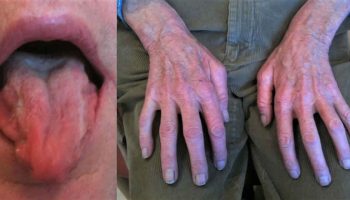
Non-toxic goiter
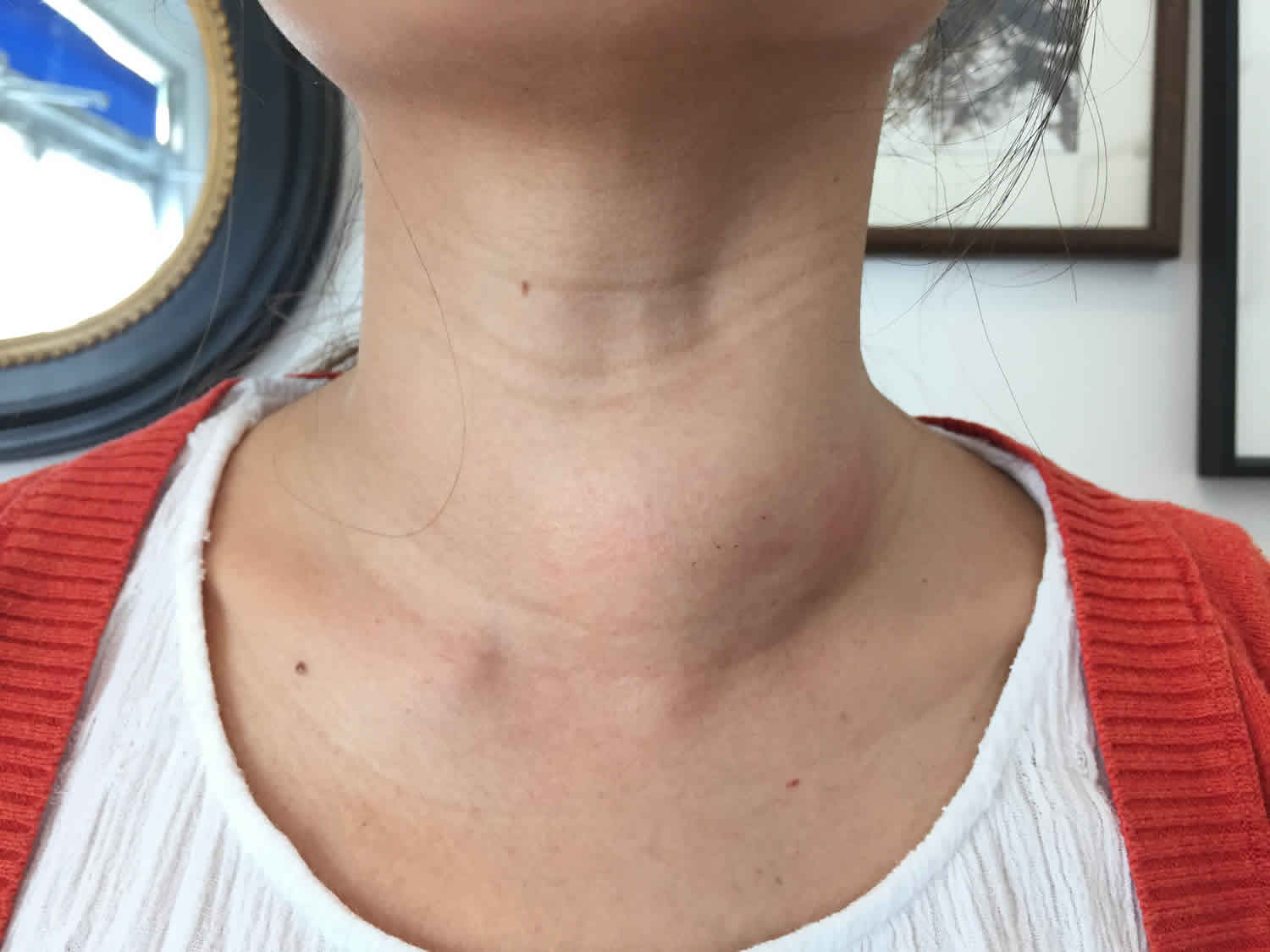
Nontoxic goiter also called euthyroid goiter, is a diffuse (non-toxic diffuse goiter) or nodular enlargement of the thyroid gland (non-toxic nodular goiter) that does not result from an inflammatory or neoplastic process and is not associated with abnormal thyroid function 1. A goiter is an abnormal enlargement of your thyroid gland. Non-toxic goiter is non-cancerous hypertrophy of the thyroid without hyperthyroidism, hypothyroidism, or inflammation 2. Except in severe iodine deficiency, thyroid function is normal and patients are asymptomatic except for an obviously enlarged, nontender thyroid. Patients with nontoxic goiters often present local compressive symptoms that require surgical treatment, including dysphagia, neck tightness, and airway obstruction 3.
Iodine deficiency goiter also known as endemic goiter, is defined as thyroid enlargement that occurs in more than 10% of a population, and sporadic goiter is a result of environmental or genetic factors that do not affect the general population. Iodine deficiency (endemic goiter) is rare in North America but remains the most common cause of goiter worldwide. Compensatory small elevations in thyroid-stimulating hormone (TSH) occur, preventing hypothyroidism, but the TSH stimulation results in goiter formation. Recurrent cycles of stimulation and involution may result in nontoxic nodular goiters. However, the true cause of most nontoxic goiters in iodine-sufficient areas is unknown.
Diagnosis of nontoxic goiter is clinical and with determination of normal thyroid function. Treatment is directed at the cause, but partial surgical removal may be required for very large goiters. The use of levothyroxine (T4) to reduce the volume of the nontoxic goiter is still a controversial treatment for large goiters and the optimal surgical procedure for nontoxic multinodular goiter is still debatable. Radioiodine (radioactive iodine) is a safe and effective treatment option when used alone or in combination with recombinant human thyroid stimulating hormone (TSH) 3.
