Lofgren syndrome
Lofgren syndrome or Löfgren syndrome is a clinically distinct phenotype of sarcoidosis 1. Sarcoidosis is a multisystem granulomatous disorder of unknown cause that commonly involves the lungs with the second most commonly affected organ being the skin 2. Cutaneous manifestations of sarcoidosis are seen in up to 33% of patients and may be the first clinical sign of Lofgren syndrome 3. In contrast to the often-insidious onset, slow disease progression and chronic disease course typical of sarcoidosis, Lofgren’s syndrome presents acutely. It typically presents in younger patients with acute onset erythema nodosum, bilateral hilar lymphadenopathy, fever, and migratory polyarthritis, and without granulomatous skin involvement. Lofgren’s syndrome portends a favorable prognosis 4.
Lofgren syndrome consistently appears to be more common in females. It occurs most commonly in European Caucasians, especially patients with Scandinavian descent. Young to middle age adults are more likely to present with this disease with a median age of 37 years old. Specific manifestations of the components of Lofgren syndrome differ based on gender, whereas erythema nodosum is found predominantly in women and arthropathy/arthritis is seen more commonly in men. It classically occurs in the spring months.
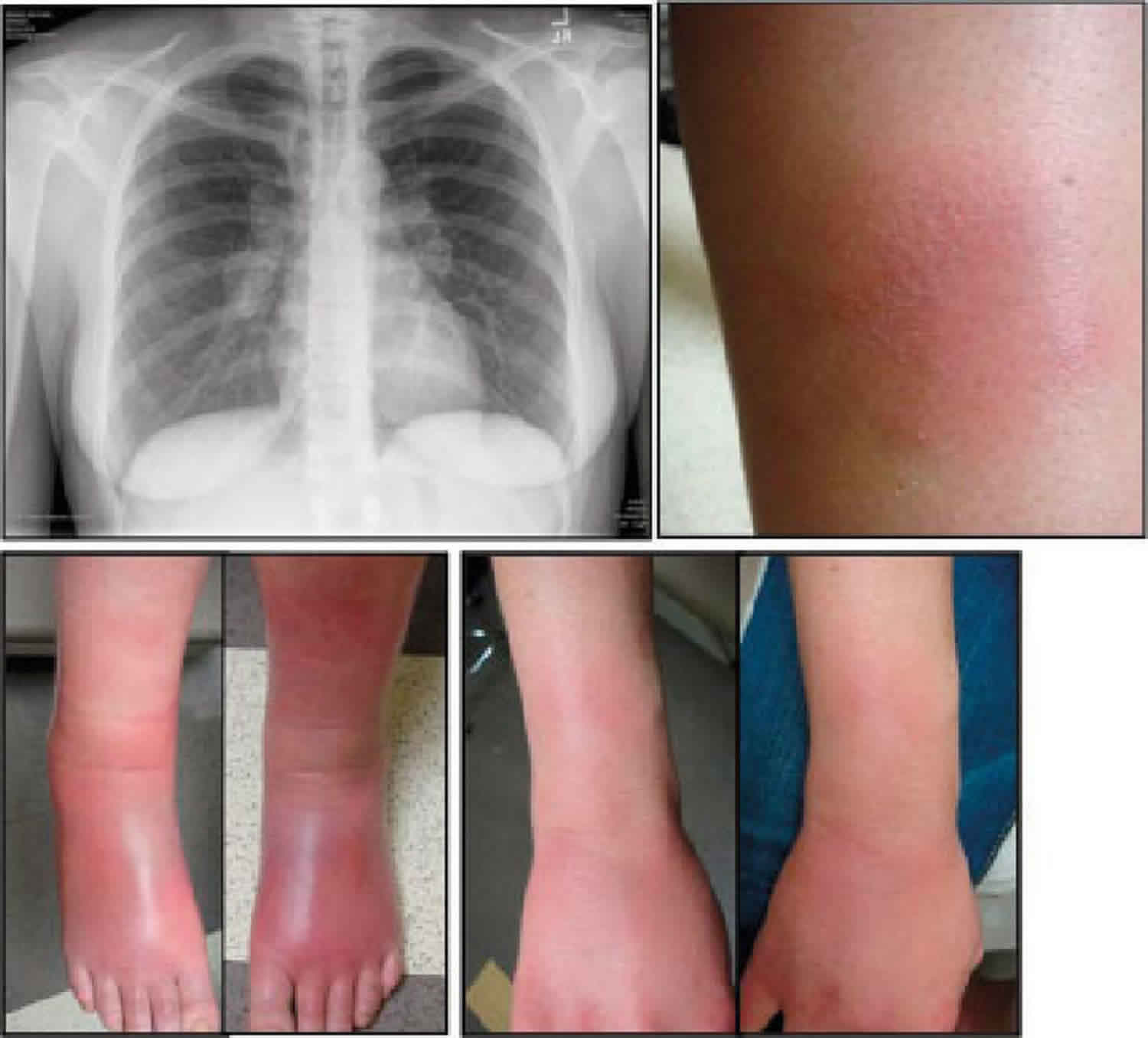
Figure 1. Lofgren syndrome
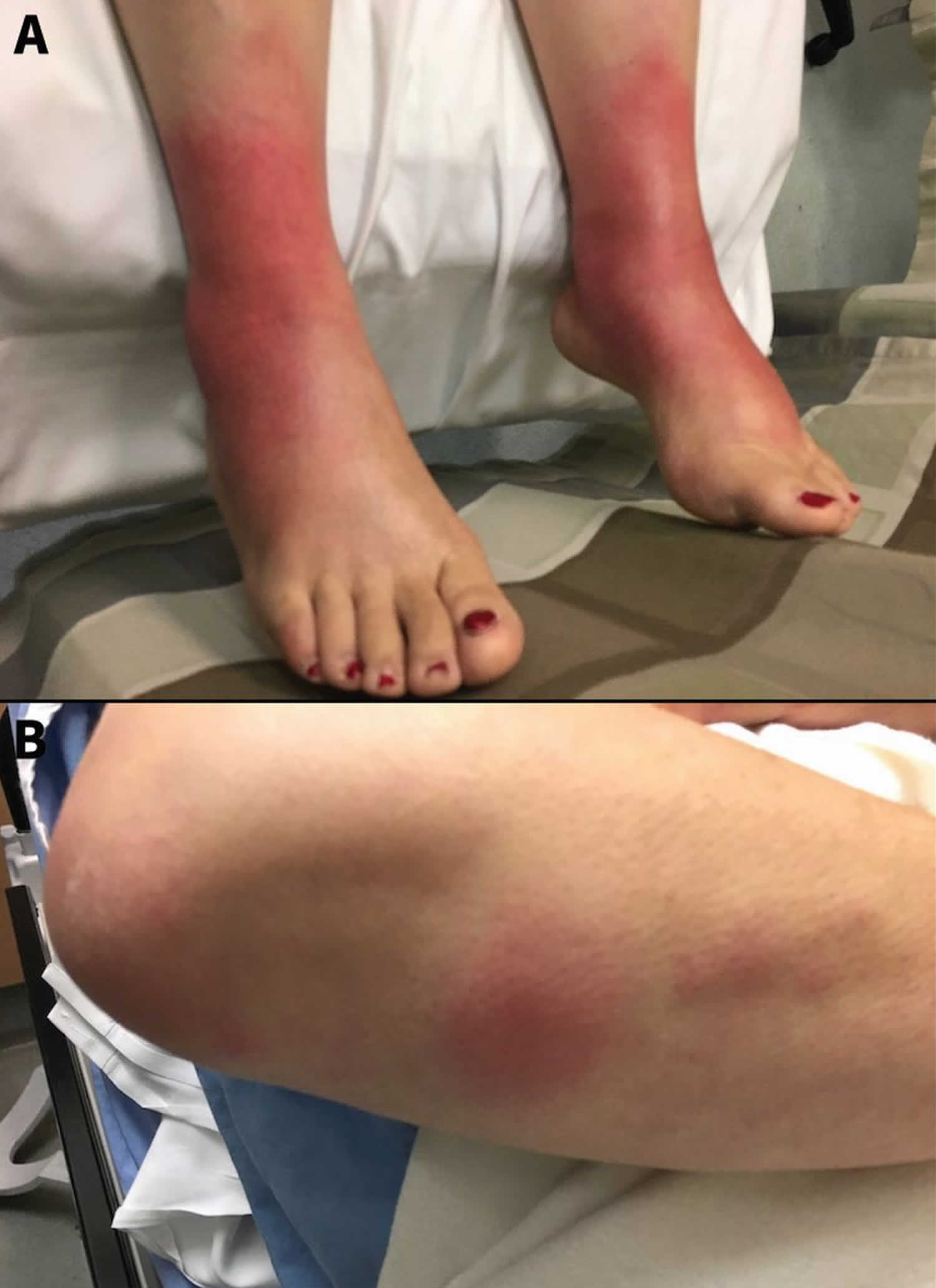
Footnote: A 61-year-old woman with (A) juxta-articular erythema and edema of both ankles characteristic of periarthritis, and multiple raised erythematous and tender nodules located over the extensor surfaces of her hands and (B) forearms that were consistent with erythema nodosum.
