Psychogenic disease
Psychogenic disease also called psychogenic illness or psychogenic disorder was formerly known as ‘hysterical’ illness, are somatic complaints for which there is no apparent physiologic cause 1. The term psychogenic usually implies that psychological factors played a key causal role in the development of the illness. Psychogenic disease may look very similar to genetic diseases of the nervous system or to illnesses caused by damage to the nerves, brain or muscles. Psychogenic diseases can result in many severe symptoms, such as painful cramps or paralysis. However, unlike organic diseases, psychogenic diseases do not have any apparent physical cause, making them difficult to diagnose and even more difficult to treat 2.
Features consistent with psychogenic disorders include 3:
- Presence of associated psychiatric disorders;
- Lack of concern (la belle indifference) or an exaggerated emotional response;
- Disease manifesting only when observed, and not when alone or thought to be unobserved;
- Bizarre or inconsistent signs;
- Presence of emotional or situational triggers;
- Multiple inexplicable physical symptoms;
- Failure to respond to therapy;
- Presence of a medical background; and
- Disease is triggered or ameliorated by placebo.
Management of psychogenic disorders is difficult, as these patients often have an underlying organic disease 4. Psychogenic disorder prognosis for functional recovery is generally poor, and successful management requires a multi-disciplinary approach featuring the psychiatrist in a pivotal role 5. Empathy and a non-judgemental manner should be used, and it is often helpful to invoke a neurobiological explanation of the symptoms in order to foster trust, acceptance, understanding and recovery 6.
Psychogenic fever
Psychogenic fever also called “neurogenic fever” 7, is one of the most common psychogenic disorders. Patients with psychogenic fever have acute or persistent core body temperature above normal range (up to 41°C or 105.8°F) in psychologically stressful situations 8, whereas others show persistent low-grade high core body temperature (98.6 – 100.4°F or 37–38°C) during situations of chronic stress, lasting months and even years, either during or after situations of chronic stress 9. Certain forms of psychogenic fever have been given additional labels, e.g., prolonged low-grade high core temperature in nervous patients has been termed “habitual hyperthermia” 10 and abrupt increases in core temperature in hysterical patients was previously called “hysterical fever” 11. Psychogenic fever is bothersome for both patients and physicians because, although many patients consider the fever to be disabling, there is no abnormal finding to account for their high core temperature and antipyretic drugs do not reduce their fever. Moreover, there are still physicians who do not recognize the fact that psychological stress can cause high core temperature.
Despite numerous case reports on psychogenic fever have been published, the mechanisms by which psychological stress increases core temperature are not yet fully understood, and epidemiological studies are limited. However, clinical case reports demonstrate that psychogenic fever is not reduced by antipyretic drugs, but by psychotropic drugs that display anxiolytic and sedative properties or by resolving patients’ difficulties via natural means or psychotherapy 12. Animal studies have demonstrated that psychological stress increases core body temperature via mechanisms distinct from infectious fever (which requires proinflammatory mediators) and that the sympathetic nervous system, particularly β3-adrenoceptor-mediated non-shivering thermogenesis in brown adipose tissue, plays an important role in the development of psychological stress-induced hyperthermia 12. Acute psychological stress induces a transient, monophasic increase in core body temperature. In contrast, repeated stress induces anticipatory hyperthermia, reduces diurnal changes in core body temperature, or slightly increases core body temperature throughout the day. Chronically stressed animals also display an enhanced hyperthermic response to a novel stress, while past fearful experiences induce conditioned hyperthermia to the fear context. The high core body temperature that psychogenic fever patients develop may be a complex of these diverse kinds of hyperthermic responses.
The age of psychogenic fever patients ranged from 3 to 56 years old and the male: female ratio was 1:1.19 13. Psychogenic fever is seen especially in adolescence in Japan 13.
Psychogenic fever is diagnosed when (1) there is no organic disease that accounts for the fever and (2) the fever develops in a psychologically stressful situation or (3) emotionally stressful stimuli induce acute or persistent increases in core temperature above the upper limit of normal body temperature (37°C) 13.
