Immune system
The immune system is made up of a complex network of cells, chemicals, tissues, organs, and the substances they make that helps your body fight infections and other diseases. The immune system includes white blood cells and organs and tissues of the lymph system, such as the thymus, spleen, tonsils, lymph nodes, lymph vessels, and bone marrow (see Figures 1 and 2 below). The immune system must recognize foreign invaders and abnormal cells (Table 1) and distinguish them from the body’s healthy cells. An underactive or overactive immune system can cause health issues. For example, autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis, happen when the body mounts an immune response against its own tissues instead of a foreign invader. In addition, allergies occur when an individual’s immune system reacts to substances in the environment that are tolerated by most people. Underactivity of the immune system or immunodeficiency, can increase your risk of infection. You may be born with an immunodeficiency (known as primary immunodeficiency or inborn errors of immunity), or acquire it from a medical treatment or another disease (known as secondary immunodeficiency). Primary immunodeficiency are a group of more than 450 rare, chronic conditions in which part of the body’s immune system is missing or does not function correctly. Primary immunodeficiencies are caused by hereditary genetic defects and can affect anyone, regardless of age, gender, or ethnicity 1. Because the most important function of the immune system is to protect against infection, children and adults with primary immunodeficiency commonly experience increased susceptibility to infection. The infections may be in the skin, sinuses, throat, ears, lungs, brain or spinal cord, or in the urinary or intestinal tracts. Increased susceptibility to infection may show up as repeated infections, infections that are unusually hard to cure or unusually severe infections (requires hospitalization or intravenous antibiotics).
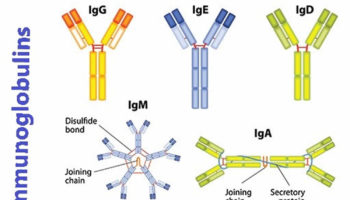
The immune system is constantly working to protect your body from infections, injury, cancers and diseases. The immune system recognizes invaders such as bacteria, viruses and fungi as well as abnormal cells. It mounts an immune response to help the body fight the invasion. When harmful microbes (tiny particles) enter and invade your body, the body produces white blood cells to fight the infection. The white blood cells identify the microbe, produce antibodies (immunoglobulins or or gamma-globulins) to fight it, and help other immune responses to occur. They also ‘remember’ the attack. There are five major classes of antibodies (IgG, IgA, IgM, IgD, and IgE). IgG has four different subclasses (IgG1, IgG2, IgG3, IgG4). IgA has two subclasses (IgA1 and IgA2). Antibodies of the IgA class are produced near mucus membranes and find their way into secretions such as tears, intestines, bile, saliva and mucus, where they protect against infection in the respiratory tract and intestines. Antibodies of the IgM class are the first antibodies formed in response to infection. They are important in protection during the early days of an infection. Antibodies of the IgE class are responsible for allergic reactions. IgD is expressed on mature B cells along with IgM and may play some role in helping B cells differentiate into plasma cells. Recently, studies have suggested that IgD may be important in the gut homeostasis by binding to mast cells and basophils to react against pathogenic bacteria in the gut.
Antibodies protect the body against infection in a number of different ways. For example, some microorganisms, such as viruses, must attach to body cells before they can cause an infection, but antibodies bound to the surface of a virus can interfere with the virus’ ability to attach to the host cell. In addition, antibodies attached to the surface of some microorganisms can cause the activation of a group of proteins called the complement system that can directly kill some bacteria. Antibody-coated bacteria are also much easier for neutrophils to ingest and kill than bacteria that are not coated with antibodies. All of these actions of antibodies prevent microorganisms from successfully invading body tissues and causing serious infections.
The immune response is split into two functional divisions: innate and acquired immunity.
- Innate immunity is the first line of defense against foreign invaders. Innate immunity involves immediate, nonspecific responses to pathogens.
- Acquired immunity also called adaptive immunity, is the second line of defense against foreign invaders. Acquired immunity involves a complex, targeted response to a specific pathogen. Exposure to a pathogen stimulates the production of certain immune cells that mark the pathogen for destruction. Upon first exposure, it takes several days or weeks to develop the acquired immune response, but the involved immune cells “remember” the encounter and respond quickly upon subsequent exposure to the same pathogen.
The components of the innate and acquired immune systems communicate and work together to protect your body from infection and disease (Table 2).
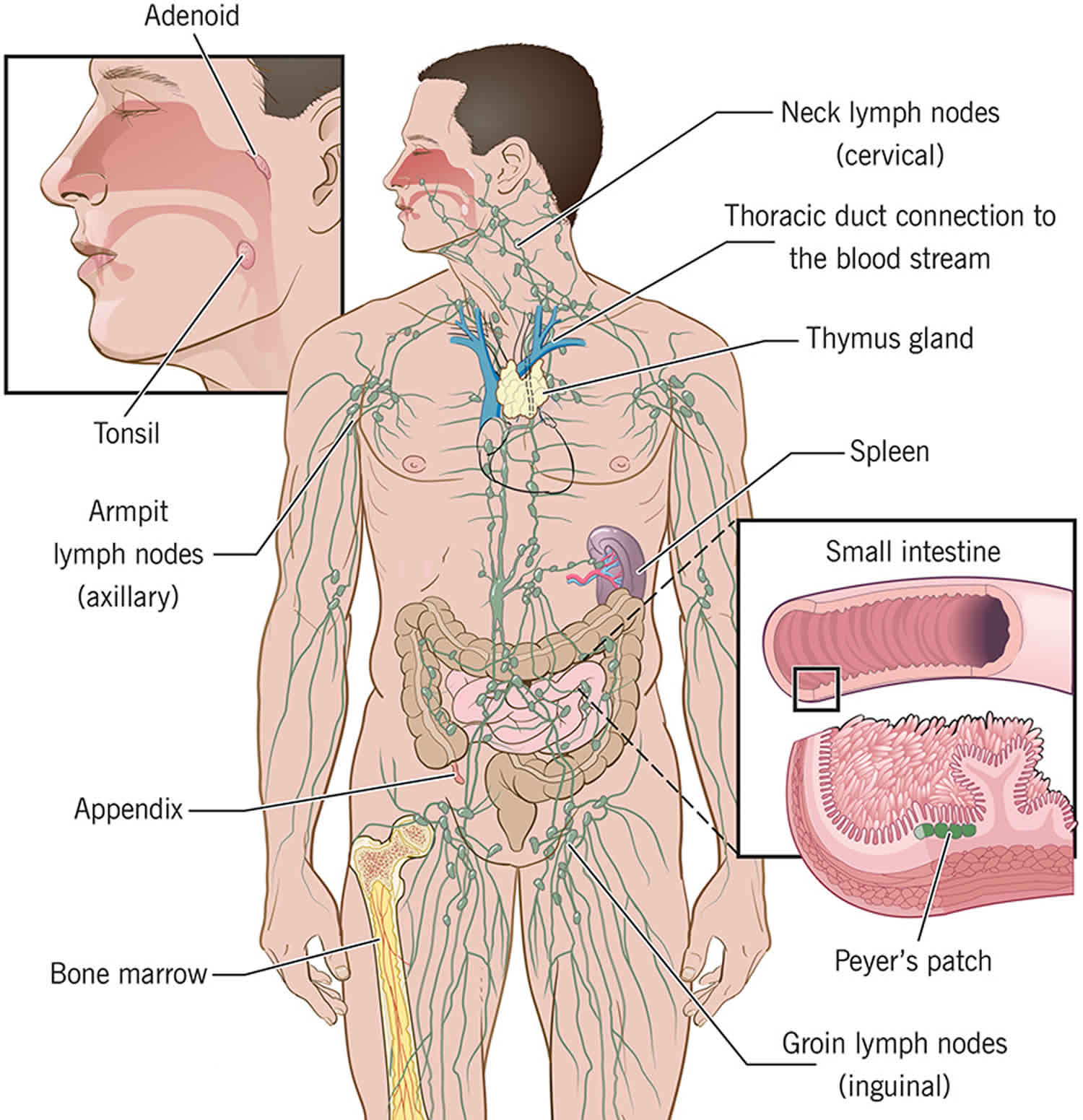
Major organs of the Immune System (Figure 1):
- Thymus: The thymus is an organ located in the upper chest where T cells mature. First, lymphocytes (a type of white blood cell) that are destined to become T cells leave the bone marrow and find their way to the thymus where they are then “educated” to become mature T cells.
- Liver: The liver is the major organ responsible for producing proteins of the complement system. In addition, it contains large numbers of phagocytic cells (a specific type of white blood cell) that ingest bacteria in the blood as it passes through the liver.
- Bone Marrow: The bone marrow is the location where all cells of the immune system begin their development from stem cells.
- Tonsils: Tonsils are collections of lymphocytes in the throat.
- Lymph Nodes: Lymph nodes are collections of B cells and T cells throughout the body. Cells congregate in lymph nodes to communicate with each other. Lymph nodes can become swollen when they are fighting an infection.
- Spleen: The spleen is a collection of B cells, T cells, and monocytes. It serves to filter the blood and provide a site for invaders/germs and cells of the immune system to interact.
- Blood: Blood is contained within the circulatory system that carries cells and proteins of the immune system from one part of the body to another.
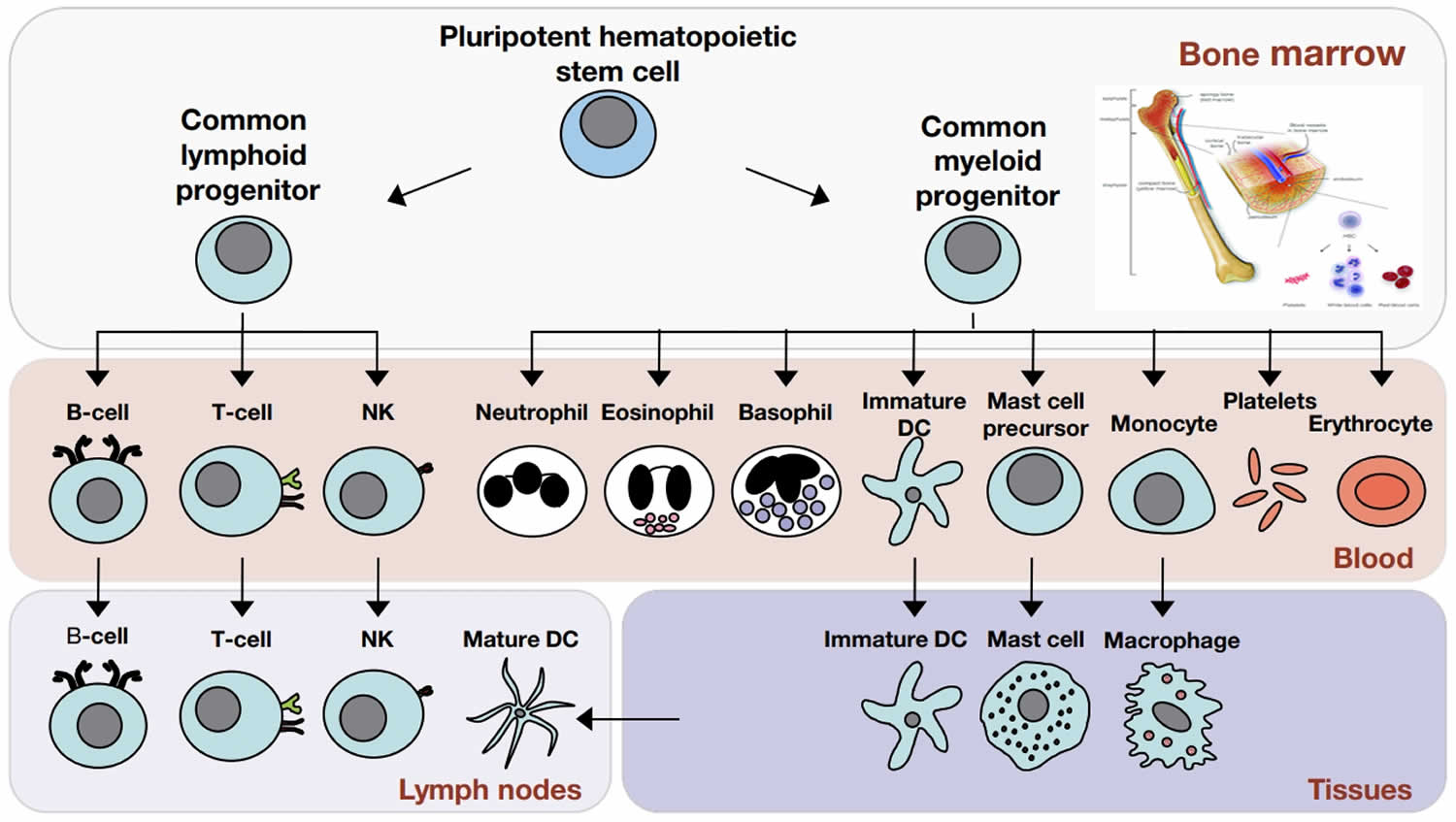
The cells that are involved in the immune system (Figure 2):
- Granulocytes include basophils, eosinophils, and neutrophils. Basophils and eosinophils are important for host defense against parasites. They also are involved in allergic reactions.
- Neutrophils (also known as polymorphonuclear cells, PMNs or granulocytes), the most numerous innate immune cell (the most numerous of all the types of white blood cells), patrol for problems by circulating in the bloodstream. Neutrophils or polymorphonuclear leukocytes are found in the bloodstream and can migrate into sites of infection within a matter of minutes. These cells, like the other cells in the immune system, develop from hematopoietic stem cells in the bone marrow. Neutrophils can phagocytose, or ingest, bacteria, degrading them inside special compartments called vesicles. Neutrophils increase in number in the bloodstream during infection and are in large part responsible for the elevated white blood cell count seen with some infections. They are the cells that leave the bloodstream and accumulate in the tissues during the first few hours of an infection and are responsible for the formation of pus. Their major role is to ingest bacteria or fungi and kill them. Their killing strategy relies on ingesting the infecting organisms in specialized pockets within the cell. Neutrophils contain toxic chemicals that fuse with the bacteria-containing pockets to kill the bacteria. Neutrophils have little role in the defense against viruses.
- Monocytes, which develop into macrophages, also patrol and respond to problems. Monocytes make up 5 to 10% of the white blood cells. They also line the walls of blood vessels in organs like the liver and spleen where they capture microorganisms in the blood as they pass by. When monocytes leave the bloodstream and enter the tissues, they change shape and size and become macrophages. Macrophages, “big eater” in Greek, are named for their ability to ingest and degrade bacteria. Macrophages are essential for killing fungi and the class of bacteria to which tuberculosis belongs (mycobacteria). Like neutrophils, macrophages ingest microbes and deliver toxic chemicals directly to the foreign invader to kill it. Macrophages live longer than neutrophils and are especially important for slow growing or chronic infections. Macrophages can be influenced by T cells and often collaborate with T cells in killing microorganisms. Macrophages also have important non-immune functions, such as recycling dead cells, like red blood cells, and clearing away cellular debris. These “housekeeping” functions occur without activation of an immune response. Upon activation, monocytes and macrophages coordinate an immune response by notifying other immune cells of the problem.
- B cells (B lymphocytes) and often named on lab reports as CD19 or CD20 cells: These lymphocytes arise in the bone marrow from stem cells. When B cells encounter foreign germs (antigens), they respond by maturing into another cell type called plasma cells. Plasma cells are the mature B cells (mature B lymphocytes) that actually produce the antibodies (also known as immunoglobulins or gamma-globulins) and are located in the spleen and lymph nodes throughout the body. B cells can also mature into memory cells, which allows a rapid response if the same infection is encountered again. The long life of plasma cells enables your body to retain immunity to viruses and bacteria that infected you many years ago. For example, once you have been fully immunized with live vaccine strains of measles virus, you will almost never catch it because your body retain the plasma cells and antibodies for many years and these antibodies prevent infection.
- T cells sometimes called T lymphocytes and often named in lab reports as CD3 cells, are another type of immune cell. Some T cells directly attack cells infected with viruses, and others act as regulators of the immune system. Each T cell reacts with one specific antigen, just as each antibody molecule reacts with one specific antigen. In fact, T cells have molecules on their surfaces that are similar to antibodies. The variety of different T cells is also so extensive that the body has T cells that can react against virtually any antigen. T cells have different abilities to recognize antigen and are varied in their function. There are killer or cytotoxic T cells (often denoted in lab reports as CD8 T cells), helper T cells (often denoted in lab reports as CD4 T cells), and regulatory T cells. Each has a different role to play in the immune system.
- Cytotoxic T cells (CD8+ T cells or Killer T cells): These lymphocytes mature in the thymus and are responsible for killing cells infected with viruses. Killer T cells protect the body from certain bacteria and viruses that have the ability to survive and even reproduce within the body’s own cells. The killer T cell must migrate to the site of infection and directly bind to its target to ensure its destruction. In addition to fighting germs, killer T cells also recognize and respond to foreign tissues in the body, such as a transplanted kidney. When T cells are fighting infections, they grow and divide, making more T cells.
- Helper T cells (CD4+ T-cell): Helper T cells assist B cells to produce antibodies and assist killer T cells in their attack on foreign substances.
- Regulatory T cells. Regulatory T cells suppress or turn off the T cells when an infection is controlled and they are no longer needed. Regulatory T cells act as the thermostat of the lymphocyte system to keep it turned on just enough—not too much and not too little. Without regulatory T cells, the immune system would keep working even after an infection has been treated. Without regulatory T cells, there is the potential for the body to overreact to the infection.
- Plasma cells: These cells develop from B cells (B lymphocytes) and are the cells that make immunoglobulin (antibodies).
- Mast cells also are important for defense against parasites. Mast cells are found in tissues and can mediate allergic reactions by releasing inflammatory chemicals like histamine.
- Dendritic cells (DC) also known as antigen-presenting cells (APCs), instruct T cells on what to attack. Dendritic cells (DC) also can develop from monocytes. Antigens are molecules from pathogens, host cells, and allergens that may be recognized by adaptive immune cells. APCs like DCs are responsible for processing large molecules into “readable” fragments (antigens) recognized by adaptive B or T cells. However, antigens alone cannot activate T cells. They must be presented with the appropriate major histocompatiblity complex (MHC) expressed on the APC. MHC provides a checkpoint and helps immune cells distinguish between host and foreign cells.
- Natural killer (NK) cells have features of both innate and adaptive immunity. They are important for recognizing and killing virus-infected cells or tumor cells. Natural killer (NK) cells are so named because they easily kill cells infected with viruses. They are said to be natural killer cells as they are always ready to fight and do not require the same thymus education that T cells require. NK cells are derived from the bone marrow and are present in relatively low numbers in the bloodstream and in tissues. They are important in defending against viruses and possibly preventing cancer as well. They contain intracellular compartments chemicals called cytotoxic granules, which are filled with proteins that can form holes in the target cell and also cause apoptosis, the process for programmed cell death. NK cells kill virus-infected cells by injecting them with a killer potion of chemicals called cytotoxic granules. It is important to distinguish between apoptosis and other forms of cell death like necrosis. Apoptosis, unlike necrosis, does not release danger signals that can lead to greater immune activation and inflammation. Through apoptosis, immune cells can discreetly remove infected cells and limit bystander damage. NK cells are particularly important in the defense against herpes viruses. This family of viruses includes the traditional cold sore form of herpes (herpes simplex) as well as Epstein-Barr virus (the cause of infectious mononucleosis or mono) and the varicella virus (the cause of chickenpox and shingles). Recently, researchers have shown in mouse models that NK cells, like adaptive cells, can be retained as memory cells and respond to subsequent infections by the same pathogen.
