Simmonds disease
Simmonds disease also known as Simmonds’ syndrome or pituitary cachexia, is a chronic deficiency of function of the pituitary gland (chiefly the anterior lobe of the pituitary gland), a form of hypopituitarism, that leads to atrophy of many of the viscera, including the heart, liver, spleen, kidneys, thyroid, adrenals, and gonads. Simmonds disease is caused by destruction of the anterior lobe of the pituitary gland from any cause 1. The most important cause of Simmonds disease is post-partum necrosis of the anterior pituitary (also known as Sheehan syndrome). Sheehan syndrome is a type of hypopituitarism that can occur in a woman who bleeds severely during childbirth. Severe bleeding during childbirth can cause tissue in the pituitary gland to die. This gland does not work properly as a result, thus the age incidence of Simmonds disease is greatest between 2o-45 years and that the disease is much commoner in women than men.
Clinically Simmonds syndrome is characterized by cachexia, premature senility, atrophy of the gonads and genitalia, with amenorrhea, atrophy of the breasts, loss of pubic and axillary hair, loss of libido, integumental changes (chiefly dryness of the skin), anorexia and constipation, hypotension and muscular weakness, hypoglycemia, decreased sugar tolerance, lowered basal metabolism and depressed specific dynamic action of proteins, anemia, lymphocytosis and sometimes eosinophilia and death if left untreated 2.
What is anterior pituitary gland?
Pituitary gland is oval and weighs 500 mg. The pituitary gland consists of two lobes 3:
- Anterior pituitary (adenohypophysis)
- Posterior pituitary (neurohypophysis)
The anterior pituitary is composed of the following parts:
- Pars distalis: This is the portion in which the majority of the hormone production occurs. It is the distal part of the pituitary and forms the majority anterior pituitary.
- Pars tuberalis: this is a tubular sheath that extends from the pars distalis and winds around the pituitary stalk. Epithelial cells arranged in cords and hypophyseal portal vessels reside in this space.
