What is Leptin Hormone ?
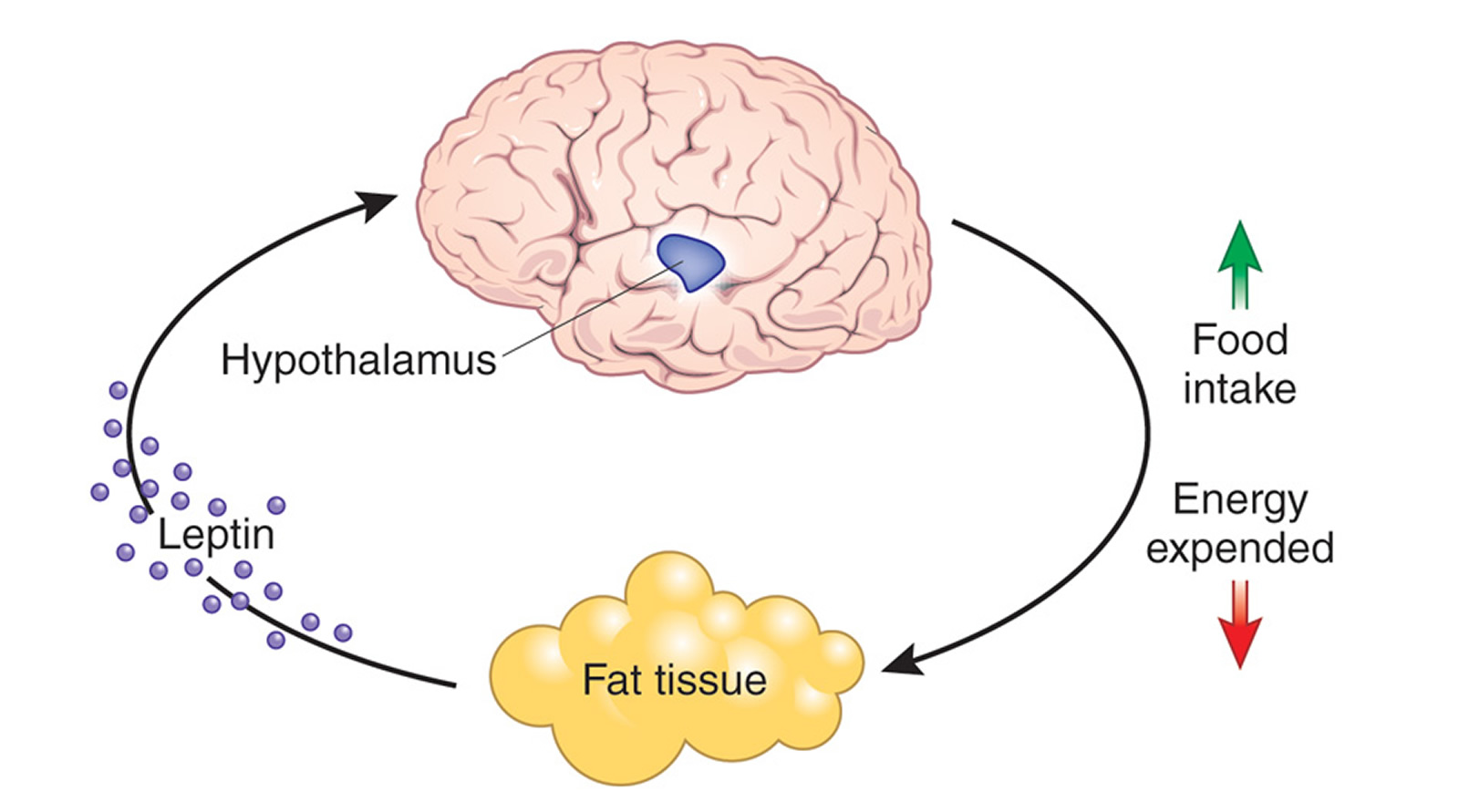
Named for the Greek word leptos, meaning “thin,” leptin is a protein that’s made in the fat cells (adipocytes), circulates in the bloodstream, and goes to the brain 1. Leptin, because it is produced by your body fatty tissue, the more fatty tissue mass (body fat) you have, the more leptin is produced 2, 3, 4. In this study, it was found that obese humans had (on average) leptin levels four times higher than non-obese individuals 5, 6. The study also confirms results of previous reports regarding a gender difference in leptin levels between males and females. Females have higher serum leptin concentrations than males 7, 8. The reason for this is unclear; the larger amounts of body fat mass, predominantly subcutaneous fat, in women and the effect of sex steroid hormones which increase leptin production have been suggested. Since leptin reflects mainly the amount of body fat, this seem to be a logical explanation for the observed gender differences 9.
| Effect on circulating leptin | |
|---|---|
| |
| Energy stores | ↑ With increase in body mass index and per cent total body fat |
| Food intake | ↑ |
| Gender | Higher in females compared with males |
| Age | ↓ With increasing age |
| Exercise | ↓ |
| Glucose uptake | ↑ |
(Source 10).
In conditions of positive energy balance such as obesity, overeating, plasma ghrelin levels decrease 11, whereas plasma leptin levels move in the opposite direction and increase 12. On the other hand, during fasting or in anorexia nervosa (is a severe psychiatric disorder affecting about 0.9% of women and 0.3% of men characterized by a marked decrease in food intake from self-induced starvation, extreme weight loss, BMI<18.5 kg/m2 and reduced body fat), which entails a lower body fat, leptin is down-regulated 13 while ghrelin is up-regulated 11, 14. Thus, circulating leptin concentrations generally reflect the status of long-term adipose tissue energy stores. Leptin is the way your fat cells tell your brain that your energy thermostat is set right—which means that the more fat you have, the higher your baseline levels of leptin will be 15. Whereas bulimia nervosa (bulimia nervosa is an eating disorder characterized by recurrent episodes of binge eating followed by inappropriate compensatory behaviors to avoid weight gain; the binge eating and inappropriate compensatory behavior both occur, on average, at least twice a week for 3 months) had ghrelin and leptin levels similar to healthy controls.
When Leptin was discovered in 1994 1, some researchers thought this would be the answer to solving the human obesity epidemic. Researchers thought that leptin would act as a simple switch that would let doctors turn off hunger pangs, solving the problem of obesity and potentially preventing millions of people from developing type 2 diabetes or putting it in remission. When the gene responsible for producing leptin was identified, it was hailed as a blockbuster discovery that the prestigious science journal Nature publicized on its cover 16.
Early work on the Leptin hormone was promising. People born without the ability to produce leptin (a very rare condition called congenital leptin deficiency that affects a few dozen people in the world) are constantly hungry and quickly become obese, apparently proving the hormone’s effect. When people with the condition were given leptin injections, they lost weight 16.
