What is Hesperidin
Hesperidin is a secondary plant metabolite and one of the principal bioflavonoids (flavanone glycoside, meaning it has a sugar molecule attached to its three-ringed flavonoid structure) of citrus fruits such as lemon, sweet orange (Citrus sinensis), and grapefruits 1. Flavonoids are a large group of plant pigments sharing the same basic chemical structure, that is a three-ringed molecule with hydroxyl (OH) groups attached. In addition to the Citrus species, hesperidin could be isolated from other plant genera like legumes 2, Papilionaceae 3, Betulaceae 4, Lamiaceae 5, Zanthoxylum species (Zanthoxylum avicennae and Zanthoxylum cuspidatum) 6 and Acanthopanax setchuenensis 7. Neohesperidin (as an isomer of hesperidin) is a bitter compound that is found in bitter orange (Citrus aurantium) 6. Lemons contain an amount of hesperidin (in mg/100 mL) comparable to that of oranges, but to drink a same volume of juice is more difficult. The flavonoid content of the red orange is mainly hesperidin (43.6 mg/100 mL), followed at a distance by narirutin (4.8 mg/100 mL) and dimidine (2.4 mg/100 mL) 8. According to a recent review, the content of hesperidin in 100 mL of juice is: orange 20–60 mg, tangerines 8–46 mg, lemon 4–41 mg, grapefruit 2–17 mg 9. This means that you can take about 100 mg of hesperidin, just in a large glass of orange juice. Based on these data, it can be said that the choice of the most suitable fruits for a better intake of hesperidin could be made between oranges, mandarins and clementines, according to individual preferences, and costs 9. Hesperidin is present mainly in the peel and in the white part (albedo) of citrus fruits, and consumption of the whole fruits may allow a greater intake than the juice 10. In fact, in fresh orange juice, the content of hesperidin is about 30 mg per 100 mL, and in commercial juice it can be a little higher 11, probably because the industrial processing incorporates more peel.
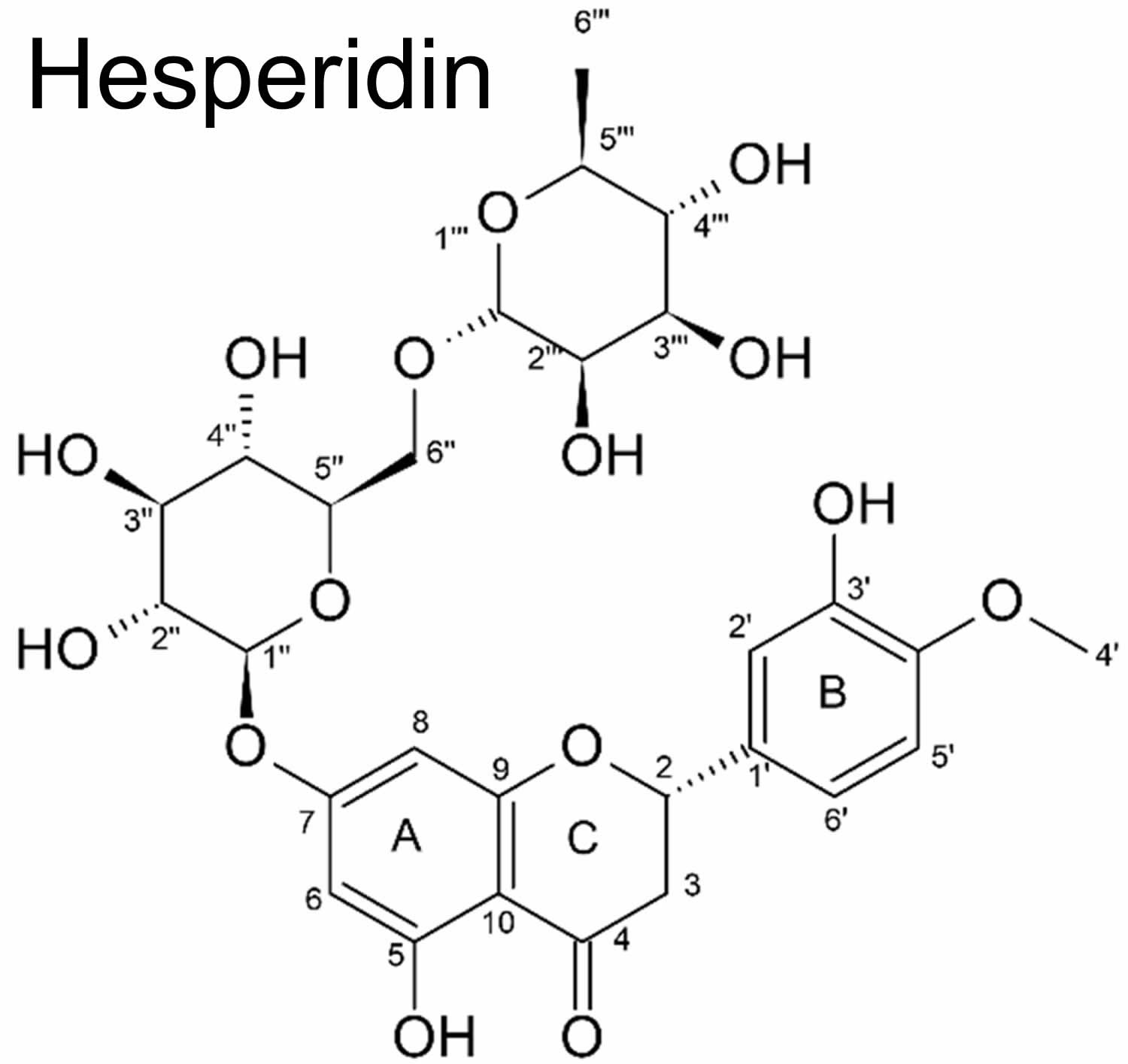
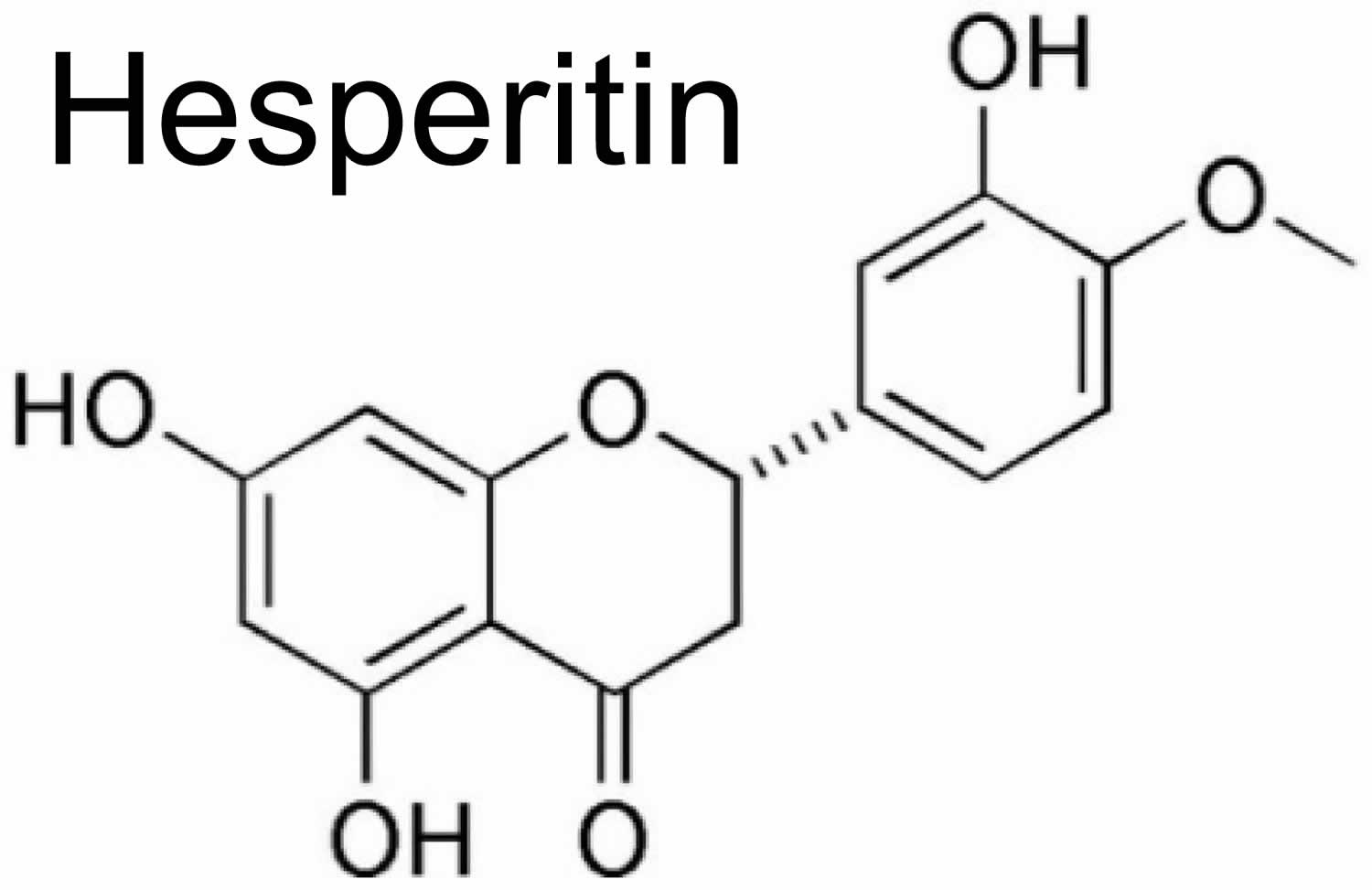
Hesperidin has an aglycone (hesperitin) bonded to rutinose [6-O-(α-l-Rhamnopyranosyl)-d-glucopyranose] and/or [6-O-(α-l-Rhamnosyl)-d-glucose], as a disaccharide, in its structure (see Figure 1) 7. Thus, hesperidin (as a non-bitter flavonoid rutinoside) can be considered a beta-7-rutinoside of hesperetin 3. Both hesperidin and its aglycone hesperitin have been reported to possess a wide range of pharmacological properties 12. Hesperidin has also been found in unripe sour oranges, Ponderosa lemon, Citrus unshiu, and calamansi 13.
Some tests have assessed the amount of hesperidin (or its metabolite hesperetin) in the blood of people drinking orange juice. Healthy volunteers drank orange juice in one intake (8 mL/kg) and blood and urine samples were collected between 0 and 24 hours after administration 14. The peak plasma concentration of hesperetin was 2.2 ± 1.6 micromol/L, with significant variations in different subjects. Elimination half-life ranged from 1.3 to 2.2 hours, indicating short-term kinetics. In another experiment 15, after a night fast, five healthy volunteers drank 0.5 or 1 L of commercial orange juice, containing 444 mg/L of hesperidin, along with a polyphenol-free breakfast. The flavanone metabolites appeared in the plasma 3 hours after the ingestion of the juice, peaked between 5 and 7 hours, then returned to the baseline value at 24 hours. The peak plasma concentration of hesperetin was 0.46 ± 0.07 micromol/L and 1.28 ± 0.13 micromol/L, after taking 0.5 and 1 L, respectively. The authors concluded that, in the case of moderate or high consumption of orange juice, flavanones represent an important part of the pool of total polyphenols in plasma 15. There is evidence that hesperidin and naringin are metabolized by intestinal bacteria, mainly in the proximal colon, with the formation of their aglycones, hesperetin and naringenin and various other small phenols 16. Studies have also shown that citrus flavanones and their metabolites are able to influence the composition and activity of the microbiota, and to exert beneficial effects on gastrointestinal function and health. Other bioavailability studies have calculated that, if the phenolic catabolites derived from the colon are added to the glucuronide and sulphate metabolites, the polyphenols derived from orange juice are much more abundant and available than previously thought 17.
Hesperidin is known for its valuable bioactivity and can act as an antioxidant 18, antibacterial 19, anti-inflammatory 20, hypolipidemic 21, vasoprotective 22, 23, anticancerogenic 24 and anti-ageing properties 25. Hesperidin, a flavonoid from citrus fruit, is known to improve vascular integrity and decrease capillary permeability 26. A dietary deficiency in hesperidin has been linked to abnormal capillary leakiness, pain and weakness in the extremities, and nocturnal leg cramps 27.
Recent animal studies have shown that hesperidin might have beneficial neuropharmacological effects including antidepressant, anticonvulsant, anti-inflammatory, and anticonvulsant properties, in addition to memory and locomotor enhancing 28. Hesperidin can effectively protect neurons from damages induced by oxidative or nitrosative stress. Moreover, it enhances cognitive functions through various mechanisms such as elevating brain-derived neurotrophic factor (BDNF, a signaling molecule secreted from activated microglia that helps to support the survival of neurons) and reversing the disruptive effect of global cerebral ischemia and reperfusion on memory.
