Curcumin
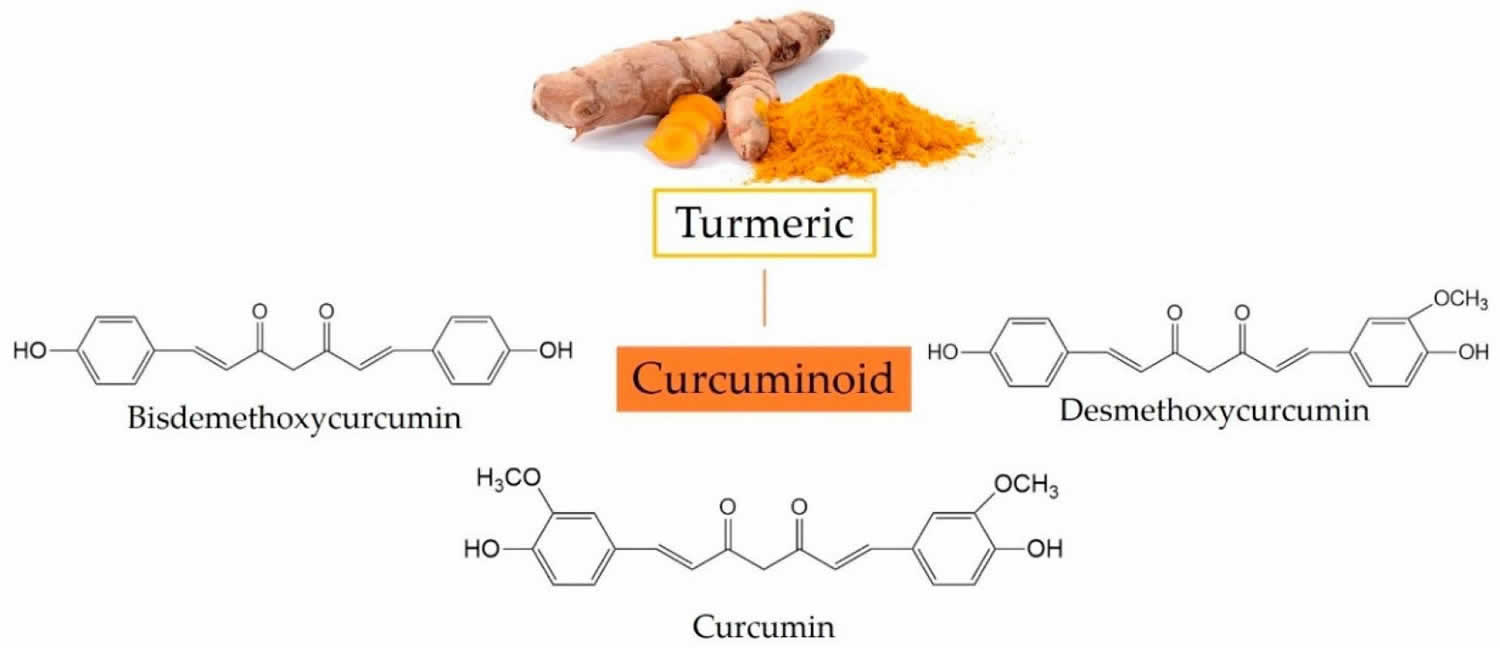
Curcumin also called Curcuma longa, Indian saffron or diferuloylmethane, is a natural bright yellow pigment obtained from the tubers of turmeric (Curcuma longa plant) in the ginger family, is native to Southeast Asia and is grown commercially in that region, primarily in India 1. Turmeric is used as a culinary spice and traditional medicine. Turmeric’s main active component — curcumin — is what gives a yellowish color to curry powder and is a major ingredient in curry powders common in many Indian and Asian dishes, and it is also used as a coloring for foods, fabrics and cosmetics. The extract of turmeric contains three major curcuminoids (Figure 1): Curcumin (60–70%), demethoxycurcumin (20–27%), and bisdemethoxycurcumin (10–15%) 2. The activities of turmeric are commonly attributed to curcuminoids (curcumin, demethoxycurcumin and others). The turmeric root can be dried and made into capsules, tablets, extracts, powders or teas. Or it can be made into a paste to apply to the skin. Turmeric may be unsafe for use during pregnancy in amounts greater than those commonly found in food. Little is known about whether it’s safe to use turmeric in amounts greater than those commonly found in food while breastfeeding.
Curcumin has been consumed for hundreds of years and is considered pharmacologically safe when taken at doses up to 100 mg/day 3. According to the Joint Food and Agriculture Organization/The World Health Organization (JECFA) report on food additives, the Acceptable Daily Intake Level (ADI) of curcumin is 0 to 0.1 mg/kg body weight 4. In clinical trials, curcumin was found to be safe and effective, and the US Food and Drug Administration (FDA) approved the curcumin as a “generally safe” (GRAS) compound 5.
Historically, turmeric was used in Ayurveda and other traditional Indian medical systems, as well as Eastern Asian medical systems such as traditional Chinese medicine. In India, it was traditionally used for disorders of the skin, upper respiratory tract, joints, and digestive system. Today, curcumin and its derivatives (curcuminoids) have received immense attention due to their biofunctional properties such as antioxidant, anti-inflammatory, immune regulatory, antiviral, antifungal, antibacterial, anticancer, antidiabetic, neuro-protective, cardiovascular protective, and hepatoprotective effects 6. These properties are attributed to the key elements in the curcumin structure 7. Turmeric is currently being promoted as a dietary supplement for a variety of conditions, including arthritis, digestive disorders, respiratory infections, allergies, liver disease, depression, and many others.
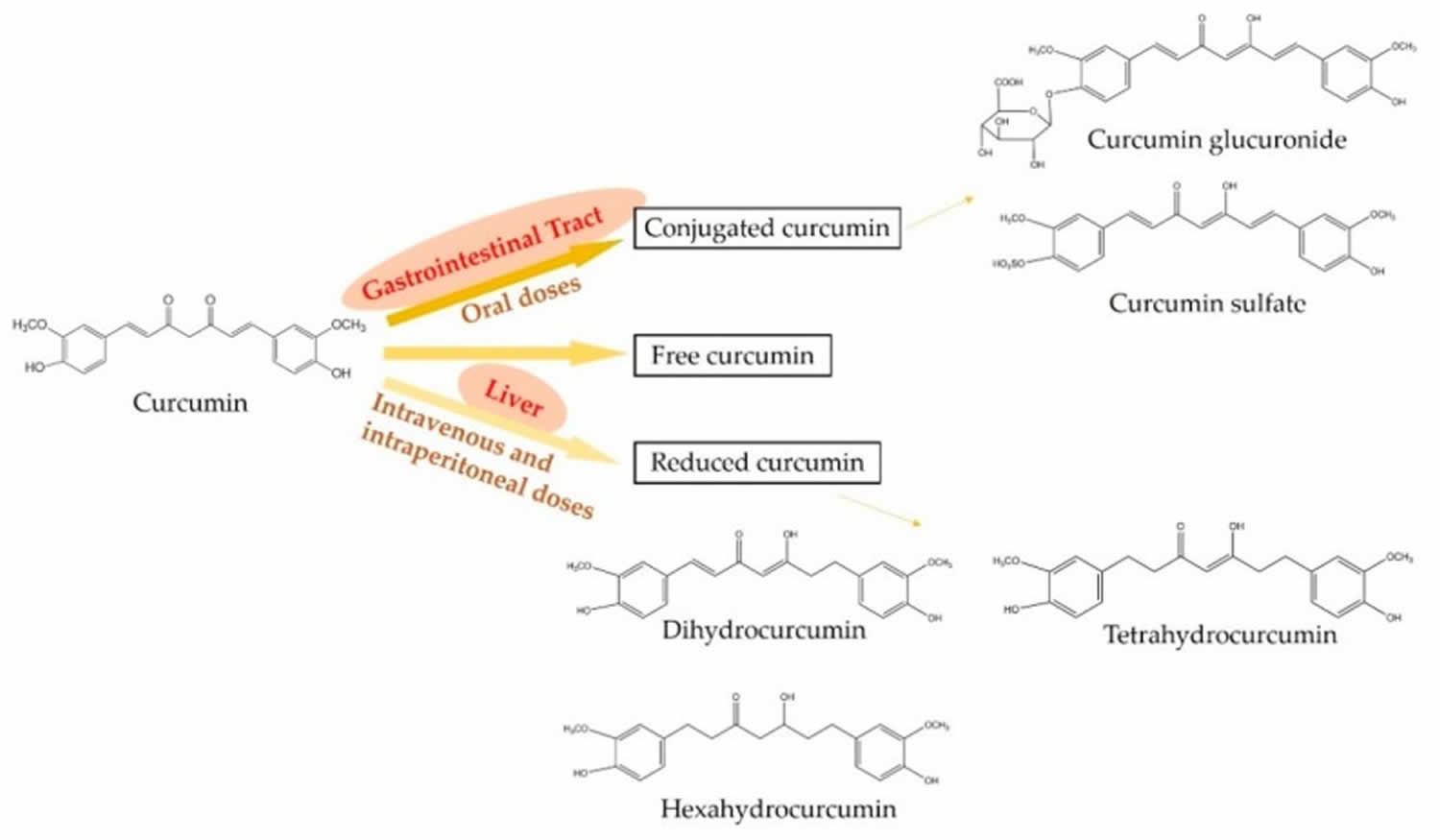
The metabolism of curcumin is critical for its potent biological activities as well as the beneficial effects on health 8. In the mammalian body, curcumin can be presented in three major forms and they are free, conjugated, and reduced curcumin states (see Figure 3) 9. Oral administration mainly metabolizes curcumin into the conjugated curcumin via glucuronidation and sulfation. Especially, the finding demonstrates that the gastrointestinal tract plays an important role in the glucuronidation of curcuminoids in human 10. In addition, intravenous or intraperitoneal administration leads to the reduction of curcumin into dihydrocurcumin, tetrahydrocurcumin, and hexahydrocurcumin 11. However, minor free and intact curcumin can be detected in plasma after the administration 12. On the other hand, the metabolism of curcuminoids mainly leads to reductive metabolites, for example, hexahydrocurcuminoids are the major reductive metabolites observed in both male and female rat liver 13.
Due to the low chemical stability and poor bioavailability of curcumin, more attention has been paid to its metabolites 14. Although the major structure of its metabolites is consistent with curcumin, the minor differences in the structure of metabolites improve their chemical stability. For example, the hexahydrocurcumin has the same phenolic groups or diketo moieties as curcumin, but has no olefinic double bonds, leading to hexahydrocurcumin being more stable than curcumin at a physiological pH of 7.4 15. In the intestine, the absorption of curcumin is poor, while the reductive and conjugated curcumin metabolites show moderate absorption 16. Hence, it is hypothesized that the biological effects of curcumin in tissues, such as liver and kidney, may be attributed to the curcumin metabolites 17. However, the metabolism or degradation of curcumin can affect the bioactivity of curcumin. A finding shows that curcumin induces arrest in the G2/M phase and mitotic catastrophe in three human cancer cell lines, while the reductive metabolism and chemical degradation inactivate the ability of curcumin to cause cancer cell death 18.
The relationship between curcumin and microbiota (community of micro-organisms) plays an important role in the gastrointestinal metabolism of curcumin 19. The main metabolic process in human intestinal microbiota includes demethoxylation, reduction, hydroxylation, methylation, and acetylation 20. A study used the bacteria strain, Bacillus megaterium DCMB-002, isolated from mice feces to treat with curcumin, and the bacteria have been found to transform curcumin into various metabolites 21. In addition, the human intestinal bacteria, Blautia sp. MRG-PMF1, have been reported to biotransform curcumin via the methyl aryl ether cleavage reaction 22.
Figure 1. Three major curcuminoids in turmeric and their chemical structures
[Source 23 ]Figure 2. Curcumin chemical structure
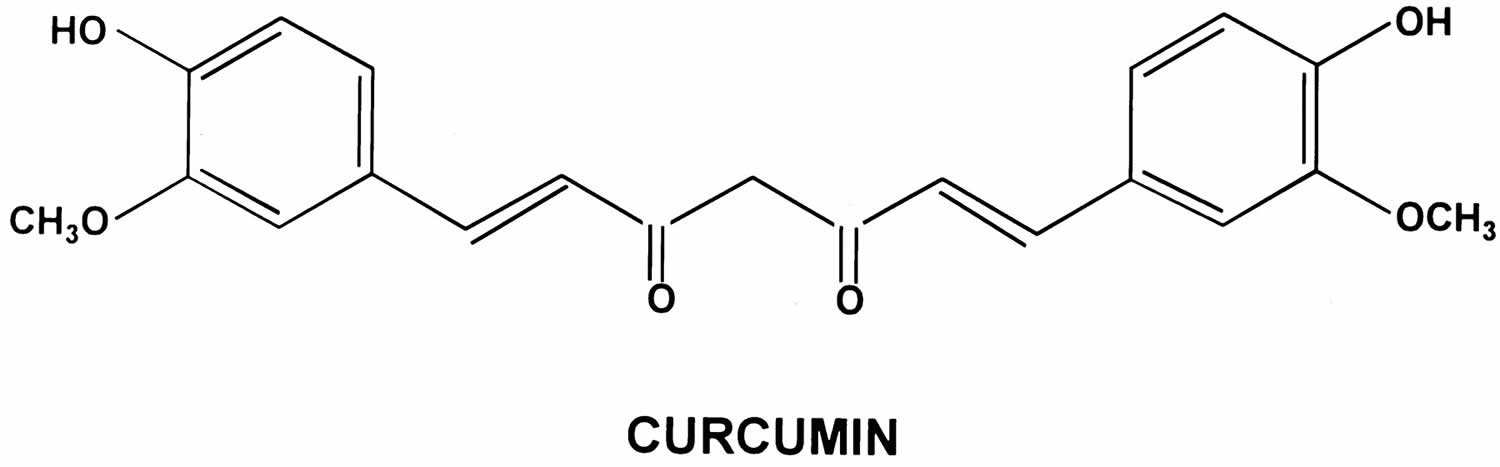
Figure 3. Metabolic pathways of curcumin
Footnotes: Oral administration mainly metabolizes curcumin into conjugated curcumin, while intravenous or intraperitoneal administration mainly leads to reduced curcumin. In addition, minor free and intact curcumin can be detected in plasma after any administration.
