Septo optic dysplasia
Septo-optic dysplasia also called De Morsier syndrome, is a disorder of early brain and eye development. Although septo optic dysplasia signs and symptoms vary, this condition is traditionally defined by three characteristic features: underdevelopment (hypoplasia) of the eye (optic) nerve, abnormal formation of structures along the midline of the brain such as the absence of the septum pellucidum and the corpus callosum, and a small pituitary (pituitary hypoplasia) 1. Signs and symptoms may include blindness in one or both eyes, an abnormal pupil dilation in response to light, nystagmus (abnormal movement of the eyes), low muscular tone and hormonal problems. Additional features may include recurring seizures, delayed development, intellectual disability, jaundice (yellow-ish skin), precocious puberty, short stature, sleep problems, obesity, lack of smell (anosmia), hearing loss and heart anomalies 2.
The first major feature, optic nerve hypoplasia, is the underdevelopment of the optic nerves, which carry visual information from the eyes to the brain. In affected individuals, the optic nerves are abnormally small and make fewer connections than usual between the eyes and the brain. As a result, people with optic nerve hypoplasia have impaired vision in one or both eyes. Optic nerve hypoplasia can also be associated with unusual side-to-side eye movements (nystagmus) and other eye abnormalities.
The second characteristic feature of septo-optic dysplasia is the abnormal development of structures separating the right and left halves of the brain. These structures include the corpus callosum, which is a band of tissue that connects the two halves of the brain, and the septum pellucidum, which separates the fluid-filled spaces called ventricles in the brain. In the early stages of brain development, these structures may form abnormally or fail to develop at all. Depending on which structures are affected, abnormal brain development can lead to intellectual disability and other neurological problems.
The third major feature of this disorder is pituitary hypoplasia. The pituitary is a gland at the base of the brain that produces several hormones. These hormones help control growth, reproduction, and other critical body functions. Underdevelopment of the pituitary can lead to a shortage (deficiency) of many essential hormones. Most commonly, pituitary hypoplasia causes growth hormone deficiency, which results in slow growth and unusually short stature. Severe cases cause panhypopituitarism, a condition in which the pituitary produces no hormones. Panhypopituitarism is associated with slow growth, low blood sugar (hypoglycemia), genital abnormalities, and problems with sexual development.
The signs and symptoms of septo-optic dysplasia can vary significantly. Some researchers suggest that septo-optic dysplasia should actually be considered a group of related conditions rather than a single disorder. About one-third of people diagnosed with septo-optic dysplasia have all three major features; most affected individuals have two of the major features. In rare cases, septo-optic dysplasia is associated with additional signs and symptoms, including recurrent seizures (epilepsy), delayed development, and abnormal movements.
Septo-optic dysplasia has a reported incidence of 1 in 10,000 newborns 1.
Although the cause is unknown in most cases, very few people with septo optic dysplasia may have variations (mutations) in the HESX1 OTX2, SOX2 or SOX3 genes 3. Other factors that may be involved are viral infections, certain medications and a blood flow disruption 4. Typically, people do not have a family history of septo-optic dysplasia. However, there have been a few cases in which multiple family members have been diagnosed. In these cases inheritance can be autosomal recessive or autosomal dominant 5. Although there is no cure for this condition, the treatment is directed toward the specific symptoms in each individual. Children with possible septo optic dysplasia must be kept under careful hormonal follow-up, and, if present, hormone deficiencies should be treated with hormone replacement therapy 3.
I have septo-optic dysplasia. I have not had a sense of smell (anosmia) for as long as I can remember. Could this be connected to the septo-optic dysplasia?
Yes, anosmia has been associated with septo optic dysplasia. Septo-optic dysplasia is a clinically diverse condition. In addition to the characteristic findings of optic nerve hypoplasia, pituitary hormone abnormalities and midline brain malformations, many patients have associated findings. In addition to the symptoms mentioned above, individuals with septo-optic dysplasia may have anosmia, diabetes insipidus, sleep disorders, autism spectrum disorders, precocious puberty, obesity, temperature regulation issues, sensorineural hearing loss, and birth defects affecting the heart, hands, and feet 6.