The immune system relies on an adequate supply of nutrients for its baseline functions as well as for ramping up its activity when necessary 2, 3, 4, 5, 6, 7. It is well established that malnutrition (protein-energy malnutrition and obesity) and deficiencies in one or more micronutrients (vitamins and nutritionally essential minerals) diminish immune function. In most instances, correcting the nutrient deficiency restores the affected immune functions. At a minimum, getting the recommended dietary allowance (RDA) for vitamin C and vitamin D is necessary for the immune system to function properly; there is some evidence that intakes above the current RDA (recommended dietary allowance) for these vitamins may be of further benefit 8. Because supplementation with iron can have unwanted side effects in those with preexisting infections, especially malaria, routine iron supplementation should be accompanied by malaria detection and treatment strategies 8. The long-chain Omega-3 Polyunsaturated Fatty Acids (PUFA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have potent anti-inflammatory effects, especially in individuals with chronic or acute inflammation 8. Increasing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) consumption increases the EPA and DHA content of immune cell membranes, mainly by displacing the long-chain Omega-6 Polyunsaturated Fatty Acids (PUFA) arachidonic acid and becoming the substrate for the enzymes that synthesize eicosanoids. EPA and DHA also give rise to anti-inflammatory compounds that “turn off” the inflammatory response. In chronic inflammatory states (e.g., rheumatoid arthritis, atherosclerosis), eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) supplements have been shown to reduce symptoms of rheumatoid arthritis and the risk of cardiac events. However, the dose of EPA plus DHA that is optimal for immune function in healthy individuals is not yet established. Ingestion of at least 500 mg/day of EPA plus DHA is recommended by the International Society for the Study of Fatty Acids and Lipids 9 and the American Heart Association recommends eating one to two servings of seafood per week to reduce your risk of some heart problems, especially if you consume the seafood in place of less healthy foods 10. For people with heart disease, the American Heart Association recommends consuming about 1 g per day EPA plus DHA, preferably from oily fish, but supplements are an option under the guidance of a health care provider 11. The AHA does not recommend omega-3 supplements for people who do not have a high risk of cardiovascular disease 12. The Linus Pauling Institute recommends that generally healthy adults eat fish twice weekly, which provides approximately 500 mg/day of EPA plus DHA; for those who do not regularly consume fish, consider taking a two-gram fish oil supplement several times a week in consultation with a physician 8. Higher daily intakes may be recommended for the treatment of specific disorders.
Table 1. The Immune System responds to Foreign Invaders and Abnormal Cells
| Foreign Invaders | Abnormal Cells |
|---|---|
| Viruses | Cancer cells |
| Bacteria | |
| Parasites | |
| Mold | |
| Allergens |
Table 2. Functional Divisions and Components of the Immune Response
| Innate Immunity (general response to a pathogen) | Acquired Immunity (specific response to a pathogen) | |||
|---|---|---|---|---|
| Barriers | Cells | Humoral Factors | Cells | Humoral Factors |
| Physical: skin, mucous membranes | Phagocytes: engulf and destroy | Eicosanoids: Eicosanoids are compounds made from 20-carbon long-chain polyunsaturated fatty acids (PUFA); the term ‘eicosanoid’ includes many compounds that can either cause, prevent or regulate inflammation | T lymphocytes: detect specific pathogens, secrete cytokines, and coordinate an immune response | Antibodies: specialized proteins that mark a pathogen for destruction |
| Chemical: acidic environment of the stomach | Mast cells: cause inflammation & symptoms of allergy | Cytokines: Cytokines are proteins made by white blood cells. Cytokines regulate inflammation. They play important roles in your body’s normal immune responses and in the immune system’s ability to respond to cancer. | B lymphocytes: produce antibodies against a specific pathogen | |
| Biological: gut microbiota | Natural killer (NK) cells: release toxic chemicals | Complement proteins: attach to and destroy bacteria; some cause inflammation | Complement proteins: attach to and destroy pathogens marked by antibodies | |
Figure 1. Immune system
Figure 2. Cells of the immune system
Footnote: The cells of the immune system originate in the bone marrow from pluripotent hematopoietic stem cells. Pluripotent hematopoietic stem cells give rise to a common lymphoid progenitor, which gives rise to all of the major lymphoid cell types (T‐cells, B‐cells, and Natural killer [NK] cells) or a common myeloid progenitor, which gives rise to all of the major myeloid cell types (neutrophils, eosinophils, basophils, dendritic cells (DCs), mast cells, and monocytes/macrophages) as well as the erythrocytes and megakaryocytes (which generate platelets).
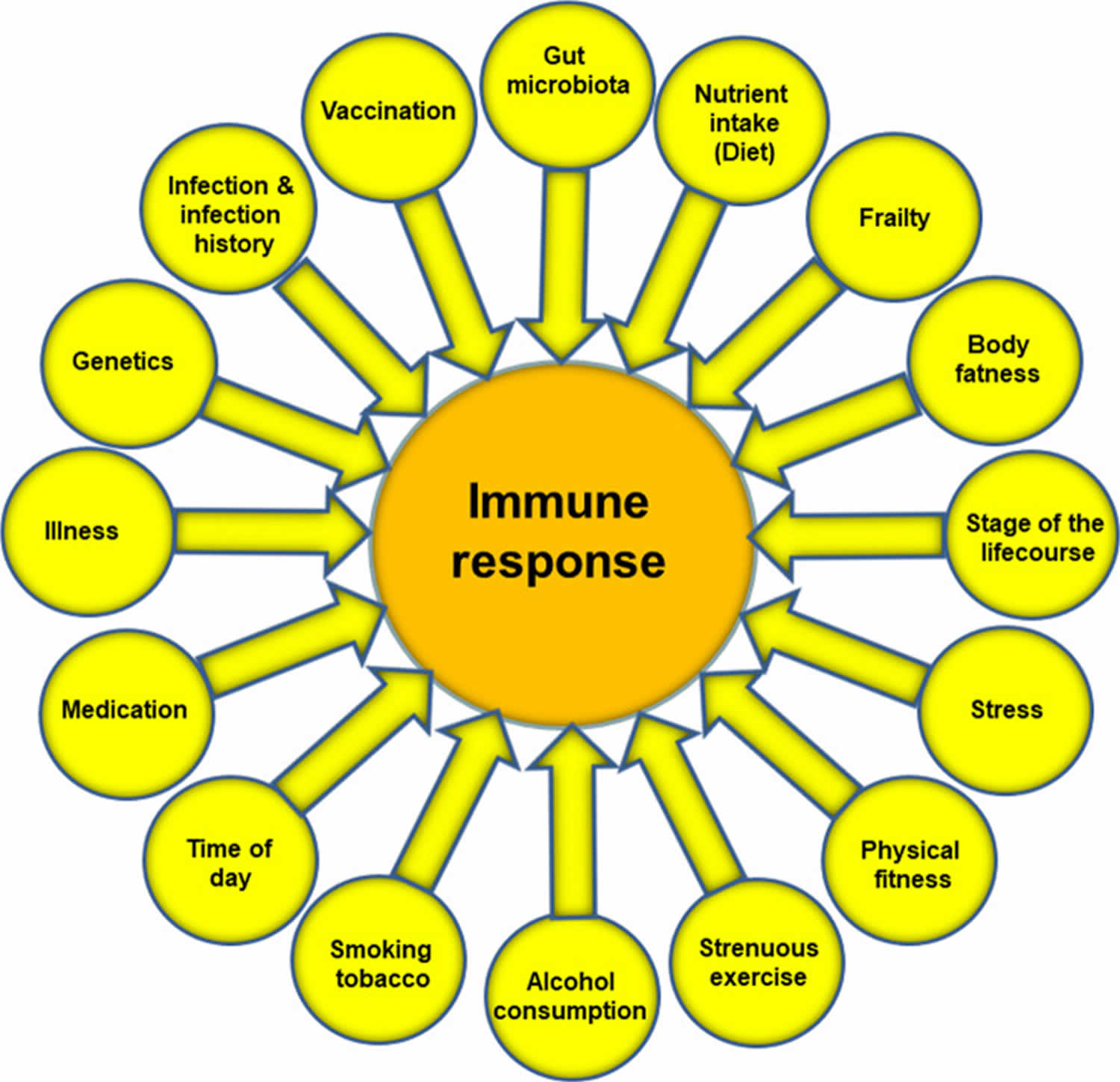
Figure 3. Factors that influence the immune response
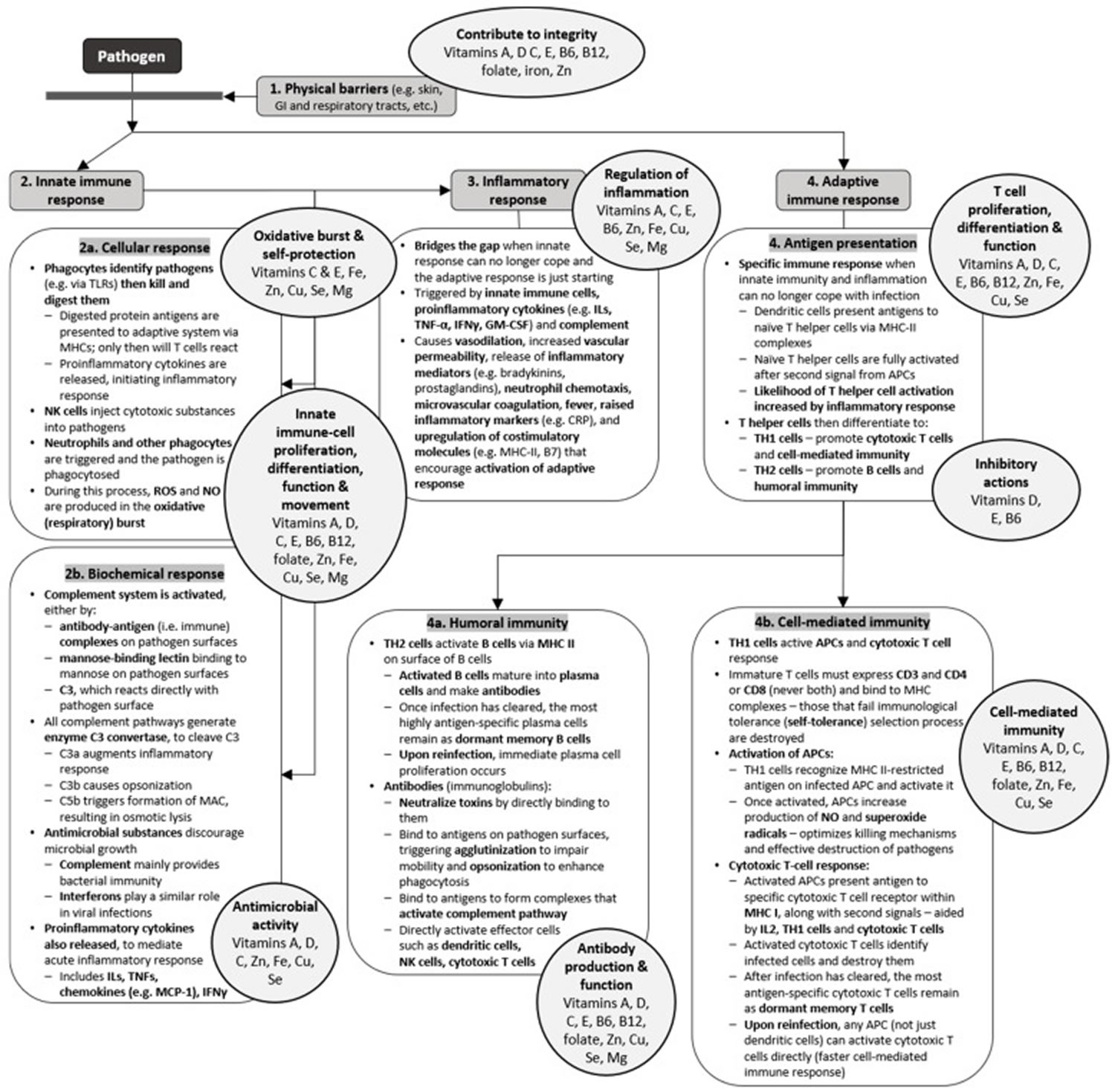
[Source 5 ]Figure 4. Vitamins and minerals for immune system
Footnotes: Vitamins and minerals have key roles at every stage of the immune response 13. This schematic summarizes important components and processes that are involved in different aspects of the innate and adaptive immune responses. The circles highlight those micronutrients that are known to affect these responses. The significant overlap between micronutrients and processes indicates the importance of multiple micronutrients in supporting proper function of the immune system.
Abbreviations: APCs = antigen-presenting cells; C3 = complement component 3; CRP = C-reactive protein; Cu = copper; Fe = iron; IFNs = interferons; Igs = immunoglobulins; ILs = interleukins; GI = gastrointestinal; GM-CSF = granulocyte-macrophage colony stimulating factor; MAC = membrane attack complex; MCP-1 = monocyte chemoattractant protein-1; Mg = magnesium; MHCs = major histocompatibility complexes; NK = natural killer; NO = nitric oxide; ROS = reactive oxygen species; Se = selenium; TLRs = toll-like receptors; TNF = tumor-necrosis factors; Zn = zinc
[Source 7 ]How does the immune system work?
The immune system involves many parts of your body. Each part plays a role in recognizing foreign microbes, communicating with other parts of your body, and working to fight the infection. Parts of the immune system are:
- Skin – The skin is usually the first line of defense against microbes. Skin cells produce and secrete important antimicrobial proteins, and immune cells can be found in specific layers of skin.
- Bone marrow – helps produce immune cells. The bone marrow contains stems cells that can develop into a variety of cell types. The common myeloid progenitor stem cell in the bone marrow is the precursor to innate immune cells—neutrophils, eosinophils, basophils, mast cells, monocytes, dendritic cells, and macrophages—that are important first-line responders to infection. The common lymphoid progenitor stem cell leads to adaptive immune cells—B cells and T cells—that are responsible for mounting responses to specific microbes based on previous encounters (immunological memory). Natural killer (NK) cells also are derived from the common lymphoid progenitor and share features of both innate and adaptive immune cells, as they provide immediate defenses like innate cells but also may be retained as memory cells like adaptive cells. B, T, and NK cells also are called lymphocytes.
- Bloodstream: Immune cells constantly circulate throughout the bloodstream, patrolling for problems. When blood tests are used to monitor white blood cells, another term for immune cells, a snapshot of the immune system is taken. If a cell type is either scarce or overabundant in the bloodstream, this may reflect a problem.
- The thymus, a small gland in your upper chest where some immune T cells mature.
- Lymphatic system is a network of of tiny vessels which allows immune cells to travel between tissues and the bloodstream. The lymphatic system contains lymphocytes (white blood cells; mostly T cells and B cells), which try to recognize any bacteria, viruses or other foreign substances in the body and fight them. They are carried in a milky fluid called lymph. Immune cells are carried through the lymphatic system and converge in lymph nodes, which are found throughout the body.
- Lymph nodes, small lumps in the groin, armpit, around the neck and elsewhere that help the lymphatic system to communicate. Lymph nodes are a communication hub where immune cells sample information brought in from the body. They can become swollen when the body mounts an immune response. For instance, if adaptive immune cells in the lymph node recognize pieces of a microbe brought in from a distant area, they will activate, replicate, and leave the lymph node to circulate and address the pathogen. Thus, doctors may check patients for swollen lymph nodes, which may indicate an active immune response.
- The spleen is an organ under the ribs behind the stomach on the left that processes information from the blood. While it is not directly connected to the lymphatic system, it is important for processing information from the bloodstream. Immune cells are enriched in specific areas of the spleen, and upon recognizing blood-borne pathogens, they will activate and respond accordingly.
- Mucous membranes, like the lining of the inside of your mouth are prime entry points for pathogens, and specialized immune hubs are strategically located in mucosal tissues like the respiratory tract and gut. For instance, Peyer’s patches are important areas in the small intestine where immune cells can access samples from the gastrointestinal tract.
Immune organs
The organ systems involved in the immune response are primarily lymphoid organs which include, spleen, thymus, bone marrow, lymph nodes, tonsils, and liver. The lymphoid organ system classifies according to the following 14:
- Primary lymphoid organs (thymus and bone marrow), where T and B cells first express antigen receptors and become mature functionally.
- Secondary lymphoid organs like the spleen, tonsils, lymph nodes, the cutaneous and mucosal immune system; this is where B and T lymphocytes recognize foreign antigens and develop appropriate immune responses.
All immune cells originate in the bone marrow, deriving from hematopoietic stem cells, but an important set of immune cells (T lymphocytes) undergo maturation in an organ known as the thymus. The thymus and bone marrow are known as primary lymphoid tissues. T lymphocytes mature in the thymus, where these cells reach a stage of functional competence while B lymphocytes mature in the bone marrow the site of generation of all circulating blood cells. Excessive release of cytokines stimulated by these organisms can cause tissue damage, such as endotoxin shock syndrome.
Secondary lymphoid tissues, namely the lymph nodes, spleen and mucosa-associated lymphoid tissues (MALT) are important sites for generating adaptive immune responses and contain the lymphocytes (key adaptive cells). The lymphatic system is a system of vessels draining fluid (derived from blood plasma) from body tissues. Lymph nodes, that house lymphocytes, are positioned along draining lymph vessels, and monitor the lymph for signs of infection. MALT tissues are important in mucosal immune responses, and reflect the particular importance of the gut and airways in immune defence. The spleen essentially serves as a ‘lymph node’ for the blood.
Immune cells communication
Immune cells communicate in a number of ways, either by cell-to-cell contact or through secreted signaling molecules. Receptors and ligands are fundamental for cellular communication.
- Receptors are protein structures that may be expressed on the surface of a cell or in intracellular compartments. The molecules that activate receptors are called ligands, which may be free-floating or membrane-bound.
- Ligand-receptor interaction leads to a series of events inside the cell involving networks of intracellular molecules that relay the message. By altering the expression and density of various receptors and ligands, immune cells can dispatch specific instructions tailored to the situation at hand.
Cytokines are small proteins with diverse functions. In immunity, there are several categories of cytokines important for immune cell growth, activation, and function.
- Colony-stimulating factors are essential for cell development and differentiation.
- Interferons (IFNs) are necessary for immune-cell activation. Type I interferons mediate antiviral immune responses, and type II interferon is important for antibacterial responses.
- Interleukins (ILs), which come in over 30 varieties, provide context-specific instructions, with activating or inhibitory responses.
- Chemokines are made in specific locations of the body or at a site of infection to attract immune cells. Different chemokines will recruit different immune cells to the site needed.
- Tumor necrosis factor (TNF) family of cytokines stimulates immune-cell proliferation and activation. They are critical for activating inflammatory responses, and as such, TNF blockers are used to treat a variety of disorders, including some autoimmune diseases.
Toll-like receptors (TLRs) are expressed on innate immune cells, like macrophages and dendritic cells. They are located on the cell surface or in intracellular compartments because microbes may be found in the body or inside infected cells. TLRs recognize general microbial patterns, and they are essential for innate immune-cell activation and inflammatory responses.
B-cell receptors (BCRs) and T-cell receptors (TCRs) are expressed on adaptive immune cells. They are both found on the cell surface, but BCRs also are secreted as antibodies to neutralize pathogens. The genes for BCRs and TCRs are randomly rearranged at specific cell-maturation stages, resulting in unique receptors that may potentially recognize anything. Random generation of receptors allows the immune system to respond to unforeseen problems. They also explain why memory B or T cells are highly specific and, upon re-encountering their specific pathogen, can immediately induce a neutralizing immune response.
Major histocompatibility complex (MHC) or human leukocyte antigen (HLA), proteins serve two general roles.
Major histocompatibility complex (MHC) proteins function as carriers to present antigens on cell surfaces. MHC class I proteins are essential for presenting viral antigens and are expressed by nearly all cell types, except red blood cells. Any cell infected by a virus has the ability to signal the problem through MHC class I proteins. In response, CD8+ T cells (also called CTLs) will recognize and kill infected cells. MHC class II proteins are generally only expressed by antigen-presenting cells like dendritic cells and macrophages. MHC class II proteins are important for presenting antigens to CD4+ T cells. MHC class II antigens are varied and include both pathogen- and host-derived molecules.