Yet when researchers tried giving leptin to ordinary overweight or obese people as a weight-loss treatment, the experiments were unsuccessful. It turned out, Leptin didn’t have that effect in obese or nonobese individuals. That’s a strong clue that leptin is just one piece in a much larger puzzle that includes genetics, environment, and lifestyle 16. This study 17 investigated the response of leptin to short-term fasting and refeeding in humans. A mild decline in subcutaneous adipocyte ob gene mRNA and a marked fall in serum leptin were observed after 36 and 60 h of fasting. Leptin began a steady decline from the baseline values after 12 h of fasting, reaching a nadir at 36 h. The subsequent restoration of normal food intake was associated with a prompt leptin rise and a return to baseline values 24 h later. This study indicates that one of the adaptive physiological responses to fasting is a fall in serum leptin. Although the mediator that brings about this effect remains unknown, it appears to be neither insulin nor ketones 17.
In fact, it’s a lot like the hormone insulin: Just as some people are resistant to insulin’s signals, forcing the pancreas to make more and more, some people are resistant to leptin’s signals. Leptin resistance is most severe in people who are obese, making them more likely to feel hungry and less likely to recognize when they’re full. That complicates the use of leptin as an obesity “cure.” Leptin resistance means no matter how much leptin you add to the system, the body still registers levels as “low.” People lacking leptin receptors in the brain—another rare medical condition—are as obese as people who don’t produce leptin. Despite some utility of this term, “leptin resistance” implies neither a single specific mechanism nor metric of leptin action. Because of the multiple effects of leptin and the numerous ways in which its action is studied, the universal application of a narrow definition of leptin resistance appears unlikely to be practical or useful in most contexts. We currently lack good predictors of, and acute assays to define, leptin responsiveness in humans 18.
Leptin Hormone Function
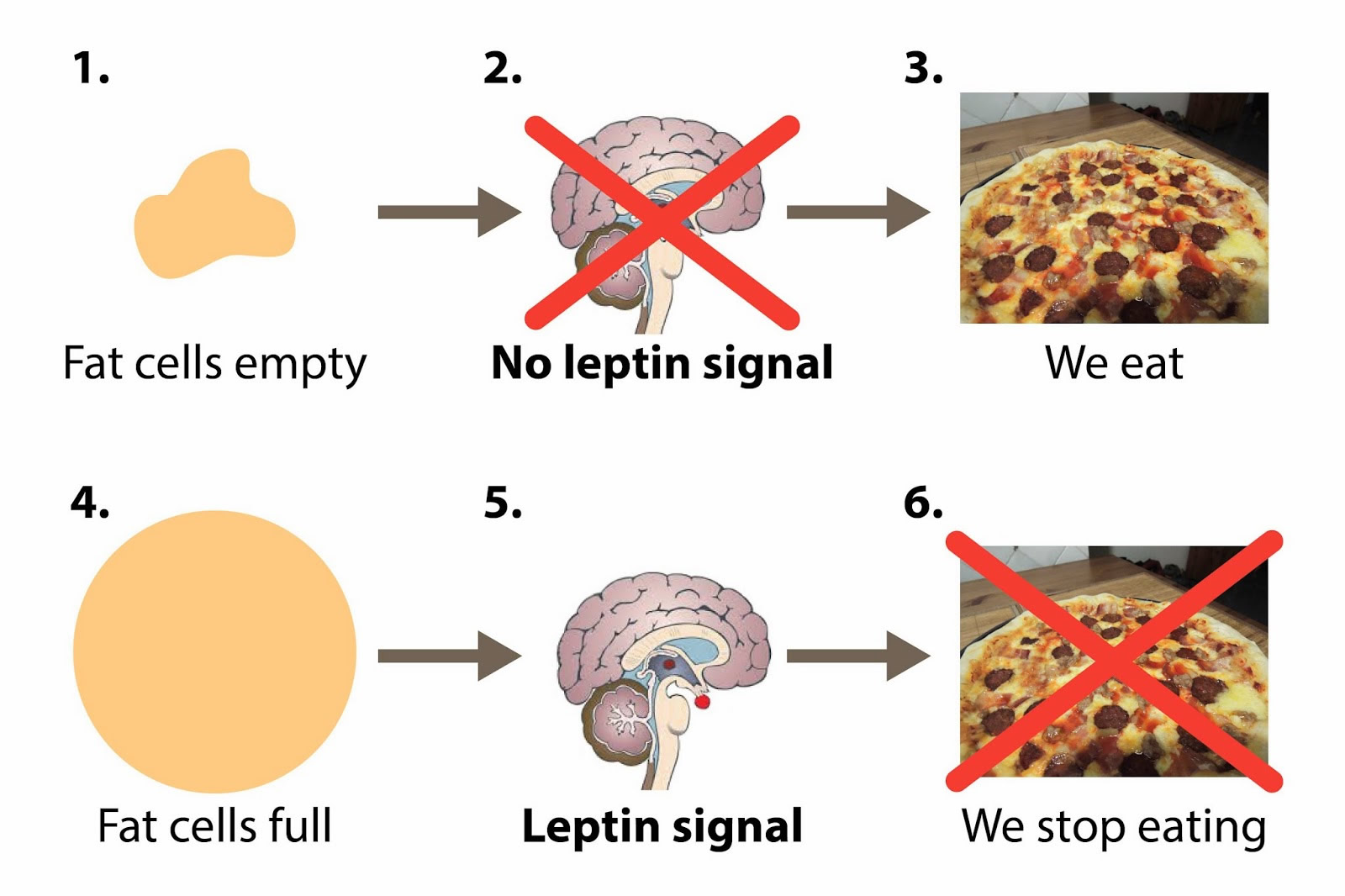
In the two decades since the discovery, our understanding of leptin’s likely role has improved. Instead of a “hunger hormone” that suppresses appetite, leptin has a role in defending body weight. Leptin functions as a feedback mechanism that signals to key regulatory centres in the brain to inhibit food intake and to regulate body weight and energy homeostasis. This has been demonstrated by many studies in rodents 19, 20. The reason people who lack leptin gain weight is that the brain is lacking the signal that there is enough body fat, so they keep eating. Leptin tells your brain that you have enough energy stored in your fat cells to engage in normal metabolic processes. In other words, when leptin levels are at a certain threshold — for each person, it’s probably genetically set — when your leptin level is above that threshold, your brain senses that you have energy sufficiency, which means you can burn energy at a normal rate, eat food at a normal amount, engage in exercise at a normal rate, and you can engage in expensive processes, like puberty and pregnancy.