[Source 23 ]Curcumin health benefits
Much research has been done on substances from turmeric, overall, several clinical trials have demonstrated the beneficial effects of curcumin in patients with inflammation, cardiovascular diseases, metabolic syndrome, or diabetes 25. Turmeric and curcumin have a variety of interesting biological activities, but they’re challenging to study because curcumin is unstable (it easily changes into other substances) and has low bioavailability (not much of it reaches the bloodstream) when it’s taken orally. For instance, the dose of curcumin given orally is 8 g/day in humans, and the data shows that curcumin is rapidly transformed into metabolites, resulting in a low level of free curcumin in plasma (<2.5 ng/mL) 26. The poor aqueous solubility and low bioavailability of curcumin are considered as the main obstacles to its clinical development, and the metabolites and derivatives may be responsible for the biological activities rather than free curcumin 27. To enhance the stability and bioavailability of curcumin, many studies focus on modifications of curcumin and its delivery systems, such as nanoparticles, micellation, and conjugation with other materials 28. Such modifications can increase its stability, solubility, in vivo uptake, bioactivity, and safety 29. In addition, curcumin products may differ in composition or contain more substances than expected, which makes the results of research on these products difficult to understand and compare. Because the actions of turmeric and its components in people are complex and not well understood, no clear conclusions have been reached about whether these substances have benefits for health conditions. Curcumin (turmeric) has been widely used as a common household remedy for cough, sore throat and respiratory ailments in Asia.
In the past five years, some clinical trials have been conducted to investigate the effects of curcumin on patients with several diseases, such as metabolic syndromes, diabetes, arthritis, and gut inflammation 30. In clinical trials, curcumin is often used in combination with other agents or alone with delivery systems, such as nanoparticles and liposomal encapsulation 31, 32.
Oxidative stress plays an important role in the progression of nondiabetic or diabetic proteinuric kidney disease. Specifically, a randomized double-blind placebo-controlled clinical trial was performed to evaluate the antioxidant effects of curcumin on chronic kidney disease (CKD). One hundred and one patients with nondiabetic or diabetic proteinuric chronic kidney disease received a placebo or 320 mg of curcumin per day for eight weeks. Results showed that curcumin attenuates lipid peroxidation in plasma in patients with nondiabetic proteinuric CKD and improved the antioxidant capacity in patients with diabetic proteinuric chronic kidney disease 33.
Due to its anti-inflammatory activity, curcumin could provide an improvement on inflammatory diseases. Thirty-six patients with rheumatoid arthritis (RA, a chronic systemic inflammatory disorder) were randomly assigned to receive a placebo, 250mg, or 500 mg of curcumin twice daily for 90 days. At the end of the study, the clinical markers of inflammation, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values, were significantly improved in the groups receiving curcumin 34. In addition, the effects of curcumin on osteoarthritis (OA) were evaluated, and 22 patients with knee osteoarthritis took three caps of bio-optimized curcumin twice a day. The treatment with curcumin significantly reduced the serum levels of Coll2-1, a cartilage-specific biomarker, attenuating knee osteoarthritis 35. Psoriasis vulgaris is a common chronic inflammatory disease. Sixty-three patients with mild-to-moderate psoriasis vulgaris were treated with 2 g of topical steroids and oral curcumin per day, or with topical steroids alone for 12 weeks. In patients treated with oral curcumin, the psoriasis area and severity index values and serum levels of IL-22 were significantly reduced and the inflammation progression was ameliorated 36. Similarly, patients with moderate-to-severe psoriasis were treated orally with 0.4 mg/kg of acitretin and 3 g of nanocurcumin or only with acitretin every day for 12 weeks. It resulted in reduced psoriasis area and severity index values and unchanged cholesterol serum levels in patients treated with curcumin and acitretin 32. Furthermore, migraines are characterized by high levels of IL-6 and CRP, which disrupt the integrity of the blood-brain barrier and induce neurogenic inflammation. Eighty episodic migraine patients were randomized to receive a combination of omega-3 fatty acids (2500 mg) and nanocurcumin (80 mg) or omega-3 alone or nanocurcumin alone for two months. Notably, the combination of nanocurcumin and omega-3 downregulated IL-6 mRNA and decreased the serum levels of IL-6 as well as high sensitivity CRP (hs-CRP) 37.
Curcumin supplements are considered as a preventive agent and an adjuvant therapy of cancers. Oral leukoplakia is a potentially malignant lesion of the oral cavity. Subjects with oral leukoplakia (n = 223) randomly took orally 3.6 g of curcumin (n = 111) or a placebo (n = 112) daily for six months. The primary endpoint was a clinical response obtained by bi-dimensional measurement of the leukoplakia size at six months of recruitment. At the end of the study, the treatment with curcumin had no safety concerns and the subjects were well tolerated and given a durable clinical response for six months 38. Additionally, curcumin has been reported to have antitumor effects on glioblastoma cells in human. Thirteen glioblastoma patients received 70 mg of micellar curcuminoids, consisting of curcumin, demethoxycurcumin, and bis-demethoxycurcumin, three times per day for four days. After the intervention of curcuminoids, the mean ratio of phosphocreatine to inorganic phosphate decreased, and the mean intratumoral pH increased, attenuating intratumoral energy metabolism 39.
In clinical trials, curcumin has also been demonstrated to show protection against cardiovascular diseases. The progression of atherosclerosis can be accelerated by alpha 1-antitrypsin-low-density lipoprotein (AT-LDL). Subjects with stages I-II chronic obstructive pulmonary disease (COPD) were randomly treated with 90 mg of Theracurmin (R) (a highly absorptive curcumin using a drug delivery system) or a placebo twice a day for 24 weeks. The level of atherosclerotic low-density lipoprotein (LDL or “bad cholesterol”) was significantly lower in the curcumin group compared with the placebo group 40. The curcumin extract was evidenced to have a lipid-lowering effect via a clinical trial of 65 patients with metabolic syndromes. Patients were randomized into two groups to take a 630 mg curcumin extract capsule or a placebo capsule thrice daily for 12 weeks. The level of high-density lipoprotein cholesterol (HDL or “good cholesterol”) were significantly increased, and the levels of LDL and triglycerides were significantly reduced after curcumin extract treatment for 12 weeks 41. A six-month clinical trial with type 2 diabetic patients shows that curcumin intervention significantly reduces pulse wave velocity, increases the level of serum adiponectin, and decreases the level of leptin, lowering the atherogenic risks 42. A randomized double-blind placebo-controlled trial including 33 patients with coronary artery disease revealed that the intake of curcumin significantly ameliorates dyslipidemia in patients. Curcumin was observed to control the blood lipid levels by decreasing the serum levels of triglycerides, LDL-cholesterol, and very low density lipoprotein-cholesterol (VLDL-C) 43.
Curcumin supplementation may exert beneficial effects on the management of diabetes. In an open-label, randomized control trial, eight type-2 diabetic patients were treated with curcumin capsules on glyburide therapy for 10 days. Results show that the levels of blood glucose, LDL, VLDL, and triglycerides were decreased significantly, and the content of HDL was increased. The findings indicate that the combination of curcumin capsules with glyburide contributes to better glycemic control in diabetic patients 44. In addition, 70 type-2 diabetic patients randomly received 80 mg of nano-micelle curcumin or a placebo daily for three months, and in the group with nano-micelle curcumin, a significant decrease was observed in glycated hemoglobin (HbA1C) and fasting blood glucose (FBG) which are biochemical parameters related to diabetes 45.
Anticancer activity of curcumin
Curcumin is shown to have anticancer potential for the prevention and treatment of different cancers 46. The underlying anticancer mechanisms of curcumin mainly depend on the inhibition of cancer cell growth, induction of cancer cell apoptosis, and suppression of cancer cell metastasis 47. Curcumin disturbs the balance in the mitochondrial membrane potential, leading to enhanced suppression of the Bcl-xL protein 48. The extrinsic apoptotic pathway works through increasing the death receptors (DRs) on cells and triggering the tumor necrosis factor (TNF)-related apoptosis. Curcumin also contributes to this pathway by upregulating the expression of death receptors DR 4 and DR 5 49. Test tube studies showed a remarkable ability of curcumin and its derivatives to induce apoptosis in different cell lines by inhibiting or downregulating intracellular transcription factors. These factors include NF-κB, activator protein 1 (AP-1), cyclooxygenase II (COX-2), nitric oxide synthase, matrix metalloproteinase-9 (MMP-9), and STAT3 50. A recent work has found a new anticancer mechanism for curcumin by decreasing the glucose uptake and lactate production (Warburg effect) in cancer cells via downregulation of pyruvate kinase M2 (PKM2). The inhibition of PKM2 was achieved by suppressing the mammalian target of rapamycin-hypoxia-inducible factor 1α (TOR-HIF1α) 51. Several studies have investigated the ability of curcumin and its derivatives to suppress multiple different carcinomas by interacting with different molecular targets.
Curcumin is also considered as an auxiliary agent, and intensifies the therapeutic effects of other cancer treatments 23. When used in radiation therapy, curcumin prevents radiation-induced activation of the NF-κB pathway, increases sensitivity to ionizing radiation and apoptosis of tumor cells, and decreases tumor cell proliferation, improving anticancer efficacy 52. Moreover, the addition of curcumin is found to potentiate 5-fluorouracil-induced reduction of proliferation and invasion in colorectal cancer cells 53 and curcumin enhances the chemosensitivity of colorectal cancer cells to 5-fluorouracil and induces apoptosis. These effects are speculated to be associated with the downregulation of NF-κB activation, inhibition of AMP-activated protein kinase (AMPK)/unc-51-like kinase (ULK1)-dependent autophagy, and the suppression of Akt activity 54.
Prostate cancer
A recent estimate reported by the American Cancer Society revealed that 2.9 million men have been diagnosed with prostate cancer in the United States 55, making it the second leading cause of cancer death in men 56. Curcumin has shown a strong ability to inhibit proliferation and induce apoptosis in prostate cancer both in vitro and in vivo 57 by interfering with a number of cellular pathways, including mitogen-activated protein kinase (MAPK), epidermal growth factor receptor (EGFR), and nuclear factor κ (NFκB) 58. A recent study has revealed the ability of curcumin to activate protein kinase D1 (PKD1), leading to attenuation of the oncogenic signaling by β-catenin and MAPK [100] and consequent inhibition of prostate cancer 59. Moreover, PKD1 was found to be severely downregulated following progression from androgen-dependent to androgen-independent prostate cancer 59 and to affect the motility and invasion of prostate cancer via interaction with E-cadherin 60. Therefore, it has been considered as a new therapeutic target for cancer in general and for prostate cancer in particular 61. In addition to curcumin, some of its derivatives have also shown anticancer activity against prostate cancer. Metallo-curcumin conjugated DNA complexes exhibited significant toxicity to prostate cancer cells (PC3, 22Rv1, TRAMP-C1, LNCaP, and DU145) 62. Dimethyl curcumin (ASC-J9) has also shown very good activity in enhancing androgen receptor degradation in androgen-dependent prostate cancer 63.
Colorectal cancer
Colorectal cancer comes third behind prostate cancer and lung cancer as the most common form of malignant cancer 64. Although patients diagnosed with colorectal carcinoma undertake surgical removal of the tumor tissue along with chemotherapy, more than half of the patients suffer from relapses 65. Administration of curcumin was found to reduce M (1) G levels in the malignant colorectal cells without changing COX-2 protein levels 66. In addition, curcumin treatment was able to downregulate miR-21 gene, which is overexpressed in colorectal cancer cells, by inhibiting AP-1 (activator protein) binding to miR-21 promoter 60. Treating HCT 116 colorectal cancer cells with curcumin resulted in a cell cycle arrest in the G2/M phase via miR-21 gene regulation and inhibited the tumor tissue growth 60. However, an in vivo study in mice with colorectal cancer demonstrated an improved response to radiation therapy when combined with curcumin due to its ability to target nuclear factor (NF-κB) 67. Another study has managed to enhance curcumin inhibition activity against colon cancer cells by combining it with ERRP, a pan-erb B inhibitor 68.
In a study in which 15 patients with advanced chemotherapy-resistant colorectal cancer were given curcumin extract in capsular form daily for up to 4 months, the intake of 440 mg/day of turmeric capsule for 29 days resulted in a reduction of lymphocytic glutathione S-transferase (GST) activity by 59%. However, the same effect was not observed at higher doses and it was emphasized that 2.2 g/day of turmeric (containing 180 mg of curcumin), which is thought to be safe, may be given to the patients, but larger clinical studies should be performed 69. In another study conducted by Sharma et al. 70, 15 patients with advanced chemotherapy-resistant colorectal cancer were given 0.45 to 3.6 g of curcumin per day for up to 4 months and it was found that 3.6 g of curcumin significantly reduced inducible Prostaglandin E2 (PGE2) production by 62% and 57%, respectively in the blood samples on the 1stand 29thdays. In the study of Garcea et al. 71, curcumin capsules were given to individuals with colorectal cancer for 7 days (3.6 g/day, 1.8 g/day or 0.45 g/day) and the levels of M(1)G, which are adenomatous burden indicators, were found two and a half times higher in the malign tissue than the normal tissue. Curcumin sulfate and curcumin glucuronide were detected in the tissue of these patients. The result of the study showed that the daily dose of 3.6 g curcumin reduced M(1)G levels in malignant colorectal tissues but did not affect the cyclooxygenase-2 (COX-2) enzyme levels. Studies show evidence of effect of curcumin on colorectal cancer, however, more clinical studies are needed for clearer results on this subject.
