Walker Warburg syndrome
Walker-Warburg syndrome also called Chemke syndrome, HARD syndrome or cerebroocular dysplasia-muscular dystrophy syndrome, is a rare form of autosomal recessive congenital muscular dystrophy associated with brain and eyes abnormalities 1. Walker-Warburg syndrome is the most severe of a group of genetic conditions known as congenital muscular dystrophies, which cause muscle weakness and wasting (atrophy) beginning very early in life. The signs and symptoms of Walker-Warburg syndrome are present at birth or in early infancy. Because of the severity of the problems caused by Walker-Warburg syndrome, most affected individuals do not survive past age 3 2.
Walker-Warburg syndrome affects the skeletal muscles, which are muscles the body uses for movement. Affected babies have weak muscle tone (hypotonia) and are sometimes described as “floppy.” The muscle weakness worsens over time.
Walker-Warburg syndrome also affects the brain; individuals with this condition typically have a brain abnormality called cobblestone lissencephaly, in which the surface of the brain lacks the normal folds and grooves and instead develops a bumpy, irregular appearance (like that of cobblestones). These individuals may also have a buildup of fluid in the brain (hydrocephalus) or abnormalities of certain parts of the brain, including a region called the cerebellum and the part of the brain that connects to the spinal cord (the brainstem). These changes in the structure of the brain lead to significantly delayed development and intellectual disability. Some individuals with Walker-Warburg syndrome experience seizures.
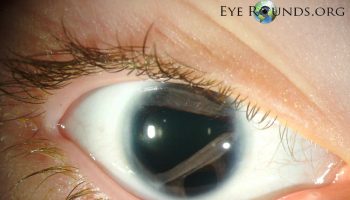
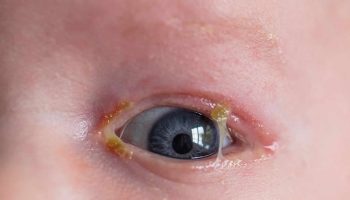
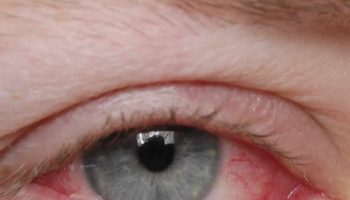
Eye abnormalities are also characteristic of Walker-Warburg syndrome. These can include unusually small eyeballs (microphthalmia), enlarged eyeballs caused by increased pressure in the eyes (buphthalmos), clouding of the lenses of the eyes (cataracts), and problems with the nerve that relays visual information from the eyes to the brain (the optic nerve). These eye problems lead to vision impairment in affected individuals.
Walker-Warburg syndrome is estimated to affect 1 in 60,500 newborns worldwide 2.
There is currently no cure or treatment for Walker-Warburg syndrome. Management is generally only supportive and preventive. Individuals who develop seizures are typically treated with anticonvulsants. A few children require surgical procedures, such as shunting for hydrocephalus or correction of encephalocele. Physical therapy can be offered to aid in development or prevent worsening of contractures; however, the benefit of this has not been established. Feeding usually needs to be monitored and in some cases, a supplemental nasogastric or gastric feeding tube may be necessary 1.
Walker Warburg syndrome causes
Walker-Warburg syndrome can be caused by mutations in at least 14 different genes that are normally responsible for making proteins involved in the glycosylation process. The most commonly mutated genes were discovered first, including POMT1, POMT2, CRPPA, FKTN, FKRP, and LARGE1. Mutations in these genes are found in about half of individuals with Walker-Warburg syndrome. Other genes, some of which have not been identified, are also involved in development of this condition.