MHC proteins also signal whether a cell is a host cell or a foreign cell. They are very diverse, and every person has a unique set of MHC proteins inherited from his or her parents. As such, there are similarities in MHC proteins between family members. Immune cells use MHC to determine whether or not a cell is friendly. In organ transplantation, the MHC or HLA proteins of donors and recipients are matched to lower the risk of transplant rejection, which occurs when the recipient’s immune system attacks the donor tissue or organ. In stem cell or bone marrow transplantation, improper MHC or HLA matching can result in graft-versus-host disease, which occurs when the donor cells attack the recipient’s body.
Complement refers to a unique process that clears away pathogens or dying cells and also activates immune cells. Complement consists of a series of proteins found in the blood that form a membrane-attack complex. Complement proteins are only activated by enzymes when a problem, like an infection, occurs. Activated complement proteins stick to a pathogen, recruiting and activating additional complement proteins, which assemble in a specific order to form a round pore or hole. Complement literally punches small holes into the pathogen, creating leaks that lead to cell death. Complement proteins also serve as signaling molecules that alert immune cells and recruit them to the problem area.
Best vitamins for immune system
Through experimental research and studies of people with immune deficiencies, a number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) have been demonstrated to have key roles in supporting the human immune system and reducing risk of infections 15. Other essential nutrients including other vitamins and trace elements, amino acids and fatty acids are also important in this regard. All of the nutrients named above have roles in supporting antibacterial and antiviral defences but zinc and selenium seem to be particularly important for the latter.
Table 3. Vitamins and minerals that affect the immune system
| Name | What It Does | Where to Get It | About Supplements |
|---|---|---|---|
| Vitamin A (Retinol) and beta-carotene | Keeps skin, lungs, and stomach healthy.
| liver, whole eggs, milk, dark green, yellow, orange, and red vegetables and fruit (like spinach, pumpkin, green peppers, squash, carrots, papaya, and mangoes). Also found in orange and yellow sweet potatoes | It’s best to get vitamin A from food. Vitamin A supplements are toxic in high doses. Supplements of beta-carotene (the form of vitamin A in fruits and vegetables) have been shown to increase cancer risk in smokers. |
| Vitamin B-group Thiamin (Vitamin B1), Riboflavin (Vitamin B2), Vitamin B6, Folate (Vitamin B9), Vitamin B12 | Keeps the immune and nervous system healthy. Vitamin B6
Folate (Vitamin B9)
Vitamin B12
| white beans, potatoes, meat, fish, chicken, watermelon, grains, nuts, avocados, broccoli, and green leafy vegetables | |
| Vitamin C | Helps protect the body from infection and aids in recovery.
| citrus fruits (like oranges, grapefruit, and lemons), tomatoes, and potatoes | |
| Vitamin D | Important for developing and maintaining healthy bones and teeth.
| fortified milk, fatty fish, sunlight | |
| Vitamin E | Protects cells and helps fight off infection.
| green leafy vegetables, vegetable oils, avocados, almonds | Limit to 400 IU per day. |
| Iron | Not having enough iron can cause anemia.
| green leafy vegetables, whole grain breads and pastas, dried fruit, beans, red meat, chicken, liver, fish, and eggs | Limit to 45 mg per day unless otherwise instructed by your doctor. Iron may be a problem for people with HIV because it can increase the activity of some bacteria. Supplements that do not contain iron may be better. |
| Copper | Copper is a cofactor for several enzymes (known as “cuproenzymes”) involved in energy production, iron metabolism, neuropeptide activation, connective tissue synthesis, and neurotransmitter synthesis. Copper is also involved in many physiologic processes, such as angiogenesis; neurohormone homeostasis; and regulation of gene expression, brain development, pigmentation, and immune system functioning. In addition, defense against oxidative damage depends mainly on the copper-containing superoxide dismutases.
| beef liver, shellfish, seeds and nuts, organ meats, wheat-bran cereals, whole-grain products, and chocolate Tap water and other beverages can also be sources of copper, although the amount of copper in these liquids varies by source (ranging from 0.0005 mg/L to 1 mg/L) | Limit to 900 mcg per day. |
| Magnesium | Important for the immune system.
| green leafy vegetables, such as spinach, legumes, nuts, seeds, and whole grains, are good sources of magnesium | Limit 420 mg (male) and 320 mg (female) per day. |
| Selenium | Important for the immune system.
| whole grains, meat, fish, poultry, eggs, peanut butter, and nuts | Limit to 400 mcg per day. |
| Zinc | Important for the immune system.
| meat, fish, poultry, beans, peanuts, and milk and dairy products | Limit to 40 mg per day. |
Abbreviations: IFN = Interferon; IL= interleukin; NK = natural killer; Th = T-helper; TNF = tumor necrosis factor
[Source 21, 5 ]Table 4. Important dietary sources of nutrients that support the immune system
| Nutrient | Good dietary sources |
| Vitamin A (or equivalents) | Milk and cheese, eggs, liver, oily fish, fortified cereals, dark orange or green vegetables (eg, carrots, sweet potatoes, pumpkin, squash, kale, spinach, broccoli), orange fruits (eg, apricots, peaches, papaya, mango, cantaloupe melon), tomato juice |
| Vitamin B6 | Fish, poultry, meat, eggs, whole grain cereals, fortified cereals, many vegetables (especially green leafy) and fruits, soya beans, tofu, yeast extract |
| Vitamin B12 | Fish, meat, some shellfish, milk and cheese, eggs, fortified breakfast cereals, yeast extract |
| Folate (Vitamin B9) | Broccoli, brussels sprouts, green leafy vegetables (spinach, kale, cabbage), peas, chick peas, fortified cereals |
| Vitamin C | Oranges and orange juice, red and green peppers, strawberries, blackcurrants, kiwi, broccoli, brussels sprouts, potatoes |
| Vitamin D | Oily fish, liver, eggs, fortified foods (spreads and some breakfast cereals) |
| Vitamin E | Many vegetable oils, nuts and seeds, wheat germ (in cereals) |
| Zinc | Shellfish, meat, cheese, some grains and seeds, cereals, seeded or wholegrain breads |
| Selenium | Fish, shellfish, meat, eggs, some nuts especially brazil nuts |
| Iron | Meat, liver, beans, nuts, dried fruit (eg, apricots), wholegrains (eg, brown rice), fortified cereals, most dark green leafy vegetables (spinach, kale) |
| Copper | Shellfish, nuts, liver, some vegetables |
| Magnesium | Green leafy vegetables, such as spinach, legumes, nuts, seeds, and whole grains |
| Essential amino acids | Meat, poultry, fish, eggs, milk and cheese, soya, nuts and seeds, pulses |
| Essential fatty acids | Many seeds, nuts and vegetable oils |
| Long chain omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) | Oily fish |
Table 5. Results of multiple scientific studies (meta-analyses) on micronutrients and respiratory infections
| Micronutrient | Sample size | Main findings | Stated conclusion in abstract | Reference |
| Vitamin A | 47 randomized controlled trials (1 223 856 children) | Vitamin A did not affect incidence of, or mortality from, respiratory disease; Note: vitamin A decreased all cause mortality and mortality from diarrhea and decreased incidence of diarrhea and measles | Vitamin A supplementation is associated with a clinically meaningful reduction in morbidity and mortality in children. | Imdad et al 22 |
| Vitamin A | 15 randomized controlled trials (3021 children) | Vitamin A did not affect mortality of children with pneumonia. Vitamin A decreased pneumonia morbidity, increased the clinical response rate, shortened clearance time of signs and shortened length of hospital stay. | Vitamin A supplementation helps to relieve clinical symptoms and signs (of pneumonia) and shorten the length of hospital stay. | Hu et al 23 |
| Vitamin C | 3 prophylactic trials (2335 participants) two therapeutic trials (197 patients) | All three trials found vitamin C decreased the incidence of pneumonia. One trial found vitamin C decreased severity and mortality from pneumonia; the other trial found vitamin C shortened duration of pneumonia. | Hemila and Louhiala 24 | |
| Vitamin C | 29 prophylactic randomized controlled trials investigating incidence (11 306 participants) 31 prophylactic randomized controlled trials investigating duration (9745 episodes) | Vitamin C did not affect incidence of the common cold in the general population (24 randomized controlled trials) but decreased incidence in people under heavy short-term physical stress (5 randomized controlled trials). Vitamin C shortened duration of common cold in all studies (31 randomized controlled trials), in adults (13 randomized controlled trials) and in children (10 randomized controlled trials) and decreased severity of colds. | Hemila and Chalker 25 | |
| Vitamin D | 11 randomized controlled trials (5660 participants) | Vitamin D decreased the risk of respiratory tract infections. | Vitamin D has a positive effect against respiratory tract infections and dosing once daily seems most effective. | Bergman et al 26 |
| Vitamin D | 25 randomized controlled trials (11 321 participants) | Vitamin D decreased the risk of acute respiratory tract infection, effects greater in those with low starting status | Vitamin D supplementation was safe and it protected against respiratory tract infection. | Martineau et al 27 |
| Vitamin D | 24 studies; 14 included in meta-analysis of risk of acute respiratory tract infections and 5 in the meta-analysis of severity | Serum vitamin D was inversely associated with risk and severity of acute respiratory tract infections. | There is an inverse non-linear association between 25-hydroxyvitamin D concentration and acute respiratory tract infection. | Pham et al 28 |
| Vitamin D | 8 observational studies (20 966 participants) | Participants with vitamin D deficiency had increased risk of community-acquired pneumonia. | There is an association between vitamin D deficiency and increased risk of community-acquired pneumonia. | Zhou et al 29 |
| Zinc, copper and iron | 13 studies in Chinese children | Children with recurrent respiratory tract infection had lower hair levels of zinc, copper and iron. | The deficiency of zinc, copper and iron may be a contributing factor for the susceptibility of recurrent respiratory tract infection in Chinese children. | Mao et al 30 |
| Zinc | 7 randomized controlled trials (575 participants) | Zinc shortened duration of common cold. | Hemila 31 | |
| Zinc | 17 randomized controlled trials (2121 adults and children) | Zinc decreased duration of common cold symptoms overall and in adults but not in children. | Oral zinc formulations may shorten the duration of symptoms of the common cold. | Science et al 32 |
| Zinc | 6 randomized controlled trials (5193 children) | Zinc decreased incidence of pneumonia. Zinc decreased prevalence of pneumonia. | Zinc supplementation in children is associated with a reduction in the incidence and prevalence of pneumonia. | Lassi et al 33 |
| Zinc | 6 randomized controlled trials (2216 adults with severe pneumonia) | Zinc given as an adjunct therapy decreased mortality. No effect of zinc on treatment failure or antibiotic treatment. | Zinc given as an adjunct to the treatment of severe pneumonia is effective in reducing mortality. | Wang and Song 34 |
List of foods containing Vitamins and Minerals that Affect the Immune System
Vitamin A
Vitamin A is name of a group of fat-soluble vitamin (retinoids, including retinol, retinal, and retinyl esters) 35, 36, 37, that is naturally present in many foods.
Vitamin A is important for normal vision, gene expression, the immune system, embryonic development, growth, and reproduction. Vitamin A also helps the heart, lungs, kidneys, and other organs work properly 38.
There are two different types of vitamin A 39.
- The first type, preformed vitamin A (retinol and its esterified form, retinyl ester), is found in meat (especially liver), poultry, fish, and dairy products.
- The second type, provitamin A carotenoids (beta-carotene, alpha-carotene and beta-cryptoxanthin), is found in fruits, vegetables, and other plant-based products (oily fruits and red palm oil). The most common type of provitamin A carotenoids in foods and dietary supplements is beta-carotene (β-carotene). The body converts these plant pigments into vitamin A.
There are a number of reviews of the role of vitamin A and its metabolites (eg, 9-cis-retinoic acid) in immunity and in host susceptibility to infection 40. Vitamin A is important for normal differentiation of epithelial tissue and for immune cell maturation and function. Thus, vitamin A deficiency is associated with impaired barrier function, altered immune responses and increased susceptibility to a range of infections. Vitamin A-deficient mice show breakdown of the gut barrier and impaired mucus secretion (due to loss of mucus-producing goblet cells), both of which would facilitate entry of pathogens. Many aspects of innate immunity, in addition to barrier function, are modulated by vitamin A and its metabolites. Vitamin A controls neutrophil maturation and in vitamin A deficiency blood neutrophil numbers are increased, but they have impaired phagocytic function. Therefore, the ability of neutrophils to ingest and kill bacteria is impaired. Vitamin A also supports phagocytic activity and oxidative burst of macrophages, so promoting bacterial killing. Natural killer cell activity is diminished by vitamin A deficiency, which would impair antiviral defences. The impact of vitamin A on acquired immunity is less clear and may depend on the exact setting and the vitamin A metabolite involved. Vitamin A controls dendritic cell and CD4+ T lymphocyte maturation and its deficiency alters the balance between T helper 1 and T helper 2 lymphocytes. Studies in experimental model systems indicate that the vitamin A metabolite 9-cis retinoic acid enhances T helper 1 responses. Retinoic acid promotes movement (homing) of T lymphocytes to the gut-associated lymphoid tissue. Interestingly, some gut-associated immune cells are able to synthesise retinoic acid. Retinoic acid is required for CD8+ T lymphocyte survival and proliferation and for normal functioning of B lymphocytes including antibody generation. Thus, vitamin A deficiency can impair the response to vaccination, as discussed elsewhere 41. In support of this, vitamin A-deficient Indonesian children provided with vitamin A showed a higher antibody response to tetanus vaccination than seen in vitamin A-deficient children 42. Vitamin A deficiency predisposes to respiratory infections, diarrhoea and severe measles. Systematic reviews and meta-analyses of trials in children with vitamin A report reduced all-cause mortality 22, reduced incidence, morbidity and mortality from measles 22 and from infant diarrhoea 22 and improved symptoms in acute pneumonia 23.
You can get recommended amounts of vitamin A by eating a variety of foods, including the following:
- Beef liver and other organ meats (but these foods are also high in cholesterol, so limit the amount you eat).
- Some types of fish, such as salmon.
- Green leafy vegetables and other green, orange, and yellow vegetables, such as broccoli, carrots, and squash.
- Fruits, including cantaloupe, apricots, and mangos.
- Dairy products, which are among the major sources of vitamin A for Americans.
- Fortified breakfast cereals.
Table 4 suggests many dietary sources of vitamin A. The foods from animal sources contain primarily preformed vitamin A, the plant-based foods have provitamin A, and the foods with a mixture of ingredients from animals and plants contain both preformed vitamin A and provitamin A.

Table 6. Selected Food Sources of Vitamin A
| Food | mcg RAE per serving | IU per serving | Percent DV* |
|---|---|---|---|
| Sweet potato, baked in skin, 1 whole | 1,403 | 28,058 | 561 |
| Beef liver, pan fried, 3 ounces | 6,582 | 22,175 | 444 |
| Spinach, frozen, boiled, ½ cup | 573 | 11,458 | 229 |
| Carrots, raw, ½ cup | 459 | 9,189 | 184 |
| Pumpkin pie, commercially prepared, 1 piece | 488 | 3,743 | 249 |
| Cantaloupe, raw, ½ cup | 135 | 2,706 | 54 |
| Peppers, sweet, red, raw, ½ cup | 117 | 2,332 | 47 |
| Mangos, raw, 1 whole | 112 | 2,240 | 45 |
| Black-eyed peas (cowpeas), boiled, 1 cup | 66 | 1,305 | 26 |
| Apricots, dried, sulfured, 10 halves | 63 | 1,261 | 25 |
| Broccoli, boiled, ½ cup | 60 | 1,208 | 24 |
| Ice cream, French vanilla, soft serve, 1 cup | 278 | 1,014 | 20 |
| Cheese, ricotta, part skim, 1 cup | 263 | 945 | 19 |
| Tomato juice, canned, ¾ cup | 42 | 821 | 16 |
| Herring, Atlantic, pickled, 3 ounces | 219 | 731 | 15 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin A, ¾–1 cup (more heavily fortified cereals might provide more of the DV) | 127–149 | 500 | 10 |
| Milk, fat-free or skim, with added vitamin A and vitamin D, 1 cup | 149 | 500 | 10 |
| Baked beans, canned, plain or vegetarian, 1 cup | 13 | 274 | 5 |
| Egg, hard boiled, 1 large | 75 | 260 | 5 |
| Summer squash, all varieties, boiled, ½ cup | 10 | 191 | 4 |
| Salmon, sockeye, cooked, 3 ounces | 59 | 176 | 4 |
| Yogurt, plain, low fat, 1 cup | 32 | 116 | 2 |
| Pistachio nuts, dry roasted, 1 ounce | 4 | 73 | 1 |
| Tuna, light, canned in oil, drained solids, 3 ounces | 20 | 65 | 1 |
| Chicken, breast meat and skin, roasted, ½ breast | 5 | 18 | 0 |
Footnote: *DV = Daily Value. DVs were developed by the FDA to help consumers compare the nutrient contents of products within the context of a total diet. The DV for vitamin A is 5,000 IU for adults and children age 4 and older. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 43]Beta Carotene
Beta-carotene is a red-orange pigment found in plants and fruits, especially carrots and colorful vegetables. It is the yellow/orange pigment that gives vegetables and fruits their rich colors.
Carotene is an orange photosynthetic pigment important for photosynthesis. It is responsible for the orange color of the carrot and many other fruits and vegetables. It contributes to photosynthesis by transmitting the light energy it absorbs to chlorophyll. Chemically, carotene is a terpene. It is the dimer of retinol (vitamin A) and comes in two primary forms: alpha- and beta-carotene. Gamma-, delta- and epsilon-carotene also exist. Carotene can be stored in the liver and converted to vitamin A as needed.
Beta-carotene in itself is not an essential nutrient, but vitamin A is.
Beta-carotene is a carotenoid that is a precursor of vitamin A and the human body converts beta-carotene into vitamin A (retinol). We need vitamin A for healthy skin and mucus membranes, our immune system, and good eye health and vision.
Beta-carotene, like all carotenoids, is an antioxidant. An antioxidant is a substance that inhibits the oxidation of other molecules; it protects the body from free radicals.
Free radicals damage cells through oxidation. Eventually, the damage caused by free radicals can cause several chronic illnesses.
Several studies have shown that antioxidants through diet help people’s immune systems, protect against free radicals, and lower the risk of developing cancer and heart disease.
Some studies have suggested that those who consume at least four daily servings of beta-carotene rich fruits and/or vegetables have a lower risk of developing cancer or heart disease.
Beta-carotene may also slow down cognitive decline. Men who have been taking beta-carotene supplements for 15 or more years are considerably less likely to experience cognitive decline than other males, researchers from Harvard Medical School reported in Archives of Internal Medicine.
B-group vitamins
There is a recent comprehensive review of B vitamins and immunity 44. B vitamins are involved in intestinal immune regulation, thus contributing to gut barrier function. Folic acid (vitamin B9) deficiency in animals causes thymus and spleen atrophy, and decreases circulating T lymphocyte numbers. Spleen lymphocyte proliferation is also reduced but the phagocytic and bactericidal capacity of neutrophils appears unchanged. In contrast, vitamin B12 deficiency decreases phagocytic and bacterial killing capacity of neutrophils, while vitamin B6 deficiency causes thymus and spleen atrophy, low blood T lymphocyte numbers and impaired lymphocyte proliferation and T lymphocyte-mediated immune responses. Vitamins B6 and B12 and folate all support the activity of natural killer cells and CD8+ cytotoxic T lymphocytes, effects which would be important in antiviral defence. Patients with vitamin B12 deficiency had low blood numbers of CD8+ T lymphocytes and low natural killer cell activity 45. In a study in healthy older humans 46, a vitamin B6-deficient diet for 21 days resulted in a decreased percentage and total number of circulating lymphocytes, and a decrease in T and B lymphocyte proliferation and IL-2 production. Repletion over 21 days using vitamin B6 at levels below those recommended did not return immune function to starting values, while repletion at the recommended intake (22.5 µg/kg body weight per day, which would be 1.575 mg/day in a 70 kg individual) did 46. Providing excess vitamin B6 (33.75 µg/kg body weight per day, which would be 2.362 mg/day in a 70 kg individual) for 4 days caused a further increase in lymphocyte proliferation and IL-2 production.
