What are phytosterols
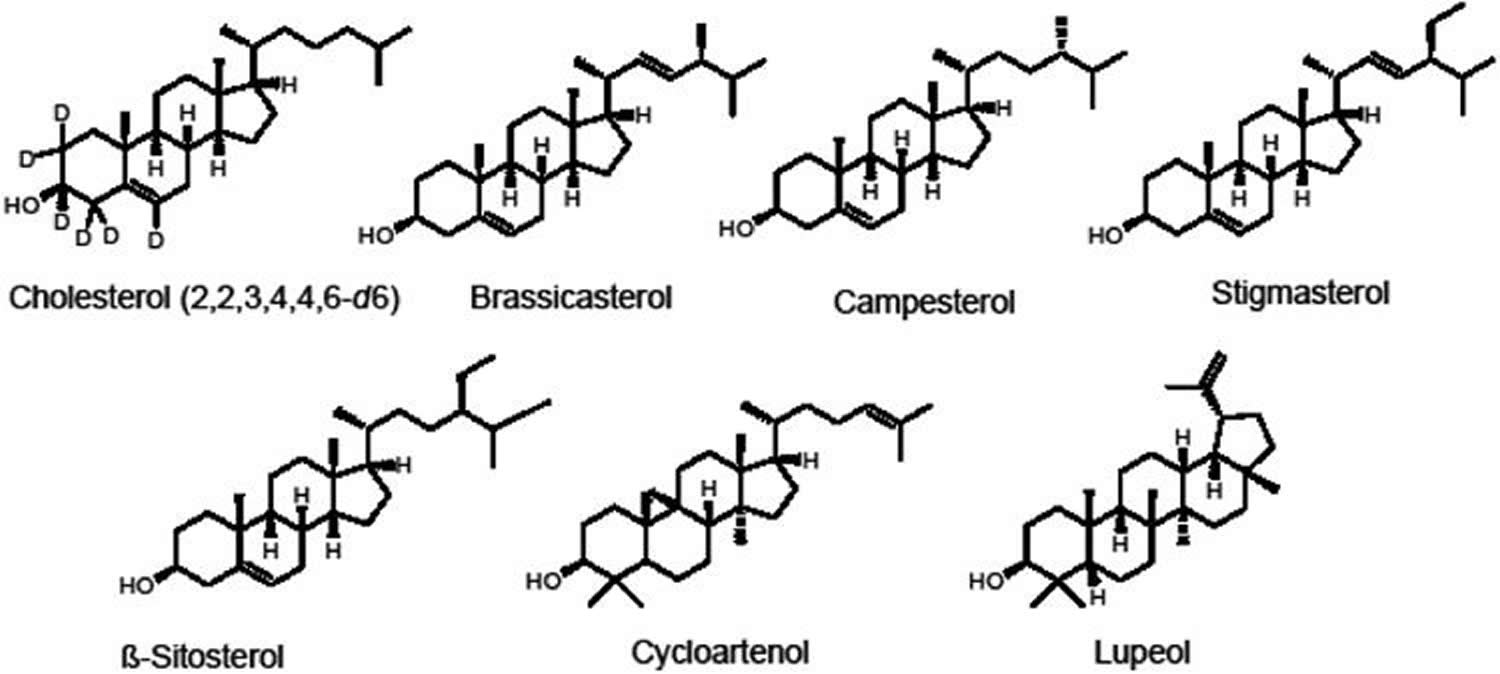
Phytosterols (plant sterols) are plant steroid alcohols (triterpenes) that are important structural components of plant membranes that are found in many food plants, nuts, seeds, vegetables, and edible oils 1. More than 250 phytosterols have been identified in botanical sources, with β-sitosterol being the most commonly reported. Phytosterols (plant sterols) can exist in plants as free form, as esters with fatty acids, as glycosides, or as acylated steryl glycosides. Other significant phytosterols and related triterpenes in edible plants include brassicasterol, campesterol, stigmasterol, cycloartenol, and lupeol (Figure 1) 2.
Phytosterols, or plant sterols, are structurally and biosynthetically related to cholesterol (a principle sterol in animal cells) with the addition of a side chain at C24. Most phytosterols contain 28 or 29 carbons and one or two carbon-carbon double bonds, typically one in the sterol nucleus and sometimes a second in the alkyl side chain. Phytostanols or plant stanols, are a fully-saturated subgroup of phytosterols (contain no double bonds) 3. Both phytosterols (plant sterols) and phytostanols (plant stanols) can be esterified to a fatty acid at the hydroxyl group to form phytosterol esters or phytostanol esters. For simplicity, “phytosterols” is used to refer to phytosterols, phytostanols and their esters throughout this article, except when noted.
Free phytosterols serve to stabilize phospholipid bilayers in plant cell membranes just as cholesterol does in animal cell membranes 3. Phytosterols regulate membrane fluidity of plant cells. Phytosterols found in fat-soluble fractions of plants have similar chemical structure and biological functions as cholesterol 4.
Early mechanism of action studies indicated that dietary phytosterols block intestinal absorption and subsequent cholesterol re-secretion into chylomicrons 5. More recently, phytosterols were also shown to reduce intestinal cholesterol absorption by regulating several transporters including the stimulation of trans-intestinal cholesterol excretion 6. Although some clinical trials have suggested that individuals with elevated low-density lipoproteins and high cardiovascular disease risk might benefit from consuming dietary supplements or foods rich in phytosterols, other studies indicate that dietary phytosterols do not reduce the risks of cardiovascular disease 5, 7, 2. Nevertheless, phytosterols remain an option for the management of serum cholesterol for those concerned about the side effects of statins 8, 9.
Figure 1. Phytosterols chemical structures
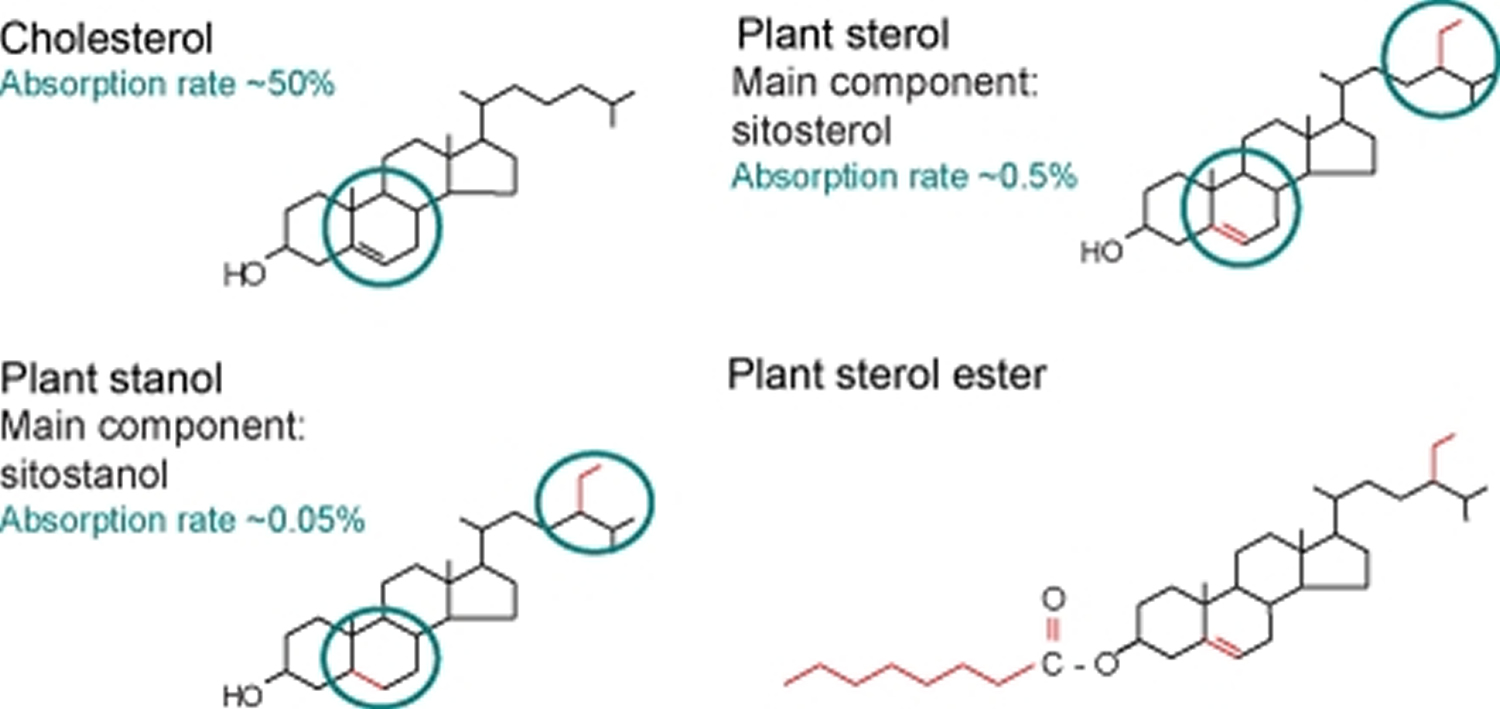
[Source 1]Figure 2. Chemical structure of cholesterol, sitosterol (plant sterol), and sitostanol (plant stanol)
Footnote: Chemical structure of cholesterol, sitosterol (plant sterol), and sitostanol (plant stanol). Both plant sterols and plant stanols differ from cholesterol only in the side chain attached to the sterol ring. Plant stanols are saturated plant sterols without a double bond in the sterol ring. Due to their saturation status, plant stanols are less effectively absorbed. Esterified plant sterols and plant stanols are supplemented in ‘nutraceuticals’ to reduce serum cholesterol levels
Phytostanols occur in trace levels in many plant species and they occur in high levels in tissues of only in a few cereal species 3. Phytosterols can be converted to phytostanols by chemical hydrogenation. More than 250 different types of phytosterols have been reported in plant species and marine materials 3.
The most abundant dietary plant sterols are sitosterol (60%), campesterol (35%) and stigmosterol 3. Phytosterols are not synthesized by the body and an estimated 200-300 mg phytosterol is obtained daily from the diet. In humans, intestinal absorption of phytosterols is low (0-10%) and from 0.02 to 0.3% for the phytostanols, compared to the >40% for cholesterol. The phytosterols and stanols are naturally occur in a variety of foods such as nuts, vegetable oils, seeds and cereals. The prime function of phytosterols is to inhibit the intestinal absorption of cholesterol. Human body uses the phytosterols to produce the hormones it needs. Phytosterols are not synthesized in human body, are poorly absorbed and are excreted faster from the liver than cholesterol.