Nontoxic goiter key points:
- Thyroid function is usually normal.
- When the cause is iodine deficiency, iodine supplementation is effective treatment.
- Blocking thyroid-stimulating hormone production by giving levothyroxine (l-thyroxine) is useful in younger patients to halt stimulation of the thyroid and shrink the goiter.
- Surgery or iodine-131 may be needed for large goiters.
What is the difference between a toxic and nontoxic goiter?
Goiters are described in a variety of ways, including the following:
- Toxic goiter: A goiter that is associated with hyperthyroidism is described as a toxic goiter. Examples of toxic goiters include diffuse toxic goiter (Graves disease), toxic multinodular goiter, and toxic adenoma (Plummer disease).
- Nontoxic goiter: A goiter without hyperthyroidism or hypothyroidism is described as a nontoxic goiter. It may be diffuse or multinodular, but a diffuse goiter often evolves into a nodular goiter. Examination of the thyroid may not reveal small or posterior nodules. Examples of nontoxic goiters include chronic lymphocytic thyroiditis (Hashimoto disease), goiter identified in early Graves disease, endemic goiter, sporadic goiter, congenital goiter, and physiologic goiter that occurs during puberty.
Non-toxic diffuse goiter
There is no ideal therapy for nontoxic diffuse goiters 4, but for patients requiring treatment, clinical management is the most frequent choice. It is unclear whether or not early treatment of nontoxic diffuse goiters can inhibit the development of nodular goiter 3.
The development of nontoxic diffuse goiters occurs from a combination of genetic and environmental factors. Prolonged TSH stimulation, which frequently occurs in association with iodine deficiency, has an important role in thyroid enlargement. In these circumstances, iodine supplementation would be an adequate approach (400 μg of iodine for 8–12 months). A significant reduction in goiter size has been observed in patients with nontoxic diffuse goiter supplemented with iodine 5. In fact, supplementation with iodine 400 μg/day has been demonstrated to be as effective as suppressive therapy with l-thyroxine 150 μg/day 6. However, except in a few European countries, iodine is no longer used for the treatment of benign non-toxic goiter.
In contrast, a beneficial effect of l-thyroxine has been shown in nontoxic diffuse goiter 7. A volume reduction of 50% or more was detected in 31% (11/35) of the patients after 6 months 7. If suppressive treatment is considered, then administration of thyroid hormone in enough doses to inhibit or reduce TSH secretion may be used. However, it should be considered that the volume of the goiter returns to pretreatment size after l-thyroxine withdrawal. As may be expected, many thyroid experts are unenthusiastic about treating nontoxic diffuse goiter, although others advocate the use of l-thyroxine favoring the notion that early treatment may prevent later development of nodular goiter. As with any issue in clinical medicine, the benefits of such therapy must be weighed against the potential risks of TSH suppression.
Regarding radioiodine therapy in nontoxic diffuse goiter, two earlier small uncontrolled studies are available 8, 9. In the first study, 11 patients with symptoms of pressure and cosmetic complaints were selected for radioiodine ablation after refusing surgical treatment 8. The mean thyroid volume reduction achieved with a single dose of radioiodine within the first year was 62%, and 2 of the 11 patients (18%) developed hypothyroidism 8. In another study, 10 patients were treated with radioiodine and were followed up for 18 months with measurements of the thyroid volume by ultrasonography 9. The volume declined by 50% within 12–18 months. During follow-up, one patient (10%) developed persistent hypothyroidism and another (who presented positive antithyroperoxidase levels) developed transient hypothyroidism 9.
Non-toxic nodular goiter
The ideal treatment for nontoxic nodular goiter is controversial (Table 1) 10, in part due to the variation in the natural history of these goiters. Some patients present an enlargement in goiter size with time along with the development of nodules, symptoms of compression, and cosmetic reasons 11. In contrast, the enlargement of the goiter may become stable or reduce spontaneously with time in around 20% of the women and 5% of the men 12.
Current alternatives for nontoxic nodular goiter treatment include:
- Clinical observation for asymptomatic patients;
- Thyroid hormone suppressive therapy;
- Radioiodine therapy alone or preceded by recombinant human TSH (rhTSH); and
- Surgery.
Among these options, treatment is chosen individually for each patient in view of the risks, benefits, and availability of the various techniques, experience of the treating physician, and patient’s personal preference (Table 2).
Table 1. Treatment choices reported by American Thyroid Association, European Thyroid Association, and Latin American Thyroid Association members based on an index case of a patient with a simple non-toxic nodular goiter, without suspicion of malignancy.
| Therapy | Non-toxic nodular goiter | ||
|---|---|---|---|
| American Thyroid Association (n = 140) | European Thyroid Association (n = 120) | Latin American Thyroid Association (n = 148) | |
| None | 36 | 28 | 39 |
| Levothyroxine | 56 | 52 | 21 |
| Radioiodinea | 1 | 6 | 7 |
| Surgery | 6 | 10 | 28 |
Footnote: The numbers correspond to the percentage of organization members who responded to the questionnaire.
a) Includes both isolated radioiodine use and radioiodine associated with recombinant human TSH (rhTSH).