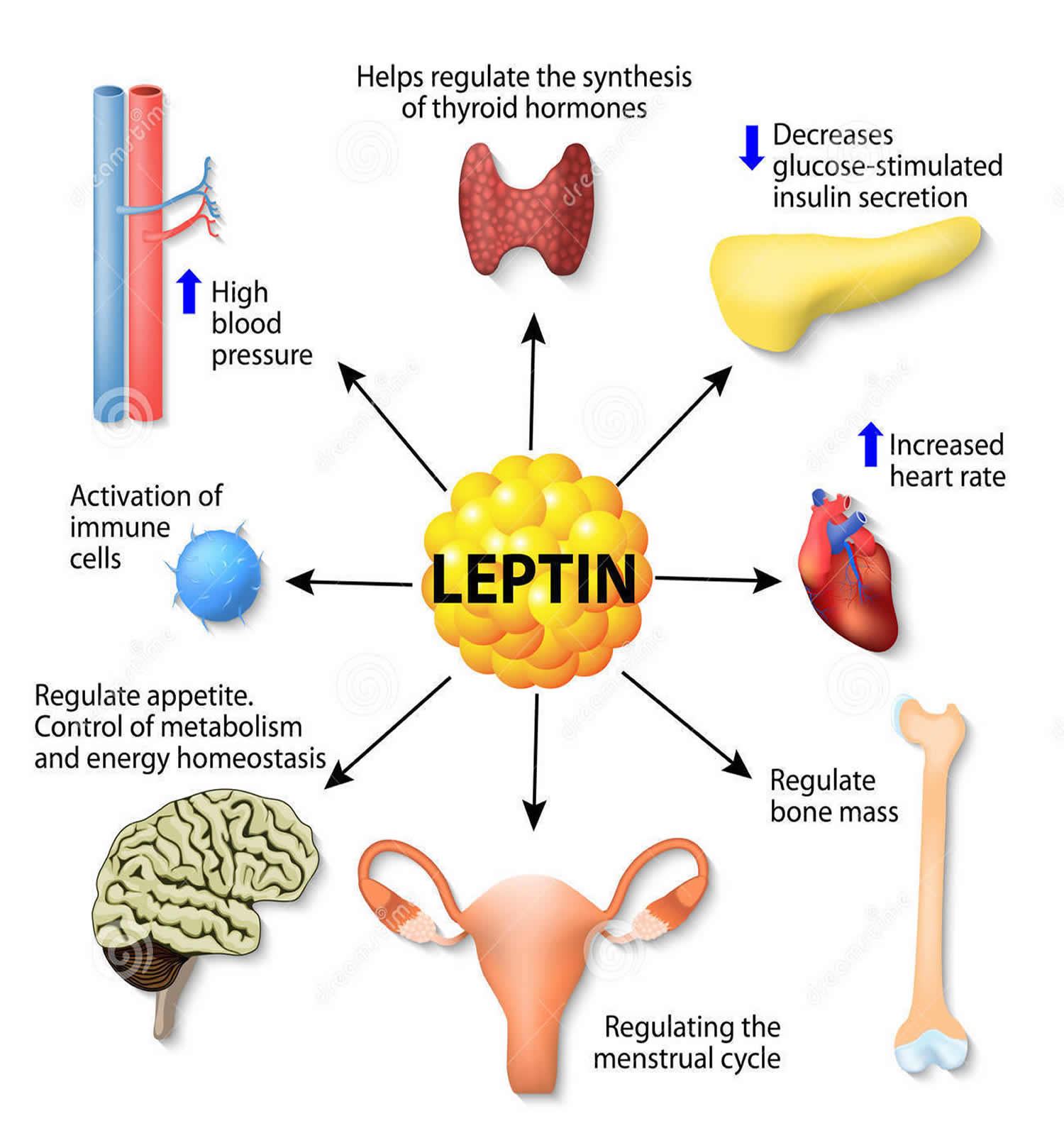
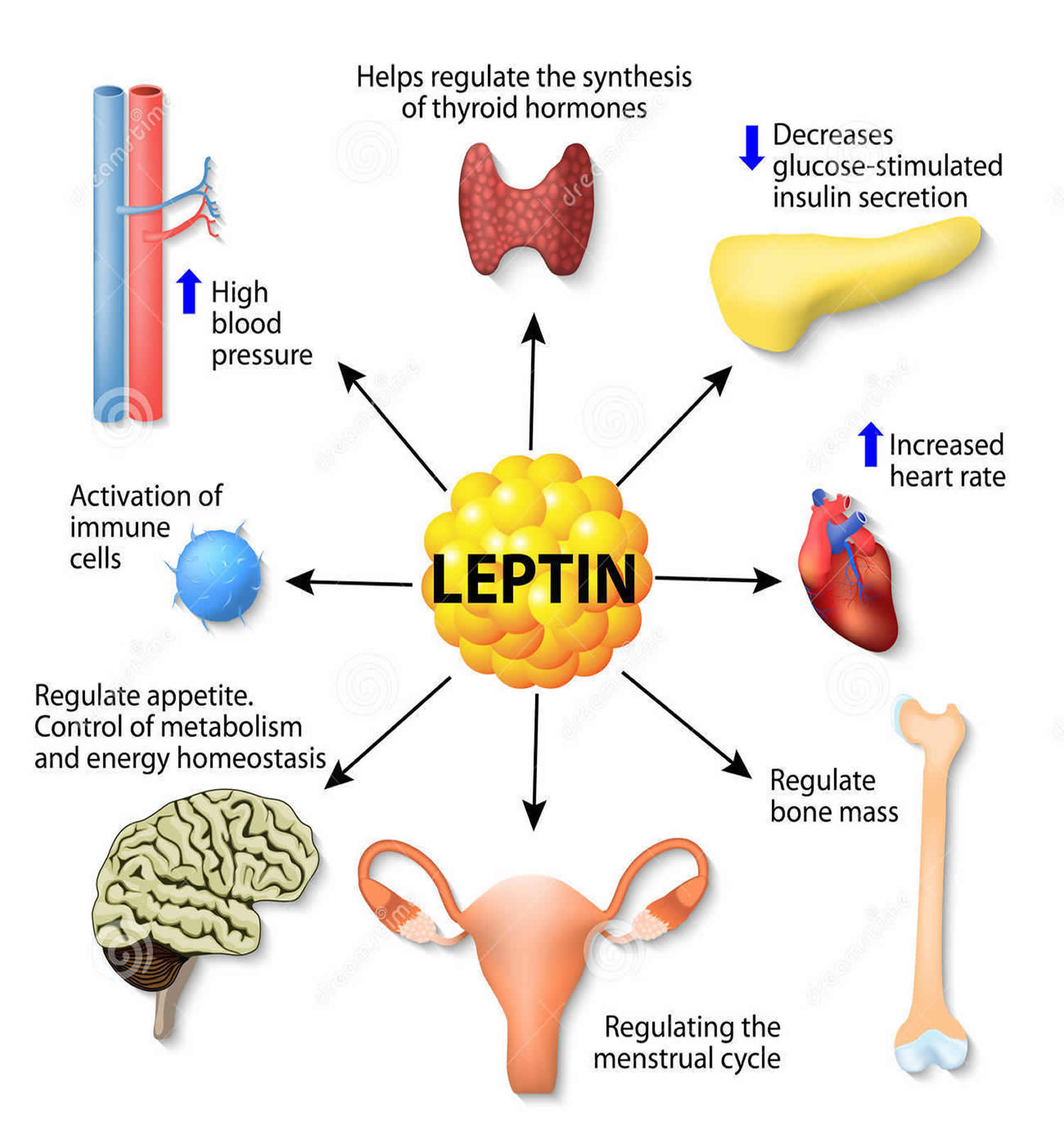
Leptin hormone also has several endocrine functions and is involved in the regulation of immune and inflammatory responses, bone formation, reproduction (initiation of human puberty), hematopoiesis (the formation of blood cells), angiogenesis (the development of new blood vessels) and wound healing 21, 22, 23, 24. Mutations in this gene and/or its regulatory regions cause severe obesity, and morbid obesity with hypogonadism. This leptin gene has also been linked to type 2 diabetes mellitus development 25.
