Hurthle cell carcinoma
Hurthle cell carcinoma also called Hurthle cell cancer of the thyroid gland is an unusual and relatively rare type of differentiated thyroid cancer 1. About 4 out of every 100 cases of thyroid cancer (4%) are Hurthle cell carcinoma. Hürthle cell thyroid cancer tends to affect more women than men. It is also most commonly found in people around the age of 50. Hurthle cell carcinomas demonstrate a wide range of aggressiveness, with some metastasizing early and others behaving in a more indolent fashion 2. Metastatic Hurthle cell carcinoma is almost invariably refractory to radioactive iodine (RAI) and is associated with clinical outcomes inferior to those of papillary and follicular thyroid carcinoma. Hurthle cell cancer accounts for only about 3-10% of all differentiated thyroid cancers; therefore, few institutions have extensive experience with Hurthle cell carcinoma. According to the World Health Organization (WHO), these Hurthle cell neoplasms are considered a variant of follicular carcinoma of the thyroid and are referred to as follicular carcinoma, oxyphilic type.
Some investigators believe that Hurthle cell carcinoma is distinct from other follicular cell neoplasms. Hürthle cells look different to follicular cells when seen under a microscope. Hurthle cells are also called oxyphilic cells so Hurthle cell cancer is also sometimes called oxyphil cell cancer.
Hürthle cells are observed in both neoplastic and nonneoplastic conditions of the thyroid gland (eg, Hashimoto thyroiditis, nodular and toxic goiter). Hürthle cells are large and polygonal in shape, with indistinct cell borders. They have a large pleomorphic hyperchromatic nucleus, a prominent nucleolus, and intensely pink, fine, granular cytoplasm with hematoxylin-eosin staining.
Hürthle cells are also found in other tissues, such as the salivary gland, parathyroid gland, esophagus, pharynx, larynx, trachea, kidney, pituitary, and liver. Controversy exists about the origin of Hürthle cells, which generally are thought to derive from the follicular epithelium.
Oncocytic cells in the thyroid are often called Hürthle cells, and oncocytic change is defined as cellular enlargement characterized by an abundant eosinophilic granular cytoplasm as a result of accumulation of altered mitochondria. This is a phenomenon of metaplasia that occurs in inflammatory disorders, such as thyroiditis, or other situations that result in cellular stress. The proliferation of oncocytes gives rise to hyperplastic and neoplastic nodules 3. The cytological features for Hürthle cell neoplasms are hypercellularity with a predominance of Hürthle cells (usually >75%), few or no lymphocytes, and scanty or absent colloid.
A Hurthle cell carcinoma is defined generally as an encapsulated thyroid lesion comprising at least 75% Hürthle cells. A benign neoplasm cannot be distinguished from a malignant neoplasm on the basis of cytologic analysis of fine-needle aspiration (FNA) biopsy. Features such as pleomorphism, anaplasia, hyperchromatism, and atypia are also observed in benign follicular adenomas; therefore, definitive differentiation of Hürthle cell carcinoma from Hürthle-cell adenoma is based on vascular invasion and/or capsular invasion, as well as on permanent histologic sections or extrathyroidal tumor spread and lymph node and systemic metastases.
In the literature, the incidence of malignancy in Hurthle cell carcinoma is variable, ranging from 13-67%. Overall, only about 33% of Hurthle cell thyroid cancer demonstrate signs of that invasive growth that indicates malignancy and the possibility of metastasizing. On balance, Hurthle cell carcinoma may be considered to be more likely to metastasize than follicular tumors. The likelihood of nodal metastases is greater in Hurthle cell carcinoma than in follicular tumors; it is, however, not as great as with papillary tumors.
Hurthle cell carcinoma tumor size is an important feature for biological behavior. A 1988 study 4 found that a Hürthle tumor that is 4 cm or larger has an 80% chance of histologic evidence of malignancy. In another study by Pisanu et al 5, in a series of 23 patients, the mean tumor size was significantly greater for carcinomas than adenomas (3.1 cm vs 1.9 cm).
In another study done at Memorial Sloan-Kettering Cancer center 6, outcomes of 56 patients with Hürthle cell cancer were analyzed. In this study, recurrence was a significant predictor of tumor-related mortality, and the most significant predictor of outcome was extent of invasion. In addition, tumor size, extrathyroidal disease extension, and initial nodal or distant metastasis were found to be associated with an adverse outcome 7.
