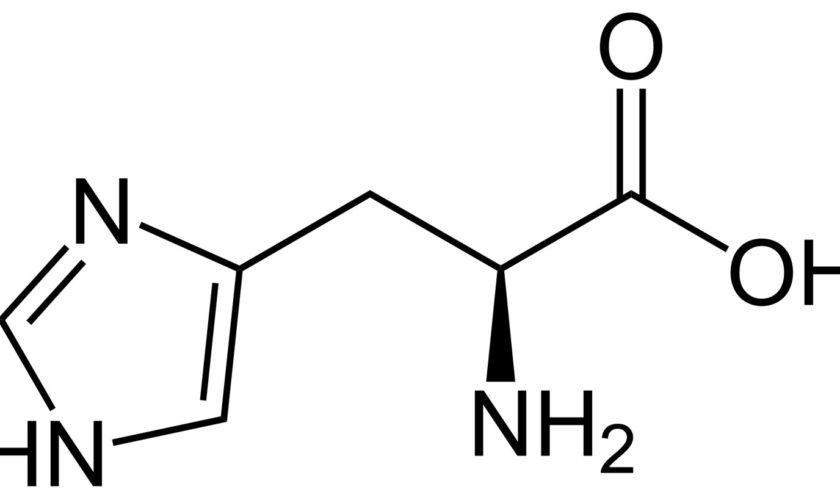
What is histidine
Histidine is a semi-essential amino acid that is required in humans for growth and tissue repair and for the synthesis of histamine. Histidine biologically active isomer is L-histidine. Histidine is the only amino acid whose side-chain can switch from an unprotonated to a protonated state under neutral pH conditions due to the pKa value of 6.0 of its side-chain 1. This characteristic enables histidine residues to act as both, a proton acceptor or a proton donor, in many cellular enzymatic reactions 2. For infants four to six months old, histidine is an essential amino acid that is required from food at 33 mg/kg body weight per day 3. However, it is not clear how adults make small amounts of histidine and dietary sources probably account for most of the histidine in the body. Histidine is abundant in red meat and fish 4. It has been reported that lower plasma concentration of histidine is associated with protein-energy wasting, inflammation and oxidative stress in chronic kidney disease patients 5. Histidine is important for maintenance of myelin sheaths that protect nerve cells and is metabolized to the neurotransmitter histamine. Histamines play many roles in immunity, gastric secretion, and sexual functions. Histidine is also required for blood cell manufacture and protects tissues against damage caused by radiation and heavy metals. Histidine is a precursor for histamine and carnosine biosynthesis. Inborn errors of histidine metabolism, including histidinemia, maple syrup urine disease, propionic acidemia, and tyrosinemia I, exist and are marked by increased histidine levels in the blood. Elevated blood histidine is accompanied by a wide range of symptoms, from mental and physical retardation to poor intellectual functioning, emotional instability, tremor, ataxia and psychosis.
Histidine and other imidazole compounds have anti-oxidant, anti-inflammatory and anti-secretory properties 6. The efficacy of L-histidine in protecting inflamed tissue is attributed to the capacity of the imidazole ring to scavenge reactive oxygen species (ROS) generated by cells during acute inflammatory response 6. Histidine, when administered in therapeutic quantities is able to inhibit cytokines and growth factors involved in cell and tissue damage.
Histidine in medical therapies has its most promising trials in rheumatoid arthritis where up to 4. 5 g daily have been used effectively in severely affected patients. Arthritis patients have been found to have low serum histidine levels, apparently because of very rapid removal of histidine from their blood 7. Other patients besides arthritis patients that have been found to be low in serum histidine are those with chronic renal failure 8. Low plasma concentrations of histidine are associated with protein-energy wasting, inflammation, oxidative stress, and greater mortality in chronic kidney disease patients 9. Bergström et al. 10 reported that the addition of histidine (included in an essential amino acid supplement) to uremia patients improved nitrogen balance and promoted net protein synthesis without any increase of the blood urea concentration. On the basis of this study 9 and the results of a study by Kopple and Swendseid 11, histidine has been considered to be an essential amino acid in uremia patients. Moreover, it was shown by Kult 12 that a daily oral supplementation of 1.5 g histidine, with no other amino acid, for 12 weeks resulted in higher concentrations of total protein, albumin, prealbumin, and the complement components C1q, C3c, C3, C1s inactivator, and C3 activator in 42 chronic kidney disease patients with a glomerular filtration rate <10 mL/min. Currently, oral and intravenous supplementation of amino acids to uremia patients and the use of amino acid-based peritoneal dialysis solutions in general includes histidine because of its status as a conditional essential amino acid in uremia and its associated beneficial effect on the nitrogen metabolism and nutritional status 9. The mechanism or mechanisms behind the deranged metabolism of amino acids and the causes of low extracellular and intracellular histidine concentrations in uremia are unclear.