- Pars intermedia: This is a section of tissue that resides between the posterior pituitary and pars distalis. This section of the anterior pituitary consists of pale cells that are large. These cells of the pars intermedia encompass follicles containing a colloidal matrix. The main hormone secreted by this portion of the anterior pituitary is melanocyte stimulating hormone (MSH).
The dimension of the pituitary gland is about 12 mm transverse and 8 mm in anterior-posterior diameter. The pituitary is located in the hypophysial fossa, in a bony depression of the sphenoid bone called the sella turcica. The sella turcica surrounds the inferior, anterior, and posterior aspects of the gland. Various structures surround the endocrine gland. The optic chiasma lies anteriorly, and the mammillary bodies lie posteriorly. Superior to the pituitary gland is the diaphragma sellae, inferior lies the sphenoidal air sinuses, and lateral is the cavernous sinuses. A fold of dura matter covers the pituitary and has an opening to allow for the infundibulum to pass through, allowing a connection to the hypothalamus.
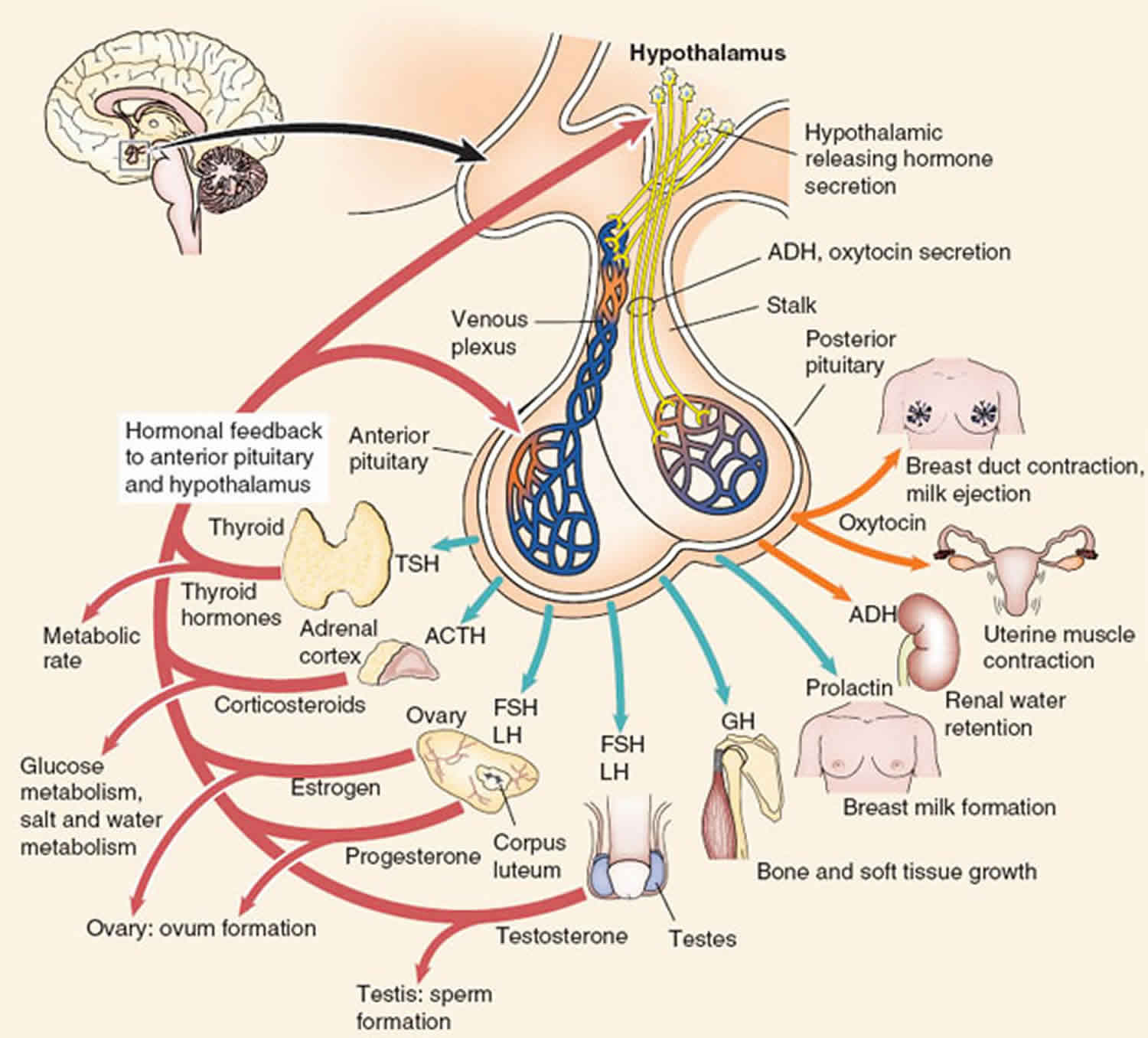
Figure 1. Anterior pituitary hormones
Figure 2. Anterior and posterior pituitary hormones
Anterior vs Posterior pituitary
The back part of the pituitary gland is called the posterior pituitary. It produces the following two hormones:
- Oxytocin: This hormone causes pregnant women to start having contractions at the appropriate time and also promotes milk flow in nursing mothers.
- Antidiuretic hormone (ADH): Commonly referred to as vasopressin, this hormone helps to regulate water balance in the body.
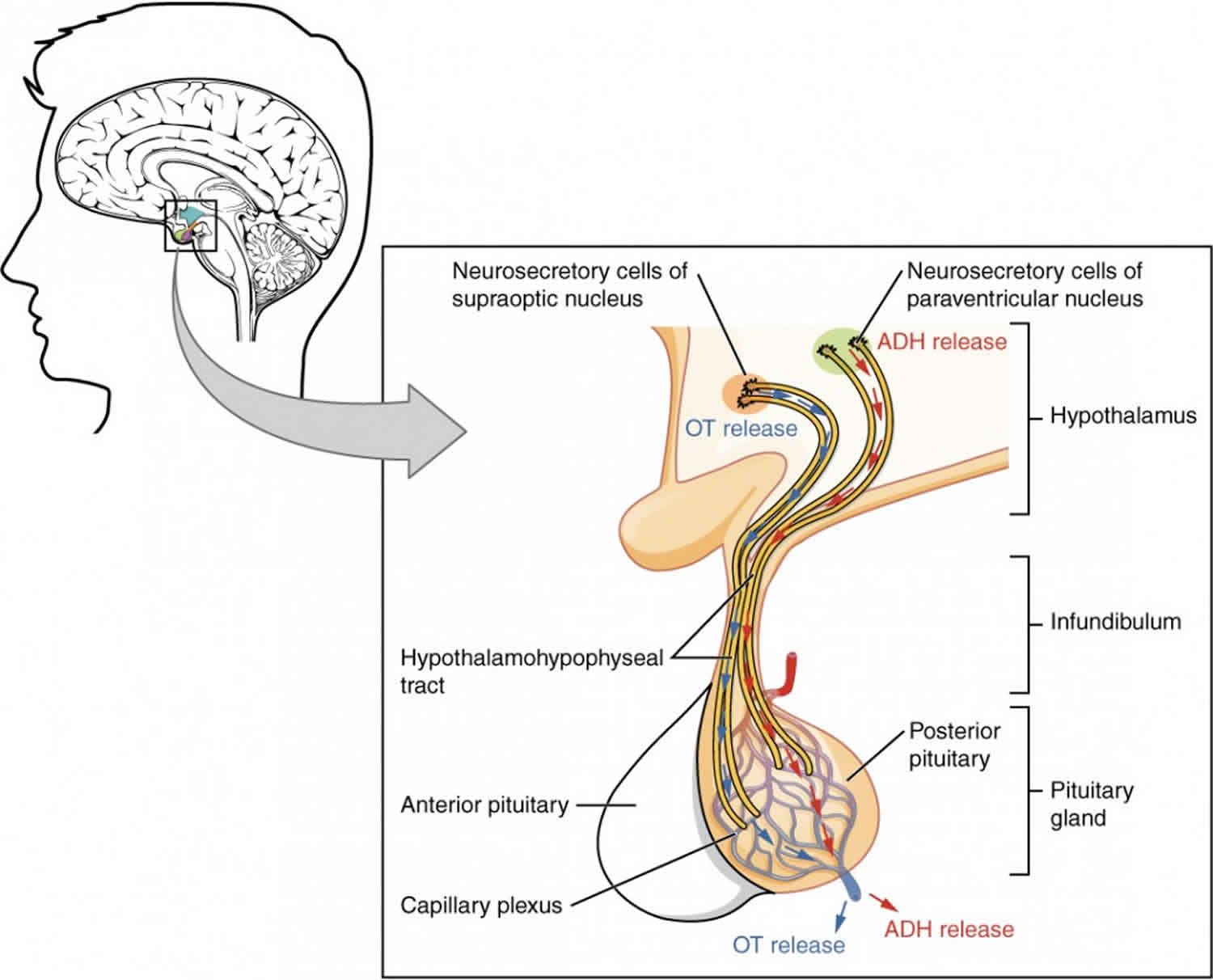
Figure 3. Posterior pituitary hormones
Oxytocin
Oxytocin is a hormone produced by the hypothalamus and secreted by the pituitary gland. This important hormone plays a crucial role in the childbirth process and also helps with male reproduction.