Hesperidin can be considered a potential candidate for the mitigation of oxidative stress, as well as for memory impairment of the Alzheimer’s disease type 28. Furthermore, hesperidin showed antidepressant activities using mechanisms that differ from those of conventional antidepressant drugs. Clinical trials showed that hesperidin-enriched dietary supplements can significantly improve cerebral blood flow, cognition, and memory performance 28. Despite the variety of in vivo (animal) mechanistic studies on the neuroprotective activity of hesperidin, the lack of clinical trials on the therapeutic effects of hesperidin is an important limitation that can be noted, deserving further research. Moreover, less is known about the clinical aspects of this compound, such as bioavailability, the appropriate dose, tolerability, and efficacy of hesperidin and its metabolites on neurodegenerative diseases 28. These limitations deserve to be surmounted before expanding hesperidin treatment into humans. This can be accomplished by conducting well-designed clinical trials in patients with different types of neurodegenerative diseases. The almost published studies of the clinical efficacy of hesperidin has only been carried out on healthy participants. Studies on the role of hesperidin, either as therapeutic or as complementary supplement, on patients with neurodegenerative diseases should be a high priority 28.
Table 1. Hesperidin content (mg/100 mL of fresh juice) in different citrus fruits
| Fruit | Hesperidin Content (mg/100 mL Juice) | |||
| mg | SD | Min. | Max. | |
| Sweet orange (Citrus sinensis) | 28.6 | 11.9 | 3.5 | 55.2 |
| Red orange (Citrus sinensis) | 43.6 | 17.9 | 18 | 66.5 |
| Commercial sweet orange juice | 37.5 | 19.2 | 4.45 | 76.3 |
| Mandarin (Citrus reticulata) | 24.3 | 18.2 | 0.81 | 45.8 |
| Clementine (Citrus clementine) | 39.9 | 29.4 | 5.21 | 86.1 |
| Lemon (Citrus limon) | 20.5 | 12.4 | 3.84 | 41 |
| Lime (Citrus aurantifolia) | 1.8 | 0.35 | 1.52 | 2 |
| Grapefruit (Citrus paradisi) | 0.9 | 0.58 | 0.25 | 1.8 |
| Commercial grapefruit juice | 2.8 | 3.9 | 0.2 | 16.4 |
Figure 1. Hesperidin chemical structure
Footnote: Chemical structure of hesperidin (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one)
[Source 25 ]Figure 2. Hesperitin chemical structure
[Source 28 ]Hesperidin benefits
Various biological and pharmacological effects have been reported for hesperidin. Hesperidin possesses the anti-oxidant, anti-inflammatory 29 and anti-cancer activities 30. Hesperidin exhibited significant mediatory effect on the extrinsic and intrinsic apoptosis of different cancerous cells 31. Hesperidin and its aglycon, hesperetin, were found to be effective on different types of cancers, including gastric cancer 32, colon cancer 33, lung cancer 34, liver cancer 35, breast cancer 36 and prostate cancer 37. Along with the anti-cancer activity of hesperidin, the effect of isoflavone on inflammation associated with cancer has been proven. It exhibited the inhibitory effect on the inflammatory-mediated cancers by regulating the level of inflammatory components like TNF-α, IL-1β, cyclooxygenase-2 (COX-2), and iNOS 38. Hesperidin was also found to have an anti-replicative activity against some viruses 39.
The effect of hesperidin administration on blood vessel disorders such as edema, bleeding, pleurisy, Henoch-Schonlein purpura and tuberculosis was observed by decreasing the capillary permeability and enhancing the capillary resistance 3. Hesperidin also exerts the antihypercholesterolemic 40, antihyperlipidaemic 41, antihypertensive, diuretic effect 42 and calcium channel blocker activity 43. The in vivo (animal study) administration of phosphorylated hesperidin in rodents caused an anti-fertility effect 44. Some other biological effects, such as immuno-modulatory activity, anti-depressant, anti-allergic effect, ultra-violet protective effect, platelet, and a cell aggregation inhibitory effect, have wound healing potential and have been attributed to hesperidin 45. Apart from the aforementioned biological activities, hesperidin possesses considerable neuroprotective property in various neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, stroke, and Huntington’s disease 46.
Despite the various animal-based studies on the role of hesperidin in neurodegenerative diseases, there have not been enough clinical studies devoted to the administration of hesperidin for human neuroprotection 28. Although a wealth of positive findings has been obtained in animal studies, the mechanism of function of hesperidin phytochemical in the human body remains to be elucidated.
In a placebo-controlled, randomized, and double-blinded clinical study, the effect of chronic administration (eight weeks) of orange juice on the cognition of 37 healthy adults (60–81 years of age) was examined 47. A group of adults consumed a juice with 549 mg/L hesperidin and 60mg/L narirutin (as a flavanone); another group drunk a juice with 64 mg/L hesperidin and 10 mg/L narirutin, 250 mL twice a day 47. At the baseline there was no significant alteration in the cognitive function of these two groups, but after eight weeks the cognitive function, executive function, and episodic memory of the group that consumed orange juice with higher hesperidin content was significantly better than the group that received a lower amount of hesperidin 47.
The most significant effect of hesperidin-rich juice was observed in the immediate recall after the follow-up period of the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) 47. Besides these effects, the chronic consumption of hesperidin-rich juice significantly decreased the diastolic blood pressure. Their study clarified that hesperidin-rich dietary interventions could prevent cognitive decline in neurodegenerative patients 47. The underlying mechanism reflecting these effects was not clear, but other studies have claimed that flavanone consumption causes increasing cerebral blood flow 48. In order to examine the possibility of this mechanism, Lamport et al. 49 carried out an acute, randomized, single-blind, placebo-controlled, clinical study on the role of citrus juice in the cognitive function and the cerebral blood flow of 44 healthy young adults (18–30 years of age). The subjects consumed 500 mL of citrus juice containing 42.15 mg hesperidin, while the control group consumed a drink containing 240 mL concentrate and 260 mL mineral water. A separate cohort of participants was chosen to examine the cerebral blood flow of functional MRI. After two hours of screening the participants, within a conscious resting state, a significant improvement was observed in the cerebral blood flow of the right frontal gyrus, as well as the Digit Symbol Substitution Test (DSST, which reflected the executive function) performance 49. Although the citrus juice could enhance these parameters individually, no direct association was found between the improvement of behavioral parameters and an increase in cerebral blood flow. The region-specific alteration in the cerebral blood flow could be attributed to the test condition, which was carried out in the conscious resting state, in which the frontal gyrus was active 49.
In another study that focused on the role of hesperidin-rich drinks in the cognitive function of healthy middle-aged men (30–65 years of age), 5.5 g of orange pomace fiber was added to 240 mL of orange juice containing 220.46 mg hesperidin 50. The beverage was then administered to the treatment group. The placebo group received a drink with a similar taste and energy, but instead included a mixture of glucose, fructose, sucrose, and 0.67% citric acid in 240 mL water. Some tests corresponding to the cognitive battery were conducted to elucidate the role of drinking on the cognitive performance after a 6 h follow-up period, while only the Continuous Performance Task (CPT, which reflected the attention and executive cognitive function of participants) and finger tapping (a criterion for psychomotor speed) enhanced significantly 6 hours post drinking hesperidin reach juice, in comparison to the placebo 50. The non-significant improvement of other cognitive measurements within 6 hours indicated that this medication could not significantly affect global performance. On the other hand, their findings revealed the role of hesperidin-rich juice on the development of objective and subjective cognitive functions and attenuating the decline of alertness 50.
The clinical role of citrus consumption on dementia was also examined in a cohort study of 13,373 Japanese elderly participants 51. The incidence of dementia in participants was closely related to the consumption of citrus in a dose-response and reverse correlation. In the area where the authors carried out the clinical study, the flavonoid-rich citrus fruits were mostly consumed, which are rich in hesperidin, neohesperidin, and other flavonoids. The multivariate-adjusted hazard ratio (HR) for the incidence of dementia was found to be 0.97 for subjects with a ≤2 times/week citrus intake, while this value was 0.92 and 0.86 for subjects with a 3–4 times/week intake and almost every day citrus intake, respectivley 51. These results indicated the protective role of citrus flavonoid in incident dementia and possibility of lowering the risk of this disorder by frequent administration of citrus fruits.
Menopausal hot flashes
Some flavonoids demonstrate a very weak estrogenic effect, which maybe why regular use can alleviate symptoms associated with menopause 52. In one clinical study, 94 menopausal women with hot flashes were given a daily formula for one month containing 900 mg hesperidin, 300 mg hesperidin methyl chalcone and 1,200mg vitamin C 53. After one month, symptoms of hot flashes were completely relieved in 53 percent and reduced in 34 percent of the women 53. No signs of toxicity have been observed with the intake of hesperidin or related compounds.
Weight loss
Mounting evidence has demonstrated that hesperidin possesses inhibitory effect against obesity diseases 54. Hesperidin regulates lipid metabolism 55 and glucose metabolism by mediating AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor (PPAR) signaling pathways, directly regulates antioxidant index and anti-apoptosis, and indirectly mediates NF-κB signaling pathway to regulate inflammation to play a role in the treatment of obesity 54. Hesperidin can also directly regulate the oxidation index, inhibit apoptosis, thereby protecting against damage caused by oxidative stress, and improving lipid peroxidation. In addition, hesperidin-enriched dietary supplements can significantly improve symptoms such as postprandial hyperglycemia and hyperlipidemia. Recent studies have shown that citrus flavonoids play an important role in the treatment of dyslipidemia, insulin resistance, hepatic steatosis, obesity and atherosclerosis 54. Citrus flavonoids, including naringenin, hesperidin, nobiletin and hesperetin, have become promising therapeutic agents for the treatment of metabolic disorders 56.
Hesperetin and hesperidin can stimulate the release of cholecystokinin (CCK), an appetite-regulating hormone, in enteroendocrine STC-1 cells, which is ultimately used to treat obesity by suppressing appetite 57. Dietary bioflavonoid hesperidin can reduce cholesterol and triglyceride levels in broiler chicken serum and pectoral muscle, and positively improve fatty acid and lipid metabolism in broiler breasts in a dose-dependent manner 58. The main types of hesperidin metabolism in rats are mainly hydrolysis, demethoxylation, dehydration, dehydrogenation, demethylation, glucuronide binding, sulfate binding and N-acetylcysteine binding 59. Hesperidin can significantly increase the level of alpha-Klotho (α-KL) in serum, liver and kidney tissues of diabetic rats, and significantly reduce the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN) and creatinine in fibroblast growth factor-23 (FGF-23) in kidney tissues and serum samples 60. High-dose hesperidin up-regulates adenosine 5ʹ-monophosphate (AMP)-activated protein kinase (AMPK) mRNA expression in mice with glycolipid metabolism disorder induced by high-fat diet, affecting insulin signaling pathway (insulin receptor, insulin receptor substrate 1 (IRS-1), GLUT2/4) and lipid metabolism-related genes (sterol regulatory element-binding protein 1c (SREBP1c) and FAS and acetyl-CoA carboxylase) gene expression also activates PPAR-α mRNA expression 61. In addition, hesperidin enhances the expression of genes encoding LDL receptors, which are some of the possible mechanisms by which hesperidin reduces blood lipids 62.
Although the hypoglycemic and lipid-lowering activities of hesperidin have been studied in some animals (such as rats) or cells, the lack of clinical trials on the therapeutic effect of hesperidin is a significant limitation that deserves further study 54.