More recently, the safety and efficacy of curcumin in familial adenomatous polyposis was evaluated in a double-blinded randomized trial by Cruz-Correa et al. 72. In this study, 44 patients with familial adenomatous polyposis with at least five intestinal adenomatous polyps who had not undergone colectomy were included in this trial and were randomly allocated to two groups receiving either pure curcumin (3 g per day orally) or placebo for 12 months. The main outcome measures were the number and size of lower gastrointestinal tract polyps, which were assessed every four months for one year. At the end of the study, no significant difference was found in the mean number or mean size of polyps between the curcumin group and the placebo group. The adverse effects were very few, and not significantly different from the placebo group. These results show the low efficacy but high safety of oral curcumin at the administered dose in patients with familial adenomatous polyposis 72.
Pancreatic cancer
In a 2010 study by Epelbaum et al. 73, patients with either advanced local or metastatic pancreatic cancer were treated with a combination of curcumin (8 g/day per oral) and gemcitabine (1000 mg/m2 intravenous once per week) for three out of four weeks of each chemotherapy cycle. The primary outcome was time to tumor progression and the main secondary outcome was toxicity profile. In the study, eight out of seventeen patients were noncompliant with curcumin due to abdominal pain, five of which discontinued treatment before two weeks and three received adjusted doses of curcumin for the rest of the study (4 g/day). One patient died during the first cycle due to cardiac problems not associated with curcumin. One patient developed grade II neutropenia and one patient grade I thrombocytopenia. The time to tumor progression was between one and twelve months (median two months), and the overall survival time was between one and 24 months (median 6). Based on these results, they concluded that the combination therapy with curcumin and gemcitabine in pancreatic cancer is feasible; however, the dose of curcumin should be less than 8 g/day 73.
Head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common form of cancer worldwide, with more than 30,000 diagnosed cases every year 74. Head and neck squamous cell carcinoma generally arises in the oral cavity, paranasal cavities, larynx, and pharynx 75. In vitro studies of curcumin in different head and neck cancer cell lines have proven its ability to inhibit cell growth due to its effects on a number of cellular pathways involved in cell proliferation, most notably NF-κB and STAT3, which are found to be overexpressed in several head and neck carcinomas 76. Curcumin was shown to downregulate NF-κB and inhibit the interleukin-6 (IL-6)-mediated phosphorylation of STAT3, thus inhibiting the proliferation of the cancer cells 77.
Kim et al. 78 performed a pilot study in patients with head and neck squamous cell carcinoma to determine the effect of curcumin on inhibiting IκB kinase β (IκKβ) activity and suppressing the proinflammatory cytokines. The patients were asked to chew curcumin tablets (2 mg), their saliva samples were collected before and after chewing the tablets, and the IκKβ activity was measured, as well as the levels of salivary cytokines interleukin (IL)-6 and IL-8. Curcumin resulted in a reduction in IκKβ activity in the salivary cells of head and neck squamous cell carcinoma patients. There was a brief reduction in IL-8 expression in eight of 21 post-curcumin samples; however, this reduction was not statistically significant. On the other hand, there was a marked decrease in the expression of other cytokines, including IL-10, IFN-γ, IL-12p70, and IL-2 clustered together, and also granulocyte macrophage colony stimulating factor (GMCSF) and TNF-α clustered together. These results show the inhibitory effect of curcumin on IκKβ activity in the salivary cells of patients with head and neck squamous cell carcinoma; therefore, they suggested considering IκKβ as a biomarker for detecting the effect of curcumin in head and neck cancer 78.
Breast cancer
Breast cancer is a leading cause of death in women 79. Despite lumpectomy, radiation therapy, chemotherapy, and endocrine therapy, the recurrence rate of breast cancer has been reported to be still high based on a meta-analysis of 21 retrospective studies 80. Therefore, there is still a need for more efficient therapeutic strategies. In a study on MCF-10A human mammary epithelial cells and MCF-7 breast cancer cells [114], a tangible drop in telomerase activity was observed as a result of treatment with curcumin in a concentration-dependent manner which was correlated to downregulation of hTERT by curcumin but not through the c-Myc mRNA pathway 81. The effect of curcumin on cell-cycle regulatory proteins, matrix metalloproteinases and NF-κB was evaluated in MDA-MB-231 and BT-483 breast cancer cell lines 82. In agreement with the previous studies on other breast cancer cell lines, this study also confirmed the ability of curcumin to downregulate NF-κB, leading to an antiproliferative effect 83. However, a decrease in cyclic D1 in MDA-MB-231 cells and a decrease in CDK4 BT-483 were observed after treatment with curcumin 82. Combining arabinogalactan and curcumin enhanced apoptosis induction by increasing ROS levels, disturbing the mitochondrial membrane and decreasing glutathione in MDA-MB-231 cell line 84. Moreover, curcumin led to the inhibition of breast tumor via overexpression of the p53 gene and reduction of antigen ki-67 levels 84. Another study on MDA-MB-231 cells has shown that curcumin also inhibits inflammatory cytokines CXCL1/2. Inhibiting CXCL1 and 2 by curcumin results in inhibiting the expression of a series of metastasis-promoting genes such as chemotactic receptor CXCR4 85. Dimethyl curcumin (ASC-J9) has also been reported to be effective against estrogen-dependent breast cancer via inhibiting several types of steroid receptors 86.
Bayet-Robert et al. 87 evaluated the feasibility and tolerability of a combination of curcumin and docetaxel in 14 patients with metastatic or locoregionally recurrent advanced breast cancer in an open-label phase 1 dose escalation clinical trial. The patients received an I.V. infusion of Docetaxel (100 mg/m²) every three weeks for six chemotherapy cycles, and oral curcumin (starting from 500 mg/day and increased until a dose-limiting toxicity would occur) for seven consecutive days in each cycle (from five days before to two days after administration of docetaxel). The primary endpoint was the maximal tolerated dose of curcumin when administered in combination with a standard dose of docetaxel in the patients. Secondary outcomes were toxicity, safety, and clinical response to the combination therapy, as well as levels of CEA tumor marker and vascular endothelial growth factor (VEGF) as a positive endogenous modulator of angiogenesis.
The maximal tolerated dose of curcumin was found to be 8 g/day as in higher doses, dose-limiting toxicities (neutropenia, anemia, and severe diarrhea) were observed, leading to the discontinuation of the trial in two patients. Other toxicities (oral cavity mucositis, hand-foot syndrome, nail changes, dermal changes, conjunctivitis, and fatigue) were either not persistent or were treated easily so did not affect the continuation of the trial. However, due to noncompliance of a number of patients with the doses higher than 6 g/day, in the end, this dose was recommended as the maximal tolerated dose to be considered for phase 2 clinical trials. In terms of clinical and biological response (decrease in CEA tumor marker across the treatment and regression of nonmeasurable lesions), some degree of improvement was observed in most patients, with five patients showing a partial response to treatment and three patients having stable disease at least six weeks after the last cycle of treatment. No disease progression was observed in any of the patients. Moreover, curcumin/docetaxel combination significantly decreased the levels of VEGF after three cycles of treatment 87.
Brain cancer and glioblastoma
Glioblastoma (GBM) is the most common malignant brain cancer in humans, accounting for about 15% of all central nervous system tumors 88. In the treatment of brain tumors and glioblastoma, surgical intervention and radiation therapy are limited due to infiltration of cancer cells into the healthy brain, leading to damaging effects after treatment 89. Therefore, alternative therapies using naturally derived compounds such as curcumin with less side effects than the conventional treatments are receiving more attention. Curcumin has multiple molecular targets, therefore, combating brain tumors may take different cellular pathways, including apoptosis, autophagy, angiogenesis, invasion, and metastasis 88. Although penetrating the blood–brain barrier (BBB) is considered the rate-limiting step for many anticancer agents, curcumin was able to cross the blood–brain barrier in high levels 90. Moreover, an animal study using human glioma U-87 cells xenografted into athymic mice showed that curcumin is able to suppress glioma angiogenesis through inhibiting MMP-9 and downregulating endothelial cell markers (CD31 and CD105 mRNA) 90. Curcumin was also able to induce G2/M cell cycle arrest by increasing protein kinase 1 (DAPK1) in U-251 malignant glioblastoma cells, which indicates that suppressing DAPK1 by curcumin does not only induce cell arrest but also inhibits STAT3 and NF-κB and activates caspase-3 91.
Antioxidant activity of curcumin
The imbalance between free radicals and the body’s defense system against oxidative stress can cause various chronic diseases 92. An excessive production of reactive oxygen species (ROS) can induce oxidative stress and damage essential biomolecules, while antioxidants, including antioxidant enzymes and antioxidant compounds, can protect the human body from free radicals and ROS effects, attenuating the progress of many chronic diseases 93. Both in vitro and in vivo studies have revealed the antioxidant activity of curcumin contributes to its diverse therapeutic effects. The research on the chemical structure of curcumin shows that electron-donating groups of curcumin, especially the phenolic hydroxyl group, are the main contributors to its antioxidant activity 94.
Curcumin mainly reduces the oxidative stress by scavenging free radicals 95. Studies have shown that curcumin can directly remove the excessive free radicals and prevent ROS production 96. In A549 cells with influenza A virus (IAV)-induced oxidant stress, curcumin treatment is found to inhibit the production of ROS as well as the activation of toll-like receptor, which may be responsible for the suppression of influenza A virus infection 97. Quinocetone causes genotoxicity and oxidative stress in human hepatocyte L02 cells, and curcumin pretreatment markedly inhibits excessive ROS generation, with the suppression of the decrease in the activity of antioxidant enzymes, like superoxide dismutase (SOD), and levels of antioxidant constituents, such as glutathione (GSH) 98. In addition, in diabetic mice, curcumin treatment inhibits ROS production and hyperglycemia-induced oxidative stress by restoring the functions of DNA methyltransferase (DNMT) 99.
On the other hand, curcumin can also increase the activities of antioxidant enzymes. Treatment of curcumin significantly increases the activity of paraoxonase 1 arylesterase (PON1), reduces the susceptibility of low-density lipoprotein (LDL or “bad cholesterol”) oxidation, and restores the abnormal biochemical parameters caused by HgCl2 100. Additionally, the administration of curcumin results in amelioration of aflatoxin B1-induced effects via increases in the level of GSH, gene expression, and activities of antioxidant enzymes, such as catalase, SOD, glutathione peroxidase (GSH-Px), and glutathione-S-transferase 101. Furthermore, curcumin significantly reverses the decreased activity of SOD induced by zymosan and attenuates the increase of the level of malondialdehyde (MDA) 102. In allergic rhinitis, the curcumin fed rats show higher tissue GSH levels in inferior turbinate tissues and GSH-Px activity in serum than those of the control 103. In addition, curcumin increases the glutathione level in erythrocytes and plasma, while simultaneously decreases the oxidant potential of plasma 104. Furthermore, an in vitro study indicates that curcumin blocks nuclear factor κB (NF-κB) activation due to its antioxidant activity 105.
Considering the potent antioxidant activity, curcumin has been found to scavenge free radicals, restore abnormal alternations induced by external factors, and repress transcription factors related to oxidation. These effects help reduce oxidative stress and lower the risk of various chronic diseases. However, it should be pointed out that a compound with antioxidant activity in test tube study cannot represent an effective antioxidant in vivo, and it can be a pro-oxidant under certain conditions, showing no health benefits 106.
Anti-inflammatory activity of curcumin
Curcumin can also protect against inflammation effectively via modulating pro-inflammatory cytokines and related signaling pathways, such as NF-κB, peroxisome proliferator-activated receptor-gamma (PPAR-γ), and myeloid differentiation protein 2-TLR 4 co-receptor (TLR4-MD2) signaling pathways 107. The inflammatory response is often accompanied by the excessive production of pro-inflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β). Therefore, the downregulation of proinflammatory cytokines may effectively reduce the incidence of inflammation 108. Pretreatment of curcumin on human genital epithelial cells abrogates the glycoprotein 120-mediated upregulation of the pro-inflammatory cytokines, TNF-α, and IL-6, as well as the chemokines, IL-8, RANTES (regulated on activation, normal T cell expressed, and secreted), and interferon γ-induced protein-10 (IP-10) 109. Moreover, curcumin-loaded solid lipid nanoparticles can effectively decrease the expression of serum pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β 110. Also, the liposomal curcumin complex effectively decreases pro-inflammatory cytokine and chemokine expression in synovial fibroblasts and macrophages without affecting cell viability, showing less toxicity compared to free curcumin 111: Toxicity and biological activity in synovial fibroblasts and macrophages. In Vivo. 2016;30:413–419.)). The degradation product of curcumin, 4-vinyl guaiacol, has been reported to decrease IL-6 gene expression in lipopolysaccharide (LPS)-stimulated mice macrophages 112.
The upregulation of anti-inflammatory cytokines is also essential for the reduction of the inflammatory response. Results show that curcumin can inhibit inflammation and increase M2-like macrophages in white adipose tissues, promoting the production of anti-inflammatory cytokines 113. Moreover, the animal study reveals that curcumin exhibits antiepileptogenic effects by upregulating the gene expression of anti-inflammatory cytokines, such as interleukin 10 receptor (IL-10R), chemokine (C-X-C motif) ligand 16 (CXCL16), and CXCL17 114. In cultured macrophages, it is observed that macrophages uptake the curcumin-loaded nanoparticles, and significantly increase the release of anti-inflammatory factors, including transforming growth factor-beta (TGF-β) and IL-10 115. In summary, curcumin exhibits anti-inflammatory activity via the regulation of pro-inflammatory cytokines, such as IL-6, TNF-α, and IL-1β, as well as anti-inflammatory cytokines, such as IL-10 and TGF-β.