Phytosterols and phytostanols have received much attention in the last few years because of their cholesterol-lowering properties. Plant sterols contain an extra methyl, ethyl group or double bond that inhibit cholesterol absorption in the small intestine 10. Early phytosterol-enriched products contained free phytosterols and relatively large dosages were required to significantly lower serum cholesterol. In the last several years two spreads, one containing phytostanyl fatty-acid esters and the other phytosteryl fatty-acid esters, have been commercialized and were shown to significantly lower serum cholesterol at dosages of 1-3 g per day. The popularity of these products has caused the medical and biochemical community to focus much attention on phytosterols and consequently research activity on phytosterols has increased dramatically.
Phytosterols and cholesterol
- Phytosterols are not synthesized in the human body.
- Phytosterols have their intestinal absorption much lower than that of cholesterol and
- Large doses of Phytosterols in diet diminish the absorption of cholesterol.
Although cholesterol and phytosterols are structurally similar, they are metabolized differently. In contrast to cholesterol, phytosterols are not synthesized in the body and are solely of dietary origin. The normal Western-type diet contains about 200–500 mg cholesterol, 200–400 mg plant sterols, and about 50 mg of plant stanols. In order to be absorbed, cholesterol and phytosterols are taken up in micelles. Sterol-laden micelles interact with the intestinal brush layer, enabling absorption in entrocytes. Phytosterols competitively inhibit intestinal absorption of cholesterol. The exact molecular mechanisms involved are not completely understood; however, it is clear that both cholesterol and phytosterols require the Niemann-Pick C1 Like 1 protein (NCP1L1) to obtain entry in enterocytes 11. In enterocytes, cholesterol is esterified via the enzyme acetyl-coenzyme A acetyltransferase 2 (ACAT2), packed into chylomycrons, and drained into the lymph system via the basolateral membrane. Non-esterified cholesterol and phytosterols are pumped back into the intestinal lumen via the ATP proteins ABCG5 and ABCG8. These complex mechanisms are responsible for the assimilation of about 50% of cholesterol, but less than 5% of plant sterols and even less than 0.5% of plant stanols 11. The major part of assimilated phytosterols is directly eliminated via the liver and the biliary system so that, in the end in healthy individuals, less than 1% is retained 12.
These findings suggest that the absorption of both dietary cholesterol and biliary cholesterol is inhibited by consumption of plant sterols 13. Plant sterols and stanols appeared to have similar effects on total cholesterol, LDL cholesterol, HDL cholesterol and triglyceride levels 14.
Phytosterols benefits
As previously noted, it is generally agreed that high blood cholesterol level (especially LDL cholesterol) is a risk factor for coronary heart disease. Oxidation of excess LDL cholesterol leads to arterial wall plaque build up (atherosclerosis), which then restricts blood flow and increases blood pressure. Unless, hypercholesterolemia and hypertension are treated, these factors are associated with increased risk of coronary heart disease (myocardial infarction) and stroke 15.
Therefore, the clinical relevance of LDL-cholesterol lowering lies in the potential for plant sterols to reduce both the total and low density lipoprotein (LDL) cholesterol are considered to be a beneficial health effect due to elevated levels of these blood lipids being risk factors for coronary heart disease.The normal range for LDL cholesterol is described as 2.0 – 3.4 mmol/L by some and <3.5 mmol/L by others. Two systematic reviews with the meta-analyses estimated that intake of 1.6 g to 2.2 g phytosterols per day resulted in an approximately 0.33 mmol/L decrease in LDL cholesterol concentration and a 0.36mmol/L decrease in total cholesterol concentration. As described, there is an impressive body of scientific data demonstrating cholesterol-lowering by plant sterols.
Dietary incorporation of plant sterols and stanols is recommended for blood cholesterol reduction 16, 17. Berger et al. 18 reviewed clinical trials on efficacy of plant sterols as cholesterol lowering agents and reported that the consumption of plant sterols/stanols have been reported to reduce low density lipoprotein (LDL) cholesterol levels by 5–15%.
In addition to their cholesterol lowering effect, plant sterols may possess anti-cancer 19, anti-atherosclerosis 20, 21, anti-inflammation 22 and anti-oxidation activities 23.
Bouic 24 and Bouic et al. 25 have reviewed the possible roles of phytosterols in the etiology or preventive role of phytosterols in various diseases and conditions, including proliferative responses of lymphocytes, pulmonary tuberculosis, feline immunodeficiency virus and HIV, stress induced immune suppression, rheumatoid arthritis, and allergic rhinitis/sinusitis. The mechanisms by which plant sterols display their anti-inflammatory activity are thought to include inhibition of secretion of inflammatory mediators such as interleukin-6, and tumor necrosis factor-α by monocytes 24. Most of the work has been conducted with animals. From these provocative results, it is not unlikely that plant sterols will be further used for purposes related to control of deveopment and spread of certain cancers in humans.
In vitro studies have shown that plant sterols are effective in preventing hyperproliferation of vascular smooth muscle cell that play a role in atherosclerosis development 26. Animal studies have shown that plant sterols also have anti-atherogenicity activity. In rabbits, sitosatanol feeding decreased plaque accumulation in coronary arteries within the ascending aorta 27. Feeding plant sterols to apo E-deficient mice decreased platelet counts as well as the susceptibility of red blood cells to hemolysis, decreased plasma fibrinogen 28 and decreased formation of atherosclerotic lesions 29. In healthy subjects who consumed 4 g/d of wood based stanol ester, the activity of antithrombin-III tended to increase compared to control group 30. Thus, plant sterols may reduce atherosclerosis development not only by reducing blood cholesterol levels but also by possessing anti-atherogenicity activity.
Anti-cancer activity
The action of plant sterols as anticancer dietary components has been recently extensively reviewed 31. Plant sterols can suppress tumor cell growth (LNCaP and HT-29) 32, 33. Compared to cholesterol, β-sitosterol caused a 24% decrease in cell growth and a 4-fold increase in apoptosis. In the latter work, the authors were interested in the effects of β-sitosterol on the sphingomyelin cycle, and measured two keys enzymes: protein phosphatase 2A (PP 2A) and phospholipase D (PLD). A 50% increase was observed in PP 2A activity in media containing 16 μM of β-sitosterol; however, there were no changes in protein levels of PP 2A. PLD activity increased in presence of phorbol myristate and β-sitosterol. This study suggests that the sphingomyelin cycle, which increases cell apoptosis, is mediated by PLD, PP 2A, and possibly, incorporation of β-sitosterol into the membrane. Another possible mechanism by which β-sitosterol can protect against cancer is through down-regulation of cholesterol synthesis, as was found in MDA-MB-231 human breast cancer cells 19. In an important in vivo study, SCID mice were xenografted with the human breast cancer cell line MDA-MB-231 34. Plant sterol-fed mice had a 33% smaller tumor size and 20% less metastases in lymph nodes and lungs than cholesterol-fed mice. This finding implied the possibility that plant sterols may retard the growth and spread of breast cancer cells. In addition to retarding the growth of breast cancer cells by plant sterols, there is some evidence that plant sterols can affect the development of prostate cancer 35. In a meta-analysis, 519 men were studied in 4 randomized, placebo-controlled, double-blind trials. β-sitosterol improved urinary symptom scores and flow measures, suggesting that non-glucosidic forms of β-sitosterol improve urinary symptoms and flow measures. Long term effectiveness, safety, and ability to prevent benign prostatic hyperplasia complications are not known 35. In another recent study, there was no evidence that plant sterol usage at dose of 300 mg/d, decreased risk of colon and rectal cancers 36. A similar conclusion was reached following a rat study in which rats were given the carcinogen methyl-nitroso-urea and then monitored for tumor development 37.
Plant sterols have also been found to have a protective effect against lung cancer 38. In this study, intake of about 144 mg/d of plant sterols was associated with reduction in risk for lung cancer even after controlling of confounding factors, i.e. tobacco smoking, vegetables, fruits, and antioxidant substances. Total dietary plant sterol intake was found to be inversely associated with breast 39 stomach 40 and esophageal 41 cancers. It was found that women with highest quartiles of total dietary intakes of plant sterols (>122 mg/d) had reduced risk of endometrial cancer 42 and intake of more than 521 mg/d reduced risk of ovarian cancer 43. On the other hand, in a prospective epidemiological study, high dietary intake was not associated with reduced risk of colon and rectal cancers 36. However, the intake of plant sterol might reduce the risk of more than one type of cancer.
Phytosterols cholesterol lowering actions
The cholesterol lowering effect of plant sterols is well documented in the literature. It is now accepted, after much earlier scientific debate and study, that 4-desmethyl plant sterols or stanols, either in their free or esterified form, decrease blood levels of total cholesterol and LDL-cholesterol through reduction of cholesterol absorption 44. Generally speaking, properly solubilized free sterols and esterified sterols possess similar cholesterol lowering ability 45, 46. Ostlund et al. 47 showed that emulsions of sitostanol, mixed with lecithin containing 0.7 g of sterol, reduced cholesterol absorption considerably, whereas less effect was seen with sitosterol in crystalline form.
Several studies 45, 46, 48, 49, 50, 51, 52 using intakes of 800–1000 mg of plant sterols per day have shown biologically/clinically significant (5% or more) reductions in LDL cholesterol levels, relative to control, or at least showed a statistically significant treatment effect relative to the starting LDL cholesterol level at the beginning of the treatment period, independent of control. Other studies 53, 54 with a similar dosage range did not meet the above criteria for biological reduction of LDL levels, or achieve statistical significance. Some studies showed that 800–1000 mg/d of free plant sterol equivalents can decrease the absorption of cholesterol, which is indicative, but not necessarily predictive, of actual LDL cholesterol lowering 48, 55.