[Source 5 ]Lofgren syndrome causes
The cause and pathophysiology of Lofgren syndrome sarcoidosis are poorly understood and attributable to both genetic and environmental factors. Though acute sarcoidosis is not infectious, there is some thought that selected cases may be due to abnormal host reaction to exposure to one or more infective agents, such as Mycobacterium tuberculosis or Propionibacterium or environmental exposures, such as beryllium, dust, or other occupational agents 6.
In general, sarcoidosis is thought to be a polygenic disorder. The impact of HLA alleles on the pathogenesis of sarcoidosis varies significantly, depending upon disease subtype and racial group. In the case of Lofgren syndrome, HLA-B8/DR3 has a strong association with the disease and also with overall disease resolution 7.
Despite a rigorous investigation, research has not unveiled a cause or proven pathophysiologic mechanism. The hypothesis is that hosts may have a genetic predisposition but still require exposure to a specific antigen whether it be exogenous or endogenous. This trigger then activates macrophages and ultimately leads to an exaggerated cellular immune response leading to granuloma formation. The pathogenesis is very complex and not yet well understood. At the core of the process, T cells play a dominant role in this immune reaction. Studies have shown that granulomas and bronchioloalveolar lavage samples from patients with pulmonary sarcoidosis shown mixed infiltrate with prominent lymphocytosis 8.
The majority of lymphocytes within sarcoidal granulomas are CD4+T helper 1 (Th1) lymphocytes, contributing to an overall elevated CD4/CD8 ratio. CD4+T cells secrete interleukin (IL)-2, IL-12, IL-18, and interferon (IFN)-gamma, which in combination with the release of tumor necrosis factor (TNF)-alpha by macrophages and some CD8+T cells, leads to persistent Th1 activity, persistent IFN-gamma elevation and macrophage accumulation within the tissue. Compartmentalization of granuloma-forming CD4+ T lymphocytes and monocytes/macrophages within peripheral tissues leads to systemic lymphopenia and decreased systemic CD4/CD8 ratio.
Despite this understanding, the evidence is emerging noting bronchioloalveolar lavage samples may have CD4 to CD8 ratio is of less importance because it can increase, be normal, and even decrease.[10] Studies have also noted patients with active sarcoidosis with elevated Th17 to Treg ratio in the peripheral blood and bronchoalveolar lavage fluid 9.
Lofgren’s syndrome symptoms
Lofgren syndrome is a constellation of the following findings: erythema nodosum, bilateral hilar lymphadenopathy, fever, and migratory polyarthritis 4. It occasionally also presents with uveitis 4.
The arthritis of Lofgren syndrome occurs more often in men, and with periarticular inflammation involving soft tissue and tenosynovitis rather than true arthritis. It typically involves ankles symmetrically but can also involve knees, wrists, and elbow.
Erythema nodosum presents as painful, bright red, subcutaneous nodules that are typically symmetric on the anterior shins, in contrast to macular/papular sarcoidosis which has a predilection for sites of repetitive trauma. These lesions along with the fever typically remit spontaneously within 6 weeks and may resolve with post-inflammatory hyperpigmentation but without scarring or atrophy. Resolution of lymphadenopathy may delay, taking up to one year, but does resolve completely in 90% of cases.
Lofgren syndrome diagnosis
The diagnosis of sarcoidosis is complex with no definitive test exists. It requires clinical-radiographic correlation, exclusion of alternative disease that can induce granuloma formation, such as tuberculosis, and histologic detection of noncaseating granulomas. There are several specific clinical situations where a presumptive diagnosis can be made based on clinic radiographic findings alone, and biopsy is not considered necessary 10. Bilateral hilar lymphadenopathy, migratory polyarthralgia, erythema nodosum, and fevers of Lofgren syndrome is one such example 11.
Chest radiography is still necessary to make this diagnosis though may see a variety of alternative pathology other than classic bilateral hilar adenopathy, including unilateral hilar adenopathy or right paratracheal lymphadenopathy with or without pulmonary involvement.
Should disease deviate from the classic course or fail to resolve within expected time definitive histologic diagnosis may be necessary. The first step in diagnosis should be to determine the most appropriate site for biopsy. Of note, lesions of erythema nodosum will not show sarcoidal granulomas and are therefore not useful biopsy sites to establishing the diagnosis. A comprehensive physical examination should be performed with extra caution taken to throughout examine periorificial sites, including parotid glands, lacrimal glands, and conjunctiva, extremities, sites of previous trauma or tattoos, and full lymph node assessment. Without an easily accessible superficial biopsy site, patients may require a more invasive biopsy method. Alternatively, minimally invasive methods have emerged including flexible bronchoscopy with bronchoalveolar lavage (BAL), endobronchial biopsy, and transbronchial biopsy to assist in the diagnosis of sarcoidosis. Several studies have noted the increased amount of activated CD4+ Th1 cells, decreased CD8+ T cells and an increase in IgG-secreting plasma cells. Consequently, a CD4/CD8 ratio greater than 3.5 has been shown to have a 94% specificity for the diagnosis of sarcoidosis.