Thiamin (Vitamin B1)
Thiamin (or thiamine) is one of the water-soluble B vitamins. It is also known as vitamin B1. Thiamin is naturally present in some foods, added to some food products, and available as a dietary supplement. This vitamin plays a critical role in energy metabolism and, therefore, in the growth, development, and function of cells 47.
Thiamin (also called vitamin B1) helps turn the food you eat into the energy you need. Thiamin is important for the growth, development, and function of the cells in your body.
Table 7. Selected Food Sources of Thiamine (vitamin B1)
| Food | Milligrams (mg) per serving | Percent DV* |
| Breakfast cereals, fortified with 100% of the DV for thiamin, 1 serving | 1.5 | 100 |
| Rice, white, long grain, enriched, parboiled, ½ cup | 1.4 | 73 |
| Egg noodles, enriched, cooked, 1 cup | 0.5 | 33 |
| Pork chop, bone-in, broiled, 3 ounces | 0.4 | 27 |
| Trout, cooked, dry heat, 3 ounces | 0.4 | 27 |
| Black beans, boiled, ½ cup | 0.4 | 27 |
| English muffin, plain, enriched, 1 muffin | 0.3 | 20 |
| Mussels, blue, cooked, moist heat, 3 ounces | 0.3 | 20 |
| Tuna, Bluefin, cooked, dry heat, 3 ounces | 0.2 | 13 |
| Macaroni, whole wheat, cooked, 1 cup | 0.2 | 13 |
| Acorn squash, cubed, baked, ½ cup | 0.2 | 13 |
| Rice, brown, long grain, not enriched, cooked, ½ cup | 0.1 | 7 |
| Bread, whole wheat, 1 slice | 0.1 | 7 |
| Orange juice, prepared from concentrate, 1 cup | 0.1 | 7 |
| Sunflower seeds, toasted, 1 ounce | 0.1 | 7 |
| Beef steak, bottom round, trimmed of fat, braised, 3 ounces | 0.1 | 7 |
| Yogurt, plain, low fat, 1 cup | 0.1 | 7 |
| Oatmeal, regular and quick, unenriched, cooked with water, ½ cup | 0.1 | 7 |
| Corn, yellow, boiled, 1 medium ear | 0.1 | 7 |
| Milk, 2%, 1 cup | 0.1 | 7 |
| Barley, pearled, cooked, 1 cup | 0.1 | 7 |
| Cheddar cheese, 1½ ounces | 0 | 0 |
| Chicken, meat and skin, roasted, 3 ounces | 0 | 0 |
| Apple, sliced, 1 cup | 0 | 0 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for thiamine is 1.5 mg for adults and children age 4 and older. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 48]Vitamin B2 (Riboflavin)
Riboflavin also called vitamin B2 is one of the B vitamins, which are all water soluble and it’s important for the growth, development, and function of the cells in your body. It also helps turn the food you eat into the energy you need.
More than 90% of dietary riboflavin is in the form of flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN); the remaining 10% is comprised of the free form and glycosides or esters 49, 50. Most riboflavin is absorbed in the proximal small intestine 51. The body absorbs little riboflavin from single doses beyond 27 mg and stores only small amounts of riboflavin in the liver, heart, and kidneys. When excess amounts are consumed, they are either not absorbed or the small amount that is absorbed is excreted in urine 50.
Bacteria in the large intestine produce free riboflavin that can be absorbed by the large intestine in amounts that depend on the diet. More riboflavin is produced after ingestion of vegetable-based than meat-based foods 49.
Riboflavin is yellow and naturally fluorescent when exposed to ultraviolet light 52. Moreover, ultraviolet and visible light can rapidly inactivate riboflavin and its derivatives. Because of this sensitivity, lengthy light therapy to treat jaundice in newborns or skin disorders can lead to riboflavin deficiency. The risk of riboflavin loss from exposure to light is the reason why milk is not typically stored in glass containers 50, 53.
Table 8. Selected Food Sources of Riboflavin (vitamin B2)
| Food | Milligrams (mg) per serving | Percent DV* |
| Beef liver, pan fried, 3 ounces | 2.9 | 171 |
| Breakfast cereals, fortified with 100% of the DV for riboflavin, 1 serving | 1.7 | 100 |
| Oats, instant, fortified, cooked with water, 1 cup | 1.1 | 65 |
| Yogurt, plain, fat free, 1 cup | 0.6 | 35 |
| Milk, 2% fat, 1 cup | 0.5 | 29 |
| Beef, tenderloin steak, boneless, trimmed of fat, grilled, 3 ounces | 0.4 | 24 |
| Clams, mixed species, cooked, moist heat, 3 ounces | 0.4 | 24 |
| Mushrooms, portabella, sliced, grilled, ½ cup | 0.3 | 18 |
| Almonds, dry roasted, 1 ounce | 0.3 | 18 |
| Cheese, Swiss, 3 ounces | 0.3 | 18 |
| Rotisserie chicken, breast meat only, 3 ounces | 0.2 | 12 |
| Egg, whole, scrambled, 1 large | 0.2 | 12 |
| Quinoa, cooked, 1 cup | 0.2 | 12 |
| Bagel, plain, enriched, 1 medium (3½”–4” diameter) | 0.2 | 12 |
| Salmon, pink, canned, 3 ounces | 0.2 | 12 |
| Spinach, raw, 1 cup | 0.1 | 6 |
| Apple, with skin, 1 large | 0.1 | 6 |
| Kidney beans, canned, 1 cup | 0.1 | 6 |
| Macaroni, elbow shaped, whole wheat, cooked, 1 cup | 0.1 | 6 |
| Bread, whole wheat, 1 slice | 0.1 | 6 |
| Cod, Atlantic, cooked, dry heat, 3 ounces | 0.1 | 6 |
| Sunflower seeds, toasted, 1 ounce | 0.1 | 6 |
| Tomatoes, crushed, canned, ½ cup | 0.1 | 6 |
| Rice, white, enriched, long grain, cooked, ½ cup | 0.1 | 6 |
| Rice, brown, long grain, cooked, ½ cup | 0 | 0 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for riboflavin is 1.7 mg for adults and children age 4 and older. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 48]Vitamin B6 (Pyridoxine)
Vitamin B6 includes a group of closely related compounds: pyridoxine, pyridoxal, and pyridoxamine. Substantial proportions of the naturally occurring pyridoxine in fruits, vegetables, and grains exist in glycosylated forms that exhibit reduced bioavailability 54. The body needs vitamin B6 for more than 100 enzyme reactions involved in metabolism. They are metabolized in the body to pyridoxal phosphate, which acts as a coenzyme in many important reactions in blood, CNS, and skin metabolism. Vitamin B6 is important in heme and nucleic acid biosynthesis and in lipid, carbohydrate, and amino acid metabolism. Vitamin B6 is also involved in brain development during pregnancy and infancy as well as immune function.
Vitamin B6 in coenzyme forms performs a wide variety of functions in the body and is extremely versatile, with involvement in more than 100 enzyme reactions, mostly concerned with protein metabolism. Both pyridoxal 5’ phosphate and pyridoxamine 5’ phosphate are involved in amino acid metabolism, and pyridoxal 5’ phosphate is also involved in the metabolism of one-carbon units, carbohydrates, and lipids 54. Vitamin B6 also plays a role in cognitive development through the biosynthesis of neurotransmitters and in maintaining normal levels of homocysteine, an amino acid in the blood 54. Vitamin B6 is involved in gluconeogenesis and glycogenolysis, immune function (for example, it promotes lymphocyte and interleukin-2 production), and hemoglobin formation 54.
The human body absorbs vitamin B6 in the jejunum. Phosphorylated forms of the vitamin are dephosphorylated, and the pool of free vitamin B6 is absorbed by passive diffusion 55.
Table 9. Selected Food Sources of Vitamin B6 (Pyridoxine)
| Food | Milligrams (mg) per serving | Percent DV* |
| Chickpeas, canned, 1 cup | 1.1 | 55 |
| Beef liver, pan fried, 3 ounces | 0.9 | 45 |
| Tuna, yellowfin, fresh, cooked, 3 ounces | 0.9 | 45 |
| Salmon, sockeye, cooked, 3 ounces | 0.6 | 30 |
| Chicken breast, roasted, 3 ounces | 0.5 | 25 |
| Breakfast cereals, fortified with 25% of the DV for vitamin B6 | 0.5 | 25 |
| Potatoes, boiled, 1 cup | 0.4 | 20 |
| Turkey, meat only, roasted, 3 ounces | 0.4 | 20 |
| Banana, 1 medium | 0.4 | 20 |
| Marinara (spaghetti) sauce, ready to serve, 1 cup | 0.4 | 20 |
| Ground beef, patty, 85% lean, broiled, 3 ounces | 0.3 | 15 |
| Waffles, plain, ready to heat, toasted, 1 waffle | 0.3 | 15 |
| Bulgur, cooked, 1 cup | 0.2 | 10 |
| Cottage cheese, 1% low-fat, 1 cup | 0.2 | 10 |
| Squash, winter, baked, ½ cup | 0.2 | 10 |
| Rice, white, long-grain, enriched, cooked, 1 cup | 0.1 | 5 |
| Nuts, mixed, dry-roasted, 1 ounce | 0.1 | 5 |
| Raisins, seedless, ½ cup | 0.1 | 5 |
| Onions, chopped, ½ cup | 0.1 | 5 |
| Spinach, frozen, chopped, boiled, ½ cup | 0.1 | 5 |
| Tofu, raw, firm, prepared with calcium sulfate, ½ cup | 0.1 | 5 |
| Watermelon, raw, 1 cup | 0.1 | 5 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for vitamin B6 is 2 mg for adults and children age 4 and older. However, the FDA does not require food labels to list vitamin B6 content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 48]Vitamin B12 (Cyanocobalamin)
Vitamin B12 is also known as Cyanocobalamin is a nutrient that helps keep the body’s nerve and blood cells healthy and helps make DNA, the genetic material in all cells. Vitamin B12 also helps prevent a type of anemia called megaloblastic anemia that makes people tired and weak.
Vitamin B12 is a water-soluble vitamin that is naturally present in some foods, added to others, and available as a dietary supplement and a prescription medication. Vitamin B12 exists in several forms and contains the mineral cobalt 56, 57, 58, 59, so compounds with vitamin B12 activity are collectively called “cobalamins”. Methylcobalamin and 5-deoxyadenosylcobalamin are the forms of vitamin B12 that are active in human metabolism 60.
Two steps are required for the body to absorb vitamin B12 from food.
- First, food-bound vitamin B12 is released in the stomach’s acid environment (hydrochloric acid and and gastric protease in the stomach separate vitamin B12 from the protein to which vitamin B12 is attached in food) and is bound to R protein (haptocorrin) 60. When synthetic vitamin B12 is added to fortified foods and dietary supplements, it is already in free form and thus, does not require this separation step.
- Second, pancreatic enzymes cleave this B12 complex (B12-R protein) in the small intestine. After cleavage, intrinsic factor (a protein made by the stomach), secreted by parietal cells in the gastric mucosa, binds with the free vitamin B12. Intrinsic factor is required for absorption of vitamin B12, which takes place in the terminal ileum 60, 61. Approximately 56% of a 1 mcg oral dose of vitamin B12 is absorbed, but absorption decreases drastically when the capacity of intrinsic factor is exceeded (at 1–2 mcg of vitamin B12) 62. Some people have pernicious anemia, a condition where they cannot make intrinsic factor. As a result, they have trouble absorbing vitamin B12 from all foods and dietary supplements.
Several food sources of vitamin B12 are listed in Table 10.
Table 10. Selected Food Sources of Vitamin B12
| Food | Micrograms (mcg) per serving | Percent DV* |
| Clams, cooked, 3 ounces | 84.1 | 1402 |
| Liver, beef, cooked, 3 ounces | 70.7 | 1178 |
| Breakfast cereals, fortified with 100% of the DV for vitamin B12, 1 serving | 6 | 100 |
| Trout, rainbow, wild, cooked, 3 ounces | 5.4 | 90 |
| Salmon, sockeye, cooked, 3 ounces | 4.8 | 80 |
| Trout, rainbow, farmed, cooked, 3 ounces | 3.5 | 58 |
| Tuna fish, light, canned in water, 3 ounces | 2.5 | 42 |
| Cheeseburger, double patty and bun, 1 sandwich | 2.1 | 35 |
| Haddock, cooked, 3 ounces | 1.8 | 30 |
| Breakfast cereals, fortified with 25% of the DV for vitamin B12, 1 serving | 1.5 | 25 |
| Beef, top sirloin, broiled, 3 ounces | 1.4 | 23 |
| Milk, low-fat, 1 cup | 1.2 | 18 |
| Yogurt, fruit, low-fat, 8 ounces | 1.1 | 18 |
| Cheese, Swiss, 1 ounce | 0.9 | 15 |
| Beef taco, 1 soft taco | 0.9 | 15 |
| Ham, cured, roasted, 3 ounces | 0.6 | 10 |
| Egg, whole, hard boiled, 1 large | 0.6 | 10 |
| Chicken, breast meat, roasted, 3 ounces | 0.3 | 5 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers determine the level of various nutrients in a standard serving of food in relation to their approximate requirement for it. The DV for vitamin B12 is 6.0 mcg. However, the FDA does not require food labels to list vitamin B12 content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 48]Folate (Vitamin B9)
Folate is also known vitamin B9 (Folacin, Folic Acid, Pteroylglutamic acid) that is naturally present in many foods. Folic Acid is a form of folate that is manufactured and used in dietary supplements and fortified foods 63. Everyone needs folic acid. Our bodies need folate to make DNA and other genetic material. Folate is also needed for the body’s cells to divide.
Folic acid and folate also help your body make healthy new red blood cells. Red blood cells carry oxygen to all the parts of your body. If your body does not make enough red blood cells, you can develop anemia. Anemia happens when your blood cannot carry enough oxygen to your body, which makes you pale, tired, or weak. Also, if you do not get enough folic acid, you could develop a type of anemia called folate-deficiency anemia 64.
Folate-deficiency anemia is most common during pregnancy. Other causes of folate-deficiency anemia include alcoholism and certain medicines to treat seizures, anxiety, or arthritis.
In women and pregnant mothers, folic acid is very important because it can help prevent some major birth defects of the baby’s brain and spine (anencephaly and spina bifida) 65.
Every woman needs folic acid every day, whether she’s planning to get pregnant or not, for the healthy new cells the body makes daily. Think about the skin, hair, and nails. These – and other parts of the body – make new cells each day.
Centers for Disease Control and Prevention (CDC) urges women to take 400 mcg of folic acid every day, starting at least one month before getting pregnant and while she is pregnant, to help prevent major birth defects of the baby’s brain and spine.
Folate is naturally present in many foods and food companies add folic acid to other foods, including bread, cereal, and pasta. You can get recommended amounts by eating a variety of foods, including the following:
- Leafy Green Vegetables (especially asparagus, Brussels sprouts, and dark green leafy vegetables such as spinach and mustard greens).
- Fruits and fruit juices (especially oranges and orange juice).
- Nuts, beans, and peas (such as peanuts, black-eyed peas, and kidney beans).
- Grains (including whole grains; fortified cold cereals; enriched flour products such as bread, bagels, cornmeal, and pasta; and rice).
- Folic acid is added to many grain-based products, enriched breads, cereals and corn masa flour (used to make corn tortillas and tamales, for example). To find out whether folic acid has been added to a food, check the product label.
Beef liver is high in folate but is also high in cholesterol, so limit the amount you eat. Only small amounts of folate are found in other animal foods like meats, poultry, seafood, eggs, and dairy products.
Table 11. Selected Food Sources of Folate and Folic Acid
| Food | mcg DFE per serving | Percent DV* |
| Beef liver, braised, 3 ounces | 215 | 54 |
| Spinach, boiled, ½ cup | 131 | 33 |
| Black-eyed peas (cowpeas), boiled, ½ cup | 105 | 26 |
| Breakfast cereals, fortified with 25% of the DV† | 100 | 25 |
| Rice, white, medium-grain, cooked, ½ cup† | 90 | 23 |
| Asparagus, boiled, 4 spears | 89 | 22 |
| Spaghetti, cooked, enriched, ½ cup† | 83 | 21 |
| Brussels sprouts, frozen, boiled, ½ cup | 78 | 20 |
| Lettuce, romaine, shredded, 1 cup | 64 | 16 |
| Avocado, raw, sliced, ½ cup | 59 | 15 |
| Spinach, raw, 1 cup | 58 | 15 |
| Broccoli, chopped, frozen, cooked, ½ cup | 52 | 13 |
| Mustard greens, chopped, frozen, boiled, ½ cup | 52 | 13 |
| Green peas, frozen, boiled, ½ cup | 47 | 12 |
| Kidney beans, canned, ½ cup | 46 | 12 |
| Bread, white, 1 slice† | 43 | 11 |
| Peanuts, dry roasted, 1 ounce | 41 | 10 |
| Wheat germ, 2 tablespoons | 40 | 10 |
| Tomato juice, canned, ¾ cup | 36 | 9 |
| Crab, Dungeness, 3 ounces | 36 | 9 |
| Orange juice, ¾ cup | 35 | 9 |
| Turnip greens, frozen, boiled, ½ cup | 32 | 8 |
| Orange, fresh, 1 small | 29 | 7 |
| Papaya, raw, cubed, ½ cup | 27 | 7 |
| Banana, 1 medium | 24 | 6 |
| Yeast, baker’s, ¼ teaspoon | 23 | 6 |
| Egg, whole, hard-boiled, 1 large | 22 | 6 |
| Vegetarian baked beans, canned, ½ cup | 15 | 4 |
| Cantaloupe, raw, 1 wedge | 14 | 4 |
| Fish, halibut, cooked, 3 ounces | 12 | 3 |
| Milk, 1% fat, 1 cup | 12 | 3 |
| Ground beef, 85% lean, cooked, 3 ounces | 7 | 2 |
| Chicken breast, roasted, ½ breast | 3 | 1 |
Footnote: * DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of products within the context of a total diet. The DV for folate is 400 mcg for adults and children aged 4 and older. However, the FDA does not require food labels to list folate content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
† Fortified with folic acid as part of the folate fortification program.
[Source 48]Vitamin C
Vitamin C also known as ascorbic acid or ascorbate, is a water-soluble vitamin that is naturally present in some foods, added to others, and available as a dietary supplement. Vitamin C is synthesized from D-glucose or D-galactose by many plants and animals. However, humans lack the enzyme L-gulonolactone oxidase required for ascorbic acid synthesis and must obtain vitamin C through food or supplements 66, 67. Vitamin C is found in many fruits and vegetables, including citrus fruits, tomatoes, potatoes, red and green peppers, kiwifruit, broccoli, strawberries, brussels sprouts, and cantaloupe. In the body, vitamin C acts as an antioxidant, helping to protect cells from the damage caused by free radicals. Free radicals are compounds formed when our bodies convert the food we eat into energy. People are also exposed to free radicals in the environment from cigarette smoke, air pollution, and ultraviolet light from the sun.
The Recommended Dietary Allowance (RDA; average daily level of intake sufficient to meet the nutrient requirement of 97–98% healthy individuals) for vitamin C ranges from 15 to 115 mg for infants and children (depending on age) and from 75 to 120 mg for nonsmoking adults; people who smoke need 35 mg more per day 68. The intestinal absorption of vitamin C is regulated by at least one specific dose-dependent, active transporter 69. Cells accumulate vitamin C via a second specific transport protein. In vitro studies have found that oxidized vitamin C, or dehydroascorbic acid, enters cells via some facilitated glucose transporters and is then reduced internally to ascorbic acid. The physiologic importance of dehydroascorbic acid uptake and its contribution to overall vitamin C economy is unknown.
Vitamin C plays a role in collagen, carnitine, hormone, and amino acid formation. It is essential for wound healing and facilitates recovery from burns. Vitamin C is also an antioxidant, supports immune function, and facilitates the absorption of iron 70. Vitamin C also plays an important role in both innate and adaptive immunity, probably because of its antioxidant effects, antimicrobial and antiviral actions, and effects on immune system modulators 71. Vitamin C helps maintain epithelial integrity, enhance the differentiation and proliferation of B cells and T cells, enhance phagocytosis, normalize cytokine production, and decrease histamine levels 72. Vitamin C might also inhibit viral replication 73.