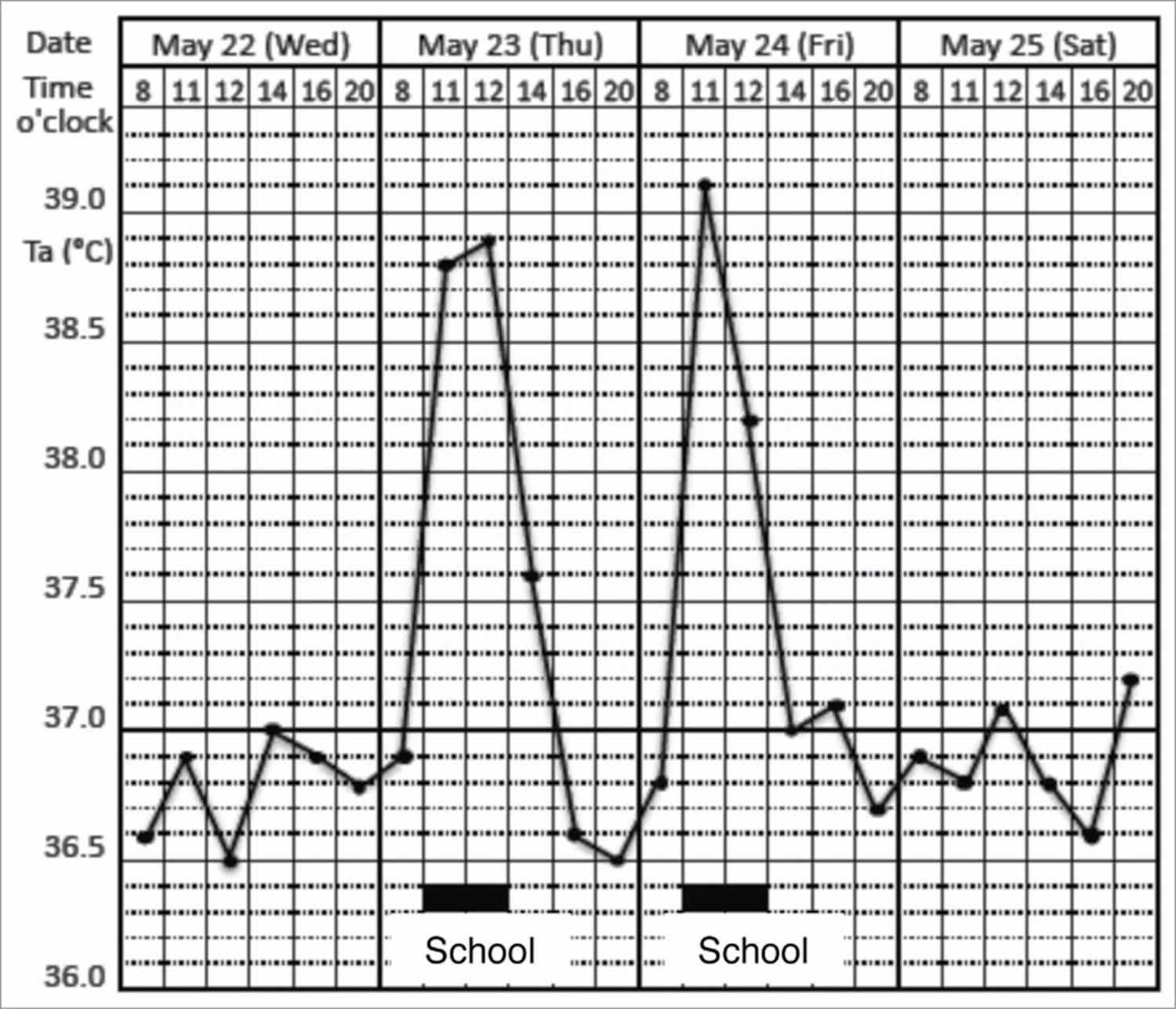
Figure 1. Psychogenic fever
Footnote: Prominent psychogenic fever observed in a 15-year-old schoolgirl. She was referred from a pediatrician to my outpatient clinic because she repeatedly developed antipyretic drug-resistant fever of unknown causes. I asked the patient to record her axillary temperature (Ta) using an electrothermometer 4 times a day (8 a.m., 12 a.m., 4 p.m., and 8 p.m.) and the events of the day in a “fever diary” to better understand mind (stressor)-body (temperature) relationships. I also asked her mother and school nurse to make sure the temperature she recorded was accurate. The fever diary demonstrated that she developed a high axillary temperature (Ta) up to 39°C only on the days when she went to school (underlined black bar).
