders or who have had weight loss surgery (bariatric surgery)
Zinc deficiency is common in people with inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn’s disease or have had weight loss surgery (bariatric surgery) involving resection of the gastrointestinal tract because of poor dietary intakes, decreased absorption, or increased urinary excretion as a result of inflammation 208, 209. Approximately 15% to 40% of people with inflammatory bowel disease (ulcerative colitis and Crohn’s disease) have zinc deficiency during active disease states and while in remission 208, 209. In patients with zinc deficiency, the risk of inflammatory bowel disease-related symptoms (including anemia, hemorrhage, and abdominal or perianal fistula) increases, and these patients are more likely to need hospitalization or surgery. Zinc supplementation might reduce these risks 208.
Approximately 50% of people with newly diagnosed celiac disease have a high risk of zinc inadequacy or deficiency; potential contributors to this risk might include zinc malabsorption and mucosal inflammation 210, 211. Zinc deficiency sometimes persist even when people with celiac disease avoid foods containing gluten 211.
Vegetarians (especially vegans)
The bioavailability of zinc from vegetarian diets is lower than from non-vegetarian diets because vegetarians typically eat large amounts of legumes and whole grains, which contain phytates that bind zinc and inhibit its absorption 4. In addition, meat is high in bioavailable zinc 212. As a result, vegetarians and vegans usually have lower dietary intakes of zinc and lower serum zinc levels than non-vegetarians 213.
Vegetarians and vegans might benefit from using certain food preparation techniques that reduce the binding of zinc by phytates and increase its bioavailability, such as soaking beans, grains, and seeds in water for several hours before cooking them 214. In addition, organic acids in fermented foods might increase zinc absorption 214. Vegetarians and vegans might also benefit from zinc supplements 215.
Women who are pregnant or lactating
During pregnancy, the amount of zinc needed increases to accommodate fetal growth, and the Food and Nutrition Board therefore recommends that pregnant people consume 3 mg/day more zinc than nonpregnant people in the same age group 2, 5. Similarly, the zinc requirement increases by 4 mg/day during lactation.
The National Health and Nutrition Examination Survey (NHANES) data from 2001–2014 show that 11% of pregnant women in the United States have total zinc intakes from foods and supplements that are below the Estimated Average Requirement (the average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals) 216. Low serum zinc concentrations during pregnancy might increase the risk of preeclampsia and low-birthweight infants 217, 218. Routine zinc supplementation during pregnancy does not appear to reduce the risk of low birthweight, stillbirth, or neonatal death, but it might lower the risk of preterm birth 219.
During lactation, some but not all studies show that adequate intakes of foods rich in zinc increase concentrations of the mineral in breast milk 220, 221, 222. Evidence is also conflicting on whether zinc supplementation during lactation increases the zinc content of breast milk 223, 224.
Older infants who are exclusively breastfed
Zinc concentrations in breast milk peak during the first month after birth and then decline by approximately 75% by the ninth month 5. Because of this sharp drop, human breast milk alone is not sufficient to meet the infant’s zinc requirement after age 6 months 5, 225. The Food and Nutrition Board recommends that in addition to breast milk, infants aged 7–12 months consume age-appropriate foods or formula containing zinc 2.
Children with sickle cell disease
Children with sickle cell disease have a high risk of zinc insufficiency or deficiency, possibly as a result of the chelation therapy used to treat iron overload 5, 226. Children with sickle cell disease and low zinc status often are shorter and weigh less than age-matched peers, and they also have a higher risk of maturation delays, vaso-occlusive pain crises (blockages of blood flow to an area of the body), and associated hospitalizations 226. Supplemental zinc might enhance growth in children with sickle cell disease and decrease the risk of bacterial infections, hospitalizations, and vaso-occlusive pain crises 227, 5, 226.
People with alcohol use disorder
Low zinc status has been observed in 30% to 50% of people with alcohol use disorder 2, 228. Ethanol consumption decreases intestinal absorption of zinc and increases urinary zinc excretion 2, 228, 229, 230. In addition, the variety and amount of food consumed by many people with alcohol use disorder is limited, leading to inadequate zinc intake 231, 232.
Zinc deficiency prevention
People should get most of their nutrients from food and beverages, according to the federal government’s Dietary Guidelines for Americans. The federal government’s 2020-2025 Dietary Guidelines for Americans notes that “Because foods contain vitamins, minerals, dietary fiber, and other components that have benefits for health, nutritional needs should be met primarily through foods. … In some cases, fortified foods and dietary supplements are useful when it is not possible otherwise to meet needs for one or more nutrients (for example, during specific life stages such as pregnancy).”
Numerous zinc supplementation trials have shown that a wide range of health benefits can be realized by increasing the intake of zinc where diets are inadequate in zinc 13, 265. The results of these trials strongly argue for the development of programs to improve zinc status in high-risk populations. The major intervention strategies are dietary diversification/modification, zinc supplementation, food fortification, and bio-fortification. These strategies are not mutually exclusive but can be used in a complementary way. Their choice depends upon the available resources and technical feasibility.
Zinc supplementation
Supplements contain several forms of zinc, including zinc gluconate, zinc sulfate, and zinc acetate 17. The percentage of elemental zinc varies by form. Zinc oxide, zinc acetate, zinc sulfate, and zinc gluconate, contain elemental zinc percentages of 80%, 30%, 23%, and 14.3%, respectively 57. For example, approximately 23% of zinc sulfate consists of elemental zinc; thus, 220 mg of zinc sulfate contains 50 mg of elemental zinc. The elemental zinc content appears in the Supplement Facts panel on the supplement container. Research has not determined whether differences exist among forms of zinc in absorption, bioavailability, or tolerability 58.
Supplementation programs are useful for targeting vulnerable population subgroups, which are at a particular high-risk of micronutrient deficiencies. The easiest way to supplement zinc could be to include it in programs already delivering daily or weekly nutrient supplements for the prevention of iron deficiency anemia and other micronutrient deficiencies. The recommended zinc dosages are 5 mg/day for children between 7 months and 3 years and 10 mg/day for older children 148, 266. When formulating multi-nutrient supplements, it is recommended that salts providing readily absorbable zinc, like zinc sulfate (ZnSO4), zinc gluconate or zinc acetate are used because they are absorbed more efficiently 259.
Supplemental zinc is also recommended as an add-on therapy during the treatment of diarrhea in children 267. The recommended daily dosage is twice the age-specific Recommended Dietary Allowance (RDA) per day for 14 days; that is 10 mg/day for children under 3 years and 20 mg for older children. Several clinical trials have demonstrated that zinc supplements reduce the severity and duration of acute and persistent diarrhea 148, 265.
Food fortification
Food fortification is a more cost-effective and sustainable strategy to overcome micronutrient malnutrition than supplementation 238. Where micronutrient deficiency is widely distributed in a population and dietary modification or diversification is difficult to achieve, fortification of centrally processed foods is an appropriate alternative. Mexico provides an example of a country with a nationwide zinc fortification program. Apart from zinc, other micronutrients are added to wheat and corn flours that are used in preparing bread and tortilla, the two principal staple foods in the country. For such multiple interventions synergistic and antagonistic interactions between micronutrients have to be taken into account during the development of appropriate formulations 268.
Fortification programs can also be specifically targeted to increase the intake of zinc in groups of high-risk such as infants and young children who consume particular type of food. In many countries, infant formulas and complementary foods are currently fortified with zinc and other micronutrients. Commercially available standard infant formulas contain zinc in concentrations of around 1 mg/L, following current recommendations. In general, the food selected for fortification should be one that is widely consumed in stable and predictable amounts. Among several zinc compounds that are available for fortification, zinc oxide and zinc sulfate are least expensive and most commonly used by the food industry. Suggested levels for fortification of flour are 30-70 mg zinc/kg 269. Zinc sulfate theoretically provides more absorbable zinc because of its greater solubility 270, but it is more expensive 259. However, there are studies in humans that do not show a difference in the absorption of zinc from zinc oxide and zinc sulfate 271. Further information is required on the bio-availability of zinc, acceptability and cost of fortifying food products with different chemical forms of zinc.
Bio-fortification
Recently, plant breeding or genetic engineering strategies that either increase the level of zinc, reduce the content of inhibitors (e.g., phytate), or increase the expression of compounds that enhance zinc absorption (e.g., amino acids) have been considered to improve the bio-availability of zinc from plant foods 272. Bio-fortification differs from ordinary fortification because it focuses on intrinsic enrichment of micronutrients in plant parts that are used for food while the plants are still growing, rather than having nutrients from external resources added to the foods when they are being processed 273. This is an improvement on ordinary fortification when it comes to providing nutrients for the rural poor, who rarely have access to commercially fortified foods 273. As such, bio-fortification is seen as an upcoming strategy for dealing with deficiencies of micronutrients in the developing world 238. Its additional benefits include higher yield where micronutrients are limiting plant growth and increased vitality of seedlings emerging from zinc-enriched seeds 238.
Dietary diversification or modification
Dietary diversification or modification is a sustainable long-term approach to improving the intake of several nutrients simultaneously. Dietary diversification or modification strategies at the community or household level have the potential to increase the intake of bio-available zinc. Such strategies include (1) Agricultural interventions (2) Production and promotion of animal-source foods through animal husbandry or aquaculture (3) Processing strategies at the commercial or household level to enhance zinc absorption from plant-based diets 274. Agricultural interventions focused on plant-based foods may have little impact on intake of bio-available zinc. Some benefit may be realized if accompanied by processing strategies to reduce the levels of substances that inhibit zinc absorption, such as phytate, however, this is likely to be insufficient to meet zinc needs of infants and young children. Animal husbandry efforts that increase red meat or liver consumption by infants and young children can have a positive impact. Milk and cheese are also important sources of dietary zinc 275. These foods generally have a higher content of readily absorbed zinc than poultry, eggs, or fish. Nutrition education to promote dietary diversification or modification can lead to greater intakes of animal-source foods and thus bioavailable zinc. Care must be taken to avoid potential adverse effects of the above strategies, such as aflatoxin contamination of germinated cereals, loss of water-soluble nutrients from soaking cereal flours, and displacement of breast milk by increased intakes of other foods 274. However, more information is required on zinc content and zinc absorption modifiers in local foods to identify suitable sources of absorbable zinc. Although, dietary modification and diversification is the most sustainable approach, change of the dietary practices and preferences is difficult and foods that provide highly bio-available zinc (such as red meat) are generally expensive.
Zinc deficiency signs and symptoms
Because zinc has many functions throughout the body, zinc deficiency affects many different tissues and organs resulting in a broad range of signs and symptoms of zinc deficiency 238. Organ systems known to be affected clinically by zinc deficiency states include the skin, bones, and the digestive, reproductive, central nervous, and immune systems 276, 238.
The signs and symptoms of zinc deficiency vary by age 238. In infants and children, diarrhea is a common sign. In older children, hair loss (alopecia), delayed growth, and frequent infections become more prevalent. In both infants and children, zinc deficiency can impair growth and lead to a loss of appetite and reproductive problems when they reach adulthood 4, 5, 239, 19. In populations with low intakes of absorbable zinc (e.g., from meat and fish), including many low-income and middle-income countries, zinc deficiency affects the health of pregnant women and their infants by increasing the risk of child morbidity (including premature birth and low birthweight) and mortality, maternal morbidity, and adverse birth outcomes 239. In addition, zinc deficiency can interfere with the senses of taste and smell 9. Zinc deficiency in older adults can cause delays in wound healing and changes in cognitive and psychological function 238.
Zinc deficiency symptoms may include:
- Frequent infections
- Hypogonadism in males
- Loss of hair (alopecia)
- Poor appetite
- Problems with the sense of taste
- Problems with the sense of smell
- Skin sores
- Slow growth
- Trouble seeing in the dark
- Wounds that take a long time to heal
Dermatitis (eczema), hair loss (alopecia), and diarrhea are typical symptoms of acquired zinc deficiency, in addition to impaired wound healing, taste disorder (dysgeusia), and night blindness 253. As with hereditary zinc deficiency, dermatitis of acquired zinc deficiency typically occurs in perioral and acral areas; skin findings are erythematous, scaly, and crusted plaques and erosions 277. There are few case reports of zinc deficiency with a facial rash 278, 279. Clinicians should consider zinc deficiency as a differential diagnosis of loss of appetite with various facial rashes.
Impaired immune system function
Adequate zinc intake is essential in maintaining the integrity of the immune system 43, specifically for normal development and function of cells that mediate both innate (neutrophils, macrophages, and natural killer cells) and adaptive (B-lymphocytes and T-lymphocytes) immune responses 55. Because pathogens also require zinc to thrive and invade, a well-established antimicrobial defense mechanism in the body sequesters free zinc away from microbes 53. Another opposite mechanism consists in intoxicating intracellular microbes within macrophages with excess zinc 54. Through weakening innate and adaptive immune responses, zinc deficiency diminishes the capacity of the body to combat pathogens 53. As a consequence, zinc-deficient individuals experience an increased susceptibility to a variety of infectious agents 56.
Diarrhea
Zinc promotes mucosal resistance to infections by supporting the activity of immune cells and the production of antibodies against invading pathogens 53. Therefore, a deficiency in zinc increases the susceptibility to intestinal infections and constitutes a major contributor to diarrheal diseases in children 56. In turn, persistent diarrhea contributes to zinc deficiency and malnutrition 56. Research indicates that zinc deficiency may also potentiate the effects of toxins produced by diarrhea-causing bacteria like E. coli 108. It is estimated that diarrheal diseases are responsible for the deaths of about 500,000 children under five years of age annually in low- and middle-income countries 280. Zinc supplementation in combination with oral rehydration therapy has been shown to significantly reduce the duration and severity of acute and persistent childhood diarrhea and to increase survival in a number of randomized controlled trials 281. A 2016 meta-analysis of randomized controlled trials found that zinc supplementation reduced the duration of acute diarrhea by one day in children aged >6 months who presented signs of malnutrition (5 trials; 419 children) 112. However, there was little evidence to suggest that zinc could be as efficacious to reduce the duration of acute diarrheal episodes in children aged <6 months and in well-nourished children aged >6 months. Zinc supplementation also reduced the duration of persistent diarrhea in children by more than half a day (5 trials; 529 children) 112.
