What is the vestibular system
The vestibular system is a complex set of structures and neural pathways that serves a wide variety of functions that contribute to your sense of proprioception and equilibrium 1. These functions include the sensation of orientation and acceleration of the head in any direction with associated compensation in eye movement and posture. These reflexes are referred to as the vestibuloocular and vestibulospinal reflexes, respectively. The centrally located vestibular system involves neural pathways in the brain that respond to afferent input from the peripheral vestibular system in the inner ear and provide efferent signals that make these reflexes possible. Current data suggest that the vestibular system also plays a role in consciousness, and dysfunctions of the system can cause cognitive deficits related to spatial memory, learning, and navigation 2.
The vestibular system is compromised of a nexus of peripheral sensory end organs and a complex network of central neurons. The peripheral anatomy and physiology are grossly responsible for sensing the degree and direction of acceleration, as well as providing a sense of orientation of the head with respect to gravity. The central connections, including most notably the vestibular nuclei, are grossly responsible for processing the numerous sensory inputs. The accurate and ubiquitous perception of movement and self-orientation occurs, in part, because of a healthy vestibular system. In many ways, this perception is both subconscious and autonomic insomuch that it occurs without intent or self-control. On its most basic level, the vestibular system is both a sensory system as well as a motor system. As a sensory system, the vestibular response not only provides an accurate representation of self motion but also is integral in constructing an “internal map” of one’s center of mass in space with respect to gravity. As a motor system, the vestibular response coordinates effective postural and ocular motor reflexes to ensure static and dynamic equilibrium with respect to one’s center of gravity as well as maintain visual acuity during head movement. It is a hierarchical system by which a proper motor response is heavily dependent upon accurate sensory perception 3.
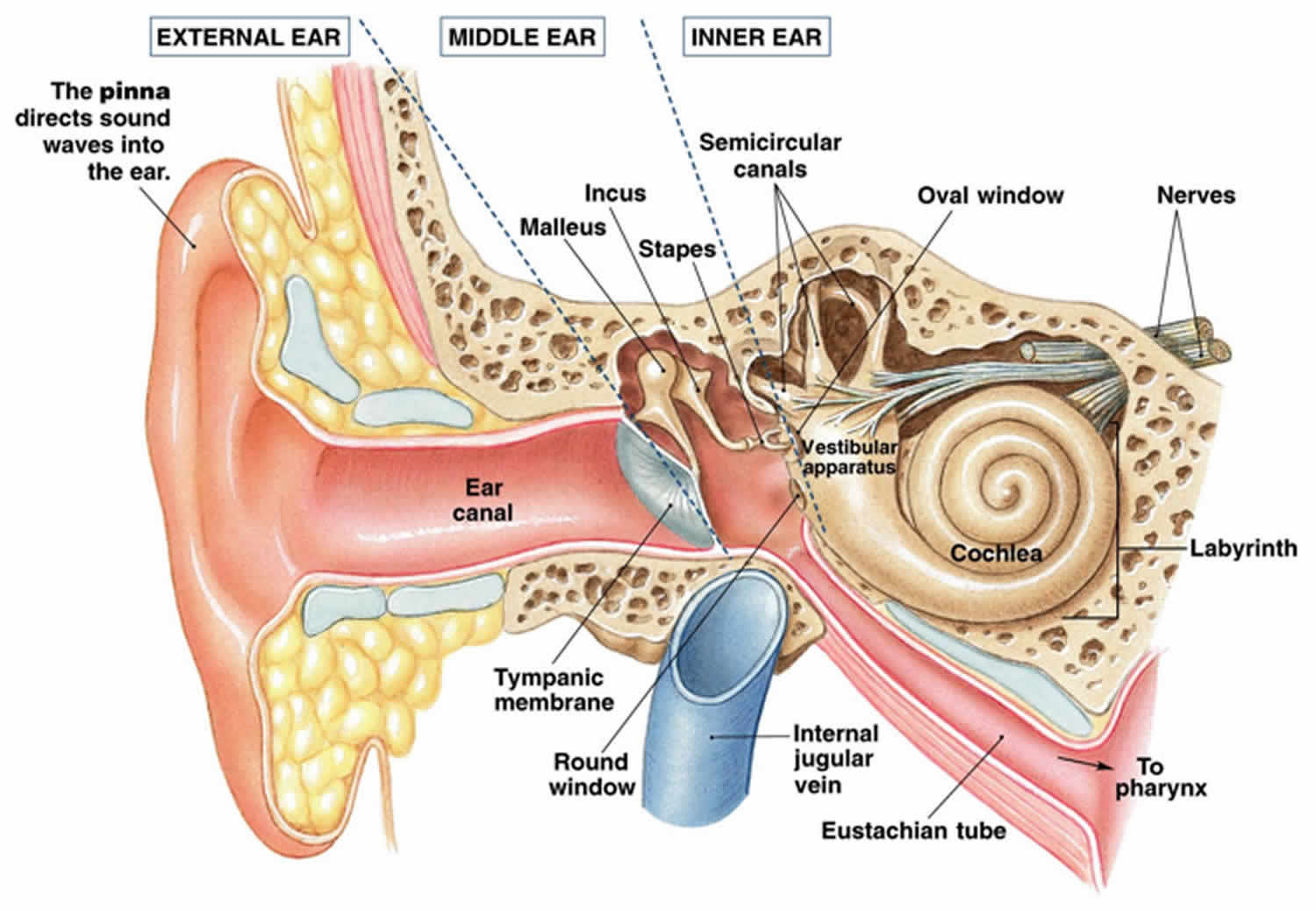
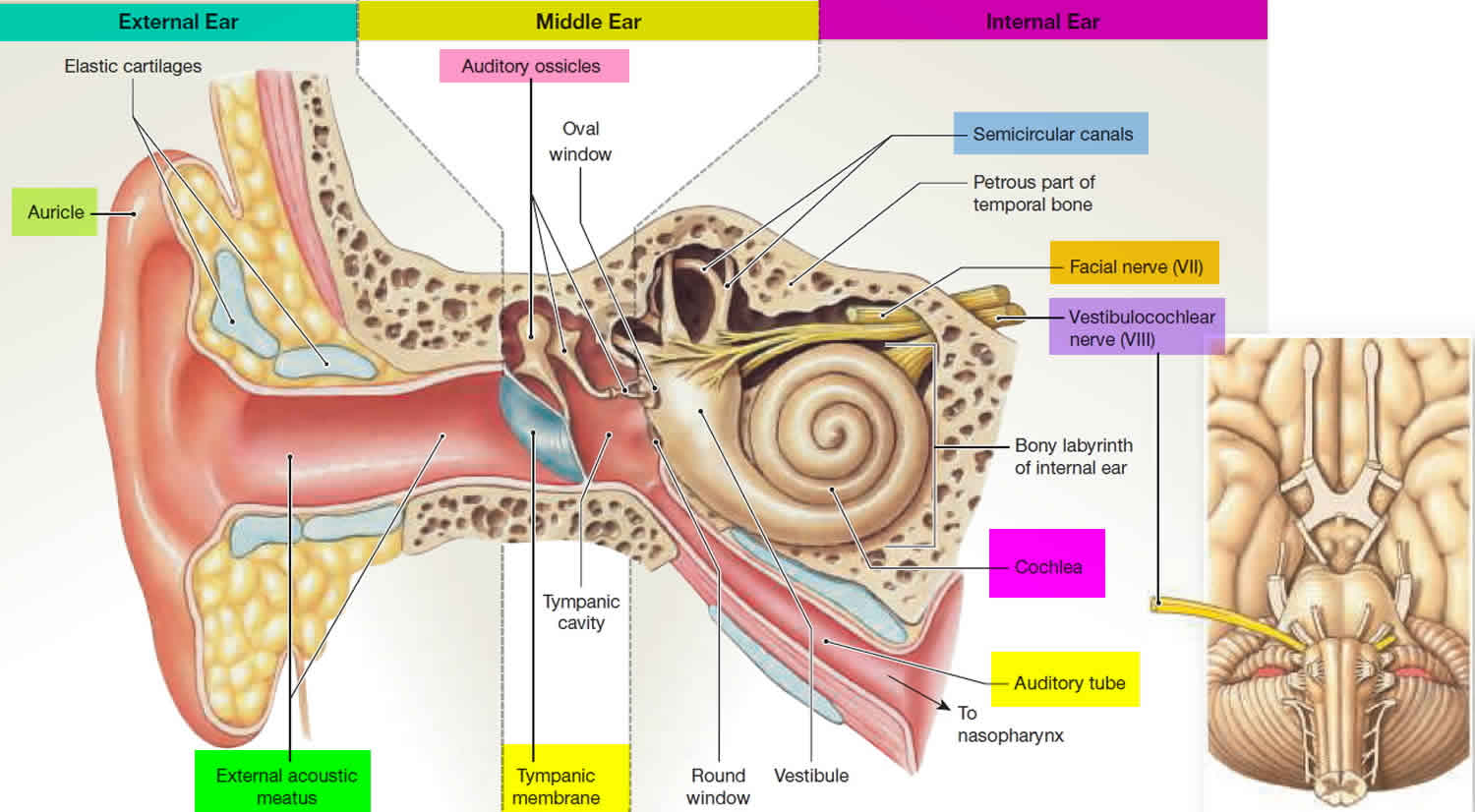
There are vast amounts of both afferent and efferent cellular connections involved in the vestibular system. Most of the afferent nerve signals come from the peripheral vestibular system found in the inner ear within the petrous temporal bone. The inner ear contains a bony labyrinth and a membranous labyrinth. The bony labyrinth is filled with a fluid known as “perilymph” which is comparable to cerebrospinal fluid and drains into the subarachnoid space. Suspended within the bony labyrinth is the membranous labyrinth that contains a fluid known as endolymph unique in composition due to its high potassium ion concentration. Endolymph within the membranous labyrinth surrounds the sensory epithelium and interacts with hair cells within the vestibular apparatus to cause nerve transmission.
The vestibular apparatus is comprised of the utricle, saccule, and superior, posterior, and lateral semicircular ducts. The sensory neuroepithelium in the utricle and saccule is the macula, and the sensory neuroepithelium in the semicircular ducts is the crista ampullaris. Both neuroepithelial structures contain specialized mechanoreceptor cells called “hair cells.” Hair cells contain a vast number of actin-myosin filaments called stereocilia that are connected at the tips by “tip links.” The stereocilia are organized in rows by length, with the tallest stereocilium connected to an immobile kinocilium. The kinocilium is made of the characteristic 9 + 2 microtubule arrangement.
Hair cells are divided into Type 1 hair cells and Type 2 hair cells. Type 1 hair cells have a high variability of resting discharge while Type 2 hair cells have a low variability of resting discharge. Acceleration of endolymph results in the movement of stereocilia, leading to either depolarization or hyperpolarization depending on the direction of the inertial drag. Movement towards the kinocilium causes potassium ion influx and depolarization that results in afferent neurotransmission to the vestibular ganglion. Also known as the Scarpa ganglion, the vestibular ganglion contains thousands of bipolar neurons that receive sensory input from hair cells within the macula and crista ampullaris. Afferent axons from the vestibular ganglion join to become the vestibular nerve. The vestibular nerve then joins the cochlear nerve to become cranial nerve VIII, the vestibulocochlear nerve. Afferent nerve signals carried by the vestibulocochlear nerve are then interpreted by the central vestibular system within the brain. The central vestibular system unites the peripheral signals from both ascending pathways to elicit eye, head, and body motor responses for control of balance and orientation 4.