In a study of overfeeding, nonexercise activity thermogenesis and the leptin levels, 16 nonobese humans before and after overfeeding were assessed for the relationship between leptin responses to overfeeding and the changes in the nonexercise activity thermogenesis 26. The researchers found adipocyte leptin expression was up-regulated with overfeeding, and leptin concentrations increased. Leptin concentrations correlated with body fat before and after overfeeding. Changes in leptin with overfeeding were strongly related to body fat mass and fat mass gain, but not to changes in nonexercise activity thermogenesis. In fact, changes in nonexercise activity thermogenesis correlated inversely with body fat gain 27. In another study, the loss of body fat by caloric restriction is in fact accompanied by decreased circulating leptin levels, increased ghrelin levels, and increased appetite 28. The study conclusion was that weight loss early in the course of dietary fat restriction occurs independently of increased plasma leptin levels, but that a later increase in amplitude of the 24-h leptin signal may contribute to ongoing weight loss. Fat restriction (low fat diet) avoids the increase in ghrelin levels caused by dietary energy restriction 28.
Cholecystokinin (CCK) is the typical gut peptide with effects on food intake. A role for cholecystokinin in feeding control was originally demonstrated in 1973 by Gibbs et al. 29 experiments demonstrating that exogenous administration of cholecystokinin resulted in dose-related suppressions of food intake in rats. Cholecystokinin has now been shown to inhibit food intake in all mammalian species in which it has been tested, including man 30. The evidence is now clear that cholecystokinin is a physiological controller of meal size. CCK is rapidly released from I cells in the upper intestine in response to the intraluminal presence of the digestive products of fats and proteins 31. Cholecystokinin acts on vagal afferent fibers to modulate neural feedback from the gastrointestinal tract to the dorsal hindbrain, resulting in the earlier termination of a meal 32. Administration of cholecystokinin antagonists results in increases in food intake and does so through increasing meal size 33.
The hormone leptin and the gut peptide cholecystokinin synergistically interact to enhance the process of satiation. Although this interaction may occur at several levels of the neuroaxis, previous results indicate that leptin can specifically enhance the satiation effect of cholecystokinin by acting on subdiaphragmatic vagal afferent neurons 34.
In fact, leptin is a lot like the hormone insulin: Just as some people are resistant to insulin’s signals (insulin resistance), forcing the pancreas to make more and more, some people are resistant to leptin’s signals. Leptin resistance is most severe in people who are obese, making them more likely to feel hungry and less likely to recognize when they’re full. That complicates the use of leptin as an obesity “cure.” Leptin resistance means no matter how much leptin you add to the system, the body still registers levels as “low.” People lacking leptin receptors in the brain—another rare medical condition—are as obese as people who don’t produce leptin.
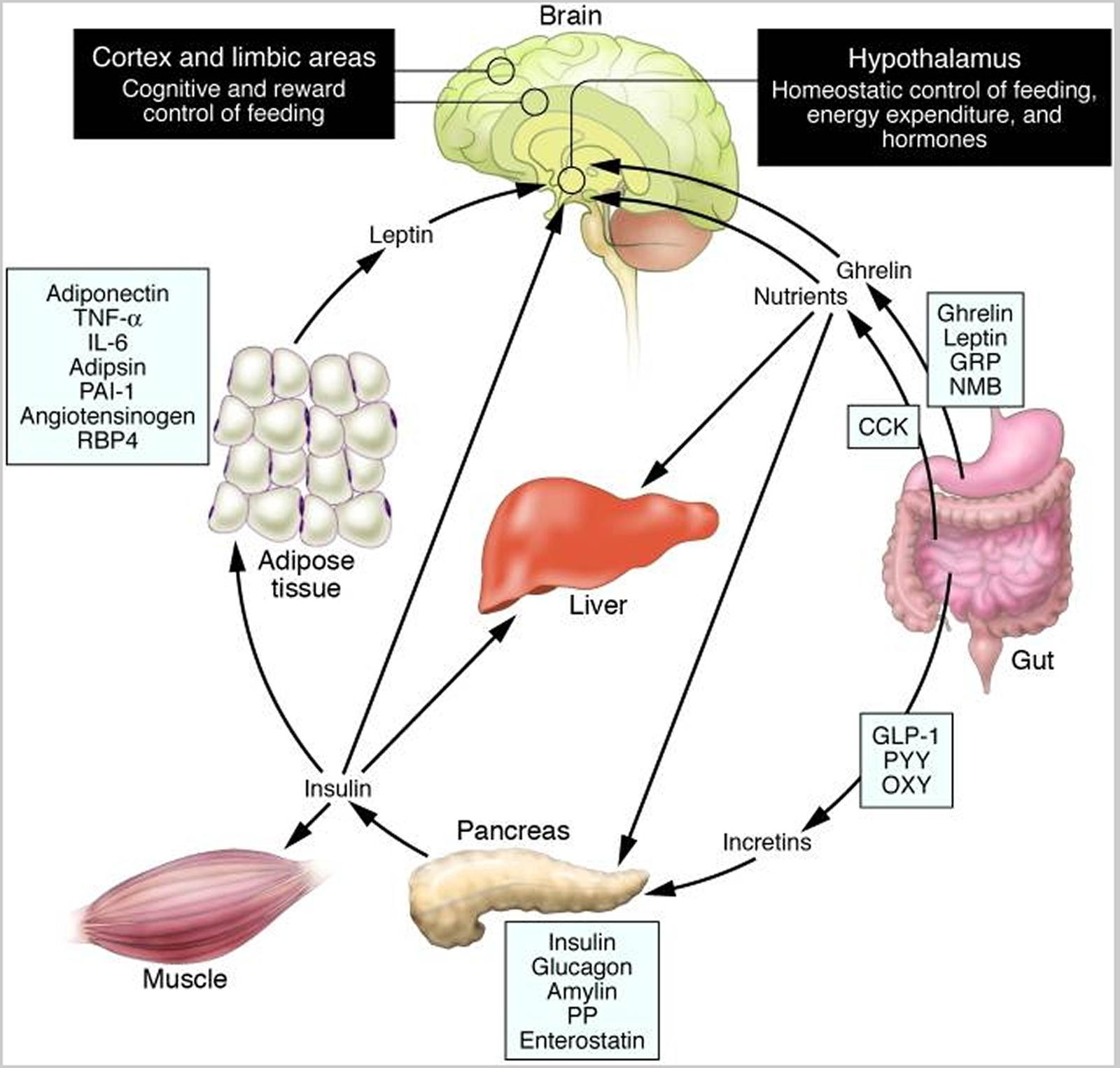
Schematic illustration of peptides secreted by the gut and adipose tissue (fat) that control energy balance. (Source 35)).