Other lab tests are not typically required and remain quite non-specific. Findings may include systemic lymphopenia, elevated levels of calcium, alkaline phosphatase, C-reactive protein (CRP), acetylcholinesterase (ACE), or gamma globulin (polyclonal). Tuberculin skin test or measurement of IFN-gamma in undiluted plasma should be performed in all patient to exclude the diagnosis of tuberculosis which can mimic both erythema nodosum and sarcoidosis 12. There are reports of thyroid dysfunction in association with cutaneous disease, consider the evaluation of thyroid function following definitive diagnosis 13.
Lofgren syndrome treatment
Treatment of Lofgren syndrome is typically supportive with spontaneous resolution occurring over 1 to 2 years. Constitutional symptoms and arthralgias are treatable with non-steroidal anti-inflammatory drug or colchicine. In rare, severe cases may warrant corticosteroids use which can usually be tapered quickly over a few weeks to months 14.
Lofgren syndrome prognosis
Lofgren syndrome portends an excellent prognosis is associated with a greater than 90% chance of spontaneous remission within 2 years.
- Brown F, Tanner LS. Lofgren Syndrome. [Updated 2019 Jul 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482315[↩]
- Siltzbach LE, James DG, Neville E, Turiaf J, Battesti JP, Sharma OP, Hosoda Y, Mikami R, Odaka M. Course and prognosis of sarcoidosis around the world. Am. J. Med. 1974 Dec;57(6):847-52.[↩]
- Wanat KA, Rosenbach M. Cutaneous Sarcoidosis. Clin. Chest Med. 2015 Dec;36(4):685-702.[↩]
- Ponhold W. [The Löfgren syndrome: acute sarcoidosis (author’s transl)]. Rontgenblatter. 1977 Jun;30(6):325-7.[↩][↩][↩]
- Löfgren syndrome in acute sarcoidosis. Alexandra P. Saltman, Bindee Kuriya. CMAJ Oct 2017, 189 (39) E1230; https://doi.org/10.1503/cmaj.170547[↩]
- Ferrara G, Valentini D, Rao M, Wahlström J, Grunewald J, Larsson LO, Brighenti S, Dodoo E, Zumla A, Maeurer M. Humoral immune profiling of mycobacterial antigen recognition in sarcoidosis and Löfgren’s syndrome using high-content peptide microarrays. Int. J. Infect. Dis. 2017 Mar;56:167-175.[↩]
- Spagnolo P, Sato H, Grunewald J, Brynedal B, Hillert J, Mañá J, Wells AU, Eklund A, Welsh KI, du Bois RM. A common haplotype of the C-C chemokine receptor 2 gene and HLA-DRB1*0301 are independent genetic risk factors for Löfgren’s syndrome. J. Intern. Med. 2008 Nov;264(5):433-41.[↩]
- Drent M, Mansour K, Linssen C. Bronchoalveolar lavage in sarcoidosis. Semin Respir Crit Care Med. 2007 Oct;28(5):486-95.[↩]
- Mortaz E, Rezayat F, Amani D, Kiani A, Garssen J, Adcock IM, Velayati A. The Roles of T Helper 1, T Helper 17 and Regulatory T Cells in the Pathogenesis of Sarcoidosis. Iran J Allergy Asthma Immunol. 2016 Aug;15(4):334-339.[↩]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 1999 Aug;160(2):736-55.[↩]
- MAYOCK RL, BERTRAND P, MORRISON CE, SCOTT JH. MANIFESTATIONS OF SARCOIDOSIS. ANALYSIS OF 145 PATIENTS, WITH A REVIEW OF NINE SERIES SELECTED FROM THE LITERATURE. Am. J. Med. 1963 Jul;35:67-89.[↩]
- LOFGREN S, LUNDBACK H. The bilateral hilar lymphoma syndrome; a study of the relation to tuberculosis and sarcoidosis in 212 cases. Acta Med Scand. 1952 Mar 19;142(4):265-73.[↩]
- Anolik RB, Schaffer A, Kim EJ, Rosenbach M. Thyroid dysfunction and cutaneous sarcoidosis. J. Am. Acad. Dermatol. 2012 Jan;66(1):167-8.[↩]
- Zisman DA, Shorr AF, Lynch JP. Sarcoidosis involving the musculoskeletal system. Semin Respir Crit Care Med. 2002 Dec;23(6):555-70.[↩]