Vitamin C deficiency impairs immune function and increases susceptibility to infections 72. Some research suggests that supplemental vitamin C enhances immune function 74, but its effects might vary depending on an individual’s vitamin C status 75.
Vitamin C deficiency is uncommon in the United States, affecting only about 7% of individuals aged 6 years and older 76. People who smoke and those whose diets include a limited variety of foods (such as some older adults and people with alcohol or drug use disorders) are more likely than others to obtain insufficient amounts of vitamin C 74.
High-Dose vitamin C, when taken by intravenous (IV) infusion, vitamin C can reach much higher levels in the blood than when it is taken by mouth. Studies suggest that these higher levels of vitamin C may cause the death of cancer cells in the laboratory. Surveys of healthcare practitioners at United States complementary and alternative medicine conferences in recent years have shown that high-dose IV vitamin C is frequently given to patients as a treatment for infections, fatigue, and cancers, including breast cancer 77.
There are reviews of the role of vitamin C in immunity and in host susceptibility to infection 78. Vitamin C is required for collagen biosynthesis and is vital for maintaining epithelial integrity. Vitamin C also has roles in several aspects of immunity, including leucocyte migration to sites of infection, phagocytosis and bacterial killing, natural killer cell activity, T lymphocyte function (especially of CD8+ cytotoxic T lymphocytes) and antibody production. Jacob et al 79 showed that a vitamin C-deficient diet in healthy young adult humans decreased mononuclear cell vitamin C content by 50% and decreased the T lymphocyte-mediated immune responses to recall antigens. Vitamin C deficiency in animal models increases susceptibility to a variety of infections 78. People deficient in vitamin C are susceptible to severe respiratory infections such as pneumonia. A meta-analysis 24 reported a significant reduction in the risk of pneumonia with vitamin C supplementation, particularly in individuals with low dietary intakes. Vitamin C supplementation has also been shown to decrease the duration and severity of upper respiratory tract infections, such as the common cold, especially in people under enhanced physical stress 25.
Vitamin C is required for the biosynthesis of collagen, L-carnitine, and certain neurotransmitters; vitamin C is also involved in protein metabolism 67, 80. Collagen is an essential component of connective tissue, which plays a vital role in wound healing. Vitamin C is also an important physiological antioxidant 81 and has been shown to regenerate other antioxidants within the body, including alpha-tocopherol (vitamin E) 82. Ongoing research is examining whether vitamin C, by limiting the damaging effects of free radicals through its antioxidant activity, might help prevent or delay the development of certain cancers, cardiovascular disease, and other diseases in which oxidative stress plays a causal role. In addition to its biosynthetic and antioxidant functions, vitamin C plays an important role in immune function 82 and improves the absorption of nonheme iron 83, the form of iron present in plant-based foods. Insufficient vitamin C intake causes scurvy, which is characterized by fatigue or lassitude, widespread connective tissue weakness, and capillary fragility 67, 80, 82, 84, 85, 86, 87.
Table 12. Selected Food Sources of Vitamin C
| Food | Milligrams (mg) per serving | Percent (%) DV* |
| Red pepper, sweet, raw, ½ cup | 95 | 158 |
| Orange juice, ¾ cup | 93 | 155 |
| Orange, 1 medium | 70 | 117 |
| Grapefruit juice, ¾ cup | 70 | 117 |
| Kiwifruit, 1 medium | 64 | 107 |
| Green pepper, sweet, raw, ½ cup | 60 | 100 |
| Broccoli, cooked, ½ cup | 51 | 85 |
| Strawberries, fresh, sliced, ½ cup | 49 | 82 |
| Brussels sprouts, cooked, ½ cup | 48 | 80 |
| Grapefruit, ½ medium | 39 | 65 |
| Broccoli, raw, ½ cup | 39 | 65 |
| Tomato juice, ¾ cup | 33 | 55 |
| Cantaloupe, ½ cup | 29 | 48 |
| Cabbage, cooked, ½ cup | 28 | 47 |
| Cauliflower, raw, ½ cup | 26 | 43 |
| Potato, baked, 1 medium | 17 | 28 |
| Tomato, raw, 1 medium | 17 | 28 |
| Spinach, cooked, ½ cup | 9 | 15 |
| Green peas, frozen, cooked, ½ cup | 8 | 13 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for vitamin C is 60 mg for adults and children aged 4 and older. The FDA requires all food labels to list the percent DV for vitamin C. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 48]Vitamin D
Vitamin D is a fat-soluble vitamin that is naturally present in very few foods, added to others, and available as a dietary supplement. It is also produced endogenously when ultraviolet rays from sunlight strike the skin and trigger vitamin D synthesis. Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylations in the body for activation 88. The first occurs in the liver and converts vitamin D to 25-hydroxyvitamin D [25(OH)D], also known as calcidiol. The second occurs primarily in the kidney and forms the physiologically active 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as calcitriol 89.
Vitamin D is a nutrient found in some foods that is needed for health and to maintain strong bones. It does so by helping the body absorb calcium (one of bone’s main building blocks) from food and supplements. People who get too little vitamin D may develop soft, thin, and brittle bones, a condition known as rickets in children and osteomalacia in adults.
Vitamin D is important to the body in many other ways as well. Muscles need it to move, for example, nerves need it to carry messages between the brain and every body part, and the immune system needs vitamin D to fight off invading bacteria and viruses. Together with calcium, vitamin D also helps protect older adults from osteoporosis. Vitamin D is found in cells throughout the body.
Vitamin D promotes calcium absorption in the gut and maintains adequate serum calcium and phosphate concentrations to enable normal mineralization of bone and to prevent hypocalcemic tetany. It is also needed for bone growth and bone remodeling by osteoblasts and osteoclasts 89, 90. Without sufficient vitamin D, bones can become thin, brittle, or misshapen. Vitamin D sufficiency prevents rickets in children and osteomalacia in adults 89. Together with calcium, vitamin D also helps protect older adults from osteoporosis.
Vitamin D has other roles in the body, including modulation of cell growth, neuromuscular and immune function, and reduction of inflammation 89, 91, 92. Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D 89. Many cells have vitamin D receptors, and some convert 25(OH)D to 1,25(OH)2D.
There are a number of reviews of the role of vitamin D and its metabolites in immunity and in host susceptibility to infection 93. The active form of vitamin D (1,25-dihydroxyvitamin D3) is referred to here as vitamin D. Vitamin D receptors have been identified in most immune cells and some cells of the immune system can synthesise the active form of vitamin D from its precursor, suggesting that vitamin D is likely to have important immunoregulatory properties. Vitamin D enhances epithelial integrity and induces antimicrobial peptide (eg, cathelicidin) synthesis in epithelial cells and macrophages, directly enhancing host defence 94. However, the effects of vitamin D on the cellular components of immunity are rather complex. Vitamin D promotes differentiation of monocytes to macrophages and increases phagocytosis, superoxide production and bacterial killing by innate immune cells. It also promotes antigen processing by dendritic cells although antigen presentation may be impaired. Vitamin D is also reported to inhibit T-cell proliferation and production of cytokines by T helper 1 lymphocytes and of antibodies by B lymphocytes, highlighting the paradoxical nature of its effects. Effects on T helper 2 responses are not clear and vitamin D seems to increase number of regulatory T lymphocytes. Vitamin D seems to have little impact on CD8+ T lymphocytes. A systematic review and meta-analysis of the influence of vitamin D status on influenza vaccination (nine studies involving 2367 individuals) found lower seroprotection rates to influenza A virus subtype H3N2 and to influenza B virus in those who were vitamin D deficient 95. Berry et al 96 described an inverse linear relationship between vitamin D levels and respiratory tract infections in a cross-sectional study of 6789 British adults. In agreement with this, data from the US Third National Health and Nutrition Examination Survey which included 18 883 adults showed an independent inverse association between serum 25(OH)-vitamin D and recent upper respiratory tract infection 97. Other studies also report that individuals with low vitamin D status have a higher risk of viral respiratory tract infections 98. Supplementation of Japanese schoolchildren with vitamin D for 4 months during winter decreased the risk of influenza by about 40% 99. Meta-analyses have concluded that vitamin D supplementation can reduce the risk of respiratory tract infections 29, 28.
Table 13. Selected Food Sources of Vitamin D
| Food | IUs per serving* | Percent DV** |
| Cod liver oil, 1 tablespoon | 1360 | 340 |
| Swordfish, cooked, 3 ounces | 566 | 142 |
| Salmon (sockeye), cooked, 3 ounces | 447 | 112 |
| Tuna fish, canned in water, drained, 3 ounces | 154 | 39 |
| Orange juice fortified with vitamin D, 1 cup (check product labels, as amount of added vitamin D varies) | 137 | 34 |
| Milk, nonfat, reduced fat, and whole, vitamin D-fortified, 1 cup | 115-124 | 29-31 |
| Yogurt, fortified with 20% of the DV for vitamin D, 6 ounces (more heavily fortified yogurts provide more of the DV) | 80 | 20 |
| Margarine, fortified, 1 tablespoon | 60 | 15 |
| Sardines, canned in oil, drained, 2 sardines | 46 | 12 |
| Liver, beef, cooked, 3 ounces | 42 | 11 |
| Egg, 1 large (vitamin D is found in yolk) | 41 | 10 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 0.75-1 cup (more heavily fortified cereals might provide more of the DV) | 40 | 10 |
| Cheese, Swiss, 1 ounce | 6 | 2 |
Footnotes: * IUs = International Units. ** DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration to help consumers compare the nutrient contents among products within the context of a total daily diet. The DV for vitamin D is currently set at 400 IU for adults and children age 4 and older. Food labels, however, are not required to list vitamin D content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 48]Vitamin E
Naturally occurring vitamin E exists in eight chemical forms (alpha-, beta-, gamma-, and delta-tocopherol and alpha-, beta-, gamma-, and delta-tocotrienol) that have varying levels of biological activity 100. Alpha- (or α-) tocopherol is the only form that is recognized to meet human requirements, but beta-, gamma-, and delta-tocopherols, 4 tocotrienols, and several stereoisomers may also have important biologic activity. These compounds act as antioxidants, which prevent lipid peroxidation of polyunsaturated fatty acids in cellular membranes 101.
Serum concentrations of vitamin E (alpha-tocopherol) depend on the liver, which takes up the nutrient after the various forms are absorbed from the small intestine.
Vitamin E is a fat-soluble antioxidant that stops the production of reactive oxygen species formed when fat undergoes oxidation. Scientists are investigating whether, by limiting free-radical production and possibly through other mechanisms, vitamin E might help prevent or delay the chronic diseases associated with free radicals.
Antioxidants protect cells from the damaging effects of free radicals, which are molecules that contain an unshared electron. Free radicals damage cells and might contribute to the development of cardiovascular disease and cancer 102. Unshared electrons are highly energetic and react rapidly with oxygen to form reactive oxygen species. The body forms reactive oxygen species endogenously when it converts food to energy, and antioxidants might protect cells from the damaging effects of reactive oxygen species. The body is also exposed to free radicals from environmental exposures, such as cigarette smoke, air pollution, and ultraviolet radiation from the sun. Reactive oxygen species are part of signaling mechanisms among cells.
The body also needs vitamin E to boost its immune system so that it can fight off invading bacteria and viruses. It helps to widen blood vessels and keep blood from clotting within them.
In addition to its activities as an antioxidant, vitamin E is involved in immune function and, as shown primarily by in vitro studies of cells, cell signaling, regulation of gene expression, and other metabolic processes 100. Alpha-tocopherol inhibits the activity of protein kinase C, an enzyme involved in cell proliferation and differentiation in smooth muscle cells, platelets, and monocytes 103. Vitamin-E–replete endothelial cells lining the interior surface of blood vessels are better able to resist blood-cell components adhering to this surface. Vitamin E also increases the expression of two enzymes that suppress arachidonic acid metabolism, thereby increasing the release of prostacyclin from the endothelium, which, in turn, dilates blood vessels and inhibits platelet aggregation 103.
There are a number of reviews of the role of vitamin E in immunity and host susceptibility to infection 104. In laboratory animals, vitamin E deficiency decreases lymphocyte proliferation, natural killer cell activity, specific antibody production following vaccination and phagocytosis by neutrophils. Vitamin E deficiency also increases susceptibility of animals to infectious pathogens. Vitamin E supplementation of the diet of laboratory animals enhances antibody production, lymphocyte proliferation, T helper 1-type cytokine production, natural killer cell activity and macrophage phagocytosis. Vitamin E promotes interaction between dendritic cells and CD4+ T lymphocytes. There is a positive association between plasma vitamin E and cell-mediated immune responses, and a negative association has been demonstrated between plasma vitamin E and the risk of infections in healthy adults over 60 years of age 105. There appears to be particular benefit of vitamin E supplementation for the elderly 106. Studies by Meydani et al 107 demonstrated that vitamin E supplementation at high doses, one study used 800 mg/day 108 and the other used doses of 60, 200 and 800 mg/day 107 enhanced T helper 1 cell-mediated immunity (lymphocyte proliferation, IL-2 production) and improved vaccination responses, including to hepatitis B virus. Supplementation of older adults with vitamin E (200 mg/day) improved neutrophil chemotaxis and phagocytosis, natural killer cell activity and mitogen-induced lymphocyte proliferation 106. Secondary analysis of data from the Alpha-Tocopherol, Beta Carotene Cancer Prevention Study identified that daily vitamin E supplements for 5 to 8 years reduced the incidence of hospital treated, community-acquired pneumonia in smokers 109. One study reported that vitamin E supplementation (200 IU/day~135 mg/day) for 1 year decreased risk of upper respiratory tract infections in the elderly 110, but another study did not see an effect of supplemental vitamin E (200 mg/day) on the incidence, duration or severity of respiratory infections in an elderly population 111.
Table 14. Selected Food Sources of Vitamin E (Alpha-Tocopherol)
| Food | Milligrams (mg) per serving | Percent DV* |
| Wheat germ oil, 1 tablespoon | 20.3 | 100 |
| Sunflower seeds, dry roasted, 1 ounce | 7.4 | 37 |
| Almonds, dry roasted, 1 ounce | 6.8 | 34 |
| Sunflower oil, 1 tablespoon | 5.6 | 28 |
| Safflower oil, 1 tablespoon | 4.6 | 25 |
| Hazelnuts, dry roasted, 1 ounce | 4.3 | 22 |
| Peanut butter, 2 tablespoons | 2.9 | 15 |
| Peanuts, dry roasted, 1 ounce | 2.2 | 11 |
| Corn oil, 1 tablespoon | 1.9 | 10 |
| Spinach, boiled, ½ cup | 1.9 | 10 |
| Broccoli, chopped, boiled, ½ cup | 1.2 | 6 |
| Soybean oil, 1 tablespoon | 1.1 | 6 |
| Kiwifruit, 1 medium | 1.1 | 6 |
| Mango, sliced, ½ cup | 0.7 | 4 |
| Tomato, raw, 1 medium | 0.7 | 4 |
| Spinach, raw, 1 cup | 0.6 | 3 |
Footnote: *DV = Daily Value. DVs were developed by the FDA to help consumers compare the nutrient content of different foods within the context of a total diet. The DV for vitamin E is 30 IU (approximately 20 mg of natural alpha-tocopherol) for adults and children age 4 and older. However, the FDA does not require food labels to list vitamin E content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 48]Iron
Iron is a mineral that our bodies need for many functions. In the human body, iron is present in all cells and has several vital functions — as a carrier of oxygen to the tissues from the lungs in the form of hemoglobin (Hb), as a facilitator of oxygen use and storage in the muscles as myoglobin, as a transport medium for electrons within the cells in the form of cytochromes, and as an integral part of enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and mortality 112, 113.
In adults, the recommended dietary allowance of iron is 8 to 11 mg per day for men and 8 to 18 mg for women in whom higher levels are recommended during pregnancy (27 mg per day) 114. Iron is poorly absorbed and body and tissue iron stores are controlled largely by modifying rates of absorption. Adequate amounts of iron are found in most Western diets, with highest levels found in red meats and moderate levels in fish, poultry, green vegetables, cereals and grains (some of which are fortified with iron).
Your body needs the right amount of iron. If you have too little iron, you may develop iron deficiency anemia. Iron deficiency is usually due to loss of iron, predominantly as a result of blood loss in the gastrointestinal tract or from menstruation and is rarely due to deficiency in intake or an inability to absorb enough iron from foods. People at higher risk of having too little iron are young children and women who are pregnant or have periods.
There are a number of reviews of the role of iron in immunity and host susceptibility to infection 115. Iron deficiency induces thymus atrophy, reducing output of naive T lymphocytes, and has multiple effects on immune function in humans. The effects are wide ranging and include impairment of respiratory burst and bacterial killing, natural killer cell activity, T lymphocyte proliferation and production of T helper 1 cytokines. T lymphocyte proliferation was lower by 50% to 60% in iron-deficient than in iron-replete housebound older Canadian women 116. These observations would suggest a clear case for iron deficiency increasing susceptibility to infection. However, the relationship between iron deficiency and susceptibility to infection remains complex 117. Evidence suggests that infections caused by organisms that spend part of their life-cycle intracellularly, such as plasmodia and mycobacteria, may actually be enhanced by iron. In the tropics, in children of all ages, iron at doses above a particular threshold has been associated with increased risk of malaria and other infections, including pneumonia. Thus, iron intervention in malaria-endemic areas is not advised, particularly high doses in the young, those with compromised immunity and during the peak malaria transmission season. There are different explanations for the detrimental effects of iron administration on infections. First, iron overload causes impairment of immune function 118. Second, excess iron favors damaging inflammation. Third, micro-organisms require iron and providing it may favor the growth of the pathogen. Perhaps for the latter reasons several host immune mechanisms have developed for withholding iron from a pathogen 115. In a recent study giving iron (50 mg on each of 4 days a week) to iron-deficient school children in South Africa increased the risk of respiratory infections 119; coadministration of omega-3 fatty acids (500 mg on each of 4 days a week) mitigated the effect of iron. Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair iron 30.
What foods provide iron?
Iron is found naturally in many foods and is added to some fortified food products. You can get recommended amounts of iron by eating a variety of foods, including the following:
- Lean meat, seafood, and poultry.
- Iron-fortified breakfast cereals and breads.
- White beans, lentils, spinach, kidney beans, and peas.
- Nuts and some dried fruits, such as raisins.
Iron in food comes in two forms: heme iron and nonheme iron. Nonheme iron is found in plant foods and iron-fortified food products. Meat, seafood, and poultry have both heme and nonheme iron.
Heme iron has higher bioavailability than nonheme iron, and other dietary components have less effect on the bioavailability of heme than nonheme iron 120. The bioavailability of iron is approximately 14% to 18% from mixed diets that include substantial amounts of meat, seafood, and vitamin C (ascorbic acid, which enhances the bioavailability of nonheme iron) and 5% to 12% from vegetarian diets 121. In addition to ascorbic acid, meat, poultry, and seafood can enhance nonheme iron absorption, whereas phytate (present in grains and beans) and certain polyphenols in some non-animal foods (such as cereals and legumes) have the opposite effect 122. Unlike other inhibitors of iron absorption, calcium might reduce the bioavailability of both nonheme and heme iron. However, the effects of enhancers and inhibitors of iron absorption are attenuated by a typical mixed western diet, so they have little effect on most people’s iron status.
Several food sources of iron are listed in Table 15. Some plant-based foods that are good sources of iron, such as spinach, have low iron bioavailability because they contain iron-absorption inhibitors, such as polyphenols 123.
Your body absorbs iron from plant sources better when you eat it with meat, poultry, seafood, and foods that contain vitamin C, like citrus fruits, strawberries, sweet peppers, tomatoes, and broccoli.