It has been shown that increasing the dosage beyond 1000 mg per day of free sterol equivalents increased LDL cholesterol lowering efficacy or consistency of response leading to more statistically significant results 54, 51. Increasing the dosage beyond 1000 mg per day of free sterol equivalents did not further increase LDL cholesterol lowering efficacy 45. Moreover, ingestion of more than 3 g/day does not lead to any further lowering of cholesterol levels 11. Of note, a diet supplementation with plant stanol esters reduces not only serum cholesterol levels, but also serum plant sterol concentrations 56. The effectiveness of cholesterol-lowering products does not only depend on the amount of dietary phytosterols, but also on genetically determined differences in sterol metabolism. At present in the EU and the US, in addition to spreads, there are numerous other foods approved for the market to which phytosterols have been added, for example, salad dressing; milk, soy, yoghurt, cheesy products; soy and fruit drinks, even sausages and breads. The combined consumption of these new types of products can lead to a cumulative intake of very high concentrations especially of plant sterols, which can affect the absorption of cartenoids and fat-soluble vitamin 57. This can, for example, constitute a hazard to children, pregnant and breastfeeding women. A labelling, therefore, advises these individuals to avoid consuming these products. Furthermore, it has been suggested as a precaution that phytosterol intake should not exceed 3 g per day 58.
In humans, there is a good likelihood that a dose of 0.8–1.0 g of free sterol equivalents per day, properly solubilized, administered in 2–3 servings with a meal, will reduce LDL cholesterol by 5% or more and that this reduction in LDL cholesterol will correlate with an approximate 6–10% reduction in coronary heart disease risk at age 70 59, 60. However, at this dosage level, it is likely that not all individuals will achieve a 5% reduction in LDL cholesterol 46.
Cholesterol absorption is a very important physiological mechanism that regulates cholesterol metabolism. Phytosterols have been shown to inhibit the uptake of both dietary and endogenously produced (biliary) cholesterol from intestinal cells. Such inhibition results in a decrease in serum total and LDL-cholesterol levels. Levels of HDL – cholesterol and triglycerides do not appear to be affected by dietary phytosterol consumption 61. Bile acid sequestrants such as phytosterols and phytostanols that waste more bile acids for the patient result in a reduction of the cholesterol pool in cells, and there is a consequent increase in de novo cholesterol synthesis, as well as in LDL receptor synthesis. Both phytosterols and phytostanols can decrease LDL concentrations by about 6 to 10% 62.
Reductions in LDL levels were greater in individuals with high baseline LDL levels compared with those with normal to borderline baseline LDL levels 61. Reductions in LDL were greater when plant sterols were incorporated into fat spreads, mayonnaise and salad dressing, milk and yoghurt comparing with other food products such as croissants and muffins, orange juice, non-fat beverages, cereal bars, and chocolate 61. Plant sterols consumed as a single morning dose did not have a significant effect on LDL cholesterol levels 61.
It has been suggested that plant sterols should be consumed at each cholesterol containing meal to achieve an optimal effect. A daily intake of 2.5 g plant stanol esters, either consumed once per day at lunch, or divided over three portions resulted in a similar decrease in serum total and LDL cholesterol levels 63. Similar efficacy with a single larger dose sterol esters has also been demonstrated in two additional studies 64. A single serving of yogurt, providing 1 g of pure free sterols, resulted in a placebo-adjusted reduction in LDL cholesterol of 6.3% 51. Consumption of a single dose of 2.4 g/d plant sterols resulted in a 9.3 and 14.6 % reductions in blood total and LDL cholesterol levels, respectively, in hypercholesterolemic individuals 64. Single doses of plant sterols may have sustained effects on cholesterol absorption via interactions with intestinal proteins.
Nevertheless, as there are a plethora of studies showing the efficacy of plant sterols distributed in 2–3 meals and only two studies to date demonstrating efficacy with a single larger serving, it seems prudent to remain consistent with the more established, conservative recommendation of consuming plant sterols in 2–3 doses with food, as adopted by the United States FDA.
Phytosterols Food sources
Plant sterols are found in all foods of plant origin especially abundant in fat-laden vegetables and vegetable products, such as vegetables oils and olive oil, but also in fruits and nuts. Foods rich in phytosterols include unrefined vegetable oils, whole grains, nuts and legumes. The main sources of Phytosterols are vegetable oils, vegetable fat spreads and margarines, cereals and cereal products (bread) and vegetables. These sources contribute to 50-80% of the total phytosterol intake. The fruits contain about 12% of phytosterol. The content of phytosterols in most vegetable oils ranges from 1.0 to 5.0 mg/g of oil. Wheat germ oil contains 17-26 mg/g of phytosterols. Lower amounts of phytosterols are found in palm oil (0.7 – 0.8 mg/g ), coconut oil (0.7 -0.8mg/g ), and olive oil (1.4 – 1.9 mg/g ). The phytosterol content in Finland rye, wheat, barley and oat are 1.0, 0.7,0.8 and 0.4 mg/g respectively.
The effects of naturally-occurring plant sterols on cholesterol metabolism have also been studied in more recent literature. It was reported that the differences between effects of different plant oils on blood lipid profiles may be related to their content of plant sterols 65, 66, 67, 68. Indeed, there has been renewed interest in the cholesterol lowering properties of speciality grains and unprocessed oils rich in plant sterols including amaranth oil 69, 70, rice bran oil 71, avocado oil 72, extra virgin olive oil 73, macadamian nut 74 and argan oil 75.
Table 1. Examples of food sources and forms of phytosterols
| Phytosterol Form | Food Sources |
|---|---|
| Free Phytosterol | wheat germ oil, corn oil, vegetable oils, all plants |
| Phytosterol Ester | wheat germ oil, corn oil, vegetable oils, whole wheat, all plants, Benecol® spreads (stanol esters), Promise activ® spreads (sterol esters) |
| Phytosterol Glycoside | unrefined plant-derived lecithin, nuts, seeds, legumes, wheat germ, whole grains, bran, fruit, vegetables |
Ostlund et al. 68 showed that doses as low as 150–300 mg of naturally present corn oil-derived phytosterols can reduce dietary cholesterol absorption. Also, it was shown that the consumption of original wheat germ, which contains about 328 mg plant sterols, reduced the cholesterol absorption by 42.8 % compared to plant sterol-free wheat germ 77. These results indicate that naturally available plant sterols are biologically effective as plant sterol supplementation in reducing cholesterol absorption, and that natural plant sterols have important effects on cholesterol metabolism 77.
Most clinical trials have been conducted using plant sterols or stanols added to spreads 44. As long as plant sterols are consumed with a meal to stimulate biliary flow, they can effectively lower LDL cholesterol on the background of various types of basal diets and food vehicles.
Plant sterols are efficacious when consumed in:
- oil: water emulsions 78, 79;
- water as lecithin micelles 47;
- yogurt 51, 80;
- low fat filled milks 81, 82, 83;
- chocolate 84;
- cereal; snack bars, breads, and beverages 85, 86; and
- beef/hamburger 87, 64.
Efficacy of a soy stanol-lecithin powder in reducing cholesterol absorption and LDL-C has been evaluated in a randomized, double-blind parallel study 88. The subjects who followed a diet consumed soy stanol-lecithin powder in a beverage. The provided daily dose of plant stanols was 1.9 g. The reductions in blood cholesterol and LDL cholesterol were 10.1 and 14.4%, respectively. In another group of subjects, cholesterol absorption was measured using 625 mg stanols provided in beverage or egg whites. Stanol-lecithin reduced cholesterol absorption by 32.1% and 38.2 % when consumed in beverage and egg white, respectively 88.
Phytosterols supplements
Commercially, plant sterols are currently contained in:
- bars (Logicol-Australia, Benecol-UK),
- vegetable oils (Ekona-Japan; NutraLease Canola Active-Israel),
- orange juice (Minute Maid Heart Wise containing Cargill CoroWise plant sterols) 89,
- mayonnaises (Logicol-Australia), milk (Benecol-UK, Logicol-Australia, SereCol-Argentenia),
- yogurt (Logicol-Australia; Benecol-UK),
- yogurt drinks (Benecol),
- soy milk (Pacific Foods),
- meat and soups (Raisio-Finland), and
- green teas (Chol zero, Korea).
Plant sterols are also being sold or developed mixed with other functional ingredients such as:
- fiber (Unilever Fruit D’or-France);
- healthy oils (Benecol Olive Spread-UK);
- non-absorbable diacylglycerol (Kao-ADM Econa Healthy Cooking Oil; Enzymotec MultOil Platform, ArteriCare products, Israel);
- almonds, soy protein and viscous fibers 90; and
- minerals like calcium, magnesium, and potassium 91, 92, 93.
There is also interest to combine plant sterols with antioxidants, such as flavonoids, quercetins, and catechin; and a spice mixture developed by Selako, and marketed as Flavomare in Scandanavia. It is only a matter of time before ingredients such as conjugated linoleic acid are mixed with plant sterols in various vehicles (e.g., Clarinol’s CLA has received GRAS status for addition to milks, yogurts, bars, etc.).
Various manufacturers also sell plant sterols in supplement form, and there is interest to develop plant sterols as drugs (e.g., Forbes’ FM-VP4 drug candidate). Plant sterols may also be combined with other drugs that lower cholesterol through different mechanisms of action, including statins and ezetimibe 94. Recent evidence suggests that patients who had previous acute coronary syndrome benefited from aggressive LDL lowering with statins to levels subtstantially below current target levels 95. This finding provides enthusiasm for developing novel plant sterol-drug and drug-drug combined strategies to aggressively lower LDL cholesterol in some populations. Despite evidence that plant sterols can effectively reduce LDL cholesterol and inhibit cholesterol absorption in vehicles other than spread type vehicles, regulatory agencies have been slow to accept plant sterols in foods other than spreads in some countries such as the USA 96 and Australia 97. Rigorous efforts underway by food companies and other highly respected organizations to allow claims for plant sterols in foods other than spreads.
A systematic review and meta-analysis comparing the LDL-cholesterol-lowering effect of plant sterols/stanols as supplements in contrast to food-based approaches showed no significant difference between the LDL-cholesterol-lowering action of plant sterols/stanols supplements vs foods enriched with plant sterols/stanols 98. The study authors concluded plant sterol/stanol supplements as part of a healthy diet represent an effective means of delivering LDL-cholesterol-lowering similar to plant sterols/stanols delivered in various food formats.