The World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) currently recommend supplementing young children with 10 to 20 mg/day of zinc as part of the treatment for acute diarrheal episodes and to prevent further episodes in the two to three months following zinc supplementation 282.
Pneumonia
Pneumonia — caused by lower respiratory tract viral or bacterial infections (LRTIs) — accounts for nearly 1 million deaths among children annually, primarily in low-and middle-income countries 280. Vaccinations against Haemophilus influenzae type B, pneumococcus, pertussis (whooping cough), and measles can help prevent pneumonia 283. According to a 2009 WHO report on disease risk factors, zinc deficiency may be responsible for 13% of all LRTI cases, primarily pneumonia and flu cases, in children younger than 5 years 284. Accordingly, a 2016 meta-analysis of six trials found that zinc supplementation in children under 5 years old reduced the risk of pneumonia by 13% 117. However, it remains unclear whether supplemental zinc, in conjunction with antibiotic therapy, is beneficial in the treatment of pneumonia. A recent randomized, placebo-controlled trial conducted in Gambian children who were not zinc deficient failed to show any benefit of zinc supplementation (10 mg/day or 20 mg/day [depending on child’s age] for 7 days) given alongside antibiotics in the treatment of severe pneumonia 122. A 2018 meta-analysis of five trials (1,822 participants) found no improvement when zinc was used as an adjunct to antibiotic treatment in children with pneumonia 123. There was, however, evidence that supplemental zinc reduced the risk of pneumonia-related mortality (3 trials; 1,318 participants) 123.
Delayed mental and psychomotor development in young children
Adequate nutrition in essential for brain growth and development, especially during the first 1,000 days of life — a critical period of development for all organs and systems spanning from conception to 24 months of age 285. Animal studies 286 have established that zinc deficiency in early life interferes with normal brain development and cognitive functions. Data on the effect of zinc supplementation during pregnancy on infants’ neurologic and psychomotor outcomes is very limited. In a randomized, placebo-controlled trial in African-American women, daily maternal supplementation with 25 mg of zinc from about 19 weeks’ gestation had no effect on neurologic development test scores in their children at five years of age 287.
Several studies have reported on the effect of postnatal zinc supplementation on mental and motor development. Two early randomized controlled trials, one conducted in India and the other in Guatemala, suggested that postnatal supplementation with 10 mg/day of zinc resulted in toddlers being more vigorous 288 and functionally active 289. In one trial conducted in Brazilian newborns from low-income families and weighing between 1,500 g and 2,499 g at birth 290, neither zinc supplementation for eight weeks with 1 mg/day or 5 mg/day improved mental and psychomotor development at 6 or 12 months of age compared to a placebo and assessed using the Bayley Scales of Infant Development (BSID) for Mental Development Index (MDI) and Psychomotor Development Index (PDI). Additionally, a randomized, placebo-controlled, double-blind trial in Chilean newborns (birth weights >2,300 g) from low-income families reported no effect of zinc supplementation (5 mg/day) on mental and psychomotor development indices at 6 and 12 months 291. Two other trials found that supplemental zinc failed to improve MDI or PDI at 12 months of age when zinc (10 mg/day) was given to six-month-old infants for six months 292 or at the end of the intervention in toddlers aged 12-18 months when zinc (30 mg/day) was given for four months 293. A 2012 Cochrane review of eight clinical trials found no evidence that postnatal zinc supplementation improves mental or motor development of infants and children from populations with presumably inadequate zinc status 294.
Impaired growth, growth retardation and development
Significant delays in linear growth and weight gain, known as growth retardation or failure to thrive, are common features of mild zinc deficiency in children. In the 1970s and 1980s, several randomized, placebo-controlled studies of zinc supplementation in young children with significant growth delays were conducted in Denver, Colorado. Modest zinc supplementation (5.7 mg/day) resulted in increased growth rates compared to placebo 295. Several meta-analyses of growth data from zinc intervention trials have confirmed the widespread occurrence of growth-limiting zinc deficiency in young children, especially in low- and middle-income countries 296. A 2018 systematic review and meta-analysis identified 54 trials 297 that examined the impact of zinc supplementation during infancy (on average, 7.6 mg/day for 30.9 weeks) or childhood (on average, 8.5 mg/day for 38.9 weeks) on child anthropometric measurements. There was evidence of a positive effect of supplemental zinc on children’s height, weight, and weight-for-age Z score (WAZ), but neither on height-for-age Z score (HAZ) or weight-for-height Z score (WHZ). In addition, zinc supplementation did not reduce the risks of underweight (WAZ<-2 standard deviation [SD]), wasting (WHZ<-2 SD), or stunting (HAZ<-2 SD) in children 297. Although the exact mechanisms for the growth-limiting effect of zinc deficiency are not known, research indicates that zinc availability affects cell-signaling systems that coordinate the response to the growth-regulating hormone, insulin-like growth factor-1 (IGF-1) 8.
Pregnancy complications and adverse pregnancy outcomes
Estimates based on national food supply indicate that dietary zinc intake is likely inadequate in most low- and middle-income countries, especially those in Sub-Saharan Africa and South Asia 298. Inadequate zinc status during pregnancy interferes with fetal development, and preterm neonates from zinc-deficient mothers suffer from growth retardation and dermatitis and are at risk of infections, necrotizing enterocolitis, chronic lung disease, and retinopathy of prematurity 23. Maternal zinc deficiency has also been associated with a number of pregnancy complications and poor outcomes. A recent case-control study conducted in an Iranian hospital reported higher odds of congenital malformations in newborns of mothers with low serum zinc concentrations during the last month of pregnancy 299. A 2016 review of 64 observational studies 217 found an inverse relationship between maternal zinc status and the severity of preeclampsia, as well as between maternal zinc intake and the risk of low-birth-weight newborns. There were no apparent associations between maternal zinc status and the risk of gestational diabetes mellitus and preterm birth. However, the conclusions of this analysis were limited by the fact that most observational studies were conducted in women from populations not at risk for zinc deficiency 217.
To date, available evidence from maternal zinc intervention trials conducted worldwide does not support the recommendation of routine zinc supplementation during pregnancy. A 2015 systematic review and meta-analysis of 21 randomized controlled trials in over 17,000 women and their babies found a 14% reduction in premature deliveries with zinc supplementation during pregnancy, mainly in low-income women 300. This analysis, however, did not find zinc supplementation to benefit other indicators of maternal or infant health, including stillbirth or neonatal death, low birth weight, small-for-gestational age, and pregnancy-induced hypertension. There was also no effect of supplemental zinc on postpartum hemorrhage, maternal infections, congenital malformations, and child development outcomes 300. A recent review of 17 trials (of which 15 were conducted in low- and middle-income countries) found that maternal supplementation with multiple micronutrients (including, among others, zinc, iron, and folic acid) reduced the risk of low-birth-weight newborns and small-for-gestational age infants when compared to supplemental iron with or without folic acid 301. While multiple micronutrient supplementation would likely benefit pregnant women with coexisting micronutrient deficiencies in low- and middle-income countries, there is no evidence to recommend zinc supplementation in isolation in pregnant women from any settings 302.
Zinc deficiency complications
Prolonged and severe zinc deficiency can lead to many complications, such as:
- Growth failure – Untreated zinc deficiency is often linked with permanently stunted growth and development 296.
- Hypogonadism
- Recurrent infections – Zinc deficiency can aggravate both acute and chronic infections and these infections, in turn, can exacerbate zinc deficiency themselves. It is established to give zinc in diarrhoeal illness; however, there is limited but growing evidence of its role in other infections, such as malaria and pneumonia 112, 303.
- Diarrhea
- Skin conditions – Skin conditions associated with zinc deficiency include acrodermatitis enteropathica, cheilitis, and dermatitis.
- Zinc deficiency is also considered a risk factor for diabetes mellitus and obesity 304. However, the causative role of zinc deficiency in these endocrine disorders is still a subject of early research.
- Delayed wound healing
- Low bone mineral density – The effect of the deficiency of zinc on bone density is not well understood. There is limited evidence adding calcium to zinc supplementation is more beneficial than giving calcium alone 305.
Zinc deficiency diagnosis
Zinc deficiency may be diagnosed by clinical history and examination 306, 245. Laboratory testing, response to zinc supplementation, and cutaneous histopathology can help to confirm the diagnosis 57. In healthy people, the amount of zinc in serum or plasma is 80 to 120 mcg/dL (12 to 18 micromol/L) 4. Serum zinc levels below 70 mcg/dL in women and 74 mcg/dL in men indicate inadequate zinc status 4. However, both serum and plasma measures have important limitations. Zinc concentrations in serum are associated with the patient’s sex and age as well as the time of the blood draw (morning vs. evening) and do not always correlate with dietary or supplemental zinc intakes 18. Zinc levels also fluctuate in response to other factors, including infections, changes in steroid hormones, and muscle catabolism during weight loss or illness 2, 5. Clinicians consider risk factors (such as inadequate caloric intake, chronic alcohol use, and malabsorptive digestive diseases) and signs of zinc deficiency (such as impaired growth in infants and children) when they assess a patient’s zinc status 2.
Serum zinc concentrations fluctuate by as much as 20% during a 24-hour period, largely due to the effects of food ingestion 307. Following a meal, there is an immediate initial increase, after which the concentration declines progressively for the next 4 hour and then rises until food is eaten again. During an overnight fast, the concentration of serum zinc increases slightly, so the highest levels of the day are generally seen in the morning 308, 309. However, diurnal variations in serum zinc concentration among fasted individuals have also been observed, whereby serum zinc decreased from morning to mid-afternoon and then began to rise again to morning levels 310.
Low serum zinc concentrations can occur in the presence of several conditions, representing a normal physiologic response and not necessarily indicative of low zinc status. Serum zinc concentrations are reduced during acute infections and inflammation, which is likely due to the redistribution of zinc from the plasma to the liver 311; cytokines released during the acute phase response activate hepatic metallothionein synthesis 312, a metal-binding protein which appears to alter the hepatic uptake of zinc 313. Elevated concentrations of C-reactive protein (CRP) or other markers of the acute phase response can be used to indicate the presence of infection and should be considered in the interpretation of results. Stress and heart attack (myocardial infarction) also reduce serum zinc levels 314. Because zinc is transported in plasma bound to albumin, diseases, such as cirrhosis and protein-energy malnutrition, that produce hypoalbuminemia result in lower serum zinc concentrations 315. Hemodilution, as observed during pregnancy, oral contraceptive use, and other hormonal treatments, also results in a lower serum zinc concentration 316. On the other hand, conditions resulting in intrinsic or extrinsic hemolysis of blood cells can result in extremely high serum zinc levels because the concentration of intracellular zinc is considerably greater than in serum.
Zinc is a cofactor for alkaline phosphatase (ALP) activity, so decreased serum alkaline phosphatase (ALP) levels may provide supportive evidence of zinc deficiency 317.
Punch biopsy and histopathology of affected skin can support the diagnosis of necrolysis seen as cytoplasmic pallor, vacuolization, ballooning degeneration, and confluent necrosis of keratinocytes in the upper epidermis. Confluent parakeratosis is often present with loss of the granular layer and with dermal edema. An associated neutrophilic crust may be present. Individual keratinocytes often have pyknotic nuclei. These findings are non-specific and are often seen with pellagra and necrolytic migratory erythema. Late lesions of zinc deficiency may mimic psoriasis. Clinical improvement to zinc supplementation can also be confirmatory 16.
Zinc deficiency treatment
Zinc deficiency treatment begins with oral zinc replacement 318. In adults, zinc 2 to 3 mg/kg per day or 20-40 mg daily dose often cures all zinc deficiency clinical manifestations within 1 to 2 weeks. Even in patients with acrodermatitis enteropathica, a disease of malabsorption, oral zinc replacement with 1 to 2 mg/kg per day is still the standard of therapy with life-long zinc supplementation 319, 320.
Formulations of zinc supplements include 318:
- Zinc sulfate
- Zinc acetate
- Zinc aspartate
- Zinc orotate
- Zinc gluconate
Recommended daily elemental zinc intake is 318:
- 3 mg/day for children less than 4 years
- 5 mg/day for children between 4 and 8 years
- 8 mg/day for children between 9 and 13 years
- 9 mg/day for women (non-pregnant and non-lactating)
- 11 mg/day for men
- 11 to 12 mg/day in pregnant and lactating women
In patients with severe zinc deficiency because of malnutrition or malabsorption in disorders such as Crohn disease or short bowel syndrome, higher doses of zinc (more than 50 mg/day) may be acutely needed 318.
For preterm infants with zinc deficiency, normal breastfeeding is usually sufficient for correction, and the zinc deficiency usually resolves within weeks with no clinical symptoms. However, maternal breast milk can be zinc deficient if the mother’s stores are depleted. Also, low maternal breast milk secretion of zinc from the SLC30A2 mutation can occur. If breast secretion is low, the infant will need zinc supplemental replacement.
