Esophageal motility disorder
Esophageal dysmotility also called esophageal motility disorder are abnormal contractions occurring in the esophagus, which propel the food bolus forward toward the stomach, causing symptoms such as difficulty in swallowing (dysphagia), heartburn, and chest pain 1. When contractions in the esophagus become irregular, unsynchronized or absent, the patient is said to have esophageal dysmotility. The areas of dysfunction in the esophagus may be in the upper esophageal sphincter, the body of the esophagus or the lower esophageal sphincter (LES).
The esophagus functions solely to deliver food from the mouth to the stomach where the process of digestion can begin. Efficient transport by the esophagus requires a coordinated, sequential motility pattern that propels food from above and clears acid and bile reflux from below. Disruption of this highly integrated muscular motion limits delivery of food and fluid, as well as causes a bothersome sense of dysphagia and chest pain.
Disorders of esophageal motility are referred to as primary or secondary esophageal motility disorders and categorized according to their abnormal manometric patterns. The spectrum of these disorders ranges from the well-defined primary esophageal motility disorders to very nonspecific disorders that may play a more indirect role in reflux disease and otherwise be asymptomatic. Esophageal motility disorders may occur as manifestations of systemic diseases, referred to as secondary motility disorders.
Disorders of esophageal motility can also be divided into two categories: hypomotility and hypermotility, either of which can affect the lower esophageal sphincter (LES) and/or the esophageal body.
Dysphagia is a condition affecting over 9 million individuals in the United States. Oropharyngeal dysphagia, specifically, happens in as much as 50% of the elderly and 50% of patients with neurological pathologies. Clinically, oropharyngeal dysphagia can present with severe symptoms such as aspiration and death. Esophageal dysphagia, on the other hand, is a rarer condition with less severe symptoms but is easier to recognize due to symptoms arising from diseases of the enteric nervous system or esophageal muscular layers.
Esophageal motility disorders are less common than mechanical and inflammatory diseases affecting the esophagus, such as reflux esophagitis, peptic strictures, and mucosal rings. The clinical presentation of a motility disorder is varied, but, classically, dysphagia and chest pain are reported. In 80% of patients, the cause of a patient’s dysphagia can be suggested from the history, including dysmotility of the esophagus. Before entertaining a diagnosis of a motility disorder, first and foremost, the physician must evaluate for a mechanical obstructing lesion.
Oropharyngeal and upper esophageal sphincter dysfunction may be caused by neurologic and neuromuscular diseases or may be of unknown cause. Oropharyngeal dysfunction may result from certain surgeries, such as tracheostomy, laryngectomy or cervical dissection.
There are primary idiopathic motor disorders that include achalasia, diffuse esophageal spasm, nutcracker esophagus, hypertensive lower esophageal sphincter and nonspecific esophageal motility disorders.
Reflux disease is associated with an lower esophageal sphincter that is not sufficiently tight allowing gastric acid to wash back into the esophagus. The contractions of the esophagus in patients with reflux are generally not abnormal, but with long-standing reflux disease, they may decrease in amplitude.
The esophagus is lined by both circular and longitudinal muscles that terminate at a 2- to 4-cm circular muscle layer called the lower esophageal sphincter. The lower esophageal sphincter accounts for approximately 90% of the basal pressure at the gastroesophageal junction, and functions as an anti-reflux barrier. Esophageal peristalsis is a very synchronized action regulated by the vagus nerve that both stimulates and inhibits neurons in the esophageal myenteric plexus. The disruption of coordination between excitatory and inhibitory signals to myenteric neurons results in esophageal motility disorder.
Hypomotility of the esophageal body ranges from low wave amplitude to complete peristaltic failure, and etiologies include rheumatologic (scleroderma, connective tissue disorders), endocrinologic (diabetes mellitus, hypothyroidism) and other diseases (alcoholism, amyloidosis).
The shared symptom of these disorders of hypomotility is gastroesophageal reflux disease (GERD). Food impaction and slow transit of pills may result in caustic esophageal damage as well. Barium esophagogram and manometry are the main diagnostic tools, and treatment varies with the etiology of the hypomotility.
Hypermotility of the lower esophageal sphincter is most commonly seen in achalasia, which is discussed in detail throughout the rest of this chapter. Hypertensive disorders of the esophageal body (which may or may not involve the lower esophageal sphincter) are termed spastic disorders. They can be further categorized as diffuse esophageal spasm and non-specific spastic disorders, and are almost universally idiopathic.
Diffuse esophageal spasm is characterized by uncoordinated or simultaneous contractions of the esophagus with abnormal peristalsis following swallowing. The other disorders may show abnormalities in the amplitude, frequency or duration of contractions and/or a hypertensive lower esophageal sphincter. One subgroup of spastic disorders is “nutcracker esophagus”, characterized by contractions of high amplitude with otherwise normal peristalsis.
Presenting symptoms of all hypermotility disorders include chest pain (in 80-90% of cases), dysphagia, regurgitation, and heartburn. A barium swallow and/or manometry are the preliminary diagnostic tests. The significance of these disorders is not fully understood however, as abnormal results can be seen in patients with and without symptoms, and the pain may not be directly attributable to dysmotility.
Fortunately, these spastic disorders are not typically progressive or fatal. Treatment risks must therefore be especially weighed against potential benefits, with consideration of low-risk treatments (e.g., proton pump inhibitor to rule out possible GERD) first. Other options range from swallowing warm liquids with meals to invasive treatments used for achalasia (see below). Bougienage and serial dilation with increasing sizes of bougies may be effective for some patients. Potential benefit may also be seen from smooth muscle relaxants (e.g., nitrates, calcium channel blockers) or antidepressants (e.g., tricyclic antidepressants, serotonin reuptake inhibitors).