Table 2. The details on weight loss effect of hesperidin
| Model | Dose And Treat Time | Described Effect | Weight Loss Mechanism | Reference |
| Sisolated perfused male wistar rats, ad libitum with a standard laboratory diet | 300µM; 0–70min | Glycogenolysis and glycolysis in the liver↑; glucose phosphorylation catalysed by GK↓ | G-6-Pase↓ | 63 |
| Rats | 1mL; 24h | Enzyme activities↓; production of pyruvate↓; hepatic gluconeogenes↓; α-ketoglutarate and the oxaloacetate↓ | Liver ALT↓; liver AST↓ | 64 |
| HIGH fat fed/streptozotocin- induced type 2 diabetic rats | 50mg/kg; 4w | Serum glucose and glycosylated hemoglobin↓; vitamin C and vitamin E↑ | NO↓; IL-6↓; TNF-α↓; serum INS↑; GSH↑; liver MDA↓; liver antioxidant enzymes↑ | 65 |
| Male wistar rats, high-cholesterol diet | 25g/d; 12w | Hepatic steatosis, adipose tissue and liver weights↓; serum TC ↓ | RBP, H-FABP, C-FABP in liver and adipose tissue↓ | 66 |
| Male wistar rats, high-fat/sucrose (western) diet | 100mg/kg; 8w | Blood lipid profle↑; hepatic lipid accumulation↓; non-alcoholic steatohepatitis↓ | SREBP1↓; PPAR-γ↓; SCD↓; FAS↓ | 67 |
| Type 2 diabetic rats, high fat diet | 50mg/kg; 4w | White blood cell count↓; neutrophils↓; monocytes↓; basophils↓ | IL-6↓; adipose tissue ACDC↑ | 68 |
| Streptozotocin-induced marginal type 1 diabetic rats | 10g/kg; 4w | Blood glucose↓; TC↓ | Serum ACDC↑; TG↓; G-6-Pase↓; GK↑; LDL-C↓; VLDL-C↓; HDL-C↑; serum INS↑; | 62 |
| Rats, high-cholesterol diet | 8mg/d; 6–12w | Body and liverand adipose tissue weights↓; cholesterol synthesis and absorption↓ | Lipid-related factors (RBP4, H-FABP and C-FABP)↓; ICAM-1↓; inflammatory-related factors (MCP1, CCR2 and TNF-α)↓ | 69 |
| Goto-Kakizaki weanling rats with type 2 diabetes | 0.01g/; 4w | Lipids in the serum and liver↓; blood glucose↓; HDL-C/TC↑ | The genes coding for PPARs↑; HMG-CoA reductase↓; the expression of genes encoding LDL receptor↑; serum ACDC↑; TG↓; INS↑ | 70 |
| Rats with diabetes induced by streptozotocin | 100mg/kg; 2w | Strong positive effects on diabetic toxicity in the liver and kidneys | Liver, kidney and serum α-KL ↑; FGF-23↓; MDA↓ | 71 |
| Rats subjected to isoproterenol- induced cardiotoxicity | 200mg/kg; 7d | TC↓ | LDL-C↓; TG↓; VLDL-C↓; FFA↓; plasma PL↓; HDL-C↑; PL in the heart and liver↑ | 72 |
| Rats, high-cholesterol diet | 100mg/kg; 5d | TC↓; HDL-C/TC↑; serum triglyceride levels↓ | GSH in the liver↑; serum and liver MDA↓ | 73 |
| Streptozotocin-induced hyperglycemic mice | 200mg/kg; 14d | Blood glucose↓; lipid peroxidation and total nitrate/nitrite↓ | Bad/Bcl-2↑; Bad/Bcl-XL↑; SOD↑; GSH↑ | 74 |
| C57BL/6J mice, high-fat diet | 100mg/kg/d; 4w | Serum total antioxidant capacity↑; liver TBARS levels↓; spleen mass↓; fat accumulation↓; liver damage↓ | IL-6↓; MCP-1↓; hs-CRP↓; LDL-C↓ | 75 |
| C57 mice, high-fat diet | 100,200,400mg/kg/d; 16w | Body weight↓; body fat deposition↓; serum glucose↓; serum lipid↓; HOMA-IR index↓ | mRNA of AMPK↑; serum INS↑; impact on signaling pathway genes↑ (INSR, IRS-1, GLUT2/4) and lipid metabolism pathway genes (SREBP1↓, FAS↓, ACC↓,PPAR-α↑) | 61 |
| Mice, high-fat diet | 0.07mg/100g; 9w | Body weight and liver and adipose tissue weight↓ | PPAR-γ↑ | 76 |
| C2C12 cells | 0.07mg/100g; 6h | Stimulated glucose↑ | PPAR-γ↑ | |
| Pre-adipocytes of mesenchymal stem cells | 1,10,25µM; 48h-8d | Anti-adipogenic and delipidating | C/EBPβ↓; SREBP1↓; perilipin↓;PPAR-γ↓ | 77 |
| Mature adipocytes from mesenchymal stem cells | 1,10,25µM; 48h-8d | Anti-adipogenic effect and delipidating | mRNA of ATGL↑; FAS↓;TG accumulation↓ | |
| 3T3-L1 pre-adipocytes | 1,10,25µM; 0-60h-8d | Lipid accumulation↓; riacylglycerol content in pre-adipocytes↓ | SREBP1↓ | 78 |
| 3T3-L1 adipocytes | 20µM; 8d | Lipid accumulation↓ | ROS↓; PPAR-γ↓; C/EBPα↓; FABP4↓ | 79 |
| 3T3-L1 cells | 0.5mg/mL; 24h | Induction of adipolytic activity↓; key adipogenic transcription factors↓ | C/EBPα↓; PPAR-γ↓; SREBP1↓ | 80 |
| 3T3-L1 cells | 10, 50, 100µM; 8d | Anti-lipogenic capacity↑ | Binding affinity for the PPAR-γ rceptor↓; SCD↓; LPL↓ | 81 |
| RAW264.7 and 3T3-L1 cells | 1.8–8.3µM; 24h | Anti-inflammatory activity↑ | ACDC↑; IL-6↓; TNF-α↓; NO↓ | 82 |
| Enteroendocrine STC-1 cells | 0.1,0.5,1.0µM; 60min | Appetite-regulating hormones↑; cholecystokinin release↑ | Intracellular Ca(2+) concentrations↑ | 57 |
| Retinal ganglial cells −5 | 12.5,25,50µmol/L; 6h | High glucose-mediated cell loss↓; mitochondrial function↑; Cell apoptosis↓ | ROS, MDA and protein carbonyl↓; SOD↑; CAT↑; GSH↑; caspase-9, caspase-3 and Bax/Bcl-2↓ | 83 |
| HepG2 cells | 100ug/mL; 48h | Lipid accumulation↓ | miR-122 and miR-33 expression↓; CPT1α↑; FAS↓ | 84 |
| HepG-2 cells | 50µM; 1min | Digestive enzyme activities↓; glycogen↑ | GK activity↑; G-6-Pase↓ | 85 |
| Porcine pancreas | 100µM; 1min | Glucose consumption↑; glycogen↑; glucokinase activity↑ | α-amylase activity↓; α-glucosidase activity↓ | |
| Caenorhaditis elegans | 50µM,100µM; 0–35d | Fat accumulation↓; the ratio of oleic acid/stearic acid↓ | SCD↓; FAT-6↓; FAT-7↓; POD-2↓; MDT-15↓; ACS-2↓; KAT-1↓ | 86 |
| Broilers | 20mg/kg; 42d | Plasma antioxidant parameters↑; TC↓; total antioxidant capacity↑ | Total SOD↑; MDA↓; TG↓ | 58 |
Footnote: ↓indicates inhibition/reduction while ↑indicates increase/promotion
Abbreviations: ACDC = adiponectin; hs-CRP = High-sensitivity C-reactive protein; INSR = Insulin receptor; IRS-1 = Insulin receptor substrate 1; ATGL = adipose triacylglyceride lipase; PL =, phospholipids; FFA = free fatty acids; TG = triglycerides; HDL-C = high density lipoprotein-cholesterol; LDL-C = low density lipoprotein-cholesterol (“bad” cholesterol); VLDL-C = very low density lipoprotein-cholesterol; MDA = malondialdehyde; GSH = glutathione; G-6-Pase = glucose-6-phosphatase; HMG-CoA = 3-hydroxy-3-methyl-glutatyl coenzyme A; α-KL = α-Klotho; FGF-23 = fibroblast growth factor-23; RBP4 = retinol-binding protein 4; CCR2 = C-C chemokine receptor type 2; MCP1 = monocyte chemoattractant protein-1; TNF-α = tumor necrosis factor alpha; TBARS = thiobarbituric acid reactive substances; ROS = reactive oxygen species; CAT = catalase; GK = glucokinase; C/EBPβ = CCAAT/enhancer-binding protein beta; PPAR-γ = peroxisome proliferator-activated receptor gamma; SREBP1 = sterol regulatory element-binding protein 1; RBP = lipid metabolism–related proteins; H-FABP = heart fatty acid–binding protein; C-FABP = cutaneous fatty acid–binding protein; IL-6 = interleukin-6; NF-κB = nuclear factor kappa B; SCD = stearoyl-CoA desaturase; TC = Total cholesterol; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CPT1α = carnitine palmitoyltransferase 1α; FAS = fatty acid synthase; LPL = lipoprotein lipase; FAT-6/7 = Fatty-acid desaturase 6/7; ACS-2 = acyl-CoA synthetase-2; KAT-1 = ketoacyl-CoA thiolase-1; POD-2 = acetyl-CoA carboxylase-2; MDT-15 = mediator subunit-15.
Use in cosmetics as anti-ageing active components
Hesperidin and hesperetin are the focus of intensive research for topical application. For example, sulfonated, acetylated, or phosphorylated hesperidin derivatives are potent inhibitors of hyaluronidase 25. Moreover, hesperidin can also act on superoxide in electron transfer as well as proton transfer in vivo (animal study). Hesperidin might act as a topical UV-protective agent, by protecting phosphatidylcholine liposomes from UV irradiation-induced peroxidation. The neohesperidin, for example, demonstrates the capacity to extend yeast’s chronical lifespan, individually or in synergism with the hesperitin, for 10 different aging factors, such as scavenging ROS (reactive oxygen species) effects, regulation of stress-related enzymes, and maintaining pH cellular value, favorable for life extension of yeast cells 87. Hesperidin was also proved as a potent anti-photoageing factor, through regulation of metalloproteinases MMP-9 via mitogen activation protein kinase (MAPK) signaling pathways 88. In the same study, Lee and colleagues 88 approved the positive effect of hesperidin on wrinkle depth on a mouse dorsal skin model and reduction in UVB-induced hydration changes and trans-epidermal water loss 89. Different studies demonstrate accelerated cutaneous diabetic or venous wounds healing and ameliorate skin epidermal barrier function already after 7 days of Hesperidin application 89. On the other hand, hesperitin was found to penetrate through the stratum corneum 90 and assays conducted in vitro (test tube) showed that the presence of lecithin and d-limonene in formulations may aid towards faster hesperetin penetration into the skin 91. Additionally, in vitro studies of the stratum corneum have demonstrated that flavonoids show differences in penetration capacities, which depend in a large part on the ingredients/vehicles present in the formulation. Therefore, the penetration rate of flavonoids, such as catechin, rutin, quercetin, and others, is influenced by moisturizing ingredients (glycerol, glycols, polyglycols, ethoxylated methyl glucoside, and urea) and by the type of cosmetic formulation (hydrogel, emulsion, microemulsion, and micellar system) 92. For example, the water in oil microemulsion formulations significantly enhances quercetin skin penetration up to 12 hour after application 93. Hesperidin-loaded nanostructured lipid vehicles show burst release in the beginning and further sustained bioflavonoid release from the lipid nanocarrier 94. Despite their interesting and beneficial skin effects, bioflavonoids are very demanding for making effective formulations. In addition, their insolubility in water complicates their use in cosmetic products and influences greatly on their extraction from fruits and plants that is usually performed by the use of organic solvents 25.
Parkinson’s disease
Parkinson’s disease is one of the neurodegenerative diseases associated with the loss of dopaminergic neurons. Oxidative stress is a prominent feature in pathogenesis of Parkinson’s disease; loss of dopaminergic neurons and the continued involvement of these features cause the appearance of non-motor symptoms of the disease (i.e., depression, mood and cognition impairment, sleep disturbance, impaired olfaction, etc.) 95. This type of neurodegenerative disease is associated with the accumulation of ROS (reactive oxygen species). The suppressive mechanisms for minimizing free radical formation and oxidative stress could involve the regulation of antioxidant enzyme levels 96. In addition to oxidative stress, the oxidative metabolism of cytosolic dopamine via interaction with monoamine oxidase (MAO) enzyme plays a prominent role in the progression of Parkinson’s disease 97.
Hesperidin, as a potent antioxidant and biomembrane stabilizer, can cause several protective effects in Parkinson’s disease models via antioxidant and dopamine-enhancing mechanisms 28. Furthermore, hesperidin exhibited modulatory activity upon kappa-opioid (κ-opioid) and serotonergic 5-HT1A receptors, leading to the reduction of depression symptoms 98. Hesperidin was found to be effective in minimizing cognitive and depressive deficits in mice by modulating neurotransmitter systems. Moreover, it potentially inhibited the depletion of dopamine and its metabolites 3,4-dihydroxyphenylacetic acid and homovanillic acid, as well as its exertion of antioxidant activity by modulating glutathione (GSH) levels, glutathione-peroxidase (GPx) and catalase (CAT) activities, inhibiting ROS formation, and attenuating glutathione reductase activity 98. Additionally, hesperidin enhanced the locomotion efficiency, inhibited the lipid peroxidation in Parkinson’s disease models (by decreasing malondialdehyde content), attenuated hypercholesterolemia (by diminishing the total cholesterol and triglycerides in plasma level), and ameliorated DNA damage (by decreasing the levels of 8-hydroxydeoxyguanosine) in Chlorpyrifos-induced models of Parkinson’s disease 99. The neuroprotective mechanism of hesperidin in Parkinson’s disease could also be due to regulation of other neurotransmitters, such as norepinephrine, serotonin, and epinephrine 100. Hesperidin consumption also can be effective on Parkinson’s disease models via down-regulating the level of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-4, and IL-10, as well as affecting glial fibrillary acidic protein (GFAP), iNOS, and COX-2 levels 101.
Hesperidin was found even more effective than the current drug, l-Dopa (levodopa). L-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), which are collectively known as catecholamines. Coadministration of hesperidin with l-Dopa increased the bioavailability of this drug in the 6-hydroxydopamine (6-OHDA) rat model of Parkinson’s disease 100 and inhibited degeneration of cytoplasmic vacuolation mediated by 6-hydroxydopamine (6-OHDA) in the striatal and mid-brain 102. The synergetic interaction of these two Parkinson’s disease medicaments caused a suppressive effect on gene expressions of synuclein alpha (SNCA, as a ubiquitously expressed protein affecting the regulation of dopamine release) and Leucine-rich repeat kinase 2 (LRRK2), as an enzyme containing kinase and GTPase function, which its mutation is the most frequent genetic cause of Parkinson’s disease 28. This combination also potentiated the expressions of parkin (a protein involved in the pathogenesis of autosomal recessive juvenile parkinsonism) and PTEN induced putative kinase1 (PINK1, a mitochondrial kinase phosphorylates parkin) 103. This combination of hesperidin with Sinemet (as one of the most common therapeutics for Parkinson’s disease) decreased the side effect of this drug in Chlorpyrifos-induced Parkinson’s disease models 99.
