Clonal hematopoiesis of indeterminate potential
Clonal hematopoiesis of indeterminate potential (CHIP) is defined as the presence of clonal hematopoiesis in the absence of cytopenias 1. CHIP is also sometimes known as age-related clonal hematopoiesis (ARCH). CHIP is a new entity in which somatic mutations are found in cells of the blood or bone marrow, but they do not have any cytopenias and do not meet the criteria for a hematologic malignancy 2. Specifically, CHIP requires the absence of morphological evidence of a hematologic neoplasm, the absence of criteria for paroxysmal nocturnal hemoglobinuria, monoclonal B-cell lymphocytosis, and monoclonal gammopathy of undetermined significance (MGUS), and the presence of a somatic mutation associated with hematologic neoplasm at a variant allele frequency of at least 2% 3. Variant allele frequency is defined as the number of reads containing the variant of interest divided by the total number of reads, is associated with somatic variants and can also be informative 4. Cases of cytopenia and clonal hematopoiesis are described as clonal cytopenias of undetermined significance (CCUS). CHIP clonal hematopoiesis and idiopathic cytopenias of undetermined significance (ICUS) have some degree of overlap (Table 1 and Figure 1). The most common mutation is on the DNMT3A gene, followed by TET2 and ASXL1 2.
Importantly, Jaiswal, et al. 5 showed that the presence of clonal hematopoiesis is associated with an increased risk of all-cause mortality, including increased risks of coronary heart disease and stroke. It is now thought that CHIP results in disordered hematopoiesis through clonal monocytes/macrophages that accelerates atherogenesis and promotes cardiovascular disease in patients with other risk factors and inflammatory disorders 6. Jaiswal, et al. 6 showed it using a mouse model, in which mice prone to hypercholesterolemia were grafted with bone marrow with a TET2 mutation via knockout. Mice engrafted with TET2-mutated allografts showed larger atherosclerotic lesions than those engrafted with normal control bone marrow allografts. They concluded that CHIP approximately doubles the risk of coronary heart disease 6. Adverse outcomes with CHIP were also reported for clonal hematopoiesis after autologous stem cell transplantation for lymphoma 7. These findings demonstrate that CHIP has clinical impact beyond increased risk for developing hematopoietic neoplasms and may have far-reaching implications in non-hematologic realms. However, it remains unclear whether other conditions are affected by clonal hematopoiesis.
Clonal hematopoiesis of indeterminate potential (CHIP) is commonly found in the elderly and is roughly 10 to 20 percent among persons aged 70 to 80 (10 to 15 percent of people over age 65 and at least 30 percent of people by age 80). Individuals with CHIP don’t have symptoms of disease or markedly abnormal blood counts, but their risk of developing a blood cancer such as leukemia is 10 times higher than average. That translates into a 0.5 to 1 percent chance per year of developing one of these diseases and thus about 13 times higher than the incidence of such neoplasias in general 8. People with CHIP also have an increased risk of cardiovascular disease and of leukemia resulting from treatment for other cancers.
CHIP is diagnosed when a test on a person’s blood or bone marrow sample shows that blood cells are carrying one of the genetic mutations associated with the condition. It’s usually discovered when an individual has a DNA test as part of treatment for another disease. If CHIP is discovered incidentally in a patient with a normal blood count, a complete blood count with differential should be repeated three months later and then at annual intervals, so that if a blood cancer develops, it can be detected and treated as early as possible. Researchers are working with cardiologists on a similar monitoring system for signs of heart disease.
Clonal hematopoiesis of indeterminate potential (CHIP) is a benign state; however, it carries a risk of progression to hematologic malignancy as well as mortality primarily because of increased cardiovascular events 9. In clinical settings, clonal hematopoiesis may be observed in cytopenic patients who do not otherwise meet the criteria for hematologic malignancy, a condition referred to as clonal cytopenias of undetermined significance (CCUS). Distinguishing clonal cytopenias of undetermined significance (CCUS) from overt myelodysplastic syndromes (MDS) or other myeloid neoplasms can be challenging because of the overlapping mutational landscape observed in these conditions. Genetic features that could be diagnostically helpful in making this distinction include the number and biological function of mutated genes as well as the observed variant allele frequency. A working knowledge of clonal hematopoiesis is essential for the diagnosis and clinical management of patients with hematologic conditions.
More recent research has helped to stratify clonal hematopoiesis into cases that are less likely to progress (or at least not as rapidly) to an overt hematologic malignancy versus those molecular profiles where transformation to a malignant state may be more imminent 3. A more robust understanding of these conditions will likely allow for highly granular associations between particular gene mutations, co-mutation patterns, and/or variant allele fractions with specific clinical phenotypes. The accuracy of myelodysplastic syndromes (MDS) diagnosis and prognostication will thereby be improved, as will the clinicians’ ability to rule out this condition with a high degree of confidence because of the high negative predictive value associated with the lack of somatic mutations in cytopenic patients 3.