Walker Warburg syndrome genes identified thus far are:
- B3GALNT2: Beta-1,3-N-acetylgalactosaminyltransferase 2 protein
- B4GAT1: Beta-1,4-glucuronyltransferase 1 protein
- DAG1: Dystrophin-associated glycoprotein 1
- FKRP: Fukutin-related protein
- FKTN: Fukutin protein
- GMPPB: GDP-mannose pyrophosphorylase B protein
- ISPD: Isoprenoid synthase domain-containing protein
- LARGE: Acetylglucosaminyltransferase-like protein
- POMT1: O-mannosyltransferase 1 protein
- POMT2: O-mannosyltransferase 2 protein
- POMGNT1: O-mannose beta-1,2-N-acetylglucosaminyltransferase protein
- POMGNT2: O-mannose beta-1,4-N-acetylglucosaminyltransferase 2 protein
- POMK: Protein-O-mannose kinase
- TMEM5: Transmembrane protein 5
The proteins produced from the genes listed above and others involved in Walker-Warburg syndrome modify a protein called alpha (α)-dystroglycan; this modification, called glycosylation, is required for α-dystroglycan to function. The α-dystroglycan protein helps anchor the structural framework inside each cell (cytoskeleton) to the lattice of proteins and other molecules outside the cell (extracellular matrix). In skeletal muscles, the anchoring function of glycosylated α-dystroglycan helps stabilize and protect muscle fibers. In the brain, it helps direct the movement (migration) of nerve cells (neurons) during early development.
Mutations in the genes associated with Walker-Warburg syndrome prevent glycosylation of α-dystroglycan, which disrupts its normal function. Without functional α-dystroglycan to stabilize muscle cells, muscle fibers become damaged as they repeatedly contract and relax with use. The damaged fibers weaken and die over time, leading to progressive weakness of the skeletal muscles.
Defective α-dystroglycan also affects the migration of neurons during the early development of the brain. Instead of stopping when they reach their intended destinations, some neurons migrate past the surface of the brain into the fluid-filled space that surrounds it. Researchers believe that this problem with neuronal migration causes cobblestone lissencephaly in children with Walker-Warburg syndrome. Less is known about the effects of the gene mutations in other parts of the body, including the eyes.