Are behavioral difficulties common among people with septo-optic dysplasia?
While limited information is available on this topic, one recent study found that children with septo-optic dysplasia are at an increased risk for having social, communication, and repetitive/restrictive behavioral difficulties 7. The following characteristics have been described as being common among children with septo-optic dysplasia, especially among those with severe vision loss 8:
- Moderate to severe delays in information processing
- Extreme tactual and auditory defensiveness
- Difficulty with transitions
- Rigid adherence to routine
- Strong interest in rhythms and music
- Restricted food preferences and eating problems related to an aversion to textured foods
- Avoidance of social interaction and engagement
- Profound distractibility
- Mild hypotonia (low muscle tone)
- Developmental delays in motor functioning
- Lack of initiative in exploring their environments
- Atypical language development
- Enjoyment of swinging
- Lack of spontaneity in verbal interactions
Septo optic dysplasia causes
In most cases of septo-optic dysplasia, the cause of the disorder is unknown. Researchers suspect that a combination of genetic and environmental factors may play a role in causing this disorder. Proposed environmental risk factors include viral infections, specific medications, and a disruption in blood flow to certain areas of the brain during critical periods of development.
At least three genes have been associated with septo-optic dysplasia, although mutations in these genes appear to be rare causes of septo-optic dysplasia. The three genes, HESX1, OTX2, and SOX2, all play important roles in embryonic development. In particular, they are essential for the formation of the eyes, the pituitary gland, and structures at the front of the brain (the forebrain) such as the optic nerves. Mutations in any of these genes disrupt the early development of these structures, which leads to the major features of septo-optic dysplasia.
Researchers are looking for additional genetic changes that contribute to septo-optic dysplasia.
Currently genetic abnormalities are identified in <1% of the patients, with the cause of septo optic dysplasia remaining unclear in the majority of patients. This has led to debate as to other factors, which may also have a role in the pathogenesis of septo optic dysplasia. Many studies have reported an increased prevalence of antenatal drug and alcohol abuse and younger maternal age in septo optic dysplasia cohorts 9. This in combination with the pattern of neurological abnormalities found in individuals with septo optic dysplasia led Lubinsky 10 to suggest that septo-optic dysplaisa occurs secondary to a vascular disruption sequence 11. It is likely that although other genetic abnormalities are likely to be identified in the future, environmental factors such as drugs, alcohol and anterior cerebral artery supply during the neonatal period may also play a significant role in the aetiology of septo optic dysplasia.
Septo-optic dysplasia inheritance pattern
Septo-optic dysplasia is usually sporadic, which means that the condition typically occurs in people with no history of the disorder in their family.
Less commonly, septo-optic dysplasia has been found to run in families. Most familial cases appear to have an autosomal recessive pattern of inheritance, which means that both copies of an associated gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition. In a few affected families, the disorder has had an autosomal dominant pattern of inheritance, which means one copy of an altered gene in each cell is sufficient to cause the condition.
Septo optic dysplasia symptoms
Typically, the symptoms develop in 3 organs, the brain (which have abnormal formation of midline structures), the eyes (due to optic nerve hypoplasia), and pituitary (due to hypoplasia. About one third of the patients present with all of the three main features. However, some symptoms may not appear until childhood or later. Symptoms may include 12:
- Blindness in one or both eyes
- Pupil dilation in response to light
- Nystagmus (a rapid, involuntary to-and-fro movement of the eyes)
- Inward and outward deviation of the eyes
- Hypotonia (low muscle tone)
- Seizures
Other common features are 12:
- Short stature due to lack of growth hormone deficiency (the most common hormonal abnormality)
- Abnormal thirst, hunger, and body temperature due to underdevelopment of the hypothalamus, a region of the brain responsible for regulating basic body functions.
- Low blood sugar
- Genital abnormalities
- Problems with sexual development or precocious puberty
- Sleep difficulties
- Obesity
- Jaundice
- Intellectual disability or learning disabilities
- Developmental delay related to vision impairment or neurological problems
- Anosmia
- Heart problems
Pituitary hormone insufficiencies may evolve over time necessitating life-long medical follow-up 2.