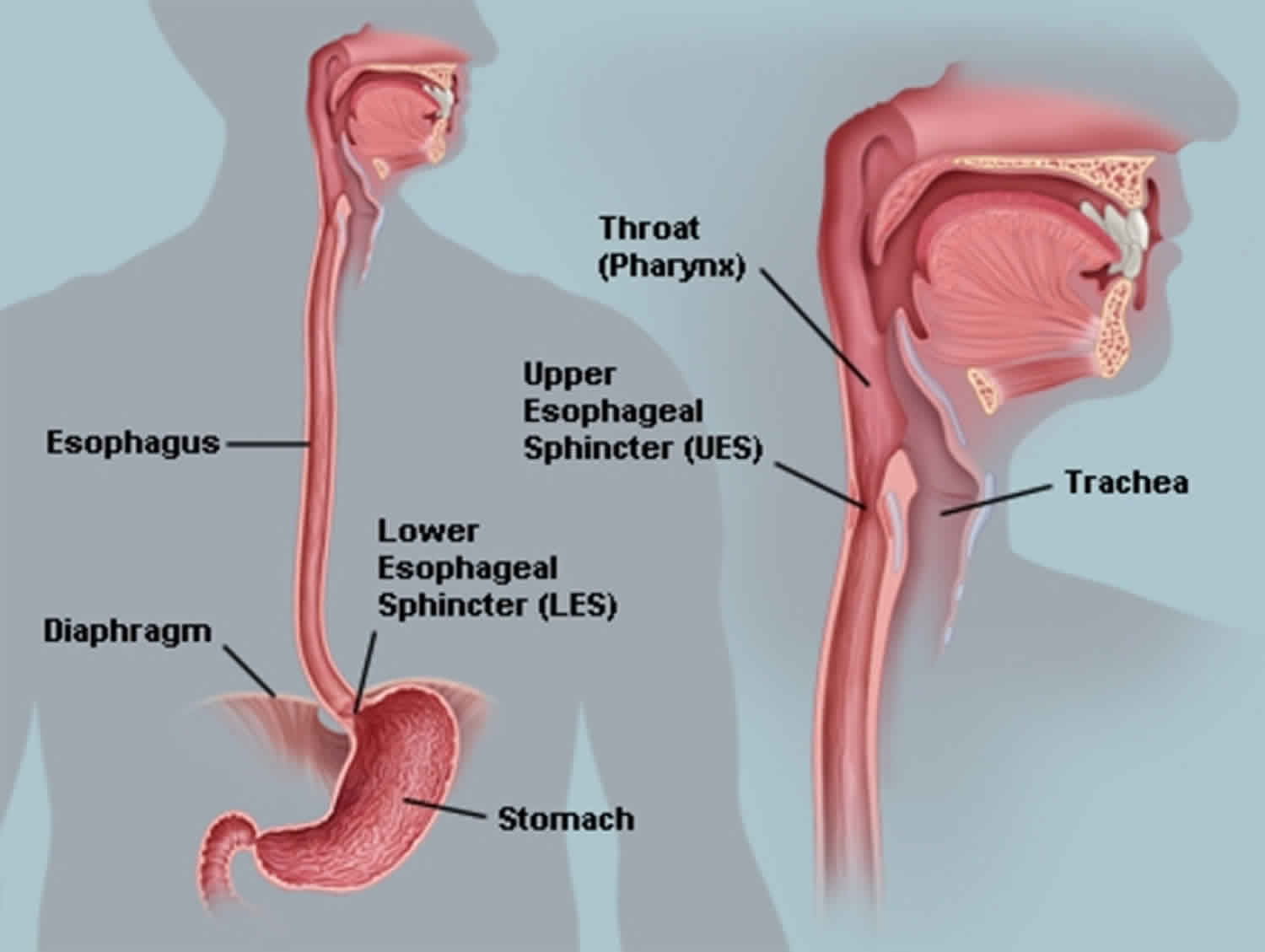
Esophagus anatomy
The tubular esophagus is a muscular organ, approximately 25 cm in length, and has specialized sphincters at proximal and distal ends. The upper esophageal sphincter (UES) is comprised of several striated muscles, creating a tonically closed valve and preventing air from entering into the gastrointestinal tract. The lower esophageal sphincter (LES) is composed entirely of smooth muscle and maintains a steady baseline tone to prevent gastric reflux into the esophagus.
The body of the esophagus is similarly composed of 2 muscle types. The proximal esophagus is predominantly striated muscle, while the distal esophagus and the remainder of the gastrointestinal tract contain smooth muscle. The mid esophagus contains a graded transition of striated and smooth muscle types. The muscle is oriented in 2 perpendicular opposing layers: an inner circular layer and an outer longitudinal layer, known collectively as the muscularis propria. The longitudinal muscle is responsible for shortening the esophagus, while the circular muscle forms lumen-occluding ring contractions.
Figure 1. Esophagus
Figure 2. Esophagus anatomy
Figure 3. Cross section view of the esophagus
Esophagus physiology
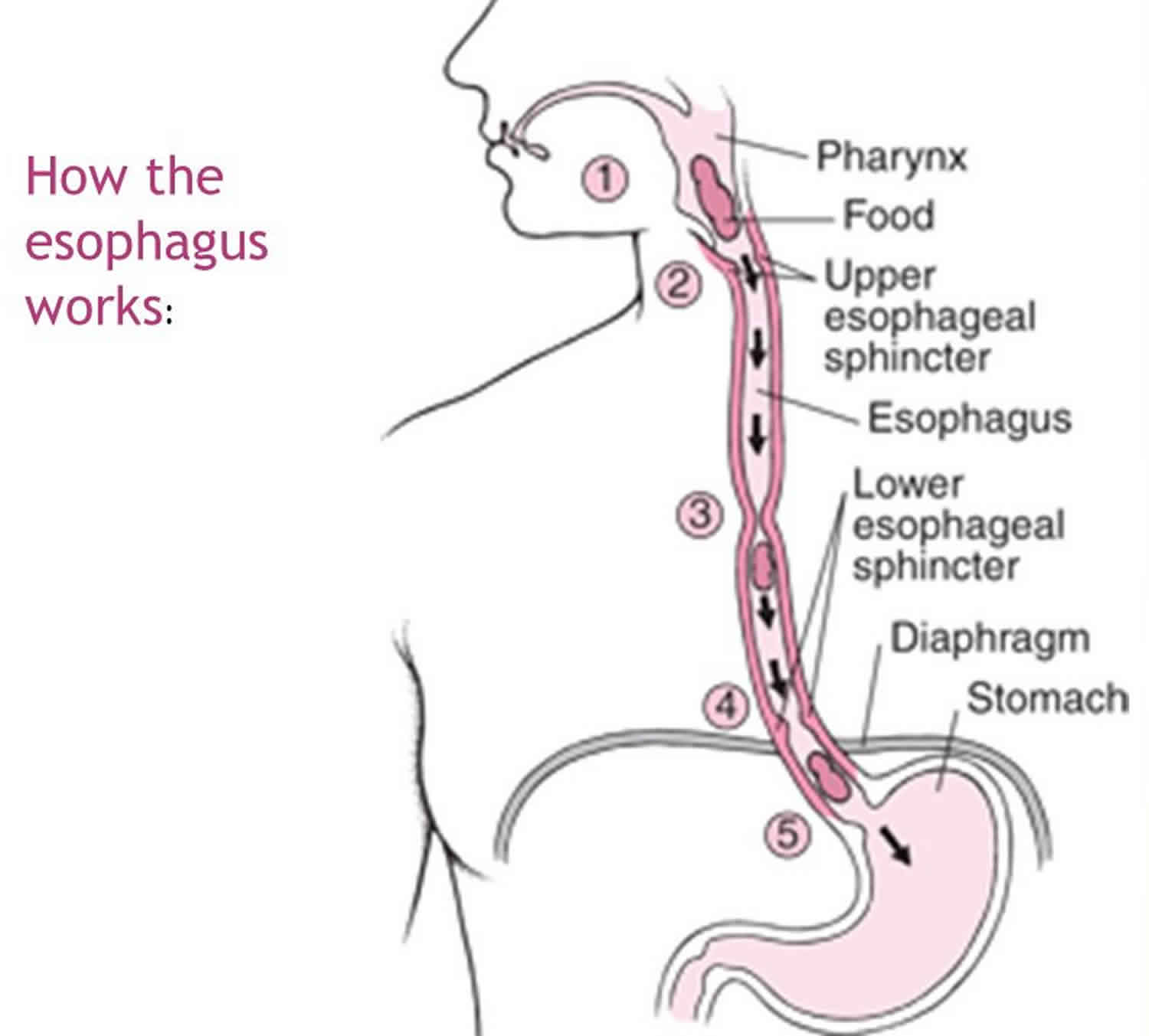
The esophagus is a conduit for the passage of a food bolus from the pharynx to the stomach. Typically, the esophagus starts at the cricopharyngeus muscle, forming part of the upper esophageal sphincter (UES), and ends at the lower esophageal sphincter, surrounded by the crural part of the diaphragm at the tenth thoracic vertebra (T10) 2. Following the relaxation of the upper esophageal sphincter and the passage of the food bolus into the esophagus, peristaltic muscular contractions propel the bolus toward the lower esophageal sphincter (LES). Relaxation of the lower esophageal sphincter in conjunction with the peristaltic propulsion of the bolus allows the entry of the bolus into the stomach.