Dementia and Alzheimer’s disease
Alzheimer’s disease is a neurodegenerative disorder in the central nervous system (brain and spinal cord) with progressive cognitive decline and memory impairment. Alzheimer’s disease is considered the most important cause of dementia 104. However, there is no definite cause for Alzheimer’s disease, even though it has been well-established that its pathogenesis is closely related to cholinergic dysfunction, mitochondrial abnormalities, and oxidative stress 6. Due to the strong memory enhancing and antioxidant effects of hesperidin, it can be considered as a potential medicament for Alzheimer’s disease and dementia 28.
The ability of hesperidin in increasing the anti-oxidative defense might be one of the mechanisms by which hesperidin improves cognitive function. Administration of hesperidin for 16 weeks helped improve learning and memory function by enhancing recognition index in the APPswe/PS1dE9 transgenic mouse model 28. It corrected the amyloid beta (Aβ)-induced mitochondrial abnormalities by reducing malondialdehyde (MDA) and hydrogen peroxide (H2O2) levels, as well as restoring depleting GSH levels and total antioxidant capacity. The mitochondrial enzyme activities were also restored by elevating the mitochondrial complex I–IV enzymes activities 105. Glycogen synthase kinase-3β (GSK-3β) is a protein kinase possesses prominent role in the mitochondrial functions and in Alzheimer’s disease. It closely affects the tau protein hyperphosphorylation and mitochondrial target 106. Activation of this protein kinase causes increasing the oxidative damage. Hesperidin potentially rescued cognitive deficits and demonstrated mitochondrial neuroprotection effect by inhibiting restoration of this kinase. It was the possible mechanism by which hesperidin decreased the level of Aβ1–40 105. Hesperidin also inhibited learning and memory impairments resultant from Aluminum chloride (AlCl3)—induced Alzheimer’s disease, acting as an acetylcholinesterase (AChE) inhibitor. Hesperidin attenuated amyloid precursor protein expression via NF-κB dependent pathway and suppressed the levels of Aβ1–40 and β- and γ-secretases (which both modulate the cleavage of amyloid precursor protein) in the hippocampus and the cortex of the brain of rats 46. In another study, the role of hesperidin on cognitive deterioration in rats with Alzheimer’s disease induced by AlCl3. Alzheimer’s disease was found to reverse the cognitive dysfunctions. By up-regulating B-cell lymphoma 2 (Bcl2) and down-regulating Bcl2-associated X proteins (Bax), expressions were included in the neuroprotective mechanism of hesperidin 107.
Beside the cognitive deficits, Alzheimer’s disease is also associated with non-cognitive and behavioral impairments. Aggregation, deposition, and neuro-inflammation of amyloid beta (Aβ) are all possible reasons for behavioral and cognitive manifestations. In addition to cognitive deficits, hesperidin was found effective in restoring the behavioral manifestation associated with Alzheimer’s disease. In a study on the amyloid precursor protein (APP)/PS1 mice model of Alzheimer’s disease, hesperidin blocked the inflammatory process, rescued amyloid precursor proteins (APPs) production and deposition of amyloid beta (Aβ) peptides in the cortex and hippocampus of the animals, and consequently enhanced the nesting ability and social interactions of the transgenic mice 108. The anti-inflammatory effect of hesperidin was found to be related to the mechanism, in which this herbal compound down-regulated the level of the transforming growth factor β1 (TGF-β1) immunoreactivity and NF-κB (which are known to be involved in the progression of Alzheimer’s disease) in the brain cortical region 109. The role of TGF-β1 in stimulating the amyloid precursor protein (APP) production and Aβ peptides deposition has been reported in a previous study conducted by Gray et al. 110.
Dementia is also a progressive dysfunction with a stepwise deterioration of memory, learning, and motor functions. The neuroprotective effect of hesperidin on the intracerebroventricular streptozotocin-induced animal model of sporadic dementia of Alzheimer’s type (SDAT) was corroborated by testing its efficacy on spatial learning, memory, and cholinergic dysfunctions. Morris water maze escape and probe tests revealed the dose-dependent effect of hesperidin to decrease escape latency and enhance memory consolidation. The decrease of acetylcholinesterase activity and lipid peroxidation (by decreasing the content of thiobarbituric acid reactive substances), enhancement of gangliosides level, and blocking inflammatory process (by inhibiting NF-κB, COX-2, and iNOS) after administration of hesperidin exhibited the promising role of this flavonoid in alleviating symptoms of sporadic dementia of Alzheimer’s type (SDAT) 111. Moreover, hesperidin exerted the protective effect on vascular dementia in the rat model of l-methionine-induced hyperhomocysteinemia (HHcy). Endothelial dysfunction in addition to cognitive deteriorates, mediated by hyperhomocysteinemia, was attenuated by hesperidin via a dose-dependent mechanism, in which nitrite and serum Hcy levels with MDA levels were decreased, acetylcholinesterase activity was inhibited, and the levels of GSH, SOD, and CAT were increased 112. Similar results were also achieved after coadministration of hesperidin and donepezil in the scopolamine induced amnesia model, corroborating the prominent role of hesperidin in ameliorating dementia and cognitive deficits of Alzheimer’s disease 113.
Huntington’s disease
Huntington’s disease is a progressive and fatal neurodegenerative disorder that is associated with a spectrum of abnormalities, such as involuntary movements, motor impairment, cognitive and memory manifestations, personality changes, neuro-psychiatric disturbances, and dementia 114. A known and well-established phenotypic Huntington’s disease inducer in both human and animal studies is 3-Nitropropionic acid (3-NP), which was used to investigate Huntington’s disease neuro-psychiatric symptoms. 3-NP intoxication was accompanied by oxidative damage, body weight deficit, mitochondrial, locomotor, and grip impairments in striatum. Hesperidin treatment necessitates overcoming these impairments and enhancing locomotor and grip strength. Hesperidin’s enhancement of striatal oxidative defense and its effect on the cellular energy stores were found to be due to the modulatory effect on nitric oxide pathway 115.
To ensure the promising role of hesperidin in attenuating nitric oxide synthase expression, scientists compared the expressions of iNOS before and after hesperidin administration to the 3-NP-intoxicated animal models of Huntington’s disease. The significant role of hesperidin in suppression of iNOS level in cortical, striatal, and hippocampal regions corroborated its nitric oxide-related mechanism of effect on the Huntington’s disease models 116. In addition to these effects, the role of hesperidin on reduction of malondialdehyde (MDA) level, enhancement of CAT activity, and prevention of prepulse inhibition of the startle response provided a strong indication that it had a beneficial role in the treatment of Huntington’s disease 116. Interestingly, a microglial pathway was found to likely be involved in the protective effect of hesperidin on Huntington’s disease 45. Coadministration of minocycline (as a microglial inhibitor) with hesperidin in rat models of quinolinic acid mediated Huntington’s disease, significantly potentiated the effect of hesperidin on excitotoxicity induced by quinolinic acid. The quinolinic acid-mediated apoptosis (increased level of caspase-3 activity), the quinolinic acid-mediated reduction of brain-derived neurotrophic factor (BDNF, a signaling molecule secreted from activated microglia that helps to support the survival of neurons) level 117 and the quinolinic acid-mediated elevation of TNF-α level were inhibited by minocycline and hesperidin 118. These results altogether suggest that the inhibitory effect of hesperidin on the activation of microglial cells and involvement of the microglial pathway in its neuroprotective effects against Huntington’s disease 28.
Multiple sclerosis
Multiple sclerosis (MS) is a chronic and complex neuro-inflammatory demyelinating disease of the central nervous system, which is the major cause of neurological disability 119. This type of neuro-inflammatory disease is commonly accompanied by axonal loss and glial scaring, in addition to the secretion of inflammatory cytokines 120. The pathogenesis of these types of central nervous system disorders include the invasion and proliferation of the CD4+ T-cells, T-cells and macrophage infiltration, and nitric oxide (NO) production in the cerebral spinal fluid (CSF) 121. The anti-inflammatory effect of flavonoids (i.e., hesperidin) and their inhibitory effect on the pro-inflammatory cytokines, together with their potential in attenuating proliferation of T-cells, makes them a promising agent in ameliorating MS. Hesperidin dose-dependently diminished demyelination in the central nervous system and ameliorated the clinical abnormalities in the myelin oligodendrocyte glycoprotein-induced C57BL/6 mice model of MS. These abnormalities include excretion of pro-inflammatory cytokines such as IL-6, IL-17, IL-23, TNF-α, and Th17 cells transcription factor (ROR-γt, retinoic acid receptor-related orphan nuclear receptor gamma) and the reduction of Treg related cytokines (IL-10 and TGF-β), as well as the FoxP3 transcription factor 122.
Other than the aforementioned abnormalities, MS models demonstrated the lipid peroxidation (elevated thiobarbituric acid reactive substances (TBARS) level) and suppression of enzymatic and non-enzymatic antioxidants. Hesperidin treatment was found beneficial to alleviate these manifestations and reversed oxidative damage and histological changes of cerebral cortex caused by experimental allergic encephalomyelitis 123. The anti-apoptotic effect of hesperidin on the neurons of a C57BL/J6 mouse model was also corroborated via down-regulating caspase3-like immunoreactivity 123.
Diabetes mellitus associated neurotoxicity
Diabetes is among the many independent risk factors of neurodegenerative diseases like Alzheimer’s disease and dementia 124. Diabetes causes vascular and neurodegenerative effects on patients, leading to the fast cognitive decline; insulin resistance causes potentiating amyloid beta (Aβ) production 125. Protein glycation and glucose autoxidation are the main reasons for damaged cell structures and disrupted cellular integrity in diabetic patients. Several studies have provided insights on the role of flavonoids as potent antioxidants with hypoglycemic and anti-inflammatory effects, in hyperglycemia of diabetes mellitus, and the incidence and progression of diabetes-induced neuro-complications 126. Hesperidin exhibited antihyperglycemic and antidyslipidemic activities in streptozotocin induced diabetes mellitus (STZ-diabetes mellitus) models and successfully attenuated the overproduction of ROS by restoring the enzymatic (glutathione-S-transferase (GST) and glutathione reductase (GR); nonenzymatic endogenous antioxidants, GSH, and Nonprotein bound thiol). Consequently, there was also the depletion of lipid peroxidation levels in a STZ diabetic rat brain 127. Furthermore, it reduces the activities of cytochrome oxidase and aldose reductase, as well as sorbitol dehydrogenase 128. The formation of xanthine oxidase (XO) in the brain of diabetic patients is included in the major pathogenesis of diabetes mellitus; hesperidin was found as one of the medicaments capable of suppressing XO levels in the diabetic brain 127. The activity of neurotoxicity markers such as AChE and Na+/K+ ATPase were also significantly affected by hesperidin treatment. This therapeutic process possessed the ability to attenuate the diabetic neurotoxicity by controlling the sodium and potassium gradients 129. These studies strongly suggest that hesperidin, as a medicament with dual anti-diabetic and Alzheimer’s disease treating attributes, is effective when targeting diabetes-induced Alzheimer’s disease.
Diabetic neuropathy is one of the most troublesome long-term complications of diabetes mellitus. Clinically, it can be distinguished by an increase in a nociceptive response with abnormal electrophysiological conduction 130. The neuroprotective effect of hesperidin against STZ-induced diabetic neuropathic pain in rats has shown that hesperidin, in conjunction with insulin, reduces the diabetic condition and reverses neuropathic pain through control over hyperglycemia and hyperlipidemia, which down-regulates ROS production, releases pro-inflammatory cytokines, and elevates membrane bound enzymes 129 . The antihyperglycemic activity of hesperidin against diabetic neuropathy is attributed to its effect on mitigating the elevated level of glycated hemoglobin (HbA1c) and overcoming the insulin resistance by increasing the level of insulin 129. In the STZ-induced diabetic neuropathy, the overproduction of TNF-α and IL-1β increase the hyperalgesia effect of polyneuropathy’s progress and maintenance 131, which could be attenuated after hesperidin treatment 129. Furthermore, hesperidin possesses a restorative effect on normal secretome and hippocampal proteome profiles in the diabetic brain 126. Interestingly, hesperidin was found to be a potent medicament for diabetic neuropathy, which is highly related to both its anti-diabetic and neuroprotective effects 28.