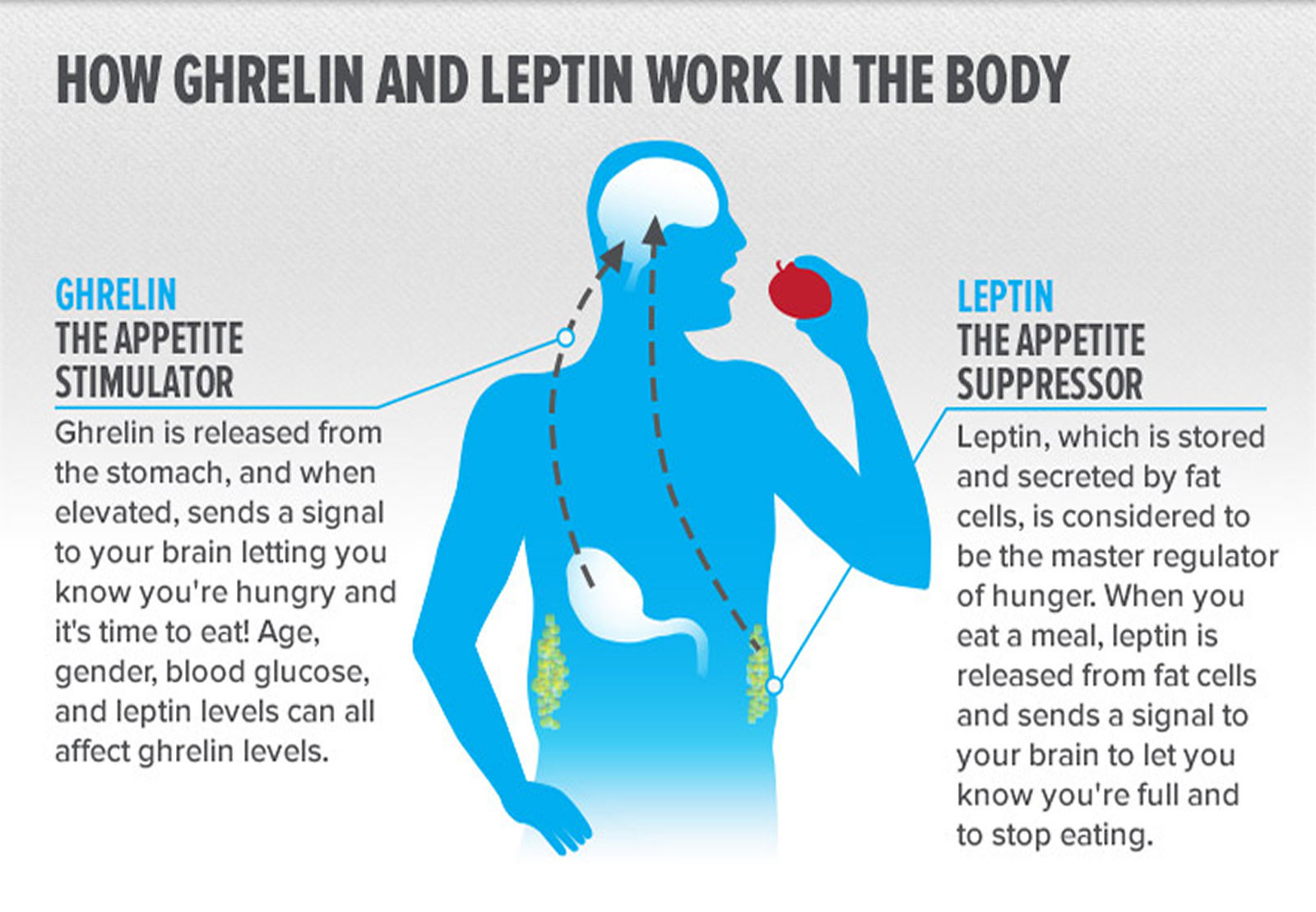
Note: Ghrelin is produced in the stomach and is a potent stimulator of appetite in the brain. In addition to increasing the uptake of nutrients by muscle, liver, and fat, insulin acts in the brain to suppress food intake. Gut-derived peptides such as GLP-1 augment insulin release from the pancreas. Leptin levels decline during weight loss and signal to the hypothalamus to stimulate feeding, reduce energy expenditure, and promote weight regain. As Rosenbaum et al. demonstrate in this issue of the The Journal of Clinical Investigation 36, low leptin levels during weight loss also increase the activity of brain areas involved in the decision-making and reward aspects of eating behavior. Thus, preventing the decline of leptin levels during weight loss by hormone replacement may be a means of overriding the homeostatic and behavioral tendencies toward energy conservation and weight regain during dieting. CCK, cholecystokinin; GLP-1, glucagon-like peptide–1; GRP, gastrin-releasing peptide; NMB, neuromedin B; OXY, oxyntomodulin; PAI-1, plasminogen activator inhibitor 1; PP, pancreatic polypeptide; PYY, peptide YY; RBP4, retinol-binding protein–4.
Overweight people have other options to aid leptin functioning, experts say.
- Reduce resistance to insulin (a hormone that controls blood sugar) and to bring down high levels of triglycerides (a blood lipid).Insulin resistance generates leptin resistance. The practical advice is: Get your insulin down.
- How do you get insulin down ? The best way is don’t let it go up. Sugar makes insulin go up. We are overdosed on sugar in this country. If you got the sugar down, your insulin resistance would improve and that would help with the weight loss.
- Reducing high triglyceride levels helps, too. Too much triglyceride interferes with leptin’s journey from the blood to the brain via a leptin transporter that allows the hormone into the brain. When you’re insulin-resistant, you have high triglyceride levels. Triglyceride seems to block leptin transport into the brain. In order to make your leptin work, you have to let the signaling occur. The only way to let the signaling occur is to get your triglyceride down.
All of this means that if you want to keep making progress, you need to keep leptin levels elevated while you’re in a caloric deficit—for now, keep these leptin-boosting tactics in mind:
- Fast at least sixteen hours a day.
- Include strategic cheat meals (maximum of one day per week).
- Perform resistance training.
- Perform long-distance cardiovascular training.
- Eat a high-protein diet.
- Don’t eat too much fructose.
- Don’t drop your calories too low on a diet (at most, drop 500–600 calories per week).
How does leptin affect weight ?