Overcorrection with zinc supplementation is rare but very large doses can cause severe side effects. Zinc supplements at doses more than 50 mg/day, gastrointestinal symptoms, such as nausea, vomiting, abdominal discomfort, gastric hemorrhage and diarrhea commonly occur 244. Furthermore, zinc doses more than 150 mg/day may adversely affect the immune status and lipid profile. These higher zinc supplements doses can also impair the absorption of iron and copper and over-treatment can lead to copper deficiency and can potentially lead to genito-urinary problems; therefore when zinc is given for extended periods of time, particularly at high doses, it is important to monitor the levels of copper in the blood.
Patients should be monitored for response to therapy and serum zinc levels should be checked after three to six months of supplementation. If there is an inadequate response, the zinc dose may be increased; however, close monitoring for toxicity should be done on higher doses.
In acrodermatitis enteropathica, zinc supplementation is taken for life. The dose of zinc should be individualized in cases where long-term zinc supplementation may be needed and it should be guided by serial serum zinc levels. Additionally, regular assessment of copper and concurrent copper supplementation may become necessary as zinc competes with copper absorption.
Parenteral zinc becomes necessary rarely, except for cases where there is an intestinal failure and/or the patient is on prolonged total parenteral nutrition 321.
Zinc deficiency prognosis
Typically cases of zinc deficiency respond to zinc supplementation and correction of any dietary factors that might lead to the condition. With treatment, there is often a rapid improvement of symptoms. Diarrhea may resolve within 24 hours, and skin lesions often heal within 1 to 2 weeks. Patients with inherited zinc deficiencies should have zinc levels, and alkaline phosphatase should be monitored 3 to 6 months after initiation of zinc replacement therapy and the zinc dose adjusted accordingly 322, 323.
Approximately 70% of patients with zinc deficiency respond positively to zinc supplementation if initiated within 6 months of onset 57. The skin lesions heal without permanent complication, but extended periods of zinc deficiency may have permanent effects on growth and development in children 57. Studies of zinc deprivation on rats demonstrated reduced motor activity, decreased brain mass, and short-term memory loss persisting from early life to adulthood. Similar experiments in mice showed stunted growth.
Untreated patients with classic acrodermatitis enteropathica usually die in the first few years of life 324, 325. Untreated infants exhibit severe growth retardation, dermatitis, alopecia, secondary bacterial and fungal infections, and neurologic and behavioral changes. However, all symptoms are reversible with zinc therapy 324, 325. Patients with acrodermatitis enteropathica uniformly respond to zinc therapy with a 100% survival rate. With zinc supplementation, various symptoms completely resolve or substantially improve.
Can too much zinc be harmful?
Yes, if you get too much. Signs of too much zinc include nausea, vomiting, dizziness, headaches, gastric distress, loss of appetite, stomach cramps and diarrhea 4, 5. When people take too much zinc for a long time, at doses of 50 mg zinc or more—typically from supplements or excessive use of denture adhesive creams that contain zinc, they sometimes have problems such as low copper levels, lower immunity, and low levels of HDL cholesterol (the “good” cholesterol) 4, 5, 326. According to a few reports, overuse of denture adhesive creams containing up to 34 mg zinc per gram of product can lead to neurological symptoms (including sensory ataxia and myelopathy) and anemia. Zinc-free formulations are available to prevent these effects 4, 21, 327.
One case report cited severe nausea and vomiting within 30 minutes of ingesting 4 g of zinc gluconate (570 mg elemental zinc) 328. Intakes of 150–450 mg of zinc per day have been associated with such chronic effects as low copper status, altered iron function, reduced immune function, and reduced levels of high-density lipoproteins 329. Reductions in a copper-containing enzyme, a marker of copper status, have been reported with even moderately high zinc intakes of approximately 60 mg/day for up to 10 weeks 27. The doses of zinc used in the AREDS study (80 mg per day of zinc in the form of zinc oxide for 6.3 years, on average) have been associated with a significant increase in hospitalizations for genitourinary causes, raising the possibility that chronically high intakes of zinc adversely affect some aspects of urinary physiology 330. Very high doses of zinc from supplements (142 mg/day) might also interfere with magnesium absorption and disrupt magnesium balance 331.
The amount of zinc obtained from food is rarely as high as 50 mg, so the zinc in foods is unlikely to cause zinc toxicity 17.
In order to prevent copper deficiency, the US Food and Nutrition Board set the tolerable upper intake level (UL) for zinc from foods, beverages, supplements, and medications for healthy adults at 40 mg/day 2. Table 3 below lists the tolerable upper intake levels (ULs) for zinc from foods, beverages, supplements, and medications for healthy adults by age group. The tolerable upper intake levels (ULs) do not apply to individuals receiving zinc for medical treatment, but such individuals should be under the care of a physician.
Table 3. Tolerable Upper Intake Levels (ULs) for Zinc
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months | 4 mg | 4 mg | ||
| 7–12 months | 5 mg | 5 mg | ||
| 1–3 years | 7 mg | 7 mg | ||
| 4–8 years | 12 mg | 12 mg | ||
| 9–13 years | 23 mg | 23 mg | ||
| 14–18 years | 34 mg | 34 mg | 34 mg | 34 mg |
| 19+ years | 40 mg | 40 mg | 40 mg | 40 mg |
Zinc and copper interactions
Taking large quantities of zinc (50 mg/day or more) over a period of weeks can interfere with copper bioavailability. High intake of zinc induces the intestinal synthesis of a copper-binding protein called metallothionein. Metallothionein traps copper within intestinal cells and prevents its systemic absorption (see Wilson’s disease). More typical intakes of zinc do not affect copper absorption, and high copper intakes do not affect zinc absorption 66.
Zinc and iron interactions
Iron and zinc compete for absorptive pathways 67. Supplemental (38-65 mg/day of elemental iron) but not dietary levels of iron may decrease zinc absorption 68. This interaction is of concern in the management of iron supplementation during pregnancy and lactation and has led some experts to recommend zinc supplementation for pregnant and lactating women taking iron supplements 69. Food fortification with iron has not been shown to negatively affect zinc absorption 71. In a placebo-controlled study, supplementation with zinc (10 mg/day) for three months in children aged eight to nine years significantly decreased serum iron concentrations, yet not to the extent of causing anemia 72. Additional randomized controlled studies have reported a worsening of nutritional iron status with chronic zinc supplementation 73.
Zinc and calcium interactions
High levels of dietary calcium impair zinc absorption in animals, but it is uncertain whether this occur in humans 66. One study showed that increasing the calcium intake of postmenopausal women by 890 mg/day in the form of milk or calcium phosphate (total calcium intake, 1,360 mg/day) reduced zinc absorption and zinc balance in postmenopausal women 76. However, another study found that increasing the calcium intake of adolescent girls by 1,000 mg/day in the form of calcium citrate malate (total calcium intake, 1,667 mg/day) did not affect zinc absorption or balance 77. Calcium in combination with phytate might affect zinc absorption, which would be particularly relevant to individuals who very frequently consume tortillas made with lime (i.e., calcium oxide). A study in 10 healthy women (age range, 21-47 years) found that high intake of dietary calcium (~1,800 mg/day) did not impair zinc absorption regardless of the phytate content of the diet 78.
Zinc and folate interactions
The bioavailability of dietary folate (vitamin B9) is increased by the action of a zinc-dependent enzyme. Accordingly, some studies found low zinc intake decreased folate absorption. It was also suggested that supplementation with folic acid — the synthetic form of folate — might impair zinc utilization in individuals with marginal zinc status 332. However, one study reported that supplementation with a relatively high dose of folic acid (800 µg/day) for 25 days did not alter zinc absorption or status in a group of students being fed a low-zinc diet (3.5 mg/day) 79.
Zinc and vitamin A interactions
Zinc and vitamin A interact in several ways. Zinc is a component of retinol-binding protein, a protein necessary for transporting vitamin A in the blood. Zinc is also required for the enzyme that converts retinol (vitamin A) to retinal. This latter form of vitamin A is necessary for the synthesis of rhodopsin, a protein in the eye that absorbs light and thus is involved in dark adaptation. Zinc deficiency has been associated with a decreased release of vitamin A from the liver, which may contribute to symptoms of night blindness that are seen with zinc deficiency 80.
Zinc Toxicity
The recommended upper limit in adults for zinc intake is 40 mg/day; the upper limit is lower for younger people. Isolated outbreaks of acute zinc toxicity have occurred as a result of the consumption of food or beverages contaminated with zinc released from galvanized containers 3. Signs of acute zinc toxicity are abdominal pain, diarrhea, nausea, and vomiting. Single doses of 225 to 450 mg of zinc usually induce vomiting. Milder gastrointestinal distress has been reported at doses of 50 to 150 mg/day of supplemental zinc 3. Metal fume fever has been reported after the inhalation of zinc oxide fumes. Specifically, profuse sweating, weakness, and rapid breathing may develop within eight hours of zinc oxide inhalation and persist for 12 to 24 hours after exposure is terminated 5, 2.
The major consequence of long-term consumption of excessive zinc is copper deficiency. Total zinc intakes of 60 mg/day (50 mg supplemental and 10 mg dietary zinc) for up to 10 weeks have been found to result in signs of copper deficiency 2. Copper deficiency has also been reported following chronic use of excessive amounts of zinc-containing denture creams (≥2 tubes per week containing 17-34 mg/g of zinc) 21.
Ingesting doses of elemental zinc ranging from 100 to 150 mg/day for prolonged periods interferes with copper metabolism and causes low blood copper levels, red blood cell microcytosis, neutropenia, and impaired immunity; higher doses should be given only for short periods of time and the patient followed closely.
Ingesting larger amounts (200 to 800 mg/day), usually by consuming acidic food or drinking from a galvanized (zinc-coated) container, can cause anorexia, vomiting, and diarrhea. Chronic toxicity may result in copper deficiency and may cause nerve damage.
Metal fume fever, also called brass-founders’ ague or zinc shakes, is caused by inhaling industrial zinc oxide fumes; it results in fever, dyspnea, nausea, fatigue, and myalgias. Symptom onset is usually 4 to 12 h after exposure. Symptoms usually resolve after 12 to 24 hours in a zinc-free environment 5.
Diagnosis of zinc toxicity is usually based on the time course and a history of exposure.
Treatment of zinc toxicity consists of eliminating exposure to zinc; no antidotes are available.
Intranasal zinc
Intranasal zinc is known to cause a loss of the sense of smell (anosmia) in laboratory animals 333 and there have been several case reports of individuals who developed loss of the sense of smell (anosmia) after using intranasal zinc gluconate 334. Since zinc-associated anosmia may be irreversible, the use of zinc nasal gels and sprays should be avoided. In June 2009, the FDA warned consumers to stop using three zinc-containing intranasal products because they might cause anosmia 84. The manufacturer recalled these products from the marketplace. Currently, these safety concerns have not been found to be associated with cold lozenges containing zinc.
References- Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000 Nov;59(4):541-52. doi: 10.1017/s0029665100000781
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222310
- Zinc. https://lpi.oregonstate.edu/mic/minerals/zinc
- Ryu M-S, Aydemir TB. Zinc. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Cambridge, Massachusetts: Wiley-Blackwell; 2020:393-408.
- King JC, Cousins RJ. Zinc. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:189-205.
- Merck Sharp & Dohme Corp., Merck Manual. Zinc. https://www.merckmanuals.com/professional/nutritional-disorders/mineral-deficiency-and-toxicity/zinc
- Frassinetti S, Bronzetti G, Caltavuturo L, Cini M, Croce CD. The role of zinc in life: a review. J Environ Pathol Toxicol Oncol. 2006;25(3):597-610. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i3.40
- MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000 May;130(5S Suppl):1500S-8S. doi: 10.1093/jn/130.5.1500S
- Kumbargere Nagraj S, George RP, Shetty N, Levenson D, Ferraiolo DM, Shrestha A. Interventions for managing taste disturbances. Cochrane Database Syst Rev. 2017 Dec 20;12(12):CD010470. doi: 10.1002/14651858.CD010470.pub3
- Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001) https://doi.org/10.17226/10026
- Prasad AS. Zinc: an overview. Nutrition. 1995 Jan-Feb;11(1 Suppl):93-9.
- Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother. 1996 Feb;30(2):186-7. doi: 10.1177/106002809603000215
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20(1):3-18. doi: 10.1016/j.jtemb.2006.01.006
- Prasad AS, Beck FW, Grabowski SM, Kaplan J, Mathog RH. Zinc deficiency: changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc Assoc Am Physicians. 1997 Jan;109(1):68-77.
- PRASAD AS, HALSTED JA, NADIMI M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med. 1961 Oct;31:532-46. doi: 10.1016/0002-9343(61)90137-1
- Prasad AS. Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol. 2014 Oct;28(4):357-63. doi: 10.1016/j.jtemb.2014.09.002
- Zinc. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional
- Hennigar SR, Lieberman HR, Fulgoni VL 3rd, McClung JP. Serum Zinc Concentrations in the US Population Are Related to Sex, Age, and Time of Blood Draw but Not Dietary or Supplemental Zinc. J Nutr. 2018 Aug 1;148(8):1341-1351. doi: 10.1093/jn/nxy105
- Krebs NF. Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab. 2013;62 Suppl 1:19-29. doi: 10.1159/000348261
- Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr. 2008 Jun;99 Suppl 3:S14-23. doi: 10.1017/S0007114508006818
- Nations SP, Boyer PJ, Love LA, Burritt MF, Butz JA, Wolfe GI, Hynan LS, Reisch J, Trivedi JR. Denture cream: an unusual source of excess zinc, leading to hypocupremia and neurologic disease. Neurology. 2008 Aug 26;71(9):639-43. doi: 10.1212/01.wnl.0000312375.79881.94
- Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006 Jan;5(1):196-201. doi: 10.1021/pr050361j
- Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, De Curtis M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients. 2015 Dec 11;7(12):10427-46. doi: 10.3390/nu7125542
- Sandstead HH. Understanding zinc: recent observations and interpretations. J Lab Clin Med. 1994 Sep;124(3):322-7.