Diosmin and hesperidin
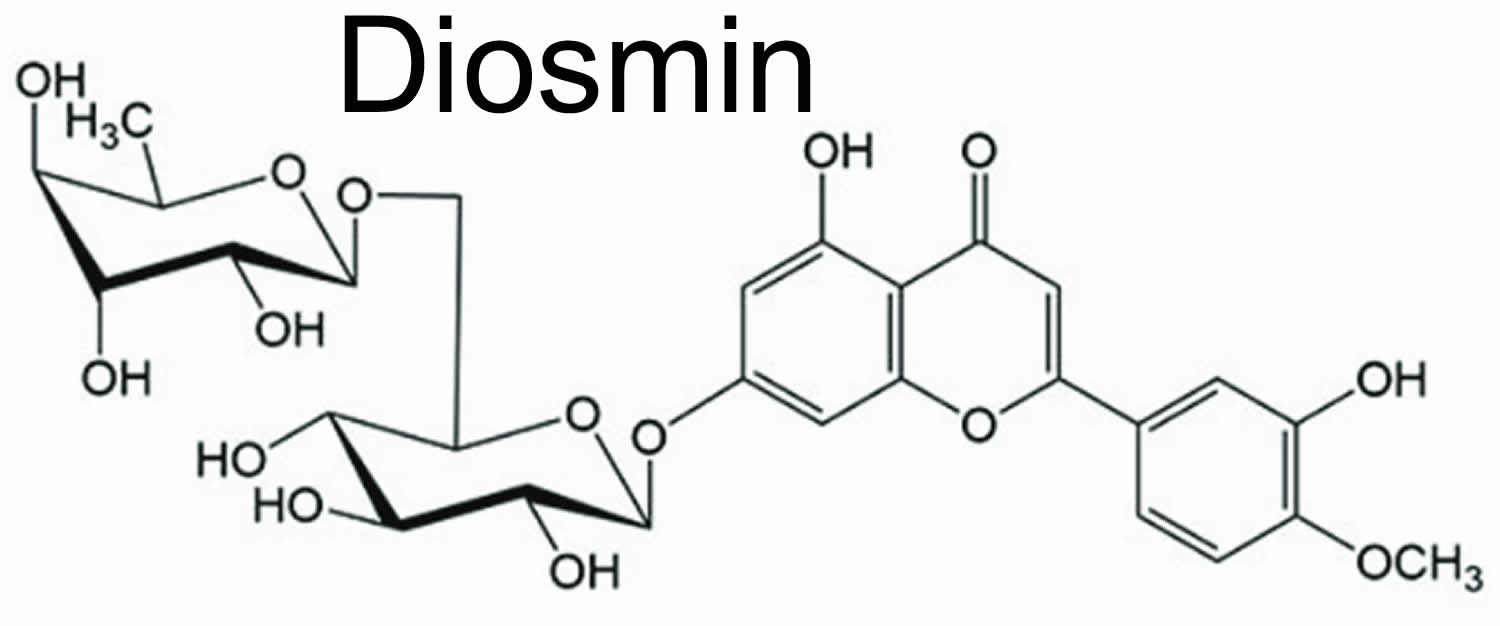
Diosmin is a naturally occurring flavonoid glycoside (diosmetin 7-rutinoside) that can be isolated from various plant sources but is contained mainly in citrus or derived from the flavonoid hesperidin 132. Diosmin differs molecularly from hesperidin by the presence of a double bond between two carbon atoms in diosmin’s central carbon ring (see Figure 3). Diosmin can be manufactured by extracting hesperidin from citrus rinds, followed by conversion of hesperidin to diosmin. Diosmin shows antihyperglycemic 133 and anticancer effects 134, in addition to anti-inflammatory and antioxidant-like actions 135. In type-2 diabetic animals, diosmin attenuated hyperglycemia and increased insulin secretion 136. Diosmin is considered to be a vascular-protecting agent used to treat chronic venous insufficiency, hemorrhoids, lymphedema and varicose veins 137. Diosmin has been used for more than 30 years as a phlebotonic and vascular-protecting agent and has recently begun to be investigated for other therapeutic purposes, including cancer, premenstrual syndrome, colitis, and diabetes. As a flavonoid, diosmin also exhibits anti-inflammatory, free-radical scavenging, and antimutagenic properties.
Diosmin’s mechanisms of action include improvement of venous tone, increased lymphatic drainage, protection of capillary bed microcirculation, inhibition of inflammatory reactions, and reduced capillary permeability 138, 139. Certain flavonoids, including diosmin, are potent inhibitors of prostaglandin E2 (PGE2)and thromboxane A2 (TxA2)7 as well as being inhibitors of leukocyte activation, migration, and adhesion. Diosmin causes a significant decrease in plasma levels of endothelial adhesion molecules and reduces neutrophil activation, thus providing protection against microcirculatory damage 140.
Studies have also investigated the use ofdiosmin for stasis dermatitis 138, wound healing 141, premenstrual syndrome 142, mastodynia 143, dermatofibrosclerosis 138, viral infections 144 and colitis 145.
Pharmacokinetic investigations have shown diosmin is rapidly transformed by intestinal flora to its aglycone form, diosmetin. Diosmetin is absorbed and rapidly distributed throughout the body with a plasma half-life of 26-43 hours. Diosmetin is degraded to phenolic acids or their glycine-conjugated derivatives and eliminated through the urine. Diosmin or diosmetin not absorbed is eliminated in the feces 146. In vitro experiments have reported that only diosmetin is more effective than diosmin 147
Figure 3. Diosmin chemical structure
Diosmin benefits
Diosmin has been widely used as a vasoprotective agent for venous vascular diseases, such as venous leg ulcers 148 and hemorrhoids 149. Diosmin also shows anti-hyperglycemic 133, anti-diabetic 150, anti-gastric ulcer 151, anti-cancer 152, anti-inflammatory, and anti-oxidant-like actions 135. These effects are achieved via decreasing the levels of hydroxyl free radicals, increasing the free thiol (SH-) group concentration and the natural scavenger capacity 153. In addition, since Diosmin has a strong anti-cancer effect, many researchers consider it as an alternative treatment for various cancers that occur in the liver, colon, and urinary system 154.
Diosmin is broken down into its aglycone, diosmetin, by enzymes in the gut bacteria before it is absorbed from the gastrointestinal tract 155. Diosmetin also has antiallergic activity due to its inhibitory effect on the release of beta-hexosaminidase, which induces an inflammatory response from RBL-2H3 cells and inhibits the production of IgE receptor-mediated IL-4 in in vitro experiments 156.
Varicose veins and chronic venous insufficiency
Chronic venous insufficiency is characterized by pain, leg heaviness, a sensation of swelling, and cramps, and is correlated with varicose veins. A multicenter international trial, carried out in 23 countries over two years, in which 5,052 symptomatic patients were enrolled, evaluated theefficacy of flavonoids in the treatment of chronic venous insufficiency 157. Patients were treated with 450 mg diosmin and 50 mg hesperidin daily for six months. Continuous clinical improvement was found throughout the study, as well as improvements in quality of life scores for participants 157.
Diosmin-containing flavonoid mixtures have also been effective in treating severe stages of chronic venous insufficiency, including venous ulceration and delayed healing 158, 139. In a randomized multicenter trial, 900 mg diosmin and 100 mg hesperidin plus standard venous ulcer management was compared with standard venous ulcer management alone 159. Standard ulcer management included cleaning, compression therapy, and skincare of the adjacent skin. Forty-seven percent of patients in the treatment group compared to 28 percent in the standard management group experienced complete healing of ulcers less than 10 cm in diameter 159.
Hemorrhoids
Several large clinical trials have demonstrated diosmin to be effective in the treatment of acute and chronic symptoms of hemorrhoids. A double-blind, placebo-controlled study of 120 patients showed improvement of pain, pruritis, discharge, edema, erythema, and bleeding on examination 160. The treatment group was given a flavonoid mixture (90% diosmin and 10% hesperidin) at a dose of two 500-mg tablets daily for two months 160.
The use of diosmin in the treatment of hemorrhoids associated with pregnancy did not adversely affect pregnancy, fetal development, birth weight, infant growth, or infant feeding. Pregnant women suffering from acute hemorrhoids were treated eight weeks before delivery and four weeks after delivery 161. More than half of the women participating in the study reported relief from symptoms by the fourth day 161. Diosmin is non-mutagenic and does not have any significant effect on reproductive function 162.
Lymphedema
Diosmin acts on the lymphatic system by increasing lymph flow and lymph oncotic pressure 163. A flavonoid mixture containing diosmin was used to treat upper limb lymphedema secondary to conventional therapy for breast cancer. Results showed improvement of symptoms and limb volume; the mean decrease in volume of the swollen limb reached 6.8 percent 164. In addition, lymphatic functional parameters assessed with scintigraphy were significantly improved. Animal studies of high-protein lymphedema, such as in burns and lung contusions, showed significant improvement with diosmin 165.
Diabetes
Diosmin has been shown to improve factors associated with diabetic complications. Blood parameters of glycation and oxidative stress were measured in type 1 diabetic patients before and after intervention with a diosmin-containing flavonoid mixture 166. A decrease in hemoglobin A1c (HbA1c) was accompanied by an increase in glutathione peroxidase, demonstrating long-term decreased blood glucose levels and increased antioxidant activity 166.
Diosmin can normalize capillary filtration rate and prevent ischemia in diabetics. Rheological studies of type 1 diabetics show diosmin can facilitate hemorheological improvements due to decreased red blood cell aggregation, which decreases blood flow resistance, resulting in reduction of both stasis and ischemia 167.
Cancer
Diosmin has been investigated in a number of animal models and human cancer cell lines and has been found to be chemopreventive and antiproliferative 168. More clinically oriented research in this area is warranted to determine effective dosages and protocols.
Diosmin dosage
The standard dose of diosmin is 500 mg twice daily. For acute dosing, a loading dose of 1,000 mg three times daily for four days is recommended, followed by 1,000 mg twice daily for three days, and a maintenance dose of 500 mg twice daily for two months 169.
Diosmin hesperidin side effects
One of the advantages of hesperidin–therapy is attributed to its safety, non-accumulative nature, and limited adverse effect, even during the pregnancy period 28. Hesperidin has been administered at doses up to 5% with no mutagenic, toxic, and carcinogenic effects on mice, even after 13 weeks 3. Diosmin is considered to have no mutagenic activity, embryo toxicity, nor any significant effect on reproductive function. Transplacental migration and passage into breast milk are minimal 170. Human studies have long shown the safety and good tolerability of hesperidin up to very high doses 9. Results from oral toxicity studies showed the absence of adverse side effects after oral hesperidin ingestion of more than 2 g /kg body weight 171. Furthermore, the oral administration of hesperidin to human caused minor adverse effects in only 10% of the patients 172. Although hesperidin is a safe phytochemical, possible interactions of this phytochemical should be considered. Coadministration of hesperidin with vincristine causes an increase in the drug uptake of this drug, in addition to hesperidin interacting with daunomycin 3. In animal studies, hesperidin showed a good safety profile, with a median lethal dose (LD50) of 4837.5 mg/kg, and in chronic administration up top, 500 mg/kg of the flavanone did not induce any abnormalities in body weight, clinical signs and symptoms and blood parameters 173. LD50 (Lethal dose 50) is the amount of an ingested substance that kills 50 percent of a test sample. It is expressed in mg/kg, or milligrams of substance per kilogram of body weight.
Daflon 500 mg is a marketed tablet dosage form containing a micronized flavonoid mixture of 50 mg of hesperidin (10% hesperidin) and 450 mg of diosmin (90% diosmin) which used as vasoprotective venotonic agent 171. Continues oral administration for hesperidin mixture to rats for 13 and 26 weeks, using a very high dose of 35-fold of the daily dosage showed no toxicity with a high LD50 value of more than 3 g/kg body weight (ie, 180 times the daily therapeutic dose). Clinical trials used more than 2850 patients treated with the hesperidin mixture for a period of 6 weeks to 1 year showed normal hematological parameters, hepatic and renal functions with no signs of toxicity 174.
Drug interactions
Diosmin can cause a decrease in red blood cell aggregation and blood viscosity 175. There are no documented cases of adverse interactions between diosmin and prescription medications, but caution should be taken when combining diosmin with aspirin or other blood-thinning medications.
Data suggest that diosmin has an inhibitory effect on cytochrome P450-mediated metabolism in healthy volunteers, which may alter the pharmacokinetics of drugs taken concomitantly. Patients given metronidazole after nine days of pretreatment with 450 mg diosmin demonstrated changes in serum concentrations of metronidazole, as well as changes in urinary concentrations of metronidazole and its metabolites compared to controls 176.