Problems in the normal physiology of the esophagus and its associated sphincters begin to arise when patients report having difficulty swallowing or dysphagia. Generally, dysphagia can be broken into two groups, obstructive lesions or motor disorders. Obstructive lesions involve a narrowing of the esophagus or an outgrowth resulting in decreased luminal diameter. Motor disorders involve atrophy of muscles, degeneration of nerves, or improper function of nerves associated with the esophageal function. Dysphagia can also be broken down based on location such as pre-esophageal/oropharyngeal dysphagia, esophageal/transport dysphagia, or postesophageal/esophagogastric dysphagia. Oropharyngeal dysphagia is particularly important because it can result in aspiration 3.
Figure 4. How the esophagus works
Upper esophageal sphincter
The upper esophageal sphincter is the high-pressure area that lies between the esophagus and the pharynx. One-third of the upper esophageal sphincter is comprised of the cricoid cartilage on the posterior surface, the arytenoid and inter arytenoid muscles in the upper part, and the cricopharyngeus muscle posteriorly and laterally. The remaining two-thirds of the upper esophageal sphincter is accounted for by the thyropharyngeus muscle. Physiologically, the upper esophageal sphincter protects against reflux of food into airways and prevents the entry of air into the digestive tract. The opening of the upper esophageal sphincter involves relaxation of the cricopharyngeus and thyropharyngeus muscles and forward movement of the larynx via contractions of the hyoid muscles 4.
The trigger for the relaxation of the upper esophageal sphincter begins with the reception of the bolus into the oropharynx which transmits signals along afferent nerves to the swallowing center of the brainstem 5. The brainstem identifies the incoming signals and produces a response sent along the efferent nerves to the muscles involved in swallowing, the opening of the upper esophageal sphincter, cessation of breathing, and peristalsis in the esophagus. The efferent signals at the cricopharyngeus muscle trigger relaxation by inhibition of signals that trigger tonic contractions rather than direct inhibitory signals. A similar mechanism of the action happens to trigger the relaxation of the thyropharyngeus muscle 4.
Efferent signals at the hyoid muscle stimulate contraction which elevates the hyoid and happens almost simultaneously with the upper esophageal sphincter relaxation. The hyoid muscles can also contract without the initiation of a pharyngeal swallow via muscular attachments to the tongue. Relaxation of the cricopharyngeus and thyropharyngeus muscles resulting in decreased upper esophageal sphincter pressure, movement of the larynx away by the hyoid muscles, and the propulsion force of the bolus allow the bolus to overcome the pressure in the upper esophageal sphincter region and pass into the esophagus 6.
The volume of the bolus also plays an important role in mediating the physiology of the upper esophageal sphincter. The bolus volume dictates the duration between the opening of the upper esophageal sphincter and pharyngeal movement. Increasing the volume of the bolus also results in the faster onset of pharyngeal movement while increasing the thickness of the bolus was also associated with increasing time differential between the opening of the upper esophageal sphincter and pharyngeal movement 6.
Peristalsis
Once the bolus has passed through the upper esophageal sphincter, it arrives into the esophagus. The upper portion of the esophagus is composed mainly of striated muscle under the control of central control mechanisms. The lower portion of the esophagus is comprised primarily of smooth muscle and is under the control of central and intrinsic control mechanisms 7.
The esophagus is comprised of a muscle layer termed the muscularis mucosa. This muscle layer can be further divided into longitudinal muscle fibers and internal circular muscle fibers. The exact role of longitudinal muscles in esophageal physiology is not known, but recent studies have shown that longitudinal muscle contraction helps to reduce the tension associated with circular muscle contractions which helps to augment peristaltic contraction. On the other hand, the role of circular muscle fibers in esophageal physiology is well known, and they contract radially to provide peristaltic propulsion of the bolus in the aboral direction 8.
Entering of the bolus into the esophagus triggers primary peristalsis. Generally, in peristalsis, the area ahead of the bolus is relaxed, and the area behind the bolus is undergoing peristaltic contraction which allows for the bolus to be propelled forward. A series of nervous inputs accomplish contraction behind the bolus on the oral side and relaxation on the aboral side of the bolus 4.
In striated muscle, upon swallowing, lower motor neurons in the nucleus ambiguous of the brainstem are activated. Each lower motor neuron is activated sequentially to create a peristaltic wave. In smooth muscle, however, upon swallowing, caudal dorsal motor nucleus (cDMN) inhibitory neurons are activated and cause simultaneous inhibition of all parts of the esophagus. This simultaneous inhibition in the smooth muscle of the esophagus is termed “deglutitive inhibition” and is the first step to generating a peristaltic wave. This inhibition lasts longer in the lower portion of the esophagus than the upper portions of the esophagus. As the inhibition ends, sequential activation of excitatory neurons in the rostral dorsal motor nucleus (rDMN) elicit peristaltic contraction. Central control mechanisms mediate the mechanisms described above 7.