Because Walker-Warburg syndrome involves a malfunction of α-dystroglycan, this condition is classified as a dystroglycanopathy.
Recent advances in genetic research and techniques, such as whole genome and whole exome sequencing technology, have permitted the identification and association of the above genes with Walker Warburg syndrome. The discovery of these genes and the characterization of symptoms they cause when mutated has revealed notable variability in the clinical presentation of Walker Warburg syndrome in affected individuals. Although the 14 above-mentioned genes have been identified as causes of Walker Warburg syndrome, they explain only half of known Walker Warburg syndrome cases, and mutations in all these genes can also cause less severe forms of dystroglycanopathies. Despite the numerous known genetic causes of Walker Warburg syndrome, genetic testing might not reveal the causative gene in every family. Therefore, it is likely that more genes associated with Walker Warburg syndrome and related conditions will be discovered in the future, which could introduce additional variability to the spectrum of Walker Warburg syndrome.
Walker Warburg syndrome inheritance pattern
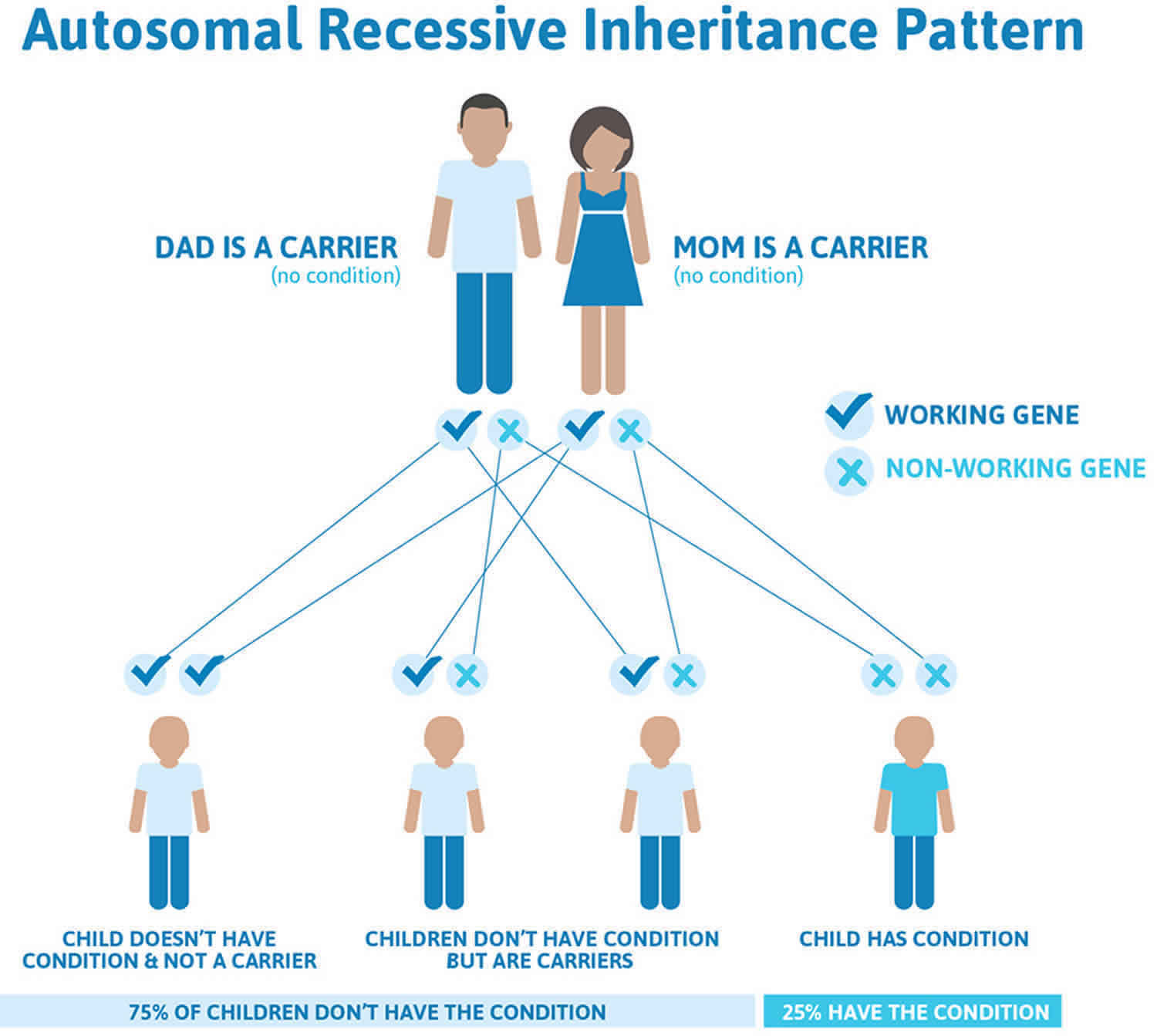
Walker Warburg syndrome is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. Walker Warburg syndrome autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Walker Warburg syndrome symptoms
The main symptoms of Walker Warburg syndrome are muscular dystrophy and abnormalities of the brain and eyes. Symptoms of Walker Warburg syndrome are congenital (present at birth), and some of the brain abnormalities can be detected by prenatal ultrasound and/or fetal MRI in the later stages of pregnancy.
Individuals with Walker Warburg syndrome have congenital muscular dystrophy, or a weakening and loss of muscle at birth. Muscular dystrophy causes affected infants to have severe hypotonia (low muscle tone), which might be noted as “floppy baby” syndrome. Muscle weakness and atrophy (wasting away) typically gets worse over time. Some affected individuals develop abnormally fixed joints (contractures) that occur when thickening and shortening of tissue, such as muscle fibers, deform and restrict movement of an affected area.