Although much is known about the vestibular system, there is much that remains unexplained and likely undiscovered regarding its physiology and sophisticated role in postural stability and locomotion. Its complex anatomy and multi-integrational physiology with other sensory systems presents difficulties in evaluating the vestibular system in its entirety. Conversely, evaluating discrete sensory end organs within each vestibular system also presents unique challenges. Secondary to the vestibular system’s complex anatomy and physiology, vestibular disease or even subtle changes in vestibular function due to aging are often difficult to differentiate and identify. Moreover, disorders of the vestibular system are not only very prevalent but often nonspecific with regards to the precise site of the lesion. This is particularly true of the elderly for whom nonspecific dizziness ranks as the most frequent health concern reported to primary care physicians at approximately eight million visits each year 5.
Figure 1. Vestibular system anatomy
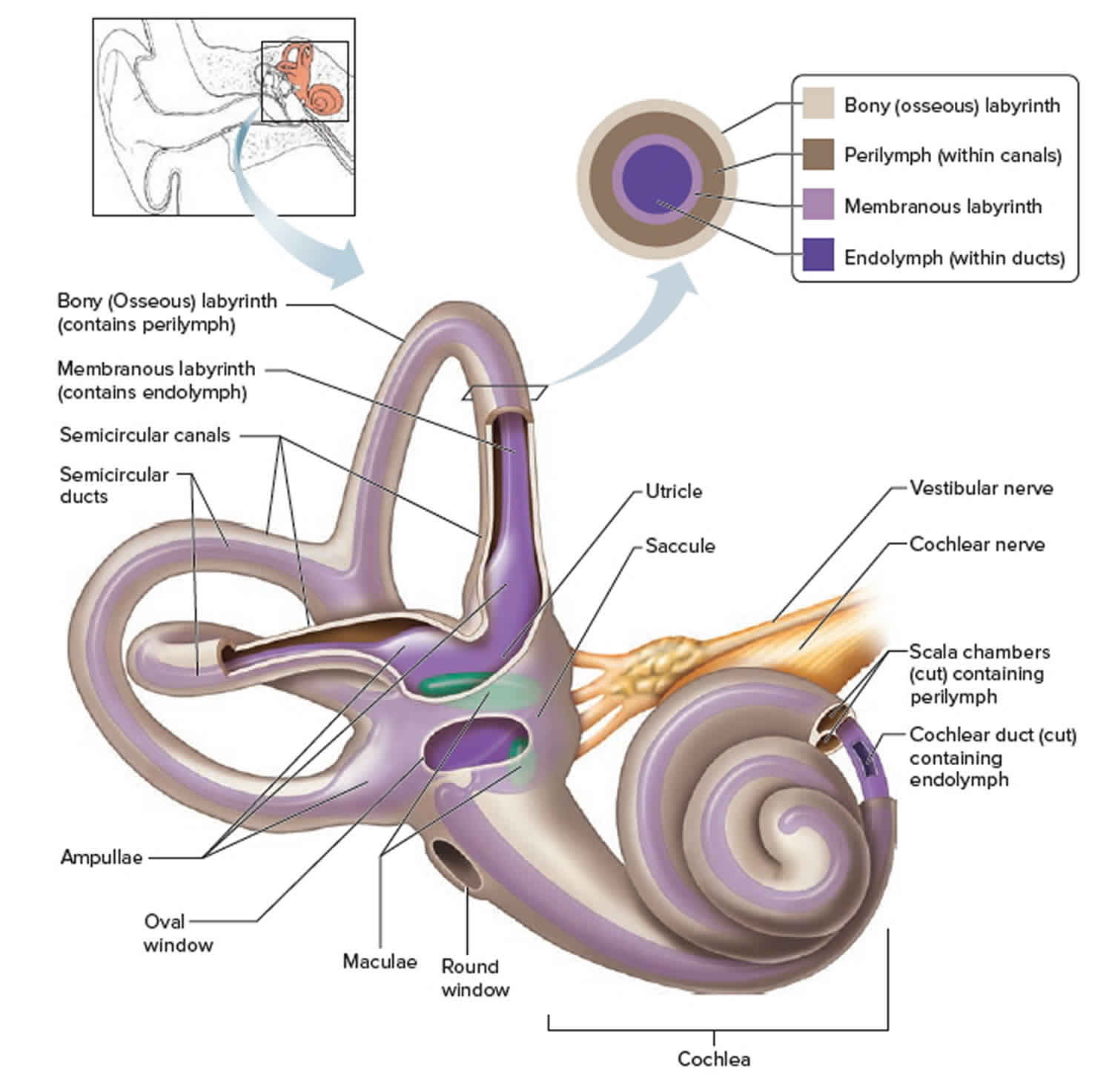 Figure 2. Vestibular system balance organs (inner ear maculae respond to changes in head position)
Figure 2. Vestibular system balance organs (inner ear maculae respond to changes in head position)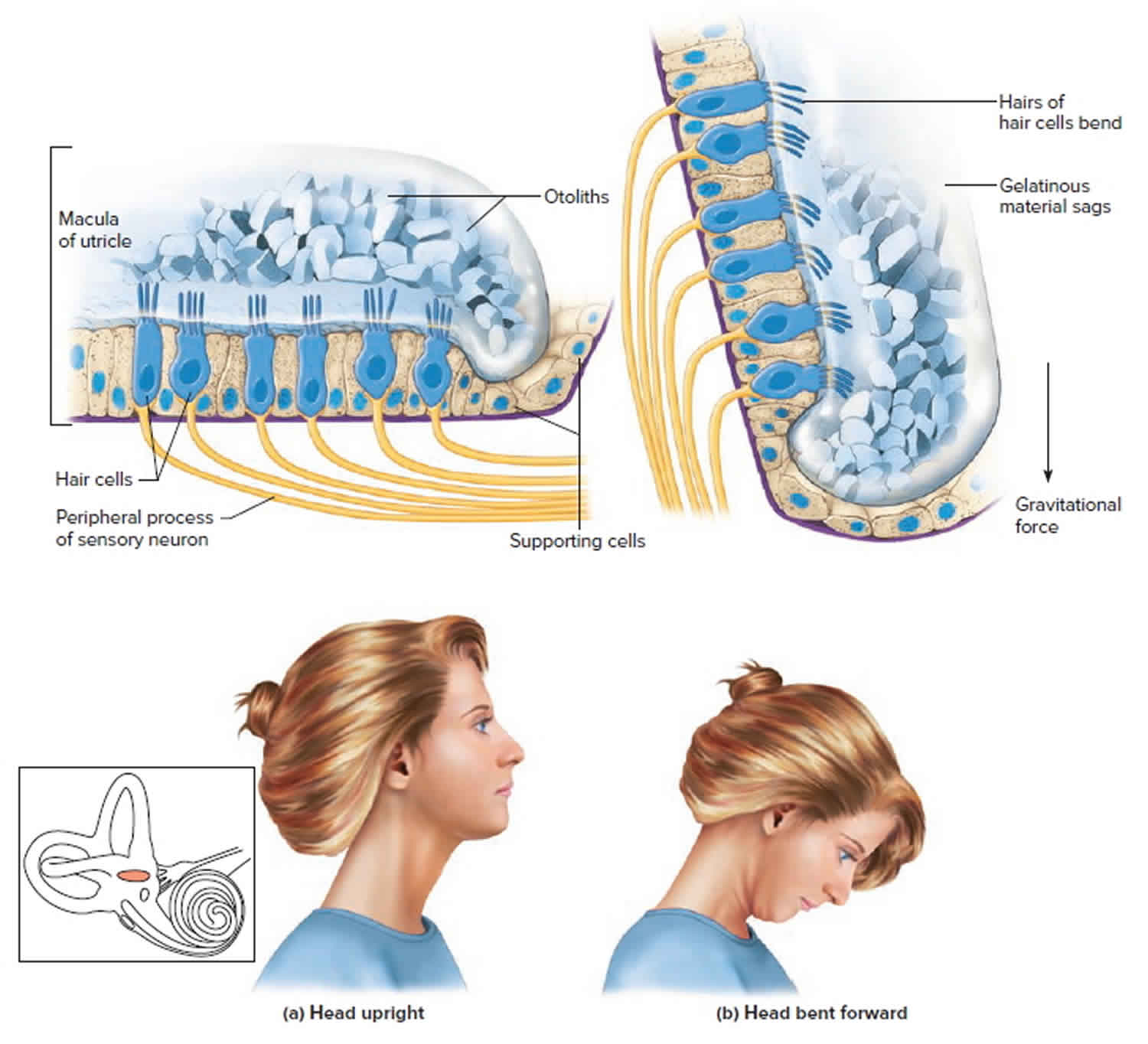 Footnote: (a) Macula of the utricle with the head in an upright position. (b) Macula of the utricle with the head bent forward.
Footnote: (a) Macula of the utricle with the head in an upright position. (b) Macula of the utricle with the head bent forward.
Figure 3. Vestibular system dynamic balance organs (dynamic inner ear balance organs (crista ampullaris) within the Semicricular ducts)
Vestibular system anatomy
There are vast amounts of both afferent and efferent cellular connections involved in the vestibular system. Most of the afferent nerve signals come from the peripheral vestibular system found in the inner ear within the petrous temporal bone. The inner ear contains a bony labyrinth and a membranous labyrinth. The bony labyrinth is filled with a fluid known as “perilymph” which is comparable to cerebrospinal fluid and drains into the subarachnoid space. Suspended within the bony labyrinth is the membranous labyrinth that contains a fluid known as endolymph unique in composition due to its high potassium ion concentration. Endolymph within the membranous labyrinth surrounds the sensory epithelium and interacts with hair cells within the vestibular apparatus to cause nerve transmission.
The vestibular apparatus is comprised of the utricle, saccule, and superior, posterior, and lateral semicircular ducts. The sensory neuroepithelium in the utricle and saccule is the macula, and the sensory neuroepithelium in the semicircular ducts is the crista ampullaris. Both neuroepithelial structures contain specialized mechanoreceptor cells called “hair cells.” Hair cells contain a vast number of actin-myosin filaments called stereocilia that are connected at the tips by “tip links.” The stereocilia are organized in rows by length, with the tallest stereocilium connected to an immobile kinocilium. The kinocilium is made of the characteristic 9 + 2 microtubule arrangement.
Hair cells are divided into Type 1 hair cells and Type 2 hair cells. Type 1 hair cells have a high variability of resting discharge while Type 2 hair cells have a low variability of resting discharge. Acceleration of endolymph results in the movement of stereocilia, leading to either depolarization or hyperpolarization depending on the direction of the inertial drag. Movement towards the kinocilium causes potassium ion influx and depolarization that results in afferent neurotransmission to the vestibular ganglion. Also known as the Scarpa ganglion, the vestibular ganglion contains thousands of bipolar neurons that receive sensory input from hair cells within the macula and crista ampullaris. Afferent axons from the vestibular ganglion join to become the vestibular nerve. The vestibular nerve then joins the cochlear nerve to become cranial nerve VIII, the vestibulocochlear nerve. Afferent nerve signals carried by the vestibulocochlear nerve are then interpreted by the central vestibular system within the brain. The central vestibular system unites the peripheral signals from both ascending pathways to elicit eye, head, and body motor responses for control of balance and orientation 4.