[Source 12 ]Psychogenic fever causes
Animal studies have demonstrated that many, but not all, types of acute psychological stress increase core temperature. For example, exposing rats or mice to stressors such as being placed into an unfamiliar space or an open field (novelty stress) 14, changing home cages (cage-change stress or cage switch stress) 15, restraint/immobilization 16, removing cage-mates (cage-mate removal stress) 17 and exposure to dominant animals (social defeat stress) 18 or an intruder 19 increases core temperature. Social defeat stress, i.e., exposing rats to a dominant conspecific, increases core temperature by up to 2°C within 30 minutes 20. As represented by this model, a single exposure to psychological stress induces a transient, monophasic increase in core temperature, known as psychological stress-induced hyperthermia (psychological stress-induced hyperthermia). Existence of psychological stress-induced hyperthermia has been observed not only in rats and mice but also in rabbits, tree shrews, sheep, squirrels, chimpanzees, impalas, Peking ducks and pigeons 12.
Animal studies have demonstrated that psychological stress increases core temperature via prostaglandinE2- and proinflammatory cytokine-independent mechanisms. This seems to be the case in patients with psychogenic fever. First, nonsteroidal antiinflammatory drugs (NSAIDs) do not attenuate the high core temperature in these patients. Second, stress interview-induced hyperthermia is not associated with changes in blood levels of prostaglandinE2 or proinflammatory cytokines 8.
In rodents, brown adipose tissue thermogenesis plays a crucial role in the development of psychological stress-induced hyperthermia. In humans, brown adipose tissue is distributed exclusively in an interscapular region in infants 21 and in thyroid/tracheal, mediastinal, paracervical/supraclavicular, parathoracical, supra- and peri-renal regions in adults 22. As in rodents, brown adipose tissue induces non-shivering thermogenesis when humans are exposed to a cold environment. Therefore, it is possible that psychological stress increases core temperature via sympathetic nerve-mediated non-shivering thermogenesis in brown adipose tissue in humans as well. Recently, Lkhagvasuren et al 23 found that patients with psychogenic fever exhibit greater heart rate response to orthostatic stress and increased prevalence of postural orthostatic tachycardia syndrome, one form of orthostatic intolerance, compared with healthy subjects, suggesting a heightened sympathetic response to stress in patients with psychogenic fever. This might account for prominent hyperthermic responses to stressful events in patients with psychogenic fever. However, a role of brown adipose tissue in psychological stress-induced hyperthermia in healthy subjects or high core temperature in patients with psychogenic fever has not yet been elucidated.
Psychological or emotional stress increases core temperature via mechanisms that are distinct from fever that animals develop when they suffer from infectious and inflammatory diseases 24. Infection- and inflammation-induced fever is induced when prostaglandinE2 acts on neurons in the preoptic area of the hypothalamus (preoptic area of the hypothalamus) 25. When animals suffer from infectious diseases, fever is initiated by the release of brain-permeable prostaglandinE2 from hepatic and pulmonary macrophages 26. Macrophages also release proinflammatory cytokines such as interleukin-1β and interleukin-6 and these cytokines stimulate synthesis and release of acute phase proteins such as C-reactive protein from hepatocytes. Furthermore, macrophage-derived proinflammatory cytokines stimulate synthesis of prostaglandinE2 from endothelial cells of the brain vessels 27 or perivascular cells 28 and cause prolonged fever 29. Activation of the dorsomedial hypothalamus–medullary raphe region (including the rostral raphe pallidus and adjacent raphe magnus nuclei)–sympathetic (hypothalamic-medullary-sympathetic, hypothalamic-medullary-sympathetic) axis increases core temperature by activating β3-adrenoceptor-mediated non-shivering thermogenesis in brown adipose tissue and α-adrenoceptor-mediated peripheral vasoconstriction to inhibit heat loss 30. Stimulation of the dorsomedial hypothalamus and the medullary raphe region also induces shivering thermogenesis in skeletal muscles 31. Usually, the preoptic area of the hypothalamus sends tonic inhibitory input to the hypothalamic-medullary-sympathetic axis. prostaglandinE2 causes fever by inhibiting the preoptic area of the hypothalamus neurons, i.e., by disinhibiting the hypothalamic-medullary-sympathetic axis 32. Consequently, fever is attenuated by nonsteroidal antiinflammatory drugs (NSAIDs), which block prostaglandinE2 synthesis.