In women, oxytocin is responsible for signaling contractions of the womb during labor. The hormone stimulates the uterine muscles to contract, so labor begins. It also increases the production of prostaglandins, which move labor along and increases the contractions even more. Because of this effect, synthetic oxytocin (pitocin) is sometimes used to induce a woman to start labor if she cannot start naturally, or it can be given to make contractions stronger if a woman’s labor is slowing.
Once the baby is born, oxytocin promotes lactation by moving the milk into the breast. When the baby sucks at the mother’s breast, oxytocin secretion causes the milk to release so the baby can feed. At the same time, oxytocin is released into the brain to stimulate further oxytocin production. Once the baby stops feeding, the production of the hormone stops until the next feeding.
For men, oxytocin function is less important, but it does have a role to play in moving sperm. It also appears to affect the production of testosterone in the testes.
Studies of oxytocin also have found that it is an important chemical messenger that controls some human behaviors and social interaction. It is oxytocin that triggers the bond between a mother and an infant, and it may also play a role in recognition, sexual arousal, trust, and anxiety. Some research shows that the hormone may affect addiction and stress as well.
How is Oxytocin production controlled?
Oxytocin production is controlled by a positive feedback mechanism. This mechanism allows the release of the oxytocin hormone when a trigger occurs. The hormone then causes an action in the body, such as the letdown of milk or the start of labor contractions, which signals more production of oxytocin. The feedback cycle continues until the action, such as childbirth or feeding the baby, is complete.
Problems with Oxytocin Production
High and low oxytocin levels are possible, but research has not yet found any implications of these conditions. Men with high levels of oxytocin sometimes develop benign prostatic hyperplasia, or the enlarging of the prostate gland. This condition can cause urinary complaints. A lack of oxytocin can prevent the milk letdown reflex and make breastfeeding difficult. Low oxytocin levels have also been linked to depression, but using oxytocin to treat mental health conditions has not yet been studied sufficiently.
Antidiuretic hormone (ADH)
Antidiuretic hormone (ADH), also called arginine vasopressin (AVP), is a hormone that helps regulate water balance in the body by controlling the amount of water the kidneys reabsorb while they are filtering wastes out of the blood.
Antidiuretic hormone (ADH) or arginine vasopressin (AVP), is produced by the hypothalamus in the brain and stored in the posterior pituitary gland at the base of the brain. Antidiuretic hormone (ADH) is normally released by the pituitary in response to sensors that detect an increase in blood osmolality (number of dissolved particles in the blood) or decrease in blood volume. The kidneys respond to antidiuretic hormone (ADH) by conserving water and producing urine that is more concentrated. The retained water dilutes the blood, lowers its osmolality, and increases blood volume and pressure. If this is not sufficient to restore the water balance, then thirst is also stimulated so that the affected person will drink more water.
There are a variety of disorders, conditions, and medications that can affect either the amount of antidiuretic hormone (ADH) released or the kidneys’ response to it. Antidiuretic hormone (ADH) deficiency and excess can cause acute and chronic symptoms that, in rare cases, may become life-threatening.
If there is too little ADH or the kidneys do not respond to ADH, then too much water is lost through the kidneys, the urine produced is more dilute than normal, and the blood becomes more concentrated. This can cause excessive thirst, frequent urination, dehydration, and – if not enough water is ingested to replace what is being lost – high blood sodium (hypernatremia).
If there is too much ADH, then water is retained, blood volume increases, and the person may experience nausea, headaches, disorientation, lethargy, and low blood sodium (hyponatremia).
The ADH test is not widely used to diagnose these conditions. Often, a diagnosis is made on the basis of clinical history and other laboratory tests, such as urine and blood osmolality and electrolytes.
Antidiuretic hormone (ADH) deficiency is called diabetes insipidus. There are two types of this disorder: central and nephrogenic.
- Central diabetes insipidus is associated with a lack of ADH production by the hypothalamus or release from the pituitary and may be due to a variety of causes, including an inherited genetic defect, head trauma, a brain tumor, or due to an infection that causes encephalitis or meningitis.
- Nephrogenic diabetes insipidus originates in the kidney and is associated with a lack of response to ADH, causing an inability to concentrate urine. It may be inherited or caused by a variety of kidney diseases.
Both types of diabetes insipidus lead to the excretion of large quantities of dilute urine.
Increased levels of ADH are often seen with “syndromes of inappropriate antidiuretic hormone” (SIADH) secretion. Syndromes of inappropriate antidiuretic hormone (SIADH) is characterized by inappropriate (that is, not due to high blood osmolality or low blood volume) production of too much ADH, resulting in water retention, low blood sodium, and decreased blood osmolality. It may be due to a wide number of diseases and conditions that either stimulate excessive ADH production and release or that prevent its suppression. Syndromes of inappropriate antidiuretic hormone (SIADH) may also be seen with cancers that produce ADH or ADH-like substances independent of the hypothalamus and pituitary glands. Regardless of the cause or source, excessive ADH causes low blood sodium and osmolality because water is retained and blood volume is increased.