There are conflicting reports on histidine levels and roles in inflammation. Asthma patients exhibit increased serum levels of histidine over normal controls 13. In a study by Watanabe and colleagues 9, histidine is low in patients with inflammation and was inversely correlated with several inflammation markers. In another study, urinary levels of histidine are reduced in pediatric patients with pneumonia. In contrast, it has been reported that histidine may have an influence on the inflammatory cascade, and histidine has been reported to have potent anti-inflammatory and antisecretory properties 14. Son et al. 15 reported that histidine in a dose-dependent manner inhibits oxidative stress and TNF-α–induced IL-8 secretion at the transcriptional level and also abolishes the NF-κB–dependent activation of IL-8 promoter induced by TNF-α. In addition, histidine supplementation could effectively down-regulate IL-6 and TNF-α in a diabetic mice model. In rheumatoid arthritis patients, oral administration of histidine was reported to improve grip strength and walking speed and to reduce the rate of erythrocyte sedimentation 16. It has been suggested that the absence of histidine led to γ-globulin aggregation and inflammation and, furthermore, that exogenous histidine would then prevent the aggregation of gamma globulin and the consequent inflammatory reaction 16. Thus, the present findings and those of previous studies suggest that histidine may have anti-inflammatory effects and that the plasma concentration of histidine may be associated with the inflammation state and is not regulated only by nutritional status in chronic kidney disease patients. This possibility is supported by observation that supplementation of amino acids, including histidine, was associated with low C-reactive protein (CRP) 9.
Serum histidine levels are lower and are negatively associated with inflammation and oxidative stress in obese women 17. Histidine supplementation has been shown to reduce insulin resistance, reduce BMI and fat mass and suppress inflammation and oxidative stress in obese women with metabolic syndrome 17. Recently, plasma histidine levels were reported to show a significant inverse association with fasting and 2 hour blood glucose levels during glucose tolerance tests in individuals without diabetes or newly diagnosed male patients with type 2 diabetes 18. Histidine was also shown to improve the hyperglycemia induced by the central administration of 2-deoxyglucose in rodents 19; however, the mechanism by which histidine lowers blood glucose levels is not clear 20. In this study 20, the intraperitoneal administration of histidine induced the phosphorylation of hepatic signal transducer and activator of transcription-3 (STAT3) and reduced blood glucose levels associated with glucose loading. Previous study 21 revealed that hepatic signal transducer and activator of transcription-3 (STAT3) decreases the expression of hepatic gluconeogenic enzymes and suppresses hepatic glucose production. Consistent with a previous study showing that histidine is not involved in the secretion of insulin 22, histidine did not significantly affect plasma insulin levels in this study 20. This study 20 demonstrates that the activation of hepatic IL-6/STAT3 signaling pathways and down regulation of hepatic gluconeogenic gene expression by histidine are mediated by central histamine action involving the histamine H1 receptor (H1R). Central histidine action, which has a central nervous system mechanism that is independent of insulin, augments the insulin-dependent suppression of glucose production in the liver. This study indicates that the central mechanism by which histidine regulates hepatic glucose metabolism and activates hepatic STAT3 is a potential target for the treatment of type 2 diabetic patients with elevated gluconeogenesis 20.
Histidine appears to suppress pro-inflammatory cytokine expression, possibly via the NF-κB pathway, in adipocytes 17. Histidine may have many other possible functions because it is the precursor of the ubiquitous neurohormone-neurotransmitter histamine. Histidine increases histamine in the blood and probably in the brain. Low blood histamine with low serum histidine occurs in rheumatoid arthritis patients. Low blood histamine also occurs in some manic, schizophrenic, high copper and hyperactive groups of psychiatric patients. Histidine maybe a useful therapy in all patients with low histamine levels.