By contrast, recent studies have demonstrated that acute psychological stress also activates the hypothalamic-medullary-sympathetic axis and increases core temperature 18, albeit via proinflammatory cytokine- and prostaglandinE2-independent mechanisms 18. Therefore, systemic administration of cyclooxygenase inhibitors, such as indomethacin, do not inhibit psychological stress-induced hyperthermia 17, while anxiolytic drugs such as diazepam or 5-hydroxytryptamine1A agonists 33 and β3-adrenoceptor antagonists, such as SR59230A, do attenuate psychological stress-induced hyperthermia 20. Other brain regions, such as the prefrontal cortex 34, the preoptic area of the hypothalamus 35, the medial amygdala 36, or the lateral habenula 37, in addition to orexin neurons 38 are also suggested to be involved in the development of psychological stress-induced hyperthermia. However, so far, it is not fully understood how psychological stress activates dorsomedial hypothalamus neurons or how other brain regions affect the hypothalamic-medullary-sympathetic axis during psychological stress.
Regardless of the source of stress, acute psychological stress-induced hyperthermia is represented by a transient, monophasic increase in core temperature, and the high core temperature returns to baseline levels within several hours if the stressor is terminated. In contrast, repeated or chronic exposure to psychological stress has complex effects on core temperature. First, repeated exposure to uncontrollable stressors such as daily confrontation with a dominant rat at fixed time intervals induces anticipatory or learned hyperthermia, i.e., core temperature becomes higher during the hour preceding the scheduled time of stress application or during the hour when animals have been exposed to dominant rats even if they are kept in their home cages without stress exposure 39. Second, repeated application of stressors (for more than several weeks) either reduces diurnal changes in core temperature, mostly by increasing core temperature in the light (inactive) period 40, or slightly increases core temperature (around 0.2–0.3°C) throughout the day 41. Third, repeated or chronic stress enhances the magnitude of the hyperthermic effect induced by a novel stressor 42 or intravenous administration of noradrenaline 43. Fourth, these rats display depressive-like behavior rather than increased anxiety-like behavior 44. Fifth, these changes can be observed even several days after cessation of the final stress exposure 41.
Hyperthermic responses in rats exposed to repeated or chronic stress do not seem to be induced by exactly the same mechanisms as acute psychological stress-induced hyperthermia. First, the hyperthermic response during conditioned fear does not appear to involve activation of brown adipose tissue 45. Contextual conditioned fear does not induce the expression of Fos, a marker of neuronal activation, in the dorsomedial hypothalamus, but does increase Fos in spinally projecting neurons in the perifornical area of the hypothalamus 46. Second, after repeated immobilization, the magnitude of the noradrenaline-induced increase in core temperature, interscapular brown adipose tissue temperature, and oxygen consumption become greater in stressed rats versus controls 43. As repeated immobilization stress induces interscapular brown adipose tissue hyperplasia 47 and increases uncoupling protein 1 (UCP-1), a protein that generates heat according to its expression and function in brown adipose tissue, these changes may lead to prominent psychological stress-induced hyperthermia 47. Thirdly, psychological stress induces microglial activation 48 and subsequent proinflammatory cytokine production 49 in the central nervous system. As brain-derived cytokines also increase core temperature 50 and induce depressive-like behavior 51, there is a possibility that hyperthermia and depressive-like behaviors in rats exposed to chronic stress are mediated, at least in part, by activated microglia and subsequent proinflammatory cytokines within the brain. However, additional studies are necessary to make sure if this is the case.
As in laboratory animals, psychological stress increases the core temperature in healthy humans. Previous studies have demonstrated that core temperature just before emotional events is higher than core temperature after these events or at the same hour of the day under non-stressful conditions 52. For example, the mean oral temperature before boxing contests (37.55°C) in 12 school boys (12–14 years old) was 0.8°C higher than that taken at home at the same hour of the day (36.75°C) 53. The mean oral temperature on movie-watching days in separate groups of females in their teens and twenties (37.55°C and 37.46°C) was 0.53°C and 0.27°C higher than that of the same hour on preceding or following days (37.03°C and 37.19°C), respectively 54. The hyperthermic effect of examination stress is reported to be weaker than the effects of the emotional events described above. For example, the mean oral temperature of 40 subjects immediately before taking a nurses’ registration examination (37.17°C) was 0.34°C higher than observed after the examination (36.83°C) 55. The mean axillary temperature of 22 residents (26 – 33 years old), 10 to 15 min before a yearly university examination (37.00°C), was 0.6°C higher than the temperature taken 2 to 3 weeks later after having sat and relaxed for at least 30 min (36.40°C) 56. The mean oral temperature of 108 medical students (18 – 27 years old) immediately before examination (37.4°C) was 0.18°C higher than what was taken at the same hour of the day 3 days after the exam (37.22°C) 57. The mean oral temperature of medical students (17 – 19 years old) 5 – 7 days before examination (36.91°C) was 0.17°C higher than that at the same time 5 – 7 days after the examination (36.74°C) 52. In contrast, one study demonstrated that exposing healthy subjects to a standardized laboratory stress task (the Trier Social Stress Test) did not change temporal artery temperature and also decreased intestinal temperature, both of which are assumed to reflect core temperature 58.