- Hwang SL, Shih PH, Yen GC. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. 2012 Feb 1;60(4):877-85. doi: 10.1021/jf204452y[↩]
- Bhalla N., Dakwale R. Chemotaxonomy of Indigofera Linn. J. Indian Bot. Soc. 1978;57:180–185.[↩]
- Garg A., Garg S., Zaneveld L., Singla A. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001;15:655–669. doi: 10.1002/ptr.1074[↩][↩][↩][↩][↩]
- Pawloska L. Flavonoids of B. pendula Roth and B. obscura Kot leaves. Acta. Soc. Bot. Pol. 1980;493:281–296.[↩]
- Kokkalou E., Kapetanidis I. Flavonoids of the aerial parts of Acinos suaveolens. Pharm. Acta Helv. 1988;636:170–173.[↩]
- Qin X.-Y., Cheng Y., Yu L.-C. Potential protection of green tea polyphenols against intracellular amyloid beta-induced toxicity on primary cultured prefrontal cortical neurons of rats. Neurosci. Let. 2012;513:170–173. doi: 10.1016/j.neulet.2012.02.029[↩][↩][↩]
- Devi K.P., Rajavel T., Nabavi S.F., Setzer W.N., Ahmadi A., Mansouri K., Nabavi S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crop Prod. 2015;76:582–589. doi: 10.1016/j.indcrop.2015.07.051[↩][↩]
- Grosso G, Galvano F, Mistretta A, Marventano S, Nolfo F, Calabrese G, Buscemi S, Drago F, Veronesi U, Scuderi A. Red orange: experimental models and epidemiological evidence of its benefits on human health. Oxid Med Cell Longev. 2013;2013:157240. doi: 10.1155/2013/157240[↩][↩]
- Meneguzzo F., Ciriminna R., Zabini F., Pagliaro M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes. 2020;8:549. doi: 10.3390/pr8050549[↩][↩][↩]
- Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem. 2006 Jan;70(1):178-92. doi: 10.1271/bbb.70.178[↩]
- Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C. Flavonoid composition of Citrus juices. Molecules. 2007 Aug 3;12(8):1641-73. doi: 10.3390/12081641[↩][↩]
- Garg A, Garg S, Zaneveld LJ, Singla AK.Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 2001;15:655-669.[↩]
- Ikan R. Natural Products: A Laboratory Guide. Elsevier; Amsterdam, The Netherlands: 2013.[↩]
- Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001 Feb;131(2):235-41. doi: 10.1093/jn/131.2.235[↩]
- Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Rémésy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr. 2003 Feb;57(2):235-42. doi: 10.1038/sj.ejcn.1601547[↩][↩]
- Stevens Y, Rymenant EV, Grootaert C, Camp JV, Possemiers S, Masclee A, Jonkers D. The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients. 2019 Jun 27;11(7):1464. doi: 10.3390/nu11071464[↩]
- Pereira-Caro G, Borges G, Ky I, Ribas A, Calani L, Del Rio D, Clifford MN, Roberts SA, Crozier A. In vitro colonic catabolism of orange juice (poly)phenols. Mol Nutr Food Res. 2015 Mar;59(3):465-75. doi: 10.1002/mnfr.201400779[↩]
- Park HK, Kang SW, Park MS. Hesperidin Ameliorates Hepatic Ischemia-Reperfusion Injury in Sprague-Dawley Rats. Transplant Proc. 2019 Oct;51(8):2828-2832. doi: 10.1016/j.transproceed.2019.02.059[↩]
- Iranshahi M, Rezaee R, Parhiz H, Roohbakhsh A, Soltani F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015 Sep 15;137:125-32. doi: 10.1016/j.lfs.2015.07.014[↩]
- Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015 Mar;29(3):323-31. doi: 10.1002/ptr.5256[↩]
- Jeon HJ, Seo MJ, Choi HS, Lee OH, Lee BY. Gelidium elegans, an edible red seaweed, and hesperidin inhibit lipid accumulation and production of reactive oxygen species and reactive nitrogen species in 3T3-L1 and RAW264.7 cells. Phytother Res. 2014 Nov;28(11):1701-9. doi: 10.1002/ptr.5186[↩]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus Flavanones: What Is Their Role in Cardiovascular Protection? J. Agric. Food Chem. 2012, 60, 8809–8822.[↩]
- Mas-Capdevila A, Teichenne J, Domenech-Coca C, Caimari A, Del Bas JM, Escoté X, Crescenti A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients. 2020 May 20;12(5):1488. doi: 10.3390/nu12051488[↩]
- Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015 Mar 1;124:64-74. doi: 10.1016/j.lfs.2014.12.030[↩]
- Stanisic, Danijela & Liu, Letícia & dos Santos, Roney & Costa, Amanda & Duran, Nelson & Tasic, Ljubica. (2020). New Sustainable Process for Hesperidin Isolation and Anti-Ageing Effects of Hesperidin Nanocrystals. Molecules (Basel, Switzerland). 25. 10.3390/molecules25194534[↩][↩][↩][↩]
- Pizzorno J, Murray M. Textbook of Natural Medicine. 2nd ed. New York, NY: Churchill Livingstone; 1999:1393.[↩]
- Philp HA. Hot flashes–a review of the literature on alternative and complementary treatment approaches. Altern Med Rev. 2003 Aug;8(3):284-302. https://altmedrev.com/wp-content/uploads/2019/02/v8-3-284.pdf[↩]
- Hajialyani, M., Hosein Farzaei, M., Echeverría, J., Nabavi, S. M., Uriarte, E., & Sobarzo-Sánchez, E. (2019). Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules (Basel, Switzerland), 24(3), 648. https://doi.org/10.3390/molecules24030648[↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩][↩]
- Tejada S, Pinya S, Martorell M, Capó X, Tur JA, Pons A, Sureda A. Potential Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Curr Med Chem. 2018;25(37):4929-4945. doi: 10.2174/0929867324666170718104412[↩]
- Roohbakhsh A., Parhiz H., Soltani F., Rezaee R., Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124(Suppl. C):64–74. doi: 10.1016/j.lfs.2014.12.030[↩]
- Bartoszewski R., Hering A., Marszałł M., Hajduk J.S., Bartoszewska S., Kapoor N., Kochan K., Ochocka R. Mangiferin has an additive effect on the apoptotic properties of hesperidin in Cyclopia sp. tea extracts. PLoS ONE. 2014;9:e92128. doi: 10.1371/journal.pone.0092128[↩]
- Park H.J., Ra J., Han M.Y., Chung J.H. Hesperidin induces apoptosis in SNU-668, human gastric cancer cells. Mol. Cell. Toxicol. 2007;3:31–35.[↩]
- Park H., Kim M.-J., Ha E., Chung J.-H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine. 2008;15:147–151. doi: 10.1016/j.phymed.2007.07.061[↩]
- Cincin Z.B., Unlu M., Kiran B., Bireller E.S., Baran Y., Cakmakoglu B. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell Oncol. 2015;38:195–204. doi: 10.1007/s13402-015-0222-z[↩]
- Julius A., Vedasendiyar R., Devakannan A., Rajaraman S., Rangasamy B., Saravanan V. Effect of Hesperidin for its Anti-Proliferative Activity on Liver Cancer and Cardio Vascular Diseases. Res. J. Pharm. Technol. 2017;10:307–308. doi: 10.5958/0974-360X.2017.00062.2[↩]
- Febriansah R., Dyaningtyas D.P., Nurulita N.A., Meiyanto E., Nugroho A.E. Hesperidin as a preventive resistance agent in MCF–7 breast cancer cells line resistance to doxorubicin. Asia. Pac. J. Trop. Biomed. 2014;4:228–233. doi: 10.1016/S2221-1691(14)60236-7[↩]
- Al-Jasabi S., Abdullah M. The role of antioxidant hesperidin in the attenuation of lung cancer caused by benzo [a] pyrene in Balb/c mice. World Appl. Sci. J. 2013;22:1106–1110.[↩]
- Tanaka T., Tanaka T., Tanaka M., Kuno T. Cancer chemoprevention by citrus pulp and juices containing high amounts of β-cryptoxanthin and hesperidin. BioMed. Res. Int. 2011;2012 doi: 10.1155/2012/516981[↩]
- Brglez Mojzer E., Knez Hrnčič M., Škerget M., Knez Ž., Bren U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21:901. doi: 10.3390/molecules21070901[↩]
- Son H.-S., Kim H.-S., Ju J.-S. Effects of rutin and hesperidin on total cholesterol concentration, transaminase and alkaline phosphatase activity in CCl4 treated rats. Appl. Biol. Chem. 1991;34:318–326.[↩]
- Choi J.S., Yokozawa T., Oura H. Antihyperlipidemic effect of flavonoids from Prunus davidiana. J. Nat. Prod. 1991;54:218–224. doi: 10.1021/np50073a022[↩]
- Galati E., Monforte M., Kirjavainen S., Forestieri A., Trovato A., Tripodo M. Biological effects of hesperidin, a citrus flavonoid.(Note I): Antiinflammatory and analgesic activity. Farmaco (Societa Chimica Italiana: 1989) 1994;40:709–712.[↩]
- Morita O., Sasaki H., Sato S. Calcium antagonists containing phenols. Pat. Jpn. Kokai Tokkyo Koho. 1992;4:822.[↩]
- Millman N., Rosen F. Failure of phosphorylated hesperidin to influence fertility in rodents. Science. 1953;118:212–213. doi: 10.1126/science.118.3060.212[↩]
- Filho C.B., Fabbro L.D., de Gomes M.G., Goes A.T.R., Souza L.C., Boeira S.P., Jesse C.R. Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test. Eur. J. Pharmacol. 2013;698:286–291. doi: 10.1016/j.ejphar.2012.11.003[↩][↩]
- Thenmozhi A.J., Raja T.R.W., Janakiraman U., Manivasagam T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res. 2015;40:767–776. doi: 10.1007/s11064-015-1525-1[↩][↩]
- Kean R.J., Lamport D.J., Dodd G.F., Freeman J.E., Williams C.M., Ellis J.A., Butler L.T., Spencer J.P. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015;101:506–514. doi: 10.3945/ajcn.114.088518[↩][↩][↩][↩][↩]
- Francis S., Head K., Morris P., Macdonald I. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006;47:2152. doi: 10.1097/00005344-200606001-00018[↩]
- Lamport D.J., Pal D., Macready A.L., Barbosa-Boucas S., Fletcher J.M., Williams C.M., Spencer J.P., Butler L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016;116:2160–2168. doi: 10.1017/S000711451600430X[↩][↩][↩]
- Alharbi M.H., Lamport D.J., Dodd G.F., Saunders C., Harkness L., Butler L.T., Spencer J.P. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016;55:2021–2029. doi: 10.1007/s00394-015-1016-9[↩][↩][↩]
- Zhang S., Tomata Y., Sugiyama K., Sugawara Y., Tsuji I. Citrus consumption and incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Br. J. Nutr. 2017;117:1174–1180. doi: 10.1017/S000711451700109X[↩][↩]
- Klinge CM, Risinger KE, Watts MB, et al. Estrogenic activity in white and red wine extracts. J Agric Food Chem 2003;51:1850-1857.[↩]
- Smith CJ. Non-hormonal control of vasomotor flushing in menopausal patients. Chic Med. 1964 Mar 7;67:193-5.[↩][↩]
- Xiong, H., Wang, J., Ran, Q., Lou, G., Peng, C., Gan, Q., Hu, J., Sun, J., Yao, R., & Huang, Q. (2019). Hesperidin: A Therapeutic Agent For Obesity. Drug design, development and therapy, 13, 3855–3866. https://doi.org/10.2147/DDDT.S227499[↩][↩][↩][↩]
- Dudhia Z, Louw J, Muller C, Joubert E, de Beer D, Kinnear C, Pheiffer C. Cyclopia maculata and Cyclopia subternata (honeybush tea) inhibits adipogenesis in 3T3-L1 pre-adipocytes. Phytomedicine. 2013 Mar 15;20(5):401-8. doi: 10.1016/j.phymed.2012.12.002[↩]
- Assini JM, Mulvihill EE, Huff MW. Citrus flavonoids and lipid metabolism. Curr Opin Lipidol. 2013;24(1):34–40. doi:10.1097/MOL.0b013e32835c07fd[↩]
- Kim HY, Park M, Kim K, Lee YM, Rhyu MR. Hesperetin Stimulates Cholecystokinin Secretion in Enteroendocrine STC-1 Cells. Biomol Ther (Seoul). 2013;21(2):121–125. doi:10.4062/biomolther.2012.077[↩][↩]
- Kamboh AA, Zhu WY. Effect of increasing levels of bioflavonoids in broiler feed on plasma anti-oxidative potential, lipid metabolites, and fatty acid composition of meat. Poult Sci. 2013;92(2):454–461. doi:10.3382/ps.2012-02584[↩][↩]
- Jiao Q, Xu L, Jiang L, Jiang Y, Zhang J, Liu B. Metabolism study of hesperetin and hesperidin in rats by UHPLC-LTQ-Orbitrap MS. Xenobiotica Fate Foreign Compd Biol Sys. 2019;57(18):1–27. doi:10.1080/00498254.2019.1567956[↩]
- Dokumacioglu E, Iskender H, Musmul A. Effect of hesperidin treatment on α-Klotho/FGF-23 pathway in rats with experimentally-induced diabetes. Biomed Pharmacother. 2019;109:1206–1210. doi:10.1016/j.biopha.2018.10.192[↩]
- Pu P. [Protection mechanisms of hesperidin on mouse with insulin resistance]. Zhongguo Zhong Yao Za Zhi. 2016;41(17):3290–3295. doi:10.4268/cjcmm20161728[↩][↩]
- Satoko A, Shin-Ichi K, Kazuharu S, Yoshiko I, Jian W, Mariko U. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr. 2010;46(1):87–92.[↩][↩]