- Vaughn AR, Foolad N, Maarouf M, Tran KA, Shi VY. Micronutrients in Atopic Dermatitis: A Systematic Review. J Altern Complement Med. 2019 Jun;25(6):567-577. doi: 10.1089/acm.2018.0363
- Ogawa Y, Kawamura T, Shimada S. Zinc and skin biology. Arch Biochem Biophys. 2016 Dec 1;611:113-119. doi: 10.1016/j.abb.2016.06.003
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001). Washington, DC: National Academy Press, 2001. https://www.nap.edu/catalog/10026/dietary-reference-intakes-for-vitamin-a-vitamin-k-arsenic-boron-chromium-copper-iodine-iron-manganese-molybdenum-nickel-silicon-vanadium-and-zinc
- Solomons NW. Mild human zinc deficiency produces an imbalance between cell-mediated and humoral immunity. Nutr Rev. 1998 Jan;56(1 Pt 1):27-8. doi: 10.1111/j.1753-4887.1998.tb01656.x
- Markell M, Siddiqi HA. Vitamins and trace elements. In: McPherson RA, Pincus MR, eds. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 24th ed. Philadelphia, PA: Elsevier; 2022:chap 27.
- Mason JB, Booth SL. Vitamins, trace minerals, and other micronutrients. In: Goldman L, Schafer AI, eds. Goldman-Cecil Medicine. 26th ed. Philadelphia, PA: Elsevier; 2020:chap 205.
- Ramu A, Neild P. Diet and nutrition. In: Naish J, Syndercombe Court D, eds. Medical Sciences. 3rd ed. Philadelphia, PA: Elsevier; 2019:chap 16.
- Zinc. https://medlineplus.gov/druginfo/natural/982.html
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993 Jan;73(1):79-118. doi: 10.1152/physrev.1993.73.1.79
- King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011 Aug;94(2):679S-84S. doi: 10.3945/ajcn.110.005744
- Cornish-Bowden A. Current IUBMB recommendations on enzyme nomenclature and kinetics. Perspectives in Science. 2014;1(1-6):74-87.
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995 Dec 15;83(6):835-9. doi: 10.1016/0092-8674(95)90199-x
- Atrián-Blasco E, Santoro A, Pountney DL, Meloni G, Hureau C, Faller P. Chemistry of mammalian metallothioneins and their interaction with amyloidogenic peptides and proteins. Chem Soc Rev. 2017 Dec 11;46(24):7683-7693. doi: 10.1039/c7cs00448f
- Hijova E. Metallothioneins and zinc: their functions and interactions. Bratisl Lek Listy. 2004;105(5-6):230-4.
- Sirangelo I, Iannuzzi C. The Role of Metal Binding in the Amyotrophic Lateral Sclerosis-Related Aggregation of Copper-Zinc Superoxide Dismutase. Molecules. 2017 Aug 29;22(9):1429. doi: 10.3390/molecules22091429
- Hershfinkel M, Moran A, Grossman N, Sekler I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11749-54. doi: 10.1073/pnas.201193398
- Ruz M, Carrasco F, Rojas P, Basfi-Fer K, Hernández MC, Pérez A. Nutritional Effects of Zinc on Metabolic Syndrome and Type 2 Diabetes: Mechanisms and Main Findings in Human Studies. Biol Trace Elem Res. 2019 Mar;188(1):177-188. doi: 10.1007/s12011-018-1611-8
- Takeda A, Tamano H. The Impact of Synaptic Zn2+ Dynamics on Cognition and Its Decline. Int J Mol Sci. 2017 Nov 14;18(11):2411. doi: 10.3390/ijms18112411
- Baum MK, Shor-Posner G, Campa A. Zinc status in human immunodeficiency virus infection. J Nutr. 2000 May;130(5S Suppl):1421S-3S. doi: 10.1093/jn/130.5.1421S
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998 Aug;68(2 Suppl):447S-463S. doi: 10.1093/ajcn/68.2.447S
- Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301-23. doi: 10.1159/000107673
- Maares M, Haase H. Zinc and immunity: An essential interrelation. Arch Biochem Biophys. 2016 Dec 1;611:58-65. doi:10.1016/j.abb.2016.03.022
- Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol. 1997 Jun;272(6 Pt 1):E1002-7. doi:10.1152/ajpendo.1997.272.6.E1002
- Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis. 2000 Sep;182 Suppl 1:S62-8. doi: 10.1086/315916
- Bahl R, Bhandari N, Hambidge KM, Bhan MK. Plasma zinc as a predictor of diarrheal and respiratory morbidity in children in an urban slum setting. Am J Clin Nutr. 1998 Aug;68(2 Suppl):414S-417S. doi: 10.1093/ajcn/68.2.414S
- Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, Diener-West M, Faruque AS, Black RE. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: randomised controlled trial. Lancet. 2005 Sep 17-23;366(9490):999-1004. doi: 10.1016/S0140-6736(05)67109-7
- Meydani SN, Barnett JB, Dallal GE, Fine BC, Jacques PF, Leka LS, Hamer DH. Serum zinc and pneumonia in nursing home elderly. Am J Clin Nutr. 2007 Oct;86(4):1167-73. doi: 10.1093/ajcn/86.4.1167. Erratum in: Am J Clin Nutr. 2008 Apr;87(4):1071.
- Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr. 2003 May;133(5 Suppl 1):1485S-9S. doi: 10.1093/jn/133.5.1485S
- Subramanian Vignesh K, Deepe GS Jr. Immunological orchestration of zinc homeostasis: The battle between host mechanisms and pathogen defenses. Arch Biochem Biophys. 2016 Dec 1;611:66-78. doi: 10.1016/j.abb.2016.02.020
- Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013 Oct 17;39(4):697-710. doi: 10.1016/j.immuni.2013.09.006
- Maares M, Haase H. Zinc and immunity: An essential interrelation. Arch Biochem Biophys. 2016 Dec 1;611:58-65. doi: 10.1016/j.abb.2016.03.022
- Fischer Walker C, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255-75. doi: 10.1146/annurev.nutr.23.011702.073054
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol. 2013 Oct;69(4):616-624.e1. https://doi.org/10.1016/j.jaad.2013.04.028
- National Institutes of Health. Office of Dietary Supplements. Zinc. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
- Donaldson M, Touger-Decker R. Vitamin and mineral supplements: friend or foe when combined with medications? J Am Dent Assoc. 2014 Nov;145(11):1153-8. doi: 10.14219/jada.2014.78
- Lomaestro BM, Bailie GR. Absorption interactions with fluoroquinolones. 1995 update. Drug Saf. 1995 May;12(5):314-33. doi: 10.2165/00002018-199512050-00004
- Chen JC, Chuang CH, Wang JD, Wang CW. Combination Therapy Using Chelating Agent and Zinc for Wilson’s Disease. J Med Biol Eng. 2015;35(6):697-708. doi: 10.1007/s40846-015-0087-7
- Suliburska J, Skrypnik K, Szulińska M, Kupsz J, Markuszewski L, Bogdański P. Diuretics, Ca-Antagonists, and Angiotensin-Converting Enzyme Inhibitors Affect Zinc Status in Hypertensive Patients on Monotherapy: A Randomized Trial. Nutrients. 2018 Sep 11;10(9):1284. doi: 10.3390/nu10091284
- Broun ER, Greist A, Tricot G, Hoffman R. Excessive zinc ingestion. A reversible cause of sideroblastic anemia and bone marrow depression. JAMA. 1990 Sep 19;264(11):1441-3. doi: 10.1001/jama.264.11.1441
- Willis MS, Monaghan SA, Miller ML, McKenna RW, Perkins WD, Levinson BS, Bhushan V, Kroft SH. Zinc-induced copper deficiency: a report of three cases initially recognized on bone marrow examination. Am J Clin Pathol. 2005 Jan;123(1):125-31. doi: 10.1309/v6gvyw2qtyd5c5pj
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001 Oct;119(10):1417-36. doi: 10.1001/archopht.119.10.1417. Erratum in: Arch Ophthalmol. 2008 Sep;126(9):1251.
- Holt RR, Uriu-Adams JY, Keen CL. Zinc. In: Erdman Jr JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington D.C.: ILSI Press; 2012:521-539.
- Sandström B. Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr. 2001 May;85 Suppl 2:S181-5.
- Zaman K, McArthur JO, Abboud MN, Ahmad ZI, Garg ML, Petocz P, Samman S. Iron supplementation decreases plasma zinc but has no effect on plasma fatty acids in non-anemic women. Nutr Res. 2013 Apr;33(4):272-8. doi: 10.1016/j.nutres.2013.02.001
- O’Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J Nutr. 2000 Sep;130(9):2251-5. doi: 10.1093/jn/130.9.2251
- Fung EB, Ritchie LD, Woodhouse LR, Roehl R, King JC. Zinc absorption in women during pregnancy and lactation: a longitudinal study. Am J Clin Nutr. 1997 Jul;66(1):80-8. doi: 10.1093/ajcn/66.1.80
- Davidsson L, Almgren A, Sandström B, Hurrell RF. Zinc absorption in adult humans: the effect of iron fortification. Br J Nutr. 1995 Sep;74(3):417-25. doi: 10.1079/bjn19950145
- de Brito NJ, Rocha ÉD, de Araújo Silva A, Costa JB, França MC, das Graças Almeida M, Brandão-Neto J. Oral zinc supplementation decreases the serum iron concentration in healthy schoolchildren: a pilot study. Nutrients. 2014 Sep 4;6(9):3460-73. doi: 10.3390/nu6093460
- Carter RC, Kupka R, Manji K, McDonald CM, Aboud S, Erhardt JG, Gosselin K, Kisenge R, Liu E, Fawzi W, Duggan CP. Zinc and multivitamin supplementation have contrasting effects on infant iron status: a randomized, double-blind, placebo-controlled clinical trial. Eur J Clin Nutr. 2018 Jan;72(1):130-135. doi: 10.1038/ejcn.2017.138
- de Oliveira Kde J, Donangelo CM, de Oliveira AV Jr, da Silveira CL, Koury JC. Effect of zinc supplementation on the antioxidant, copper, and iron status of physically active adolescents. Cell Biochem Funct. 2009 Apr;27(3):162-6. doi: 10.1002/cbf.1550
- Whittaker P. Iron and zinc interactions in humans. Am J Clin Nutr. 1998 Aug;68(2 Suppl):442S-446S. doi: 10.1093/ajcn/68.2.442S
- Wood RJ, Zheng JJ. High dietary calcium intakes reduce zinc absorption and balance in humans. Am J Clin Nutr. 1997 Jun;65(6):1803-9. doi: 10.1093/ajcn/65.6.1803
- McKenna AA, Ilich JZ, Andon MB, Wang C, Matkovic V. Zinc balance in adolescent females consuming a low- or high-calcium diet. Am J Clin Nutr. 1997 May;65(5):1460-4. doi: 10.1093/ajcn/65.5.1460
- Hunt JR, Beiseigel JM. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am J Clin Nutr. 2009 Mar;89(3):839-43. doi: 10.3945/ajcn.2008.27175
- Kauwell GP, Bailey LB, Gregory JF 3rd, Bowling DW, Cousins RJ. Zinc status is not adversely affected by folic acid supplementation and zinc intake does not impair folate utilization in human subjects. J Nutr. 1995 Jan;125(1):66-72. doi: 10.1093/jn/125.1.66
- Boron B, Hupert J, Barch DH, Fox CC, Friedman H, Layden TJ, Mobarhan S. Effect of zinc deficiency on hepatic enzymes regulating vitamin A status. J Nutr. 1988 Aug;118(8):995-1001. doi: 10.1093/jn/118.8.995
- Christian P, West KP Jr. Interactions between zinc and vitamin A: an update. Am J Clin Nutr. 1998 Aug;68(2 Suppl):435S-441S. doi: 10.1093/ajcn/68.2.435S
- Jafek BW, Linschoten MR, Murrow BW. Anosmia after intranasal zinc gluconate use. Am J Rhinol. 2004 May-Jun;18(3):137-41.