Vestibular system peripheral sensory end organs
The sense of equilibrium (balance) is really two senses:
- Static equilibrium and
- Dynamic equilibrium—that come from different sensory organs.
The organs of static equilibrium sense the position of the head, maintaining balance, stability and posture when the head and body are still. When the head and body suddenly move or rotate, the organs of dynamic equilibrium detect such motion and aid in maintaining balance.
Static Equilibrium
The organs of static equilibrium are in the vestibule, a bony chamber between the semicircular canals and the cochlea. The membranous labyrinth inside the vestibule consists of two expanded chambers—a utricle and a saccule (see Figure 1).
The saccule and utricle each have a tiny structure called a macula. Maculae have many hair cells, which serve as sensory receptors. The hairs of the hair cells project into a mass of gelatinous material, which has grains of calcium carbonate (otoliths) embedded in it. These particles add weight to the gelatinous structure.
Bending the head forward, backward, or to either side tilts the gelatinous masses of the maculae, and as they sag in response to gravity, the hairs projecting into them bend. This action causes the hair cells to signal the sensory neurons associated with them in a manner similar to that of hair cells associated with hearing. The resulting action potentials are conducted into the central nervous system on the vestibular branch of the vestibulocochlear nerve, informing the brain of the head’s new position. The brain responds by adjusting the pattern of motor impulses to skeletal muscles, which contract or relax to maintain balance (Figure 2).
Sensory receptors
The vestibular sensory organ is a paired structure located symmetrically on either side of the head within the inner ear. Inside each end organ are the hair cells, the detection units for both linear and angular acceleration. Extending from each hair cell are fine, hairlike cilia; displacement of the cilia alters the electrical potential of the cell. Bending the cilia in one direction causes the cell membrane to depolarize, while hyperpolarization is induced by movement in the opposite direction. Changes in membrane potential induce alteration in the firing of nerve impulses by the afferent neurons supplying each hair cell.
The two types of acceleration are detected by two types of vestibular end organ. Linear acceleration is sensed by a pair of organs—the saccule and utricle—while there are three receptor organs—called semicircular canals—in each vestibular apparatus for the detection of angular acceleration.
Saccule and utricle
Each saccule and utricle has a single cluster, or macula, of hair cells located in the vertical and horizontal planes, respectively. Resting upon the hair cells is a gelatinous membrane in which are embedded calcareous granules called otoliths. Changes in linear acceleration alter the pressure on the otoliths, causing displacement of the cilia and providing an adequate stimulus for membrane depolarization. Within each macula the hair cells are arranged in two groups oriented in opposite directions, so that the receptor functions in a push-pull fashion within each organ. Since many of the nerve fibres traveling from the hair cells to the brain are constantly active, this arrangement makes the receptors a highly sensitive detection system for both vertical and horizontal linear acceleration.
Semicircular canals
The angular acceleration detectors within the semicircular canals function in a different way. The three canals—which in fact are considerably more than a semicircle in circumference—are oriented at approximately right angles to one another. Two are vertically placed, and one is at about 30° to the horizontal. In this arrangement the anterior canal of one side of the head is in the plane of the posterior canal of the other side. A ridge, or crista, covered by sensory hair cells is located at the end of each canal within an expanded chamber called the ampulla. Rotation of the canals about an imaginary axis passing through the centre of each semicircle causes endolymphatic fluid to flow toward or away from the crista, generating a force that bends the cilia by displacement of a gelatinous plate resting upon the hairs. The cells of the vertical canals are oriented in such a way that centrifugal movement away from the cristae depolarizes the hair cell membranes of the vertical canals, while the opposite applies to the horizontal canal.
Nerve supply
As in the case of the utricle and saccule, some of the nerve fibres conveying information from the cells are constantly active. The hair cells receive nerve impulses from the brain (via efferent fibres) and send them to the central nervous system (via afferent fibres). Excitatory efferent fibres increase the sensitivity of the hair cells, while inhibitory fibres decrease sensitivity. This system gives the semicircular canals a plasticity that is essential to maintaining optimal activity under different environmental conditions—including such extraordinary states as space travel.
The vestibular apparatus is supplied by neurons that make up the vestibular portion of the vestibulocochlear, or eighth cranial, nerve. The somata, or cell bodies, of the afferent fibres lie in the vestibular ganglia near the end organ. Most of the nerve fibres pass from there to vestibular nuclei in the pons, while others pass directly to the cerebellum. The efferent fibres of the vestibular nerve arise from nuclei in the pons.
Dynamic Equilibrium
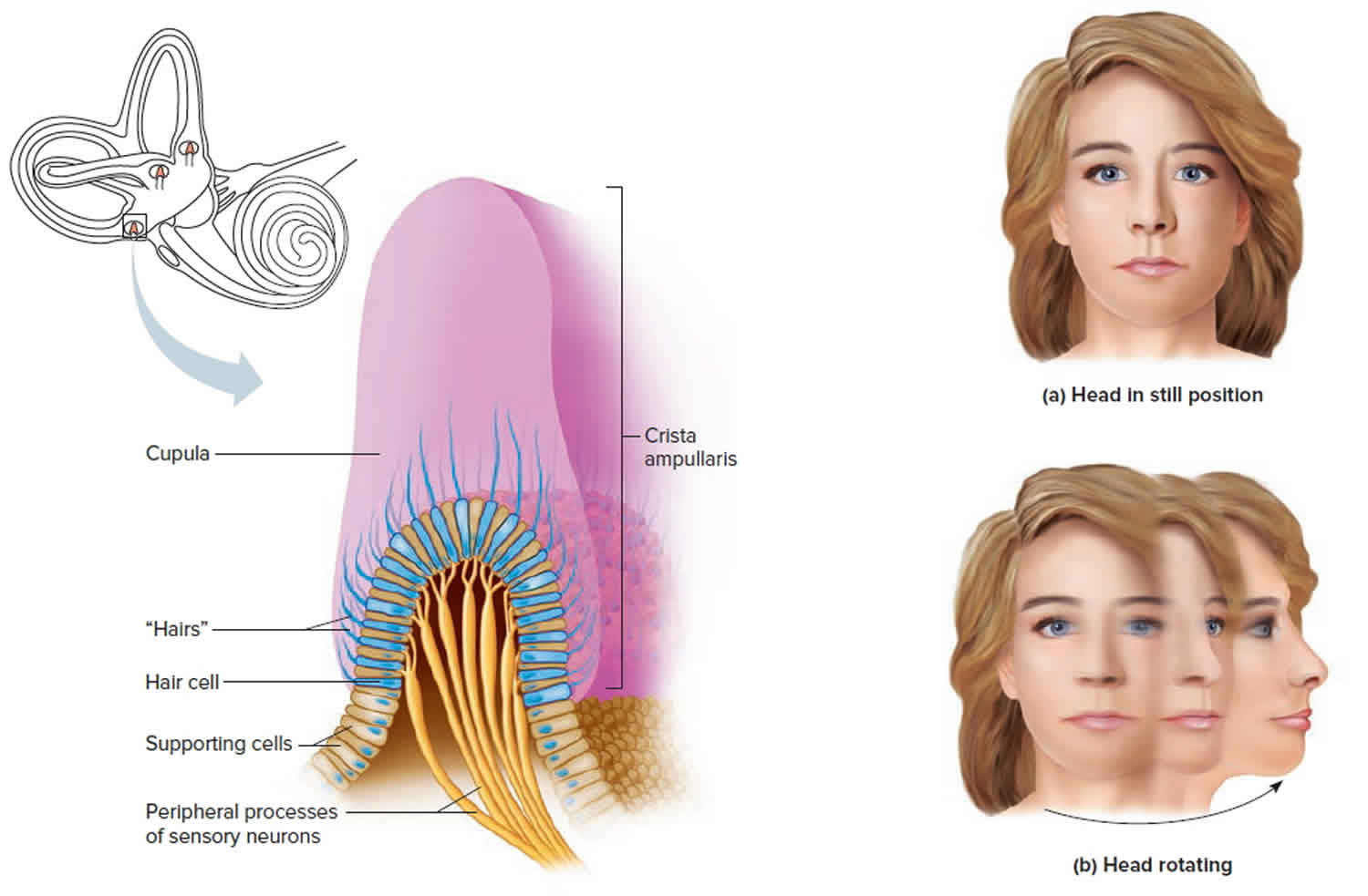
The organs of dynamic equilibrium are the three semicircular canals in the labyrinth. They detect motion of the head and aid in balancing the head and body during sudden movement. These canals lie at right angles to each other (see Figure 1).
Suspended in the perilymph of the bony portion of each semicircular canal is a membranous semicircular duct that ends in a swelling called an ampulla, which
houses the sensory organs of the semicircular canals. Each of these sensory organs, called a crista ampullaris, contains a number of sensory hair cells and supporting cells. Like the hairs of the maculae, the hair cells of the crista ampullaris extend upward into a dome-shaped, gelatinous mass called the cupula (Figure 3). When the head is stationary, the cupula of the crista ampullaris remains upright. When the head is moving rapidly, the cupula bends opposite the motion of the head, stimulating sensory receptors.
Rapid movement of the head or body stimulates the hair cells of the crista ampullaris (Figure 3). At such times, the semicircular canals move with the head or body, but the fluid inside the membranous ducts remains stationary. Imagine turning rapidly while holding a full glass of water. This action bends the cupula in one or more of the canals in a direction opposite that of the head or body movement, and the hairs embedded in it also bend. The stimulated hair cells signal their associated neurons, which conduct impulses to the brain. The brain interprets these impulses as a movement in a particular direction.
Parts of the cerebellum are particularly important in interpreting impulses from the semicircular canals. Analysis of such information allows the brain to predict the consequences of rapid body movements. By modifying signals to appropriate skeletal muscles, the cerebellum can maintain balance.
Other sensory structures aid in maintaining equilibrium. For example, certain mechanoreceptors (proprioceptors), particularly those associated with the joints of the neck, inform the brain about the position of body parts. In addition, the eyes detect changes in position that result from body movements. Such visual information is so important that even if the organs of equilibrium are damaged, a person may be able to maintain normal balance by keeping the eyes open and moving slowly.
The nausea, vomiting, dizziness, and headache of motion sickness arise from sensations that don’t make sense. The eyes of a person reading in a moving car, for example, signal the brain that the person is stationary, because the print doesn’t move. However, receptors in the skin detect bouncing, swaying, starting, and stopping as the inner ear detects movement. The contradiction triggers the symptoms. Similarly, in a passenger of an airplane flying through heavy turbulence, receptors in the skin and inner ear register the chaos outside, but the eyes focus on the immobile seats and surroundings.
To prevent or lessen the misery of motion sickness, focus on the horizon or an object in the distance ahead. Medications are available by pill (diphenhydramine and dimenhydrinate) and, for longer excursions, in a skin patch (scopolamine).