The excitatory pathway in smooth muscle for the generation of primary peristalsis includes vagal preganglionic neurons of the rostral part in the DMN of the brainstem. The DMN is a cranial nerve nucleus for the vagus nerve in the medulla that lies ventral to the floor of the fourth ventricle. The rDMN pre-ganglionic neurons attach to the excitatory postganglionic neurons that release acetylcholine (ACh) and substance P. The inhibitory pathway includes preganglionic vagal fibers in the cDMN. These fibers project onto postganglionic inhibitory neurons that contain nitric oxide (NO), vasoactive intestinal peptide (VIP), adenosine triphosphate (ATP), and substance P 7.
Interestingly, the esophagus can also undergo secondary peristalsis which is initiated by the intrinsic nervous system and vaso-vagal responses if there is residual food in the esophagus. Secondary peristalsis can have the same strength and speed as primary peristalsis but is generated in the absence of a swallow 5. In skeletal muscle, secondary peristalsis is centrally mediated and occurs in a similar method to primary peristalsis with nerve innervations arising from the nucleus ambiguus. In smooth muscle, secondary peristalsis is due to a peripheral mechanism. The peripheral mechanism involves the activation of sensory neurons by stimulation from distention or the presence of a food bolus in the esophagus. The excited sensory neuron transmits the signal to an interneuron which relays the signal to a motor neuron. Subsequently, the motor neuron then releases acetylcholine orally and nitric oxide aborally to create a secondary peristaltic wave. This peristaltic wave in smooth muscle is locally contained and generated by the peripheral mechanism 9.
Lower esophageal sphincter
Once the bolus has arrived at the end of the esophagus, it must pass through the lower esophageal sphincter to arrive in the stomach. The lower esophageal sphincter and crural diaphragm constitute the high-pressure zone between the esophagus and stomach. The lower esophageal sphincter and the crural diaphragm function as anti-reflex barriers to protect the esophagus but also allow for the passage of the bolus into the stomach 10.
The lower esophageal sphincter is comprised mainly of smooth muscle and has no dilator muscles to help open the lower esophageal sphincter. The opening of the lower esophageal sphincter is triggered by direct inhibitory innervation resulting in relaxation of the smooth muscles in the lower esophageal sphincter in conjunction with the movement of the bolus through the relaxed sphincter 7.
lower esophageal sphincter pressure is dependent on the myogenic tone, inhibitory nitrergic nerves, and excitatory cholinergic nerves. The myogenic tone is responsible for the tonic contraction of the lower esophageal sphincter and is due to the specialized properties of the smooth muscle cells at the lower esophageal sphincter. The lower esophageal sphincter smooth muscle cells are thought to have more depolarized resting membrane potentials resulting in spontaneous spike-like action potentials and generation of basal tone 10.
Excitatory cholinergic nerves (ACh) and the tonic, myogenic property of the lower esophageal sphincter favor contraction, whereas the inhibitory nitrergic (nitric oxide) pathway favors inhibition. The lower esophageal sphincter remains contracted due to its myogenic property even when it is entirely denervated as in advanced achalasia 10.
Presence of a bolus in the pharynx triggers receptors which relay signals that eventually induce esophageal peristalsis and lower esophageal sphincter relaxation. The sensory stimulus travels to the nucleus of tractus solitarius which connects with the dorsal motor nucleus (DMN). The vagal efferent nerves from the dorsal motor nucleus, however, do not innervate the smooth muscle but instead innervate the myenteric plexus which mediates lower esophageal sphincter relaxation. The myenteric plexus is composed of inhibitory and excitatory motorneurons, and the location of stimulus dictates inhibitory or excitatory actions since the inhibitory pathway neurons arise from the caudal dorsal motor nucleus (cDMN) while the excitatory pathway neurons arise from the rostral dorsal motor nucleus (rDMN). Important excitatory postganglionic neurotransmitters are acetylcholine (ACh) and tachykinins while nitric oxide (NO) is the most important inhibitory postganglionic neurotransmitter 10.
Another important physiological component of the lower esophageal sphincter other than relaxation during swallowing is transient lower esophageal sphincter relaxation. Transient lower esophageal sphincter relaxation is a physiological mechanism designed to allow the venting of gas from the stomach 11. Transient lower esophageal sphincter relaxation afferent nerves arise from the pharynx, larynx, and the stomach while the efferent nerves use the same pathway as the swallow reflex to trigger lower esophageal sphincter relaxation 12.
The other component that constitutes the high-pressure zone between the esophagus and stomach is the crural diaphragm. The crural diaphragm also plays important roles in helping to regulate the rate of reflux into the esophagus and allowing the passage of a food bolus into the stomach. The crural diaphragm is anchored to the lower esophageal sphincter by the phrenoesophageal ligament which means that the two structures move together on inspiration and expiration but can separate during peristalsis and transient lower esophageal sphincter relaxation 13. For the food bolus to pass into the stomach and allow for reflux from the stomach into the esophagus, the crural diaphragm must relax. Although the exact mechanism of relaxation for the crural diaphragm is not known, the crural diaphragm is controlled by the phrenic nerve via nicotinic cholinergic receptors which induce contraction 14.
Esophageal motility disorder causes
There are several mechanisms by which dysphagia can occur including physical obstructions or motor issues. The physical obstruction or motor issue can additionally be localized at the level of the upper esophageal sphincter, esophagus, or lower esophageal sphincter. Some motor causes of dysphagia include diffuse esophageal spasms, achalasia, scleroderma, or diabetes mellitus. Some physical obstructions in the esophagus causing dysphagia include esophageal carcinoma, reflux esophagitis, peptic strictures, or Schatski’s rings. The upper esophageal sphincter can also be a cause of motor dysphagia but occurs in the oropharyngeal portion of the gastrointestinal tract 15.
Primary esophageal causes of esophageal dysmotility include:
- Achalasia
- Diffuse esophageal spasm. In including diffuse esophageal spasm (DES), muscular hypertrophy or hyperplasia has been described in the distal two thirds of the esophagus. Muscle wall thickening has been described in patients who are asymptomatic and, conversely, has been absent in some patients with typical symptoms and manometric findings. This controversial finding causes difficulty in attributing symptoms or manometric abnormalities to muscle structure changes. In addition, anxiety states may also play a role in some patients.