- Davidson TM. Re: Alexander TH, Davidson TM. Intranasal zinc and anosmia: the zinc-induced anosmia syndrome. Laryngoscope 2006;116:217-220. Laryngoscope. 2006 Sep;116(9):1721-2; discussion 1722-3. doi: 10.1097/01.mlg.0000231313.29500.f2
- U.S. Food and Drug Administration. Warnings on Three Zicam Intranasal Zinc Products. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm166931.htm
- Spain RI, Leist TP, De Sousa EA. When metals compete: a case of copper-deficiency myeloneuropathy and anemia. Nat Clin Pract Neurol. 2009 Feb;5(2):106-11. doi: 10.1038/ncpneuro1008
- Rhinoviruses. https://www.cdc.gov/ncird/rhinoviruses-common-cold.html
- Singh M, Das RR. Zinc for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No.: CD001364. DOI: 10.1002/14651858.CD001364.pub3 http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD001364.pub3/full
- DeGeorge KC, Ring DJ, Dalrymple SN. Treatment of the Common Cold. Am Fam Physician. 2019 Sep 1;100(5):281-289. https://www.aafp.org/pubs/afp/issues/2019/0901/p281.html
- Treating the Common Cold in Children. https://www.aafp.org/pubs/afp/issues/2019/0901/p281-s2.html
- Suara RO, Crowe JE Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004 Mar;48(3):783-90. doi: 10.1128/AAC.48.3.783-790.2004
- Novick SG, Godfrey JC, Godfrey NJ, Wilder HR. How does zinc modify the common cold? Clinical observations and implications regarding mechanisms of action. Med Hypotheses. 1996 Mar;46(3):295-302. doi: 10.1016/s0306-9877(96)90259-5
- Hulisz D. Efficacy of zinc against common cold viruses: an overview. J Am Pharm Assoc (2003). 2004 Sep-Oct;44(5):594-603. doi: 10.1331/1544-3191.44.5.594.hulisz
- Caruso TJ, Prober CG, Gwaltney JM Jr. Treatment of naturally acquired common colds with zinc: a structured review. Clin Infect Dis. 2007 Sep 1;45(5):569-74. doi: 10.1086/520031
- Rao G, Rowland K. PURLs: Zinc for the common cold–not if, but when. J Fam Pract. 2011 Nov;60(11):669-71. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3273967
- Hunter J, Arentz S, Goldenberg J, Yang G, Beardsley J, Myers SP, Mertz D, Leeder S. Zinc for the prevention or treatment of acute viral respiratory tract infections in adults: a rapid systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2021 Nov 2;11(11):e047474. doi: 10.1136/bmjopen-2020-047474
- Hemilä H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017 May 2;8(5):2054270417694291. doi: 10.1177/2054270417694291
- Hemilä H. Zinc lozenges may shorten the duration of colds: a systematic review. Open Respir Med J. 2011;5:51-8. doi: 10.2174/1874306401105010051
- Science M, Johnstone J, Roth DE, Guyatt G, Loeb M. Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized controlled trials. CMAJ. 2012 Jul 10;184(10):E551-61. doi: 10.1503/cmaj.111990
- Prasad AS, Beck FW, Bao B, Snell D, Fitzgerald JT. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J Infect Dis. 2008 Mar 15;197(6):795-802. doi: 10.1086/528803
- Turner RB, Cetnarowski WE. Effect of treatment with zinc gluconate or zinc acetate on experimental and natural colds. Clin Infect Dis. 2000 Nov;31(5):1202-8. doi: 10.1086/317437
- Mak CM, Lam CW. Diagnosis of Wilson’s disease: a comprehensive review. Crit Rev Clin Lab Sci. 2008;45(3):263-90. doi: 10.1080/10408360801991055
- Poujois A, Woimant F. Wilson’s disease: A 2017 update. Clin Res Hepatol Gastroenterol. 2018 Dec;42(6):512-520. doi: 10.1016/j.clinre.2018.03.007
- Shimizu N, Fujiwara J, Ohnishi S, Sato M, Kodama H, Kohsaka T, Inui A, Fujisawa T, Tamai H, Ida S, Itoh S, Ito M, Horiike N, Harada M, Yoshino M, Aoki T. Effects of long-term zinc treatment in Japanese patients with Wilson disease: efficacy, stability, and copper metabolism. Transl Res. 2010 Dec;156(6):350-7. doi: 10.1016/j.trsl.2010.08.007
- Brewer GJ, Dick RD, Johnson VD, Fink JK, Kluin KJ, Daniels S. Treatment of Wilson’s disease with zinc XVI: treatment during the pediatric years. J Lab Clin Med. 2001 Mar;137(3):191-8. doi: 10.1067/mlc.2001.113037
- Eda K, Mizuochi T, Iwama I, Inui A, Etani Y, Araki M, Hara S, Kumagai H, Hagiwara SI, Murayama K, Murakami J, Shimizu N, Kodama H, Yasuda R, Takaki Y, Yamashita Y. Zinc monotherapy for young children with presymptomatic Wilson disease: A multicenter study in Japan. J Gastroenterol Hepatol. 2018 Jan;33(1):264-269. doi: 10.1111/jgh.13812
- Gupta P, Choksi M, Goel A, Zachariah U, Sajith KG, Ramachandran J, Chandy G, Kurian G, Rebekah G, Eapen CE. Maintenance zinc therapy after initial penicillamine chelation to treat symptomatic hepatic Wilson’s disease in resource constrained setting. Indian J Gastroenterol. 2018 Jan;37(1):31-38. doi: 10.1007/s12664-018-0829-x
- Sinha S, Taly AB. Withdrawal of penicillamine from zinc sulphate-penicillamine maintenance therapy in Wilson’s disease: promising, safe and cheap. J Neurol Sci. 2008 Jan 15;264(1-2):129-32. doi: 10.1016/j.jns.2007.08.006
- Wapnir RA. Zinc deficiency, malnutrition and the gastrointestinal tract. J Nutr. 2000 May;130(5S Suppl):1388S-92S. doi: 10.1093/jn/130.5.1388S
- Lazzerini M. Oral zinc provision in acute diarrhea. Curr Opin Clin Nutr Metab Care. 2016 May;19(3):239-43. doi: 10.1097/MCO.0000000000000276
- Diarrhoeal disease. https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease
- Black RE. Progress in the use of ORS and zinc for the treatment of childhood diarrhea. J Glob Health. 2019 Jun;9(1):010101. doi: 10.7189/jogh.09.010101
- Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016 Dec 20;12(12):CD005436. doi: 10.1002/14651858.CD005436.pub5
- Florez ID, Veroniki AA, Al Khalifah R, Yepes-Nuñez JJ, Sierra JM, Vernooij RWM, Acosta-Reyes J, Granados CM, Pérez-Gaxiola G, Cuello-Garcia C, Zea AM, Zhang Y, Foroutan N, Guyatt GH, Thabane L. Comparative effectiveness and safety of interventions for acute diarrhea and gastroenteritis in children: A systematic review and network meta-analysis. PLoS One. 2018 Dec 5;13(12):e0207701. doi: 10.1371/journal.pone.0207701
- Clinical management of acute diarrhoea: WHO/Unicef joint statement. https://apps.who.int/iris/bitstream/handle/10665/68627/WHO_FCH_CAH_04.7.pdf
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015 Jan 31;385(9966):430-40. doi: 10.1016/S0140-6736(14)61698-6. Erratum in: Lancet. 2015 Jan 31;385(9966):420. Erratum in: Lancet. 2016 Jun 18;387(10037):2506.
- Pneumonia in children. https://www.who.int/news-room/fact-sheets/detail/pneumonia
- Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016 Dec 4;12(12):CD005978. doi: 10.1002/14651858.CD005978.pub3
- Saleh NY, Abo El Fotoh WMM. Low serum zinc level: The relationship with severe pneumonia and survival in critically ill children. Int J Clin Pract. 2018 Jun;72(6):e13211. doi: 10.1111/ijcp.13211
- Sakulchit T, Goldman RD. Zinc supplementation for pediatric pneumonia. Can Fam Physician. 2017 Oct;63(10):763-765. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5638472
- Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013 Apr 20;381(9875):1405-1416. doi: 10.1016/S0140-6736(13)60222-6
- Global health risks : mortality and burden of disease attributable to selected major risks. https://apps.who.int/iris/bitstream/handle/10665/44203/9789241563871_eng.pdf
- Howie S, Bottomley C, Chimah O, Ideh R, Ebruke B, Okomo U, Onyeama C, Donkor S, Rodrigues O, Tapgun M, Janneh M, Oluwalana C, Kuti B, Enwere G, Esangbedo P, Doherty C, Mackenzie G, Greenwood B, Corrah T, Prentice A, Adegbola R, Zaman S. Zinc as an adjunct therapy in the management of severe pneumonia among Gambian children: randomized controlled trial. J Glob Health. 2018 Jun;8(1):010418. doi: 10.7189/jogh.08.010418
- Wang L, Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: A meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J. 2018 Mar;12(3):857-864. doi: 10.1111/crj.12646
- Brown N, Kukka AJ, Mårtensson A. Efficacy of zinc as adjunctive pneumonia treatment in children aged 2 to 60 months in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Paediatr Open. 2020 Jul 12;4(1):e000662. doi: 10.1136/bmjpo-2020-000662
- Lai H, Lai S, Shor-Posner G, Ma F, Trapido E, Baum MK. Plasma zinc, copper, copper:zinc ratio, and survival in a cohort of HIV-1-infected homosexual men. J Acquir Immune Defic Syndr. 2001 May 1;27(1):56-62. doi: 10.1097/00126334-200105010-00010
- Wellinghausen N, Kern WV, Jöchle W, Kern P. Zinc serum level in human immunodeficiency virus-infected patients in relation to immunological status. Biol Trace Elem Res. 2000 Feb;73(2):139-49. doi: 10.1385/BTER:73:2:139
- Mocchegiani E, Muzzioli M. Therapeutic application of zinc in human immunodeficiency virus against opportunistic infections. J Nutr. 2000 May;130(5S Suppl):1424S-31S. doi: 10.1093/jn/130.5.1424S
- Baum MK, Lai S, Sales S, Page JB, Campa A. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV-infected adults. Clin Infect Dis. 2010 Jun 15;50(12):1653-60. doi: 10.1086/652864
- Zeng L, Zhang L. Efficacy and safety of zinc supplementation for adults, children and pregnant women with HIV infection: systematic review. Trop Med Int Health. 2011 Dec;16(12):1474-82. doi: 10.1111/j.1365-3156.2011.02871.x
- Hadadi A, Ostovar A, Edalat Noor B, Rasoolinejad M, Haji Abdolbaghi M, Yousefi S, Khalili H, Manshoori G, Khashayar P, Alipour Z, Safari N. The effect of selenium and zinc on CD4(+) count and opportunistic infections in HIV/AIDS patients: a randomized double blind trial. Acta Clin Belg. 2020 Jun;75(3):170-176. doi: 10.1080/17843286.2019.1590023
- Irlam JH, Siegfried N, Visser ME, Rollins NC. Micronutrient supplementation for children with HIV infection. Cochrane Database Syst Rev. 2013 Oct 11;(10):CD010666. doi: 10.1002/14651858.CD010666
- Visser ME, Durao S, Sinclair D, Irlam JH, Siegfried N. Micronutrient supplementation in adults with HIV infection. Cochrane Database Syst Rev. 2017 May 18;5(5):CD003650. doi: 10.1002/14651858.CD003650.pub4
- Fawzi WW, Villamor E, Msamanga GI, Antelman G, Aboud S, Urassa W, Hunter D. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV-1-infected women in Tanzania. Am J Clin Nutr. 2005 Jan;81(1):161-7. doi: 10.1093/ajcn/81.1.161
- Villamor E, Aboud S, Koulinska IN, Kupka R, Urassa W, Chaplin B, Msamanga G, Fawzi WW. Zinc supplementation to HIV-1-infected pregnant women: effects on maternal anthropometry, viral load, and early mother-to-child transmission. Eur J Clin Nutr. 2006 Jul;60(7):862-9. doi: 10.1038/sj.ejcn.1602391
- Bobat R, Coovadia H, Stephen C, Naidoo KL, McKerrow N, Black RE, Moss WJ. Safety and efficacy of zinc supplementation for children with HIV-1 infection in South Africa: a randomised double-blind placebo-controlled trial. Lancet. 2005 Nov 26;366(9500):1862-7. doi: 10.1016/S0140-6736(05)67756-2
- Srinivasan MG, Ndeezi G, Mboijana CK, Kiguli S, Bimenya GS, Nankabirwa V, Tumwine JK. Zinc adjunct therapy reduces case fatality in severe childhood pneumonia: a randomized double blind placebo-controlled trial. BMC Med. 2012 Feb 8;10:14. doi: 10.1186/1741-7015-10-14
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015 Feb 2;2015(2):CD000230. doi: 10.1002/14651858.CD000230.pub5. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD000230.