Table 15. Selected Food Sources of Iron
| Food | Milligrams per serving | Percent DV* |
| Breakfast cereals, fortified with 100% of the DV for iron, 1 serving | 18 | 100 |
| Oysters, eastern, cooked with moist heat, 3 ounces | 8 | 44 |
| White beans, canned, 1 cup | 8 | 44 |
| Chocolate, dark, 45%–69% cacao solids, 3 ounces | 7 | 39 |
| Beef liver, pan fried, 3 ounces | 5 | 28 |
| Lentils, boiled and drained, ½ cup | 3 | 17 |
| Spinach, boiled and drained, ½ cup | 3 | 17 |
| Tofu, firm, ½ cup | 3 | 17 |
| Kidney beans, canned, ½ cup | 2 | 11 |
| Sardines, Atlantic, canned in oil, drained solids with bone, 3 ounces | 2 | 11 |
| Chickpeas, boiled and drained, ½ cup | 2 | 11 |
| Tomatoes, canned, stewed, ½ cup | 2 | 11 |
| Beef, braised bottom round, trimmed to 1/8” fat, 3 ounces | 2 | 11 |
| Potato, baked, flesh and skin, 1 medium potato | 2 | 11 |
| Cashew nuts, oil roasted, 1 ounce (18 nuts) | 2 | 11 |
| Green peas, boiled, ½ cup | 1 | 6 |
| Chicken, roasted, meat and skin, 3 ounces | 1 | 6 |
| Rice, white, long grain, enriched, parboiled, drained, ½ cup | 1 | 6 |
| Bread, whole wheat, 1 slice | 1 | 6 |
| Bread, white, 1 slice | 1 | 6 |
| Raisins, seedless, ¼ cup | 1 | 6 |
| Spaghetti, whole wheat, cooked, 1 cup | 1 | 6 |
| Tuna, light, canned in water, 3 ounces | 1 | 6 |
| Turkey, roasted, breast meat and skin, 3 ounces | 1 | 6 |
| Nuts, pistachio, dry roasted, 1 ounce (49 nuts) | 1 | 6 |
| Broccoli, boiled and drained, ½ cup | 1 | 6 |
| Egg, hard boiled, 1 large | 1 | 6 |
| Rice, brown, long or medium grain, cooked, 1 cup | 1 | 6 |
| Cheese, cheddar, 1.5 ounces | 0 | 0 |
| Cantaloupe, diced, ½ cup | 0 | 0 |
| Mushrooms, white, sliced and stir-fried, ½ cup | 0 | 0 |
| Cheese, cottage, 2% milk fat, ½ cup | 0 | 0 |
| Milk, 1 cup | 0 | 0 |
Footnote: * DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for iron is 18 mg for adults and children age 4 and older. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 48]Copper
Copper is an essential mineral that you need to stay healthy. Your body uses copper to carry out many important functions, including making energy, connective tissues, and blood vessels. Copper also helps maintain the nervous, pigmentation, and immune systems, and activates genes. Your body also needs copper for brain development 124. In addition, defense against oxidative damage depends mainly on the copper-containing superoxide dismutases 125. Copper is a cofactor for several enzymes known as “cuproenzymes” involved in energy production, iron metabolism, neuropeptide activation, connective tissue synthesis, and neurotransmitter synthesis 124. One abundant cuproenzyme is ceruloplasmin, which plays a role in iron metabolism and carries more than 95% of the total copper in healthy human plasma 126.
There are a number of reviews of the role of copper in immunity and host susceptibility to infection 127. Copper itself has antimicrobial properties. Copper supports neutrophil, monocyte and macrophage function and natural killer cell activity. It promotes T lymphocyte responses such as proliferation and IL-2 production. Copper deficiency in animals impairs a range of immune functions and increases susceptibility to bacterial and parasitic challenges. Human studies show that subjects on a low copper diet have decreased lymphocyte proliferation and IL-2 production, with copper administration reversing these effects 128. Children with Menke’s syndrome, a rare congenital disease with complete absence of the circulating copper-carrying protein caeruloplasmin, show immune impairments and have increased bacterial infections, diarrhea and pneumonia 129. Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair copper 30.
A wide variety of plant and animal foods contain copper, and the average human diet provides approximately 1,400 mcg/day for men and 1,100 mcg/day for women that is primarily absorbed in the upper small intestine 130. Almost two-thirds of the body’s copper is located in the skeleton and muscle 124.
Only small amounts of copper are typically stored in the body, and the average adult has a total body content of 50–120 mg copper 124. Most copper is excreted in bile, and a small amount is excreted in urine. Total fecal losses of copper of biliary origin and nonabsorbed dietary copper are about 1 mg/day 124. Copper levels in the body are homeostatically maintained by copper absorption from the intestine and copper release by the liver into bile to provide protection from copper deficiency and toxicity 131.
Copper status is not routinely assessed in clinical practice, and no biomarkers that accurately and reliably assess copper status have been identified 132. Human studies typically measure copper and cuproenzyme activity in plasma and blood cells because individuals with known copper deficiency often have low blood levels of copper and ceruloplasmin 132. However, plasma ceruloplasmin and copper levels can be influenced by other factors, such as estrogen status, pregnancy, infection, inflammation, and some cancers 132. Normal serum concentrations are 10–25 mcmol/L (63.5–158.9 mcg/dL) for copper and 180–400 mg/L for ceruloplasmin 133.
The amount of copper you need each day depends on your age. Typical diets in the United States meet or exceed the copper recommended dietary allowance (RDA), which is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals. Mean dietary intakes of copper from foods range from 800 to 1,000 mcg per day for children aged 2–19 134. In adults aged 20 and older, average daily intakes of copper from food are 1,400 mcg for men and 1,100 mcg for women. Total intakes from supplements and foods are 900 to 1,100 mcg/day for children and 1,400 to 1,700 mcg/day for adults aged 20 and over.
Copper deficiency is uncommon in humans 132. Based on studies in animals and humans, the effects of copper deficiency include anemia, hypopigmentation, hypercholesterolemia, connective tissue disorders, osteoporosis and other bone defects, abnormal lipid metabolism, ataxia, and increased risk of infection 135.
Copper is available in dietary supplements containing only copper, in supplements containing copper in combination with other ingredients, and in many multivitamin/multimineral products 136. These supplements contain many different forms of copper, including cupric oxide, cupric sulfate, copper amino acid chelates, and copper gluconate. To date, no studies have compared the bioavailability of copper from these and other forms 137. The amount of copper in dietary supplements typically ranges from a few micrograms to 15 mg (about 17 times the daily value [DV] for copper) 136.
Table 16. Selected Food Sources of Copper
| Food | Micrograms (mcg) per serving | Percent DV* |
| Beef, liver, pan fried (3 ounces) | 12400 | 1378 |
| Oysters, eastern, wild, cooked, 3 ounces | 4850 | 539 |
| Baking chocolate, unsweetened, 1 ounce | 938 | 104 |
| Potatoes, cooked, flesh and skin, 1 medium potato | 675 | 75 |
| Mushrooms, shiitake, cooked, cut pieces, ½ cup | 650 | 72 |
| Cashew nuts, dry roasted, 1 ounce | 629 | 70 |
| Crab, Dungeness, cooked, 3 ounces | 624 | 69 |
| Sunflower seed kernels, toasted, ¼ cup | 615 | 68 |
| Turkey, giblets, simmered, 3 ounces | 588 | 65 |
| Chocolate, dark, 70%-85% cacao solids, 1 ounce | 501 | 56 |
| Tofu, raw, firm, ½ cup | 476 | 53 |
| Chickpeas, mature sees, ½ cup | 289 | 32 |
| Millet, cooked, 1 cup | 280 | 31 |
| Salmon, Atlantic, wild, cooked, 3 ounces | 273 | 30 |
| Pasta, whole wheat, cooked, 1 cup (not packed) | 263 | 29 |
| Avocado, raw, ½ cup | 219 | 24 |
| Figs, dried, ½ cup | 214 | 24 |
| Spinach, boiled, drained, ½ cup | 157 | 17 |
| Asparagus, cooked, drained, ½ cup | 149 | 17 |
| Seseame seeds, ¼ cup | 147 | 16 |
| Turkey, ground, cooked, 3 ounces | 128 | 14 |
| Cereals, Cream of Wheat, cooked with water, stove-top, 1 cup | 104 | 12 |
| Tomatoes, raw, chopped, ½ cup | 53 | 6 |
| Yogurt, Greek, plain, lowfat, 7-ounce container | 42 | 5 |
| Milk, nonfat, 1 cup | 27 | 3 |
| Apples, raw, with skin, ½ cup slices | 17 | 2 |
Footnote: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for copper is 0.9 mg (900 mcg) for adults and children age 4 years and older [13]. The FDA does not require food labels to list copper content unless copper has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 48]Selenium
Selenium is a trace element that is naturally present in many foods, added to others, and available as a dietary supplement. Selenium, which is nutritionally essential for humans, is a constituent of more than two dozen selenoproteins that play critical roles in reproduction, thyroid hormone metabolism, DNA synthesis, and protection from oxidative damage and infection 138.
Selenium exists in two forms:
- inorganic (selenate and selenite) and
- organic (selenomethionine and selenocysteine) 139.
Both forms can be good dietary sources of selenium 140. Soils contain inorganic selenites and selenates that plants accumulate and convert to organic forms, mostly selenocysteine and selenomethionine and their methylated derivatives.
Selenium is found naturally in many foods. The amount of selenium in plant foods depends on the amount of selenium in the soil where they were grown. The amount of selenium in animal products depends on the selenium content of the foods that the animals ate. You can get recommended amounts of selenium by eating a variety of foods, including the following:
- Seafood
- Meat, poultry, eggs, and dairy products
- Breads, cereals, and other grain products.
Selenium is important for reproduction, thyroid gland function, DNA production, and protecting the body from damage caused by free radicals and from infection 141, 142. Selenium is incorporated into selenoproteins that have a wide range of pleiotropic effects, ranging from antioxidant and anti-inflammatory effects to the production of active thyroid hormone 143. In the past 10 years, the discovery of disease-associated polymorphisms in selenoprotein genes has drawn attention to the relevance of selenoproteins to health. Low selenium status has been associated with increased risk of mortality, poor immune function, and cognitive decline. Higher selenium status or selenium supplementation has antiviral effects, is essential for successful male and female reproduction, and reduces the risk of autoimmune thyroid disease. Prospective studies have generally shown some benefit of higher selenium status on the risk of prostate, lung, colorectal, and bladder cancers, but findings from trials have been mixed, which probably emphasises the fact that supplementation will confer benefit only if intake of a nutrient is inadequate. Supplementation of people who already have adequate intake with additional selenium might increase their risk of type-2 diabetes. The crucial factor that needs to be emphasised with regard to the health effects of selenium is the inextricable U-shaped link with status; whereas additional selenium intake may benefit people with low status, those with adequate-to-high status might be affected adversely and should not take selenium supplements.
There are a number of reviews of the role of selenium in immunity and host susceptibility to infection 144. Selenium deficiency in laboratory animals adversely affects several components of both innate and acquired immunity, including T and B lymphocyte function including antibody production and increases susceptibility to infections. Lower selenium concentrations in humans have been linked with diminished natural killer cell activity and increased mycobacterial disease. Selenium deficiency was shown to permit mutations of coxsackievirus, polio virus and murine influenza virus increasing virulence 145. These latter observations suggest that poor selenium status could result in the emergence of more pathogenic strains of virus, thereby increasing the risks and burdens associated with viral infection. Selenium supplementation (100 to 300 µg/day depending on the study) has been shown to improve various aspects of immune function in humans 146, including in the elderly 147. Selenium supplementation (50 or 100 µg/day) in adults in the UK with low selenium status improved some aspects of their immune response to a poliovirus vaccine 148.
Table 17. Selected Food Sources of Selenium
| Food | Micrograms (mcg) per serving | Percent DV* |
| Brazil nuts, 1 ounce (6–8 nuts) | 544 | 777 |
| Tuna, yellowfin, cooked, dry heat, 3 ounces | 92 | 131 |
| Halibut, cooked, dry heat, 3 ounces | 47 | 67 |
| Sardines, canned in oil, drained solids with bone, 3 ounces | 45 | 64 |
| Ham, roasted, 3 ounces | 42 | 60 |
| Shrimp, canned, 3 ounces | 40 | 57 |
| Macaroni, enriched, cooked, 1 cup | 37 | 53 |
| Beef steak, bottom round, roasted, 3 ounces | 33 | 47 |
| Turkey, boneless, roasted, 3 ounces | 31 | 44 |
| Beef liver, pan fried, 3 ounces | 28 | 40 |
| Chicken, light meat, roasted, 3 ounces | 22 | 31 |
| Cottage cheese, 1% milkfat, 1 cup | 20 | 29 |
| Rice, brown, long-grain, cooked, 1 cup | 19 | 27 |
| Beef, ground, 25% fat, broiled, 3 ounces | 18 | 26 |
| Egg, hard-boiled, 1 large | 15 | 21 |
| Puffed wheat ready-to-eat cereal, fortified, 1 cup | 15 | 21 |
| Bread, whole-wheat, 1 slice | 13 | 19 |
| Baked beans, canned, plain or vegetarian, 1 cup | 13 | 19 |
| Oatmeal, regular and quick, unenriched, cooked with water, 1 cup | 13 | 19 |
| Spinach, frozen, boiled, 1 cup | 11 | 16 |
| Milk, 1% fat, 1 cup | 8 | 11 |
| Yogurt, plain, low fat, 1 cup | 8 | 11 |
| Lentils, boiled, 1 cup | 6 | 9 |
| Bread, white, 1 slice | 6 | 9 |
| Spaghetti sauce, marinara, 1 cup | 4 | 6 |
| Cashew nuts, dry roasted, 1 ounce | 3 | 4 |
| Corn flakes, 1 cup | 2 | 3 |
| Green peas, frozen, boiled, 1 cup | 2 | 3 |
| Bananas, sliced, 1 cup | 2 | 3 |
| Potato, baked, flesh and skin, 1 potato | 1 | 1 |
| Peaches, canned in water, solids and liquids, 1 cup | 1 | 1 |
| Carrots, raw, 1 cup | 0 | 0 |
| Lettuce, iceberg, raw, 1 cup | 0 | 0 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for selenium is 70 mcg for adults and children aged 4 and older. Foods providing 20% or more of the DV are considered to be high sources of a nutrient. The U.S. Department of Agriculture’s (USDA’s) Nutrient Database Web site 149 lists the nutrient content of many foods and provides a comprehensive list of foods containing selenium arranged by nutrient content and by food name.
[Source 48]Zinc
Zinc is involved in numerous aspects of cellular metabolism. It is required for the catalytic activity of approximately 100 enzymes, including many nicotinamide adenine dinucleotide (NADH) dehydrogenases, RNA and DNA polymerases, and DNA transcription factors as well as alkaline phosphatase, superoxide dismutase, and carbonic anhydrase 150, 151 and it plays a role in immune function 152, 153, protein synthesis 153, wound healing 154, DNA synthesis 151, 153 and cell division 153. Zinc also supports normal growth and development during pregnancy, childhood, and adolescence 155, 156, 157 and is required for proper sense of taste and smell 158. A daily intake of zinc is required to maintain a steady state because the body has no specialized zinc storage system 159.
Most Americans get enough zinc from the foods they eat.
There are a number of reviews of the role of zinc in immunity and host susceptibility to infection 160. Of note, Read et al 161 have recently provided a very insightful evaluation of the role of zinc in antiviral immunity. Zinc inhibits the RNA polymerase required by RNA viruses, like coronaviruses, to replicate 162, suggesting that zinc may play a key role in host defence against RNA viruses. In vitro replication of influenza virus was inhibited by the zinc ionophore pyrrolidine dithiocarbamate 163 and there are indications that zinc might inhibit replication of SARS-associated coronavirus (SARS-CoV) in vitro 164. In addition, as discussed by Read et al 161, the zinc-binding metallothioneins seem to play an important role in antiviral defence 165. Zinc deficiency has a marked impact on bone marrow, decreasing the number immune precursor cells, with reduced output of naive B lymphocytes and causes thymic atrophy, reducing output of naive T lymphocytes. Therefore, zinc is important in maintaining T and B lymphocyte numbers. Zinc deficiency impairs many aspects of innate immunity, including phagocytosis, respiratory burst and natural killer cell activity. Zinc also supports the release of neutrophil extracellular traps that capture microbes 166. There are also marked effects of zinc deficiency on acquired immunity. Circulating CD4+ T lymphocyte numbers and function (eg, IL-2 and IFN-γ production) are decreased and there is a disturbance in favour of T helper 2 cells. Likewise, B lymphocyte numbers and antibody production are decreased in zinc deficiency. Zinc supports proliferation of CD8+ cytotoxic T lymphocytes, key cells in antiviral defence. Many of the in vitro immune effects of zinc are prevented by zinc chelation 167. Moderate or mild zinc deficiency or experimental zinc deficiency in humans result in decreased natural killer cell activity, T lymphocyte proliferation, IL-2 production and cell-mediated immune responses which can all be corrected by zinc repletion 168. In patients with zinc deficiency related to sickle cell disease, natural killer cell activity is decreased, but can be returned to normal by zinc supplementation 169. Patients with the zinc malabsorption syndrome acrodermatitis enteropathica display severe immune impairments 170 and increased susceptibility to bacterial, viral and fungal infections. Zinc supplementation (30 mg/day) increased T lymphocyte proliferation in elderly care home residents in the USA, an effect mainly due to an increase in numbers of T lymphocytes 171. The wide ranging impact of zinc deficiency on immune components is an important contributor to the increased susceptibility to infections, especially lower respiratory tract infection and diarrhoea, seen in zinc deficiency. Correcting zinc deficiency lowers the likelihood of diarrhea and of respiratory and skin infections, although some studies fail to show benefit of zinc supplementation in respiratory disease 172. Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair zinc 30. Recent systematic reviews and meta-analyses of trials with zinc report shorter duration of common cold in adults 31, reduced incidence and prevalence of pneumonia in children 33 and reduced mortality when given to adults with severe pneumonia 34.
Table 18. Selected Food Sources of Zinc
| Food | Milligrams (mg) per serving | Percent DV* |
| Oysters, cooked, breaded and fried, 3 ounces | 74 | 493 |
| Beef chuck roast, braised, 3 ounces | 7 | 47 |
| Crab, Alaska king, cooked, 3 ounces | 6.5 | 43 |
| Beef patty, broiled, 3 ounces | 5.3 | 35 |
| Breakfast cereal, fortified with 25% of the DV for zinc, ¾ cup serving | 3.8 | 25 |
| Lobster, cooked, 3 ounces | 3.4 | 23 |
| Pork chop, loin, cooked, 3 ounces | 2.9 | 19 |
| Baked beans, canned, plain or vegetarian, ½ cup | 2.9 | 19 |
| Chicken, dark meat, cooked, 3 ounces | 2.4 | 16 |
| Yogurt, fruit, low fat, 8 ounces | 1.7 | 11 |
| Cashews, dry roasted, 1 ounce | 1.6 | 11 |
| Chickpeas, cooked, ½ cup | 1.3 | 9 |
| Cheese, Swiss, 1 ounce | 1.2 | 8 |
| Oatmeal, instant, plain, prepared with water, 1 packet | 1.1 | 7 |
| Milk, low-fat or non fat, 1 cup | 1 | 7 |
| Almonds, dry roasted, 1 ounce | 0.9 | 6 |
| Kidney beans, cooked, ½ cup | 0.9 | 6 |
| Chicken breast, roasted, skin removed, ½ breast | 0.9 | 6 |
| Cheese, cheddar or mozzarella, 1 ounce | 0.9 | 6 |
| Peas, green, frozen, cooked, ½ cup | 0.5 | 3 |
| Flounder or sole, cooked, 3 ounces | 0.3 | 2 |
Footnote: * DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration to help consumers compare the nutrient contents of products within the context of a total diet. The DV for zinc is 15 mg for adults and children age 4 and older. Food labels, however, are not required to list zinc content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 48 ]Magnesium
Magnesium (Mg) is an abundant mineral in your body that your body needs to stay healthy and is naturally present in many foods, added to other food products, available as a dietary supplement, and present in some medicines (such as antacids and laxatives) 173. Magnesium is important for many processes in your body, including regulating muscle and nerve function, energy production, blood sugar levels, and blood pressure and making protein, bone, and DNA. Magnesium is a cofactor in more than 300 enzyme systems that regulate diverse biochemical reactions in the body, including protein synthesis, muscle and nerve function, blood glucose control, and blood pressure regulation 174, 175, 176, 177, 178. Low magnesium levels (hypomagnesemia) don’t cause symptoms in the short term. However, chronically low magnesium levels can increase your risk of high blood pressure, heart disease, type 2 diabetes and osteoporosis 179.