Here’s why Leptin is important for weight control : one of the master hormones, leptin influences the production and secretion of other hormones that regulate metabolism, such as thyroid hormones T3 and T4. When leptin levels are high, your production of T3 and T4 will also be relatively high, allowing you to burn fat faster; when leptin levels drop, these other hormones go too.
The fact that leptin is produced in fat cells is an important reason why it’s easier to drop weight when you have more excess weight to lose. However, leptin levels also share a direct relationship with caloric intake—when you eat fewer calories, your leptin levels drop considerably. This, in turn, lowers your other fat-burning hormones, bringing your fat loss to a crawl.
For this reason, leptin is often called the anti-starvation hormone—your body is slowing your metabolism to keep you alive when food is scarce. This means that leptin decreases your hunger. That’s great for survival, but it creates a pretty clear fat-loss catch-22: you need to eat less to burn fat, but eating less compromises your body’s ability to produce leptin. And the less leptin you produce, the hungrier you become and the more likely you are to eat more than you need.
Not only is leptin part of the hunger system, it’s also part of the reward system. When your leptin levels are low, food is even more rewarding. When your leptin levels are high, that’s supposed to extinguish the reward system so that you don’t need to eat so much, and food doesn’t look nearly as good.
The problem is that overweight people have large amounts of leptin – leptin levels are high in obese subjects indicating resistance to the actions of leptin, that is their brains aren’t getting the important signal to stop eating.
How come the brain doesn’t get it ? That phenomenon is called ‘leptin resistance. Leptin resistance is similar to insulin resistance in type 2 diabetes, in which the pancreas produces large amounts of insulin, but the body doesn’t respond to it properly.
In leptin resistance, your leptin is high, which means you’re fat, but your brain can’t see it. In other words, your brain is starved, while your body is obese. And that’s what obesity is: it’s brain starvation.
Leptin levels can keep going higher as people get fatter. We all have a leptin floor; the problem is, we don’t have a leptin ceiling.
In leptin-resistant people, the reward system doesn’t cue a person to stop eating when leptin levels rise. The leptin is being made by the fat cells, the fat cells are trying to tell the brain, ‘Hey, I don’t need to eat so much,’ but the brain can’t get the signal. You feel hungrier and the reward doesn’t get extinguished. It only gets fostered, and so you eat more and you keep going and it becomes a vicious cycle. If your brain can’t see the leptin signal, you’re going to get obese.
High leptin levels have been associated with increased cardiovascular and metabolic risks, but it is not clear if increased leptin or leptin resistance contributes to the increased cardiovascular risk. Further, even though leptin receptors are present in fat tissue, leptin’s role in fat tissue functions are not completely investigated in humans. Based on preliminary research data, researchers hypothesize that resistance to leptin action in obese adipose tissue is responsible for altering the expression of adipose tissue proteins which contribute to the development of cardiovascular and metabolic dysfunction. With the more recent understanding of leptin resistance, it makes no sense to give people leptin if they have an impaired response.
Leptin appears to have many functions that scientists are still exploring. The hormone plays a role in heart and bone health. We know that leptin is very important in keeping the immune system happy and that chronic inflammation occurs in the face of inadequate leptin signaling, and that’s part of cardiovascular disease.
The leptin science has only been unraveling since 1994, so there are a lot of unanswered questions.
How to Increase Leptin Hormone Level
Food intake can have significant effects on circulating leptin and ghrelin levels. Overfeeding results in an increase in adipocyte leptin expression and circulating leptin in healthy human subjects 37, 38. Fasting (for 20 or 36 h or 3 days) results in a decrease of adipocyte leptin mRNA and serum leptin levels, with a greater decline in leptin levels in lean subjects than in obese subjects 39, 40, 41. Refeeding is again associated with a rise in serum leptin levels, and leptin levels return to baseline after 24 h 41, 39. On the other hand, fasting results in an increase in plasma ghrelin levels, with a nearly twofold increase immediately before each meal 42, 43. This preprandial increase in ghrelin levels correlates with hunger scores in humans 44. Feeding results in a decrease in plasma ghrelin levels within 1 to 2 h 42, 45.
Not only the size and frequency of meals have an effect on circulating leptin and ghrelin levels, but also the composition of a meal is a determinant of leptin and ghrelin levels in humans (Table 2). For example, low-fat/high-carbohydrate meals result in an increase in circulating leptin concentrations, which is larger, compared with high-fat/low-carbohydrate meals 46. In addition, high-fat meals lower 24-h circulating leptin levels relative to high-carbohydrate meals 47. Hydrolysed guar fibre or protein intake does not seem to have influence on circulating leptin concentrations 48, 49.
Table 2. Effects of diet composition on circulating leptin and ghrelin levels
| Diet | Effect on circulating leptin | Effect on circulating ghrelin |
|---|---|---|
| ||
| High-fat | 24-h circulating leptin levels ↓ relative to high-carbohydrate meal | ↓ |
| High-carbohydrate | ↓ (Larger drop compared with high-fat diet) | |
| Low-fat/high-carbohydrate | ↑(Larger compared with high-fat/low-carbohydrate meal) | No increase |
| High-fat/low-carbohydrate | ↑ | |
| Protein | No effect | Conflicting results |
| Hydrolysed guar fibre | No effect | |
| Non-caloric Psyllian fibres | ↓ | |
A low-fat diet seems to have an inhibitory effect on ghrelin levels, as one study reported that a low-fat/high-carbohydrate diet resulted in weight loss, without an increase in plasma ghrelin levels 50. Another study demonstrated that a high-carbohydrate diet caused a larger drop in ghrelin levels than a high-fat diet in healthy women 51. The effect of protein ingestion on ghrelin levels gives conflicting results 49, 52, 53. Finally, the use of non-caloric Psyllian fibres results in a decrease of plasma ghrelin levels in healthy women 54. Together, these data indicate that for obese subjects it is important to follow a specific diet in order to regulate food intake and body weight.
- Ensure enough sleep.
- Avoid eating too much sugar.
- Don’t eat too often.
- Eat Low Fat/high carb (high GI) diet.
- Eat more fiber.
- Get Physically Active.
- Eat more protein.
- Practice intermittent fasting.
What about leptin supplements ?
Because leptin is a digestible protein that doesn’t enter the bloodstream, it can’t be taken in supplement form. If you were to take it as a pill, it’s just like eating chicken or beef. It’s a protein and your body would just break it up, so you wouldn’t absorb it from a pill.
Summary
As diet and exercise have significant effects on energy homeostasis. Orzano and Scott already showed that the most effective treatment is provided by a combination of diet and exercise 55. Taken together, the best strategy to accomplish long-term changes in body weight seems to be the use of potential anti-obesity agents in combination with a low-fat, high fiber diet and sufficient exercise and sleep.