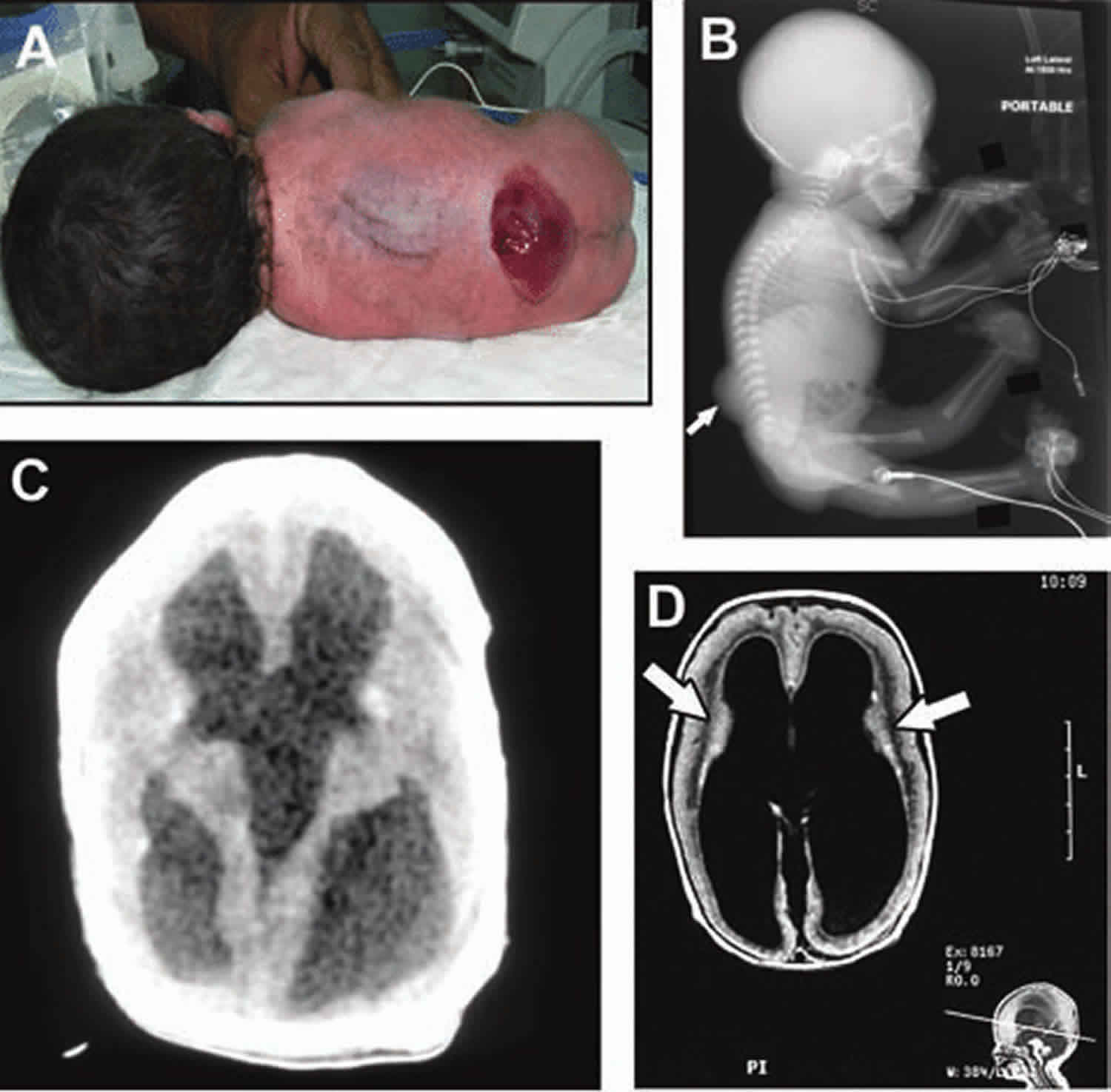
Affected infants usually have a variety of serious brain findings, including lissencephaly (smooth brain), hydrocephalus (enlarged ventricles) and malformations in the back of the brain. The type of lissencephaly involved in Walker Warburg syndrome is described as type 2, or cobblestone lissencephaly. This is because the surface of the brain is smooth and has a cobblestone appearance due to the collection of clumps of brain cells (neurons) at the surface. Hydrocephalus, which is characterized by having too much cerebrospinal fluid in the ventricles of the brain causing an enlargement, can be quite severe and lead to an abnormally large head. Malformations of the back portions of the brain can include underdevelopment (hypoplasia) of the cerebellum and brainstem. The cerebellum helps coordinate voluntary muscle movements; while the brainstem helps control basic functions such as breathing, salivation and heart rate. These posterior malformations can involve an abnormally enlarged space at the back of the brain, sometimes referred to as Dandy-Walker malformation. In some individuals with Walker Warburg syndrome, there is protrusion of part of the brain through the skull bone (encephalocele) and/or absence of the corpus callosum, which is the band of white matter that normally connects the two brain hemispheres.