Histidine foods
Table 1. Foods high in histidine (ordered from highest to low)
| Description | Histidine (g) Value Per 100 grams |
| Whale, beluga, meat, dried (Alaska Native) | 3 |
| Soy protein isolate | 2.3 |
| Soy protein isolate, potassium type | 2.3 |
| Egg, white, dried, stabilized, glucose reduced | 2.05 |
| Egg, white, dried, powder, stabilized, glucose reduced | 1.87 |
| Fish, cod, Atlantic, dried and salted | 1.85 |
| Egg, white, dried | 1.83 |
| Egg, white, dried, flakes, stabilized, glucose reduced | 1.75 |
| Cheese, parmesan, shredded | 1.61 |
| Pork, cured, bacon, cooked, microwaved | 1.59 |
| Soy protein concentrate, produced by alcohol extraction | 1.58 |
| Soy protein concentrate, produced by acid wash | 1.58 |
| Seeds, cottonseed flour, low fat (glandless) | 1.57 |
| Seeds, cottonseed meal, partially defatted (glandless) | 1.55 |
| Beverages, Protein powder soy based | 1.51 |
| Game meat, deer, cooked, roasted | 1.49 |
| Seeds, sesame flour, low-fat | 1.48 |
| Game meat, boar, wild, cooked, roasted | 1.44 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 1.41 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 1.4 |
| Game meat, antelope, cooked, roasted | 1.4 |
| Tofu, dried-frozen (koyadofu) | 1.39 |
| Tofu, dried-frozen (koyadofu), prepared with calcium sulfate | 1.39 |
| Pork, cured, bacon, cooked, broiled, pan-fried or roasted, reduced sodium | 1.39 |
| Pork, cured, bacon, pre-sliced, cooked, pan-fried | 1.39 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.39 |
| Cheese, parmesan, hard | 1.38 |
| Beef, plate steak, boneless, inside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.38 |
| Game meat, beaver, cooked, roasted | 1.37 |
| Beef, top loin filet, boneless, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 1.35 |
| Pork, cured, bacon, cooked, baked | 1.34 |
| Beef, loin, top loin steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 1.34 |
| Beef, loin, top loin steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.34 |
| Seeds, sunflower seed flour, partially defatted | 1.33 |
| Beef, round, eye of round steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.33 |
| Veal, leg (top round), separable lean only, cooked, braised | 1.33 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 1.33 |
| Beef, loin, top sirloin petite roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 1.32 |
| Beef, rib, back ribs, bone-in, separable lean only, trimmed to 0″ fat, select, cooked, braised | 1.32 |
| Peanut flour, defatted | 1.32 |
| Veal, leg (top round), separable lean and fat, cooked, braised | 1.31 |
| Beef, rib eye steak, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 1.31 |
| Beef, rib eye steak, boneless, lip off, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.31 |
| Pork, fresh, loin, center loin (chops), boneless, separable lean only, cooked, pan-broiled | 1.3 |
| Veal, shoulder, arm, separable lean only, cooked, braised | 1.3 |
| Beef, rib eye roast, boneless, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, roasted | 1.3 |
| Beef, plate steak, boneless, outside skirt, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.29 |
| Seeds, cottonseed flour, partially defatted (glandless) | 1.29 |
| Beef, top loin petite roast, boneless, separable lean only, trimmed to 1/8″ fat, select, cooked, roasted | 1.29 |
| Beef, ribeye petite roast, boneless, separable lean only, trimmed to 0″ fat, select, cooked, roasted | 1.28 |
| Beef, loin, top sirloin cap steak, boneless, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 1.28 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, choice, cooked, grilled | 1.27 |
| Beef, round, top round steak, boneless, separable lean and fat, trimmed to 0″ fat, select, cooked, grilled | 1.27 |
| Soy flour, defatted | 1.27 |
| Veal, cubed for stew (leg and shoulder), separable lean only, cooked, braised | 1.27 |
| Pork, fresh, loin, top loin (chops), boneless, separable lean only, with added solution, cooked, pan-broiled | 1.27 |
| Beef, loin, tenderloin steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.27 |