The anti-inflammatory property of curcumin is also involved in other signaling pathways. Curcumin is also found to induce degranulation in human neutrophils by increasing the cell surface expression of cluster of differentiation 35 (CD35) (secretory vesicle), CD63 (azurophilic granules), and CD66b (gelatinase granules) 116. The control of neutrophils may be a potential anti-inflammatory mechanism of curcumin. In addition, curcumin exhibits anti-inflammatory activity via the PPAR-γ. A study finds that curcumin decreases the production of NO and suppresses the proliferation of vascular smooth muscle cells by elevating PPAR-γ activity, so as to attenuate angiotensin II-induced inflammatory responses 117. Additionally, it has been reported that the TLR4-MD2 signaling complex is inhibited by curcumin and its analogs. It is supposed that curcumin and its analogs can compete with lipopolysaccharide for binding on MD2 and finally reduce the inflammation 107. Overall, curcumin also exhibits anti-inflammatory activity by interacting with inflammation-related signaling pathways, such as NF-κB, PPAR-γ, and TLR4-MD2 signaling pathways.
Immune-regulatory activity of curcumin
Numerous studies have indicated that curcumin is beneficial to the immune system, and it interacts with immune cells to protect against immune-related diseases by modulating various immune cells, such as various T lymphocyte subsets, macrophages, dendritic cells, B lymphocytes, and natural killer cells, and improving the aberrant alternations of immunological parameters 118. Curcumin can reduce the numbers of neutrophil and eosinophil, and increase the lymphocyte counts 119. In addition, Th1 cells produce IFN-γ, TNF-α, and IL-1β, while Th2 cells produce IL-4, IL-10, and TGF-β. Th17 cells can produce IL-17, and the immune suppressive Treg cells can prevent autoimmune disease. The balance of this subpopulation of T cells is crucial for the modulation of the immune system 120. Meanwhile, pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages correspond to Th1 and Th2 cells, respectively. The levels of M1 and M2 macrophages are of great importance for the immune system homeostasis 121. Curcumin poses a stimulatory effect on Th1 cells and an inhibitory effect on Th2 cells, regulating the Th1/Th2 balance in ovalbumin-sensitized rats. Curcumin exhibits protective effects against immunotoxicity induced by 2-amino-3-methylimidazole (4,5-f) quinoline, which diminishes the percentage of blood lymphocytes 122. It has also been reported that curcumin attenuates experimental autoimmune myasthenia gravis via the regulation of various immune cells 123. Specifically, it inhibits the expression of T cell co-stimulatory molecules, shifts the balance from Th1/Th17 toward Th2/Treg, increases the number of NKR-P1+ cells, and promotes the differentiation of B cells into a subset of B10 cells. Also, an in vitro study of bone marrow-derived mesenchymal stem cells with curcumin shows an increasing trend towards M2 macrophage polarization, providing a favorable immune microenvironment for cutaneous wound healing 121. Moreover, curcumin mediates a lower level of macrophage infiltration and inhibits NF-κB activation in macrophages 124. An in silico study indicates that the antioxidant property and strong binding affinity with CD4 and CD8 receptors of curcumin inhibit the thymic apoptosis induced by deltamethrin 125. Furthermore, curcumin has been reported to provide a protection of immunity by reversing type-2 cytokine bias, reducing the Treg cell population, and inhibiting T cell apoptosis 126.
In fish fed with the dietary curcumin, it has been observed that the lysozyme activity, total IgG, and IgM levels increase after curcumin treatment, suggesting an effect on improving the immune responses to Aeromonas hydrophila 127. For the weaned piglets orally administered with the enterotoxigenic Escherichia coli, the treatment of curcumin increases the secretory immunoglobulin (sIgA), improving the immune status 128. Moreover, curcumin is able to modulate the production of immune mediators. For example, it can induce the increase of IFN-γ as well as the decrease of IL-1β, IL-4, IL-6, IL-17A, and TNF-α 129.
Overall, curcumin mainly improves the immune system via the interaction with immune cells, such as lymphocytes and macrophages. Also, curcumin induces immune responses by modulating immune molecules, such as IgG, IgM, and sIgA.
Neuroprotective activity of curcumin
Curcumin has shown the potential as a neuroprotective agent due to its antioxidant and anti-inflammatory activities and the ability to maintain the chemical balance in the brain 95. Scientists have revealed that curcumin pretreatment is effective to reduce H2O2-induced neurotoxicity in PC12 cells by attenuating caspase activation, poly (ADP-ribose) polymerase (PARP) cleavage, DNA damage, and the accumulation of ROS, and dysregulation of the MAPK and Akt pathways. These protective effects can be used in the therapy for human neurodegenerative diseases 130. Furthermore, the neuroprotective property of curcumin can reduce H2O2-induced damage in the SH-SY5Y human neuroblastoma cell line, and enhance cell viability 131. Anti-neuroinflammatory results elucidate that curcumin inhibits the secretion of pro-inflammatory mediators and cytokines, induces HO-1 transcription and translation, and modulates NF-κB and MAPK signaling pathways, reducing inflammation in microglial cells 132. The suppression of inflammation by curcumin leads to the reduction in astrocyte hypertrophy and the activation of microglia in the hippocampus, contributing to a better memory and mood function 133. Furthermore, the pretreatment of curcumin is also effective to attenuate brain trauma and improve neurological function, alleviate neuropathic pain, mitigate axonal injury, and protect against ischemia and reperfusion injury 134.
Test tube and animal studies have shown the potential of curcumin as an adjuvant for the treatment of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease. In neurodegenerative diseases, curcumin treatment can bind to some proteins or limit the aggregation of other protein to maintain homeostasis of the inflammatory system and facilitate the heat shock system 135. Numerous studies acknowledge that the accumulation of amyloid-β protein and hyperphosphorylated tau proteins is the hallmark of Alzheimer’s disease. Curcumin may directly bind to PPAR-γ and increase transcriptional activity and protein levels of PPAR-γ, which alleviates amyloid-β-induced neuroinflammation and improves neuronal status in the rat model of Alzheimer’s disease 136. The toxicity of the amyloid-β peptide damages spatial learning and memory through the c-Jun N-terminal kinase (JNK) signaling pathway in Alzheimer’s disease. After treating with curcumin, Alzheimer’s disease rats show restored spatial learning and memory deficits, and JNK-3 phosphorylation is inhibited 137. In mice with Alzheimer’s disease, active avoidance and locomotor activity are improved by curcumin, which protects against neurodegeneration 138. For Parkinson’s disease, increasing evidence indicates that the accumulation of α-synuclein protein plays a vital role in the occurrence of Parkinson’s disease 139, suggesting that curcumin can reduce the accumulation of A53T α-synuclein by downregulating the mTOR/ribosomal protein S6 kinase (p70S6K) signaling, and restoring macroautophagy. Curcumin prevents α-synuclein from aggregating in the dopaminergic neurons at both the gene level and protein expression level 140. Meanwhile, curcumin inhibits astrocytic activation, and reduces iron deposition on the dopaminergic neurons. Moreover, the cognitive function is impaired in mice with Parkinson’s disease, while curcumin treatment in mice reduces the enhanced acetylcholine esterase enzyme levels, and restores the moto deficits, improving the cognitive functions 141. Curcumin provides protection for neurons by reducing p-p28 expression, caspase-3 activation, and toxic quinoprotein formation in 6-hydroxydopamine treated SH-SY5Y cells, ameliorating oxidative stress-related neurodegeneration, like Parkinson’s disease 142.
It has been documented that the exposure to some heavy metals, medications, and chemicals can induce nerve injuries, and even neuropathology of several neurodegenerative disorders. Curcumin diminishes copper-induced neurotoxicity in vivo by upregulating tyrosine hydroxylase expression and restoring locomotor performance 143. In addition, curcumin treatment can ameliorate arsenic-induced mitochondrial dysfunctions and modulate proteins related to apoptosis to reduce cholinergic deficits 144. Additionally, curcumin has been reported to abrogate the neurotoxicity induced by some drugs. For example, curcumin is found to remarkably ameliorate the abnormal alternations caused by cisplatin, including thermal hypoalgesia, reduced sciatic motor nerve conduction velocity, and nuclear and nucleolar atrophy with the loss of neurons 145. Bupivacaine, a widely-used anesthetic, has neurotoxicity in SH-SY5Y cells, and curcumin has been observed to protect against neuronal injury by increasing the level of Akt phosphorylation 146. With the intervention of curcumin on pregnant mice, cultured neurons, and neural progenitor cells, results show that curcumin can mitigate the toxic effects of celecoxib on fetal brain development by suppressing the proliferation of neuronal progenitor cells via activation of the Wnt/β-catenin pathway 147. Furthermore, the treatment of curcumin has been reported to reduce colistin-induced neurotoxicity in neuroblastoma-2a through regulating NF-κB signaling, leading to antioxidative and anti-apoptotic responses 148. Furthermore, toxic chemicals, like quinolinic acid, also impair the nervous system. Curcumin prevents rotation behavior, striatal morphological alterations, and neurodegeneration induced by quinolinic acid, and activates the Nrf2 cytoprotective pathway to induce antioxidant responses 149.
Cardiovascular protection
It has been documented that the intake of curcumin is beneficial to cardiovascular diseases 150. Curcumin can protect heart muscle cells (cardiomyocytes). For instance, curcumin protects against norepinephrine-induced hypertrophic stress and maintains extracellular matrix remodeling by inhibiting the increased collagen content under hypertrophic stress, and suppressing enhanced gelatinase B expression and gelatinolytic activity in H9c2 cardiomyocytes 151. It has also been found that curcumin prevents cardiomyocytes from norepinephrine-induced apoptosis and restores their physiological status 152. In addition, the administration of curcumin can attenuate the cytotoxicity of hemiscorpius lepturus venom, which damages the mitochondrial respiratory chain and results in ATP depletion, death signaling, and consequent cardiomyocytes apoptosis 153. Furthermore, curcumin increases the viability of H9c2 cardiomyoblasts exposed to H2O2 by upregulating the HO-1 protein, inhibiting cleaved caspase-3 protein expression, and increasing the Bcl-2/Bax ratio 154. Additionally, curcumin prevents cardiomyocytes from high glucose-induced apoptosis via the inhibition of NADPH-mediated oxidative stress by modulating the PI3K/Akt signaling pathway 150.
It is reported that curcumin can ameliorate cardiac hypertrophy and preserve cardiac function by upregulating Na+/Ca2+ exchanger expression levels. The left ventricle (LV) end-systolic and diastolic dimensions are reduced, and the LV ejection fraction and LV fractional shortening are enhanced in vivo by curcumin 155. Another work uses high doses of glucose and insulin to induce hypertrophy in cardiomyocytes and the treatment of curcumin decreases the increased ANF mRNA expression, total protein content, and cell surface area, resulting in attenuated cardiomyocyte hypertrophy 156. Meanwhile, curcumin increases PPAR-γ and Akt protein expressions, endothelial NO synthase (eNOS) mRNA expression, eNOS content, and NO concentration in cardiomyocytes. Curcumin is also found to suppress hypertrophic responses in cardiomyocytes by disrupting the formations of the zinc finger transcription factor, GATA4, and functional proteins, such as intrinsic histone acetyltransferase, and p300 157. Additionally, curcumin can attenuate chronic heart failure by increasing Dickkopf-related protein 3, p38 MAPK, JNK, and apoptosis signal-regulating kinase 1 (ASK1) 158. Moreover, curcumin has been demonstrated to have hypocholesterolemic activity both in vitro and in vivo. Curcumin treatment is found to reduce cholesterol absorption in Caco-2 cells via downregulation of expressions of Niemann-Pick C1-like 1 (NPC1L1) and sterol regulatory element binding protein-2 (SREBP-2) 159.
The occurrence of cardiovascular diseases is closely related with unbalanced diets. For curcumin, it protects the cardiomyocytes from injuries, and decreases cardiac hypertrophy, chronic heart failure, and cholesterol absorption.
Anti-diabetic effect of curcumin
Diabetes mellitus has reached pandemic status and there have been numerous studies on the development of anti-diabetic drugs with fewer side effects 160. Curcumin has been found to exhibit promising anti-diabetic activity, and curcumin intervention on prediabetic individuals can significantly lower the risk of developing type 2 diabetes 161. Additionally, curcumin can be a potential alternative to prevent and treat diabetes as well as certain complications, such as diabetic retinopathy 162.
The anti-diabetic activity of curcumin commonly reflects on the control of hyperglycemia by downregulating α-glucosidase and α-amylase activity. In addition, curcumin is beneficial for insulin-producing and insulin-responsive tissue, such as liver, skeletal muscle, and adipose tissues 163. Molecular docking in silico shows that curcumin poses a better inhibitory effect on α-amylase than other natural compounds, such as quercetin and berberine 164. After hyperglycaemic rats were administered with curcumin, the mean blood glucose level was significantly reduced 165. Additionally, the glucose tolerance and insulin sensitivity of diabetic rats were enhanced after being treated with curcumin 166. Meanwhile, the levels of Akt phosphorylation and glucose transporter type 4 (GLUT4) translocation in skeletal muscles are increased by curcumin. Moreover, in diabetic mice treated with curcumin for 12 weeks, pancreatic islets have no lymphocytes infiltration and there is an increase in the numbers of small islets of Langerhans near the ducts in the pancreas, which indicates an improvement of pancreatic islets 167.