- Hypertensive lower esophageal sphincter
- Nutcracker esophagus
- Eosinophilic esophagitis
- Nonspecific esophageal motility disorder (inefficient esophageal motility disorder)
Primary esophageal motility disorders are idiopathic in nature, but postviral, infectious, environmental, and genetic factors have been hypothesized. The pathophysiology of the primary esophageal motility disorders is poorly defined, with the exception of achalasia. The underlying cause of all the primary motility disorders remains elusive. The secondary motility disorders, such as scleroderma esophagus or esophageal motility disorder of diabetes, are better understood from the standpoint of the preexisting underlying disorders.
Systemic disorders causing esophageal dysmotility include:
- Systemic sclerosis or scleroderma. In scleroderma, the primary defect in this systemic process is related to smooth muscle atrophy and fibrosis. Esophageal dysmotility develops as the smooth muscle of the esophagus is replaced by scar tissue, gradually leading to progressive loss of peristalsis and a weakening of lower esophageal sphincter. Motility is preserved at the proximal striated muscle portion of the esophagus.
- Chagas disease
- Diabetes mellitus,
- Alcohol consumption,
- Psychiatric disorders,
- Presbyesophagus.
Many generalized disorders of neuromuscular function (eg, myasthenia gravis, amyotrophic lateral sclerosis, stroke, Parkinson disease) can affect swallowing but are not typically classified as esophageal motility disorders.
Esophageal spasms are characterized by uncoordinated contractions of the esophagus resulting in dysphagia. Characteristic x-ray findings are described as “corkscrew” or rosary bead” esophagus and manometry is used to evaluate the motor function of the esophagus 16.
Achalasia is also known as cardiospasm, achalasia cardiae, spastic achalasia, esophageal achalasia, and esophageal aperistalsis. Achalasia is a chronic motility disorder caused by impaired relaxation of the lower esophageal sphincter (LES) and usually aperistalsis of the distal two-thirds of the esophagus, which prevents the ability of the bolus to pass onto the next stage of the gastrointestinal tract. Achalasia results from dysfunction of the nitric oxide producing neurons that relax the distal esophagus. This leads to impaired muscle relaxation and hypercontractility in the distal esophagus and lower esophageal sphincter. The result is an often dramatic presentation of dysphagia, regurgitation and chest pain. Although the underlying etiology is still not known, viral, genetic, autoimmune, and chronic degenerative processes have been suggested. Primary achalasia should be differentiated from secondary achalasia which is typically indistinguishable from the primary form in both clinical presentation and diagnostic testing. Secondary achalasia is seen in some multisystem diseases like Chagas disease or is iatrogenic from fundoplication or gastric banding. Generally, achalasia is associated with the absence of peristalsis on diagnostic testing and diagnosis is made based on barium swallow or esophageal manometry testing 17.
Scleroderma also called systemic scleroderma or systemic sclerosis, is also due to reduced smooth muscle contractions but because the smooth muscle has atrophied and been replaced by collagen fibers. This fibrosis of smooth muscle results in hypomotility. Manometry is the gold standard for diagnosis of this condition 18.
Diabetes mellitus causes dysphagia by inducing mechanic-structural remodeling in the esophagus. Remodeling is triggered because of esophageal sensorimotor abnormalities and symptoms encountered by diabetes patients. The sensory dysfunction appears to arise due to demyelination and progressive axonal atrophy of parasympathetic nerves in the esophagus. Increased autonomic neuropathy also leads to a decreased lower esophageal sphincter tone which results in gastroesophageal reflux 19.
Esophageal carcinomas are usually either squamous cell carcinomas or adenocarcinomas. The exact mechanism for dysphagia in esophageal carcinomas is not known. Endoscopy with a biopsy is used for diagnosis 20.
Peptic strictures are the narrowing of the esophagus due to prolonged inflammation and scarring from stomach acid or exposure to other irritants. These are normally diagnosed based on barium swallow or endoscopy with a biopsy 21.
A Schatzki’s ring is a ring of redundant mucosa over the normal mucosa resulting in a decreased luminal diameter of the esophagus. A Schatzki’s ring is formed due to in-folding of the esophagus during transient shortenings of the esophagus. Diagnosis is normally made based on esophagogastroduodenoscopy (EGD) or barium swallow studies. It is often associated with hernias 22.
Lack of upper esophageal sphincter relaxation can also trigger dysphagia. For the bolus to pass the upper esophageal sphincter, normal muscle tension must be overcome. The tension of the upper esophageal sphincter is reduced by the contraction of the cricopharyngeus and thyropharyngeus muscles. This means that the timing of the muscle contraction and proper force are necessary for the bolus to pass to the esophagus. Any diseases or disorders that disrupt these muscles or increase the baseline tension that must be overcome will prevent the bolus from passing through the upper esophageal sphincter. One such case is the improper relaxation of the cricopharyngeus muscle resulting in a Zenker’s diverticulum. A Zenker’s diverticulum is a diverticulum occurring just between the cricopharyngeus muscle and the inferior pharyngeal constrictor muscles due to excessive pressure in the lower pharynx causing the weakest portion of the of the pharyngeal wall to balloon out. Barium swallow studies diagnose this. Dysphagia in Zenker’s diverticulum can be due to the incomplete opening of the upper esophageal sphincter and/or the compression of the esophagus by the diverticulum itself 23.
Esophageal motility disorder symptoms
Symptoms of esophageal motility disorders depend on the cause but typically include difficulty swallowing (dysphagia), chest pain, and/or heartburn.
Achalasia
Note the following:
- Progressive dysphagia for both solids and liquids is the hallmark of achalasia. Dysphagia for solids is more common than for liquids.
- Retrospectively, symptoms are present on average as long as 6 years prior to presentation.
- Regurgitation of food retained in the proximal dilated esophagus is a common occurrence, especially at night, requiring patients to sleep using multiple pillows or upright in a chair. This symptom worsens as the esophagus dilates with time.
- Chest pain may be another early symptom, characterized by a squeezing retrosternal pain radiating to the neck, jaw, arms, or back. The chest pain may worsen with food and can awaken patients from sleep.
- A sensation of heartburn may be reported by 30% of patients and is assumed to be related to retained food fermentation and lactic acid.
- Emotional stress or rapid eating may worsen all of the symptoms described above.
- Weight loss is common with achalasia; however, the loss is usually slight.
Dysphagia for both liquids and solids that is refractory to proton pump inhibitor therapy is the presenting symptom in almost all cases of achalasia. The degree of dysphagia may become so severe over time that patients report trying postural changes (e.g., raising their arms up, standing straight) to aid their swallowing.