| Pork, Leg sirloin tip roast, boneless, separable lean and fat, cooked, braised | 1.26 |
| Beef, rib eye steak, bone-in, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 1.26 |
| Pork, fresh, loin, sirloin (chops), bone-in, separable lean only, cooked, braised | 1.26 |
| Beef, round, top round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 1.26 |
| Pork, ground, 96% lean / 4% fat, cooked, pan-broiled | 1.25 |
| Veal, rib, separable lean only, cooked, braised | 1.25 |
| Beef, ribeye filet, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.25 |
| Beef, round, eye of round roast, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, roasted | 1.25 |
| Beef, loin, tenderloin roast, separable lean only, boneless, trimmed to 0″ fat, select, cooked, roasted | 1.24 |
| Beef, New Zealand, imported, brisket point end, separable lean only, cooked, braised | 1.24 |
| Pork, fresh, loin, top loin (chops), boneless, separable lean only, cooked, braised | 1.24 |
| Pork, fresh, loin, top loin (chops), boneless, separable lean and fat, with added solution, cooked, pan-broiled | 1.24 |
| Beef, loin, top sirloin filet, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 1.24 |
| Pork, fresh, loin, top loin (chops), boneless, separable lean only, cooked, pan-fried | 1.24 |
| Game meat, bison, chuck, shoulder clod, separable lean only, cooked, braised | 1.24 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, choice, cooked, braised | 1.24 |
| Beef, round, top round, separable lean only, trimmed to 0″ fat, select, cooked, braised | 1.24 |
| Pork, fresh, loin, sirloin (roasts), boneless, separable lean only, cooked, roasted | 1.24 |
| Beef, short loin, t-bone steak, bone-in, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 1.24 |
| Veal, sirloin, separable lean only, cooked, braised | 1.23 |
| Cheese, romano | 1.23 |
| Pork, fresh, loin, center loin (chops), bone-in, separable lean only, cooked, braised | 1.23 |
| Beef, short loin, porterhouse steak, separable lean only, trimmed to 1/8″ fat, select, cooked, grilled | 1.23 |
| Beef, round, top round steak, boneless, separable lean and fat, trimmed to 0″ fat, all grades, cooked, grilled | 1.22 |
| Veal, shoulder, whole (arm and blade), separable lean only, cooked, braised | 1.22 |
| Veal, shoulder, arm, separable lean and fat, cooked, braised | 1.22 |
| Beef, rib eye roast, bone-in, lip-on, separable lean only, trimmed to 1/8″ fat, select, cooked, roasted | 1.22 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, choice, cooked, braised | 1.22 |
| Beef, round, top round, separable lean and fat, trimmed to 0″ fat, select, cooked, braised | 1.22 |
| CRACKER BARREL, grilled sirloin steak | 1.22 |
| Veal, loin, separable lean only, cooked, braised | 1.22 |
| Beef, plate steak, boneless, inside skirt, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 1.22 |
| Pork, fresh, loin, tenderloin, separable lean only, cooked, broiled | 1.22 |
| Soy meal, defatted, raw | 1.21 |
| Beef, round, top round roast, boneless, separable lean and fat, trimmed to 0″ fat, select, cooked, roasted | 1.21 |
| Beef, round, eye of round steak, boneless, separable lean only, trimmed to 0″ fat, all grades, cooked, grilled | 1.21 |
| Beef, round, top round steak, boneless, separable lean and fat, trimmed to 0″ fat, choice, cooked, grilled | 1.21 |
| Pork, cured, ham with natural juices, slice, bone-in, separable lean only, heated, pan-broil | 1.21 |
| Beef, round, top round steak, boneless, separable lean only, trimmed to 0″ fat, select, cooked, grilled | 1.21 |
| Beef, New Zealand, imported, bolar blade, separable lean only, cooked, fast roasted | 1.21 |
| Pork, ground, 96% lean / 4% fat, cooked, crumbles | 1.21 |
| Pork, fresh, loin, sirloin (roasts), boneless, separable lean and fat, cooked, roasted | 1.21 |
| Veal, leg (top round), separable lean only, cooked, pan-fried, not breaded | 1.2 |
- Nelson DL, Cox MM. Lehninger Biochemie. Berlin, Germany: Springer; 2009.
- The catalytic triad of serine peptidases. Polgár L. Cell Mol Life Sci. 2005 Oct; 62(19-20):2161-72.