Vestibular system function
The vestibular system functions to detect the position and movement of our head in space. This allows for the coordination of eye movements, posture, and equilibrium. The vestibular apparatus found in the inner ear helps to accomplish this task by sending afferent nerve signals from its individual components. The utricle and the saccule are responsible for sensing linear acceleration, gravitational forces, and tilting of the head. The neuroepithelium found in the utricle and saccule is the macula which provides neural feedback about horizontal motion from the utricle and vertical motion from the saccule. Embedded within the macula are small calcium carbonate crystals known as otoliths that assist in hair cell response to the inertial drag of endolymph. Angular acceleration and rotation of the head in various planes are sensed by the three semicircular ducts that are oriented at right angles to one another. Each of the semicircular ducts contains a dilation near the opening to the utricle. This dilation is called the ampulla which contains a neuroepithelial structure called the “crista ampullaris.” The crista ampullaris is coated by a gelatinous substance known as the cupula which holds the hair cells in place. Unlike the macula, the crista ampullaris does not contain otoliths.
In addition to the functions associated with the peripheral vestibular system, the central vestibular system allows for processing and interpretation of afferent signals and output of efferent signals. Efferent signals include the vestibuloocular reflex, which allows the eyes to remain fixed on an object while the head is moving. This is accomplished by coordinating movement between both eyes involving the parapontine reticular formation and output to various extraocular eye muscles involving the oculomotor and abducens nerves. The vestibulospinal reflex maintains balance and posture through the coordination of spinal musculature with head movement. Cognitive functions that involve the central vestibular system are based on established neural pathways, although many pathways are still unknown. The known central vestibular connections include the vestibulo-thalamo-cortical tract, dorsal tegmental nucleus to entorhinal cortex tract, and nucleus reticularis pontis oralis to hippocampus tract. These tracts form a series of complex connections that play a functional role in self-motion perception, spatial navigation, spatial memory, and object recognition memory 6.
The mechanism involved with the function of the peripheral vestibular system involves acceleration of endolymph within the various structures of the vestibular apparatus. Head movement in various directions is responsible for this acceleration that results in stimulation of the stereocilia of hair cells. When the head stops accelerating, hair cells return to their baseline position which allows them to respond to further changes in endolymph acceleration. Depending on the direction of acceleration, the inertial drag of the endolymph will push the stereocilia either towards or away from the fixed kinocilium. Movement towards the kinocilium results in depolarization of the hair cell and movement away from the kinocilium results in hyperpolarization and an overall reduction in afferent firing rates. Depolarization results in potassium ion channel opening, followed by calcium channel opening. Calcium channel opening results in neurotransmitter release across the synaptic cleft, leading to nerve transmission to the vestibular ganglion. Nerve signals pass through the 20,000 bipolar neurons in the vestibular ganglion and leave along the vestibular nerve. The vestibular nerve joins the cochlear nerve and enters the brainstem at the pontomedullary junction. The primary processor of vestibular signals is the vestibular nucleus complex that extends from the rostral medulla to the caudal pons. Many signals are sent from the vestibular nucleus to either the thalamus, cortex, or cerebellum that help to process and adjust efferent signals to postural or ocular muscles. Of note, the hippocampus plays an important role in spatial memory, including the functions of navigation and orientation 4.
Although balance and equilibrium are heavily dependent upon other central nervous system processes, especially the visual and somatosensory systems, the contributions of the vestibular system are undoubtedly critical. This is particularly evident when functioning in vestibular-dependent environments where visual and somatosensory cues are compromised 7. The importance of an accurate sensory perception of one’s environment is most apparent when the vestibular system fails to translate an appropriate signal. An aberrant or absent vestibular response results in significant debilitating balance deficits and often produces an array of symptoms including dizziness, disequilibrium, vertigo, nausea, pallor, diaphoresis, general malaise, and even emesis. Depending on the severity, these symptoms can often lead to physical, mental, and even social isolation.
Vestibular system testing
Many tests can help determine if the vestibular system is functioning properly. The oculocephalic reflex is a simple test used to determine if the brainstem of a comatose patient is intact using the reflexes of the vestibular system. The test involves rotating a patient’s head horizontally, which should activate the vestibular system on the ipsilateral side of rotation. This results in the patient’s eyes slowly deviating to the side opposite of head movement if the brainstem is intact. If the brainstem is not intact, the eyes will follow the movement of the head to the ipsilateral side.
Caloric testing is also used to determine brainstem function in patients; it involves the injection of a small volume of either warm or cold water to the external auditory meatus. Patients with an intact vestibular system will exhibit nystagmus to the same side as the irrigation if warm water is used, and nystagmus to the opposite side of irrigation if cold water is used.
More specific tests for components of vestibular function include videonystagometry, the most common vestibular function test. The test is divided into three parts including ocular motor, positional testing, and caloric testing. Other diagnostic modalities include rotation tests and video head impulse testing. Both of these tests use devices to monitor eye movements when the head is rotated in various directions to test the integrity of the vestibuloocular reflex 8.
Vestibular system disorders
Dysfunction of the vestibular system can manifest symptomatically as vertigo, nausea, vomiting, visual disturbance, hearing changes, and various cognitive deficits. The relationship of the vestibular system to cognition is not well understood, but many patients with vestibular dysfunction exhibit impairment of spatial navigation, learning, memory, and object recognition. The pathophysiology of vertigo can be defined as peripheral or central. Peripheral vertigo is more common than central vertigo, and three of the most common etiologies include benign paroxysmal positional vertigo, Meniere disease, and viral labyrinthitis.
Benign paroxysmal positional vertigo (BPPV) is the most common cause of peripheral vertigo and lasts for seconds to minutes. The pathophysiology is believed to be due to displaced otoconia in the posterior semicircular canal that cause an inappropriate sensation of movement. BPPV is diagnosed based on a thorough history and use of the Dix-Hallpike test with associated reproduction of vertigo symptoms and nystagmus. There are many movement techniques used to treat BPPV; however, the Epley maneuver is often cited as being one of the most effective. The technique involves rotating and tilting the head and body in various ways to reposition displaced otoconia in the inner ear. For acute, severe exacerbations of BPPV, anti-vertigo medications are indicated to help with symptom control.
Meniere disease is another cause of peripheral vertigo that can last for hours and also manifest with symptoms of hearing loss and tinnitus. The pathophysiology of Meniere disease is endolymphatic hydrops, or expansion of endolymph volume within the membranous labyrinth. This volume expansion impacts both the vestibular apparatus and cochlea that are both filled with endolymph. Expansion of endolymph within the cochlear duct results in hearing symptoms that differentiate it from BPPV. Meniere disease is diagnosed based on clinical criteria, and currently, there are no curative treatments exist. Symptoms are managed with anti-vertigo medications, low-salt diet, and surgical decompression of the endolymphatic sac as a last option.
Viral labyrinthitis, also known as “vestibular neuritis,” is another cause of peripheral vertigo that can be attributed to inflammation of the vestibular nerve, secondary to a viral infection. Symptoms include a simultaneous loss of hearing and balance function in the affected ear that can last from days to weeks. Treatment involves symptom control with anti-vertigo medications, and symptoms will typically resolve in one to three weeks.
In addition to the pathologies listed, vestibular function in the elderly has been well studied as a contributing factor to dizziness and imbalance leading to falls. This is due to the significant loss of both Type 1 and Type 2 hair cells that occurs most prominently between the ages of 65 and 70 9.
Inner ear vertigo
Vertigo, a type of dizziness, is an unpleasant disturbance of spatial orientation or to the erroneous perception of movement 10. Vertigo involves a perceived movement either of one’s own body, such as swaying or rotation, or of the environment, or both. Alongside headache, dizziness and vertigo are among the more common symptoms with which patients present to physicians in general, not just to neurologists. Their lifetime prevalence is approximately 20% to 30% 11. Dizziness, a common symptom that affects more than 90 million Americans, has been reported to be the most common complaint in patients 75 years of age or older 12.
The relative frequencies of various syndromes presenting with dizziness and vertigo are listed in table 1.
Table 1. The relative frequencies of different dizziness and vertigo syndromes
| Diagnosis | Number of patients | Percent |
| Benign paroxysmal positioning vertigo | 1336 | 18.6 |
| Phobic postural vertigo | 1127 | 15.6 |
| Central vestibular vertigo | 893 | 12.4 |
| Basilar/vestibular migraine | 738 | 10.2 |
| Menière’s disease | 677 | 9.4 |
| Vestibular neuritis | 531 | 7.4 |
| Bilateral vestibulopathy | 367 | 5.1 |
| Vestibular paroxysmia | 284 | 3.9 |
| Psychogenic dizziness | 228 | 3.2 |
| Perilymph fistula | 44 | 0.6 |
| Dizziness syndromes of unclear etiology | 239 | 3.3 |
| Other | 741 | 10.3 |
| Overall | 7205 |
The important criteria for distinguishing among them are as follows 13:
- The type of dizziness/vertigo: rotatory vertigo resembles the sensation of being on a merry-go-round (in vestibular neuritis and other disorders), while postural vertigo resembles the sensation of riding in a boat (e.g., in bilateral vestibulopathy). Many patients use the term “dizziness” for lightheadedness without any sensation of movement (e.g., in drug intoxication).
- The duration of dizziness/vertigo: attacks may last for seconds or minutes (as in vestibular paroxysm) or hours (as in Menière’s disease or vestibular migraine). Persistent vertigo lasting days or weeks is seen in vestibular neuritis, among other conditions. Attacks of postural vertigo lasting minutes to hours can be produced, for example, by brainstem transient ischemic attacks.
- Precipitating and exacerbating factors of dizziness and vertigo: the symptoms arise at rest in some conditions (e.g., vestibular neuritis); they can also arise when the patient walks (as in bilateral vestibulopathy) or be induced by turning the head to the right or left (as in vestibular paroxysm). Other possible precipitating factors include turning in bed (as in benign paroxysmal positioning vertigo [BPPV]), coughing, pressing, and loud tones of a particular frequency (Tullio’s phenomenon, seen in perilymph fistula), as well as certain social or environmental conditions (e.g., phobic postural vertigo).
- The accompanying symptoms, if present, may arise from the inner ear – e.g., attacks of intense tinnitus, hearing impairment, and a pressure sensation in the ear, which are typical of Menière’s disease. Diplopia, sensory disturbances, dysphagia, dysarthria, and paralysis of arms and legs are symptoms of central origin that usually arise in the brainstem. Headache or a history of migraine may point to the diagnosis of vestibular migraine but can also be caused by brainstem ischemia or posterior fossa hemorrhage.
Acute Severe Dizziness
The patient who presents with sudden onset severe dizziness – in the absence of prior similar episodes – has the “acute severe dizziness” presentation 14. Patients with acute severe dizziness appear ill due to the dizziness and accompanying nausea and vomiting. Impaired ability to walk is also common. Though rigorous epidemiological studies are lacking, the most common cause is an acute lesion – presumed viral in origin – of the vestibular nerve on one side, so-called vestibular neuritis 15. The mechanism underlying vestibular neuritis is the eighth cranial nerve is affected in vestibular neuritis. Patients with vestibular neuritis nearly always report true vertigo, which is characteristically described as visualized spinning of the environment. The symptoms are typically severe for 1-2 days with gradual resolution over weeks to months. It is exceedingly rare to have more than one bout of vestibular neuritis, so an alternative diagnosis should be considered whenever more than one episode is reported.
It is now clear that a small stroke within the posterior fossa can present as acute severe dizziness, closely mimicking vestibular neuritis 16, 17, 18. The first step to distinguishing vestibular neuritis from stroke is asking the patient about other neurological symptoms such as focal numbness, focal weakness, or slurred speech. Mild double vision can result from a peripheral vestibular lesion so this symptom is not a reliable discriminator. The next step is the physical examination. Patients with vestibular neuritis have highly characteristic exam features. Only in an extremely rare case can all of the vestibular neuritis exam features be mimicked by a stroke.