Septo optic dysplasia diagnosis
Septo-optic dysplasia can present at birth in association with multiple congenital abnormalities or much later on when growth failure occurs in a child also noted to have visual abnormalities (although not necessarily as may just have growth failure with mild undetected visual abnormalities) 13. The child may present with strabismus, nystagmus, or other visual abnormalities. In the majority of cases, the earlier the diagnosis is made the better the outcome, as untreated hormonal abnormalities place an additional neurodevelopmental burden on a child already compromised by visual impairment, and also place the patient at risk of hypoglycaemia, adrenal crises and consequently death. Clinically the diagnosis should be suspected in newborns with hypoglycaemia, jaundice, a microphallus with or without undescended testes and nystagmus with or without associated midline abnormalities such as cleft palate. In these infants, baseline endocrine tests should be performed as outlined below and an ophthalmology referral made. MRI brain images in conjunction with dynamic studies of pituitary function can then be used to confirm the diagnosis. Hormonal insufficiencies should be treated, and early referral both to a neurodevelopmental team with an interest in visual impairment and to a tertiary centre with expertise in managing these children made. A summary of key points to note while taking the history, undertaking ophthalmology assessment, interpreting MRI brain images and investigating pituitary function are outlined below.
Associated features to ask about when taking history 14:
- Consanguinity.