Anterior pituitary function
The anterior pituitary produces and secretes a majority of the hormones of the pituitary gland 4.
These hormones are secreted in response to the prohormones that are secreted by the hypothalamus.
The anterior pituitary is characterized by well-demarcated acini. These acini are composed of a mixture of different hormone-producing cells. The different cells can be differentiated via hemotoxin and eosin (H and E) stains and the PAS-OG preparations. There are three distinct cell types seen based on these stains.
- Acidophils: The cytoplasm of these cells stain red or orange. Acidophils usually contain polypeptide hormones. The cells that are acidophils are somatotropes and lactotropes. These cells have the largest size granules.
- Basophils: These cells have cytoplasm that stains a bluish or purple color. Basophils contain glycoprotein hormones. The basophils that secrete glycoprotein hormones are thyrotropes, gonadotropes, and corticotropes.
- Chromophobes: These cells do not stain because they have very minimal or no hormonal content. Many of the chromophobes may have been acidophils and basophils that have degranulated and have released their hormones. Some of the chromophobes are thought to be stem cells that have not differentiated into hormone-producing cells.
About 50% of anterior pituitary secretory cells are somatotrophs (synthesizing somatotrophin or growth hormone), 10–25% lactotrophs (making prolactin), 15–20% corticotrophs (ACTH), 10–15% gonadotrophs (LH and FSH), and 3–5% thyrotrophs (TSH). Some cells, usually chromophobes, do not stain with any of the antibodies to the various anterior pituitary hormones although electron microscopy reveals that these cells contain secretory granules.
Adrenocorticotropin (ACTH)
ACTH hormone is secreted in response from corticotropin-releasing hormone (CRH) from the hypothalamus gland. The corticotropin-releasing hormone (CRH)would travel from the hypothalamus to its portal system. Upon reaching the anterior pituitary, it stimulates the cleavage of the Proopiomelanocortin (POMC) into several molecules. The three major ones are ACTH, melanocyte stimulating hormone (MSH), and Beta-endorphins. ACTH would travel via the bloodstream to stimulate the adrenal cortex to help stimulate the production of cortisol. Cortisol also exerts negative feedback to inhibit the release of corticotropin-releasing hormone (CRH) and ACTH.
Prolactin (PRL)
The control of prolactin is primarily under the control of the hypothalamus. Dopamine is the predominant hormone in inhibiting the secretion of prolactin. When the hypothalamus receives afferent sensory nerve cell input from the nipple stimulation of lactating women, it will cause the feedback loop of inhibition of prolactin to reverse. Due to the suckling, there would be inhibition of dopamine release. This would allow an unopposed release of prolactin. Prolactin helps with the growth and development of the breasts and the maintenance of lactation in women.
Lutenizing hormone (LH) and follicle-stimulating hormone (FSH)
These two hormones are produced by the gonadotrophin cells in the anterior pituitary.
In women: LH acts on the ovaries to stimulate steroid hormone production. FSH stimulates the follicular development of the ovary in women, more specifically the granulosa cells.
In men: LH acts by stimulating the Leydig cells in the testes to secrete testosterone. FSH stimulates the Sertoli cells to secrete androgen-binding proteins to initiate spermatogenesis.
In both males and females, these hormones help stimulate the maturation of primordial germ cells.
The control of LH and FSH is under the control of gonadotropin-releasing hormone (GnRH) produced by the hypothalamus. There is a negative feedback loop for LH and FSH from steroid sex hormones. Inhibin allows for a more specific feedback for FSH.
Growth Hormone (GH) or Somatotropin
The growth hormone (GH) exerts its effects on many tissues. Growth hormone promotes linear growth via:
- The stimulation of protein synthesis
- Increasing the transport of amino acid through the cells
Growth hormone (GH) stimulates the many tissues and the liver to produce somatomedins. Somatomedin helps stimulates cell division and cell proliferation. Somatomedins have the following effects:
- Enhance T-cell proliferation
- Increase in the absorption of calcium from the intestines and decreases the loss from urinary excretion
- Stimulates hepatic glycogenolysis to raise levels of glucose in the blood
- Increase in the growth of soft and skeletal tissues
- Increase uptake of non-esterified fatty acids by the muscle.
Growth hormone-releasing hormone (GHRH) is released by the hypothalamus, on which it reacts with the cells in the anterior pituitary. There is negative feedback on GH from the increase in the circulating concentrations of GH and IGF-1.
Thyroid-stimulating hormone (TSH)
The purpose of thyroid-stimulating hormone (TSH) is to stimulate the thyroid gland to help in the production and the release of T3 and T4.
Thyrotropin-releasing hormone (TRH) is released via the hypothalamus to the anterior pituitary. The thyrotropin-releasing hormone (TRH) stimulates thyrotrophic cells to secrete thyroid-stimulating hormone (TSH).