Psychogenic fever symptoms
Patients with psychogenic fever have acute or persistent core body temperature above normal range (up to 41°C or 105.8°F) in psychologically stressful situations 8, whereas others show persistent low-grade high core body temperature (98.6 – 100.4°F or 37–38°C) during situations of chronic stress, lasting months and even years, either during or after situations of chronic stress 9.
The clinical significance of high core temperature in patients with psychogenic fever is different from psychological stress-induced hyperthermia in healthy subjects in several ways 59. Remarkable differences include the magnitude of increase in core temperature and the associated symptoms. First, in healthy subjects, although emotional events increase core temperature, its magnitude is <1°C and the maximal core temperature they show is <37.5°C in most cases 52. By contrast, in some patients with psychogenic fever, emotional events increase core temperature to 39–41°C 60. Such differences may arise according to the severity of stressors. However, as was shown in animal studies, it is also possible that chronic stressors that the patient has experienced cause the induction of a prominent hyperthermic response when the patient is exposed to emotional events. Second, healthy subjects do not complain of symptoms even when they exhibit high core temperature. By contrast, although some patients have no complaints except for the high core temperature, others complain of numerous symptoms in addition to high core temperature. These symptoms include insomnia, fatigue, headache, nausea, and/or abdominal pain. As increases in core temperature are frequently associated with these symptoms, patients consider the high core temperature disabling. Some patients are neurotic and have high anxiety 7. Psychogenic fever is also observed in patients who have traumatic experiences in their early lives 11 and with psychiatric disorders such as anxiety (panic and post-traumatic stress) disorders 60, mood (depressive and bipolar) disorders 61, somatoform (conversion) disorders 11, catatonia 62 and borderline personality disorders 63. For these reasons, they worry about their high core temperature and may consult their physicians asking for treatment.
Psychogenic fever diagnosis
There are no standard tests for psychogenic fever. Diagnosis usually involves assessment of existing symptoms and ruling out any infectious or other medical condition that could cause the symptoms.
Psychogenic fever treatment
Previous clinical case reports have demonstrated that patients who exhibit persistent low-grade high core temperature were treated successfully with drugs that produce sedative effects such as opium 10 or phenobarbital 63, serotonergic tricyclic antidepressants such as amitriptyline and clomipramine 64, selective serotonin reuptake inhibitor (SSRI) such as paroxetine 65 or serotonin 1A receptor agonists such as tandospirone 66. Furthermore, relaxation training 60, solving their difficulties, and psychotherapy to ventilate suppressed negative emotion and conflicts verbally 63 or non-verbally 67 are also helpful strategies.
- Purcell TB. The somatic patient. Emerg Med Clin N Am 1991;9: 137-59[↩]
- University College London. Psychogenic diseases linked to abnormal brain activity. https://www.ucl.ac.uk/news/2013/feb/psychogenic-diseases-linked-abnormal-brain-activity[↩]
- Alsaadi TM, Marquez AV. Psychogenic nonepileptic seizures. Am Fam Physician 2005;72: 849-56[↩]
- Miyasaki JM, Sa DS, Galvez-Jimenez N, Lang AE. Psychogenic movement disorders. Can J Neurol Sci 2003;30(suppl 1): S94-100[↩]
- Lim EC, Seet RC. What is the place for placebo in the management of psychogenic disease?. J R Soc Med. 2007;100(2):60-61. doi:10.1177/014107680710000202 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1790983[↩]