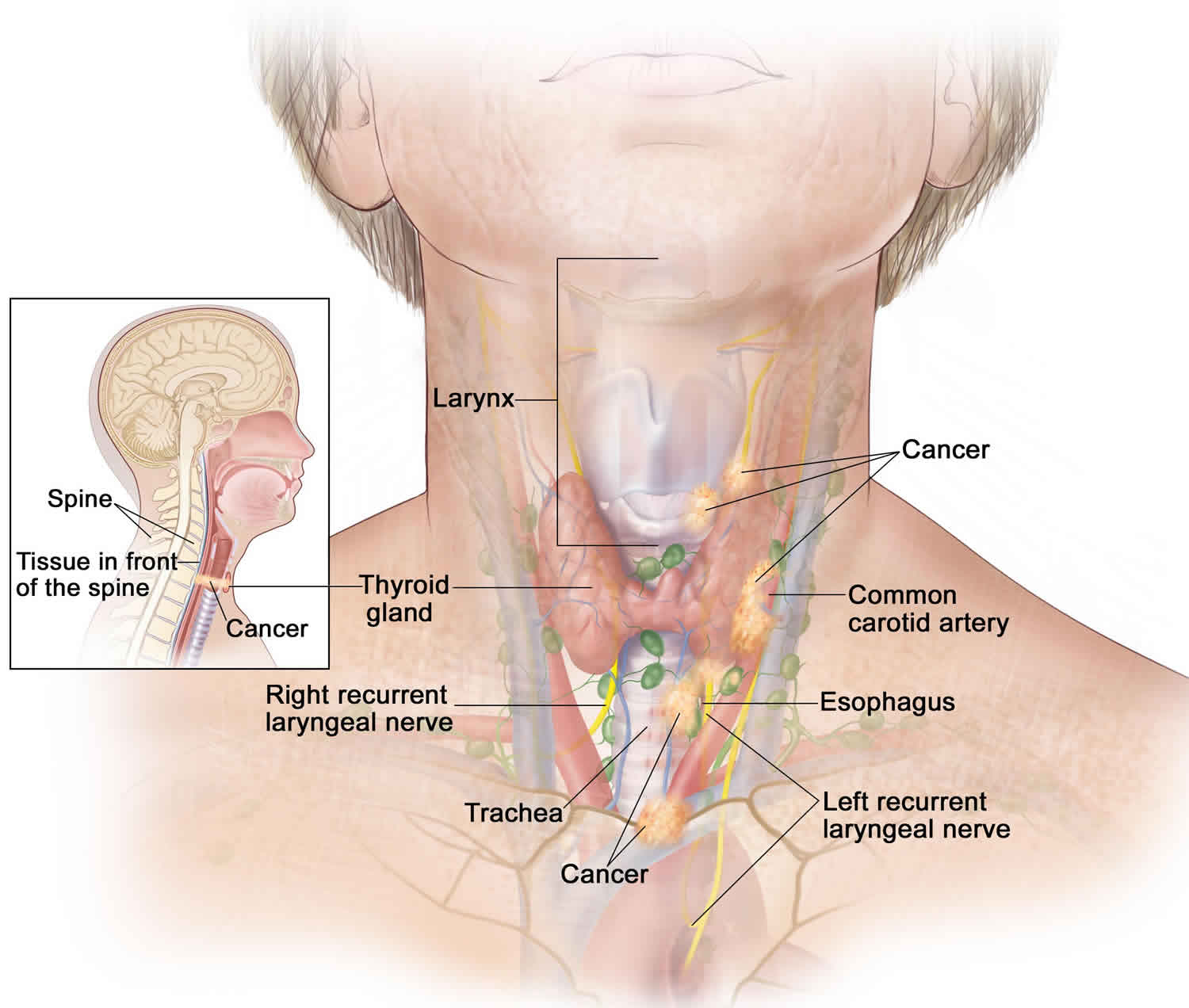
Hurthle cell carcinoma of thyroid has the highest incidence of metastasis among the differentiated thyroid cancers. Metastatic disease is reported at the time of initial diagnosis in 10-20% of patients and in 34% of the patients overall. Metastasis usually occurs hematogenously, but lymph node metastasis is also not uncommon and typically involves the regional lymph nodes. Some studies suggest that lymph node metastases at initial diagnosis may not be an unfavorable prognostic factor 8. The lungs, bones, and central nervous system are the most prevalent sites of metastases.
Hurthle cell carcinoma causes
It’s not clear what causes Hurthle cell cancer.
Doctors know that cancer begins when a cell develops errors in its DNA — the genetic material that contains instructions for biochemical processes in your body. When DNA is altered or damaged, these genes may not function properly, causing cells to grow out of control and eventually form a mass (tumor) of cancerous (malignant) cells.