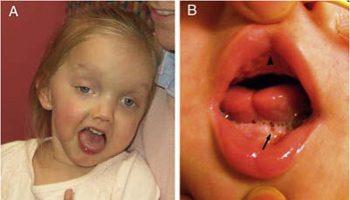
The eye (ocular) abnormalities associated with Walker Warburg syndrome vary widely from person to person and can include any of the following: abnormally small eyes (microphthalmia), absent or underdeveloped optic nerves (optic nerve hypoplasia), malformations of the fluid-filled space within the eyes behind the cornea and in front of the iris, and malformation of the retina, which could cause the retina to become detached (retinal dysplasia). Additional eye symptoms can include cataracts, a cleft or loss of tissue of the retina or iris (colobomas), abnormally large and protruding eyes (buphthalmos), or increased pressure within the eyes (glaucoma). Most of these abnormalities lead to partial or complete blindness.
The brain malformations associated with Walker Warburg syndrome cause serious, life-threatening complications during infancy. Children born with Walker Warburg syndrome display varying degrees of intellectual disability and often have seizures. The combined brain and muscle abnormalities lead to significant delays in reaching developmental milestones (e.g. sitting up, grabbing objects, crawling, talking) and can be so severe as to cause difficulties in breathing and swallowing. Occasionally, additional symptoms in different body systems could also be present. In some affected children, genitourinary abnormalities can occur, causing urinary tract blockage and kidney pelvic dilation (hydronephrosis), or failure of the testes to descend into the scrotum in males (cryptorchidism). Some affected children can have other features, such as low-set or prominent ears, cleft palate or cleft lip.
Walker Warburg syndrome diagnosis
A diagnosis of Walker Warburg syndrome can be suspected via routine ultrasound and/or fetal MRI during the late stages of pregnancy and confirmed at or shortly after birth following thorough clinical evaluation and identification of characteristic findings that might require a variety of tests.
Identification of the brain findings is best made using magnetic resonance imaging (MRI), which provides detailed pictures of the brain. However, the enlarged ventricles that often accompany a diagnosis of Walker Warburg syndrome can also be detected by ultrasound and computed tomography (CT) of the head. A biopsy and microscopic evaluation of muscle tissue might be needed to reveal characteristic changes in muscle fibers. A blood test for creatine kinase (CK) levels is also commonly done, as CK is a measure of the breakdown of muscle tissue. The detection of elevated CK levels can confirm that muscle is damaged or inflamed, but cannot confirm a diagnosis of Walker Warburg syndrome specifically. A careful eye exam can also identify the characteristic eye findings of Walker Warburg syndrome.
Genetic testing can be done in an effort to provide molecular confirmation of a clinical diagnosis of Walker Warburg syndrome. Genetic confirmation occurs when two mutations are identified in a known causative gene. Genetic testing can be done in a variety of ways. One genetic testing method is the sequencing of a single known Walker Warburg syndrome gene and could be done if one particular gene is suspected. It is also possible to test for multiple known Walker Warburg syndrome or CMD genes at once via a multiple gene panel test. Another genetic testing method is called whole exome sequencing, in which every known gene can be analyzed for mutations at once. Each of these methods has benefits and limitations, and individual circumstances might suggest one method over another. If the affected individual is of Ashkenazi Jewish descent, the FKTN gene should be tested first, since a specific mutation in this gene is common in this population. Otherwise, there is significant overlap of symptoms caused by mutations in all known Walker Warburg syndrome genes, so it is difficult to determine a specific order for genetic testing.