- Hearing abnormality – sensorineural.
- Developmental delay.
- Anosmia.
- Other congenital anomalies such as cardiac, oesophageal atresia and micropenis.
- Seizures – commonly related to underlying abnormality of the brain anatomy, important to rule out hypoglycaemia as a cause.
- Stereotypical behaviours – for example, mannerisms suggestive of a diagnosis of autistic spectrum disorder.
- Sleep disorder – arrhythmicity 15.
- Excessive appetite.
- Temperature instability.
- Symptoms suggestive of hormonal deficiencies such as fatigue, polyuria and polydipsia, constipation, dry skin, hair loss and sweating.
Ophthalmology
- Is there strabismus or nystagmus, and if so is it unilateral or bilateral?
- Perform ophthalmological examination to identify clinical signs of optic nerve hypoplasia, which include a double-ring sign, pale and/or small optic discs/neuroretinal rim area.
- Are optic nerve hypoplasia or dysplasia, microphthalmia, coloboma and/or other ophthalmological signs present?
- Assess degree of visual impairment.
MRI of the brain
- Are hypothalamo-pituitary axis appearances normal, checking specifically for:
- Anterior pituitary size.
- Presence and location of posterior pituitary.
- Presence and thickness of infundibulum.
- Is the septum pellucidum present?
- Are the optic nerves and chiasm normal?
- Is the appearance of the corpus callosum normal?
- Are there any other associated brain abnormalities such as schizencephaly, cavum septum pellucidum, cerebellar hypoplasia and aplasia of the fornix.
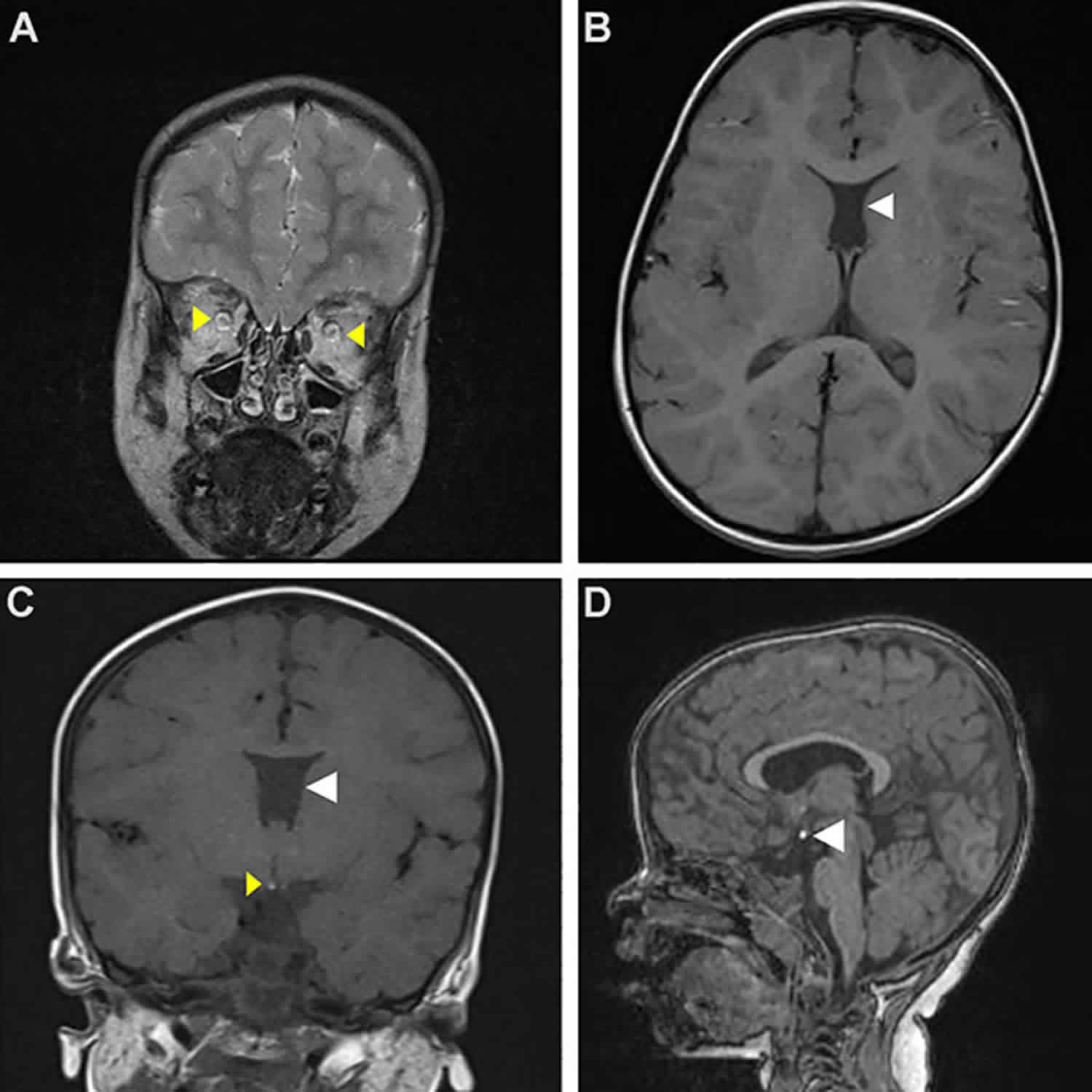
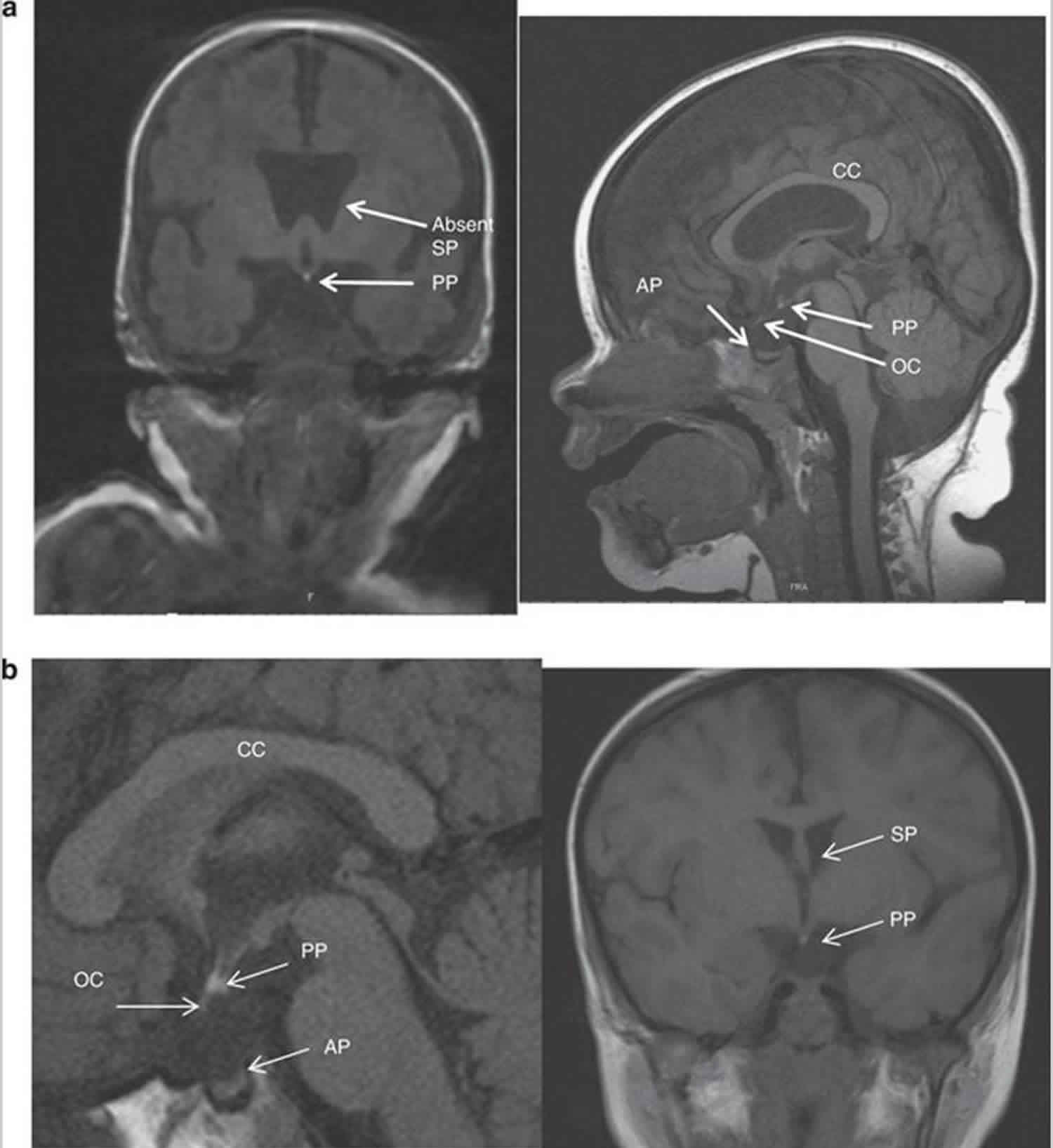