- Do Nascimento GS, Constantin RP, Gilglioni EH. et al. The acute effects of citrus flavanones on the metabolism of glycogen and monosaccharides in the isolated perfused rat liver. Toxicol Lett. 2018;291:S0378427418301279. doi:10.1016/j.toxlet.2018.04.001[↩]
- Zareei S, Boojar MMA, Amanlou M. Inhibition of liver alanine aminotransferase and aspartate aminotransferase by hesperidin and its aglycone hesperetin: an in vitro and in silico study. Life Sci. 2017;178:49–55. doi:10.1016/j.lfs.2017.04.001[↩]
- Mahmoud AM, Ashour MB, Abdel A. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats – journal of diabetes and its complications. J Diabetes Complications. 2012;26(6):483–490. doi:10.1016/j.jdiacomp.2012.06.001[↩]
- Wang X, Hasegawa J, Kitamura Y, et al. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J Pharmacol Sci. 2011;117:129–138. doi:10.1254/jphs.11097FP[↩]
- Mosqueda-Solís A, Sánchez J, Reynés B, et al. Hesperidin and capsaicin, but not the combination, prevent hepatic steatosis and other metabolic syndrome-related alterations in western diet-fed rats. Sci Rep. 2018;8:15100. doi:10.1038/s41598-018-32875-4[↩]
- Mahmoud AM. Hematological alterations in diabetic rats – role of adipocytokines and effect of citrus flavonoids. Excli J. 2013;12:647–657.[↩]
- Qian W, Hasegawa J, Cai X. Components of boiogito suppress the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. Yonago Acta Med. 2016;59(1):67–80.[↩]
- Akiyama S, Katsumata SI, Suzuki K. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem. 2009;73(12):2779–2782. doi:10.1271/bbb.90576[↩]
- Chagwedera DN, Ang QY, Bisanz JE, et al. Nutrient sensing in CD11c cells alters the gut microbiota to regulate food intake and body mass. Cell Metab. 2019. doi:10.1016/j.cmet.2019.05.002[↩]
- Selvaraj P, Pugalendi KV. Efficacy of hesperidin on plasma, heart and liver tissue lipids in rats subjected to isoproterenol-induced cardiotoxicity. Experimental Toxicol Pathol. 2012;64(5):449–452. doi:10.1016/j.etp.2010.10.012[↩]
- Yuce B, Danis O, Sener G, Bulut M, Yarat A. Antioxidative and lipid lowering effects of 7,8-dihydroxy-3- (4-methylphenyl) coumarin in hyperlipidemic rats. Arzneimittelforschung. 2009;59(03):129–134. doi:10.1055/s-0031-1296375[↩]
- Parmar M, Syed I, Gray J. Curcumin, hesperidin, and rutin selectively interfere with apoptosis signaling and attenuate streptozotocin-induced oxidative stress-mediated hyperglycemia. <![CDATA[Current Neurovascular Research]]>. 2015;12(4):363–374. doi:10.2174/1567202612666150812150249[↩]
- Ferreira PS, Spolidorio LC, Manthey JA. Citrus flavanones prevent systemic inflammation and ameliorate oxidative stress in C57BL/6J mice fed high-fat diet. Food Funct. 2016;7(6):2675–2681. doi:10.1039/C5FO01541C[↩]
- Shin EJ, Hur HJ, Sung MJ. Ethanol extract of the Prunus mume fruits stimulates glucose uptake by regulating PPAR-γ in C2C12 myotubes and ameliorates glucose intolerance and fat accumulation in mice fed a high-fat diet. Food Chem. 2013;141(4):4115–4121. doi:10.1016/j.foodchem.2013.06.059[↩]
- Gómez-Zorita S, Lasa A, Abendaño N. Phenolic compounds apigenin, hesperidin and kaempferol reduce in vitro lipid accumulation in human adipocytes. J Transl Med. 2017;15(1):237. doi:10.1186/s12967-017-1343-0[↩]
- Mosqueda-Solís A, Lasa A, Gómez-Zorita S, Eseberri I, Picó C, Portillo MP. Screening of potential anti-adipogenic effects of phenolic compounds showing different chemical structure in 3T3-L1 preadipocytes. Food Funct. 2017;8(10):3576–3586. doi:10.1039/C7FO00679A[↩]
- Jeon H-J, Seo M-J, Choi H-S. Gelidium elegans, an edible red seaweed, and hesperidin inhibit lipid accumulation and production of reactive oxygen species and reactive nitrogen species in 3T3-L1 and RAW264.7 cells. Phytother Res. 2014;28(11):1701–1709. doi:10.1002/ptr.5186[↩]
- Lim H, Yeo E, Song E, et al. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr Res Pract. 2015;9(6):599–605. doi:10.4162/nrp.2015.9.6.599[↩]
- Aranaz P, Navarro-Herrera D, Zabala M, et al. Phenolic compounds inhibit 3T3-L1 adipogenesis depending on the stage of differentiation and their binding affinity to PPARγ. Molecules. 2019;24(6):1045. doi:10.3390/molecules24061045[↩]
- Nakajima VM, Moala T, Caria C, et al. Biotransformed citrus extract as a source of anti-inflammatory polyphenols: effects in macrophages and adipocytes. Food Res Int. 2017;97:37–44. doi:10.1016/j.foodres.2017.03.034[↩]
- Liu W, Shorong-Shii L, Tang-Yao H, I-Min L. Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients. 2017;9(12):1312. doi:10.3390/nu9121312[↩]
- Su D, Liu H, Qi X, Dong L, Zhang R, Zhang J. Citrus peel flavonoids improve lipid metabolism by inhibiting miR-33 and miR-122 expression in HepG2 cells. Biosci Biotechnol Biochem. 2019;83(9):1–9. doi:10.1080/09168451.2019.1608807[↩]
- Shen W, Xu Y, Lu Y-H. Inhibitory effects of citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J Agric Food Chem. 2012;60(38):9609–9619. doi:10.1021/jf3032556[↩]
- Peng H, Wei Z, Luo H. Inhibition of fat accumulation by hesperidin in caenorhabditis elegans. J Agric Food Chem. 2016;64(25):5207–5214. doi:10.1021/acs.jafc.6b02183[↩]
- Guo, C.; Zhang, H.; Guan, X.; Zhou, Z. The Anti-Aging Potential of Neohesperidin and Its Synergistic Effects with Other Citrus Flavonoids in Extending Chronological Lifespan of Saccharomyces Cerevisiae BY4742. Molecules 2019,24, 4093.[↩]
- Lee, H.J.; Im, A.-R.; Kim, S.-M.; Kang, H.-S.; Lee, J.D.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Complem. Altern. Med.2018, 18, 1–20.[↩][↩]
- Man, M.-Q.; Yang, B.; Elias, P.M. Benefits of Hesperidin for Cutaneous Functions. Evid. Based Complementary Altern. Med.2019, 2019, 2676307.[↩][↩]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid Hsd. Phytother. Res. 2001,15, 655–669.[↩]
- Saija, A.; Tomaino, A.; Trombetta, D.; Giacchi, M.; Pasquale, A.; de Bonina, F. Influence of different penetration enhancers on in vitro skin permeation and in vivo photoprotective effect of flavonoids. Int. J. Pharm. 1998,175, 85–94.[↩]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002,13, 572–584.[↩]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Cosmetology, 4th ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2014.[↩]
- Duran, N.; Costa, A.F.; Stanisic, D.; Bernardes, J.S.; Tasic, L. Nanotoxicity and Dermal Application of Nanostructured Lipid Carrier Loaded with Hesperidin from Orange Residue. J. Phys. Conf. Ser. 2019, 1323, 012021.[↩]
- Mottay D., Neergheen-Bhujun V.S. Anticholinesterase and antioxidant effects of traditional herbal medicines used in the management of neurodegenerative diseases in mauritius. Arch. Med. Biomed. Res. 2015;2:114–130. doi: 10.4314/ambr.v2i4.2[↩]
- Girdhar S., Girdhar A., Verma S.K., Lather V., Pandita D. Plant derived alkaloids in major neurodegenerative diseases: From animal models to clinical trials. J. Ayurved. Herb. Med. 2015;1:91–100.[↩]
- Kivrak İ., Duru M.E., Öztürk M., Mercan N., Harmandar M., Topçu G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009;116:470–479. doi: 10.1016/j.foodchem.2009.02.069[↩]
- Antunes M.S., Goes A.T.R., Boeira S.P., Prigol M., Jesse C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30:1415–1422. doi: 10.1016/j.nut.2014.03.024[↩][↩]
- Salem H.R.A., El-Raouf A., Saleh E.M., Shalaby K.A. Influence of hesperidin combined with Sinemet on genetical and biochemical abnormalities in rats suffering from Parkinson’s disease. Life. Sci. J. 2012;9:930–945.[↩][↩]
- Nagappan P., Krishnamurthy V., Sereen K. Investigation of the neuroprotective effect of hesperidin on behavioural activities in 6-OHDA induced Parkinson model. Int. J. Pharm. Biol. Sci. 2014:570–577.[↩][↩]
- Tamilselvam K., Nataraj J., Janakiraman U., Manivasagam T., Essa M.M. Antioxidant and anti-inflammatory potential of hesperidin against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced experimental Parkinson’s disease in mice. Int. J. Nutr. Pharm. Neurol. Dis. 2013;3:294.[↩]
- Nagappan P., Krishnamurthy V. Anti-Parkinson Effect of Hesperidin in Combination with L-DOPA on 6-OHDA Induced Parkinsonism in Wistar Rats-A Neurochemical, Histopathological and Immunohistochemical Analysis. Int. J. Pharm. Tech. Res. 2016;9:266–273.[↩]
- Santos G., Giraldez-Alvarez L.D., Ávila-Rodriguez M., Capani F., Galembeck E., Neto A.G., Barreto G.E., Andrade B. SUR1 Receptor Interaction with Hesperidin and Linarin Predicts Possible Mechanisms of Action of Valeriana officinalis in Parkinson. Front. Aging Neurosci. 2016;2:97. doi: 10.3389/fnagi.2016.00097[↩]
- Jiménez-Aliaga K., Bermejo-Bescós P., Benedí J., Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life. Sci. 2011;89:939–945. doi: 10.1016/j.lfs.2011.09.023[↩]
- Wang D., Liu L., Zhu X., Wu W., Wang Y. Hesperidin Alleviates Cognitive Impairment, Mitochondrial Dysfunction and Oxidative Stress in a Mouse Model of Alzheimer’s Disease. Cell Mol. Neurobiol. 2014;34:1209–1221. doi: 10.1007/s10571-014-0098-x[↩][↩]
- Badalzadeh R., Mohammadi M., Yousefi B., Farajnia S., Najafi M., Mohammadi S. Involvement of glycogen synthase kinase-3β and oxidation status in the loss of cardioprotection by postconditioning in chronic diabetic male rats. Adv. Pharm. Bull. 2015;5:321. doi: 10.15171/apb.2015.045[↩]
- Justin Thenmozhi A., William Raja T.R., Manivasagam T., Janakiraman U., Essa M.M. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr. Neurosci. 2017;20:360–368. doi: 10.1080/1028415X.2016.1144846[↩]
- Li C., Zug C., Qu H., Schluesener H., Zhang Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015;281(Suppl. C):32–42. doi: 10.1016/j.bbr.2014.12.012[↩]
- Ghorbani A., Nazari M., Jeddi-Tehrani M., Zand H. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: Involvement of PPARγ-dependent mechanism. Eur. J. Nutr. 2012;51:39–46. doi: 10.1007/s00394-011-0187-2[↩]
- Gray C.W., Patel A.J. Regulation of β-amyloid precursor protein isoform mRNAs by transforming growth factor-β1 and interleukin-1β in astrocytes. Mol. Brain Res. 1993;19:251–256. doi: 10.1016/0169-328X(93)90037-P[↩]
- Javed H., Vaibhav K., Ahmed M.E., Khan A., Tabassum R., Islam F., Safhi M.M., Islam F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J. Neurol. Sci. 2015;348:51–59. doi: 10.1016/j.jns.2014.10.044[↩]
- Hemanth Kumar B., Dinesh Kumar B., Diwan P.V. Protective effects of natural dietary antioxidants fisetin and hesperidin on chronic mild hyperhomocysteinemia-induced vascular dementia in wistar rats. J. Neurol. Sci. 2017;381:319. doi: 10.1016/j.jns.2017.08.905[↩]
- Habibyar A.F., Sharma N., Khurana N. PASS assisted prediction and pharmacological evaluation of hesperidin against scopolamine induced amnesia in mice. Eur. J. Pharmacol. 2016;789(Suppl. C):385–394. doi: 10.1016/j.ejphar.2016.07.013[↩]
- Rosas H.D., Lee S.Y., Bender A.C., Zaleta A.K., Vangel M., Yu P., Fischl B., Pappu V., Onorato C., Cha J.-H. Altered white matter microstructure in the corpus callosum in Huntington’s disease: Implications for cortical “disconnection” Neuroimage. 2010;49:2995–3004. doi: 10.1016/j.neuroimage.2009.10.015[↩]
- Kumar P., Kumar A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington’s like symptoms in rats: Possible role of nitric oxide. Behav. Brain Res. 2010;206:38–46. doi: 10.1016/j.bbr.2009.08.028[↩]
- Menze E.T., Tadros M.G., Abdel-Tawab A.M., Khalifa A.E. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. Neurotoxicology. 2012;33:1265–1275. doi: 10.1016/j.neuro.2012.07.007[↩][↩]