Nystagmus in Acute Severe Dizziness
Nystagmus is a term used to describe alternating slow and fast movements of the eyes. These alternating movements give the appearance that the eyes are beating toward one or more directions. Patients with vestibular neuritis have a peripheral vestibular pattern of nystagmus. In this setting, the peripheral vestibular pattern is a unidirectional, principally horizontal pattern of nystagmus. This description means that the nystagmus beats is in only one direction (i.e., a left-beating nystagmus never converts to right-beating, or a right-beating nystagmus never converts to left-beating). Patients with a peripheral disorder demonstrate nystagmus to the contralateral side which suppresses with visual fixation. Nystagmus improves with gaze towards the lesion and worsens with gaze opposite the lesion 19. Conversely, bi-directional gaze-evoked nystagmus (i.e., right beating nystagmus present with gaze toward the right, and left-beating nystagmus present with gaze toward the left side) is a central nervous system pattern of nystagmus. Other central nervous system patterns are pure torsional nystagmus or spontaneous vertical (typically downbeat) nystagmus. With an acute peripheral vestibular lesion, the only pattern of nystagmus that can result is unidirectional nystagmus. In acute severe dizziness presentations, any other pattern should be considered a central nervous system sign. Patients often prefer to keep their eyes closed early on, but the eyes should be opened and the pattern of nystagmus defined.
Nystagmus in vestibular neuritis is spontaneous (i.e., present in primary gaze) for at least the first several hours of symptoms. Following this initial time period, the nystagmus may only be identified during gaze testing (i.e., having the patient look to each side) or if visual fixation is blocked. Patients can suppress peripheral vestibular nystagmus by visual fixation on a target, so removing the patient’s ability to fixate can bring out the spontaneous nystagmus. The simplest way to block fixation is to place a blank sheet of paper a few inches in front of the patient and then observe for spontaneous nystagmus from the side.
The reason for the characteristic pattern of nystagmus in vestibular neuritis is an imbalance in the peripheral vestibular signals to the brain. Normally, the peripheral vestibular system on each side has a baseline firing rate of action potentials that functions to drive the eyes toward the other side. When the peripheral vestibular system on each side is intact, the input from each side is balance so the eyes remain stationary. When an acute lesion occurs on one side, the input from the opposite side is unopposed. As a result, the eyes will be “pushed” toward the lesioned side. This movement of the eyes is the slow phase of nystagmus. When the eyes reach a critical point off center, the brain responds by generating a corrective eye movement to move the eyes back. This is the fast phase of nystagmus. Since the direction of the fast phase gives the appearance that the eyes are beating in that direction, an acute left peripheral vestibular lesion leads to spontaneous right beating nystagmus. Over time, the asymmetry resolves or the brain compensates for the asymmetry.
Vascular Causes of Acute Severe Dizziness
Although vestibular neuritis is the most common cause of the acute dizziness presentation, no laboratory or imaging test exists to confirm a viral etiology. A peripheral vestibular lesion can be caused by a vascular occlusion of the blood supply to the peripheral vestibular components, though presumably this cause is much less common.
Stroke should be a serious consideration in the patient who presents with the acute dizziness presentation. Dizziness is a symptom of stroke in 50% of stroke presentations 20. Most stroke patients that report dizziness as a symptom have other prominent central nervous system features, but a small stroke of the cerebellum or brain stem can present with isolated dizziness (i.e., dizziness without other accompanying central nervous system signs or symptoms). In a population-based study, about 3% of patients with dizziness had a stroke etiology, but less than 1% of patients with isolated dizziness had stroke as the etiology 21. However, a prospective study of 24 patients with acute severe dizziness reported 6 patients (25%) with stroke etiology 16. Patients with stroke presenting as isolated dizziness may report imbalance, true vertigo, a more vague dizziness sensation, or a combination of these. Nausea and vomiting are also common, as they are with vestibular neuritis. Unfortunately, computerized tomography (CT) scans are an extremely insensitive test for acute stroke presentations in general 22, and particularly so for infarction within the posterior fossa 23. A stroke within the posterior fossa may not appear on a CT scan for days or weeks because of artifacts or poor resolution. Because of this, CT should never be considered as a means of excluding stroke. Magnetic resonance imaging (MRI) is a much more sensitive test, but is not a practical test to screen for stroke in emergency department dizziness presentations. Like CT, the sensitivity of the test is the lowest for stroke of the posterior fossa 24.
The key features discriminating stroke from vestibular neuritis are the pattern of nystagmus and the results of the head thrust test. Down-beating nystagmus or bidirectional gaze-evoked nystagmus are both immediate indications that the localization must be in the central nervous system. These patterns are not caused by lesions of the peripheral vestibular system. This is the reason that an examination of ocular movements is required before a diagnosis is even considered. Another highly suspicious pattern of nystagmus is a pure torsional pattern. There are now case reports of patients who have unidirectional horizontal nystagmus and a stroke etiology so the pattern of nystagmus should not be the sole criterion.3-5 A patient with unidirectional nystagmus, a positive head thrust in the direction opposite the fast phase of nystagmus, and no other neurological features can be diagnosed with vestibular neuritis with a high level of certainty. It would take a well placed and small stroke to cause the peripheral vestibular pattern of nystagmus and a corresponding positive head thrust test without any other central nervous system features. Though all patients with vestibular neuritis are unsteady walking, the inability to walk is another red flag.5 Finally, a person’s risk for stroke based on stroke risk factors should be considered. Though no validated scale exists to grade stroke risk based on stroke risk factors in this population, a stroke work-up is reasonable in patients with a high risk for stroke. One should not be over reliant on stroke risk factors as discriminators, however, since other stroke mechanisms – such as arterial dissection – occur in the absence of stroke risk factors.
Management of Acute Severe Dizziness
The management of the acute dizziness presentation begins with supportive care. If stroke is suspected then a neuro-imaging study should be considered. Though CT could serve as the initial study, a normal result on CT should provide little confidence that stroke can be excluded. In this situation, an MRI or hospital admission for close observation should be considered. If stroke is confirmed to be the cause and the patient presents within three hours of onset, thrombolytic treatment should be considered. A short course of corticosteroids should be considered for patients with vestibular neuritis. A randomized controlled trial showed that patients with vestibular neuritis treated with corticosteroids within three days of symptom onset had a higher likelihood of recovery of the peripheral vestibular caloric response at 12 months 25. However this study did not test whether the patient’s functional or symptomatic outcome improved, and corticosteroids are not without potential side effects. After the initial severe symptomatic time period, it is important that patients resume activities because this helps the brain to compensate for the asymmetry of vestibular signals. A formal vestibular therapy program has been shown in a randomized trial to improve outcomes in patients with vestibular neuritis 26.
Treatment for vertigo
The treatment of dizziness and vertigo 13 may include medication, physical therapy, and psychotherapy; a few limited cases may require surgical treatment. Before the treatment is begun, the patient should be told that the prognosis is generally good: many of these conditions have a favorable spontaneous course, both because peripheral vestibular dysfunction tends to improve and because there is central vestibular compensation for asymmetrical peripheral vestibular tone. Moreover, most of these conditions can be treated successfully.
Labyrinthitis
Labyrinthitis is an inflammatory disorder of the membranous labyrinth, affecting both the vestibular and cochlear end organs 19. It may present unilaterally or bilaterally, and similar to vestibular neuronitis, it is often preceded by an upper respiratory infection. This disorder occurs when infectious microorganisms or inflammatory mediators invade the membranous labyrinth, damaging the vestibular and auditory end organs. Potential etiologies include viral pathogens, bacterial invasion, bacterial toxins, and systemic disease 19.
Viral labyrinthitis usually occurs in adults in their fourth to seventh decades of life. Bacterial labyrinthitis may result from both otogenic and meningitic infection, progressing to involve the labyrinth. Labyrinthitis of otogenic origin can be observed in any age group and may result from cholesteatoma or otitis media 19. Meningitic labyrinthitis is more common in children less than 2 years of age, who are more susceptible to developing meningitis 19. Otogenic infections typically cause unilateral symptoms while meningitic infections cause bilateral symptoms 19.
Unlike vestibular neuronitis, patients with labyrinthitis present with complaints indicative of both vestibular and cochlear damage. Vertigo presents suddenly and is accompanied by hearing loss 19. Electronystagmography may reveal nystagmus, and audiometry will reveal a sensorineural hearing loss or mixed hearing loss if middle ear effusion is present 19. Depending on the source of infection, patients may also present with findings consistent with otitis media, mastoiditis, or meningitis.
Treatment is aimed primarily at eradication of the underlying infection and supportive care 19. Middle ear effusions and mastoiditis should be drained and treated with antibiotics. Meningitis should be treated with culture-directed antibiotics with central nervous system penetration and appropriate consultation. Anti-emetics and anti-nausea medications are helpful during the acute phase.
Peripheral vestibular vertigo
A functional classification of peripheral vestibular disorders divides them into three main types, which can be distinguished on the basis of their typical symptoms and signs (table 2):
- Chronic, bilateral dysfunction of the vestibular nerve or the peripheral vestibular organs;
- Acute, unilateral vestibular dysfunction;
- Paroxysmal pathological excitation or inhibition of the vestibular nerve or vestibular organs.
Table 2. The presenting manifestations and causes of peripheral vestibular types of vertigo
| Type of disorder | Presenting manifestations | Examples and causes |
|---|---|---|
| Chronic, bilateral peripheral vestibular dysfunction |
| Bilateral vestibulopathy due to (e.g.):
|
| Acute/subacute unilateral vestibular dysfunction (labyrinth and/or vestibular nerve) with asymmetrical vestibular tone |
|
due to reactivation of a latent herpes simplex virus type 1 infection |
| Inappropriate unilateral paroxysmal excitation or loss of function of the peripheral vestibular system |
|
|
1)Benign paroxysmal positioning vertigo (BPPV)
This is the most common type of vertigo; it mainly affects older patients (Table 1) and has a lifetime prevalence of 2.4% 11. It is characterized by brief attacks of rotational vertigo, accompanied by vertical positioning nystagmus that rotates toward the lower of the two ears and beats toward the forehead. Nystagmus is a term used to describe alternating slow and fast movements of the eyes 14. These alternating movements give the appearance that the eyes are beating toward one or more directions. The attacks are precipitated by reclination of the head, or by lateral positioning of the head or body, with the affected ear downward 10. After a change in position of one of these types, rotational vertigo and nystagmus arise after a latency of a few seconds and then take a characteristic crescendo-decrescendo course, lasting a total of 30 to 60 seconds 10. The nystagmus corresponds to a so-called ampullofugal excitation of the affected posterior vertical semicircular canal of the affected (lower) ear.
More than 90% of cases are idiopathic 10; the remaining, symptomatic cases are most commonly due to head trauma, vestibular neuritis, or Menière’s disease 27. Benign paroxysmal positioning vertigo is called “benign” because it usually resolves spontaneously within a few weeks or months; in some cases, however, it can last for years. If left untreated, it persists in about 30% of patients. Benign paroxysmal positioning vertigo also arises with greater than usual frequency after prolonged bed rest necessitated by other diseases, or after surgery. Benign paroxysmal positioning vertigo of the horizontal semicircular canal is rare and is precipitated by rotation of the head in the recumbent position.