Anterior pituitary hormones
Table 1. Hormones secreted by the anterior pituitary gland and their control
| Cell type | Hormone | % Pituitary cell population | Hypothalamic hormone | Predominant hypothalamic nucleus of synthesis |
|---|---|---|---|---|
| Thyrotroph† | TSH | 3–5% | TRH (+) | Paraventricular, anterior periventricular |
| omatostatin (-) | ||||
| Corticotroph † | ACTH | 15–20% | CRH (+) | Paraventricular, supraoptic |
| Vasopressin (augments CRH) | ||||
| Gonadotroph† | LH FSH | 10–15% | GnRH (+) | Arcuate |
| Somatotroph* | GH | 40–50% | GHRH (+) | Arcuate, anterior periventricular |
| Somatostatin (-) | ||||
| Lactotroph* | Prolactin | 10–25% | Dopamine (-) | Arcuate, paraventricular, unknown |
| TRH (+) | ||||
| PRF’s (+) |
Footnote:
† Basophils – stain with basic dyes
* Acidophils – stain with acidic dyes
(+), stimulatory; (-), inhibitory
Abbreviations: TRH = thyrotropin-releasing hormone ; CRH = corticotropin-releasing hormone; GnRH = gonadotropin-releasing hormone; TSH = thyroid-stimulating hormone; ACTH = adrenocorticotropic hormone; GH = growth hormone; PRF = prolactin releasing factor
[Source 5 ]Adrenocorticotropin (ACTH)
Adrenocorticotropic hormone (ACTH) is a hormone that stimulates the production of cortisol. Cortisol is a steroid hormone made by the adrenal glands that is important for regulating glucose, protein, and lipid metabolism, suppressing the immune system’s response, and helping to maintain blood pressure.
ACTH is produced by the anterior pituitary gland. Located below the brain in the center of the head, the pituitary gland is part of the endocrine system, a network of glands that work together to produce hormones that act on organs, tissues, and other glands to regulate systems throughout the body.
Normally, ACTH levels increase when cortisol is low and fall when cortisol is high. In response to a fall in the blood cortisol level, the hypothalamus produces corticotropin-releasing hormone (CRH). This stimulates the production of ACTH by the pituitary, which in turn stimulates the production of cortisol by the adrenal glands, small organs located at the top of each kidney. To make the appropriate amounts of cortisol, the hypothalamus, pituitary, and adrenal glands must be functioning properly.
Conditions that affect the hypothalamus, pituitary, or adrenal glands can interfere with regulating ACTH and cortisol production, increasing or decreasing how much of the hormones the glands produce. This can cause signs and symptoms associated with an excess or deficiency of cortisol. Conditions that affect ACTH include Cushing disease, Addison disease, and hypopituitarism. Some tumors found outside of the pituitary in locations such as the lungs can also increase cortisol concentrations by producing ACTH.
Prolactin (PRL)
Prolactin is a hormone whose primary role is to promote breast milk production (lactation). It is normally elevated in women during pregnancy and just after childbirth. It is normally low in men and non-pregnant women.
The brain chemical dopamine and hormone estrogen control prolactin production and release from the pituitary gland. During pregnancy, the hormones prolactin, estrogen, and progesterone stimulate breast development and milk production. Following childbirth, prolactin helps initiate and maintain the breast milk supply. If a woman does not breastfeed, her prolactin level soon drops back to pre-pregnancy levels. If she does nurse, suckling by the infant plays an important role in the release of prolactin. There is a feedback mechanism between how often the baby nurses and the amount of prolactin released by the pituitary as well as the amount of milk produced.
A common cause of an abnormally elevated prolactin level is a prolactinoma, a tumor of the pituitary gland that causes excess production of prolactin. Prolactinoma is the most common type of pituitary tumor and is usually benign. They develop more frequently in women but are also found in men. Symptoms can arise both from the unintended effects of excess prolactin, such as milk production in a woman who is not pregnant or nursing and, rarely, in a man (galactorrhea), as well as from the size and location of the tumor.
If the anterior pituitary gland and/or the tumor enlarge significantly, it can put pressure on the optic nerve, causing headaches and problems with vision. It can also interfere with the other hormones that the pituitary gland produces. In women, prolactinomas can cause infertility and irregularities in menstruation, while in men these tumors can cause a gradual loss in sexual function and libido. Left untreated, prolactinomas may eventually damage the surrounding tissues.
Lutenizing hormone (LH)
Luteinizing hormone (LH) is a hormone associated with reproduction and the stimulation of the release of an egg from the ovary (ovulation) in women and testosterone production in men.
Control of luteinizing hormone (LH) production is a complex system involving the hypothalamus in the brain, the pituitary gland, and the hormones produced by the ovaries and testicles.
In premenopausal women, several hormones rise and fall in a specific sequence during each menstrual cycle. During the cycle, luteinizing hormone (LH) stimulates ovulation and the production of other hormones, estradiol and progesterone.
Womens’ menstrual cycles are divided into follicular and luteal phases, with each phase lasting about 14 days. Near the end of the follicular phase, there is a mid-cycle surge of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). This surge triggers ovulation, causing the rupture of the egg follicle on the ovary and the release of the egg.
During the luteal phase, the site where the egg follicle ruptured becomes a “corpus luteum.” Luteinizing hormone (LH) secretion stimulates the corpus luteum to start producing progesterone. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels decline, while progesterone and estradiol concentrations increase. These hormone levels decrease in turn after several days if the egg is not fertilized. Menstruation starts and when it ends, the cycle begins again.
As a woman ages and menopause approaches, ovarian function wanes and eventually ceases. As this occurs, FSH and LH levels rise.
In men, LH stimulates Leydig cells in the testicles to produce testosterone. LH levels are relatively constant in men after puberty. A high testosterone level provides negative feedback to the pituitary gland and the hypothalamus, thus decreasing the amount of LH secreted.