Liver protective effect of curcumin
Numerous studies indicate that curcumin exhibits hepatoprotective effects on acute and chronic liver injuries induced by pollutants, drugs, and alcohol, nonalcoholic fatty liver disease, and liver fibrosis 168. Carbon tetrachloride (CCl4), a well-known hepatotoxin pollutant, can induce acute or chronic liver injuries, and result in a high level of ROS, mitochondrial dysfunction, and cellular apoptosis 169. Nanoparticulated curcumin has been evidenced to maintain cellular ROS levels, increase cellular antioxidant enzymes, prevent excessive mitochondrial destruction, and decrease fatty changes and inflammation in CCl4-treated rat hepatocytes 170. Enzymes, such as aspartate aminotransferase (AST) and alanine transaminase (ALT), in serum, are the main liver transaminases for liver damage assessment, and the hepatic GSH content indicates oxidative stress in the liver 171. Turmeric extract and curcumin are found to suppress CCl4-induced hepatic oxidative stress. They decrease the activities of serum aspartate aminotransferase (AST) and alanine transaminase (ALT) and the level of lipid peroxidase, while increasing the hepatic GSH content 172. Additionally, curcumin is able to reduce the number of apoptotic hepatocytes induced by cadmium accumulation 173.
Curcumin has been reported to ameliorate streptozotocin-induced liver injury of diabetic rats by decreasing hepatic endoplasmic reticulum stress marker protein and the sub-arm of unfolded protein response signaling protein, and inhibiting TNF-α, IL1β, phospho-p38 MAPK, and ASK1 in liver 174. Paracetamol-induced mitochondrial dysfunction in hepatocytes is also attenuated by curcumin. It decreases oxygen consumption in the membrane potential, ATP synthesis, aconitase activity, and the activity of respiratory complexes, I, III, and IV 175.
Alcoholic liver disease is characterized by the disturbance of alcohol and lipid metabolism. A metabolomic pathway analysis shows that curcumin attenuates ethanol-induced liver injury by inhibiting the biosynthesis of unsaturated fatty acids, and interconversions of pentose and glucuronate 176. In addition, curcumin dose-dependently attenuates the hepatocyte necroptosis and alcohol-induced decrease in hepatic Nrf2 expression in alcoholic mice 177.
Recently, curcumin has been reported to possess therapeutic efficacy in nonalcoholic fatty liver disease (NAFLD) experimental models, including non-alcoholic steatohepatitis (NASH) 178. Oral administration of curcumin effectively protects against the progression of NAFLD induced by a high-fat diet through alternations in the metabolism, and the intrahepatic CD4+ cell accumulation 179. Furthermore, high mobility group box 1 (HMGB1) is demonstrated to interact with TLR4, and induce nuclear translocation of activated NF-κB, releasing pro-inflammatory cytokines 180. Curcumin can reduce cytoplasmic translocation of HMGB1, the level of TLR4 protein expression, and the nuclear translocation of NF-κB 181. Moreover, some studies elucidate that curcumin exhibits its hepatoprotective property by inhibiting transient receptor potential melastatin 2 (TRPM2) channels, and curcumin can restore Ca2+ levels, reduce oxidative stress, and lower the risk of NASH 182.
Further investigations reveal that curcumin inhibits the angiogenesis in liver fibrosis. Curcumin reduces the angiogenic properties of hepatic stellate cells, disrupts the platelet-derived growth factor- receptor (PDGF-R)/ERK and mammalian target of rapamycin (mTOR) pathways, and regulates activation of PPAR-γ 183. In addition, curcumin can suppress liver fibrosis via miRNA-mediated epigenetic regulation. It has been reported that curcumin upregulates miR-29b expression with the downregulation of DNA methyltransferase 3b as well as the upregulation of PTEN, which inhibits the activated hepatic stellate cells 184.
Curcumin and turmeric summary
In light of the instability of curcumin, many animals and human studies, especially preclinical trails, still support the therapeutic and protective effects of curcumin, while more clinical trials are necessary to clarify its effects on human. Although there is still a debate about curcumin beneficial effects on humans, numerous preclinical studies still support its health benefits due to their versatile bioactivity. Therefore, curcumin can be still a good natural ingredient for the development of relevant functional foods as promising alternatives for the prevention of certain chronic diseases.
In conclusion, curcumin has been reported to exhibit versatile bioactivity and provide diverse health benefits to humans, such as antioxidant, anti-inflammatory, and anticancer activities. Furthermore, it is promising to use the nanotechnology to encapsulate curcumin to improve its stability, bioavailability, bioactivity, and health benefits.
- Alibeiki, F., Jafari, N., Karimi, M., & Peeri Dogaheh, H. (2017). Potent anti-cancer effects of less polar Curcumin analogues on gastric adenocarcinoma and esophageal squamous cell carcinoma cells. Scientific reports, 7(1), 2559. https://doi.org/10.1038/s41598-017-02666-4[↩]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The Essential Medicinal Chemistry of Curcumin. J Med Chem. 2017 Mar 9;60(5):1620-1637. doi: 10.1021/acs.jmedchem.6b00975[↩]
- Zlotogorski, A.; Dayan, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. (2013). Nutraceuticals as new treatment approaches for oral cancer -I: Curcumin. Oral Oncology 49: 187-191.[↩]
- WHO FOOD ADDITIVES SERIES: 52. CURCUMIN. https://apps.who.int/iris/bitstream/handle/10665/42849/WHO_TRS_922.pdf;jsessionid=0712A5359CCD928747F01055C86D8E11?sequence=1[↩]
- https://www.fda.gov/media/132575/download[↩]
- Cao J, Wang T, Wang M. Investigation of the anti-cataractogenic mechanisms of curcumin through in vivo and in vitro studies. BMC Ophthalmol. 2018 Feb 17;18(1):48. doi: 10.1186/s12886-018-0711-8[↩]
- Aggarwal, B. B., Deb, L., & Prasad, S. (2014). Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses. Molecules (Basel, Switzerland), 20(1), 185–205. https://doi.org/10.3390/molecules20010185[↩]
- Pulido-Moran, M., Moreno-Fernandez, J., Ramirez-Tortosa, C., & Ramirez-Tortosa, M. (2016). Curcumin and Health. Molecules (Basel, Switzerland), 21(3), 264. https://doi.org/10.3390/molecules21030264[↩]
- Jankun J, Wyganowska-Świątkowska M, Dettlaff K, Jelińska A, Surdacka A, Wątróbska-Świetlikowska D, Skrzypczak-Jankun E. Determining whether curcumin degradation/condensation is actually bioactivation (Review). Int J Mol Med. 2016 May;37(5):1151-8. doi: 10.3892/ijmm.2016.2524[↩]
- Hoehle SI, Pfeiffer E, Metzler M. Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol Nutr Food Res. 2007 Aug;51(8):932-8. doi: 10.1002/mnfr.200600283[↩]
- Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014 Jan;46(1):2-18. doi: 10.4143/crt.2014.46.1.2[↩]
- Tsuda T . Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018 Feb 21;9(2):705-714. doi: 10.1039/c7fo01242j[↩]
- Hoehle SI, Pfeiffer E, Sólyom AM, Metzler M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J Agric Food Chem. 2006 Feb 8;54(3):756-64. doi: 10.1021/jf058146a[↩]
- Pfeiffer E, Hoehle SI, Walch SG, Riess A, Sólyom AM, Metzler M. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem. 2007 Jan 24;55(2):538-44. doi: 10.1021/jf0623283[↩]
- Huang Y, Cao S, Zhang Q, Zhang H, Fan Y, Qiu F, Kang N. Biological and pharmacological effects of hexahydrocurcumin, a metabolite of curcumin. Arch Biochem Biophys. 2018 May 15;646:31-37. doi: 10.1016/j.abb.2018.03.030[↩]
- Dempe JS, Scheerle RK, Pfeiffer E, Metzler M. Metabolism and permeability of curcumin in cultured Caco-2 cells. Mol Nutr Food Res. 2013 Sep;57(9):1543-9. doi: 10.1002/mnfr.201200113[↩]
- Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Biofactors. 2013 Jan-Feb;39(1):14-20. doi: 10.1002/biof.1042[↩]
- Dempe JS, Pfeiffer E, Grimm AS, Metzler M. Metabolism of curcumin and induction of mitotic catastrophe in human cancer cells. Mol Nutr Food Res. 2008 Sep;52(9):1074-81. doi: 10.1002/mnfr.200800029[↩]
- de Oliveira MR, Jardim FR, Setzer WN, Nabavi SM, Nabavi SF. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol Adv. 2016 Sep-Oct;34(5):813-826. doi: 10.1016/j.biotechadv.2016.04.004[↩]
- Lou Y, Zheng J, Hu H, Lee J, Zeng S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Mar 15;985:38-47. doi: 10.1016/j.jchromb.2015.01.014[↩]
- An C.-Y., Sun Z.-Z., Shen L., Ji H.-F. Biotransformation of food spice curcumin by gut bacterium Bacillus megaterium DCMB-002 and its pharmacological implications. Food Nutr. Res. 2017;61 doi: 10.1080/16546628.2017.1412814[↩]
- Burapan S, Kim M, Han J. Curcuminoid Demethylation as an Alternative Metabolism by Human Intestinal Microbiota. J Agric Food Chem. 2017 Apr 26;65(16):3305-3310. doi: 10.1021/acs.jafc.7b00943[↩]
- Xu, X. Y., Meng, X., Li, S., Gan, R. Y., Li, Y., & Li, H. B. (2018). Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients, 10(10), 1553. https://doi.org/10.3390/nu10101553[↩][↩][↩]
- Chemopreventive Effect of Curcumin, a Naturally Occurring Anti-Inflammatory Agent, during the Promotion/Progression Stages of Colon Cancer. Toshihiko Kawamori, Ronald Lubet, Vernon E. Steele, Gary J. Kelloff, Robert B. Kaskey, Chinthalapally V. Rao and Bandaru S. Reddy. Cancer Res February 1 1999 (59) (3) 597-601. https://cancerres.aacrjournals.org/content/59/3/597[↩]
- Xu, X. Y., Meng, X., Li, S., Gan, R. Y., Li, Y., & Li, H. B. (2018). Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients, 10(10), 1553. https://doi.org/10.3390/nu10101553[↩]
- Kunati S.R., Yang S.M., William B.M., Xu Y. An LC-MS/MS method for simultaneous determination of curcumin, curcumin glucuronide and curcumin sulfate in a phase II clinical trial. J. Pharm. Biomed. Anal. 2018;156:189–198. doi: 10.1016/j.jpba.2018.04.034[↩]
- Jankun J., Wyganowska-Swiatkowska M., Dettlaff K., Jelinska A., Surdacka A., Watrobska-Swietlikowska D., Skrzypczak-Jankun E. Determining whether curcumin degradation/condensation is actually bioactivation (review) Int. J. Mol. Med. 2016;37:1151–1158. doi: 10.3892/ijmm.2016.2524[↩]
- Khayyal M.T., El-Hazek R.M., El-Sabbagh W.A., Frank J., Behnam D., Abdel-Tawab M. Micellar solubilisation enhances the antiinflammatory activities of curcumin and boswellic acids in rats with adjuvant-induced arthritis. Nutrition. 2018;54:189–196. doi: 10.1016/j.nut.2018.03.055[↩]
- Akbar M.U., Zia K.M., Nazir A., Iqbal J., Ejaz S.A., Akash M.S.H. Pluronic-based mixed polymeric micelles enhance the therapeutic potential of curcumin. AAPS PharmSciTech. 2018;19:2719–2739. doi: 10.1208/s12249-018-1098-9[↩]
- Mantzorou M., Pavlidou E., Vasios G., Tsagalioti E., Giaginis C. Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytother. Res. 2018;32:957–975. doi: 10.1002/ptr.6037[↩]
- Greil R., Greil-Ressler S., Weiss L., Schonlieb C., Magnes T., Radl B., Bolger G.T., Vcelar B., Sordillo P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin [lipocurc TM] in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018;82:695–706. doi: 10.1007/s00280-018-3654-0[↩]
- Bilia A.R., Bergonzi M.C., Isacchi B., Antiga E., Caproni M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J. Pharm. Pharmacol. 2018;70:919–928. doi: 10.1111/jphp.12910[↩][↩]
- Jimenez-Osorio A.S., Garcia-Nino W.R., Gonzalez-Reyes S., Alvarez-Mejia A.E., Guerra-Leon S., Salazar-Segovia J., Falcon I., de Oca-Solano H.M., Madero M., Pedraza-Chaverri J. The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: A pilot study. J. Renal Nutr. 2016;26:237–244. doi: 10.1053/j.jrn.2016.01.013[↩]