Classically MRI brain images show hypoplasia of the optic nerves (unilateral or bilateral) and optic chiasm, agenesis of the septum pellucidum, abnormalities of the corpus callosum and hypothalamo-pituitary axis (Figure 1). However, MRI findings vary considerably between patients 16.
Figure 1. Septo optic dysplasia MRI
Footnote: MRI appearances of septo-optic dysplasia. (a) Anterior pituitary hypoplasia and absent infundibulum associated with bilateral optic nerve hypoplasia. The posterior pituitary is ectopic. There is an absence of the septum pellucidum. (b) Ectopic posterior pituitary, anterior pituitary hypoplasia, absence of the infundibulum and partial absence of the septum pellucidum associated with optic nerve hypoplasia.
Abbreviations: CC = corpus callosum; AP = anterior pituitary; PP = posterior pituitary; SP = septum pellucidum; OC = optic chiasm.
[Source 14 ]Tests of pituitary function
- Thyroid function (low TSH of secondary hypothyroidism may be missed by neonatal screening).
- Cortisol
- Random cortisol sufficient in neonate, 8 am cortisol appropriate in individuals aged >1 year.
- If abnormal cortisol, proceed to synacthen test or 24-h cortisol and glucose profile.
- Growth hormone
- Ask for symptoms of hypoglycaemia and measure glucose profile on adequate hydrocortisone replacement. If hypoglycaemia detected measure IGF-1 and IGFBP-3, and consider GH replacement.
- Monitor growth and if poor growth velocity measure GH response to provocation testing in patients over the age of one year.
- Monitor pubertal status – septo optic dysplasia can be associated with precocious puberty secondary to hypothalamic dysfunction, or hypogonadotrophic hypogonadism secondary to LH and FSH deficiency.