In infants and children, LH levels rise shortly after birth and then fall to very low levels (by 6 months in boys and 1-2 years in girls). At about 6-8 years, levels again rise before the beginning of puberty and the development of secondary sexual characteristics.
Follicle-stimulating hormone (FSH)
Follicle-stimulating hormone (FSH) is a hormone associated with reproduction and the development of eggs in women and sperm in men.
Control of follicle-stimulating hormone (FSH) production is a complex system involving the hypothalamus in the brain, the pituitary gland, and the hormones produced by the ovaries or testicles. The hypothalamus releases gonadotropin-releasing hormone (GnRH), which stimulates the pituitary to release FSH and luteinizing hormone (LH), a closely related hormone also involved in reproduction.
- In women, follicle-stimulating hormone (FSH) stimulates the growth and maturation of eggs (follicles) in the ovaries during the follicular phase of the menstrual cycle. The menstrual cycle is divided into the follicular and the luteal phases, with each phase lasting about 14 days. During this follicular phase, follicle-stimulating hormone (FSH) initiates the production of estradiol by the follicle, and the two hormones work together in the further development of the egg follicle. Near the end of the follicular phase, there is a surge of FSH and luteinizing hormone. Release of the egg from the ovary (ovulation) occurs shortly after this surge of hormones. The hormone inhibin as well as estradiol and progesterone help control the amount of FSH released by the pituitary gland. Follicle-stimulating hormone (FSH) also facilitates the ability of the ovary to respond to luteinizing hormone (LH). As a woman ages and menopause approaches, ovarian function wanes and eventually ceases. As this occurs, FSH and LH levels rise.
- In men, follicle-stimulating hormone (FSH) stimulates the testicles to produce mature sperm and also promotes the production of androgen binding proteins. FSH levels are relatively constant in men after puberty.
- In infants and children, follicle-stimulating hormone (FSH) levels rise shortly after birth and then fall to very low levels by 6 months in boys and 1-2 years in girls. Concentrations begin to rise again before the beginning of puberty and the development of secondary sexual characteristics.
Disorders affecting the hypothalamus, pituitary, and/or the ovaries or testicles can cause the production of too much or too little FSH, resulting in a variety of conditions such as infertility, abnormal menstrual cycles, or early (precocious) or delayed sexual maturation (puberty).
Growth hormone (GH)
Growth hormone (GH) is a hormone that is essential for normal growth and development in children. It promotes proper linear bone growth from birth through puberty. In both children and adults, growth hormone helps regulate the rate at which the body both produces energy from food (metabolism) and makes lipids, proteins, and glucose (sugar). Growth hormone (GH) also helps regulate the production of red blood cells and muscle mass.
Growth hormone (GH) is normally released into the bloodstream in pulses throughout the day and night with peaks that occur mostly during the night. Because of this, a single measurement of the level of GH in blood is difficult to interpret and not usually clinically useful. The value will be higher if the sample is taken during a pulse and lower if it is taken during a period between pulses. GH stimulation and suppression tests are therefore often used to diagnose GH abnormalities.
Growth hormone (GH) deficiency
Children with insufficient growth hormone (GH) production grow more slowly and are smaller in size for their age. Some children have growth hormone deficiency at birth (congenital), but some may develop a deficiency later due, for example, to a brain injury or tumor. These conditions can affect the pituitary gland, causing a decrease in pituitary function, resulting in a lowered production of pituitary hormones (hypopituitarism). Sometimes, the cause of the deficiency is not known.
In adults, growth hormone plays a role in regulating bone density, muscle mass, and glucose and lipid metabolism. It can also affect heart and kidney function. Deficiencies may have begun in childhood or develop in adulthood. A deficiency can develop, for example, because of damage to the pituitary gland caused by a head injury, brain tumor, or surgery or radiation treatment. This can result in a decrease in pituitary hormones (hypopituitarism). The deficiency in growth hormone can lead to decreased bone density, less muscle mass, and altered lipid levels. However, testing for GH deficiency is not routine in adults who have decreased bone density and/or muscle strength or increased lipids. Growth hormone deficiency is a very rare cause of these disorders.
Thyroid-stimulating hormone (TSH)
Thyroid-stimulating hormone (TSH) is produced by the anterior pituitary gland, a small organ located below the brain and behind the sinus cavities. TSH stimulates the thyroid, a small butterfly-shaped gland located inside the neck in front of the windpipe, by binding to the TSH receptor to release the hormones thyroxine (T4) and triiodothyronine (T3) into the blood.
Thyroxine (T4) and triiodothyronine (T3) help control the rate at which the body uses energy. Most of the hormone produced by the thyroid is T4. This hormone is relatively inactive, but it is converted into the much more active T3 in the liver and other tissues.
Thyroid-stimulating hormone (TSH), along with its regulatory hormone thyrotropin releasing hormone (TRH), which comes from the hypothalamus, is part of the feedback system that the body uses to maintain stable amounts of thyroid hormones in the blood.
- When thyroid hormone levels decrease in the blood, the pituitary gland produces more thyroid-stimulating hormone (TSH) in response to thyrotropin releasing hormone (TRH) stimulation. Thyroid-stimulating hormone (TSH) in turn stimulates the thyroid to produce and release more T4 and T3.
- When thyroid hormone levels increase in the blood, the pituitary gland produces less thyroid-stimulating hormone (TSH), and the thyroid produces less T4 and T3.