- Amalraj A., Varma K., Jacob J., Divya C., Kunnumakkara A.B., Stohs S.J., Gopi S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm, and parallel-group study. J. Med. Food. 2017;20:1022–1030. doi: 10.1089/jmf.2017.3930[↩]
- Henrotin Y., Gharbi M., Dierckxsens Y., Priem F., Marty M., Seidel L., Albert A., Heuse E., Bonnet V., Castermans C. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement. Altern. Med. 2014;14:159. doi: 10.1186/1472-6882-14-159[↩]
- Antiga E., Bonciolini V., Volpi W., Del Bianco E., Caproni M. Oral curcumin (Meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. BioMed Res. Int. 2015;2015:283634. doi: 10.1155/2015/283634[↩]
- Abdolahi M., Sarraf P., Javanbakht M.H., Honarvar N.M., Hatami M., Soveyd N., Tafakhori A., Sedighiyan M., Djalali M., Jafarieh A., et al. A novel combination of omega-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity c-reactive protein serum levels in patients with migraine: A randomized clinical trial study. CNS Neurol. Disord. Drug Targets. 2018;17:430–438. doi: 10.2174/1871527317666180625101643[↩]
- Kuriakose M.A., Ramdas K., Dey B., Iyer S., Rajan G., Elango K.K., Suresh A., Ravindran D., Kumar R.R., Prathiba R., et al. A randomized double-blind placebo-controlled phase iib trial of curcumin in oral leukoplakia. Cancer Prev. Res. 2016;9:683–691. doi: 10.1158/1940-6207.CAPR-15-0390[↩]
- Duetzmann S., Schiborr C., Kocher A., Pilatus U., Hattingen E., Weissenberger J., Gessler F., Quick-Weller J., Franz K., Seifert V., et al. Intratumoral concentrations and effects of orally administered micellar curcuminoids in glioblastoma patients. Nutr. Cancer. 2016;68:943–948. doi: 10.1080/01635581.2016.1187281[↩]
- Funamoto M., Sunagawa Y., Katanasaka Y., Miyazaki Y., Imaizumi A., Kakeya H., Yamakage H., Satoh-Asahara N., Komiyama M., Wada H., et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild copd. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:2029–2034. doi: 10.2147/copd.s104490[↩]
- Yang Y.S., Su Y.F., Yang H.W., Lee Y.H., Chou J.I., Ueng K.C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2014;28:1770–1777. doi: 10.1002/ptr.5197[↩]
- Chuengsamarn S., Rattanamongkolgul S., Phonrat B., Tungtrongchitr R., Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014;25:144–150. doi: 10.1016/j.jnutbio.2013.09.013[↩]
- Mirzabeigi P., Mohammadpour A.H., Salarifar M., Gholami K., Mojtahedzadeh M., Javadi M.R. The effect of curcumin on some of traditional and non-traditional cardiovascular risk factors: A pilot randomized, double-blind, placebo-controlled trial. Iran. J. Pharm. Res. 2015;14:479–486.[↩]
- Neerati P., Devde R., Gangi A.K. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother. Res. 2014;28:1796–1800. doi: 10.1002/ptr.5201[↩]
- Rahimi H.R., Mohammadpour A.H., Dastani M., Jaafari M.R., Abnous K., Mobarhan M.G., Oskuee R.K. The effect of nano-curcumin on HBA1C, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. Phytomed. 2016;6:567–577.[↩]
- Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W, Yang D, Yang A, Yu Y. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep. 2018 Mar;39(3):1523-1531. doi: 10.3892/or.2018.6188[↩]
- Feng S, Wang Y, Zhang R, Yang G, Liang Z, Wang Z, Zhang G. Curcumin exerts its antitumor activity through regulation of miR-7/Skp2/p21 in nasopharyngeal carcinoma cells. Onco Targets Ther. 2017 May 2;10:2377-2388. doi: 10.2147/OTT.S130055[↩]
- Balasubramanian S, Eckert RL. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J Biol Chem. 2007 Mar 2;282(9):6707-15. doi: 10.1074/jbc.M606003200[↩]
- Ashour AA, Abdel-Aziz AA, Mansour AM, Alpay SN, Huo L, Ozpolat B. Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells. Apoptosis. 2014 Jan;19(1):241-58. doi: 10.1007/s10495-013-0927-2[↩]
- Hahn, Y. I., Kim, S. J., Choi, B. Y., Cho, K. C., Bandu, R., Kim, K. P., Kim, D. H., Kim, W., Park, J. S., Han, B. W., Lee, J., Na, H. K., Cha, Y. N., & Surh, Y. J. (2018). Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Scientific reports, 8(1), 6409. https://doi.org/10.1038/s41598-018-23840-2[↩]
- Siddiqui FA, Prakasam G, Chattopadhyay S, Rehman AU, Padder RA, Ansari MA, Irshad R, Mangalhara K, Bamezai RNK, Husain M, Ali SM, Iqbal MA. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci Rep. 2018 May 29;8(1):8323. doi: 10.1038/s41598-018-25524-3[↩]
- Orr WS, Denbo JW, Saab KR, Ng CY, Wu J, Li K, Garner JM, Morton CL, Du Z, Pfeffer LM, Davidoff AM. Curcumin potentiates rhabdomyosarcoma radiosensitivity by suppressing NF-κB activity. PLoS One. 2013;8(2):e51309. doi: 10.1371/journal.pone.0051309. Epub 2013 Feb 7. Retraction in: PLoS One. 2019 Aug 30;14(8):e0222130[↩]
- Shakibaei M, Kraehe P, Popper B, Shayan P, Goel A, Buhrmann C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer. 2015 Apr 10;15:250. doi: 10.1186/s12885-015-1291-0[↩]
- Zhang P, Lai ZL, Chen HF, Zhang M, Wang A, Jia T, Sun WQ, Zhu XM, Chen XF, Zhao Z, Zhang J. Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J Exp Clin Cancer Res. 2017 Dec 22;36(1):190. doi: 10.1186/s13046-017-0661-7[↩]
- Cheng M.A., Chou F.-J., Wang K., Yang R., Ding J., Zhang Q., Li G., Yeh S., Xu D., Chang C. Androgen receptor (AR) degradation enhancer ASC-J9® in an FDA-approved formulated solution suppresses castration resistant prostate cancer cell growth. Cancer Lett. 2018;417:182–191. doi: 10.1016/j.canlet.2017.11.038[↩]
- Ahmed A., Ali S., Sarkar F.H. Advances in androgen receptor targeted therapy for prostate cancer. J. Cell. Physiol. 2014;229:271–276. doi: 10.1002/jcp.24456[↩]
- Dorai T., Cao Y.C., Dorai B., Buttyan R., Katz A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293–303. doi: 10.1002/pros.1074[↩]
- McCarty M.F. Targeting multiple signaling pathways as a strategy for managing prostate cancer: Multifocal signal modulation therapy. Integr. Cancer Ther. 2004;3:349–380. doi: 10.1177/1534735404270757[↩]
- Sundram V., Chauhan S.C., Jaggi M. Emerging roles of protein kinase D1 in cancer. Mol. Cancer Res. 2011;9:985–996. doi: 10.1158/1541-7786.MCR-10-0365[↩][↩]
- Mudduluru G., George-William J.N., Muppala S., Asangani I.A., Kumarswamy R., Nelson L.D., Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011;31:185–197. doi: 10.1042/BSR20100065[↩][↩][↩]
- LaValle C.R., George K.M., Sharlow E.R., Lazo J.S., Wipf P., Wang Q.J. Protein kinase D as a potential new target for cancer therapy. Biochim. Biophys. Acta. 2010;1806:183–192. doi: 10.1016/j.bbcan.2010.05.003[↩]
- Vellampatti S., Chandrasekaran G., Mitta S.B., Lakshmanan V.-K., Park S.H. Metallo-Curcumin-Conjugated DNA Complexes Induces Preferential Prostate Cancer Cells Cytotoxicity and Pause Growth of Bacterial Cells. Sci. Rep. 2018;8:14929. doi: 10.1038/s41598-018-33369-z[↩]
- Soh S.F., Huang C.K., Lee S.O., Xu D., Yeh S., Li J., Yong E.L., Gong Y., Chang C. Determination of androgen receptor degradation enhancer ASC-J9R in mouse sera and organs with liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014;88:117–122. doi: 10.1016/j.jpba.2013.08.020[↩]
- Nautiyal J., Banerjee S., Kanwar S.S., Yu Y., Patel B.B., Sarkar F.H., Majumdar A.P. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int. J. Cancer. 2011;128:951–961. doi: 10.1002/ijc.25410[↩]
- Carrato A. Adjuvant treatment of colorectal cancer. Gastrointest. Cancer Res. 2008;2:S42–S46.[↩]
- Garcea G., Berry D.P., Jones D.J., Singh R., Dennison A.R., Farmer P.B., Sharma R.A., Steward W.P., Gescher A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Prev. Biomark. 2005;14:120–125.[↩]
- Kunnumakkara A.B., Diagaradjane P., Guha S., Deorukhkar A., Shentu S., Aggarwal B.B., Krishnan S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 2008;14:2128–2136. doi: 10.1158/1078-0432.CCR-07-4722[↩]
- Xu H., Yu Y., Marciniak D., Rishi A.K., Sarkar F.H., Kucuk O., Majumdar A.P. Epidermal growth factor receptor (EGFR)-related protein inhibits multiple members of the EGFR family in colon and breast cancer cells. Mol. Cancer Ther. 2005;4:435–442. doi: 10.1158/1535-7163.mct-04-0280[↩]
- Sharma, R.A.; Mclelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. (2001). Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clinical Cancer Research 7: (7), 1894-1900.[↩]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; Pirmohamed, M.; Gescher, A.J.; Steward, W.P. (2004). Phase I clinical trial of oral curcumin: biomkers of systemic activity and compliance. Clinical Canser Research 10: (20), 6847-6854.[↩]
- Garcea, G.; Berry, D.P.; Jones, D.J.L. (2005). Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiology, Biomarkers & Prevention 14: (1), 120-125.[↩]
- Cruz-Correa M., Hylind L.M., Marrero J.H., Zahurak M.L., Murray-Stewart T., Casero R.A., Jr., Montgomery E.A., Iacobuzio-Donahue C., Brosens L.A., Offerhaus G.J., et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients with Familial Adenomatous Polyposis. Gastroenterology. 2018;155:668–673. doi: 10.1053/j.gastro.2018.05.031[↩][↩]
- Epelbaum R., Schaffer M., Vizel B., Badmaev V., Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer. 2010;62:1137–1141. doi: 10.1080/01635581.2010.513802[↩][↩]
- okes E.E., Weichselbaum R.R., Lippman S.M., Hong W.K. Head and neck cancer. N. Engl. J. Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306[↩]
- Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharm. 2017;174:1325–1348. doi: 10.1111/bph.13621[↩]
- Chakravarti N., Myers J.N., Aggarwal B.B. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int. J. Cancer. 2006;119:1268–1275. doi: 10.1002/ijc.21967[↩]
- LoTempio M.M., Veena M.S., Steele H.L., Ramamurthy B., Ramalingam T.S., Cohen A.N., Chakrabarti R., Srivatsan E.S., Wang M.B. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin. Cancer Res. 2005;11:6994–7002. doi: 10.1158/1078-0432.CCR-05-0301[↩]
- Kim S.G., Veena M.S., Basak S.K., Han E., Tajima T., Gjertson D.W., Starr J., Eidelman O., Pollard H.B., Srivastava M., et al. Curcumin treatment suppresses IKKbeta kinase activity of salivary cells of patients with head and neck cancer: A pilot study. Clin. Cancer Res. 2011;17:5953–5961. doi: 10.1158/1078-0432.CCR-11-1272[↩][↩]
- Ananthakrishnan P., Balci F.L., Crowe J.P. Optimizing surgical margins in breast conservation. Int. J. Surg. Oncol. 2012;2012:585670. doi: 10.1155/2012/585670[↩]
- Houssami N., Macaskill P., Marinovich M.L., Dixon J.M., Irwig L., Brennan M.E., Solin L.J. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur. J. Cancer. 2010;46:3219–3232. doi: 10.1016/j.ejca.2010.07.043[↩]
- Ramachandran C., Fonseca H.B., Jhabvala P., Escalon E.A., Melnick S.J. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002;184:1–6. doi: 10.1016/S0304-3835(02)00192-1[↩]
- Liu Q., Loo W.T.Y., Sze S.C.W., Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFκB, cyclinD and MMP-1 transcription. Phytomedicine. 2009;16:916–922. doi: 10.1016/j.phymed.2009.04.008[↩][↩]
- Bachmeier B., Nerlich A.G., Iancu C.M., Cilli M., Schleicher E., Vene R., Dell’Eva R., Jochum M., Albini A., Pfeffer U. The chemopreventive polyphenol Curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell. Physiol. Biochem. 2007;19:137–152. doi: 10.1159/000099202[↩]
- Moghtaderi H., Sepehri H., Attari F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed. Pharmacother. 2017;88:582–594. doi: 10.1016/j.biopha.2017.01.072[↩][↩]
- Bachmeier B.E., Mohrenz I.V., Mirisola V., Schleicher E., Romeo F., Hohneke C., Jochum M., Nerlich A.G., Pfeffer U. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29:779–789. doi: 10.1093/carcin/bgm248[↩]
- Yang Z., Chang Y.J., Yu I.C., Yeh S., Wu C.C., Miyamoto H., Merry D.E., Sobue G., Chen L.M., Chang S.S., et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 2007;13:348–353. doi: 10.1038/nm1547[↩]