The canalolithiasis hypothesis explains all of the manifestations of positioning vertigo and nystagmus 28. According to this hypothesis, the condition is due to the presence of agglomerates of many otoconia (small crystals of calcium carbonate in the saccule and utricle of the inner ear) that nearly fill the lumen of the semicircular canal and are freely mobile within it, instead of the small pieces of particulate matter that adhere firmly to the cupula (so-called cupulolithiasis).
Benign paroxysmal positioning vertigo is treated with positioning maneuvers: rapid repositioning of the head can move the otoconial agglomerate out of the semicircular canal so that it can no longer cause positioning vertigo 10. The treatments of choice are the Semont 29 and Epley maneuvers. For the Semont maneuver, see Figure 4; the Epley maneuver involves rotation of the patient in the recumbent position with the head hanging down. Most patients can perform these maneuvers themselves after brief training. The two are equally effective, and the cure rate is more than 95% within a few days, as shown by multiple controlled studies and meta-analyses 30. The rate of recurrence of benign paroxysmal positioning vertigo is about 15% to 30% per year. The symptoms eventually recur at some time after effective treatment in about 50% of patients 31 but can then be treated effectively a second time in the same manner.
Epley Maneuver to treat BPPV Vertigo
Deep Head Hanging Maneuver to treat BPPV Vertigo
Lempert (BBQ) Maneuver to treat BPPV Vertigo
Half Somersault Maneuver to treat BPPV Vertigo
Figure 4. The treatment of benign paroxysmal positioning vertigo with the Semont maneuver

Note: The illustration shows the treatment of benign paroxysmal positioning vertigo (BPPV) due to canalolithiasis of the right posterior semicircular canal.
a) In the initial, sitting position, the head is turned 45° to the side of the unaffected (“healthy”) ear.
b) The patient is laid on the right side, i.e., on the side of the affected ear, while the head is kept in 45° of rotation to the other side. This induces movement of the particulate matter in the posterior semicircular canal by gravity, leading to rotatory nystagmus toward the lower ear that extinguishes after a brief interval. The patient should maintain this position for about one minute.
c) While the head is still kept in 45° of rotation toward the side of the healthy ear, the patient is rapidly swung over to the side of the unaffected ear, so that the nose now points downward. The particulate matter in the semicircular canal now moves toward the exit from the canal. This position, too, should be maintained for at least one minute.
d) The patient returns slowly to the initial, sitting position. The particulate matter settles in the utricular space, where it can no longer induce rotatory vertigo. This sequence (a-d) should be performed three times in a row three times per day, in the morning, at noon, and at night. Most patients are free of symptoms after doing this for three days.
[Source 10]2) Vestibular neuritis
The clinical syndrome of vestibular neuritis is characterized by the following:
- Persistent rotational vertigo with a pathological inclination of the visual vertical axis toward the side of the affected labyrinth
- Spontaneous, horizontally rotating nystagmus toward the unaffected side, producing apparent movement of the environment (“oscillopsia”)
- Gait deviation and falling tendency toward the affected side
- Nausea and vomiting
- Unilateral dysfunction of the horizontal semicircular canal, as revealed by the Halmagyi-Curthoys head impulse test 32 for the function of the vestibulo-ocular reflex, as well as by caloric testing.
A viral and/or autoimmune as cause for vestibular neuritis is probable but has not yet been proven. Autopsy studies have revealed inflammatory degeneration of the vestibular nerve, the presence of viral DNA from herpes simplex virus type I, and the so-called “latency-associated transcript” (LAT) in vestibular ganglion cells 33. The treatment is symptomatic, causal, and physiotherapeutic:
- Symptomatic treatment: antivertiginous medications, such as 100 to 300 mg of dimenhydrinate, should be given only in the first three days and only if necessary to treat severe nausea and vomiting, because they delay the development of central compensation mechanisms.
- “Causal” treatment: a four-armed, placebo-controlled trial was performed, based on the assumption that vestibular neuritis is caused by the reactivation of a latent herpes simplex virus type 1 infection. The trial revealed that monotherapy with a glucocorticoid-methylprednisolone at an initial dose of 100 mg daily, reduced in 20-mg steps every four days, significantly improved the recovery of peripheral vestibular function. The administration of valacyclovir alone had no effect, nor did its administration in combination with the glucocorticoid have any additional effect 34.
- Physical therapy: a further principle of treatment is the promotion of central compensation by physical therapy. Equilibrium training significantly lessens the time required for vestibulospinal compensation and postural regulation to develop 35. Voluntary eye movements and fixation are exercised in order to improve impaired visual fixation; furthermore, active head movements are exercised to realign the vestibular reflex, as well as balance tasks and goal-directed movements.
3) Menière’s disease
This condition is probably due to labyrinthine endolymphatic hydrops with periodic rupturing of the membrane that separates the endolymphatic and perilymphatic spaces. These ruptures precipitate the paroxysmal attacks that last a few minutes to hours 36. The ultimate etiology is impaired resorption in the endolymphatic sac due to perisaccular fibrosis or to obliteration of the endolymphatic duct. Attacks are produced when rupture of the endolymphatic tube causing calcium-induced depolarization of the vestibulocochlear nerve. A classic Menière’s attack consists of rotatory vertigo, tinnitus, hearing impairment, and pressure sensation in one ear. The lifetime prevalence of this condition is approximately 0.5% 11. It usually begins on one side, and the frequency of attacks is highly variable. Menière’s disease becomes bilateral in 50% of cases 37 and is the second most common cause of bilateral vestibulopathy.
Its treatment is based on two principles:
- Treatment of individual attacks: vertigo and nausea can be improved with antivertiginous medications just as in the treatment of other types of acute labyrinthine dysfunction. For example, 100 mg dimenhydrate suppositories can be used.
- Attack prophylaxis: this type of treatment is aimed at improving the underlying endolymphatic hydrops. Despite the high prevalence of Menière’s disease and the large number of clinical studies that have been performed, there is still no treatment of this type that has been conclusively shown to be effective. The spectrum of recommendations ranges from a sodium-free diet to diuretics, transtympanic gentamicin instillation (20 to 40 mg given repeatedly, at intervals of several weeks, until symptoms improve), betahistine, and surgical procedures 36. A beneficial effect on the frequency of attacks has been reported for transtympanic gentamicin 30 and for the prolonged high-dose administration of betahistine hydrochloride (48 mg tid for 12 months). The latter dose of betahistine hydrochloride is currently recommended on the basis of a recently reported observational treatment study in 112 patients who were treated for at least 12 months at doses of 16, 24, or 48 mg tid 38. The highest dose led to a statistically significantly greater reduction of attack frequency and was well tolerated.
4. Vestibular Schwannoma
Vestibular schwannoma is the most common intracranial neoplasm producing vestibular symptoms, affecting one in every 100,000 people per year 39. These are usually slow-growing, benign tumors that originate from the Schwann cells lining the vestibular portion of cranial nerve VIII 19. Occasionally these tumors arise from the cochlear branch of the eighth nerve, but this is reported in less than 5% of cases 40. Patients may present with either unilateral or bilateral vestibular schwannoma. Bilateral vestibular schwannoma is associated with neurofibromatosis II, which is additionally characterized by glioma, meningioma, subcapsular ventricular opacities, and less frequently, peripheral neurofibromata and café au lait spots.
Patients may present with episodic or positional vertigo, disequilibrium, tinnitus, and usually asymmetric hearing loss. Early in the disease, when the tumor is small, patients complain of dizziness, hearing loss, and tinnitus, due to compression of the vestibulocochlear nerve. The slow growth often allows for central compensation, alleviating vertigo. With continued growth, the tumor can press against the facial or trigeminal nerve causing facial weakness and numbness, respectively. Eventually, the tumor grows to a size where it compresses the brainstem and cerebellum causing truncal ataxia, dysmetria, disequilibrium, and possibly death.
Diagnosis begins with a thorough history and physical examination. An audiogram is important in documenting hearing loss and any asymmetries which may exist. If you suspect vestibular schwannoma, then imaging is necessary. Computed tomography of the head with contrast is helpful, but magnetic resonance imaging with and without enhancement is the preferred imaging modality.
Once vestibular schwannoma is confirmed radiographically, a decision should be made on how to proceed with treatment. Treatment options include surgical excision, radiation therapy, and observation with serial magnetic resonance imaging. In making this decision, one should consider the size of the lesion, age and health of the patient, and what symptoms are present. These patients should be referred to a neuro-otologist for management of their care.
Central vestibular syndromes
Central vestibular syndromes are mainly caused by lesions of the vestibular pathways, which arise in the vestibular nuclei in the caudal portion of the brainstem and proceed to the cerebellum, thalamus, and vestibular cortex, or by damage to the vestibulocerebellum 10. Pathological excitation is a rare cause, as occurs, for example, in the paroxysmal brainstem attacks with ataxia that can be produced by multiple sclerosis or vestibular epilepsy. The common causes of central vestibular vertigo include vestibular migraine and ischemic lesions in the brainstem 10. Furthermore, central vestibular disturbances arise in the setting of certain ocular motor disorders such as downbeat and upbeat nystagmus, as attacks in episodic ataxia type 2, and in vestibular migraine. These individual disorders, and the treatment of each, will be discussed in the following sections.
Motion sickness
Motion sickness is attributed to an incongruence in the sensory input from the vestibular, visual, and somato-sensory systems 41. Motion sickness occurs while riding in a car, boat, or airplane if the vestibular and somato-sensory systems sense movement, but the visual system does not.
On the first sensation of motion sickness, efforts should be made to bring vestibular, visual, and somato-sensory input back in congruence. For example, a person on a boat who starts to feel seasick should immediately watch the horizon. Seasickness can be prevented by applying a scopolamine patch (Transderm-Scop) behind one ear at least four hours before boating 42.
Vertebrobasilar Ischemic Stroke
The blood supply to the brainstem, cerebellum, and inner ear is derived from the vertebrobasilar system 19. Occlusion of any of the major branches of this system may result in vertigo. Symptoms of vertebrobasilar ischemic stroke are highly variable and depend on which of the three major circumferential branches are occluded; the posterior inferior cerebellar artery, anterior inferior cerebellar artery, or superior cerebellar artery. Numerous processes may occlude the vertebrobasilar system. The most common are atherosclerosis, emboli, and vertebral artery dissection. Vertebral artery dissection can result from trauma or neck manipulation, or can occur spontaneously. Less common causes include subclavian steal syndrome, hypercoagulation disorders, and inflammatory conditions.
As mentioned earlier, the symptoms associated with ischemic stroke in this area are highly variable and greatly dependent upon which branch of the system is occluded. Occlusion of the posterior inferior cerebellar artery will cause a lateral medullary infarction and result in lateral medullary syndrome, also known as Wallenberg’s syndrome. Expected manifestations include vertigo, nystagmus, gait disturbance, ipsilateral limb ataxia and facial pain or numbness, contralateral body hemianesthesia, Horner’s syndrome, dysphagia, hoarseness, and rarely, facial nerve paralysis.