Safety concerns of phytosterols supplements
It has been concluded that plant sterols, within the range that causes desirable reduction in blood levels of total cholesterol and LDL-cholesterol, are clinically safe. This conclusion has been reported in short-term studies 45, 99, 51, 100 as well as in long-term study that lasted for 1 year 101. Since plant sterols decrease the absorption of cholesterol, they might also affect the absorption of fat-soluble vitamins. The scientific evidence for the impact of phytosterols on carotenoid status and fat soluble vitamins is a controversial issue. In some studies, plant sterols consumption has been shown to significantly reduce levels of carotenoids 102, 54, 103, 101, 100, 104, tocopherol 54, and lycopene 102, 103. Other studies reported that the consumption of plant sterols does not affect blood levels of carotenoids 99, 86, 105, 106, tocopherol 107 and lycopene 107.
In a recent trial comparing equal free sterol equivalent amounts (2.2 g/d) of esterified sterols and free sterols in milk, both forms of sterols decreased the absorption of β-carotene and α-tocopherol in normocholesterolemic men. The reduction in β-carotene bioavailability was significantly less pronounced with free plant sterols than with plant sterol esters However, there was no difference in cholesterol absorption between the two forms of plant sterols 83. Esters are presumed to have more of an effect on fat soluble vitamins because they partition into the oil phase of the intestine, whereas free sterol would partition into the micellar phase 108.
During plant sterol consumption, increasing the consumption of fruits and vegetables of more than five servings and including one or more carotenoid rich source would be enough to avoid reduction in carotenoid levels resulted from plant sterol intake 109.
Phytosterols and Familial Hypercholesterolaemias
Familial hypercholesterolaemia, defined as the heritable occurrence of severe hypercholesterolaemia due to the accumulation of LDL cholesterol in the plasma with cholesterol deposits in tendons and occasionally in skin and a high risk of atherosclerosis manifesting almost exclusively as coronary artery disease, is caused by at least four genes in sterol and lipoprotein pathways and displays varying gene-dose effects 62. Primary mutations causing familial hypercholesterolaemia are either due to defects in the low density lipoprotein-receptor gene (LDLR), apolipoprotein B-100 gene (APOB), or proprotein convertase subtilisin/kexin type 9 gene (PCSK9), singly or in combination 110. The most prevalent of these genetic defects are defects in the LDLR gene with approximately 1600 known (till date) mutations in the LDLR gene causing almost 85% to 90% cases of FH. Defects in the APOB gene account for 5% to 10% of familial hypercholesterolaemia in northern European population (less in other populations). The PCSK9 gene defects account for about 5% of cases of familial hypercholesterolaemia 111. The most severe form is related to total lack of receptors (receptor-negative mutations), while ‘receptor-defective’ mutations that comprise most of the mutations are usually accompanied by lesser symptoms 112. All of these disorders have in common defective clearance of LDL within a complex system of lipid and lipoprotein metabolism and regulation, but their inheritance may be autosomal dominant or recessive and not all dominant disorders have a gene-dose effect 62.
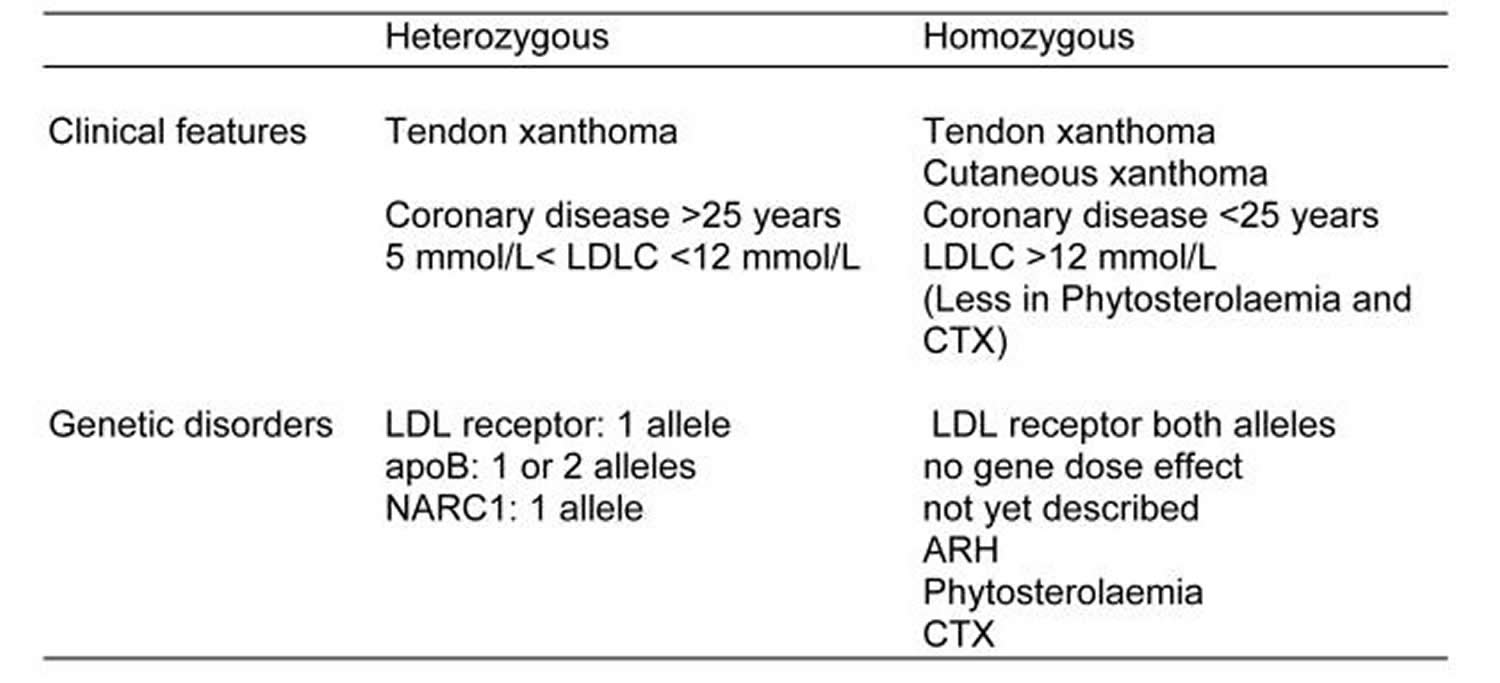
Familial hypercholesterolaemias may be loosely classified into an “heterozygous” and an “homozygous” clinical phenotype (Table 2). The heterozygous phenotype is inherited in an autosomal dominant manner and has a LDL-cholesterol concentration between range of 9 to 14.2 mmol/l (350 to 550 mg/dL), tendon xanthomata are common and coronary heart disease before 55 years of age is also common. The leading cause of the heterozygous phenotype is a mutation in the LDL receptor. Occasionally a mutation disrupting the binding of apoB to the LDL receptor is encountered. Recently a newly discovered protein, neural apoptosis regulated convertase 1 (NARC1), the product of the gene proprotein convertase subtilisin/kexin type 9 (PCSK9) has been referred to as autosomal dominant familial hypercholesterolaemias 3 62.
The homozygous phenotype has more severe LDL hypercholesterolaemia from 16.8 to 25.9 mmol/l (650 to 1000 mg/dL) 113, 114 and typically there are also xanthomata in the skin and tendons. On westernised diets, the LDL-cholesterol concentration usually exceeds 15 mmol/L. Included in this phenotype of cutaneous and tendinous xanthomata are other sterol disorders that do not result in the same degree of hypercholesterolaemia: phytosterolaemia and cerebrotendinous xanthomatosis 62. The genetic causes of the homozygous phenotype include the concurrence of two LDL receptor defects, but the same phenotype is not seen with concurrence of binding defective apoB. The homozygous phenotype is recessively inherited when mutations disrupt the function of an adaptor protein, now known by its property to confer autosomal recessive hypercholesterolaemia 62. Phytosterolaemia is inherited in an autosomal recessive manner as a result of mutations in adenosine binding cassette transporter proteins G5 and G8. Whilst phytosterolaemia can have significant hypercholesterolaemia, it seldom exceeds the heterozygous familial Hypercholesterolemia range. Cerebrotendinous xanthomatosis, also autosomal recessively inherited, is due to mutations in 27-hydroxylase (CYP27). Cerebrotendinous xanthomatosis is not associated severe hypercholesterolaemia.
This disorder is one of the most common congenital metabolic disorders; the prevalence of heterozygous familial hypercholesterolaemia is approximately 1 in 300 to 500 with much higher incidence in certain populations, such as the Afrikaners, Christian Lebanese, Finns, and French-Canadians 115.
Table 2. Familial hypercholesterolaemias phenotypes and their genetic causes
It is recommended that in children under 16 years of age, diagnosis of familial hypercholesterolaemia is based on a total cholesterol level of above 6.7 mmol/l (260 mg/dl) and a LDL cholesterol of above 4.0 mmol/l (155 mg/dl) on two measurements taken one month apart 116. In the 1994 revision of the Simon Broome Register Group definition, cases are categorised as ‘definite’ or ‘possible’ 117. According to the revision, ‘DNA based evidence of an LDL-receptor mutation or familial defective apoB-100’ was added as a sufficient criteria for ‘definite’ familial hypercholesterolaemia diagnosis. The aim of treatment in children and adults is the reduction of blood LDL cholesterol concentrations in order to reduce the risk of ischaemic heart disease.
Management of familial hypercholesterolaemia aims at lowering LDL by ≥ 50% or to < 3.36 mmol/l (130mg/dL). Statins are the most preferred pharmacological agents recommended for the treatment of familial hypercholesterolaemia along with diet and physical activity management in all age groups 118, 119. Four statins (lovastatin, simvastatin, pravastatin and atorvastatin) have also been approved by U.S. Food and Drug Administration (US FDA) for use in children with familial hypercholesterolaemia. Children who do not achieve the LDL cholesterol goal after prescribed initial statin dosing need higher dose of statin or addition of another lipid lowering agent. Ezetimibe, a cholesterol absorption inhibitor, is recommended as a monotherapy or in combination with statins in children and adolescents 120. Bile acid sequestrants cholestyramine and cholestipol are not recommended for use in pediatric age group due to severe gastrointestinal side effects and poor palatability. Colesvelam, another bile acid sequastrant, can be used in boys aged 7 to 10 years and in postmenarchal girls as monotherapy or as adjuvant to statins. Niacin and fibrates are not recommended in the pediatric age group due to their adverse effects 121.