Since it is likely that not all genes for Walker Warburg syndrome have yet been identified, genetic testing could be negative. Therefore, a negative result does not exclude a diagnosis of Walker Warburg syndrome, which is made primarily based on clinical symptoms, and in these cases, re-evaluation of genetic testing opportunities (to include newly identified Walker Warburg syndrome genes) could be considered periodically in the future.
Genetic counseling for families can help their understanding of autosomal recessive inheritance, the current and ever-changing state of genetic testing for Walker Warburg syndrome, what the results of genetic testing for a family mean, and the impact of this information on individuals in the family.
Walker Warburg syndrome diagnostic criteria
Walker Warburg syndrome diagnosis is established on the basis of four criteria 3:
- Congenital muscular dystrophy characterized by hypoglycosylation of α-dystroglycan
- High creatine kinase level
- Anterior or posterior eye anomalies
- Migrational brain defect with type II lissencephaly and hydrocephalus
- Abnormal brainstem/cerebellum
The diagnostic criteria for Walker Warburg syndrome were established on the basis of data obtained from 21 patients and an additional 42 patients from the literature 4. Four abnormalities were present in all patients checked for these anomalies: type II lissencephaly (21/21), cerebellar malformation (20/20), retinal malformation (18/18), and congenital muscular dystrophy (14/14). Two other frequently observed abnormalities, ventricular dilatation with or without hydrocephalus (20/21) and anterior chamber malformation (16/21), were helpful but not obligatory for diagnosis because they were not constant. All other abnormalities occurred less frequently. Congenital macrocephaly with hydrocephalus (11/19) was more common than congenital microcephaly (3/19). Dandy-Walker malformation (10/19) was sometimes associated with posterior cephalocele (5/21). Additional abnormalities included slit-like ventricles (1/21), microphthalmia (8/21), ocular colobomas (3/15), congenital cataracts (7/20), and genital anomalies in males (5/8).
Walker Warburg syndrome treatment
There is no cure for Walker Warburg syndrome at this time and treatment is individualized based on specific symptoms. Medical management can require the coordinated efforts of a team of specialists including pediatricians, geneticists, orthopedic surgeons, neurologists, eye specialists, and other health care professionals to systematically and comprehensively plan an affected child’s treatment.
Treatments might include anti-seizure medication, surgery for hydrocephalus, such as the implantation of shunts to drain excess cerebrospinal fluid and reduce pressure, and physical therapy to improve muscle strength and prevent contractures. Some affected infants might need a gastric tube to assist with feeding difficulties. Other symptomatic and supportive treatments could also be necessary. Due to the severe brain and muscle abnormalities, life expectancy of children with classic Walker Warburg syndrome is always reduced.
Walker Warburg syndrome prognosis
Walker Warburg syndrome is usually lethal within the first few months of life, with almost all children dying by the age of three.
- Vajsar, J., Schachter, H. Walker-Warburg syndrome. Orphanet J Rare Dis 1, 29 (2006). https://doi.org/10.1186/1750-1172-1-29[↩][↩]
- Walker-Warburg syndrome. https://ghr.nlm.nih.gov/condition/walker-warburg-syndrome[↩][↩]
- Vajsar J, Schachter H. Walker-Warburg syndrome. Orphanet J Rare Dis. 2006;1:29. Published 2006 Aug 3. doi:10.1186/1750-1172-1-29 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1553431[↩]
- Dobyns WB, Pagon RA, Armstrong D, Curry CJ, Greenberg F, Grix A, Holmes LB, Laxova R, Michels VV, Robinow M, Zimmerman RL. Diagnostic criteria for Walker-Warburg syndrome. Am J Med Genet. 1989;32:195–210. doi: 10.1002/ajmg.1320320213[↩]