Magnesium is required for energy production, oxidative phosphorylation, and glycolysis 180. It contributes to the structural development of bone and is required for the synthesis of DNA, RNA, and the antioxidant glutathione. Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to nerve impulse conduction, muscle contraction, and normal heart rhythm 176. Many people don’t get enough magnesium in their diets. However, before you reach for a supplement, though, you should know that just a few servings of magnesium-rich foods a day can meet your need for this important nutrient.
An adult body contains approximately 25 g magnesium, with 50% to 60% present in the bones and most of the rest in soft tissues 181. Less than 1% of total magnesium is in blood serum, and these levels are kept under tight control through its absorption, reservoir, and excretion process by various organs such as the gut, bone, and kidney 182. Besides these organs, several hormones, namely vitamin D, parathyroid hormone, and estrogen, are involved in the regulation of normal level of magnesium 183. Normal serum magnesium concentrations range between 0.75 and 0.95 millimoles (mmol)/L 174, 184. Hypomagnesemia is defined as a serum magnesium level less than 0.75 mmol/L 185. Magnesium homeostasis is largely controlled by the kidney, which typically excretes about 120 mg magnesium into the urine each day 175. Urinary excretion is reduced when magnesium status is low 174. Hypomagnesemia is characterized by tetany (involuntary contraction of muscles), convulsion (seizures), and cardiac arrhythmia 186. Clinical and preclinical studies revealed that the magnesium level is found to be low in various pathological conditions such as migraine, diabetes, osteoporosis, asthma, preeclampsia, cardiovascular diseases and its correction is an important treatment strategy for these conditions 187, 188, 189, 190, 191.
The diets of many people in the United States provide less than the recommended amounts of magnesium. Men older than 70 and teenage girls and boys are most likely to have low intakes of magnesium. When the amount of magnesium people get from food and dietary supplements is combined, however, total intakes of magnesium are generally above recommended amounts.
Assessing magnesium status is difficult because most magnesium is inside cells or in bone 176. The most commonly used and readily available method for assessing magnesium status is measurement of serum magnesium concentration, even though serum levels have little correlation with total body magnesium levels or concentrations in specific tissues 185. Other methods for assessing magnesium status include measuring magnesium concentrations in erythrocytes, saliva, and urine; measuring ionized magnesium concentrations in blood, plasma, or serum; and conducting a magnesium-loading (or “tolerance”) test. No single method is considered satisfactory 192. Some experts 181 but not others 176 consider the tolerance test (in which urinary magnesium is measured after parenteral infusion of a dose of magnesium) to be the best method to assess magnesium status in adults. To comprehensively evaluate magnesium status, both laboratory tests and a clinical assessment might be required 185.
Magnesium is important in acquired immunity via regulating lymphocyte growth 193. An in vitro study (test tube study) carried out in chicken B cell line DT40 revealed that the removal of magnesium channel, TRPM7, results in cell death and can be partially corrected by magnesium supplementation 194. Mutation in a magnesium transporter, MagT1, is reported in patients with X-linked immunodeficiency diseases, Epstein–Barr virus infection, and neoplasia 195. Low CD4+ T cells and defective activation of T-lymphocytes are due to the decreased magnesium influx, which fails to activate PLCγ1 196. The importance of magnesium for CD4+ activation is also evident from reported studies conducted in asthma patients 197. However, further studies are essential to conclude the effect of magnesium on T cell signaling.
Magnesium has an important role in synthesizing and releasing immune cells and other associated processes like cell adhesion and phagocytosis 198. Magnesium acts as a natural calcium antagonist, the molecular basis for inflammatory response could be the result of modulation of intracellular calcium concentration 199. Besides, magnesium acts as a cofactor for the synthesis of immunoglobulin, CI 3 convertase, antibody-dependent cytolysis, macrophage responses to lymphocyte, IgM lymphocyte binding, T helper B cell adherence, substance P binding with lymphoblast, and binding of antigen to macrophage 200, 201. Magnesium deficiency affects various immune functions like the decline in NK cell level, monocytes and T cell ratio, increased oxidative stress after strenuous exercise, and elevated cytokine IL-6 level and inflammatory events. Deficiency of magnesium may be prone to recurrent bacterial and fungal infection 202. Many studies have demonstrated that in humans, a moderate or subclinical magnesium deficiency can induce chronic low-grade inflammation or exacerbate inflammatory stress caused by other factors 203. This low-grade inflammation increases the secretion of pro-inflammatory cytokines, which stimulate the resorption of bone by the induction of the differentiation of osteoclasts from their precursors 204. The ability of magnesium to decrease the inflammatory response and oxidative stress, as well as improving lung inflammation, possibly by inhibiting IL-6 pathway, NF-κB pathway, and L-type calcium channels 205, has raised the hypothesis of a possible magnesium supplementation in the prevention and treatment of COVID-19 patients, as suggested in the recent papers by Tang et al 206 and Iotti 207. Based Tang et al 208 basic and clinical research study, it is evident that magnesium effectively treats respiratory diseases like asthma and pneumonia because of its anti-inflammatory, antioxidant, and smooth muscle relaxant properties. A substantial decrease in the need for oxygen or intensive treatment assistance is reported in elderly COVID-19 patients upon the intake of vitamin D, magnesium, and vitamin B12 in combination 209. Iran’s clinical trial registry 210 confirmed that magnesium sulphate inhalation is effective in improving respiratory symptoms such as shortness of breath, cough and oxygen saturation in COVID-19 patients.
Magnesium deficient animal model exhibits inflammation as the first noticeable change with elevated levels of pro-inflammatory mediators like TNFα with declined anti-inflammatory cytokine levels 211, 201. The activation of immune cells like monocyte, macrophages, and polymorphonuclear cells are involved in the release of inflammatory mediators like cytokine, free radical and eicosanoids 201. Administration of magnesium reduces leukocyte activation and oxidative damage to peripheral blood lymphocyte DNA in athletes and sedentary young men 19. Thus, magnesium is an important factor for optimum immune cell functioning by regulating the proliferation and function of lymphocytes 198. In vitro studies also prove the role of magnesium in reducing leukocyte activation through its calcium antagonistic action 200. Magnesium deficiency results in the stress condition that activate the sympathetic system and hypothalamic-pituitary axis causes fat accumulation and release of neuropeptides; results in the immune response followed by inflammatory cascades 212.
Magnesium can inhibit cytokine storm and hyper oxidative stress in COVID-19 patients through various mechanisms such as its antioxidant, immune-modulatory, and anti-inflammatory activities 213.
Table 19. Magnesium Rich Foods
| Food | Milligrams (mg) per serving | Percent Daily Value (DV)* |
| Almonds, dry roasted, 1 ounce | 80 | 20 |
| Spinach, boiled, ½ cup | 78 | 20 |
| Cashews, dry roasted, 1 ounce | 74 | 19 |
| Peanuts, oil roasted, ¼ cup | 63 | 16 |
| Cereal, shredded wheat, 2 large biscuits | 61 | 15 |
| Soymilk, plain or vanilla, 1 cup | 61 | 15 |
| Black beans, cooked, ½ cup | 60 | 15 |
| Edamame, shelled, cooked, ½ cup | 50 | 13 |
| Peanut butter, smooth, 2 tablespoons | 49 | 12 |
| Bread, whole wheat, 2 slices | 46 | 12 |
| Avocado, cubed, 1 cup | 44 | 11 |
| Potato, baked with skin, 3.5 ounces | 43 | 11 |
| Rice, brown, cooked, ½ cup | 42 | 11 |
| Yogurt, plain, low fat, 8 ounces | 42 | 11 |
| Breakfast cereals, fortified with 10% of the DV for magnesium | 40 | 10 |
| Oatmeal, instant, 1 packet | 36 | 9 |
| Kidney beans, canned, ½ cup | 35 | 9 |
| Banana, 1 medium | 32 | 8 |
| Salmon, Atlantic, farmed, cooked, 3 ounces | 26 | 7 |
| Milk, 1 cup | 24–27 | 6–7 |
| Halibut, cooked, 3 ounces | 24 | 6 |
| Raisins, ½ cup | 23 | 6 |
| Chicken breast, roasted, 3 ounces | 22 | 6 |
| Beef, ground, 90% lean, pan broiled, 3 ounces | 20 | 5 |
| Broccoli, chopped and cooked, ½ cup | 12 | 3 |
| Rice, white, cooked, ½ cup | 10 | 3 |
| Apple, 1 medium | 9 | 2 |
| Carrot, raw, 1 medium | 7 | 2 |
Footnote: *DV = Daily Value. DVs were developed by the U.S. Food and Drug Administration (FDA) to help consumers compare the nutrient contents of products within the context of a total diet. The DV for magnesium is 400 mg for adults and children aged 4 and older. However, the FDA does not require food labels to list magnesium content unless a food has been fortified with this nutrient. Foods providing 20% or more of the DV are considered to be high sources of a nutrient.
[Source 214 ] References- About Primary Immunodeficiencies. https://primaryimmune.org/about-primary-immunodeficiencies
- Marcos A. Editorial: A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2021 Nov 22;13(11):4180. doi: 10.3390/nu13114180
- Gorji A, Khaleghi Ghadiri M. Potential roles of micronutrient deficiency and immune system dysfunction in the coronavirus disease 2019 (COVID-19) pandemic. Nutrition. 2021 Feb;82:111047. doi: 10.1016/j.nut.2020.111047
- James PT, Ali Z, Armitage AE, Bonell A, Cerami C, Drakesmith H, Jobe M, Jones KS, Liew Z, Moore SE, Morales-Berstein F, Nabwera HM, Nadjm B, Pasricha SR, Scheelbeek P, Silver MJ, Teh MR, Prentice AM. The Role of Nutrition in COVID-19 Susceptibility and Severity of Disease: A Systematic Review. J Nutr. 2021 Jul 1;151(7):1854-1878. doi: 10.1093/jn/nxab059
- Calder PC. Nutrition and immunity: lessons for COVID-19. Eur J Clin Nutr. 2021 Sep;75(9):1309-1318. doi: 10.1038/s41430-021-00949-8
- Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020 Apr 23;12(4):1181. doi: 10.3390/nu12041181
- Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020 Jan 16;12(1):236. doi: 10.3390/nu12010236
- Immunity In Brief. https://lpi.oregonstate.edu/mic/health-disease/immunity-in-brief
- Rice HB, Bernasconi A, Maki KC, Harris WS, von Schacky C, Calder PC. Conducting omega-3 clinical trials with cardiovascular outcomes: Proceedings of a workshop held at ISSFAL 2014. Prostaglandins Leukot Essent Fatty Acids. 2016 Apr;107:30-42. https://doi.org/10.1016/j.plefa.2016.01.003
- Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation. 2019 Sep 17;140(12):e673-e691. doi: 10.1161/CIR.0000000000000709
- Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation. 2017 Apr 11;135(15):e867-e884. doi: 10.1161/CIR.0000000000000482
- Rimm EB, Appel LJ, Chiuve SE, Djoussé L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation. 2018 Jul 3;138(1):e35-e47. doi: 10.1161/CIR.0000000000000574
- Maggini S., Pierre A., Calder P.C. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10:1531. doi: 10.3390/nu10101531
- Justiz Vaillant AA, Sabir S, Jan A. Physiology, Immune Response. [Updated 2020 Sep 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539801
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. Efsa J 2016;14:4369.
- Forrest H Nielsen, Henry C Lukaski . Update on the relationship between magnesium and exercise. Magnesium Research. 2006;19(3):180-189. doi:10.1684/mrh.2006.0060
- F. C. Mooren, S.W. Golf, and K. Völker . Effect of magnesium on granulocyte function and on the exercise induced inflammatory response . Magnesium Research. 2003;16(1):49-58.
- Maria José Laires, Cristina Monteiro . Exercise, magnesium and immune function. Magnesium Research. 2008;21(2):92-96. doi:10.1684/mrh.2008.0136
- Petrović J, Stanić D, Dmitrašinović G, Plećaš-Solarović B, Ignjatović S, Batinić B, Popović D, Pešić V. Magnesium Supplementation Diminishes Peripheral Blood Lymphocyte DNA Oxidative Damage in Athletes and Sedentary Young Man. Oxid Med Cell Longev. 2016;2016:2019643. doi: 10.1155/2016/2019643
- Bussière FI, Mazur A, Fauquert JL, Labbe A, Rayssiguier Y, Tridon A. High magnesium concentration in vitro decreases human leukocyte activation. Magnes Res. 2002 Mar;15(1-2):43-8.
- Vitamins and minerals that affect the immune system. https://www.hiv.va.gov/patient/daily/diet/vitamin-mineral-chart.asp
- Imdad A, Mayo-Wilson E, Herzer K, et al. . Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev 2017;3:CD008524. 10.1002/14651858.CD008524.pub3
- Hu N, QB L, Zou SY. Effect of vitamin A as an adjuvant therapy for pneumonia in children: a meta analysis. Zhongguo Dang Dai Er. Ke. Za Zhi 2018;20:146–53.
- Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev 2013:CD005532. 10.1002/14651858.CD005532.pub3
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2013:CD000980. 10.1002/14651858.CD000980.pub4
- Bergman P, Lindh Åsa U., Björkhem-Bergman L, et al. . Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2013;8:e65835 10.1371/journal.pone.0065835
- Martineau AR, Jolliffe DA, Hooper RL, et al. . Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583 10.1136/bmj.i6583
- Pham H, Rahman A, Majidi A, et al. . Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis. Int J Environ Res Public Health 2019;16:3020 10.3390/ijerph16173020
- Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine 2019;98:17252.
- Mao S, Zhang A, Huang S. Meta-Analysis of Zn, Cu and Fe in the hair of Chinese children with recurrent respiratory tract infection. Scand J Clin Lab Invest 2014;74:561–7. 10.3109/00365513.2014.921323
- Hemilä H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open 2017;8:205427041769429. 10.1177/2054270417694291
- Science M, Johnstone J, Roth DE, et al. . Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized controlled trials. Can Med Assoc J 2012;184:E551–61. 10.1503/cmaj.111990
- Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev 2016;12:CD005978. 10.1002/14651858.CD005978.pub3
- Wang L, Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J 2018;12:857–64. 10.1111/crj.12646
- Johnson EJ, Russell RM. Beta-Carotene. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:115-20.
- Ross CA. Vitamin A. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:778-91.
- Ross A. Vitamin A and Carotenoids. In: Shils M, Shike M, Ross A, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006:351-75.
- Institute of Medicine, US Panel on Micronutrients. Dietary reference intakes for vitamin A, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academies Press. Washington, DC, 2001. PMID: 25057538 www.ncbi.nlm.nih.gov/pubmed/25057538.
- National Institute of Health. Vitamin A. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional
- Oliveira LdeM, Teixeira FME, Sato MN. Impact of retinoic acid on immune cells and inflammatory diseases. Mediators Inflamm 2018;2018:1–17. 10.1155/2018/3067126
- Ross AC. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol 1996;80:S63–72. 10.1006/clin.1996.0143
- Semba RD, Scott AL, et al. , Muhilal . Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr 1992;122:101–7. 10.1093/jn/122.1.101
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 24. Nutrient Data Laboratory Home Page, 2011. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/
- Yoshii K, Hosomi K, Sawane K, et al. . Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019;6:48 10.3389/fnut.2019.00048
- Tamura J, Kubota K, Murakami H, et al. . Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol 1999;116:28–32. 10.1046/j.1365-2249.1999.00870.x
- Meydani SN, Ribaya-Mercado JD, Russell RM, et al. . Vitamin B − 6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am J Clin Nutr 1991;53:1275–80. 10.1093/ajcn/53.5.1275
- Said HM. Thiamin. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:748-53.
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27. Nutrient Data Laboratory home page, 2014. https://ndb.nal.usda.gov/ndb/
- Said HM, Ross AC. Riboflavin. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:325-30.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline 1998. https://www.nap.edu/catalog/6015/dietary-reference-intakes-for-thiamin-riboflavin-niacin-vitamin-b6-folate-vitamin-b12-pantothenic-acid-biotin-and-choline
- McCormick DB. Riboflavin. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:280-92.
- Rivlin RS. Riboflavin. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:691-9.
- Gaylord AM, Warthesen JJ, Smith DE. Influence of milk fat, milk solids, and light intensity on the light stability of vitamin A and riboflavin in lowfat milk. J Dairy Sci 1986;69:2779-84. https://www.ncbi.nlm.nih.gov/pubmed/3805455?dopt=Abstract
- Mackey A, Davis S, Gregory J. Vitamin B6. In: Shils M, Shike M, Ross A, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005.
- McCormick D. Vitamin B6. In: Bowman B, Russell R, eds. Present Knowledge in Nutrition. 9th ed. Washington, DC: International Life Sciences Institute; 2006.
- Herbert V. Vitamin B12 in Present Knowledge in Nutrition. 17th ed. Washington, DC: International Life Sciences Institute Press, 1996.
- Herbert V, Das K. Vitamin B12 in Modern Nutrition in Health and Disease. 8th ed. Baltimore, MD: Williams & Wilkins, 1994.
- Combs G. Vitamin B12 in The Vitamins. New York: Academic Press, Inc., 1992.
- Zittoun J, Zittoun R. Modern clinical testing strategies in cobalamin and folate deficiency. Sem Hematol 1999;36:35-46. https://www.ncbi.nlm.nih.gov/pubmed/9930567?dopt=Abstract
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press, 1998.
- Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clin Chem 2000;46:1277-83. https://www.ncbi.nlm.nih.gov/pubmed/10926922?dopt=Abstract
- Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood.2008;112:2214-21. https://www.ncbi.nlm.nih.gov/pubmed/18606874?dopt=Abstract
- U.S. National Library of Medicine, MedlinePlus – Folic Acid – https://medlineplus.gov/folicacid.html
- Office on Women’s Health, U.S. Department of Health and Human Services – Folic acid – https://www.womenshealth.gov/a-z-topics/folic-acid
- Centers for Disease Control and Prevention – Facts About Folic Acid – https://www.cdc.gov/ncbddd/folicacid/about.html
- Naidu KA: Vitamin C in human health and disease is still a mystery? An overview. Nutr J 2: 7, 2003. https://www.ncbi.nlm.nih.gov/pubmed/14498993?dopt=Abstract
- Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007;137:2171-84. https://www.ncbi.nlm.nih.gov/pubmed/17884994?dopt=Abstract
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids Washington, DC: National Academy Press; 2000.
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care 2002;5:66-74. https://www.ncbi.nlm.nih.gov/pubmed/12134712
- Merck Sharp & Dohme Corp., Merck Manual. Vitamin C (Ascorbic Acid). https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-c
- Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020 Dec 7;12(12):3760. doi: 10.3390/nu12123760
- Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017 Nov 3;9(11):1211. doi: 10.3390/nu9111211
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD000980. doi: 10.1002/14651858.CD000980.pub4
- Johnston CS. Vitamin C. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition 11th ed. Cambridge, MA: Elsevier; 2020:155-69.