- Visek WJ. An update of concepts of essential amino acids. Annu Rev Nutr 1984;4:137–55.
- Li Y-C, Li C-L, Qi J-Y, et al. Relationships of Dietary Histidine and Obesity in Northern Chinese Adults, an Internet-Based Cross-Sectional Study. Nutrients. 2016;8(7):420. doi:10.3390/nu8070420. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4963896/
- Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. Watanabe M, Suliman ME, Qureshi AR, Garcia-Lopez E, Bárány P, Heimbürger O, Stenvinkel P, Lindholm B. Am J Clin Nutr. 2008 Jun; 87(6):1860-6.
- Anti-inflammatory and antisecretory potential of histidine in Salmonella-challenged mouse small intestine. Lab Invest. 1998 May;78(5):523-34. https://www.ncbi.nlm.nih.gov/pubmed/9605177
- Gerber DA. Low free serum histidine concentration in rheumatoid arthritis. A measure of disease activity. Journal of Clinical Investigation. 1975;55(6):1164-1173. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC301869/pdf/jcinvest00170-0038.pdf
- Watanabe M, Suliman ME, Qureshi AR et al (2008) Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality. Am J Clin Nutr 87:1860–1866
- Makoto Watanabe, Mohamed E Suliman, Abdul Rashid Qureshi, Elvia Garcia-Lopez, Peter Bárány, Olof Heimbürger, Peter Stenvinkel, Bengt Lindholm; Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality, The American Journal of Clinical Nutrition, Volume 87, Issue 6, 1 June 2008, Pages 1860–1866, https://doi.org/10.1093/ajcn/87.6.1860
- Bergström J, Furst P, Josephson B, Noree LO. Improvement of nitrogen balance in a uremic patient by the addition of histidine to essential amino acid solutions given intravenously. Life Sci II 1970;9:787–94.
- Kopple JD, Swendseid ME. Evidence that histidine is an essential amino acid in normal and chronically uremic man. J Clin Invest 1975;55:881–91.
- Kult J. The action of histidine supplementation on plasma protein metabolism in uremia. In: Kluthe R, Katz NR. eds. Histidine: metabolism, clinical aspects and therapeutic use. First International workshop. Freiburg, Germany: Georg Thieme Publishers Stuttgart, 1978.
- Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin Exp Allergy. 2013 Apr;43(4):425-33. doi: 10.1111/cea.12089. https://doi.org/10.1111/cea.12089
- Tanaka S. [Physiological function mediated by histamine synthesis]. Yakugaku Zasshi 2003;123:547–59
- Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett 2005;579:4671–7.
- Stifel FB, Herman RH. Is histidine an essential amino acid in man? Am J Clin Nutr 1972;25:182–5.
- Histidine supplementation improves insulin resistance through suppressed inflammation in obese women with the metabolic syndrome: a randomised controlled trial. Feng, R.N., Niu, Y.C., Sun, X.W. et al. Diabetologia (2013) 56: 985. https://doi.org/10.1007/s00125-013-2839-7
- Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Stancáková A, Civelek M, Saleem NK, Soininen P, Kangas AJ, Cederberg H, Paananen J, Pihlajamäki J, Bonnycastle LL, Morken MA, Boehnke M, Pajukanta P, Lusis AJ, Collins FS, Kuusisto J, Ala-Korpela M, Laakso M. Diabetes. 2012 Jul; 61(7):1895-902
- Possible role of L-carnosine in the regulation of blood glucose through controlling autonomic nerves. Nagai K, Niijima A, Yamano T, Otani H, Okumra N, Tsuruoka N, Nakai M, Kiso Y. Exp Biol Med (Maywood). 2003 Nov; 228(10):1138-45.
- Kimura K, Nakamura Y, Inaba Y, et al. Histidine Augments the Suppression of Hepatic Glucose Production by Central Insulin Action. Diabetes. 2013;62(7):2266-2277. doi:10.2337/db12-1701. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3712067/
- Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M, Teshigawara K, Matsuki Y, Watanabe E, Hiramatsu R, Notohara K, Katayose K, Okamura H, Kahn CR, Noda T, Takeda K, Akira S, Inui A, Kasuga M. Cell Metab. 2006 Apr; 3(4):267-75.
- Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest 1966;45:1487–1502
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/search/list