- Bayet-Robert M., Kwiatkowski F., Leheurteur M., Gachon F., Planchat E., Abrial C., Mouret-Reynier M.A., Durando X., Barthomeuf C., Chollet P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392[↩][↩]
- Klinger N.V., Mittal S. Therapeutic potential of curcumin for the treatment of brain tumors. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/9324085[↩][↩]
- Chintala S.K., Tonn J.C., Rao J.S. Matrix metalloproteinases and their biological function in human gliomas. Int. J. Dev. Neurosci. 1999;17:495–502. doi: 10.1016/S0736-5748(99)00010-6[↩]
- Perry M.C., Demeule M., Regina A., Moumdjian R., Beliveau R. Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Mol. Nutr. Food Res. 2010;54:1192–1201. doi: 10.1002/mnfr.200900277[↩][↩]
- Wu B., Yao H., Wang S., Xu R. DAPK1 modulates a curcumin-induced G2/M arrest and apoptosis by regulating STAT3, NF-κB, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2013;434:75–80. doi: 10.1016/j.bbrc.2013.03.063[↩]
- Asouri M., Ataee R., Ahmadi A.A., Amini A., Moshaei M.R. Antioxidant and free radical scavenging activities of curcumin. Asian J. Chem. 2013;25:7593–7595.[↩]
- Derochette S., Franck T., Mouithys-Mickalad A., Ceusters J., Deby-Dupont G., Lejeune J.P., Neven P., Serteyn D. Curcumin and resveratrol act by different ways on NADPH oxidase activity and reactive oxygen species produced by equine neutrophils. Chem.-Biol. Interact. 2013;206:186–193. doi: 10.1016/j.cbi.2013.09.011[↩]
- Zheng Q.T., Yang Z.H., Yu L.Y., Ren Y.Y., Huang Q.X., Liu Q., Ma X.Y., Chen Z.K., Wang Z.B., Zheng X. Synthesis and antioxidant activity of curcumin analogs. J. Asian Nat. Prod. Res. 2017;19:489–503. doi: 10.1080/10286020.2016.1235562[↩]
- Dikmen M., Kaya-Tilki E., Engur S., Ozturk Y. Neuritogenic activity of epigallocatechin gallate and curcumin combination on rat adrenal pheochromocytoma cells. Fresenius Environ. Bull. 2017;26:4726–4733.[↩][↩]
- Tapia E., Sanchez-Lozada L.G., Garcia-Nino W.R., Garcia E., Cerecedo A., Garcia-Arroyo F.E., Osorio H., Arellano A., Cristobal-Garcia M., Loredo M.L., et al. Curcumin prevents maleate-induced nephrotoxicity: Relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free Radic. Res. 2014;48:1342–1354. doi: 10.3109/10715762.2014.954109[↩]
- Dai J., Gu L., Su Y., Wang Q., Zhao Y., Chen X., Deng H., Li W., Wang G., Li K. Inhibition of curcumin on influenza a virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/jnk mapk and NF-κB pathways. Int. Immunopharmacol. 2018;54:177–187. doi: 10.1016/j.intimp.2017.11.009[↩]
- Dai C.S., Tang S.S., Li D.W., Zhao K.N., Xiao X.L. Curcumin attenuates quinocetone-induced oxidative stress and genotoxicity in human hepatocyte l02 cells. Toxicol. Mech. Methods. 2015;25:340–346. doi: 10.3109/15376516.2015.1045659[↩]
- Maugeri A., Mazzone M.G., Giuliano F., Vinciguerra M., Basile G., Barchitta M., Agodi A. Curcumin modulates DNA methyltransferase functions in a cellular model of diabetic retinopathy. Oxid. Med. Cell. Longev. 2018;2018:5407482. doi: 10.1155/2018/5407482[↩]
- Joshi D., Mittal D.K., Shukla S., Srivastav S.K., Dixit V.A. Curcuma longa Linn. Extract and curcumin protect CYP 2E1 enzymatic activity against mercuric chloride-induced hepatotoxicity and oxidative stress: A protective approach. Exp. Toxicol. Pathol. 2017;69:373–382. doi: 10.1016/j.etp.2017.02.009[↩]
- El-Bahr S.M. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin b1. Phytother. Res. 2015;29:134–140. doi: 10.1002/ptr.5239[↩]
- Goc Z., Semla M., Kapusta E., Gren A., Muchacka R., Lukacova J. The effect of curcumin on the activity of antioxidant enzymes in the liver, pancreas and kidneys of Swiss mice; Proceedings of the 12th International Scientific Conference on Animal Physiology; Boretice, Czech Republic. 13–15 June 2016[↩]
- Altintoprak N., Kar M., Acar M., Berkoz M., Muluk N.B., Cingi C. Antioxidant activities of curcumin in allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2016;273:3765–3773. doi: 10.1007/s00405-016-4076-4[↩]
- Singh P., Kesharwani R.K., Misra K., Rizvi S.I. Modulation of erythrocyte plasma membrane redox system activity by curcumin. Biochem. Res. Int. 2016;2016:6025245. doi: 10.1155/2016/6025245[↩]
- Xu D.P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., Zhang J.J., Li H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017;18:96. doi: 10.3390/ijms18010096[↩]
- Pompella A., Sies H., Wacker R., Brouns F., Grune T., Biesalski H.K., Frank J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition. 2014;30:791–793. doi: 10.1016/j.nut.2013.12.002[↩]
- Zhang Y.L., Liu Z.G., Wu J.Z., Bai B., Chen H.J., Xiao Z.X., Chen L.F., Zhao Y.J., Lum H., Wang Y., et al. New MD2 inhibitors derived from curcumin with improved anti-inflammatory activity. Eur. J. Med. Chem. 2018;148:291–305. doi: 10.1016/j.ejmech.2018.02.008[↩][↩]
- Wu N.C., Wang J.J. Curcumin attenuates liver warm ischemia and reperfusion-induced combined restrictive and obstructive lung disease by reducing matrix metalloprotease 9 activity. Transplant. Proc. 2014;46:1135–1138. doi: 10.1016/j.transproceed.2013.12.020[↩]
- Ferreira V.H., Nazli A., Dizzell S.E., Mueller K., Kaushic C. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PLoS ONE. 2015;10:e0124903. doi: 10.1371/journal.pone.0124903[↩]
- Wang J., Wang H.Y., Zhu R.R., Liu Q., Fei J., Wang S.L. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1 beta transgenic mice subjected to the, lipopolysaccharide-induced sepsis. Biomaterials. 2015;53:475–483. doi: 10.1016/j.biomaterials.2015.02.116[↩]
- Kloesch B., Gober L., Loebsch S., Vcelar B., Helson L., Steiner G. In vitro study of a liposomal curcumin formulation (lipocurc (TM[↩]
- Esatbeyoglu T., Ulbrich K., Rehberg C., Rohn S., Rimbach G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015;6:887–893. doi: 10.1039/C4FO00790E[↩]
- Song Z.L., Revelo X., Shao W.J., Tian L.L., Zeng K.J., Lei H., Sun H.S., Woo M., Winer D., Jin T. Dietary curcumin intervention targets mouse white adipose tissue inflammation and brown adipose tissue ucp1 expression. Obesity. 2018;26:547–558. doi: 10.1002/oby.22110[↩]
- Hui-Yin Y., Ahmad N., Azmi N., Makmor-Bakry M. Curcumin: The molecular mechanisms of action in inflammation and cell death during kainate-induced epileptogenesis. Indian J. Pharm. Educ. 2018;52:32–41. doi: 10.5530/ijper.52.1.4[↩]
- Wang J., Kang Y.X., Pan W., Lei W., Feng B., Wang X.J. Enhancement of anti-inflammatory activity of curcumin using phosphatidylserine-containing nanoparticles in cultured macrophages. Int. J. Mol. Sci. 2016;17:969. doi: 10.3390/ijms17060969[↩]
- Antoine F., Girard D. Curcumin increases gelatinase activity in human neutrophils by a p38 mitogen-activated protein kinase (MAPK)-independent mechanism. J. Immunotoxicol. 2015;12:188–193. doi: 10.3109/1547691X.2014.917749[↩]
- Li H.Y., Yang M., Li Z., Meng Z. Curcumin inhibits angiotensin II-induced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-gamma activity and reducing oxidative stress. Int. J. Mol. Med. 2017;39:1307–1316. doi: 10.3892/ijmm.2017.2924[↩]
- Abdollahi E., Momtazi A.A., Johnston T.P., Sahebkar A. Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades? J. Cell. Physiol. 2018;233:830–848. doi: 10.1002/jcp.25778[↩]
- Shakeri F., Boskabady M.H. Anti-inflammatory, antioxidant, and immunomodulatory effects of curcumin in ovalbumin-sensitized rat. BioFactors. 2017;43:567–576. doi: 10.1002/biof.1364[↩]
- Han F., Luo B., Shi R., Han C., Zhang Z., Xiong J., Jiang M., Zhang Z. Curcumin ameliorates rat experimental autoimmune neuritis. J. Neurosci. Res. 2014;92:743–750. doi: 10.1002/jnr.23357[↩]
- Yang Z., He C.M., He J.Y., Chu J., Liu H.P., Deng X.Y. Curcumin-mediated bone marrow mesenchymal stem cell sheets create a favorable immune microenvironment for adult full-thickness cutaneous wound healing. Stem Cell. Res. Ther. 2018;9:21. doi: 10.1186/s13287-018-0768-6[↩][↩]
- Afia M., Alshehri M., Alfaifi M., Shakor A.B.A. Repressive effect of curcumin against 2-amino-3-methylimidazo 4, 5-f quinoline induced hepato- and immunotoxicity in mice. Indian J. Exp. Biol. 2017;55:365–371.[↩]
- Wang S., Li H., Zhang M., Yue L.T., Wang C.C., Zhang P., Liu Y., Duan R.S. Curcumin ameliorates experimental autoimmune myasthenia gravis by diverse immune cells. Neurosci. Lett. 2016;626:25–34. doi: 10.1016/j.neulet.2016.05.020[↩]
- Liu Y., Chen L.Y., Shen Y., Tan T., Xie N.Z., Luo M., Li Z.H., Xie X.Y. Curcumin ameliorates ischemia-induced limb injury through immunomodulation. Med. Sci. Monit. 2016;22:2035–2042. doi: 10.12659/MSM.896217[↩]
- Kumar A., Sasmal D., Jadav S.S., Sharma N. Mechanism of immunoprotective effects of curcumin in DLM-induced thymic apoptosis and altered immune function: An in silico and in vitro study. Immunopharmacol. Immunotoxicol. 2015;37:488–498. doi: 10.3109/08923973.2015.1091004[↩]
- Bose S., Panda A.K., Mukherjee S., Sa G. Curcumin and tumor immune-editing: Resurrecting the immune system. Cell Div. 2015;10:6. doi: 10.1186/s13008-015-0012-z[↩]
- Mahmoud H.K., Al-Sagheer A.A., Reda F.M., Mahgoub S.A., Ayyat M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to aeromonas hydrophila in oreochromis niloticus. Aquaculture. 2017;475:16–23. doi: 10.1016/j.aquaculture.2017.03.043[↩]
- Xun W.J., Shi L.G., Zhou H.L., Hou G.Y., Cao T., Zhao C.P. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharmacol. 2015;27:46–52. doi: 10.1016/j.intimp.2015.04.038[↩]
- Lee G., Chung H.S., Lee K., Lee H., Kim M., Bae H. Curcumin attenuates the scurfy-induced immune disorder, a model of ipex syndrome, with inhibiting Th1/Th2/Th17 responses in mice. Phytomedicine. 2017;33:1–6. doi: 10.1016/j.phymed.2017.01.008[↩]
- Fu X.Y., Yang M.F., Cao M.Z., Li D.W., Yang X.Y., Sun J.Y., Zhang Z.Y., Mao L.L., Zhang S., Wang F.Z., et al. Strategy to suppress oxidative damage-induced neurotoxicity in PC12 cells by curcumin: The role of ROS-mediated DNA damage and the MAPK and Akt pathways. Mol. Neurobiol. 2016;53:369–378. doi: 10.1007/s12035-014-9021-1[↩]
- Szczepanowicz K., Jantas D., Piotrowski M., Staron J., Leskiewicz M., Regulska M., Lason W., Warszynski P. Encapsulation of curcumin in polyelectrolyte nanocapsules and their neuroprotective activity. Nanotechnology. 2016;27:355101. doi: 10.1088/0957-4484/27/35/355101[↩]
- Jin M.L., Park S.Y., Shen Q., Lai Y.H., Ou X.M., Mao Z., Lin D.X., Yu Y.Y., Zhang W.Z. Anti-neuroinflammatory effect of curcumin on Pam3CSK4-stimulated microglial cells. Int. J. Mol. Med. 2018;41:521–530. doi: 10.3892/ijmm.2017.3217[↩]
- Kodali M., Hattiangady B., Shetty G.A., Bates A., Shuai B., Shetty A.K. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 2018;69:499–514. doi: 10.1016/j.bbi.2018.01.009[↩]
- Huang L.F., Chen C.W., Zhang X., Li X., Chen Z.P., Yang C., Liang X.L., Zhu G.C., Xu Z. Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J. Mol. Neurosci. 2018;64:129–139. doi: 10.1007/s12031-017-1006-x[↩]
- Hu S.X., Maiti P., Ma Q.L., Zuo X.H., Jones M.R., Cole G.M., Frautschy S.A. Clinical development of curcumin in neurodegenerative disease. Expert Rev. Neurother. 2015;15:629–637. doi: 10.1586/14737175.2015.1044981[↩]
- Liu Z.J., Li Z.H., Liu L., Tang W.X., Wang Y., Dong M.R., Xiao C. Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s disease. Front. Pharmacol. 2016;7:261. doi: 10.3389/fphar.2016.00261[↩]
- Wang Y.L., Li J.F., Wang Y.T., Xu C.Y., Hua L.L., Yang X.P., Geng S., Wang S.S., Wang Z., Yin H.L. Curcumin reduces hippocampal neuron apoptosis and JNK-3 phosphorylation in rats with A beta-induced Alzheimer’s disease: Protecting spatial learning and memory. J. Neurorestoratol. 2017;5:117–123. doi: 10.2147/JN.S125567[↩]