Familial hypercholesterolaemia can’t be treated by diet and exercise alone. In the 2014 Cochrane Review of dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia, the authors could not make any conclusions about the effectiveness of a cholesterol-lowering diet, or any of the other dietary interventions suggested for familial hypercholesterolaemia, for the primary outcomes: evidence and incidence of ischaemic heart disease, number of deaths and age at death, due to the lack of data on these 122. The review authors added that large, parallel, randomised controlled trials are needed to investigate the effectiveness of a cholesterol-lowering diet (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) and the addition of omega-3 fatty acids, plant sterols or stanols, soya protein, dietary fibers to a cholesterol-lowering diet.
Treatment usually involves a statin drug. Often familial hypercholesterolaemia patients require more than one medication, and sometimes more than two. When statins don’t lower cholesterol enough, more cholesterol-lowering medications like ezetimibe are used. People with extremely high LDL, like those with the homozygous form, may undergo LDL apheresis. This is a dialysis-like procedure that’s done every few weeks to remove cholesterol from the blood.
Another class of lipid-lowering medications (bile acid sequestrants) like cholestyramine or colesevelam may also be used. They reduce the amount of cholesterol absorbed by the intestines. This lowers the amount of cholesterol that gets into the blood.
Two PCSK9 inhibitors are newly developed injectable antibodies that lower cholesterol levels 123. They target and block the PCSK9 protein. This makes more receptors on the liver available to remove LDL cholesterol from blood. Although FDA-approved, they’re still a new, costly treatment with limited use 123.
Phytosterols and Sitosterolemia
Phytosterolemia (sitosterolemia) is a rare autosomal recessive sterol storage disease caused by mutations in either of the adenosine triphosphate (ATP) binding cassette transporter genes; (ABC)G5 or ABCG8 located on human chromosome 2p21 124. These genes encode the heterodimer transporter ABCG5/G8, which is expressed in enterocytes (cells lining the intestine), in the proximal small intestine, and hepatocytes (liver cells) 125, 126, and function to rapidly excrete cholesterol, plant sterols and their saturated derivatives (stanols) from the body. The ABCG5/G8 transporters work to limit intestinal uptake of these sterols, promoting their biliary secretion, and subsequently preventing their accumulation in the body 126, 127, 128, 129. Sitosterolemia is caused impaired elimination of plant sterols and stanols characterized by significant elevation of serum plant sterols and stanols 130, 131 with potential plant sterols build-up in the arteries, skin (xanthomas), and tissues 132. Total cholesterol level can be normal to moderately in some sitosterolemia patients 128 although in some cases it can be extremely high 133. Individuals with sitosterolemia may present with tendon xanthomas and in some cases the tendency to develop coronary disease at an early age 134.
Hematologic abnormalities including macrothrombocytopenia, stomatocytosis and hemolysis are frequently observed in sitosterolemia patients. Currently, ezetimibe, a sterol absorption inhibitor, is used as the routine treatment for sitosterolemia, with reported improvement in plant sterol levels and hemolytic parameters. Extremely elevated plasma phytosterols levels in sitosterolemia have been associated with increased risk of premature atherosclerosis. Treatment of sitosterolemia includes the use of bile acid sequestrants, ileal bypass surgery and ezetimibe 135.
- Mo S, Dong L, Hurst WJ, van Breemen RB. Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. Lipids. 2013;48(9):949-956. doi:10.1007/s11745-013-3813-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4073239/[↩][↩]
- Phytosterols and cardiovascular health. Marangoni F, Poli A. Pharmacol Res. 2010 Mar; 61(3):193-9. https://www.ncbi.nlm.nih.gov/pubmed/20067836/[↩][↩]
- Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Moreau RA, Whitaker BD, Hicks KB. Prog Lipid Res. 2002 Nov; 41(6):457-500. https://www.ncbi.nlm.nih.gov/pubmed/12169300/[↩][↩][↩][↩][↩]
- Piironen V, Lindsay DG, Miettinen TA, Toivo J, Lampi A-M. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J Sci Food Agric. 2000;80:939–966. doi: 10.1002/(SICI)1097-0010(20000515)80:7<939::AID-JSFA644>3.3.CO;2-3.[↩]
- Phytosterol ester processing in the small intestine: impact on cholesterol availability for absorption and chylomicron cholesterol incorporation in healthy humans. Amiot MJ, Knol D, Cardinault N, Nowicki M, Bott R, Antona C, Borel P, Bernard JP, Duchateau G, Lairon D. J Lipid Res. 2011 Jun; 52(6):1256-64. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3090246/[↩][↩]
- Effects of plant sterols and stanols on intestinal cholesterol metabolism: suggested mechanisms from past to present. De Smet E, Mensink RP, Plat J. Mol Nutr Food Res. 2012 Jul; 56(7):1058-72. https://www.ncbi.nlm.nih.gov/pubmed/22623436/[↩]
- Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Genser B, Silbernagel G, De Backer G, Bruckert E, Carmena R, Chapman MJ, Deanfield J, Descamps OS, Rietzschel ER, Dias KC, März W. Eur Heart J. 2012 Feb; 33(4):444-51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3279314/[↩]
- Controversial role of plant sterol esters in the management of hypercholesterolaemia. Weingärtner O, Böhm M, Laufs U. Eur Heart J. 2009 Feb; 30(4):404-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2642922/[↩]
- Subjects with elevated LDL cholesterol and metabolic syndrome benefit from supplementation with soy protein, phytosterols, hops rho iso-alpha acids, and Acacia nilotica proanthocyanidins. Lerman RH, Minich DM, Darland G, Lamb JJ, Chang JL, Hsi A, Bland JS, Tripp ML. J Clin Lipidol. 2010 Jan-Feb; 4(1):59-68. https://www.ncbi.nlm.nih.gov/pubmed/21122628/[↩]
- Nigon F, Serfaty-Lacrosnière C, Beucler I, Chauvois D, Neveu C, Giral P, Chapman MJ, Bruckert E. Plant sterol-enriched margarine lowers plasma LDL in hyperlipidemic subjects with low cholesterol intake: effect of fibrate treatment. Clin Chem Lab Med. 2001 Jul;39(7):634-40. https://www.ncbi.nlm.nih.gov/pubmed/11522112[↩]
- Weingärtner O, Böhm M, Laufs U. Controversial role of plant sterol esters in the management of hypercholesterolaemia. European Heart Journal. 2009;30(4):404-409. doi:10.1093/eurheartj/ehn580. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2642922/[↩][↩][↩]
- Phytosterols and vascular disease. Patel MD, Thompson PD. Atherosclerosis. 2006 May; 186(1):12-9. https://www.ncbi.nlm.nih.gov/pubmed/16325823/[↩]
- Hallikainen MA, Uusitupa MI. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am J Clin Nutr 1999;69:403–10.[↩]
- Talati R, Sobieraj DM, Makanji SS, Phung OJ, Coleman CI. The comparative efficacy of plant sterols and stanols on serum lipids: a systematic review and meta-analysis. J Am Diet Assoc. 2010 May;110(5):719-26. doi: 10.1016/j.jada.2010.02.011. https://www.ncbi.nlm.nih.gov/pubmed/20430133[↩]
- Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367–372. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2539460/pdf/bmj00426-0021.pdf[↩]
- National Cholesterol Education Program Expert Panel. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–421. http://circ.ahajournals.org/content/106/25/3143.long[↩]
- Fletcher B, Berra K, Ades P, Braun LT, Burke LE, Durstine JL, Fair JM, Fletcher GF, Goff D, et al. Managing abnormal blood lipids – A collaborative approach. Circulation. 2005;112:3184–209. http://circ.ahajournals.org/content/112/20/3184.long[↩]
- Berger A, Jones PJ, Abumweis SS. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004;3:5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC419367/[↩]
- Awad AB, Roy R, Fink CS. Beta-sitosterol, a plant sterol, induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol Rep. 2003;10:497–500. https://www.ncbi.nlm.nih.gov/pubmed/12579296[↩][↩]
- Moghadasian MH, McManus BM, Pritchard PH, Frohlich JJ. “Tall oil”-derived phytosterols reduce atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1997;17:119–126. http://atvb.ahajournals.org/content/17/1/119.long[↩]
- Moghadasian MH, McManus BM, Godin DV, Rodrigues B, Frohlich JJ. Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: possible mechanisms of action. Circulation. 1999;99:1733–1739. http://circ.ahajournals.org/content/99/13/1733.long[↩]
- Bouic PJ. The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Curr Opin Clin Nutr Metab Care. 2001;4:471–475. doi: 10.1097/00075197-200111000-00001. https://www.ncbi.nlm.nih.gov/pubmed/11706278[↩]
- van Rensburg SJ, Daniels WM, van Zyl JM, Taljaard JJ. A comparative study of the effects of cholesterol, beta-sitosterol, beta-sitosterol glucoside, dehydroepiandrosterone sulphate and melatonin on in vitro lipid peroxidation. Metab Brain Dis. 2000;15:257–265. doi: 10.1023/A:1011167023695. https://www.ncbi.nlm.nih.gov/pubmed/11383550[↩]
- The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Bouic PJ. Curr Opin Clin Nutr Metab Care. 2001 Nov; 4(6):471-5. https://www.ncbi.nlm.nih.gov/pubmed/11706278/[↩][↩]