[Source 13 ]Table 2. Therapeutic options for individuals with non-toxic multinodular goiter
| Treatment | Advantages | Disadvantages | Comments |
| Levothyroxine | Outpatient use Low cost Prevent formation of other nodules? | Low efficacy Main effect on perinodular volume Continuous suppression of TSH, which leads to side events associated with subclinical hyperthyroidism, as well as unpredictable bone and cardiac side effects | Declining due to adverse effects and lack of efficacy |
| Surgery | Substantial reduction in goiter size Rapid relief of compression of vital cervical structures Allows pathological assessment | Not applicable to all individuals Post-surgical hemorrhage (1%) Damage to the recurrent laryngeal nerve (1–2%) Temporary (0.5%) or definitive (0.6%) hypoparathyroidism Goiter recurrence depending on the extent of resection Post-surgical tracheomalacia (rare) Increased morbidity in cases with large goiters, intrathoracic extension or reoperation | Standard treatment for large goiters or when rapid decompression of cervical structures is required Total thyroidectomy should be considered the preferred therapy to prevent goiter recurrence |
| Radioiodine | Thyroid volume reduction by half in 1 year Improves respiratory capacity in the long term Frequent outpatient use Can be successfully repeated Few side effects | Gradual reduction in goiter size The larger the goiter, the lower the effect Slight risk of short-term increase in goiter size 3% risk of thyroiditis 5% risk of development of Graves’ disease 15–20% risk of hypothyroidism at 12 months Small risk of radiation-induced ophthalmopathy Requires retreatment in some cases Risk of radiation-induced malignancy has not been established | In some European countries, radioiodine has become the standard therapy, replacing surgery May be considered in place of surgical intervention in patients who refuse or are unable to undergo surgery, or in those with large goiters (except in cases that require large radioiodine doses) Can be preceded by rhTSH with lower radioiodine dose |
Footnote: Recombinant human TSH (rhTSH).
[Source 13 ]Nontoxic goiter signs and symptoms
The patient may have a history of low iodine intake or overingestion of food goitrogens, but these phenomena are rare in North America. In the early stages, the goiter is typically soft, symmetric, and smooth. Later, multiple nodules and cysts may develop.
Nontoxic goiter diagnosis
The diagnosis of nontoxic goiter may include:
- Physical exam. Your doctor examines your eyes to see if they’re irritated or protruding and looks to see if your thyroid gland is enlarged. Your doctor will check your pulse and blood pressure and look for signs of tremor.
- Blood sample. Your doctor will order blood tests to determine your levels of thyroid-stimulating hormone (TSH), the pituitary hormone that normally stimulates the thyroid gland, as well as levels of thyroid hormones such as thyroxine (T4) and triiodothyronine (T3). Thyroid antibodies are measured to rule out Hashimoto thyroiditis.
- Thyroidal radioactive iodine uptake. Your body needs iodine to make thyroid hormones. By giving you a small amount of radioactive iodine and later measuring the amount of it in your thyroid gland with a specialized scanning camera, your doctor can determine the rate at which your thyroid gland takes up iodine. The amount of radioactive iodine taken up by the thyroid gland helps determine if nontoxic goiter or another condition is the cause of the goiter. This test may be combined with a radioactive iodine scan to show a visual image of the uptake pattern.
- Ultrasound. Ultrasound uses high-frequency sound waves to produce images of structures inside the body. Ultrasound can show if the thyroid gland is enlarged, and is most useful in people who can’t undergo radioactive iodine uptake, such as pregnant women. Thyroid ultrasonography is done to determine whether there are nodules that are suggestive of cancer.
- Imaging tests. Your doctor may order an imaging test, such as CT scan, a specialized X-ray technology that produces thin cross-sectional images. Magnetic resonance imaging (MRI), which uses magnetic fields and radio waves to create either cross-sectional or 3-D images, also may be used.
In the early stages, thyroidal radioactive iodine uptake may be normal or high with normal thyroid scans. Thyroid function test results are usually normal.
In endemic goiter, serum TSH may be slightly elevated, and serum T4 may be low-normal or slightly low, but serum T3 is usually normal or slightly elevated.
Nontoxic goiter treatment
Non-toxic goiter treatment depends on cause.
Clinical observation, including thyroid function monitoring and ultrasonographic assessment at regular intervals, is an alternative in cases of small goiters not causing compressive symptoms and associated with normal thyroid function. If clinical observation is chosen, the possibility of malignancy should be excluded by fine-needle aspiration biopsy (FNAB) that is guided by ultrasonography.
Asymptomatic euthyroid patients with benign non-toxic goiter and without cosmetic symptoms may be simply observed with clinical and laboratory evaluation and thyroid imaging tests. In most cases, patients with asymptomatic non-toxic nodular goiter may be evaluated yearly with careful thyroid palpation and measurement of serum TSH level. Ultrasonography may be performed when the palpation of the thyroid is uncertain, whereas nodules that appear to enlarge should be evaluated with fine-needle aspiration biopsy. Evaluation with neck computed tomography or magnetic resonance imaging (MRI) may be required when the goiter is subesternal.
The American Thyroid Association recommends a standard follow-up interval of 6–18 months for patients with non-toxic nodular goiter, which may be gradually prolonged if no substantial changes are observed during the first 3–5 years 14.
In iodine-deficient areas, the following will eliminate iodine deficiency:
- Iodine supplementation of salt
- Oral administration of iodized oil
- Intramuscular administration of iodized oil yearly
- Iodination of water, crops, or animal fodder
Goitrogens being ingested should be stopped.
In other instances, suppression of the hypothalamic-pituitary axis with thyroid hormone blocks TSH production (and hence stimulation of the thyroid). Moderate doses of l-thyroxine (100 to 150 mcg orally once a day depending on the serum TSH) are useful in younger patients to reduce the serum TSH to the low-normal range.
Levothyroxine (l-thyroxine) is contraindicated in older patients with nontoxic nodular goiter, because these goiters rarely shrink and may harbor areas of autonomy so that l-thyroxine therapy can result in hyperthyroidism.
Large goiters occasionally require surgery or iodine-131 to shrink the gland enough to prevent interference with respiration or swallowing or to correct cosmetic problems.
Suppressive therapy with levothyroxine
Although levothyroxine (l-thyroxine) is broadly used in the United States, Europe, and Latin America, as shown in different surveys 10, the use of thyroid hormones to treat nontoxic nodular goiters is controversial, and its efficacy is dependent on the degree of TSH suppression. Considering that non-toxic nodular goiter patients frequently have normal serum TSH levels, the enlargement of the thyroid in these patients is probably associated with the prolonged action of different growth factors (including TSH) on thyroid follicular cells with different synthetic and growth potentials. Advantages of this treatment modality include low cost, administration on an outpatient basis, and inhibition of the development of new nodules. In contrast, suppressive therapy has little effectiveness, as it requires permanent treatment and may have potential undesirable effects on bone (demineralization) 15 and heart (arrhythmias), especially in older individuals 16.
In a randomized placebo-controlled trial, a ≥25% goiter volume reduction was seen in 58% of the individuals with nontoxic nodular goiter and non-toxic diffuse goiter during levothyroxine (l-thyroxine) suppressive therapy over 9 months, with a return to the original volume after treatment withdrawal. Over the same period, patients randomized to the placebo group showed a 20% increase in thyroid volume 17.
In another randomized trial comparing suppressive therapy with radioiodine, patients in the radioiodine group showed a 35% reduction in goiter volume at 1 year and 44% at 2 years, whereas those who received levothyroxine (l-thyroxine) presented 7 and 1% reduction at 1 and 2 years, respectively. A response to the therapy was found in 97% of the individuals who received radioiodine and 43% of those undergoing levothyroxine (l-thyroxine) therapy 18. A recent meta-analysis showed lack of substantial benefits and a relative risk of only 1.9 (95% confidence interval, 0.95–3.81) of a decrease in nodular size with levothyroxine (l-thyroxine) therapy 19. Thus, according to these randomized controlled trials, it seems that thyroid hormone suppression can interfere with the process of goitrogenesis (goiter growth and new nodule formation), with only some goiters responding and others growing despite levothyroxine (l-thyroxine) treatment 17.
The occurrence of adverse events is more evident in older patients, a population that comprises most patients with nontoxic nodular goiter. Also, about 22% of the individuals with nontoxic nodular goiter may harbor areas with functional autonomy, which increase the concerns with levothyroxine (l-thyroxine) therapy because of risks of bone loss and atrial fibrillation. Therefore, treatment with thyroid hormone should be avoided, especially in patients with prior serum TSH concentrations below the normal range.
Patients who start levothyroxine (l-thyroxine) suppression must continue the therapy for a long time. The reduction in goiter size with levothyroxine (l-thyroxine) seen in some individuals is probably due to decreased TSH secretion, especially in patients who live in areas with borderline or low iodine levels. Any decrease in nontoxic nodular goiter size that may occur with levothyroxine (l-thyroxine) suppression is lost when the treatment is interrupted, and the nodules and goiter may grow again in size 17. In addition, keeping the serum TSH concentration in the lowest reference range or slightly below the normal range, rather than below 0.01 μU/mL, appears to be efficacious. Pending further studies, this strategy may relieve the adverse effects of levothyroxine (l-thyroxine) therapy 20. Accordingly, an levothyroxine (l-thyroxine) dose adjusted to obtain a non-suppressive level of serum TSH in the range of 0.5–0.8 μU/mL has been shown to significantly reduce the growth of nodules within multinodular goiters in 165 out of 356 female patients during a follow-up period of 9 years 21.
A potential benefit of thyroid hormone therapy is a reduction in the risk of thyroid oncogenesis 22. However, this hypothesis is still speculative, and more studies are still needed.
In general, since the volume of the thyroid reduces in only 30% of the patients with nontoxic nodular goiters treated with levothyroxine (l-thyroxine) suppression, recommendations for this type of treatment have decreased 11.
Therapy with radioiodine
Therapy with radioiodine may be recommended in cases of nontoxic nodular goiter affecting patients who refuse or have contraindications for surgery. Over the past years, this type of treatment has increased in patients with nodular goiter and is associated with a substantial decrease in glandular volume, reaching 30–40% in the first year, and 50–60% in the fourth year. Obstructive symptoms improve in most individuals 23, with reports of a single dose administered orally restoring euthyroidism over a period of 2–4 months 24.
Radioiodine for the treatment of goiter was introduced approximately three decades ago. In an initial report, 25 individuals with a mean glandular volume of 73 cm3, who received 100 μCi of radioiodine per gram of thyroid tissue corrected to 100% uptake in 24 h, showed an approximate reduction in goiter volume of 41% after 1 year of follow-up 25. The larger the volume of the gland and the lower the radioiodine uptake (RAIU), the higher should be the radioiodine activity to be administered. Subsequent studies have unanimously corroborated this observation 26. Patients with very large goiters (>100 cm3) have a smaller decrease in glandular volume (about 35%) even with administration of similar radioiodine doses 23.
In some European countries such as Denmark and the Netherlands (to some degree), radioiodine has currently replaced surgery as the treatment of choice for nontoxic nodular goiter 11. Depending on each country’s regulations, radioiodine administered for treatment purposes may be delivered in fractions on an outpatient basis, reducing the costs of hospitalization 27.
Some patients develop temporary mild thyrotoxicosis within the initial 2 weeks of treatment, and about 45% of them develop hypothyroidism, requiring thyroid hormone replacement for life 18. The occurrence of hyperthyroidism due to Graves’ disease associated with increased serum concentrations of TSH receptor antibodies has also been described in patients with increased baseline levels of thyroid peroxidase antibodies after radioiodine treatment for nontoxic nodular goiter 28.
Based on measurements of whole-body radiation exposure, the theoretical lifetime risk of development of cancer outside the thyroid gland has been calculated as 1.6%. This is due to the use of high doses of radioiodine in patients with very large goiters (the mean goiter volume used in the calculation was ~220 g). When administered to individuals aged 65 years or older, the estimated risk is ~0.5% 29.
Recombinant human TSH-stimulated Radioiodine Therapy
Low isotope accumulation in inactive and partially suppressed areas around the nodule is a limitation of radioiodine treatment in patients with nontoxic nodular goiters. This problem may be solved by increasing the radioiodine uptake (RAIU) in such nodular goiters 30. For this purpose, recent studies using recombinant human TSH (rhTSH) in preparation for radioiodine therapy have shown good results 31.
Several studies have assessed the adjuvant role of recombinant human TSH in the radioiodine treatment of nontoxic nodular goiter. Administration of recombinant human TSH is associated with a twofold to fourfold increase in RAIU by the thyroid 32. It should be noted that the baseline serum TSH may be a confounding factor since the increase in the 24-h thyroid RAIU correlates inversely with this variable. Since patients with nontoxic nodular goiter often present low serum TSH, the radioiodine is only taken up by some “hot” areas encircled by suppressed thyroid tissue that is inactive on scintigraphy. This phenomenon can be explained by a suppression of the paranodular parenchyma as a consequence of the low TSH level. Upon stimulation with recombinant human TSH , these dormant areas, which concentrate radioiodine weakly, reactivate and eventually amplifies the effect of the radioiodine in the gland, promoting a further reduction in the volume of the goiter. In fact, recombinant human TSH has been shown to distribute the radioiodine more homogeneously in the goiter, allowing a decrease in the dose of radioiodine to be administered 33. Most studies analyzing recombinant human TSH in this setting have used fixed doses of radioiodine ranging from ~14 to ~42 mCi 23 and treatment with one or two recombinant human TSH doses ranging from 0.1 to 0.3 mg administered 24 h prior to the radioiodine. However, the dose with ideal efficacy and safety is yet to be defined 34.
Combined therapy with recombinant human TSH and radioiodine is well tolerated, and potential side effects that occur are similar to those observed in individuals receiving radioiodine alone. However, patients may experience a short increase in thyroid hormone levels within 48 h from the administration of radioiodine, leading to transient mild thyrotoxicosis, which may be followed by hypothyroidism during the first 30 days after the therapy 35. Other acute adverse events that have been reported include painful transient thyroiditis, thyroid swelling, compression of the trachea, and, often, heart-related symptoms. Administration of glucocorticoids and β-blockers may help minimize these symptoms 36.
Recent studies 23 have shown that these adverse events may be dependent on the dose and are negligible with lower doses of recombinant human TSH. Bonnema et al. 33 have shown that the ideal recombinant human TSH dose to improve treatment with radioiodine may be about 0.03–0.1 mg. This dose range increases the thyroid RAIU substantially, with a minimal risk of thyroid swelling and transient thyrotoxicosis.
Studies assessing the long-term adverse effects of the combined use of recombinant human TSH and radioiodine therapy showed an increased rate of permanent hypothyroidism. Three randomized controlled studies have reported 1-year rates of permanent hypothyroidism between 21 and 65% in rhTSH-pretreated patients compared with rates between 7 and 21% in patients in whom recombinant human TSH was not administered 37. Due to its effect on the thyroid, recombinant human TSH can potentially trigger an autoimmune response. Following treatment with radioiodine stimulated with recombinant human TSH, 8 of 15 subjects with nontoxic nodular goiter developed antiperoxidase antibodies. However, no differences in autoimmunity were observed at 12 months between the subjects who underwent therapy with radioiodine alone versus those pretreated with recombinant human TSH before radioiodine 38.
No studies have specifically assessed the risk of malignancy after nontoxic nodular goiter treatment with radioiodine with recombinant human TSH prestimulation. However, by increasing the uptake of radioiodine by the thyroid, recombinant human TSH results in less radioactivity delivered to the thyroid and to the rest of the body, which potentially reduces the theoretical risk of radioiodine-induced malignancy 23.
To avoid unintentional stimulation of the thyroid, a “modified-release recombinant human TSH” (MRrhTSH) has been recently introduced. This compound has a slightly different serum profile than that of recombinant human TSH, with a more delayed peak and, therefore, a possible lower risk of goiter enlargement. A phase II study of modified-release recombinant human TSH use in patients with nontoxic nodular goiter undergoing radioiodine therapy was recently published. After 6 months, patients pre-stimulated with modified-release recombinant human TSH 0.01 mg or placebo before radioiodine therapy had a 23% reduction in the goiter volume, whereas the reduction was 33% in individuals pre-stimulated with modified-release recombinant human TSH 0.03 mg 39.
Both recombinant human TSH and modified-release recombinant human TSH are not approved by the US Food and Drug Administration or European Medicines Agency to treat nontoxic nodular goiter in association with radioiodine; so, their use for this purpose is currently off-label.
An alternative approach to increase the thyroid RAIU is to administer methimazole to stimulate the secretion of endogenous TSH. Albino et al. 40 pretreated nine patients with nodular goiter and subclinical hyperthyroidism with methimazole, aiming at increasing the serum level of TSH above 6 μU/mL. When this level was achieved, radioiodine 30 mCi was administered. The 24-h thyroid RAIU increased from 21 to 78%, and the mean goiter reduction at 1 year was 46%. In eight individuals (89%) with subclinical hyperthyroidism, the thyroid function normalized after 1 year. Five patients (56%) developed overt hypothyroidism, and no adverse clinical events were observed. However, whether a marginal hypothyroid state obtained with methimazole is as effective as recombinant human TSH to increase the thyroid RAIU and to augment the reduction in goiter size after radioiodine therapy remains to be clarified with controlled trials.
In summary, individuals with non-toxic diffuse goiter should receive clinical rather than surgical treatment. nontoxic nodular goiter is a highly prevalent disease, even in regions without iodine deficiency. Many individuals are asymptomatic, and when symptoms are present, the most common clinical manifestations result from local compressive effects. Individuals with non-toxic diffuse goiter should be thoroughly evaluated to exclude malignancy. They should then receive individualized therapy after assessment of risks and benefits of each treatment option and discussion with their physicians. The first therapeutic option is total thyroidectomy, followed by treatment with radioiodine alone or after recombinant human TSH stimulation to increase the radioiodine efficacy.
It is unclear if suppressive therapy with thyroid hormone is effective in patients with nontoxic nodular goiter. The choice of this therapy has decreased because of concerns regarding eventual long-term side events due to subclinical hyperthyroidism, and due to the fact after therapy interruption, most goiters grow again in size. All these therapies have potential advantages and disadvantages and are associated with short-term and long-term side events.
Surgery
The best surgical procedure to treat patients with nontoxic nodular goiter is still controversial 41. A recent meta-analysis that included 1305 participants evaluated the impact of total/near-total thyroidectomy versus subtotal thyroidectomy in adults with non-toxic multinodular goiter. The key results of this study were (a) the recurrence of goiter was lower in patients who underwent thyroidectomy compared with those who underwent subtotal thyroidectomy, with rates of goiter recurrence of 84 in 1000 patients with subtotal thyroidectomy and 5 in 1000 patients with total thyroidectomy; (b) no clear benefits or harms were observed with subtotal or total thyroidectomy in patients undergoing surgical intervention due to recurrence in goiter size, complications such as permanent recurrent laryngeal nerve palsy, or occurrence of thyroid carcinoma; and (c) cancer detection was lower (6.1%) in patients undergoing subtotal thyroidectomy compared with 7.3% of those undergoing total thyroidectomy, but this difference was not significant 41.
In most patients with large obstructive goiters, cosmetic complaints, or retrosternal nontoxic nodular goiters for whom surgery is recommended, total thyroidectomy is preferable over subtotal thyroidectomy 42. In the long term, patients undergoing subtotal thyroidectomy have higher recurrence rates than those undergoing total thyroidectomy, and 2.5–42% are required to undergo a new intervention. In addition, 3.5% of the nontoxic nodular goiter patients undergoing subtotal thyroidectomy require another surgical intervention to remove remaining thyroid tissue because of incidental thyroid carcinoma 43. Rates of permanent complications, such as hypoparathyroidism and vocal palsy, are similar with both total and subtotal surgeries; however, total thyroidectomy is preferred due to an increased risk of these complications associated with a new intervention 44. In patients with unilateral nontoxic nodular goiter, some authors recommend unilateral thyroidectomy based on a low rate of recurrence (2%) and high rate of maintenance of euthyroidism (73%) 45.
Post-surgical recurrence rates are directly proportional to the volume of the remaining thyroid tissue. Hegedüs et al. 46 studied 202 consecutive patients who underwent surgical resection due to benign non-toxic goiter. They found a recurrence rate of 35% detected by ultrasonography during a median follow-up of 10 years 46. In a retrospective study, the assessment of 112 individuals 30 years after surgery showed rates of disease recurrence of 40–45% during a 30-year follow-up period 47. In another study, the recurrence rate of the goiters was 18% when assessed by ultrasonography 9 years after surgery 48.
This high probability of goiter recurrence after subtotal thyroidectomy resulted in a preventive use of levothyroxine. However, only one 49 out of four randomized trials 46 has demonstrated levothyroxine to be effective in this setting.
A cervical incision is often used to approach intrathoracic goiters; however, 10–30% of the patients require sternotomy or thoracotomy 50.
After total thyroidectomy, patients must start levothyroxine replacement at a dose of 1.4–2.2 μg/kg/day 51. Adjustments in levothyroxine dose must be based on the patient’s age 52, according to the usual recommendations for patients with hypothyroidism. However, in patients undergoing partial thyroidectomy, treatment with levothyroxine should be implemented after the establishment of hypothyroidism and not preventively against goiter recurrence, because this benefit has not been confirmed in randomized studies 53.
The benefits and risks of a surgical procedure must be carefully considered, since the incidence of nontoxic nodular goiter increases with age, affecting particularly elderly individuals who often have other comorbidities. As discussed above, recombinant human TSH-stimulated radioiodine may be a treatment alternative for these patients.
- Nontoxic goiter. https://emedicine.medscape.com/article/120392-overview[↩]
- Simple Nontoxic Goiter. https://www.msdmanuals.com/en-au/professional/endocrine-and-metabolic-disorders/thyroid-disorders/simple-nontoxic-goiter[↩]
- Knobel M. Which Is the Ideal Treatment for Benign Diffuse and Multinodular Non-Toxic Goiters?. Front Endocrinol (Lausanne). 2016;7:48. Published 2016 May 23. doi:10.3389/fendo.2016.00048 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4876491/[↩][↩][↩]
- Samuels MH. Evaluation and treatment of sporadic nontoxic goiter – some answers and more questions [editorial]. J Clin Endocrinol Metab (2001) 86:994–7.10.1210/jcem.86.3.7384[↩]
- Peters H, Hackel D, Schleusener H. Treatment of euthyroid struma. Comparable volume reduction with 400 micrograms iodine, 100 micrograms levothyroxine combined with 100 micrograms iodine or individually dosed levothyroxine. Med Klin (Munich) (1997) 92:63–7.10.1007/BF03042286[↩]
- Hintze G, Kobberling J. Treatment of iodine deficiency goiter with iodine, levothyroxine or a combination of both. Thyroidology (1992) 4:37–40.[↩]
- Güllü S, Gürses MA, Başkal N, Uysal AR, Kamel AN, Erdoğan G. Suppressive therapy with levothyroxine for euthyroid diffuse and nodular goiter. Endocr J (1999) 46:221–6.10.1507/endocrj.46.221[↩][↩]
- Hegedüs L, Bennedbaek FN. Radioiodine for non-toxic diffuse goitre. Lancet (1997) 350:409–10.10.1016/S0140-6736(05)64132-3[↩][↩][↩]
- Nygaard B, Farber J, Veje A, Hansen JE. Thyroid volume and function after 131I treatment of diffuse non-toxic goitre. Clin Endocrinol (Oxf) (1997) 46:493–6.10.1046/j.1365-2265.1997.1760987.x[↩][↩][↩]
- Diehl LA, Garcia V, Bonnema SJ, Hegedüs L, Albino CC, Graf H, et al. Management of the nontoxic multinodular goiter in Latin America: comparison with North America and Europe, an electronic survey. J Clin Endocrinol Metab (2005) 90:117–23.10.1210/jc.2004-1722[↩][↩]
- Hegedüs L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev (2003) 24:102–32.10.1210/er.2002-0016[↩][↩][↩]
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) (1995) 43:55–68.10.1111/j.1365-2265.1995.tb01894.x[↩]
- Knobel M. Which Is the Ideal Treatment for Benign Diffuse and Multinodular Non-Toxic Goiters?. Front Endocrinol (Lausanne). 2016;7:48. Published 2016 May 23. doi:10.3389/fendo.2016.00048 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4876491[↩][↩]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009) 19:1167–214.10.1089/thy.2009.0110[↩]
- Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab (1996) 81:4278–89.10.1210/jcem.81.12.8954028[↩]
- Rieu M, Bekka S, Sambor B, Berrod JL, Fombeur JP. Prevalence of subclinical hyperthyroidism and relationship between thyroid hormonal status and thyroid ultrasonographic parameters in patients with non-toxic nodular goitre. Clin Endocrinol (Oxf) (1993) 39(1):67–71.10.1111/j.1365-2265.1993.tb01752.x[↩]
- Berghout A, Wiersinga WM, Drexhage HA, Smits NJ, Touber JL. Comparison of placebo with L-thyroxine alone or with carbimazole for treatment of sporadic non-toxic goitre. Lancet (1990) 336:193–7.10.1016/0140-6736(90)91730-X[↩][↩][↩]
- Wesche MF, Tiel-V Buul MM, Lips P, Smits NJ, Wiersinga WM. A randomized trial comparing levothyroxine with radioactive iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab (2001) 86:998–1005.10.1210/jcem.86.3.7244[↩][↩]
- Castro MR, Caraballo PJ, Morris JC. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a meta-analysis. J Clin Endocrinol Metab (2002) 87:4154–9.10.1210/jc.2001-011762[↩]
- Grussendorf M, Reiners C, Paschke R, Wegscheider K, LISA Investigators . Reduction of thyroid nodule volume by levothyroxine and iodine alone and in combination: a randomized, placebo-controlled trial. J Clin Endocrinol Metab (2011) 96:2786–95.10.1210/jc.2011-0356[↩]
- Puzziello A, Carrano M, Angrisani E, Marotta V, Faggiano A, Zeppa P, et al. Evolution of benign thyroid nodules under levothyroxine non-suppressive therapy. J Endocrinol Invest (2014) 37:1181–6.10.1007/s40618-014-0128-z[↩]
- Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27914 patients. Endocr Relat Cancer (2010) 17:231–9.10.1677/ERC-09-0251[↩]
- Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev (2012) 33:920–80.10.1210/er.2012-1030[↩][↩][↩][↩][↩]
- Knobel M. Etiopathology, clinical features, and treatment of diffuse and multinodular nontoxic goiters. J Endocrinol Invest (2016) 39:357–73.10.1007/s40618-015-0391-7[↩]
- Hegedüs L, Hansen BM, Knudsen N, Hansen JM. Reduction of size of thyroid with radioactive iodine in multinodular non-toxic goitre. BMJ (1988) 297:661–2.10.1136/bmj.297.6649.661[↩]
- Bonnema SJ, Bertelsen H, Mortensen J, Andersen PB, Knudsen DU, Bastholt L, et al. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J Clin Endocrinol Metab (1999) 84:3636–41.10.1210/jcem.84.10.6052[↩]
- Howarth DM, Epstein MT, Thomas PA, Allen LW, Akerman R, Lan L. Outpatient management of patients with large multinodular goitres treated with fractionated radioiodine. Eur J Nucl Med (1997) 24:1465–9.10.1007/s002590050175[↩]
- Nygaard B, Knudsen JH, Hegedüs L, Scient AV, Hansen JE. Thyrotropin receptor antibodies and Graves’ disease, a side-effect of 131I treatment in patients with nontoxic goiter. J Clin Endocrinol Metab (1997) 82:2926–30.10.1210/jc.82.9.2926[↩]
- Huysmans DA, Buijs WC, van de Ven MT, van den Broek WJ, Kloppenborg PW, Hermus AR, et al. Dosimetry and risk estimates of radioiodine therapy for large, multinodular goiters. J Nucl Med (1996) 37:2072–9[↩]
- Bonnema SJ, Fast S, Hegedüs L. The role of radioiodine therapy in benign nodular goiter. Best Pract Res Clin Endocrinol Metabol (2014) 28:619–31.10.1016/j.beem.2014.02.00[↩]
- Medeiros-Neto G, Marui S, Knobel M. An outline concerning the potential use of recombinant human thyrotropin for improving radioiodine therapy of multinodular goiter. Endocrine (2008) 33:109–17.10.1007/s12020-008-9077-7[↩]
- Nielsen VE, Bonnema SJ, Boel-Jørgensen H, Veje A, Hegedüs L. Recombinant human thyrotropin markedly changes the 131I kinetics during 131I therapy of patients with nodular goiter: an evaluation by a randomized double-blinded trial. J Clin Endocrinol Metab (2005) 90:79–83.10.1210/jc.2004-1550[↩]
- Bonnema SJ, Fast S, Hegedüs L. The role of radioiodine therapy in benign nodular goiter. Best Pract Res Clin Endocrinol Metabol (2014) 28:619–31.10.1016/j.beem.2014.02.001[↩][↩]
- Graf H. Recombinant human TSH and radioactive iodine therapy in the management of benign multinodular goiter. Eur J Endocrinol (2015) 172:R47–52.10.1530/EJE-14-0608[↩]
- Romao R, Rubio IGS, Tomimori EK, Camargo RY, Knobel M, Medeiros-Neto G. High prevalence of side effects after rhTSH stimulated radioiodine treatment with 30 mCi in patients with multinodular goiter and subclínical/clínical hyperthyroidism. Thyroid (2009) 19:945–51.10.1089/thy.2008.0394[↩]
- Fast S, Nielsen VE, Bonnema SJ, Hegedüs L. Time to reconsider nonsurgical therapy of benign non-toxic multinodular goitre: focus on recombinant human TSH augmented radioiodine therapy. Eur J Endocrinol (2009) 160:517–28.10.1530/EJE-08-0779[↩]
- Fast S, Nielsen VE, Grupe P, Boel-Jørgensen H, Bastholt L, Andersen PB, et al. Prestimulation with recombinant human thyrotropin (rhTSH) improves the long-term outcome of radioiodine therapy for multinodular nontoxic goiter. J Clin Endocrinol Metab (2012) 97:2653–60.10.1210/jc.2011-3335[↩]
- Rubio IG, Perone BH, Silva MN, Knobel M, Medeiros-Neto G. Human recombinant TSH preceding a therapeutic dose of radioiodine for multinodular goiters has no significant effect in the surge of TSH-receptor and TPO antibodies. Thyroid (2005) 15:134–9.10.1089/thy.2005.15.134[↩]
- Graf H, Fast S, Pacini F, Pinchera A, Leung A, Vaisman M, et al. Modified-release recombinant human TSH (MRrhTSH) augments the effect of 131I therapy in benign multinodular goiter. Results from a multicenter international, randomized, placebo-controlled study. J Clin Endocrinol Metab (2011) 96:1368–76.10.1210/jc.2010-1193[↩]
- Albino CC, Graf H, Sampaio AP, Vigário A, Paz-Filho GJ. Thiamazole as an adjuvant to radioiodine for volume reduction of multinodular goiter. Expert Opin Investig Drugs (2008) 17:1781–6.10.1517/13543780802501325[↩]
- Cirocchi R, Trastulli S, Randolph J, Guarino S, Di Rocco G, Arezzo A, et al. Total or near-total thyroidectomy versus subtotal thyroidectomy for multinodular non-toxic goitre in adults. Cochrane Database Syst Rev (2015) 7(8):CD010370.10.1002/14651858.CD010370.pub2[↩][↩]
- Agarwal G, Aggarwal V. Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review. World J Surg (2008) 32:1313–24.10.1007/s00268-008-9579-8[↩]
- Thomusch O, Machens A, Sekulla C, Ukkat J, Lippert H, Gastinger I, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg (2000) 24:1335–41.10.1007/s002680010221[↩]
- Yoldas T, Makay O, Icoz G, Kose T, Gezer G, Kismali E, et al. Should subtotal thyroidectomy be abandoned in multinodular goiter patients from endemic regions requiring surgery? Int Surg (2015) 100:9–14.10.9738/INTSURG-D-13-00275.1[↩]
- Bauer PS, Murray S, Clark N, Pontes DS, Sippel RS, Chen H. Unilateral thyroidectomy for the treatment of benign multinodular goiter. J Surg Res (2013) 184:514–8.10.1016/j.jss.2013.04.045[↩]
- Hegedüs L, Nygaard B, Hansen JM. Is routine thyroxine treatment to hinder postoperative recurrence of nontoxic goiter justified? J Clin Endocrinol Metab (1999) 84:756–60.10.1210/jcem.84.2.5478[↩][↩][↩]
- Rojdmark J, Jarhult J. High long term recurrence rate after subtotal thyroidectomy for nodular goitre. Eur J Surg (1995) 161:725–7[↩]
- Berghout A, Wiersinga WM, Drexhage HA, van Trotsenburg P, Smits NJ, van der Gaag RD, et al. The long-term outcome of thyroidectomy for sporadic non-toxic goitre. Clin Endocrinol (Oxf) (1989) 31:193–9.10.1111/j.1365-2265.1989.tb01242.x[↩]
- Miccoli P, Antonelli A, Iacconi P, Alberti B, Gambuzza C, Baschieri L. Prospective, randomized, double-blind study about effectiveness of levothyroxine suppressive therapy in prevention of recurrence after operation: result at the third year of follow-up. Surgery (1993) 114:1097–101[↩]
- Mercante G, Gabrielli E, Pedroni C, Formisano D, Bertolini L, Nicoli F, et al. CT cross-sectional imaging classification system for substernal goiter based on risk factors for an extracervical surgical approach. Head Neck (2010) 33:792–9.10.1002/hed.21539[↩]
- Baehr KM, Lyden E, Treude K, Erickson J, Goldner W. Levothyroxine dose following thyroidectomy is affected by more than just body weight. Laryngoscope (2012) 122:834–8.10.1002/lary.23186[↩]
- Pieracci FM, Fahey TJ, 3rd. Substernal thyroidectomy is associated with increased morbidity and mortality as compared with conventional cervical thyroidectomy. J Am Coll Surg (2007) 205:1–7.10.1016/j.jamcollsurg.2007.03.010[↩]
- Ross DS. Thyroid hormone suppressive therapy of sporadic nontoxic goiter. Thyroid (1992) 2:263–9.10.1089/thy.1992.2.263[↩]