What becomes clear from this review is that both leptin and ghrelin play major roles in the control system for energy balance in humans. However, leptin is primarily involved in long-term regulation of energy balance; it is released into the circulatory system as a function of energy stores, whereas ghrelin is a fast-acting hormone, of which the circulatory levels show clear meal-related changes 10. One other difference is that, in contrast to leptin, ghrelin does not seem to be critical for normal appetite and growth. Interestingly, leptin and ghrelin functioning in the system for energy homeostasis involves several overlapping pathways. At present, it is still not clear whether abnormalities in the leptin or ghrelin systems contribute to the development of obesity. Nevertheless, disturbances in both systems seem to play a role in the maintenance of obesity 10.
Most importantly, obese patients are leptin-resistant and it is therefore necessary to develop a treatment that overcomes leptin insensitivity or bypasses normal central leptin functioning, for example, by developing novel forms of leptin with stronger physiological properties. The Fc-leptin immunofusins used by Lo et al. were shown to have positive effects on body weight in mice 56. Additional studies are warranted to assess the effects of these compounds in humans. Also, ghrelin is still recognized as a potential drug target for weight regulation. When obese patients lose weight, ghrelin levels show an increase, as if to compensate for this weight loss 57.
Furthermore, the peptides downstream of leptin and ghrelin constitute possible targets for therapeutic interventions. For example, Makimura et al. demonstrated that a reduction of hypothalamic AgRP results in an increase of metabolic rate and a decrease of body weight without affecting food intake in mice. This suggests that agents antagonizing the effect of AgRP may be a useful strategy to treat obesity, without producing unacceptable loss of appetite 58. Interestingly, Belsham et al. created a number of hypothalamic neuronal cell lines, which can be used as models to study the regulation of neuropeptides associated with the control of feeding behaviour. Eventually, such studies may provide information that is necessary for the design of anti-obesity agents 59.
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–432. https://www.ncbi.nlm.nih.gov/pubmed/7984236[↩][↩]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. https://www.ncbi.nlm.nih.gov/pubmed/7489415[↩]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. https://www.ncbi.nlm.nih.gov/pubmed/8532024[↩]
- Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents: Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–1663. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC185793/[↩]
- Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 1996; 2: 589–593. https://www.ncbi.nlm.nih.gov/pubmed/8616722[↩]
- Serum immunoreactive-leptin concentrations in normal-weight and obese humans. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. N Engl J Med. 1996 Feb 1; 334(5):292-5. https://www.ncbi.nlm.nih.gov/pubmed/8532024/[↩]
- Effects of gender, body composition, and menopause on plasma concentrations of leptin. Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, Leibel RL. J Clin Endocrinol Metab. 1996 Sep; 81(9):3424-7. https://www.ncbi.nlm.nih.gov/pubmed/8784109/[↩]
- Sex differences in circulating human leptin pulse amplitude: clinical implications. Licinio J, Negrão AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, Negro PP, Mulla A, Veldhuis JD, Cearnal L, Flier JS, Gold PW. J Clin Endocrinol Metab. 1998 Nov; 83(11):4140-7. https://www.ncbi.nlm.nih.gov/pubmed/9814504/[↩]
- Gender differences in both spontaneous and stimulated leptin secretion by human omental adipose tissue in vitro: dexamethasone and estradiol stimulate leptin release in women, but not in men. Casabiell X, Piñeiro V, Peino R, Lage M, Camiña J, Gallego R, Vallejo LG, Dieguez C, Casanueva FF. J Clin Endocrinol Metab. 1998 Jun; 83(6):2149-55. https://www.ncbi.nlm.nih.gov/pubmed/9626154/[↩]
- Obesity Reviews Volume 8, Issue 1, January 2007. Pages 21–34. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. http://onlinelibrary.wiley.com/doi/10.1111/j.1467-789X.2006.00270.x/full[↩][↩][↩]
- Ghrelin levels in obesity and anorexia nervosa: effect of weight reduction or recuperation. Soriano-Guillén L, Barrios V, Campos-Barros A, Argente J. J Pediatr. 2004 Jan; 144(1):36-42. https://www.ncbi.nlm.nih.gov/pubmed/14722516/[↩][↩]
- The physiology and brain mechanisms of feeding. Rowland NE, Morien A, Li BH. Nutrition. 1996 Sep; 12(9):626-39. https://www.ncbi.nlm.nih.gov/pubmed/8878173/[↩]
- Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. Miljic D, Pekic S, Djurovic M, Doknic M, Milic N, Casanueva FF, Ghatei M, Popovic V. J Clin Endocrinol Metab. 2006 Apr; 91(4):1491-5. https://www.ncbi.nlm.nih.gov/pubmed/16449333/[↩]
- Plasma obestatin, ghrelin, and ghrelin/obestatin ratio are increased in underweight patients with anorexia nervosa but not in symptomatic patients with bulimia nervosa. Monteleone P, Serritella C, Martiadis V, Scognamiglio P, Maj M. J Clin Endocrinol Metab. 2008 Nov; 93(11):4418-21. https://www.ncbi.nlm.nih.gov/pubmed/18728162/[↩]
- Ahima R.S., et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. https://www.ncbi.nlm.nih.gov/pubmed/8717038[↩]
- American Diabetes Association. How Hormones and the Brain Affect Your Appetite and Weight. http://www.diabetesforecast.org/2017/jul-aug/how-hormones-and-the-brain.html[↩][↩][↩]
- Diabetes Nov 1996, 45 (11) 1511-1515; DOI: 10.2337/diab.45.11.1511. Responses of Leptin to Short-Term Fasting and Refeeding in Humans: A Link With Ketogenesis but Not Ketones Themselves. http://diabetes.diabetesjournals.org/content/45/11/1511.long[↩][↩]
- Cell Metab. 2012 Feb 8; 15(2): 150–156. Defining Clinical Leptin Resistance – Challenges and Opportunities. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3281561/[↩]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995; 269: 540–543. https://www.ncbi.nlm.nih.gov/pubmed/7624776[↩]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269: 543–546. https://www.ncbi.nlm.nih.gov/pubmed/7624777[↩]
- Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab 1997; 82: 1066–1070. https://www.ncbi.nlm.nih.gov/pubmed/9100574[↩]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998; 394: 897–901. https://www.ncbi.nlm.nih.gov/pubmed/9732873[↩]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol 2000; 68: 437–446. https://www.ncbi.nlm.nih.gov/pubmed/11037963[↩]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002; 111: 305–317. https://www.ncbi.nlm.nih.gov/pubmed/12419242[↩]