- Plant sterol/sterolin supplement use in a cohort of South African HIV-infected patients–effects on immunological and virological surrogate markers. Bouic PJ, Clark A, Brittle W, Lamprecht JH, Freestone M, Liebenberg RW. S Afr Med J. 2001 Oct; 91(10):848-50. https://www.ncbi.nlm.nih.gov/pubmed/11732455/[↩]
- Plant sterols regulate rat vascular smooth muscle cell growth and prostacyclin release in culture. Awad AB, Smith AJ, Fink CS. Prostaglandins Leukot Essent Fatty Acids. 2001 Jun; 64(6):323-30. https://www.ncbi.nlm.nih.gov/pubmed/11427042/[↩]
- Dietary sitostanol reduces plaque formation but not lecithin cholesterol acyl transferase activity in rabbits. Ntanios FY, Jones PJ, Frohlich JJ. Atherosclerosis. 1998 May; 138(1):101-10. https://www.ncbi.nlm.nih.gov/pubmed/9678775/[↩]
- Proatherogenic and antiatherogenic effects of probucol and phytosterols in apolipoprotein E-deficient mice: possible mechanisms of action. Moghadasian MH, McManus BM, Godin DV, Rodrigues B, Frohlich JJ. Circulation. 1999 Apr 6; 99(13):1733-9. https://www.ncbi.nlm.nih.gov/pubmed/10190884/[↩]
- Effects of various amounts of dietary plant sterol esters on plasma and hepatic sterol concentration and aortic foam cell formation of cholesterol-fed hamsters. Ntanios FY, van de Kooij AJ, de Deckere EA, Duchateau GS, Trautwein EA. Atherosclerosis. 2003 Jul; 169(1):41-50. https://www.ncbi.nlm.nih.gov/pubmed/12860249/[↩]
- Vegetable oil based versus wood based stanol ester mixtures: effects on serum lipids and hemostatic factors in non-hypercholesterolemic subjects. Plat J, Mensink RP. Atherosclerosis. 2000 Jan; 148(1):101-12. https://www.ncbi.nlm.nih.gov/pubmed/10580176/[↩]
- Phytosterols as anticancer dietary components: evidence and mechanism of action. Awad AB, Fink CS. J Nutr. 2000 Sep; 130(9):2127-30. http://jn.nutrition.org/content/130/9/2127.long[↩]
- Awad AB, von Holtz RL, Cone JP, Fink CS, Chen YC. beta-sitosterol inhibits growth of Ht-29 human colon cancer cells by activating the sphingomyelin cycle. Anticancer Res. 1998;18:471–473. https://www.ncbi.nlm.nih.gov/pubmed/9568122[↩]
- Awad AB, Gan Y, Fink CS. Effect of b-sitosterol, a plant sterol, on growth, protein phosphatase 2A, and phospholipase D in LNCaP cells. Nutr Cancer. 2000;36:74–78. doi: 10.1207/S15327914NC3601_11. https://www.ncbi.nlm.nih.gov/pubmed/10798219[↩]
- Awad AB, Downie A, Fink CS, Kim U. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Res. 2000;20:821–824. https://www.ncbi.nlm.nih.gov/pubmed/10810360[↩]
- Wilt T, Ishani A, MacDonald R, Stark G, Mulrow C, Lau J. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst. Rev 2000; CD001043 Review. https://www.ncbi.nlm.nih.gov/pubmed/10796740[↩][↩]
- Normén AL, Brants HA, Voorrips LE, Andersson HA, van den Brandt PA, Goldbohm RA. Plant sterol intakes and colorectal cancer risk in the Netherlands Cohort Study on Diet and Cancer. Am J Clin Nutr. 2001;74:141–148. http://ajcn.nutrition.org/content/74/1/141.long[↩][↩]
- Quilliot D, Boman F, Creton C, Pelletier X, Floquet J, Debry G. Phytosterols have an unfavourable effect on bacterial activity and no evident protective effect on colon carcinogenesis. Eur J Cancer Prev. 2001;10:237–243. doi: 10.1097/00008469-200106000-00006. https://www.ncbi.nlm.nih.gov/pubmed/11432710[↩]
- Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, Carzoglio J, Ronco A. Phytosterols and risk of lung cancer: a case-control study in Uruguay. Lung Cancer. 1998;21:37–45. doi: 10.1016/S0169-5002(98)00044-0. https://www.ncbi.nlm.nih.gov/pubmed/9792052[↩]
- Ronco A, De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Leborgne F. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer. 1999;35:111–119. doi: 10.1207/S15327914NC352_3. https://www.ncbi.nlm.nih.gov/pubmed/10693163[↩]
- De Stefani E, Boffetta P, Ronco AL, Brennan P, Deneo-Pellegrini H, Carzoglio JC, Mendilaharsu M. Plant sterols and risk of stomach cancer: a case-control study in Uruguay. Nutr Cancer. 2000;37:140–144. doi: 10.1207/S15327914NC372_4. https://www.ncbi.nlm.nih.gov/pubmed/11142085[↩]
- De Stefani E, Brennan P, Boffetta P, Ronco AL, Mendilaharsu M, Deneo-Pellegrini H. Vegetables, fruits, related dietary antioxidants, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer. 2000;38:23–29. doi: 10.1207/S15327914NC381_4. https://www.ncbi.nlm.nih.gov/pubmed/11341040[↩]
- McCann SE, Freudenheim JL, Marshall JR, Brasure JR, Swanson MK, Graham S. Diet in the epidemiology of endometrial cancer in western New York (United States) Cancer Causes Control. 2000;11:965–974. doi: 10.1023/A:1026551309873. https://www.ncbi.nlm.nih.gov/pubmed/11142531[↩]
- McCann SE, Freudenheim JL, Marshall JR, Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr. 2003;133:1937–1942. http://jn.nutrition.org/content/133/6/1937.long[↩]
- Berger A, Jones PJ, Abumweis SS. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids in Health and Disease. 2004;3:5. doi:10.1186/1476-511X-3-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC419367/[↩][↩]
- Hendriks HF, Weststrate JA, van Vliet T, Meijer GW. Spreads enriched with three different levels of vegetable oil sterols and the degree of cholesterol lowering in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr. 1999;53:319–327. doi: 10.1038/sj.ejcn.1600728. https://www.ncbi.nlm.nih.gov/pubmed/10334658[↩][↩][↩][↩]
- Sierksma A, Weststate JA, Meijer GW. Spreads enriched with plant sterols, either esterified 4,4 dimethylsterols or free 4 desmethylsterols, and plasma total and LDL cholesterol concentrations. Br J Nutr. 1999;82:273–282. https://www.ncbi.nlm.nih.gov/pubmed/10655976[↩][↩][↩]
- Ostlund RE, Spilburg CA, Stenson WF. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am J Clin Nutr. 1999;70:826–831. http://ajcn.nutrition.org/content/70/5/826.long[↩][↩]
- Miettinen TA, Vanhanen H. Dietary sitostanol related to absorption, synthesis and serum level of cholesterol in different apolipoprotein E phenotypes. Atherosclerosis. 1994;105:217–226. https://www.ncbi.nlm.nih.gov/pubmed/8003098[↩][↩]
- Vanhanen HT, Kajander J, Lehtovirta H, Miettinen TA. Serum levels, absorption efficiency, faecal elimination and synthesis of cholesterol during increasing doses of dietary sitostanol esters in hypercholesterolaemic subjects . Clin Sci. 1994;87:61–67. https://www.ncbi.nlm.nih.gov/pubmed/8062521[↩]
- Pelletier X, Belbraouet S, Mirabel D, Mordret F, Perrin JL, Pages X, Debry G. A diet moderately enriched in phytosterols lowers plasma cholesterol concentrations in normocholesterolemic humans . Ann Nutr Metab. 1995;39:291–295. https://www.ncbi.nlm.nih.gov/pubmed/8585698[↩]
- Volpe R, Niittynen L, Korpela R, Sirtori C, Bucci A, Fraone N, Pazzucconi F. Effects of yoghurt enriched with plant sterols on serum lipids in patients with moderate hypercholesterolaemia. Br J Nutr. 2001;86:233–239. https://www.ncbi.nlm.nih.gov/pubmed/11502237[↩][↩][↩][↩][↩]
- Maki KC, Davidson MH, Umporowicz DM, Schaefer EJ, Diklin MR, Ingram KA, Chen S, McNamara Jr W, Gebhart B, Ribaya-Mercado JD, Perrone G, Robins SJ, Franke WC. Lipid responses to plant sterol-enriched reduced-fat spreads incorporated into a National Cholesterol Education step 1 diet. Am J Clin Nutr. 2001;74:33–43. http://ajcn.nutrition.org/content/74/1/33.long[↩]
- Vanhanen HT, Miettinen TA. Effects of unsaturated and saturated dietary plant sterols on their serum contents . Clin Chim Acta. 1992;205:97–107. https://www.ncbi.nlm.nih.gov/pubmed/1521345[↩]
- Hallikainen MA, Sarkkinen ES, Uusitupa MI. Plant stanol esters affect serum cholesterol concentrations of hypercholesterolemic men and women in a dose-dependent manner. J Nutr. 2000;130:767–776. http://jn.nutrition.org/content/130/4/767.long[↩][↩][↩][↩]
- Mattson FH, Grundy SM, Crouse JR. Optimizing the effect of plant sterols on cholesterol absorption in man. Am J Clin Nutr. 1982;35:697–700. https://www.ncbi.nlm.nih.gov/pubmed/7072622[↩]
- Comparison of efficacy of plant stanol ester and sterol ester: short-term and longer-term studies. O’Neill FH, Sanders TA, Thompson GR. Am J Cardiol. 2005 Jul 4; 96(1A):29D-36D. https://www.ncbi.nlm.nih.gov/pubmed/15992513/[↩]
- Simulation of prospective phytosterol intake in Germany by novel functional foods. Kuhlmann K, Lindtner O, Bauch A, Ritter G, Woerner B, Niemann B. Br J Nutr. 2005 Mar; 93(3):377-85. https://www.ncbi.nlm.nih.gov/pubmed/15877878/[↩]
- A report from the data collection and exposure unit in response to a request from the European Commission. Consumption of food and beverages with added plant sterols in the European Union. The EFSA Journal. 2008;133:1–21. http://www.efsa.europa.eu[↩]
- Law MR, Wald NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? BMJ. 1994;308:367–372. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2539460/[↩]
- Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction . N Engl J Med. 1991;325:373–381. https://www.ncbi.nlm.nih.gov/pubmed/2062328[↩]
- AbuMweis SS, Barake R, Jones PJH. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food & Nutrition Research. 2008;52:10.3402/fnr.v52i0.1811. doi:10.3402/fnr.v52i0.1811. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2596710/[↩][↩][↩][↩]