Factors that may cause Hürthle cell carcinomas include the following:
- Radiation to the neck
- Iodide deficiency
- Overexpression of the p53 oncogene
- Activating mutations of genes encoding the thyrotropin receptor and the alpha subunit of the stimulatory of G protein are reported in some follicular carcinomas
- Somatic mutations of genes important in growth control
- Oncogene activation, particularly by mutation or translocation of the ras oncogene
- Mitochondrion-related alterations (eg, mutations in mitochondrial DNA) also are described.
A study by Maximo et al 9 linked somatic and germline mutation in GRIM-19 (a dual-function gene involved in mitochondrial metabolism and cell death) to Hürthle cell tumors of the thyroid. This is the first nuclear gene mutation described for a subgroup of Hürthle cell carcinomas.
Risk factors of developing thyroid cancer
Factors that increase the risk of developing thyroid cancer include:
- Being female
- Being older
- Having a history of radiation treatments to the head and neck
Hurthle cell cancer symptoms
Hurthle cell cancer doesn’t always cause symptoms, and it’s sometimes detected during a physical examination or an imaging test done for some other reason.
Signs and symptoms of Hurthle cell cancer may include:
- A lump in your neck, just below your Adam’s apple
- Pain in your neck or throat
- Hoarseness or other changes in your voice
- Shortness of breath
- Swallowing difficulty
These signs and symptoms don’t necessarily mean you have Hurthle cell cancer. They may be indications of other medical conditions — such as inflammation of the thyroid gland or a noncancerous enlargement of the thyroid (goiter).
Hurthle cell carcinoma complications
Possible complications of Hurthle cell cancer include:
- Problems with swallowing and breathing. They can occur if the tumor grows and presses on the food tube (esophagus) and windpipe (trachea).
- Spread of the cancer. Hurthle cell cancer can spread (metastasize) to other tissues and organs, making treatment and recovery more difficult.
Hurthle cell carcinoma diagnosis
Tests and procedures used to diagnose Hurthle cell carcinoma include:
- Physical exam. Your doctor will examine your neck, checking the size of your thyroid and seeing whether your lymph nodes are swollen.
- Blood tests. Blood tests may reveal abnormalities in your thyroid function that give your doctor more information about your condition.
- Imaging tests. Imaging tests, including ultrasound and CT, can help your doctor determine whether an abnormal growth is present in the thyroid.
- Thyroid uptake and scan: Typical finding is an area of decreased uptake, which corresponds to the tumor.
- Thyroid ultrasound: Ultrasound usually demonstrates a solid mass, as well as characterizes the solid/cystic nature of the lesion, and is also helpful in diagnosing enlarged lymph nodes in the neck.
- MRI of the neck: MRI will provide more detailed information about the tumor and its relation to the other neck structures.
- CT scan of the neck: CT scan provides more detailed information about the tumor and its relation to the other neck structures. CT scan is also helpful for assessment of calcifications.
- Octreotide scintigraphy: This study can be considered for patients with metastatic Hürthle cell carcinoma because evidence suggests that some Hürthle cell neoplasms can express somatostatin receptors.
- Positron emission tomography with 18F-2-fluoro-2-deoxy-D-glucose (18F-FDG PET) has been shown to be helpful in diagnosing metastatic disease in Hürthle cell carcinomas, particularly with tumors that have low iodine avidity. In a study by Pryma et al, 18F-FDG PET was shown to increase diagnostic accuracy over CT and radioactive iodine scan. In addition, in this study, intense 18F-FDG uptake in lesions were an indicator of poor prognosis 10.
- Removing a sample of thyroid tissue for testing (biopsy). During a thyroid biopsy, a fine needle is passed through the skin of your neck guided by ultrasound images. The needle is attached to a syringe, which withdraws a sample of thyroid tissue. The sample is analyzed in a laboratory for signs of cancer.
Hurthle cell carcinoma treatment
Treatment for Hurthle cell cancer usually requires surgery to remove the thyroid. Radiation therapy and chemotherapy may be options.
Surgery
Total or near-total removal of the thyroid (thyroidectomy) is the most common treatment for Hurthle cell cancer.
During thyroidectomy, the surgeon removes all or nearly all of the thyroid gland and leaves tiny edges of thyroid tissue near small adjacent glands (parathyroid glands) to lessen the chance of injuring them. The parathyroid glands regulate your body’s calcium level.
Surrounding lymph nodes may be removed if there’s suspicion that the cancer has spread to them.