- National Center for Biotechnology Information, U.S. National Library of Medicine. Leptin Gene. https://www.ncbi.nlm.nih.gov/gene/3952[↩]
- J Clin Endocrinol Metab. 1999 Aug;84(8):2751-4. Leptin responses to overfeeding: relationship with body fat and nonexercise activity thermogenesis. https://www.ncbi.nlm.nih.gov/pubmed/10443673[↩]
- J Clin Endocrinol Metab. 1999 Aug;84(8):2751-4.Leptin responses to overfeeding: relationship with body fat and nonexercise activity thermogenesis. https://www.ncbi.nlm.nih.gov/pubmed/10443673[↩]
- J Clin Endocrinol Metab. 2003 Apr;88(4):1577-86. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. https://www.ncbi.nlm.nih.gov/pubmed/12679442[↩][↩]
- Cholecystokinin decreases food intake in rats. Gibbs J, Young RC, Smith GP. J Comp Physiol Psychol. 1973 Sep; 84(3):488-95. https://www.ncbi.nlm.nih.gov/pubmed/4745816/[↩]
- C-terminal octapeptide of cholecystokinin decreases food intake in man. Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. Am J Clin Nutr. 1981 Feb; 34(2):154-60. https://www.ncbi.nlm.nih.gov/pubmed/6259918/[↩]
- Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. J Clin Invest. 1985 Apr; 75(4):1144-52. https://www.ncbi.nlm.nih.gov/pubmed/2580857/[↩]
- Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Smith GP, Jerome C, Norgren R. Am J Physiol. 1985 Nov; 249(5 Pt 2):R638-41. https://www.ncbi.nlm.nih.gov/pubmed/4061684/[↩]
- Blockade of type A, but not type B, CCK receptors postpones satiety in rhesus monkeys. Moran TH, Ameglio PJ, Peyton HJ, Schwartz GJ, McHugh PR. Am J Physiol. 1993 Sep; 265(3 Pt 2):R620-4. https://www.ncbi.nlm.nih.gov/pubmed/8214156/[↩]
- American Journal of Physiology – Regulatory, Integrative and Comparative Physiology Published 1 June 2006 Vol. 290 no. 6, R1544-R1549 DOI: 10.1152/ajpregu.00811.2005. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. http://ajpregu.physiology.org/content/290/6/R1544.long[↩]
- Gastroenterology 2007. 132(Issue 6[↩]
- Rosenbaum M., Sy M., Pavlovich K., Leibel R.L., Hirsch J. Leptin reverses weight loss–induced changes in regional neural activity responses to visual food stimuli. J. Clin. Invest. 2008;118:2583–2591. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2430499/[↩]
- Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab 1996; 81: 4162–4165. https://www.ncbi.nlm.nih.gov/pubmed/8923877[↩]
- Levine JA, Eberhardt NL, Jensen MD. Leptin responses to overfeeding: relationship with body fat and nonexercise activity thermogenesis. J Clin Endocrinol Metab 1999; 84: 2751–2754. https://www.ncbi.nlm.nih.gov/pubmed/10443673[↩]
- Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, Myint M, Caro JF. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes 1996; 45: 1511–1515. https://www.ncbi.nlm.nih.gov/pubmed/8866554[↩][↩]
- Korbonits M, Trainer PJ, Little JA, Edwards R, Kopelman PG, Besser GM, Svec F, Grossman AB. Leptin levels do not change acutely with food administration in normal or obese subjects, but are negatively correlated with pituitary-adrenal activity. Clin Endocrinol 1997; 46: 751–757. https://www.ncbi.nlm.nih.gov/pubmed/9274707[↩]
- Weigle DS, Duell PB, Connor WE, Steiner RA, Soules MR, Kuijper JL. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J Clin Endocrinol Metab 1997; 82: 561–565. https://www.ncbi.nlm.nih.gov/pubmed/9024254[↩][↩]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–1719. https://www.ncbi.nlm.nih.gov/pubmed/11473029[↩][↩]
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 2001; 86: 4753–4758. https://www.ncbi.nlm.nih.gov/pubmed/11600536[↩]
- Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab 2004; 287: E297–E304. https://www.ncbi.nlm.nih.gov/pubmed/15039149[↩]
- Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 2001; 24: RC19–RC21. https://www.ncbi.nlm.nih.gov/pubmed/11434675[↩]
- Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 1999; 48: 334–341. https://www.ncbi.nlm.nih.gov/pubmed/10334310[↩]
- Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc 2000; 59: 359–371. https://www.ncbi.nlm.nih.gov/pubmed/10997652[↩]
- Heini AF, Lara-Castro C, Schneider H, Kirk KA, Considine RV, Weinsier RL. Effect of hydrolyzed guar fiber on fasting and postprandial satiety and satiety hormones: a double-blind, placebo-controlled trial during controlled weight loss. Int J Obes Relat Metab Disord 1998; 22: 906–909. https://www.ncbi.nlm.nih.gov/pubmed/9756250[↩]
- Groschl M, Knerr I, Topf HG, Schmid P, Rascher W, Rauh M. Endocrine responses to the oral ingestion of a physiological dose of essential amino acids in humans. J Endocrinol 2003; 179: 237–244. https://www.ncbi.nlm.nih.gov/pubmed/14596675[↩][↩]
- Weigle DS, Cummings DE, Newby PD, Breen PA, Frayo RS, Matthys CC, Callahan HS, Purnell JQ. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab 2003; 88: 1577–1586. https://www.ncbi.nlm.nih.gov/pubmed/12679442[↩]
- Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J Clin Endocrinol Metab 2003; 88: 5510–5514. https://www.ncbi.nlm.nih.gov/pubmed/14602798[↩]
- Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept 2003; 116: 101–107. https://www.ncbi.nlm.nih.gov/pubmed/14599721[↩]
- Greenman Y, Golani N, Gilad S, Yaron M, Limor R, Stern N. Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin Endocrinol 2004; 60: 382–388. https://www.ncbi.nlm.nih.gov/pubmed/15009005[↩]
- Nedvidkova J, Krykorkova I, Bartak V, Papezova H, Gold PW, Alesci S, Pacak K. Loss of meal-induced decrease in plasma ghrelin levels in patients with anorexia nervosa. J Clin Endocrinol Metab 2003; 88: 1678–1682. https://www.ncbi.nlm.nih.gov/pubmed/12679456[↩]
- Orzano AJ, Scott JG. Diagnosis and treatment of obesity in adults: an applied evidence-based review. J Am Board Fam Pract 2004; 17: 359–369. https://www.ncbi.nlm.nih.gov/pubmed/15355950[↩]
- Lo KM, Zhang J, Sun Y, Morelli B, Lan Y, Lauder S, Brunkhorst B, Webster G, Hallakou-Bozec S, Doare L, Gillies SD. Engineering a pharmacologically superior form of leptin for the treatment of obesity. Protein Eng Des Sel 2005; 18: 1–10. https://www.ncbi.nlm.nih.gov/pubmed/15790575[↩]
- Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord 2004; 28: 879–885. https://www.ncbi.nlm.nih.gov/pubmed/15111983[↩]
- Makimura H, Mizuno TM, Mastaitis JW, Agami R, Mobbs CV. Reducing hypothalamic AGRP by RNA interference increases metabolic rate and decreases body weight without influencing food intake. BMC Neurosci 2002; 3: 18. https://www.ncbi.nlm.nih.gov/pubmed/12423556[↩]
- Belsham DD, Cai F, Cui H, Smukler SR, Salapatek AM, Shkreta L. Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology 2004; 145: 393–400. https://www.ncbi.nlm.nih.gov/pubmed/14551229[↩]