- Hemilä H. Vitamin C and Infections. Nutrients. 2017 Mar 29;9(4):339. doi: 10.3390/nu9040339
- Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009 Nov;90(5):1252-63. doi: 10.3945/ajcn.2008.27016
- National Cancer Institute. High-Dose Vitamin C–Patient Version. https://www.cancer.gov/about-cancer/treatment/cam/patient/vitamin-c-pdq#link/_5
- Hemilä H. Vitamin C and infections. Nutrients 2017;9:339 10.3390/nu9040339
- Jacob RA, Kelley DS, Pianalto FS, et al. . Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr 1991;54:1302S–9. 10.1093/ajcn/54.6.1302s
- Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr 1999;69:1086-107. https://www.ncbi.nlm.nih.gov/pubmed/10357726?dopt=Abstract
- Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377-81. Ascorbate is an outstanding antioxidant in human blood plasma. https://www.ncbi.nlm.nih.gov/pubmed/2762330%20?dopt=Abstract
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care 2002;5:66-74. https://www.ncbi.nlm.nih.gov/pubmed/12134712?dopt=Abstract
- Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev 1993;51:313-26. https://www.ncbi.nlm.nih.gov/pubmed/8108031?dopt=Abstract
- Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics 2001;108:E55. https://www.ncbi.nlm.nih.gov/pubmed/11533373?dopt=Abstract
- Wang AH, Still C. Old world meets modern: a case report of scurvy. Nutr Clin Pract 2007;22:445-8. https://www.ncbi.nlm.nih.gov/pubmed/17644699?dopt=Abstract
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000. https://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med 2001;21:235-7. https://www.ncbi.nlm.nih.gov/pubmed/11604276
- National Institute of Health. Vitamin D. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010
- Cranney C, Horsely T, O’Donnell S, Weiler H, Ooi D, Atkinson S, et al. Effectiveness and safety of vitamin D. Evidence Report/Technology Assessment No. 158 prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-02.0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality, 2007. https://www.ncbi.nlm.nih.gov/pubmed/18088161?dopt=Abstract
- Holick MF. Vitamin D. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease, 10th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Norman AW, Henry HH. Vitamin D. In: Bowman BA, Russell RM, eds. Present Knowledge in Nutrition, 9th ed. Washington DC: ILSI Press, 2006.
- Medrano M, Carrillo-Cruz E, Montero I, et al. . Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci 2018;19:2663 10.3390/ijms19092663
- Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 2009;4:1151–65. 10.2217/fmb.09.87
- Lee M-D, Lin C-H, Lei W-T, et al. . Does vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients 2018;10:409 10.3390/nu10040409
- Berry DJ, Hesketh K, Power C, et al. . Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr 2011;106:1433–40. 10.1017/S0007114511001991
- Ginde AA, Mansbach JM, Camargo CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third National Health and Nutrition Examination Survey. Arch Intern Med 2009;169:384–90. 10.1001/archinternmed.2008.560
- Sabetta JR, DePetrillo P, Cipriani RJ, et al. . Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One 2010;5:e11088 10.1371/journal.pone.0011088
- Urashima M, Segawa T, Okazaki M, et al. . Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010;91:1255–60. 10.3945/ajcn.2009.29094
- Traber MG. Vitamin E. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006;396-411.
- Merck Sharp & Dohme Corp., Merck Manual. Vitamin E (Tocopherol). https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-e
- Verhagen H, Buijsse B, Jansen E, Bueno-de-Mesquita B. The state of antioxidant affairs. Nutr Today 2006;41:244-50.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000. https://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids
- Lee G, Han S. The role of vitamin E in immunity. Nutrients 2018;10:614 10.3390/nu10111614
- Chavance M, Herbeth B, Fournier C, et al. . Vitamin status, immunity and infections in an elderly population. Eur J Clin Nutr 1989;43:827–35.
- De la Fuente M, Hernanz A, Guayerbas N, et al. . Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res 2008;42:272–80. 10.1080/10715760801898838
- Meydani SN, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. JAMA 1997;277:1380–6. 10.1001/jama.1997.03540410058031
- Meydani SN, Barklund MP, Liu S, et al. . Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr 1990;52:557–63. 10.1093/ajcn/52.3.557
- Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging 2016;11:1379–85. 10.2147/CIA.S114515
- Meydani SN, Leka LS, Fine BC, et al. . Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA 2004;292:828–36. 10.1001/jama.292.7.828
- Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA 2002;288:715–21. 10.1001/jama.288.6.715
- U.S. National Library of Medicine. Medline Plus. Iron. https://medlineplus.gov/iron.html#cat_51
- Centers for Disease Control and Prevention. Recommendations to Prevent and Control Iron Deficiency in the United States. https://www.cdc.gov/mmwr/preview/mmwrhtml/00051880.htm
- U.S. National Library of Medicine. Iron. https://livertox.nlm.nih.gov/Iron.htm
- Nairz M, Dichtl S, Schroll A, et al. . Iron and innate antimicrobial immunity-Depriving the pathogen, defending the host. J Trace Elem Med Biol 2018;48:118–33. 10.1016/j.jtemb.2018.03.007
- Ahluwalia N, Sun J, Krause D, et al. . Immune function is impaired in iron-deficient, homebound, older women. Am J Clin Nutr 2004;79:516–21. 10.1093/ajcn/79.3.516
- Nairz M, Theurl I, Swirski FK, et al. . “Pumping iron”—how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch – Eur J Physiol 2017;469:397–418. 10.1007/s00424-017-1944-8
- Ganz T. Iron and infection. Int J Hematol 2018;107:7–15. 10.1007/s12185-017-2366-2
- Malan L, Baumgartner J, Calder PC, et al. . n–3 long-chain PUFAs reduce respiratory morbidity caused by iron supplementation in iron-deficient South African schoolchildren: a randomized, double-blind, placebo-controlled intervention. Am J Clin Nutr 2015;101:668–79. 10.3945/ajcn.113.081208
- Murray-Kolbe LE, Beard J. Iron. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:432-8.
- Aggett PJ. Iron. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:506-20.
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr 2010;91:1461S-7S. http://ajcn.nutrition.org/content/91/5/1461S.long
- Rutzke CJ, Glahn RP, Rutzke MA, Welch RM, Langhans RW, Albright LD, et al. Bioavailability of iron from spinach using an in vitro/human Caco-2 cell bioassay model. Habitation 2004;10:7-14. https://www.ncbi.nlm.nih.gov/pubmed/15880905
- Collins JF. Copper. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:206-16.
- Allen KG, Klevay LM. Copper: an antioxidant nutrient for cardiovascular health. Curr Opin Lipidol. 1994 Feb;5(1):22-8. doi: 10.1097/00041433-199402000-00005
- Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439-58. doi: 10.1146/annurev.nutr.22.012502.114457
- Li C, Li Y, Ding C. The role of copper homeostasis at the host-pathogen axis: from bacteria to fungi. Int J Mol Sci 2019;20:175 10.3390/ijms20010175
- Hopkins RG, Failla ML. Copper deficiency reduces interleukin-2 (IL-2) production and IL-2 mRNA in human T-lymphocytes. J Nutr 1997;127:257–62. 10.1093/jn/127.2.257
- Vyas D, Chandra RK. Thymic factor activity, lymphocyte stimulation response and antibody producing cells in copper deficiency. Nutr Res 1983;3:343–9. 10.1016/S0271-5317(83)80084-0
- Klevay LM. Is the Western diet adequate in copper? J Trace Elem Med Biol. 2011 Dec;25(4):204-12. doi: 10.1016/j.jtemb.2011.08.146
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academies Press; 2001.
- Prohaska JR. Copper. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:540-53.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America, 2013-2014. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables
- Prohaska JR. Impact of copper deficiency in humans. Ann N Y Acad Sci. 2014 May;1314:1-5. doi: 10.1111/nyas.12354
- National Institutes of Health. Dietary Supplement Label Database. 2021. https://dsld.od.nih.gov/dsld
- Rosado JL. Zinc and copper: proposed fortification levels and recommended zinc compounds. J Nutr. 2003 Sep;133(9):2985S-9S. doi: 10.1093/jn/133.9.2985S
- Sunde RA. Selenium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2012:225-37
- Sunde RA. Selenium. In: Bowman B, Russell R, eds. Present Knowledge in Nutrition. 9th ed. Washington, DC: International Life Sciences Institute; 2006:480-97
- Terry EN, Diamond AM. Selenium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:568-87
- Environ Health Prev Med. 2008 Mar; 13(2): 102–108. Selenium: its role as antioxidant in human health. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2698273/
- Br J Nutr. 2008 Aug;100(2):254-68. doi: 10.1017/S0007114508939830. Epub 2008 Mar 18. Food-chain selenium and human health: emphasis on intake. https://www.ncbi.nlm.nih.gov/pubmed/18346308?dopt=Abstract
- Lancet. 2012 Mar 31;379(9822):1256-68. doi: 10.1016/S0140-6736(11)61452-9. Epub 2012 Feb 29. Selenium and human health. https://www.ncbi.nlm.nih.gov/pubmed/22381456?dopt=Abstract
- Guillin OM, Vindry C, Ohlmann T, et al. . Selenium, selenoproteins and viral infection. Nutrients 2019;11:2101 10.3390/nu11092101
- Beck M, Handy J, Levander O. Host nutritional status: the neglected virulence factor. Trends Microbiol 2004;12:417–23. 10.1016/j.tim.2004.07.007
- Hawkes WC, Kelley DS, Taylor PC. The effects of dietary selenium on the immune system in healthy men. Biol Trace Elem Res 2001;81:189–213. 10.1385/BTER:81:3:189
- Roy M, Kiremidjian-Schumacher L, Wishe HI, et al. . Supplementation with selenium restores age-related decline in immune cell function. Exp Biol Med 1995;209:369–75. 10.3181/00379727-209-43909
- Broome CS, McArdle F, Kyle JAM, et al. . An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 2004;80:154–62. 10.1093/ajcn/80.1.154
- https://ndb.nal.usda.gov/ndb/
- Sandstead HH. Understanding zinc: recent observations and interpretations. J Lab Clin Med 1994;124:322-7. https://www.ncbi.nlm.nih.gov/pubmed/8083574?dopt=Abstract
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001). Washington, DC: National Academy Press, 2001. https://www.nap.edu/catalog/10026/dietary-reference-intakes-for-vitamin-a-vitamin-k-arsenic-boron-chromium-copper-iodine-iron-manganese-molybdenum-nickel-silicon-vanadium-and-zinc
- Solomons NW. Mild human zinc deficiency produces an imbalance between cell-mediated and humoral immunity. Nutr Rev 1998;56:27-8. https://www.ncbi.nlm.nih.gov/pubmed/9481116?dopt=Abstract
- Prasad AS. Zinc: an overview. Nutrition 1995;11:93-9. https://www.ncbi.nlm.nih.gov/pubmed/7749260?dopt=Abstract
- Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother 1996;30:186-7. https://www.ncbi.nlm.nih.gov/pubmed/8835055?dopt=Abstract
- Simmer K, Thompson RP. Zinc in the fetus and newborn. Acta Paediatr Scand Suppl 1985;319:158-63. https://www.ncbi.nlm.nih.gov/pubmed/3868917?dopt=Abstract
- Fabris N, Mocchegiani E. Zinc, human diseases and aging. Aging (Milano) 1995;7:77-93. https://www.ncbi.nlm.nih.gov/pubmed/7548268?dopt=Abstract
- Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 2006;20:3-18. https://www.ncbi.nlm.nih.gov/pubmed/16632171?dopt=Abstract
- Prasad AS, Beck FW, Grabowski SM, Kaplan J, Mathog RH. Zinc deficiency: changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc Assoc Am Physicians 1997;109:68-77. https://www.ncbi.nlm.nih.gov/pubmed/9010918?dopt=Abstract
- Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc 2000;59:541-52. https://www.ncbi.nlm.nih.gov/pubmed/11115789?dopt=Abstract
- Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int J Mol Sci 2017;18:2222 10.3390/ijms18102222
- Read SA, Obeid S, Ahlenstiel C, et al. . The role of zinc in antiviral immunity. Adv Nutr 2019;10:696–710. 10.1093/advances/nmz013
- Kaushik N, Subramani C, Anang S, et al. . Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J. Virol 2017;91i:e00754–17
- Uchide N, Ohyama K, Bessho T, et al. . Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res 2002;56:207–17. 10.1016/S0166-3542(02)00109-2
- te Velthuis AJW, van den Worm SHE, Sims AC, et al. . Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 2010;6:e1001176 10.1371/journal.ppat.1001176
- Subramanian Vignesh K, Deepe Jr. G. Metallothioneins: emerging modulators in immunity and infection. Int J Mol Sci 2017;18:2197 10.3390/ijms18102197
- Hasan R, Rink L, Haase H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun 2013;19:253–64. 10.1177/1753425912458815
- Hasan R, Rink L, Haase H. Chelation of free Zn²⁺ impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol Trace Elem Res 2016;171:79–88. 10.1007/s12011-015-0515-0
- Kahmann L, Uciechowski P, Warmuth S, et al. . Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res 2008;11:227–37. 10.1089/rej.2007.0613
- Tapazoglou E, Prasad AS, Hill G, et al. . Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J Lab Clin Med 1985;105:19–22.
- Sandström B, et al. Acrodermatitis enteropathica, zinc metabolism, copper status, and immune function. Arch Pediatr Adolesc Med 1994;148:980–5. 10.1001/archpedi.1994.02170090094017
- Barnett JB, Dao MC, Hamer DH, et al. . Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2016;103:942–51. 10.3945/ajcn.115.115188
- Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients 2017;9:624. 10.3390/nu9060624
- National Institutes of Health. Magnesium. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- Institute of Medicine (IOM). Food and Nutrition Board. Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: National Academy Press, 1997.
- Rude RK. Magnesium. In: Coates PM, Betz JM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, eds. Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare; 2010:527-37.
- Rude RK. Magnesium. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, Mass: Lippincott Williams & Wilkins; 2012:159-75.
- Pham PCT, Pham PAT, Pham SV, Pham PTT, Pham PMT, Pham PTT. Hypomagnesemia: a clinical perspective. Int J Nephrol Renovasc Dis. 2014 doi: 10.2147/IJNRD.S42054
- Bharadwaj J, Darbari A, Naithani M. Magnesium: the fifth electrolyte. J Med Nutr Nutraceuticals. 2014;3:186. doi: 10.4103/2278-019x.131955
- Ahmed F, Mohammed A. Magnesium: the forgotten electrolyte—a review on hypomagnesemia. Med Sci. 2019;7:56. doi: 10.3390/medsci7040056
- Fiorentini D, Cappadone C, Farruggia G, Prata C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients. 2021 Mar 30;13(4):1136. doi: 10.3390/nu13041136
- Volpe SL. Magnesium. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Ames, Iowa; John Wiley & Sons, 2012:459-74.
- Mathew AA, Panonnummal R. ‘Magnesium’-the master cation-as a drug-possibilities and evidences. Biometals. 2021 Oct;34(5):955-986. doi: 10.1007/s10534-021-00328-7
- Al Alawi AM, Majoni SW, Falhammar H. Magnesium and human health: perspectives and research directions. Int J Endocrinol. 2018 doi: 10.1155/2018/9041694
- Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res 2010;23:1-5. https://www.ncbi.nlm.nih.gov/pubmed/20736141?dopt=Abstract
- Gibson, RS. Principles of Nutritional Assessment, 2nd ed. New York, NY: Oxford University Press, 2005.
- Alexander RT, Hoenderop JG, Bindels RJ. Molecular determinants of magnesium homeostasis: insights from human disease. J Am Soc Nephrol. 2008;19:1451–1458. doi: 10.1681/ASN.2008010098
- Dolati S, Rikhtegar R, Mehdizadeh A, Yousefi M. The role of magnesium in pathophysiology and migraine treatment. Biol Trace Elem Res. 2019 doi: 10.1007/s12011-019-01931-z
- Gröber U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7:8199–8226. doi: 10.3390/nu7095388
- DiNicolantonio JJ, O’Keefe JH, Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Hear. 2018 doi: 10.1136/openhrt-2017-000668
- Li X, Han X, Yang J, Bao J, Di X, Zhang G, Liu H. Magnesium sulfate provides neuroprotection in eclampsia-like seizure model by ameliorating neuroinflammation and brain edema. Mol Neurobiol. 2017;54:7938–7948. doi: 10.1007/s12035-016-0278-4
- Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40:1169–1175. doi: 10.1161/STROKEAHA.108.527788
- Witkowski M, Hubert J, Mazur A. Methods of assessment of magnesium status in humans: a systematic review. Magnesium Res 2011;24:163-80. https://www.ncbi.nlm.nih.gov/pubmed/22064327?dopt=Abstract
- Tam M, Gómez S, González-Gross M, Marcos A. Possible roles of magnesium on the immune system. Eur J Clin Nutr. 2003;57:1193–1197. doi: 10.1038/sj.ejcn.1601689
- Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7-gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009
- Ravell J, Chaigne-Delalande B, Lenardo M. X-linked immunodeficiency with magnesium defect, Epstein-Barr virus infection, and neoplasia disease: a combined immune deficiency with magnesium defect. Curr Opin Pediatr. 2014;26:713–719. doi: 10.1097/MOP.0000000000000156
- Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011 Jul 27;475(7357):471-6. doi: 10.1038/nature10246
- Liang RY, Wu W, Huang J, Jiang SP, Lin Y. Magnesium affects the cytokine secretion of CD4+ T lymphocytes in acute asthma. J Asthma. 2012;49:1012–1015. doi: 10.3109/02770903.2012.739240
- Kubena KS. The role of magnesium in immunity. J Nutr Immunol. 1994;2(3):107–126. doi: 10.1300/J053v02n03_07
- Mazur A., Maier J.A., Rock E., Gueux E., Nowacki W., Rayssiguier Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007;458:48–56. doi: 10.1016/j.abb.2006.03.031
- Laires MJ, Monteiro C. Exercise, magnesium and immune function. Magnes Res. 2008;21(2):92–96. doi: 10.1684/mrh.2008.0136
- Galland L. Magnesium and immune function: an overview. Magnesium. 1988;7(5-6):290-9.
- Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm Res. 2018;11:25–34. doi: 10.2147/JIR.S136742
- Nielsen F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018;11:25–34. doi: 10.2147/JIR.S136742
- Klein G.L. The Role of Calcium in Inflammation-Associated Bone Resorption. Biomolecules. 2018;8:69. doi: 10.3390/biom8030069
- Güzel A., Doğan E., Türkçü G., Kuyumcu M., Kaplan İ., Çelik F., Yıldırım Z.B. Dexmedetomidine and Magnesium Sulfate: A Good Combination Treatment for Acute Lung Injury? J. Investig. Surg. 2019;32:331–342. doi: 10.1080/08941939.2017.1422575
- Tang C.-F., Ding H., Jiao R.-Q., Wu X.-X., Kong L.-D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharmacol. 2020;886:173546. doi: 10.1016/j.ejphar.2020.173546
- Iotti S., Wolf F., Mazur A., Maier J.A. The COVID-19 pandemic: Is there a role for magnesium? Hypotheses and perspectives. Magnes. Res. 2020;33:21–27. doi: 10.1684/mrh.2020.0465
- Tang CF, Ding H, Jiao RQ, Xing Xin Wu, Kong LD. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur J Pharmacol. 2020;886:173546. doi: 10.1016/j.ejphar.2020.173546
- Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, et al. A cohort study to evaluate the effect of combination vitamin D, magnesium and vitamin B12 (DMB) on progression to severe outcome in older COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.06.01.20112334
- Pourdowlat G, Mousavinasab SR, Farzanegan B, Kashefizadeh A, Meybodi ZA, Jafarzadeh M, Baniasadi S. Evaluation of the efficacy and safety of inhaled magnesium sulphate in combination with standard treatment in patients with moderate or severe COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:60. doi: 10.1186/s13063-021-05032-y
- Schmitz C, Anne-Laure P. Molecular, genetic, and nutritional aspects of major and trace minerals. In: Collins J, editor. Magnesium and the immune response. Amsterdam: Elsevier Inc; 2017. pp. 319–331.
- Rayssiguier Y, Libako P, Nowacki W, Rock E. Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnes Res. 2010;23(2):73–80. doi: 10.1684/mrh.2010.0208
- Moh’d Nour MBY, Alshawabkeh AD, Jadallah ARR, et al. Magnesium sulfate extended infusion as an adjunctive treatment for complicated Covid-19 infected critically ill patients. EAS J Anesthesiol Crit Care. 2020 doi: 10.36349/easjacc.2020.v02i03.17
- United States Department of Agriculture Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb/