Rift Valley fever
Rift Valley fever is a mosquito-borne zoonotic fever-causing viral disease most commonly observed in domesticated animals (such as cattle, buffalo, sheep, goats, and camels), with the ability to infect and cause illness in humans that are endemic to Africa and the Arabian Peninsula 1. High rates of abortion among infected ruminants and hemorrhagic fever in infected humans are major public health concerns. Commercially available veterinary Rift Valley fever vaccines are important for preventing the spread of the Rift Valley fever virus in endemic countries; however, Rift Valley fever virus outbreaks continue to occur frequently in endemic countries in the 21st century. In the U.S., the live-attenuated MP-12 vaccine has been developed for both animal and human vaccination 1. This vaccine strain is well attenuated, and a single dose induces neutralizing antibodies in both ruminants and humans 1.
Rift Valley fever is caused by a mosquito-borne Rift Valley fever virus, a member of the genus Phlebovirus in the family Bunyaviridae 2. Rift Valley fever was first reported in livestock (sheep, cattle and goats) by veterinary officers in Kenya’s Rift Valley in the early 1910s 3. Rift Valley fever disease was characterized by a high mortality rate (more than 90%) of newborn lambs and high abortion rates among pregnant ewes, cattle, and goats. Daubney and Hudson 3 proposed that Rift Valley fever virus is transmitted by mosquitoes, and in the 1940s, Smithburn et al. 4 accidentally isolated Rift Valley fever virus (Entebbe strain) from a pool of Aedes and Eretmapodites spp. mosquitoes, which were caught for the investigation of Yellow fever, in a forest uninhabited by humans in Uganda. From the 1950s to the 1990s, Rift Valley fever outbreaks occurred in various countries throughout the African continent, such as Kenya, South Africa, Zimbabwe, Namibia, Mozambique, Madagascar, Mauritania, Senegal, Egypt, and Sudan 5. During this period, it became evident that human infection with Rift Valley fever virus can occasionally lead to hemorrhagic fever, encephalitis, or retinitis with partial or complete blindness, in addition to febrile illness 6.
Approximately 1% of humans infected with Rift Valley fever virus die of the disease 7. By contrast, case-fatality proportions for infected livestock are significantly higher, with Rift Valley fever virus infection causing abortion in nearly 100% of pregnancies 7.
For humans, studies have shown that spending time in rural areas and sleeping outdoors at night in regions where outbreaks occur could be a risk factor for exposure to mosquito and other insect vectors. Animal herdsmen, abattoir workers, veterinarians, and other individuals who work with potentially-infected animals in Rift Valley fever-endemic areas (areas where the virus is present) have an increased risk for infection. International travelers increase their chances of getting the disease when they visit Rift Valley fever-endemic locations during periods when sporadic cases or epidemics are occurring.
Rift Valley fever is generally found in regions of eastern and southern Africa where sheep and cattle are raised, but the virus exists in most of sub-Saharan Africa, including west Africa and Madagascar. In September 2000, a Rift Valley fever outbreak was reported in Saudi Arabia and subsequently, Yemen. This outbreak represents the first cases of Rift Valley fever identified outside Africa 7.
Outbreaks of Rift Valley fever can have major societal impacts, including significant economic losses and trade reductions. The virus most commonly affects livestock, causing disease and abortion in domesticated animals, an important income source for many. Outbreaks of disease in animal populations are called “epizootics.” The most notable Rift Valley fever epizootic occurred in Kenya in 1950-1951, resulting in the death of an estimated 100,000 sheep.
Additionally, epizootic outbreaks of Rift Valley fever increase the likelihood of contact between diseased animals and humans, which can lead to epidemics of Rift Valley fever in humans. One example occurred in 1977 when the virus was detected in Egypt (possibly imported from infected domestic animals from Sudan) and caused a large outbreak of Rift Valley fever among both animals and humans resulting in over 600 human deaths. Another example of Rift Valley fever spillover into human populations occurred in west Africa in 1987, and was linked to construction of the Senegal River Project. The project caused flooding in the lower Senegal River area, altering ecological conditions and interactions between animals and humans. As a result, a large Rift Valley fever outbreak occurred in animals and humans.
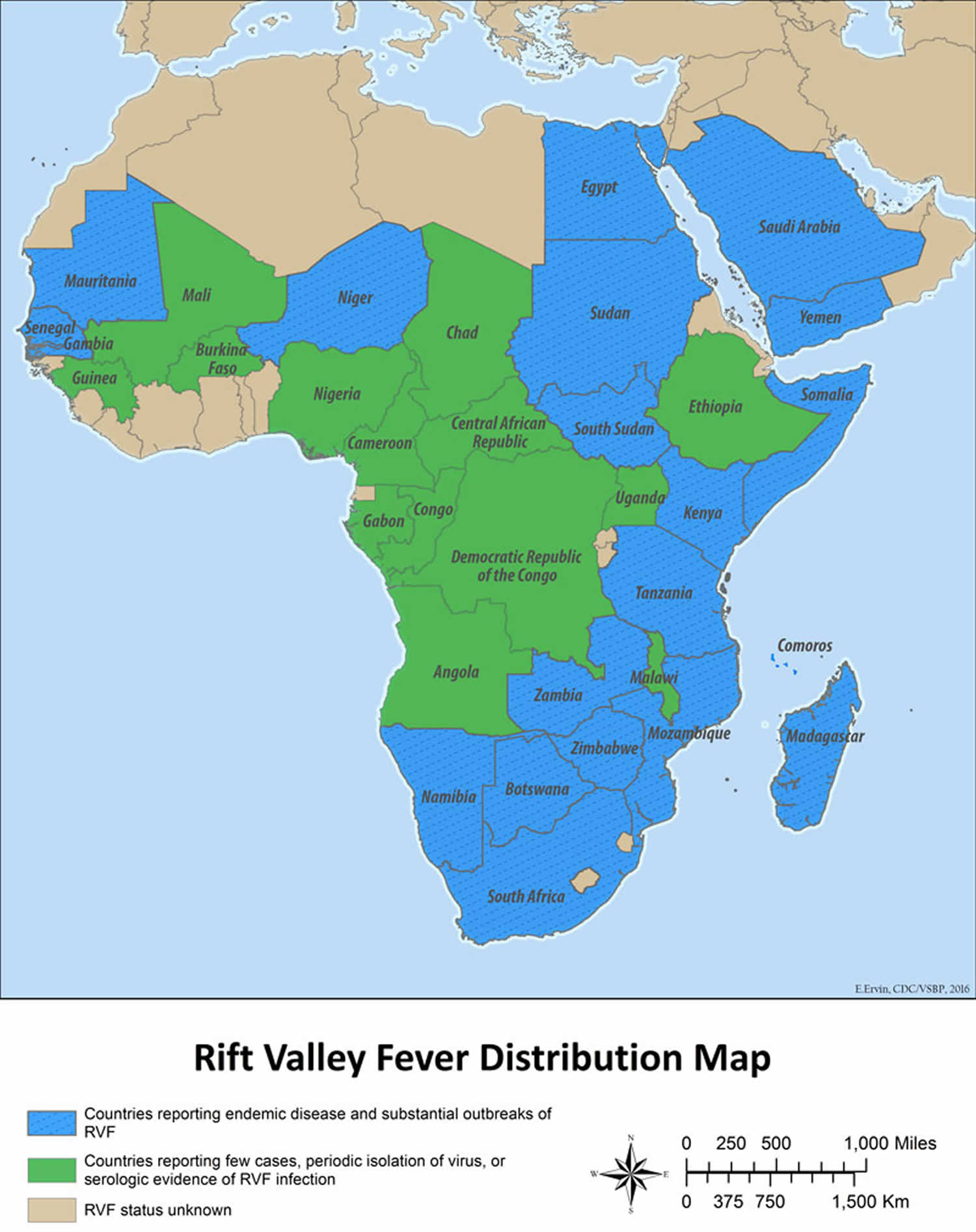
Figure 1. Rift Valley fever distribution map
Footnote:
- Countries reporting endemic disease and substantial outbreaks of Rift Valley fever: Egypt, Saudi Arabia, Yemen, Mauritania, Senegal, the Gambia, Sudan, South Sudan, Kenya, Tanzania, Zambia, Zimbabwe, Mozambique, Madagascar, Namibia, South Africa
- Countries reporting few cases, periodic isolation of virus, or serologic evidence of Rift Valley fever infection: Mali, Chad, Ethiopia, Somalia, Guinea, Burkina Faso, Nigeria, Cameroon, Central African Republic, Gabon, Republic of Congo, Democratic Republic of Congo, Uganda, Angola, Botswana, Niger
- Rift Valley fever status unknown: All other countries
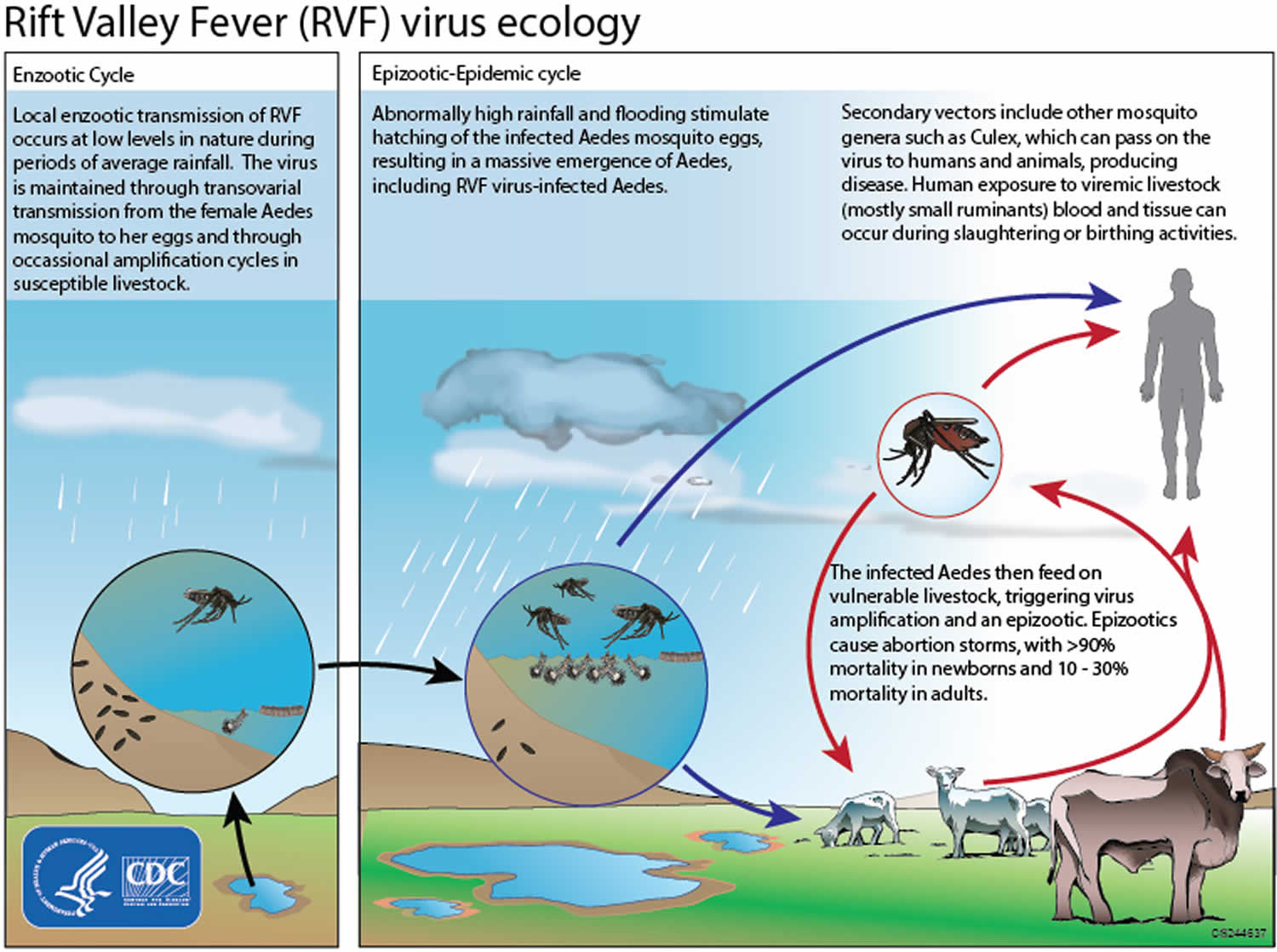
Figure 2. Rift Valley fever virus life cycle
Rift Valley fever key points 1
- Rift Valley fever virus is endemic to Africa and the Arabian Peninsula, and infected mosquitoes can transmit viruses to ruminants and humans.
- Rift Valley fever virus is teratogenic in ruminants, such as sheep, cattle, and goats.
- Rift Valley fever vaccines for animals are available in endemic areas, yet the development of further safe and efficacious vaccines for pregnant animals remains important.
- The live-attenuated MP-12 vaccine is attenuated and efficacious for both animals and humans, and is conditionally licensed for animal use in the U.S.
- Effective control of a Rift Valley fever outbreak should be considered using suitable Rift Valley fever vaccines in different scenarios.
Rift Valley fever transmission
People are infected with Rift Valley fever virus through contact with blood, body fluids, or tissues of Rift Valley fever virus-infected animals, mainly livestock. This direct contact with infected animals can occur during slaughter or veterinary procedures, like assisting the animal with giving birth. Infection through breathing in droplets contaminated with Rift Valley fever virus (aerosol transmission) has occurred in the laboratory setting. Less commonly, people can be infected with Rift Valley fever virus from bites of infected mosquitoes and, rarely, from other biting insects that have the virus on their mouthparts. Spread from person to person has not been documented.
Several mosquito species can spread Rift Valley fever virus and these vary by region. Environmental influences, particularly rainfall, are an important risk factor for outbreaks in both animals and people. Rift Valley fever outbreaks have been observed during years with unusually heavy rainfall and localized flooding.
The transmission cycle of Rift Valley fever virus can look like this:
- The Rift Valley fever virus can be spread from female mosquitos to their offspring through the eggs (vertical transmission).
- In the eggs, the virus remains viable (infectious) for several years during dry conditions.
- Excessive rainfall enables more mosquito eggs to hatch.
- As mosquito populations increase, the potential for the virus to spread to the animals and people also increases
- Rift Valley fever virus outbreaks in animals, most commonly livestock, lead to increased handling of infected animals, which then increases risk of exposure to the virus for people.
Rift Valley fever prevention
A person’s chances of becoming infected can be reduced by taking measures to decrease contact with blood, body fluids, or tissues of infected animals and protecting themselves against mosquitoes and other bloodsucking insects. Use of mosquito repellents and bednets are two effective methods. For persons working with animals in Rift Valley fever-endemic areas, wearing protective equipment to avoid any exposure to blood or tissues of animals that may potentially be infected is an important protective measure.
A number of questions and challenges remain in the control and prevention of Rift Valley fever. Knowledge regarding virus maintenance and transmission within different mosquito species and the risk factors associated with severe cases of Rift Valley fever in humans are still under investigation. Potentially, establishing environmental monitoring and case surveillance systems may aid in the prediction and control of future Rift Valley fever outbreaks.
Rift Valley fever virus is pathogenic to both animals and humans, and vaccination is one of the most effective measures to prevent the disease. No vaccines are currently available for human vaccination (see below).
Different types of vaccines for veterinary use are available. The killed vaccines are not practical in routine animal field vaccination because of the need of multiple injections. Live vaccines require a single injection but are known to cause birth defects and abortions in sheep and induce only low-level protection in cattle. The live-attenuated vaccine, MP-12, has demonstrated promising results in laboratory trials in domesticated animals, but more research is needed before the vaccine can be used in the field. The live-attenuated clone 13 vaccine was recently registered and used in South Africa. Alternative vaccines using molecular recombinant constructs are in development and show promising results.
In addition, surveillance (close monitoring for Rift Valley fever infection in animal and human populations) is essential to learning more about how Rift Valley fever virus infection is transmitted and to formulate effective measures for reducing the number of infections.
MP-12 vaccine to human use
The formalin-inactivated Rift Valley fever vaccine (TSI-GSD-200) was developed from the pathogenic Entebbe strain, and the vaccine requires three repeated vaccinations and periodical boosters to maintain its protective efficacy 9. The MP-12 vaccine is an Investigational New Drug, and its safety and immunogenicity have been tested in clinical trials 10.
In the initial human trial, three out of four volunteers administered with the MP-12 vaccine (104.4 pfu dose, s.c.) developed neutralizing antibodies, whereas all four showed transient mild to moderate elevation of serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) 11. In a subsequent dose escalation study, 22 humans were administered with the MP-12 vaccine (either 104.4, 103.4, 102.4, or 101.4 pfu dose, s.c.) 11. Within the 104.4 pfu dose group, seroconversion (positive for IgM or IgG via either PRNT50 or PRNT80, or ELISA) occurred in two of four individuals, in addition to injection site tenderness (3 of 4), severe AST elevation (1 of 4), or elevated creatine phosphokinase (CPK) (3 of 4).
In another clinical trial for dose escalation and route comparison, a comparative analysis of MP-12 vaccination in humans via the s.c. route (104.7 pfu, n = 10) or i.m. route (103.4 pfu, n = 6, or 104.4 pfu, n = 27) was performed 11. Overall, 93% of subjects (40 of 43) showed an increase in neutralizing antibody titers (PRNT80 ≥20) from 14 to 21 dpv. Peak IgM and IgG ELISA titers were higher in the s.c. injection group than in the i.m. group, whereas PRNT50 and PRNT80 titers were higher and more sustained in the i.m. (104.4 pfu dose) injection group (peak geometric PRNT80 titer was 1:277) than in the s.c. injection group. PRNT50 and PRNT80 titers of ≥ 1:20 were maintained in 87% of individuals in the i.m. group with 104.4 pfu dose for one year, indicating a better vaccine immunogenicity than that of TSI-GSD-200 (formalin-inactivated Entebbe strain) 12. One volunteer (104.4 pfu, i.m.) developed viremia of 1.3 pfu/ml at 9 dpv. Adverse effects within the i.m. group (104.4 pfu) were mild to moderate: chills (7.4%), dizziness (11.1%), fever (3.7%), headache (29.6%), malaise (22.2%), or vomiting (7.4%). According to the guideline of the U.S. Food and Drug Administration 13, the abnormality grading of liver enzymes (ALT and AST) is defined as the relative increase compared to the upper limit of the normal range: i.e., 1.1× – 2.5× (mild, Grade 1), 2.6× – 5.0× (moderate, Grade 2), 5.1× – 10× (severe, Grade 3), >10× (potentially life threatening, Grade 4), and that of CPK as 1.25× – 1.5× (mild, Grade 1), 1.6× – 3.0× (moderate, Grade 2), 3.1× – 10× (severe, Grade 3), >10× (potentially life threatening, Grade 4). Symptomatic transient elevation of serum ALT, AST, CPK, and lactate dehydrogenase (LDH) were found in all groups (40% in s.c. group, 50% in i.m. 103.4 pfu dose group, and 44% in i.m. 104.4 pfu dose group). Although most cases were mild or moderate (Grade 1 or 2) increases, one vaccinee in the i.m. 103.4 pfu dose group had 4,139 U/L of LDH (Grade 3) and 587 U/L of AST (Grade 4). Grade 4 CPK elevation was also recorded in one in the i.m. 103.4 pfu dose group and two in the placebo group, all of which were recorded after physical exercise with muscle fatigue. The values of CPK, AST, ALT, and LDH were returned to normal within a few days without clinical significance in all cases. Further evaluation will be required to ensure the safety of MP-12 vaccine candidate in humans.
Since vaccination via the i.m. (intramuscular) route induced higher neutralizing titers than that via the s.c. route, further clinical trials for vaccine immunogenicity and viral genetic stability were performed. A total of 19 volunteers were vaccinated with the MP-12 vaccine (1×105 pfu, i.m.) and 18 (95%) had increased PRNT80 titers of ≥ 1:20 by 28 dpv, and maintained 1:20 or higher titers for 12 months afterwards 14. Follow-up evaluation of those volunteers showed that eight of nine tested sera still maintained neutralizing antibodies of ≥ 1:20 at five years post vaccination. Adverse effects were reported including headache (58%), fatigue (42%), nausea (26%), flu-like symptoms (21%), fever (16%), and local tenderness (48%). Ophthalmologic examination (28 dpv) or electrocardiogram (three months post vaccination) showed normal results. Virus isolation was attempted from plasma (1 ml) and buffy coat (0.2 ml) specimens collected at 1 to 14 dpv. Vero cells were adsorbed with those clinical specimens in T-25 flasks at 37°C for one hour, and further incubated with fresh media for five days. After additional medium replacement, cells were incubated for an additional five days. The freeze-thawed cells were added into fresh Vero cells, and a second 10-day incubation was performed with medium replacement at 5 dpi. All negative flasks were tested for viral RNA via RT-PCR. When cells showed cytopathic effects (CPE), the supernatants derived from freeze-thawed cell samples containing viruses were further amplified in fresh Vero cells. Virus titers of culture supernatants were measured by plaque assay. As a result, nine MP-12 isolates were amplified from the plasma samples of five vaccinees at 4 to 9 dpv, and two isolates were made from buffy coat samples at 7 and 8 dpv. A total of 10 mutations (two silent mutations and eight amino acid substitutions) were found, yet none were reversion mutations. LD50 of each of the 11 isolates in 19-day-old CD1 mice, a mouse model susceptible to MP-12 in an age-dependent manner, was greater than 1×101.88 pfu/ml, indicating no increase in the virulence of the MP-12 strain due to those mutations. Overall, clinical trials demonstrated that the MP-12 vaccine induces neutralizing antibodies with a single dose for at least several years. Due to a lack of Rift Valley fever in the U.S., the vaccine efficacy study in humans is difficult. Thus, an alternative approach to claim a valid efficacy may be applicable using more than one appropriate animal models under Good Laboratory Practice and the Animal Welfare Act under the Animal Rule of the FDA 15.
Protective efficacy of the MP-12 vaccine against aerosol exposure to the pathogenic Rift Valley fever virus strain
Because Rift Valley fever virus exposure via aerosol (e.g., bioterrorism, laboratory exposure) may alter the pathogenesis of disease, it is important to evaluate the protective efficacy of the MP-12 vaccine against aerosol exposure challenge. Rhesus monkeys were vaccinated with the MP-12 vaccine (6×103 pfu, i.m.) and challenged with a small particle (<5 μm) aerosol exposure of the pathogenic ZH501 strain (~ 5×105 pfu) 16. Viremia of <2.0 log10 pfu/ml was detected in three of nine vaccinated monkeys, and the virus was also isolated from oropharyngeal swabs at 4 or 7 dpv. Neutralizing antibody titers (PRNT80) of ≥ 1:20 were detected at 7 dpv, and increased to between 1:320 and 1:2,560 by 126 dpv. Slight increases in AST and CK were observed in vaccinated animals from 1 to 3 dpv, but all vaccinated animals survived the challenge without detectable clinical signs or viremia, whereas all control animals developed viremia after the challenge. Thus, vaccination of MP-12 via the i.m. route was shown to be efficacious to protect against aerosol exposure.
Another study also showed the efficacy of the MP-12 vaccine via the aerosol or intranasal vaccination route 17. Rhesus monkeys were vaccinated with MP-12 (~ 1×105 pfu via aerosol, or 5×104 pfu via intranasal vaccination route) and challenged with a small particle (<5 μm) aerosol exposure of the pathogenic ZH501 strain at 56 dpv. Vaccinated monkeys did not develop viremia, and increased titers of neutralizing antibody to 1:40 or more by 28 dpv. Vaccinated monkeys were fully protected from aerosol challenge of the ZH501 strain. Thus, this study demonstrated the efficacy of the MP-12 vaccine via the aerosol or intranasal route. Because none of the vaccinated monkeys showed clinical signs, safe vaccination with the MP-12 vaccine via the mucosal route could now be considered for massive vaccination without using a needle.
Expert commentary
In endemic countries, mosquitoes potentially harbor pathogenic Rift Valley fever virus, regardless of any ongoing Rift Valley fever outbreak 18. In such an environment, Rift Valley fever vaccination may be focused on: (1) the containment of active Rift Valley fever outbreaks and (2) long-term protection from Rift Valley fever exposure to infected mosquitoes. Because Rift Valley fever vaccination performed during a Rift Valley fever outbreak increases the risk of co-infection of the pathogenic Rift Valley fever virus strain and live-attenuated Rift Valley fever virus strain in animals, it is ideal that endemic countries continue regular vaccination of susceptible animals with commercially available efficacious Rift Valley fever vaccines before new outbreaks start 1. Although the Smithburn vaccine has been used for a long period of time in endemic areas, residual virulence in pregnant animals hampers safe vaccination of highly susceptible animal populations. Unfortunately, mosquitoes transmit Rift Valley fever virus into offspring without the involvement of animals and thus, regular vaccination may be the only practically effective approach to protect susceptible livestock animals from continuous Rift Valley fever virus exposure by infected mosquitoes, unless infected mosquitoes can be eradicated in the endemic area. Further safe vaccines, including other live-attenuated vaccines, subunit vaccines, or replicon/vector vaccines, can better support the safe vaccination of ruminants in endemic countries. It will therefore be important to accelerate the communication between vaccine manufacturers in endemic countries and vaccine stakeholders, to facilitate the introduction of better Rift Valley fever vaccines in endemic countries. The MP-12 vaccine has never been used in endemic countries; however, if the MP-12 vaccine were to become available in such places, the long-term protection of ruminants, including newborn animals through colostrum, as well as viral spill-over into mosquitoes, should be evaluated on a large scale. In non-endemic countries, including the U.S., Canada, and European countries, no vaccination will be required until the first Rift Valley fever virus case is confirmed, as early containment of viral spread will be important for minimizing the impact on society. A national stockpile vaccine, such as the MP-12 vaccine in the U.S., will allow immediate vaccination of susceptible animals in case of an unexpected Rift Valley fever outbreak. Because the MP-12 vaccine lacks a DIVA marker, subunit vaccines or replicon or vector vaccines will be more useful than the MP-12 vaccine on the occasion when rapid protection is less important, and surveillance of viral spread is required.
Rift Valley fever symptoms
Pathogenesis of Rift Valley fever in human patients is different from that in ruminants, and is characterized by febrile illness, hemorrhagic fever, encephalitis, and blindness, rather than abortion or fetal malformation. Rift Valley fever virus has an incubation period of 2-6 days following infection and can cause several different disease syndromes. Most commonly, people with Rift Valley fever have either no symptoms or a mild illness associated with fever and liver abnormalities. Patients who become ill usually experience fever, generalized weakness, back pain, and dizziness at the onset of the illness. Typically, patients recover within two days to one week after onset of illness.
However, a small percentage (8-10%) of people infected with Rift Valley fever virus develop much more severe symptoms, including:
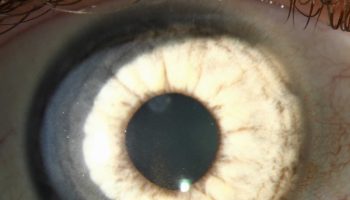
- Ocular disease (diseases affecting the eye), which sometimes accompanies the mild symptoms described above. Lesions on the eyes may occur 1-3 weeks after onset of initial symptoms with patients reporting blurred and decreased vision. For many patients, lesions disappear after 10-12 weeks; however, for those with lesions occurring in the macula, approximately 50% of patients will have permanent vision loss.
- Encephalitis, or inflammation of the brain, which can lead to headaches, coma, or seizures. This occurs in less than 1% of patients and presents 1-4 weeks after first symptoms appear. Though death from this is rare, neurological deficits, sometimes severe, may persist.
- Hemorrhagic fever, which occurs in less than 1% of overall Rift Valley fever patients, but fatality for those who do develop these symptoms, is around 50%. Symptoms of hemorrhaging may begin with jaundice and other signs of liver impairment, followed by vomiting blood, bloody stool, or bleeding from gums, skin, nose, and injection sites. These symptoms appear 2-4 days and death usually occurs 3-6 days after.
Rift Valley fever diagnosis
During the early phase of illness in the blood and in postmortem tissue, the virus may be detected by virus isolation in cell culture, antigen-detection ELISA, and molecular techniques (PCR). Antibody testing using enzyme-linked immunoassay (ELISA) can be used to confirm presence of IgM antibodies, which appear as an early, transient response, and IgG antibodies, which persist for several years. Both IgM and IgG antibodies are specific to Rift Valley fever virus.
Rift Valley fever treatment
Because most human cases of Rift Valley fever are mild and self-limiting, a specific treatment for Rift Valley fever has not been established. The rare, but serious, cases are generally limited to supportive care.
The most common complication associated with Rift Valley fever is inflammation of the retina (a structure connecting the nerves of the eye to the brain). As a result, approximately 1% – 10% of affected patients may have permanent vision loss.
- Ikegami T. Rift Valley fever vaccines: an overview of the safety and efficacy of the live-attenuated MP-12 vaccine candidate. Expert Rev Vaccines. 2017;16(6):601–611. doi:10.1080/14760584.2017.1321482 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5658789[↩][↩][↩][↩][↩]
- Elliott R. Bunyaviruses: general features. In: Mahy BW, van Regenmortel MH, editors. Encyclopedia of virology. London: Elsevier-Academic Press; 2008. p.390-9.[↩]
- Daubney R, Hudson JR. Enzootic hepatitis or Rift Valley fever: An undescribed virus disease of sheep cattle and man from east Africa. J Path Bact. 1931;34:545–579.[↩][↩]
- Smithburn KC, Haddow AJ, Gillett JD. Rift Valley fever; isolation of the virus from wild mosquitoes. Br J Exp Pathol. 1948;29(2):107–121.[↩]
- Swanepoel R, Coetzer JAW. Rift Valley fever. In: Coetzer JAW, Tustin RC, editors. Infectious diseases of livestock with special reference to southern Africa. 2. Cape Town, South Africa: Oxford University Press; 2004. pp. 1037–1070. A historical review of Rift Valley fever in Africa.[↩]
- Ikegami T, Makino S. The Pathogenesis of Rift Valley Fever. Viruses. 2011;3(5):493–519.[↩]
- Rift Valley fever. https://www.cdc.gov/vhf/rvf/[↩][↩][↩]
- Rift Valley fever distribution map. https://www.cdc.gov/vhf/rvf/outbreaks/distribution-map.html[↩]
- Pittman PR, Liu CT, Cannon TL, et al. Immunogenicity of an inactivated Rift Valley fever vaccine in humans: a 12-year experience. Vaccine. 1999;18(1–2):181–189.[↩]
- Morrill JC, Peters CJ. Pathogenicity and neurovirulence of a mutagen-attenuated Rift Valley fever vaccine in rhesus monkeys. Vaccine. 2003;21(21–22):2994–3002.[↩]
- Pittman PR, McClain D, Quinn X, et al. Safety and immunogenicity of a mutagenized, live attenuated Rift Valley fever vaccine, MP-12, in a Phase 1 dose escalation and route comparison study in humans. Vaccine. 2016;34(4):424–429. Clinical trial of MP-12 vaccine including the evaluation of adverse effects.[↩][↩][↩]
- Rusnak JM, Gibbs P, Boudreau E, Clizbe DP, Pittman P. Immunogenicity and safety of an inactivated Rift Valley fever vaccine in a 19-year study. Vaccine. 2011;29(17):3222–3229.[↩]
- Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. Guidance for Industry Updated June 2016 https://www.fda.gov/media/82945/download[↩]
- Pittman PR, Norris SL, Brown ES, et al. Rift Valley fever MP-12 vaccine Phase 2 clinical trial: Safety, immunogenicity, and genetic characterization of virus isolates. Vaccine. 2016;34(4):523–530. Clinical trial of MP-12 vaccine including the analysis of MP-12 vaccine isolates from vaccinees.[↩]
- Snoy PJ. Establishing efficacy of human products using animals: the US food and drug administration’s “animal rule” Vet Pathol. 2010;47(5):774–778.[↩]
- Morrill JC, Peters CJ. Protection of MP-12-vaccinated rhesus macaques against parenteral and aerosol challenge with virulent rift valley fever virus. J Infect Dis. 2011;204(2):229–236.[↩]
- Morrill JC, Peters CJ. Mucosal Immunization of Rhesus Macaques With Rift Valley Fever MP-12 Vaccine. J Infect Dis. 2011;204(4):617–625.[↩]
- Liu W, Sun FJ, Tong YG, Zhang SQ, Cao WC. Rift Valley fever virus imported into China from Angola. Lancet Infect Dis. 2016;16(11):1226[↩]