- Huang H.C., Zheng B.W., Guo Y., Zhao J., Zhao J.Y., Ma X.W., Jiang Z.F. Antioxidative and neuroprotective effects of curcumin in an Alzheimer’s disease rat model co-treated with intracerebroventricular streptozotocin and subcutaneous D-galactose. J. Alzheimers Dis. 2016;52:899–911. doi: 10.3233/JAD-150872[↩]
- Jiang T.F., Zhang Y.J., Zhou H.Y., Wang H.M., Tian L.P., Liu J., Ding J.Q., Chen S.D. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70s6K signaling and the recovery of macroautophagy. J. Neuroimmune Pharmacol. 2013;8:356–369. doi: 10.1007/s11481-012-9431-7[↩]
- Sharma N., Nehru B. Curcumin affords neuroprotection and inhibits alpha-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology. 2018;26:349–360. doi: 10.1007/s10787-017-0402-8[↩]
- Khatri D.K., Jnvekar A.R. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2016;150:39–47. doi: 10.1016/j.pbb.2016.09.002[↩]
- Meesarapee B., Thampithak A., Jaisin Y., Sanvarinda P., Suksamrarn A., Tuchinda P., Morales N.P., Sanvarinda Y. Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells. Phytother. Res. 2014;28:611–616. doi: 10.1002/ptr.5036[↩]
- Abbaoui A., Chatoui H., El Hiba O., Gamrani H. Neuroprotective effect of curcumin-i in copper-induced dopaminergic neurotoxicity in rats: A possible link with Parkinson’s disease. Neurosci. Lett. 2017;660:103–108. doi: 10.1016/j.neulet.2017.09.032[↩]
- Srivastava P., Yadav R.S., Chandravanshi L.P., Shukla R.K., Dhuriya Y.K., Chauhan L.K.S., Dwivedi H.N., Pant A.B., Khanna V.K. Unraveling the mechanism of neuroprotection of curcumin in arsenic induced cholinergic dysfunctions in rats. Toxicol. Appl. Pharmacol. 2014;279:428–440. doi: 10.1016/j.taap.2014.06.006[↩]
- Agthong S., Kaewsema A., Charoensub T. Curcumin ameliorates functional and structural abnormalities in cisplatin-induced neuropathy. Exp. Neurobiol. 2015;24:139–145. doi: 10.5607/en.2015.24.2.139[↩]
- Fan Y.L., Li H.C., Zhao W., Peng H.H., Huang F., Jiang W.H., Xu S.Y. Curcumin attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells via activation of the Akt signaling pathway. Neurochem. Res. 2016;41:2425–2432. doi: 10.1007/s11064-016-1955-4[↩]
- Wang R., Tian S.Q., Yang X., Liu J.J., Wang Y.H., Sun K. Celecoxib-induced inhibition of neurogenesis in fetal frontal cortex is attenuated by curcumin via Wnt/beta-catenin pathway. Life Sci. 2017;185:95–102. doi: 10.1016/j.lfs.2017.07.028[↩]
- Dai C.S., Ciccotosto G.D., Cappai R., Tang S.S., Li D.W., Xie S.L., Xiao X.L., Velkov T. Curcumin attenuates colistin-induced neurotoxicity in N2a cells via anti-inflammatory activity, suppression of oxidative stress, and apoptosis. Mol. Neurobiol. 2018;55:421–434. doi: 10.1007/s12035-016-0276-6[↩]
- Carmona-Ramirez I., Santamaria A., Tobon-Velasco J.C., Orozco-Ibarra M., Gonzalez-Herrera I.G., Pedraza-Chaverri J., Maldonado P.D. Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. J. Nutr. Biochem. 2013;24:14–24. doi: 10.1016/j.jnutbio.2011.12.010[↩]
- Yu W., Zha W.L., Ke Z.Q., Min Q., Li C.R., Sun H.R., Liu C. Curcumin protects neonatal rat cardiomyocytes against high glucose-induced apoptosis via PI3K/Akt signalling pathway. J. Diabetes Res. 2016;2016:4158591. doi: 10.1155/2016/4158591[↩][↩]
- Kohli S., Chhabra A., Jaiswal A., Rustagi Y., Sharma M., Rani V. Curcumin suppresses gelatinase B mediated norepinephrine induced stress in H9c2 cardiomyocytes. PLoS ONE. 2013;8:e76519. doi: 10.1371/journal.pone.0076519[↩]
- Manghani C., Gupta A., Tripathi V., Rani V. Cardioprotective potential of curcumin against norepinephrine-induced cell death: A microscopic study. J. Microsc. 2017;265:232–244. doi: 10.1111/jmi.12492[↩]
- Naserzadeh P., Mehr S.N., Sadabadi Z., Seydi E., Salimi A., Pourahmad J. Curcumin protects mitochondria and cardiomyocytes from oxidative damage and apoptosis induced by hemiscorpius lepturus venom. Drug Res. 2018;68:113–120. doi: 10.1055/s-0043-119073[↩]
- Yang X.B., Jiang H., Shi Y. Upregulation of heme oxygenase-1 expression by curcumin conferring protection from hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblasts. Cell Biosci. 2017;7:523. doi: 10.1186/s13578-017-0146-6[↩]
- Bai X.J., Hao J.T., Wang J., Zhang W.F., Yan C.P., Zhao J.H. Curcumin inhibits cardiac hypertrophy and improves cardiovascular function via enhanced Na+/Ca2+ exchanger expression after transverse abdominal aortic constriction in rats. Pharmacol. Rep. 2018;70:60–68. doi: 10.1016/j.pharep.2017.07.014[↩]
- Chen R.C., Peng X.F., Du W.M., Wu Y., Huang B., Xue L., Wu Q., Qiu H.M., Jiang Q.S. Curcumin attenuates cardiomyocyte hypertrophy induced by high glucose and insulin via the PPAR gamma/Akt/NO signaling pathway. Diabetes Res. Clin. Pract. 2015;108:235–242. doi: 10.1016/j.diabres.2015.02.012[↩]
- Katanasaka Y., Sunagawa Y., Hasegawa K., Morimoto T. Application of curcumin to heart failure therapy by targeting transcriptional pathway in cardiomyocytes. Biol. Pharm. Bull. 2013;36:13–17. doi: 10.1248/bpb.b212022[↩]
- Cao Q., Zhang J.X., Gao L., Zhang Y.J., Dai M.Y., Bao M.W. Dickkopf-3 upregulation mediates the cardioprotective effects of curcumin on chronic heart failure. Mol. Med. Rep. 2018;17:7249–7257. doi: 10.3892/mmr.2018.8783[↩]
- Feng D., Zou J., Zhang S.S., Li X.C., Lui M.Q. Hypocholesterolemic activity of curcumin is mediated by down-regulating the expression of Niemann-Pick C1-like 1 in hamsters. J. Agric. Food Chem. 2017;65:276–280. doi: 10.1021/acs.jafc.6b04102[↩]
- Meng B., Li J., Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013;19:2101–2113.[↩]
- Chuengsamarn S., Rattanamongkolgul S., Luechapudiporn R., Phisalaphong C., Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35:2121–2127. doi: 10.2337/dc12-0116[↩]
- Li J., Wang P.P., Ying J., Chen Z., Yu S.P. Curcumin attenuates retinal vascular leakage by inhibiting calcium/calmodulin-dependent protein kinase ii activity in streptozotocin-induced diabetes. Cell. Physiol. Biochem. 2016;39:1196–1208. doi: 10.1159/000447826[↩]
- Wojcik M., Krawczyk M., Wojcik P., Cypryk K., Wozniak L.A. Molecular mechanisms underlying curcumin-mediated therapeutic effects in type 2 diabetes and cancer. Oxid. Med. Cell. Longev. 2018;2018:9698258. doi: 10.1155/2018/9698258[↩]
- Jhong C.-H., Riyaphan J., Lin S.-H., Chia Y.-C., Weng C.-F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. BioFactors. 2015;41:242–251. doi: 10.1002/biof.1219[↩]
- Akolade J.O., Oloyede H.O.B., Onyenekwe P.C. Encapsulation in chitosan-based polyelectrolyte complexes enhances antidiabetic activity of curcumin. J. Funct. Foods. 2017;35:584–594. doi: 10.1016/j.jff.2017.06.023[↩]
- Gutierres V.O., Campos M.L., Arcaro C.A., Assis R.P., Baldan-Cimatti H.M., Peccinini R.G., Paula-Gomes S., Kettelhut I.C., Baviera A.M., Brunetti I.L. Curcumin pharmacokinetic and pharmacodynamic evidences in streptozotocin-diabetic rats support the antidiabetic activity to be via metabolite(s) Evid. Based Complement. Alternat. Med. 2015;2015:678218. doi: 10.1155/2015/678218[↩]
- Chanpoo M., Petchpiboonthai H., Panyarachun B., Anupunpisit V. Effect of curcumin in the amelioration of pancreatic islets in streptozotocin-induced diabetic mice. J. Med. Assoc. Thai. 2010;93(Suppl. 6):S152–S159[↩]
- Abd-Allah G.A., El-Bakry K.A., Bahnasawy M.H., El-Khodary E.R. Protective effects of curcumin and ginger on liver cirrhosis induced by carbon tetrachloride in rats. Int. J. Pharmacol. 2016;12:361–369. doi: 10.3923/ijp.2016.361.369[↩]
- Peng X.Y., Dai C.S., Liu Q.W., Li J.K., Qiu J.R. Curcumin attenuates on carbon tetrachloride-induced acute liver injury in mice via modulation of the Nrf2/HO-1 and TGF-1/Smad3 pathway. Molecules. 2018;23:215. doi: 10.3390/molecules23010215[↩]
- Choudhury S.T., Das N., Ghosh S., Ghosh D., Chakraborty S., Ali N. Vesicular (liposomal and nanoparticulated) delivery of curcumin: A comparative study on carbon tetrachloride-mediated oxidative hepatocellular damage in rat model. Int. J. Nanomed. 2016;11:2179–2193. doi: 10.2147/ijn.s101886[↩]
- Tung B.T., Hai N.T., Son P.K. Hepatoprotective effect of phytosome curcumin against paracetamol-induced liver toxicity in mice. Braz. J. Pharm. Sci. 2017;53 doi: 10.1590/s2175-97902017000116136[↩]
- Lee H.Y., Kim S.W., Lee G.H., Choi M.K., Jung H.W., Kim Y.J., Kwon H.J., Chae H.J. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement. Altern. Med. 2016;16:316. doi: 10.1186/s12906-016-1307-6[↩]
- Bayindir N., Esrefoglu M., Kumas M., Iraz M., Kesgin S., Kilic E. Protective effect of curcumin on cadmium-induced liver apoptosis in rats. Bezmialem Sci. 2016;4:99–105. doi: 10.14235/bs.2016.714[↩]
- Afrin R., Arumugam S., Soetikno V., Thandavarayan R.A., Pitchaimani V., Karuppagounder V., Sreedhar R., Harima M., Suzuki H., Miyashita S., et al. Curcumin ameliorates streptozotocin-induced liver damage through modulation of endoplasmic reticulum stress-mediated apoptosis in diabetic rats. Free Radic. Res. 2015;49:279–289. doi: 10.3109/10715762.2014.999674[↩]
- Granados-Castro L.F., Rodriguez-Rangel D.S., Fernandez-Rojas B., Leon-Contreras J.C., Hernandez-Pando R., Medina-Campos O.N., Eugenio-Perez D., Pinzon E., Pedraza-Chaverri J. Curcumin prevents paracetamol-induced liver mitochondrial alterations. J. Pharm. Pharmacol. 2016;68:245–256. doi: 10.1111/jphp.12501[↩]
- Guo C., Ma J.F., Zhong Q.H., Zhao M.Y., Hu T.X., Chen T., Qiu L.X., Wen L.P. Curcumin improves alcoholic fatty liver by inhibiting fatty acid biosynthesis. Toxicol. Appl. Pharmacol. 2017;328:1–9. doi: 10.1016/j.taap.2017.05.001[↩]
- Lu C.F., Xu W.X., Zhang F., Shao J.J., Zheng S.Z. Nrf2 knockdown disrupts the protective effect of curcumin on alcohol-induced hepatocyte necroptosis. Mol. Pharm. 2016;13:4043–4053. doi: 10.1021/acs.molpharmaceut.6b00562[↩]
- Zabihi N.A., Pirro M., Johnston T.P., Sahebkar A. Is there a role for curcumin supplementation in the treatment of non-alcoholic fatty liver disease? The data suggest yes. Curr. Pharm. Des. 2017;23:969–982. doi: 10.2174/1381612822666161010115235[↩]
- Inzaugarat M.E., De Matteo E., Baz P., Lucero D., Garcia C.C., Ballerga E.G., Daruich J., Sorda J.A., Wald M.R., Chernavsky A.C. New evidence for the therapeutic potential of curcumin to treat nonalcoholic fatty liver disease in humans. PLoS ONE. 2017;12:e0172900. doi: 10.1371/journal.pone.0172900[↩]
- Watanabe K., Karuppagounder V., Arumugam S., Thandavarayan R.A., Pitchaimani V., Sreedhar R., Afrin R., Harima M., Suzuki H., Suzuki K., et al. Pruni cortex ameliorates skin inflammation possibly through HMGB1-NF kappa B pathway in house dust mite induced atopic dermatitis NC/NGA transgenic mice. J. Clin. Biochem. Nutr. 2015;56:186–194. doi: 10.3164/jcbn.14-75[↩]
- Afrin R., Arumugam S., Rahman A., Wahed M.I.I., Karuppagounder V., Harima M., Suzuki H., Miyashita S., Suzuki K., Yoneyama H., et al. Curcumin ameliorates liver damage and progression of nash in NASH-HCC mouse model possibly by modulating HMGB1-Nf-kappa B translocation. Int. Immunopharmacol. 2017;44:174–182. doi: 10.1016/j.intimp.2017.01.016[↩]
- Kheradpezhouh E., Barritt G.J., Rychkov G.Y. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes. Redox Biol. 2016;7:1–7. doi: 10.1016/j.redox.2015.11.001[↩]
- Zhang F., Zhang Z.L., Chen L., Kong D.S., Zhang X.P., Lu C.F., Lu Y., Zheng S.Z. Curcumin attenuates angiogenesis in liver fibrosis and inhibits angiogenic properties of hepatic stellate cells. J. Cell. Mol. Med. 2014;18:1392–1406. doi: 10.1111/jcmm.12286[↩]
- Zheng J.J., Wu C.Z., Lin Z., Guo Y., Shi L., Dong P.H., Lu Z.Q., Gao S.M., Liao Y., Chen B.C., et al. Curcumin up-regulates phosphatase and tensin homologue deleted on chromosome 10 through microRNA-mediated control of DNA methylation—A novel mechanism suppressing liver fibrosis. FEBS J. 2014;281:88–103. doi: 10.1111/febs.12574[↩]