Risks associated with thyroidectomy include:
- Injury to the nerve that controls the voice box (recurrent laryngeal nerve), which could cause temporary or permanent hoarseness or a loss of your voice
- Damage to the parathyroid glands
- Excessive bleeding
After surgery, your doctor will prescribe the hormone levothyroxine (Synthroid, Unithroid, others) to replace the hormone produced by your thyroid. You’ll need to take this hormone for the rest of your life.
Radioactive iodine therapy
Radioactive iodine I 131 therapy involves swallowing a capsule that contains a radioactive iodine I 131 liquid.
Radioactive iodine I 131 therapy may be recommended after surgery because it can help destroy any remaining thyroid tissue, which can contain traces of cancer. Radioactive iodine I 131 treatment is usually administered if postoperative iodine scanning shows uptake, in the thyroid bed or elsewhere. Radioactive iodine I 131 therapy may also be used if Hurthle cell cancer has spread to other parts of the body.
Radioactive iodine I 131 therapy is used after surgery for three reasons. First, radioactive iodide destroys any remaining normal thyroid tissue, thereby enhancing the sensitivity of subsequent radioactive iodine I 131 total-body scanning and increasing the specificity of measurements of serum thyroglobulin for the detection of persistent or recurrent disease. Second, radioactive iodine I 131 therapy may destroy occult microscopic carcinoma. Third, the use of a large amount of radioactive iodine I 131 allows for total-body scanning, which is a more sensitive test for detecting persistent carcinoma.
Compared with other thyroid carcinomas, Hürthle cell cancer has a lower avidity for radioactive iodine I 131; therefore, treatment with radioactive iodide has limited efficacy. Reportedly, approximately 10% of metastases take up radioiodine, compared with 75% of metastases from follicular carcinoma; thus, radioactive iodide treatment, which is the most useful nonsurgical therapy for recurrent well-differentiated thyroid carcinoma, is not always useful in patients with Hürthle cell carcinoma. This causes difficulty in the treatment of recurrences. Nevertheless, radioactive iodide treatment is used for most patients with Hürthle cell cancers after total and near-total thyroidectomy and in the treatment of patients with recurrent and metastatic Hürthle cell carcinoma.
Jillard et al reported that post-thyroidectomy radioactive iodine I 131 therapy improves survival in patients with Hürthle cell carcinoma. In their review of 1909 cases, patients who received radioactive iodine I 131 (n=1162) had superior 5-year and 10-year survival compared with patients who did not (88.9 vs. 83.1% and 74.4 vs. 65.0%, respectively). These authors conclude that their finding suggest that radioactive iodine therapy should be advocated for patients with tumors >2 cm, and those with nodal and distant metastatic disease 11.
There is limited evidence in the literature that redifferentiation therapy with retinoic acid may restore radioactive iodine I 131 uptake in some thyroid carcinomas that have lost their capability for radioiodine concentration; however, the benefits of this approach remain uncertain 12. Retinoic acid therapy also may be considered in patients with Hürthle cell carcinoma that does not take up radioactive iodide, although this is not yet a standard form of therapy.
Temporary side effects of radioiodine therapy can include:
- Dry mouth
- Decrease in taste sensations
- Neck tenderness
- Nausea
Levothyroxine treatment
The growth of thyroid tumor cells is controlled by thyroid-stimulating hormone (TSH), and the inhibition of TSH secretion with levothyroxine (T4) lowers recurrence rates and improves survival; therefore, T4 should be administered to all patients with thyroid carcinoma, regardless of the extent of thyroid surgery and other treatments.
Levothyroxine treatment is started after the treatment dose of radioactive iodine I 131 is administered. The effective dose of T4 in adults is 2.2-2.8 mcg/kg; children require higher doses. The adequacy of therapy is monitored by measuring serum TSH about 8-12 weeks after the treatment begins. The initial goal is a serum TSH concentration of 0.1 µU/mL or less and a serum triiodothyronine concentration within the reference range. When these guidelines are followed, T4 therapy does not have deleterious effects on the heart or bone.
Radiation therapy
Radiation therapy uses high-powered energy beams, such as X-rays or protons, to kill cancer cells. During radiation therapy, you’re positioned on a table and a machine moves around you, delivering the radiation to specific points on your body.
Radiation therapy may be an option if cancer cells remain after surgery and radioactive iodine treatment or if Hurthle cell cancer spreads.
Side effects may include:
- Sore throat
- Sunburn-like skin rash
- Fatigue
Targeted drug therapy
Targeted drug treatments use medications that attack specific abnormalities within cancer cells. Targeted therapy may be an option if your Hurthle cell cancer returns after other treatments or if it spreads to distant parts of your body.