Other common symptoms include heartburn, regurgitation, retrosternal chest discomfort and weight loss (later in disease). Patients often complain of non-esophageal symptoms including sore throat, hoarseness, and a nocturnal cough as a result of aspiration. Dysphagia typically precedes respiratory complaints by approximately 2-years alluding to the chronic and progressive nature of the disease.
Patients with achalasia tend to have a higher incidence of autoimmune diseases then the general population.
Diagnosis of achalasia is relatively simple, with classic symptoms of dysphagia and regurgitation that are not due to gastroesophageal reflux disease (GERD). A diagnosis should be pursued only when an esophagogastroduodenoscopy does not reveal mechanical obstruction or an inflammatory cause of these symptoms.
If achalasia is suspected, a barium swallow with fluoroscopy should be performed to look for poor peristalsis of the esophageal body and failure of the lower esophageal sphincter to relax, possibly a dilated esophagus and the pathognomonic “bird’s beak” esophagus. The average patient is diagnosed after 4-6 years of symptoms. Early cases may have falsely negative radiographic studies, and later cases may be less symptomatic as the esophagus dilates. Manometry may also be indicated if radiographic studies are unrevealing.
Spastic esophageal motility disorders
Chest pain is the hallmark of spastic esophageal motility disorders, although patients with spastic esophageal motility disorders also may report dysphagia. Similar to the chest pain of achalasia, it may mimic angina. The mechanism is not clear but may be related to transient esophageal muscle ischemia, luminal distension, or altered visceral sensation.
Dysphagia is not necessarily related to chest pain. Dysphagia for solids and liquids is a common symptom and especially seen in diffuse esophageal spasm. Dysphagia may be intermittent and nonprogressive in nature, typically not prolonging mealtime or causing weight loss.
Patients also commonly report heartburn, regurgitation, or other esophageal complaints of reflux disease due to ineffective acid clearance from the esophagus.
Weight loss is common with achalasia; however, the loss is usually slight.
Scleroderma esophagus
Scleroderma involves the esophagus in more than 75% of patients, regardless of clinical type. Two forms of this disease exist–(1) progressive systemic sclerosis, characterized by diffuse scleroderma, and a more fulminant form with early involvement of internal organs or (2) CREST syndrome, characterized by calcinosis, Raynaud phenomenon, esophageal dysfunction, sclerodactyly, and telangiectasia. The severity of esophageal involvement does not correlate necessarily with severity of involvement of other organs. In fact, dysphagia may be the presenting clinical symptom in some patients.
The esophageal symptoms of scleroderma usually reflect the severity of acid reflux disease, including heartburn, regurgitation, and dysphagia.
Erosive esophagitis is observed in as many as 60% of patients, and the incidence of Barrett esophagus and adenocarcinoma of the esophagus is increased.
Dysphagia usually is due to diminishing peristalsis, peptic strictures, or a combination of both.
Esophageal motility disorder diagnosis
The patient’s history suggests the diagnosis almost 80% of the time. The only physical findings in esophageal disorders are cervical and supraclavicular lymphadenopathy caused by metastasis, swellings in the neck caused by large pharyngeal diverticula or thyromegaly, white plaques in the posterior oropharynx caused by Candida infection, and prolonged swallowing time (the time from the act of swallowing to the sound of the bolus of fluid and air entering the stomach—normally ≤ 12 seconds—heard by auscultation with the stethoscope over the epigastrium). Watching the patient swallow may help diagnose aspiration or nasal regurgitation. Most esophageal disorders require specific tests for diagnosis.
Evaluation of esophageal motility disorders depends on the patient’s presenting symptoms and may include upper gastrointestinal endoscopy, barium swallow, high-resolution manometry (HRM), pH measurement, and impedance monitoring of the esophagus. Endoscopies, barium swallow studies, and high-resolution manometry are the more frequently used tests to evaluate esophageal function 24. Endoscopies are used to evaluate the mucosa and submucosa of the esophagus and frequently involve taking biopsies to better evaluate the tissue. A barium swallow study involves the administration of barium followed by sequential usage of x-rays to determine the movement of barium through the upper gastrointestinal (GI) tract. This helps to evaluate the physiology of the upper esophageal sphincter, the esophagus, lower esophageal sphincter, or for the presence of any potential anatomical defects. Barium studies, however, are problematic because it is difficult to determine what is the normal luminal diameter of the esophagus 24. High-resolution manometry involves the use of pressure sensitive catheters to evaluate esophageal motor function and sphincter function. The catheter is moved sequentially down the esophagus and its associated sphincters and when the sphincters open/close or peristalsis occurs, the pressure sensitive catheters pick up the pressure changes 25. Ultrasound may also be used to test for the function of the esophagus, but it has not gained wide popularity 24.
Esophageal motility disorder treatment
Esophageal motility disorder treatment involves treating the underlying cause.
Diffuse esophageal spasm is treated with smooth muscle relaxants or surgically with a long myotomy with or without the anti-reflux procedure.
Patients with nonspecific esophageal motility disorders are usually evaluated for severe gastroesophageal reflux and treatment for gastroesophageal reflux disease (GERD) is instituted.
Primary esophageal spasm is rarely life threatening, and the most important element in treatment is often reassurance. However when dysphagia or chest pain is frequent or severe, drugs that decrease smooth muscle contractility are often used. Unfortunately, in addition to lowering pressure in the esophagus, these medications also lower blood pressure. Recently it has been recognized that patients with chest pain presumed to be of esophageal origin are often unusually sensitive to esophageal stimulation. In this case, tricyclic antidepressant drugs have been effective in some patients. The effect of these drugs occurs at low doses and does not appear to relate to the presence or absence of depression. This suggests that the drugs may act on pain recognition rather than mood alteration.
Esophageal strictures treatment
There are a variety of conditions that narrow the esophagus. Among the most common are webs and rings, which are thin bands of tissue that form a shelf-like constriction of the esophagus. Inflammatory strictures are a product of esophageal wall thickening resulting from a combination of active inflammation and subsequent scarring. Strictures may also result from malignant tumors involving the esophagus.
Strictures may cause difficulty in swallowing solid foods. Tougher cuts of meat, stringy vegetables and doughy foods such as breads or pasta may increase the severity of symptoms. To best manage symptoms, it is important to cut food into small pieces, adequately chew before swallowing and eat slowly. A varied menu should be sought when dining out. Appropriate food selection and careful attention to cutting and chewing will decrease the frequency and severity of symptoms.