- Marais AD. Familial Hypercholesterolaemia. The Clinical Biochemist Reviews. 2004;25(1):49-68. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1853359/[↩][↩][↩][↩][↩][↩][↩]
- Plat J, Van Onselen ENM, Van Heugten MMA, Mensink RP. Effects on serum lipids, lipoproteins and fat soluble antioxidant concentrations of consumption frequency of margarines and shortenings enriched with plant stanol esters . Eur J Clin Nutr. 2000;54:671–677. doi: 10.1038/sj.ejcn.1601071. https://www.ncbi.nlm.nih.gov/pubmed/11002377[↩]
- Matvienko OA, Lewis DS, Swanson M, Arndt B, Rainwater DL, Stewart J, Alekel DL. A single daily dose of soybean phytosterols in ground beef decreases serum total cholesterol and LDL cholesterol in young, mildly hypercholesterolemic men. Am J Clin Nutr. 2002;76:57–64. http://ajcn.nutrition.org/content/76/1/57.long[↩][↩][↩]
- Howard BV, Gidding SS, Liu K. Association of apolipoprotein E phenotype with plasma lipoproteins in African-American and white young adults. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1998;148:859–868. https://www.ncbi.nlm.nih.gov/pubmed/9801016[↩]
- Pedersen A, Baumstark MW, Marckmann P, Gylling H, Sandstrom B. An olive oil-rich diet results in higher concentrations of LDL cholesterol and a higher number of LDL subfraction particles than rapeseed oil and sunflower oil diets. J Lipid Res. 2000;41:1901–1911. http://www.jlr.org/content/41/12/1901.long[↩]
- Wagner KH, Tomasch R, Elmadfa I. Impact of diets containing corn oil or olive/sunflower oil mixture on the human plasma and lipoprotein lipid metabolism. Eur J Nutr. 2001;40:161–167. doi: 10.1007/s003940170004. https://www.ncbi.nlm.nih.gov/pubmed/11905957[↩]
- Ostlund RE, Jr, Racette SB, Okeke A, Stenson WF. Phytosterols that are naturally present in commercial corn oil significantly reduce cholesterol absorption in humans. Am J Clin Nutr. 2002;75:1000–1004. http://ajcn.nutrition.org/content/75/6/1000.long[↩][↩]
- Berger A, Gremaud G, Baumgartner M, Rein D, Monnard I, Kratky E, Geiger W, Burri J, Dionisi F, Allan M, Lambelet P. Cholesterol-lowering properties of amaranth grain and oil in hamsters. Int J Vitam Nutr Res. 2003;73:39–47. https://www.ncbi.nlm.nih.gov/pubmed/12690910[↩]
- Berger A, Monnard I, Dionisi F, Gumy D, Lambelet P, Hayes KC. Preparation of amaranth flakes, crude oils, and refined oils for evaluation of cholesterol-lowering properties in hamster. Food Chem. 2003;81:119–124. doi: 10.1016/S0308-8146(02)00387-4.[↩]
- Trautwein EA, Schulz C, Rieckhoff D, Kunath-Rau A, Erbersdobler HF, de Groot WA, Meijer GW. Effect of esterified 4-desmethylsterols and -stanols or 4,4′-dimethylsterols on cholesterol and bile acid metabolism in hamsters. Br J Nutr. 2002;87:227–238. doi: 10.1079/BJNBJN2001509. https://www.ncbi.nlm.nih.gov/pubmed/12064331[↩]
- Kritchevsky D, Tepper SA, Wright S, Czarnecki SK, Wilson TA, Nicolosi RJ. Cholesterol vehicle in experimental atherosclerosis 24: avocado oil. J Am Coll Nutr. 2003;22:52–55. https://www.ncbi.nlm.nih.gov/pubmed/12569114[↩]
- Perona JS, Canizares J, Montero E, Sanchez-Dominguez JM, Ruiz-Gutierrez V. Plasma lipid modifications in elderly people after administration of two virgin olive oils of the same variety (Olea europaea var. hojiblanca) with different triacylglycerol composition. Br J Nutr. 2003;89:819–826. doi: 10.1079/BJN2003852. https://www.ncbi.nlm.nih.gov/pubmed/12828801[↩]
- Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003;133:1060–1063. http://jn.nutrition.org/content/133/4/1060.long[↩]
- Khallouki F, Younos C, Soulimani R, Oster T, Charrouf Z, Spiegelhalder B, Bartsch H, Owen RW. Consumption of argan oil (Morocco) with its unique profile of fatty acids, tocopherols, squalene, sterols and phenolic compounds should confer valuable cancer chemopreventive effects. Eur J Cancer Prev. 2003;12:67–75. doi: 10.1097/00008469-200302000-00011. https://www.ncbi.nlm.nih.gov/pubmed/12548113[↩]
- Racette SB, Spearie CA, Phillips KM, Lin X, Ma L, Ostlund RE. Phytosterol-deficient and high-phytosterol diets developed for controlled feeding studies. Journal of the American Dietetic Association. 2009;109(12):2043-2051. doi:10.1016/j.jada.2009.09.009. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2833354/[↩]
- Ostlund R E, Jr, Racette SB, Stenson WF. Inhibition of cholesterol absorption by phytosterol-replete wheat germ compared with phytosterol-depleted wheat germ. Am J Clin Nutr. 2003;77:1385–1389. http://ajcn.nutrition.org/content/77/6/1385.long[↩][↩]
- Gremaud G, Piguet C, Baumgartner M, Pouteau E, Decarli B, Berger A, Fay LB. Simultaneous assessment of cholesterol absorption and synthesis in humans using on-line gas chromatography/combustion and gas chromatography/pyrolysis/isotope-ratio mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:1207–1213. doi: 10.1002/rcm.365. https://www.ncbi.nlm.nih.gov/pubmed/11445904[↩]
- Gremaud G, Dalan E, Piguet C, Baumgartner M, Ballabeni P, Decarli B, Leser ME, Berger A, Fay LB. Effects of non-esterified stanols in a liquid emulsion on cholesterol absorption and synthesis in hypercholesterolemic men. Eur J Nutr. 2002;41:54–60. doi: 10.1007/s003940200008. https://www.ncbi.nlm.nih.gov/pubmed/12083314[↩]
- Mensink RP, Ebbing S, Lindhout M, Plat J, van Heugten MMA. Effects of plant stanol esters supplied in low-fat yoghurt on serum lipids and lipoproteins, non-cholesterol sterols and fat soluble antioxidant concentrations. Atherosclerosis. 2002;160:205–213. doi: 10.1016/S0021-9150(01)00562-7. https://www.ncbi.nlm.nih.gov/pubmed/11755939[↩]
- Thomsen AB, Hansen HB, Christiansen C, Green H, Berger A. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. Eur J Clin Nutr. 2004. https://www.ncbi.nlm.nih.gov/pubmed/15164106[↩]
- Pouteau E, Monnard I, Piguet-Welsch C, Groux MJA, Sagalowicz L, Berger A. Non-esterified plant sterols solubilized in low fat milks inhibit cholesterol absorption: a stable isotope double-blind crossover study. Eur J Nutr. 2003;42:154–164. https://www.ncbi.nlm.nih.gov/pubmed/12811473[↩]
- Richelle M, Enslen M, Hager C, Groux M, Tavazzi I, Godin JP, Berger A, Métairon S, Quaile S, Piguet C, Sagalowicz L, Green H, Fay LB. Both free and esterified plant sterols decrease the bioavailability of β-carotene and β-tocopherol, in normocholesterolemic humans. Am J Clin Nutr. 2004. p. http://ajcn.nutrition.org/content/80/1/171.long[↩][↩]
- De Graaf J, De Sauvage Nolting PR, Van Dam M, Belsey EM, Kastelein JJ, Pritchard PH, Stalenhoef AF. Consumption of tall oil-derived phytosterols in a chocolate matrix significantly decreases plasma total and low-density lipoprotein-cholesterol levels. Br J Nutr. 2002;88:479–488. doi: 10.1079/BJN2002690. https://www.ncbi.nlm.nih.gov/pubmed/12425728[↩]
- Maki KC, Shinnick F, Seeley MA, Veith PE, Quinn LC, Hallissey PJ, Temer A, Davidson MH. Food products containing free tall oil-based phytosterols and oat beta-glucan lower serum total and LDL cholesterol in hypercholesterolemic adults. J Nutr. 2003;133:808–813. http://jn.nutrition.org/content/133/3/808.long[↩]
- Nestel P, Cehun M, Pomeroy S, Abbey M, Weldon G. Cholesterol-lowering effects of plant sterol esters and non-esterified stanols in margarine, butter and low-fat foods. Eur J Clin Nutr. 2001;55:1084–1090. doi: 10.1038/sj.ejcn.1601264. https://www.ncbi.nlm.nih.gov/pubmed/11781675[↩][↩]
- Carr TP, Cornelison RM, Illston BJ, Stuefer-Powell CL, Gallaher DD. Plant sterols alter bile acid metabolism and reduce cholesterol absorption in hamsters fed a beef-based diet . Nutr Res. 2002;22:745–754. doi: 10.1016/S0271-5317(02)00389-5.[↩]
- Spilburg CA, Goldberg AC, McGill JB, Stenson WF, Racette SB, Bateman J, McPherson TB, Ostlund R E, Jr. Fat-free foods supplemented with soy stanol-lecithin powder reduce cholesterol absorption and LDL cholesterol. J Am Diet Assoc. 2003;103:577–581. doi: 10.1053/jada.2003.50110. https://www.ncbi.nlm.nih.gov/pubmed/12728215[↩][↩]
- Devaraj S, Jialal I, Vega S. Plant sterol-fortified orange juice effectively lowers cholesterol levels in mildly hypercholesterolemic healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:e25. doi: 10.1161/01.ATV.0000120784.08823.99. http://atvb.ahajournals.org/content/24/3/e25.long[↩]
- Jenkins DJ, Kendall CW, Marchie A, Faulkner D, Vidgen E, Lapsley KG, Trautwein EA, Parker TL, Josse RG, Leiter LA, Connelly PW. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism. 2003;52:1478–1483. doi: 10.1016/S0026-0495(03)00260-9. https://www.ncbi.nlm.nih.gov/pubmed/14624410[↩]
- Tikkanen MJ, Hogstrom P, Tuomilehto J, Keinanen-Kiukaanniemi S, Sundvall J, Karppanen H. Effect of a diet based on low-fat foods enriched with nonesterified plant sterols and mineral nutrients on serum cholesterol. Am J Cardiol. 2001;88:1157–1162. doi: 10.1016/S0002-9149(01)02053-7. https://www.ncbi.nlm.nih.gov/pubmed/11703963[↩]
- Vaskonen T, Mervaala E, Krogerus L, Karppanen H. Supplementation of plant sterols and minerals benefits obese zucker rats fed an atherogenic diet. J Nutr. 2002;132:231–237. https://www.ncbi.nlm.nih.gov/pubmed/11823583[↩]
- Vaskonen T, al. et. Effects of calcium and plant sterols on serum lipids in obese Zucker rats on a low-fat diet. Br J Nutr. 2002;87:239–246. doi: 10.1079/BJNBJN2001508. https://www.ncbi.nlm.nih.gov/pubmed/12064332[↩]