- Ask about fluid intake: if excessive, measure paired fasting plasma and urine osmolalities; may need to perform water deprivation test to confirm diagnosis of diabetes insipidus.
Septo optic dysplasia treatment
Patients with septo optic dysplasia need regular (at least six-monthly) ongoing follow-up by a multi-disciplinary team. Treatment is directed toward the specific symptoms in each individual and may require a team of specialists including pediatricians, ophthalmologists, neurologists, and endocrinologists. If present, hormone deficiencies may be treated with hormone replacement therapy. Vision problems are generally not treatable. Special services that may be useful include vision, physical, and occupational therapies 17.
Septo optic dysplasia life expectancy
The life expectancy for individuals with septo-optic dysplasia varies according to the presence and severity of symptoms. Early diagnosis and treatment of hormone deficiencies (when present) allows for a better outcome for some associated symptoms 17.
- Septo-optic dysplasia. https://ghr.nlm.nih.gov/condition/septo-optic-dysplasia[↩][↩]
- Webb EA & Dattani MT. Septo-optic dysplasia. European Journal of Human Genetics. 2010; 18(4):393-397. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2987262[↩][↩]
- Saranac L & Gucev Z. New insights into septo-optic dysplasia. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2014; 35(1):123-7. https://www.ncbi.nlm.nih.gov/pubmed/24802313[↩][↩]
- Garne E, Rissmann A, Addor MC et al. Epidemiology of septo-optic dysplasia with focus on prevalence and maternal age – A EUROCAT study.. Eur J Med Genet. May 10, 2018; 1769-7212(18):30149-6. https://www.ncbi.nlm.nih.gov/pubmed/29753093[↩]
- Optic Nerve Hypoplasia. https://rarediseases.org/rare-diseases/optic-nerve-hypoplasia[↩]
- Septo-optic dysplasia spectrum. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=3157[↩]
- Parr JR, Dale NJ, Shaffer LM, Salt AT. Social communication difficulties and autism spectrum disorder in young children with optic nerve hypoplasia and/or septo-optic dysplasia. ev Med Child Neurol. October 2010; 52(10):917-921. http://www.ncbi.nlm.nih.gov/pubmed?term=20370811[↩]
- Bahar C, Brody J, McCann ME, Mendiola R, Slott G.. A Multidisciplinary Approach to Educating Preschool Children with Optic Nerve Hypoplasia and Septo-Optic Nerve Dysplasia. RE: view. 2003; 35(1):15. https://eric.ed.gov/?id=EJ672974[↩]
- Riedl S, Vosahlo J, Battelino T, et al. Refining clinical phenotypes in septo-optic dysplasia based on MRI findings. Eur J Pediatr. 2008;167:1269–1276.[↩]
- Lubinsky MS. Hypothesis: septo-optic dysplasia is a vascular disruption sequence. Am J Med Genet. 1997;69:235–236.[↩]
- Stevens CA, Dobyns WB. Septo-optic dysplasia and amniotic bands: further evidence for a vascular pathogenesis. Am J Med Genet A. 2004;125A:12–16.[↩]
- Septo-optic dysplasia spectrum. https://rarediseases.info.nih.gov/diseases/7627/septo-optic-dysplasia-spectrum[↩][↩]
- Hellstrom A, Aronsson M, Axelson C, et al. Children with septo-optic dysplasia – how to improve and sharpen the diagnosis. Horm Res. 2000;53 Suppl1:19–25.[↩]
- Webb EA, Dattani MT. Septo-optic dysplasia. Eur J Hum Genet. 2010;18(4):393–397. doi:10.1038/ejhg.2009.125 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2987262[↩][↩]
- Rivkees SA. Arrhythmicity in a child with septo-optic dysplasia and establishment of sleep-wake cyclicity with melatonin. J Pediatr. 2001;139:463–465.[↩]
- Mehta A, Hindmarsh PC, Mehta H, et al. Congenital hypopituitarism: clinical, molecular and neuroradiological correlates Clin Endocrinole-pub ahead of print, 6 March 2009.[↩]
- Septo-Optic Dysplasia Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Septo-Optic-Dysplasia-Information-Page[↩][↩]