Side effects depend on the particular drug, but may include:
- Diarrhea
- Fatigue
- High blood pressure
- Liver problems
Targeted drug therapy is an active area of cancer research. Doctors are studying many new targeted therapy drugs for use in people with thyroid cancer. The drugs include multikinase inhibitors, selective kinase inhibitors, and combination therapies. Examples include sorafenib, gefitinib, axitinib, motesanib, sunitinib, and pazopanib. Sorafenib is approved by the US Food and Drug Administration (FDA) for advanced differentiated thyroid cancer 13.
Hurthle cancer survival rate
The survival statistics below are from a large European study. They are based on people treated between 2000 and 2007. Treatments improve over time, so people treated now may have a better outlook.
These are general statistics based on large groups of patients. They can’t tell you what will happen in your individual case.
No one can tell you exactly how long you’ll live. It depends on:
- the type and stage of cancer
- presence of cancer in the groin nodes
- your level of fitness
- previous treatment
Your outcome depends on the stage of the thyroid cancer when it was diagnosed. This means how big it is and whether it has spread.
The type and grade of thyroid cancer also affects your likely survival. Grade means how abnormal the cells look under the microscope.
Your likely survival is also affected by your age. Survival is better in younger men and women.
Your likely survival is also affected if you have other medical conditions that might influence the treatment you can have.
The 10-year survival rate for Hurthle cell cancer that is confined to the thyroid gland is 75% but is closer to 50% if it has spread to the cervical lymph nodes, and is lower still if spread to distant sites.
- Hurthle cell carcinoma. https://emedicine.medscape.com/article/279462-overview[↩]
- SPORE in Thyroid Cancer. Sloan-Kettering Institute for Cancer Research. https://trp.cancer.gov/spores/abstracts/mskcc_thyroid.htm#h05[↩]
- Asa SL. My approach to oncocytic tumours of the thyroid. J Clin Pathol. 2004 Mar. 57(3):225-32.[↩]
- Bronner MP, Clevenger CV, Edmonds PR, Lowell DM, McFarland MM, LiVolsi VA. Flow cytometric analysis of DNA content in Hürthle cell adenomas and carcinomas of the thyroid. Am J Clin Pathol. 1988 Jun. 89(6):764-9.[↩]
- Pisanu A, Sias L, Uccheddu A. Factors predicting malignancy of Hürthle cell tumors of the thyroid: influence on surgical treatment. World J Surg. 2004 Aug. 28(8):761-5.[↩]
- Stojadinovic A, Ghossein RA, Hoos A, Urist MJ, Spiro RH, Shah JP. Hürthle cell carcinoma: a critical histopathologic appraisal. J Clin Oncol. 2001 May 15. 19(10):2616-25.[↩]
- Strazisar B, Petric R, Sesek M, Zgajnar J, Hocevar M, Besic N. Predictive factors of carcinoma in 279 patients with Hürthle cell neoplasm of the thyroid gland. J Surg Oncol. 2010 Jun 1. 101(7):582-6.[↩]
- Guerrero MA, Suh I, Vriens MR, Shen WT, Gosnell J, Kebebew E, et al. Age and tumor size predicts lymph node involvement in Hürthle Cell Carcinoma. J Cancer. 2010 Jun 2. 1:23-6.[↩]
- Máximo V, Botelho T, Capela J, Soares P, Lima J, Taveira A, et al. Somatic and germline mutation in GRIM-19, a dual function gene involved in mitochondrial metabolism and cell death, is linked to mitochondrion-rich (Hurthle cell) tumours of the thyroid. Br J Cancer. 2005 May 23. 92(10):1892-8.[↩]
- Pryma DA, Schoder H, Gonen M, Robbins RJ, Larson SM, Yeung HW, et al. Diagnostic accuracy and prognostic value of 18F-FDG PET in Hurthle cell thyroid cancer patients. J Nucl Med. 2006 Aug. 47(8):1260-6.[↩]
- Jillard C, Youngwirth L, Scheri R, Roman S, Sosa JA. Radioactive Iodine Treatment is Associated with Improved Survival for Patients with Hürthle Cell Carcinoma. Thyroid. 2016 May 5.[↩]
- Fernández CA, Puig-Domingo M, Lomeña F, Estorch M, Camacho Martí V, Bittini AL, et al. Effectiveness of retinoic acid treatment for redifferentiation of thyroid cancer in relation to recovery of radioiodine uptake. J Endocrinol Invest. 2009 Mar. 32(3):228-33[↩]
- Haugen BR, Sherman SI. Evolving approaches to patients with advanced differentiated thyroid cancer. Endocr Rev. 2013 Jun. 34(3):439-55.[↩]