- Manhas A, Farmer JA. Hypolipidemic therapy and cholesterol absorption. Curr Atheroscler Rep. 2004;6:89–93. https://www.ncbi.nlm.nih.gov/pubmed/15023291[↩]
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Comparison of Intensive and Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. N Engl J Med. 2004;350:15. doi: 10.1056/NEJMoa040583. http://www.nejm.org/doi/full/10.1056/NEJMoa040583[↩]
- FDA Food labeling: health claims; plant sterol/stanol esters and coronary heart disease. Food and Drug Admin Fed . https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm277265.pdf[↩]
- Food Standards Australia and New Zealand. Systematic Review of the Evidence for a Relationship between Phytosterols and Blood Cholesterol. http://www.foodstandards.gov.au/publications/Documents/EU%20health%20claims%20reviews/Systematic%20review%20phytosterols%20and%20cholesterol.pdf[↩]
- Amir Shaghaghi M, Abumweis SS, Jones PJ. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta-analysis. J Acad Nutr Diet. 2013 Nov;113(11):1494-503. doi: 10.1016/j.jand.2013.07.006. https://www.ncbi.nlm.nih.gov/pubmed/24144075[↩]
- Christiansen LI, Lähteenmäki PLA, Mannelin MR, Seppänen-Laakso TE, Hiltunen RVK, Yliruusi JK. Cholesterol-lowering effect of spreads enriched with microcrystalline plant sterols in hypercholesterolemic subjects. Eur J Nutr. 2001;40:66–73. doi: 10.1007/s003940170017. https://www.ncbi.nlm.nih.gov/pubmed/11518201[↩][↩]
- Davidson MH, Maki KC, Umporowicz DM, Ingram KA, Dicklin MR, Schaefer E, Lane RW, McNamara JR, Ribaya-Mercado JD, Perrone G, Robins SJ, Franke WC. Safety and tolerability of esterified phytosterols administered in reduced-fat spread and salad dressing to healthy adult men and women. J Am Coll Nutr. 2001;20:307–319. https://www.ncbi.nlm.nih.gov/pubmed/11506058[↩][↩]
- Hendriks HF, Brink EJ, Meijer GW, Princen HM, Ntanios FY. Safety of long-term consumption of plant sterol esters-enriched spread. Eur J Clin Nutr. 2003;57:681–692. doi: 10.1038/sj.ejcn.1601598. https://www.ncbi.nlm.nih.gov/pubmed/12771969[↩][↩]
- Westrate JA, Meijer GW. Plant sterol-enriched margarines and reduction of plasma total and LDL-cholesterol concentrations in normocholesterolaemic and midly hypercholesterolaemic subjects. Eur J Clin Nutr. 1998;52:334–343. doi: 10.1038/sj.ejcn.1600559. https://www.ncbi.nlm.nih.gov/pubmed/9630383[↩][↩]
- Maki KC, Davidson MH, Umporowicz DM, Schaefer EJ, Dicklin MR, Ingram KA, Chen S, McNamara JR, Gebhart BW, Ribaya-Mercado JD, Perrone G, Robins SJ, Franke WC. Lipid responses to plant-sterol-enriched reduced-fat spreads incorporated into a National Cholesterol Education Program Step I diet. Am J Clin Nutr. 2001;74:33–43. http://ajcn.nutrition.org/content/74/1/33.long[↩][↩]
- Plat J, Mensink RP. Effects of plant stanol esters on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase mRNA expression in mononuclear blood cells of healthy men and women. FASEB J. 2002;16:258–260. doi: 10.1096/fj.01-0718hyp. https://www.ncbi.nlm.nih.gov/pubmed/11772951[↩]
- Hallikainen MA, Sarkkinen ES, Uusitupa MIJ. Effects of low-fat stanol ester enriched margarines on concentrations of serum carotenoids in subjects with elevated serum cholesterol concentrations. Eur J Clin Nutr. 1999;53:966–969. doi: 10.1038/sj.ejcn.1600882. https://www.ncbi.nlm.nih.gov/pubmed/10602355[↩]
- Ntanios FY, Duchateau GS. A healthy diet rich in carotenoids is effective in maintaining normal blood carotenoid levels during the daily use of plant sterol-enriched spreads. Int J Vitam Nutr Res. 2002;72:32–39. https://www.ncbi.nlm.nih.gov/pubmed/11887750[↩]
- Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Jones PJ. No changes in serum fat-soluble vitamin and carotenoid concentrations with the intake of plant sterol/stanol esters in the context of a controlled diet. Metabolism. 2002;51:652–656. doi: 10.1053/meta.2002.32021. https://www.ncbi.nlm.nih.gov/pubmed/11979401[↩][↩]
- Nissinen M, Gylling H, Vuoristo M, Miettinen TA. Micellar distribution of cholesterol and phytosterols after duodenal plant stanol ester infusion. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1009–10015. http://ajpgi.physiology.org/content/282/6/G1009.long[↩]
- Noakes M, Clifton P, Ntanios F, Shrapnel W, Record I, McInerney J. An increase in dietary carotenoids when consuming plant sterols or stanols is effective in maintaining plasma carotenoid concentrations. Am J Clin Nutr. 2002;75:79–86. http://ajcn.nutrition.org/content/75/1/79.long[↩]
- Rader DJ, Cohen J, Hobbs HH. Monogenic hypercholesterolemia: new insights in pathogenesis and treatment. Journal of Clinical Investigation 2003;111(12):1795-1803. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC161432/[↩]
- Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. Familial Hypercholesterolemias:prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. Journal of Clinical Lipidology 2011;5(3 suppl):S9–S17.[↩]
- Austin MA, Hutter CM, Zimmern RL, Humphries SE. Familial hypercholesterolemia and coronary heart disease: a HuGE association review. American Journal of Epidemiology 2004;160(5):421-9. https://www.ncbi.nlm.nih.gov/pubmed/15321838[↩]
- Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial Hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients. Journal of Clinical Lipidology 2011;5(3 suppl):51-8.[↩]
- Scientific Steering Committee on behalf of the Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ 1991;303(6807):893-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1671226/pdf/bmj00148-0031.pdf[↩]
- Marais AD. Familial hypercholesterolaemia. Clinical Biochemist Reviews 2004;25(1):49-68. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1853359/[↩]
- Wray R, Neil H, Rees J. Screening for hyperlipidaemia in childhood. Recommendations of the British Hyperlipidaemia Association. Journal of Royal College of Physicians London 1996;30(2):115-8.[↩]
- Marks D, Thorogood M, Neil AW, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis 2003;168(1):1-14. https://www.ncbi.nlm.nih.gov/pubmed/12732381[↩]
- Avis HJ, Vissers MN, Stein EA, Wijburg FA, Frip MD, Kastelein JJ, et al. A systematic review and meta-analysis of statin therapy in children with familial hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology 2007;27(8):1803-10. http://atvb.ahajournals.org/content/27/8/1803.long[↩]
- Shafiq N, Bhasin B, Pattanaik S, Pandhi P, Venkateshan SP, Singh M, et al. A meta-analysis to evaluate the efficacy of statins in children with familial hypercholesterolemia. International Journal of Clinical Pharmacology and Therapeutics 2007;45(10):548-55. https://www.ncbi.nlm.nih.gov/pubmed/17966840[↩]
- Yeste D, Chacon P, Clemente M, Albisu MA, Gussinye M, Carracosa A. Ezetimibe as monotherapy in the treatment of hypercholesterolemia in children and adolescents. Journal of Pediatric Endocrinology and Metabolism 2009;22(6):487-92.[↩]
- Tonstad S. A rational approach to treating hypercholesterolaemia in children. Weighing the risks and benefits. Drug safety 1997;16(5):330-41. https://www.ncbi.nlm.nih.gov/pubmed/9187532[↩]
- The Cochrane Library 10 June 2014. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD001918.pub3/full[↩]
- American Heart Association. Familial Hypercholesterolemia (FH). http://www.heart.org/HEARTORG/Conditions/Cholesterol/CausesofHighCholesterol/Familial-Hypercholesterolemia-FH_UCM_493541_Article.jsp[↩][↩]
- Mapping a gene involved in regulating dietary cholesterol absorption. The sitosterolemia locus is found at chromosome 2p21. Patel SB, Salen G, Hidaka H, Kwiterovich PO, Stalenhoef AF, Miettinen TA, Grundy SM, Lee MH, Rubenstein JS, Polymeropoulos MH, Brownstein MJ. J Clin Invest. 1998 Sep 1; 102(5):1041-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC508970/[↩]
- Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Science. 2000 Dec 1; 290(5497):1771-5. https://www.ncbi.nlm.nih.gov/pubmed/11099417/[↩]
- Wang J, Mitsche MA, Luetjohann D, Cohen JC, Xie XS, Hobbs HH. J Lipid Res 2014.[↩][↩]
- Klett EL, Patel S. Curr Opin Lipidol. 2003;14:341–345. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1201437/[↩]
- Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. J Lipid Res. 1992;33:945–957. http://www.jlr.org/content/33/7/945.long[↩][↩]
- Lee MH, Lu K, Patel SB. Curr Opin Lipidol. 2001;12:141–149. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1350992/[↩]
- Rao MKG, Perkins EG, Connor WE, Bhattacharyya AK. Lipids. 1975;10:566–568. https://www.ncbi.nlm.nih.gov/pubmed/1177671[↩]
- Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Arterioscler Thromb. 1992;12:563–568. http://atvb.ahajournals.org/content/12/5/563.long[↩]
- Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Nat Genet. 2001;27:79–83. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1350991/[↩]
- Park JH, Chung IH, Kim DH, Choi MH, Garg A, Yoo EG. J Clin Endocrin Metab. 2014;99:1512–1518. https://www.ncbi.nlm.nih.gov/pubmed/24423340[↩]
- Sudhop T, von Bergmann K. Z Kardiol. 2004;93:921–928. https://www.ncbi.nlm.nih.gov/pubmed/15599566[↩]
- Ajagbe BO, Othman RA, Myrie SB. Plant Sterols, Stanols, and Sitosterolemia. Journal of AOAC International. 2015;98(3):716-723. doi:10.5740/jaoacint.SGEAjagbe. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4514516/[↩]