- Ford B, Williams DT, Fahn S. Treatment of psychogenic movement disorders. In: Kurlan R, ed. Treatment of Movement Disorders. Philadelphia: JB Lippincott, 1995: 475-85[↩]
- Kintner AR, Rowntree LG. Long continued, low grade, idiopathic fever. JAMA 1934;102:889-92; http://dx.doi.org/10.1001/jama.1934.02750120001001[↩][↩]
- Oka T, Kanemitsu Y, Sudo N, Hayashi H, Oka K. Psychological stress contributed to the development of low-grade fever in a patient with chronic fatigue syndrome: a case report. Biopsychosoc Med 2013; 7:7; http://dx.doi.org/10.1186/1751-0759-7-7[↩][↩][↩]
- Kaneda Y, Tsuji S, Oka T. Age distribution and gender differences in psychogenic fever patients. Biopsychosoc Med 2009; 3:6; http://dx.doi.org/10.1186/1751-0759-3-6[↩][↩]
- Reimann HA. The problem of long, continued, low grade fever. JAMA 1936;107:1089-94; http://dx.doi.org/10.1001/jama.1936.02770400001001[↩][↩]
- Duras FP. Hyperpyrexia due to hysteria. Lancet 1942; 11:39; http://dx.doi.org/10.1016/S0140-6736(00)62135-9[↩][↩][↩]
- Oka T. Psychogenic fever: how psychological stress affects body temperature in the clinical population. Temperature (Austin). 2015;2(3):368-378. Published 2015 Jun 3. doi:10.1080/23328940.2015.1056907 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4843908[↩][↩][↩][↩]
- Oka T, Oka K. Age and gender differences of psychogenic fever: a review of the Japanese literature. Biopsychosoc Med. 2007;1:11. Published 2007 May 19. doi:10.1186/1751-0759-1-11 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1888691[↩][↩][↩]
- Butterweck V, Prinz S, Schwaninger M. The role of interleukin-6 in stress-induced hyperthermia and emotional behaviour in mice. Behav Brain Res 2003; 144:49-56; http://dx.doi.org/10.1016/S0166-4328(03)00059-7[↩]
- Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol 2003; 551:945-54; http://dx.doi.org/10.1113/jphysiol.2003.048140[↩]
- Ootsuka Y, Blessing WW, Nalivaiko E. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress 2008; 11:125-33; http://dx.doi.org/10.1080/10253890701638303[↩]
- Vinkers CH, van Bogaert MJ, Klanker M, Korte SM, Oosting R, Hanania T, Hopkins SC, Olivier B, Groenink L. Translational aspects of pharmacological research into anxiety disorders: the stress-induced hyperthermia (SIH) paradigm. Eur J Pharmacol 2008; 585:407-25; http://dx.doi.org/10.1016/j.ejphar.2008.02.097[↩][↩]
- Lkhagvasuren B, Oka T, Nakamura Y, Hayashi H, Sudo N, Nakamura K. Distribution of Fos-immunoreactive cells in rat forebrain and midbrain following social defeat stress and diazepam treatment. Neuroscience 2014; 272:34-57; http://dx.doi.org/10.1016/j.neuroscience.2014.04.047[↩][↩][↩]
- Mohammed M, Ootsuka Y, Blessing W. Brown adipose tissue thermogenesis contributes to emotional hyperthermia in a resident rat suddenly confronted with an intruder rat. Am J Physiol Regul Integr Comp Physiol 2014; 306:R394-400; http://dx.doi.org/10.1152/ajpregu.00475.2013[↩]
- Lkhagvasuren B, Nakamura Y, Oka T, Sudo N, Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur J Neurosci 2011; 34:1442-52; http://dx.doi.org/10.1111/j.1460-9568.2011.07863.x[↩][↩]
- Enerback S. Human brown adipose tissue. Cell Metab 2010; 11:248-52; http://dx.doi.org/10.1016/j.cmet.2010.03.008[↩]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al.. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360:1518-25; http://dx.doi.org/10.1056/NEJMoa0808949[↩]
- Lkhagvasuren B, Tanaka H, Sudo N, Kubo C, Oka T. Characteristics of the orthostatic cardiovascular response in adolescent patients with psychogenic fever. Psychother Psychosom 2014; 83:318-9; PMID:25116930; http://dx.doi.org/10.1159/000360999[↩]
- Vinkers CH, Groenink L, van Bogaert MJ, Westphal KG, Kalkman CJ, van Oorschot R, Oosting RS, Olivier B, Korte SM. Stress-induced hyperthermia and infection-induced fever: two of a kind? Physiol Behav 2009; 98:37-43; http://dx.doi.org/10.1016/j.physbeh.2009.04.004[↩]
- Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci 2004; 9:1977-93; http://dx.doi.org/10.2741/1383[↩]
- Steiner AA, Ivanov AI, Serrats J, Hosokawa H, Phayre AN, Robbins JR, Roberts JL, Kobayashi S, Matsumura K, Sawchenko PE, et al.. Cellular and molecular bases of the initiation of fever. PLoS Biol 2006; 4:e284; http://dx.doi.org/10.1371/journal.pbio.0040284[↩]
- Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci 2003; 6:1137-8; http://dx.doi.org/10.1038/nn1137[↩]
- Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci 2003; 8:s1321-9; http://dx.doi.org/10.2741/1211[↩]
- Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol 2002; 92:2648-55; http://dx.doi.org/10.1152/japplphysiol.01005.2001[↩]
- Rathner JA, Madden CJ, Morrison SF. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am J Physiol Regul Integr Comp Physiol 2008; 295:R343-54; http://dx.doi.org/10.1152/ajpregu.00115.2008[↩]
- Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol 2011; 589:3641-58; http://dx.doi.org/10.1113/jphysiol.2011.210047[↩]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci 2011; 16:74-104; http://dx.doi.org/10.2741/3677[↩]
- Olivier B, Bouwknecht JA, Pattij T, Leahy C, van Oorschot R, Zethof TJ. GABAA-benzodiazepine receptor complex ligands and stress-induced hyperthermia in singly housed mice. Pharmacol Biochem Behav 2002; 72:179-88; http://dx.doi.org/10.1016/S0091-3057(01)00759-6[↩]
- Pecoraro N, de Jong H, Ginsberg AB, Dallman MF. Lesions of the medial prefrontal cortex enhance the early phase of psychogenic fever to unexpected sucrose concentration reductions, promote recovery from negative contrast and enhance spontaneous recovery of sucrose-entrained anticipatory activity. Neuroscience 2008; 153:901-17; http://dx.doi.org/10.1016/j.neuroscience.2008.03.043[↩]
- Egawa M, Yoshimatsu H, Bray GA. Preoptic area injection of corticotropin-releasing hormone stimulates sympathetic activity. Am J Physiol 1990; 259:R799-806[↩]
- Vinkers CH, Bijlsma EY, Houtepen LC, Westphal KG, Veening JG, Groenink L, Olivier B. Medial amygdala lesions differentially influence stress responsivity and sensorimotor gating in rats. Physiol Behav 2010; 99:395-401; http://dx.doi.org/10.1016/j.physbeh.2009.12.006[↩]
- Ootsuka Y, Mohammed M. Activation of the habenula complex evokes autonomic physiological responses similar to those associated with emotional stress. Physiol Rep 2015; 3:e12297; http://dx.doi.org/10.14814/phy2.12297[↩]
- Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol 2010; 588:4117-29; http://dx.doi.org/10.1113/jphysiol.2010.195099[↩]
- Pardon MC, Kendall DA, Perez-Diaz F, Duxon MS, Marsden CA. Repeated sensory contact with aggressive mice rapidly leads to an anticipatory increase in core body temperature and physical activity that precedes the onset of aversive responding. Eur J Neurosci 2004; 20:1033-50; http://dx.doi.org/10.1111/j.1460-9568.2004.03549.x[↩]
- Kant GJ, Bauman RA, Pastel RH, Myatt CA, Closser-Gomez E, D’Angelo CP. Effects of controllable vs. uncontrollable stress on circadian temperature rhythms. Physiol Behav 1991; 49:625-30; http://dx.doi.org/10.1016/0031-9384(91)90289-Z[↩]
- Endo Y, Shiraki K. Behavior and body temperature in rats following chronic foot shock or psychological stress exposure. Physiol Behav 2000; 71:263-8; http://dx.doi.org/10.1016/S0031-9384(00)00339-5[↩][↩]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol 2006; 18:13-24; http://dx.doi.org/10.1111/j.1365-2826.2005.01375.x[↩]
- Nozu T, Okano S, Kikuchi K, Yahata T, Kuroshima A. Effect of immobilization stress on in vitro and in vivo thermogenesis of brown adipose tissue. Jpn J Physiol 1992; 42:299-308; http://dx.doi.org/10.2170/jjphysiol.42.299[↩][↩]
- Rygula R, Abumaria N, Havemann-Reinecke U, Ruther E, Hiemke C, Zernig G, Fuchs E, Flugge G. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav Pharmacol 2008; 19:183-96; http://dx.doi.org/10.1097/FBP.0b013e3282fe8871[↩]