Simple dilation (stretching), performed during upper endoscopy, provides prompt and often sustained relief of symptoms from rings or webs. Recurrent symptoms generally respond to repeat dilations or placement of an esophageal stent. Strictures also respond to dilation but are likely to recur fairly rapidly unless the cause of inflammation is controlled. Re-stricturing may continue even after the underlying process is recognized and removed due to ongoing scar formation. However, over time, the rate of re-stricturing and the accompanying need for dilation should decrease. Malignant strictures are best managed surgically. When this is not possible, radiation therapy, laser therapy and stent placement (a rigid tube that holds the channel open) are among the therapeutic approaches.
Achalasia treatment
Achalasia is a condition in which the nerves in the esophageal wall have been damaged. The cause of this injury is unknown, but its effects are well recognized. With this condition, the esophagus is unable to contract in a coordinated manner and the lower esophageal sphincter does not relax with swallowing. This results in food remaining in the esophagus above a closed sphincter segment. Treatment is directed toward weakening the sphincter muscle, after which food will empty by gravity. Routine dilation, such as that used for strictures is usually ineffective. Effective treatments include dilation with a large size balloon dilator, botulinum toxin (Botox) injection in the lower esophageal sphincter or surgical myotomy (a procedure in which the lower esophageal sphincter is cut). The best approach depends upon the situation, local expertise and the patient’s personal preference. The choice should be made after a consultation in which these factors can be considered and discussed.
The basic algorithm for treatment of achalasia begins with two groups, patients at low surgical risk and patients at high risk or who decline surgery. For the latter group, treatment options are limited to botulinum toxin injections or medications. For the former group, either mechanical dilation or surgical intervention is offered first, possibly depending on the success rate of surgeries at an institution.
Medications
Nitrates and calcium channel blockers may be used to relax smooth muscle and improve symptoms and are given sublingually because of patients’ dysmotility and poor absorption. These medications are effective in lowering lower esophageal sphincter pressure by up to 47% to 64%, however, they inconsistently relieve symptoms of dysphagia. The effectiveness is unpredictable and often short-lived, and side effects include headaches, hypotension and edema.
In patients who cannot tolerate oral calcium channel blockers or nitrates, 5-phosphodieserase inhibitors such as sildenafil have been shown to lower lower esophageal sphincter pressures, however, long-term efficacy data is very limited. Thus, these medications are reserved for patients with relatively asymptomatic disease or significant comorbidities.
Botulinum toxin injection
Botulinum toxin type A, derived from Clostridium botulinum, can be injected directly into the lower esophageal sphincter to decrease basal tone. The toxin adheres to cholinergic nerves, irreversibly blocking acetylcholine release. Although many patients may respond initially, the effect is short-lived (often only 6 months), and repeated injections are decreasingly effective.
Patients may eventually require surgery, but with repeated injections, the area forms scar tissue, making surgical intervention much more difficult. This expensive therapy should be used only in patients who cannot undergo dilation or surgery, or possibly in difficult diagnoses of achalasia.
Mechanical dilation
Dilation, one of the oldest treatments for achalasia, is still an effective method. During endoscopy, polyethylene balloons of increasing size are sequentially inflated inside the lower esophageal sphincter, disrupting the tight muscular fibers. Randomized control trials have demonstrated a reduction in dysphagia symptoms that range from 62% to 90%. Pneumatic dilation has also been shown to have excellent long-term efficacy where only a third of patients will relapse in 4 to 6 years. Patients who do not respond to initial dilation are unlikely to respond to repeat procedures. The procedure’s most feared complication is esophageal perforation and occurs at a rate of less than 1% with newer techniques. An esophagogram should be performed following the procedure, using a water-soluble contrast to rule out perforation.
Like botulinum toxin injections, repeated dilations may be decreasingly effective. Unlike botulinum toxin injections, however, there is no increase in scar tissue or complications should the patient require surgical correction in the future.
Surgery
There have been many advancements and modifications in surgical treatment that have made this intervention the most effective for achalasia. Although there are several operative approaches, most consist of a Heller myotomy, where the lower esophageal sphincter is severed to better food passage to the stomach, followed by a procedure (such as a Dor fundoplication) to prevent acid from refluxing into the esophagus once the lower esophageal sphincter is no longer an effective barrier. Ninety to ninety-five percent of patients will have excellent improvement in their symptoms following surgery, with an estimated 80-85% still well controlled 10 years later.
The results are thought to be highly dependent on surgical experience with a significant learning curve. Laparoscopic techniques and robotic arms are decreasing surgical time, hospital stays and invasiveness, making surgery a favorable option for those healthy enough.
A newer treatment approach is the per-oral endoscopic myotomy (POEM) which is performed intraluminally. The procedure involves creating an incision in the mid-esophagus extending to the gastric cardia and creating a selective myotomy in the lower esophageal sphincter. The advantages of POEM are that it is less invasive than a laparoscopic approach, it leads to shorter hospital stays and a myotomy can be performed at any length of the esophagus. Therefore, the patient with achalasia that does not respond to a laparoscopic approach due an incomplete dissection on the gastric side, can undergo POEM where the endoscopist can create an extended-length myotomy from the esophagus to the gastric cardia.
A growing amount of data has shown that POEM has similar short-term efficacy compared to laparoscopic myotomy. There is promising long-term data that show 2-month, 1- to 2-year, and 3-year overall success rates of 91.3%, 91.0%, and 88.5% respectively. Other studies have shown sustained success rates of POEM in over 90% over patients ranging from 1 to 3 years.
When myotomy and dilation fail, or in patients with a significantly dilated esophagus, complete esophagectomy may be indicated.
Common pitfalls and side-effects of management
Caution with choosing the initial treatment for achalasia is indicated, as it can influence the success rate of future interventions. For example, repeated botulinum toxin injections may cause scar tissue and worsen future surgical outcomes. Repeated injections or repeated dilations may be decreasingly successful. Medications are limited in their usefulness and have side effects such as hypotension and headaches.
Pneumatic dilations have a risk of perforation, which requires emergent surgical correction, so patients who are not good surgical candidates are usually not considered for dilations either. Because of the high risk of GERD following myotomy of the lower esophageal sphincter, an anti-reflux procedure is generally recommended in tandem.
The incidence of adverse effects from POEM is 3.2% and includes pneumothorax, severe bleeding, mucosal injuries including perforation, postoperative hematoma, and pleural effusion. However, as endoscopists become more competent with this novel technique, the incidence of morbidity should decrease.
Medication
If medications are used, the following doses are recommended:
- Isosorbide dinitrate, 5-10mg sublingually before meals
- Nifedipine 10-20mg sublingually before meals
Other calcium channel blockers can be used (e.g., diltiazem, verapamil), and are most effective when given sublingually immediately before a meal.
Esophageal motility disorder prognosis
Achalasia
Achalasia is a progressive disease that requires chronic therapy. Depending on the rate and extent of disease progression, therapy might include endoscopic and surgical interventions. Advanced achalasia can lead to malnutrition, dehydration, and aspiration.