Table 1. Comparison of ICUS, CHIP, CCUS, and myeloid malignancy features
| ICUS | CHIP | CCUS | Myeloid malignancy | |
|---|---|---|---|---|
| Cytopenias | + | – | + | + |
| Clonality | – | + | + | + |
| Number of Mutated genes | 0 | 1–2 | 1–3 | ≥2 |
| Variant allele frequency | NA | <5–10% | Varies | >20% |
| Morphological features of malignancy* | – | – | – | + |
| Risk to developing myeloid malignancy† | <1% | <1% | Low-risk pattern <1% | NA |
| High risk pattern ~10% |
Footnotes: * Morphological features of myeloid malignancy include the presence of significant dyspla sia, increased blasts, and/or atypical morphology diagnostic of a myeloid malignancy as per the WHO classification of myeloid neoplasms 10;
† The risk of developing a myeloid malignancy refers to the overall risk per year, with the <1% risk for CHIP based on the study by Steensma, et al. 3 and the risk for clonal cytopenias of undetermined significance (CCUS) estimated based on the study by Malcovati, et al. 11.
Abbreviations: ICUS = idiopathic cytopenias of undetermined significance; CHIP = clonal hematopoiesis of indeterminate potential; CCUS = clonal cytopenias of undetermined significance; NA = not applicable.
[Source 9 ]Table 2. Molecular characteristics of genes commonly mutated in clonal hematopoiesis of indeterminate potential (CHIP)
| Gene | Location | Function | Types of mutations | Mutational effect |
|---|---|---|---|---|
| DNMT3A | 2p23.3 | DNA methylation | Loss of function, frameshift or nonsense mutations, commonly involving Arginine 882 hot spot | Hypomethylation of DNA due to loss of methyltransferase enzyme activity |
| TET2 | 4q24 | DNA demethylation | Variety of loss of function, deletion, frameshift, or nonsense mutations | Hypermethylation of DNA resulting from disruption of the function of TET family of dioxygenases |
| ASXL1 | 20q11.21 | Chromatin modification | Recurrent truncation loss of function Exon 11 or 12 nonsense or frameshift mutations resulting | Abnormal epigenetic regulation via interaction with polycomb recessive complex |
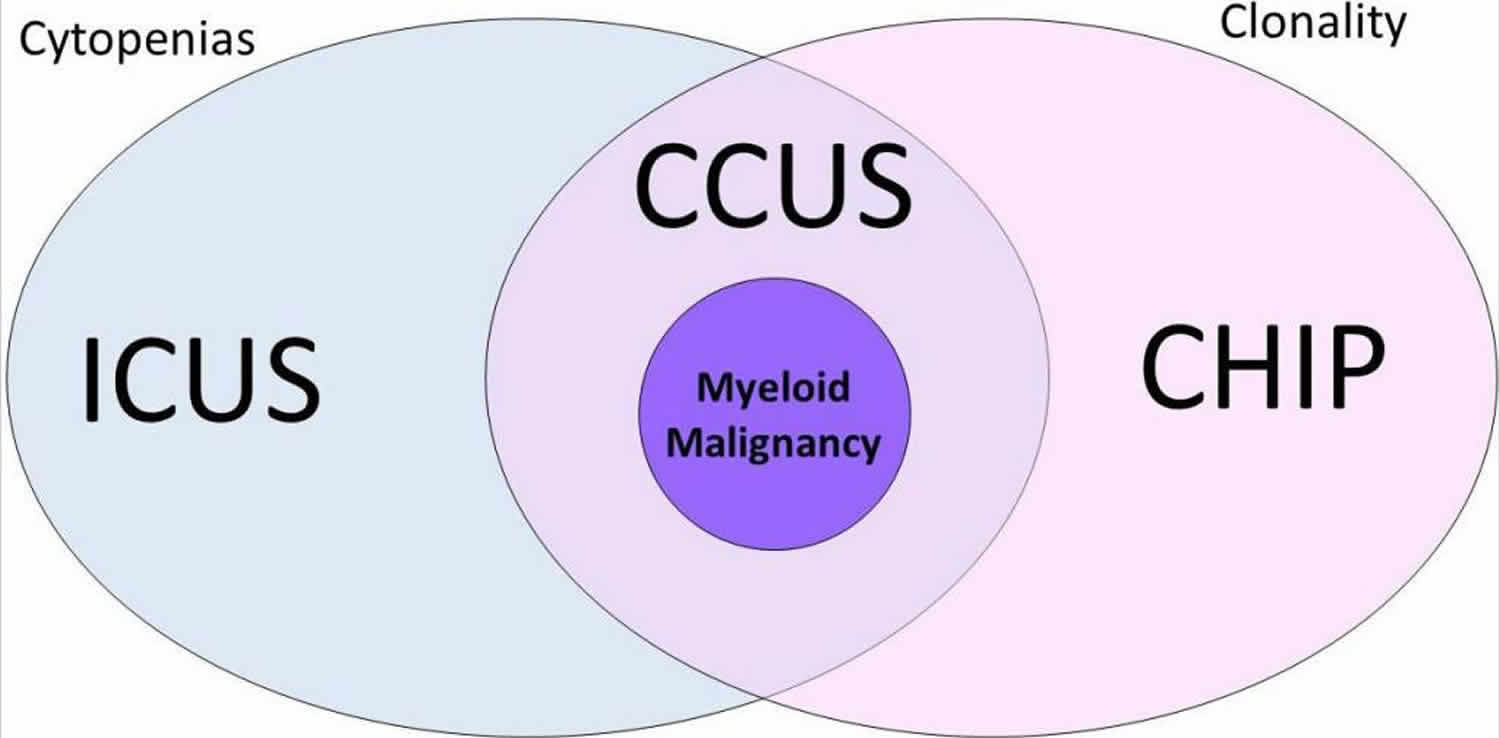
Figure 1. Clonal hematopoiesis of indeterminate potential
Footnote: Venn diagram showing the relationship between idiopathic cytopenias of undetermined significance (ICUS), clonal cytopenia of undetermined significance (CCUS), clonal hematopoiesis of indeterminate potential (CHIP), and myeloid malignancy. Patients with cytopenias without clonality are designated as ICUS, whereas patients with clonality without significant cytopenias are designated as CHIP. Patients with both cytopenia(s) and clonality are designated as CCUS. A subset of patients within this group will have morphological features diagnostic of myeloid malignancy; such features include significant dysplasia (≥10% dysplasia within one or more cell lineages), with or without increased blasts, and with or without other morphological atypia.
[Source 9 ]Clonal hematopoiesis of indeterminate potential treatment
Clonal hematopoiesis plays a significant role in both asymptomatic patients and those with cytopenias. These potential outcomes raise concerns regarding diagnostic strategies and whether large-scale screening of patients should be conducted. As CHIP is present in 10–15% of patients >70 years old, the clinical management of such patients also poses significant challenges. Management of such patients with clonal cytopenia of undetermined significance (CCUS) includes monitoring disease progression and consideration of cardiovascular risk factors 12. For patients with CHIP alone (i.e., without cytopenias), management should focus on cardiovascular risk factors, given the increased risk of stroke and coronary heart disease 13.