Lateral pontomedullary infarction secondary to occlusion of the anterior inferior cerebellar artery will result in lateral inferior pontine syndrome. This syndrome is characterized by symptoms similar to Wallenberg’s syndrome with notable differences. Involvement of cranial nerves VII and VIII results in ipsilateral facial paralysis and tinnitus and hearing loss, respectively. Dysphagia and hoarseness, however, are not apparent as cranial nerves IX and X nuclei are uninvolved with occlusion of the anterior inferior cerebellar artery.
Lateral superior pontine syndrome occurs when the superior cerebellar artery is occluded. With this syndrome, one can expect vertigo, nystagmus, gait disturbance, ipsilateral limb ataxia and facial pain or numbness, contralateral body hemianesthesia, and Horner’s syndrome. Distinguishing this syndrome is the finding of contralateral impairment of vibration and temperature due to medial lemniscus involvement.
A high index of suspicion must be kept with any patient presenting with spontaneous vertigo to avoid missing the diagnosis of ischemic stroke. It is essential to consider stroke in any acutely vertiginous patient with concomitant neurological signs and symptoms. Once vertebrobasilar ischemic stroke is suspected, an expeditious work-up is necessary. This should include a thorough physical examination, imaging, and neurology consultation for both evaluation and treatment.
Vertebrobasilar Insufficiency
Vertebrobasilar insufficiency is synonymous with a transient ischemic attack (TIA) of the vertebrobasilar system. By definition, patients experience symptoms similar to those detailed above, but the symptoms resolve within 24 hours. If left untreated, the disease process will eventually progress to stroke with permanent or long-lasting seqeulae. Risk factors and causes are identical to those for VIS. Forty-eight percent of patients who suffer a vertebrobasilar ischemic stroke report a transient ischemic attack in the preceding days or weeks 43. In fact, 29% of patients suffer from at least one episode of vertigo, a symptom of vertebrobasilar insufficiency, prior to their vertebrobasilar insufficiency 44. Patients suffering a vertebrobasilar transient ischemic attack are likely to progress to stroke more quickly than those experiencing transient ischemic attacks in the anterior territory.
Vertebrobasilar insufficiency is a common cause of vertigo in the elderly. Symptoms may last from minutes to hours, but typically average 8 minutes in duration. In as many as one third of patients, vertigo is the only manifestation of their disease. Although this disease should always be in the differential, several months of recurrent vertigo unaccompanied by other neurological signs suggests another disorder. The likelihood of immediate stroke is less in patients presenting with only episodic vertigo. Patients who also present with paresis, blindness, or altered consciousness, however, should be evaluated urgently for fear of impending stroke. Evaluation is similar to that of an ischemic stroke. Treatment includes antiplatelets, anticoagulation, possible thrombolysis and percutaneous transluminal angioplasty, and neurological consultation.
The sudden onset of vertigo in a patient with additional neurologic symptoms (e.g., diplopia, dysarthria, dysphagia, ataxia, weakness) suggests the presence of vascular ischemia.
Treatment of transient ischemic attack and stroke includes preventing future events through blood pressure control, cholesterol-level lowering, smoking cessation, inhibition of platelet function (e.g., aspirin, clopidogrel [Plavix], aspirin-dipyridamole [Aggrenox]) and, possibly, anticoagulation (warfarin [Coumadin]).
Acute vertigo caused by a cerebellar or brainstem stroke is treated with vestibular suppressant medication and minimal head movement for the first day. As soon as tolerated, medication should be tapered, and vestibular rehabilitation exercises should be initiated 45, 46.
Placement of vertebrobasilar stents may be considered in a patient with symptomatic critical vertebral artery stenosis that is refractory to medical management 47. Rarely, infarction or hemorrhage in the cerebellum or brainstem may present with acute vertigo as the only neurologic symptom 48. Given the risk of brainstem compression with a large cerebellar stroke, neurosurgical decompression may be indicated.
Downbeat and upbeat nystagmus
Two types of vertically beating central nystagmus are of special importance: downbeat nystagmus and upbeat nystagmus, each named after the direction of the rapid, beating phase. Downbeat nystagmus is the most common type of acquired, persistent nystagmus 49. Both types manifest themselves above all with swaying nystagmus and unsteadiness of gait and only secondarily with oscillopsia, i.e., apparent movement of the environment due to oscillation of the retinal image. In distinction to spontaneous nystagmus such as in vestibular neurits, downbeat nystagmus and upbeat nystagmus are types of fixation nystagmus, i.e., their intensity increases with visual fixation. Both downbeat nystagmus and upbeat nystagmus always indicate the presence of a central disturbance and possess special localizing significance. Downbeat nystagmus is usually due to bilateral dysfunction of the flocculus 50; its three common causes are cerebellar atrophy, ischemia, and Arnold-Chiari malformation 49. Upbeat nystagmus – which, unlike downbeat nystagmus, generally persists for no more than a few weeks – can be caused by paramedian medullary or pontomesenchephalic lesions, e.g., brainstem infarct or hemorrhage.
A randomized, placebo-controlled study of downbeat nystagmus has shown that the potassium-channel blockers 3,4-diaminopyridine 51 and 4-aminopyridine can significantly improve this type of nystagmus 52. The dosage is 5 to 10 mg tid; follow-up ECG is necessary. The effectiveness of this treatment has since been confirmed by multiple studies. 4-Aminopyridine seems to be effective against upbeat nystagmus as well, but this has been documented to date only in a single case study 53.
Episodic ataxia type 2
The familial episodic ataxias are rare genetic diseases of autosomal dominant transmission. There are at least two well-defined varieties. Type 2 (EA 2) is characterized by recurrent attacks of dizziness and ataxia that are precipitated by physical activity, stress, or alcohol and usually last for hours. In between attacks, more than 90% of patients have marked central ocular motor disturbances, often downbeat nystagmus. EA 2 is caused by mutations in the CACNA1A gene (PQ calcium channel gene). Most patients can be treated successfully with acetazolamide. If this treatment is ineffective, or if adverse effects such as kidney stones develop, patients with EA 2 can also be treated with 4-aminopyridine (5 mg tid) 54.
Aminopyridines are thus an effective treatment for downbeat nystagmus, upbeat nystagmus, and episodic ataxias type 2 which is well tolerated at the low dose that is generally used. These studies have also led to the development of a new principle of treatment; activation of cerebellar Purkinje cells through potassium-channel blockade enhances the cerebellar inhibitory influence on the vestibular and cerebellar nuclei.
Vestibular migraine or migraine with vestibular aura
Vestibular migraine is characterized by recurrent attacks that last minutes to hours and usually consist of rotatory vertigo 55, 56. It is the most common cause of spontaneously occurring attacks of vertigo (Table 1). Its lifetime prevalence is 0.98% 11. In more than 60% of patients, these attacks are associated with headache and/or photophobia or phonophobia; the remaining patients have attacks of vertigo alone. Most patients also have migraine attacks with or without an aura; this fact makes the condition easier to diagnose. In some patients, the diagnosis can be made only on the basis of a positive response to the treatment of the individual attacks with medication and to pharmacological prophylaxis. The prophylactic treatment of vestibular migraine is analogous to that of migraine with aura and consists of the administration of beta-blockers, valproic acid, and topiramate. No randomized, controlled studies on the efficacy of medications for vestibular migraine have yet been published.
Phobic postural vertigo
Phobic postural vertigo is the second most common diagnosis in a specialized neurological ambulatory clinic for dizziness and vertigo. This disorder is not found in the diagnostic repertoire of most neurologists and ENT specialists. Patients with phobic postural vertigo usually complain of swaying vertigo, lightheadedness, and gait unsteadiness that are continually present but fluctuate in severity. These symptoms are often accompanied by anxiety and are situationally dependent. The precipitating factor may be the presence of a large crowd, or waiting in the check-out line at a store; often, avoidance behavior results 13. The symptoms typically improve when the patient participates in sports or has had a small amount of alcohol to drink. The affected patients often have an obsessive-compulsive personality, in the sense of “accentuated” personality traits, with a marked tendency toward introspection and a need to “have everything under control.” The central problem in phobic postural vertigo is the patient’s attempt to establish conscious control over body equilibrium, which leads to a “spiral of self-observation.” When this happens, the body’s own movements may be perceived as movements of the outside world. The main features of this disorder and its treatment are summarized in the box. The clinical neurological examination and ancillary tests reveal no relevant pathological findings.
Clinical features
- The patient has postural vertigo with unsteadiness of stance and gait; the neurological examination and ancillary tests are generally unremarkable
- Fluctuating unsteadiness of stance and gait with attacks of fear of falling, but without an actual fall
- Anxiety and autonomic disturbances sometimes occur during or just after the attacks
- The attacks are precipitated or exacerbated by typical situations, e.g., crowds, empty spaces, driving
- The symptoms often improve during sporting activity or after the consumption of a small amount of alcohol
- Increasingly severe avoidance behavior is common
The patient’s personality is usually of an obsessive-compulsive or reactive-depressive type. At the onset of the disorder, there is often a vestibular disturbance (25%) or a situation giving rise to particular stress (70%).
Treatment
- A thorough diagnostic assessment to allay the patient’s fear of having a serious organic disease
- Psycho-educative therapy to inform the patient about the pathological mechanism and the precipitating factors and situations
- Desensitization by self-exposure, i.e., the deliberate seeking out of situations that precipitate vertigo. Light sporting activities are also helpful.
- If the symptoms persist, pharmacotherapy, e.g., with selective serotonin reuptake inhibitors, and/or cognitive behavioral therapy are indicated
Treatment markedly improves symptoms in about 70% of patients 57.