- McHenry MS, Dixit A, Vreeman RC. A Systematic Review of Nutritional Supplementation in HIV-Infected Children in Resource-Limited Settings. J Int Assoc Provid AIDS Care. 2015 Jul-Aug;14(4):313-23. doi: 10.1177/2325957414539044
- Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012 Jul 16;10(8):525-37. doi: 10.1038/nrmicro2836
- Alker W, Haase H. Zinc and Sepsis. Nutrients. 2018 Jul 27;10(8):976. doi: 10.3390/nu10080976
- Hoeger J, Simon TP, Beeker T, Marx G, Haase H, Schuerholz T. Persistent low serum zinc is associated with recurrent sepsis in critically ill patients – A pilot study. PLoS One. 2017 May 4;12(5):e0176069. doi: 10.1371/journal.pone.0176069
- Banupriya N, Vishnu Bhat B, Benet BD, Sridhar MG, Parija SC. Efficacy of zinc supplementation on serum calprotectin, inflammatory cytokines and outcome in neonatal sepsis – a randomized controlled trial. J Matern Fetal Neonatal Med. 2017 Jul;30(13):1627-1631. doi: 10.1080/14767058.2016.1220524
- Banupriya N, Bhat BV, Benet BD, Catherine C, Sridhar MG, Parija SC. Short Term Oral Zinc Supplementation among Babies with Neonatal Sepsis for Reducing Mortality and Improving Outcome – A Double-Blind Randomized Controlled Trial. Indian J Pediatr. 2018 Jan;85(1):5-9. doi: 10.1007/s12098-017-2444-8
- Newton B, Bhat BV, Dhas BB, Mondal N, Gopalakrishna SM. Effect of Zinc Supplementation on Early Outcome of Neonatal Sepsis–A Randomized Controlled Trial. Indian J Pediatr. 2016 Apr;83(4):289-93. doi: 10.1007/s12098-015-1939-4
- Mehta K, Bhatta NK, Majhi S, Shrivastava MK, Singh RR. Oral zinc supplementation for reducing mortality in probable neonatal sepsis: a double blind randomized placebo controlled trial. Indian Pediatr. 2013 Apr;50(4):390-3. doi: 10.1007/s13312-013-0120-2
- Black MM. Zinc deficiency and child development. Am J Clin Nutr. 1998 Aug;68(2 Suppl):464S-469S. doi: 10.1093/ajcn/68.2.464S
- Shankar AH. Nutritional modulation of malaria morbidity and mortality. J Infect Dis. 2000 Sep;182 Suppl 1:S37-53. doi: 10.1086/315906
- Müller O, Becher H, van Zweeden AB, Ye Y, Diallo DA, Konate AT, Gbangou A, Kouyate B, Garenne M. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomised double blind placebo controlled trial. BMJ. 2001 Jun 30;322(7302):1567. doi: 10.1136/bmj.322.7302.1567
- Zinc Against Plasmodium Study Group. Effect of zinc on the treatment of Plasmodium falciparum malaria in children: a randomized controlled trial. Am J Clin Nutr. 2002 Oct;76(4):805-12. doi: 10.1093/ajcn/76.4.805
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Dutta A, Dhingra U, Stoltzfus RJ, Othman MK, Kabole FM. Effect of zinc supplementation on mortality in children aged 1-48 months: a community-based randomised placebo-controlled trial. Lancet. 2007 Mar 17;369(9565):927-34. doi: 10.1016/S0140-6736(07)60452-8
- Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull. 2009 Mar;30(1 Suppl):S12-40. doi: 10.1177/15648265090301S103
- Darling AM, Mugusi FM, Etheredge AJ, Gunaratna NS, Abioye AI, Aboud S, Duggan C, Mongi R, Spiegelman D, Roberts D, Hamer DH, Kain KC, Fawzi WW. Vitamin A and Zinc Supplementation Among Pregnant Women to Prevent Placental Malaria: A Randomized, Double-Blind, Placebo-Controlled Trial in Tanzania. Am J Trop Med Hyg. 2017 Apr;96(4):826-834. doi: 10.4269/ajtmh.16-0599
- Hodkinson CF, Kelly M, Alexander HD, Bradbury I, Robson PJ, Bonham MP, O’Connor JM, Coudray C, Strain JJ, Wallace JM. Effect of zinc supplementation on the immune status of healthy older individuals aged 55-70 years: the ZENITH Study. J Gerontol A Biol Sci Med Sci. 2007 Jun;62(6):598-608. doi: 10.1093/gerona/62.6.598
- Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009 Jun 12;6:9. doi: 10.1186/1742-4933-6-9
- Bogden JD, Oleske JM, Lavenhar MA, Munves EM, Kemp FW, Bruening KS, Holding KJ, Denny TN, Guarino MA, Holland BK. Effects of one year of supplementation with zinc and other micronutrients on cellular immunity in the elderly. J Am Coll Nutr. 1990 Jun;9(3):214-25. doi: 10.1080/07315724.1990.10720372
- Bogden JD, Oleske JM, Lavenhar MA, Munves EM, Kemp FW, Bruening KS, Holding KJ, Denny TN, Guarino MA, Krieger LM, et al. Zinc and immunocompetence in elderly people: effects of zinc supplementation for 3 months. Am J Clin Nutr. 1988 Sep;48(3):655-63. doi: 10.1093/ajcn/48.3.655
- Provinciali M, Montenovo A, Di Stefano G, Colombo M, Daghetta L, Cairati M, Veroni C, Cassino R, Della Torre F, Fabris N. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: a randomized controlled trial. Age Ageing. 1998 Nov;27(6):715-22. doi: 10.1093/ageing/27.6.715
- Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008 May;43(5):370-7. doi: 10.1016/j.exger.2007.10.013
- Fortes C, Forastiere F, Agabiti N, Fano V, Pacifici R, Virgili F, Piras G, Guidi L, Bartoloni C, Tricerri A, Zuccaro P, Ebrahim S, Perucci CA. The effect of zinc and vitamin A supplementation on immune response in an older population. J Am Geriatr Soc. 1998 Jan;46(1):19-26. doi: 10.1111/j.1532-5415.1998.tb01008.x
- Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu D, Dallal GE, Jacques PF, Schreiber R, Kong E, Meydani SN. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2016 Mar;103(3):942-51. doi: 10.3945/ajcn.115.115188
- Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab 2007;51:301-23. https://www.ncbi.nlm.nih.gov/pubmed/17726308?dopt=Abstract
- Anderson I. Zinc as an aid to healing. Nurs Times 1995;91:68, 70. https://www.ncbi.nlm.nih.gov/pubmed/7644377?dopt=Abstract
- Wilkinson EA, Hawke CI. Does oral zinc aid the healing of chronic leg ulcers? A systematic literature review. Arch Dermatol 1998;134:1556-60. https://www.ncbi.nlm.nih.gov/pubmed/9875193?dopt=Abstract
- Wilkinson EA, Hawke CI. Oral zinc for arterial and venous leg ulcers. Cochrane Database Syst Rev 2000;(2):CD001273. https://www.ncbi.nlm.nih.gov/pubmed/10796629?dopt=Abstract
- The Cochrane Collaboration 9 September 2014. Oral zinc supplements for treating leg ulcers. http://www.cochrane.org/CD001273/WOUNDS_oral-zinc-supplements-for-treating-leg-ulcers
- Age-Related Macular Degeneration (AMD). https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/age-related-macular-degeneration
- Cho E, Stampfer MJ, Seddon JM, Hung S, Spiegelman D, Rimm EB, Willett WC, Hankinson SE. Prospective study of zinc intake and the risk of age-related macular degeneration. Ann Epidemiol. 2001 Jul;11(5):328-36. doi: 10.1016/s1047-2797(01)00217-4
- van Leeuwen R, Boekhoorn S, Vingerling JR, Witteman JC, Klaver CC, Hofman A, de Jong PT. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005 Dec 28;294(24):3101-7. doi: 10.1001/jama.294.24.3101
- VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol. 1998 Jul 15;148(2):204-14. doi: 10.1093/oxfordjournals.aje.a009625
- Evans JR. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD000254. doi: 10.1002/14651858.CD000254.pub2. Update in: Cochrane Database Syst Rev. 2012;11:CD000254
- Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD; Age-Related Eye Disease Study Research Group. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013 Aug;120(8):1604-11.e4. doi: 10.1016/j.ophtha.2013.01.021. Epub 2013 Apr 10. Erratum in: Ophthalmology. 2016 Dec;123(12 ):2634.
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013 May 15;309(19):2005-15. doi: 10.1001/jama.2013.4997. Erratum in: JAMA. 2013 Jul 10;310(2):208.
- Aronow ME, Chew EY. Age-related Eye Disease Study 2: perspectives, recommendations, and unanswered questions. Curr Opin Ophthalmol. 2014 May;25(3):186-90. doi: 10.1097/ICU.0000000000000046
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2017 Jul 31;7(7):CD000254. doi: 10.1002/14651858.CD000254.pub4
- Chong EW, Wong TY, Kreis AJ, Simpson JA, Guymer RH. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ. 2007 Oct 13;335(7623):755. doi: 10.1136/bmj.39350.500428.47
- Fernández-Cao JC, Warthon-Medina M, H Moran V, Arija V, Doepking C, Serra-Majem L, Lowe NM. Zinc Intake and Status and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients. 2019 May 8;11(5):1027. doi: 10.3390/nu11051027
- El Dib R, Gameiro OL, Ogata MS, Módolo NS, Braz LG, Jorge EC, do Nascimento P Jr, Beletate V. Zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance. Cochrane Database Syst Rev. 2015 May 28;2015(5):CD005525. doi: 10.1002/14651858.CD005525.pub3
- Asbaghi O, Sadeghian M, Fouladvand F, Panahande B, Nasiri M, Khodadost M, Shokri A, Pirouzi A, Sadeghi O. Effects of zinc supplementation on lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020 Jul 24;30(8):1260-1271. doi: 10.1016/j.numecd.2020.03.021
- Pompano LM, Boy E. Effects of Dose and Duration of Zinc Interventions on Risk Factors for Type 2 Diabetes and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Adv Nutr. 2021 Feb 1;12(1):141-160. doi: 10.1093/advances/nmaa087. Erratum in: Adv Nutr. 2021 Jun 1;12(3):1049.
- Wang X, Wu W, Zheng W, Fang X, Chen L, Rink L, Min J, Wang F. Zinc supplementation improves glycemic control for diabetes prevention and management: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019 Jul 1;110(1):76-90. doi: 10.1093/ajcn/nqz041
- Li X, Zhao J. The influence of zinc supplementation on metabolic status in gestational diabetes: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2021 Jul;34(13):2140-2145. doi: 10.1080/14767058.2019.1659769
- Momen-Heravi M, Barahimi E, Razzaghi R, Bahmani F, Gilasi HR, Asemi Z. The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Wound Repair Regen. 2017 May;25(3):512-520. doi: 10.1111/wrr.12537
- Moore ZE, Corcoran MA, Patton D. Nutritional interventions for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2020 Jul 17;7(7):CD011378. doi: 10.1002/14651858.CD011378.pub2
- Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, Jacqueminet S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. 2017 Apr;60(4):636-644. doi: 10.1007/s00125-017-4206-6
- Karamali M, Heidarzadeh Z, Seifati SM, Samimi M, Tabassi Z, Talaee N, Bahardoost H, Asemi Z. Zinc Supplementation and the Effects on Pregnancy Outcomes in Gestational Diabetes: a Randomized, Double-blind, Placebo-controlled Trial. Exp Clin Endocrinol Diabetes. 2016 Jan;124(1):28-33. doi: 10.1055/s-0035-1564146
- Karamali M, Heidarzadeh Z, Seifati SM, Samimi M, Tabassi Z, Hajijafari M, Asemi Z, Esmaillzadeh A. Zinc supplementation and the effects on metabolic status in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2015 Nov-Dec;29(8):1314-9. doi: 10.1016/j.jdiacomp.2015.07.001
- Karamali M, Bahramimoghadam S, Sharifzadeh F, Asemi Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. 2018 Jun;43(6):565-570. doi: 10.1139/apnm-2017-0521
- Ostadmohammadi V, Samimi M, Mobini M, Zarezade Mehrizi M, Aghadavod E, Chamani M, Dastorani M, Asemi Z. The effect of zinc and vitamin E cosupplementation on metabolic status and its related gene expression in patients with gestational diabetes. J Matern Fetal Neonatal Med. 2019 Dec;32(24):4120-4127. doi: 10.1080/14767058.2018.1481952. Update in: J Matern Fetal Neonatal Med. 2022 Oct;35(20):4030.
- Li DD, Zhang W, Wang ZY, Zhao P. Serum Copper, Zinc, and Iron Levels in Patients with Alzheimer’s Disease: A Meta-Analysis of Case-Control Studies. Front Aging Neurosci. 2017 Sep 15;9:300. doi: 10.3389/fnagi.2017.00300
- Ventriglia M, Brewer GJ, Simonelli I, Mariani S, Siotto M, Bucossi S, Squitti R. Zinc in Alzheimer’s Disease: A Meta-Analysis of Serum, Plasma, and Cerebrospinal Fluid Studies. J Alzheimers Dis. 2015;46(1):75-87. doi: 10.3233/JAD-141296
- Ventriglia M, Bucossi S, Panetta V, Squitti R. Copper in Alzheimer’s disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J Alzheimers Dis. 2012;30(4):981-4. doi: 10.3233/JAD-2012-120244
- Brewer GJ. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors. 2012 Mar-Apr;38(2):107-13. doi: 10.1002/biof.1005
- Maserejian NN, Hall SA, McKinlay JB. Low dietary or supplemental zinc is associated with depression symptoms among women, but not men, in a population-based epidemiological survey. J Affect Disord. 2012 Feb;136(3):781-8. doi: 10.1016/j.jad.2011.09.039
- Nowak G, Siwek M, Dudek D, Zieba A, Pilc A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: a preliminary placebo-controlled study. Pol J Pharmacol. 2003 Nov-Dec;55(6):1143-7.
- Siwek M, Dudek D, Paul IA, Sowa-Kućma M, Zieba A, Popik P, Pilc A, Nowak G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord. 2009 Nov;118(1-3):187-95. doi: 10.1016/j.jad.2009.02.014
- Alaimo K, McDowell MA, Briefel RR, et al. Dietary intake of vitamins, minerals, and fiber of persons ages 2 months and over in the United States: Third National Health and Nutrition Examination Survey, Phase 1, 1986-91. https://www.cdc.gov/nchs/data/ad/ad258.pdf
- Interagency Board for Nutrition Monitoring and Related Research. Third Report on Nutrition Monitoring in the United States. Washington, DC: U.S. Government Printing Office, 1995.
- Ervin RB, Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr 2002;132:3422-7. https://www.ncbi.nlm.nih.gov/pubmed/12421862?dopt=Abstract
- Ribar DS, Hamrick KS. Dynamics of Poverty and Food Sufficiency. Food Assistance and Nutrition Report Number 36, 2003. Washington, DC: U.S. Department of Agriculture, Economic Research Service. https://www.ers.usda.gov/publications/pub-details/?pubid=46766
- Dixon LB, Winkleby MA, Radimer KL. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988-1994. J Nutr 2001;131:1232-46. https://www.ncbi.nlm.nih.gov/pubmed/11285332?dopt=Abstract
- Zinc. https://ods.od.nih.gov/factsheets/Zinc-Consumer
- Sandstrom B. Bioavailability of zinc. Eur J Clin Nutr 1997;51 (1 Suppl):S17-9. https://www.ncbi.nlm.nih.gov/pubmed/9023474?dopt=Abstract
- Wise A. Phytate and zinc bioavailability. Int J Food Sci Nutr 1995;46:53-63. https://www.ncbi.nlm.nih.gov/pubmed/7712343?dopt=Abstract
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/
- Hambidge KM, Mild zinc deficiency in human subjects. In: Mills CF, ed. Zinc in Human Biology. New York, NY: Springer-Verlag, 1989:281-96.