- Coull J.A.M., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M.W., De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223[↩]
- Kumar A., Chaudhary T., Mishra J. Minocycline modulates neuroprotective effect of hesperidin against quinolinic acid induced Huntington’s disease like symptoms in rats: Behavioral, biochemical, cellular and histological evidences. Eur. J. Pharmacol. 2013;720:16–28. doi: 10.1016/j.ejphar.2013.10.057[↩]
- Ontaneda D., Thompson A.J., Fox R.J., Cohen J.A. Progressive multiple sclerosis: Prospects for disease therapy, repair, and restoration of function. Lancet. 2017;389:1357–1366. doi: 10.1016/S0140-6736(16)31320-4[↩]
- Farzaei M.H., Shahpiri Z., Bahramsoltani R., Najafi F., Rahimi R. Efficacy and Tolerability of Phytomedicines in Multiple Sclerosis Patients: A Review. CNS Drugs. 2017;31:867–889. doi: 10.1007/s40263-017-0466-4[↩]
- Muili K.A., Gopalakrishnan S., Meyer S.L., Eells J.T., Lyons J.-A. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS ONE. 2012;7:e30655. doi: 10.1371/journal.pone.0030655[↩]
- Haghmorad D., Mahmoudi M.B., Salehipour Z., Jalayer Z., Rastin M., Kokhaei P., Mahmoudi M. Hesperidin ameliorates immunological outcome and reduces neuroinflammation in the mouse model of multiple sclerosis. J. Neuroimmun. 2017;302:23–33. doi: 10.1016/j.jneuroim.2016.11.009[↩]
- Ciftci O., Ozcan C., Kamisli O., Cetin A., Basak N., Aytac B. Hesperidin, a citrus flavonoid, has the ameliorative effects against experimental autoimmune encephalomyelitis (EAE) in a C57BL/J6 mouse model. Neurochem. Res. 2015;40:1111–1120. doi: 10.1007/s11064-015-1571-8[↩][↩]
- Ahtiluoto S., Polvikoski T., Peltonen M., Solomon A., Tuomilehto J., Winblad B., Sulkava R., Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia A population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8[↩]
- Craft S. Alzheimer disease: Insulin resistance and AD–extending the translational path. Nat. Rev. Neurol. 2012;8:360–362. doi: 10.1038/nrneurol.2012.112[↩]
- Khowal S., Mustufa M.M., Chaudhary N.K., Naqvi S.H., Parvez S., Jain S.K., Wajid S. Assessment of the therapeutic potential of hesperidin and proteomic resolution of diabetes-mediated neuronal fluctuations expediting Alzheimer’s disease. RSC Adv. 2015;5:46965–46980. doi: 10.1039/C5RA01977J[↩][↩]
- Ashafaq M., Varshney L., Khan M.H.A., Salman M., Naseem M., Wajid S., Parvez S. Neuromodulatory effects of hesperidin in mitigating oxidative stress in streptozotocin induced diabetes. BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/249031[↩][↩]
- Ibrahim S.S. Protective effect of hesperidin, a citrus bioflavonoid, on diabetes-induced brain damage in rats. J. Appl. Sci. Res. 2008;4:84.[↩]
- Visnagri A., Kandhare A.D., Chakravarty S., Ghosh P., Bodhankar S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014;52:814–828. doi: 10.3109/13880209.2013.870584[↩][↩][↩][↩]
- Miranda H., Sierralta F., Jorquera V., Poblete P., Prieto J., Noriega V. Antinociceptive interaction of gabapentin with minocycline in murine diabetic neuropathy. Inflammopharmacology. 2017;25:91–97. doi: 10.1007/s10787-017-0308-5[↩]
- Gustafson-Vickers S.L., Lu V.B., Lai A.Y., Todd K.G., Ballanyi K., Smith P.A. Long-term actions of interleukin-1β on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. Mol. Pain. 2008;4:63. doi: 10.1186/1744-8069-4-63[↩]
- Nogata Y., Sakamoto K., Shiratsuchi H., Ishii T., Yano M., Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006;70:178–192. doi: 10.1271/bbb.70.178[↩]
- Pari L., Srinivasan S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010;64:477–481. doi: 10.1016/j.biopha.2010.02.001[↩][↩]
- Maksimovic Z.V., Maksimovic M., Jadranin D., Kuzmanovic I., Andonovic O. Medicamentous treatment of chronic venous insufficiency using semisynthetic diosmin—A prospective study. Acta Chir. Iugosl. 2008;55:53–59. doi: 10.2298/ACI0804053M[↩]
- Firuzi O., Miri R., Tavakkoli M., Saso L. Antioxidant therapy: Current status and future prospects. Curr. Med. Chem. 2011;18:3871–3888. doi: 10.2174/092986711803414368[↩][↩]
- Srinivasan S., Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem. Biol. Interact. 2012;195:43–51. doi: 10.1016/j.cbi.2011.10.003[↩]
- Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018 Apr;37(2):143-154. doi: 10.23736/S0392-9590.18.03975-5[↩]
- Ramelet AA. Clinical benefits of Daflon 500mg in the most severe stages of chronic venous insufficiency. Angiology 2001;52:S49-S56.[↩][↩][↩]
- Bergan JJ, Schmid-Schonbein GW, Takase S.Therapeutic approach to chronic venousinsufficiency and its complications: place of Daflon 500 mg. Angiology 2001;52:S43-S47.[↩][↩]
- Manthey JA. Biological properties of flavonoids pertaining to inflammation. Microcirculation 2000;7:S29-S34.[↩]
- Hasanoglu A, Ara C, Ozen S, et al. Efficacy of micronized flavonoid fraction in healing of clean and infected wounds. Int J Angiol 2001;10:41-44.[↩]
- Serfaty D, Magneron AC. Premenstrualsyndrome in France: epidemiology and therapeutic effectiveness of 1000 mg of micronized purified flavonoid fraction in 1,473 gynecological patients. Contracept Fertil Sex 1997;25:85-90. [Article in French][↩]
- Meggiorini ML, Cascialli GL, Luciani S, et al. Randomized study of the use of synthetic diosmin in premenstrual and vascular dysplastic mastodynia. Minerva Ginecol 1990;42:421-425. [Article in Italian][↩]
- Bae EA, Han MJ, Lee M, Kim DH. In vitro inhibitory effect of some flavonoids on rotavirus infectivity. Biol Pharm Bull 2000;23:1122-1124.[↩]
- Crespo ME, Galvez J, Cruz T, et al. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med 1999;65:651-653.[↩]
- Lyseng-Williamson KA, Perry CM.Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003;63:71-100.[↩]
- Villa P, Cova D, De Francesco L, Guaitani A, Palladini G, Perego R. Protective effect of diosmetin on in vitro cell membrane damage and oxidative stress in cultured rat hepatocytes. Toxicology. 1992;73(2):179-89. doi: 10.1016/0300-483x(92)90101-j[↩]
- Guilhou JJ, Dereure O, Marzin L, Ouvry P, Zuccarelli F, Debure C, Van Landuyt H, Gillet-Terver MN, Guillot B, Levesque H, Mignot J, Pillion G, Février B, Dubeaux D. Efficacy of Daflon 500 mg in venous leg ulcer healing: a double-blind, randomized, controlled versus placebo trial in 107 patients. Angiology. 1997 Jan;48(1):77-85. doi: 10.1177/000331979704800113[↩]
- Diana G, Catanzaro M, Ferrara A, Ferrari P. Attività della diosmina pura nel trattamento della malattia emorroidaria [Activity of purified diosmin in the treatment of hemorrhoids]. Clin Ter. 2000 Sep-Oct;151(5):341-4. Italian.[↩]
- Ahmed S, Mundhe N, Borgohain M, Chowdhury L, Kwatra M, Bolshette N, Ahmed A, Lahkar M. Diosmin Modulates the NF-kB Signal Transduction Pathways and Downregulation of Various Oxidative Stress Markers in Alloxan-Induced Diabetic Nephropathy. Inflammation. 2016 Oct;39(5):1783-97. doi: 10.1007/s10753-016-0413-4[↩]
- Arab HH, Salama SA, Omar HA, Arafa el-SA, Maghrabi IA. Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS One. 2015 Mar 30;10(3):e0122417. doi: 10.1371/journal.pone.0122417[↩]
- Maksimovic Z.V., Maksimovic M., Jadranin D., Kuzmanovic I., Andonovic O. Medicamentous treatment of chronic venous insufficiency using semisynthetic diosmin: A prospective study. Acta Chir. Iugosl. 2008;55:53–59. doi: 10.2298/ACI0804053M[↩]
- Rapavi E., Kocsis I., Fehér E., Szentmihályi K., Lugasi A., Székely E., Blázovics A. The effect of citrus flavonoids on the redox state of alimentary-induced fatty liver in rats. Nat. Prod. Res. 2007;21:274–281. doi: 10.1080/14786410500518545[↩]
- Perumal S, Langeshwaran K, Selvaraj J, Ponnulakshmi R, Shyamaladevi B, Balasubramanian MP. Effect of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Mol Cell Biochem. 2018 Dec;449(1-2):27-37. doi: 10.1007/s11010-018-3339-3[↩]
- Kanaze F. I., Bounartzi M. I., Niopas I. A validated HPLC determination of the flavone aglycone diosmetin in human plasma. Biomed. Chromatogr. 2004;18:800–804. doi: 10.1002/bmc.391[↩]
- Mastuda H., Morikawa T., Ueda K., Managi H., Yoshikawa M. Structural requirements of flavonoids for inhibition of antigen-Induced degranulation, TNF-alpha and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2002;10:3123–3128. doi: 10.1016/S0968-0896(02)00227-4[↩]
- Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assEssment and quaLity of lIfe improvEment with micronized Flavonoids. Angiology. 2002 May-Jun;53(3):245-56. doi: 10.1177/000331970205300301[↩][↩]
- Ramelet AA. Clinical benefits of Daflon 500mg in the most severe stages of chronic venousinsufficiency. Angiology 2001;52:S49-S56.[↩]
- Glinski W, Chodynicka B, Roszkiewicz J, etal. The beneficial augmentative effect of micronised purified flavonoid fraction (MPFF) on the healing of leg ulcers: an open, multicenter, controlled, randomized study. Phlebology 1999; 14:151-157.[↩][↩]
- Godeberge P. Daflon 500 mg in the treatmentof hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology 1994;45:574-578.[↩][↩]
- Buckshee K, Takkar D, Aggarwal N. Micronized flavonoid therapy in internal hemorrhoids of pregnancy. Int J Gynaecol Obstet 1997;57:145-151.[↩][↩]
- Meyer OC. Safety and security of Daflon 500mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994; 45:579-584.[↩]
- Pecking AP, Fevrier B, Wargon C, Pillion G. Efficacy of Daflon 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer). Angiology 1997;48:93-98.[↩]
- Pecking AP. Evaluation by lymphoscintigraphy of the effect of a micronized flavonoid fraction (Daflon 500 mg) in the treatment of upper limb lymphedema. Int Angiology 1995;14:39-43.[↩]
- Casley-Smith JR, Casley-Smith JR. The effectsof diosmin (a benzo-pyrone) upon some high-protein oedemas: lung contusion, and burn and lymphoedema of rat legs. Agents Actions 1985;17:14-20.[↩]
- Manuel Y, Keenoy B, Vertommen J, De LeeuwI. The effect of flavonoid treatment on the glycation and antioxidant status in type 1 diabetic patients. Diabetes Nutr Metab 1999;12:256-263.[↩][↩]
- Valensi PE, Behar A, de Champvallins MM, etal. Effects of a purified micronized flavonoid fraction on capillary filtration in diabetic patients. Diabet Med 1996;13:882-888.[↩]
- Kuntz S, Wenzel U, Daniel H. Comparativeanalysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr 1999;38:133-142.[↩]
- Diosmin. Alternative Medicine Review Volume 9, Number 3, 2004. https://altmedrev.com/wp-content/uploads/2019/02/v9-3-308.pdf[↩]
- Meyer OC. Safety and security of Daflon 500mg in venous insufficiency and in hemorrhoidal disease. Angiology 1994;45:579-584.[↩]
- Nagasako-Akazome Y. Chapter 58 – Safety of high and long-term intake of polyphenols. In: Watson R.R., Preedy V.R., Zibadi S., editors. Polyphenols in Human Health and Disease. Academic Press; San Diego: 2014. pp. 747–756.[↩][↩]
- Meyer O.C. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology. 1994;45:579–584. doi: 10.1177/000331979404500614[↩]
- Li Y, Kandhare AD, Mukherjee AA, Bodhankar SL. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul Toxicol Pharmacol. 2019 Jul;105:77-85. doi: 10.1016/j.yrtph.2019.04.001[↩]
- Meyer OC. Safety and security of Daflon 500 mg in venous insufficiency and in hemorrhoidal disease. Angiology. 1994 Jun;45(6 Pt 2):579-84. doi: 10.1177/000331979404500614[↩]
- Lacombe C, Bucherer C, Lelievre JC. Hemorheological improvement after Daflon 500 mg treatment in diabetes. Int Angiol 1988;7:21-24.[↩]
- Rajnarayana K, Reddy MS, Krishna DR. Diosmin pretreatment affects bioavailability of metronidazole. Eur J Clin Pharmacol 2003;58:803-807.[↩]