- Marks A, Vianna DM, Carrive P. Nonshivering thermogenesis without interscapular brown adipose tissue involvement during conditioned fear in the rat. Am J Physiol Regul Integr Comp Physiol 2009; 296:R1239-47; http://dx.doi.org/10.1152/ajpregu.90723.2008[↩]
- Carrive P, Gorissen M. Premotor sympathetic neurons of conditioned fear in the rat. Eur J Neurosci 2008; 28:428-46; http://dx.doi.org/10.1111/j.1460-9568.2008.06351.x[↩]
- Kuroshima A, Habara Y, Uehara A, Murazumi K, Yahata T, Ohno T. Cross adaption between stress and cold in rats. Pflugers Arch 1984; 402:402-8; http://dx.doi.org/10.1007/BF00583941[↩][↩]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 2013; 23:1784-97; http://dx.doi.org/10.1093/cercor/bhs151[↩]
- Johnson JD, Zimomra ZR, Stewart LT. Beta-adrenergic receptor activation primes microglia cytokine production. J Neuroimmunol 2013; 254:161-4; http://dx.doi.org/10.1016/j.jneuroim.2012.08.007[↩]
- Oka T, Oka K, Hosoi M, Hori T. Intracerebroventricular injection of interleukin-6 induces thermal hyperalgesia in rats. Brain Res 1995; 692:123-8; http://dx.doi.org/10.1016/0006-8993(95)00691-I[↩]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65:732-41; http://dx.doi.org/10.1016/j.biopsych.2008.11.029[↩]
- Shah SJ, PAtel HM. Effect of examination stress on parameters of autonomic functions in medical students. Int J Sci Res 2014; 3:273-6[↩][↩][↩]
- Renbourn ET. Body temperature and pulse rate in boys and young men prior to sporting contests. A study of emotional hyperthermia: with a review of the literature. J Psychosom Res 1960; 4:149-75; http://dx.doi.org/10.1016/0022-3999(60)90008-8[↩]
- Kleitman N. The effect of motion pictures on body temperature. Science 1945; 102:430-1; http://dx.doi.org/10.1126/science.102.2652.430[↩]
- Wynn FR. The psychic factor as an element in temperature disturbance. JAMA 1919; 73:31-4; http://dx.doi.org/10.1001/jama.1919.02610270035010[↩]
- Marazziti D, Di Muro A, Castrogiovanni P. Psychological stress and body temperature changes in humans. Physiol Behav 1992; 52:393-5; http://dx.doi.org/10.1016/0031-9384(92)90290-I[↩]
- Briese E. Emotional hyperthermia and performance in humans. Physiol Behav 1995; 58:615-8; http://dx.doi.org/10.1016/0031-9384(95)00091-V[↩]
- Vinkers CH, Penning R, Hellhammer J, Verster JC, Klaessens JH, Olivier B, Kalkman CJ. The effect of stress on core and peripheral body temperature in humans. Stress 2013; 16:520-30; http://dx.doi.org/10.3109/10253890.2013.807243[↩]
- Oka T, Oka K. Mechanisms of psychogenic fever. Adv Neuroimmune Biol 2012; 3:3-17[↩]
- Timmerman RJ, Thompson J, Noordzij HM, van der Meer JW. Psychogenic periodic fever. Neth J Med 1992; 41:158-60[↩][↩][↩]
- Nozu T, Uehara A. The diagnoses and outcomes of patients complaining of fever without any abnormal findings on diagnostic tests. Intern Med 2005; 44:901-2; http://dx.doi.org/10.2169/internalmedicine.44.901[↩]
- Bohorfoush JG, Craig JB, Patterson HS. Catatonia as a cause of fever of undetermined origin. J Med Assoc Ga 1965; 54:324-5[↩]
- McNeil GN, Leighton LH, Elkins AM. Possible psychogenic fever of 103.5 degrees F in a patient with borderline personality disorder. Am J Psychiatry 1984; 141:896-7; http://dx.doi.org/10.1176/ajp.141.7.896[↩][↩][↩]
- Timmerman RJ, Thompson J, Noordzij HM, van der Meer JW. Psychogenic periodic fever. Neth J Med. 1992;41(3-4):158-160[↩]
- Oka T, Kaneda Y, Takenaga M, Hayashida S, Tamagawa Y, Kodama N, Tsuji S. Efficacy of paroxetine for treating chronic stress-induced low-grade fever. Jpn J Psychosom Intern Med 2006; 10:5-8[↩]
- Oka T, Hayashida S, Kaneda Y, Kodama N, Hashimoto T, Tsuji S. Why do chronic stress-induced hyperthermia patients worry about slightly elevated body temperature? Jpn J Psychosom Intern Med 2006; 10:243-6[↩]
- Araki T, Oka T, Oyama N, Akamine M, Kubo C. A case of psychogenic fever treated successfully with art therapy. Jpn J Psychosom Med 2004; 44:289-94[↩]