- King JC, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins, RJ, eds. Modern Nutrition in Health and Disease, 10th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2005:271-85.
- Prasad AS. Zinc deficiency in women, infants and children. J Am Coll Nutr 1996;15:113-20. https://www.ncbi.nlm.nih.gov/pubmed/8778139?dopt=Abstract
- Siva S, Rubin DT, Gulotta G, Wroblewski K, Pekow J. Zinc Deficiency is Associated with Poor Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017 Jan;23(1):152-157. doi: 10.1097/MIB.0000000000000989
- Ehrlich S, Mark AG, Rinawi F, Shamir R, Assa A. Micronutrient Deficiencies in Children With Inflammatory Bowel Diseases. Nutr Clin Pract. 2020 Apr;35(2):315-322. doi: 10.1002/ncp.10373
- Wierdsma NJ, van Bokhorst-de van der Schueren MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013 Sep 30;5(10):3975-92. doi: 10.3390/nu5103975
- Rondanelli M, Faliva MA, Gasparri C, Peroni G, Naso M, Picciotto G, Riva A, Nichetti M, Infantino V, Alalwan TA, Perna S. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina (Kaunas). 2019 Jul 3;55(7):337. doi: 10.3390/medicina55070337
- Bakaloudi DR, Halloran A, Rippin HL, Oikonomidou AC, Dardavesis TI, Williams J, Wickramasinghe K, Breda J, Chourdakis M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin Nutr. 2021 May;40(5):3503-3521. doi: 10.1016/j.clnu.2020.11.035
- Foster M, Chu A, Petocz P, Samman S. Effect of vegetarian diets on zinc status: a systematic review and meta-analysis of studies in humans. J Sci Food Agric. 2013 Aug 15;93(10):2362-71. doi: 10.1002/jsfa.6179
- Agnoli C, Baroni L, Bertini I, Ciappellano S, Fabbri A, Papa M, Pellegrini N, Sbarbati R, Scarino ML, Siani V, Sieri S. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr Metab Cardiovasc Dis. 2017 Dec;27(12):1037-1052. doi: 10.1016/j.numecd.2017.10.020
- Foster M, Samman S. Vegetarian diets across the lifecycle: impact on zinc intake and status. Adv Food Nutr Res. 2015;74:93-131. doi: 10.1016/bs.afnr.2014.11.003
- Bailey RL, Pac SG, Fulgoni VL 3rd, Reidy KC, Catalano PM. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw Open. 2019 Jun 5;2(6):e195967. doi: 10.1001/jamanetworkopen.2019.5967
- Wilson RL, Grieger JA, Bianco-Miotto T, Roberts CT. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients. 2016 Oct 15;8(10):641. doi: 10.3390/nu8100641
- He L, Lang L, Li Y, Liu Q, Yao Y. Comparison of serum zinc, calcium, and magnesium concentrations in women with pregnancy-induced hypertension and healthy pregnant women: A meta-analysis. Hypertens Pregnancy. 2016 May;35(2):202-9. doi: 10.3109/10641955.2015.1137584
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015 Feb 2;2015(2):CD000230. doi: 10.1002/14651858.CD000230.pub5. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD000230
- Bzikowska-Jura A, Sobieraj P, Michalska-Kacymirow M, Wesołowska A. Investigation of Iron and Zinc Concentrations in Human Milk in Correlation to Maternal Factors: An Observational Pilot Study in Poland. Nutrients. 2021 Jan 21;13(2):303. doi: 10.3390/nu13020303
- Keikha M, Shayan-Moghadam R, Bahreynian M, Kelishadi R. Nutritional supplements and mother’s milk composition: a systematic review of interventional studies. Int Breastfeed J. 2021 Jan 4;16(1):1. doi: 10.1186/s13006-020-00354-0
- Aumeistere L, Ciproviča I, Zavadska D, Bavrins K, Borisova A. Zinc Content in Breast Milk and Its Association with Maternal Diet. Nutrients. 2018 Oct 5;10(10):1438. doi: 10.3390/nu10101438
- Abe SK, Balogun OO, Ota E, Takahashi K, Mori R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst Rev. 2016 Feb 18;2(2):CD010647. doi: 10.1002/14651858.CD010647.pub2
- Katayama K, Hosui A, Sakai Y, Itou M, Matsuzaki Y, Takamori Y, Hosho K, Tsuru T, Takikawa Y, Michitaka K, Ogawa E, Miyoshi Y, Ito T, Ida S, Hamada I, Miyoshi K, Kodama H, Takehara T. Effects of Zinc Acetate on Serum Zinc Concentrations in Chronic Liver Diseases: a Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial and a Dose Adjustment Trial. Biol Trace Elem Res. 2020 May;195(1):71-81. doi: 10.1007/s12011-019-01851-y
- Ackland ML, Michalczyk AA. Zinc and infant nutrition. Arch Biochem Biophys. 2016 Dec 1;611:51-57. doi: 10.1016/j.abb.2016.06.011
- Martyres DJ, Vijenthira A, Barrowman N, Harris-Janz S, Chretien C, Klaassen RJ. Nutrient Insufficiencies/Deficiencies in Children With Sickle Cell Disease and Its Association With Increased Disease Severity. Pediatr Blood Cancer. 2016 Jun;63(6):1060-4. doi: 10.1002/pbc.25940
- Swe KM, Abas AB, Bhardwaj A, Barua A, Nair NS. Zinc supplements for treating thalassaemia and sickle cell disease. Cochrane Database Syst Rev. 2013 Jun 28;2013(6):CD009415. doi: 10.1002/14651858.CD009415.pub2
- Skalny AV, Skalnaya MG, Grabeklis AR, Skalnaya AA, Tinkov AA. Zinc deficiency as a mediator of toxic effects of alcohol abuse. Eur J Nutr. 2018 Oct;57(7):2313-2322. doi: 10.1007/s00394-017-1584-y
- Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med. 2005 Aug-Oct;26(4-5):391-404. doi: 10.1016/j.mam.2005.07.002
- McClain C, Vatsalya V, Cave M. Role of Zinc in the Development/Progression of Alcoholic Liver Disease. Curr Treat Options Gastroenterol. 2017 Jun;15(2):285-295. doi: 10.1007/s11938-017-0132-4
- Navarro S, Valderrama R, To-Figueras J, Giménez A, López JM, Campo E, Fernandez-Cruz L, Ros E, Caballería J, Parés A. Role of zinc in the process of pancreatic fibrosis in chronic alcoholic pancreatitis. Pancreas. 1994 Mar;9(2):270-4. doi: 10.1097/00006676-199403000-00020
- Menzano E, Carlen PL. Zinc deficiency and corticosteroids in the pathogenesis of alcoholic brain dysfunction–a review. Alcohol Clin Exp Res. 1994 Aug;18(4):895-901. doi: 10.1111/j.1530-0277.1994.tb00057.x
- Perafán-Riveros C, França LF, Alves AC, Sanches JA., Jr Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol 2002. Sep-Oct;19(5):426-431. 10.1046/j.1525-1470.2002.00200.x
- Kaur S, Sangwan A, Sahu P, Dayal S, Jain VK. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol 2016. Jan;17(1):35 . 10.4103/2319-7250.173153
- Acrodermatitis enteropathica. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica
- Acrodermatitis enteropathica. https://rarediseases.info.nih.gov/diseases/5723/acrodermatitis-enteropathica
- Küry S, Dréno B, Bézieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002 Jul;31(3):239-40. doi: 10.1038/ng913
- Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013 Feb;18(2):144-57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3724376
- Gupta S, Brazier AKM, Lowe NM. Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet. 2020 Oct;33(5):624-643. doi: 10.1111/jhn.12791
- Snow C, Heard S, Karnes J, Harpell G. Progressive Rash in an Infant. Am Fam Physician. 2022 Mar 1;105(3):319-320. https://www.aafp.org/pubs/afp/issues/2022/0300/p319.html
- Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, Fazel N. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007 Jan;56(1):116-24. doi: 10.1016/j.jaad.2006.08.015
- Al Naamani A, Al Lawati T. Acrodermatitis Enteropathica: A Case Report. Oman Med J. 2020 Nov 23;35(6):e201. doi: 10.5001/omj.2020.97
- Al-Mendalawi MD. Transient Symptomatic Zinc Deficiency: An Overlooked Diagnosis in Acrodermatitis Enteropathica like Eruption in an Exclusively Breastfed Preterm Infant. Oman Med J. 2022 Mar 22;37(2):e364. doi: 10.5001/omj.2022.51
- Skalny AV, Aschner M, Tinkov AA. Zinc. Adv Food Nutr Res. 2021;96:251-310. doi: 10.1016/bs.afnr.2021.01.003
- Hess SY. National Risk of Zinc Deficiency as Estimated by National Surveys. Food Nutr Bull. 2017 Mar;38(1):3-17. doi: 10.1177/0379572116689000
- Oldewage-Theron WH, Samuel FO, Venter CS. Zinc deficiency among the elderly attending a care centre in Sharpeville, South Africa. J Hum Nutr Diet. 2008 Dec;21(6):566-74. doi: 10.1111/j.1365-277X.2008.00914.x
- Schneider JM, Fujii ML, Lamp CL, Lönnerdal B, Zidenberg-Cherr S. The prevalence of low serum zinc and copper levels and dietary habits associated with serum zinc and copper in 12- to 36-month-old children from low-income families at risk for iron deficiency. J Am Diet Assoc. 2007 Nov;107(11):1924-9. doi: 10.1016/j.jada.2007.08.011
- Pitchik HO, Fawzi WW, McCoy DC, Darling AM, Abioye AI, Tesha F, Smith ER, Mugusi F, Sudfeld CR. Prenatal nutrition, stimulation, and exposure to punishment are associated with early child motor, cognitive, language, and socioemotional development in Dar es Salaam, Tanzania. Child Care Health Dev. 2018 Nov;44(6):841-849. doi: 10.1111/cch.12605
- Vuralli D, Tumer L, Hasanoglu A. Zinc deficiency in the pediatric age group is common but underevaluated. World J Pediatr. 2017 Aug;13(4):360-366. doi: 10.1007/s12519-017-0007-8
- Trace elements. In: Kleinman RE, Greer FR; American Academy of Pediatrics Committee on Nutrition. Pediatric Nutrition. 8th ed. American Academy of Pediatrics; 2020:591. https://ebin.pub/qdownload/pediatric-nutrition-8nbsped-1610023609-9781610023603-2018968584-9781610023610.html
- Zaladonis CA, Safeer LZ, Hanson DC, Erickson-Parsons L, Krakowski AC. Zinc Deficiency in a Preterm Infant. J Pediatr. 2022 Jan;240:304-306. https://doi.org/10.1016/j.jpeds.2021.08.020
- Kothari R, Khare S. Dietary Zinc Deficiency-Associated Dermatitis in a Child. N Engl J Med. 2023 May 11;388(19):1799. https://www.nejm.org/doi/full/10.1056/NEJMicm2213071
- Kunitomo, K. (2020), Acquired zinc deficiency. J Gen Fam Med, 21: 82-83. https://doi.org/10.1002/jgf2.303
- Hambidge M. Human zinc deficiency. J Nutr. 2000 May;130(5S Suppl):1344S-9S. doi: 10.1093/jn/130.5.1344S
- Lönnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000 May;130(5S Suppl):1378S-83S. doi: 10.1093/jn/130.5.1378S
- Andriollo-Sanchez M, Hininger-Favier I, Meunier N, Toti E, Zaccaria M, Brandolini-Bunlon M, Polito A, O’Connor JM, Ferry M, Coudray C, Roussel AM. Zinc intake and status in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005 Nov;59 Suppl 2:S37-41. doi: 10.1038/sj.ejcn.1602296
- Van Wouwe JP. Clinical and laboratory diagnosis of acrodermatitis enteropathica. Eur J Pediatr. 1989 Oct;149(1):2-8. doi: 10.1007/BF02024322
- Brown KH, Peerson JM, Allen LH. Effect of zinc supplementation on children’s growth: A meta-analysis of intervention trials. In: Sandström B, Walter P, editors. Role of Trace Elements for Health Promotion and Disease Prevention. California: S Karger Pub; 1998. pp. 76–83.
- International Zinc Nutrition Consultative Group (IZiNCG); Brown KH, Rivera JA, Bhutta Z, Gibson RS, King JC, Lönnerdal B, Ruel MT, Sandtröm B, Wasantwisut E, Hotz C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004 Mar;25(1 Suppl 2):S99-203.
- Maret W. Zinc biochemistry, physiology, and homeostasis: Recent insights and current trends. BioMetals. 2001;14:187–90.
- Ciampo I, Sawamura R, Ciampo LAD, Fernandes MIM. Acrodematitis enteropathica: clinical manifestations and pediatric diagnosis. Rev Paul Pediatr. 2018;36(2):238-241. doi: 10.1590/1984-0462/;2018;36;2;00010
- Glutsch V, Hamm H, Goebeler M. Zinc and skin: an update. J Dtsch Dermatol Ges. 2019; 17(6): 589–96.
- Fischer Walker CL, Ezzati M, Black RE. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur J Clin Nutr. 2009 May;63(5):591-7. doi: 10.1038/ejcn.2008.9
- Prasad AS. Discovery of human zinc deficiency: 50 years later. J Trace Elem Med Biol. 2012 Jun;26(2-3):66-9. doi: 10.1016/j.jtemb.2012.04.004
- Shankar AH, Genton B, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Bannon D, Tielsch JM, West KP Jr, Alpers MP. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg. 2000 Jun;62(6):663-9. doi: 10.4269/ajtmh.2000.62.663
- Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002 Jun;75(6):1062-71. doi: 10.1093/ajcn/75.6.1062
- Fontaine O. Effect of zinc supplementation on clinical course of acute diarrhoea. J Health Popul Nutr. 2001 Dec;19(4):339-46.