When all three organs (hypothalamus, pituitary and thyroid) are functioning normally, thyroid production is regulated to maintain relatively stable levels of thyroid hormones in the blood.
If the thyroid releases inappropriately large amounts of T4 and T3, the affected person may experience symptoms associated with overactive thyroid (hyperthyroidism), such as rapid heart rate, weight loss, nervousness, hand tremors, irritated eyes, and difficulty sleeping. Graves disease is the most common cause of hyperthyroidism. It is a chronic autoimmune disorder in which the affected person’s immune system produces autoantibodies that act like TSH, bind and activate the TSH receptor, leading to the production of excessive amounts of thyroid hormone. In response, the pituitary produces less TSH, usually leading to a low level in the blood.
If there is decreased production of thyroid hormones by the thyroid (underactive thyroid or hypothyroidism), the person may experience symptoms such as weight gain, dry skin, constipation, cold intolerance, and fatigue. Hashimoto thyroiditis is the most common cause of hypothyroidism in the U.S. It is a chronic autoimmune condition in which the immune response causes inflammation and damage to the thyroid as well as the production of autoantibodies. However, the autoantibodies do not cause the hypothyroidism. The detection of thyroid-related autoantibodies (e.g., thyroperoxidase autoantibodies and/or thyroglobulin autoantibodies) indicate that thyroid autoimmunity is present. These autoantibodies can be detected in Graves disease or Hashimoto thyroiditis. With Hashimoto thyroiditis, the thyroid produces low levels of thyroid hormone. In response, the pituitary normally produces more TSH, usually resulting in a high level in the blood.
However, the level of TSH alone does not always predict or reflect thyroid hormone levels. Some people with pituitary disease produce an abnormal form of TSH that does not function properly. They often have hypothyroidism despite having normal or even mildly elevated TSH levels.
Rarely, pituitary dysfunction may result in increased or decreased amounts of TSH. In addition to pituitary dysfunction, hyperthyroidism or hypothyroidism can occur if there is a problem with the hypothalamus (insufficient or excessive thyrotropin releasing hormone [TRH]).
Simmonds disease cause
Simmonds disease is caused by destruction of the anterior lobe of the pituitary gland from any cause 1. The most common cause of Simmonds’ disease is post-partum necrosis of the anterior lobe of the pituitary. The original case described by Simmonds was of this type. Sheehan has stressed the importance of collapse at the time of the significant delivery, in the production of post-partum necrosis of the anterior pituitary. A history of a delivery complicated by severe ante-partum or post-partum hemorrhage can usually be obtained in cases of post-partum Simmonds disease. Many examples of Simmonds syndrome have been described in the literature. Destruction of the anterior lobe of the pituitary can be accomplished in other ways; trauma, especially bullet wounds and fractures of the base of the skull, may cause destruction of the parenchyma of the anterior pituitary. Acceptable cases of Simmonds disease due to trauma have been described. Tuberculosis and syphilis may destroy completely the pituitary gland and cases due to these causes have been described. Tumors of the pituitary and the supra-sellar cysts may, by compression, destroy the gland cells of the anterior lobe. Numerous examples of hypopituitarism due to the effects of tumors have been described.
Simmonds disease due to causes other than post-partum necrosis is much less common, but may be found in men. An incomplete syndrome usually results with hypogonadism as the predominating feature 1.
Simmonds disease prevention
Severe loss of blood during childbirth can often be prevented by proper medical care. Otherwise, Simmonds disease is not preventable.
Simmonds disease symptoms
Although cachexia may be present, Simmonds disease is characterized by symptoms due to decreased gonadal, thyroidal and adrenal function. These include:
- amenorrhea or oligomenorrhea, impotence, loss of libido
- tiredness, hypotension
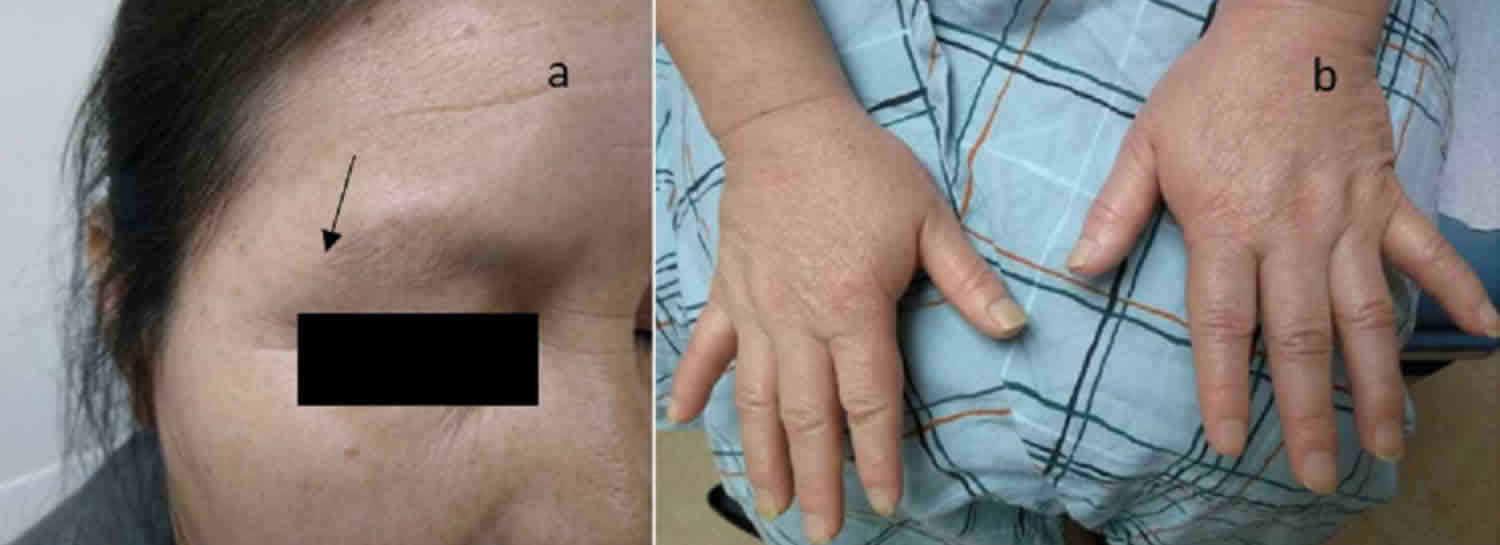
- waxy skin, loss of body hair
Other symptoms that may occur with Simmonds disease:
- Face swelling
- Hair loss
- Hoarseness or changing voice