The clinical utility of targeted next-generation sequencing panels that provide comprehensive assessment of CHIP-related mutations is increasingly apparent in the context of cytopenic patients, for whom a diagnosis of myelodysplastic syndromes (MDS) is being considered 14. While further studies relating somatic mutations to clinical impact are necessary, accumulating data may facilitate the designation of “MDS-associated somatic mutations” (or patterns of co-mutation) enabling a definitive diagnosis of MDS in the absence of morphological criteria, similar to the “MDS-associated cytogenetic abnormalities” established in the current WHO classification for MDS 15. Numerous somatic mutations also carry prognostic value in MDS; however, they are yet to be incorporated into existing prognostic scoring systems 16. This contrasts with age-related clonal hematopoiesis in the absence of cytopenias, where the concern is not hematologic malignancy, but rather a cardiovascular risk factor. Further understanding of the pathways driving expansion of clonal hematopoiesis and its interaction with atherogenesis may lead to better intervention strategies.
- Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9-16. https://doi.org/10.1182/blood-2015-03-631747[↩]
- Heuser M, Thol F, Ganser A. Clonal Hematopoiesis of Indeterminate Potential. Dtsch Arztebl Int. 2016;113(18):317-322. doi:10.3238/arztebl.2016.0317 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4961884[↩][↩]
- Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16.[↩][↩][↩][↩]
- Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150:264–278.[↩]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498.[↩]
- Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377(2):111-121. doi:10.1056/NEJMoa1701719 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6717509[↩][↩][↩]
- Gibson CJ, Lindsley RC, Tchekmedyian V, Mar BG, Shi J, Jaiswal S, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35:1598–1605.[↩]
- Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129:3371-3378. https://doi.org/10.1182/blood-2017-01-763425[↩]
- Karner K, George TI, Patel JL. Current Aspects of Clonal Hematopoiesis: Implications for Clinical Diagnosis. Ann Lab Med. 2019;39(6):509-514. doi:10.3343/alm.2019.39.6.509 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6660325[↩][↩][↩][↩]
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405.[↩]
- Malcovati L, Gallì A, Travaglino E, Ambaglio I, Rizzo E, Molteni E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129:3371–3378.[↩]
- Steensma DP. Clinical implications of clonal hematopoiesis. Mayo Clin Proc. 2018;93:1122–1130.[↩]
- Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2018;2:3404–3410.[↩]
- Bejar R. Clinical and genetic predictors of prognosis in myelodysplastic syndromes. Haematologica. 2014;99:956–964.[↩]
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405.[↩]
- Schanz J, Tüchler H, Solé F, Mallo M, Luño E, Cervera J, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–829.[↩]