- 2nd ed. Bangkok, Thailand: 2004. FAO/WHO. Expert Consultation on Human Vitamin and Mineral Requirements, Vitamin and mineral requirements in human nutrition: Report of joint FAO/WHO expert consolation; p. 341.
- Guideline: fortification of wheat flour with vitamins and minerals as a public health strategy. https://www.who.int/publications/i/item/9789240043398
- Wolfe SA, Gibson RS, Gadowsky SL, O’Connor DL. Zinc status of a group of pregnant adolescents at 36 weeks gestation living in southern Ontario. J Am Coll Nutr. 1994 Apr;13(2):154-64. doi: 10.1080/07315724.1994.10718389
- Hotz C, DeHaene J, Woodhouse LR, Villalpando S, Rivera JA, King JC. Zinc absorption from zinc oxide, zinc sulfate, zinc oxide + EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas. J Nutr. 2005 May;135(5):1102-5. doi: 10.1093/jn/135.5.1102
- Lönnerdal B. Genetically modified plants for improved trace element nutrition. J Nutr. 2003 May;133(5 Suppl 1):1490S-3S. doi: 10.1093/jn/133.5.1490S
- Banuelos G, Lin ZQ. Florida: CRC Press, Boca Raton; 2009. Phytoremediation of selenium-contaminated soil and water produces biofortified products and new agricultural byproducts. Development and Uses of Biofortified Agricultural Products; pp. 57–70.
- Gibson RS, Anderson VP. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr Bull. 2009 Mar;30(1 Suppl):S108-43. doi: 10.1177/15648265090301S107
- Roohani N, Hurrell R, Wegmueller R, Schulin R. Zinc and phytic acid in major foods consumed by a rural and a suburban population in central Iran. J Food Comp Anal. 2012;28:8–15.
- Hambidge KM, Walravens PA. Disorders of mineral metabolism. Clin Gastroenterol. 1982 Jan;11(1):87-117.
- Glutsch V, Hamm H, Goebeler M. Zinc and skin: an update. J Dtsch Dermatol Ges. 2019 Jun;17(6):589-596. doi: 10.1111/ddg.13811
- Weismann K, Wanscher B, Knudsen L. Diagnose und Therapie des Zinkmangelsyndroms während totaler parenteraler Ernährung. Eine Ubersicht mit einem Fallbericht [Diagnosis and therapy of the zinc deficiency syndrome during total parenteral feeding. Review with report of a case]. Hautarzt. 1977 Nov;28(11):578-82.
- Bernstein B, Leyden JJ. Zinc deficiency and acrodermatitis after intravenous hyperalimentation. Arch Dermatol. 1978 Jul;114(7):1070-2.
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015 Jan 31;385(9966):430-40. doi: 10.1016/S0140-6736(14)61698-6. Epub 2014 Sep 30. Erratum in: Lancet. 2015 Jan 31;385(9966):420. Erratum in: Lancet. 2016 Jun 18;387(10037):2506
- Black RE. Progress in the use of ORS and zinc for the treatment of childhood diarrhea. J Glob Health. 2019 Jun;9(1):010101. doi: 10.7189/09.010101
- Clinical management of acute diarrhoea. WHO/Unicef joint statement 2004. https://www.who.int/maternal_child_adolescent/documents/who_fch_cah_04_7/en
- Pneumonia. https://www.who.int/news-room/fact-sheets/detail/pneumonia
- World Health Organization. (2009). Global health risks : mortality and burden of disease attributable to selected major risks. World Health Organization. https://apps.who.int/iris/handle/10665/44203
- https://thousanddays.org/
- Bhatnagar S, Taneja S. Zinc and cognitive development. Br J Nutr. 2001 May;85 Suppl 2:S139-45. doi: 10.1079/bjn2000306
- Tamura T, Goldenberg RL, Ramey SL, Nelson KG, Chapman VR. Effect of zinc supplementation of pregnant women on the mental and psychomotor development of their children at 5 y of age. Am J Clin Nutr. 2003 Jun;77(6):1512-6. doi: 10.1093/ajcn/77.6.1512
- Sazawal S, Bentley M, Black RE, Dhingra P, George S, Bhan MK. Effect of zinc supplementation on observed activity in low socioeconomic Indian preschool children. Pediatrics. 1996 Dec;98(6 Pt 1):1132-7.
- Bentley ME, Caulfield LE, Ram M, Santizo MC, Hurtado E, Rivera JA, Ruel MT, Brown KH. Zinc supplementation affects the activity patterns of rural Guatemalan infants. J Nutr. 1997 Jul;127(7):1333-8. doi: 10.1093/jn/127.7.1333
- Ashworth A, Morris SS, Lira PI, Grantham-McGregor SM. Zinc supplementation, mental development and behaviour in low birth weight term infants in northeast Brazil. Eur J Clin Nutr. 1998 Mar;52(3):223-7. doi: 10.1038/sj.ejcn.1600553
- Castillo-Durán C, Perales CG, Hertrampf ED, Marín VB, Rivera FA, Icaza G. Effect of zinc supplementation on development and growth of Chilean infants. J Pediatr. 2001 Feb;138(2):229-35. doi: 10.1067/mpd.2001.110530
- Lind T, Lönnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, Persson LA. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr. 2004 Sep;80(3):729-36. doi: 10.1093/ajcn/80.3.729
- Taneja S, Bhandari N, Bahl R, Bhan MK. Impact of zinc supplementation on mental and psychomotor scores of children aged 12 to 18 months: a randomized, double-blind trial. J Pediatr. 2005 Apr;146(4):506-11. doi: 10.1016/j.jpeds.2004.10.061
- Gogia S, Sachdev HS. Zinc supplementation for mental and motor development in children. Cochrane Database Syst Rev. 2012 Dec 12;12:CD007991. doi: 10.1002/14651858.CD007991.pub2
- Walravens PA, Hambidge KM, Koepfer DM. Zinc supplementation in infants with a nutritional pattern of failure to thrive: a double-blind, controlled study. Pediatrics. 1989 Apr;83(4):532-8.
- Imdad A, Bhutta ZA. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool. BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S22. doi: 10.1186/1471-2458-11-S3-S22
- Liu E, Pimpin L, Shulkin M, Kranz S, Duggan CP, Mozaffarian D, Fawzi WW. Effect of Zinc Supplementation on Growth Outcomes in Children under 5 Years of Age. Nutrients. 2018 Mar 20;10(3):377. doi: 10.3390/nu10030377
- Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7(11):e50568. doi: 10.1371/journal.pone.0050568
- Moghimi M, Ashrafzadeh S, Rassi S, Naseh A. Maternal zinc deficiency and congenital anomalies in newborns. Pediatr Int. 2017 Apr;59(4):443-446. doi: 10.1111/ped.13176
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015 Feb 2;2015(2):CD000230. https://doi.org/10.1002/14651858.CD000230.pub5
- Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2017 Apr 13;4(4):CD004905. doi: 10.1002/14651858.CD004905.pub5. Update in: Cochrane Database Syst Rev. 2019 Mar 14;3:CD004905
- Petry N, Olofin I, Boy E, Donahue Angel M, Rohner F. The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients. 2016 Nov 30;8(12):773. doi: 10.3390/nu8120773
- Haider BA, Bhutta ZA. The effect of therapeutic zinc supplementation among young children with selected infections: a review of the evidence. Food Nutr Bull. 2009 Mar;30(1 Suppl):S41-59. doi: 10.1177/15648265090301S104
- Fukunaka A, Fujitani Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int J Mol Sci. 2018 Feb 6;19(2):476. doi: 10.3390/ijms19020476
- Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006;46(8):621-8. doi: 10.1080/10408390500466174
- Han YM, Yoon H, Lim S, Sung MK, Shin CM, Park YS, Kim N, Lee DH, Kim JS. Risk Factors for Vitamin D, Zinc, and Selenium Deficiencies in Korean Patients with Inflammatory Bowel Disease. Gut Liver. 2017 May 15;11(3):363-369. doi: 10.5009/gnl16333
- Hambidge KM, Goodall MJ, Stall C, Pritts J. Post-prandial and daily changes in plasma zinc. J Trace Elem Electrolytes Health Dis. 1989 Mar;3(1):55-7.
- Couturier E, van Onderbergen A, Bosson D, Neve J. Circadian variations in plasma zinc and cortisol in man. J Trace Elem Electrolytes Health Dis. 1988 Dec;2(4):245-9.
- Goode HF, Robertson DA, Kelleher J, Walker BE. Effect of fasting, self-selected and isocaloric glucose and fat meals and intravenous feeding on plasma zinc concentrations. Ann Clin Biochem. 1991 Sep;28 ( Pt 5):442-5. doi: 10.1177/000456329102800503
- Guillard O, Piriou A, Gombert J, Reiss D. Diurnal variations of zinc, copper and magnesium in the serum of normal fasting adults. Biomedicine. 1979 Nov;31(7):193-4.
- Singh A, Smoak BL, Patterson KY, LeMay LG, Veillon C, Deuster PA. Biochemical indices of selected trace minerals in men: effect of stress. Am J Clin Nutr. 1991 Jan;53(1):126-31. doi: 10.1093/ajcn/53.1.126
- Schroeder JJ, Cousins RJ. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3137-41. doi: 10.1073/pnas.87.8.3137
- Rofe AM, Philcox JC, Coyle P. Trace metal, acute phase and metabolic response to endotoxin in metallothionein-null mice. Biochem J. 1996 Mar 15;314 ( Pt 3)(Pt 3):793-7. doi: 10.1042/bj3140793
- Prasad AS. Laboratory diagnosis of zinc deficiency. J Am Coll Nutr. 1985;4(6):591-8. doi: 10.1080/07315724.1985.10720101
- Solomons NW. On the assessment of zinc and copper nutriture in man. Am J Clin Nutr. 1979 Apr;32(4):856-71. doi: 10.1093/ajcn/32.4.856
- Hobisch-Hagen P, Mörtl M, Schobersberger W. Hemostatic disorders in pregnancy and the peripartum period. Acta Anaesthesiol Scand Suppl. 1997;111:216-7.
- Lombeck I, Fuchs A. Zinc and copper in infants fed breast-milk or different formula. Eur J Pediatr. 1994 Oct;153(10):770-6. doi: 10.1007/BF01954500
- Maxfield L, Shukla S, Crane JS. Zinc Deficiency. [Updated 2022 Nov 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493231
- Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis. 2017 May;13(5):727-741. doi: 10.1016/j.soard.2016.12.018
- Freitas BA, Lima LM, Moreira ME, Priore SE, Henriques BD, Carlos CF, Sabino JS, Franceschini Sdo C. Micronutrient supplementation adherence and influence on the prevalences of anemia and iron, zinc and vitamin A deficiencies in preemies with a corrected age of six months. Clinics (Sao Paulo). 2016 Aug;71(8):440-8. doi: 10.6061/clinics/2016(08)06
- Sriram K, Lonchyna VA. Micronutrient supplementation in adult nutrition therapy: practical considerations. JPEN J Parenter Enteral Nutr. 2009 Sep-Oct;33(5):548-62. doi: 10.1177/0148607108328470
- Narváez-Caicedo C, Moreano G, Sandoval BA, Jara-Palacios MÁ. Zinc Deficiency among Lactating Mothers from a Peri-Urban Community of the Ecuadorian Andean Region: An Initial Approach to the Need of Zinc Supplementation. Nutrients. 2018 Jul 5;10(7):869. doi: 10.3390/nu10070869
- Dao DT, Anez-Bustillos L, Cho BS, Li Z, Puder M, Gura KM. Assessment of Micronutrient Status in Critically Ill Children: Challenges and Opportunities. Nutrients. 2017 Oct 28;9(11):1185. doi: 10.3390/nu9111185
- Pediatric Acrodermatitis Enteropathica. https://emedicine.medscape.com/article/912075-overview#a2
- Acrodermatitis Enteropathica. https://emedicine.medscape.com/article/1102575-overview#a2
- Plum LM, Rink L, Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010 Apr;7(4):1342-65. doi: 10.3390/ijerph7041342
- Doherty K, Connor M, Cruickshank R. Zinc-containing denture adhesive: a potential source of excess zinc resulting in copper deficiency myelopathy. Br Dent J. 2011 Jun 10;210(11):523-5. doi: 10.1038/sj.bdj.2011.428
- Lewis MR, Kokan L. Zinc gluconate: acute ingestion. J Toxicol Clin Toxicol. 1998;36(1-2):99-101. doi: 10.3109/15563659809162595
- Hooper PL, Visconti L, Garry PJ, Johnson GE. Zinc lowers high-density lipoprotein-cholesterol levels. JAMA. 1980 Oct 24-31;244(17):1960-1.
- Johnson AR, Munoz A, Gottlieb JL, Jarrard DF. High dose zinc increases hospital admissions due to genitourinary complications. J Urol. 2007 Feb;177(2):639-43. doi: 10.1016/j.juro.2006.09.047
- Spencer H, Norris C, Williams D. Inhibitory effects of zinc on magnesium balance and magnesium absorption in man. J Am Coll Nutr. 1994 Oct;13(5):479-84. doi: 10.1080/07315724.1994.10718438
- Food and Nutrition Board, Institute of Medicine. Zinc. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, D.C.: National Academy Press; 2001:442-501.
- McBride K, Slotnick B, Margolis FL. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem Senses. 2003 Oct;28(8):659-70. doi: 10.1093/chemse/bjg053
- DeCook CA, Hirsch AR. Anosmia due to inhalational zinc: a case report. Chem Senses. 2000;25(5):659.