- Casale J, Gupta G. Physiology, Vestibular System. [Updated 2018 Dec 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532978[↩]
- Zalewski CK. Aging of the Human Vestibular System. Semin Hear. 2015 Aug;36(3):175-96.[↩]
- Horak F. Philadelphia, PA: F.A. Davis Company; 2008. Role of the Vestibular System in Postural Control. 3rd ed.[↩]
- Corns LF, Johnson SL, Roberts T, Ranatunga KM, Hendry A, Ceriani F, Safieddine S, Steel KP, Forge A, Petit C, Furness DN, Kros CJ, Marcotti W. Mechanotransduction is required for establishing and maintaining mature inner hair cells and regulating efferent innervation. Nat Commun. 2018 Oct 01;9(1):4015[↩][↩][↩]
- Marchetti G F Whitney S L Older adults and balance dysfunction Neurol Clin 2005233785–805., vii[↩]
- Dieterich M, Brandt T. The bilateral central vestibular system: its pathways, functions, and disorders. Ann. N. Y. Acad. Sci. 2015 Apr;1343:10-26.[↩]
- Peterka R J. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097–1118[↩]
- Zalewski CK. Aging of the Human Vestibular System. Semin Hear. 2015 Aug;36(3):175-96[↩]
- Kingma H, van de Berg R. Anatomy, physiology, and physics of the peripheral vestibular system. Handb Clin Neurol. 2016;137:1-16[↩]
- Strupp M, Brandt T. Diagnosis and Treatment of Vertigo and Dizziness. Deutsches Ärzteblatt International. 2008;105(10):173-180. doi:10.3238/arztebl.2008.0173. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2696792/[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Epidemiology of vertigo. Neuhauser HK. Curr Opin Neurol. 2007 Feb; 20(1):40-6. https://www.ncbi.nlm.nih.gov/pubmed/17215687/[↩][↩][↩][↩]
- Dizziness in primary care. Results from the National Ambulatory Medical Care Survey. Sloane PD. J Fam Pract. 1989 Jul; 29(1):33-8. https://www.ncbi.nlm.nih.gov/pubmed/2738548/[↩]
- Brandt T, Dieterich M, Strupp M. Vertigo – Leitsymptom Schwindel. Darmstadt: Steinkopff; 2003.[↩][↩][↩]
- Kerber KA. Vertigo and Dizziness in the Emergency Department. Emergency medicine clinics of North America. 2009;27(1):39-viii. doi:10.1016/j.emc.2008.09.002. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2676794/[↩][↩]
- Clinical practice. Vestibular neuritis. Baloh RW. N Engl J Med. 2003 Mar 13; 348(11):1027-32. https://www.ncbi.nlm.nih.gov/pubmed/12637613/[↩]
- Norrving B, Magnusson M, Holtas S. Isolated acute vertigo in the elderly; vestibular or vascular disease? Acta Neurol Scand. 1995 Jan;91(1):43–48. https://www.ncbi.nlm.nih.gov/pubmed/7732773[↩][↩]
- Lee H, Cho YW. A case of isolated nodulus infarction presenting as a vestibular neuritis. J Neurol Sci. 2004 Jun 15;221(12):117–119. https://www.ncbi.nlm.nih.gov/pubmed/15178226[↩]
- Lee H, Sohn SI, Cho YW, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006 Oct 10;67(7):1178–1183. https://www.ncbi.nlm.nih.gov/pubmed/17030749[↩]
- Thompson TL, Amedee R. Vertigo: A Review of Common Peripheral and Central Vestibular Disorders. The Ochsner Journal. 2009;9(1):20-26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3096243/[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Kleindorfer DO, Miller R, Moomaw CJ, et al. Designing a message for public education regarding stroke: does FAST capture enough stroke? Stroke. 2007 Oct;38(10):2864–2868. http://stroke.ahajournals.org/content/38/10/2864.long[↩]
- Kerber KA, Brown DL, Lisabeth LD, Smith MA, Morgenstern LB. Stroke Among Patients With Dizziness, Vertigo, and Imbalance in the Emergency Department: A Population-Based Study. Stroke; a journal of cerebral circulation. 2006;37(10):2484-2487. doi:10.1161/01.STR.0000240329.48263.0d. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1779945/[↩]
- Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi:10.1016/S0140-6736(07)60151-2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1859855/[↩]
- Wasay M, Dubey N, Bakshi R. Dizziness and yield of emergency head CT scan: Is it cost effective? Emergency Medicine Journal : EMJ. 2005;22(4):312. doi:10.1136/emj.2003.012765. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1726733/pdf/v022p00312.pdf[↩]
- Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol. 2000 Sep;21(8):1434–1440. http://www.ajnr.org/content/21/8/1434.long[↩]
- Methylprednisolone, valacyclovir, or the combination for vestibular neuritis. Strupp M, Zingler VC, Arbusow V, Niklas D, Maag KP, Dieterich M, Bense S, Theil D, Jahn K, Brandt T. N Engl J Med. 2004 Jul 22; 351(4):354-61. http://www.nejm.org/doi/full/10.1056/NEJMoa033280[↩]
- Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Strupp M, Arbusow V, Maag KP, Gall C, Brandt T. Neurology. 1998 Sep; 51(3):838-44. https://www.ncbi.nlm.nih.gov/pubmed/9748036/[↩]
- What inner ear diseases cause benign paroxysmal positional vertigo ? Karlberg M, Hall K, Quickert N, Hinson J, Halmagyi GM. Acta Otolaryngol. 2000 Mar; 120(3):380-5. https://www.ncbi.nlm.nih.gov/pubmed/10894413/[↩]
- Current view of the mechanism of benign paroxysmal positioning vertigo: cupulolithiasis or canalolithiasis ? Brandt T, Steddin S. J Vestib Res. 1993 Winter; 3(4):373-82. https://www.ncbi.nlm.nih.gov/pubmed/8275271/[↩]
- Curing the BPPV with a liberatory maneuver. Semont A, Freyss G, Vitte E. Adv Otorhinolaryngol. 1988; 42():290-3. https://www.ncbi.nlm.nih.gov/pubmed/3213745/[↩]
- Strupp M, Cnyrim C, Brandt T. Vertigo and dizziness: Treatment of benign paroxysmal positioning vertigo, vestibular neuritis and Menère’s disease. In: Candelise L, editor. Evidence-based Neurology – management of neurological disorders. Oxford: Blackwell Publishing; 2007. pp. 59–69.[↩][↩]
- Benign paroxysmal positioning vertigo: a long-term follow-up (6-17 years) of 125 patients. Brandt T, Huppert D, Hecht J, Karch C, Strupp M. Acta Otolaryngol. 2006 Feb; 126(2):160-3. https://www.ncbi.nlm.nih.gov/pubmed/16428193/[↩]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. https://www.ncbi.nlm.nih.gov/pubmed/3390028[↩]
- Prevalence of HSV-1 LAT in human trigeminal, geniculate, and vestibular ganglia and its implication for cranial nerve syndromes. Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, Brandt T. Brain Pathol. 2001 Oct; 11(4):408-13. https://www.ncbi.nlm.nih.gov/pubmed/11556685/[↩]
- Strupp M, Zingler VC, Arbusow V, et al. Methylprednisolone, valacyclovir, or the combination for vestibular neuritis. N Engl J Med. 2004;351:354–361. http://www.nejm.org/doi/full/10.1056/NEJMoa033280[↩]
- Strupp M, Arbusow V, Maag KP, Gall C, Brandt T. Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Neurology. 1998;51:838–844. https://www.ncbi.nlm.nih.gov/pubmed/9748036[↩]
- Ménière’s disease. Minor LB, Schessel DA, Carey JP. Curr Opin Neurol. 2004 Feb; 17(1):9-16. https://www.ncbi.nlm.nih.gov/pubmed/15090872/[↩][↩]
- Ménière’s disease: a long-term follow-up study of bilateral hearing levels. Takumida M, Kakigi A, Takeda T, Anniko M. Acta Otolaryngol. 2006 Sep; 126(9):921-5. https://www.ncbi.nlm.nih.gov/pubmed/16864488/[↩]
- Strupp M, Huppert D, Frenzel C, Wagner J, Zingler VC, Mansmann U, Brandt T. Long-term prophylactic treatment of attacks of vertigo in Menière’s disease-comparison of a high with a low dosage of betahistine in an open trial. Acta Otolaryngol. 2008 May;128(5):520-4. doi: 10.1080/00016480701724912. https://www.ncbi.nlm.nih.gov/pubmed/18421605[↩]
- Epidemiology of acoustic neuromas. Tos M, Thomsen J. J Laryngol Otol. 1984 Jul; 98(7):685-92. https://www.ncbi.nlm.nih.gov/pubmed/6747450/[↩]
- Khrais T., Romano G., Sanna M. Nerve origin of vestibular schwannoma: a prospective study. J Laryngol Otol. 2008;122:128–131. https://www.ncbi.nlm.nih.gov/pubmed/18039415[↩]
- Fife TD. Episodic vertigo. In: Rakel RE, ed. Conn’s Current therapy, 1999: latest approved methods of treatment for the practicing physician. 51st ed. Philadelphia: Saunders, 1999:923–30.[↩]
- Flake ZA, Scalley RD, Bailey AG. Practical selection of antiemetics. Am Fam Physician. 2004;69:1169–74.[↩]
- Kim H. Y., Chung C. S., Moon S. Y., et al. Complete nonvisualization of basilar artery on MR angiography in patients with vertebrobasilar ischemic stroke: favorable outcome factors. Cerebrovasc Dis. 2004;18:269–276. https://www.ncbi.nlm.nih.gov/pubmed/15331872[↩]
- Grad A., Baloh R. W. Vertigo of vascular origin. Clinical and electronystagmographic features in 84 cases. Arch Neurol. 1989;46:281–284. https://www.ncbi.nlm.nih.gov/pubmed/2919982[↩]
- Quigley EM, Hasler WL, Parkman HP. AGA technical review on nausea and vomiting. Gastroenterology. 2001;120:263–86.[↩]
- Baloh RW. Vertigo in older people. Curr Treat Options Neurol. 2000;2:81–9.[↩]
- Levy EI, Hanel RA, Bendok BR, Boulos AS, Hartney ML, Guterman LR, et al. Staged stent-assisted angioplasty for symptomatic intracranial vertebrobasilar artery stenosis. J Neurosurg. 2002;97:1294–301.[↩]
- Norrving B, Magnusson M, Holtas S. Isolated acute vertigo in the elderly; vestibular or vascular disease?. Acta Neurol Scand. 1995;91:43–8.[↩]
- Downbeat nystagmus: aetiology and comorbidity in 117 patients. Wagner JN, Glaser M, Brandt T, Strupp M. J Neurol Neurosurg Psychiatry. 2008 Jun; 79(6):672-7. https://www.ncbi.nlm.nih.gov/pubmed/17872983/[↩][↩]
- Detection of floccular hypometabolism in downbeat nystagmus by fMRI. Kalla R, Deutschlander A, Hufner K, Stephan T, Jahn K, Glasauer S, Brandt T, Strupp M. Neurology. 2006 Jan 24; 66(2):281-3. https://www.ncbi.nlm.nih.gov/pubmed/16434677/[↩]
- Treatment of downbeat nystagmus with 3,4-diaminopyridine: a placebo-controlled study. Strupp M, Schüler O, Krafczyk S, Jahn K, Schautzer F, Büttner U, Brandt T. Neurology. 2003 Jul 22; 61(2):165-70. https://www.ncbi.nlm.nih.gov/pubmed/12874393/[↩]
- 4-aminopyridine restores vertical and horizontal neural integrator function in downbeat nystagmus. Kalla R, Glasauer S, Büttner U, Brandt T, Strupp M. Brain. 2007 Sep; 130(Pt 9):2441-51. https://www.ncbi.nlm.nih.gov/pubmed/17664175/[↩]
- 4-aminopyridine restores visual ocular motor function in upbeat nystagmus. Glasauer S, Kalla R, Büttner U, Strupp M, Brandt T. J Neurol Neurosurg Psychiatry. 2005 Mar; 76(3):451-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1739571/pdf/v076p00451.pdf[↩]
- Treatment of episodic ataxia type 2 with the potassium channel blocker 4-aminopyridine. Strupp M, Kalla R, Dichgans M, Freilinger T, Glasauer S, Brandt T. Neurology. 2004 May 11; 62(9):1623-5. https://www.ncbi.nlm.nih.gov/pubmed/15136697/[↩]
- The interrelations of migraine, vertigo, and migrainous vertigo. Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. Neurology. 2001 Feb 27; 56(4):436-41. https://www.ncbi.nlm.nih.gov/pubmed/11222783/[↩]
- Episodic vertigo related to migraine (90 cases): vestibular migraine ? Dieterich M, Brandt T. J Neurol. 1999 Oct; 246(10):883-92. https://www.ncbi.nlm.nih.gov/pubmed/10552234/[↩]
- Phobic postural vertigo–a long-term follow-up (5 to 15 years) of 106 patients. Huppert D, Strupp M, Rettinger N, Hecht J, Brandt T. J Neurol. 2005 May; 252(5):564-9. https://www.ncbi.nlm.nih.gov/pubmed/15742115/[↩]