Even after therapy, patients continue to have mild symptoms related to aperistaltic esophagus and, thus, will want to still follow careful eating habits.
Scleroderma esophagus
Scleroderma is a systemic disease with a progressive nature. Systemic complications are the major cause of mortality.
Significant acid reflux might lead to disabling symptoms, caused by reflux or its complications.
Spastic esophageal motility disorders
Whether or not symptomatic relief is achieved, the prognosis in patients with spastic esophageal motility disorders is favorable. Life expectancy is not affected, and weight loss is rare.
If symptoms progress, then the workup should be repeated because DES can progress to achalasia.
Mortality and morbidity
Achalasia is associated with significant and progressive symptomatic discomfort. When advanced, this condition can lead to such severe dysphagia that malnutrition, weight loss, and dehydration can develop. Increased incidence of both esophageal squamous cell and adenocarcinoma is observed in patients with long-standing achalasia. Therapeutic procedures and operations are associated with a small but significant risk of mortality and morbidity.
Spastic esophageal motility disorders are associated with symptomatic discomfort but do not lead to the severity of dysphagia observed in patients with achalasia. Chest pain is, in fact, a more common complaint that may precipitate emergency room visits and cardiologic evaluations.
Scleroderma esophagus is associated with severe and progressive acid reflux symptoms and complications. Associated complications, including strictures, Barrett esophagus, and adenocarcinoma of the esophagus, are the concern.
Complications
Achalasia and squamous cell carcinoma
With achalasia, the risk of squamous cell carcinoma of the esophagus is higher than that of the general population. However, no studies to date have shown convincing evidence that surveillance is worthwhile.
The pathogenesis is not well documented, but chronic mucosal irritation is incriminated.
Squamous cell carcinoma usually develops several years after the diagnosis of achalasia. The risk typically starts increasing after approximately 10 years of having the disease process. At the time of diagnosis, the esophagus usually is dilated, and the tumor is advanced.
- Primary Esophageal Motility Disorders. Mayo Clinic proceedings, ISSN: 0025-6196, Vol: 76, Issue: 2, Page: 195-200 https://doi.org/10.4065/76.2.195[↩]
- Bajwa SA, Kasi A. Physiology, Esophagus. [Updated 2019 Apr 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519011[↩]
- Wolf DC. Dysphagia. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; Boston: 1990.[↩]
- Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am. J. Med. 2000 Mar 06;108 Suppl 4a:27S-37S.[↩][↩][↩]
- Shaw SM, Martino R. The normal swallow: muscular and neurophysiological control. Otolaryngol. Clin. North Am. 2013 Dec;46(6):937-56.[↩][↩]
- Mendell DA, Logemann JA. Temporal sequence of swallow events during the oropharyngeal swallow. J. Speech Lang. Hear. Res. 2007 Oct;50(5):1256-71.[↩][↩]
- Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J. Clin. Gastroenterol. 2008 May-Jun;42(5):610-9.[↩][↩][↩][↩]
- Brasseur JG, Nicosia MA, Pal A, Miller LS. Function of longitudinal vs circular muscle fibers in esophageal peristalsis, deduced with mathematical modeling. World J. Gastroenterol. 2007 Mar 07;13(9):1335-46.[↩]
- Shiina T, Shima T, Wörl J, Neuhuber WL, Shimizu Y. The neural regulation of the mammalian esophageal motility and its implication for esophageal diseases. Pathophysiology. 2010 Apr;17(2):129-33.[↩]
- Farré R, Sifrim D. Regulation of basal tone, relaxation and contraction of the lower oesophageal sphincter. Relevance to drug discovery for oesophageal disorders. Br. J. Pharmacol. 2008 Mar;153(5):858-69.[↩][↩][↩][↩]
- Kim HI, Hong SJ, Han JP, Seo JY, Hwang KH, Maeng HJ, Lee TH, Lee JS. Specific movement of esophagus during transient lower esophageal sphincter relaxation in gastroesophageal reflux disease. J Neurogastroenterol Motil. 2013 Jul;19(3):332-7.[↩]
- Mittal RK. Motor Function of the Pharynx, Esophagus, and its Sphincters. Morgan & Claypool Life Sciences; San Rafael (CA): 2011.[↩]
- Mittal RK. Regulation and dysregulation of esophageal peristalsis by the integrated function of circular and longitudinal muscle layers in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016 Sep 01;311(3):G431-43.[↩]
- Pickering M, Jones JF. The diaphragm: two physiological muscles in one. J. Anat. 2002 Oct;201(4):305-12.[↩]
- Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am. J. Med. 2000 Mar 06;108 Suppl 4a:27S-37S[↩]
- Roman S, Kahrilas PJ. Management of spastic disorders of the esophagus. Gastroenterol. Clin. North Am. 2013 Mar;42(1):27-43.[↩]
- Ates F, Vaezi MF. The Pathogenesis and Management of Achalasia: Current Status and Future Directions. Gut Liver. 2015 Jul;9(4):449-63.[↩]
- Ebert EC. Esophageal disease in scleroderma. J. Clin. Gastroenterol. 2006 Oct;40(9):769-75.[↩]
- Zhao J, Gregersen H. Diabetes-induced mechanophysiological changes in the esophagus. Ann. N. Y. Acad. Sci. 2016 Sep;1380(1):139-154.[↩]
- Martin RE, Letsos P, Taves DH, Inculet RI, Johnston H, Preiksaitis HG. Oropharyngeal dysphagia in esophageal cancer before and after transhiatal esophagectomy. Dysphagia. 2001 Winter;16(1):23-31.[↩]
- Mamazza J, Schlachta CM, Poulin EC. Surgery for peptic strictures. Gastrointest. Endosc. Clin. N. Am. 1998 Apr;8(2):399-413.[↩]
- DeVault KR. Lower esophageal (Schatzki’s) ring: pathogenesis, diagnosis and therapy. Dig Dis. 1996 Sep-Oct;14(5):323-9.[↩]
- Law R, Katzka DA, Baron TH. Zenker’s Diverticulum. Clin. Gastroenterol. Hepatol. 2014 Nov;12(11):1773-82; quiz e111-2.[↩]
- Mittal RK. Esophageal function testing: beyond manometry and impedance. Gastrointest. Endosc. Clin. N. Am. 2014 Oct;24(4):667-85.[↩][↩][↩]
- Carlson DA, Pandolfino JE. High-Resolution Manometry in Clinical Practice. Gastroenterol Hepatol (N Y). 2015 Jun;11(6):374-84.[↩]