- Joint stiffness
- Weight gain
The classical concept of the appearance of a patient suffering from Simmonds disease is of an emaciated, prematurely senile woman, but the true appearance is vastly different. These patients are of average nutrition and have a pale, dry, rather sallow skin. Their appearance suggests a degree of anemia much more severe than is found when hematological examinations are carried out. Progeria is unusual. The head hair is normal in amount and little altered in texture. The eye brows tend to be thin. The appearance of these patients suggests a diagnosis of anemia, with the possibility of a sub-thyroid condition 1. Anorexia nervosa is frequently confused with Simmonds disease, because emaciation is regarded as one of the principal signs of anterior hypopituitarism.
Symptoms of hypopituitarism include any of the following:
- Abdominal pain
- Decreased appetite
- Lack of sex drive (in men or women)
- Dizziness or fainting
- Excessive urination and thirst
- Failure to release milk (in women)
- Fatigue, weakness
- Headache
- Infertility (in women) or stopping of menstrual periods
- Loss of armpit or pubic hair
- Loss of body or facial hair (in men)
- Low blood pressure
- Low blood sugar
- Sensitivity to cold
- Short height (less than 5 feet or 1.5 meters) if onset is during a growth period
- Slowed growth and sexual development (in children)
- Vision problems
- Weight loss
Symptoms may develop slowly and may vary greatly, depending upon:
- The number of hormones that are missing and the organs they affect
- The severity of the disorder
Simmonds disease diagnosis
To diagnose Simmonds disease, there must be low hormone levels due to a problem with the pituitary gland. The diagnosis must also rule out diseases of the organ that is affected by this hormone.
Tests may include:
- Brain CT scan
- Pituitary MRI
- ACTH
- Cortisol
- Estradiol (estrogen)
- Follicle-stimulating hormone (FSH)
- Insulin-like growth factor 1 (IGF-1)
- Luteinizing hormone (LH)
- Osmolality tests for blood and urine
- Testosterone level
- Thyroid-stimulating hormone (TSH)
- Thyroid hormone (T4)
- Biopsy of the pituitary
Level of a pituitary hormone may be high in the bloodstream if you have a pituitary tumor that is producing too much of that hormone. The tumor may crush other cells of the pituitary, leading to low levels of other hormones.
Simmonds disease treatment
Simmonds disease treatment involves treating the underlying cause and replacement of the anterior pituitary hormones. If Simmonds disease is caused by a tumor, you may need surgery to remove the tumor. Radiation therapy may also be needed.
You will need lifelong hormone medicines to replace hormones that are no longer made by organs under the control of the pituitary gland. These may include:
- Corticosteroids (cortisol)
- Growth hormone
- Sex hormones (testosterone for men and estrogen for women)
- Thyroid hormone
- Desmopressin
Drugs are also available to treat related infertility in men and women.
If you take glucocorticoid medicines for pituitary ACTH deficiency, be sure you know when to take a stress dose of your medicine. Discuss this with your health care provider.
Always carry medical ID (card, bracelet, or necklace) that says you have adrenal insufficiency. The ID should also say the type of medicine and dosage you need in case of an emergency caused by adrenal insufficiency.
Simmonds disease prognosis
Hypopituitarism is usually permanent. It requires lifelong treatment with one or more medicines. But you can expect a normal life span with early diagnosis and treatment.
- SUMMERS VK. The diagnosis and treatment of Simmonds’ disease. Postgrad Med J. 1947;23(263):441-443. doi:10.1136/pgmj.23.263.441 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2529616/pdf/postmedj00634-0047.pdf[
][
][
][
]
- SIMMONDS’ DISEASE (PITUITARY CACHEXIA); REPORT OF A CASE. JASON E. FARBER. Annals of Internal Medicine 1940 13:11, 2171-2177 [
]
- Ilahi S, Ilahi TB. Anatomy, Adenohypophysis (Pars Anterior, Anterior Pituitary) [Updated 2018 Dec 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519039[
]
- Jirikowski GF. Diversity of central oxytocinergic projections. Cell Tissue Res. 2019 Jan;375(1):41-48[
]
- Nussey S, Whitehead S. Endocrinology: An Integrated Approach. Oxford: BIOS Scientific Publishers; 2001. Chapter 7, The pituitary gland. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27[
]